BioImpacts. 6(3):119-124.
doi: 10.15171/bi.2016.18
Original Article
The effects of valsartan on renal glutathione peroxidase expression in alleviation of cyclosporine nephrotoxicity in rats
Sina Raeisi 1, 2, 3, Amir Ghorbanihaghjo 1, *, Hassan Argani 4, Siavoush Dastmalchi 5, Babollah Ghasemi 6, Teimour Ghazizadeh 2, Nadereh Rashtchizadeh 5, Mehran Mesgari Abbasi 1, Nasrin Bargahi 5, Mahboob Nemati 7, Ali Mota 2, Amir Mansour Vatankhah 1
Author information:
1Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Department of Biochemistry and Clinical Laboratories, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
3Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
4Urology and Nephrology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
5Biotechnology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
6Division of Clinical Laboratory, Tabriz Children’s Hospital, Tabriz University of Medical Sciences, Tabriz, Iran
7Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
Nephrotoxicity as a side effect caused by the immunosuppressive drug, cyclosporine-A (CsA), can be a major problem in transplant medicine. Oxidative stress may play an important role in the CsA-induced nephrotoxicity. It has been shown that the antihypertensive drug, valsartan (Val), has also renoprotective effects but, its molecular mechanism is largely unknown. In the present study, it was aimed to evaluate the Val effect in the alleviation of CsA nephrotoxicity via probable renal glutathione peroxidase (GPx) upregulation and oxidative stress decrease.
Methods:
Thirty-two Sprague-Dawley rats were divided into four groups based on CsA and/or Val administration: group A (Control, 1 mL/kg/day of olive oil as vehicle), group B (CsA, 30 mg/kg/day), group C (CsA+Val, 30+30 mg/kg/day), and group D (Val, 30 mg/kg/day). After the administration period (six weeks), renal GPx expression was evaluated by real-time polymerase chain reaction (PCR). Plasma levels of GPx and 8-Hydroxydeoxyguanosine (8-OHdG) were measured by enzyme-linked immunosorbent assay (ELISA). Malondialdehyde (MDA) and protein carbonyl groups (PCG) were measured by spectrophotometer. Plasma levels of urea and creatinine were measured by an autoanalyzer.
Results:
CsA treatment led to the decrease in renal expression and plasma levels of GPx in comparison to other study groups. Rats received CsA were detected to have significantly (p<0.05) higher plasma 8-OHdG, MDA, PCG, urea, and creatinine levels in comparison to other groups. Plasma urea and creatinine levels were negatively correlated with renal GPx expression and positively correlated with the oxidative stress markers.
Conclusion:
Administration of Val may result in attenuating the nephrotoxic side effect of CsA via probable renal GPx upregulation, and subsequently oxidative stress decrease.
Keywords: Cyclosporine-A, Glutathione peroxidase, Oxidative stress, Transplantation, Valsartan
Copyright and License Information
© 2016 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Transplantation medicine is a preferable treatment for patients with end-stage renal diseases. It has achieved excellent short-term outcomes, but long-term outcomes have not improved since the introduction of the cyclosporine-A (CsA) in the late 1970s.
1,2
CsA, as an immunosuppressive drug with a short-term outcome, has been widely used to avoid organ transplant rejection.
2
Nephrotoxic side effect of CsA can be the Achilles’ heel of this immunosuppressive drug.
2,3
CsA nephrotoxicity that may eventually lead to nephropathy can be an important cause of renal dysfunction, the most important reason of graft loss.
4
Although CsA-induced nephrotoxicity is a multifactorial complex,
5
based on previous studies, oxidative stress and high reactive oxygen species (ROS) production may play an important role in its pathogenesis.
6
Glutathione peroxidase (GPx) as an antioxidant enzyme is found in all mammalian organs. It can protect cells against oxidative damage by reducing hydrogen peroxide and organic peroxides with reduced glutathione. The level of GPx expression varies according to the types of the tissues. High amounts of Gpx are detected in kidneys.
7
Therefore, it may play an important role in renal protection against oxidative stress.
There are some present and potential strategies to diminish CsA-induced nephrotoxicity.
2
It has been shown that antihypertensive drug, valsartan (Val), has also renoprotective effects as documented by reduced urinary albumin and protein excretion in patients with diabetes and/or chronic kidney disease.
8-10
Val is an angiotensin II receptor blocker (ARB) which blocks angiotensin II type 1 (AT1) receptor and mediates the blood pressure (BP) elevating the effect of angiotensin.
11
Effects of different ARBs on BP are close to each other, however some studies have shown that Val is the most specific, most effective, and the safest drug of all. Although hypertension is a prominent cause and also outcome of kidney diseases,
12
it has been recognized that renal protection of Val is independent from its BP lowering effect.
9,10
The molecular mechanisms responsible for Val renal protection have not yet been clear.
13
According to some previous studies, Val may attenuate oxidative stress
14-16
; therefore, it may lead to alleviating the nephrotoxic side effect of CsA. In the present study, we aimed to evaluate the Val effects in diminishing the CsA nephrotoxicity via probable upregulation of renal GPx gene, and subsequent decrease of oxidative stress.
Materials and methods
Animals
In this study, 32 male 12-week-old Sprague-Dawley rats weighing 220 to 280 g were purchased from Pasteur Institute of Iran (Tehran, Iran). According to Guide for Care and Use of Laboratory Animals [U.S. Department of Health, Education, and Welfare (DHEW), Publication number 78-23, National Institutes of Health (NIH), revised 1978] and local guidelines for compassionate use of animals in research, two rats were housed per cage, provided with free access to tap water and compact standard chow. The animals were kept in similar laboratory conditions (18°C to 23°C room temperature and controlled humidity) with alternating 12-h light and dark cycles.
