Radiolabeled theranostics: magnetic and gold nanoparticles
Review
Blood-brain barrier transport machineries and targeted therapy of brain diseases
Jaleh Barar1,2,
Mohammad A. Rafi3,
Mohammad M. Pourseif1,
Yadollah Omidi1,2,*
1
Research Center for Pharmaceutical Nanotechnology, Tabriz University of Medical Sciences, Tabriz, Iran
2
Department of Pharmaceutics, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
3
Department of Neurology, Sidney Kimmel College of Medicine, Thomas Jefferson University, Philadelphia, PA, USA
*Corresponding author: Yadollah Omidi, Email:yomidi@tbzmed.ac.ir
© 2016 The Author(s). This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by-nc/4.0). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Abstract
Introduction:
Desired clinical outcome of pharmacotherapy of brain diseases largely depends upon the safe drug delivery into the brain parenchyma. However, due to the robust blockade function of the blood-brain barrier (BBB), drug transport into the brain is selectively controlled by the BBB formed by brain capillary endothelial cells and supported by astrocytes and pericytes.
Methods:
In the current study, we have reviewed the most recent literature on the subject to provide an insight upon the role and impacts of BBB on brain drug delivery and targeting.
Results:
All drugs, either small molecules or macromolecules, designated to treat brain diseases must adequately cross the BBB to provide their therapeutic properties on biological targets within the central nervous system (CNS). However, most of these pharmaceuticals do not sufficiently penetrate into CNS, failing to meet the intended therapeutic outcomes. Most lipophilic drugs capable of penetrating BBB are prone to the efflux functionality of BBB. In contrast, all hydrophilic drugs are facing severe infiltration blockage imposed by the tight cellular junctions of the BBB. Hence, a number of strategies have been devised to improve the efficiency of brain drug delivery and targeted therapy of CNS disorders using multimodal nanosystems (NSs).
Conclusions:
In order to improve the therapeutic outcomes of CNS drug transfer and targeted delivery, the discriminatory permeability of BBB needs to be taken under control. The carrier-mediated transport machineries of brain capillary endothelial cells (BCECs) can be exploited for the discovery, development and delivery of small molecules into the brain. Further, the receptor-mediated transport systems can be recruited for the delivery of macromolecular biologics and multimodal NSs into the brain.
Keywords: Blood-brain barrier, Brain drug delivery, Brain drug targeting, Carrier-mediated transport, Endocytosis, Receptor-mediated transport, Transcytosis
Introduction
Microcirculation of nutrients and drugs into the brain is selectively controlled by a unique biological barrier called blood-brain barrier (BBB). Such biological barrier is formed by the brain capillary endothelial cells (BCECs) whose maturation and functions is largely dependent upon intercellular interaction with brain astrocytes and pericytes.1-3 Of the BBB cooperating cells, the astrocytes are members of the glial family, which are able to interact with neurons and other brain cells (e.g., microglia and endothelial cells) directly and/or indirectly through the exchange of soluble materials. At the BBB, the astrocytic foot process covers 99% of the abluminal surface of the capillary basement membrane, while the brain capillary pericytes share the basement membrane with BCECs (Fig. 1). The BCECs form a co-operating complex with astrocytes and pericytes (the “Neurovascular Unit”) to: (a) maintain brain homeostasis, (b) selectively control the delivery of nutrients and blood-borne solutes to the brain parenchyma.1 In addition to other modulation roles of astrocytes and pericytes on BCECs (e.g., restrictive tight junction (TJ) regulation and differentiation), they are also involved in modulating the functional expression of transport machineries required for the selective inward/outward transportation of nutrients and drugs.4

Fig. 1. Schematic representation of the blood-brain barrier, astrocytes and pericytes. Image was adapted with permission from our previously published work.1 Note: Not drawn to scale.
In contrast polar hydrophilic small molecules (e.g., amino acids and carbohydrates) exploit the facilitative or passive SLC transport systems, while macromolecular biomolecules such as insulin, transferrin (Tf) and targeted nanoscale drug delivery systems (DDSs) use VTS pathways potentially involving a receptor-mediated transporter (RMT).8,9
In addition, it extracellular vesicles (exosomes and ectosomes) are engaged in macromolecular trafficking and cellular communications and can be used for targeted therapy of diseases such as CNS disorders.10-14
In this current review article, we provide an overview on the biological features of BBB relevant to brain drug delivery and targeting by highlighting the potential therapeutic importance of BBB transport machinery.
Experimental models for BBB
In order to evaluate the specific functions of BBB, in vivo and in vitro as well as in silico approaches have been employed by a number of research groups. Although the in vivo investigations provides much more reliable and robust physiological context measures of BBB functions, conducting animal based studies may encounter with some pivotal limitations (e.g., ethical issues of animal uses, cost and time restrictions, need for genetically modified animal models). Therefore, in vitro cell systems can serve as a complementray model to reduce and maybe refine later in-vivo investigations.15 Cell-based models have capacity to provide relatively high trans-endothelial electrical resistance (TEER) values (i.e., about 1000 Ω.cm2), which offer some discrimination between transcellular and paracellular routes of permeation. We have previously examined several cell culture systems and found that the isolated primary cultures of brain capillary endothelial cells from porcine and bovine offer reliable outcomes.16-19, Indeed the ability to use primary cell models or at the very least early low passage cells appears particularly important in BBB investigations. Clearly for human cell work much potential may arise through developments with induced pluripotent stem cells (iPSC).20 Beyond permeation per se, cell models offer the potential for mechanistic transporter investigations.18,21
Brain drug delivery
A large number of studies on brain drug delivery and targeting, using in vitro cell-based BBB models15-19 as well as animal models, have sought to address how the brain capillary endothelium can be safely and efficiently crossed by experimental therapeutics and DDS. As shown in Fig. 2, the blood-borne substances, nutrients and drugs can enter into the CNS through one or more pathways each of which has been explored as a mean to overcome the BBB restrictiveness. The penetration of drugs into the brain may occur by following routs:

Fig. 2. Different mechanisms of drug trafficking from blood into the brain. Image was adapted with permission from our previously published work.1 Note: Not drawn to scale.
transcellular passive diffusion that is a primary route for small lipophilic substances, which could be optimized by altering drug physicochemical properties,
SLC-mediated transport exploiting the diverse members of the SLC family of transporters preset within the BBB (e.g., various amino acids),8,26
endocytosis through RMT (e.g., monoclonal antibodies (mAbs) specific to various receptors at the BBB like Tf receptor, targeted nanomedicines),27-29 and
adsorptive endocytosis or pinocytosis (e.g., albumin) a relatively low capacity route with non-receptor bound material shuttled to the lysosomes for degradation.
It should be noted that conjugation of a drug with a targeting peptide/protein or with an antibody (Ab)/aptamer (Ap) provides nanoconjugates that can specifically shuttle the conjugated drugs into the brain through RMTs.30,31
Besides, targeted nanosystems (NSs) such as polymeric/lipidic nanoparticles (NPs) decorated with homing entities also provide robust tools for crossing BBB that can be used to treat life-threatening diseases such as glioma.32-34
ABC transporters
The direct energy-dependent ABC active transporters, are primarily localized on the luminal membrane of brain microvasculatur cells. ABC transporters possess a common structure of transmembrane domains (TMDs) and cytosolic nucleotide binding domains (NBDs). The ABC superfamily encompasses about 48 distinct transporters categorized into 7 families (ABC-A,-B,- C,-D,-E,-F, and G). Of these, the most investigated transporter in the BBB is that coded by the ABCB1 gene, ie. P-glycoprotein (P-gp or MDR1) (Fig. 3). As a phosphorylated glycoprotein (170 kDa), it plays extremely important role in tumor cells resulting in their resistance to anticancer drugs by effluxing xenobiotics and metabolites out of the cell. P-gp is expressed in both epithelial and endothelial cells that are involved in formation of biological barriers such as BBB. Table 1 represents ABC transporters function and clinical impacts. The functional expression of these transport machineries play key roles in the maintenance of brain homeostasis, in which traverse of blood-borne substances should be specifically regulated.
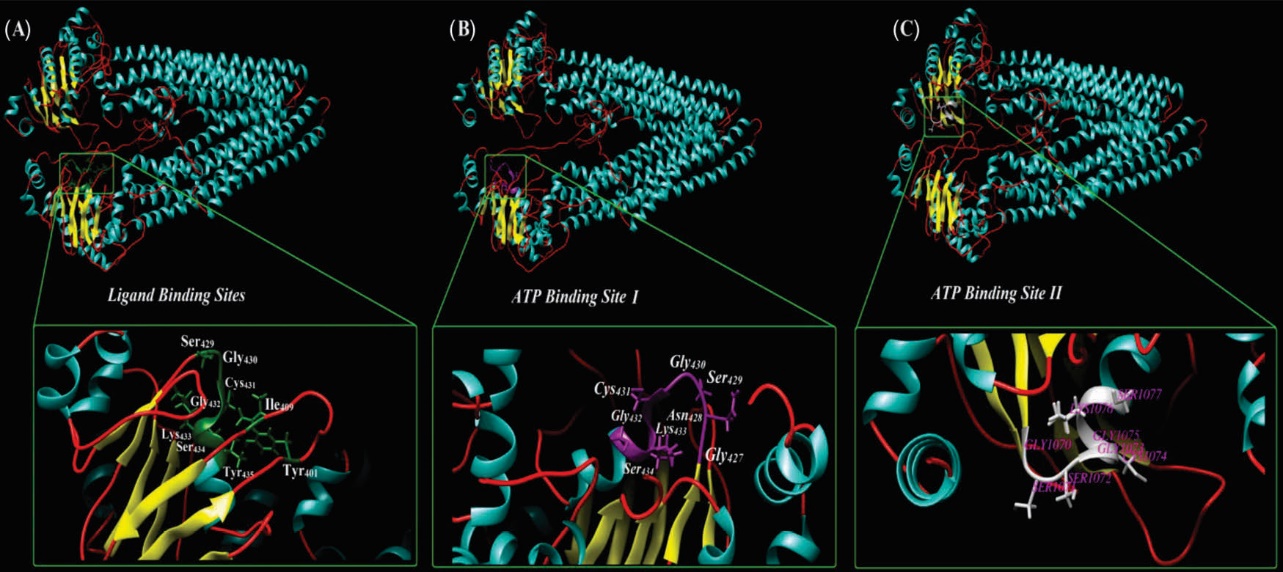
Fig. 3. Ribbon style representation of the modeled multidrug resistance protein 1 (MDR1). (A) The residues that are correlated ligand binding site are magnified. Panel B and C show the two ATP-specific binding sites in MDR1 structure. The UCSF Chimera v1.11 standalone software (freely available at: http://www.rbvi.ucsf.edu/chimera) and Visual Molecular Dynamics (VMD) (freely available at: https://www-s.ks.uiuc.edu/Research/vmd/) were used for visualization of MDR1.
|
Table 1. ATP-binding cassette transporters’ functions and clinical significances
|
|
Family
|
Members
|
Function
|
Clinical relevance
|
| ABCA |
Subgroup I: ABCA1, 2, 3, 4, 7, 12, and 13
Subgroup II: ABCA5, 6, 8, 9 and 10
|
Transportation of cholesterol and lipids |
Mutation in ABCA1 causes Tangier’s disease (hypoalphalipoproteinemia)
35
; Mutation in ABCA3 are associated to cataract-microcornea syndrome
36
Mutation in ABCA4 causes autosomal-recessive disease Stargardt macular dystrophy (STGD)
37
; ABCA7 involves in Alzheimer's disease
38
; Mutation in ABCA5 cause excessive hair overgrowth
39
; Mutations in ABCA12 cause severe congenital skin disease harlequin ichthyosis
40
|
| ABCB |
ABCB1 (multidrug resistance protein 1 (MDR1)/CD243, the so-called P-glycoprotein 1 (P-gp) |
Export of divers xenobiotics and toxic metabolites |
P-gp transports various substrates across the BBB |
| ABCC |
ABCC also known as multidrug resistance proteins (MRPs) members are MRP1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 and 13 |
Anions and conjugated metabolites (e.g., glucuronides, glutathione) efflux |
Involved in ion transport, toxin secretion, and signal transduction at BBB (MRP1, 2, 4, 5, 6); Deficiency in functional expression of ABCC7 (CFTR protein) causes cystic fibrosis
41
; Overexpression of MRP2 at BBB contributes to drug resistance in epilepsy
42
; MRP4 involves in efflux of oseltamivir
43
|
| ABCD |
ABCD1 |
Transportation of long chain fatty acids |
Mutations in ABCD1 cause accumulation of very long chain fatty acids; ABCD1 dysfunction at BBB contributes to the increased trafficking of leucocytes across the BBB as seen in cerebral adrenouleukodystrophy
44
|
| ABCE |
ABCE1 |
ABCE1 inhibits the action of ribonuclease L |
Overexpression of ABCE1 promotes growth and inhibits apoptosis of tumor cells
45
|
| ABCF |
ABCF1 |
Export of some drugs |
ABCF1 associates with Graves' ophthalmopathy
46
; ABCF1 may play a role in mediating antidepressant response
47
|
| ABCG |
ABCG1, 2, 3 and 4 |
Transportation of lipids, steroids, cholesterol and different drugs |
Loss of ABCG1/ABCG4 functions in the CNS alters metabolic pathways and elicits behavior changes
48
; Functional expression of ABCG2 at BBB results in markedly high efflux of divers drug substances
49
|
Impacts of solute carriers (SLCs)
The BBB carrier-mediated transporters belonging to the SLC superfamily are comprised of two functional groups that include the mediation of solute influx or efflux from the cell.
The SLC transporters involved in influx at the BBB may be divided into different groups, as follows:
Hexose transport system for glucose and mannose such as glucose transporters (i.e., Glut-1 and Glut-3)50,51
Monocarboxylic acid transport (MCT) system such as MCT1 and MCT 8 for transportation of lactate, short-chain fatty acids, biotin, salicylic acid, valproic acid, phenylbutyrate and 3,5,3’-triiodo-L-thyronine52-56
Neutral amino acids transporter (NAAT), system L, divided into large (LAT1) and small (LAT2) neutral amino acid transporter systems for transportation of neutral amino acids and some drugs such as L-dopa57-59
Anionic amino acids transporters for transportation of aspartate and glutamate60-62
Cationic amino acids transporter, system y+, for transportation of L-lysine, L-arginine, and L-ornithine63,64
Beta amino acid transporter for transportation of β-alanine and taurine65-67
Choline transport system for transportation of choline and thiamine through organic cation transporter (OCT) family68-70
Nucleoside transport system71
Peptide transporters, including oligopeptide transporters (e.g., PepT1, PepT2) and polypeptide transport system (e.g., Oatp2, OAT-K1, OATP)72,73
Medium-chain fatty acid transporter.74
In the following, some brief discussion is provided on the clinical or biological importance of some of these SLCs at the BBB.
