Abstract
Introduction:
Superparamagnetic iron oxide nanoparticles (SPIONs) can be functionalized with various agents (e.g., targeting and therapeutic agents) and used for targeted imaging/therapy of cancer. In the present study, we engineered doxorubicin (DOX)-conjugated anti-mucin -1 (MUC-1) aptamer (Ap)-armed PEGylated SPIONs for targeted delivery of DOX molecules to the breast cancer MCF-7 cells.
Methods:
The SPIONs were synthesized using the thermal decomposition method and modified by polyethylene glycol (PEG) to maximize their biocompatibility and minimize any undesired cytotoxicity effects. Subsequently, DOX molecules were loaded onto the SPIONs, which were further armed with amine-modified MUC-1 aptamer by EDC/NHS chemistry.
Results: The morphologic and size analyses of nanoparticles (NPs) by transmission electron microscopy (TEM) and dynamic light scattering (DLS) revealed spherical and monodisperse MNPs with a size range of 5-64 nm. The FT-IR spectrophotometry and 1HNMR analysis confirmed the surface modification of NPs. The cytotoxicity assay of the aptamer-armed MNPs exhibited a higher death rate in the MUC-1 over-expressing MCF-7 cells as compared to the MUC-1 under-expressing MDA-MB-231 cells. The flow cytometry analysis of the engineered Ap-armed SPIONs revealed a higher uptake as compared to the SPIONs alone.
Conclusion: Based on our findings, the anti-MUC-1 Ap-armed PEGylated SPIONs loaded with DOX molecules could serve as an effective multifunctional theranostics for simultaneous detection and eradication of MUC-1-positive breast cancer cells.
Keywords: Breast cancer, Mucin-1 aptamer, Nanomedicine, SPION, Targeted drug delivery, Theranostics
Introduction
Breast cancer is one of the high-prevalence malignancies among females with relatively high mortality worldwide. According to some global reports, approximately 1.7 million new cases of metastatic/advanced breast cancers are diagnosed each year.1 To this end, a broad diversity of cancer detection approaches such as mammography, positron emission tomography (PET), single-photon emission computed tomography (SPECT) and magnetic resonance imaging (MRI) are used for the precise diagnosis and effective eradication of the malignant sites by improving the efficacy of cancer treatment modalities.2-4 Currently, various imaging and therapeutic approaches are used for the treatment of breast cancer.5-7
It should be noted that some of the currently used conventional therapeutic modalities are invasive and may inadvertently induce undesired side effects in the other healthy organs, tissues and cells. Such complications need to be controlled by specifically targeting of the diseased cells/tissue with the nanoscaled targeted drug delivery systems (DDSs), the so-called multimodal nanomedicines and theranostics.8
In fact, for the selectively and specifically enhancing of the effects of chemotherapies on the target cells/tissue, a number of researchers have capitalized on the development of all-in-one seamless nanosystems (NSs) for simultaneous targeting, imaging, and treatment of cancer. For this purpose, several advanced nanoparticles (NPs) have been developed and further decorated with various targeting agents such as antibodies (Abs), aptamers (Aps) or ligands as well as imaging agents such as radionuclides or fluorescent agents.9,10
So far, a large number of organic and inorganic NPs have been engineered and examined for their potential to serve as advanced nanoscaled DDSs. We have developed various NPs and NSs for targeted delivery of anticancer cytotoxic agents such doxorubicin (DOX), mitoxantrone (MTN), erlotinib (ELT), methotrexate (MTX), shikonin (SHK) and cisplatin (CDDP).11-19 Among different types of NPs, magnetic nanoparticles (MNPs) and superparamagnetic iron oxide nanoparticles (SPIONs) possess distinct features, including biocompatibility, high surface area, magnetic resonance (MR) imaging, and contrasting capability. SPIONs are considered as one of the most attractive NPs to serve as a DDS of different anticancer pharmaceuticals.20-23 In addition, they can be employed through either passive or active targeting mechanisms. Tumor cells, in coop with stromal cells, are able to form a permissive milieu, so-called tumor microenvironment (TME).
In solid tumors, anomalous biological phenomena occur within the TME, including aberrant metabolism of glucose and amino acids such as L-tryptophan by cancer cells, expression of several distinct molecular machineries by cancer cells, cooperation of the immune system with cancer cells, irregular angiogenesis with leaky endothelium and interstitial fluid with high pressure. The leaky tumor microvasculature (TMV) results in an enhanced permeability and retention (EPR) effect that is the basis of the passive targeting mechanism since NPs can accumulate within the TME through pores and gaps of endothelial cells of the TMV.24-26
In the active targeting mechanism, NPs such as SPIONs are armed with an appropriate homing agent (e.g., Ab, Ap, ligand), upon which the engineered NSs get an ability to target the diseased cells/tissues specifically. Various chemotherapy agents such as DOX, MTX, and CDDP can be loaded onto the NPs. These chemotherapies can induce profound toxicity in the human cells nonspecifically, while their formulation as nanoscaled targeted DDSs can significantly reduce their undesired toxicity and adverse reactions in the healthy cells/tissues.
Of the chemotherapy agents, DOX is an antineoplastic drug that is metabolized to doxorubicinol (DOXol), which is the major metabolite of DOX and can preferentially accumulate in the heart during the course of chronic administration of DOX, resulting in a profound cardiotoxicity. DOXol can interfere with iron by aconitase 1 and affect the regulation of calcium by interfering with Na+/K+ pumps of sarcoplasmic reticulum, F0F1 ATPase proton pump of mitochondria, ATP2A2 and Ryanodine receptor 2.27-29 The interactions/regulations of DOX metabolites with the some of the normal functions of organs can result in some degrees of toxicity (e.g., cardiotoxicity).
Further, multidrug-resistance (MDR) can also be activated that may limit the clinical benefits of DOX.30-33 On the verge of such undesired toxicity, many researchers have aimed at reducing the unwanted toxicity of DOX through the development of nanoformulation loaded with DOX molecules.
For the production of targeted nanoformulation, various cancer biomarkers have been exploited, including folate receptors and mucin 1 (MUC1). Of the cancer markers, MUC, as a well-studied transmembrane glycoprotein, shows at least 10-fold overexpression in most of the malignant adenocarcinomas such as breast cancer.34 This transmembrane oncoprotein was shown to associate with the HER2-overexpressing breast cancer cells, and hence, functionalization of NPs with a MUC1 targeting ligand can result in the receptor-mediated endocytosis of NPs. The resultant targeted NPs can specifically interact with cancer cells, resulting in an increased intracellular concentration of drug molecules inside the cancerous cells.35
On the basis of these fundamental concepts, we prepared PEGylated SPIONs armed with an aptamer specific to MUC1. The nanoscaled targeted NS loaded with DOX was designed for the pH-triggered release of DOX molecules in the target cells, and hence, an evaluated cytotoxic impact on the human breast cancer MDA-MB-231 and MCF-7 cells.
