The study of association between reduced folate carrier 1 (RFC1) polymorphism and non-syndromic cleft lip/palate in Iranian population
Bioimpacts, 7(4), 263-268; DOI:10.15171/bi.2017.31
Original Research
The study of association between reduced folate carrier 1 (RFC1) polymorphism and non-syndromic cleft lip/palate in Iranian population
Behnoosh Soghani1, Asghar Ebadifar1,2 ,*, Hamid Reza Khorram Khorshid3, Koorosh Kamali4, Roya Hamedi5, Fatemeh Aghakhani Moghadam3
1
Dentofacial Deformities Research Center Research Institute of Dental Sciences, Faculty of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2
Department of Orthodontic, Faculty of Dentistry, Shahid Behehsti University of Medical Sciences, Tehran, Iran
3
Genetic Research Centre, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran
4
Department of Public Health, School of Public Health, Zanjan University of Medical Sciences, Zanjan, Iran
5
Dental Carries Prevention Research Center, Qazvin University of Medical Sciences, Qazvin, Iran
*Corresponding author: Asghar Ebadifar, Email: a.ebadifar@sbmu.ac.ir
Abstract
Introduction:
Cleft lip/palate is one of the most common congenital defects and is supposed to have multifactorial etiology, including a complex interaction between genetics and environment. Reduced folate carrier 1 (RFC1) gene takes part in folate transportation within the cells. In this study, the association of A80G polymorphism in the RFC1 gene with the non-syndromic cleft lip/palate (nsCL/P) was investigated in Iranian infants for the first time.
Methods: In this case-control survey, 122 Iranian infants with nsCL/P and 164 healthy infants were investigated for RFC1 polymorphism by PCR and RFLP methods. The results were statistically compared with control group, odds ratios with 95% CI were estimated by univariate and multivariate logistic regression model and a P <0.05 was considered statistically significant.
Results: The RFC1 G allele was significantly higher (P=0.001; OR=7, 95% CI: 4.7-10.2) in the cases (60.3%) compared with the controls (17.9%). Not only the RFC1 AG genotype was significantly higher (P<0.001; OR=44, 95% CI: 14.6-133) in cases (67.8%) than the controls (27.4%), but also GG genotype (P<0.001; OR=85, 95% CI: 20.5-352) was much higher in cases (26.4%) than the controls (4.3%).
Conclusion: Our study indicated that the RFC1 (A80G) polymorphism was associated with the nsCL/P in Iranian population. Moreover, 80GG homozygosity was significant in the cases. The presence of G allele can be considered as a risk factor for the nsCL/P. Infants with the GG and AG genotypes were more prone to cleft lip/palate as compared to the AA ones. This finding emphasizes the role of RFC1 gene and the intracellular levels of folate.