Group design (drug treatment)
After a two-week acclimation period, the weight-matched rats were randomly divided into four groups (eight rats per group): group A (control) received daily subcutaneous injection of vehicle (1 mL/kg of olive oil, Sigma Co.); group B (CsA) received daily subcutaneous injection of CsA (Novartis Pharma) diluted in olive oil (15 mg/mL) at the dose of 30 mg/kg; group C (Val+CsA) received both Val (Novartis Pharma; 30 mg/kg/day, in drinking water) and CsA (30 mg/kg/day, subcutaneous injection); and group D (Val) received daily administrations of Val (30 mg/kg, in drinking water). The administration period was six weeks in all study groups. The conditions of the administrations were based on previous studies.
17-19
After the administration period, all rats were weighed and anesthetized by a single dose (100 mg/kg) intraperitoneal injection of ketamine. Afterwards, intravenous blood samples were collected in ethylenediaminetetraacetic acid (EDTA); the plasma samples were separated by centrifugation at 3000 g (unit of gravity) for 15 min and stored at -80°C until the analyses were carried out. Then, the rats were killed with a single lethal dose (200 mg/kg) of ketamine and the kidneys were excised and weighed separately. The right kidneys were collected and fixed in 10% formalin for histological assessments. The left kidneys were snap-frozen in liquid nitrogen and stored at -80°C until the analyses were performed.
Analysis of renal GPx expression
Quantitative real-time polymerase chain reaction
Total RNA was extracted from the kidney tissues using RNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Then, 1 μg of the total RNA was used for complementary DNA (cDNA) synthesis with Oligo (dT) primers using the RevertAid first-strand cDNA synthesis kit (Thermo Fisher Scientific Inc., Waltham, MA USA) according to the manufacturer’s instruction. Quantitative real-time polymerase chain reaction (PCR) analysis was performed in duplicate using SYBR Premix Ex Taq II (Takara Bio Inc., Shiga, Japan) on the Rotor-Gene 6000 real-time PCR detection system (Corbett Research, Sydney, Australia). Results were normalized to the gene expression of rat glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the reference gene. The used specific primer-sequences were 5’-CGGTTTCCCGTGCAATCAGT-3’ as the forward and 5’-ACACCGGGGACCAAATGATG-3’ as reverse primer. PCR program consisted of a preincubation step at 94°C for 7min, followed by 40 cycles of denaturation (94°C, 20s), annealing (64°C, 30s), and extension (72°C, 30s) steps with the final extension step at 72˚C for 10 min. The 2−ΔΔCt formula was used to calculate the gene expression ratio of GPx to GAPDH with respect to the control group.
Biochemical parameters
Plasma concentrations of urea and creatinine (Cr) were determined by a colorimetry method using commercial reagents in an automated chemical analyzer (Roche Cobas Mira, Basel, Switzerland). Furthermore, deteriorated renal failure (DRF) index was calculated by “(Cr + urea)/2” formula as a way to better estimate the glomerular filtration rate (GFR).
Oxidative stress markers
Plasma levels of GPx were measured by a rat enzyme-linked immunosorbent assay (ELISA) kit (CUSABIO, Wuhan, China). 8-hydroxydeoxyguanosine (8-OHdG), a marker of oxidative stress to DNA, was also measured by ELISA (Hangzhou Eastbiopharm CO., LTD.; Hangzhou, China; CK-E90285). Malondialdehyde (MDA) level as a lipid peroxidation marker was measured by spectrophotometric method using thiobarbituric acid reactive substances (TBARS) assay according to the method of Lapenna et al.
20
Protein oxidation was evaluated spectrometrically by measuring protein carbonyls according to the method described by Reznick et al.
21
Histological assessment
Formalin fixed kidney samples were subjected to dehydration in the ascending grades of ethanol and were kept in xylene overnight for complete dehydration. After embedding in paraffin, the tissue sections of 5 µm thick were cut and subsequently stained with hematoxylin and eosin. Thereafter, at least 10 fields of each slide were examined by a pathologist blinded to the grouping of the rats at ×200 magnification under light microscope.
Statistical analysis
Data are shown as mean ± standard deviation (SD). Statistical comparisons of the groups were carried out by one-way analysis of variance (ANOVA) and Bonferroni post hoc analysis. Pearson correlation coefficient was also calculated. A p value of less than 0.05 was considered statistically significant. The analyses were performed in SPSS 16.0 software.
Results
Body weights and plasma biochemical parameters
The basic parameters of the study groups after the treatment period are depicted in Table 1. The rats were weight matched before the intervention (p>0.05), but after the intervention, group B (CsA) had significantly lower mean body weight (BW) than the other groups (p<0.05). Furthermore, the rats receiving CsA (group B) were detected to have significantly (p<0.05) higher plasma urea, Cr and DRF-index levels than the other study groups.
Table 1
.