Materials and Methods
Materials
N, N-dicyclohexylcarbodiimide (DCC), fluorescein isothiocyanate (FITC), N-hydroxysuccinimidde (NHS), poly(ethylene glycol) bis(carboxymethyl) ether-600 and poly(ethylene glycol) methyl ether 2000 were purchased from Sigma-Aldrich Chemie GmbH (Munich, Germany) and used without further purification. The human breast cancer, MCF-7, and MDA-MB-231 cell lines were obtained from National Cell Bank of Iran, Pasteur Institute (Tehran, Iran). All media and cell culture components were purchased from Invitrogen (Karlsruhe, Germany).
Aptamer (5′-NH2(C6)GGGAGACAAGAATAAACGCTCAAGCAGTTGATCCTTTGGATACCCTGGTTCGACAGGAGGCTCACAACAGGC-3′) was purchased from Takapoo Zist Co. (Tehran, Iran).
Instrumentation
Dynamic light scattering (DLS) and ξ-potential of the synthesized SPIONs were carried out using Nanotrac Wave (Microtrac Inc, Montgomeryville, PA, USA). The size and morphology were measured by a transmission electron microscope (TEM) Carl Zeiss, LEO 906E (Jena, Germany). The FT-IR analysis was performed using Bruker FT-IR, (Bruker Optik GmbH, Ettlingen, Germany) to confirm the functional group(s) in the range of 400–4000 cm−1. The 1HNMR spectra were recorded using 400 MHz spectrometer (Bruker Optik GmbH, Ettlingen, Germany). The UV/Vis analysis was performed using Cecil spectrophotometer (Cecil, Cambridge, UK) in the range of 200–800 nm.
Preparation of poly(ethylene glycol) bis(carboxymethyl)-600 NHS ester (PEG600-2NHS)
The solution of N,N'-dicyclohexylcarbodiimide (0.667 g, 0.367 mmol) in dichloromethane (5 mL) was added dropwise to a stirred solution of poly(ethylene glycol) bis(carboxymethyl) ether-600 (1 g, 1.67 mmol) and N-hydroxysuccinimide (422 mg, 0.367 mmol) in 20 mL dichloromethane. The reaction mixture was stirred overnight at 25°C under the N2 flow, then the mixture poured onto the pad of silica gel and eluted with n-hexane/ethyl acetate (8:2) to obtain PEG600-2NHS (product II, shown in Fig. 1) as a yellow oil (1.2 g, 90%). The FT-IR νmax(solution in CH2Cl2): 2948, 2876, 1772, 1706, 1656, 1545, 1431, 1354, 1213, 1083cm-1.The 1HNMR (400 MHz; CDCl3): 2.7 (8H, m, NCO–CH2–CH2–CON), 3.75-377 (10H,–O–CH2–CH2–O–) and 4.16 (4H, s, –O–CH2–COO–).
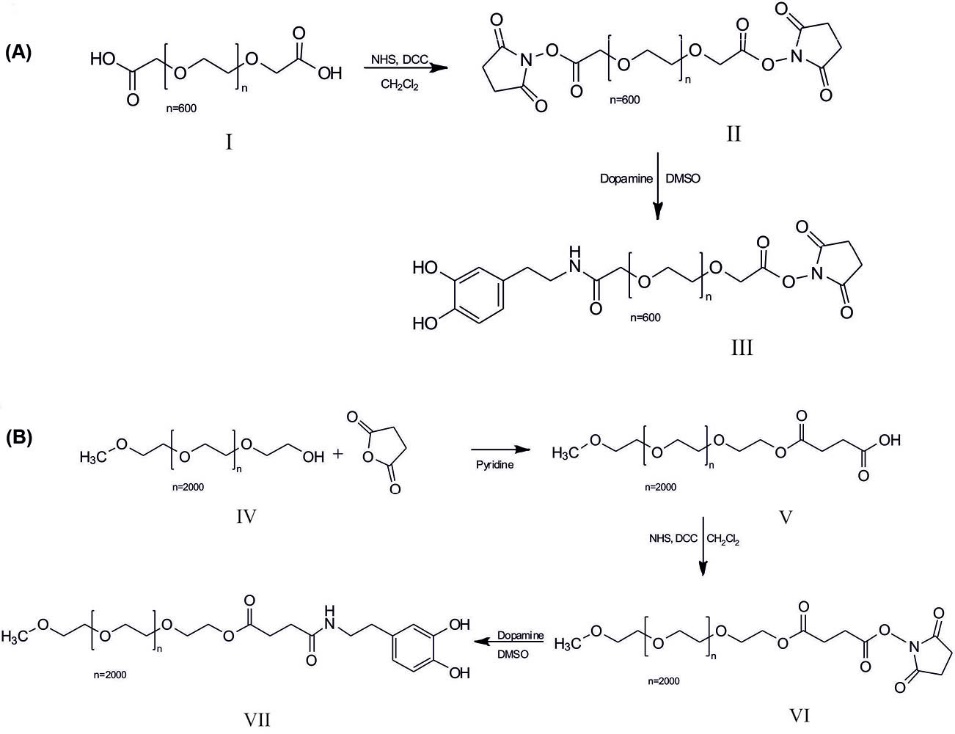
Synthesis of DOPA-PEG600-NHS (a) and DOPA-SA-PEG2000 (b). I, Poly(ethylene glycol) bis(carboxymethyl) ether-600; II, Poly(ethylene glycol) bis(carboxymethyl)-600 NHS ester (PEG600-2NHS); III, Dopamine-PEG600-NHS (DOPA-PEG600-NHS); IV, Poly(ethylene glycol) methyl ether 2000; V, poly(ethylene glycol) methyl ether 2000 succinic acid (PEG2000-SA); VI, PEG2000-SA-NHS ester (PEG2000-SA-NHS); VII, Dopamine- PEG2000-SA(DOPA-SA-PEG2000).
Synthesis of dopamine-PEG600-NHS (DOPA-PEG600-NHS)
Dopamine (DOPA) (184 mg, 1.2 mmol) was dissolved in 10 mL CH2Cl2, then the solution of PEG600-2NHS (1 g, 1.2 mmol) in 10 mL CH2Cl2 was added. The mixture was stirred overnight at 25°C under the N2 flow. The insoluble compounds were filtered, and then the filtrate was precipitated using diethyl ether. The yield of DOPA-PEG600-NHS (product III, shown in Fig. 1) was 0.82 g (79%). The FT-IR νmax(solution in CH2Cl2): 3441, 3997, 2914, 1657, 1422, 1210, 1031 cm-1. The 1HNMR (400 MHz; CDCl3) 2.61 (2H, t,–CH2–CH2N–), 2.81 (4H, m, NCO–CH2–CH2–CON), 3.27 (2H, t, –Ph–CH2–CH2–), 3.36–3.63 (10H, –O–CH2–CH2–O–), 4.08 (2H, s, O–CH2–CO-NH-), 4.26 (2H, t, O–CH2–COO–N), 6.52 (1H, d, Ph) and 6.71 (2H, m, Ph).
