BioImpacts. 8(4):263-270.
doi: 10.15171/bi.2018.29
Original Research
Nafion-coated cadmium pentacyanonitrosylferrate-modified glassy carbon electrode for detection of dopamine in biological samples
Mohammad Johari-Ahar 1, 2, Jaleh Barar 2, 3, *  , Pari Karami 1, 2, Davoud Asgari 2, 3, Soodabeh Davaran 3, Mohammad-Reza Rashidi 3, *
, Pari Karami 1, 2, Davoud Asgari 2, 3, Soodabeh Davaran 3, Mohammad-Reza Rashidi 3, *
Author information:
1Biosensors and Bioelectronics Research Center, Ardabil University of Medical Sciences, Ardabil, Iran
2Research Center for Pharmaceutical Nanotechnology, Biomedicine Institute, Tabriz University of Medical Sciences, Tabriz, Iran
3Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction
: Dopamine is one of the key neurotransmitters (NTs) in nature, which plays a crucial role in the mammalian central nervous system (CNS). Its selective determination in the biological fluids is an essential need in the field of biomedicine studies.
Methods
: In this work, an amperometric sensor was developed using Nafion-coated cadmium pentacyanonitrosylferrate (CdPCNF) modified glassy carbon (GC) electrode (Nafion|CdPCNF|GC electrode) as an electrocatalyst to detect dopamine (DA) in human serum samples. To develop this sensor, the surface of bare GC electrode was coated with the film of CdPCNF through an electropolymerization method and then the modified electrode was coated with Nafion to minimize interferences, especially those arising from the presence of anionic compounds. The electrocatalytic behavior of the modified electrodes was studied using the cyclic voltammetry and amperometry, and then the ability of the sensor for the determination of DA in synthetic and biological samples was investigated.
Results
: The modified electrode was showed a significant electrocatalytic activity for the oxidation of DA at pH 7.4. The limit of detection (LOD) was 0.7 µM and also no interference effects arose from ascorbic acid (AA), uric acid (UA) or the other biological NTs was observed in the DA detection using the modified Nafion|CdPCNF|GC electrode.
Conclusion
: In comparison with the bare electrode, the Nafion|CdPCNF|GC electrode could determine DA in the biological samples with adequate sensitivity and selectivity. Therefore, we propose that the modified electrode is utilizable as an amperometric DA sensor for the biological sample analysis.
Keywords: Amperometric sensor, Dopamine, Nafion, Cadmium pentacyanonitrosylferrate
Copyright and License Information
© 2018 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Neurotransmitters (NTs) are endogenous chemicals that transmit electrical signals from one neuron to another across the synapse. This synaptic transmission proceeds through the interaction of NTs with the specific receptors onto the membrane of the target cells.
1
The interaction between NT and its receptor is a key event in the action of all NTs and is considered as one of the major modes of communication between neurons. Of important NTs, dopamine (DA) is responsible for transmitting signals in the mammalian central nervous system (CNS). Since its discovery in the 1950s, it has been shown that DA level fluctuations may lead to several CNS diseases such as Schizophrenia, Parkinson's disease
2,3
and Tourette's syndrome.
4-6
Therefore, it is important to quantify the content of DA in the clinical samples of patients with CNS disorders using a simple and rapid method of quantitative detection.
DA concentration in the biological samples is fairly low (1-100 ng/mL).
7
Plasma dopamine is originated from a variety of tissues including sympathetic nerves and adrenal. It is present in free form in a concentration approximately equivalent to that of epinephrine and about 25% that of norepinephrine.
8
Following some sympathetic activities such as stress, exercise, standing, or hypovolemia the plasma concentration of DA increase, however this nervous responses may have a significantly smaller magnitude than those for plasma norepinephrine and epinephrine.
8
There still exist increasing demands for the selective and specific detections of DA in the biological samples. However, has not paid sufficiently on the assessment of DA level in the human blood samples, and the majority of previously reported sensors have been applied towards the detection of DA content in the pharmaceutical formulations. Moreover, a number of studies conducted for determination of DA quantitatively in the biological media, Direct electrochemical methods with high selectivity and specificity are capable for DA detection in the highly complex media, and therefore, are of great interest of scientists in the area of analytical chemistry. Because of the overlapping oxidation potentials of some endogenous biomolecules in the biological fluids (e.g., ascorbic acid [AA] and uric acid [UC]) they can interfere with detection of DA. Hence, the detection of DA in the presence of AA/UC through electroanalytical approaches is a challenging issue.
9
In recent years, several strategies have been employed to improve the selectivity of DA detection such as polymer film modified electrodes.
10-13
Recently, much attention has been paid to the ion-exchange polymers due to their exclusive properties in removing interferences.
14
Nafion, a negatively charged copolymer due to the presence of sulfonated groups in its own backbone, has widely been used as an ion-exchange material to eliminate interferences caused by anions.
15,16
At physiological pH, DA (pKa, 8.87) exists mostly in the cationic form, while AA (pKa, 4.17) and UA (pKa, 3.70) exist as anions. Thus, Nafion coated GC electrode allows detection of DA and eliminates interferences of AA and UA.
15
There is a substantial interest in terms of redox properties of Prussian blue (PB) type metal hexacyanoferrates with respect to their fundamental importance and also possible applications. Preparation of some analogs of PB modified electrodes and their electrochemical behavior have been reported based upon different transition of metal hexacyanoferrates such as chromium,
17
nickel,
18
cobalt,
19
copper,
20
palladium,
21
molybdenum,
22
vanadium,
23
and platinum.
24
Among many metal hexacyanoferrates, cobalt hexacyanoferrate showed a similar electrochemical behavior to the PB. The other PB analogs indicated only one redox center due to hexacyanoferrate (III)/(II) system.
In the present study, an amperometric DA sensor is developed by using of Nafion|CdPCNF|GC electrode to detect dopamine electrochemically with high sensitivity and selectivity.
Materials and Methods
Chemicals
Sodium pentacyanonitrosylferrate, cadmium chloride (CdCl2), sodium nitrate (NaNO3), citric acid, oxalic acid, Titrisol®(for phosphate buffer solution), and tartaric acid were purchased from Merck Co. (Rahway, New Jersey, USA) in the analytical grade. DA, AA, and UA were obtained from ACROS Co. (New Jersey, USA) and were used without further purification. Nafion was obtained from DuPont Co. (Wilmington, Delaware, USA). The serum samples were taken from the healthy people and were then provided from Imam Reza hospital (Tabriz, Iran). All aqueous solutions were prepared using the deionized (DI) water.
