Bioimpacts. 7(3):139-145.
doi: 10.15171/bi.2017.17
Original Research
The effect of cell penetrating peptides on transfection activity and cytotoxicity of polyallylamine
Sarvenaz Sabouri-Rad 1, Reza Kazemi Oskuee 2, Asma Mahmoodi 1, Leila Gholami 3, Bizhan Malaekeh-Nikouei 4, *
Author information:
1School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran
2Targeted Drug Delivery Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
3Department of Modern Sciences and Technologies, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
4Nanotechnology Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
Abstract
Introduction:
Cationic polymers have the potential to be modified to achieve an ideal gene vector lacking viral vector defects. The aim of the present study was to improve polyallylamine (PAA) transfection efficiency and to reduce cytotoxicity by incorporating of cell-penetrating peptides (CPPs).
Methods:
To prepare the peptide-based polyplexes, PAA (15 kDa) was modified with 2 peptides (TAT and CyLoP-1) by covering the 0.5% and 1% of amines. Buffer capacity and DNA condensation ability of modified polymer, particle size and zeta potential of nanoparticles, cell viability, and transfection activity of vectors were evaluated.
Results:
In low carrier to plasmid (C/P) weight ratios such as 0.5 and 1, the unmodified polymer was more capable to condense the DNA compared to the synthesized vectors. In C/P ratio of 2, the plasmid was fully condensed in all vectors. The size of polyplexes ranged from 195 to 240 nm. The zeta potential was almost as the same as PAA and varied from 25 to 27 mV. All polyplexes increased the buffer capacity compared to PAA. The transfection efficiency was improved compared to unmodified polymer especially in the vectors modified with 1% of TAT or CyLoP-1 peptides in C/P ratio of 2. The cytotoxicity of prepared vectors was less than PAA. In most ratios, the cytotoxicity of the CyLoP-1 modified samples was less than the TAT modified ones.
Conclusion:
Modification of PAA with CPPs improved the transfection activity of vector.
Keywords: Cell penetrating peptides, Polyallylamine, Polyplexes, Transfection
Copyright and License Information
© 2017 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
An enormous challenge against the therapeutic methods of gene therapy is finding of a suitable vector to transfer the DNA or RNA to the target cells. All the necessary features for a vector have not been supported by the recently introduced carriers. A suitable vector should supply a high degree of transfection in a long period of time without leading to systemic toxicity and immunogenicity.
1,2
Many efforts have been made to select a proper choice among a variety of biological and non-biological vectors such as viruses (retroviruse, herpes simplex viruses, and adenoviruses) and non-viral vectors including dendrimers, cationic lipids and polymers.
Despite the high transfection activity of viral vectors, their damage to the host genes, immune system stimulation and potential of infection limit their application in gene therapy.
3
However, the tendency to synthetic vectors has been increased but the main obstacle with these kinds of a vector is the low efficiency of transfection.
4
Many studies have concentrated on improving of the transfection of non-viral vectors with minimal toxicity. Cationic polymers and lipids are largely considered in the recent researches because of their merits in gene delivery.
5
Cationic polymers with their high positive charge can interact with negatively charged DNA. The formed electrostatic bindings lead to condensed nanoparticles which are able to penetrate the target cells.
6
Polyethyleneimine (PEI) has indicated a high efficiency in gene delivery.
7
Existence of the tertiary amines in the structure of polymer leads to high buffering capacity which is highly effective in endosomal escape and access to the nucleus.
8
The other polymer is poly L-lysine which is biodegradable because of its peptide-like structure.
9
The high molecular weight of this polymer has indicated high transfection efficiency and cellular toxicity.
3
Polyallylamine (PAA) is the other cationic polymer with a high density of the primary amines helping to condense the plasmid effectively. In spite of the effective DNA condensation, considerable cytotoxicity due to high positive charge and lack of buffering capacity makes this cationic polymer unsuitable for the gene therapy.
10,11
To improve the properties of non-viral vectors, many techniques are applied to overcome the cellular barriers. One of these methods is the application of cell-penetrating peptides (CPPs).
12
These peptides are capable to transfer macromolecules to the cytoplasm and nucleus.
13
The aim of the present study was to improve the PAA transfection activity through binding of two kinds of CPPs to the primary amine groups of the polymer.
Materials and Methods
Materials
PAA with the molecular weight of 15 kDa was ordered from Polyscience Inc (USA) and TAT (YGRKKRRQRRR) and CyLoP-1 (CRWRWKCCKK) peptides were purchased from Pepmic (China). Neuro2A (murine neuroblastoma) cells (ATCC CCL-131) were maintained in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with fetal bovine serum (10%), streptomycin (100 μg/mL), and penicillin (100 U/ mL). The cells were incubated under 5% CO2 at 37°C. 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS) were obtained from Sigma-Aldrich (USA). GFP encoding plasmid DNA was ordered from Promega (USA).