Preparation of poly(ethylene glycol) methyl ether 2000 succinic acid (PEG2000-SA)
The poly(ethylene glycol) methyl ether 2000 (9 g, 4.5 mmol) was dissolved in 20 mL pyridine, then succinic anhydride (SA) (2.25 g, 22.5 mmol) was added and the reaction mixture refluxed overnight under vigorous stirring. The solvent was evaporated under vacuum and the residue was extracted by dichloromethane and re-precipitated using diethyl ether. The yield of PEG2000-SA was 7.46 g (79%).The FT-IR νmax(solution in CH2Cl2): 2853, 1962, 1740, 1463, 1356, 953 cm-1. The 1HNMR (400 MHz; CDCl3) 2.65 (2H, t, –COO–CH2–CH2–COO–), 2.87 (2H, t,–COO–CH2–CH2–COO–), 3.19 (3H, s, O–CH3), 3.37–3.67 (34 H, –O–CH2–CH2–O–), 4.54 (2H, t, COO–CH2–CH2–O–).
Synthesis of PEG2000-SA-NHS ester (PEG2000-SA-NHS)
The solution of N,N'-dicyclohexylcarbodiimide (205 mg, 99.3 mmol) in dichloromethane (10 mL) was added dropwise to a stirred solution of PEG2000-SA (2 g, 95.2 mmol) and N-hydroxysuccinimide (115 mg, 99.3 mmol) in 100 mL dichloromethane. After 24 hours, the mixture was filtered using a filter paper and purified using a pad of silica gel, which was then eluted with n-hexane/ethyl acetate (9:1) to obtain PEG2000-SA-NHS (product VI, shown in Fig. 2), yield (1.56 g, 74%). The FT-IR νmax(solution in CH2Cl2): 2879, 1731, 1459, 1345, 1100, 952 cm-1. The 1HNMR (400 MHz; CDCl3) 2.63 (2H, t, –COO–CH2–CH2–COO–), 2.88 (4H, m, NCO–CH2–CH2–CON) 2.91 (2H, t,–COO–CH2–CH2–COO–), 3.19 (3H, s, O–CH3), 3.36–3.64 (34H, –O–CH2–CH2–O–), 4.54 (2H, t, COO–CH2–CH2–O–).
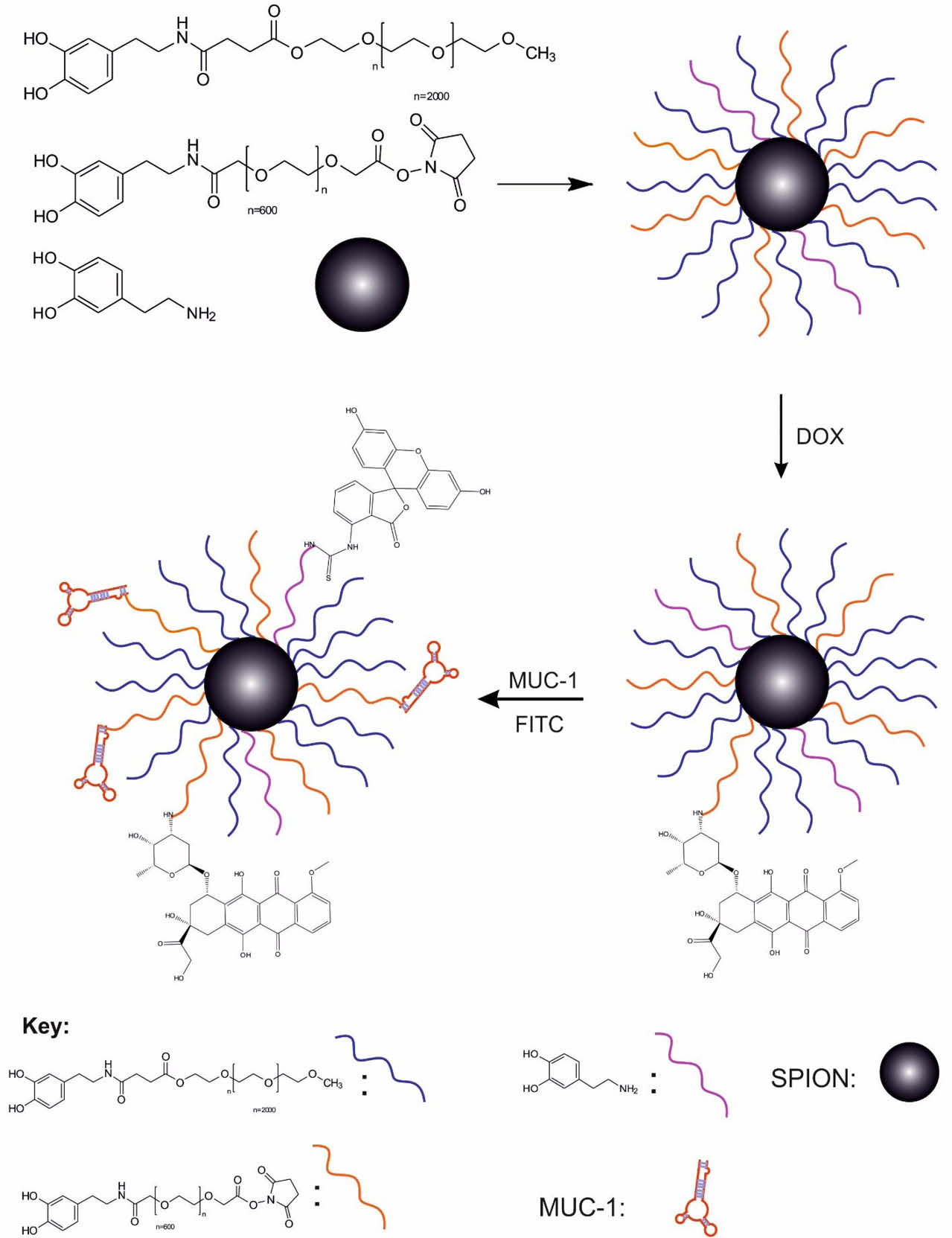
Schematically represents preparation of magnetic nanopartciles.
