BioImpacts. 7(1):49-57.
doi: 10.15171/bi.2017.07
Review
Perspective highlights on biodegradable polymeric nanosystems for targeted therapy of solid tumors
Marziyeh Fathi 1, Jaleh Barar 1, 2, *
Author information:
1Research Center for Pharmaceutical Nanotechnology, Tabriz University of Medical Sciences, Tabriz, Iran
2Department of Pharmaceutics, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
Polymeric nanoparticles (NPs) formulated using biodegradable polymers offer great potential for development of de novo drug delivery systems (DDSs) capable of delivering a wide range of bioactive agents. They can be engineered as advanced multifunctional nanosystems (NSs) for simultaneous imaging and therapy known as theranostics or diapeutics.
Methods:
A brief prospective is provided on biomedical importance and applications of biodegradable polymeric NSs through reviewing the recently published literature.
Results:
Biodegradable polymeric NPs present unique characteristics, including: nanoscaled structures, high encapsulation capacity, biocompatibility with non-thrombogenic and non-immunogenic properties, and controlled-/sustained-release profile for lipophilic and hydrophilic drugs. Once administered in vivo, all classes of biodegradable polymers (i.e., synthetic, semi-synthetic, and natural polymers) are subjected to enzymatic degradation; and hence, transformation into byproducts that can be simply eliminated from the human body. Natural and semi-synthetic polymers have been shown to be highly stable, much safer, and offer a non-/less-toxic means for specific delivery of cargo drugs in comparison with synthetic polymers. Despite being biocompatible and enzymatically-degradable, there are some drawbacks associated with these polymers such as batch to batch variation, high production cost, structural complexity, lower bioadhesive potential, uncontrolled rate of hydration, and possibility of microbial spoilage. These pitfalls have bolded the importance of synthetic counterparts despite their somewhat toxicity.
Conclusion:
Taken all, to minimize the inadvertent effects of these polymers and to engineer much safer NSs, it is necessary to devise biopolymers with desirable chemical and biochemical modification(s) and polyelectrolyte complex formation to improve their drug delivery capacity in vivo.
Keywords: Biodegradable polymers, Synthetic and semi-synthetic polymers, Natural polymers, Targeted therapy, Advanced drug delivery systems
Copyright and License Information
© 2017 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Biodegradable polymeric nanoparticles (NPs) and nanosystems (NSs) are deemed to be very efficient drug delivery systems (DDSs) that are extremely safer than any other non-biodegradable polymers and lipids used for gene/drug delivery.
1-4
The biodegradable polymers are also bioactive and hence can be used as polymer-therapeutics,
5-8
which can also be exploited for targeted delivery of a wide range of small and large molecules (e.g., human growth hormone,
9
insulin,
10
anti-tumor agents,
11
contraceptives,
12
vaccines,
13
anticancer drugs,
14
and antibiotics
15
) in a controlled, sustained or pulsatile manner.
16
It should be highlighted that the liberation of encapsulated/incorporated drugs from these polymers can be carefully controlled and the drug concentration in the target site is maintained within the therapeutic window.
17
Biodegradable polymers are considered as ideal biomaterials for the development of controlled-/sustained-release DDSs as well as therapeutic devices such as degradable implants, impermanent prostheses, and degradable 3D scaffolds for tissue engineering. To develop effective therapeutic devices, one needs to use the most compatible biodegradable polymers depending on the endpoint biological uses based on their specific physicochemical, biomechanical and enzymatic/hydrolytic degradation properties.
18
In fact, significant efforts, time and resources are required to engineer biomaterial with unique properties towards development of sophisticated biotherapeutics. Further, the currently implemented biomaterials in various clinical settings need to be revisited to address all the issues associated with application of biopolymers in vivo. Most of these issues are in close association with the physicochemical interaction of the applied biopolymers with the target tissue/cells. Of these, for instance, inadvertent immunologic reactions can dramatically limit their uses while such drawback can be beneficial when the endpoint objective is the activation of immune system by vaccination/immunization. Besides, compelling evidence on long term safety of these materials seems to be necessary. In this review, we highlight the importance of biodegradable polymers and the key issues that have crucially contributed to their limits/slow evolution towards thier applications in biomedical/pharmaceutical fields.
Structural properties of biodegradable polymers
Structurally, biodegradable polymers possess bonds (i.e., ester, amide, or ether bonds) which are cleavable enzymatically or hydrolytically. Based upon their synthesis methodologies, biodegradable polymers can be categorized into (i) natural (e.g., fibrin, collagen, gelatin, cellulose, hyaluronan, pectin), (ii) semisynthetic (e.g., chemically modified natural polymers such as chitosan), and (iii) synthetic [e.g., poly(lactic acid)(PLA), poly(glycolic acid)(PGA), poly(lactic-co-glycolic acid)(PLGA), poly(ℇ-caprolactone)(PCL), poly(dioxanone)(PDO), poly(anhydrides), poly(trimethylene carbonate), poly(ortho esters) and poly(phosphazenes)].
It should be stated that a polymer with a C-C backbone can resist the degradation, while heteroatom-containing polymers can show some degrees of biodegradability depending on the structural properties of the polymer. Hence, inclusion of degradable chemical linkages (e.g., ester, amide and anhydride) can improve the degradation processes. Technically, several intrinsic physicochemical and formulation properties (e.g., structural chemistry, molecular weight, hydrophilicity/hydrophobicity, water absorption, surface charge, type and morphology of formulation, surface modification, and degradation and erosion mechanism) of degradable polymers can affect their compatibility and interaction with biological settings. Fig. 1 illustrates the chemical structures of some selected important biodegradable polymers used in various biomedical applications.
