BioImpacts. 6(3):155-167.
doi: 10.15171/bi.2016.22
Review
Russian olive (Elaeagnus angustifolia) as a herbal healer
Zeinab Amiri Tehranizadeh 1, Ali Baratian 2, Hossein Hosseinzadeh 3, *
Author information:
1Department of Medicinal Chemistry, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran
2Department of Pharmaceutics, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran
3Department of Pharmacodynamics and Toxicology, Pharmaceutical Research Center, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran
Abstract
Introduction:
Elaeagnus spp. is one in the family of riparian trees growing near the rivers or water corridors. In this family, Elaeagnus angustifolia (Russian olive) is famous because of its medical applications.
Methods:
A comprehensive review was performed to extract the related data from published literature.
Results:
Traditionally, it has been used as an analgesic, antipyretic and diuretic herbal medicine. A large number of compounds have been derived from Russian olive and made this plant a source of flavonoids, alkaloids, minerals and vitamins. Although the purpose of most studies is to use this plant for preparation of herbal medicines and as an ingredient for drug formulation, there is no available drug dosage form commercially.
Conclusion:
This review aimed to provide the most important documentary information on the active components of Elaeagnus spp. and their relation to the pharmacological properties and compare them with reported medicinal effects.
Keywords:
Elaeagnus angustifolia
, Russian olive, Inflammation, Traditional remedy, Flavonoid
Copyright and License Information
© 2016 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Elaeagnus spp. (Plantae>Rosales>Elaeagnaceae>Elaeagnus) is in the family of riparian trees growing near rivers or water corridors.
1
It is mostly found in central Asia
2
including Iran,
3
Uzbekistan,
2
Syria
4
and north-west of China
5
and exotically in river banks of central Spain,
6
Canada
7
and west of United-states.
1
The family Elaeagnaceae, consists of three genera and 50 species. Elaeagnus angustifolia (Russian olive) (Fig. 1) and E. pungens are partially adapted to the center of Asia. Shepherdia canadensis belongs to the Unites-states and Canada. Hippophae rhamnoides is endemic in Europe.
8
In this family; E. angustifolia is famous for its medical benefits. Traditionally, it has been used as an analgesic, antipyretic and diuretic herbal medicine. A large number of compounds have been derived from Russian olive and made this plant a source of flavonoids, alkaloids, minerals and vitamins. Several experimental studies have been done and some advances in drug formulation and herbal medicine have been achieved. This review tries to gather the most important documentary information on its active components and their relation to the Russian olive pharmacological properties and compare them with reported medicinal effects. Another review on this plant has been published recently, but we tried to check more articles with conflicting reports for writing better and more comprehensive systematic review.
9
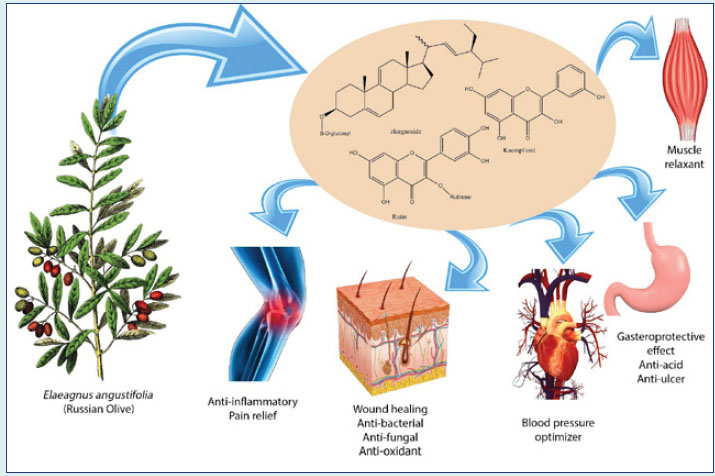
Fig. 1
.
A brief review of E. angustifolia.
.
A brief review of E. angustifolia.
Active ingredients
The aqueous and non-aqueous extracts of E. angustifolia are full of medically significant active ingredients. The extracts contain a variety of compounds such as flavonoids and alkaloids, simple sugars and complicated sterols (Fig. 2).
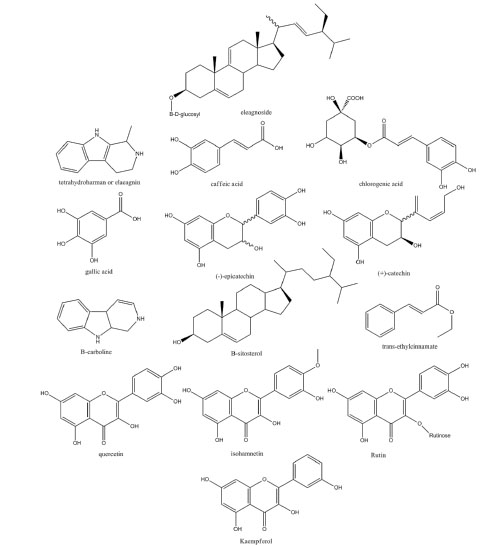
Fig. 2
.
Fig. 2. Major constituents of E.angustifolia.
.
Fig. 2. Major constituents of E.angustifolia.
Flavonoids
Flavonoids (which are commonly referred to vitamin P) are a large set of polyphenolic compounds with a benzo-γ-pyrone structure and are found exclusively in plants. They can be categorized in different classes like: flavones, flavonols, flavanones, flavanonols, isoflavones and flavan-3-ols.
10
In the pulp of Russian olive, 4 glycosylated flavonoids
10
identified: Quercetin 3,4’-O-β-D-diglucoside, Isorhamnetin-3-O-β-D-galactopyranoside, Quercetin 3-O-β-D-Galactopyranoside- 4’-O-β-D-glucopyranoside and Isorhamnetin 3-O-β-D-Galactopyranoside-4’-O-β-D-glucopyranoside.
11
Rutin glycosylated kampferols such as kaempferol 7-p-coumaroyl-3-D-glucoside, kaempferol-3-D-glucopyranoside (astragalin) and kaempferol 3-O-D-glucosido-41-p-coumaroyl-7-O-D- acyl galactoside are other available flavonols of the fruits.
12
Rutin eleagnoside and kampferol are also available in the flavonoid fraction of the fruit. In acetone extract from the bark of Russian olive, (flavan-3-ol
10
) obtained two catechins as well: (+)-catechin and (-)-epicatechin.
13
Glycosylated flavonoids have difficulty in absorption; after hydrolysis by lactase phlorizin hydrolase (LPH), they should transfer from Na+-dependent glucose cotransporter in small intestine. Mostly, the glycosylated flavonoids pass the colon and exit. For these reasons, E. angustifolia flavonoids may also exit the colon and have no benefits. So, it should be taken into account whether the amount of absorption is enough for medical benefits or not.
10
However, they have different roles in plants; for example, they play a major role in oxidative stress response and are also used as growth regulators. But, talking about the exact amount of the flavonoids in each plant is something difficult. Recent studies showed that geographical location may influence the quantity and quality of flavonoids.
10
Fatty acids
Fatty acids of this family first recognized by Obodovski and Devyatnin. Total lipid content of E. angustifolia varies between 0.8% in pericarp to 26% in seeds per mass.
12
Fruit methanol extract from Russian olive contains different kinds of fatty acids. In an experiment by Kusova et al. oleic acid and linoleic acid made up 92.8% of the petroleum extract of the fruit accompanied by a low concentration of fat-soluble vitamins.
14
In another study which published in the journal of the chemistry of natural compounds on the Russian olive seeds, six epoxy acids and five hydroxy acids were collected: Cl8:0, C18:1 and C18:2 diepoxy acids and C18-hydroxy acids with double bonds at the 9-C atoms.
15
Palmitic acid (16:1) is also present in trace amount (3%-10%)
16
in both seeds and fruits,
16,18
but, it is not a chemotaxonomic compound for this tree.
16
In general, flesh of the fruit is low in lipids but seeds and pericarps of Russian olive are statured with these compounds. Triacylglycerol with linoleic acid is the main lipid of the seeds. Saturated free fatty acids (octadecanoic acid 1.63% and hexadecanoic acid 3.91%)
17
are eminent; however, the highest concentration among free fatty acids belongs to essential linoleic acid (49.12%).
17
These amounts are somehow identical to the pericarp, except for the amount of triacylglycerol which is lower (18%-30%) in the pericarp.
16
The amount of fatty acids and their derivatives are different from tree to tree based on cultivation soil, tree age and planting area.
16
However, what is important is whether the amount of fatty acids is commercially efficient or not; far seeds with 26% fatty acid per total mass, it seems applicable but for the rest (pericarp, leaves and flowers), it needs cost-benefit experiments.
Sterols
The most significant sterol of Russian olive is β-sitosterol.
16,18
It is found mainly in seeds, leaves and tree branches. Elaeagnoside with a sterol-like structure is derived from the name of this family and present in the fruits of Russian olive.
12,19
Carbohydrates
Usually, free sugars are the first descriptors of the fruits. In Russian olive, fructose and glucose are the predominant monosaccharaides. The percentage of weight of fructose and glucose to the total dried weight of the fruit is 32%-34% and 23%-24% respectively.
20
In the ripened fruits there is no significant amount of sucrose; it is said that this might be due to the effects of invertase during ripening process
20
; In another study, it became clear that raw fruits have variable amount of sucrose but during the process of ripening, sucrose cleaves to fructose and glucose.
21
Sugars can also be seen in combination with flavonoids; derivatives of isoharmentin, like isoharmentin 3-β-D galactopyranoside that are present in the fruits.
21
Among reducing sugars, xylose, mannose and rhamnose are also present in fruit extracts.