Hexose transporters
The functional expression of glucose transporters (GLUTs) was initially identified and characterized using D-glucose specific competitor cytochalasin B in the cerebral microvessels of rat and pig.75 Later on, Pardridge and coworkers showed that the GLUT1 isoform is selectively expressed at the BBB by means of Western blotting, cytochalasin B binding, and in situ hybridization in bovine brain cortex. As a result, they concluded that the GLUT1 gene is selectively expressed in the brain capillary endothelial cells, where 100% of the glucose transporter binding sites at the BBB can be attributed to the GLUT1 isoform.51 Morgello et al. examined human and monkey brain sections recruiting immunoelectron microscopy. They showed GLUT1 expression in the gray matter and white matter endothelial cells, astrocyte foot processes surrounding gray matter blood vessels, and astrocytes adjacent to synaptic contacts. While the immunoblots of adult human frontal cortex were reported to have two forms of GLUT1 (45 and 52 kDa), the immunoblots of vessel-depleted frontal lobe showed the 45 kDa form solely in gray matter fractions. Such findings confirmed linkage to GLUT1 in both astrocyte and astrocyte-associated neuronal energy metabolism.76 The modeled structure of GLUT1 is shown in Fig. 4. From a clinical viewpoint GLUT1 is highly upregulated at the BBB of patients with seizures,77 and downregulated in Alzheimer’s disease and diabetes.78-79 Dysfunctional mutations in GLUT1 may lead to some deficiencies in the transportation of glucose in the related diseases.80
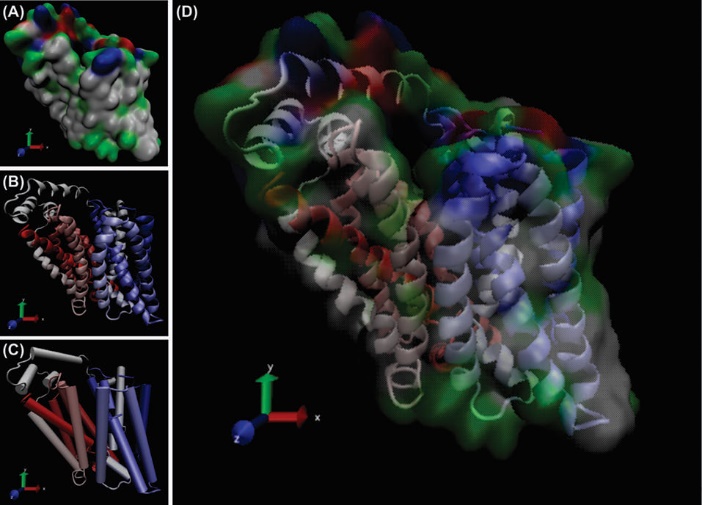
Fig. 4. The modeled structure of GLUT1 as an ABC inward transporter. Panels A, B and C represent different modes of the protein with 12 transmembrane helices. Panel D shows the superimposed image of the space filling structure (panel A) and the ribbon style model (panel B). The visual molecular dynamics (freely available at: https://www-s.ks.uiuc.edu/Research/vmd/) was used to visualize the GLUT1 in different modes.81
Monocarboxylic acid transport system
Monocarboxylate transporters (MCTs) consist of different isoforms. Of these, the MCT1, 2, 3 and 4 catalyze the proton-linked transport of monocarboxylates (e.g., L-lactate) across the plasma membrane,82 the expression of MCT4 seems to be largely dependent upon the oxygen level of the cell as a hypoxia-dependent process.83 Hypoxia may affect the expression of MCT1 and 2 and is mediated by the hypoxia inducible factor 1α (HIF-1α) that is overexpressed in tumor cells.84 These transporters play pivotal metabolic roles in most tissues, including the brain and other tissues. The MCT8 and MCT10 are respectively involved in the transportation of thyroid hormones as well as some aromatic AAs through the BBB.56,85,86 Inhibition of MCT1 by its specific inhibitors may provide a new treatment horizon in cancer chemotherapy in particular for glioblastomas (GBM).87,88 This type of malignancy with high cellular heterogeneity and conspicuous necrotic regions is largely associated with hypoxia, in which the functional expressions of MCT1 and GBM hypoxia marker carbonic anhydrase 9 (CAIX) play key roles. It seems that dysregulated pH in the tumor microenvironment (TME) may pose a significant role in metabolic path of such malignancies.89,90
Amino acid transporters
Several amino acid transporters function at the BBB to maintain brain homeostasis. Of these, the glycoprotein-associated amino acid transporters (gpaATs) play an important role in AAs homeostasis in brain parenchyma. These transporters are basically part of the amino acid/polyamine/choline (APC) superfamily that is considered as one of the largest families of transporters. Table 2 lists some of the key amino acid transporters. The function of neutral amino acid transporter(s) is of note due to their involvement in several crucial events in CNS including synthesis of the neurotransmitters and peptides/proteins. Based upon the binding affinity of a particular transporter to AAs, along with its dependency to sodium ion, amino acid transporters can be classified into different groups such as system L, y+L, (consisting of xCT and 4F2hc), asc, and b0,0. These transporters show some commonalities and differences in terms of binding affinity to different amino acid substrates. For example, alanine can be transported into the brain through systems L and/or asc, which is an overlapped function between these transporters. For instance, system L plays a key role upon traverse of the neutral AAs (NAAs) in and out of the brain, maintaining desired concentrations of NAAs and hence affecting the NAA-related disorders such as depression. System L is a member of the gpaATs family, and possesses putative 12-transmembrane segment topology and comprises of light (LAT) and heavy (4F2hc) chains and two types of LAT1/4F2hc and LAT2/4F2hc.57 System L and other members of gpaATs including y+LAT1, y+LAT2 and xCT share somewhat commonalities in terms of amino acid identity (around 40%-89%).
|
Table 2. The glycoprotein-associated amino acid transporters at BBB
|
|
Transporter system
|
Light chain subunit
|
Heavy chain subunit
|
Tissue/Localization
|
Function
|
Transport type
|
Pathology
|
|
Influx
|
Efflux
|
|
L
|
LAT1 (SLC7A5)
|
4F2/CD98
(SLC3A2)
|
Ubiquitous: ovary, placenta, brain>spleen, testis; activated lymphocytes; some tumor cells |
L-type AAT 1; Na+-independent, prefers L over A, accepts BCH, functions as an exchanger
|
L,H,I,F,Y,W,
V,(M,Q)
|
L, others n.d. |
n.d. |
|
LAT2 (SLC7A8)
|
4F2/CD98
(SLC3A2)
|
Kidney proximal tubule, small intestine>>ovary, placenta, brain |
L-type AAT 2; higher affinity to small NAAs (e.g. A) than LAT1, functions as an exchanger; basolateral in transporting epithelia |
F,Y,W>T,I,C,S,V,L,Q,
A,H,N,M>(G)
|
F,I,L,
others
n.d.
|
n.d. |
|
y
+
L
|
y+LAT1 (SLC7A7)
|
4F2/CD98
(SLC3A2)
|
Kidney, small intestine, leukocytes, lung, erythrocytes, placenta/ basolateral, brain |
Na+-dependent large NAAs/ dibasic AAs exchanger
|
Na+-dependent: L,Q,M,H0, (A,F); Na+-independent: (R,K,H+)
|
R, no L, others n.d. |
Lysinuric protein intolerance (y+LAT1) |
|
Y+LAT2 (SLC7A6)
|
4F2/CD98
(SLC3A2)
|
Brain (glia, neurons), small intestine, testis, parotis, heart, kidney, lung, liver/ basolateral |
Na+-dependent NAAs/dibasic AAs exchanger; glutamine/arginine exchanger
|
similar to y+LAT1
|
n.d. |
n.d. |
|
X-c
|
xCT (SLC7A11)
|
4F2/CD98
(SLC3A2)
|
Macrophages, liver, kidney, brain, retinal pigment cells/basolateral |
Glutamate/cystine exchanger |
C-C>E |
E |
n.d. |
|
asc
|
ascAT1
(SLC7A10)
|
4F2/CD98
(SLC3A2)
|
Brain, lung, placenta, small intestine, kidney/basolateral |
Na+-independent exchanger of small NAAs, also D-serine, D-glycine
|
A,G |
n.d. |
n.d. |
|
B
0,+
|
b0,1AT
(SLC7A9)
|
rBAT
(SLC3A1)
|
Kidney, small intestine, brain/apical |
Na+-independent exchanger of neutral/dibasic AAs; cystine, arginine, lysine, and ornithine reabsorption
|
C-C,R,K,>L,Y,A |
L,R,A other n.d. |
Cystinuria type I (rBAT) and non-type I (b0,1AT) |
|
The gene name for each transport system is presented in the bracket. The capital single letter identity was used to present amino acids. C-C: L-cystine; H
+: the protonated L-histidine; H
0: the zwitterionic form of L-histidine. AAs: amino acids; AAT: amino acid transporter; NAAs: neutral amino acids; diAAs: diamino acids; n.d.: not determined. For detailed information, readers are referred to the following cited articles.
91-94
|
Furthermore, some of these transporters act as obligatory exchangers in an intricate holistic manner and play key roles in the mental health and neurological diseases through maintaining required concentrations. For example, xCT/4F2hc functions as the exchanger of L-cystine and L-glutamic acid.95 Of these amino acid transporters, system y+L functions as a Na+-dependent carrier for transportation of NAAs. Further, emergence of mutations in systems b0,0 and y+L transporters can respectively lead to cystinuria and lysinuric protein intolerance that are considered as inevitable hereditary diseases.96 For example, two distinct genetic disorders, cystinura and Hartnup disease, are related to the sodium-dependent neutral and basic amino acid transport protein rBAT (SLC3A1) and neutral amino acid transporter B0 AT1 (SLC6A19).97-101 In regards to the sodium-dependency of the ABC transporters, System L, System y+ and System Xc– are further reviewed in the following sections.
System L
The system L functions as a Na+-independent bidirectional transporter that is responsible for both branched and aromatic neutral amino acids in almost all types of cells with different affinity to a range of large and small neutral amino acids.57,58,102,103 As shown in Table 3, LAT1 is a ubiquitous transporter with a high affinity for LNAAs and with Km values in the µM range, whereas LAT2 shows a lower affinity to LNAAs and higher affinity to SNAAs and possessing a Km value in the mM range for NAAs.58,102,104,105 Fig. 5 represents the membrane topology model of glycoprotein-associated amino acid transporters (gpaATs, i.e., SLC7A5-11) including system L (LAT1/4F2hc and LAT2/4F2hc heterodimers).103 The heterodimeric complexation of 4F2hc with LAT1 or LAT2, through a covalent disulfide band, is in favor of functionality of the light chains and their co-expression as heterodimer complex - essential for transport functionality of the system. Table 2 represents AAs transporters whose associated heavy chain is the 4F2hc/rBAT.
|
Table 3. Affinity of system L transporters to different amino acids and drug/chemical substances
|
|
Amino acid
|
Affinity (K
M
value)
|
Condition/model
|
Ref.
|
| LAT1/4F2hc |
LAT2/4F2hc |
| Thyroxin (T4) |
7.9 µM |
− |
Xenopus laevis oocyte and presence of choline chloride
|
108
|
| Triiodothyronine (T3) |
0.8 µM |
− |
Xenopus laevis oocyte and presence of choline chloride
|
108
|
| Reverse triiodothyronine (rT3) |
12.5 µM |
− |
Xenopus laevis oocyte and presence of choline chloride
|
108
|
| 3,3'-diiodothyronine (T2) |
7.9 µM |
− |
Xenopus laevis oocyte and presence of choline chloride
|
108
|
| L-Phenylalanine |
14.2 µM |
45.0 µM |
Xenopus oocyte
|
109,110
|
|
55.2 µM |
− |
T24 human bladder carcinoma cells |
104
|
| L-Isoleucine |
25.1 µM |
96.7 µM |
Xenopus oocyte
|
109,110
|
| L-Methionine |
20.2 µM |
204.0 µM |
Xenopus oocyte
|
109,110
|
| L-Valine |
47.2 µM |
124.0 µM |
Xenopus oocyte
|
109,110
|
| L-Histidine |
12.7 µM |
181.0 µM |
Xenopus oocyte
|
109,110
|
| L-Tyrosine |
28.3 µM |
35.9 µM |
Xenopus oocyte
|
109,110
|
|
60.4 µM |
− |
T24 human bladder carcinoma cells |
104
|
|
16.4 µM |
− |
Human fibroblasts |
111
|
| L-Leucine |
19.7 µM |
151.0 µM |
Xenopus oocyte
|
112
|
| L-Tryptophan |
21.4 µM |
57.6 µM |
Xenopus oocyte
|
109,110
|
| L-Asparagine |
1.6 mM |
80.7 µM |
Xenopus oocyte
|
109,110
|
| L-Glutamine |
2.2 mM |
275.0 µM |
Xenopus oocyte
|
109,110
|
| L-Threonine |
− |
68.6 µM |
Xenopus oocyte
|
110
|
| L-Alanine |
10.0 mM |
978.0 µM |
Xenopus oocyte
|
110
|
| L-Glycine |
− |
265.0 µM |
Xenopus oocyte
|
110
|
| L-Serine |
− |
116.0 µM |
Xenopus oocyte
|
110
|
| L-Dopa |
34.2 µM |
− |
T24 human bladder carcinoma cells |
104
|
| 3-O-Methyldopa |
96.5 µM |
− |
T24 human bladder carcinoma cells |
104
|
| α-Methyltyrosine |
153.0 µM |
− |
T24 human bladder carcinoma cells |
104
|
| α-Methyldopa |
216.0 µM |
− |
T24 human bladder carcinoma cells |
104
|
| Gabapentin |
191.0 µM |
− |
T24 human bladder carcinoma cells |
104
|
| Melphalan |
73.5 µM |
− |
T24 human bladder carcinoma cells |
104
|
| BCH |
156.0 µM |
|
T24 human bladder carcinoma cells |
104
|
|
For detailed information, readers are also referred to the cited articles. BCH: 2-Aminobicyclo-[2, 2,1]heptane-2-carboxylic acid.
|
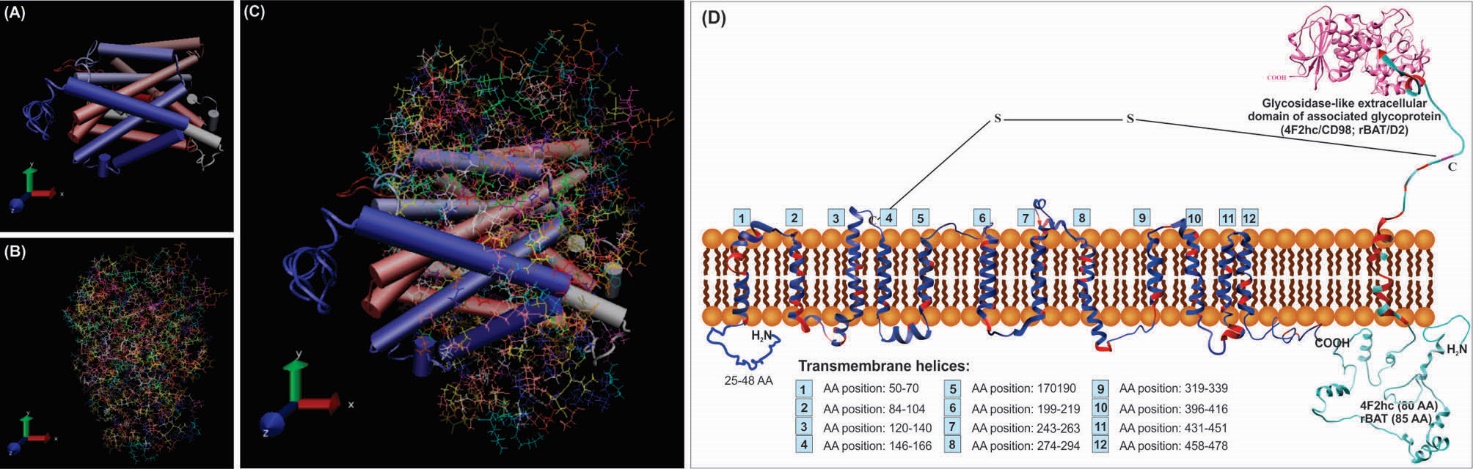
Fig. 5. Predicted membrane topology of the glycoprotein-associated amino acid transporters (gpaATs) in association with type II surface glycoprotein (4F2hc/CD98 or rBAT/ D2). Panels A, B and C represent 3D models of LAT1 (SLC7A5), 4F2hc (SLC3A2) and their interacted structures, respectively. Panel D shows the assumed interaction of gpaATs and CD98/D2 with cell membrane. The conserved regions of gpaATs are drawn as red color. The amino acids (AAs) of the aligned sequences (human and mouse LAT1, LAT2 and y+LAT1; human y+LAT2; mouse xCT; human, rat, mouse and rabbit b0,+AT) show some commonalities and differences. There are some AAs that are not present in all aligned sequences. Numbers 1-12 show the predicted position of the putative transmembrane helices. The two-third predicted transmembrane segments could take an α-helical structure. For function of gpaATs, they need to be coupled with their related heavy chain (4F2hc/rBAT). As showed in panel D; gpaATs have an intramolecular covalent disulfide bridge (S–S) with their associated type II glycoprotein. Human rBAT and 4F2hc have six and three possible N-glycosylation sites, respectively. For detailed information, readers are referred to the following cited articles.91 I-TASSER web-server has been utilized for homology modeling of this complex protein. Transmembrane sections were also identified by use of UniProt detailed annotations (http://www.uniprot.org/). The UCSF Chimera v1.11 standalone software (freely available at: http://www.rbvi.ucsf.edu/chimera) and Visual Molecular Dynamics (VMD) (freely available at: https://www-s.ks.uiuc.edu/Research/vmd/) were used for visualization of LAT1/4F2hc.81 Note: Not drawn/modeled to scale.
In the absence of 4F2hc, the LAT1 is found in the intracellular compartments. Upon complexation with 4F2hc, it can reach to the plasma membrane, while the 4F2hc itself reaches the plasma membrane independently. The surface conformation of 4F2hc seems to be altered in the presence of LAT1, confining it to cell-cell adhesion sites. The 4F2hc comprises 529 amino acids (94 kDa, glycosylated; 72 kDa unglycosylated). The light chain LAT1 is widely expressed in non-epithelial cells including brain, spleen, thymus, testis, skin, liver, placenta, skeletal muscle, and stomach.
