BioImpacts. 7(2):83-89.
doi: 10.15171/bi.2017.11
Original Research
Early endocrine disruptors exposure acts on 3T3-L1 differentiation and endocrine activity
Sofiane Boudalia 1, 2, 3, 4, 5, *, Christine Belloir 1, 2, 3, Marie-Louise Miller 1, 2, 3, Marie-Chantal Canivenc-Lavier 1, 2, 3
Author information:
1INRA, UMR1324, Centre des Sciences du Goût et de l'Alimentation, F-21000 Dijon, France
2CNRS, UMR6265, Centre des Sciences du Goût et de l'Alimentation, F-21000 Dijon, France
3Université de Bourgogne, Centre des Sciences du Goût et de l'Alimentation, F-21000 Dijon, France
4Département d’Ecologie et Génie de l’Environnement, Université 8 Mai 1945, Guelma, Algérie
5Laboratoire de Biologie, Eau et Environnement, Université 8 Mai 1945, Guelma, Algérie
Abstract
Introduction:
Data from last years suggested that early exposure to endocrine disruptors (EDs) can predispose newborns to endocrine dysfunction of adipocytes, obesity, and associated disorders. The implication of EDs at low doses on adipocyte development has been poorly investigated. For instance, vinclozolin (V) is a dicarboximide fungicide widely used in agriculture since the 90's, alone or in mixture with genistein (G), an isoflavonoid from Leguminosae. This study aims to identify the effect of vinclozolin alone or with genistein, on adipose tissue properties using cell culture.
Methods:
In steroid-free conditions, 3T3-L1 pre-adipocytes were induced to differentiate in the presence of EDs, singularly or in mixtures, for 2 days. DNA and triglyceride (TG) levels were measured on days 0, 2 and 8 of differentiation. Leptin secretion was measured only on the eighth day.
Results:
We show that low doses of G (25 µM) and V (0.1 µM) inhibit pre-adipocytes differentiation. This inhibition has been represented by a decreasing in DNA content (µg/well) and decreasing in TG accumulation (mg/mL) in 3T3-L1 cells. Nevertheless, V increased the anti-adipogenic properties of G.
Conclusion:
This study confirms that EDs singularly or in mixtures, introduced during early stages of life, could affect the differentiation and the endocrine activity of adipocytes, and can act as potential factors for obesity.
Keywords: Adipose tissue, Endocrine disruptors, Genistein, Triglyceride, Leptin, Vinclozolin
Copyright and License Information
© 2017 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Both distributions of adipose tissues and food intake differ between males and females, and steroid hormones appear to be important in these differences.
1-3
The number of adipocytes at birth and their functional characteristics depend on endocrine and nutritional conditions during in utero development.
4
In addition, the adipose tissue synthesizes leptin, which plays an important role in the control of fat reserve, food intake and satiety.
5,6
However, recent studies in rodents have revealed that the exposure to some dietary and environmental EDs could lead to later fat depot enlargement, suggesting the influence of EDs on the development of fatty mass, mainly in prenatal period.
7,8
EDs can indirectly act through changes in maternal endocrine status (i.e. plasma hormone levels) and/or nutritional behavior.
9
Therefore, this can predispose newborns to nutritional and endocrine imbalances, adipocyte activity dysfunction, obesity and related disorders.
10
Also, EDs like bisphenol A can act directly as agonists on peroxisome proliferator-activated receptors (PPARγ), a family of transcription factors, and disrupt physiological functions of adipocytes.
11
In the same way, other EDs like Mono-(2-ethylhexyl) phthalate (MEHP), Diethylstilbestrol (DES) and 4-Nonylphenol (4-NP) can act on 3T3-L1 differentiation, and activate the expression of estrogen receptor (ER) and PPARγ.
12-14
G and V are two EDs well known for their estrogenic and anti-androgenic properties respectively. These molecules are able to mimic or to disturb the action of endogenous hormones, particularly those implicated in reproduction.
15-17
G and V can also modulate food behavior and body weight.
15
V causes alterations in consumption of saccharin-flavored solutions.
18
In addition, G inhibits the differentiation of adipocyte which is accompanied by a reduction of lipids accumulation in dose a dependent manner,
16,19
mainly in early stages of adipocyte development.
20
G inhibits the glucose metabolism responsible for decreasing of leptin release.
21
It is also able to counteract the anti-lipolytic action of insulin, which may contribute to the decrease of TG accumulation in adipose tissue.
22
The effects of V are not well described; recently we have shown the effect of V on sweet taste preference.
23
Our results are in concordance with the results published by Flynn et al.
18
Additionally, flutamide (known as to be another anti-androgen drug) increases leptin levels in treated women.
24
In everyday life, we are not exposed to just one ED, but rather simultaneously to mixtures of EDs, generally at low levels. To the best of our knowledge, the effects of the combination of G and V at low doses have been poorly investigated, we have already shown in vivo studies that EDs mixtures (G and V) can induce more pronounced effects on salivary glands morphology and gene expression,
25
testis transcriptome,
26
mammary gland development and ERα, AR expression,
27
and gene expression involved in muscle development, differentiation and morphogenesis of mammary gland.
28
In the same way and from literature, Vilela et al, have shown that in utero exposure to the G at realistic daily exposure cause hypospadias in a mouse model. Also, the addition of the V at a dose below that which cause observable effects increases hypospadias rate.