Synthesis of dopamine- PEG2000-SA(DOPA-SA-PEG2000)
The solution of PEG2000-SA-NHS (1 g, 45.15 mmol) in 10 mL CH2Cl2 was added to the solution of dopamine (70 mg, 45.2 mmol) and triethylamine (TEA) (10 µL) in 50 mL CH2Cl2. The mixture was stirred overnight at RT under the N2 flow. The insoluble compounds were filtered and the filtrate was precipitated using diethyl ether. The yield of DOPA-PEG600-NHS (product VII, shown in Fig. 2) was 0.85 g (83%). The FT-IR νmax (KBr): 3404, 2887, 1719, 1019, 1425, 1227, 1657, cm-1.The 1HNMR (400 MHz; CDCl3) 2.60 (2H, t, –Ph–CH2–CH2–), 2.75 (4H, m, NCO–CH2–CH2–COO), 3.19 (3H, s, O–CH3), 3.39 (2H, t,–CH2–CH2NH–), 3.27 (2H, t, –Ph–CH2–CH2–), 3.5–3.63 (34H, –O–CH2–CH2–O–), 4.54 (2H, t, COO–CH2–CH2–O–), 6.52 (1H, d, Ph) and 6.72 (2H, m, Ph).
Preparation of SPIONs
Iron (III) acetylacetonate (Fe(acac)3) (0.11 g, 0.31 mmol) was dissolved in the mixture of oleylamine and benzyl ether (2 mL:2 mL) and dehydrated at 110°C for 1 hour under the N2 flow. Then, the temperature of the reaction mixture was raised to 290°C for 3 hours under the N2 flow. After cooling to the room temperature, 50 mL ethanol was added to the mixture and precipitated by centrifugation at 9000 ×g for 10-15 minutes.
The NPs were dispersed in 10 mL n-hexane and stored at 4°C. The yield was about 0.85 g (80%). The FT-IR νmax (solution in CH2Cl2): 3473, 3011, 2919, 2853, 1772, 1738, 1650, 1543, 1455, 1298, 692, 453 cm-1.22
SPIONs functionalization by DOPA, DOPA-PEG600-NHS, and DOPA-SA-PEG
2000
The separate solutions of DOPA-PEG600-NHS (50 mg), DOPA-SA-PEG2000 (100 mg), and dopamine (5 mg, 32.64 mmol) in 10 mL dichloromethane were added to a stirred suspension of SPIONs (40 mg) in 10 mL chloroform. The resulting mixture was sonicated at 25°C for 2 hours. Then, the PEG-modified NPs were precipitated by centrifuging at 5000 rpm for 15-20 minutes. The SPION-DOPA-PEG NPs rinsed using 10 mL ethanol (×3) and again was centrifuged, and then, dispersed in 10 mL n-hexane and stored at 4°C. The yield was approximately 80%. The FT-IR νmax (KBr): 3404, 3100, 2920, 2850, 1707, 1648, 1607, 1516, 1435, 1227, 1008, 706, 450 cm-1.
Conjugation of a MUC-1 aptamer to MNPs (SPION-DOPA-PEG-DOX-MUC1)
About 100 nM of a solution of amine-terminated MUC1 aptamer in DNase/RNase free water was added to the suspension of NHS-activated SPION-DOPA-PEG-DOX (5mg/mL) in 2-(N-morpholino)ethanesulfonic acid (MES) buffer (pH = 7.4) and incubated under shaking with constant mixing at 25°C for 4 hours. Then, the MUC1 Ap-conjugated SPIONs were collected by the magnetic bead separation system and washed (×3) with phosphate buffered saline (PBS) and re-suspended in DNase/RNase free water at 4°C before use.
Conjugation of fluorescein isothiocyanate (FITC) to MNPs
For the conjugation of the fluorophore, the engineered SPIONs (100 mg) was suspended in 10 mL DMSO, and then, 20 µL of TEA was added to the suspension. Afterward, the solution of fluorescein isothiocyanate (FITC) (20 mg, 0.032 mmol) was added to the reaction flask and stirred at RT overnight under the N2 flow. Finally, the product was collected using the magnetic bead separation system, washed with PBS (×3) and used for the cytotoxicity studies.
Chemical loading of DOX
The TEA (5 μL) was added to the suspension of SPION-DOPA-PEG (150 mg) in 10 mL of DMSO and the reaction mixture was stirred for 30 min under the N2 flow. In another flask, the solution of DOX (50 mg, 92 µmol) and TEA (5 µL) was stirred for 30 min. Then, these suspension and solution were mixed and stirred overnight at 25°C under the N2 blanket. The engineered SPION-DOPA-PEG-DOX NPs were collected using the magnetic bead separation system. The loading efficiency was calculated by means of a calibration curve using a UV/vis spectrophotometer at 480 nm. (80%). The FT-IR νmax (KBr): 3430, 3100, 2998, 2910, 2850, 1714, 1581, 1518, 1429, 1305, 1214, 1104, 1021, 708, 453 cm-1. Yield: 189 mg.
In vitro release studies
To evaluate the non-enzymatic release of DOX conjugated on MNPs, 2 mL of SPION-DOPA-PEG-DOX suspension (2.5 mg/mL) was placed into a pre-swollen dialysis bag (Sigma Aldrich, cut-off: 2000 Da). The suspended particles were immersed in 198 mL of PBS (pH 5.4, 6.4 and 7.4) and incubated at 37°C to confirm the pH-sensitive release trends of the NPs in the physiological condition. The incubation was continued for 240 hours. At the designated time points, the aliquots (2 mL) were taken out from the suspension while the total volume of media tank was kept constant by adding the same volume from fresh PBS after each sampling. The amount of released DOX from MNPs was estimated by a quantitative UV spectroscopy analysis at 480 nm and calculated according to the following equation: Loading efficiency (%)= [(amount of loaded DOX)/(amount of added DOX)]× %100.
Cell culture
The MCF-7 and MDA-MB-231 human breast cancer cells were cultured at a seeding density of 7.0 × 103 cells/well in Roswell Park Memorial Institute medium (RPMI-1640) supplemented with 10% fetal bovine serum (FBS) and streptomycin (100 mg/mL), penicillin (100 mg/mL), and L-glutamine (2 mM). The cells were kept at 37°C and 5% CO2 in a water-saturated atmosphere in a CO2 incubator.
Cytotoxicity analysis
The toxicities of SPION-DOPA-PEG, SPION-DOPA-PEG-MUC1, free DOX, and DOX-loaded SPION-DOPA-PEG, as well as SPION-DOPA-PEG-MUC1 were investigated in the MCF-7 and MDA-MB-231 cells. About 24 hours post-seeding, the cells were treated with varying concentrations of NPs and drug-loaded NPs – equivalent to the DOX alone. At the designated time points (24, 48 and 72 hours of incubation periods), the media was removed and 50 µL (3-(4,5-dimethylthiazol-2-yl)-2,5diphenyltetrazolium bromide (MTT) solution (2 mg/mL in complete media) plus 150 µL of the fresh media were added to each well.36 Then, the cells were incubated at 37°C for 4 hours, the media was removed and the formazan products formed by oxidation of the MTT dye were dissolved in DMSO (200 µL) plus Sorenson’s buffer (40 µL). The absorbance of formazan product was read at 570 nm using a plate reader spectrophotometer, ELx808 (BioTek Instruments, Winooski, VT, USA).