13
To sum up, in every Russian olive trees, fructose and glucose seemed to be the major composition of the sugars but the amount of sugars does not depend on the origin or age of the trees.
21
Alkaloids
Alkaloids were detected by TLC of different fractions of Russian olive. They are mainly condensed in the root, bark and aerial part of the plant. The most famous alkaloid of Russian olive is elaeagnin or calligonin which is structurally a tetrahydroharman. In the bark of this plant, N-methyl harmol, N-methyl tetrahydroharmol, Harman, dihydroharman, 2-methyl-1,2,3,4- tetrahydro-β-carboline, harmin and harmol are available as well.
There are some unlabeled medical usages of elaeagnin and β-carbolinesuch as the reduction of blood pressure and antimalarial effect, but none of them were investigated for the extracts of this plant.
12,22-24
Other ingredients
Vitamins and minerals play a major role in the medical properties of E. angustifolia. Vitamin A and K are oleophilic vitamins available in the methanol extract of the flowers of the plant. B vitamins are also present in the flowers.
25
The highest concentration of a mineral element in this plant belongs to potassium with a concentration of 8504 mg/kg. Second and third highest levels belong to sodium and phosphorus with 1731 and 635 mg/kg, respectively.
26
Calcium is also found with high levels in this plant and traditionally the flowers of Russian olive were considered a good source of Ca2+ among people of Iran.
25
Steroidal glycosides (saponins) can be detected in the fruits by Fontan-Candel method (foam-forming reaction). The saponins of Russian olive do not cause hemolysis of erythrocytes in isotonic saline. Aglycone part of saponins can be either neutral or acidic. Total amount of saponins in the fruits of EA estimated to be 1.96 % of the net weight of the fruit.
13
Free acids are also present in the fruits of this plant: benzoic acid, 4-hydroxy benzoic acid and vanilic acid are the benzoic derivatives. The derivatives of cinamic acid, like caffeic acid and ferrolic acid are available as well. In this list,4-hydroxy benzoic acid and caffeic acid are found in highest concentration, respectively.
20
Amino acids such as aspargic acid, threonine, serine, glutamine, proline, glycine, alanine, valine and some others were observed as well (Table 1).
13
Table 1
.
Percentages of the active ingredients of dried ripe fruits of
Elaeagnus angustifolia
13
|
Elaeagnus spp. is in the family riparian trees growing near the rivers or water corridors
|
Percentage in dried ripe fruits of
E. angustifolia
|
| Reducing sugars |
50.67-55.75% |
| Total sugar |
60 ± 5% |
| Pectic polysaccharides |
3.58 ± 0.3% |
| Total flavonoids and polycarboxilic acids |
1.35 ± 0.15% |
| Total saponins |
1.96 ± 0.52% |
| Ascorbic acid |
5.6 mg% |
| B-carotene |
17.5 mg% |
| Tannin |
5.03 ± 0.05% |
Traditional remedies
Traditionally, Russian olive was used as an anti-ulcer remedy for wound healing or sometimes gastric disorders.
27
E. angustifolia fruits were also famous in Turkish folklore as tonic, antipyretic, kidney disorder healing (anti-inflammatory and/or kidney stone treatment) and anti-diarrhea (astringent).
16,28
In ancient Iran, the fruit decoction of Russian olive was taught to be used a good remedy for fever, jaundice, asthma, tetanus and rheumatoid arthritis by Iranian apothecaries. In general, it was used as a substitute of any anti-inflammatory and analgesic agent, in the first line.
29
The leaves and fruits of the plant were also famous as diuretics and antipyretic agents. In Turkey, it was common to eat the fruits an hour before the meal as an appetizer.
21
In Table 2 the traditional uses of this plant summarized. In the next sections, we will discuss some parts in detail.
Table 2
.
Traditional remedies of Elaeagnus angustifolia
|
Extract/Country
|
Application
|
Rout of administration
|
| Iran |
|
|
| Infusion of the dried flowers |
Anti-pyretic |
oral
30
|
| Infusion of the dried leaves |
Astringent |
oral
30
|
| Turkey |
|
|
| Fresh branch in hot water |
To remove wart |
External
31
|
| Decoction of barks |
Gall bladder problems and kidney stones |
Drink one teacup twice a day for 20 days
32
|
| Extract of leaves with the ash of Salix sp. bark |
To treat abscess |
external
33
|
| Fruits |
Kidney stones |
oral
34
|
| Tea or decoction of root barks |
Dysuria |
Oral
35
|
|
Extract of leaves with Juglansregia and Menthalongifolia
|
Sun block |
External
36
|
| Azerbaijan |
|
|
| Infusion of the aerobic parts |
Treatment of alimentary canal
|
Oral (NAPALERT) |
Experimental and clinical studies
Anti-inflammatory and analgesic effects
Animal studies have shown the effectiveness of the aqueous and ethanol extract of Russian olive in pain and inflammation treatment. Flavonoids play the main role in this matter,
37
although anthocyanins, saponins
38
and terpenoids may take part as well.
39
There is a controversy on the success of the extract of this plant on acute or chronic pain. Formalin test is one of the reliable tests in both acute and chronic pain. In the first phase of formalin test, 5 minutes after injection, the direct influence of the compound on the pain fibers is tested and mostly called as an acute phase. 20 to 30 minutes after injection, the chronic phase or the inflammatory pain starts. For some medicines such as morphine and codeine with a central effect both phases can be suppressed but for some others like non steroid anti-inflammatory drugs (NSAIDs) and steroids only the chronic phase can be effected.
40,41
While Farahbakhsh et al.
39
believe in ineffectiveness of the aqueous extract of the Russian olive in the acute phase; Hosseinzadeh et al.
18
and Ahmadiani et al.
29
proved (p <0.001) the comparable effect of the fruit extract with both NSAIDs and narcotics. Altogether, it can be inferred that the fruit extract of Russian olive with low doses (20-40 mg/kg) only suppresses the chronic pain
29
and high doses (130-450 mg/kg) can be successfully used against both chronic and acute pain.
37,38
On the other hand, the mechanism of its analgesic effects was also tested in hot-plate and writhing test. In comparison to indomethacin with a proven peripheral mechanism of pain relief in writhing test, the flavonoid extract of the Russian olive (134-402 mg/kg) showed both peripheral and central effects in hot-plate and writhing tests.
38
Quercetin is one of the most famous flavonoids with an analgesic effect available in Russian olive. It can block the activity of cyclooxygenase (COX) and lipooxygenase. In vitro, it has an inhibitory effect on the secretion of the immunoglobulins.
10
Bioflavonoids with an ability to block the release of the bradykinin and arachidonic acid play an important role in the mechanism of controlling the chronic pain. Sometimes, muscle relaxant ability of the plant is known as an axillary mechanism for pain relief. Available alkaloids, such as harman and harmaline, inhibit mono amino oxidase (MAO) and increase serotonin and noradrenaline (like tricyclic antidepressants) at the synaptic sites. This mechanism can also be a part of analgesic effect of the plant.
37
Based on pharmacological information, flavonoids can block N-Methyl-D-aspartate (NMDA) receptor and reduce the amount of intracellular calcium and lead to reduced enzymatic activity of nitric oxide and phospholipase A2-Calcium-dependant protein.
42
Flavonoids fill the ATP binding sites of kinases (serine/threonine or tyrosine kinese). These results obtained in less activation of phospholipase A2 and less production of COX. Some other flavonoids can block cAMP phosphodiestrase in the platelets. The reduction of cAMP changes cytoskeletal rearrangements and deactivates special protein kinases. The substrates of the kinases like vasodilator-stimulated phosphoproteins remain inactive and finally platelet secretion, adhesion and aggregation stops. This is one of the important mechanisms in controlling inflammation.
43
Linoleic acid can also suppress the gene expression inflammatory cytokines. The inflammation is mediated by inhibition of NF-κB (a protein complex that controls cytokine production) activation through PPAR-gamma pathway.
44
Recently in a clinical study, Russian olive extract (medulla powder and whole fruit, 15 g/kg, 8 weeks) showed a significant improvement in pain and inflammation management of knee osteoarthritis in women. It can reduce serum levels of inflammatory cytokines (TNF-α, MMP-1, etc) and also enhance patients’ presentation of the disease; but the study was only carried out in obese women (according to reported body mass index) having chronic arthritis pain. It may need another comprehensive study containing at least pregnant women or athletes bearing acute pain and a range of dosages corresponding to previous laboratory studies.
45
Two separated randomized, double bind clinical studies, effectiveness of aqueous extract of Russian olive (300-600 mg/kg) which contained at least 0.21% kaempferol, was compared with ibuprofen (800 mg/kg) in women with knee osteoarthritis. E. angustifolia extract was very safe and tolerable in 2 doses and was so beneficial in reducing symptoms of osteoarthritis.
46,47
In Table 3 most of the available studies are summarized. What we can infer is that analgesic effects of E. angustifolia are the most important medical applications of this plant. It is concentrated mainly in fruits and specially seeds of the trees. The mechanism does not relate to the opioid receptors, but the polyphenolic extracts can attenuates pains as good as the opioids. Gastroprotective effect
Table 3
.