29
Here, we used 3T3-L1 to identify and compare the low dose effects of the phytoestrogen G alone or in the mixture, with an anti-androgen V on the differentiation and endocrine activity (TG and leptin) of 3T3-L1 pre-adipocytes.
Materials and Methods
Molecules exposure
G was synthesized and provided by Pr. B. Bennetau from the Laboratory of Organic and Organometallic Chemistry (UMR 5802 CNRS-University of Bordeaux I, France), with a purity of 99%. It was obtained by demethylation of biochanin A, and purified by liquid chromatography on a silica gel column.
30
Vinclozolin (V) was provided by Dr. Cravedi from the UMR 1089 Xenobiotics (INRA Toulouse, France). It was purified from the commercial product (RonilanTM). Its purity was verified by HPLC-diode-array (from 192 to 400 nm) and gas chromatography (GC) coupled to mass spectrometer (MS). Results showed purity greater than 96%.
31
Doses used
Doses used were chosen on the basis of results obtained from our previous study,
20
and from cytotoxic assays. The concentrations selected for these experiments were: 25 µM G alone or in combination with: 0.1 µM or 1 µM or 10 µM of V.
V 10 µM: this concentration was also used alone.
Materials
The cell culture media Dulbecco's Modified Eagle's Medium (DMEM) high glucose concentration (4.5 g/L), the GlutaMAXTM, sodium pyruvate, PBS (Phosphate-Buffered Saline), bovine insulin and trypsin solution obtained from Invitrogen (Cergy Pontoise, France). The foetal calf serum gold (with hormones), and dialysis (without hormones) are provided by PAA Laboratories (Mureaux, France). Consumable plastic (culture flasks, multi-well plates, pipettes) come from BD (BD Biosciences, Pont de Claix, France). The dimethylsulfoxide (DMSO), neutral red, oil-red O (ORO), 3-isobutyl-1-methylxanthine (IBMX), dexamethasone and calf thymus DNA solution was supplied by Sigma-Aldrich (Saint Quentin Fallavier, France). The ELISA kit to assay mouse leptin (Mediagnost, Germany) and the kit used for the enzymatic assay of triglycerides (TG PAP 150) products of Biomérieux (Craponne, France).
Cell culture
The cell line 3T3-L1 mouse embryo fibroblasts was obtained from the American Type Culture Collection (ATCC-LGC Promchem, Molsheim, France). The cells are grown in T75 flasks containing 10 ml of DMEM medium with high concentration of glucose supplemented with GlutaMAXTM (2 mM), sodium pyruvate (1 mM), and Fetal bovine serum (FBS) gold (10%, v/v). They are incubated at 37 °C under humid atmosphere with 10.2% (v/v) CO2 to maintain a physiological pH of 7.35. The culture medium was renewed every 48 hours until a confluent monolayer. Until the ninth passage, after treatment with trypsin, the cells were suspended in medium DMEM supplemented with dialyzed serum (10%; v/v). This dialyzed serum makes it possible to be free from any residual hormonal which can affect adipocytes differentiation. They are then sown with a density of 25 000 cells/well in plates 24 wells, the medium was renewed every 48 hours. To induce differentiation at day 0 cells were treated with the hormonal cocktail of 0.5 mM IBMX, 0.25 µM dexamethasone and insulin 1 µg/mL for 48 hours.
Two days later (day 2), the medium of differentiation was replaced by a medium supplemented with dialyzed serum (10%; v/v) and of insulin 1 µg/mL during 2 days more. Then from day 4 until day 8, the medium is only supplemented with serum dialyzed (10%; v/v) and changed every 2 days. In the aim to study EDs effects on the early stages of adipogenesis, cells were treated simultaneously with the differentiation mix (IBMX, dexamethasone and insulin) from day 0 to day 2. G and/or V were dissolved in DMSO 0.1% (v/v).
Cytotoxicity assay
Cell viability was determined by the neutral red assay.
32
The fluorescence of each well was read (λexcitation = 535 nm / λemission = 580 nm) with VICTOR3V (Perlin-Elmer, Courtaboeuf, France). Results were expressed as the percentage of viable control cells (co-treated with 0.1% DMSO) at the end of the induction period (day 2). A compound was considered cytotoxic if the number of viable cells after EDs exposure was less than or equal to 75%.
Cellular DNA content
The cellular DNA content was determined by the DABA (Diaminobenzoic acid dihydrochloride) fluoremitric assay using calf thymus DNA as standard.
33
The fluorescence of each well was determined (λexcitation=405 nm, λemission=485 nm) with fluorilite 1000 spectrofluorimeter (Dynatech, Hauts-de-Seine, France).
Oil Red O staining
The lipids synthesised and accumulated by the adipocyte during its maturation can be visualised after specific colouring by red oil.
34
The cells are rinsed with 1 mL PBS and fixed for 2 minutes with 500 µL para-formaldehyde (3.7%; m/v) in PBS. They are then incubated with 0.5 mL/well of red oil solution (0.5%; m/v) during 30 minutes at ambient temperature. After 2 rinsing by PBS (1 mL/well), the wells are photographed to visualize the intensity of coloring. Then, the addition of isopropyl alcohol (0.5 mL/well) and agitation during 15 minutes allows red oil extraction from the vacuoles of the adipocyte. The red quantity of salted out oil is measured by spectrophotometry (λ = 590 Nm). The oil red concentration was related to cells number which is represented by cellular DNA content after DABA assay to take into account cellular proliferation and toxicity of our molecules.