Keywords: Cleft lip/palate, Polymorphism, RFC1 gene
Introduction
Cleft lip with/without cleft palate (CL/P) is one of the most common congenital abnormalities with multifactorial etiology, including environmental and genetic factors.1 CL/P shows a prevalence of 3.73 per 1000 in Iranian newborns.2 Genetic factors play a very important role in this disorder because whole genome analysis shows 18 genetic risk loci related to these defects.3 While the gene identification process for this defect is premature, the steps are rapidly increasing. By the aid of identifying candidate genes of non-syndromic CL/P (nsCL/Ps); not only prediction but also prevention will be possible.4
Various studies demonstrate the pathogenicity of folate decreasing in periconceptional period and role of folic acid in preventing neural tube defects.5,6 Among genes taking part in the folic acid metabolism, the reduced folate carrier (RFC1), also known as SLC19A1, has been shown to be associated with the nsCL/P.7Folate is a highly hydrophilic molecule that cannot cross the biological membranes by diffusion solely. This gene have a role as an organic anion exchanger in folic acid absorption by the bi-directional action of transporting 5-methyltetrahydrofolate and thiamine monophosphate.8,9 5-methyltetrahydrofolate is the metabolic active version of folate which inserts into cells and constructs intracellular folate concentration.10
RFC1 (SLC19A1) gene is located on chromosome 21 (21q22.2-q22.3).11SLC19A1 polymorphism consisting of substitution of adenine to guanine in exon 2 at nucleotide position 80 (A80G), changes the 27th amino acid of protein from histidine (CAC) to arginine (CGC).12,13
Several associations have demonstrated the relationship between polymorphism of RFC1 gene (Fig. 1) and the risk of nsCL/P, conotruncal heart defects, Down syndrome,15 spina bifida,16 neural tube defects,17 and lymphoblastic leukemia.18
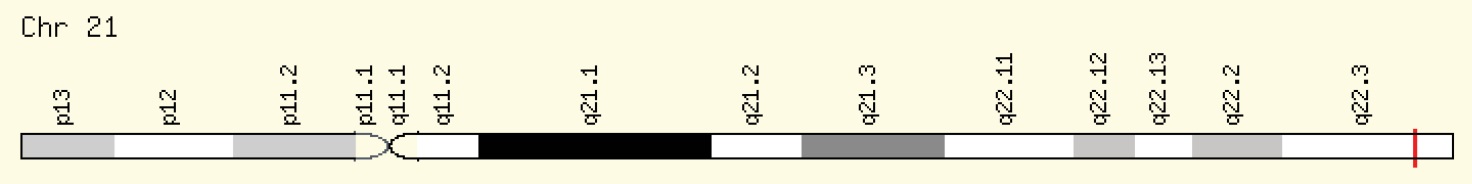
Fig. 1.
RFC1 Gene in genomic location.14
Some previous researches have suggested the association between the nsCL/P and G allele in the RFC1 gene, thus it could be a candidate gene for the nsCL/P.19-22
In 2004, Schultz et al examined fifteen candidate regions for their relationships to nsCL/P. Analysis of 126 Filipino cases and 218 controls revealed a borderline significant association between RFC1 gene and nsCL/P.23 Wang et al in a Chinese population,20 Girardi et al in Italian population21and Lakkakula et al in south Indian population22 indicated that the RFC1 gene variant increases occurrence risk of nsCL/P. However, this is contrary to the studies which were done by Shaw et al in Californian infants,24 Pei et al in Chinese population25 and Mostowska et al in Polish population,26 demonstrating the role of race and genetic differences in different populations. The literature review of association studies of RFC1 polymorphism and nsCL/P have been conflicting.6,16,19-23,25,27 Numerous researches evaluated the correlation between RFC1 gene polymorphism and ns CL/P but there is no evidence of Iranian population examined. The main aim of the present case-control study was to investigate the association between RFC1 gene polymorphism (A80G) and the risk of ns CL/P in Iranian population for the first time.
Materials and Methods
Subjects
In this survey, samples were recruited from Mofid Pediatric hospital in Tehran, Iran, from 2013 to 2016. A sample of 122 newborns with a primary diagnosis of ns CL/P was collected. Control group consisting 164 Iranian newborns, simulated to cases regarding socioeconomic, traits and age, were gathered and their blood samples were collected. Cases that had other facial or skeletal anomalies (such as bifida uvula and lip pits), conotruncal heart defects, anomalies of the other organs or metabolic disorders and mother’s history of methotrexate consumption in pregnancy were excluded.
Genotyping
Five mL of peripheral blood of infants were gathered in the tube containing 200 μL of 0.5 M EDTA and stored at -80°C, then DNA was extracted using simple salting out method.28
Genotyping of the RFC1 (rs1051266, A80G) polymorphism was accomplished by polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) methods with Hha1 enzyme (Fermentas, Germany).29,30 The sequences are displayed in Table 1.