Apparatus
All electrochemical experiments were carried out using Potentiostat-Galvanostat Autolab station PGSTAT 302N (Echo Chemie B.V., Metrohm Co., Schiedam, Netherlands) equipped with frequency response analyzer (FRA) module. Electrochemical impedance spectroscopy (EIS) data were analyzed and fitted by Nova 1.8 software. 744-pH meter from Metrohm Co. (Herisau, Switzerland) was used for pH measurement. A conventional three-electrode system was employed to perform the electrochemical analysis. Glassy carbon (GC) electrode (3 mm in diameter), Ag/AgCl (saturated KCl) electrode and Platinum wire were used as the working, reference, and auxiliary electrode, respectively.
Fabrication of CdPCNF|GC electrode
The bare GC electrode was polished successively with 0.3 and 0.05 μm Al2O3 slurry on emery paper prior to modification. The GC electrode was rinsed with DI water. The CdPCNF|GC electrode was prepared as described before.
25
Briefly, the potential ranging from -0.2 V to 1 V at the scan rate of 0.1 V/s was applied to the GC electrode through cyclic voltammetry 21 times in a solution (pH=3) containing 0.1 M KNO3, 5.0 mM CdCl2 and 5.0 mM Na2[Fe(CN)5NO]∙2H2O.
Optimization of the amount of Nafion coated on the CdPCNF|GC electrode
It is expected that functionality of the modified electrode to be deeply dependent on the amount of Nafion deposited on working areas of the electrode. Deposition of various amounts of Nafion on the surface of electrode was evaluated. This interaction has imposed effects on the performance of the modified electrode. Further, to study the diffusion limitations, the impacts of additional polymer layers were examined.
pH optimization and selection of supporting electrolytes
To evaluate the effect of pH on the performance of Nafion|CdPCNF|GC electrode, Britton–Robinson (B.R) buffer (0.1 M) solutions with various pH values ranging from 2 to 12 was used for the electrochemical measurements. Moreover, the effect of different kinds of buffers (e.g., acetate [AC], citrate [CT], and phosphate [PB]) was surveyed at the optimized pH.
Optimal rotation speed of electrode
The rotation speed of the electrode is an important parameter that determines a mass transfer rate, which can be optimized by tuning of the rotation speed in a certain range. At lower rotation speed, the mass transfer rate is low and may be a rate-limiting step of the electrocatalytic process. Whenever the rotation speed is optimal, the mass transfer reaches to the equilibration and the efficient electrocatalytic reaction remains almost unchanged.
Electrochemical impedance spectroscopy analysis
The EIS approach was employed to measure the electron transfer resistance (Rct) of the electrode, in which each step of modification and the obtained EIS data was represented by the Nyquist plot. In this analysis, the diameter of the obtained semicircles indicates the interfacial Rct and strait line denotes Warburg-type impedance (Zw).
Interference study
Determination of DA using Nafion|CdPCNF|GC electrode was carried out in the presence of various interfering species including ascorbic acid (AA), uric acid (UA), tyrosine (Try), tryptophan (Trp), norepinephrine (NE), epinephrine (EP), serotonin (Ser), citric acid (CA), oxalic acid (OA), glucose (Glu) and nicotinamide adenine dinucleotide (NADH). These interfering substances can be oxidized at a potential close to that of DA at most solid electrodes and appear to be the major sources of interferences in DA detections, which can be resulted in the overlapping voltammetric responses. Amperometric responses of DA were recorded through the CdPCNF|GC and Nafion|CdPCNF|GC electrodes and then compared with each other before and after addition of interfering species.
Detection of DA in human serum samples
The electrochemical studies were performed in human serum samples and PBS solution (pH= 7.4) containing various concentrations of DA. The current was monitored at the fixed potential (0.7 V) and the hydrodynamic current-time data of DA detection at different concentrations ranging from 1.7 µM to 150 µM were recorded through Nafion|CdPCNF|GC electrode. Further, the limit of detection (LOD) and the limit of quantification (LOQ) of DA detection in the buffer and serum samples were calculated using YLOD = YB + 3sB and YLOQ = YB + 10sB equations, where YLOD is LOD, YB is the blank signal, sB is as standard deviation (SD) of the blank, and YLOQ is the limit of quantification.
26
To obtain the recovery of DA, 6 DA pharmaceutical injections (Caspian Tamin Pharmaceutical Co., Iran), containing 200 mg/5 mL DA, were spiked with 30 mg of DA and DA levels were determined using Nafion|CdPCNF|GC electrode. In addition, four human serum samples from healthy volunteers were spiked with 10 µM DA in the presence of 5 mM AA and 10 mM UA. The recovery of DA from each sample was measured using the proposed method.
Stability and reproducibility of Nafion|CdPCNF|GC electrode
Nafion|CdPCNF|GC electrode was dried by nitrogen flow, kept at room temperature (25°C) for 12 weeks and then analyzed for changes in electrochemical responses once a week. The results were compared with the initial response of the electrode through the coefficient variation analysis so as to assess the long-term stability of Nafion|CdPCNF|GC electrode.
Results
General characteristics of the modified electrode
The CdPCNF layer was deposited through CV method and it was observed that the thickness of the deposited layer increased after each cycle. After 21 cycles, while no changes in the CV spectrum were detected, we selected 21 potential cycles of CV for the final preparation of CdPCNF|GC electrode (see Fig. S1, Supplementary file 1). Moreover, we observed that the amount of deposited Nafion on the CdPCNF|GC electrode affected the performance of the modified electrode. After a series of attempts, 5 µL Nafion (0.5 wt. %) dispersion was considered as an optimal amount.
The CV spectra of GC, CdPCNF|GC, and Nafion|CdPCNF|GC electrodes in the absence and presence of DA are represented in Fig. 1. In the absence of DA, no redox peaks were observed by using the bare GC electrode in the potential range of 0-1 V (Fig. 1A ; see curve a). In the presence of DA (Fig. 1B ; curve a) quasi-reversible voltammetric redox peaks were observed at 0.68 V (anodic) and 0.56 V (cathodic) using the bare GC electrode. Due to the coating of the electrode surface by oxidized products, the peak-to-peak separation presumably suggests slow electron transfer kinetics. As illustrated in Fig. 1 (panel B, curve c) , using Nafion|CdPCNF|GC electrode, a pair of symmetrical redox peaks appeared at 0.573 and 0.459 V. The mean peak potential E0= (Epa+Epc)/2 was calculated to be 0.516 V (vs. Ag|AgCl) and the peak potential separation was found to be 0.114 V at the scan rate of 0.1 V/s. This redox peak is related to the reduction and oxidation of the redox couple of Fe3+/Fe2+ in a matrix of PCNF.