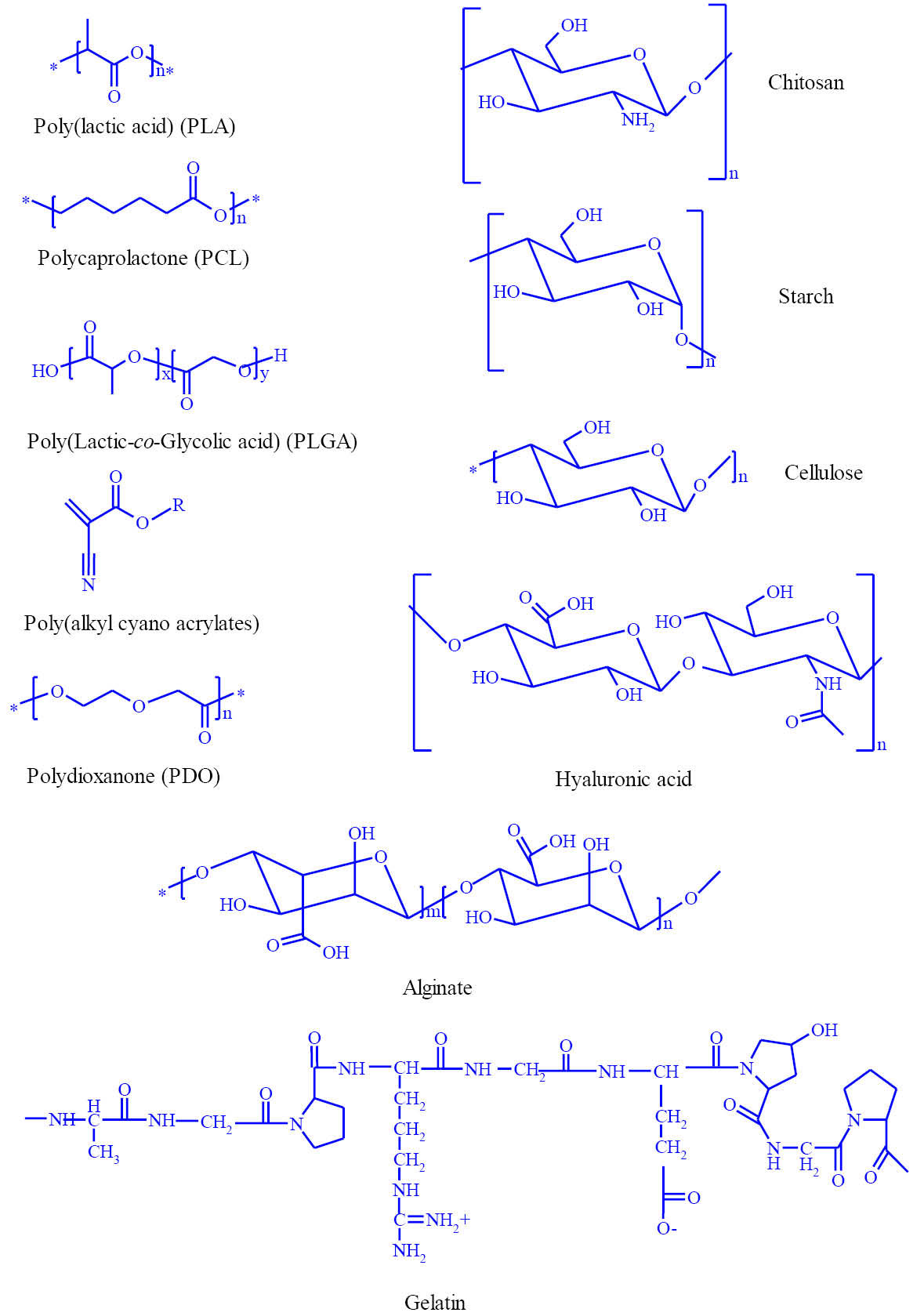
Fig. 1.
Chemical structures of some widely used biodegradable polymers in various biomedical/pharmaceutical applications.
.
Chemical structures of some widely used biodegradable polymers in various biomedical/pharmaceutical applications.
Natural biopolymers
The natural biodegradable polymers, known as biopolymers, encompass various classes of counterparts such as polysaccharides (e.g., starch, cellulose, chitin, chitosan) and naturally existing proteins (e.g., collagen, laminin and fibronectin). Nowadays, many researches have been directed towards the use of natural polymers,
19
which are particularly suitable for medical and pharmaceutical applications due to their biocompatibility and biodegradibility.
20
Fig. 1 epitomizes some commonly used natural biodegradable polymers.
17,21
Natural polymers blending with synthetic polymers (e.g., poly(vinyl alcohol), poly(ethylene oxide), poly(vinyl pyrrolidone)) provide possibility for fabrication of bioartificial/biosynthetic polymers as a new class of advanced materials with improved mechanical properties and biocompatibility in comparison with those of single components. This class of biomaterials can be tailored to adequately mimic human tissue components, and are applicable in cell-based transplantation, tissue engineering and gene therapy.
22
For example, chemically modified hyaluronic acid and gelatin is investigated to deliver the mesenchymal stem cells to repair the osteochondral defects in a rabbit model. After 12 weeks, defects were completely ameliorated with the cartilage and repaired.
23
Synthetic degradable polymers
There exist various synthetic biodegradable polymers such as (poly(hydroxylbutyrate), poly anhydride copolymers, poly(orthoester)s, polyphosphazenes, poly(amidoester)s, poly(cyano acrylate)s and PLGA.
14,24,25
PLGA is a widely used polymer that has been approved by the United State Food and Drug Administration (FDA) for various therapeutic/diagnostic applications. This class of polymers offer enormous potentials as drug carriers, in large part due to their biodegradability, biocompatibility, and possibility for development of sustained-/controlled-/pulsatile-release and targeted delivery.
14,24,26
Table 1 represents some clinical trials that have used PLGA as delivery system. It should be noted that PLGA undergoes hydrolytic degradation in aqueous environment where ester linkages along with the polymer backbone are randomly hydrolyzed. The ratio of lactic acid (LA) to glycolic acid (GA) plays an important role in degradation mechanism of the PLGA. Additionally, degradation rate and accordingly drug release rate can be manipulated by varying the ratio of LA to GA. For instance, PLGA 50:50 degrades at a faster rate in comparison with PLGA 85:15 due to the higher hydrophilic GA content of the copolymer.
25,27-29
The attractive features of PLGA-based NPs/NSs (e.g., small size, high structural integrity, stability, ease of fabrication, tunable properties, sustained-/controlled-release capability, and surface functionalization characteristics) make them versatile therapeutic delivery vehicles. However, there exist some drawbacks for the PLGA-based NPs in terms of physiochemical and biological properties that limit their applications in pharmaceutical/biomedical fields. These pitfalls include (a) poor loading efficiency for hydrophobic drugs, (b) high burst release, (c) uptake by the reticuloendothelial system (RES), (d) poor stability in water, (e) difficulties in producing particles below 100 nm in diameter, (f) less circulation time in the body, (g) aggregation, and (h) manufacturing scale-up issues. To resolve such constraints, the main focus is now on the development of hybrid PLGA NPs.
30-32
Technically, PLGA NPs can be formulated by emulsification–diffusion, solvent emulsion–evaporation, interfacial deposition, or nanoprecipitation methods.
33
However the scale-up process of PLGA NPs’ formulation by means of these methods appears to be costly.
Table 1.
Application of PLGA in clinical trialsa
|
Clinical trial identifier and description
|
Application
|
Drug/Device
|
Phase; status
|
Formulation
|
| NCT02487186: Locally Delivered Doxycycline Adjunct to Nonsurgical Periodontal Therapy |
Periodontal
disease
|
Doxicicline |
Phase IV; completed |
PLGA microspheres |
| NCT02138110: Probable Benefit of the Neuro- Spinal Scaffold for Treatment of AIS A |
Traumatic acute spinal cord injury |
Neuro-Spinal Scaffold |
Phase III;
currently recruiting participants
|
PLGA scaffold |
| NCT00836797: Radiographic Assessment of Bone Regeneration in Alveolar Sockets with PLGA Scaffold After Teeth Extraction |
Assessment of bone regeneration |
Alveolar Sockets |
Phase I;
completed
|
PLGA bioscaffold |
| NCT01729195: Ankle Syndesmosis Fixation by Antibiotic Releasing Bioabsorbable Screw |
Ankle fracture |
A ciprofloxacin containing bioabsorbable PLGA bone screw |
Phase II;
completed
|
PLGA-based bioabsorbable thread (4.5 mm in diameter) |
| NCT02255188: Experimental Study of the Vascular Prosthesis Manufactured by Electrospinning |
Arterial occlusive disease |
Vascular Prosthesis |
Phase I; currently recruiting participants |
Graft type - PLGA/PCL/gelatin poorly permeable layer |
| NCT01681381: Evaluate Safety and Effectiveness of the Tivoli® DES and the Firebird2® DES for Treatment Coronary Revascularization |
Ischemic heart disease; coronary artery lesions; acute coronary syndrome |
Tivoli® DES rapamycin-Eluting stent |
currently recruiting participants |
Stent coated with a biodegradable polymer (PLGA) |
| NCT01753089: Dendritic Cell Activating Scaffold in Melanoma |
Melanoma |
WDVAX |
Phase I; currently recruiting participants |
PLGA scaffold |
| NCT02017275: Comparison of BuMA eG Based Bio Degradable Polymer Stent with EXCEL Biodegradable Polymer Sirolimus-eluting Stent in "Real-World" Practice (PANDA-III) |
Coronary artery disease |
BuMA and EXCEL Stent |
Phase IV; completed |
Biodegradable PLGA coating |
aData were obtained from clinicaltrials.gov on January 2017.