Concisely, a total volume of 25 μL including 30 ng of genomic DNA, 5 μM of each primer, 1 μL dNTPs mix (Fermentas, Germany), 2.5 μL 10×buffer and 0.5 U of Taq DNA polymerase (Fermentas, Germany) with 1.5 mM MgCl2 was formulated in the 0.5 mL microtube for amplification of the objective sequences. Amplification conditions began with an inceptive denaturation step of 4 minutes at 95°C, proceeded by 33 cycles of 45 seconds denaturation (94°C), 30 seconds annealing (60°C) and 40 seconds extension (72°C), terminated by an ultimate extension for 5 minutes (72°C) and eventually chilling to 4°C. The PCR product of the rs1051266 polymorphism was broken down with the IUHha1 restriction enzymes at 37°C overnight (New England BioLabs, Beverly, USA). All PCR products were subjected to 8% polyacrylamide gel electrophoresis then colored by silver nitrate. The model of restriction fragments for Hha1 is shown in Table 1. All genotyping was executed blinded.
|
Table 1.
Primer sequences and their PCR product sizes, restriction enzymes, and RFLP fragments for the RFC1 rs1051266 A/G polymorphism
|
|
SNPs
|
Primer sequence (5’→3)
|
Product size
|
RFLP Fragments (bp)
|
Cuts positions
|
|
RFC1 (rs1051266 A/G)
|
F: AGCGTCACCTTCGTCCC |
230 |
A allele:162+68 |
162 |
| R: TCCCGCGTGAAGTTCTTG |
G allele=125+37+68 |
125, 162 |
Statistical analysis
Statistical analyses were accomplished using SPSS version 11.5 (SPSS Inc, Chicago, USA). Chi-square (χ2) test was used to compare the allele frequencies between the study groups. Odds Ratios with 95% confidence Interval were estimated by univariate and multivariate (sex, mother age, parents smoking, folic acid consumption during pregnancy and other CL/P in the family) logistic regression model. The P < 0.05 was considered for statistically significant results.
Results
Cases consisted of 122 Iranian infants with cleft lip with or without cleft palate and 164 healthy infants as the control group. The nsCL/P samples consisted of 70 (57%) males and 52 (43%) females. The distributions of genotypes in the entire population, and in female and male subsets, using chi-square showed they were in Hardy-Weinberg equilibrium. The dispersal of genotypes and allele frequencies of the RFC1 (A80G) polymorphism are presented in Table 2.
|
Table 2
. The genotype and allele frequencies of the RFC1 (A80G) polymorphism in nsCL/P case and control
|
|
Variable
|
Controls, No. (%)
|
Cases, No. (%)
|
P
value
|
OR (95% CI)
|
|
Allele
|
| A |
269 (82.0) |
96 (39.6) |
Reference Allele |
| G |
59 (17.9) |
146 (60.3) |
0.001 |
7 (4.7-10.2) |
|
Genotype
|
| AA |
112 (68.3) |
7 (5.8) |
Reference Genotype |
| AG |
45 (27.4) |
82 (67.8) |
<0.001* |
44 (14.6-133) |
| GG |
7 (4.3) |
32 (26.4) |
<0.001* |
85 (20.5-352) |
| Yes |
0 (0) |
10(8.2) |
|
|
Results of this study demonstrated significant differences in allele frequency and genotype distribution of RFC1 polymorphism between the case and control groups. The RFC1 G allele was significantly higher (P=0.001; OR=7, 95% CI: 4.7-10.2) in the cases (60.3%) compared with the control group (17.9%).
According to univariate logistic regression analysis, not only the RFC1 AG genotype was significantly higher (P < 0.001; OR = 44, 95% CI: 14.6-133) in case group (67.8%) than the control group (27.4%), but also GG genotype (P < 0.001; OR = 85, 95% CI: 20.5-352) was much higher in case group (26.4%) than the control group (4.3%). Therefore, AG and GG genotypes are risk factors for CL/P. But, by adjustment the effect of genotypes by sex, mother age, parents smoking, folic acid consumption during pregnancy and other CL/P in family, AG and GG genotypes were also significantly associated with CL/P with multivariate logistic regression. Also, the frequency of homozygosity for G allele in the cases is much greater than controls. This study showed the association ofA80G polymorphism in the RFC1 gene (SLC19A1), with nsCL/P in Iranian infants for the first time.