SPIONs uptake evaluation by flow cytometry
The flow cytometry was used for the quantitative analysis of the cellular uptake of NPs by the MCF-7 and MDA-MB-231 cells. Briefly, six-well plates were seeded with a seeding density of 1.0 × 105 cells/well and incubated for 24 h. Then, the FITC-labeled MNPs dispersions were prepared under sterile conditions by diluting the stock in the corresponding FBS-supplemented growth media to the required concentration, immediately before their addition to the plates. The cultured cells were treated with the fluorophore-labeled non-targeted SPIONs, the Ap-conjugated SPIONs alone, and the DOX-loaded Ap-conjugated SPIONs at 37°C in a CO2 incubator for 2 hours.12,13 The cells were washed (×3) with PBS, and then, trypsinized, centrifuged at 160 ×g for 5 min. The cells pellet re-suspended in PBS and analyzed by FACS Calibur® flow cytometer (Becton Dickinson, San Jose, CA, USA) using a minimum number of 1.0×104 cells per event.
Statistical analysis
The results are expressed as the mean ± standard deviation (SD). The data were statistically assessed by the analysis of variance (ANOVA) using GraphPad Prism 7.0 software (GraphPad Software Inc, San Diego, CA, USA). A P value of less than 0.05 was considered statistically significant.
Results
As shown in Figs. 1 and 2, for the development of the PEG600 and PEG2000 terminated dopamine molecules (DOPA-PEG600, DOPA-PEG2000) were synthesized through the amide bond. To increase the biocompatibility of NPs, SPIONs were functionalized with DOPA-PEG molecules via a ligand exchange reaction. Then, the functionalized SPIONs were loaded with DOX molecules and conjugated with the amine-modified MUC1 aptamer. After characterization of the engineered NSs by various techniques, in vitro cell experiments were conducted to study the biological impacts of the NSs.
FT-IR characterizations
The surface modifications of SPIONs with oleylamine and dopamine conjugated PEG were confirmed using FT-IR spectroscopy. The main peaks for oleylamine are: 3473 (NH2), 3011(=C–H) and 2919, 2853(C–H) cm-1 and the peaks related to SPION are about 692, 453 (Fe–O) cm-1. The peak at 1543 represents the Fe–N bond between NH2 of oleylamine and Fe(III) of Fe3O4. Dopamine as an anchoring agent could replace the oleylamine on the surface of SPIONs.
The formation of SPION-DOPA-PEG NPs was validated by the presence of functional groups at 1008 (C–O–C, PEG), 2850, 2920 (CH2), and the presence of phenyl ring of dopamine was confirmed by the peak at 3100 (=C–H). Moreover, the absorption peak at 1516 cm1 indicates the amine functional group of dopamine. The results of 1500–1707 (–COO) and 1648 cm1 confirm the conjugation of PEG-DOPA-NHS to the SPIONs. In the FT-IR spectrum of SPION-DOPA-PEG-DOX, the peak at 1581 cm1 confirms the conjugation of NHS-activated PEG on the surface of SPIONs with DOX via the amide bonds (Fig. 3).
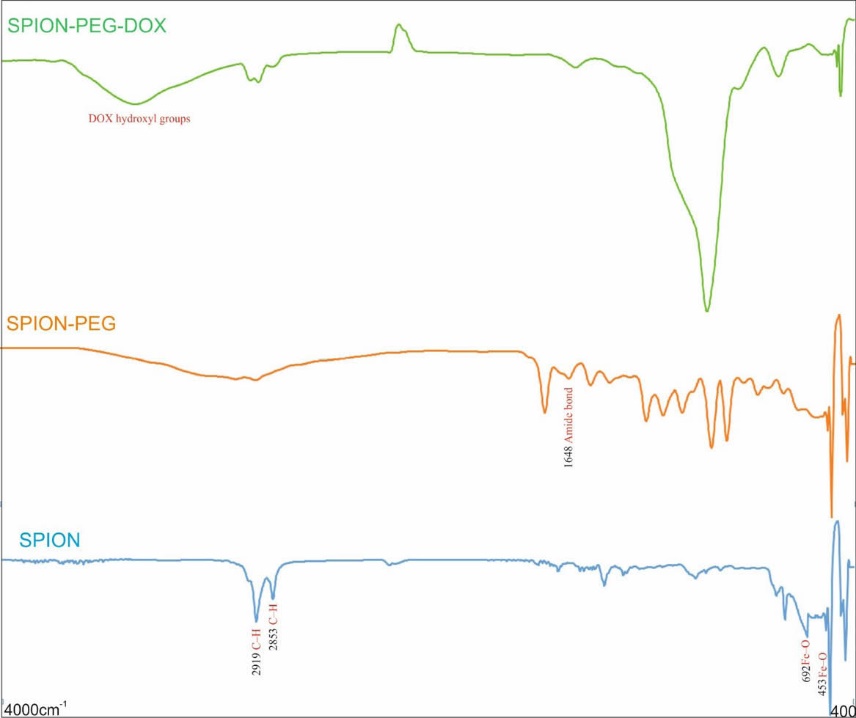
FT-IR spectra of SPION, SPION-PEG, and SPION-PEG-DOX.
Morphological studies
A typical TEM image of the SPION-DOPA-PEG-DOX, as shown in Fig. 4, revealed a spherical shape with smaller size, which was further confirmed by the DLS. The slight difference between the TEM and DLS results may be due to the dehydration or attenuation of the NPs during the electron microscopy imaging process.
