BioImpacts. 7(2):75-82.
doi: 10.15171/bi.2017.10
Original Research
An in vitro ethnopharmacological study on Prangos ferulacea: a wound healing agent
Keyvan Yousefi 1, 2, Sanaz Hamedeyazdan 3, Darya Hodaei 1, 2, Farzaneh Lotfipour 4, Behzad Baradaran 5, Mona Orangi 5, Fatemeh Fathiazad 1, 3, *
Author information:
1Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
3Department of Pharmacognosy, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
4Department of Drug and Food Control, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
5Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
Traditionally Prangos ferulacea root is being used as an effective wound healing agent especially for pus-filled wounds both in human and stocks in the western north of Iran. Regarding the subject we decided to study P. ferulacea roots essential oil (PFE) for its antimicrobial and wound healing activities.
Methods:
The in vitro wound healing activity of PFE was evaluated in the mouse fibroblast cell line L929 using MTT assay of cell viability and cytotoxicity indices. Scratch assay as an in vitro model of wound healing assay was also conducted in this study. Moreover, the type I collagen content was used as an indicator of progress in wound healing process using Sircol collagen assay. Besides, PFE was subjected to GC/MS to identify the chemical constituents, and antimicrobical property was also evaluated against S. aureus, S. epidermidis, E. coli, P. aeruginosa, S. paratyphi and C. albicans using agar dilution method.
Results:
GC/MS analysis showed that the monoterpene hydrocarbones dominated in PFE, amounting to a total percentage of 95.1% with the major constituents: β-Phellandrene (32.1%), m-Tolualdehyde (26.2%), and δ-3-carene (25.8%). PFE inhibited the growth of S. aureus and P. aeruginusa with the MIC value of 20 µg/mL. In addition, at the second day of treatment, PFE at concentrations of 4 and 16 µg/mL significantly (P<0.001) enhanced the migration rate of L929 cells by 87.05±2.4 and 63.5±0.08 %, respectively. Moreover, the collagen production by L929 cells was increased greatly (P<0.001).
Conclusion:
It is proposed that the excellent antimicrobial activity along with the significant increase of migration rate and collagen production by fibroblast cells might be associated with the high content and synergistic effect of the monoterpens, corroborating the traditional usage of this plant as a wound healing agent.
Keywords: Prangos ferulacea, Eessential oil, MTT assay, Scratch assay, Sircol collagen assay, Mouse fibroblast cell line
Copyright and License Information
© 2017 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
The Prangos genus belongs to the family Apiaceae. This genus includes about fifty species distributed in the Mediterranean and Middle-East regions including Iran. Fifteen species of this genus are growing wildly in many regions of Iran.
1
The plants of this genus have been used as emollient, carminative, tonic, anti-flatulent and anthelmintic in the traditional medicine of Iran.
2
According to the literature and also our observations from local people, the freshly crushed root oily exudate of Prangos ferulacea has been used (as topical ointment) as an effective wound healing agent especially for pus-filled wounds both in human and stocks in the northern west of Iran.
2
Fig. 1 depicts the oil secreted from fresh plant’s root by local people of Toujaleh and Mishadeh villages, Piranshahr, West Azarbayjan province, Iran. In this study, we aimed to evaluate the potential antimicrobial and wound healing activity of the essential oil of P. ferulacea roots obtained from the western north region of Iran. Additionally, by using GC-MS, we could identify the components of the essential oil, to which the antimicrobial and healing effects could be attributed.

Fig. 1.
The fresh oil exudes from the roots of Prangos ferulacea
traditionally used as a wound healing agent by local people of
western north of Iran (Mishadeh and Tujaleh, Piranshahr, Iran).
.
The fresh oil exudes from the roots of Prangos ferulacea
traditionally used as a wound healing agent by local people of
western north of Iran (Mishadeh and Tujaleh, Piranshahr, Iran).
Essential oils of Apiaceae taxa are well-known for their variability with respect to the growing site and time of harvest.
3
There are several studies investigating the chemical composition of the essential oils of different parts of P. ferulacea such as aerial parts, fruits, seeds, flowers, stems and leaves from different locations. Monoterpene compounds have been well established to be the major component in the essential oil of leaves, stems, flowers and fruits of P. ferulacea,
4,5
and reported to possess antibacterial activity.
6
Sajjadi et al, for the first time, carried out the GC/MS analysis of the essential oil of the roots of P. ferulacea from centre of Iran and reported the oil to be consist of monoterpene hydrocarbons (78.4%), oxygenated monoterpenes (9.4%), sesquiterpene hydrocarbons (5.3%) and three oxygenated sesquiterpenes.
7
In addition, a phytochemical screening of the root extract of P. ferulacea resulted in the identification of coumarin compounds, including osthole, isoimperatiorin, oxypeucedanin, psoralen and gosferol, some of which with reported anti-HSV and cytotoxic effects. Moreover, the compound osthole showed anti-inflammatory property
8
and in vivo antispasmodic
9,10
and antitumor
11,12
activities.
There are some reports evaluating the antimicrobial property of the essential oil from different parts of P. ferulacea. In spite of significant antibacterial activity reported for the fruits, leaves, stems and flowers of P. ferulacea essential oil against both gram-positive and gram-negative bacteria, it has been demonstrated that methanol, ethanol and n-hexane extracts of aerial parts of P. ferulacea showed only slight antimicrobial activity against some gram-positive bacteria.
4,5,13
Razavi et al. reported high antibacterial effect of the fruits and umbels of P. ferulacea essential oil especially against gram-positive bacteria Bacillus cereus.