Triglyceride assay
The intracellular TGs content was determined with TG PAP 150 enzymatic kit (BioMérieux, France) according to the manufacturer’s instructions. The concentration of TG is measured by spectrophotometry (λ = 510 nm). The TG content was related to cells number which is represented by cellular DNA content after DABA assay to take into account cellular proliferation and toxicity of our molecules.
Leptin assay
According to the manufacturer’s instructions, leptin presence in the culture media was determined on day 8 after 48 hours using mediagnost mouse leptin ELISA kit. The concentration of leptin was measured by a spectrophotometer (λ = 450 nm, filter reference = 590 nm). The leptin concentration was related to cells number which is represented by cellular DNA content after DABA assay to take into account cellular proliferation and toxicity of our molecules.
Statistical analysis
The results were expressed in the form of the mean ± standard error of mean (SEM). DNA content, intracellular TGs amounts, the proportion of Oil-red O-positive cells per well and leptin concentration were measured in triplicates for each treatment and in three separate experiments. Also, to take into account cellular proliferation and toxicity of our molecules, results are normalized to the number of viable cells.
All results were analyzed on the basis of a comparison of the mean and an analysis of variance (ANOVA), followed by the test of Dunnett, when the conditions of normality and homogeneity of the variances are observed, or by the nonparametric test of multiple comparison of Kruskal-Wallis followed by the Newman–Keuls test if necessary.
Statistical significance was considered at P < 0.05 using Statistica software version 10 (StatSoft, Maisons-Alfort, France).
Results
Our results confirm our hypothesis: - decrease observed through the amount of DNA - disruption of the endocrine activity (TG and leptin synthesis).
3T3-L1 viability
Two days after induction of adipocyte differentiation, we can observe that our compounds added individually (genistein 25 µM, vinclozolin 10 µM) or in combination (25 µM +0.1 µM/ 25 µM+1 µM/ 25 µM+10 µM genistein and vinclozolin respectively) for two days have not affected cell viability (viability >75%) (Supplementary file).
Cellular DNA containing
Treatments with EDs were added for 2 days in the early phase of adipocyte differentiation, and we can observe that our compounds have not induced apoptosis at day 8. However, the treatments combination induced a decrease in cellular DNA content (P < 0.05) (Fig. 1). V increased the apoptotic properties of G. Our treatments do not have any effects at day 2 (data not shown).
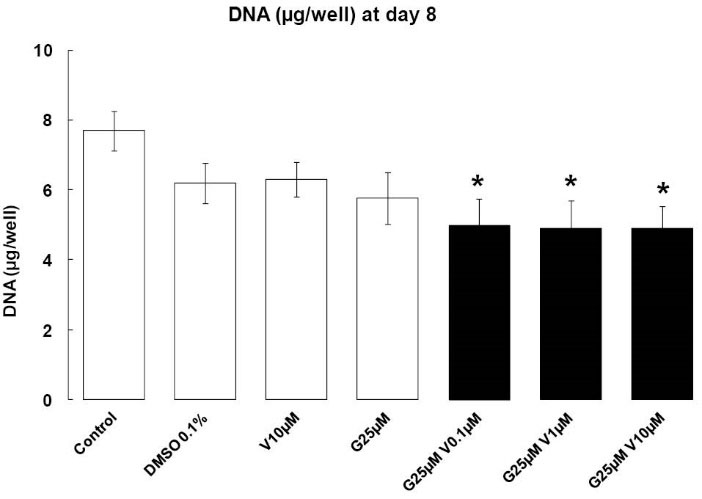
Fig. 1.
Cellular DNA containing. Effects of endocrine disruptors
treatments on DNA quantification (µg/well) in 3T3-L1 at day 8th of
culture; Treatments were administered for 2 days simultaneously
to the Mix of differentiation induction. Each bar represent the
mean of DNA quantity ± SEM. Means which are denoted by *
differ from the DMSO group 0.1% (v/v) (Dunnett, P<0.05) (n = 3
independent experiments in triplicate)
.
Cellular DNA containing. Effects of endocrine disruptors
treatments on DNA quantification (µg/well) in 3T3-L1 at day 8th of
culture; Treatments were administered for 2 days simultaneously
to the Mix of differentiation induction. Each bar represent the
mean of DNA quantity ± SEM. Means which are denoted by *
differ from the DMSO group 0.1% (v/v) (Dunnett, P<0.05) (n = 3
independent experiments in triplicate)
Oil Red staining
With our treatment, we were not able to see any change at day 2 (data not shown). At eight days post-induction, the appearance of rounded, lipid droplet-containing cells occurred in every well. However, in genistein-treated wells (singulary or in a mixture), a low proportion of cells still presented a fibroblastic-like undifferentiated morphology, and differentiated adipocytes contained smaller vesicles than in control, DMSO and V alone wells (Fig. 2). After oil-red extraction, we can observe a tendency lipid decrease accumulation with G alone or in mixture with V 10 µM (Fig. 3), the statistics results are not significant.