Preparation of modified PAA with TAT peptide
Initially, the aqueous solution of polymer was prepared. The peptide solutions were also produced according to peptide-type and coverage percentage by diluting the estimated volumes of peptide solutions with the deionized water. However, the carboxylic group of peptides should be activated to interact with the primary amines of the polymer to cover the 0.5 and 1% of them so two reagents of EDC and NHS were added to both solutions of TAT and CyLoP-1 peptides being prepared in four different concentrations. The mixtures were incubated at 25°C for 30 min under stirring with the speed of 120 rpm. After 30 minutes, the mixtures were slowly added to the polymer solution. Thereafter, this solution containing peptide and polymer were placed on shaker-incubator with the speed of 120 rpm under the temperature of 25°C for 20 minutes. Finally, 4 different vectors were synthesized to test their ability as a capable gene carrier.
Purification of modified polymer
To purify the modified polymer, the final solutions were dialyzed in dialysis tubing (molecular weight cut off 12 kDa) for 3 days at 25°C in the dark against the deionized water. The water exchange was done after 3, 24, 48 and 72 hours. After dialysis, the frozen samples were lyophilized and stored at 4°C.
Buffering capacity
To compare this capability between PAA and the synthetic vectors, acid-base titration was used. The solutions of the samples (PAA and peptide modified PAA) were prepared in the concentration of 0.4 µg/µL. Thereafter, the initial pH of solutions was approximately set in 12 by adding NaOH 1 N. Then, 5 µL aliquots of 1M HCl were subsequently added until the pH was reduced to 2. Then, the trend of pH changes in the range of 2-12 was recorded in each step.
Gel retardation assay
To test the condensation ability of modified polymer, we applied the gel retardation assay. The samples were prepared in C/P ratios of 0.5, 1 and 2. The Green viewer Dye was added to each sample and electrophoresed on agarose gel 1% at 100 mV for 30 minutes in the prepared buffer (EDTA, Tris base, boric acid). The bands were visualized under a UV transilluminator at wavelength of 254 nm.
Preparation of nanoparticles
For the preparation of nanoparticles, different amounts of the modified PAA were separately diluted into HEPES buffered glucose solution (20 mM HEPES in 5% aqueous glucose solution). This solution was mixed with pDNA at different carrier to pDNA weight ratios (C/P) ranging from 0.5:1 to 2:1. The mixture was incubated at room temperature for 30 minutes before use.
Determination of size and zeta potential of nanoparticles
Mean size and zeta potential were measured by Zetasizer (Malvern, UK) at C/P ratio of 2. The sample of plasmid was prepared in HBG buffer. The polymer solution was slowly added to plasmid solution and placed in room temperature for 20 minutes to form the polyplexes. Then, size and zeta potential were recorded after a suitable dilution with deionized water.
In vitro transfection efficiency
Neuro2A cells were seeded in a 96-well plate at the concentration of 104 cells per well and incubated for 24 hours. The polyplexes were prepared in three C/P ratios of 0.5, 1 and 2. The complexes were added to the cells in serum free-medium for 4 hours and then this medium was replaced with the fresh serum-containing DMEM medium. After changing the medium, the incubation continued for another 18 hours. Finally, after removing the medium, the lysis buffer was added to each well. After 30 minutes, the fluorescence intensity of green fluorescent protein (GFP) was measured at 498 nm (excitation wavelength) and 535 nm (emission wavelength).
The green fluorescence intensities of cells were qualified using fluorescence microscopy, the images were acquired with a fluorescence microscope (Hund, Germany) equipped with a 60× objective lens, Scale bar: 20 μm with the exposure time of 1/15 seconds.
Cytotoxicity evaluation
The toxicity of polyplexes was evaluated by MTT assay. Cells were treated as described in the transfection experiment. After 18 hours of incubation, 20 µL of MTT indicator was added to each well. After 2 hours incubation in the temperature of 37°C, the formazan crystals were dissolved in 100 µL of DMSO. The color intensity of the samples was recorded at wavelength of 540 nm by a plate reader (BioTek, USA). Cell viability was expressed as the percentage of living cells compared to untreated cells with 100% viability. Transfection and MTT assays were performed by adding 10 µL of polyplex solution (the equivalent of 200 ng pDNA) to the wells of 96-well plates.
Statistical analysis
The data analysis was performed by Tukey-Kramer test using InStat software (version 3.1) with P value < .05 as the level of significance.
Results
DNA condensation
The electrostatic interaction between the phosphate groups of DNA and positive charge of polymer results in polyplex formation. According to gel retardation images, the condensation ability of the vectors in C/P 0.5 was the same as PAA except for the PAA-TAT-1%. In C/P 1, this ability slightly reduced in PAA-TAT-0.5% and PAA-CyLop1-1%. In C/P 2, all the vectors condensed the DNA completely (Fig. 1).
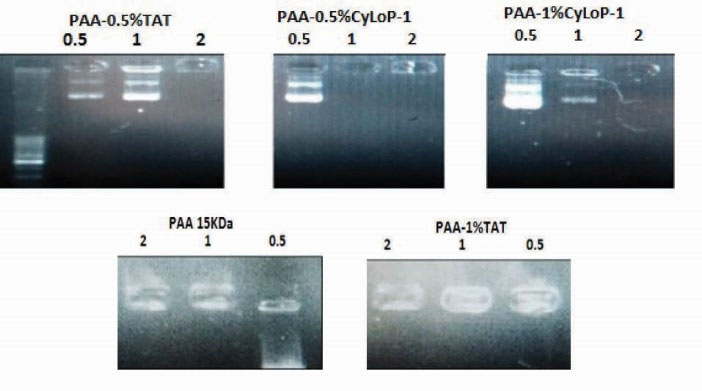
Figure 1.