14
Basically, regarding its traditional use as a wound healing agent, this study was undertaken to evaluate the wound healing activity and antimicrobial effect of the essential oil of P. ferulacea roots from the western north of Iran. For the first time, we aimed to analyze its chemical composition to which these biological activities could be correlated. However, the traditional use of this plant by local people as a wound healing agent is the basis of this study.
Materials and Methods
Plant material
The roots of P. ferulacea were collected form the mountainous area between Mishadeh and Toujaleh; Piranshahr; West Azarbaijan; Iran (36°34'0"N, 45°21'23"E, 2000 m) in June 2012. Voucher specimens (No. Tbz-Fph-732) have been deposited at the herbarium of the Department of Pharmacognosy, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran.
Isolation of the essential oil
After harvesting, 100 g of fresh and crushed roots were immediately subjected to hydro-distillation in a Clevenger type apparatus for 3 hours for extraction of the essential oil. The oil was collected, de-hydrated with anhydrous sodium sulphate, measured (as 1.2%) and kept cool in the dark prior to analysis.
Analysis of volatile compounds
The GC/MS analysis was performed using Agilent 19091S-433 Quadropole gas chromatograph (Agilent Santa Clara, CA, USA). The components were separated with a HP-5MS (30 cm × 0.25 mm; film thickness 0.25 µm) capillary column and a flame ionization detector (FID), which was operated in EI mode at 70 eV. Injector temperature, 260°C; detector temperature, 290°C; column temperature was programmed 60-280°C at a rate of 4°C/min; Volume injected, 1 µL of the essential oil solution in hexane; Split ratio, 1:29. The MS operating parameters were as follows: EI mode 70 eV; Helium was the carrier gas with a flow rate of 1.8 mL/min; ion source temperature, 270°C; quadrupole 100°C; Solvent delay 2 minutes; scan speed 2000 amu/s; scan range 30-600 amu and EV voltage 3000 V.
Identification of the compounds
The constituents of the essential oil were identified by their retention indices under temperature-programmed conditions for n-alkanes (C8–C21) and the essential oil on a HP-5MS column under the same chromatographic conditions. The identification of compounds was based on direct comparison of the retention times and mass spectral data with those for the standards and by computer matching with the Wiley 229, Nist 107, Nist 21 Library, as well as by comparing the fragmentation patterns of the mass spectra with those reported in the literature.
15,16
For quantification purpose, relative area percentages obtained by FID were used without the use of correction factors.
Microbial strains and inoculum preparation
The essential oil, methanol and chloroform extracts of P. ferulacea were investigated for the antimicrobial activity against six microorganisms. The reference strains were: Staphylococcus aureus ATCC (6538), Staphylococcus epidermidis ATCC (12228), Escherichia coli ATCC (8739), Pseudomonas aeruginosa ATCC (9027), Salmonella paratyphi ATCC (4420) and Candida albicans ATCC (10231). These strains were obtained in lyophilized form from Institute of pasture (Tehran, Iran), and activated by culturing in Luria Bertuni agar medium (Hi Media, India) and incubation for 24 hours at 37°C. Single colony from the plate was transferred into 4 mL fluid LB medium and incubated over night at 37°C and 200 rpm in shaking incubator. The cells were harvested by centrifugation at 3000 rpm for 15 min and 4°C. Subsequently, they were washed twice and re-suspended in saline solution to provide bacterial concentrations between 107–108 CFU/mL.
17
Antimicrobial assay
In order to determine the antimicrobial activity of the essential oil of the roots of P. ferulacea, the agar disc diffusion method was employed. Firstly, Sterile Muller Hinton agar medium was prepared and poured into sterile plates, which were incubated at 37oC for 24 hours to check for any signs of contamination. Subsequently, 100 mL of each inoculum was thoroughly spread on the corresponding Muller Hinton agar plate. Succeeding that sterile filter paper discs (whatman filter paper) of 6 mm diameter were soaked in the essential oil (10 µL) and then placed on the inoculated plates. After that the Petri dishes were incubated at 37°C for 24 hours and the diameters of the inhibition zones were measured in millimeters. Amikacin (30 µg/disc) standard was used as positive control. The inhibition zone was determined by measuring the minimum dimensions of the zone of no microbial growth around the disc. An average of four independent determinations was recorded.
Determinations of minimum inhibitory concentration (MIC)
The agar dilution method was employed to determine MIC of the essential oil according to CLSI.
17
The prepared inoculum was added to Muller Hinton broth medium to reach the final concentration of 106 CFU/mL. One milliliter of the inoculated medium was added to 8 sterile tubes include seven tests and a control tubes. Dimethylsulfoxide (DMSO) was used as the solvent for mixing essential oil with the medium. A serial doubling dilution of the essential oil was added in the tubes over the range 2.5-80 µg/mL of the essential oil. The tubes were incubated at 37oC for 24 hours. After that, from the content of each tube a streak culture was grown on Mueller Hinton Agar plates. The first concentration with no sign of bacterial growth on plates was considered as the MIC. DMSO without essential oil was used as a negative control. All experiments were performed in three separate occasions.
Cell cultures
The mouse fibroblast cell line L929 were purchased from the cell bank of Pasteur Institute (Tehran, Iran). The cells were grown and maintained in a humidified incubator at 37°C and in 5% CO2 atmosphere. RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO, USA) was supplemented with 10% heat inactivated Fetal Calf Serum (FCS), 100 units/ml penicillin, and 100 μg/mL streptomycin (all from Invitrogen Gibco, Carlsbad, CA, USA) for cell cultures. Upon reaching confluency, the cells were passaged. After being harvested from sterile T75 culture flasks (Nunc, Denmark), the cells were counted using a hemocytometer and cell viability was determined by trypan blue exclusion. Ten thousand cells from log phase cultures were seeded in 100 μL of RPMI medium supplemented with 10% fetal bovine serum per well of 96-well flat-bottom culture plates (Nunc, Denmark).