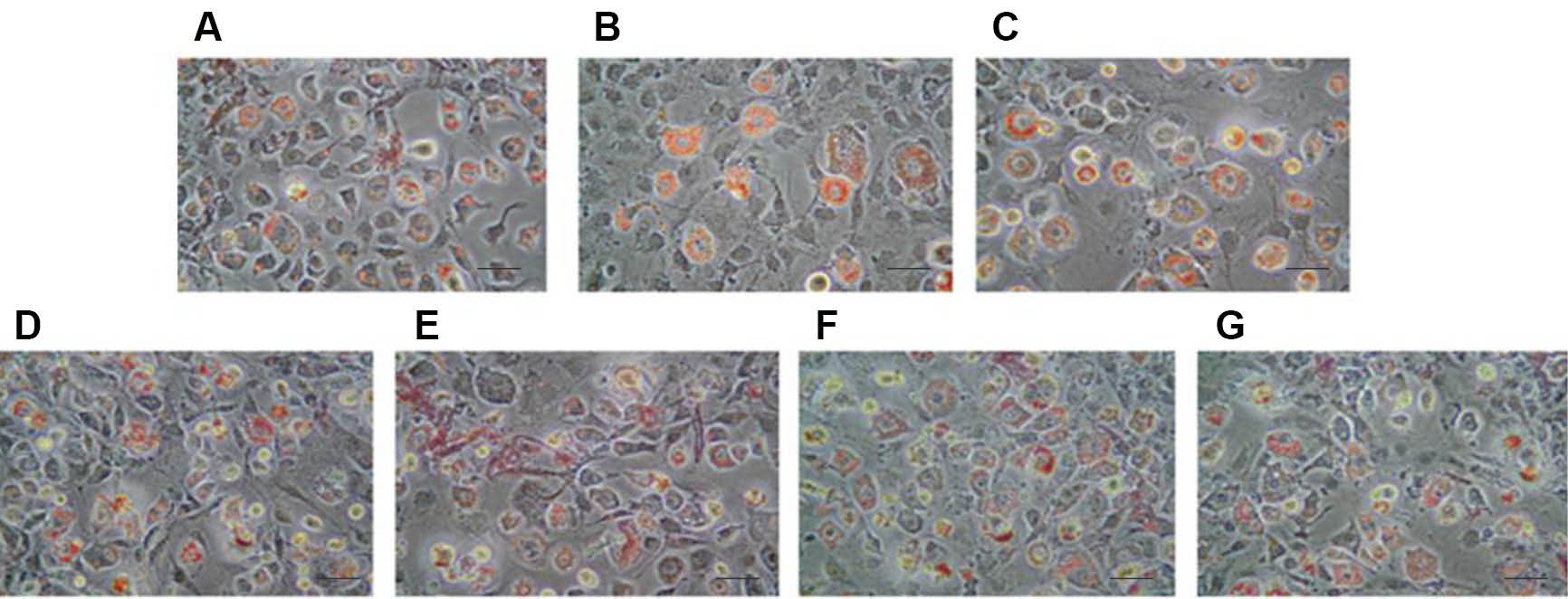
Fig. 2.
Morphological aspects of differentiated 3T3-L1 (Oil red O staining). Effects of endocrine disruptor’s treatments on the
morphological aspects of differentiated 3T3-L1 cells at the day 8 of culture (Determined by Oil red O staining of the cells, magnification
×200, and scale bar represents 100 µm). Treatments were administered for 2 days simultaneously to the Mix of differentiation induction.
(A) Control; (B) DMSO 0.1%; (C) V10 µM; (D) G25 µM; (E) G25 µM, V 0.1 µM; (F) G25 µM, V1 µM; (G) G25 µM, V10 µM.
.
Morphological aspects of differentiated 3T3-L1 (Oil red O staining). Effects of endocrine disruptor’s treatments on the
morphological aspects of differentiated 3T3-L1 cells at the day 8 of culture (Determined by Oil red O staining of the cells, magnification
×200, and scale bar represents 100 µm). Treatments were administered for 2 days simultaneously to the Mix of differentiation induction.
(A) Control; (B) DMSO 0.1%; (C) V10 µM; (D) G25 µM; (E) G25 µM, V 0.1 µM; (F) G25 µM, V1 µM; (G) G25 µM, V10 µM.
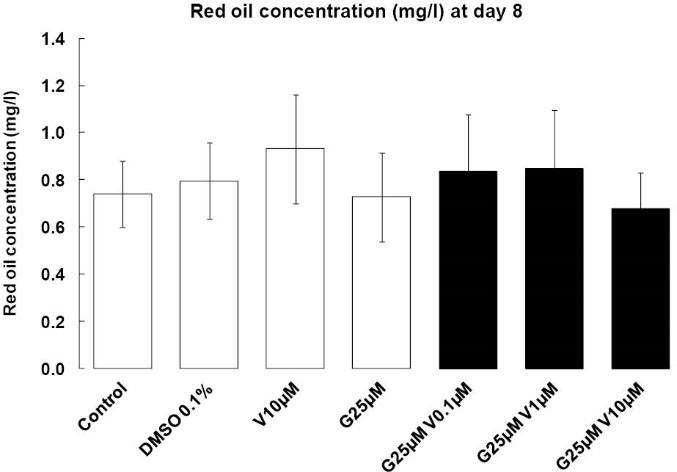
Fig. 3.