Biodegradable synthetic polymers with three-dimensional scaffolds are widely used in tissue engineering. Ultrasonically blended suspension of cellulose-nanofibers (CNFs) with PLGA have been fabricated and the obtained scaffolds appeared to possess suitable mechanical strength and biocompatibility for the cultivation of NIH 3T3 cells, which have been used in tissue engineering.
34
For example, PLGA coated beta-tricalcium phosphate (β-TCP) scaffold loaded with vascular endothelial growth factor was synthesized and cell proliferation and attachment was investigated. It was found that the scaffold with sustained-and/or localized-release of VEGF could be favorable for bone regeneration in vitro.
35
Semisynthetic degradable polymers
The morphological and chemical modifications of natural polymers produce semisynthetic polymers that are better suited for processing and production of materials with potential of mineralization and conversion to biomass.
36
Chitosan is a semisynthetic natural based polymer that is primarily obtained from chitin, which is the second abundant polysaccharide in nature.
37
Chitosan is obtained by deacetylation of chitin in alkaline condition mostly from the shell waste of shrimps, crabs, krills and lobsters.
21
Chitosan is soluble in 0.1 N acetic acid and is a positively-charged linear polymer, which can be formulated as homogenous NPs through simple mixing of the polymer with negatively-charged drugs or nucleic acids. Chitosan NPs have shown great advantages, in large part due to their non-immunogenicity and possibility of introducing larger size of genes into host cells in comparison with the viral vectors.
20,38-41
To improve the applicability of chitosan and its various derivatives (e.g., carboxylated, thiolated and acylated structures) for pharmaceutical/biomedical applications, they have so far been decorated with various functional groups such as polyelectrolyte/polyionic complexes.
19
Polyelectrolyte complex of chitosan and gelatin hydrogels prepared at pH 6.5 could be optimized for tree-dimensional bioprinting at room temperature.
42
Also, the modified chitosan with diacetate and triacetate is used as novel matrix to sustained release of doxorubicin (DOX). NPs loaded with DOX indicated high encapsulation efficiency, sustained-release pattern and enhanced cellular accumulation. Further, chitosan-based NPs could improve the oral bioavailability of DOX.
43
Targeted therapy of solid tumors
Development and progression of solid tumors are in close relation with Warburg effect and aberrant glucose metabolism through glycolysis resulting in excess production of acidic byproducts whose efflux can deregulate the pH of tumor microenvironment (TME). In solid tumors, cancerous cells upregulate the expression of glucose transporter (GLUT-1) and some key enzymes and transporters (MCT-1, NHE-1, CA IX and H+ pump V-ATPase) to fulfill the high energy requirement. This deviant phenomenon provokes the efflux of protons into extracellular fluid (ECF), acidifying ECF (pH ~6.6), while the pH of cancer cells holds up to about 7.4.
44
These anomalous phenomena form a permissive milieu in favor of cancer cells survival, proliferation and invasion. The cocktail of various enzymes with acidic pH can remodel the extracellular matrix (ECM) that favors (a) the epithelial–mesenchymal transition (EMT) process necessary for survival of cancer cells, (b) migration of cancer cells form its primary setting to distant organs/tissues, and (c) formation of TME with altered interstitium in which the interstitial fluid pressure (IFP) is markedly high opposing the permeation and penetration of anticancer agents into the core of solid tumors.
45
It is believed that, during metastasis, the EMT process assists cancer cells to avoid anoikis through various mechanisms enabling them to circulate within the blood stream or even lymphatic routes and colonize beyond its primary niches.
46,47
Further, neovascularization within solid tumors are often incomplete encompassing non-integrated endothelial cells with pores and gaps (120-1200 nm) between these cells, upon which the tumor vasculature shows an enhanced permeability and retention (EPR) effect.
48,49
This latter phenomenon have widely been used for passive targeting of solid tumors
50
despite opposing impact(s) of high IFP of TME.
51,52
Stimuli-responsive polymers are able to alter their physical properties in response to environmental changes (e.g., temperature, pH, light, ultrasound, etc.), at which they are considered as superior carriers for targeted delivery of drugs to and on-demand drug release at the site of solid tumors.
53
The pH difference of TME with normal tissues seems to be the main driving force for development of pH-sensitive DDSs. Biodegradable pH-responsive polymeric carriers in the form of micelles, vesicles or NPs have great potential to provide selective/on-demand drug release at tumor sites, which can be then rapidly degraded with no/trivial undesired impacts. Poly (β-amino ester) as a biodegradable cationic polymer is used in development of pH-sensitive DDSs. At low levels of pH (≤6.5), the polymer dissolves rapidly and releases the drug.
54
Hydrogels based on PCL, methacrylic acid (MAA), and Pluronic is developed as a potential biodegradable polymer for drug delivery uses. The hydrolytic degradation behavior of hydrogel was shown to be enhanced with the increase of PCL mainly due to the acid cleavage of ester bonds.
55
Biodegradable polymeric micelles composed of PEG and polycarbonate functionalized with disulfide and carboxylic group can be synthesized as pH and redox dual responsive DDS. The DOX-loaded micelles with small particle size and narrow size distribution indicate high drug loading capacity. When NPs were exposed to the endosomal pH of 5.0, DOX release rate was found to be accelerated by at least two-fold. The DOX-loaded micelles showed enhanced cytotoxicity in nude mice bearing BT-474.
56
Hydrophilic thermo-sensitive biodegradable polymeric nanocarriers, as another example of smart DDSs, are collapsed at hyperthermic condition of 42°C which causes greater drug release and may lead to a synergistic effect of chemotherapy and hyperthermia for treatment of solid tumors.
57,58
A new pH-/temperature-sensitive, biocompatible, biodegradable, and injectable hydrogel based on poly(ethylene glycol)-poly(amino carbonate urethane) (PEG-PACU) copolymers is developed for the sustained delivery of human growth hormone (hGH). The prepared copolymer is sol at the low pH and temperature (pH 6.0, 23°C), while it forms gel in the physiological condition (pH 7.4, 37°C). In vivo investigation of the prepared hydrogel confirmed the in situ gel formation and controlled degradation at the injection site.