18
MTT cell viability assay
The assay detects the reduction of MTT [3-(4, 5-dimethylthiazolyl)-2, 5-diphenyl-tetrazolium bromide, Sigma] (a colorimetric technique) by mitochondrial dehydrogenase to blue formazan product, which reflects the normal function of mitochondria and hence for measuring the cytotoxicity cell and viability. About 1.0×104 viable cells/well of L929 line were plated into the 96-well tissue culture plates (Nunc, Denmark), and then incubated at 37°C overnight. The next day when cells reached over 80% confluence, the media were replaced with 200 mL of fresh complete medium containing 1-132 µg/mL of PFE, no oil was added to the negative control well. After 24 h, the supernatants were removed and cell layers were washed with phosphate buffered saline (PBS, Invitrogen, Gibco) and incubated with MTT (50 µL, 2 mg/mL) in RPMI 1640 without FCS for 4 h in a humidified atmosphere at 37°C according to the manufacturer’s protocol. The cell cultures were centrifuged at 1000 ×g for 5 minutes and the supernatants were discarded. Subsequently, 200 mL of DMSO (Sigma-Aldrich) and 25 mL Sorenson buffer were added to dissolve the formazan crystals formed. The optical density (OD) colored solution was quantified at 570 nm by an enzyme linked immunoabsorbent assay reader (ELISA Reader, BIO-RAD, Hercules, CA, USA). The absorbance of untreated cells was considered as 100%. All the experiments were assayed in quadruplicate in three independent experiments.
19
Eventually, the most effective concentrations by which the viability of fibroblasts was increased were selected to be evaluated in the in vitro scratch wound healing model.
In vitro scratch assay
As an in vitro model of wound healing assay, scratch test was performed to evaluate the ability of PFE to enhance the spreading and migration of fibroblast cells.
20,21
Briefly, L929 cells (5.0×103 cells/cm2) were seeded in 6-well plates and when the confluent monolayer was obtained a linear scratch was generated using a sterile pipette tip. After that, cellular residues were washed out by PBS and 2 mL of fresh RPMI-1640 medium containing 4 and 16 µg/mL (concentrations were selected from the results of the MTT assay) PFE was added to the wells. No oil was added to the control wells. Samples were in quadruplicates. Plates were incubated at 37°C with 5% CO2 and photographs were taken at a 4x magnification on days 0, 1 and 2. Using computing software Image J 147, for each day, the distance of each scratch closure was determined and the migration rate percentage was calculated according to the following formula
22
where DBS is the average distance between scratch:
Migration rate % = (DBS (day 0) – DBS (day 1 or 2))/ DBS (day 0).
Type I collagen production assessment
The type I collagen content, which has been used as an indicator of progress in wound healing process,
23
was determined using Sircol™ Collagen Assay (Biocolor Life Science Assays, Carrickfergus, UK). Briefly, L929 fibroblasts (3.3×104 cells/cm2) were seeded in 96-well plates and after one day were treated with PFE at a concentration range of 2-16 µg/mL. No oil was added to the control wells. Subsequently, wells incubated at 37oC with 5% CO2 for 72 hours. Then, supernatants were collected and the total soluble collagen content was quantified according to manufacturer’s instruction.
Statistics
Data were presented as mean ± SEM. One-way-ANOVA was used to make comparisons between the groups. If the ANOVA analysis indicated significant differences, a Student–Newman–Keuls post hoc test was performed to compare the mean values between the treatment groups and the control. Statitical differences between groups were considered significant at P<0.05.
Results
GC/MS analysis of PFE
Roots of P. ferulacea yielded 1.2% V/W of a pale yellow volatile oil. The chemical constituents and their percentage are given in Table 1 (GC/MS chromatogram and Mass spectrum has been included as supplementary file). Fourteen compounds, representing 95.1% of the total essential oil were identified with the help of GC-MS. Monoterpene hydrocarbons predominated the essential oil (67.3%), with β-Phellandrene (32.1%), m-Tolualdehyde (26.2%), and δ-3-carene (25.8%) and α-Pinene (4.7%) as the major components.
Table 1.
Chemical compositions of Prangos ferulacea root essential oil
|
No.
|
RI
a
|
RT
b
|
Molecular formula
|
Compounds
|
Area (%)
|
| 1 |
937 |
4.14 |
C10H16
|
α-Pinene |
4.7 |
| 2 |
950 |
4.42 |
C10H16
|
Camphene |
0.9 |
| 3 |
974 |
4.94 |
C10H16
|
Sabinene |
0.5 |
| 4 |
990 |
5.34 |
C10H16
|
β-Myrcene
|
2.1 |
| 5 |
1010 |
5.93 |
C10H16
|
δ -3-Carene |
25.8 |
| 6 |
1030 |
6.49 |
C10H16
|
β-Phellandrene |
32.1 |
| 7 |
1042 |
7.10 |
C10H16
|
γ-Terpinene
|
1.2 |
| 8 |
1051 |
7.52 |
C8H6O
|
Coumarone |
0.9 |
| 9 |
1059 |
8.09 |
C8H8O
|
m-Tolualdehyde |
26.2 |
| 12 |
1217 |
10.55 |
C10H16O
|
trans-Carveol |
0.2 |
| 13 |
1228 |
14.02 |
C12H20O2
|
Fenchyl acetate |
0.2 |
| 14 |
1298 |
15.54 |
C12H18O2
|
Sabinyl acetate |
0.5 |
| Total |
|
|
|
|
95.1% |
| Monoterpene hydrocarbons |
|
|
|
|
67.3%
|
aRI: Retention indices as determined on a HP-5MS column using the homologous series of alkanes (C8-C21).
bRT: retention time.