Anti-inflammatory and analgesic effects of E. angustifolia
|
Extract and doses
|
Compared medicine and doses
|
Experiment
|
Results and considerations
|
References
|
|
Seed (aqueous, ethanol and
polyphenolic fraction)
100-1000 mg/kg
|
Indomethacin (1 mg/kg)
Morphine (5 mg/kg)
|
Hotplate test
Writhing test
|
Aqueous and ethanolic extracts were effective
in concentration above 500 mg/kg
Polyphenolic extract was effective in 300 mg/
kg
The role of polyphenolic groups in pain relief
were proved
|
38
|
|
Total fruit (aqueous fraction)
1000 mg/kg
|
Sodium salicylate (100-300 mg/
kg)
|
Formalin test
Tail-flick test
Antiinflammatory
test
|
Acute phase of pain: peritoneal injection was
effective
Chronic phase of pain: like NSAIDs have pain
relief effects even orally
In both phases, naloxone could not reverse
the effects
Central mechanism was proved
|
29
|
|
Total fruit (aqueous fraction)
50 mg/kg
|
Morphine (10 mg/kg)
Indomethacin (10 mg/kg)
Dexamethasone (10 mg/kg)
|
Formalin test
|
No suppression in acute phase (because of
low doses of extract)
Pain relief in chronic phase with an inhibition
of COX enzymes
naloxone couldn’t reverse the effects
|
39
|
|
Fruit (aqueous fraction)
20 mg/kg
|
Diclofenac (10 mg/kg)
|
Xylene-induced
ear edema
|
Seeds have the most anti-inflammatory effect
in chronic phase
|
18
|
|
Fruit (aqueous fraction)
250-500-700 mg/kg
|
Indomethacin (5 mg/kg)
|
Writhing test |
Comparable visceral pain relief with NSAIDs |
37
|
|
Fruit (aqueous fraction)
1-1.5 g/kg
|
Imipramine (40 mg/kg)
|
Hot-plate test |
Comparable pain relief in chronic phase with
imipramine
|
37
|
From ancient remedies, Russian olive is famous for its antiulcer effect. In vivo studies in rats proved that the alcoholic extracts of this plant in low doses (400-800 mg/kg) and high doses (3150 mg/kg) can improve lesions of NSAIDs and ethanol, respectively. These properties are as effective as misoprostol and less operative than omeprazole. In histopathological examinations, no protective effect on stomach mucosa has been observed. In another study, aqueous extract of Russian olive imposed positive effects on pregnant mice. With doses as low as 640 mg/kg, fetal stomach cells became hypertrophic by gene regulation. In other words, E. angustifolia extract could reinforce fetus digestive system. There are also some evidences that Russian olive fruit extract can reduce gastric acid secretion simulated by cholinergic system in a dose and time dependent manner. Russian olive was shown to relaxe the muscle contraction of lumen and with its antioxidant effects; it works as a barrier on the surface of stomach against gastric mucosa.
28,48-50
We can infer from this section that Russian olive ethanol extract is comparable with misoprostol on gastric ulcer lesions; it can reduce mucosal damage formed by aspirin, NSAIDs and alcohol like H2 receptor blockers, but not as powerful as proton-pump inhibitors. With less side effects (especially no oxytocic property), Russian olive dosage forms or extracts can be administered before NSAIDs-intake or for pregnant women in controlled quantities.
5
Wound treatment
In folk remedies, the extracts of Russian olive are known as a wound healing accelerator. If treatment of inflammation and proliferation is considered as the main steps of wound treatment, Russian olive fruit extracts can help in wound closure. It can increase the hydroxyproline content, improve the histological scores (epidermis regeneration, collagen deposition and proliferation), control the pain and inflammation and inhibit the cyclooxygenases I and II. Moreover, E. Angustifolia has a potent antibacterial property comparable with mupirocin 2% with added antioxidant activity. Phenolic compounds, mostly flavonoids are responsible for these effects. Vitamin A also plays important roles; the acceleration of cell replication, more collagen precipitation and induction of hyaluronate synthesis are the main effects of this vitamin in wound healing.
39,52-55
Antibacterial and antifungal effect
In general, this plant shows lower overall antimicrobial effects in comparison to other similar species.
56
However, in some cases, extraordinary effects have been reported. Reported antifungal and antibacterial effects vary based on the dose used and the extraction method recruited. In an in vitro study, its leaf extract was used to treat mastitis pathogens.
57
These pathogens cause severe illnesses in domestic animals and by triggering steep decline in milk production result in vast economical loss.
58
Such illnesses are also classified as threatening to public human health.
59
They are caused by a group of bacteria such as Staphylococcus aureus and Coagulase Negative Staphylococci (CNS) which are susceptible to this plant’s extracts.
60
In another study, four hospital germs were collected and tested in a disc diffusion method, and two soft extracts of leaves and flowers of Russian olive proved to have an intense antibacterial activity against wound germs (S. aureus), pharynx exudates (Streptococcus pyogenes), saliva (Klebsiella pneumoniae) and urine (Escherichia coli).
61
A brief review of antibacterial and antifungal activity of Russian olive is gathered in Table 4. In accordance with the importance of this plant in the traditional remedies, we considered some of its applications were related to its fungicidal and bactericidal properties. In ancient apothecary, it was said that E. angustifolia was administered to control the gull bladder problems and diarrheas. Gull bladder problems may be E.coli related or the table-listed gram negative bacteria could be responsible for the mentioned diarrhea and so on.
Table 4
.
Different extracts of Elaeagnus angustifolia and their effects on pathogens
|
|
Aqueous
|
Ethanolic
|
Chloroform
|
Crude
|
n-Hexane
|
Methanolic
|
|
Mastitis pathogen
60
|
|
|
|
|
|
√ |
|
Staphylococcus aureus
62
|
|
√ |
|
|
|
|
|
Escherichia coli
62
|
√ |
|
|
|
|
|
|
Salmonella thyphimurium
57
|
√ |
√ |
|
|
√ (The most effective extract) |
√ |
|
Bascillusthurigiensis
56
|
|
√ |
|
|
|
|
|
Pseudomonas aeroginosa
62
|
|
√ |
|
|
|
|
|
Pseudomonas extorquous
56
|
|
√ |
|
|
|
|
|
Yersinia enterocolitica
63
|
|
|
|
|
|
√ |
|
Candida albicans
57
|
√ |
√ |
|
|
√ (The most effective extract) |
|
|
Aspergillus fumagatus
62
|
|
|
|
√ |
√ (The most effective extract) |
|
|
Aspergillus favis
62
|
√ |
√ |
|
|
√ (The most effective extract) |
|
|
Aspergillus niger
62
|
|
√ |
|
|
√ (The most effective extract) |
|
|
Alternariasolani
64
|
|
|
|
|
|
√ |
|
Bacillus subtilis
65
|
√ |
√ |
|
|
√ |
|
|
Shigelladysentriae
65
|
√ |
√ |
|
|
√ |
|
|
Vibrio cholerae
65
|
√ |
√ |
|
|
√ |
|
The exact mechanism of these properties is not clear, but most scientists believe in phenolic compounds,
52,62
alkaloids
62
and essential oils
60
as predominant reasons of being germicide in medical plants. Amongst its flavonoids, flavones like kaempferol, quercetin and isohamentin are deemed to provide such pharmacologic effects.
52
Flavonoids especially the ones with more lipophilic structures can penetrate into the cell wall of the bacteria easily and spoil its condensed structure. The intracellular components permeate and the bacteria are being killed. Another proposed mechanism is related to the complex of the flavonoids with active proteins of the bacteria with specific (covalent) or nonspecific (hydrogen or van der Waals) bonds and make critical changes in bacteria’s function that may finally lead to the death of the microorganism.
43
Antioxidant effect
Most studies on E. angustifolia is related to its antioxidant capacity of phenolic compounds and antocyanosides. The highest content of total phenolic compounds can be extracted on the first 10 days of October, therefor the best time for harvesting Russian olive is exactly at this time in cases highest antioxidant properties are desired.
66,67
Apart from the seasonal changes, genotype is also important in the amount of antioxidant capacity. For E. angustifolia we know at least 7 genotypes (IEa1 to 7) in Iran.
68
Polyphenols can suppress the oxidative stress produced by reactive oxygen species (ROS) with their hydroxyl groups. They can also chelate metals.
69
Antioxidant activity of raw materials can be mainly evaluated in two methods: antioxidant capacity against of free radical species comprising of hydrogen atoms transfer reactions model (HAT), single electron transfer reactions model (SET) and a hydrogen-electron transfer models which is a mixture of HAT and SET methods; and antioxidant capacity against biological markers and characterized substrates.
70
Oxygen radical absorbance capacity assay (ORAC), a HAT mechanism, is a core method in antioxidant studies. The senior method referes to the usage of β-phycoerythrin, a fluorescent hydrophilic probe, which was photo sensitive and caused false positive results. Fluorescin, eosin and 6-carboxyfluorescin with higher stabilities and more specific interactions with antioxidants are the probes of ORAC method.
71
Phenolic compounds and even vitamins of Russian olive can hydrogenate peroxyl radicals of AAPH and increases the fluorescent emission strength. Among the extracts, water/ethanol (1:1) extracts of the leaves has the most potent antioxidant property. All in all, this method has exclusively been characterized to evaluate the antioxidant capacity of water-soluble phytochemicals and may not cover all aspects of this plant.
72,73
Furthermore, 2, 2-Diphenyl-1-picryhydrazyl (DPPH) radical assay based on Benvenutti et al. method is an available and cheap antioxidant analysis experiment. The stable and commercially accessible DPPH is a free radical that can accept hydrogens and electrons from polyphenols and also is one of the comparable methods of antioxidant measurement (Fig. 3).
70,74
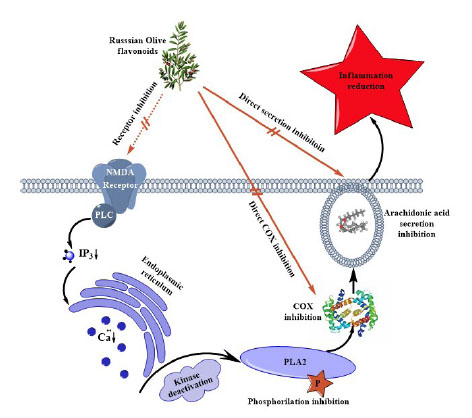
Fig. 3
.