59
Wang et al prepared a thermo-sensitive hydrogel of chitosan/hydroxypropyl methylcellulose/glycerol and the hydrogel showed the in situ gel formation at physiological condition (pH ranging from 6.8 to 6.9 at 37°C). The synthesized hydrogel indicated low toxicity, good fluidity, thermosensitivity, biodegradability and controlled-release of bovine serum albumin.
60
Furthermore, the biodegradable polymeric carriers have been modified by tumor targeting agents such as specific ligands (e.g. folic acid),
61
antibodies
28
and aptamers
62
to enhance the NPs translocation into tumor cells. PEG-PCL-PEG thermo-sensitive hydrogel containing a tumor-targeted biodegradable folate-poly(ester amine)/DNA complexes has been synthesized and investigated for targeted gene delivery. The hydrogel composite indicates slight cytotoxicity with high transfection efficiency in vitro. The synthesized hydrogel with sustained gene release and local gene delivery could met the demand for the effective tumor-targeted gene delivery system.
63
Taken all these issues into consideration, it seems that the effective therapy of cancer demands specific targeting of the cancerous cells by smart NSs and delivery of anticancer agents to the target cells but not the healthy normal cells. An efficient delivery of anticancer drugs specifically to solid tumors requires implementation of nanocarriers with high payload capacity and suitable permeability and degradability within the TME.
64,65
Advanced biodegradable NSs armed with homing devices have the ability to penetrate into TME and target the cancerous cells solely while they impose no/little effects on the healthy cells and immunosurveillance activity of the immune system.
14,45,
66-68
Fig. 2 represents schematic structures of advanced DDSs and multifunctional NSs used for targeted therapy of cancer.
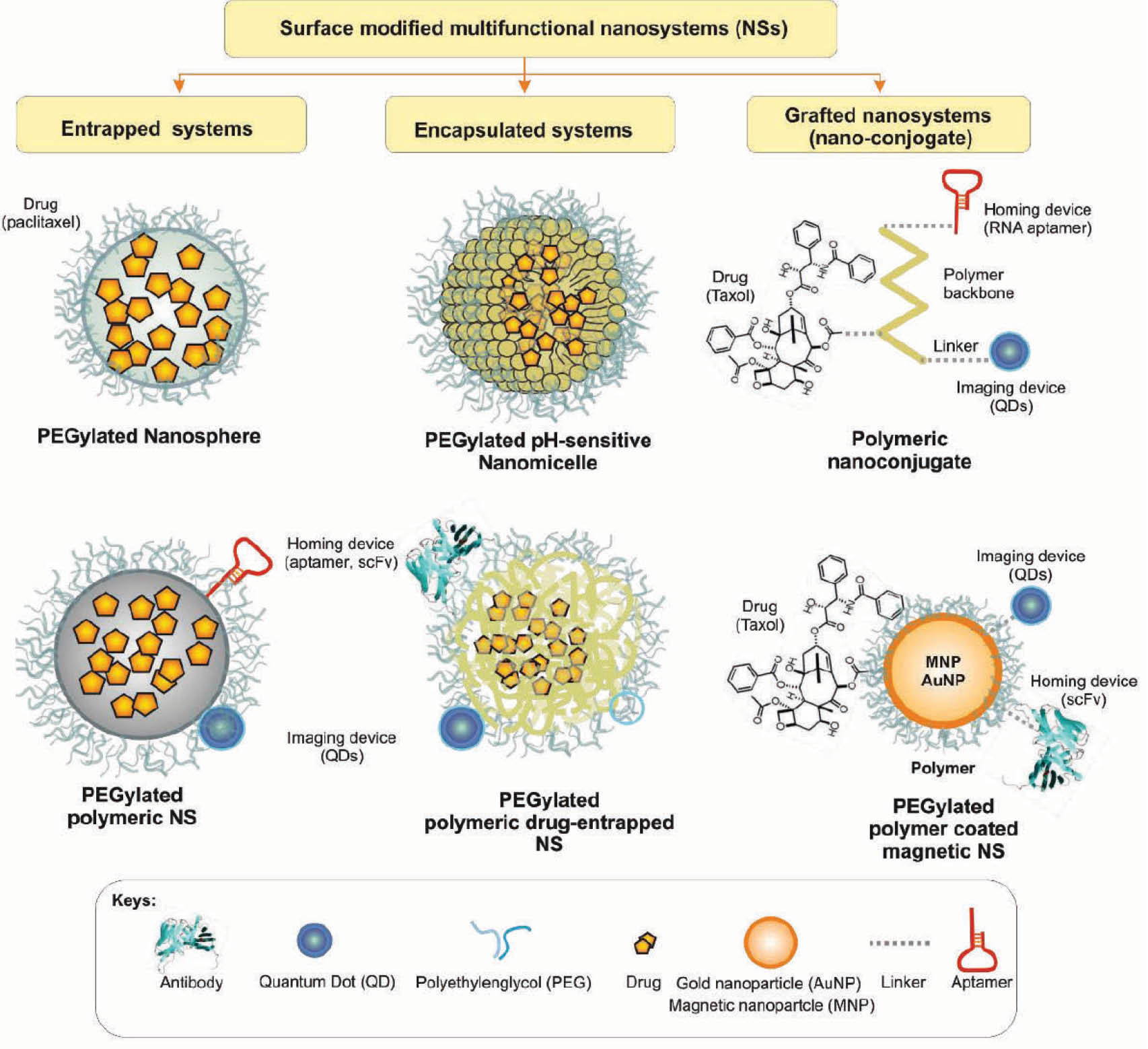
Fig. 2.
Schematic illustration of advanced multifunctional drug delivery systems. Image was adapted with permission from our previous publication.
58
.
Schematic illustration of advanced multifunctional drug delivery systems. Image was adapted with permission from our previous publication.
58
It should be also pointed out that because of the wide-range applications and different properties of the polymeric biomaterials, there is no ideal polymer with universal use. Hence, depending on the endpoint use of the biopolymer, the right structure and formulation must be selected/devised while there exists an array of macromolecular biomaterials that may meet the need(s) for development of therapeutic NSs. Biodegradable polymers, no matter synthetic or natural, can be degradable in vivo into biocompatible by-products through enzymatic transformation (e.g., hydrolysis).
17
Several factors (chemical structure and composition, distribution of repeat units in multimers, presence of ionic groups, structural configuration, molecular weight, morphology and pH) can affect the biodegradation process of polymeric system.
25,70
Hence a better understanding about all these influencing factors can facilitate the development of advanced DDSs and multimodal NSs. As shown in Table 2, several biodegradable polymeric nano-formulations are under investigation for treatment of the wide spectra of diseases. Further, the biodegradability of these polymers make them as the safest implantable systems that can be degraded, and hence need no subsequent surgical operation to removal of transplanted system.
17
A multilayer cylindrical implant made of PLGA was used for the controlled- and extended-release of DOX molecules in murine breast cancer. This implant system compared to the DOX traditional IV route, could delivered greater amount of DOX with better coverage of local tumor, preventing metastatic spread and less drug toxicity without weight loss, splenomegaly and cardiac toxicity.
79
Table 2.