In brain, LAT1 expression is found predominantly in capillary endothelial cells that form the BBB. In human, the LAT1 protein consists of 507 AAs with a molecular mass of 55 kDa, whose association with 4F2hc (LAT1/4F2hc heterodimeric complex) induces transportation of LNAAs into the brain.58,106 Affinity of LAT1/4F2hc and LAT2/4F2hc transporters vary for different AAs. In addition to its role in neuronal cell proliferation (neurogenesis) in brain, system L shows affinity to thyroxin (T4) and triiodothyronine (T3). Table 2 represents the Km values for some AAs in both subunits of the system L. Depending on the site of the biological membrane studied, luminal and abluminal transportation of AAs show different pattern. Since system L functions bi-directionally, the inward and outward traverses of AAs appear to differ one another, significantly. For example, the outward trafficking of L-phenylalanine seems to be greater than L-leucine whose inward transportation happens to be markedly high. A number of studies have shown that the subunits of system L (LAT1 and LAT2) act as an obligatory exchanger, so that the inward transportation of one AA may associate with the outward transportation of other AA.107 Such exchange functionality, which may be indirectly related to ATP-dependent transporters activities, seems to provide the driving force for the Na+-independent AA transport systems including system L. Nevertheless, the molecular mechanism(s) of this process is yet to be fully understood.
Of note, L-glutamine (Gln) is known as one of the most copious AAs in plasma and cerebrospinal fluid. It is a precursor for the CNS neurotransmitters such as gamma-aminobutyric acid (GABA). In an interesting study, Dolgodilina et al. analyzed the concentrations of Gln and also 13 other brain interstitial fluid AAs in mice by means of hippocampal microdialysis technique. Given that the interstitial fluid levels for all AAs including Gln were found to be approximately 5-10 times lower than in cerebrospinal fluid, they showed that the competitive inhibition of systems L transporter by 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH) could significantly increase the concentration of Gln in interstitial fluid in comparison with inhibition of system A. They proposed an independent homeostatic regulation of AAs through system L transport machinery (LAT1/4F2hc) in the interstitial fluid in comparison with the cerebrospinal fluid.113 Since LAT1 is an important transporter for drug delivery into the brain through CMT machinery, Ylikangas et al. developed a comparative molecular field analysis (CoMFA) model for the guided synthesis of LAT1-specific novel prodrugs which were examined using an in situ rat brain perfusion technique. These researchers concluded that L-tryptophan (Trp) can be used as the promoiety for the creation of novel LAT1-specific prodrugs with high-affinity.114 Likewise, in a previous study, four phenylalanine derivatives of valproic acid were designed and examined for their potential to utilize the LAT1-mediated transportation. In this study, a novel meta-substituted phenylalanine analogue promoiety with high affinity to LAT1 was introduced – a proof of application for the LAT1-mediated brain targeting.115 In fact, despite ubiquitous expression of the LAT1 and LAT2 transporters by BCECs, only a few drugs (e.g., L-DOPA, alpha-methyldopa, melphalan, and gabapentin) have been developed as the substrates of LAT1 and LAT2. This could be due to possible ambiguousness in the pharmacokinetics of LAT1 and LAT2 transporters because of involvement of other amino acid transporters.59,116
Like any other AA transporters expressed by the BCECs, the impacts of system L transport machinery in healthy and diseased brain are yet to be fully elucidated. In fact, CNS disorders such as schizophrenia, depression and Parkinson appear to be largely dependent upon the brain uptake of LNAAs such as Trp and L-tyrosine (Tyr) through LAT1 and homeostasis of serotonin. Therefore, any malfunctioning of system L (e.g., mutations or other genetic defects) can affect the homeostasis of LNNAs that are precursors of various neurotransmitters. What is more, it seems that LAT1 and LAT2 functions are complementary, in which LAT2 can mediate the outward transportation of various AAs such as LNAAs that are not competently transported through the unidirectional transporter located in the same membrane.
It appears that a complementary functioning transport systems such as y+LAT1 are involved in maintaining the CNS homeostasis from AA concentration viewpoints.112
Further, on the ground of anomalous overexpression of the LAT1 on the membrane of different types of cancerous cells,117-121 this transporter is considered as a potential cancer marker molecule for targeted therapy.122-124 For example, qRT-PCR and Western blot analyses of the expression of three cancer-associated CMTs involved in the traverse of AAs (i.e., LAT1, ASCT2 and SN2) have revealed that the LAT1 was markedly upregulated in human ovarian cancer cells (SKOV3, IGROV1, A2780, and OVCAR3 cell lines as compared to the normal ovarian epithelial IOSE397 cells), whereas the functional expression of ASCT2 and SN2 transporters was not altered.117 Therefore, the LAT1 has been considered as a target for the combinational therapy of cancer using anti-proliferative agents such as aminopeptidase inhibitors.
In 2015, Wu et al. developed a LAT1-mediated chemotherapeutic agent using aspartate-conjugated doxorubicin (Asp-DOX) with high affinity to LAT1. These researchers showed that the Asp-DOX could substantially inhibit the LAT1 overexpressing cancer cells. Such findings were further confirmed by pharmacokinetic data through significant accumulation of Asp-DOX within the tumor tissue in HepG2 and HCT116 tumor-bearing mice most likely through LAT1 uptake. Upon which, the Asp-DOX was proposed as a new treatment strategy for the LAT1 overexpressing cancer cells.124
Owing to high expression of the LAT1 at the BBB, prodrugs can be developed to mimic the AAs that are substrates of LAT1. These prodrugs should ideally possess both the negatively charged alpha-carboxyl and the positively charged alpha-amino groups.125
System y+
Transportation of cationic amino acids (CAAs) is mediated through two families of proteins that are abundant in different cells/tissues. These families are (a) system y+ so-called CAA transporters (CAT), and (b) broad-scope amino acid transporters (BAT) consisting of systems b0,+, B0,+, and y+L. The functional expression and distribution of CATs and BATs by BCECs have not been fully addressed. Plasma membranes of bovine brain microvessels have been isolated and used to identify the CAAs transporters at BBB; as a result, the system y+ was found to be the only transporter present, mostly on the abluminal membrane. It showed voltage dependency and KM values at µM range for AAs such as L-lysine, L-arginine, and L-ornithine. The system y+, in the presence of Na+, was inhibited by several essential neutral AAs with Ki values of 3-10 times greater than the plasma concentrations. However, some small nonessential AAs (i.e., serine, glutamine, alanine, and glycine) were able to inhibit the system y+ with Ki values similar to their plasma concentrations, implying that the system y+ might be involved in BBB traverse of these AAs. Further, the system y+ could be a key transporter in traverse of arginine that is necessary for the synthesis of nitric oxide (NO) in cerebral endothelial cells.63 Taken all, it seems that this system is the only CAA transport machineries at BBB.63,64 It should be also stated that y+LAT1 (SLC7A7) is highly expressed at the basolateral membrane of epithelial cells in the intestine and kidney. It is involved in transportation of dibasic AAs, and emergence of mutations in this transporter can lead to lysinuric protein intolerance (LPI). It is an inherited aminoaciduria that is a rare recessive disorder with certain symptoms such as growth retardation, muscle hypotonia and hepatosplenomegaly. Technically, LPI patients are compound heterozygote with a missense mutation(s) in one allele and a frameshift mutation in the other.126,127 Defective functionality of the y+LAT1 was reported to be influential on the functional expression of y+LAT2 (SLC7A6), perhaps through interference with its compensatory functions in LPI patients.128 The pathological consequences of such mutations at the BBB are yet to be fully understood.
System Xc
–
As an ABC transporter the system Xc– (SLC7A11) possess 12 transmembrane helices and extracellular and cytoplasmic domains (Fig. 6). It is critical in AA exchanger function in various cell types such as BCECs.
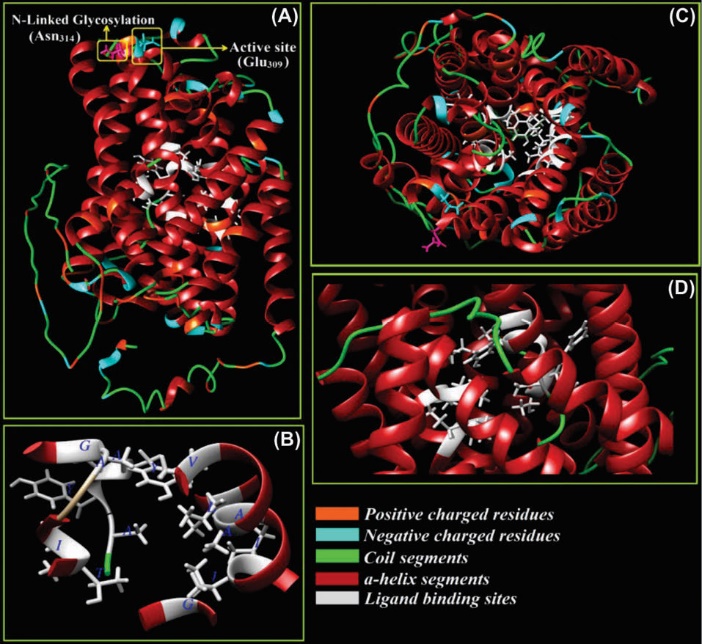
Fig. 6. Three-dimensional structure of ATP-binding cassette transporter, system Xc–. The Na+-independent system Xc– functions as L-cysteine/L-glutamate antiporter. Panel A shows an active site (Glu309) and N-glycosylation residue (Asn314). Panels B, C and D show the ligand binding site residues (Thr56, Ile57, Ala60, Gly61, Ile133, Ile134, Ala137, Ala138, Val141, Tyr244, Ala245, Tyr246, Ala247, Gly334, and Lue392) from different prospects. The positive and negative charged amino acids, α-helix and coil regions of the model are also indicated. The structure is modeled through I-TASSER web server and based on threading and homology modeling approaches and is visualized by UCSF Chimera v1.11 software (our unpublished data).

Fig. 7. Inward and outward trafficking of L-cysteine and L-glutamine by the brain capillary endothelial cells. System Xc– acts as the exchanger for amino acids such as L-cysteine and L-glutamine, and in close cooperation with excitatory amino acid transporters (EAATs) traverse of glutamate is controlled by its efflux from brain and production of glutathione. Glutamate as the most abundant excitatory neurotransmitter can be transported among brain capillary endothelial cells, neurons and astrocytes, where the endothelial cells of the blood-brain barrier play pivotal role in maintenance of glutamate in brain. Glutamate is stored in vesicles at the chemical synapses, where the impulses of nerve is able to activate the liberation of the glutamate molecules from the presynaptic cell. Its concentration in the extracellular space is controlled through Na+-dependent glutamate transporters (i.e., EAAT1, 2 or 3) found in neuronal and glial membranes. Any malfunctioning of EAATs may result in inadvertent accumulation of glutamate molecules causing inevitable excitotoxicity and interfering with the normal activity of neurons (our unpublished data). Note: Not drawn to scale.
Given the importance of glutathione (GSH) inside and outside of the CNS, several mechanisms are involved in its regulation, including Nrf2-ARE signaling pathway.129 Further, the synthesis of GSH is attributed to some enzymes as well as CMTs such as system Xc– and excitatory amino acid transporters (EAATs) such as EAAT1, 2 and 3.130
Fig. 7 represents the inward and outward trafficking of L-cysteine and L-glutamine by the brain capillary endothelial cells. At the BBB, the endothelial cells functionally express the EAATs on the abluminal membrane to shift the glutamate molecules from the extracellular fluid (ECF). This function controls the traverse of glutamate molecules in the CNS, resulting in selective maintenance of the low glutamate concentrations in the ECF within the CNS. As a result, the BBB remains almost impermeable to L-glutamate even at high concentrations. However, some diseases (e.g., diabetes131) can compromise the integrity of BBB.132 It should be noted that the excessive amounts of glutamate can result in toxic impacts in the neurons, while the EAATs in cooperation with system Xc– recycle the extra amount through efflux and production of GSH. Based upon the glutamate-glutamine cycle, the neurotransmitter glutamate is adequately maintained in the CNS, where the neurons are not able to synthesize the neurotransmitter as well as the γ-aminobutyric acid (GABA) from glucose. There is a glutamate-glutamine cycle between neurons and astrocytes, at which the liberated glutamate/GABA molecules from neurons can be taken up by the astrocytes that are able to convert it to glutamine.133 The latter biomolecules can be used by neurons as precursors for the synthesis of glutamate or GABA. However, in the case of ischemic brain damages, the EAATs function reversely resulting in the accumulation of glutamate molecules in the ECF and hence the blockage of neurotransmission. At the BBB, the system Xc–pumps the glutamine molecules out of the ECF of CNS into the endothelial cells and then to the blood. Further, the BCECS are also able to hydrolyze glutamine, and use it for production of GSH. In such process, harmonically orchestrated functions of various AA transport machineries such as Na+-dependent systems N (astrocytes) and A (neurons) as well as facilitative glutamate transporters maintain such homeostasis.133 The role of BBB and transportation of glutamate has comprehensively been discussed recently, readers are referred to the cited article.132
Nucleoside trafficking
Trafficking of nucleosides and related anticancer and antiviral nucleoside drugs across the BBB is mediated through the nucleoside transporters (NTs) located on the plasma membrane of the BCECs. These transport machineries play pivotal role in maintaining the homeostasis of the nucleosides within the CNS, in particular the level of adenosine, to keep it available to bind to receptors, by which a variety of physiological processes are modulated.134
Recently, Lepist et al. studied the transportation of tecadenoson, which is a selective A1 adenosine receptor agonist with similar structure to that of adenosine, into the CNS. Based on their findings, the binding affinities of the hENTs and hCNTs to tecadenoson largely varied showing the following pattern: hENT1 > hCNT1 > hCNT3 > hENT2 > hCNT2. They concluded that the hENT1 is the main transporter of the tecadenoson at the BBB.142 In fact, the hENT1 is a ubiquitous transporter found in various human cells/tissues. The hENT2 is expressed in a variety of tissues such as the skeletal muscle where it plays a predominant role. Its functional expression in the brain microvasculature endothelial cells is yet to be fully addressed.
It should be highlighted that both pyrimidine and purine nucleoside analogs are currently used in clinic. Accordingly, various prodrugs can be developed to mimic these transporters capacities.143,144 Of the pyrimidin analog drugs used in clinics, cytarabine (Cytosar-U®, Ara-C, DepoCyt®) is an analog of deoxycytidine (1-β-d-arabinofuranosylcytosine(. It is used in the combination chemotherapy against chronic myelogenous, leukemia, multiple myeloma, Hodgkin’s lymphoma and non-Hodgkin’s lymphomas. Gemcitabine (Gemzar®, Eli Lilly and Company, USA) has broad spectra applications in treatment of various malignancies such as pancreatic and bladder cancers. Capecitabine (Xeloda®, Roche, Switzerland) is a prodrug and analog of pyrimidin used orally for the treatment of metastatic gastric, colorectal, oesophageal and breast cancers. Of the purine nucleoside analogs, fludarabine (Fludara®) is used for the treatment of low-grade lymphomas. Cladribine (Leustatin®) is used for the treatment of hairy cell leukemia and for B-cell chronic lymphocytic leukemia.
Efflux CMTs
In addition to influx systems of CMTs at the BBB, brain capillary microvasculature embraces some key transport machineries that act as major hindrance against the entry of a number of different therapeutic drugs into the brain parenchyma – a function known as the efflux pumps of BBB. Of these, multidrug resistance 1 (MDR1/ABCB1)/P-gp as an ATP-binding cassette (ABC) drug transport protein seems to be the major efflux system at BBB that is predominantly found in the luminal sides of different endothelial and epithelial cells as well as polarized BCECs.145,146 The putative transmembrane biostructure of human MDR1/P-gp is primarily found in the plasma membrane cell as 12 transmembrane segments that form a three-dimensional structure in the cell plasma membrane blocking the entry of various compounds. This polypeptide consists of two similar halves with six putative transmembrane segments and intracellular ATP-binding site (Fig. 3B, C). The hydrolysis of ATP biomolecules appears to maintain the active export of substrates by this transporter.