Anti-inflammatory mechanism of E. angustifolia: Flavonoids
of E. angustifolia can directly block active sites of COX and the
secretion of the arachidonic acid. Thus, arachidonic acid cannot
change into prostaglandins and leukotrienes, the most important
mediators of vasodilation, platelet activation and inflammation
(filled arrows). Another proposed mechanism describes the
importance of flavonoids in blocking NMDA receptors. NMDA
receptor inactivation leads to a significant calcium secretion
reduction from PLC pathway and finally no kinase, PLA2 and
COX activation, respectively (dashed arrow). (N-methyl-Daspartate:
NMDA, phospholipase C: PLC, inositol trisphosphate:
IP3, phospholipase A2: PLA2, cyclooxygenase: COX)
.
Anti-inflammatory mechanism of E. angustifolia: Flavonoids
of E. angustifolia can directly block active sites of COX and the
secretion of the arachidonic acid. Thus, arachidonic acid cannot
change into prostaglandins and leukotrienes, the most important
mediators of vasodilation, platelet activation and inflammation
(filled arrows). Another proposed mechanism describes the
importance of flavonoids in blocking NMDA receptors. NMDA
receptor inactivation leads to a significant calcium secretion
reduction from PLC pathway and finally no kinase, PLA2 and
COX activation, respectively (dashed arrow). (N-methyl-Daspartate:
NMDA, phospholipase C: PLC, inositol trisphosphate:
IP3, phospholipase A2: PLA2, cyclooxygenase: COX)
With an increase in the concentration and polarity of ethyl acetate extracts of Russian olive, its polyphenolic contents can clear DPPH radicals out up to 94%. It has been estimated and shown that its antioxidant ability is comparable with α-tocopherol and BHT in terms of IC50. Polysaccharide contents of Russian olive comprising of monomers such as rhamnose, xylose, mannose, glucose and galactose can reduce DPPH free radicals up to 88.1%. From the concentration point of view, polyphenols radical scavenging activityg is more than ten times stronger than polysaccharides’.
75-78
As a suggestion, it is better to define antioxidant ability of the raw materials with a standard chemical like Trolox instead of IC50 or percentage. Trolox which can be the positive control in research projects and the minimum inhibitory concentration, can become a measure of comparison in chains of studies.
63
However, what we are sure about is that the antioxidant capacity of this plant has a linear correlation with the amount of phenolic compounds.
79
Phenolic compounds can be extracted from the methanol or ethanol extracts. They can donate hydrogen or electron and remove the free radicals. They reduce the amount of ROS by controlling the free-radical-producer enzymes like NADH oxidase, glutathione s-transferase and microsomal monooxygenase or chelating trace elements. Some of active ingredients of E. angustifolia such as epicatechin, catechin, rutin, quercetin and kaempferol with a hydroxyl group in seventh position of the 4-chromanone ring of the base structure have the maximum efficacy against oxidative groups.
8
Anti-neoplastic effect
With an increase in concentration and polarity of E. angustifolia extracts, its anti-tumor effects level up. In an in vivo study on mice, elevation in immunity with increase in spleen index and thymus index manifestation has been reported as the main anticancer mechanism of Russian olive extracts. It has been shown that the anti-cancer properties are the effects of essential oils (ethyl cinnamate, 2-phenyl-ethyl benzoate, 2-phenyl-ethyl isovalerate, nerolidole, squalene and acetaphenone), flavonoids and pro anthocyanosides.
75,81,82
Moreover, the efficacy of Russian olive in inhibition of angiogenesis in Human umbilical vein endothelial cells may result in control of diabetes, retinopathy, rheumatoid arthritis and skin diseases like psoriasis in addition to malignancies.
83,84
Documented studies on Russian olive extracts in anti-neoplastic effects have been summarized in Table 5.
Table 5
.
Anti-neoplastic effects of Eaeagnus angustifolia
|
Type of extract
|
Cell line
|
Considerations
|
References
|
|
Hydroalcoholic of flowers
|
Human umbilical vein endothelial
cells(HUVEC)
|
In vitro inhibition of angiogenesis
|
84
|
|
Phenolic extract
|
Human colorectal adenocarcinoma cells(HT29) |
In vitro control of colorectal cancers in different
stages
|
85, 86
|
|
Ethanolic of leaves
|
Breast adenocarcinoma cell(MCF7) |
In vitro studies showed no significant difference
with control group
|
87
|
|
Ethyl acetate of the whole plant
|
Human cervical carcinoma cells (Hela) |
In vitro study
|
75
|
|
Edible parts of the plants
|
Human hepatoma cells(HepG2) |
In vitro and in vivo studies on H22 cells-bearing
mice
|
81
|
|
Tincture of flowers
|
Walker 256 carcinoma cells |
In vivo study on Wistar rats
|
69
|
Flavonoids can down regulate mutant p53 genes (p53 wild gene is a regulatory gene that codes special proteins which control cell cycles and known as a tumor suppressor gene) and arrest the cell cycle in G2 or M in malignant tumors. At the same time, flavonoids can suppress the expression of Ras protein and control the heat shock proteins especially in leukemia and colorectal cancers. Quercetin, one of the main flavonoid compositions of E. angustifolia is known as an anti-proliferative agent because it can block the cell surface tyrosine kinases and stops the transfer of growth messages to the nucleus.
4
Muscle relaxant
In traditional medicine, muscle relaxant effects of EA has been noted upon in different literature.
16
An in vivo study on mice to investigate such effects showed that polar extracts of this plant can exhibit dose dependent effects comparable to diazepam (2 mg/kg). It was suggested that flavones available in such extracts possess a partial agonistic effect on benzodiazepine (BDZ) receptors.
88
Flavones especially the 6-substitution compounds have strong affinity with GABAA receptors; however flavones which are naturally synthesized, have partial agonistic effect on BDZ receptors. This mechanism is mainly described for the sedative compounds; but in this filed, the extract shows a pharmacological effect comparing to diazepam for controlling muscle contractions. After the activation of GABA receptors, chloride channels open and hyperpolarize cell membrane which prevents further excitations.
89
On the other hand, the same muscle relaxant effect is produced in smooth muscles of the intestine, due to the acetylcholine antagonizing effects of the said extract. This provides a means to treat intestinal colic pains.
9
Cardiovascular effects
Elaeagnin, one of the alkaloids of E. angustifolia, was reported as a blood pressure optimizer. It has a tetrahydroharman structure resembling reserpine on the basis of overlay studies of bioinformatics (Fig. 4). It can bind reversibly or irreversibly to the human monoamino oxidase A (MAO-A) active site and lower the blood pressure. As elaeagnin can fit with X-ray structure of harmine in MAO-A active site, theoretically, it would be effective as a blood pressure controller like reserpine (harmine in white and elaeagnin in yellow). On the other hand, aqueous leaf extracts of E. angustifolia with antioxidant property, can improve oxidative stress state brought by ischemic/reperfusion (I/R) in rats. This extract (0.5 mg/mL and 1 mg/mL) can reduce cardio toxicity of I/R and increase myocardial biochemical parameters. Altogether, this plant with possible reduction of blood pressure and enhancing the recovery of I/R can be a good choice in cardiac diseases but more studies needed.
37,91
ROS production is one of the main mechanisms of myocardial dysfunction and necrosis after I/R. superoxide dismutase (SOD) enzymes can control the radical species or ROS in myocardium. But, the problem triggers when the amount of ROS exceed the threshold limit of SOD. The leaves extract of E. angustifolia with polyphenolic compositions can reduce the activity of ROS, serum level of carbonyl groups and malondialdehydes with the molecular mechanism described in Fig. 5 and increase the ability of SOD to control the oxidative stress.
92
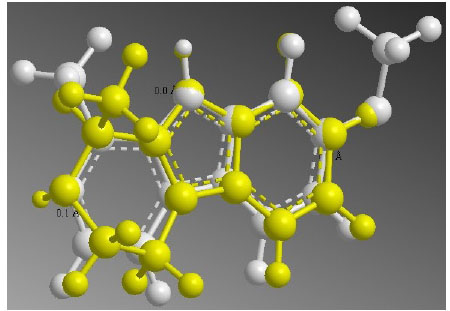
Fig. 4
.
Overlay of elaeagnin (the active component of E. angustifolia) and harmine
.
Overlay of elaeagnin (the active component of E. angustifolia) and harmine
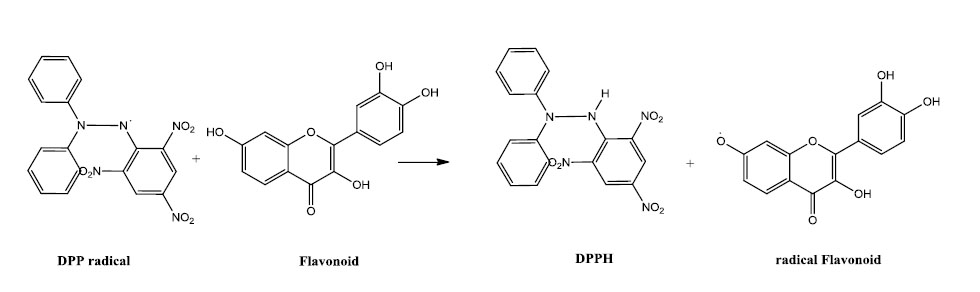
Fig. 5
.
Free radical scavenging mechanism of flavonoids.
.
Free radical scavenging mechanism of flavonoids.