Antimicrobial activity of PFE
PFE was screened for antimicrobial activity against the selected strains using the agar disc diffusion method and the results are presented in Table 2. According to the results, PFE exhibited remarkable antimicrobial activity against all the tested strains. Interestingly, after treating with PFE (10 µg/disc) the inhibition zone of three strains including P. aeruginusa, S. aureus and S. epidermidis appeared greater than those for Amikacin (30 µg/disc) as the positive control.
Moreover, MIC of PFE was determined using the agar dilution method and the results are depicted in Table 2 as well. As it can be seen in Table 2, P. ferulacea inhibited the growth of S. aureus and P. aeruginosa with the MIC value of 20 µg/mL. Interesting MIC values were also obtained in the screening against S. paratyphi (10 µg/mL), E. coli (5 µg/mL) and C. albicans (5 µg/mL).
Table 2.
The antimicrobial activity of the essential oil of Prangos ferulacea roots (PFE)
|
Strain
|
ZI
a
of PFE
(10 µg/disc) (mm±SD)
|
ZI of Amikacin
(30 µg/disc) (mm±SD)
|
MIC
b
for PFE (µg/mL)
|
MIC for Amikacin (µg/mL)
|
|
Pseudomonas aeruginosa ATCC (9027)
|
12±0.8 |
10±1 |
20 |
15 |
|
Staphylococcus aureus ATCC (6538)
|
21.5±1.1 |
15±1.2 |
20 |
15 |
|
Staphylococcus epidermidis ATCC (12228)
|
19±0.9 |
14±1 |
20 |
25 |
|
Candida albicans ATCC (10231)
|
14±0.4 |
18±0.5 |
5 |
5 |
|
Salmonella paratyphi ATCC (4420)
|
14.5±0.1 |
17±0.7 |
10 |
5 |
|
Escherichia coli ATCC (8739)
|
14±0.3 |
16±1.1 |
5 |
5 |
Values are mean±SD, (n=4). aZI: Zones of inhibition,bMinimum Inhibitory Concentration, Disc diameter = 6 mm
Effect of PFE on viability of L929 fibroblast cells
The effect of treatment with PFE on fibroblast cells, at a concentration rage of 1-132 µg/mL, was studied by MTT assay. According to the results, as shown in Fig. 2, PFE not only showed no toxic effect on L929 fibroblast cells, but also demonstrated a viability increasing effect at a range of 4-16 µg/mL. Accordingly, for the next step, 4 and 16 µg/mL doses were selected to be evaluated in scratch test for their wound healing potential.
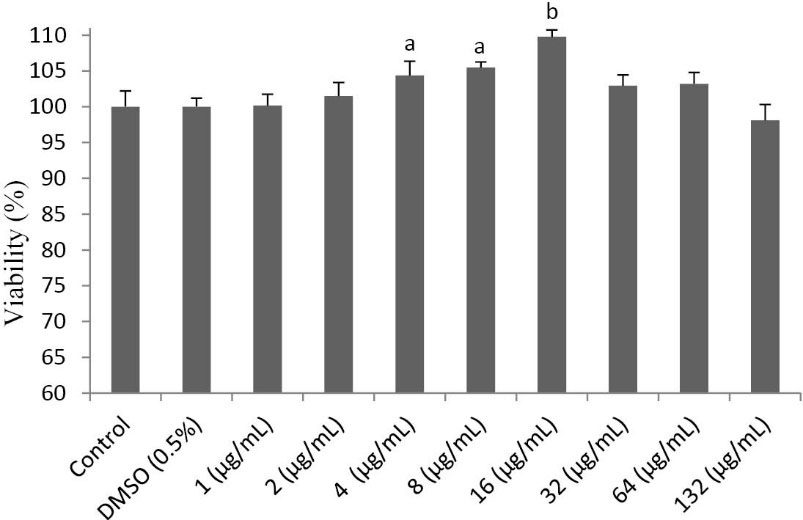
Fig. 2.
The effect of the essential oil of P. ferulacea roots (PFE) on the viability of L929 fibroblasts. PFE at different concentratioons (1-
132 µg/mL) was added to fibroblast cells and the viability of cells after treatment was calculated using MTT assay. At doses 4-16 µg/mL,
PFE treatment led to a moderate increase in the viability of fibroblast cells.
.
The effect of the essential oil of P. ferulacea roots (PFE) on the viability of L929 fibroblasts. PFE at different concentratioons (1-
132 µg/mL) was added to fibroblast cells and the viability of cells after treatment was calculated using MTT assay. At doses 4-16 µg/mL,
PFE treatment led to a moderate increase in the viability of fibroblast cells.
The effect of PFE on in vitro scratch test
Scratch test, as a standard in vitro method for wound healing studies, was performed to investigate the impact of PFE on the migration of L929 cells to the scratched area, so-called the wounded section. According to the quantitative results listed in Table 3, PFE, at doses of 4 and 16 µg/mL, was able to increase substantially (P < 0.001) the migration of fibroblast cells into the wounded site by 15.4-57.05%. Fig. 3 illustrates the results of scratch assay.
Table 3.