Discussion
Reduced folate carrier1 (RFC1) is one of the enzymes in the metabolism of folic acid which transports the active form of folic acid (5-methyltetrahydrofolate) into cells.10 A change of A allele to G allele results in a mutant protein that can affect enzymatic activity and has a potential role as a risk factor for homocysteinemia, Down Syndrome, cardiovascular diseases,31,32 neural tube defects and nonsyndromic cleft lip/palate,21 in contrast to conflicting literature.
Cleft lip with or without cleft palate (CL/P) is one of the most common congenital abnormalities with multifactorial etiology including environmental and genetic factors. Among genes taking part in folic acid metabolism, RFC1, also known as SLC19A1, has been shown associated with nsCL/P.6 Individuals carrying a specific polymorphism of SLC19A1 (G 80A) have lower levels of folate. As folate transport across cell membranes is mediated in part by RFC1v (Fig. 2 and 3),9 variants within this gene may influence nsCL/P risk via an effect on folate and/or homocysteine levels.
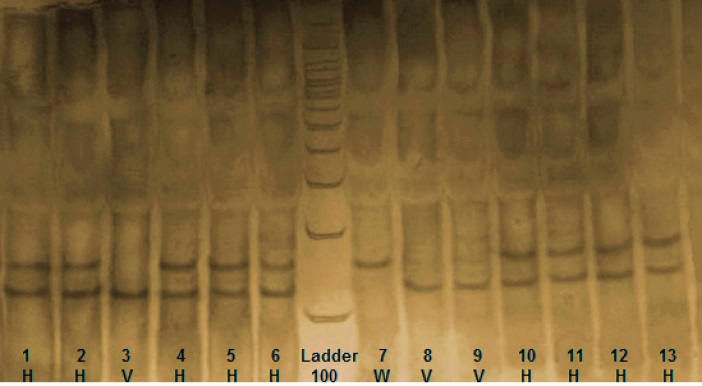
Fig. 2 .
RFC1 (SLC19A1) RFLP. Three genotypes from nsCL/P cases indicating the wild type (W), heterovariant (H) and homovariant (V). After digestion of PCR product with the restriction enzyme Hha1, one specific band of 162 bp was indicated in wild genotype, two specific bands of 162 bp and 125 bp were indicated in heterovariant genotype and one specific band of 125 bp was indicated in homovariant genotype.
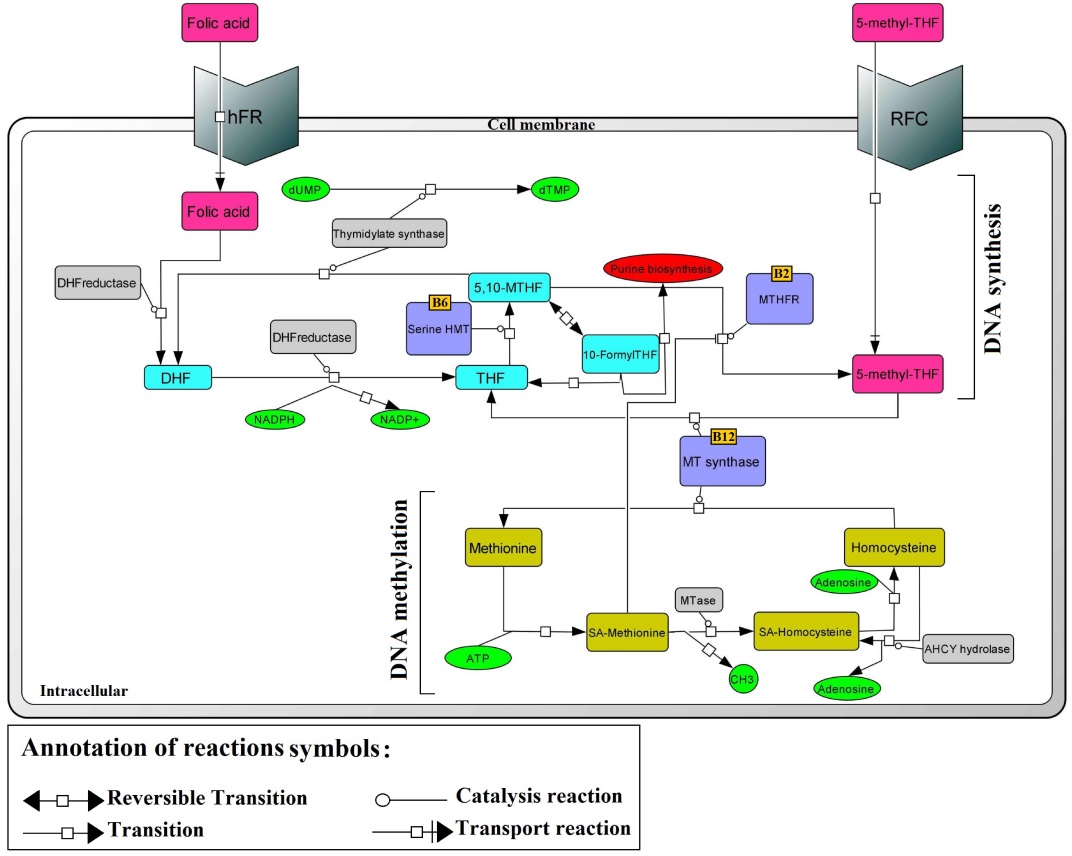
Fig. 3 .
Schematic diagram of RFC1 in folate metabolism. RFC, reduced folate carrier; hFR, human folate receptor; MTR, methionine synthase; MTHFR, methylenetetrahydrofolate reductase; SHMT, serine hydroxymethlytransferase; TS, thymidylate synthase; THF, tetrahydrofolate; DHF, dihydrofolate; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; dUMP, deoxyuridine monophosphate; dTMP, deoxythymidine monophosphate.33
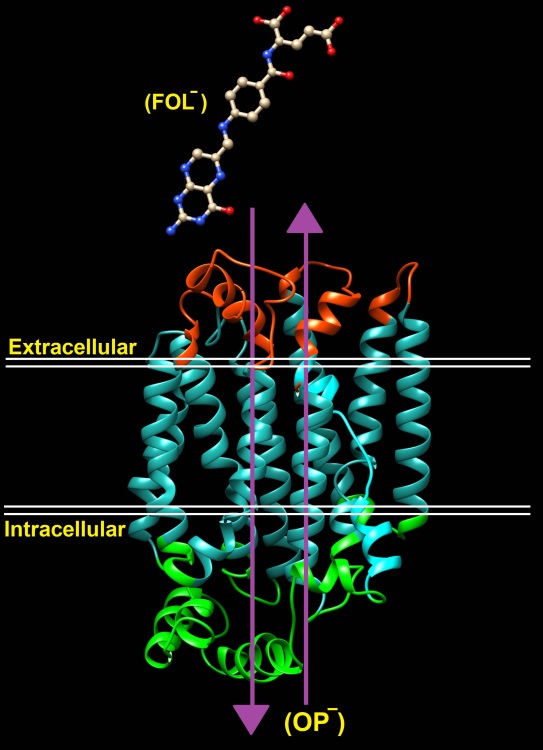
Fig. 4 .
Overview of folate transporter (RFC (reduced folate carrier/SLC19A1), organic phos- phate [OP -]).