Other medical applications
In animal models, aqueous extract of E. angustifolia can improve cognitive disorders. Tamtaji et al. treated the scopolamine-induced alzheimeric rats with increased doses (50-100-200-400 mg/kg) of Russian olive and resulted in significant improvements of special learning and memory.
93
In a randomized clinical trial, females with orgasmic disorders received flower extract of E. angustifolia (4.5 g/D in 2 doses) and sildenafil citrate (50 mg, 1 h before intercourse), female sexual function and the levels of prolactin and TSH was measured after 4 weeks’ treatment. Hormonal levels were found to remain even. Although Russian olive was less functional than sildenafil, it was effective in reduction of the frequencies of orgasmic disorders. The mechanism is not clear, but it may be the result of NO rise and subsequently cGMP increase in cells. It may cause vaginal smooth muscle relaxation, artery vasodilation and finally swelling of genital system.
94
The effects of Russian olive in treatment of gastrointestinal problems have been proved. It may also be operative in ulcerative colitis and help to prevent cancers. Histopathological studies in rats with induced ulcerative colitis presented a significant improvement with edible extract (600 mg) and less improvement with the enema gel containing 20 percent extracts of Russian olive.
95
Drug formulation
Using herbal compounds as drug carriers due to their lower side effects is a good step forward.
96
In this category, several studies have been carried out using dried extract to transport calcium carbonate, naproxen and ibuprofen trying to increase drug wetting, prevent caking, increase particle surface and increase drug dissolution speed. These efforts are expected to result in increased blood concentration of medicines. Nanocapsules containing calcium carbonate and EA in addition to other formulation improvements in comparison to calcium carbonate nanocapsules and calcium carbonate tablets result in higher calcium blood levels. Solid dispersions containing naproxen and ibuprofen show higher analgesic and anti-inflammatory effects. Despite its lower efficacy, in comparison to chemicals such as cross povidone, extracts of this plant are still more favorable, in large part due to less documented side effects.
97-99
Moreover, 6% soft extract of Russian olive can be useful in cream formulations. In addition to the antioxidant and anti-inflammatory effects for wound healing, it helps the spread capacity and thixotropic properties of cream or ointments bases. The best formulation for the extract insertion is an anionic base (oil/water emulsions).
69,100,101
Russian olive extracts have also been inserted in topical gels for oral lesions. Clinical studies on 28 patients with oral lichen planus resulted in considerable decrease of lesion size and pain. Minerals and vitamins especially vitamin K with its efficacy in blood coagulation play main rolls in this matter.
10
Use in pregnancy controversies
Previously, in traditional benefits of Russian olive, it was described that its extract can be useful for the treatment of rheumatoid arthritis. Pregnant women were among the suggested groups by the alchemists to use this extract against pain, inflammation and all problems in their joins and bones. Recent studies have shown the disadvantages of the fruit extract on the embryo osteogenesis and chondrogenesis. Although this extract does not reduce the amount of calcium, it reduces the volume of bones and cartilages. In contrary, mouse embryos showed a significant increase in femur length. The exact mechanism is not clear, but we can say phytoestrogens like flavonoids and tannins may inhibit non-classic estrogen signaling pathway, G protein receptor 30 (GRP30), and exert these effects in a dose depending manner. To end up, Russian olive extract can improve osteogenesis and chondrogenesis, but may decrease bone mass in mouse embryos, although it makes no craniofacial malformation or limb abnormalities.
48
Therefore, in this case, pregnant mothers should avoid the traditional remedies for joint and bone disorders.
103
Possible adverse effects
Russian olive is among the safest trees in the world and apart from possible allergies, there is no other hazard reports. Even though it is rich in heavy metals such as Cr, Pb, Zn, Cu, Ni and Co, some researches proved that in any condition of harvesting, the amount of heavy metals in all parts of the tree will not exceed the WHO limits (based on WHO guideline: the permissible limit of Cr, Pb, Zn, Cu, Ni and Co in medicinal plants is 1.5, 10, 50, 10, 1.5 and 0.2 part per million, respectively).
62,104
Even in an investigation on pregnant mice, E. angustifolia extracts made no significant differences in weight and CRL of mice fetus; but, because of the effects of concentrated extracts on bones and cartridge volumes, especially in pregnant women, more consideration may be needed.
48
Allergenicity
Russian olive is common as a wind-cutting tree in American and European countries, so its pollens would spread widely in the cities especially in the pollination season, spring. In an investigation, Ole e 1 and Ole e 4-like allergens, major allergens in olive pollen,
105,106
were recognized in this plant. With a prick test, 30 % of patients with rhinitis and rhinoconjunctivitis were sensitive to Russian olive pollens and 44 percent of patients with sensitivity to pollens have positive Prick tests. With these findings and the fact that its pollen size is much greater than grass and olive pollens (40-50 μm), it can be concluded that E. angustifolia is one of the major causes of allergies in the cities.
107
The mechanism which triggers these reactions is an IgE-mediated type II allergic type.
108
Moreover, dermatitis caused by contact with Elaeagnus sp. has already been reported; but, mostly among florists with repeated contact.
109
Concluding remarks
Utilizing the unlimited natural sources of medicines with the least restrictions of safety is one of the human beings goals. In this area, E. angustifolia can fulfill these objectives. This plant is a riparian tree considered as a N2-fixer herb, increases the nitrification of the soil (inorganic nitrogen).
110
It is rich in water and fat soluble vitamins, flavonoids, carbohydrates, alkaloids and biological active lipids. This review aimed to gather ethnopharmacological properties of E. angustifolia with focus on active constituents and medical benefits, and also make suggestions for future plans. For this purpose, almost all the medical articles from 1970 to 2016 were collected from various databases such as Embase, NCBI, Science Direct and Scopus. The most important and peer-reviewed information were analyzed and classified judiciously.
For years, E. angustifolia was used as an astringent, kidney stone removal, anti-inflammatory and pain relief agent. It was a well-known remedy for fever, jaundice, asthma, tetanus and rheumatoid arthritis. In turkey, E. angustifolia was an appetizer and a source of nutrition. It can be a food additive; as in an experiment by Cakmakc et al. addition of the flour and crust of the fruits as a flavor and formulation optimizer (viscosity enhancement) in an ice cream have showed perfect results. In food industry, natural plants with antioxidant properties can result in vast commercial advertisements.
111
Effects of Russian olive on inflammation and pain have been studied the most. More than 4 clinical trials are available and all of them have reported a comparable efficacy as NSAIDs. Total fruit has muscle relaxant capacity in a dose-dependent manner comparable to that of diazepam, COX inhibition property like indomethacin and ibuprofen, and is full of kaempferol that can suppress inflammation mediators.
A number of experiments have evaluated its active constituents for their applications in malignancies, ulcers, gastric problems and osteoarthritis. Previous studies showed that even in injection forms in mice and rats, there is no significant immunological responses (as the corticosteroid levels were even),
39
this plant can even be applied in developed injection formulations for special applications like intra-articular vials.
In “experimental and clinical studies” section, the E. angustifolia extract was suggested as blood pressure optimizer, because of special structure similarity of elaeagnin to hamine and reserpine. Till now, no supported studies have proved it; perhaps due to the time-consuming mechanism of reserpine in controlling blood pressure. As known, reserpine blocks MAO irreversibly and then increases the amount of neurotransmitters in the synaptic cavity; this elevation of neurotransmitters lead to down regulation of the vascular receptors and decreases blood pressure subsequently. The aforementioned process is a long-lasting effect. None of the clinical studies of E. angustifolia extract consider time as a major factor in cardiovascular protective properties of the plant.
112
Flavonoids of this plant are so hydrophobic. Lots of E. angustifolia medicinal benefits are related to this property of flavonoids. Anti-bacterial effects are one of them. Flavonoids can penetrate the cell membrane of the bacteria and exert their destructive effects. In most cases, aggregation of bactria was seen. Some of its flavonoids like kaempferol and quercetin show slight β-lactamase inhibitory effects, as well. As presented in Table 4, the main antibacterial properties are related to the methanol or ethanol extracts.
113
The lack of evidence on the relation of the medical applications and detailed structure of constituents, conceal its exclusive properties. There are no reports on experimental studies of pharmacologic/toxicologic effects of a few compounds like elaeagnine or elaeagnoside that are found in this plant. The gastroprotective effects of the plant appear to lead us towards development of medicines comparable with misoprostol. As for the anti-neoplastic effects or bactericidal effects, this plant can be a source of inspiration for rational drug design and medicinal chemistry. As a matter of fact, around 85 percent of chemotherapy agents have natural sources.
114
However, the lack of clinical studies on this plant has limited its availability in drug forms. It is certain that further studies would be necessary to fill such information paucity and gap.
Ethical approval
There is none to be declared.
Competing interests
There is none to be declared.
Acknowledgments
This review article was supported by research center of Mashhad University of Medical Sciences.
Review Highlights
What is current knowledge?
simple
-
√ E. angustifolia can be a source of bioflavonoids that are so
applicable in controlling pain and inflammation.
-
√ Active ingredients of E. angustifolia can even be useful as
anti-cancer, anti-bacterial and anti-fungal medicines.
-
√ The protective properties of E. angustifolia extract in
gastrointestinal system became clear.
What is new here?
simple
-
√ The relationship between its active ingredients and medical
properties are not mechanistically clear; but there are some
evidences in participation of the flavonoids of Russian olive
in controlling enzymes (COX) or blocking the receptors
(NMDA-R or BDZ-R).
-
√ One of the possible mechanisms in which active ingredients
of Russian olive may decrease the blood pressure, have been
discussed.
-
√ No critical and significant side effects of this plant (even in
high doses) have been reported.