Biochemical parameters in the study groups
|
Parameters
|
Group A (Control)
|
Group B (CsA)
|
Group C (Val+CsA)
|
Group D (Val)
|
|
BW b.i (g)
|
209.80 ± 13.78 |
208.24 ± 11.13 |
207.77 ± 7.00 |
210.12 ± 12.39 |
|
BW a.i (g)
|
231.00 ± 16.04 |
192.47 ± 18.68a
|
225.61 ± 24.55b
|
242.22 ± 21.13c
|
| Cr (mg/dL) |
1.05 ± 0.21 |
1.99 ± 0.81d
|
1.17 ± 0.71e
|
0.85 ± 0.77f
|
| Urea (mg/dL) |
60.14 ± 14.48 |
107.87 ± 47.66g
|
68.86 ± 24.97h
|
57.66 ± 23.70i
|
| DRF index |
30.59 ± 7.13 |
54.93 ± 19.22j
|
35.01 ± 11.33k
|
29.25 ± 6.81l
|
Abbreviations: a.i., after intervention; b.i., before intervention; BW, body weight; Cr, creatinine; CsA, Cyclosporine-A; DRF, deteriorated renal failure; Val, valsartan.
a
p<0.001 vs. control; bp=0.021 vs. CsA; cp<0.001 vs. CsA; dp=0.010 vs. control; ep=0.041 vs. CsA; fp=0.001 vs. CsA; gp=0.001 vs. control; hp=0.040 vs. CsA; ip=0.001 vs. CsA; jp=0.001 vs. control; kp=0.035 vs. CsA; lp=0.001 vs. CsA.
Data are shown as Mean ± SD
Renal expression and plasma levels of GPx
The results of real-time PCR revealed that the renal expression of GPx was significantly (p<0.05) lower in CsA group than in other study groups (Fig. 1A). It is remarkable that the changes in plasma levels of GPx were also conformed to the results of expression alteration (Fig. 1B).
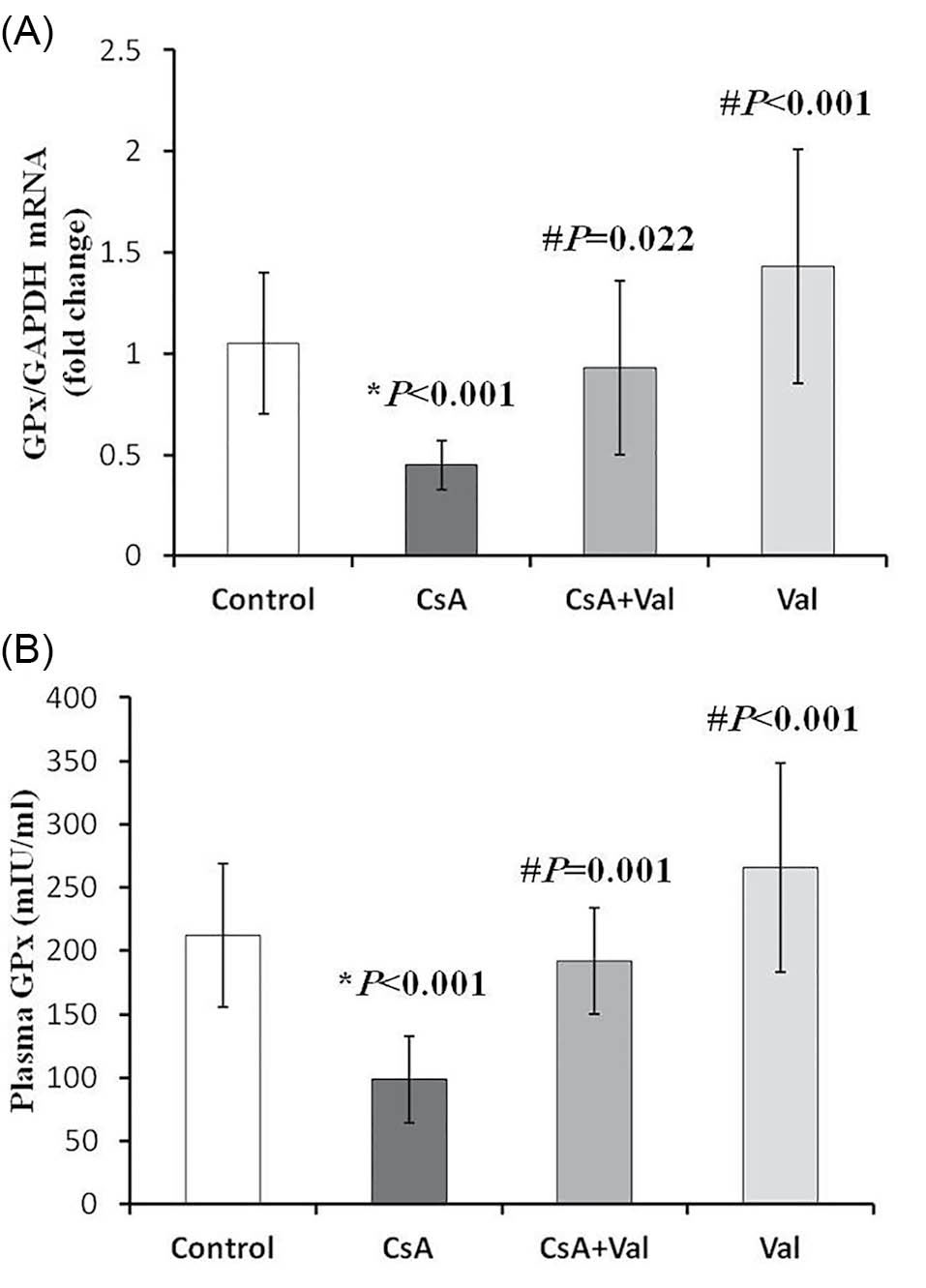
Fig. 1
.
Renal expression and plasma levels of glutathione
peroxidase (GPx) affected by cyclosporine (CsA) and/
or valsartan (Val) in the study groups.