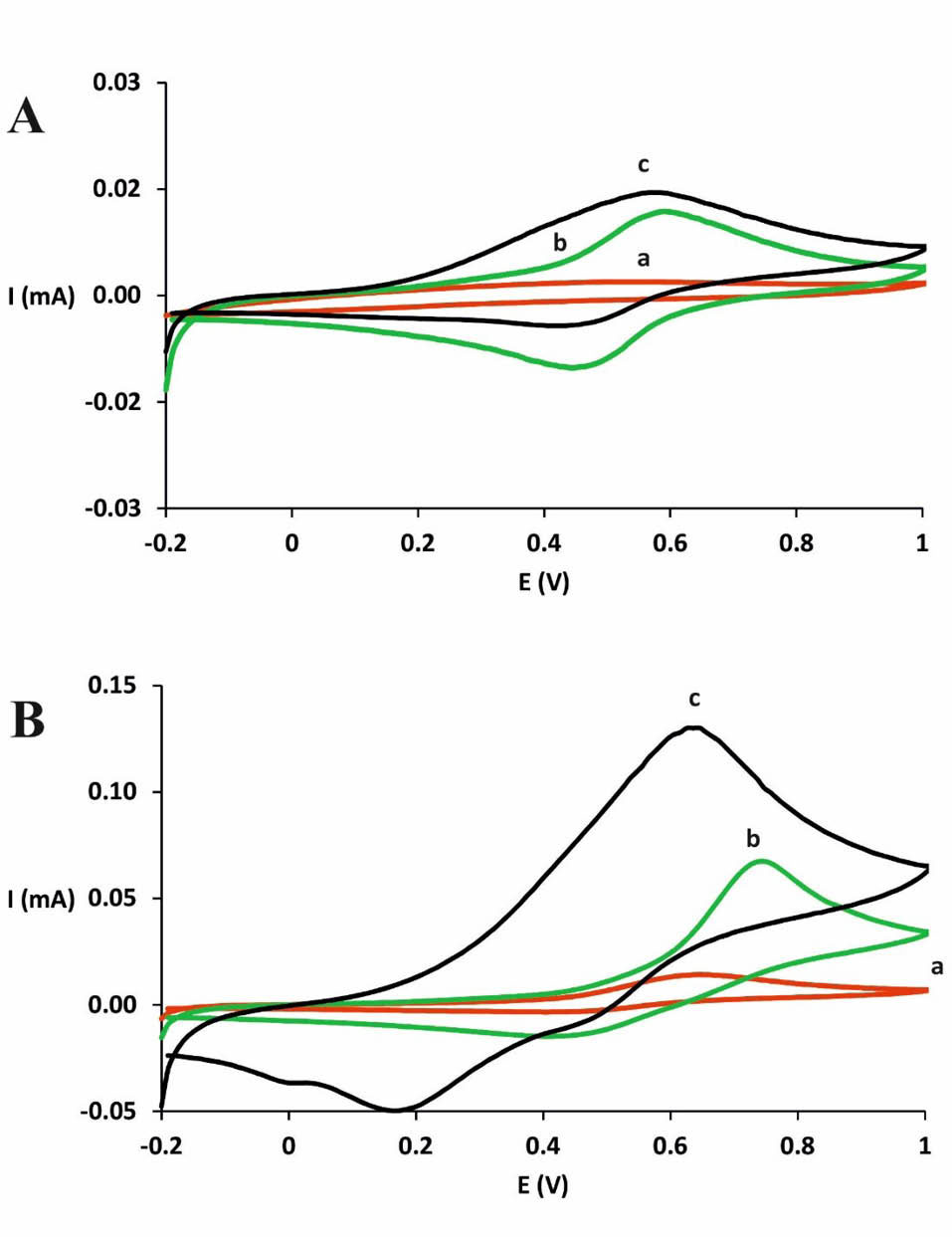
Fig. 1.
CV spectra of GC electrode (a), CdPCNF|GC electrode (b) and Nafion|CdPCNF|GC electrode (c) in the absence (A) and presence (B) of DA (5.0 mM) in PBS (pH 7.4) at the potential scan rate of 0.1 V/s.
.
CV spectra of GC electrode (a), CdPCNF|GC electrode (b) and Nafion|CdPCNF|GC electrode (c) in the absence (A) and presence (B) of DA (5.0 mM) in PBS (pH 7.4) at the potential scan rate of 0.1 V/s.
The anodic peak current (Ipa) changes are proportional to the square root of potential scan rates, indicating that the electrochemical reaction is a reversible and diffusion controlled process. At higher scan rates the electrochemical process is found to be dominated by kinetic controlled parameters of the electrochemical reaction (Fig. S2, Supplementary file 1). The calibration plot of DA determination clearly shows that the peak current values are linearly changed with DA concentrations in the range of 1-40 mM (Fig. S3, Supplementary file 1). For concentrations more than 40 mM, the variation of peak currents deviated from the linearity. The LOD was found to be 0.12 mM, which is too large for some analytical purposes. To improve the LOD, we investigated the catalytic activity of the modified electrode using the constant potential amperometric technique in the next sections of this paper.
Nafion|CdPCNF|GC electrode was stable enough in the broad range of pH (2-10). The pH of supporting electrolyte also affected the voltammetric behavior of the modified electrode. Therefore, the anodic peak current (Ipa) was increased when the pH was incremented from 2 to 7.4 and decreased up to 12 (Fig. 2). Nafion|CdPCNF|GC electrode showed maximum electrocatalytic behavior at pH 7.4. Taken all, pH 7.4 was selected for the electrochemical determination of DA.
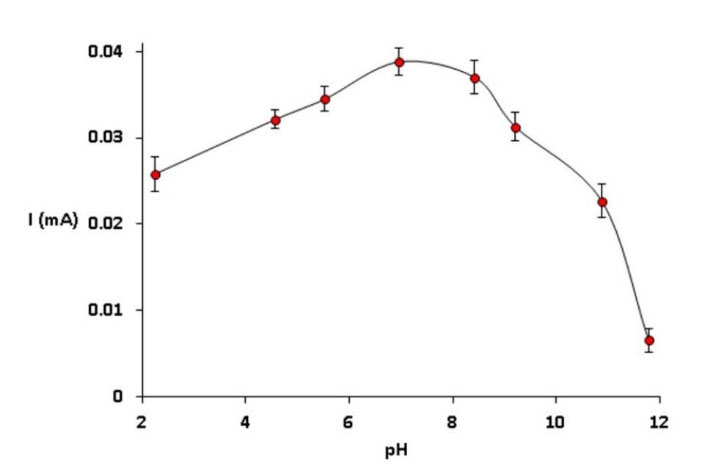
Fig. 2.
The response of Nafion|CdPCNF|GC electrode to the addition of 5.0 mM DA at various pH ranging from 2 to 12 in B. R. buffer solutions at the scan rate of 0.1 V/s.
.