This study was executed to determine whether the RFC1 (rs1051266, A80G) polymorphism has a relationship with increased risk of nsCL/P in Iranian infants consisting 122 cases and 164 controls. The results proved that substitution of A allele by G allele in RFC1 was associated with nsCL/P in Iranian population. The frequency of RFC1 AG genotype in cases (67.8%) was nearly 2.5 times more than that of controls (27.4%). The frequency of RFC1 GG genotype in cases (26.4%) was approximately 6 times more than that of controls (4.3%). The RFC1 G allele was much higher in the nsCL/P infants (60.3%) compared with control infants (17.9%). These results recommend the possible role of G allele as a risk factor for nsCL/P in the Iranian population with a strong effect.
Chango et al found lower plasma folate and higher homocysteine level in the cases who had GG genotype for RFC113 and it was an important step for later researches of RFC1.
Shaw et al studied the association between RFC1 (A80G) in Californian population and realized the risk of spina bifida and conotruncal heart defects among infants who had GG genotype of RFC1 and their mothers did not consume vitamins during pregnancy.29 These findings demonstrated gene-nutrient relations.
Vieira et al investigated the association of RFC1 polymorphism with orofacial clefts in Californian population. They found the relation of RFC1 with cleft lip only.19 Lakkakula et al in the Indian population evaluated the association between the RFC1 polymorphism and nsCL/P. Their samples consisted of 142 cases and 141 controls. They concluded that G allele (P = 0.050; OR = 1.40, 95% CI: 1.00-1.97) was associated with the nsCL/P.21 This finding seems to similar to our results based on the population (both Iran and India located at Asia). Therefore, G allele of RFC1 may be associated with nsCL/P in Asia; nevertheless, more studies should be carried out to address this issue.
Conclusion
Briefly, our study indicated a strong association between RFC1 (A80G) polymorphism and nsCL/P in Iranian population. Moreover, AG heterozygosity and GG homozygosity were significant among the Iranian cases. Presence of G allele can be considered as a risk factor for nsCL/P in Iranian infants. By considering the role of RFC1 in folate pathway and the importance of intracellular folate level, the results are indictable. Because gene-environment interaction and folate’s level play important roles in the nsCL/P etiology, further studies with different methods such as intracellular folate measurement should be taken into consideration.
Conflict of interests
All the authors declare that they have no competing interests.
Ethical approval
The survey was approved by Ethics Committee of the Dental Research Center in Shahid Beheshti University for dentofacial deformities (Code No. IR.SBMU.RIDS.REC.1395.195). The survey was also preceded by completing written consent forms by all parents.
Acknowledgments
The authors would like to thank the infants and their parents participating in this survey for their cooperation and completing informed consent, and Mofid Hospital staff for their kind helps in recruiting study subjects. The research was supported by Dentofacial Deformities Research Center, Research Institute of Dental Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran. Their financial support is therefore highly appreciated.
References
- Dixon MJ, Marazita ML, Beaty TH, Murray JC. Cleft lip and palate: understanding genetic and environmental influences. Nat Rev Genet 2011; 12:167-178. doi: 10.1038/nrg2933. [Crossref]
- Taher AA. Cleft lip and palate in Tehran. Cleft Palate Cranio J. 1992; 29:15-16.
- Peng HH, Chang NC, Chen KT, Lu JJ, Chang PY, Chang SC, et al. Nonsynonymous variants in MYH9 and ABCA4 are the most frequent risk loci associated with nonsyndromic orofacial cleft in Taiwanese population. BMC Med Genet 2016; 17:59. doi: 10.1186/s12881-016-0322-2. [Crossref]
- Aylward A, Cai Y, Lee A, Blue E, Rabinowitz D, Haddad J, et al. Using Whole Exome Sequencing to Identify Candidate Genes With Rare Variants In Nonsyndromic Cleft Lip and Palate. Genet Epidemiol 2016; 40:432-441. doi: 10.1002/gepi.21972. [Crossref]
- De-Regil Luz M, Peña-Rosas Juan P, Fernández-Gaxiola Ana C, Rayco-Solon P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev 2015; Dec 14;(12):CD007950. doi: 10.1002/14651858. [Crossref]
- Ebadifar A, Khorshid HRK, Kamali K, Zeinabadi, MS, Khoshbakht T, Ameli N. Maternal supplementary folate intake, methylenetetrahydrofolate reductase (MTHFR) C677t and A1298C polymorphisms and the risk of orofacial cleft in Iranian children. Avicenna J Med Biotechnol. 2015;7(2):80-84.