(A) Real-time PCR
analyses revealed that renal expression of GPx were significantly
downregulated in CsA group (0.45 ± 0.12; fold change) compared
to control (1.05 ± 0.35; fold change), CsA+Val (0.93 ± 0.43;
fold change), and Val groups (1.43 ± 0.58; fold change). (B)
Plasma levels of GPx in CsA group (98.57 ± 34.11; mIU/mL) were
significantly lower than those in control (212.14 ± 56.75; mIU/mL),
CsA+Val (192.00 ± 42.14; mIU/mL), and Val groups (265.55 ±
82.43; mIU/mL). Data are presented as mean ± SD. *p<0.05 vs.
control; #p<0.05 vs. CsA.
.
Renal expression and plasma levels of glutathione
peroxidase (GPx) affected by cyclosporine (CsA) and/
or valsartan (Val) in the study groups.
(A) Real-time PCR
analyses revealed that renal expression of GPx were significantly
downregulated in CsA group (0.45 ± 0.12; fold change) compared
to control (1.05 ± 0.35; fold change), CsA+Val (0.93 ± 0.43;
fold change), and Val groups (1.43 ± 0.58; fold change). (B)
Plasma levels of GPx in CsA group (98.57 ± 34.11; mIU/mL) were
significantly lower than those in control (212.14 ± 56.75; mIU/mL),
CsA+Val (192.00 ± 42.14; mIU/mL), and Val groups (265.55 ±
82.43; mIU/mL). Data are presented as mean ± SD. *p<0.05 vs.
control; #p<0.05 vs. CsA.
Plasma levels of oxidative stress markers
Plasma levels of 8-OHdG, MDA, and PCG as oxidative stress markers were measured and compared among the study groups. As presented in Fig. 2, the rats receiving CsA were detected to have significantly (p<0.05) higher plasma 8-OHdG, MDA, and PCG levels than the other study groups.
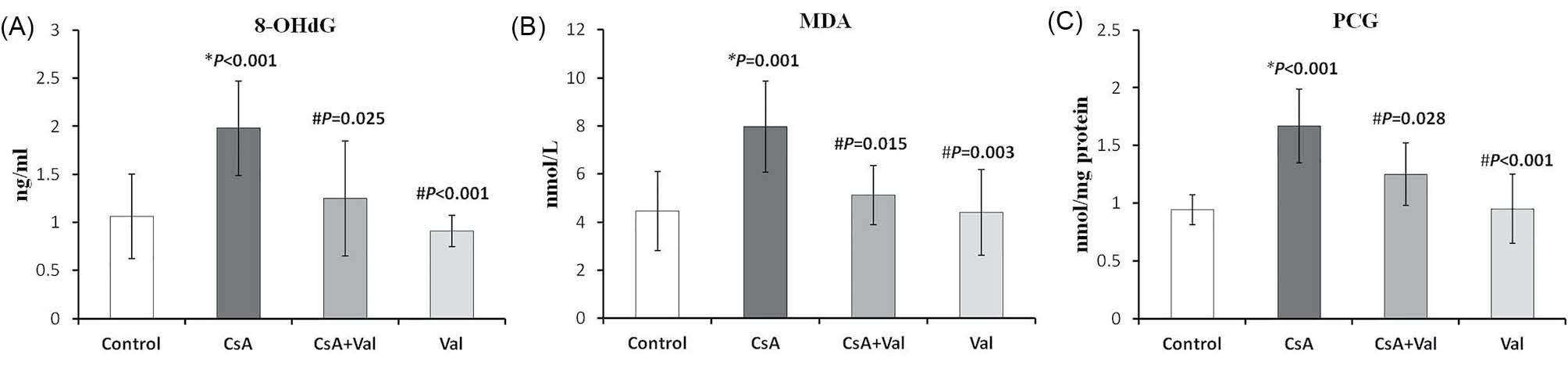
Fig. 2
.
Plasma levels of 8-Hydroxydeoxyguanosine (8-OHdG), Malondialdehyde (MDA), and protein carbonyl groups (PCG) affected by cyclosporine (CsA) and/or valsartan (Val) in the study groups. (A) Plasma levels of 8-OHdG in CsA group (1.98 ± 0.49
ng/mL) were significantly higher than those in the other study groups (control, 1.06 ± 0.44; CsA+Val, 1.25 ± 0.6; Val, 0.91 ± 0.16; ng/
mL). (B) Plasma levels of MDA in CsA group (7.98 ± 1.89 nmol/L) were significantly higher than those in the other study groups (control,
4.46 ± 1.65; CsA+Val, 5.12 ± 1.23; Val, 4.4 ± 1.78; nmol/L). (C) Plasma levels of PCG in CsA group (1.67 ± 0.32 nmol/mg protein) were
significantly higher than those in the other study groups (control, 0.94 ± 0.13; CsA+Val, 1.25 ± 0.27; Val, 0.95 ± 0.3; nmol/mg protein). Data
are expressed as mean ± SD. *p
<0.05 vs. control; #p<0.05 vs. CsA.
.