Oil Red O staining. Effects of endocrine disruptors
treatments on the proportion of Oil-red O (mg/L) by 3T3-L1 cell
at the day 8th of culture. Treatments were administered for 2
days simultaneously to the Mix of differentiation induction. Each
bar represents the mean ± SEM. Means which are denoted by *
differ from the DMSO group 0.1% (v/v) (Dunnett, P<0.05). (n = 3
independent experiments in triplicate).
.
Oil Red O staining. Effects of endocrine disruptors
treatments on the proportion of Oil-red O (mg/L) by 3T3-L1 cell
at the day 8th of culture. Treatments were administered for 2
days simultaneously to the Mix of differentiation induction. Each
bar represents the mean ± SEM. Means which are denoted by *
differ from the DMSO group 0.1% (v/v) (Dunnett, P<0.05). (n = 3
independent experiments in triplicate).
TG accumulation
When 3T3-L1 was treated with G 25 µM individually, TG accumulation decreased at day 8 after induction of differentiation (P < 0.05), but V 10 µM had no effects. When the 2 compounds were added in combination, TG accumulation decreased (P < 0.05), and V increased the anti-adipogenic properties of G (Fig. 4).
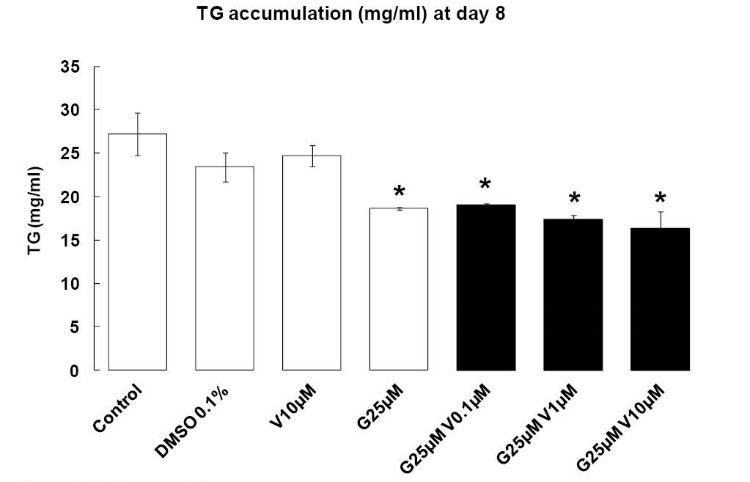
Fig. 4.
TG accumulation. Effects of endocrine disruptors
treatments on triglycerides synthesis (mg/L) by 3T3-L1 cell at
the day 8th of culture. Treatments were administered for 2 days
simultaneously to the Mix of differentiation induction. Each bar
represents the mean ± SEM. Means which are denoted by *
differ from the DMSO group 0.1% (v/v) (Dunnett, P<0.05). (n = 3
independent experiments in triplicate).
.
TG accumulation. Effects of endocrine disruptors
treatments on triglycerides synthesis (mg/L) by 3T3-L1 cell at
the day 8th of culture. Treatments were administered for 2 days
simultaneously to the Mix of differentiation induction. Each bar
represents the mean ± SEM. Means which are denoted by *
differ from the DMSO group 0.1% (v/v) (Dunnett, P<0.05). (n = 3
independent experiments in triplicate).
The increase of apoptosis by G and V was associated with a decrease in TG accumulation.
Leptin synthesis
Leptin concentration was measured in the serum on day 8, it is considered as a late indicator of adipocyte maturation, and a key mediator of adipose tissue endocrine function. When compounds were added individually, or in combination with the low concentration of V (0.01 µM and 1 µM) we have not seen any effects. But G at 25 µM combined with V at 10 µM lead to an increase of leptin concentration compared to DMSO group (P < 0.05) (Fig. 5).
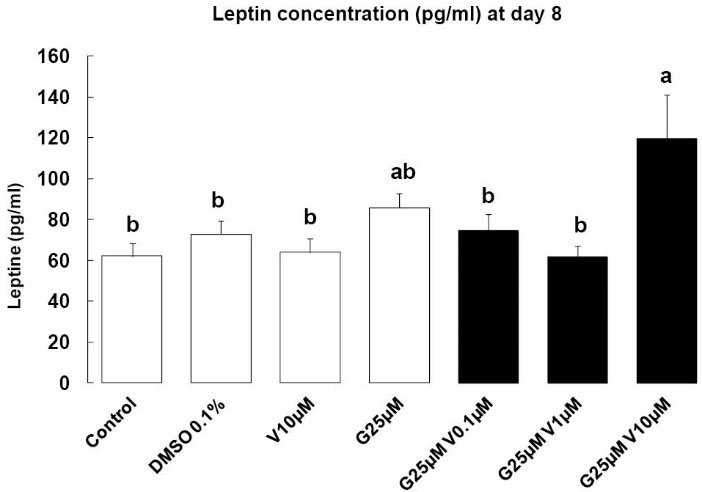
Fig. 5.
Leptin syntheses. Effects of endocrine disruptors
treatments on leptin synthesis (pg/mL) by 3T3-L1 cell at the
day 8th of culture. Treatments were administered for 2 days
simultaneously to the mix of differentiation induction. Each
bar represents the mean ± SEM. Means which are denoted
by: different letters (a, b) indicate significantly different values
(Newman-Keuls, P<0.05). (n = 3 independent experiments in
triplicate)
.