- Vieira AR, Murray JC, Trembath D, Orioli IM, Castilla EE, Cooper ME, et al. Studies of reduced folate carrier 1 (RFC1) A80G and 5,10-methylenetetrahydrofolate reductase (MTHFR) C677T polymorphisms with neural tube and orofacial cleft defects. Am J Med Genet A 2005; 135:220-223. doi: 10.1002/ajmg.a.30705. [Crossref]
- Zierhut H, Linet MS, Robison LL, Severson RK, Spector L. Family history of cancer and non-malignant diseases and risk of childhood acute lymphoblastic leukemia: A Children's Oncology Group Study. Cancer epidemiol 2012; 36:45-51. doi: 10.1016/j.canep.2011.06.004. [Crossref]
- Matherly LH, Hou Z, Deng Y. Human reduced folate carrier: translation of basic biology to cancer etiology and therapy. Cancer Metastasis Rev 2007; 26:111-128. doi: 10.1007/s10555-007-9046-2. [Crossref]
- Antony AC. The biological chemistry of folate receptors. Blood 1992; 79:2807-2820.
- Moscow JA, Gong M, He R, Sgagias MK, Dixon KH, Anzick SL, et al. Isolation of a gene encoding a human reduced folate carrier (RFC1) and analysis of its expression in transport-deficient, methotrexate-resistant human breast cancer cells. Cancer Res 1995; 55:3790-3794.
- Tolner B, Roy K, Sirotnak F. Structural analysis of the human RFC-1 gene encoding a folate transporter reveals multiple promoters and alternatively spliced transcripts with 5′ end heterogeneity. Gene 1998; 211:331-341.
- Chango A, Emery-Fillon N, de Courcy GP, Lambert D, Pfister M, Rosenblatt DS, et al. A polymorphism (80G-> A) in the reduced folate carrier gene and its associations with folate status and homocysteinemia. Mol Genet Metab 2000; 70:310-315. doi: 10.1006/mgme.2000.3034. [Crossref]
- Gong M, Cowan KH, Gudas J, Moscow JA. Isolation and characterization of genomic sequences involved in the regulation of the human reduced folate carrier gene (RFC1). Gene 1999;233: 21-31.
- Coppedè F, Lorenzoni V, Migliore L. The reduced folate carrier (RFC-1) 80A> G polymorphism and maternal risk of having a child with Down syndrome: a meta-analysis. Nutrients 2013; 5:2551-2563. doi: 10.3390/nu5072551. [Crossref]
- Shaw GM, Zhu H, Lammer EJ, Yang W, Finnell RH. Genetic variation of infant reduced folate carrier (A80G) and risk of orofacial and conotruncal heart defects. Am J Epidemiol 2003; 158:747-752.
- Shang Y, Zhao H, Niu B, Li WI, Zhou R, Zhang T, et al. Correlation of polymorphism of MTHFRs and RFC‐1 genes with neural tube defects in China. Birth Defects Res A Clin Mol Teratol 2008; 82:3-7. doi: 10.1002/bdra.20416. [Crossref]
- Dawidowska M, Kosmalska M, Sędek Ł, Szczepankiewicz A, Twardoch M, Sonsala A, et al. Association of germline genetic variants in RFC, IL15 and VDR genes with minimal residual disease in pediatric B-cell precursor ALL. Sci Rep 2016; 6:29427. doi: 10.1038/srep29427. [Crossref]
- Vieira AR, Cooper ME, Marazita ML, Castilla EE, Orioli IM. Reduced folate carrier 1 (RFC1) is associated with cleft of the lip only. Braz J Med Biol Res 2008; 41:689-693.