The response of Nafion|CdPCNF|GC electrode to the addition of 5.0 mM DA at various pH ranging from 2 to 12 in B. R. buffer solutions at the scan rate of 0.1 V/s.
As represented in Fig. S4 (see Supporting Information), among the buffers, PBS gave us the best responses and peak shape for oxidation of DA, and hence PBS was employed for the further analysis. Moreover, with an increase in the rotation speed from 0 to 1000 rpm, the electrochemical response of 50 µM DA continuously improved while no further improvement was observed above 1000 rpm. Thus, this rotation speed was chosen as the optimal value.
EIS analysis
Fig. 3 shows the EIS spectra of the bare GC electrode, CdPCNF|GC electrode and Nafion|CdPCNF|GC electrode in the frequency range of 0.01–105 Hz represented by the Nyquist plot. The Randle’s circuit (Fig. 3, inset) was used to fit the obtained impedance data including the electron transfer resistance (Rct) and the Warburg-type impedance (Zw). The diameter of the semicircle in the Nyquist plot shows the Rct value. As represented in Fig. 3, analyses of the bare GC electrode exhibited a very small semicircle showing low Rct value and also straight line corresponding to the diffusion process at the low-frequency region.
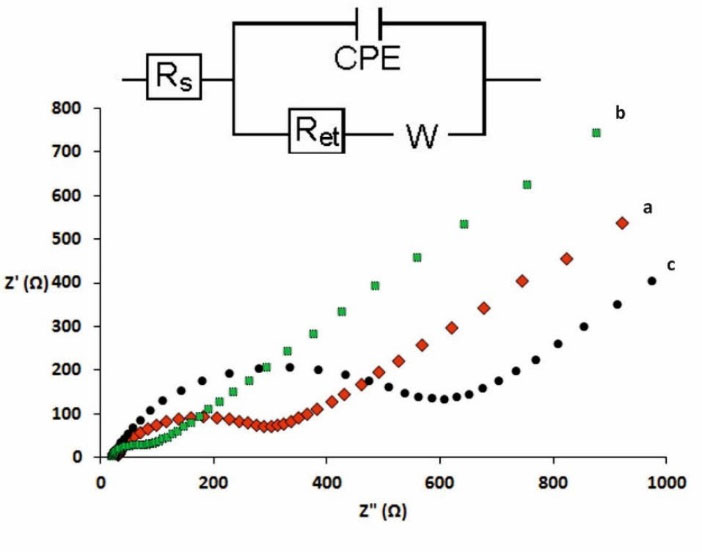
Fig. 3.
EIS spectra (Nyquist plot) of the bare GC electrode (a), CdPCNF|GC electrode (b) and Nafion|CdPCNF|GC electrode (c) obtained in the frequency range of 0.01-105 Hz using Fe (CN)63−/Fe (CN)64− (5.0 mM).
.
EIS spectra (Nyquist plot) of the bare GC electrode (a), CdPCNF|GC electrode (b) and Nafion|CdPCNF|GC electrode (c) obtained in the frequency range of 0.01-105 Hz using Fe (CN)63−/Fe (CN)64− (5.0 mM).
The calibration plot and LOD
Amperometry technique was used for DA quantification using Nafion|CdPCNF|GC electrode. The current response was measured through the rotating disk electrode at the fixed potential of 0.7 V. Fig. 4 shows the amperometric response of Nafion|CdPCNF|GC electrode, while exhibiting a rapid response (less than 2 seconds) to the changes in the DA concentration. Furthermore, the electrochemical response of the electrode for changes in DA concentrations was linear in the range of 1.7 (-92.1 µM) and LOD and LOQ were found to be 0.7 and 2.3 µM, respectively. In Table 1, a number of previously reported DA sensors with their important features, including types of modifiers, LOD, linear ranges, and method of analysis are represented. With having relatively more selectivity of detection and simplicity of electrode preparation than reported DA sensors, our proposed DA sensor is capable to detect DA with fairly better LOD but undesirably narrow linear range of detection.
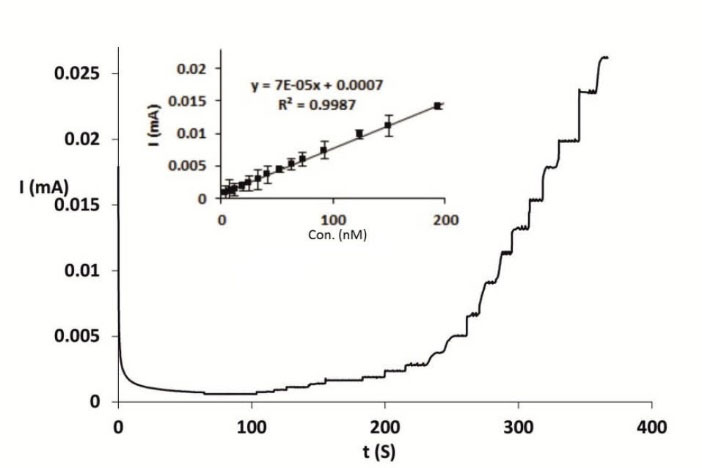
Fig. 4.
Current-time cure for the Nafion|CdPCNF|GC electrode obtained on the addition of different amount of DA ranging from 1.7 to 150 µM into human serum sample. Inset: Calibration curve.
.
Current-time cure for the Nafion|CdPCNF|GC electrode obtained on the addition of different amount of DA ranging from 1.7 to 150 µM into human serum sample. Inset: Calibration curve.
Table 1.
Comparison of our report with previous reports for DA detection
|
Electrode type
|
Modifier
|
LOD (µM)
|
Linear range (µM)
|
Method
|
Reference
|
| Au-µA |
PB-PEDOT |
4.3 |
5 – 10 |
CV |
27
|
| GC electrode |
HTAB-GO-MWCNT |
1.5 |
5 – 500 |
A |
28
|
| GC electrode |
ZrO2-GO
|
9 |
9 – 237 |
LSV |
29
|
| CCE |
Ag |
1.4 |
6.6 – 1200 |
A |
30
|
| GC electrode |
AgNPs-rGO |
5.4 |
10 – 800 |
LSV |
31
|
| ITO |
AuNPs |
3.45 |
10 – 900 |
SWV |
32
|
| GC electrode |
Nafion|CdPCNF |
0.7 |
1.7 – 92.1 |
A |
This work |
AgNPs: Silver nanoparticles, AuNPs: Gold nanoparticles, Au-µA: Gold disk microelectrode arrays, CCE: Ceramic–graphite composite, GCE: Glassy carbon electrode, GO: Graphene oxide, HTAB: Hexadecyl trimethyl ammonium bromide, ITO: Indium tin oxide, LSV: Linear sweep voltammetry, MWCNT: multiwalled carbon nanotubes, PB: Prussian blue, PEDOT: Poly(3,4-ethylenedioxythiophene), rGO: Reduced graphene oxide, SWV: Square wave voltammetry, ZrO2: Highly loaded zirconium oxide nanoparticles, CV: cyclic voltammetry, A: amperometry,
Interference study
CdPCNF|GC electrode showed a strong response to the presence of AA and UA, a very faint response to tyrosine (Try), tryptophan (Trp), norepinephrine (NE), epinephrine (EP), serotonin. however, it represented no response to CA, Glu, and NADH. Further, Nafion|CdPCNF|GC electrode displayed no response to the presence of any of these types of interferences, the result express the ability of Nafion to eliminate the possible interferences, completely (Fig. 5).