It has been shown that mouse and human P-gp transporters are able to actively export a number of compounds such as ivermectin, dexamethasone, digoxin, cyclosporin A and morphine across the polarized epithelial cells in vitro. In wild-type mice, the efflux of digoxin and cyclosporine was 20- to 50-fold higher than that of the mdr1a knockout mice.147 Of the ABC transporters, in addition to MDR1/P-gp, the MRPs and cancer resistance protein (BCRP) expressed by BCECs can also pump out a large number of various compounds.7 For example, it has recently been shown that both HIV-1 protease inhibitors (HPIs) and nucleoside reverse transcriptase inhibitors (NRTIs) are prone to be taken out by the efflux function of BBB through MRPs (e.g., MRP1 and MRP2).148 Further, it has also been reported that the brain accumulation of sunitinib, as a multi-targeted tyrosine kinase inhibitor (TKI), is markedly restricted by both ABCB1 and ABCG2 efflux activities. Given that the full inhibition of these transporters can significantly increase the accumulation of sunitinib within the brain, combined oral administration of sunitinib with the P-gp inhibitor elacridar can be of great benefit in treatment of brain tumors.149 While sunitinib is metabolized by CYP3A4, perhaps rationalized combination of the CYP3A4 inhibitor (ritonavir) to such combination therapy could augment the impacts of pharmacotherapy, an extended exposure of BCECs to ritonavir was reported to impose a concentration-dependent increase in P-gp activity and expression. It has been shown that cyclosporin A (CysA) as a potent P-gp and MRP modulator, is able to moderate the concentration of methotrexate (MTX) in the rat brain. As a result, it is proposed as a useful tool to achieve an appropriate MTX concentration in brain in the case of brain tumors.150 As another instance, it has been shown that antiepileptic drugs (AEDs) can only treat 30-40% of patients with epilepsy effectively. Such failure is deemed to be in large part due to the impacts of efflux functions of BCECs on outward transportation of AEDs which may be considered as a mechanism for resistance to AEDs in epileptic patients.151 Of the AEDs, lacosamide (LCM) has been tested for its liability towards efflux functions in cell models by concentration equilibrium transport assays (CETAs). In addition, LCM can be taken up by MDR1 expressing cells through a passive diffusion, however it could be a substrate of P-gp in CETA.151
Peptide transporters
Peptides (mostly di-/tri-peptides) are normally crossed the BBB through peptide transporters found as an integral part of the plasma membrane proteins which also mediate the trafficking of peptide-mimetic drugs. It should be noted that the electrochemical Na+ gradient, the so-called sodium-motive force (SMF) is the main driving force for the active/facilitated transportations of a variety of biomolecules such as AAs and carbohydrates in the most of living cells as well as the mammalian cells. The peptide transport process, nevertheless, seems to be an evolutionary shift in the coupling ion and favors H+, through which a proton-motive force (PMF) energizes active traverse of peptides through cell membrane. Interestingly, various cellular transportation activities in mammalian cells are coupled with the Na+/H+ exchangers (NHEs), Na+/K+ pump and H+ pump in terms of energy transduction.152,153 Regulation of these cellular machineries orchestrate the solutes concentrations within the cells as well as the ECF, playing central roles in health and diseases. For example, in addition to the key role of NHE1 (SLC9A1) in dysregulation of pH in the tumor microenvironment (TME),89 ischemia-reperfusion was shown to induce cardiac injuries that are mediated by NHE1. Its upregulation can modulate the activity of the plasmalemmal Na+/Ca2+ exchanger.152 While NHE1 and NHE2 (SLC9A2) are the keystones in maintaining the volume and ionic compositions of brain interstitial fluid,154 their roles in brain ischemic disease can be expected.155
PepT1 can be used for peptidomimetic oral drug delivery through intestine.161 Accordingly, the bioavailability of prodrugs specific to PepT1 was shown to be higher than those of the corresponding parent compounds, in large part because of the improved transportation. Such phenomenon have emerged a new field of peptide transporter mediated targeted therapy, so-called PepT1-targeted prodrugs.162 Valganciclovir, a valyl ester of ganciclovir, was clinically shown to possess markedly higher bioavailability than ganciclovir when taken orally by patients with cytomegalovirus infection, in large part for being substrate of both PepT1 and PepT2.163 A number of drugs seem to be substrate to these transporters, including amoxicillin, benazepril, captopril, cefixime, cephalexin, enalapril, levodopa, oxacillin, Ramipril, tolbutamide and valaciclovir. They are relatively stereoselective, while showing greater affinity to the peptides formed from L isomer amino acids than those possess D isomer amino acids. Kinetically, depending upon the substrate, PepT1 is considered as a low-affinity and high-capacity system which yields apparent affinity constants, Km, ranging from 0.2-10 mM. However, PepT2 is also considered as a high-affinity and low-capacity transport system showing Km values of 5–500 μM.
Endocytosis, transcytosis and RMTs
Cellular trafficking of macromolecular biomolecules is mediated through membranous vesicular machineries that are comprised of numerous components including lipid rafts, membranous caveolae and CCPs/clathrin-coated vesicles (CCVs). Various cell micro-/nano-machineries are involved in vesicular trafficking, including Rab GTPases. Of various Rab family of proteins as a member of the Ras superfamily of monomeric G proteins, different Rab GTPases are responsible for regulation of different processes of the membrane trafficking such as the formation and travelling of vesicles within the cytoskeleton’s actin and tubulin networks and even invagination and fusion processes. The newly biosynthesized protein in ER is trafficked across the Golgi (cis, medial and trans sections) to be matured and reach to other cell compartments or outwardly to the ECF through exocytosis, during which Rab GTPases play key roles. Fig. 8 shows schematic representation for the vesicular trafficking.

Fig. 8. Schematic representation for the diversity of vesicular internalization and trafficking. Macromolecular entities (e.g., bacteria, viruses) can be internalized through phagocytosis (I). Engulfed particles within the phagosomes are prone to fusion with lysosomes. Cells can internalize surrounding substances by a phenomenon called pinocytosis/fluid phase endocytosis (II). Transcytosis is the main route for crossing of macromolecules through cells (III). Lipid rafts play a key role in signal transduction and macromolecules (e.g., cholera toxin, glycophosphatidylinositol (GPI)-anchored proteins and SV40 viral particles) internalization (IV). Endocytosis can occur through membranous caveolae and clathrin coated pits (V). The clathrin mediated endocytosis can be categorized as coated pits endocytosis (transferrin (Tf), low-density lipoprotein (LDL), some toxins, free fatty acids and viruses) and cholesterol-dependent clathrin-mediated endocytosis (e.g., toxins such as tetanus and anthrax toxins, PRPC, IL-2 receptor and APP). Once internalized, depending on the type vesicle, it can interact with other cellular compartments or vesicles such as endosomes and lysosomes and exocytose (VI) or even interact with the neighboring cells (VII). In the case of newly synthesized proteins, the secretory proteins such as glycoproteins are basically traversed across the membranes of endoplasmic reticulum (ER) and cis-face of Golgi apparatus through coat proteins (COP) I/II. The matured peptides/proteins traverse through the cisternae and transporter out of the cells through vesicular trafficking from trans-face of Golgi apparatus (red box). During such biosynthesis, maturation and post translational modification processes in the ER and Golgi apparatus, the sorting machineries get involved by recruiting a number of adaptor, retrieval and coat proteins such as and soluble N-ethylmaleimide sensitive attachment protein receptor (SNARE). These bio-elements are implemented for the fusion of vesicles (see Fig. 9) and various Rab GTPases. For detailed information on vesicular trafficking readers are referred to the following citation.134,164,165
The specific endocytic path is entirely dependent upon the receptor–ligand pairing process, in which solute molecules bind to its cognate receptor on the cell membrane. Once paired with the canonical receptor, solute are internalized into the cytoplasm through either a constitutive (class I) or ligand-stimulated (class II) internalization processes.164 In the constitutive endocytosis, the continual plasma membrane turnover with the receptor internalized in an endocytic vesicle mediates the endocytosis process in the presence or even absence of the ligands such as Tf or LDL. In the ligand-stimulated endocytosis, the ligand-receptor pairing process is the main driving force for the initiation of the internalization of the ligands such as insulin and epidermal growth factors (EGFs).
In the RMTs, several key receptors are specifically responsible for the transportation of various biomolecules including insulin, insulin-like growth factor, leptin Tf and LDL.
Endosomal trafficking
Vesicles, after reacting into the cytoplasm, must be sorted out and directed to their specific itinerary. As a result, in a short period of time (<10 min), the internalized vesicles fuse with the early endosomes that act as an initial sorting station and express molecular markers such as the early endosome antigen-1 (EEA-1). In the early endosomes, where the pH is acidic (<pH 6.0), the transported cargo molecules dissociate from their specific receptors. This process is essential step for transportation of ligands to lysosomes for their degradation. The acidic condition of lysosomes enhances the binding affinity of IgG molecules in attaching to the neonatal Fc receptors (FcRns) that are expressed by variety of cells in particular endothelial cells such as BCECs,172 and rescue the IgG molecules from degradation.164 In polarized cells such as BCECs, the endocytic activities occur both in luminal and abluminal sections with distinct specifications that is in favor of either blood-to-brain or brain-to-blood traverse of substances, depending on biological prerequisites and activities of the brain cells. Such process also favors the recycling process of the endocytosed receptors, which allow cells to constantly recycle the receptor and dispose them back in the original membrane, unless they possess necessary signals for degradation or transcytosis. For example, the epidermal growth factor and its cognate receptor (EGF-EGFR) complex is basically trafficked to the lysosomes to be destructed, while other ligand-receptor pairs may possess signals for transcytosis. Trafficking of albumin following binding to its cognate receptor gp60 results in transcytosis of albumin.173,174 FcRn-IgG complex undergoes transcytosis at the BBB in both directions, i.e., apical-to-basal (A-to-B) and basal-to-apical (B-to-A).175 The B-to-A transportation (the so-called reverse transcytosis) of IgG molecules seems to depict the efflux of therapeutic immunotherapies from CNS in rat brain,176 resulting in failure of the treatment modalities in CNS diseases. It is now clear that FcRn play a critical role in controlling IgG pharmacokinetics as well as serum albumin trafficking,177 by which the improved pharmacokinetics of albumin-conjugated therapeutics can be explicated. In a study, to investigate the FcRn impact of the efflux of Abs, Cooper et al. treated rats with two mAbs specific to FcRn with markedly different binding affinities (IgG1 N434A and H435A) by intra-nasal-to-CNS and intra-cranial administrations. Upon intra-nasal delivery, mAbs reached the low cerebral hemisphere within 20 min, where the Ab with higher affinity to FcRn (N434A) showed a faster clearance trend. Having bilateral injection into the rat cortex, decreased and increased levels of N434A was detected respectively in the cerebral hemispheres and in serum, indicating the profound role of FcRn in efflux of IgG from the rat brain.176
In tack of selective retrieval of the endocytosed substances, they undergo further vesicular trafficking towards the late endosomes that are multivesicular bodies enriched in lysobisphosphatidic acid and others in phosphatidylinositol 3-phosphate (pH, 5.5–6.0) and potential to have homotypic/heterotypic fusion with lysosomes.178 This is a process by which the endocytosed cargo molecules undergo the proteolytic degradation by lysosomes (pH, 5.0) that is known by the expression of the lysosome associated membrane protein-1. Once in the lysosome, the endocytosed molecules are exposed to array of hydrolytic enzymes such as proteases, lipases nucleases, glycosidases and phospholipases.179-181
For the endosomal trafficking, RMT follows different routes such as membranous caveolae, CCPs and lipid rafts that will be further discussed.
Caveolae-mediated endocytosis
Membranous caveolae are flask-shaped invaginations of the plasma membrane is described as “smooth” vesicles with sizes ranging from 50 to 100 nm and coated by a 22 kDa structural protein caveolin, whose N and C termini remain cytoplasmic.182 Initially detected in endothelial cells, caveolae tend to mediate the selective uptake of various small molecules and macromolecules such as albumin and lipoproteins. Functional expression of caveolin is linked with the maintenance of health and progression of various diseases such as cancers, hence it is considered as a new molecular marker for the targeted therapy of related diseases.183,184 Caveolae has initially been detected in endothelial cells and is highly enriched in biomolecules such as glycosphingolipids, cholesterol, sphingomyelin, and lipid-anchored membrane proteins. Of these biomolecules, cholesterol plays a crucial role of in the biogenesis of caveolae. It has been validated by treating cells with cholesterol binding agents (e.g., nystatin, filipin, or cyclodextrin). This results in obliteration of caveolae structures. Further, the subcellular transition of the main protein of caveolae vesicles, caveolin, is intricately linked to the transportation of cholesterol. Given the fact that cholesterol is predominantly transported to the plasma membrane through a Golgi-independent vesicular path,185,186 and the high binding capacity of cholesterol to caveolin-1, caveolae vesicles appear to be the main mediator of the cholesterol transportation.
It seems to elicit similar effect(s) on the biogenesis formation of the clathrin-coated endocytic vesicles and synaptic vesicles. It should be noted that the caveolin-1 is capable of forming extremely robust detergent-resistant homo-oligomeric complexes (comprised of ~14 to 16 monomers) that can further create higher oligomer-oligomer complexes through interactions with the respective C-terminal domains – a process in favor of bending of the plasma membrane similar to that of the triskelion in the CCPs.182 Depending on the cell/tissue, the caveolae show different morphology such as grape-like clusters (abundant in developing skeletal muscle cells), rosettes (adipocytes), and detached vesicles/tubules in endothelial cells, as well as fused form as elongated tubules or transcellular channels.182 Caveolae-mediated vesicles are involved in endocytosis, transcytosis and potocytosis. While for the endocytosis, caveolae bud off from the plasma membrane and fuse with various intracellular compartments such as caveosomes interaction with ER, during the transcytosis, caveolae vesicles deliver proteins from one side to the other side of endothelial cells. As for the potocytosis (cellular drinking), caveolae vesicles are involved in the uptake of small solutes (<1 kDa) such as folate, in which the vesicles are pinched off and remained associated with the cell membrane, then undergo the caveolar fission/fusion through the SNARE complex and GTP hydrolysis (Figs. 8 and 9).

Fig. 9. Vesicular fusion through the SNARE complex and GTP hydrolysis.
While some recently conducted investigations implicated that caveolae and caveolin-mediated vesicles plays a check-and-balance role in the cerebral ischemia pathophysiology through incorporation with cholesterol molecules,187 it can be molecular target for treatment of these diseases. Further, the upregulation of caveolin in various malignancies make it an important cancer marker,188 while the endocytic capacity of the caveolar vesicles make this vesicular transportation route as an attractive mechanism for the targeted therapy, nonetheless caveolin-1 has been reported to be downregulated in some tumors. This vesicular mechanism has been shown to be beneficial path for macromolecular trafficking as reported for the endocytosis of atorvastatin calcium loaded BSA (ATV-BSA) nanoparticles (NPs), polyethylenimine (PEI), polyamidoamine (PAMAM) polyplexes and silica-coated iron oxide NPs.189-191
Clathrin-mediated endocytosis
Clathrin-mediated endocytosis (CME) is considered as the main RMTs process. During this vesicular trafficking phenomenon, CCPs/CCVs containing cargo molecules bud off from the plasma membrane and enter into the cytoplasm. Fig. 10 shows schematic illustration and transmission electron microscopy micrograph of the CCV.
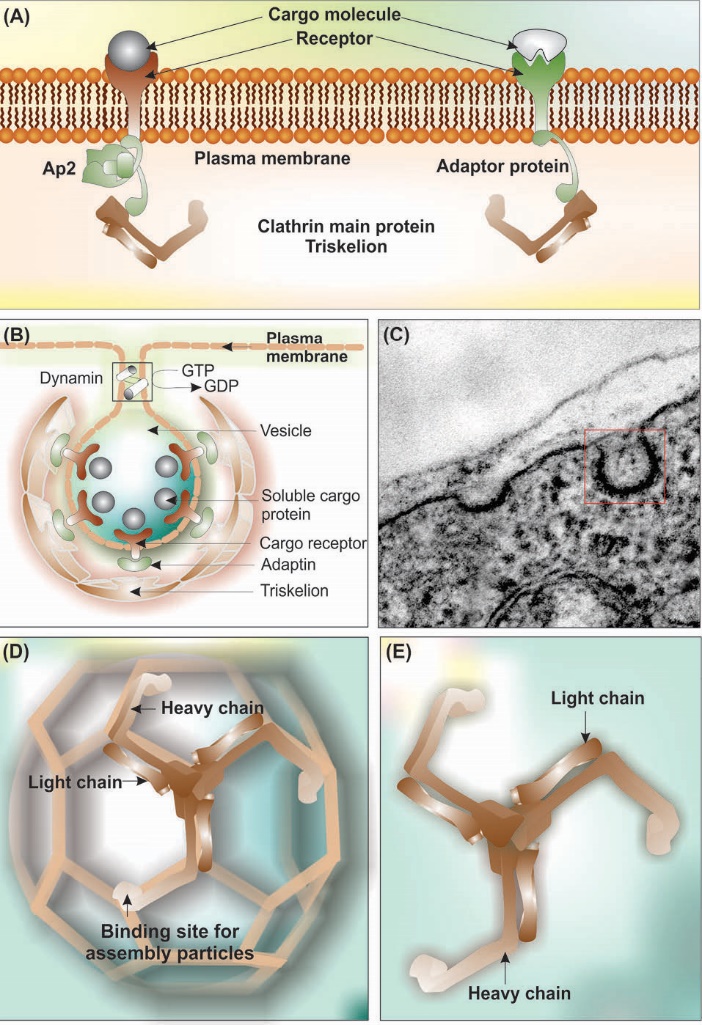
Fig. 10. Clathrin coated pits and clathrin-mediated endocytosis. (A) Schematic representation of plasma membrane interaction with cargo receptor, adaptor proteins and clathrin. (B) Clathrin forms a non-covalently bound triskelton structure composed of three heavy chains (192 kDa each) and three light chains. (C) The transmission electron microscopy micrograph of clathrin-coated pit in the brain capillary endothelial cell. (D) Schematic representation of assembled triskelion heavy and light chains forming pentagonic and hexagonic structure. (E) Triskelion heavy and light chains. Note: Not drown to scale.