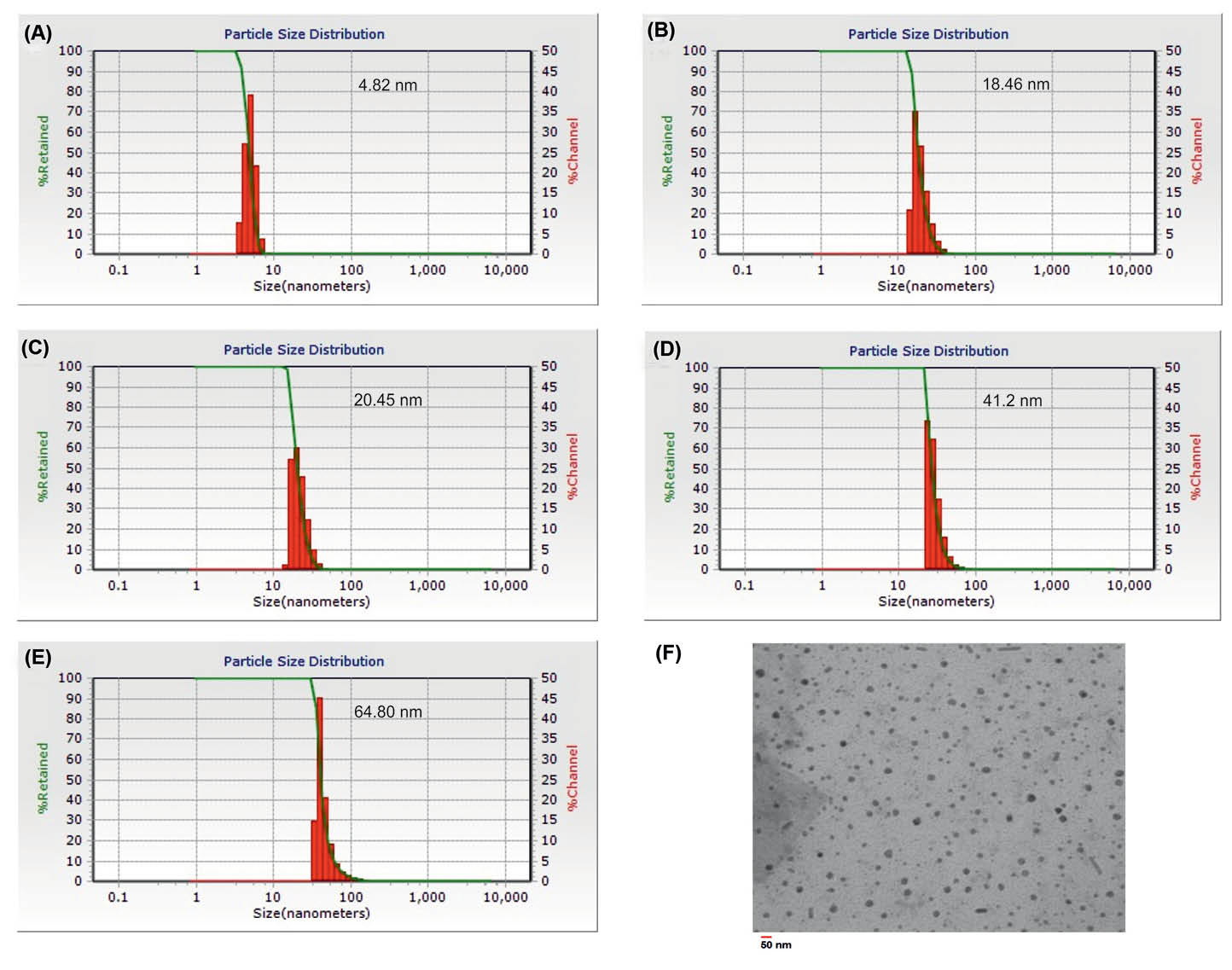
The particle size of nanoparticles. (a) SPION. (b) SPION-DOPA-PEG. (c) SPION-DOPA-PEG-MUC1. (d) SPION-DOPA-PEG-DOX. (e) SPION-DOPA-PEG-DOX-MUC1. (f) Transmission electron microscopy image of SPION-DOPA-PEG-DOX.
DLS analysis
The size and ξ-potential of synthesized MNPs attained by the DLS analysis resulted in a mean particle size of about 5±0.83 nm (PDI = 0.05) with a zeta potential of +25 mV for the engineered SPIONs alone.
The mean particle size of NPs was increased by the modification of SPIONs using DOPA-PEG, drug loading, and Ap conjugation processes. As a result, as presented in Fig. 4, the SPION-DOPA-PEG, SPION-DOPA-PEG-MUC1, SPION-DOPA-PEG-DOX and SPION-DOPA-PEG-DOX-MUC1 displayed the sizes about of 19±1.01 nm (PDI = 0.08), 21±1.83 nm (PDI = 0.07), 41±2.26 nm (PDI = 0.04) and 65±3.11 nm (PDI = 0.09), respectively. Additionally, the surface charge of all the NPs was found to be positive except the SPION-DOPA-PEG NPs because of containing carboxylic functional moieties on its surface.
Drug loading and release profile
For the loading of DOX molecules, the NHS-activated PEG600 was used for the conjugation of DOX onto the SPIONs through the formation of amide bonds. Further, because of the probability of interactions between the PEGylated SPIONs and DOX, some of the cargo loading capacity might be considered due to the physical loading. The results of drug release from NPs showed a pH-responsive behavior.
As shown in Fig. 5, the release of DOX molecules from NPs was found to be increased by reducing of the pH from the physiologic pH of 7.4 to the pH values of endosomal compartments (6.4 and 5.4).
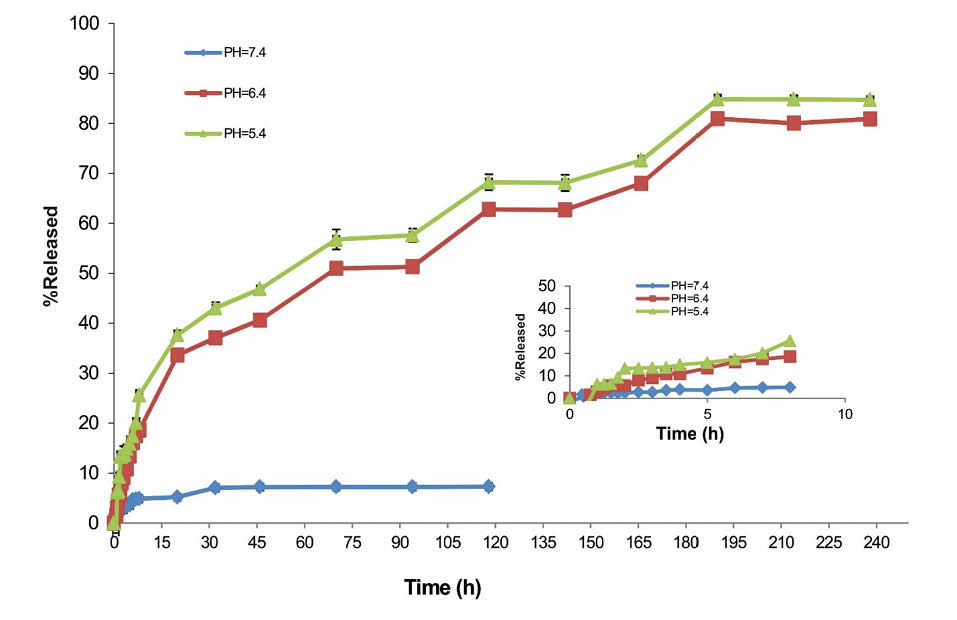
Release of doxorubicin from SPION-DOPA-PEG-DOX-MUC1 nanoparticles based on UV absorption in buffer solutions (pH 5.4, 6.4, and 7.4 at 37ºC).