Leptin syntheses. Effects of endocrine disruptors
treatments on leptin synthesis (pg/mL) by 3T3-L1 cell at the
day 8th of culture. Treatments were administered for 2 days
simultaneously to the mix of differentiation induction. Each
bar represents the mean ± SEM. Means which are denoted
by: different letters (a, b) indicate significantly different values
(Newman-Keuls, P<0.05). (n = 3 independent experiments in
triplicate)
Discussion
In our study, EDs treatments (singularly or in mixtures) affected the differentiation and the endocrine activity (synthesis of TG and leptin) of 3T3-L1 cells.
G at 100 μM inhibits the proliferation of postconfluent pre-adipocytes, blocks differentiation, and activates lipolysis when administered in early adipocyte differentiation.
35
The anti-adipogenic effects of G can be observed at low concentrations, for example at 25 μM G can decrease significantly the synthesis of TG by the 3T3-L1.
36
The effect of G (estrogenic compound) on lipogenesis is associated with inhibition of the activity of glycerol-3-phosphate dehydrogenase, transcriptional regulation of fatty acid synthase, the lipoprotein-lipase, and leptin.
37
Concerning our experimental conditions, the G at 25 μM did not affect the synthesis of leptin in 3T3-L1.
V administered alone during the induction phase of differentiation did not affect 3T3-L1 differentiation or the ability of adipocytes to synthesise leptin or TG. In contrast, the mixture produced an anti-adipogenic from the first 48 hours, which depends on V concentrations. This effect wsa the same when treated with G at 100 μM.
20
This could be related to hormonal effects of V metabolites. In the rat, V is metabolized into 6 metabolites: M1, M2, M3, M4, M5, and M6, with anti-androgenic properties described for the M1 and M2 but also M2, is partial agonist in androgen receptor (AR). Such as V, the 2 metabolites M1 and M2 are in addition agonists to estrogen receptor (ER), with a slight affinity to the ERβ.
17,31
This could explain the amplification of anti-adipogenic effect of G in our study.
The effects of mixtures are described with flavonoids. At low doses of G, quercetin (Q) and resveratrol (R), there was no effect on differentiation and the endocrine activity of 3T3-L1. The combination of these molecules shows an anti-adipogenic effect.
16
In our conditions, the endocrine activity of adipocytes is not affected by the molecules alone, but with the mixtures G at 25 µM and V at 10 µM increase leptin synthesis. These effects probably result from an estrogenic action, because estradiol and dexamethasone have a stimulating effect on the leptin synthesis.
38
Leptin synthesis was increased in the cells exposed to G + V. It seems unlikely, knowing the very short half-life of leptin mRNA in vitro (2 hours),
39
that these effects observed at day 8, a result of a direct transcriptional activation by the treatment during the first 2 days of differentiation. Thus, these alterations occur at a critical stage of cell maturation, which may affect some endocrine functions in a persistent way.
On the whole, our results show that EDs mixtures (G and V) can induce more pronounced effects on 3T3-L1 cells. These results are in agreement with the results obtained in "in vivo" studies conducted previously using the same molecules (G and /or V), and several targets have been analysed: salivary glands morphology,
25
testis transcriptome,
26
mammary gland development.
27
Conclusion
Our study shows G and/or V effect, on one of the crucial factors involved in regulation of food intake and sweet taste modulation (leptin).
Also in this study, we show that EDs in mixtures at doses close to the no-observed-adverse-effect level (NOAEL) may affect the differentiation and the endocrine activity of adipose tissue (TG, and leptin synthesis).
Our data suggest that dietary and environmental EDs in mixtures can lead to an abnormal food behavior, associated with subsequent morbidity (obesity, diabetes, metabolic syndromes, etc).
The results are all in vitro data, effects of G and V on adipocytes need to be evaluated in vivo, and additional studies are necessary to establish the mechanisms by which EDs mixtures can affect leptin synthesis and 3T3-L1 differentiation.
Ethical approval
There is none to be declared.
Competing interests
The authors declare no conflict interests.
Acknowledgment
We thank the members of the relevant thesis committees, namely JP Cravedi (INRA Toxalim, Toulouse) and P Besnard (INSERM U866, Dijon), for their fruitful discussions.
Supplementary materials
Supplementary file contains Fig. S1.
()
Research Highlights
What is current knowledge?
simple
-
√ Endocrine disruptors (EDs) can predispose newborns to
endocrine dysfunction of adipocytes, obesity, and related
disorders.
-
√ EDs mixtures can induce more pronounced effects on
reproductive system.
What is new here?
simple
-
√ Genistein and/or vinclozolin can affect 3T3-L1 differentiation.
-
√ Genistein and/or vinclozolin can affect 3T3-L1 leptin
synthesis.
-
√ Genistein and/or vinclozolin at low doses close to the
NOAEL can affect 3T3-L1 development.