Gel retardation assay of PAA/pDNA, PAA-TAT/pDNA and PAA-CyLoP-1/pDNA complexes prepared in three C/P of 0.5, 1 and 2. Polymer was modified by CPPs at 0.5% and 1%.
.
Gel retardation assay of PAA/pDNA, PAA-TAT/pDNA and PAA-CyLoP-1/pDNA complexes prepared in three C/P of 0.5, 1 and 2. Polymer was modified by CPPs at 0.5% and 1%.
Buffering capacity
The aim of this experiment is the evaluation of the endosomal rupture by the vectors. In all four peptide-modified samples, the buffering capacity has increased compared to PAA 15 kDa (Figs. 2 and 3).
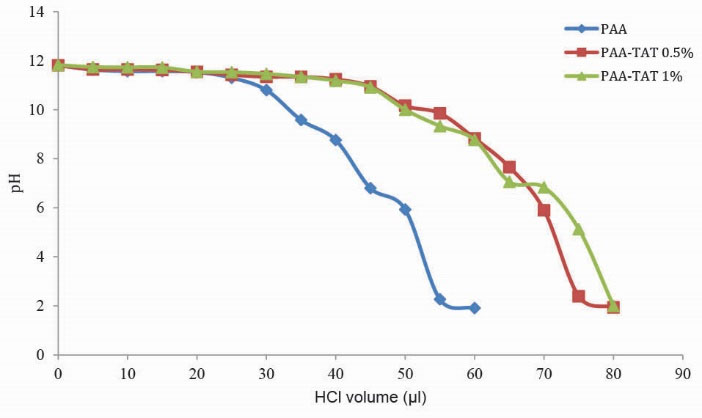
Figure 2.
Comparison of buffering capacity of PAA and TAT modified samples. Polymer was modified by TAT peptide at 0.5 and 1%.
.
Comparison of buffering capacity of PAA and TAT modified samples. Polymer was modified by TAT peptide at 0.5 and 1%.
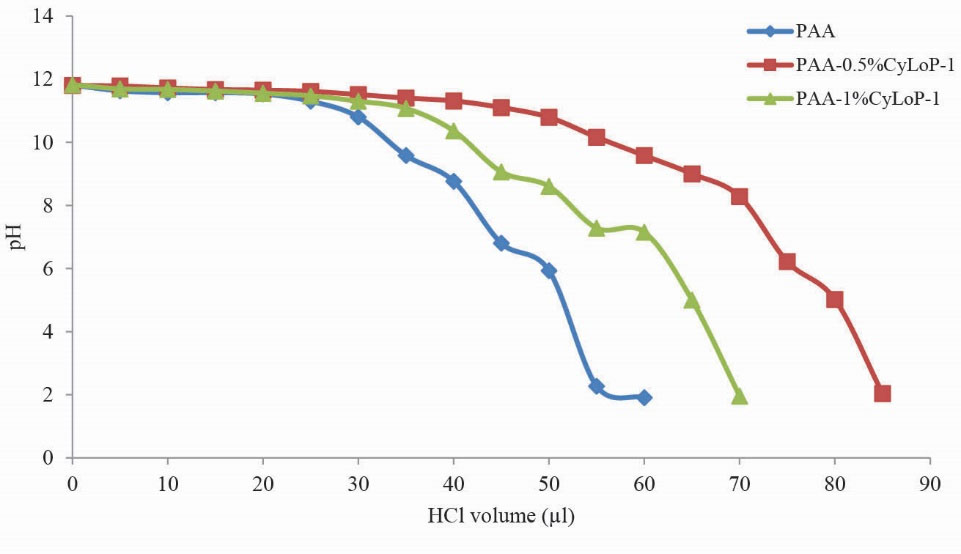
Figure 3.
Comparison of buffering capacity of PAA and CyLoP-1 modified samples. Polymer was modified by CyLoP-1 peptide at 0.5 and 1%.
.
Comparison of buffering capacity of PAA and CyLoP-1 modified samples. Polymer was modified by CyLoP-1 peptide at 0.5 and 1%.
Particle size and surface charge
The particle size of the samples was in the range of 194-240 nm (Table 1). Size of nanoparticles composed of unmodified PAA was under 200 nm. In PAA-TAT1% and PAA-CyLop1-1%, the size significantly increased compared to PAA. However, the vectors with 0.5% coverage of amines in both peptides had the similar size with PAA. The zeta potential of synthetic vectors was the same as PAA and the surface charge ranged from 25 to 27 mV.
Table 1.