The effect of the essential oil of Prangos ferulacea roots (PFE) on the migration of fibroblast cells in the in vitro scratch test
|
Day
|
Group
|
Length between scratch (µm)
|
Migration Rate (%)
|
| Control |
0 |
195±3.6 |
- |
| 1 |
175.5±2.3 |
10±0.36 |
| 2 |
136±5.2 |
30.2±1.3 |
| PFE (4 µg/mL) |
0 |
208±4.5 |
- |
| 1 |
155±2.8a
|
25.4±1.1a
|
| 2 |
76±4.1a
|
63.5±0. 8a
|
| PFE (16 µg/mL) |
0 |
200.8±1.5 |
- |
| 1 |
92.9±1.7a
|
53.7±1.3a
|
| 2 |
26±5.2a
|
87.05±2.4a
|
Values are mean ± SD (n = 4). aP<0.001 as compared with normal control group of same day using one way ANOVA with Student-Newman-Keuls post hoc test.
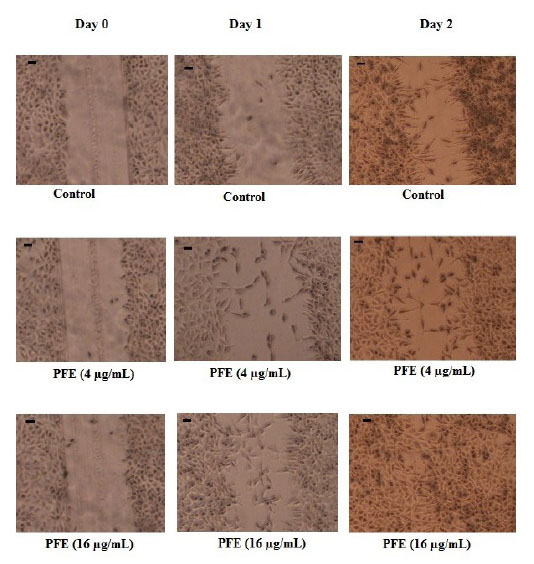
Fig. 3.
The effect of the essential oil of P. ferulacea roots (PFE)
in scratch wound healing model using L929 fibroblasts. The
migration rate of cells into the scratched site evaluated in groups
including Control (no treatment) and PFE groups (treated with 4
and 16 µg/mL) at days 0, 1 and 2 after incubation. Photographs
were taken at a 4x magnification. Scale bar indicates 0.1 inches.
.
The effect of the essential oil of P. ferulacea roots (PFE)
in scratch wound healing model using L929 fibroblasts. The
migration rate of cells into the scratched site evaluated in groups
including Control (no treatment) and PFE groups (treated with 4
and 16 µg/mL) at days 0, 1 and 2 after incubation. Photographs
were taken at a 4x magnification. Scale bar indicates 0.1 inches.
The effect of PFE on the production of collagen by L929 fibroblast cells
To realize the effect of PFE on the total amount of type I collagen produced by L929 fibroblast cells, the standard collagen assay kit was used as described earlier. As shown in Fig. 4, PFE increased significantly (P < 0.001) the total concentration of type I collagen in a dose-dependent manner.
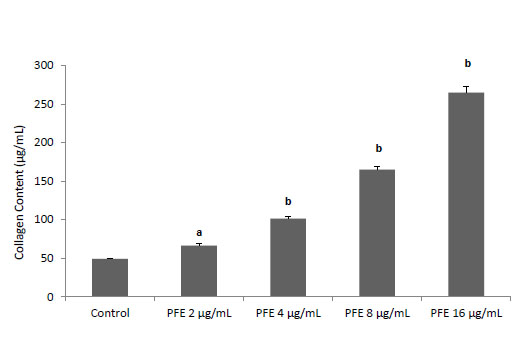
Fig. 4.
The effect of the essential oil of P. ferulacea roots (PFE)
on the production of collagen by L929 fibroblast cells. Values
are mean±S.D. (n = 4). a
P<0.01 and b
P<0.001 as compared
with normal control group using one way ANOVA with StudentNewman-Keuls
post hoc test.
.
The effect of the essential oil of P. ferulacea roots (PFE)
on the production of collagen by L929 fibroblast cells. Values
are mean±S.D. (n = 4). a
P<0.01 and b
P<0.001 as compared
with normal control group using one way ANOVA with StudentNewman-Keuls
post hoc test.
Discussion
Wound healing process is an intricate procedure which concerns many events including angiogenesis, reepithelialization, granulation tissue formation, and remodeling of extracellular matrix.
24
Fibroblasts’ role, as the cornerstone cells of the architecture of tissue repair, is of utmost importance. In the earlier days of healing process, fibroblasts proliferate and migrate to the wounded site. Then, from third day after wounding, fibroblasts obtain a new responsibility, collagen production, which is continued until about three weeks.
25,26
Collagen is another important factor during healing process and is an imperative element for events like angiogenesis and extracellular matrix remodeling. The total amount of collagen produced by fibroblasts in a wound is directly associated with the final tensile strength and integrity of newly formed blood vessels.
22
Hence, using fibroblast cells in the in vitro wound models, like scratch assay, and evaluating collagen production by these cells is of significant value in studying the wound healing potential of natural products. On the other hand, assessment of proliferative activity or in contrast, toxicity of natural products against fibroblast cells, could be a valuable examination which somehow shows the potency and the safety of the tested product.