Plasma levels of 8-Hydroxydeoxyguanosine (8-OHdG), Malondialdehyde (MDA), and protein carbonyl groups (PCG) affected by cyclosporine (CsA) and/or valsartan (Val) in the study groups. (A) Plasma levels of 8-OHdG in CsA group (1.98 ± 0.49
ng/mL) were significantly higher than those in the other study groups (control, 1.06 ± 0.44; CsA+Val, 1.25 ± 0.6; Val, 0.91 ± 0.16; ng/
mL). (B) Plasma levels of MDA in CsA group (7.98 ± 1.89 nmol/L) were significantly higher than those in the other study groups (control,
4.46 ± 1.65; CsA+Val, 5.12 ± 1.23; Val, 4.4 ± 1.78; nmol/L). (C) Plasma levels of PCG in CsA group (1.67 ± 0.32 nmol/mg protein) were
significantly higher than those in the other study groups (control, 0.94 ± 0.13; CsA+Val, 1.25 ± 0.27; Val, 0.95 ± 0.3; nmol/mg protein). Data
are expressed as mean ± SD. *p
<0.05 vs. control; #p<0.05 vs. CsA.
Correlations of GPx, oxidative stress markers, and DRF index
As shown in Table 2, GPx was negatively correlated with oxidative stress markers and DRF index as well. The plasma level of MDA was positively correlated with 8-OHdG as well as PCG levels. Plasma PCG and 8-OHdG levels were also positively correlated. Each of these oxidative stress markers was positively correlated with DRF index.
Table 2
.
The correlations of GPx, oxidative stress markers, and DRF index
|
Parameters
|
GPx
|
8-OHdG
|
MDA
|
PCG
|
DRF index
|
| GPx |
- |
-0.724, <0.001 |
-0.688, <0.001 |
-0.644, <0.001 |
-0.701, <0.001 |
|
8-OHdG (r, p-value)
|
-0.724, <0.001 |
- |
0.884, <0.001 |
0.610, <0.001 |
0.761, <0.001 |
|
MDA (r, p-value)
|
-0.688, <0.001 |
0.884, <0.001 |
- |
0.681, <0.001 |
0.791, <0.001 |
|
PCG (r, p-value)
|
-0.644, <0.001 |
610, <0.001 |
0.681, <0.001 |
- |
0.530, <0.001 |
|
DRF index (r, p-value)
|
-0.701, <0.001 |
0.761, <0.001 |
0.791, <0.001 |
0.530, <0.001 |
- |
Abbreviations: GPx, glutathione peroxidase; MDA, malondialdehyde; 8-OHdG, 8-hydroxydeoxyguanosine; PCG, protein carbonyl groups; r, correlation coefficient.
Histological findings
Histological changes including hyperemia, inflammatory cell infiltration, and vacuolization were exhibited in the CsA treated rats. Val ameliorated these histological changes (Fig. 3).
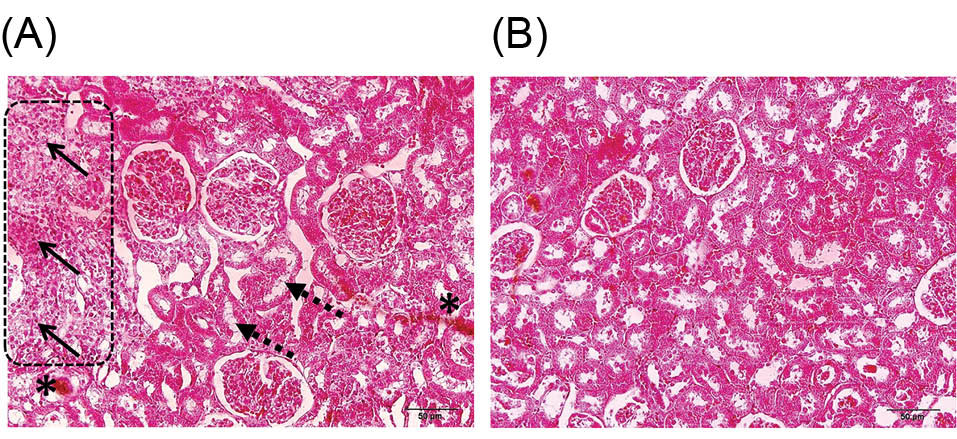
Fig. 3
.
Hematoxylin and eosin (H&E) staining of the rats’ kidney-tissues for histologic assessment (magnification, ×200). (A) Hyperemia (asterisks), inflammatory cell infiltration (arrows), and vacuolization (dashed arrow) were exhibited in the cyclosporine- (CsA) treated rats. (B) valsartan (Val) ameliorated these histological changes. There were no histologic alterations in Val treated rats.
.
Hematoxylin and eosin (H&E) staining of the rats’ kidney-tissues for histologic assessment (magnification, ×200). (A) Hyperemia (asterisks), inflammatory cell infiltration (arrows), and vacuolization (dashed arrow) were exhibited in the cyclosporine- (CsA) treated rats. (B) valsartan (Val) ameliorated these histological changes. There were no histologic alterations in Val treated rats.
Discussion
CsA as a calcineurin inhibitor prevents calcineurin, a protein-phosphatase involved in the activation of T-cells, and reduces the activity of the immune system. As an immunosuppressant drug, CsA is widely used to prevent organ transplantation rejection, but its nephrotoxic side effect may lead to dramatic problems post-transplantation.
22
There is much evidence that suggests oxidative stress may have an important role in CsA-induced toxicity.
6,23-25
In the present study, we demonstrated that the plasma levels of 8-OHdG, a direct indicator of oxidative DNA damage; MDA, a marker determining the degree of lipid peroxidation; and also protein oxidation marker, PCG, were significantly higher in CsA group compared with the other study groups. Plasma levels and renal expression of GPx were also decreased by CsA treatment. This result can confirm the oxidative stress-inducing effect of CsA. GPx acts in the scavenging and inactivating of ROS, thereby protecting the body against oxidative stress.
7
Reduced renal GPx level in the present study might be a reason of oxidative stress elevation by CsA.
Another mechanisms can also be assumed. Yoon et al.