Size and zeta potential of PAA/DNA, PAA-TAT/DNA and PAA-CyLoP-1/DNA
|
Vector type
|
Z-Average (nm)
|
PDI
|
Zeta potential (mV)
|
| PAA 15kD |
197.5 ± 7.74 |
0.44 |
25.1 ± 0.5 |
| PAA-0.5%TAT |
195.2 ± 1.6 |
0.39 |
27.3 ± 0.6 |
| PAA-1%TAT |
236.4 ± 8.1 |
0.49 |
25.5 ± 2.5 |
| PAA-0.5%CyLoP-1 |
200.1 ± 8.0 |
0.48 |
24.8 ± 2.7 |
| PAA-1%CyLoP-1 |
229.2 ± 13.4 |
0.53 |
25.1 ± 0.6 |
In vitro transfection assay
To evaluate the transfection ability, the amount of GFP expression was measured in Neuro2a cells in three C/P ratios of 0.5, 1 and 2 (Fig. 4). The transfection activity of prepared vectors improved compared to unmodified PAA in all vectors except for the PAA-0.5%TAT and PAA-0.5%CyLoP-1 in C/P ratio of 2. The gene expression by 2 vectors of PAA-1%TAT and PAA-1%CyLoP-1 was more than the gold standard of gene transfer, PEI 25 kDa. The transfection improvement was significantly more in PAA-1%TAT compared to PEI 25 kDa (P < .05). The transfection efficiency reduced in vectors with 0.5% coverage of amines in C/P ratio of 2. Regarding the effect of C/P in transfection activity, there was no significant difference between the C/P ratio of 0.5 and 1 in case of PAA-1%TAT and PAA-1%CyLoP-1. However, the gene expression in C/P ratio 2 was significantly more than the other C/P ratios. The transfection efficiency in PAA-0.5% TAT and PAA-0.5% CyLoP-1in C/P 2 was less than the other C/P ratios. In fact, in C/P ratio of 2, the increase in the concentration of the peptides resulted in transfection improvement. In order to visualize the transfection results of vectors, the green fluorescent of Neuro2A cells expressing EGFP protein was provided in Fig. 5.
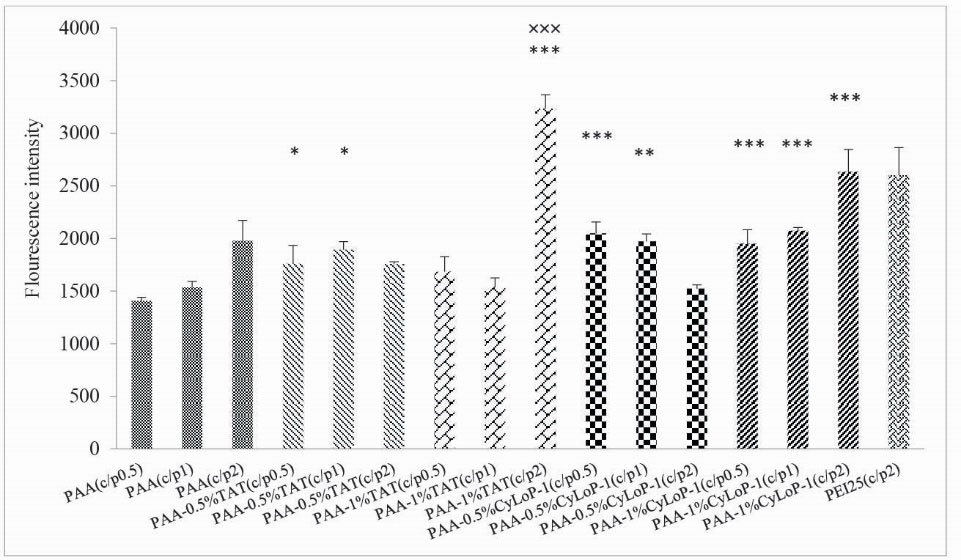
Figure 4.
Transfection efficiency of polyplexes in N2A cells in 3 C/P ratios of 0.5, 1 and 2 (mean ± SD, n = 3, *** P < .001, ** P < .01, * P < .05 comparison between synthetic vectors and unmodified PAA in identical C/P ratios) (×××P < .001 comparison between synthetic vectors and PEI 25 kDa).
.
Transfection efficiency of polyplexes in N2A cells in 3 C/P ratios of 0.5, 1 and 2 (mean ± SD, n = 3, *** P < .001, ** P < .01, * P < .05 comparison between synthetic vectors and unmodified PAA in identical C/P ratios) (×××P < .001 comparison between synthetic vectors and PEI 25 kDa).
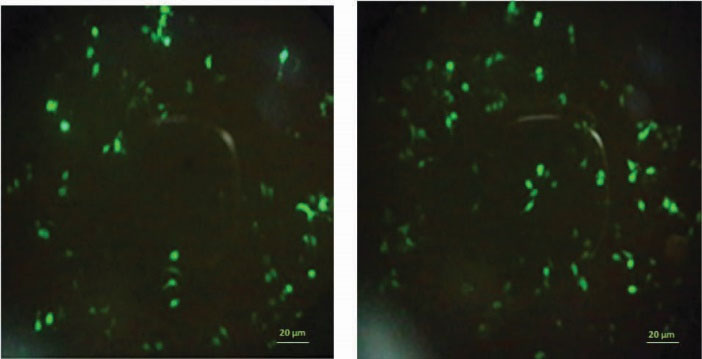
Figure 5.