In this study, even after treating with high concentrations of PFE (up to 132 µg/mL), the viability of L929 fibroblast cells was remained close to that of normal control cells (Fig. 2). Besides, within the concentration range of 4-16 µg/mL, an increase in the viability of L929 cells was observed; therefore, we tested these doses in scratch assay. Our results showed that PFE at 4 and 16 µg/mL was able to increase significantly (P < 0.001) the population of L929 fibroblast cells in scratched area which could be due to immigration of fibroblasts or proliferation of migrated cells. Furthermore, collagen production by fibroblasts which was assessed after treatment with different concentrations of PFE, increased markedly (P < 0.001) in a dose-dependent manner. This finding can be considered as a consequent event of fibroblast proliferation induced by PFE and confirms the wound healing activity of PFE.
Additionally, monoterpenes were shown to be major constituent of PFE. There are some controversial studies on the impact of monoterpenes in different types of cells. In some studies, they act as antiproliferative,
27,28
in contrast, there are some other reports showing their proliferative effect.
29
It is highly important to note that, depending on the cell type, the same compound can elicit different actions. However, we propose that the increased proliferation and migration rate of fibroblast cells by PFE seen in this study could be attributed to its high monoterpene content. On the other hand, non-toxicity of PFE was a valuable finding demonstrating its safety for topical application.
The chemical compositions of the essential oil of different parts of P. ferulacea have been previously investigated. In almost all parts of the plant, monoterpene hydrocarbons have been found to be the most abundant compounds of which 2, 3, 6-trimethyl benzaldehyde, δ-3-carene, α-pinene, sabinene, limonene and phellandrene were the main constituents of the essential oils.
4,5,14
In the present study, PFE was characterized by a high content of the monoterpene hydrocarbons. Thus, this result is consistent with the previous studies. We found that monoterpene hydrocarbons predominated in the oil of P. ferulacea roots (67.3%) with β-Phellandrene (32.1%), m-Tolualdehyde (26.2%), and δ-3-carene (25.8%) and α-Pinene (4.7%) as the major components.
Some hurdles can appear in the wound healing process path. Wound contamination by different categories of microorganisms is a serious and undesirable problem which can result in wound infection and as a consequent, complicating the wound healing process. The situation is more deteriorating because of the growing emerges of multidrug-resistant strains. To combat the shortcomings of the commonly used synthetic antibiotics, there has been an upward global trend in development and application of naturally originating antimicrobial agents since the past few decades. Natural remedies have been widely used in traditional medicine in Iran and it backs to more than 1000 years ago.
2
The oil extracted from the roots of P. ferulacea has been used as a potent wound healing remedy especially for pus-filled both in human and stocks in the western north of Iran where its effectiveness practically has been proven. Therefore, we hypothesized that essential oil of P. ferulacea roots could have strong antimicrobial activity especially against the strains involving in wound infections so this way, it helps wound healing process.
The normal indigenous skin microbiota such as staphylococci, micrococci and propionibacteria are the very first sources by which the wound is colonized. In addition, exogenous microorganisms originated from the environment predominantly pseudomonas spp. have a high probability to contaminate wounds and worsen the patient's treatment situation.
30
Among the contaminant bacteria sources, S. aureus and P. aeruginosa are the most mutant to multidrug-resistant forms.
31
According to Tammelin et al, 12.5% of S. aureus isolates and 21.7% of Pseudomonas species were found to be resistant to a clinically relevant synthetic antibiotic.
32
In addition, strains from Entrobacteriaceae, especially E. coli, have the potential to contaminate wounds. For example, the mostfrequent pathogens isolated from the post-operation infected wounds of 676 surgery patients were S. aureus (28.2%), P. aeruginosa (25.2%), E. coli (7.8%), and S. epidermidis (7.1%).
33
Now, care professionals unanimously believe in that aerobic or facultative pathogens such as S. aureus, P. aeruginosa, and beta-hemolytic streptococci are the primary causes of delayed healing and infection in both acute and chronic wounds.
30
According to our antimicrobial results, the essential oil of P. ferulacea roots remarkably suppressed the growth of S. aureus and P. aeruginosa in agar disc diffusion assay with MIC values of 20 µg/mL (Table 2) reflecting its significant role in wound healing activity.
The antibacterial activity of essential oils rich in monoterpenes has been frequently reported.
34-39
For instance, the essential oil of Melaleuca alternifolia (tea tree oil), which consists largely of cyclic monoterpenes, exhibited a broad-spectrum antimicrobial activity against S. aureus, E. coli and C. albicans.
36
Therefore, the high monoterpene content, which was revealed in the essential oil of this plant to be as 67.3% of the total oil, and the probability of synergistic effect of the individual monoterpene constituents, is proposed to be responsible for its strong antimicrobial activity.
Conclusion
Taken all together, the present study demonstrated the promising wound healing property of the essential oil of P. ferulacea roots from western north of Iran, as evidenced by its potent antibacterial activity along with its ability to induce proliferation, migration and collagen production of fibroblasts and these findings corroborate its traditional use as a wound healing agent. The observed effects could be attributed to the high monoterpene content of the essential oil. Lastly, we suggest that further phytochemical and in vivo studies are needed to elucidate the components responsible for its healing activity and to clarify the actual effect of the oil on skin after its topical application.
Ethical approval
There is no ethical issue to be considered.
Competing interests
The authors declare that they have no conflict of interest.
Acknowledgment
Authors are thankful to Dr. Marco Leonti (Dipartimento Farmaco Chimico Tecnologico, Facoltà di Farmacia, Università di Cagliari, Cagliari, Italy) for revising the manuscript and also to Mr. Ahmad Yousefi (from Piranshahr) and Mr. Esmaeil Padash (villager from Mishadeh) for their help in collecting the plant. Financial support of this study by the Research Vice-Chancellor of Tabriz University of Medical Sciences is faithfully acknowledged.