References
- Mineau MM, Baxter GV, Marcarelli AM, Minshall GW. An invasive riparian tree reduces stream ecosystem efficiency via a recalcitrant organic matter subsidy. Ecology 2012; 93:1501-8. [ Google Scholar]
- Lamers JP, Khamzina A. Seasonal quality profile and production of foliage from trees grown on degraded cropland in arid Uzbekistan, Central Asia. J Anim Physiol Anim Nutr (Berl) 2010; 94:e77-85. doi: 10.1111/j.1439-0396.2009.00983.x [Crossref] [ Google Scholar]
- Mehrabani Natanzi M, Pasalar P, Kamalinejad M, Dehpour AR, Tavangar SM, Sharifi R. Effect of aqueous extract of Elaeagnus angustifolia fruit on experimental cutaneous wound healing in rats. Acta Med Iran 2012; 50:589-96. [ Google Scholar]
- Kurdali F, Al-Shamma’a M. Natural abundances of 15N and 13C in leaves of some N2-fixing and non-N2-fixing trees and shrubs in Syria. Isotopes Environ Health Stud 2009; 45:198-207. doi: 10.1080/10256010903084126 [Crossref] [ Google Scholar]
- Huang Z, Liu M, Chen B, Uriankhai T, Xu C, Zhang M. Distribution and interspecific correlation of root biomass density in an arid Elaeagnus angustifolia–Achnatherum splendens community. Acta Ecolo Sin 2010; 30:45-9. doi: 10.1016/j.chnaes.2009.12.008 [Crossref] [ Google Scholar]
- Gonzalez-Munoz N, Castro-Diez P, Fierro-Brunnenmeister N. Establishment success of coexisting native and exotic trees under an experimental gradient of irradiance and soil moisture. Environ Manage 2011; 48:764-73. doi: 10.1007/s00267-011-9731-3 [Crossref] [ Google Scholar]
- Rood SB, Braatne JH, Goater LA. Favorable fragmentation:river reservoirs can impede downstream expansion of riparian weeds. Ecol Appl 2010; 20:1664-77. [ Google Scholar]
-
Fritz Hans Schweingruber AB, Ernst-Detlef Schulze. Elaeagnaceae. Atlas of Stem Anatomy in Herbs, Shrubs and Trees. New York: Springer; 2011. p. 152-5.
- Farzaei MH, Bahramsoltani R, Abbasabadi Z, Rahimi R. A comprehensive review on phytochemical and pharmacological aspects of Elaeagnus angustifolia L. J Pharm Pharmacol 2015; 67:1467-80. [ Google Scholar]
- Kumar R, Kaur M, Silakari O. Chemistry and biological activities of thioacridines/thioacridones. Mini Rev Med Chem 2013; 13:1220-30. [ Google Scholar]
-
Wang Y, Guo T, Li JY, Zhou SZ, Zhao P, Fan MT. Four flavonoid glycosides from the pulps of Elaeagnus angustifolia and their antioxidant activities. In: Cai SZ, Zhang QF, eds. Advanced Materials Research. Nanjing, Jiangsu; 2013. p. 16-20.
- Bekker NP, Glushenkova AI. Components of Certain Species of the Elaeagnaceae Family. Chem Nat Compd 2001; 37:97-116. doi: 10.1023/a:1012395332284 [Crossref] [ Google Scholar]
- Abizov EA, Tolkachev ON, Mal’Tsev SD, Abizova EV. Composition of biologically active substances isolated from the fruits of Russian olive (Elaeagnus angustifolia) introduced in the European part of Russia. Pharm Chem J 2008; 42:696-8. [ Google Scholar]
- Kusova RD, Luk’yanchikov MS. Fatty acid composition of the fruit oil of Elaeagnus angustifolia. Chem Nat Compd 1989; 25:718. doi: 10.1007/bf00598274 [Crossref] [ Google Scholar]
- Goncharova NP, Plugar VN, Rashkes YV, Isamukhamedov AS, Glushenkova AI. Oxygenated fatty acids of the seeds of Elaeagnus angustifolia. Chem Nat Compd 1994; 30:661-5. doi: 10.1007/bf00630597 [Crossref] [ Google Scholar]
- Goncharova NP, Glushenkova AI. Lipids of elaeagnus fruit. Chem Nat Compd 1990; 26:12-5. doi: 10.1007/bf00605188 [Crossref] [ Google Scholar]
- Zhang N, Bao D, Yang C, Huang X. Ultrasound-assisted extraction of Elaeagnus angustifolia seeds oil and its physical-chemical properties. Journal of the Chinese Cereals and Oils Association 2013; 28:82-5. [ Google Scholar]
- Hosseinzadeh H, Rahimi R. Anti-inflammatory effects of Elaeagnus angustifolia L fruits in mice and rats. Iran J Basic Med Sci 1999; 24:143-7. [ Google Scholar]
- Ayaz M, Riaz M, Malik A, Ahmad E, Fatima I, Arif Lodhi M. Elaeagnoside, chymotrypsin inhibiting steroidal glucoside from Elaeagnus orientalis. Nat Prod Res 2009; 23:409-14. doi: 10.1080/14786410601083506 [Crossref] [ Google Scholar]
- Ayaz FA, Bertoft E. Sugar and phenolic acid composition of stored commercial oleaster fruits. J Food Compost Anal 2001; 14:505-11. doi: 10.1006/jfca.2001.1004 [Crossref] [ Google Scholar]
- AYAZ FA. AYAZ FASoluble Sugar Composition of Elaeagnus angustifoliaLvarorientalis (L) Kuntze (Russian olive) Fruits. Tr J Bot 1999; 23:349-354. [ Google Scholar]
- Nikolaeva AG. Alkaloids of Elaeagnus angustifolia. Chem Nat Compd 1970; 6:659. doi: 10.1007/bf00563479 [Crossref] [ Google Scholar]
- Allen JR, Holmstedt BR. The simple β-carboline alkaloids. Phytochemistry 1980; 19:1573-82. [ Google Scholar]
- Ancolio C, Azas N, Mahiou V, Ollivier E, Di Giorgio C, Keita A. Antimalarial activity of extracts and alkaloids isolated from six plants used in traditional medicine in Mali and Sao Tome. Phytother Res 2002; 16:646-9. [ Google Scholar]
- Esmaeili A, Niknam S. Characterization of nanocapsules containing Elaeagnus angustifolia L extract prepared using an emulsion-diffusion process. Flavour Fragr J 2013; 28:309-15. doi: 10.1002/ffj.3164 [Crossref] [ Google Scholar]
- Cansev A, Sahan Y, Celik G, Taskesen S, Ozbey H. Chemical Properties and Antioxidant Capacity of Elaeagnus angustifolia L Fruits. Asian J Chem 2011; 23:2661-5. [ Google Scholar]
- Yeşilada E, Gürbüz İ. A compilation of the studies on the Anti-ulcerogenic effects of medicinal plants Recent Progress in Medicinal Plants. Phytochem Pharmacol 2002; 2:111-74. [ Google Scholar]
- Gürbüz I, Üstün O, Yesilada E, Sezik E, Kutsal O. Anti-ulcerogenic activity of some plants used as folk remedy in Turkey. J Ethnopharmacol 2003; 88:93-7. [ Google Scholar]
- Ahmadiani A, Hosseiny J, Semnanian S, Javan M, Saeedi F, Kamalinejad M. Antinociceptive and anti-inflammatory effects of Elaeagnus angustifolia fruit extract. J Ethnopharmacol 2000; 72:287-92. doi: 10.1016/s0378-8741(00)00222-1 [Crossref] [ Google Scholar]
-
A. Z. Medicinal plants. 5th ed. Tehran, Iran: Tehran University Publications; 1992.
- Sezik E, Yeşilada E, Honda G, Takaishi Y, Takeda Y, Tanaka T. Traditional medicine in Turkey X Folk medicine in Central Anatolia. J Ethnopharmacol 2001; 75:95-115. [ Google Scholar]
- Kültür S. Medicinal plants used in Ki{dotless}rklareli Province (Turkey). J Ethnopharmacol 2007; 111:341-64. [ Google Scholar]
- Yesilada E, Honda G, Sezik E, Tabata M, Fujita T, Tanaka T. Traditional medicine in Turkey V Folk medicine in the inner Taurus Mountains. J Ethnopharmacol 1995; 46:133-52. [ Google Scholar]
- Honda G, Yesilada E, Tabata M, Sezik E, Fujita T, Takeda Y. Traditional medicine in Turkey VI Folk medicine in west Anatolia:Afyon, Kutahya, Denizli, Mugla, Aydin provinces. J Ethnopharmacol 1996; 53:75-87. doi: 10.1016/S0378-8741(96)01426-2 [Crossref] [ Google Scholar]
- Fujita T, Sezik E, Tabata M, Yesilada E, Honda G, Takeda Y. Traditional medicine in Turkey VII Folk medicine in middle and west Black Sea regions. Econ Bot 1995; 49:406-22. [ Google Scholar]
- Sezik E, Yeşİlada E, Tabata M, Honda G, Takaishi Y, Fujita T. Traditional medicine in turkey viii Folk medicine in east Anatolia; Erzurum, Erzíncan, Ağri, Kars, Iğdir provinces. Econ Bot 1997; 51:195-211. [ Google Scholar]
- Karimi G, Hosseinzadeh H, Rassoulzadeh M, Razavi BM, Taghiabadi E. Antinociceptive effect of Elaeagnus angustifolia fruits on sciatic nerve ligated mice. Iran J Basic Med Sci 2010; 13:97-101. [ Google Scholar]
- Ramezani M, Hosseinzadeh H, Daneshmand N. Antinociceptive effect of Elaeagnus angustifolia fruit seeds in mice. Fitoterapia 2001; 72:255-62. doi: 10.1016/s0367-326x(00)00290-2 [Crossref] [ Google Scholar]
- Farahbakhsh S, Arbabian S, Emami F, Moghadam BR, Ghoshooni H, Noroozzadeh A. Inhibition of cyclooxygenase type 1 and 2 enzyme by aqueous extract of Elaeagnus Angustifolia in mice. Basic Clin Neurosci 2011; 2:31-7. [ Google Scholar]
- Abbadie C, Taylor BK, Peterson MA, Basbaum AI. Differential contribution of the two phases of the formalin test to the pattern of c-fos expression in the rat spinal cord:studies with remifentanil and lidocaine. Pain 1997; 69:101-10. [ Google Scholar]
- Hunskaar S, Hole K. The formalin test in mice:dissociation between inflammatory and non-inflammatory pain. Pain 1987; 30:103-14. [ Google Scholar]
-
Rang HP, Ritter JM. Text Book of Pharmacology. 7th ed. New York2012.