26
in their study showed that CsA could downregulate the antioxidant enzymes, manganese superoxide dismutase (MnSOD) and hemeoxygenase-1.
Based on previous studies, the adverse effects of CsA can be alleviated by some agents such as SKF 106203 (a leukotriene receptor antagonist)
27
and paricalcitol (an active and nonhypercalcemic analog of vitamin D).
28
Moreover, recently hydrogen sulphide was introduced as a new therapeutic agent that can reverse the vasoconstriction changes associated with CsA treatment during reperfusion.
29
Due to the renal protective effect of Val, we initially hypothesized that it might also lead to the diminution of the nephrotoxicity of CsA. It has been shown that Val treatment does not interfere with immunosuppressive therapy.
30
Val can therapeutically be administered to patients with kidney diseases, as hypertension is a prominent cause and also outcome of kidney diseases.
12
Effects of different ARBs on BP are close to each other, but some studies have shown that Val is the most specific, most effective, and the safest drug of all.
11,30
These factors may make Val to be distinct among other agents in alleviating the CsA nephrotoxicity. The exact mechanism of Val renal protection has not yet been clear.
13
Some previous studies have demonstrated that the renoprotective effect of Val is independent from BP changes.
9,10
In our study, the dose of Val was 30 mg/kg/day; based on previous studies, this dose of Val does not change BP in the rats.
19,31
Due to the elimination of BP lowering role, the other potential mechanisms for the renal protection of Val against CsA nephrotoxicity could be possible. Jiao et al.
14
and Wu et al.
16
in their study demonstrated that Val might attenuate oxidative stress. In the present study, Val could diminish the effect of CsA in renal GPx downregulation and oxidative stress enhancement as well.
In the present study, effects of CsA and Val administrations on kidney tissue (histological assessment) and function were also evaluated. DRF index as a marker of kidney function in CsA group was significantly higher than that in the other study groups. According to the negative and positive correlations of DRF index with oxidative stress markers and GPx respectively, Val-alleviating CsA-induced renal damage might be mediated by upregulating GPx and decreasing oxidative stress levels, subsequently. Histological assessment showed that CsA could cause histological changes indicating its nephrotoxic effect. Based on the results, there was not any histologic alteration in the kidney samples of Val-treated rats that indicates Val may ameliorate CsA nephrotoxicity.
GPx upregulation may be not the only possible mechanism of the renal protection effect of Val. It has been shown that transforming growth factor beta (TGF-β), proinflammatory cytokine, and monocyte chemoattractant protein-1 (MCP-1) play important roles in nephropathy and kidney damage.
14
ROS may act as integral signaling molecules in the nephropathy. Protein kinase C (PKC) activation by ROS and the subsequent mitogen-activated protein kinases (MAPKs) play critical roles in kidney damage. PKC activation increases the expression of TGF-β, which can cause an increase in mesangial matrix deposition and glomerular basement membrane thickening and may promote glomerular mesangial cells’ apoptosis. In addition, PKC activation can induce MCP-1 synthesis. Since MCP-1 is the strongest chemotactic factor for the monocytes, its over-expression would lead to the increase of monocyte immigration and monocyte activity and exacerbate interstitial fibrosis, thereby deteriorating renal function.
14
Jiao et al. in their study
14
showed that Val could decrease TGF-β1 and MCP-1 expression and oxidative stress in glomerular mesangial cells and glomerular epithelial cells cultured in high-glucose and oxidative stress conditions. These mechanisms might be related with the renal protection of Val.
Conclusion
In the present study, it was demonstrated that CsA could deteriorate the renal function possibly through the downregulation of renal GPx and enhancement of oxidative stress enhancement. Val treatment might ameliorate CsA-induced nephrotoxicity via renal GPx upregulation that could eventually lead to oxidative stress decrease (Fig. 4). Further studies are needed to better understand the mechanisms involved in CsA nephrotoxicity and Val renal protection as well.
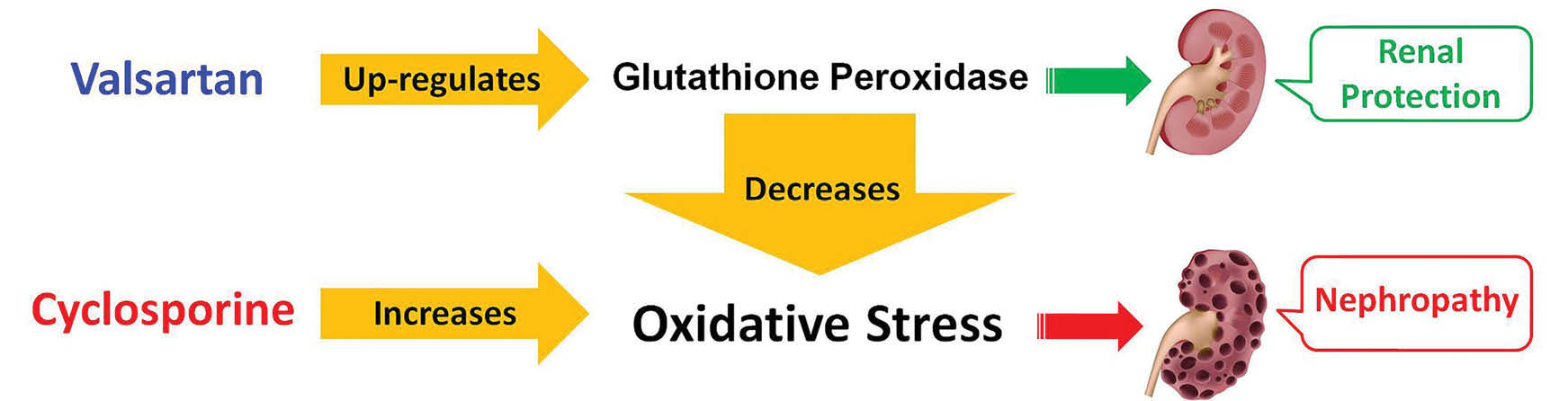
Fig. 4
.