Research Highlights
What is current knowledge?
simple
-
√ Antibiotic resistant microbial strain is a matter of fact in
modern drug therapy.
-
√ Traditionally Prangos ferulacea roots are being used as
an effective wound healing agent especially for pus-filled
wounds among people.
What is new here?
simple
-
√ This is the very first study on PEE wound healing activity
in vitro.
-
√ Monoterpenes were shown to be major constituent of PFE.
-
√ Collagen production by fibroblasts after treatment with
PFE, was increased markedly (P<0.001) in a dose-dependent
manner.
-
√ Collective antimicrobial effects together with wound
healing potency of PEE could shorten the length of recovery
period.
References
-
Trease GE, Evans WC. Trease and Evans Pharmacognosy. W. B. Saunders Edinburgh London ed. New York: Philadelphia St. Louis Sydney Toronto; 2002.
-
Zargari A. Medicinal Plants. Tehran: Tehran University Publications; 1988.
-
Hegnauer R. Chemical Patterns and Relationships of Umbelliferae. In: The Biology and Chemistry of the Umbelliferae. Heywood VH, ed. New York: Academic Press; 1971.
- Masumi MA, Fazeli MR, Alavi SHR, Ajani Y. Chemical Constituents and Antibacterial Activity of Essential Oil of Prangos ferulacea (L) Lindl Fruits. Iranian Journal of Pharmaceutical Sciences 2007; 3:171-6. [ Google Scholar]
- Akbari MT, Esmaeili A, Zare AH, Saad N, F Bagheri. Chemical composition and antibacterial activity of essential oil from leaves, stems and flowers of Prangos ferulacea (L) Lindl. Bulgarian Chemical Communications 2010; 42:36-9. [ Google Scholar]
- Griffin SG, Wyllie SG, Markham JL, Leach DN.
The role of structure and molecular properties of terpenoids in determining
their antimicrobial activity
. Flavour Fragr J 1999; 14:322-32. doi: 10.1002/(SICI)1099-1026(199909/10)14:5<322::AIDFFJ837>3.0.CO;2-4 [Crossref] [ Google Scholar]
- Sajjadi SE, Shokoohinia Y, Gholamzadeh S. Chemical composition of essential oil of Prangos ferulacea (L) Lindl roots. Chemija 2011; 22:178-80. [ Google Scholar]
- Liu J, Zhang W, Zhou L, Wang X, Lian Q.
Antiinflammatory effect
and mechanism of osthole in rats
. Zhong Yao Cai 2005; 28:1002-6. [ Google Scholar]
- Li L, Zhuang FE, Zhao GS, Zhoa DK. Effects of osthole on the isolated guinea-pig ileum and taeniae coli. Yao Xue Xue Bao 1993; 28:899-904. [ Google Scholar]
- Teng CM, Lin CH, Ko FN, Wu TS, Huang TF. The relaxant action of osthole isolated from Angelicapubescensin guinea-pig trachea. Naunyn Schmiedebergs Arch Pharmacol 1994; 349:202-8. doi: 10.1007/BF00169838 [Crossref] [ Google Scholar]
- Kuo P L, Hsu Y L, Chang C H, Chang J K. Osthole mediatedcell differentiation through bone morphogenetic protein-2/p38 and extracellular signal-regulated kinase 1/2 pathway in human osteoblast cells. J Pharmacol Exp Ther 2005; 14:1290-9. doi: 10.1124/jpet.105.085092 [Crossref] [ Google Scholar]
- Okamoto T, Kobayashi T, Yoshida S. Chemical aspects of coumarin compounds for the prevention of hepatocellular carcinomas. Curr Med Chem Anticancer Agents 2005; 5:47-51. doi: 10.2174/1568011053352622 [Crossref] [ Google Scholar]
- Durmaz H, Sagun E, Tarakci Z, Ozgokce F. Antibacterial activities of Allium vineale, Chaerophyllum macropodum and Prangos ferulacea. African Journal of Biotechnology 2008; 5:1795-8. doi: 10.5897/AJB06.395 [Crossref] [ Google Scholar]
- Razavi SM, Nazemiyeh H, Zarrini G, Asna-Asharii S, Dehghan G. Chemical composition and antimicrobial activity of essential oil of Prangos ferulaceae (L) Lindl from Iran. Nat Prod Res 2010; 24:530-3. doi: 10.1080/14786410802379539 [Crossref] [ Google Scholar]
-
Adams RP. Identification of essential oil components by gas chromatography/Quadrople Mass Spectroscopy. USA: Illinois: Allured Publishing Corporation; 2004.
-
McLafferty F, W,, Stauffer D, B,. The Wiley/NBS Registry of Mass Spectral Data. New York: Wiley and Sons; 1989.
-
CLSI. Performance standards for antimicrobial disk susceptibility tests for the bacteria that grow aerobically; approved standard. 7th ed. Pennsylvania, Wayne: Clinical and Laboratory Standards Institute; 2006.