In vivo investigation of some selected biodegradable polymeric nano-formulations for clinical application
|
Polymer
|
Encapsulant
|
Formulation method
|
In vivo biological impacts
|
Ref.
|
| PLA |
Hemoglobin |
Double emulsion method |
Reduced lever accumulation |
60
|
| PLA |
Ellagic acid |
Emulsion diffusion evaporation method |
NPs protected the cyclosporin induced nephrotoxicity in rats |
61
|
| PCL |
Tamoxifen |
Solvent displacement |
Increased level of accumulation of the drug within tumor with time and extended their presence in circulation |
62
|
| Gelatin |
Paclitaxel |
Desolvation method |
Paclitaxel-loaded NPs were active against human RT4 bladder transitional cancer cells |
63
|
| Alginate/Chitosan |
Insulin |
Ionotropic
gelation method
|
NPs adhere to intestinal epithelium and internalized by intestinal mucosa |
64
|
| Chitosan |
Cyclosporin A |
Ionic gelation
method
|
Cyclosporin A concentration were higher incornea than conjunctiva |
65
|
|
Poly(n-butyl cyanoacrylate)
|
Indomethacin |
Interfacial polymerization |
Permeate through rats skin in a period of 8 h |
66
|
| Glycol chitosan |
Doxorubicin |
Self-assembling |
Self-aggregates loaded with doxorubicin exhibited lower toxicity than free doxorubicin |
67
|
Biodegradable scaffolds composed of PLA and β-tricalcium phosphate is developed for complex maxillofacial reconstruction. Biocompatibility tests with mesenchymal stem cells indicated better proliferation, without toxicity. The porous interconnected structures make possible cellular adhesion and vascular proliferation. The in vivo investigation in rats led to complete bone ingrowth within 30 days with minimal inflammatory impacts.
80
Final remarks
To engineer the most compatible biomaterials, a number of central characteristics need to be met. These materials must (a) pose no/trivial inflammatory response; (b) possess a degradation time coinciding with their function; (c) have appropriate mechanical properties for their intended use; (d) produce nontoxic degradation products that can be readily reabsorbed or excreted; and (e) include appropriate permeability and processability for designed application.
81
These properties are greatly affected by a number of features of degradable polymeric biomaterials including, but not limited to: material chemistry, molecular weight, hydrophobicity, surface charge, water adsorption, degradation and erosion mechanism.
Due to the wide range use of polymeric biomaterials, a single, ideal polymer or polymeric family does not exist. Instead a library of materials is available to researchers that can be synthesized and engineered to best match the specifications of the material’s desired biomedical function. Current efforts in biodegradable polymer synthesis have been focused on custom designing and synthesizing polymers with tailored properties for specific applications by: (i) developing novel synthetic polymers with unique chemistries to increase the diversity of polymer structure, (ii) developing biosynthetic processes to form biomimetic polymer structures and (iii) adopting combinatorial and computational approaches in biomaterial design to accelerate the discovery of novel resorbable polymers.
Taken all, it should be taken into consideration that an ideal biodegradable polymeric DDS must be tailored in a way that it provides a number of imperative characteristics such as (a) suitable permeability and drug release profile based on physicochemical properties (e.g., lipophilicity and hydrophilicity) of cargo molecules, (b) biodegradability and biocompatibility, (c) tensile strength, and (d) possibility for surface modification and decoration.
17
It should be also pointed out that, for broadening the potential applications of biodegradable polymers, they should be modified utilizing several methods such as random and block copolymerization, grafting, blending and composites forming, which lead to new advanced biomaterials with unique properties including high performance, low cost, and good processability.
82
Given the fact that various non-biodegradable polymers and lipids used as DDSs and/or gene delivery systems (GDSs) impose intrinsic inadvertent cytotoxic and genotoxic impacts
1-4,
83-87
and some inevitable downsides of the currently used biodegradable polymers, we need to advance DDSs/GDSs towards mimicking the natural polymers found in human body. Perhaps, it is the right time to move on and implement the biopolymers of the human body, and engineer human-origin polymeric scaffolds to be able to specifically deliver drugs into the target cells/tissue without any detrimental impacts on the healthy normal cells by delivery vehicles per se.
Ethical issues
There is none to be declared.
Competing interests
No competing interests to be disclosed.
Acknowledgments
Authors would like to acknowledge the Iranian National Science Foundation (INSF) for the financial support (Grant No: 93030668).
Review Highlights
What is current knowledge?
simple
-
√ Biodegradable polymers (synthetic, semi-synthetic and natural) used for development of NPs possess unique characteristics including nanoscaled structures, high encapsulation capacity, biocompatibility and controlled-/sustained-release profile for lipophilic/hydrophilic drugs.
-
√ PLGA based NPs as a synthetic biodegradable polymeric system, is FDA approved and used widely.
-
√ Chitosan as a semisynthetic natural based polymer can be chemically modified to improve its applicability for various pharmaceutical/biomedical applications.
What is new here?
simple
-
√ Advanced biodegradable NSs armed with homing devices have ability to penetrate into TME and target the cancerous cells solely with no/little effects on the healthy cells.
-
√ Stimuli-responsive biopolymeric NSs offer selective drug release at tumor sites.
References
- Kafil V, Omidi Y. Cytotoxic impacts of linear and branched polyethylenimine nanostructures in a431 cells. Bioimpacts 2011; 1:23-30. doi: 10.5681/bi.2011.004 [Crossref] [ Google Scholar]
- Omidi Y, Barar J, Heidari HR, Ahmadian S, Yazdi HA, Akhtar S. Microarray analysis of the toxicogenomics and the genotoxic potential of a cationic lipid-based gene delivery nanosystem in human alveolar epithelial a549 cells. Toxicol Mech Methods 2008; 18:369-78. doi: 10.1080/15376510801891286 [Crossref] [ Google Scholar]