CME, as the most widely investigated vesicular transportation system, is a key step not only for the uptakes of nutrients and substances, but also for the intracellular signaling and membranous recycling. It should be noted that the binding of the receptors to the ligand molecules and activation of the receptors demand binding of intracellular part of the receptors to the adaptor proteins (Fig. 10A).192 Adaptor proteins play central roles in the assembly of the clathrin protein onto the plasma membrane.192 Of these, AP2 (subunits, α, β2, σ2, and μ2,) is one of the well-studied adapter proteins, which has a number of binding partners such as phosphatidylinositol 4,5-bisphosphate (PIP2), clathrin, different endocytic accessory proteins, and two signaling motifs present on some cargo receptors (Fig. 10). The G-protein-coupled receptors (GPCRs) interact with various ligands (e.g., ions, amines, peptides, proteins, lipids, nucleotides, etc.), at which the receptor is activated with certain conformational changes towards plausible conversion of the receptor to the protein kinase substrate. The latter process results in binding of β-arrestins to the receptor, which is a necessary step for the internalization of cargo molecules through GPCR-mediated AP-2 adaptor protein complex and assisted by CME.192,193
Structurally, as shown in Fig. 10 (panel B), the clathrin-mediated vesicles is characterized by three distinct layers as (i) the inner layer in which cargo molecules are interacted with their cognate receptor in association with adaptor protein, (ii) the internal layer consisting of lipid bilayer interacted with integrated proteins, and (iii) the outer layer containing triskelion assembled as the pentagons and hexagons.
In general, the CME begins with (a) the interaction of cargo molecules with their cognate receptors, (b) the formation of vesicles on the inner surface of the cytoplasmic that possesses clathrin elements, (c) involvement of the AP2 adaptor protein complex and accessory proteins.
It should be highlighted that the subunits of AP2 adaptor complex initiate the formation of the clathrin matrix, in which some helper proteins (e.g., β-arrestins) are also involved. Once the α-subunit of AP2 attached to phosphatidylinositol-4,5-bisphosphate, it binds to the designated endocytic motifs through phosphorylation of μ2-subunit that results in binding of the μ2-subunit to the cargo protein undergoing endocytosis. All these events materialize the formation of CCVs, after which other accessory proteins such as clathrin assembly lymphoid myeloid leukemia (CALM) are literally involved to amalgamate the formation of clathrin lattice.194 Concurrent with the emergence of clathrin matrix, various other proteins (dynamin and the actin cytoskeleton, rab5 guanine nucleotide exchange factor, Rme-6) are engaged to control the CCVs budding off, trafficking and recycling.195,196
CME participates in vesicular trafficking of blood-borne macromolecular substances into the brain by specialized functions at the BBB. Accordingly, the functional macromolecular trafficking through CCVs is important phenomenon not only for the maintenance of CNS homeostasis, but also for the targeted therapy of brain diseases such as brain tumors. For example, OX26 mAb has been recruited for crossing the BBB, which is a RME mechanism implementing TfR-mediated CCVs. Accordingly, various OX26 functionalized NPs have been developed including solid lipid NPs, PEGylated liposomes and polymers such as chitosan and poly(lactic-co-glycolic acid).197-204
Transcytosis
As a well-known transport process, transcytosis was first introduced in capillary permeability studies. In addition to the paracellular entry of the macromolecules through normal or anomalous opening of the biological barriers (Fig. 2), transcytosis is the main route for the entrance of macromolecules into the tissue/organ from the surrounding biological barrier. As shown in Figs. 2 and 8, transcytosis is an important process through which the cargo molecules move from one side of the cells to the other side bidirectionally (i.e., A-to-B and B-to-A).
In the brain microvasculature endothelia, at least three mechanisms are involved in entry of the particulate substances into the brain, including:
caveolae, in which the pits (50–100 nm and flask shape) formed plasma membrane (Fig. 8) enriched with cholesterol and sphingolipids similar to that of the lipid raft components, then get coated with flexible caveolin-1/2 and bud off from the membrane by the aid of GTPase dynamin to carry cargo molecules (e.g., folate receptor, tetanus toxins and alkaline phosphatase),
clathrin, in which spherical pits (60–200 nm) bud off from plasma (Fig. 10) by the aid of AP2 forming semi-rigid matrix that is cleaved by the GTPase dynamin to transport cargo molecules (e.g., LDL, LRP1, transferrin, insulin); and
micropinocytosis, in which irregular spherical/tubular vesicles (0.2-5 mM) perturb from the plasma membrane and are provoked by inflammation/lipopolysaccharide and shuffle various macromolecular entities.27
At the BBB, up to 20 receptors have been recognized with ability to initiate receptor-mediated transcytosis, including: TfR, heparin-binding EGF, scavenger receptors AI, BI, EGF receptor, tumor necrosis factor, insulin and insulin-like growth factor receptors, apolipoprotein E receptor 2, leptin receptor, melanotransferrin receptor, LDL receptors. Most of these receptors are associated with CCVs to initiate transcytosis.27 Therefore, CCVs have been the main route for targeted delivery of nanoparticles and nanoconjugates into the brain.197,201,205-209
Multifunctional nanomedicines for brain targeting
While the currently used treatment modalities for brain diseases target the symptoms solely as noncurative agents, the nanoscaled DDSs show promising clinical outcomes by facilitating the drug delivery into the brain and improving the pharmacokinetics profile. However, these strategies need further investigations prior to their translation into clinical applications in human subjects. In fact, we need to improve our trifling understanding upon possible inadvertent neurotoxicity and detrimental effects of nanosized DDSs on neuronal cells. Targeting the cells within the central dogma brain is quit important for several formidable diseases such as neurodegenerative diseases and brain tumor gliomas. The latter disease has been classified into three major classes of astrocytomas, oligodendrogliomas and mixed gliomas (oligoastrocytomas), which cause a great deal of mortalities worldwide. Indeed, the targeted nanomedicines (e.g., polymeric/lipidic biodegradable NSs and inorganic nanoconjugates armed with targeting devices such as Tf or folic acid), with capability to cross BBB through active targeting mechanisms, can offer great benefits to patients with brain tumor.210 In 2014, Su et al. capitalized on a dual-targeting DDS drug delivery system using bovine serum albumin nanoparticles (BSA-NPs) modified with both lactoferrin (Lf) and mPEG2000. The engineered NPs were loaded with DOX molecules and examined in both in vitro and in vivo models. While the in vivo pharmacokinetics analysis in healthy rats showed longer circulation in rat, the PEGylated Lf-armed DOX-loaded NPs induced profound toxicity in BCECs and C6 cells. As a result, the engineered NS was proposed as an effective targeting therapy against brain gliomas.211
In a study, brain-targeted peptide angiopep-2 (ANG) was used for surface decoration of poly(lactic-co-glycolic acid) (PLGA) NPs encapsulating DOX and anti-EGFR siRNA. This multimodal NS was found to efficiently deliver DOX and siRNA into U87MG cells, causing marked cytotoxicity and apoptosis and suppressing EGFR. Once used in the animal, the analysis of brain orthotopic U87MG glioma xenograft mice revealed that the ANG-armed PLGA NS was able to prolong the life span of the glioma-bearing mice.205 Clark and Davis decorated Gold NPs (AuNPs) with Tf through an acid-cleavable linkage or anti-TfR Ab. They showed high affinity and avidity of Tf-armed AuNPs to TfRs on the blood side of the BBB. Once internalized, the multidentate Tf-TfR interactions was easily dissociated in large part because of acidification during the vesicular trafficking. Such mechanism was shown to profoundly enhance the entrance of NPs into the brain parenchyma of mice. While these researchers confirmed the trafficking of high-affinity anti-TfR Abs through lysosomal compartments of BCECs, they showed that the high-avidity Tf-armed AuNPs with the acid-cleavable linkage was able to avoid the major endothelium retention in large part through shedding surface Tf during their transcytosis.212
Recently, an “autocatalytic” approach was projected to enhance the transportation of NPs into the brain based upon entrance of a small number of NPs and liberation of specific modulators with capability to mediate the entry of more NPs into the brain.213 The autocatalytic brain tumor-targeting poly(amine-co-ester) terpolymer NPs was used with results showing their ability to preferentially highly accumulate in brain tumors though through a passive targeting mechanism.
In 2014, Zong et al. developed a dual-targeting liposomal NS loaded with DOX and conjugated with cell-penetrating peptide (TAT) and Tf. Having examined in both in vitro and in vivo models, these researchers showed significantly high penetration of the dual-targeting NS across BCECs and marked accumulation in tumor cells. Based upon their findings by in vivo imaging, the engineered NS was able to reach the core of the tumor spheroids, resulting in marked increase in survival time of tumor-bearing mice after treatment in comparison with free drug molecules.214
Another study was later on conducted to investigate the effectiveness of similar NS in targeted delivery of anti-EGFR siRNA to tumor bearing mice.215 And, substantially long survival period and great downregulation in the expression of EGFR was reported in mice treated with such NS. As a result, it seems that the Tf-TAT conjugated NPs may be considered as a promising NS for shuttling drugs/genes into the brain. In fact, various biological capacities of BBB such as CMTs and RMTs can be exploited for the specific targeting of brain diseases using different strategies.216
Concluding remakes
The BBB selectively/specifically controls/regulates the traverse of blood-circulating substances into the brain and vice versa, by which the CNS homeostasis is conservatively maintained. Such biological barrier also selectively controls the entrance of medicines necessary for diagnosis and/or therapy of the CNS diseases. The CMT and RMT machineries of the BBB can clinically be of great importance not only in terms of pathobiology of brain capillary endothelial cells and surrounding parenchymal cells, but also for drug discovery, delivery and targeting. In fact, our current challenge in CNS dreg delivery dilemma is how to exploit the naturally occurring vesicular trafficking systems and the receptor-mediated transfer machinery in safely crossing the BBB. We may also consider focusing our endeavors on exploiting multifunctional nanoscaled drug delivery systems (DDSs) to efficiently cross the restricted barrier and safely carry out the designated diagnostic or deliver therapeutic medicines to the target site(s) within the brain without imposing undesired impacts.217 There have been a large number of investigations on developing smart multimodal brain drug delivery and targeting systems. While discussion on this subject was beyond the objective of this review, to the best of our knowledge, such DDSs are yet to be developed. Although multifunctional nanomedicines are supposed to be able to enter the brain exploiting the endocytic machineries mainly through functionalized ligands, their safety and nonspecific impacts on the brain parenchymal entities yet to be fully evaluated.
Acknowledgments
Authors like to acknowledge Tabriz University of Medical Sciences for the financial support of the brain drug delivery and targeting project.
Ethical approval
There is none to be disclosed.
Competing interests
The authors like to declare that JB, MR and YO are the current editors of the journal. The publication process (blind peer-review) of the current article has been performed in accordance with the COPE guidelines.
References
- Omidi Y, Barar J. Impacts of blood-brain barrier in drug delivery and targeting of brain tumors. Bioimpacts 2012; 2: 5-22. doi: 10.5681/bi.2012.002. [Crossref]
- Willis CL. Imaging in vivo astrocyte/endothelial cell interactions at the blood-brain barrier. Methods Mol Biol 2012; 814: 515-29. doi: 10.1007/978-1-61779-452-0_34. [Crossref]
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006; 7: 41-53. doi: 10.1038/nrn1824. [Crossref]
- Omidi Y, Barar J, Ahmadian S, Heidari HR, Gumbleton M. Characterization and astrocytic modulation of system L transporters in brain microvasculature endothelial cells. Cell Biochem Funct 2008; 26: 381-91. doi: 10.1002/cbf.1455. [Crossref]
- Pardridge WM. Molecular biology of the blood-brain barrier. Mol Biotechnol 2005; 30: 57-70. doi: 10.1385/MB:30:1:057. [Crossref]
- Miller DS. Regulation of ABC transporters at the blood-brain barrier. Clin Pharmacol Ther 2015; 97: 395-403. doi: 10.1002/cpt.64. [Crossref]
- Begley DJ. ABC transporters and the blood-brain barrier. Curr Pharm Des 2004; 10: 1295-312.
- Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab 2012; 32: 1959-72. doi: 10.1038/jcbfm.2012.126. [Crossref]
- Pardridge WM. Drug targeting to the brain. Pharm Res 2007; 24: 1733-44. doi: 10.1007/s11095-007-9324-2. [Crossref]
- Wood MJ, O’Loughlin AJ, Samira L. Exosomes and the blood-brain barrier: implications for neurological diseases. Ther Deliv 2011; 2: 1095-9.
- Rafi MA, Omidi Y. A prospective highlight on exosomal nanoshuttles and cancer immunotherapy and vaccination. Bioimpacts 2015; 5: 117-22. doi: 10.15171/bi.2015.22. [Crossref]
- O’Loughlin AJ, Woffindale CA, Wood MJ. Exosomes and the emerging field of exosome-based gene therapy. Curr Gene Ther 2012; 12: 262-74.
- Grapp M, Wrede A, Schweizer M, Huwel S, Galla HJ, Snaidero N, et al. Choroid plexus transcytosis and exosome shuttling deliver folate into brain parenchyma. Nat Commun 2013; 4: 2123. doi: 10.1038/ncomms3123. [Crossref]
- Sun D, Zhuang X, Zhang S, Deng ZB, Grizzle W, Miller D, et al. Exosomes are endogenous nanoparticles that can deliver biological information between cells. Adv Drug Deliv Rev 2013; 65: 342-7. doi: 10.1016/j.addr.2012.07.002. [Crossref]
- Gumbleton M, Audus KL. Progress and limitations in the use of in vitro cell cultures to serve as a permeability screen for the blood-brain barrier. J Pharm Sci 2001; 90: 1681-98.
- Smith M, Omidi Y, Gumbleton M. Primary porcine brain microvascular endothelial cells: biochemical and functional characterisation as a model for drug transport and targeting. J Drug Target 2007; 15: 253-68. doi: 10.1080/10611860701288539. [Crossref]
- Barar J, Gumbleton M, Asadi M, Omidi Y. Barrier functionality and transport machineries of human ECV304 cells. Med Sci Monit 2010; 16: BR52-60.
- Nakhlband A, Omidi Y. Barrier functionality of porcine and bovine brain capillary endothelial cells. Bioimpacts 2011; 1: 153-9. doi: 10.5681/bi.2011.021. [Crossref]
- Omidi Y, Campbell L, Barar J, Connell D, Akhtar S, Gumbleton M. Evaluation of the immortalised mouse brain capillary endothelial cell line, b.End3, as an in vitro blood-brain barrier model for drug uptake and transport studies. Brain Res 2003; 990: 95-112.
- Katt ME, Xu ZS, Gerecht S, Searson PC. Human Brain Microvascular Endothelial Cells Derived from the BC1 iPS Cell Line Exhibit a Blood-Brain Barrier Phenotype. PLoS One 2016; 11: e0152105. doi:10.1371/journal.pone.0152105. [Crossref]
- Helms HC, Abbott NJ, Burek M, Cecchelli R, Couraud PO, Deli MA, et al. In vitro models of the blood-brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J Cereb Blood Flow Metab 2016; 36: 862-90. doi: 10.1177/0271678X16630991. [Crossref]
- Rapoport SI. Osmotic opening of the blood-brain barrier. Ciba Found Symp 1978; 237-55.
- Easton AS, Abbott NJ. Bradykinin increases permeability by calcium and 5-lipoxygenase in the ECV304/C6 cell culture model of the blood-brain barrier. Brain Res 2002; 953: 157-69.
- Sanovich E, Bartus RT, Friden PM, Dean RL, Le HQ, Brightman MW. Pathway across blood-brain barrier opened by the bradykinin agonist, RMP-7. Brain Res 1995; 705: 125-35.
- Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol 2000; 20: 131-47.
- Pardridge WM. Blood-brain barrier endogenous transporters as therapeutic targets: a new model for small molecule CNS drug discovery. Expert Opin Ther Targets 2015; 19: 1059-72. doi: 10.1517/14728222.2015.1042364. [Crossref]
- Preston JE, Joan Abbott N, Begley DJ. Transcytosis of macromolecules at the blood-brain barrier. Adv Pharmacol 2014; 71: 147-63. doi: 10.1016/bs.apha.2014.06.001. [Crossref]
- Wang YY, Lui PC, Li JY. Receptor-mediated therapeutic transport across the blood-brain barrier. Immunotherapy 2009; 1: 983-93. doi: 10.2217/imt.09.75. [Crossref]
- de Boer AG, Gaillard PJ. Drug targeting to the brain. Annu Rev Pharmacol Toxicol 2007; 47: 323-55. doi: 10.1146/annurev.pharmtox.47.120505.105237. [Crossref]
- Peluffo H, Unzueta U, Negro-Demontel ML, Xu Z, Vaquez E, Ferrer-Miralles N, et al. BBB-targeting, protein-based nanomedicines for drug and nucleic acid delivery to the CNS. Biotechnol Adv 2015; 33: 277-87. doi: 10.1016/j.biotechadv.2015.02.004. [Crossref]
- Patil R, Ljubimov AV, Gangalum PR, Ding H, Portilla-Arias J, Wagner S, et al. MRI virtual biopsy and treatment of brain metastatic tumors with targeted nanobioconjugates: nanoclinic in the brain. ACS Nano 2015; 9: 5594-608. doi: 10.1021/acsnano.5b01872. [Crossref]
- Gao H, Yang Z, Zhang S, Pang Z, Liu Q, Jiang X. Study and evaluation of mechanisms of dual targeting drug delivery system with tumor microenvironment assays compared with normal assays. Acta Biomater 2014; 10: 858-67. doi: 10.1016/j.actbio.2013.11.003. [Crossref]
- Gao H, Yang Z, Zhang S, Pang Z, Jiang X. Internalization and subcellular fate of aptamer and peptide dual-functioned nanoparticles. J Drug Target 2014; 22: 450-9. doi: 10.3109/1061186X.2014.886038. [Crossref]
- Ma H, Gao Z, Yu P, Shen S, Liu Y, Xu B. A dual functional fluorescent probe for glioma imaging mediated by blood-brain barrier penetration and glioma cell targeting. Biochem Biophys Res Commun 2014; 449: 44-8. doi: 10.1016/j.bbrc.2014.04.148. [Crossref]
- Ordovas JM. ABC1: the gene for Tangier disease and beyond. Nutr Rev 2000; 58: 76-9.
- Chen P, Dai Y, Wu X, Wang Y, Sun S, Xiao J, et al. Mutations in the ABCA3 gene are associated with cataract-microcornea syndrome. Invest Ophthalmol Vis Sci 2014; 55: 8031-43. doi: 10.1167/iovs.14-14098. [Crossref]
- Maeda A, Maeda T, Golczak M, Palczewski K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem 2008; 283: 26684-93. doi: 10.1074/jbc.M804505200. [Crossref]
- Steinberg S, Stefansson H, Jonsson T, Johannsdottir H, Ingason A, Helgason H, et al. Loss-of-function variants in ABCA7 confer risk of Alzheimer’s disease. Nat Genet 2015; 47: 445-7. doi: 10.1038/ng.3246. [Crossref]
- DeStefano GM, Kurban M, Anyane-Yeboa K, Dall’Armi C, Di Paolo G, Feenstra H, et al. Mutations in the cholesterol transporter gene ABCA5 are associated with excessive hair overgrowth. PLoS Genet 2014; 10: e1004333. doi: 10.1371/journal.pgen.1004333. [Crossref]
- Kelsell DP, Norgett EE, Unsworth H, Teh MT, Cullup T, Mein CA, et al. Mutations in ABCA12 underlie the severe congenital skin disease harlequin ichthyosis. Am J Hum Genet 2005; 76: 794-803. doi: 10.1086/429844. [Crossref]
- Girodon-Boulandet E, Cazeneuve C, Goossens M. Screening practices for mutations in the CFTR gene ABCC7. Hum Mutat 2000; 15: 135-49. doi: 10.1002/(SICI)1098-1004(200002)15:2<135::AIDHUMU2>3.0.CO;2-H [Crossref]
- Hoffmann K, Gastens AM, Volk HA, Loscher W. Expression of the multidrug transporter MRP2 in the blood-brain barrier after pilocarpine-induced seizures in rats. Epilepsy Res 2006; 69: 1-14. doi: 10.1016/j.eplepsyres.2005.12.005. [Crossref]
- Ose A, Ito M, Kusuhara H, Yamatsugu K, Kanai M, Shibasaki M, et al. Limited brain distribution of [3R,4R,5S]-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate phosphate (Ro 64-0802), a pharmacologically active form of oseltamivir, by active efflux across the blood-brain barrier mediated by organic anion transporter 3 (Oat3/Slc22a8) and multidrug resistance-associated protein 4 (Mrp4/Abcc4). Drug Metab Dispos 2009; 37: 315-21. doi: 10.1124/dmd.108.024018. [Crossref]
- Musolino PL, Gong Y, Snyder JM, Jimenez S, Lok J, Lo EH, et al. Brain endothelial dysfunction in cerebral adrenoleukodystrophy. Brain 2015; 138: 3206-20. doi: 10.1093/brain/awv250. [Crossref]
- Huang B, Zhou H, Lang X, Liu Z. siRNAinduced ABCE1 silencing inhibits proliferation and invasion of breast cancer cells. Mol Med Rep 2014; 10: 1685-90. doi: 10.3892/mmr.2014.2424. [Crossref]
- Liu YH, Chen YJ, Wu HH, Wang TY, Tsai FJ. Single nucleotide polymorphisms at the PRR3, ABCF1, and GNL1 genes in the HLA class I region are associated with Graves’ ophthalmopathy in a gender-dependent manner. Ophthalmology 2014; 121: 2033-9. doi: 10.1016/j.ophtha.2014.04.027. [Crossref]
- Powell TR, Tansey KE, Breen G, Farmer AE, Craig IW, Uher R, et al. ATP-binding cassette sub-family F member 1 (ABCF1) is identified as a putative therapeutic target of escitalopram in the inflammatory cytokine pathway. J Psychopharmacol 2013; 27: 609-15. doi: 10.1177/0269881113490329. [Crossref]
- Bojanic DD, Tarr PT, Gale GD, Smith DJ, Bok D, Chen B, et al. Differential expression and function of ABCG1 and ABCG4 during development and aging. J Lipid Res 2010; 51: 169-81. doi: 10.1194/M900250-JLR200. [Crossref]
- Nicolazzo JA, Katneni K. Drug transport across the blood-brain barrier and the impact of breast cancer resistance protein (ABCG2). Curr Top Med Chem 2009; 9: 130-47.
- Mantych GJ, James DE, Devaskar SU. Jejunal/kidney glucose transporter isoform (Glut-5) is expressed in the human blood-brain barrier. Endocrinology 1993; 132: 35-40. doi: 10.1210/endo.132.1.8419132. [Crossref]
- Pardridge WM, Boado RJ, Farrell CR. Brain-type glucose transporter (GLUT-1) is selectively localized to the blood-brain barrier. Studies with quantitative western blotting and in situ hybridization. J Biol Chem 1990; 265: 18035-40.
- Halestrap AP. Monocarboxylic acid transport. Compr Physiol 2013; 3: 1611-43. doi: 10.1002/cphy.c130008. [Crossref]
- Miller LP, Oldendorf WH. Regional kinetic constants for blood-brain barrier pyruvic acid transport in conscious rats by the monocarboxylic acid carrier. J Neurochem 1986; 46: 1412-6.
- Oldendorf WH. Carrier-mediated blood-brain barrier transport of short-chain monocarboxylic organic acids. Am J Physiol 1973; 224: 1450-3.
- Conn AR, Fell DI, Steele RD. Characterization of alpha-keto acid transport across blood-brain barrier in rats. Am J Physiol 1983; 245: E253-60.
- Ceballos A, Belinchon MM, Sanchez-Mendoza E, Grijota-Martinez C, Dumitrescu AM, Refetoff S, et al. Importance of monocarboxylate transporter 8 for the blood-brain barrier-dependent availability of 3,5,3’-triiodo-L-thyronine. Endocrinology 2009; 150: 2491-6. doi: 10.1210/en.2008-1616. [Crossref]
- Kido Y, Tamai I, Uchino H, Suzuki F, Sai Y, Tsuji A. Molecular and functional identification of large neutral amino acid transporters LAT1 and LAT2 and their pharmacological relevance at the blood-brain barrier. J Pharm Pharmacol 2001; 53: 497-503.
- Kageyama T, Nakamura M, Matsuo A, Yamasaki Y, Takakura Y, Hashida M, et al. The 4F2hc/LAT1 complex transports L-DOPA across the blood-brain barrier. Brain Res 2000; 879: 115-21.
- Rautio J, Gynther M, Laine K. LAT1-mediated prodrug uptake: a way to breach the blood-brain barrier? Ther Deliv 2013; 4: 281-4. doi: 10.4155/tde.12.165. [Crossref]
- Sakaeda T, Siahaan TJ, Audus KL, Stella VJ. Enhancement of transport of D-melphalan analogue by conjugation with L-glutamate across bovine brain microvessel endothelial cell monolayers. J Drug Target 2000; 8: 195-204. doi: 10.3109/10611860008996865. [Crossref]
- O’Kane RL, Martinez-Lopez I, DeJoseph MR, Vina JR, Hawkins RA. Na(+)-dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) of the blood-brain barrier. A mechanism for glutamate removal. J Biol Chem 1999; 274: 31891-5.
- Benrabh H, Lefauconnier JM. Glutamate is transported across the rat blood-brain barrier by a sodium-independent system. Neurosci Lett 1996; 210: 9-12.
- O’Kane RL, Vina JR, Simpson I, Zaragoza R, Mokashi A, Hawkins RA. Cationic amino acid transport across the blood-brain barrier is mediated exclusively by system y+. Am J Physiol Endocrinol Metab 2006; 291: E412-9. doi: 10.1152/ajpendo.00007.2006. [Crossref]
- Stoll J, Wadhwani KC, Smith QR. Identification of the cationic amino acid transporter (System y+) of the rat blood-brain barrier. J Neurochem 1993; 60: 1956-9.
- Benrabh H, Bourre JM, Lefauconnier JM. Taurine transport at the blood-brain barrier: an in vivo brain perfusion study. Brain Res 1995; 692: 57-65.
- Tamai I, Senmaru M, Terasaki T, Tsuji A. Na(+)- and Cl(-)-dependent transport of taurine at the blood-brain barrier. Biochem Pharmacol 1995; 50: 1783-93.
- Komura J, Tamai I, Senmaru M, Terasaki T, Sai Y, Tsuji A. Brain-to-blood active transport of beta-alanine across the blood-brain barrier. FEBS Lett 1997; 400: 131-5.
- Murakami H, Sawada N, Koyabu N, Ohtani H, Sawada Y. Characteristics of choline transport across the blood-brain barrier in mice: correlation with in vitro data. Pharm Res 2000; 17: 1526-30.
- Kang YS, Terasaki T, Ohnishi T, Tsuji A. In vivo and in vitro evidence for a common carrier mediated transport of choline and basic drugs through the blood-brain barrier. J Pharmacobiodyn 1990; 13: 353-60.
- Karlsson C, Maepea O, Alm A. Choline transport through the blood-retinal and the blood-brain barrier in vivo. Acta Ophthalmol (Copenh) 1984; 62: 763-6.
- Masereeuw R, Jaehde U, Langemeijer MW, de Boer AG, Breimer DD. In vitro and in vivo transport of zidovudine (AZT) across the blood-brain barrier and the effect of transport inhibitors. Pharm Res 1994; 11: 324-30.
- Tsuji A, Tamai I. Organic anion transporters. Pharm Biotechnol 1999; 12: 471-91.
- Tsuji A, Tamai II. Carrier-mediated or specialized transport of drugs across the blood-brain barrier. Adv Drug Deliv Rev 1999; 36: 277-90.
- Adkison KD, Shen DD. Uptake of valproic acid into rat brain is mediated by a medium-chain fatty acid transporter. J Pharmacol Exp Ther 1996; 276: 1189-200.
- Dick AP, Harik SI, Klip A, Walker DM. Identification and characterization of the glucose transporter of the blood-brain barrier by cytochalasin B binding and immunological reactivity. Proc Natl Acad Sci U S A 1984; 81: 7233-7.
- Morgello S, Uson RR, Schwartz EJ, Haber RS. The human blood-brain barrier glucose transporter (GLUT1) is a glucose transporter of gray matter astrocytes. Glia 1995; 14: 43-54. doi: 10.1002/glia.440140107. [Crossref]
- Cornford EM, Nguyen EV, Landaw EM. Acute upregulation of blood-brain barrier glucose transporter activity in seizures. Am J Physiol Heart Circ Physiol 2000; 279: H1346-54.
- Shah K, Desilva S, Abbruscato T. The role of glucose transporters in brain disease: diabetes and Alzheimer’s Disease. Int J Mol Sci 2012; 13: 12629-55. doi: 10.3390/ijms131012629. [Crossref]
- Winkler EA, Nishida Y, Sagare AP, Rege SV, Bell RD, Perlmutter D, et al. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci 2015; 18: 521-30. doi: 10.1038/nn.3966. [Crossref]
- Seidner G, Alvarez MG, Yeh JI, O’Driscoll KR, Klepper J, Stump TS, et al. GLUT-1 deficiency syndrome caused by haploinsufficiency of the blood-brain barrier hexose carrier. Nat Genet 1998; 18: 188-91. doi:10.1038/ng0298-188. [Crossref]
- Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph 1996; 14: 33-8, 27-8.
- Halestrap AP, Wilson MC. The monocarboxylate transporter family--role and regulation. IUBMB Life 2012; 64: 109-19. doi: 10.1002/iub.572. [Crossref]
- Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem 2006; 281: 9030-7. doi: 10.1074/jbc.M511397200. [Crossref]
- Perez de Heredia F, Wood IS, Trayhurn P. Hypoxia stimulates lactate release and modulates monocarboxylate transporter (MCT1, MCT2, and MCT4) expression in human adipocytes. Pflugers Arch 2010; 459: 509-18. doi: 10.1007/s00424-009-0750-3. [Crossref]
- Kinne A, Schulein R, Krause G. Primary and secondary thyroid hormone transporters. Thyroid Res 2011; 4 Suppl 1: S7. doi: 10.1186/1756-6614-4-S1-S7. [Crossref]
- Van Herck SL, Delbaere J, Bourgeois NM, McAllan BM, Richardson SJ, Darras VM. Expression of thyroid hormone transporters and deiodinases at the brain barriers in the embryonic chicken: Insights into the regulation of thyroid hormone availability during neurodevelopment. Gen Comp Endocrinol 2015; 214: 30-9. doi: 10.1016/j.ygcen.2015.02.021. [Crossref]
- Grillon E, Farion R, Fablet K, De Waard M, Tse CM, Donowitz M, et al. The spatial organization of proton and lactate transport in a rat brain tumor. PLoS One 2011; 6: e17416. doi: 10.1371/journal.pone.0017416. [Crossref]
- Miranda-Goncalves V, Granja S, Martinho O, Honavar M, Pojo M, Costa BM, et al. Hypoxia-mediated upregulation of MCT1 expression supports the glycolytic phenotype of glioblastomas. Oncotarget 2016. doi: 10.18632/oncotarget.10114. [Crossref]
- Barar J, Omidi Y. Dysregulated pH in Tumor Microenvironment Checkmates Cancer Therapy. Bioimpacts 2013; 3: 149-62. doi: 10.5681/bi.2013.036. [Crossref]
- Omidi Y, Barar J. Targeting tumor microenvironment: crossing tumor interstitial fluid by multifunctional nanomedicines. Bioimpacts 2014; 4: 55-67. doi: 10.5681/bi.2014.021. [Crossref]
- Verrey F, Meier C, Rossier G, Kuhn LC. Glycoprotein-associated amino acid exchangers: broadening the range of transport specificity. Pflugers Arch 2000; 440: 503-12.
- Wagner CA, Broer A, Albers A, Gamper N, Lang F, Broer S. The heterodimeric amino acid transporter 4F2hc/LAT1 is associated in Xenopus oocytes with a non-selective cation channel that is regulated by the serine/threonine kinase sgk-1. J Physiol 2000; 526 Pt 1: 35-46.