Mechanism of valsartan (Val) effect in alleviation of cyclosporine (CsA) nephrotoxicity. CsA could deteriorate renal function
possibly through renal GPx downregulation and oxidative stress enhancement. Val treatment might ameliorate CsA-induced nephrotoxicity
via renal GPx upregulation that could eventually lead to oxidative stress decrease.
.
Mechanism of valsartan (Val) effect in alleviation of cyclosporine (CsA) nephrotoxicity. CsA could deteriorate renal function
possibly through renal GPx downregulation and oxidative stress enhancement. Val treatment might ameliorate CsA-induced nephrotoxicity
via renal GPx upregulation that could eventually lead to oxidative stress decrease.
Ethical approval
The Animal protocol in the present study was based on the Guide for Care and Use of Laboratory Animals [U.S. Department of Health, Education, and Welfare (DHEW), Publication number 78-23, National Institutes of Health (NIH), revised 1978] and local guidelines for compassionate use of animals in research.
Competing interests
Authors declare no conflict of interests.
Acknowledgments
This study is a project supported by grants from the Drug Applied Research Center (DARC) at Tabriz University of Medical Sciences and Urology and Nephrology Research Center at Shahid Beheshti University of Medical Sciences. A part of the results of the present study was presented as an abstract at “15th International Congress on Nephrology, Dialysis and Transplantation, 29 September-2 October 2015, Mashhad, Iran”. It was selected as one of the best papers for awards.
Research Highlights
What is current knowledge?
simple
-
√ CGPx and oxidative stress may have important roles in CsA nephrotoxicity and Val renal protection as well.
What is new here?
simple
-
√ Val could alleviate the CsA-induced nephrotoxicity via the upregulation of renal GPx and reduction of oxidative stress.
References
- Issa N, Kukla A, Ibrahim HN. Calcineurin inhibitor nephrotoxicity: a review and perspective of the evidence. Am J Nephrol 2013; 37:602-12. doi: 10.1159/000351648 [Crossref] [ Google Scholar]
- Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol 2009; 4:481-508. doi: 10.2215/cjn.04800908 [Crossref] [ Google Scholar]
- Sarró E, Tornavaca O, Plana M, Meseguer A, Itarte E. Phosphoinositide 3-kinase inhibitors protect mouse kidney cells from cyclosporine-induced cell death. Kidney Int 2008; 73:77-85. doi: 10.1038/sj.ki.5002638 [Crossref] [ Google Scholar]
- Xiao Z, Li C, Shan J, Luo L, Feng L, Lu J. Interventions to improve chronic cyclosporine A nephrotoxicity through inhibiting renal cell apoptosis: a systematic review. Chinese Med J 2012; 126:3767-74. [ Google Scholar]
- Jennings P, Koppelstaetter C, Aydin S, Abberger T, Wolf AM, Mayer G. Cyclosporine A induces senescence in renal tubular epithelial cells. Am J Physiol Renal Physiol 2007; 293:F831-F8. doi: 10.1152/ajprenal.00005.2007 [Crossref] [ Google Scholar]
- DeHornedo JP, De Arriba G, Fernández MC, Benito S, Cid TP. Cyclosporin A causes oxidative stress and mitochondrial dysfunction in tubular renal cells. Nefrologia 2007; 27:565-73. [ Google Scholar]
- de Haan JB, Stefanovic N, Nikolic-Paterson D, Scurr LL, Croft KD, Mori TA. Kidney expression of glutathione peroxidase-1 is not protective against streptozotocin-induced diabetic nephropathy. Am J Physiol Renal Physiol 2005; 289:F544-F51. doi: 10.1152/ajprenal.00088.2005 [Crossref] [ Google Scholar]
- Black HR, Bailey J, Zappe D, Samuel R. Valsartan: more than a decade of experience. Drugs 2009; 69:2393-414. doi: 10.2165/11319460-000000000-00000 [Crossref] [ Google Scholar]
- Katayama S, Yagi S, Yamamoto H, Yamaguchi M, Izumida T, Noguchi Y. Is renoprotection by angiotensin receptor blocker dependent on blood pressure?: the Saitama Medical School, Albuminuria Reduction in Diabetics with Valsartan (STAR) study. Hypertens Res 2007; 30:529-33. doi: 10.1291/hypres.30.529 [Crossref] [ Google Scholar]
- Viberti G, Wheeldon NM. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus a blood pressure–independent effect. Circulation 2002; 106:672-8. [ Google Scholar]
- Abraham I, MacDonald K, Hermans C, Aerts A, Lee C, Brié H. Real-world effectiveness of valsartan on hypertension and total cardiovascular risk: review and implications of a translational research program. Vasc Health Risk Manag 2011; 7:209. doi: 10.2147/vhrm.s9434 [Crossref] [ Google Scholar]
- Tedla F, Brar A, Browne R, Brown C. Hypertension in chronic kidney disease: navigating the evidence. Int J Hypertens 2011; 2011:132405. doi: 10.4061/2011/132405 [Crossref] [ Google Scholar]