Expression of green fluorescent protein in Neuro-2a cells transfected with polyplexes prepared from an EGFP expressing plasmid DNA and (A) PEI 25 kDa C/P 2 or (B) PAA-1%-TAT C/P 2 that were most active in the transfection study.
.
Expression of green fluorescent protein in Neuro-2a cells transfected with polyplexes prepared from an EGFP expressing plasmid DNA and (A) PEI 25 kDa C/P 2 or (B) PAA-1%-TAT C/P 2 that were most active in the transfection study.
MTT assay
The cytotoxicity of polyplexes was examined by the MTT assay. The color intensity has a correlation with the living cell number. In most groups, the viability had no correlation with C/P ratio increase (Fig. 6). In most C/P ratios, the cell viability was 60%-80%. In general, the cell toxicity in synthetic vectors compared to unmodified PAA reduced in most vectors with similar C/P ratios. In PAA-0.5% CyLoP-1 (C/P 1 and 2) and PAA-1%CyLoP-1 (C/P 0.5 and 2), the cell viability was above 90%. The cell toxicity in CyLoP-1 modified samples was less than TAT modified ones in most C/P ratios.
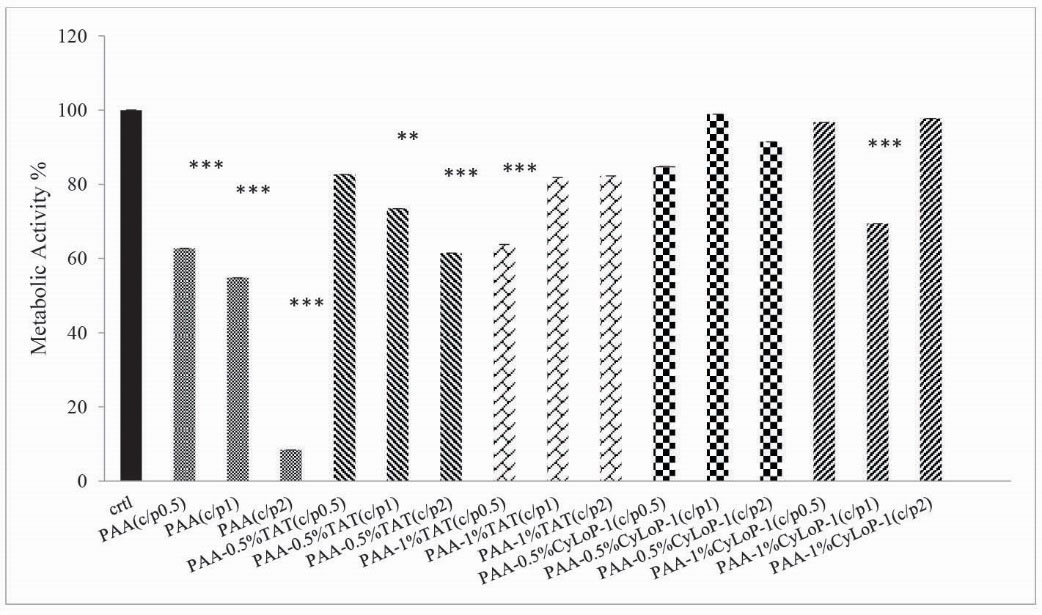
Figure 6.
Comparison of cell viability between unexposed cells and vector- treated cells in cell line of Neuro2A (mean ± SD, n = 3, *** P < .001, ** P < .01, * P < .05 comparison between polyplexes and control group).
.
Comparison of cell viability between unexposed cells and vector- treated cells in cell line of Neuro2A (mean ± SD, n = 3, *** P < .001, ** P < .01, * P < .05 comparison between polyplexes and control group).
Discussion
In recent decades, the viral vectors have indicated high efficiency in gene delivery. However, due to pathogenicity, immunogenicity and low loading capacity, the investigations about the non-viral systems have begun.
14,15
The major obstacle in the application of non-viral vectors is low transfection with high cytotoxicity.
16
PAA as a non-viral vector is not known very well. This polymer contains the high density of the primary amines.
17
In a comparative study, among the cationic polymers, PDMEM (polydimethylaminoethylmethacrylate), PLL (poly-L-lysine), PAA, PVA (polyvinylamine) and PTMAEM (poly-(N,N,N-trimethylammonio)ethylmethacrylate), the PAA was one of the most efficient vectors in gene transfection.
18
The substitution of the amine groups in this polymer may help to acquire a suitable gene carrier.
19
In 2 different studies, glycosylation and dextran substitution have improved the transfection in this polymer.
11,17
Also, the nanoparticles composed of PAA and imidazolyl group crosslinking with PEG also indicated an increase in transfection.
19
Application of non-viral vectors is limited by weak endosomal escape and nuclear delivery.
16
In recent years, several peptide-based systems to overcome these obstacles have been proposed.
20
In this study, we tried to bind two kinds of CPPs to PAA 15 kDa to improve the transfection efficiency and reduce the cytotoxicity of the vector.
CPPs are polypeptides with less than 30 amino acids mediating the cellular uptake by energy-dependent endocytosis and energy independent direct penetration.
21,22
TAT peptide is one of the most known CPPs interacting with the cell membrane due to its positive charge.