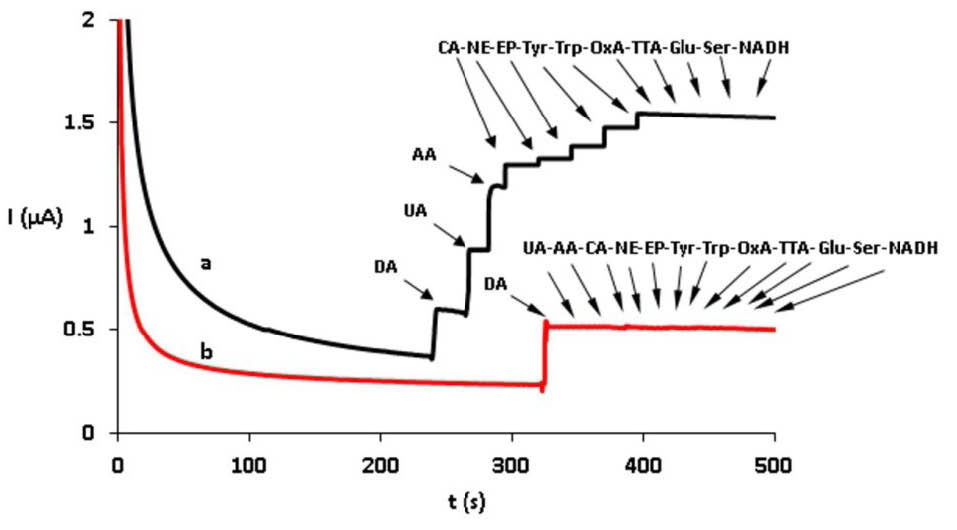
Fig. 5.
Amperometric response of CdPCNF|GC electrode (a) and Nafion|CdPCNF|GC electrode (b) for the DA detection in the presence of interfering substances
.
Amperometric response of CdPCNF|GC electrode (a) and Nafion|CdPCNF|GC electrode (b) for the DA detection in the presence of interfering substances
Determination of DA in pharmaceutical injections and spiked human serum samples
Determination of DA content at biological samples was carried out using Nafion|CdPCNF|GC electrode based on an amperometric technique through the standard addition method. Fig. 6 represents Nafion|CdPCNF|GC electrode responses to the existing DA in spiked serum samples with the LOD and LOQ values of 0.72 and 2.40 µM, respectively (relative standard deviation (RSD) = 5.7 %, N = 6). The LOD and LOQ were unexpectedly close to that of the DA analysis (i.e., LOD and LOQ of 0.68 and 2.27 µM, respectively) performed on buffer solutions. Moreover, a nonlinear response was observed at a concentration above 100 μM.
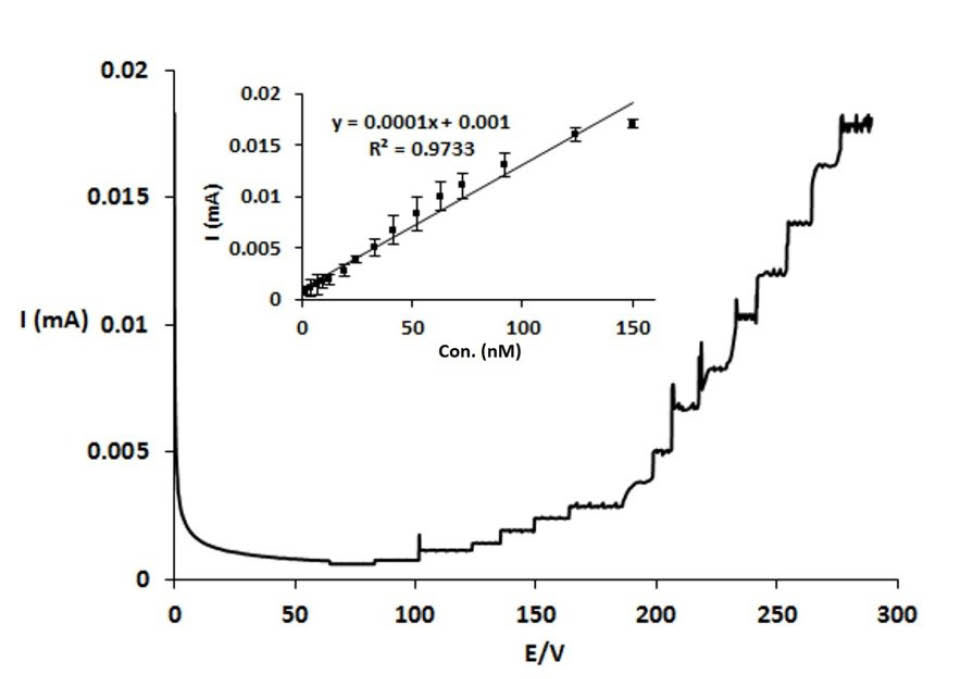
Fig. 6.
Current-time cure for the Nafion|CdPCNF|GC electrode Obtained on the addition of different amount of DA ranging from 1.7 to 150 µM into human serum sample. Inset: Calibration curve .
.
Current-time cure for the Nafion|CdPCNF|GC electrode Obtained on the addition of different amount of DA ranging from 1.7 to 150 µM into human serum sample. Inset: Calibration curve .
Recovery values of DA from pharmaceutical injections and serum samples is presented in Table 2. In our tests, no DA was detected in healthy serum samples. When samples were spiked with determined DA concentrations, acceptable recovery values were obtained in the range of 97.2-100.8% and also the presence of AA, UC and the other interferences did not interfere with the determination of DA. This finding implies a promising feature for the applicability of the modified electrode for the direct determination of DA in pharmaceutical and real samples.
Table 2.