References
- Ferguson SA, Delclos KB, Newbold RR, Flynn KM. Dietary ethinyl estradiol exposure during development causes increased voluntary sodium intake and mild maternal and offspring toxicity in rats. Neurotoxicol Teratol 2003; 25:491-501. [ Google Scholar]
- Geary N. Estradiol, CCK and satiation. Peptides 2001; 22:1251-63. [ Google Scholar]
- Curtis KS, Davis LM, Johnson AL, Therrien KL, Contreras RJ. Sex differences in behavioral taste responses to and ingestion of sucrose and NaCl solutions by rats. Physiol Behav 2004; 80:657-64. [ Google Scholar]
- Symonds ME, Mostyn A, Pearce S, Budge H, Stephenson T. Endocrine and nutritional regulation of fetal adipose tissue development. J Endocrinol 2003; 179:293-9. [ Google Scholar]
- Kawai K, Sugimoto K, Nakashima K, Miura H, Ninomiya Y. Leptin as a modulator of sweet taste sensitivities in mice. Proc Natl Acad Sci U S A 2000; 97:11044-9. [ Google Scholar]
- Rocha M, Bing C, Williams G, Puerta M. Physiologic estradiol levels enhance hypothalamic expression of the long form of the leptin receptor in intact rats. J Nutr Biochem 2004; 15:328-34. doi: 10.1016/j.jnutbio.2004.01.003 [Crossref] [ Google Scholar]
- Boudalia S, Berges R, Chabanet C, Folia M, Decocq L, Pasquis B. A multi-generational study on low-dose BPA exposure in Wistar rats: effects on maternal behavior, flavor intake and development. Neurotoxicol Teratol 2014; 41:16-26. doi: 10.1016/j.ntt.2013.11.002 [Crossref] [ Google Scholar]
- Miyawaki J, Sakayama K, Kato H, Yamamoto H, Masuno H. Perinatal and postnatal exposure to bisphenol a increases adipose tissue mass and serum cholesterol level in mice. J Atheroscler Thromb 2007; 14:245-52. [ Google Scholar]
- Newbold RR, Padilla-Banks E, Snyder RJ, Jefferson WN. Perinatal exposure to environmental estrogens and the development of obesity. Mol Nutr Food Res 2007; 51:912-7. doi: 10.1002/mnfr.200600259 [Crossref] [ Google Scholar]
- Bispham J, Gopalakrishnan GS, Dandrea J, Wilson V, Budge H, Keisler DH. Maternal endocrine adaptation throughout pregnancy to nutritional manipulation: consequences for maternal plasma leptin and cortisol and the programming of fetal adipose tissue development. Endocrinology 2003; 144:3575-85. doi: 10.1210/en.2003-0320 [Crossref] [ Google Scholar]
- Riu A, Grimaldi M, le Maire A, Bey G, Phillips K, Boulahtouf A. Peroxisome proliferator-activated receptor gamma is a target for halogenated analogs of bisphenol A. Environ Health Perspect 2011; 119:1227-32. doi: 10.1289/ehp.1003328 [Crossref] [ Google Scholar]
- Hao C, Cheng X, Xia H, Ma X. The endocrine disruptor mono-(2-ethylhexyl) phthalate promotes adipocyte differentiation and induces obesity in mice. Biosci Rep 2012; 32:619-29. doi: 10.1042/BSR20120042 [Crossref] [ Google Scholar]
- Hao CJ, Cheng XJ, Xia HF, Ma X. The endocrine disruptor 4-nonylphenol promotes adipocyte differentiation and induces obesity in mice. Cell Physiol Biochem 2012; 30:382-94. doi: 10.1159/000339032 [Crossref] [ Google Scholar]
- Hao CJ, Cheng XJ, Xia HF, Ma X. The endocrine disruptor diethylstilbestrol induces adipocyte differentiation and promotes obesity in mice. Toxicol Appl Pharmacol 2012; 263:102-10. doi: 10.1016/j.taap.2012.06.003 [Crossref] [ Google Scholar]
- Flynn KM, Ferguson SA, Delclos KB, Newbold RR. Effects of genistein exposure on sexually dimorphic behaviors in rats. Toxicol Sci 2000; 55:311-9. [ Google Scholar]
- Park HJ, Yang JY, Ambati S, Della-Fera MA, Hausman DB, Rayalam S. Combined effects of genistein, quercetin, and resveratrol in human and 3T3-L1 adipocytes. J Med Food 2008; 11:773-83. doi: 10.1089/jmf.2008.0077 [Crossref] [ Google Scholar]
- Molina-Molina JM, Hillenweck A, Jouanin I, Zalko D, Cravedi JP, Fernandez MF. Steroid receptor profiling of vinclozolin and its primary metabolites. Toxicol Appl Pharmacol 2006; 216:44-54. doi: 10.1016/j.taap.2006.04.005 [Crossref] [ Google Scholar]
- Flynn KM, Delclos KB, Newbold RR, Ferguson SA. Behavioral responses of rats exposed to long-term dietary vinclozolin. J Agric Food Chem 2001; 49:1658-65. [ Google Scholar]
- Kandulska K, Nogowski L, Szkudelski T. Effect of some phytoestrogens on metabolism of rat adipocytes. Reprod Nutr Dev 1999; 39:497-501. [ Google Scholar]
- Phrakonkham P, Viengchareun S, Belloir C, Lombes M, Artur Y, Canivenc-Lavier MC. Dietary xenoestrogens differentially impair 3T3-L1 preadipocyte differentiation and persistently affect leptin synthesis. J Steroid Biochem Mol Biol 2008; 110:95-103. doi: 10.1016/j.jsbmb.2008.02.006 [Crossref] [ Google Scholar]