23
This peptide is derived from the transduction domain of HIV1 TAT protein having several Arg and Lys in its sequence making it so cationic.
24,25
To evaluate the effect of TAT incorporation with other gene vectors, TAT peptide could transfer the proteins with the weight of more than 100 KD, 40 nm particles and 200 nm liposomes into cells.
26
CyLoP-1, a cysteine rich peptide, also provides the intracellular access for the macromolecules.
27
In this study, four vectors of PAA-0.5%TAT, PAA-1%TAT, PAA-0.5%CyLoP-1 and PAA-1%CyLoP-1 were synthesized which IR and NMR spectrums confirmed the accuracy of the synthesis reaction (data not shown). The gel retardation assay revealed that in most synthetic vectors, the condensation ability reduced compared to unmodified polymer in C/P ratios of 0.5 and 1 which can be the result of the amine group coverage. The other reason could be the reduction of amines charge due to pH increase because the cationic polymers interact with pDNA in a pH-dependent manner.
28
In C/P 2, all the vectors completely condense the pDNA. Probably, a suitable balance between the charge of amine groups and peptides should be exhibited to get an appropriate condensation. The slight reduction in plasmid condensation can be useful since the strong connection may prevent the cytoplasmic release. Hu et al in a study on Man-PEI 1800 kD-CPP showed the amine substitution with mannose resulted in charge reduction to interact with the plasmid.
29
The major obstacle to the efficient gene delivery is the plasmid degradation in the endosomal compartment. The titration graphs indicated the buffering capacity increase in all synthetic vectors. The positive charge of particles may interact with the negative charge membrane resulting in the endosomal membrane lysis.
30
In CyLoP-1 peptide, the hydrophobicity of the tryptophans resulted in the endosomal membrane lysis.
27
In a study of Rudolph et al, Arg-rich oligomers of TAT promoted the cellular uptake of the particle through the endosomal pathway.
31
Furthermore, Rahmat et al in 2012 pointed out the TAT role in zeta potential increase and endosomal lysis.
30
Size and surface charge are 2 essential factors affecting the mechanism of entry. Recently, several internalization mechanisms are introduced by CPPs.
32
The size average of PAA was below 200 nm. In PAA-1%TAT and PAA-1%CyLoP-1, the particle size significantly increased. The size range of polyplexes was between 194-240 nm. The zeta potential was the same as PAA due to low concentration of peptides ranging from 25 to 27 mV. The size increase might happen in 1% coverage of amines due to repulsive electrostatic force of polymer and peptides.
11
The suggested internalization mechanisms by the CPPs are categorized into 2 groups; endocytosis and direct penetration.
22
In transfection experiment, polymer modification by the peptides resulted in transfection improvement in C/P 0.5 and 1 compared to PAA in same C/P ratios. The transfection efficiency in PAA-1%CyLoP-1 was almost the same as PEI 25KD and in PAA-1%TAT was significantly higher than PEI25KD. The reason of this improvement can be the suitable size and cationic charge for interaction with the cell membrane. TAT peptide has a high affinity to the heparan sulfate of the cell membrane and also stimulates the receptor dependent endocytosis.
30,33
TAT peptide acts as a transduction domain and nucleus localization signal
34
so probably can transfer the polyplexes into cells and nucleus efficiently. CyLoP-1 peptide also can interact with the membrane and the tryptophans resulted in the membrane instability
27
. In C/P 2 in vectors with 0.5% coverage of amines, the transfection rate reduced compared to PAA so the concentration increase of the peptides resulted in transfection improvement. Recently, several internalization mechanisms for TAT peptide are suggested such as lipid-raft mediated macropinocytosis, caveolae mediated endocytosis and clathrin-dependent endocytosis.
35-37
In PAA-TAT and PAA-CyLoP-1 vectors, the polyplexe may enter the cell by clathrin-dependent endocytosis and direct penetration. In lower C/P ratios of 0.5 and 1, the CyLoP-1 peptide acted more successfully than TAT peptide in transfection because the cytosol targeting even in low concentrations is a specific characteristic of CyLoP-1 peptide.
27
PAA due to cationic structure has cytotoxicity
11
. In this investigation, the cell viability was between 60%-80%. Application of peptides with this polymer has reduced the cytotoxicity especially in C/P 2. Generally, the CyLoP-1 modified samples were less toxic than TAT modified ones. Maybe, the hydrophilic structure of TAT peptide resulted in more cytotoxicity.
38
Conclusion
Briefly, TAT and CyLoP-1 peptides has improved the transfection and reduced the cytotoxicity compared to PAA 15 kDa in most C/P ratios. Two vectors of PAA-1%CyLoP-1 and PAA-1%TAT had better transfection compared to PEI 25 kDa but the PAA-1%CyLoP-1 is a better choice for gene therapy due to lower cytotoxicity.
Acknowledgments
This study was supported by a Grant from the Vice Chancellor for Research of Mashhad University of Medical Science, Mashhad, Iran.
Competing interests
There is no conflict of interest in this study.
Ethical approval
There is nothing to be declared.
Research Highlights
What is current knowledge?
simple
-
√ Many techniques are applied to overcome the cellular
barriers in gene delivery.