Determination of DA in pharmaceutical injection and human serum samples
|
Sample
|
DA (Detected)
|
DA (Added)*
|
DA (Found)*
|
Recovery %
|
| Pharmaceutical injection |
4.0 |
30 |
34.2±0.76** |
100.5 |
|
|
5.1 |
30 |
35.2±0.82 |
100.2 |
|
|
3.8 |
30 |
33.1±0.67 |
97.9 |
|
|
7.2 |
30 |
37.1±0.93 |
99.7 |
|
|
5.4 |
30 |
35.6±0.137 |
100.5 |
|
|
5.3 |
30 |
35.1±0.148 |
99.4 |
| Serum samples |
0 |
5 |
5.1±0.13 |
102.0 |
|
|
0 |
10 |
9.9±0.29 |
99.0 |
|
|
0 |
15 |
15.2±0.48 |
101.3 |
|
|
0 |
20 |
19.8±0.38 |
99.0 |
*The unit of DA in the pharmaceutical injection and serum samples was µg/mL and µM, respectively.
** The results are expressed as 95% confidence interval for the mean of founded DA. Six replicate measurements were made on each sample.
Stability and reproducibility of Nafion|CdPCNF|GC electrode
The electrochemical DA sensor exhibited no obvious decrease in current responses in the first week and maintained about 94% of its initial values up to 6 weeks. Afterward, electrochemical responses reached to 80% of original values, which it reflects the acceptable stability of the prepared electrode. In addition, the RSD of DA (50 µM) analysis for 6 measurements was calculated to be 5.4% when it was determined for reproducibility assessments, indicating the satisfactory reproducibility of measurements carried out by using Nafion|CdPCNF|GC electrode.
Discussion
Dopamine plays a vital role in the mammalian CNS and its selective determination in the biological fluids is of tremendous importance in the field of biomedical sciences. AA, UA and NTs present in the biological fluids can interfere with detection of DA. Therefore, development of a modified electrode with capability for detecting DA in the presence of these interfering species appears to be significantly important. To develop DA sensor, a GC electrode was modified with CdPCNF and Nafion. A small amount of Nafion formed a thin layer on the CdPCNF|GC electrode that resulted in poor sensitivity for DA detection. While using a too much Nafion led to the formation a relatively thick film and this decreased the mass transfer rate of DA in the Nafion film. Besides, the excess deposition of Nafion profoundly decreases the mass transfer rate and limits the diffusion of the analyte through the polymer layer.
Using Nafion|CdPCNF|GC electrode the current redox peak increased 10 times in comparison with the bare GC electrode. An increase in the sensitivity of the DA detection reflected in the observation of two reduction peaks in the DA CV spectrum recorded through Nafion|CdPCNF|GC electrode. These two peaks were not observed in the CV spectrum of the bare GC electrode. It has been proposed that the reduction peaks at 0.162 V and 0.011 V arise from the reduction of dopamine quinone and leucodopaminechrome (a closed ring product formed from dopamine quinone) to DA respectively (Scheme 1).
33
It appears that on the surface of Nafion|CdPCNF|GC electrode, DA is oxidized to dopaminquinone, which was converted into leucodopaminechrome next (Scheme 2). However, it is clear that the electron transfer kinetic of the electrochemical reaction was remarkably improved after the modification of the GC electrode.
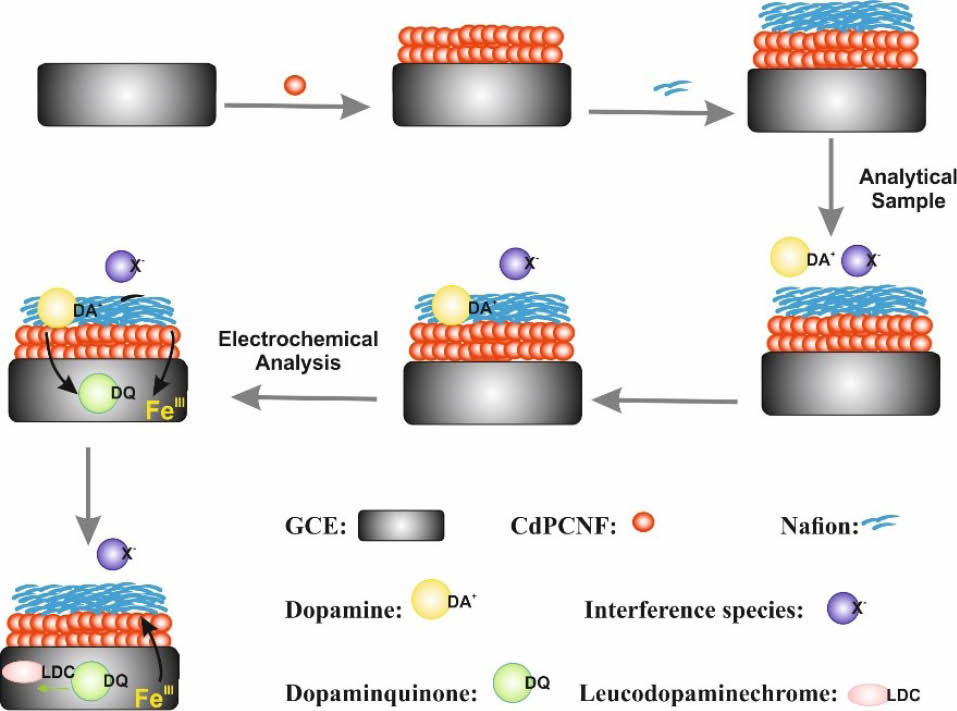
Scheme 1.
Illustration of Nafion|CdPCNF|GC electrode preparation steps and electrochemical oxidation process of DA.
.
Illustration of Nafion|CdPCNF|GC electrode preparation steps and electrochemical oxidation process of DA.
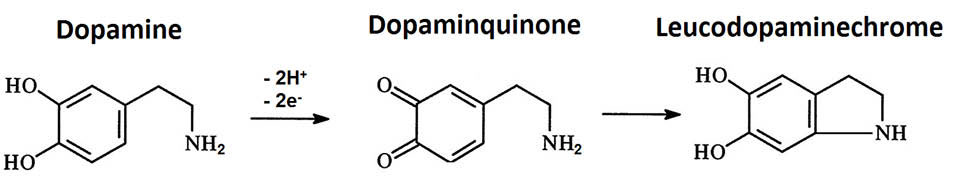
Scheme 2.
Chemicals produced in the electrochemical oxidation of DA at the surface of electrode.
.
Chemicals produced in the electrochemical oxidation of DA at the surface of electrode.
However, it was found that the selectivity and sensitivity of DA detection using CdPCNF|GC electrode were improved after modification with Nafion in comparing with bare and CdPCNF|GC electrodes and the Nafion modified electrode showed a sharp anodic peak for DA detection with the clear peak potential shift of 65 mV towards theanodic region. We assume that this effect is due to the cation exchanging characteristics of Nafion, by which negatively charged analytes are repelled from the surface of the electrode. When dopamine exists as a protonated form in the solution, it is attracted towards the electrode surface due to interactions between negatively charged sulfonate groups of Nafion and positively charged dopamine molecules. Sulfonate groups of Nafion are relatively fixed at the electrode surface. Therefore, the neutrality of the interface is achieved mainly through the cation’s concentration variation within the double layer and thus leading to the pre-concentration of DA at the electrode surface.