Cytotoxicity effects of MNPs
The MTT assay confirmed the cytotoxicity of the prepared nanoformulations. As shown in Fig. 6, the cytotoxicity of the DOX-loaded PEGylated SPIONs were high in both cell lines as compared to the free DOX.
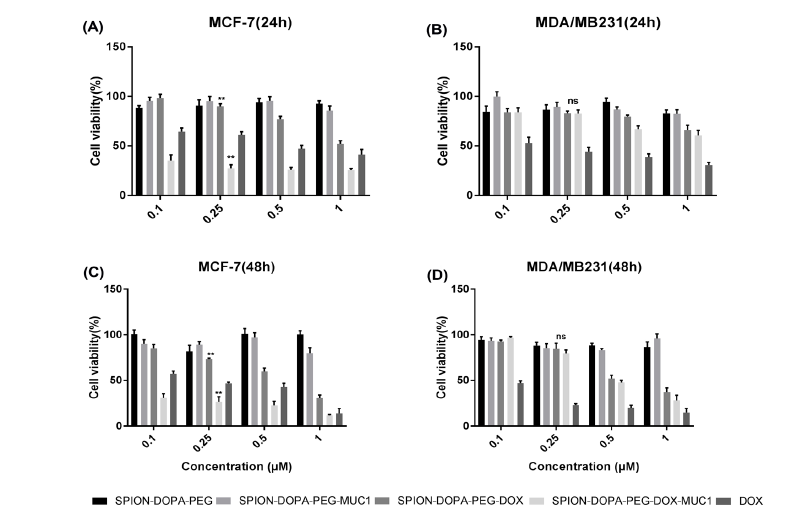
The cell viability of the MUC1 high-expressing MCF-7 cells (panels a and c), and the MUC1 low-expressing MDA-MB-231 cells (panels b and d) after treatment with NPs. Cells were treated with different NPs, including SPION-DOPA-PEG, SPION-DOPA-PEG-MUC1, SPION-DOPA-PEG-DOX, SPION-DOPA-PEG-DOX-MUC1, and DOX for 24 and 48 h. **P < 0001, ns: not significant.
The viability of the MUC1-high-expressing MCF-7 cells treated with SPION-DOPA-PEG-DOX-MUC1 NPs was considerably lower than that of the MUC1-low-expressing MDA-MB-231 cells. While DOX molecules alone induced a significant cytotoxicity in both cell lines, the SPION-DOPA-PEG-MUC1 and SPION-DOPA-PEG did not elicit noticeable cytotoxic effects even after 48 h in both MCF-7 and MDA-MB-231 cells. Expectedly, both treated cell lines were more viable at low concentrations of SPIONs alone while the cell viability was slightly decreased by the increase in the NPs concentration.
Of note, the DOX-loaded SPION-DOPA-PEG-MUC1 NPs exhibited an enhanced cytotoxicity in the MUC1-high-expressing MCF-7 cells as compared to the DOX molecules alone, but not in the MUC1-low-expressing MDA-MB-231 cells.
The cell viability study confirmed that the DOX-loaded SPION-DOPA-PEG-MUC1 NPs could induce significantly greater cytotoxicity in the MUC1-expressing MCF-7 cells. Moreover, under the same condition, the non-targeted DOX-loaded SPION-DOPA-PEG-MUC1 NPs showed lower toxicity than the targeted NPs in the MCF-7 cells, in large part due to the inefficient cellular uptake and relatively low release rate of the DOX molecules from the NPs.
FACS flow cytometry analysis of the cellular uptake
The cellular uptake of SPION-DOPA-PEG-DOX and Ap-conjugated NPs by the MUC1-high-expressing MCF-7 and the MUC-low-expressing MDA-MB-231 cells were further assessed by the flow cytometry method. As shown in Fig. 7, the MCF-7 cells treated with the SPIONs armed with Ap revealed a significantly enhanced internalization of NPs in these cells as compared to the non-targeted SPIONs.
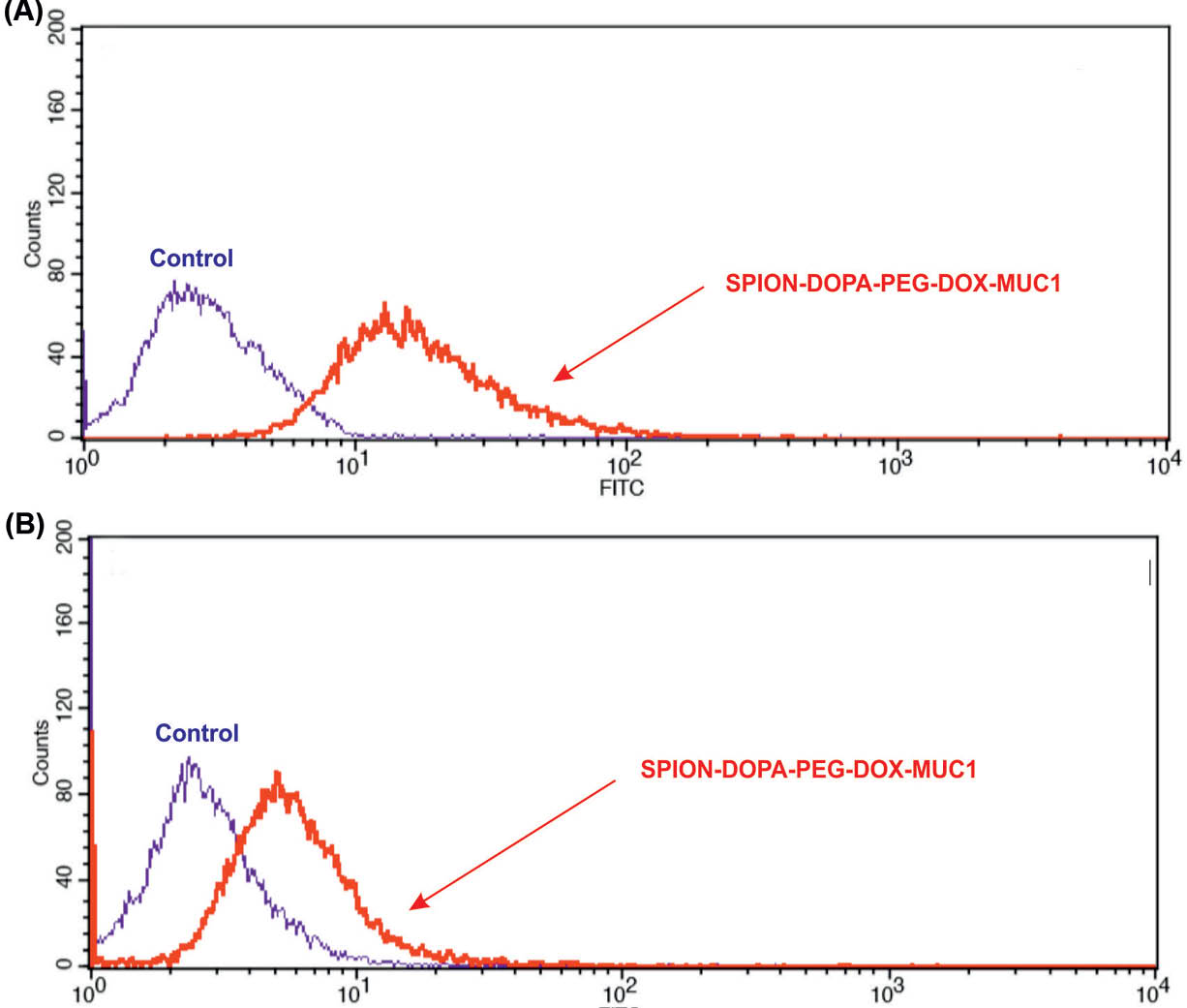
Cellular uptake of SPION-DOPA-PEG-DOX-MUC1 nanoparticles by the MUC1 high-expressing MCF-7 cells (panel a) and the MUC1 low-expressing MDA-MB-231 cells (panel b). Cells were treated with NPs at the concentration of 10 µM for 2 h.