- Kumar S, Pandey AK. Chemistry and biological activities of flavonoids:an overview. ScientificWorldJournal 2013; 2013:162750. doi: 10.1155/2013/162750 [Crossref] [ Google Scholar]
- Zhao G, Etherton TD, Martin KR, Vanden Heuvel JP, Gillies PJ, West SG. Anti-inflammatory effects of polyunsaturated fatty acids in THP-1 cells. Biochem Biophys Res Commun 2005; 336:909-17. doi: 10.1016/j.bbrc.2005.08.204 [Crossref] [ Google Scholar]
- Nikniaz Z, Ostadrahimi A, Mahdavi R, Ebrahimi AA, Nikniaz L. Effects of Elaeagnus angustifolia L supplementation on serum levels of inflammatory cytokines and matrix metalloproteinases in females with knee osteoarthritis. Complement Ther Med 2014; 22:864-9. [ Google Scholar]
- Ebrahimi A, Ebrahimi F, Nikniaz Z, Mahdavi R, Ostadrahimi A, Nikniaz L. Effect of elaeagnus angustifolia L Medulla fruit powder on pain of females with knee osteoarthritis. Int J Rheum Dis 2015; 18:79-80. [ Google Scholar]
- Panahi Y, Alishiri GH, Bayat N, Hosseini SM, Sahebkar A. Efficacy of Elaeagnus angustifolia extract in the treatment of knee osteoarthritis:A randomized controlled trial. EXCLI J 2016; 15:203-10. [ Google Scholar]
- Malihezaman M, Mahbobe M, Maryam SL. The ultrastructural and stereological study of aqueous extracts of Zataria multiflora Boiss and Elaeagnus angustifolia on the mouse fetus stomach. J Biol Sci 2007; 7:648-52. [ Google Scholar]
- Eliassi A, Ghodrati M, Kamalinrjad M. Effect of oral (systemic) Elaeagnus angustifolia L fruit on stimulated gastric acid secretion in unconscious rat and on basal acid secretion in conscious rat. Journal of Medicinal Plants 2009; 8:123-30. [ Google Scholar]
- Eliassi A, Mandipour M, Kamalinejad M. Intragastric effect of elaeagnus angustifolia L fruit on gastric acid secretion in a rat pylorus - Ligated model. Journal of Medicinal Plants 2008; 7:82-91. [ Google Scholar]
- Watkinson G, Hopkins A, Akbar F. The therapeutic efficacy of misoprostol in peptic ulcer disease. Postgrad Med J 1987; 64:60-77. [ Google Scholar]
- Ghosh PK, Gaba A. Phyto-extracts in wound healing. J Pharm Pharm Sci 2013; 16:760-820. [ Google Scholar]
- Natanzi MM, Pasalar P, Kamalinejad M, Dehpour AR, Tavangar SM, Sharifi R. Effect of aqueous extract of Elaeagnus angustifolia fruit on experimental cutaneous wound healing in rats. Acta Med Iran 2012; 50:589-96. [ Google Scholar]
- Guo Sa, DiPietro LA. Factors affecting wound healing. J Dent Res 2010; 89:219-29. [ Google Scholar]
- Burgess C. Topical vitamins. J Drugs Dermatol 2008; 7:s2-s6. [ Google Scholar]
- Biyik H. Antimicrobial activity of the ethanol extracts of some plants natural growing in Aydin, Turkey. Afr J Microbiol Res 2010; 4:2318-23. [ Google Scholar]
- Hambaba L, Boudjellal K, Abdeddaim M, Aberkane MC, Boudiaf K. In vitro investigation of antimicrobial and antioxidant activities of Elaeagnus angustifolia L fruit’s extracts. Phytotherapie 2012:1-7. doi: 10.1007/s10298-012-0737-7 [Crossref]
- Sharma N, Maiti S, Koley K. Studies on the incidence of sub clinical mastitis in buffaloes of Rajnandgaon district of Chhattisgarh state. Vet Pract 2004; 5:123-4. [ Google Scholar]
- Roberts D. Microbiological aspects of goat’s milk A Public Health Laboratory Service survey. J Hyg (Lond) 1985; 94:31-44. [ Google Scholar]
- Okmen G, Turkcan O. The antibacterial activity of elaeagnus angustifolia l Against mastitis pathogens and antioxidant capacity of the leaf methanolic extracts. J Anim Vet Adv 2013; 12:491-6. doi: 10.3923/javaa.2013.491.496 [Crossref] [ Google Scholar]
- Bucur L, Badea V, Istudor V. Antibacterial activity for two soft extracts of Elaeagnus angustifolia L. Archives of the Balkan Medical Union 2006; 41:127-31. [ Google Scholar]
- Khan SU, Khan AU, Shah AUHA, Shah SM, Hussain S, Ayaz M. Heavy metals content, phytochemical composition, antimicrobial and insecticidal evaluation of Elaeagnus angustifolia. Toxicol Ind Health 2016; 32:154-61. [ Google Scholar]
- Okmen G, Turkcan O. A study on antimicrobial, antioxidant and antimutagenic activities of Elaeagnus angustifolia L leaves. Afr J Tradit Complement Altern Med 2014; 11:116-20. [ Google Scholar]
- Bahraminejad S, Abbasi S, Amiri R. In Vitro Antifungal Activity of Various Methanolic Plant Extracts Against Alternaria Solani. Iran J Pharm Res 2013; 12:1470. [ Google Scholar]
- Malik F, Hussain S, Mirza T, Hameed A, Ahmad S, Riaz H. Screening for antimicrobial activity of thirty-three medicinal plants used in the traditional system of medicine in Pakistan. Journal of Medicinal Plants Research 2011; 5:3052-60. [ Google Scholar]
- Wang Y, Guo T, Zhao C, Zhao P, Fan M. Changes in total phenolic and flavonoid contents and antioxidant activities of the fruit from Elaeagnus angustifolia during an 80-day study period. Agro Food Ind Hi Tech 2014; 25:7-10. [ Google Scholar]
- Shi C, Sun Z, Xie B. Extraction, purification and antioxidation of proanthocyanidins of Elaeagnus angustifolia L Jujubes. Nongye Gongcheng Xuebao/Transactions of the Chinese Society of Agricultural Engineering 2006; 22:158-61. [ Google Scholar]
- Faramarz S, Dehghan G, Jahanban-Esfahlan A. Antioxidants in different parts of oleaster as a function of genotype. Bioimpacts 2015; 5:79-85. [ Google Scholar]
- Bucur L, Ţarǎlungǎ G, Istudor V, Badea V. Pharmacological studies on Elaeagnus angustifolia L tinctures and extracts Note 2 Experimental research about the flowers tincture antitumoral activity. Archives of the Balkan Medical Union 2008; 43:107-12. [ Google Scholar]
-
Giardi MT, Rea G, Berra B. Bio-Farms for Nutraceuticals: Functional Food and Safety Control by Biosensors. Springer Science & Business Media; 2011.
- Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem 2001; 49:4619-26. [ Google Scholar]
- Yalcin G, Sogut O. Antioxidant capacity of Elaeagnus angustifolia L and investigation of eosin y as the fluorescent probe in ORAC method. J Food Agric Environ 2014; 12:51-4. [ Google Scholar]
- Yalcin G, Sogut O. Influence of extraction solvent on antioxidant capacity value of oleaster measured by ORAC method. Nat Prod Res 2014; 28:1513-7. [ Google Scholar]
- Benvenuti S, Pellati F, Melegari Ma, Bertelli D. Polyphenols, anthocyanins, ascorbic acid, and radical scavenging activity of Rubus, Ribes, and Aronia. J Food Sci 2004; 69:FCT164-FCT9. [ Google Scholar]
- Ya W, Shang-Zhen Z, Chun-Meng Z, Tao G, Jian-Ping M, Ping Z. Antioxidant and Antitumor Effect of Different Fractions of Ethyl Acetate Part from Elaeagnus angustifolia L. Adv J Food Sci Technol 2014; 6:707-10. [ Google Scholar]
-
Si CL, Qin PP, Lu YY, Wu L, Wang HH, Hui LF, et al. GC-MS analysis of chemical composition and free radical scavenging activity of elaeagnus angustifolia bark. Harbin; 2011. p. 854-8.