For the EIS analyses of the CdPCNF|GC electrode, the Rct value was smaller than that for the bare GC electrode, whereas modification of CdPCNF|GC electrode with Nafion led to a small increase in the Rct value. These results indicate that deposition of Nafion on the CdPCNF|GC electrode has no significant effects on the electron transfer rate. Thus, it can be used as a selective barrier for the anionic interferences and also as a selective and passive transporter for the DA molecules.
DA (pKa= 5.13) exits mostly in the cationic form in human plasma, while AA (pKa1= 4.17, PKa2= 11.57) and UA (pKa1= 5.70, pKa2= 9.80) as the most problematic interfering species exist mainly as anions.
16
Nafion is a negatively charged polymer and it can prevent the negatively-charged species that are reaching the electrode surface
34
. In contrast, DA alone can easily pass through the interface. This implies that Nafion|CdPCNF|GC electrode can be used for detection of DA existing in human plasma (pH= 7.4).
Conclusion
Key roles of DA as a functional NT in the CNS are of paramount importance to mammalians especially humans. In this regard, quantitative determination of DA in biological matrices has recently emerged as a major issue in the bioanalytical sciences. In the current study, we aimed to develop a simple and robust sensor for sensitive and selective determination of DA existing in the biological mediums. To develop this sensor, bare GC electrodes were modified with the electropolymerized film of CdPCNF and then coated with Nafion to minimize interferences. The developed sensor exhibited satisfactory responses as compared with the bare electrode and previously reported sensors with adequate sensitivity and selectivity to the presence of DA in the biological samples. However, it successfully determined DA in the presence of interfering species, indicating that major difficulties arising from overlapping electrochemical signals of these substances can be overcome with utilizing a very thin film of Nafion deposited on the electrode. The other advantages of the present sensor (e.g., the simplicity of preparation, rapidity of determination, and low cost of production) are. suggest that it can be served for the practical applications.
Acknowledgments
In addition, we express our deepest blessing as a memorial to Ms. Maryam Harasi, who sadly passed away recently, for her valuable collaboration on this study.
Funding sources
This study was funded by Research Center for Pharmaceutical Nanotechnology (RCPN) at Tabriz University of Medical Sciences in Iran.
Ethical statement
None.
Competing interests
There is none to be declared
Authors contribution
MJA: conceptualization and experiments. PK: data handling, data analysis, data presentation and study validation. JB: provision of equipment and supervision. DA: consultation, draft preparation, draft writing, and draft reviewing. SD: study consultation, provision of study materials and equipment. MRR: project administration, draft reviewing.
Supplementary Materials
Supplementary file 1 contains Figs. S1-S4.
(pdf)
Research Highlights
What is the current knowledge?
simple
-
√ Dopamine (DA) plays pivotal roles in CNS health and disorders.
-
√ Selective determination of DA in the biological fluids is of tremendous importance.
-
√ Various biossensors have been developed for the sensing
of DA.
What is new here?
simple
-
√ Nafion-coated Cadmium Pentacyanonitrosylferrate Modified Glassy Carbon (CdPCNF|GC) electrode was engineered.
-
√ Dopamine was detected in biological samples using cyclic voltammetry and amperometry.
-
√ No interfering effects through ascorbic acid (AA), uric acid (UA) and neurotransmitters on dopamine detection were observed.
References
- Michael DJ, Wightman RM. Electrochemical monitoring of biogenic amine neurotransmission in real time. J Pharm Biomed Anal 1999; 19:33-46. doi: 10.1016/S0731-7085(98)00145-9 [Crossref] [ Google Scholar]
- Wightman RM, May LJ, Michael AC. Detection of dopamine dynamics in the brain. Anal Chem 1988; 60:769A-79A. doi: 10.1021/ac00164a001 [Crossref] [ Google Scholar]
- Mo JW, Ogorevc B. Simultaneous measurement of dopamine and ascorbate at their physiological levels using voltammetric microprobe based on overoxidized poly(1,2-phenylenediamine)-coated carbon fiber. Anal Chem 2001; 73:1196-202. doi: 10.1021/ac0010882 [Crossref] [ Google Scholar]
- Tohgi H, Abe T, Takahashi S. The effects of L-threo-3,4-dihydroxyphenylserine on the total norepinephrine and dopamine concentrations in the cerebrospinal fluid and freezing gait in parkinsonian patients. J Neural Transm Park Dis Dement Sect 1993; 5:27-34. doi: 10.1007/BF02260912 [Crossref] [ Google Scholar]
- O'Neill RD. Microvoltammetric techniques and sensors for monitoring neurochemical dynamics in vivo A review. Analyst 1994; 119:767-79. doi: 10.1039/AN9941900767 [Crossref] [ Google Scholar]
- Maruyama W, Nakahara D, Naoi M. A new metabolic pathway of L-threo-3,4-dihydroxyphenylserine, a precursor amino acid of norepinephrine, in the brain Studies by in vivo microdialysis. J Neural Transm Park Dis Dement Sect 1994; 7:21-33. doi: 10.1007/BF02252660 [Crossref] [ Google Scholar]
- Tomita H, FUSE S, CHIBA S. Plasma concentration and acute clinical effects of docarpamine, orally active dopamine prodrug, in infants. Pediatr Int 1996; 38:440-3. doi: 10.1111/j.1442-200X.1996.tb03523.x [Crossref] [ Google Scholar]
- Van Loon GR, Sole MJ. Plasma dopamine: source, regulation, and significance. Metabolism 1980; 29:1119-23. doi: 10.1016/0026-0495(80)90020-7 [Crossref] [ Google Scholar]
- Kumar S, Mathiyarasu J, Phani K, Jain Y, Yegnaraman V. Determination of Uric Acid in the Presence of Ascorbic Acid Using Poly(3,4-ethylenedioxythiophene)-Modified Electrodes. Electroanal 2005; 17:2281-6. doi: 10.1002/elan.200503375 [Crossref] [ Google Scholar]