Discussion
Solid tumors, as complex systems, show unique characteristics such as adaptation and evolution. The cancer cells get the capability of forming lenient microenvironment (TME), in which they are in some sort of coop with the immune system's cells and the other stromal components.8,24 In fact, one single cancerous cell can evolve expressing different biological traits, which continue during its development to a number of cancerous cells and finally the formation of TME. Further progression of cancer may result in its invasion and metastasizing to neighboring cells and tissues. During this process, the epithelial cancer cells undergo a transition towards the formation of mesenchymal type cells (the so-called cancer stem cells). The emergence of such capacity bestows a single cancer stem cell great capability of invading other tissues with a great potential of avoiding anoikis – a programmed cell death process that occurs in the unanchored detached cells.37-39, Upon the treatment with anticancer chemotherapy, the cancer cells are further evolved, emerging the drug-resistance potential by the overexpression of molecular machineries such as MDR and multidrug resistance proteins (MRPs). Of particular relevance to the breast cancer chemotherapies include the functional expression of efflux machineries, including P-glycoprotein (P-gp), MRPs, and breast cancer resistance protein (BCRP). It has been reported that over 80% of currently used anticancer drugs are subjected to these efflux transporters.40,41 The good news, however, is that the anticancer nanomedicine formulations show a great capability of overcoming such drug resistance potential of cancer cells.42-44 If equipped with the targeting agents (Ab, Ap or ligand), the targeted nanomedicines can specifically/selectively detect the cancer cells and deliver the anticancer drugs to the diseased cells per se.45-47 Once decorated with imaging agents, the targeted nanomedicines can be used for simultaneous diagnosis and therapy of cancer – a platform so-called diapeutics or theranostics.8 We have previously developed and evaluated various types of nanoscaled DDSs using organic and inorganic entities, which have shown promising diagnostic and/or therapeutic outcomes.48-51
Our previous results on the MUC1 Ap-armed gold coated SPIONs highlighted the usefulness of these advanced NPs for the magnetic resonance (MR) imaging and photothermal therapy (PTT) of the colon cancer.20 In this current study, we aimed at using Ap-decorated DOX-loaded PEGylated SPIONs for the targeted MR-imaging and specific delivery of cargo molecules to the diseased cells. The central focus of the current study was to develop an Ap-conjugated PEGylated SPIONs loaded with DOX (Figs. 1 and 2) for the targeted delivery of drug molecules to the breast cancer cells overexpressing the MUC1 molecular marker. The synthesis of NPs was carried out by the preparation of DOPA-PEG600-NHS (50 mg) and DOPA-SA-PEG2000, which were then grafted to SPIONs synthesized using Fe(acac)3. The PEG600 di-acid was functionalized for the conjugation of MUC-1 and DOX molecules onto the SPIONs via dopamine. Moreover, on the account of the capability of PEG as a hydrophilic polymer in minimizing the possibility of NPs interactions with cells, we functionalized SPIONs with PEG2000 to solubilize NPs and reduce the undesired immune clearance of NPs by the reticuloendothelial system (RES). The engineered NPs were then grafted with MUC1 aptamer and loaded with DOX molecules (SPION-DOPA-PEG-DOX-MUC1 NPs). The resultant NS was characterized (Figs. 3 and 4) and examined for the release of DOX molecules from cargo NPs. The results revealed a faster release of DOX molecules from the SPION-DOPA-PEG-DOX-MUC1 NPs with a size of around 65 nm in an acidic media (Fig. 5). This finding confirms that the drug release might occur significantly in the endosomal compartment after the internalization of NPs via vesicular trafficking. We have previously discussed the cellular trafficking of NPs.52 Further, the MUC1-mediated internalization has been shown to occur via clathrin-coated pits endocytosis as a dynamin- and Rab5-dependent process.53,54
The use of SPION-DOPA-PEG-DOX-MUC1 NPs against the MUC1-overexpressing breast cancer MCF-7 cells and the MUC1-underexpressing MDA-MB-231 cells revealed a markedly high internalization and toxicity in the MUC1-overexpressing MCF-7 cells, but not the MUC1-underexpressing MDA-MB-231 cells (Figs. 6 and 7).
Further, based on the internalization and cytotoxicity, the MUC1-high-expressing MCF-7 cells showed a significant difference between the uptakes of the targeted and non-targeted SPIONs. However, the MUC1-underexpressing MDA-MB-231 cells showed no significant difference between the cellular uptakes of the targeted and non-targeted SPIONs. It seems that targeted delivery through the MUC1 provides compelling evidence for the active targeting of the cancer cells that overexpress the MUC1 oncomarker. In accordance with our findings, it has been reported that the MUC1 Ap-armed mesoporous silica NPs can effectively target the breast cancer cells.55 Further, the MUC1-Ap functionalized hybrid NPs have successfully been used for the targeted delivery of miRNA-29b to the non-small cell lung cancer.56 Altogether, based on our findings, it can be speculated that the anti-MUC1 Ap-armed PEGylated SPIONs can be specifically taken up by the MUC1-positive cancer cells through clathrin-coated pits endocytosis, upon which the anticancer cargo molecules are delivered to the cancerous cells exclusively. As a result, the engineered nanosystem is envisioned to impose maximum therapeutic effect with minimum side effects in the solid tumors such as breast cancer.