- Gu C, Zhou G, Noble NA, Border WA, Cheung AK, Huang Y. Targeting reduction of proteinuria in glomerulonephritis: Maximizing the antifibrotic effect of valsartan by protecting podocytes. J Renin Angiotensin Aldosterone Syst 2014; 15:177-89. doi: 10.1177/1470320312466127 [Crossref] [ Google Scholar]
- Jiao B, Wang Y, Cheng Y, Gao J, Zhang Q. Valsartan attenuated oxidative stress, decreased MCP-1 and TGF-β 1 expression in glomerular mesangial and epithelial cells induced by high-glucose levels. Biosci Trends 2011; 5:173-81. doi: 10.5582/bst.2011.v5.4.173 [Crossref] [ Google Scholar]
- Goyal S, Bharti S, Sahoo KC, Sharma AK, Arya DS. Valsartan, an angiotensin II receptor blocker, attenuates cardiac dysfunction and oxidative stress in isoproterenol-induced cardiotoxicity. Cardiovasc toxicol 2011; 11:148-56. [ Google Scholar]
- Wu B, Lin R, Dai R, Chen C, Wu H, Hong M. Valsartan attenuates oxidative stress and NF-κB activation and reduces myocardial apoptosis after ischemia and reperfusion. Eur J Pharmacol 2013; 705:140-7. [ Google Scholar]
- Hara S, Umino D, Someya T, Fujinaga S, Ohtomo Y, Murakami H. Protective effects of Mizoribine on Cyclosporine A nephropathy in rats. Pediatr Res 2009; 66:524-7. [ Google Scholar]
- Yoon HE, Ghee JY, Piao S, Song J-H, Han DH, Kim S. Angiotensin II blockade upregulates the expression of Klotho, the anti-ageing gene, in an experimental model of chronic cyclosporine nephropathy. Nephrol Dial Transplant 2011; 26:800-13. [ Google Scholar]
- Nishiyama A, Kobori H, Fukui T, Zhang G-X, Yao L, Rahman M. Role of angiotensin II and reactive oxygen species in cyclosporine A–dependent hypertension. Hypertension 2003; 42:754-60. doi: 10.1161/01.hyp.0000085195.38870.44 [Crossref] [ Google Scholar]
- Mitobe M, Yoshida T, Sugiura H, Shirota S, Tsuchiya K, Nihei H. Oxidative stress decreases klotho expression in a mouse kidney cell line. Nephron Exp Nephrol 2005; 101:e67-e74. [ Google Scholar]
- Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 1994; 233:357-63. doi: 10.1016/s0076-6879(94)33041-7 [Crossref] [ Google Scholar]
- Perico N, Ruggenenti P, Gotti E, Gaspari F, Cattaneo D, Valente U. In renal transplantation blood cyclosporine levels soon after surgery act as a major determinant of rejection: insights from the MY SS trial. Kidney Int 2004; 65:1084-90. [ Google Scholar]
- Wolf A, Trendelenburg C-F, Diez-Fernandez C, Prieto P, Houy S, Trommer WE. Cyclosporine A-induced oxidative stress in rat hepatocytes. J Pharmacol Exp Ther 1997; 280:1328-34. [ Google Scholar]
- Hagar HH. The protective effect of taurine against cyclosporine A-induced oxidative stress and hepatotoxicity in rats. Toxicol Lett 2004; 151:335-43. doi: 10.1016/j.toxlet.2004.03.002 [Crossref] [ Google Scholar]
- Doh KC, Lim SW, Piao SG, Jin L, Heo SB, Zheng YF. Ginseng treatment attenuates chronic cyclosporine nephropathy via reducing oxidative stress in an experimental mouse model. Am J Nephrol 2013; 37:421-33. doi: 10.1159/000342117 [Crossref] [ Google Scholar]
- Yoon HE, Lim SW, Piao SG, Song J-H, Kim J, Yang CW. Statin upregulates the expression of klotho, an anti-aging gene, in experimental cyclosporine nephropathy. Nephron Exp Nephrol 2012; 120:e123-e33. doi: 10.1159/000342117 [Crossref] [ Google Scholar]
- Butterly DW, Spurney RF, Ruiz P, Griffiths R, Albrightson C, Coffman TM. A role for leukotrienes in cyclosporine nephrotoxicity. Kidney Int 2000; 57:2586-93. [ Google Scholar]
- Park JW, Bae EH, Kim IJ, Ma SK, Choi C, Lee J. Paricalcitol attenuates cyclosporine-induced kidney injury in rats. Kidney Int 2010; 77:1076-85. doi: 10.1038/ki.2010.69 [Crossref] [ Google Scholar]
- Lee G, Hosgood SA, Patel MS, Nicholson ML. Hydrogen sulphide as a novel therapy to ameliorate cyclosporine nephrotoxicity. J Surg Res 2015; 197:419-26. doi: 10.1016/j.jss.2015.02.061 [Crossref] [ Google Scholar]
- Andres A, Morales E, Morales J, Bosch I, Campo C, Ruilope L editors. Efficacy and safety of valsartan, an angiotensin II receptor antagonist, in hypertension after renal transplantation: a randomized multicenter study. Transplant Proc 2006; 38(8):2419-23. doi: 10.1016/j.transproceed.2006.08.066 [Crossref] [ Google Scholar]
- Tominaga N, Robert A, Izuhara Y, Ohtomo S, Dan T, Chihara K. Very high doses of valsartan provide renoprotection independently of blood pressure in a type 2 diabetic nephropathy rat model. Nephrology 2009; 14:581-7. [ Google Scholar]