- Wang Y, Song X, Guo J, Zhu W. [Relationship between genetic polymorphisms of RFC1 A80G and nonsymdromic cleft lip with or without palate]. Wei Sheng Yan Jiu 2009; 38:276-279.
- Girardi A, Martinelli M, Cura F, Palmieri A, Carinci F, Sesenna E, et al. RFC1 and non-syndromic cleft lip with or without cleft palate: an association based study in Italy. J Craniomaxillofac Surg 2014; 42:1503-1505.
- Lakkakula B, Murthy J, Gurramkonda VB. Relationship between reduced folate carrier gene polymorphism and non-syndromic cleft lip and palate in Indian population. J Matern Fetal Neonatal Med 2015; 28:329-332. doi: 10.3109/14767058.2014.916677. [Crossref]
- Schultz RE, Cooper ME, Daack-Hirsch S, Shi M, Nepomucena B, Graf KA, et al. Targeted scan of fifteen regions for nonsyndromic cleft lip and palate in Filipino families. Am J Med Genet A 2004; 125A:17-22.
- Shaw GM, Lammer EJ, Zhu H, Baker MW, Neri E, Finnell RH. Maternal periconceptional vitamin use, genetic variation of infant reduced folate carrier (A80G), and risk of spina bifida. Am J Med Genet 2002; 108:1-6.
- Pei L, Zhu H, Zhu J, Ren A, Finnell RH, Li Z. Genetic variation of infant reduced folate carrier (A80G) and risk of orofacial defects and congenital heart defects in China. Ann Epidemiol 2006; 16:352-356. doi: 10.1016/j.annepidem.2005.02.014. [Crossref]
- Mostowska A, Hozyasz KK, Jagodzinski PP. Maternal MTR genotype contributes to the risk of non-syndromic cleft lip and palate in the Polish population. Clin Genet 2006; 69:512-517.
- Pei LJ, Ren AG, Hao L, Zhu HP, Zhu JH, Zhao WR, et al. [Study on the association between reduced folate carrier gene polymorphism and congenital heart defects and cleft lip with or without cleft palate]. Zhonghua Liu Xing Bing Xue Za Zhi 2004; 25:1063-1067.
- Rivero ER, Neves AC, Silva-Valenzuela MG, Sousa SO, Nunes FD. Simple salting-out method for DNA extraction from formalin-fixed, paraffin-embedded tissues. Pathol Res Pract 2006; 202:523-529. doi: 10.1016/j.prp.2006.02.007. [Crossref]
- Shaw GM, Zhu H, Lammer EJ, Yang W, Finnell RH. Genetic variation of infant reduced folate carrier (A80G) and risk of orofacial and conotruncal heart defects. Am J Epidemiol. 2003; 158(8):747-52.
- Kumari P, Ali A, Sukla KK, Singh SK, Raman R. Lower incidence of nonsyndromic cleft lip with or without cleft palate in females: Is homocysteine a factor? J Biosci 2013; 38:21-26.
- Sukla K, Raman R. Association of MTHFR and RFC1 gene polymorphism with hyperhomocysteinemia and its modulation by vitamin B12 and folic acid in an Indian population. Eur J Clin Nutr 2012; 66:111-118. doi: 10.1038/ejcn.2011.152. [Crossref]
- Scala I, Granese B, Sellitto M, Salomè S, Sammartino A, Pepe A, et al. Analysis of seven maternal polymorphisms of genes involved in homocysteine/folate metabolism and risk of Down syndrome offspring. Genet Med 2006; 8:409-416. doi: 10.109701.gim.0000228206.21793.82. [Crossref]
- De Silva N, Davis B. Iron, B 12 and folate. Medicine 2013; 41: 204-7.