- Hollins AJ, Omidi Y, Benter IF, Akhtar S. Toxicogenomics of drug delivery systems: Exploiting delivery system-induced changes in target gene expression to enhance siRNA activity. J Drug Target 2007; 15:83-8. doi: 10.1080/10611860601151860 [Crossref] [ Google Scholar]
- Omidi Y, Hollins AJ, Benboubetra M, Drayton R, Benter IF, Akhtar S. Toxicogenomics of non-viral vectors for gene therapy: a microarray study of lipofectin- and oligofectamine-induced gene expression changes in human epithelial cells. J Drug Target 2003; 11:311-23. doi: 10.1080/10611860310001636908 [Crossref] [ Google Scholar]
- Duncan R. Polymer therapeutics: Top 10 selling pharmaceuticals - what next?. J Control Release 2014; 190:371-80. doi: 10.1016/j.jconrel.2014.05.001 [Crossref] [ Google Scholar]
- Duncan R, Vicent MJ. Polymer therapeutics-prospects for 21st century: the end of the beginning. Adv Drug Deliv Rev 2013; 65:60-70. doi: 10.1016/j.addr.2012.08.012 [Crossref] [ Google Scholar]
- Duncan R. Polymer therapeutics as nanomedicines: new perspectives. Curr Opin Biotechnol 2011; 22:492-501. doi: 10.1016/j.copbio.2011.05.507 [Crossref] [ Google Scholar]
- Duncan R, Ringsdorf H, Satchi-Fainaro R. Polymer therapeutics--polymers as drugs, drug and protein conjugates and gene delivery systems: past, present and future opportunities. J Drug Target 2006; 14:337-41. doi: 10.1080/10611860600833856 [Crossref] [ Google Scholar]
- Kim HK, Chung HJ, Park TG. Biodegradable polymeric microspheres with "open/closed" pores for sustained release of human growth hormone. J Control Release 2006; 112:167-74. doi: 10.1016/j.jconrel.2006.02.004 [Crossref] [ Google Scholar]
- Fernandez-Urrusuno R, Calvo P, Remunan-Lopez C, Vila-Jato JL, Alonso MJ. Enhancement of nasal absorption of insulin using chitosan nanoparticles. Pharm Res 1999; 16:1576-81. doi: 10.1023/A:1018908705446 [Crossref] [ Google Scholar]
- Khosroushahi AY, Naderi-Manesh H, Yeganeh H, Barar J, Omidi Y. Novel water-soluble polyurethane nanomicelles for cancer chemotherapy: physicochemical characterization and cellular activities. J Nanobiotechnology 2012; 10:2. doi: 10.1186/1477-3155-10-2 [Crossref] [ Google Scholar]
- Jackanicz TM, Nash HA, Wise DL, Gregory JB. Polylactic acid as a biodegradable carrier for contraceptive steroids. Contraception 1973; 8:227-34. doi: 10.1016/0010-7824(73)90033-4 [Crossref] [ Google Scholar]
- Pavot V, Berthet M, Resseguier J, Legaz S, Handke N, Gilbert SC. Poly(lactic acid) and poly(lactic-co-glycolic acid) particles as versatile carrier platforms for vaccine delivery. Nanomedicine (Lond) 2014; 9:2703-18. doi: 10.2217/nnm.14.156 [Crossref] [ Google Scholar]
- Matthaiou EI, Barar J, Sandaltzopoulos R, Li C, Coukos G, Omidi Y. Shikonin-loaded antibody-armed nanoparticles for targeted therapy of ovarian cancer. Int J Nanomedicine 2014; 9:1855-70. doi: 10.2147/IJN.S51880 [Crossref] [ Google Scholar]
- Moogooee M, Ramezanzadeh H, Jasoori S, Omidi Y, Davaran S. Synthesis and in vitro studies of cross-linked hydrogel nanoparticles containing amoxicillin. J Pharm Sci 2011; 100:1057-66. doi: 10.1002/jps.22351 [Crossref] [ Google Scholar]
- Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release 2001; 70:1-20. doi: 10.1016/S0168-3659(00)00339-4 [Crossref] [ Google Scholar]
- Joshi J, Patel RP. Role of biodegradable polymers in drug delivery. Int J Curr Pharm Res 2012; 4:74-81. [ Google Scholar]
- Ulery BD, Nair LS, Laurencin CT. Biomedical Applications of Biodegradable Polymers. J Polym Sci B Polym Phys 2011; 49:832-64. doi: 10.1002/polb.22259 [Crossref] [ Google Scholar]
- Brar V, Kaur G. Biopolymers as Carriers for Nasal Drug Delivery. Polym Plast Technol Eng 2014; 53:1518-31. doi: 10.1080/03602559.2014.912327 [Crossref] [ Google Scholar]
- Hosseinkhani H, He W-J, Chiang C-H, Hong P-D, Yu D-S, Domb AJ. Biodegradable nanoparticles for gene therapy technology. J Nanopart Res 2013; 15:1-15. doi: 10.1007/s11051-013-1794-z [Crossref] [ Google Scholar]
- Germershaus O, Lühmann T, Rybak J-C, Ritzer J, Meinel L. Application of natural and semi-synthetic polymers for the delivery of sensitive drugs. Int Mater Rev 2015; 60:101-31. doi: 10.1179/1743280414Y.0000000045 [Crossref] [ Google Scholar]
- Sionkowska A. Current research on the blends of natural and synthetic polymers as new biomaterials: Review. Prog Polym Sci 2011; 36:1254-76. doi: 10.1016/j.progpolymsci.2011.05.003 [Crossref] [ Google Scholar]
- Liu Y, Shu XZ, Prestwich GD. Osteochondral Defect Repair with Autologous Bone Marrow–Derived Mesenchymal Stem Cells in an Injectable, In Situ, Cross-Linked Synthetic Extracellular Matrix. Tissue engineering 2006; 12:3405-16. [ Google Scholar]
- Mooguee M, Omidi Y, Davaran S. Synthesis and in vitro release of adriamycin from star-shaped poly(lactide-co-glycolide) nano- and microparticles. J Pharm Sci 2010; 99:3389-97. doi: 10.1002/jps.22106 [Crossref] [ Google Scholar]
- Kapoor DN, Bhatia A, Kaur R, Sharma R, Kaur G, Dhawan S. PLGA: a unique polymer for drug delivery. Ther Deliv 2015; 6:41-58. doi: 10.4155/tde.14.91 [Crossref] [ Google Scholar]
- Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release 2012; 161:505-22. doi: 10.1016/j.jconrel.2012.01.043 [Crossref] [ Google Scholar]
- Hans M, Lowman A. Biodegradable nanoparticles for drug delivery and targeting. Curr Opin Solid State Mater Sci 2002; 6:319-27. doi: 10.1016/S1359-0286(02)00117-1 [Crossref] [ Google Scholar]
- Shargh VH, Hondermarck H, Liang M. Antibody-targeted biodegradable nanoparticles for cancer therapy. Nanomedicine (Lond) 2016; 11:63-79. doi: 10.2217/nnm.15.186 [Crossref] [ Google Scholar]
- Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev 2003; 55:329-47. doi: 10.1016/S0169-409X(02)00228-4 [Crossref] [ Google Scholar]
- Locatelli E, Franchini MC. Biodegradable PLGA-b-PEG polymeric nanoparticles: synthesis, properties, and nanomedical applications as drug delivery system. J Nanoparticle Res 2012; 14:1-17. doi: 10.1007/s11051-012-1316-4 [Crossref] [ Google Scholar]
-
Ohya Y, Takahashi A, Nagahama K. Biodegradable polymeric assemblies for biomedical materials. Polymers in Nanomedicine: Springer; 2012. p. 65-114.