- Chen Q, Chen J, Du H, Li Q, Chen J, Zhang G. Structural characterization and antioxidant activities of polysaccharides extracted from the pulp of Elaeagnus angustifolia L. Int J Mol Sci 2014; 15:11446-55. [ Google Scholar]
- Caliskan E, Elmastas M, Gokce I. Evaluation of antioxidant properties of elaeagnus angustifolia flowers. Asian J Chem 2010; 22:2840-8. [ Google Scholar]
- Bucur L, Negreanu-Pîrjol T, Giurginca M, Istudor V. Some new Elaeagnus angustifolia L Extracts and the pharmaceutical products’ antioxidant activities determined by the chemiluminiscence method. Rev Roum Chim 2008; 53:961-4. [ Google Scholar]
- Bendaikha S, Gadaut M, Harakat D, Magid A. Acylated flavonol glycosides from the flower of Elaeagnus angustifolia L. Phytochemistry 2014; 103:129-36. [ Google Scholar]
- Wang Y, Fan M, Li J, Guo T. Antitumor effect of edible part of elaeagnus angustifolia L in vivo and in vitro. Journal of Chinese Institute of Food Science and Technology 2013; 13:26-31. [ Google Scholar]
- Bucur L, Stanciu G, Istudor V. The GC-MS analysis of Elaeagnus angustifolia L flowers essential oil. Revista de Chimie 2007; 58:1027-9. [ Google Scholar]
- Creamer D, Sullivan D, Bicknell R, Barker J. Angiogenesis in psoriasis. Angiogenesis 2002; 5:231-6. [ Google Scholar]
- Badrhadad A, Kh P, Mansouri K. In vitro anti-angiogenic activity fractions from hydroalcoholic extract of Elaeagnus angustifolia L flower and Nepeta crispa L arial part. Journal of Medicinal Plants Research 2012; 6:4633-9. [ Google Scholar]
- Srirama R, Ramesha B, Ravikanth G, Uma Shaanker R, Ganeshaiah K. Are plants with anti-cancer activity resistant to crown gall?:a test of hypothesis. Curr Sci 2008; 95:1407-8. [ Google Scholar]
- Gill CI, Boyd A, McDermott E, McCann M, Servili M, Selvaggini R. Potential anti‐cancer effects of virgin olive oil phenolson colorectal carcinogenesis models in vitro. Int J Cancer 2005; 117:1-7. [ Google Scholar]
- Abu-Dahab R, Afifi F. Antiproliferative activity of selected medicinal plants of Jordan against a breast adenocarcinoma cell line (MCF7). Sci Pharm 2007; 75:121. [ Google Scholar]
- Hosseinzadeh H, Ramezani M, Namjo N. Muscle relaxant activity of Elaeagnus angustifolia L fruit seeds in mice. J Ethnopharmacol 2003; 84:275-8. doi: 10.1016/s0378-8741(02)00331-8 [Crossref] [ Google Scholar]
- Wasowski C, Marder M. Flavonoids as GABAA receptor ligands:the whole story?. J Exp Pharmacol 2012; 4:9. [ Google Scholar]
- Mohammed FI, Al-Essa MK, Shafagoj YA, Afifi FU. Investigation of the direct effects of the alcoholic extract of Elaeagnus angustifolia L (Elaeagnaceae) on dispersed intestinal smooth muscle cells of guinea pig. Sci Pharm 2006; 74:21-30. doi: 10.3797/scipharm.2006.74.21 [Crossref] [ Google Scholar]
- Semenov B, Novikov K, Spitsin A, Azev V, Kachala V. Diastereotopic synthesis of 1-and 1, 1-substituted 4-phenyl-2, 3, 4, 9-tetrahydro-1H-β-carbolines. Chem Nat Compd 2004; 40:585-90. [ Google Scholar]
- Wang B, Qu H, Ma J, Sun X, Wang D, Zheng Q. Protective Effects of Elaeagnus angustifolia Leaf Extract against Myocardial Ischemia/Reperfusion Injury in Isolated Rat Heart. J Chem 2014; 2014:6. doi: 10.1155/2014/693573 [Crossref] [ Google Scholar]
- Tamtaji OR, Taghizadeh M, Takhtfiroozeh SM, Talaei SAR. The effect of elaeagnus angustifolia water extract on Scopolamine-Induced memory impairment in rats. Majallahi Ilmi Pizhuhishii Danishgahi Ulumi Pizishki Khadamati Bihdashtii Darmanii Zanjan 2014; 22:101-11. [ Google Scholar]
- Akbarzadeh M, Zeinalzadeh S, Zolghadri J, Mohagheghzadeh A, Faridi P, Sayadi M. Comparison of Elaeagnus angustifolia extract and sildenafil citrate on female orgasmic disorders:A randomized clinical trial. J Reprod Infertil 2014; 15:190-8. [ Google Scholar]
- Khodakarm-Tafti A, Mehrabani D, Homafar L, Farjanikish G. Healing effects of Elaeagnus angustifolia extract in experimentally induced ulcerative colitis in rats. J Pharmacol Toxicol 2015; 10:29-35. [ Google Scholar]
- Khafagy E-S, Morishita M, Onuki Y, Takayama K. Current challenges in non-invasive insulin delivery systems:a comparative review. Adv Drug Deliv Rev 2007; 59:1521-46. [ Google Scholar]
- Kaouah F, Boumaza S, Berrama T, Trari M, Bendjama Z. Preparation and characterization of activated carbon from wild olive cores (oleaster) by H3PO4 for the removal of Basic Red 46. J Clean Prod 2013; 54:296-306. doi: 10.1016/j.jclepro.2013.04.038 [Crossref] [ Google Scholar]
- Mohajjel Nayebi AR, Barzegar Jalali M, Pourmohammad S. Study of analgesic effect of naproxen solid dispersions in crosspovidone and Elaeagnus Angustifolia fruit powder by using formalin test. Bioimpacts 2009; 15:125-32. [ Google Scholar]
- Mohammadi G, Barzegar-Jalali M, Valizadeh H, Nazemiyeh H, Barzegar-Jalali A, Shadbad MRS. Reciprocal powered time model for release kinetic analysis of ibuprofen solid dispersions in oleaster powder, microcrystalline cellulose and crospovidone. J Pharm Pharm Sci 2010; 13:152-61. [ Google Scholar]
- Bucur L, Tarălungă G, Alexandrescu R, Negreanu T, Istudor V. Evaluation of biological activity of a dermatological preparation with elaeagnus angustifolia flowers soft extract. Rev Med Chir Soc Med Nat Iasi 2007; 112:1098-103. [ Google Scholar]
- Bucur L, Hirjau V, Istudor V. Elaeagnus angustifolia L flowers soft extract valorification in a dermatological preparation Note 1 Development of dermatological preparation and preliminary quality control. Farmacia 2008; 56:215. [ Google Scholar]
- Taheri JB, Anbari F, Maleki Z, Boostani S, Zarghi A, Pouralibaba F. Efficacy of Elaeagnus angustifolia topical gel in the treatment of symptomatic oral lichen planus. J Dent Res Dent Clin Dent Prospects 2010; 4:29-32. [ Google Scholar]
- Talaei-Khozani T, Vojdani Z, Dehghani F, Heidari E, Kharazinejad E, Panjehshahin MR. Toxic effects of Elaeagnus angustifolia fruit extract on chondrogenesis and osteogenesis in mouse limb buds. Tokai J Exp Clin Med 2011; 36:63-70. [ Google Scholar]
- Khan JA, Khan MS. Metal analysis, phytotoxic, insecticidal and cytotoxic activities of selected medicinal plants of Khyber Pakhtunkhwa. Pak J Pharm Sci 2012; 25:51-8. [ Google Scholar]
- Quiralte J, Florido F, Arias de Saavedra J, Gómez A,
Saenz de San Pedro
B
, Gonzalez E. Olive allergen‐specific IgE responses in patients with Olea europaea pollinosis. Allergy 2002; 57:47-52. [ Google Scholar]
- Carnés Sánchez J, Iraola V, Sastre J, Florido F, Boluda L, Fernández‐Caldas E. Allergenicity and immunochemical characterization of six varieties of Olea europaea. Allergy 2002; 57:313-8. [ Google Scholar]
- Sastre J, Lluch‐Bernal M, Bustillo A, Carnes J, Maranon F, Casanovas M. Allergenicity and cross‐reactivity of Russian olive pollen (Eleagnus angustifolia). Allergy 2004; 59:1181-6. [ Google Scholar]
- Songnuan W. Wind-pollination and the roles of pollen allergenic proteins. Asian Pac J Allergy Immunol 2013; 31:261. [ Google Scholar]
- Fonia A, White IR, White JML. Allergic contact dermatitis to Elaeagnus plant (Oleaster). Contact Dermatitis 2009; 60:178-9. doi: 10.1111/j.1600-0536.2008.01485.x [Crossref] [ Google Scholar]
- Shah JJF, Harner MJ, Tibbets TM. Elaeagnus angustifolia elevates soil inorganic nitrogen pools in riparian ecosystems. Ecosystems 2010; 13:46-61. doi: 10.1007/s10021-009-9299-4 [Crossref] [ Google Scholar]
- Çakmakçı S, Topdaş EF, Kalın P, Han H, Şekerci P, Köse L. Antioxidant capacity and functionality of oleaster (Elaeagnus angustifolia L) flour and crust in a new kind of fruity ice cream. Int J Food Sci Technol 2015; 50:472-81. doi: 10.1111/ijfs.12637 [Crossref] [ Google Scholar]
-
Shore PA, Giachetti A. Reserpine:basic and clinical pharmacology. In: Handbook of psychopharmacology. Springer; 1978. p. 197-219.
- Daglia M. Polyphenols as antimicrobial agents. Curr Opin Biotechnol 2012; 23:174-81. [ Google Scholar]
- Newman DJ, Cragg GM. Natural Products as Sources of New Drugs over the Last 25 Years. J Nat Prod 2007; 70:461-77. [ Google Scholar]