- Ciszewski A, Milczarek G. Polyeugenol-modified platinum electrode for selective detection of dopamine in the presence of ascorbic Acid. Anal Chem 1999; 71:1055-61. doi: 10.1021/ac9808223 [Crossref] [ Google Scholar]
- Roy PR, Okajima T, Ohsaka T. Simultaneous electroanalysis of dopamine and ascorbic acid using poly (N,N-dimethylaniline)-modified electrodes. Bioelectrochemistry 2003; 59:11-9. doi: 10.1016/S1567-5394(02)00156-1 [Crossref] [ Google Scholar]
- Pihel K, Walker QD, Wightman RM. Overoxidized polypyrrole-coated carbon fiber microelectrodes for dopamine measurements with fast-scan cyclic voltammetry. Anal Chem 1996; 68:2084-9. doi: 10.1021/ac960153y [Crossref] [ Google Scholar]
- Ulubay S, Dursun Z. Cu nanoparticles incorporated polypyrrole modified GCE for sensitive simultaneous determination of dopamine and uric acid. Talanta 2010; 80:1461-6. doi: 10.1016/j.talanta.2009.09.054 [Crossref] [ Google Scholar]
- Crespi F. In vivo voltammetry with micro-biosensors for analysis of neurotransmitter release and metabolism. J Neurosci Methods 1990; 34:53-65. doi: 10.1016/0165-0270(90)90042-E [Crossref] [ Google Scholar]
- Wang HS, Li TH, Jia WL, Xu HY. Highly selective and sensitive determination of dopamine using a Nafion/carbon nanotubes coated poly(3-methylthiophene) modified electrode. Biosens Bioelectron 2006; 22:664-9. doi: 10.1016/j.bios.2006.02.007 [Crossref] [ Google Scholar]
- Zen JM, Hsu CT. A selective voltammetric method for uric acid detection at Nafion(R)-coated carbon paste electrodes. Talanta 1998; 46:1363-9. doi: 10.1021/ac9703562 [Crossref] [ Google Scholar]
- Lin MS, Shih WC. Chromium hexacyanoferrate based glucose biosensor. Anal Chim Acta 1999; 381:183-9. doi: 10.1016/S0003-2670(98)00745-4 [Crossref] [ Google Scholar]
- Joseph J, Gomathi H, Prabhakara Rao G. Electrochemical characteristics of thin films of nickel hexacyanoferrate formed on carbon substrates. Electrochim Acta 1991; 36:1537-41. doi: 10.1016/0013-4686(91)85003-P [Crossref] [ Google Scholar]
- Joseph J, Gomathi H, Rao GP. Electrodes modified with cobalt hexacyanoferrate. J Electroanal Chem Interfacial Electrochem 1991; 304:263-9. doi: 10.1016/0022-0728(91)85509-N [Crossref] [ Google Scholar]
- Gao Z, Zhang Y, Tian M, Zhao Z. Electrochemical study of copper-heptacyanonitrosylferrate film modified electrodes: Preparation, properties and applications. J Electroanal Chem 1993; 358:161-76. doi: 10.1016/0022-0728(93)80436-L [Crossref] [ Google Scholar]
- Jiang M, Zhao Z. A novel stable electrochromic thin film: a Prussian Blue analogue based on palladium hexacyanoferrate. J Electroanal Chem Interfacial Electrochem 1990; 292:281-7. doi: 10.1016/0022-0728(90)87343-I [Crossref] [ Google Scholar]
- Dong S, Jin Z. Molybdenum hexacyanoferrate film modified electrodes. J Electroanal Chem Interfacial Electrochem 1988; 256:193-8. doi: 10.1016/0022-0728(88)85017-4 [Crossref] [ Google Scholar]
- Liu C, Dong S. Electrochemistry of vanadium hexacyanoferrate film. Electroanalysis 1997; 9:838-42. doi: 10.1002/elan.1140091107 [Crossref] [ Google Scholar]
- Liu S, Li H, Jiang M, Li P. Platinum hexacyanoferrate: a novel Prussian Blue analogue with stable electroactive properties. J Electroanal Chem 1997; 426:27-30. doi: 10.1016/S0022-0728(97)00021-1 [Crossref] [ Google Scholar]
- Razmi H, Heidari K. Preparation and electrochemistry of cadmium pentacyanonitrosylferrate film modified glassy carbon electrode. Electrochim Act 2005; 50:4048-56. doi: 10.1016/j.electacta.2004.12.038 [Crossref] [ Google Scholar]
-
Miller JN, Miller JC, editors. Statistics and Chemometrics for Analytical Chemistry. Fifth Edition ed. Essex Pearson Education Limited; 2005.
- Lupu S, del Campo FJ, Muñoz FX. Development of microelectrode arrays modified with inorganic–organic composite materials for dopamine electroanalysis. J Electroanal Chem 2010; 639:147-53. doi: 10.1016/j.jelechem.2009.12.003 [Crossref] [ Google Scholar]
- Wang Z, Liu J, Liang Q, Wang Y, Luo G. Carbon nanotube-modified electrodes for the simultaneous determination of dopamine and ascorbic acid. Analyst 2002; 127:653-8. doi: 10.1039/B201060G [Crossref] [ Google Scholar]
- Ezhil Vilian A, Rajkumar M, Chen S-M. In situ electrochemical synthesis of highly loaded zirconium nanoparticles decorated reduced graphene oxide for the selective determination of dopamine and paracetamol in presence of ascorbic acid. Colloids Surf B Biointerfaces 2014; 115:295-301. doi: 10.1016/j.colsurfb.2013.12.014 [Crossref] [ Google Scholar]
- Ravi Shankaran D, Uehara N, Kato T. Sol–gel derived metal dispersed ceramic–graphite composite electrode for amperometric determination of dopamine. Anal Chim Acta 2003; 478:321-7. doi: 10.1016/S0003-2670(02)01511-8 [Crossref] [ Google Scholar]
- Kaur B, Pandiyan T, Satpati B, Srivastava R. Simultaneous and sensitive determination of ascorbic acid, dopamine, uric acid, and tryptophan with silver nanoparticles-decorated reduced graphene oxide modified electrode. Colloids Surf B Biointerfaces 2013; 111:97-106. doi: 10.1016/j.colsurfb.2013.05.023 [Crossref] [ Google Scholar]
- Goyal RN, Gupta VK, Oyama M, Bachheti N. Gold nanoparticles modified indium tin oxide electrode for the simultaneous determination of dopamine and serotonin: application in pharmaceutical formulations and biological fluids. Talanta 2007; 72:976-83. doi: 10.1016/j.talanta.2006.12.029 [Crossref] [ Google Scholar]
- Zhao H, Zhang Y, Yuan Z. Study on the electrochemical behavior of dopamine with poly(sulfosalicylic acid) modified glassy carbon electrode. Anal Chim Acta 2001; 441:117-22. doi: 10.1016/S0003-2670(01)01086-8 [Crossref] [ Google Scholar]
- Dam ME, Schrøder KH. Mercury film electrodes coated with negatively charged polymer films in speciation studies of trace amounts of lead. Electroanalysis 1996; 8:1040-50. doi: 10.1002/elan.1140081112 [Crossref] [ Google Scholar]