- Phelan M C. Basic techniques for mammalian to cell tissue culture. Curr Protoc Cell Biol 1998; 7:1-10. doi: 10.1002/0471143030.cb0101s66 [Crossref] [ Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65:55-63. doi: 10.1016/0022-1759(83)90303-4 [Crossref] [ Google Scholar]
- Adetutu A, Morgan WA, Corcoran O. Ethnopharmacological survey and in vitro evaluation of wound-healing plants used in South-western Nigeria. J Ethnopharmacol 2011; 137:50-6. doi: 10.1016/j.jep.2011.03.073 [Crossref] [ Google Scholar]
- Fronza M, Heinzmann B, Hamburger M, Laufer S, Merfort I. Determination of the wound healing effect of Calendula extracts using the scratch assay with 3T3 fibroblasts. J Ethnopharmacol 2009; 126:463-7. doi: 10.1016/j.jep.2009.09.014 [Crossref] [ Google Scholar]
- Balekar N, Katkam NG, Nakpheng T, Jehtae K, Srichana T. Evaluation of the wound healing potential of Wedelia trilobata (L) leaves. J Ethnopharmacol 2012; 141:817-24. doi: 10.1016/j.jep.2012.03.019 [Crossref] [ Google Scholar]
- Thakur R, Jain N, Pathak R, Sandhu SS. Practices in Wound Healing Studies of Plants. Evid Based Complement Alternat Med 2011; 2011:1-17. doi: 10.1155/2011/438056 [Crossref] [ Google Scholar]
- Gurtner CG, Werner S, Barrandon Y, Longanker MT. Wound repair and regeneration. Nature 2008; 453:314-21. doi: 10.1038/nature07039 [Crossref] [ Google Scholar]
- Schafer M, Werner S. Transcriptional control of wound repair. Annu Rev Cell Dev Biol 2007; 23:69-92. doi: 10.1146/annurev.cellbio.23.090506.123609 [Crossref] [ Google Scholar]
- Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2007; 2:329-33. doi: 10.1038/nprot.2007.30 [Crossref] [ Google Scholar]
-
de Cássia da Silveira e Sá
R
, Nalone Andrade L, Pergentino de Sousa D. A Review on Anti-Inflammatory Activity of Monoterpenes. Molecules 2013; 18:1227-54. doi: 10.3390/molecules18011227 [Crossref] [ Google Scholar]
- Bardon S, Foussard V, Fournel S, Loubat A. Monoterpenes inhibit proliferation of human colon cancer cells bymodulating cell cycle-related protein expression. Cancer Lett 2002; 181:187-94. doi: 10.1016/S0304-3835(02)00047-2 [Crossref] [ Google Scholar]
- Micucci P, Di-Leo-Lira P, Zettler G, Turner S, Ferraro G, Davicino R. Comparative study of Tilia x viridis extract and isolated compounds on the proliferation of two lymphoma cell lines. Molecular Medicinal Chemistry 2010; 21:53-9. [ Google Scholar]
- Bowler PG, Duerden BI, Armstrong DG. Wound Microbiology and Associated Approaches to Wound Management. Clin Microbiol Rev 2001; 14:244-69. doi: 10.1128/CMR.14.2.244-269.2001 [Crossref] [ Google Scholar]
- Kirketerp-Møller K, Jensen PØ, Fazli M, Madsen KG, Pedersen J, Moser C. Distribution, Organization, and Ecology of Bacteria in Chronic Wounds. J Clin Microbiol 2008; 46:2717-22. doi: 10.1128/JCM.00501-08 [Crossref] [ Google Scholar]
- Tammelin A, Lindholm C, Hambraeus A. Chronic ulcers and antibiotic treatment. J Wound Care 1998; 7:435-7. [ Google Scholar]
- Giacometti A, Cirioni O, Schimizzi AM, Prete MSD, Barchiesi F, D'Errico MM. Epidemiology and Microbiology of Surgical Wound Infections. J Clin Microbiol 2000; 38:918-22. [ Google Scholar]
- Jerković I, Gašo-Sokač D, Pavlović H, Marijanović Z, Gugić M, Petrović I. Volatile Organic Compounds from Centaurium erythraea Rafn (Croatia) and the Antimicrobial Potential of Its Essential Oil. Molecules 2012; 17:2058-72. doi: 10.3390/molecules17022058 [Crossref] [ Google Scholar]
- Mariateresa C, Manuela DA, Giuseppina M, Francesco C, Maria G, Sarpietro , Dorotea M. Interaction of Four Monoterpenes Contained in Essential Oils with Model Membranes: Implications for Their Antibacterial Activity. J Agric Food Chem 2007; 55:6300-8. doi: 10.1021/jf070094x [Crossref] [ Google Scholar]
- Cox S, Mann C, Markham J, Gustafson J, Warmington J, Wyllie S. Determining the Antimicrobial Actions of Tea Tree Oil. Molecules 2001; 6:87-91. doi: 10.3390/60100087 [Crossref] [ Google Scholar]
- Bassolé IHN, Lamien-Meda A, Bayala B, Tirogo S, Franz C, Novak J. Composition and Antimicrobial Activities of Lippia multiflora Moldenke, Mentha x piperita L and Ocimum basilicum L Essential Oils and Their Major Monoterpene Alcohols Alone and in Combination. Molecules 2010; 15:7825-39. doi: 10.3390/molecules15117825 [Crossref] [ Google Scholar]
- Rocha P, Rodilla J, Díez D, Elder H, Guala M, Silva L. Synergistic Antibacterial Activity of the Essential Oil of Aguaribay (Schinus molle L). Molecules 2012; 17:12023-36. doi: 10.3390/molecules171012023 [Crossref] [ Google Scholar]
- Elaissi A, Rouis Z, Mabrouk S, Salah KBH, Aouni M, Khouja ML. Correlation Between Chemical Composition and Antibacterial Activity of Essential Oils from Fifteen Eucalyptus Species Growing in the Korbous and Jbel Abderrahman Arboreta (North East Tunisia). Molecules 2012; 17:3044-57. doi: 10.3390/molecules17033044 [Crossref] [ Google Scholar]