-
√ Cell penetrating peptides are capable to transfer
macromolecules to the cytoplasm and nucleus.
What is new here?
simple
-
√ Modification of PAA with TAT and CyLoP-1 peptides has
improved the transfection activity of vector.
-
√ Cytotoxicity of prepared vectors was less than PAA.
References
- Sharma R, Khajuria R, Sharma C, Kapoor B, Goswami K, Kohli K. Gene therapy: Current concepts. JK Science 2004; 6:62-6. [ Google Scholar]
- Mehier-Humbert S, Guy RH. Physical methods for gene transfer: improving the kinetics of gene delivery into cells. Adv Drug Deliv Rev 2005; 57:733-53. doi: 10.1016/j.addr.2004.12.007 [Crossref] [ Google Scholar]
- Rezaee M, Kazemi Oskuee R, NassirliH NassirliH, Malaekeh-NikoueiB Malaekeh-NikoueiB. Progress in the development of lipopolyplexes as efficient non-viral gene delivery systems. J Control Release 2016; 236:1-14. doi: 10.1016/j.jconrel.2016.06.023 [Crossref] [ Google Scholar]
- Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chem Rev 2009; 109:259-302. doi: 10.1021/cr800409e [Crossref] [ Google Scholar]
- Boussif O, Zanta M, Behr J. Optimized galenics improve in vitro gene transfer with cationic molecules up to 1000-fold. Gene Ther 1996; 3:1074-80. [ Google Scholar]
- El-Aneed A. An overview of current delivery systems in cancer gene therapy. J Control Release 2004; 94:1-14. doi: 10.1016/j.jconrel.2003.09.013 [Crossref] [ Google Scholar]
- Fischer D, Bieber T, Li Y, Elsässer H-P, Kissel T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharm Res 1999; 16:1273-79. doi: 10.1023/A:1014861900478 [Crossref] [ Google Scholar]
- Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA 1995; 92:7297-301. [ Google Scholar]
- Kwoh DY, Coffin CC, Lollo CP, Jovenal J, Banaszczyk MG, Mullen P. Stabilization of poly-L-lysine/DNA polyplexes for in vivo gene delivery to the liver. Biochim Biophys Acta 1999; 1444:171-90. doi: 10.1016/S0167-4781(98)00274-7 [Crossref] [ Google Scholar]
- Chen DJ, Majors BS, Zelikin A, Putnam D. Structure–function relationships of gene delivery vectors in a limited polycation library. J Control Release 2005; 103:273-83. doi: 10.1016/j.jconrel.2004.11.028 [Crossref] [ Google Scholar]
- Nimesh S, Kumar R, Chandra R. Novel polyallylamine–dextran sulfate–DNA nanoplexes: highly efficient non-viral vector for gene delivery. Int J Pharm 2006; 320:143-49. doi: 10.1016/j.ijpharm.2006.03.050 [Crossref] [ Google Scholar]
- Munyendo WL, Lv H, Benza-Ingoula H, Baraza LD, Zhou J. Cell penetrating peptides in the delivery of biopharmaceuticals. Biomolecules 2012; 2:187-202. doi: 10.3390/biom2020187 [Crossref] [ Google Scholar]
- Pooga M, Hällbrink M, Zorko M. Cell penetration by transportan. The FASEB J 1998; 12:67-77. [ Google Scholar]
- Verma IM, Somia N. Gene therapy-promises, problems and prospects. Nature 1997; 389:239-42. doi: 10.1038/38410 [Crossref] [ Google Scholar]
- Song YK, Liu F, Chu S, Liu D. Characterization of cationic liposome-mediated gene transfer in vivo by intravenous administration. Hum Gene Ther 1997; 8:1585-94. doi: 10.1089/hum.1997.8.13-1585 [Crossref] [ Google Scholar]
- Luo D, Saltzman WM. Synthetic DNA delivery systems. Nat Biotechnol 2000; 18:33-7. doi: 10.1038/71889 [Crossref] [ Google Scholar]
- Boussif O, Delair T, Brua C, Veron L, Pavirani A, Kolbe HV. Synthesis of polyallylamine derivatives and their use as gene transfer vectors in vitro. Bioconjug Chem 1999; 10:877-83. doi: 10.1021/bc9900439 [Crossref] [ Google Scholar]
- Slita A, Kasyanenko N, Nazarova O, Gavrilova I, Eropkina E, Sirotkin A. DNA–polycation complexes effect of polycation structure on physico-chemical and biological properties. J Biotechnol 2007; 127:679-93. doi: 10.1016/j.jbiotec.2006.07.016 [Crossref] [ Google Scholar]
- Pathak A, Aggarwal A, Kurupati RK, Patnaik S, Swami A, Singh Y. Engineered polyallylamine nanoparticles for efficient in vitro transfection. Pharm Res 2007; 24:1427-40. doi: 10.1007/s11095-007-9259-7 [Crossref] [ Google Scholar]
- Gariépy J, Kawamura K. Vectorial delivery of macromolecules into cells using peptide-based vehicles. Trends Biotechnol 2001; 19:21-8. doi: 10.1016/S0167-7799(00)01520-1 [Crossref] [ Google Scholar]
- Zhang D, Wang J, Xu D. Cell-penetrating peptides as noninvasive transmembrane vectors for the development of novel multifunctional drug-delivery systems. J Control Release 2016; 229:130-39. doi: 10.1016/j.jconrel.2016.03.020 [Crossref] [ Google Scholar]
- Trabulo S, Cardoso AL, Mano M, De Lima MCP. Cell-penetrating peptides-mechanisms of cellular uptake and generation of delivery systems. Pharmaceuticals 2010; 3:961-93. doi: 10.3390/ph3040961 [Crossref] [ Google Scholar]
- Järver P, Langel Ü. The use of cell-penetrating peptides as a tool for gene regulation. Drug Discov Today 2004; 9:395-402. doi: 10.1016/S1359-6446(04)03042-9 [Crossref] [ Google Scholar]
- Tünnemann G, Ter‐Avetisyan G, Martin RM, Stöckl M, Herrmann A, Cardoso MC. Live‐cell analysis of cell penetration ability and toxicity of oligo‐arginines. J Peptide Sci 2008; 14:469-76. doi: 10.1002/psc.968 [Crossref] [ Google Scholar]
- Snyder EL, Dowdy SF. Cell penetrating peptides in drug delivery. Pharm Res 2004; 21:389-93. doi: 10.1023/B:PHAM.0000019289.61978.f5 [Crossref] [ Google Scholar]
- Berry C. Berry CIntracellular delivery of nanoparticles via the HIV-1 tat peptide .