- Pandita D, Kumar S, Lather V. Hybrid poly(lactic-co-glycolic acid) nanoparticles: design and delivery prospectives. Drug Discov Today 2015; 20:95-104. doi: 10.1016/j.drudis.2014.09.018 [Crossref] [ Google Scholar]
- Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf, B 2010; 75:1-18. doi: 10.1016/j.colsurfb.2009.09.001 [Crossref] [ Google Scholar]
- Tang A, Li J, Zhao S, Liu T, Wang Q, Wang J. Biodegradable Tissue Engineering Scaffolds Based on Nanocellulose/PLGA Nanocomposite for NIH 3T3 Cell Cultivation. Journal of Nanoscience and Nanotechnology 2017; 17:3888-95. [ Google Scholar]
- Khojasteh A, Fahimipour F, Eslaminejad MB, Jafarian M, Jahangir S, Bastami F. Development of PLGA-coated β-TCP scaffolds containing VEGF for bone tissue engineering. Materials Science and Engineering: C 2016; 69:780-8. doi: 10.1016/j.msec.2016.07.011 [Crossref] [ Google Scholar]
- Heller J. Biodegradable polymers in controlled drug delivery. Crit Rev Ther Drug Carrier Syst 1983; 1:39-90. [ Google Scholar]
- Dash M, Chiellini F, Ottenbrite R, Chiellini E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog Polym Sci 2011; 36:981-1014. doi: 10.1016/j.progpolymsci.2011.02.001 [Crossref] [ Google Scholar]
- Sarvaiya J, Agrawal Y. Chitosan as a suitable nanocarrier material for anti-Alzheimer drug delivery. Int J Biol Macromolec 2015; 72:454-65. doi: 10.1016/j.ijbiomac.2014.08.052 [Crossref] [ Google Scholar]
- Anitha A, Sowmya S, Kumar PS, Deepthi S, Chennazhi K, Ehrlich H. Chitin and chitosan in selected biomedical applications. Prog Polym Sci 2014; 39:1644-67. doi: 10.1016/j.progpolymsci.2014.02.008 [Crossref] [ Google Scholar]
- Barikani M, Oliaei E, Seddiqi H, Honarkar H. Preparation and application of chitin and its derivatives: a review. Iran Polym J 2014; 23:307-26. doi: 10.1007/s13726-014-0225-z [Crossref] [ Google Scholar]
- Shukla SK, Mishra AK, Arotiba OA, Mamba BB. Chitosan-based nanomaterials: A state-of-the-art review. Int J Biol Macromolec 2013; 59:46-58. doi: 10.1016/j.ijbiomac.2013.04.043 [Crossref] [ Google Scholar]
-
Ng WL, Yeong WY, Naing MW. Polyelectrolyte gelatin-chitosan hydrogel optimized for 3D bioprinting in skin tissue engineering. International Journal of Bioprinting 2016; 2.
- Khdair A, Hamad I, Alkhatib H, Bustanji Y, Mohammad M, Tayem R. Modified-chitosan nanoparticles: Novel drug delivery systems improve oral bioavailability of doxorubicin. European Journal of Pharmaceutical Sciences 2016; 93:38-44. doi: 10.1016/j.ejps.2016.07.012 [Crossref] [ Google Scholar]
- Barar J, Omidi Y. Dysregulated pH in Tumor Microenvironment Checkmates Cancer Therapy. Bioimpacts 2013; 3:149-62. doi: 10.5681/bi.2013.036 [Crossref] [ Google Scholar]
- Omidi Y, Barar J. Targeting tumor microenvironment: crossing tumor interstitial fluid by multifunctional nanomedicines. Bioimpacts 2014; 4:55-67. doi: 10.5681/bi.2014.021 [Crossref] [ Google Scholar]
- Coates JM, Galante JM, Bold RJ. Cancer therapy beyond apoptosis: autophagy and anoikis as mechanisms of cell death. J Surg Res 2010; 164:301-8. doi: 10.1016/j.jss.2009.07.011 [Crossref] [ Google Scholar]
- Paoli P, Giannoni E, Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta 2013; 1833:3481-98. doi: 10.1016/j.bbamcr.2013.06.026 [Crossref] [ Google Scholar]
- Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today 2006; 11:812-8. doi: 10.1016/j.drudis.2006.07.005 [Crossref] [ Google Scholar]
- Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul 2001; 41:189-207. doi: 10.1016/S0065-2571(00)00013-3 [Crossref] [ Google Scholar]
- Adiseshaiah PP, Hall JB, McNeil SE. Nanomaterial standards for efficacy and toxicity assessment. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2010; 2:99-112. doi: 10.1002/wnan.66 [Crossref] [ Google Scholar]
- Baronzio G, Parmar G, Baronzio M. Overview of Methods for Overcoming Hindrance to Drug Delivery to Tumors, with Special Attention to Tumor Interstitial Fluid. Front Oncol 2015; 5:165. doi: 10.3389/fonc.2015.00165 [Crossref] [ Google Scholar]
- Khawar IA, Kim JH, Kuh HJ. Improving drug delivery to solid tumors: priming the tumor microenvironment. J Control Release 2015; 201:78-89. doi: 10.1016/j.jconrel.2014.12.018 [Crossref] [ Google Scholar]
- Na K, Lee KH, Bae YH. pH-sensitivity and pH-dependent interior structural change of self-assembled hydrogel nanoparticles of pullulan acetate/oligo-sulfonamide conjugate. J Control Release 2004; 97:513-25. doi: 10.1016/j.jconrel.2004.04.005 [Crossref] [ Google Scholar]
- Shenoy D, Little S, Langer R, Amiji M. Poly (ethylene oxide)-modified poly (β-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs: part 2 In vivo distribution and tumor localization studies. Pharmaceutical research 2005; 22:2107-14. [ Google Scholar]
- Wang K, Xu X, Liu T, Fu S, Guo G, Gu Y. Synthesis and characterization of biodegradable pH-sensitive hydrogel based on poly (ε-caprolactone), methacrylic acid, and Pluronic (L35). Carbohydrate Polymers 2010; 79:755-61. [ Google Scholar]
- Teo JY, Chin W, Ke X, Gao S, Liu S, Cheng W. pH and redox dual-responsive biodegradable polymeric micelles with high drug loading for effective anticancer drug delivery. Nanomedicine: Nanotechnology, Biology and Medicine 2017; 13:431-42. doi: 10.1016/j.nano.2016.09.016 [Crossref] [ Google Scholar]
- Na K, Lee KH, Lee DH, Bae YH. Biodegradable thermo-sensitive nanoparticles from poly(L-lactic acid)/poly(ethylene glycol) alternating multi-block copolymer for potential anti-cancer drug carrier. Eur J Pharm Sci 2006; 27:115-22. doi: 10.1016/j.ejps.2005.08.012 [Crossref] [ Google Scholar]
- Fathi M, Entezami AA, Arami S, Rashidi M-R. Preparation of N-isopropylacrylamide/itaconic acid magnetic nanohydrogels by modified starch as a crosslinker for anticancer drug carriers. Int J Polym Mater Polym Biomater 2015; 64:541-9. [ Google Scholar]
-
Phan VG, Thambi T, Duong HTT, Lee DS. Poly (amino carbonate urethane)-based biodegradable, temperature and pH-sensitive injectable hydrogels for sustained human growth hormone delivery. Scientific Reports 2016; 6.