- Szkudelski T, Nogowski L, Pruszynska-Oszmalek E, Kaczmarek P, Szkudelska K. Genistein restricts leptin secretion from rat adipocytes. J Steroid Biochem Mol Biol 2005; 96:301-7. doi: 10.1016/j.jsbmb.2005.04.033 [Crossref] [ Google Scholar]
- Szkudelska K, Nogowski L, Szkudelski T. Genistein, a plant-derived isoflavone, counteracts the antilipolytic action of insulin in isolated rat adipocytes. J Steroid Biochem Mol Biol 2008; 109:108-14. doi: 10.1016/j.jsbmb.2007.12.006 [Crossref] [ Google Scholar]
- Kouidhi W, Berges R, Tiffon C, Desmetz C, El May M, Auger J. Perinatal xenohormone exposure impacts sweet preference and submandibular development in male rats. Oral Dis 2013; 19:812-23. doi: 10.1111/odi.12078 [Crossref] [ Google Scholar]
- Sahin I, Serter R, Karakurt F, Demirbas B, Guler S, Culha C. Leptin levels increase during flutamide therapy in women with polycystic ovary syndrome. Horm Res 2003; 60:232-6. [ Google Scholar]
- Kouidhi W, Desmetz C, Nahdi A, Berges R, Cravedi JP, Auger J. In utero and lactational exposure to low-dose genistein-vinclozolin mixture affects the development and growth factor mRNA expression of the submandibular salivary gland in immature female rats. Toxicol Pathol 2012; 40:593-604. doi: 10.1177/0192623311436183 [Crossref] [ Google Scholar]
- Eustache F, Mondon F, Canivenc-Lavier MC, Lesaffre C, Fulla Y, Berges R. Chronic dietary exposure to a low-dose mixture of genistein and vinclozolin modifies the reproductive axis, testis transcriptome, and fertility. Environ Health Perspect 2009; 117:1272-9. doi: 10.1289/ehp.0800158 [Crossref] [ Google Scholar]
- El Sheikh Saad H, Meduri G, Phrakonkham P, Berges R, Vacher S, Djallali M. Abnormal peripubertal development of the rat mammary gland following exposure in utero and during lactation to a mixture of genistein and the food contaminant vinclozolin. Reprod Toxicol 2011; 32:15-25. doi: 10.1016/j.reprotox.2011.03.001 [Crossref] [ Google Scholar]
- El Sheikh Saad H, Toullec A, Vacher S, Pocard M, Bieche I, Perrot-Applanat M. In utero and lactational exposure to vinclozolin and genistein induces genomic changes in the rat mammary gland. J Endocrinol 2013; 216:245-63. doi: 10.1530/JOE-12-0395 [Crossref] [ Google Scholar]
- Vilela ML, Willingham E, Buckley J, Liu BC, Agras K, Shiroyanagi Y. Endocrine disruptors and hypospadias: role of genistein and the fungicide vinclozolin. Urology 2007; 70:618-21. doi: 10.1016/j.urology.2007.05.004 [Crossref] [ Google Scholar]
- Pelissero C, Bennetau B, Babin P, Le Menn F, Dunogues J. The estrogenic activity of certain phytoestrogens in the Siberian sturgeon Acipenser baeri. J Steroid Biochem Mol Biol 1991; 38:293-9. [ Google Scholar]
- Bursztyka J, Debrauwer L, Perdu E, Jouanin I, Jaeg JP, Cravedi JP. Biotransformation of vinclozolin in rat precision-cut liver slices: comparison with in vivo metabolic pattern. J Agric Food Chem 2008; 56:4832-9. doi: 10.1021/jf0728045 [Crossref] [ Google Scholar]
- Rat P, Korwin-Zmijowska C, Warnet JM, Adolphe M. New in vitro fluorimetric microtitration assays for toxicological screening of drugs. Cell Biol Toxicol 1994; 10:329-37. [ Google Scholar]
- Kissane JM, Robins E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem 1958; 233:184-8. [ Google Scholar]
- Vankoningsloo S, Piens M, Lecocq C, Gilson A, De Pauw A, Renard P. Mitochondrial dysfunction induces triglyceride accumulation in 3T3-L1 cells: role of fatty acid beta-oxidation and glucose. J Lipid Res 2005; 46:1133-49. doi: 10.1194/jlr.M400464-JLR200 [Crossref] [ Google Scholar]
- Harmon AW, Harp JB. Differential effects of flavonoids on 3T3-L1 adipogenesis and lipolysis. Am J Physiol Cell Physiol 2001; 280:C807-13. [ Google Scholar]
- Rayalam S, Della-Fera MA, Yang JY, Park HJ, Ambati S, Baile CA. Resveratrol potentiates genistein's antiadipogenic and proapoptotic effects in 3T3-L1 adipocytes. J Nutr 2007; 137:2668-73. [ Google Scholar]
- Park HJ, Della-Fera MA, Hausman DB, Rayalam S, Ambati S, Baile CA. Genistein inhibits differentiation of primary human adipocytes. J Nutr Biochem 2009; 20:140-8. doi: 10.1016/j.jnutbio.2008.01.006 [Crossref] [ Google Scholar]
- Kristensen K, Pedersen SB, Richelsen B. Regulation of leptin by steroid hormones in rat adipose tissue. Biochem Biophys Res Commun 1999; 259:624-30. doi: 10.1006/bbrc.1999.0842 [Crossref] [ Google Scholar]
- Leroy P, Dessolin S, Villageois P, Moon BC, Friedman JM, Ailhaud G. Expression of ob gene in adipose cells. Regulation by insulin J Biol Chem 1996; 271:2365-8. [ Google Scholar]