Nanomedicine (Lond)
2008; 3:357-65. doi: 10.1021/bi700416h [Crossref] [ Google Scholar]
- Jha D, Mishra R, Gottschalk S, Wiesmüller KH, Ugurbil K, Maier ME, Engelmann J. CyLoP-1: a novel cysteine-rich cell-penetrating peptide for cytosolic delivery of cargoes. Bioconjug Chem 2011; 22:319-28. doi: 10.1021/bc100045s [Crossref] [ Google Scholar]
- Rungsardthong U, Ehtezazi T, Bailey L, Armes SP, Garnett MC, Stolnik S. Effect of polymer ionization on the interaction with DNA in nonviral gene delivery systems. Biomacromolecules 2003; 4:683-90. doi: 10.1021/bm025736y [Crossref] [ Google Scholar]
- Hu Y, Xu B, Ji Q, Shou D, Sun X, Xu J. A mannosylated cell-penetrating peptide-graft-polyethylenimine as a gene delivery vector. Biomaterials 2014; 35:4236-46. doi: 10.1186/s11671-016-1337-5 [Crossref] [ Google Scholar]
- Rahmat D, Khan MI, Shahnaz G, Sakloetsakun D, Perera G, Bernkop-Schnürch A. Synergistic effects of conjugating cell penetrating peptides and thiomers on non-viral transfection efficiency. Biomaterials 2012; 33:2321-26. doi: 10.1016/j.biomaterials.2011.11.046 [Crossref] [ Google Scholar]
- Rudolph C, Plank C, Lausier J, Schillinger U, Müller RH, Rosenecker J. Oligomers of the arginine-rich motif of the HIV-1 TAT protein are capable of transferring plasmid DNA into cells. J Biol Chem 2003; 278:11411-18. doi: 10.1074/jbc.M211891200 [Crossref] [ Google Scholar]
- Zaro JL, Shen W-C. Quantitative comparison of membrane transduction and endocytosis of oligopeptides. Biochem Biophys Res Commun 2003; 307:241-7. doi: 10.1016/S0006-291X(03)01167-7 [Crossref] [ Google Scholar]
- Ziegler A, Seelig J. Interaction of the protein transduction domain of HIV-1 TAT with heparan sulfate: binding mechanism and thermodynamic parameters. Biophys J 2004; 86:254-63. doi: 10.1016/S0006-3495(04)74101-6 [Crossref] [ Google Scholar]
- Tkachenko AG, Xie H, Liu Y, Coleman D, Ryan J, Glomm WR. Cellular trajectories of peptide-modified gold particle complexes: comparison of nuclear localization signals and peptide transduction domains. Bioconjug Chem 2004; 15:482-90. doi: 10.1021/bc034189q [Crossref] [ Google Scholar]
- Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med 2004; 10:310-5. doi: 10.1038/nm996 [Crossref] [ Google Scholar]
- Ferrari ME, Nguyen CM, Zelphati O, Tsai Y, Felgner PL. Analytical methods for the characterization of cationic lipid-nucleic acid complexes. Hum Gene Ther 1998; 9:341-51. doi: 10.1089/hum.1998.9.3-341 [Crossref] [ Google Scholar]
- Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ. Cell-penetrating peptides A reevaluation of the mechanism of cellular uptake. J Biol Chem 2003; 278:585-90. doi: 10.1074/jbc.M209548200 [Crossref] [ Google Scholar]
- Brooks H, Lebleu B, Vivès E. Tat peptide-mediated cellular delivery: back to basics. Adv Drug Deliv Rev 2005; 57:559-77. doi: 10.1016/j.addr.2004.12.001 [Crossref] [ Google Scholar]