- Wang T, Chen L, Shen T, Wu D. Preparation and properties of a novel thermo-sensitive hydrogel based on chitosan/hydroxypropyl methylcellulose/glycerol. International Journal of Biological Macromolecules 2016; 93, Part A:775-82. doi: 10.1016/j.ijbiomac.2016.09.038 [Crossref] [ Google Scholar]
- Yoo HS, Park TG. Folate receptor targeted biodegradable polymeric doxorubicin micelles. J Control Release 2004; 96:273-83. doi: 10.1016/j.jconrel.2004.02.003 [Crossref] [ Google Scholar]
- Xu G, Yu X, Zhang J, Sheng Y, Liu G, Tao W. Robust aptamer-polydopamine-functionalized M-PLGA-TPGS nanoparticles for targeted delivery of docetaxel and enhanced cervical cancer therapy. Int J Nanomedicine 2016; 11:2953-65. doi: 10.2147/IJN.S103513 [Crossref] [ Google Scholar]
- Yang Y, Zhao H, Jia Y, Guo Q, Qu Y, Su J. A novel gene delivery composite system based on biodegradable folate-poly (ester amine) polymer and thermosensitive hydrogel for sustained gene release. Scientific reports 2016; 6:21402. [ Google Scholar]
- Kirtane AR, Kalscheuer SM, Panyam J. Exploiting nanotechnology to overcome tumor drug resistance: Challenges and opportunities. Adv Drug Deliv Rev 2013; 65:1731-47. doi: 10.1016/j.addr.2013.09.001 [Crossref] [ Google Scholar]
- Miao L, Huang L. Exploring the tumor microenvironment with nanoparticles. Cancer Treat Res 2015; 166:193-226. doi: 10.1007/978-3-319-16555-4_9 [Crossref] [ Google Scholar]
- Parveen S, Sahoo SK. Polymeric nanoparticles for cancer therapy. J Drug Target 2008; 16:108-23. doi: 10.1080/10611860701794353 [Crossref] [ Google Scholar]
- Zhong Y, Meng F, Deng C, Zhong Z. Ligand-directed active tumor-targeting polymeric nanoparticles for cancer chemotherapy. Biomacromolecules 2014; 15:1955-69. doi: 10.1021/bm5003009 [Crossref] [ Google Scholar]
- Han S, Liu Y, Nie X, Xu Q, Jiao F, Li W. Efficient delivery of antitumor drug to the nuclei of tumor cells by amphiphilic biodegradable poly(L-aspartic acid-co-lactic acid)/DPPE co-polymer nanoparticles. Small 2012; 8:1596-606. doi: 10.1002/smll.201102280 [Crossref] [ Google Scholar]
- Barar J, Omidi Y. Surface modified multifunctional nanomedicines for simultaneous imaging and therapy of cancer. Bioimpacts 2014; 4:3-14. doi: 10.5681/bi.2014.011 [Crossref] [ Google Scholar]
- Chandra R, Rustgi R. Biodegradable polymers. Prog Polym Sci 1998; 23:1273-335. [ Google Scholar]
- Sheng Y, Yuan Y, Liu C, Tao X, Shan X, Xu F. In vitro macrophage uptake and in vivo biodistribution of PLA–PEG nanoparticles loaded with hemoglobin as blood substitutes: effect of PEG content. Mater Med 2009; 20:Mater Med 2009; 20. doi: 10.1007/s10856-009-3746-9 [Crossref] [ Google Scholar]
- Sonaje K, Italia J, Sharma G, Bhardwaj V, Tikoo K, Kumar MR. Development of biodegradable nanoparticles for oral delivery of ellagic acid and evaluation of their antioxidant efficacy against cyclosporine A-induced nephrotoxicity in rats. Pharm Res 2007; 24:899-908. doi: 10.1007/s11095-006-9207-y [Crossref] [ Google Scholar]
- Devalpally H, Shenoy D, Little S, Langer R, Amiji M. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs: part 3 Therapeutic efficacy and safety studies in ovarian cancer xenograft model. Cancer Chemother Pharmacol 2007; 59:477. doi: 10.1007/s00280-006-0287-5 [Crossref] [ Google Scholar]
- Lu Z, Yeh T-K, Tsai M, Au JL-S, Wientjes MG. Paclitaxel-loaded gelatin nanoparticles for intravesical bladder cancer therapy. Clin Cancer Res 2004; 10:7677-84. doi: 10.1158/1078-0432.CCR-04-1443 [Crossref] [ Google Scholar]
- Sarmento B, Ribeiro A, Veiga F, Sampaio P, Neufeld R, Ferreira D. Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm Res 2007; 24:2198-206. doi: 10.1007/s11095-007-9367-4 [Crossref] [ Google Scholar]
- De Campos AM, Sánchez A, Alonso MaJ. De Campos AM, Sánchez A, Alonso MaJChitosan nanoparticles: a new vehicle for the improvement of the delivery of drugs to the ocular surfaceApplication to cyclosporin A. Int J Pharm 2001; 224:159-68. doi: 10.1016/S0378-5173(01)00760-8 [Crossref] [ Google Scholar]
- Miyazaki S, Takahashi A, Kubo W, Bachynsky J, Löbenberg R. Poly n-butylcyanoacrylate (PNBCA) nanocapsules as a carrier for NSAIDs: in vitro release and in vivo skin penetration. J Pharm Pharm Sci 2003; 6:238-45. [ Google Scholar]
- Park JH, Kwon S, Lee M, Chung H, Kim J-H, Kim Y-S. Self-assembled nanoparticles based on glycol chitosan bearing hydrophobic moieties as carriers for doxorubicin: in vivo biodistribution and anti-tumor activity. Biomaterials 2006; 27:119-26. doi: 10.1016/j.biomaterials.2005.05.028 [Crossref] [ Google Scholar]
- Elzey B, Torregrosa-Allen S, Li P, Ramsey B, Shaw M. A Totally Absorbable Multilayer PLGA Implant Device Containing Doxorubicin Inhibited Tumor Growth and Metastasis without Systemic Toxicity in Murine Breast Cancer and an Ideal Pharmacological Paradigm for Regional Chemotherapy. Journal of Biosciences and Medicines 2016; 4:66. [ Google Scholar]
-
Smeets R, Barbeck M, Hanken H, Fischer H, Lindner M, Heiland M, et al. Selective laser‐melted fully biodegradable scaffold composed of poly (d, l‐lactide) and β‐tricalcium phosphate with potential as a biodegradable implant for complex maxillofacial reconstruction: In vitro and in vivo results. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2016.
- Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control release 2001; 70:1-20. doi: 10.1016/S0168-3659(00)00339-4 [Crossref] [ Google Scholar]
- Hamad K, Kaseem M, Ko YG, Deri F. Biodegradable polymer blends and composites: An overview. Polym Sci A 2014; 56:812-29. doi: 10.1134/S0965545X14060054 [Crossref] [ Google Scholar]
- Omidi Y, Barar J, Akhtar S. Toxicogenomics of cationic lipid-based vectors for gene therapy: impact of microarray technology. Curr Drug Deliv 2005; 2:429-41. doi: 10.2174/156720105774370249 [Crossref] [ Google Scholar]
- Omidi Y, Hollins AJ, Drayton RM, Akhtar S. Polypropylenimine dendrimer-induced gene expression changes: the effect of complexation with DNA, dendrimer generation and cell type. J Drug Target 2005; 13:431-43. doi: 10.1080/10611860500418881 [Crossref] [ Google Scholar]
-
Ahmadian S, Barar J, Saei AA, Fakhree MA, Omidi Y. Cellular toxicity of nanogenomedicine in MCF-7 cell line: MTT assay. J Vis Exp 2009.
- Hamidi A, Sharifi S, Davaran S, Ghasemi S, Omidi Y, Rashidi MR. Novel aldehyde-terminated dendrimers; synthesis and cytotoxicity assay. Bioimpacts 2012; 2:97-103. doi: 10.5681/bi.2012.014 [Crossref] [ Google Scholar]
- Omidi Y, Barar J. Induction of human alveolar epithelial cell growth factor receptors by dendrimeric nanostructures. Int J Toxicol 2009; 28:113-22. doi: 10.1177/1091581809335177 [Crossref] [ Google Scholar]