- Broer A, Friedrich B, Wagner CA, Fillon S, Ganapathy V, Lang F, et al. Association of 4F2hc with light chains LAT1, LAT2 or y+LAT2 requires different domains. Biochem J 2001; 355: 725-31.
- Wagner CA, Lang F, Broer S. Function and structure of heterodimeric amino acid transporters. Am J Physiol Cell Physiol 2001; 281: C1077-93.
- Hosoya K, Tomi M, Ohtsuki S, Takanaga H, Saeki S, Kanai Y, et al. Enhancement of L-cystine transport activity and its relation to xCT gene induction at the blood-brain barrier by diethyl maleate treatment. J Pharmacol Exp Ther 2002; 302: 225-31.
- Verrey F. System L: heteromeric exchangers of large, neutral amino acids involved in directional transport. Pflugers Arch 2003; 445: 529-33. doi: 10.1007/s00424-002-0973-z. [Crossref]
- Seow HF, Broer S, Broer A, Bailey CG, Potter SJ, Cavanaugh JA, et al. Hartnup disorder is caused by mutations in the gene encoding the neutral amino acid transporter SLC6A19. Nat Genet 2004; 36: 1003-7. doi: 10.1038/ng1406. [Crossref]
- Broer S. The role of the neutral amino acid transporter B0AT1 (SLC6A19) in Hartnup disorder and protein nutrition. IUBMB Life 2009; 61: 591-9. doi: 10.1002/iub.210. [Crossref]
- Saadi I, Chen XZ, Hediger M, Ong P, Pereira P, Goodyer P, et al. Molecular genetics of cystinuria: mutation analysis of SLC3A1 and evidence for another gene in type I (silent) phenotype. Kidney Int 1998; 54: 48-55. doi: 10.1046/j.1523-1755.1998.00956.x. [Crossref]
- Koulivand L, Mohammadi M, Ezatpour B, Salehi R, Markazi S, Dashti S, et al. Mutation analysis of SLC3A1 and SLC7A9 genes in patients with cystinuria. Urolithiasis 2015; 43: 447-53. doi: 10.1007/s00240-015-0794-0. [Crossref]
- Markazi S, Kheirollahi M, Doosti A, Mohammadi M, Koulivand L. A Novel Mutation in SLC3A1 Gene in Patients With Cystinuria. Iran J Kidney Dis 2016; 10: 44-7.
- Matsuo H, Tsukada S, Nakata T, Chairoungdua A, Kim DK, Cha SH, et al. Expression of a system L neutral amino acid transporter at the blood-brain barrier. Neuroreport 2000; 11: 3507-11.
- Nii T, Segawa H, Taketani Y, Tani Y, Ohkido M, Kishida S, et al. Molecular events involved in up-regulating human Na+-independent neutral amino acid transporter LAT1 during T-cell activation. Biochem J 2001; 358: 693-704.
- Kim DK, Kanai Y, Choi HW, Tangtrongsup S, Chairoungdua A, Babu E, et al. Characterization of the system L amino acid transporter in T24 human bladder carcinoma cells. Biochim Biophys Acta 2002; 1565: 112-21.
- Kanai Y, Endou H. Functional properties of multispecific amino acid transporters and their implications to transporter-mediated toxicity. J Toxicol Sci 2003; 28: 1-17.
- Boado RJ, Li JY, Nagaya M, Zhang C, Pardridge WM. Selective expression of the large neutral amino acid transporter at the blood-brain barrier. Proc Natl Acad Sci U S A 1999; 96: 12079-84.
- Meier C, Ristic Z, Klauser S, Verrey F. Activation of system L heterodimeric amino acid exchangers by intracellular substrates. EMBO J 2002; 21: 580-9.
- Friesema EC, Docter R, Moerings EP, Verrey F, Krenning EP, Hennemann G, et al. Thyroid hormone transport by the heterodimeric human system L amino acid transporter. Endocrinology 2001; 142: 4339-48. doi: 10.1210/endo.142.10.8418. [Crossref]
- Yanagida O, Kanai Y, Chairoungdua A, Kim DK, Segawa H, Nii T, et al. Human L-type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim Biophys Acta 2001; 1514: 291-302.
- Segawa H, Fukasawa Y, Miyamoto K, Takeda E, Endou H, Kanai Y. Identification and functional characterization of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem 1999; 274: 19745-51.
- Vumma R, Wiesel FA, Flyckt L, Bjerkenstedt L, Venizelos N. Functional characterization of tyrosine transport in fibroblast cells from healthy controls. Neurosci Lett 2008; 434: 56-60. doi: 10.1016/j.neulet.2008.01.028. [Crossref]
- Rossier G, Meier C, Bauch C, Summa V, Sordat B, Verrey F, et al. LAT2, a new basolateral 4F2hc/CD98-associated amino acid transporter of kidney and intestine. J Biol Chem 1999; 274: 34948-54.
- Dolgodilina E, Imobersteg S, Laczko E, Welt T, Verrey F, Makrides V. Brain interstitial fluid glutamine homeostasis is controlled by blood-brain barrier SLC7A5/LAT1 amino acid transporter. J Cereb Blood Flow Metab 2015. doi: 10.1177/0271678X15609331. [Crossref]
- Ylikangas H, Malmioja K, Peura L, Gynther M, Nwachukwu EO, Leppanen J, et al. Quantitative insight into the design of compounds recognized by the L-type amino acid transporter 1 (LAT1). ChemMedChem 2014; 9: 2699-707. doi: 10.1002/cmdc.201402281. [Crossref]
- Peura L, Malmioja K, Laine K, Leppanen J, Gynther M, Isotalo A, et al. Large amino acid transporter 1 (LAT1) prodrugs of valproic acid: new prodrug design ideas for central nervous system delivery. Mol Pharm 2011; 8: 1857-66. doi: 10.1021/mp2001878. [Crossref]
- del Amo EM, Urtti A, Yliperttula M. Pharmacokinetic role of L-type amino acid transporters LAT1 and LAT2. Eur J Pharm Sci 2008; 35: 161-74. doi: 10.1016/j.ejps.2008.06.015. [Crossref]
- Fan X, Ross DD, Arakawa H, Ganapathy V, Tamai I, Nakanishi T. Impact of system L amino acid transporter 1 (LAT1) on proliferation of human ovarian cancer cells: a possible target for combination therapy with anti-proliferative aminopeptidase inhibitors. Biochem Pharmacol 2010; 80: 811-8. doi: 10.1016/j.bcp.2010.05.021. [Crossref]
- Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, et al. Prognostic significance of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (CD98) expression in surgically resectable stage III non-small cell lung cancer. Exp Ther Med 2010; 1: 799-808. doi: 10.3892/etm.2010.117. [Crossref]
- Kaira K, Takahashi T, Murakami H, Shukuya T, Kenmotsu H, Naito T, et al. Relationship between LAT1 expression and response to platinum-based chemotherapy in non-small cell lung cancer patients with postoperative recurrence. Anticancer Res 2011; 31: 3775-82.
- Kaira K, Arakawa K, Shimizu K, Oriuchi N, Nagamori S, Kanai Y, et al. Relationship between CD147 and expression of amino acid transporters (LAT1 and ASCT2) in patients with pancreatic cancer. Am J Transl Res 2015; 7: 356-63.
- Huttunen KM, Gynther M, Huttunen J, Puris E, Spicer JA, Denny WA. A Selective and Slowly Reversible Inhibitor of l-Type Amino Acid Transporter 1 (LAT1) Potentiates Antiproliferative Drug Efficacy in Cancer Cells. J Med Chem 2016; 59: 5740-51. doi: 10.1021/acs.jmedchem.6b00190. [Crossref]
- Fukumoto S, Hanazono K, Fu DR, Endo Y, Kadosawa T, Iwano H, et al. A new treatment for human malignant melanoma targeting L-type amino acid transporter 1 (LAT1): a pilot study in a canine model. Biochem Biophys Res Commun 2013; 439: 103-8. doi: 10.1016/j.bbrc.2013.08.020. [Crossref]
- Rosilio C, Nebout M, Imbert V, Griessinger E, Neffati Z, Benadiba J, et al. L-type amino-acid transporter 1 (LAT1): a therapeutic target supporting growth and survival of T-cell lymphoblastic lymphoma/T-cell acute lymphoblastic leukemia. Leukemia 2015; 29: 1253-66. doi: 10.1038/leu.2014.338. [Crossref]
- Wu W, Dong Y, Gao J, Gong M, Zhang X, Kong W, et al. Aspartate-modified doxorubicin on its N-terminal increases drug accumulation in LAT1-overexpressing tumors. Cancer Sci 2015; 106: 747-56. doi: 10.1111/cas.12672. [Crossref]
- Rautio J, Karkkainen J, Huttunen K, Gynther M. Amino acid ester prodrugs conjugated to the alpha-carboxylic acid group do not display affinity for the L-type amino acid transporter 1 (LAT1). Eur J Pharm Sci 2014; 66C: 36-40. doi: 10.1016/j.ejps.2014.09.025. [Crossref]
- Shoji Y, Noguchi A, Shoji Y, Matsumori M, Takasago Y, Takayanagi M, et al. Five novel SLC7A7 variants and y+L gene-expression pattern in cultured lymphoblasts from Japanese patients with lysinuric protein intolerance. Hum Mutat 2002; 20: 375-81. doi: 10.1002/humu.10140. [Crossref]
- Torrents D, Mykkanen J, Pineda M, Feliubadalo L, Estevez R, de Cid R, et al. Identification of SLC7A7, encoding y+LAT-1, as the lysinuric protein intolerance gene. Nat Genet 1999; 21: 293-6. doi: 10.1038/6809. [Crossref]
- Sperandeo MP, Paladino S, Maiuri L, Maroupulos GD, Zurzolo C, Taglialatela M, et al. A y(+)LAT-1 mutant protein interferes with y(+)LAT-2 activity: implications for the molecular pathogenesis of lysinuric protein intolerance. Eur J Hum Genet 2005; 13: 628-34. doi: 10.1038/sj.ejhg.5201376. [Crossref]
- Chen G, Fang Q, Zhang J, Zhou D, Wang Z. Role of the Nrf2-ARE pathway in early brain injury after experimental subarachnoid hemorrhage. J Neurosci Res 2011; 89: 515-23. doi: 10.1002/jnr.22577. [Crossref]
- Cohen-Kashi-Malina K, Cooper I, Teichberg VI. Mechanisms of glutamate efflux at the blood-brain barrier: involvement of glial cells. J Cereb Blood Flow Metab 2012; 32: 177-89. doi: 10.1038/jcbfm.2011.121. [Crossref]
- Sickmann HM, Waagepetersen HS. Effects of diabetes on brain metabolism--is brain glycogen a significant player? Metab Brain Dis 2015; 30: 335-43. doi: 10.1007/s11011-014-9546-z. [Crossref]
- Hawkins RA. The blood-brain barrier and glutamate. Am J Clin Nutr 2009; 90: 867S-74S. doi: 10.3945/ajcn.2009.27462BB. [Crossref]
- Broer S, Brookes N. Transfer of glutamine between astrocytes and neurons. J Neurochem 2001; 77: 705-19.
- Omidi Y, Gumbleton M. Biological membranes and barriers. Biomaterials for Delivery and Targeting of Proteins Nucleic Acids, Mahato, RI (Ed): CRC Press, New York; 2005. p. 232-74.
- Boleti H, Coe IR, Baldwin SA, Young JD, Cass CE. Molecular identification of the equilibrative NBMPR-sensitive (es) nucleoside transporter and demonstration of an equilibrative NBMPR-insensitive (ei) transport activity in human erythroleukemia (K562) cells. Neuropharmacology 1997; 36: 1167-79.
- Chishty M, Begley DJ, Abbott NJ, Reichel A. Interaction of nucleoside analogues with nucleoside transporters in rat brain endothelial cells. J Drug Target 2004; 12: 265-72. doi: 10.1080/10611860410001731398. [Crossref]
- Choi JS, Berdis AJ. Nucleoside transporters: biological insights and therapeutic applications. Future Med Chem 2012; 4: 1461-78. doi: 10.4155/fmc.12.79. [Crossref]
- Mangravite LM, Xiao G, Giacomini KM. Localization of human equilibrative nucleoside transporters, hENT1 and hENT2, in renal epithelial cells. Am J Physiol Renal Physiol 2003; 284: F902-10. doi: 10.1152/ajprenal.00215.2002. [Crossref]
- Rodriguez-Mulero S, Errasti-Murugarren E, Ballarin J, Felipe A, Doucet A, Casado FJ, et al. Expression of concentrative nucleoside transporters SLC28 (CNT1, CNT2, and CNT3) along the rat nephron: effect of diabetes. Kidney Int 2005; 68: 665-72. doi: 10.1111/j.1523-1755.2005.00444.x. [Crossref]
- Ritzel MW, Ng AM, Yao SY, Graham K, Loewen SK, Smith KM, et al. Molecular identification and characterization of novel human and mouse concentrative Na+-nucleoside cotransporter proteins (hCNT3 and mCNT3) broadly selective for purine and pyrimidine nucleosides (system cib). J Biol Chem 2001; 276: 2914-27. doi: 10.1074/jbc.M007746200. [Crossref]
- Damaraju S, Zhang J, Visser F, Tackaberry T, Dufour J, Smith KM, et al. Identification and functional characterization of variants in human concentrative nucleoside transporter 3, hCNT3 (SLC28A3), arising from single nucleotide polymorphisms in coding regions of the hCNT3 gene. Pharmacogenet Genomics 2005; 15: 173-82.
- Lepist EI, Damaraju VL, Zhang J, Gati WP, Yao SY, Smith KM, et al. Transport of A1 adenosine receptor agonist tecadenoson by human and mouse nucleoside transporters: evidence for blood-brain barrier transport by murine equilibrative nucleoside transporter 1 mENT1. Drug Metab Dispos 2013; 41: 916-22. doi: 10.1124/dmd.112.049858. [Crossref]
- Siccardi D, Gumbleton M, Omidi Y, McGuigan C. Stereospecific chemical and enzymatic stability of phosphoramidate triester prodrugs of d4T in vitro. Eur J Pharm Sci 2004; 22: 25-31. doi: 10.1016/j.ejps.2004.02.006. [Crossref]
- Balzarini J, Cahard D, Wedgwood O, Salgado A, Velazquez S, Yarnold CJ, et al. Marked inhibitory activity of masked aryloxy aminoacyl phosphoramidate derivatives of dideoxynucleoside analogues against visna virus infection. J Acquir Immune Defic Syndr Hum Retrovirol 1998; 17: 296-302.
- Schinkel AH. P-Glycoprotein, a gatekeeper in the blood-brain barrier. Adv Drug Deliv Rev 1999; 36: 179-94.
- Borst P, Schinkel AH. P-glycoprotein ABCB1: a major player in drug handling by mammals. J Clin Invest 2013; 123: 4131-3. doi: 10.1172/JCI70430. [Crossref]
- Schinkel AH, Wagenaar E, van Deemter L, Mol CA, Borst P. Absence of the mdr1a P-Glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Invest 1995; 96: 1698-705. doi: 10.1172/JCI118214. [Crossref]
- Eilers M, Roy U, Mondal D. MRP (ABCC) transporters-mediated efflux of anti-HIV drugs, saquinavir and zidovudine, from human endothelial cells. Exp Biol Med (Maywood) 2008; 233: 1149-60. doi: 10.3181/0802-RM-59. [Crossref]
- Tang SC, Lagas JS, Lankheet NA, Poller B, Hillebrand MJ, Rosing H, et al. Brain accumulation of sunitinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by oral elacridar and sunitinib coadministration. Int J Cancer 2012; 130: 223-33. doi: 10.1002/ijc.26000. [Crossref]
- Ogushi N, Sasaki K, Shimoda M. Effect of cyclosporin on distribution of methotrexate into the brain of rats. J Vet Med Sci 2015. doi: 10.1292/jvms.14-0671. [Crossref]
- Zhang C, Chanteux H, Zuo Z, Kwan P, Baum L. Potential role for human P-glycoprotein in the transport of lacosamide. Epilepsia 2013; 54: 1154-60. doi: 10.1111/epi.12158. [Crossref]
- Padan E, Landau M. Sodium-Proton (Na(+)/H(+)) Antiporters: Properties and Roles in Health and Disease. Met Ions Life Sci 2016; 16: 391-458. doi: 10.1007/978-3-319-21756-7_12. [Crossref]
- Ganapathy V, Leibach FH. Proton-coupled solute transport in the animal cell plasma membrane. Curr Opin Cell Biol 1991; 3: 695-701.
- Lam TI, Wise PM, O’Donnell ME. Cerebral microvascular endothelial cell Na/H exchange: evidence for the presence of NHE1 and NHE2 isoforms and regulation by arginine vasopressin. Am J Physiol Cell Physiol 2009; 297: C278-89. doi: 10.1152/ajpcell.00093.2009. [Crossref]
- Mokgokong R, Wang S, Taylor CJ, Barrand MA, Hladky SB. Ion transporters in brain endothelial cells that contribute to formation of brain interstitial fluid. Pflugers Arch 2014; 466: 887-901. doi: 10.1007/s00424-013-1342-9. [Crossref]
- Nassl AM, Rubio-Aliaga I, Fenselau H, Marth MK, Kottra G, Daniel H. Amino acid absorption and homeostasis in mice lacking the intestinal peptide transporter PEPT1. Am J Physiol Gastrointest Liver Physiol 2011; 301: G128-37. doi: 10.1152/ajpgi.00017.2011. [Crossref]
- Bockman DE, Ganapathy V, Oblak TG, Leibach FH. Localization of peptide transporter in nuclei and lysosomes of the pancreas. Int J Pancreatol 1997; 22: 221-5. doi: 10.1007/BF02788388. [Crossref]
- Zhou X, Thamotharan M, Gangopadhyay A, Serdikoff C, Adibi SA. Characterization of an oligopeptide transporter in renal lysosomes. Biochim Biophys Acta 2000; 1466: 372-8.
- Momburg F, Neefjes JJ, Hammerling GJ. Peptide selection by MHC-encoded TAP transporters. Curr Opin Immunol 1994; 6: 32-7.
- Roelse J, Gromme M, Momburg F, Hammerling G, Neefjes J. Trimming of TAP-translocated peptides in the endoplasmic reticulum and in the cytosol during recycling. J Exp Med 1994; 180: 1591-7.
- Tsuji A. Tissue selective drug delivery utilizing carrier-mediated transport systems. J Control Release 1999; 62: 239-44.
- Zhang Y, Sun J, Sun Y, Wang Y, He Z. Prodrug design targeting intestinal PepT1 for improved oral absorption: design and performance. Curr Drug Metab 2013; 14: 675-87.
- Sugawara M, Huang W, Fei YJ, Leibach FH, Ganapathy V, Ganapathy ME. Transport of valganciclovir, a ganciclovir prodrug, via peptide transporters PEPT1 and PEPT2. J Pharm Sci 2000; 89: 781-9. doi: 10.1002/(SICI)1520-6017(200006)89:6<781::AIDJPS10>3.0.CO;2-7 [Crossref]
- Smith MW, Gumbleton M. Endocytosis at the blood-brain barrier: from basic understanding to drug delivery strategies. J Drug Target 2006; 14: 191-214. doi: 10.1080/10611860600650086. [Crossref]
- Rajendran L, Knolker HJ, Simons K. Subcellular targeting strategies for drug design and delivery. Nat Rev Drug Discov 2010; 9: 29-42. doi: 10.1038/nrd2897. [Crossref]
- Omidi Y, Hollins AJ, Benboubetra M, Drayton R, Benter IF, Akhtar S. Toxicogenomics of non-viral vectors for gene therapy: a microarray study of lipofectin- and oligofectamine-induced gene expression changes in human epithelial cells. J Drug Target 2003; 11: 311-23. doi: 10.1080/10611860310001636908. [Crossref]
- Omidi Y, Barar J, Akhtar S. Toxicogenomics of cationic lipid-based vectors for gene therapy: impact of microarray technology. Curr Drug Deliv 2005; 2: 429-41.
- Omidi Y, Barar J, Heidari HR, Ahmadian S, Yazdi HA, Akhtar S. Microarray analysis of the toxicogenomics and the genotoxic potential of a cationic lipid-based gene delivery nanosystem in human alveolar epithelial a549 cells. Toxicol Mech Methods 2008; 18: 369-78. doi: 10.1080/15376510801891286. [Crossref]
- Nomani A, Haririan I, Rahimnia R, Fouladdel S, Gazori T, Dinarvand R, et al. Physicochemical and biological properties of self-assembled antisense/poly(amidoamine) dendrimer nanoparticles: the effect of dendrimer generation and charge ratio. Int J Nanomedicine 2010; 5: 359-69.
- Kafil V, Omidi Y. Cytotoxic impacts of linear and branched polyethylenimine nanostructures in a431 cells. Bioimpacts 2011; 1: 23-30. doi: 10.5681/bi.2011.004. [Crossref]
- Barar J, Aghanejad A, Fathi M, Omidi Y. Advanced drug delivery and targeting technologies for the ocular diseases. Bioimpacts 2016; 6: 49-67. doi: 10.15171/bi.2016.07. [Crossref]
- Schlachetzki F, Zhu C, Pardridge WM. Expression of the neonatal Fc receptor (FcRn) at the blood-brain barrier. J Neurochem 2002; 81: 203-6.
- Schnitzer JE. gp60 is an albumin-binding glycoprotein expressed by continuous endothelium involved in albumin transcytosis. Am J Physiol 1992; 262: H246-54.
- Vogel SM, Minshall RD, Pilipovic M, Tiruppathi C, Malik AB. Albumin uptake and transcytosis in endothelial cells in vivo induced by albumin-binding protein. Am J Physiol Lung Cell Mol Physiol 2001; 281: L1512-22.
- Caram-Salas N, Boileau E, Farrington GK, Garber E, Brunette E, Abulrob A, et al. In vitro and in vivo methods for assessing FcRn-mediated reverse transcytosis across the blood-brain barrier. Methods Mol Biol 2011; 763: 383-401. doi: 10.1007/978-1-61779-191-8_26. [Crossref]
- Cooper PR, Ciambrone GJ, Kliwinski CM, Maze E, Johnson L, Li Q, et al. Efflux of monoclonal from rat brain by neonatal Fc receptor, FcRn. Brain Res 2013; 1534: 13-21. doi: 10.1016/j.brainres.2013.08.035. [Crossref]
- Christianson GJ, Sun VZ, Akilesh S, Pesavento E, Proetzel G, Roopenian DC. Monoclonal antibodies directed against human FcRn and their applications. MAbs 2012; 4: 208-16. doi: 10.4161/mabs.4.2.19397. [Crossref]
- Piper RC, Luzio JP. Late endosomes: sorting and partitioning in multivesicular bodies. Traffic 2001; 2: 612-21.
- Dice JF. Selective degradation of cytosolic proteins by lysosomes. Ann N Y Acad Sci 1992; 674: 58-64.
- Luzio JP, Pryor PR, Gray SR, Gratian MJ, Piper RC, Bright NA. Membrane traffic to and from lysosomes. Biochem Soc Symp 2005; 77-86.
- Dice JF, Terlecky SR. Targeting of cytosolic proteins to lysosomes for degradation. Crit Rev Ther Drug Carrier Syst 1990; 7: 211-33.
- Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev 2002; 54: 431-67.
- Gumbleton M. Caveolae-mediated membrane transport. Adv Drug Deliv Rev 2001; 49: 217-21.
- Gumbleton M, Hollins AJ, Omidi Y, Campbell L, Taylor G. Targeting caveolae for vesicular drug transport. J Control Release 2003; 87: 139-51.
- DeGrella RF, Simoni RD. Intracellular transport of cholesterol to the plasma membrane. J Biol Chem 1982; 257: 14256-62.
- Kaplan MR, Simoni RD. Transport of cholesterol from the endoplasmic reticulum to the plasma membrane. J Cell Biol 1985; 101: 446-53.
- Xu L, Guo R, Xie Y, Ma M, Ye R, Liu X. Caveolae: molecular insights and therapeutic targets for stroke. Expert Opin Ther Targets 2015; 19: 633-50. doi: 10.1517/14728222.2015.1009446. [Crossref]
- Bertino EM, Williams TM, Nana-Sinkam SP, Shilo K, Chatterjee M, Mo X, et al. Stromal Caveolin-1 Is Associated With Response and Survival in a Phase II Trial of nab-Paclitaxel With Carboplatin for Advanced NSCLC Patients. Clin Lung Cancer 2015; 16: 466-74. doi: 10.1016/j.cllc.2015.05.004. [Crossref]
- Sripriyalakshmi S, Anjali CH, George PD, Rajith B, Ravindran A. BSA nanoparticle loaded atorvastatin calcium--a new facet for an old drug. PLoS One 2014; 9: e86317. doi: 10.1371/journal.pone.0086317. [Crossref]
- Hwang ME, Keswani RK, Pack DW. Dependence of PEI and PAMAM Gene Delivery on Clathrin- and Caveolin-Dependent Trafficking Pathways. Pharm Res 2015; 32: 2051-9. doi: 10.1007/s11095-014-1598-6. [Crossref]
- Bohmer N, Jordan A. Caveolin-1 and CDC42 mediated endocytosis of silica-coated iron oxide nanoparticles in HeLa cells. Beilstein J Nanotechnol 2015; 6: 167-76. doi: 10.3762/bjnano.6.16. [Crossref]
- Popova NV, Deyev IE, Petrenko AG. Clathrin-mediated endocytosis and adaptor proteins. Acta Naturae 2013; 5: 62-73.
- Godlee C, Kaksonen M. Review series: From uncertain beginnings: initiation mechanisms of clathrin-mediated endocytosis. J Cell Biol 2013; 203: 717-25. doi: 10.1083/jcb.201307100. [Crossref]
- Miller SE, Mathiasen S, Bright NA, Pierre F, Kelly BT, Kladt N, et al. CALM regulates clathrin-coated vesicle size and maturation by directly sensing and driving membrane curvature. Dev Cell 2015; 33: 163-75. doi: 10.1016/j.devcel.2015.03.002. [Crossref]
- Zhu J, Zhou K, Hao JJ, Liu J, Smith N, Zhan X. Regulation of cortactin/dynamin interaction by actin polymerization during the fission of clathrin-coated pits. J Cell Sci 2005; 118: 807-17. doi: 10.1242/jcs.01668. [Crossref]
- Smythe E. Role of the rab5 guanine nucleotide exchange factor, Rme-6, in the regulation of clathrin-coated vesicle uncoating. Methods Mol Biol 2015; 1298: 283-93. doi: 10.1007/978-1-4939-2569-8_24. [Crossref]
- Monsalve Y, Tosi G, Ruozi B, Belletti D, Vilella A, Zoli M, et al. PEG-g-chitosan nanoparticles functionalized with the monoclonal antibody OX26 for brain drug targeting. Nanomedicine (Lond) 2015; 10: 1735-50. doi: 10.2217/nnm.15.29. [Crossref]
- Liu Z, Zhao H, Shu L, Zhang Y, Okeke C, Zhang L, et al. Preparation and evaluation of Baicalin-loaded cationic solid lipid nanoparticles conjugated with OX26 for improved delivery across the BBB. Drug Dev Ind Pharm 2015; 41: 353-61. doi: 10.3109/03639045.2013.861478. [Crossref]
- Liu Z, Zhang L, He Q, Liu X, Okeke CI, Tong L, et al. Effect of Baicalin-loaded PEGylated cationic solid lipid nanoparticles modified by OX26 antibody on regulating the levels of baicalin and amino acids during cerebral ischemia-reperfusion in rats. Int J Pharm 2015; 489: 131-8. doi: 10.1016/j.ijpharm.2015.04.049. [Crossref]
- Yue PJ, He L, Qiu SW, Li Y, Liao YJ, Li XP, et al. OX26/CTX-conjugated PEGylated liposome as a dual-targeting gene delivery system for brain glioma. Mol Cancer 2014; 13: 191. doi: 10.1186/1476-4598-13-191. [Crossref]
- Bao H, Jin X, Li L, Lv F, Liu T. OX26 modified hyperbranched polyglycerol-conjugated poly(lactic-co-glycolic acid) nanoparticles: synthesis, characterization and evaluation of its brain delivery ability. J Mater Sci Mater Med 2012; 23: 1891-901. doi: 10.1007/s10856-012-4658-7. [Crossref]
- Pang Z, Lu W, Gao H, Hu K, Chen J, Zhang C, et al. Preparation and brain delivery property of biodegradable polymersomes conjugated with OX26. J Control Release 2008; 128: 120-7. doi: 10.1016/j.jconrel.2008.03.007. [Crossref]
- Aktas Y, Yemisci M, Andrieux K, Gursoy RN, Alonso MJ, Fernandez-Megia E, et al. Development and brain delivery of chitosan-PEG nanoparticles functionalized with the monoclonal antibody OX26. Bioconjug Chem 2005; 16: 1503-11. doi: 10.1021/bc050217o. [Crossref]
- Gosk S, Vermehren C, Storm G, Moos T. Targeting anti-transferrin receptor antibody (OX26) and OX26-conjugated liposomes to brain capillary endothelial cells using in situ perfusion. J Cereb Blood Flow Metab 2004; 24: 1193-204. doi: 10.1097/01.WCB.0000135592.28823.47. [Crossref]
- Wang L, Hao Y, Li H, Zhao Y, Meng D, Li D, et al. Co-delivery of doxorubicin and siRNA for glioma therapy by a brain targeting system: angiopep-2-modified poly(lactic-co-glycolic acid) nanoparticles. J Drug Target 2015; 23: 832-46. doi: 10.3109/1061186X.2015.1025077. [Crossref]
- Cabezon I, Manich G, Martin-Venegas R, Camins A, Pelegri C, Vilaplana J. Trafficking of Gold Nanoparticles Coated with the 8D3 Anti-Transferrin Receptor Antibody at the Mouse Blood-Brain Barrier. Mol Pharm 2015; 12: 4137-45. doi: 10.1021/acs.molpharmaceut.5b00597. [Crossref]
- Zhang B, Sun X, Mei H, Wang Y, Liao Z, Chen J, et al. LDLR-mediated peptide-22-conjugated nanoparticles for dual-targeting therapy of brain glioma. Biomaterials 2013; 34: 9171-82. doi: 10.1016/j.biomaterials.2013.08.039. [Crossref]
- Cui Y, Xu Q, Chow PK, Wang D, Wang CH. Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment. Biomaterials 2013; 34: 8511-20. doi: 10.1016/j.biomaterials.2013.07.075. [Crossref]
- Tosi G, Badiali L, Ruozi B, Vergoni AV, Bondioli L, Ferrari A, et al. Can leptin-derived sequence-modified nanoparticles be suitable tools for brain delivery? Nanomedicine (Lond) 2012; 7: 365-82. doi: 10.2217/nnm.11.98. [Crossref]
- Tzeng SY, Green JJ. Therapeutic nanomedicine for brain cancer. Ther Deliv 2013; 4: 687-704. doi: 10.4155/tde.13.38. [Crossref]
- Su Z, Xing L, Chen Y, Xu Y, Yang F, Zhang C, et al. Lactoferrin-modified poly(ethylene glycol)-grafted BSA nanoparticles as a dual-targeting carrier for treating brain gliomas. Mol Pharm 2014; 11: 1823-34. doi: 10.1021/mp500238m. [Crossref]
- Clark AJ, Davis ME. Increased brain uptake of targeted nanoparticles by adding an acid-cleavable linkage between transferrin and the nanoparticle core. Proc Natl Acad Sci U S A 2015; 112: 12486-91. doi: 10.1073/pnas.1517048112. [Crossref]
- Han L, Kong DK, Zheng MQ, Murikinati S, Ma C, Yuan P, et al. Increased Nanoparticle Delivery to Brain Tumors by Autocatalytic Priming for Improved Treatment and Imaging. ACS Nano 2016; 10: 4209-18. doi: 10.1021/acsnano.5b07573. [Crossref]
- Zong T, Mei L, Gao H, Cai W, Zhu P, Shi K, et al. Synergistic dual-ligand doxorubicin liposomes improve targeting and therapeutic efficacy of brain glioma in animals. Mol Pharm 2014; 11: 2346-57. doi: 10.1021/mp500057n. [Crossref]
- Wei L, Guo XY, Yang T, Yu MZ, Chen DW, Wang JC. Brain tumor-targeted therapy by systemic delivery of siRNA with Transferrin receptor-mediated core-shell nanoparticles. Int J Pharm 2016; 510: 394-405. doi: 10.1016/j.ijpharm.2016.06.127. [Crossref]
- Zhang TT, Li W, Meng G, Wang P, Liao W. Strategies for transporting nanoparticles across the blood-brain barrier. Biomater Sci 2016; 4: 219-29. doi: 10.1039/c5bm00383k. [Crossref]
- Cheng Y, Morshed RA, Auffinger B, Tobias AL, Lesniak MS. Multifunctional nanoparticles for brain tumor imaging and therapy. Adv Drug Deliv Rev 2014; 66: 42-57. doi: 10.1016/j.addr.2013.09.006. [Crossref]