BioImpacts. 7(2):99-108.
doi: 10.15171/bi.2017.13
Original Research
Co-liquefaction with acetone and GC analysis of volatile compounds in exhaled breath as lung cancer biomarkers
Abolghasem Jouyban 1, 2, *, Djavanshir Djozan 3, Parastou Mohammadandashti 3, Aliakbar Alizadeh-Nabil 4, Hooshangh Ghorbanpour 4, Maryam Khoubnasabjafari 5, Mohammad Mohammadzadeh 6
Author information:
1Pharmaceutical Analysis Research Center and Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
2Kimia Idea Pardaz Azarbayjan (KIPA) Science Based Company, Tabriz University of Medical Sciences, Tabriz, Iran
3Department of Chemistry, College of Sciences, East Azarbayjan Sciences and Research Branch, Islamic Azad University, Tabriz, Iran
4Food and Drug Safety Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
5Tuberculosis and Lung Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
6Department of Radiotherapy, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
A simple, rapid and low cost method for enrichment of volatile organic compounds (VOCs) from exhaled breath (EB) is presented.
Methods:
A 1000 mL home-made extraction device was filled with EB. The VOCs were extracted and condensed in 0.5 mL acetone. Recognition of volatiles in the real studied EB samples was performed by a GC-MS.
Results:
The method displays an extraction efficiency of >86% with the enrichment factor of 1929 for octanal. Limits of detection and quantification, and linear dynamic range were 0.008, 0.026 and 0.026-400 ng/mL respectively. Analysis of real samples showed the existence of more than 100 compounds in EB of healthy volunteers and patients with lung cancer before and after treatment. Exhaled octanal concentration was significantly higher in lung cancer patient than in healthy volunteers and lung cancer patient after treatment.
Conclusion:
Having used the proposed approach, high extraction recovery (up to 86%) was attained for the lung cancer marker, octanal, as an important biomarker. Our findings on smaples of EB of healthy controls and patients with lung cancer before and after treatment provide complelling evidence upon the effectiveness of the developed method.
Keywords: Co-liquefaction, Exhaled breath, Volatile compounds, Cancer biomarkers, Lung cancer
Copyright and License Information
© 2017 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Volatile organic compounds (VOCs) attracted more attentions in various disciplines including environmental, food, flavor and fragrances, pharmaceutical, forensic and particularly medical sciences.
1-6
Growing awareness of the impact of VOCs on human health encouraged medical researchers to focus on VOCs. It is estimated that 10,000 types of low molecular weight VOCs are involved in the odor of artificial or natural products, of which only about 5% play a role in imparting smell.
7
Itching, vomiting, and rashes are the more common symptoms of exposure to VOCs. Some VOCs are suspected as human carcinogens according to the US Environmental Protection Agency.
8
About 3,000 types of VOCs are contained in a human exhaled breath (EB), however, 20 to 30 of VOCs are more investigated in medical area and observed in human subjects.
9
VOCs in EB are divided into two groups, namely exogenous volatiles and endogenously produced compounds. Exogenous volatiles include those inhaled from the environment, the oral ingestion of food, and smoking cigarettes. Endogenously produced volatiles include materials newly made from cells in the body and those made by intestinal bacteria. These compounds present in very low concentrations from malignant cells of human organs, e.g. lung diseases.
10-12
VOCs in EB provide valuable information on human health/disease conditions. The composition of the breath is affected by the types of diseases. For example, sweet smell indicates diabetes, while the odor of rotten eggs, which are caused by sulfur-containing compounds, suggests liver problems.
13,14
Currently, intensive search are carried out for compounds that could be the potential biomarkers of cancer to facilitate the diagnosis in the future.
15,16
A number of papers have been published on diagnosis of lung cancer by VOCs in EB.
13,17,19,20
During last decade of twentieth century, branched and oxygenated aliphatic compounds were proposed as cancer biomarkers. However, some of the compounds described as cancer biomarkers are related to smoking and disinfectants or emanate from medical material. Unsaturated or aromatic compounds such as acrolein, heterocyclic amines and aza-arenes are constituents of cigarette smoke,
21,22
and cyclic hydrocarbons such as diethylhexyl phthalate or cyclohexanone are originated from plastic material and fuel combustion.
24
In contrast, the relation of carbonyl compounds such as ketones and aldehydes with oxidative stress and up regulation of cell proliferation
19,2225-28
is well established. Exhaled C1-C10 aldehydes have been detected in all healthy volunteers, smokers and lung cancer patients. Concentrations ranged from 7 pmol/L for butanal and to 71 nmol/L for formaldehyde. Highest inspired concentrations were found for formaldehyde and acetaldehyde (0–55 nmol/Land 0–13 nmol/L, respectively). Acetaldehyde, propanal, butanal, heptanal and decanal concentrations showed no significant differences for cancer patients, smokers and healthy volunteers. Exhaled pentanal, hexanal, octanal and nonanal concentrations were significantly higher in lung cancer patients than in smokers and healthy controls. Lung cancer patients could be therefore screened by means of exhaled pentanal, hexanal, octanal and nonanal concentrations.
28,29
Breath analysis is a promising approach
18,19,30
that allows development of a method for diagnosis of cancer using a non-invasive sampling. Breath analysis requires sophisticated and expensive equipment such as gas chromatography coupled with mass spectrometry (GC-MS) and excellent skilled operators, because the target compounds are only found in traces (e.g. 10-12 mol/L or
10-9 mol/L); a pre-concentration procedure is therefore crucial.
31,32
In addition to EB samples which traps gaseous analytes, the exhaled breath condensate (EBC) contains gaseous and also dissolved analytes in the micro scaled droplets of lung lining fluid
33
and contains very small analytes such as sodium ions up to macromolecules such as proteins. Analyses of EB and EBC samples were attracted more attention and a number of works were reported in the literature.
34-38
EBC samples are collected by using a freezing cold traps.
39
In addition to monitoring biomarkers in EBC,
40,41
determination of drug concentrations in EBC
42-44
provides a new non-invasive method for medical investigations.
Nowadays, specific attention is paid for development and optimization of a reliable, robust, simple and no-expensive method for isolation and enrichment of exhaled VOCs, especially aldehydes prior to their analyses. A lot of new methods such as active and passive sample enrichment on solid adsorption (sorbent trapping),
45-48
static SPME
15
dynamic SPME,
29
on fiber derivatization SPME,
18,28,29,49
colorimetric sensor
50
and silicon coated microchips
20
were developed during last decades. These methods have been used to extraction and monitoring of some VOCs such as aliphatic and aromatic hydrocarbons
49,51
and also carbonyl compounds
52
from human breath. However, some of these methods have restricted capacity for extraction of analytes. The compounds with higher concentration or higher distribution coefficient will be extracted in preference and saturate the extracting phases and therefore the analytes cannot be extracted despite their presence in the sample. When the main goal of analysis is focused on the determination of real profile of VOCs in real samples, these methods cannot be used as reliable sample preparation procedures. There are a number of other methods which are time consuming or require especial materials to be operated.
The present study describes a rapid, simple, inexpensive, universal and integrative gas sampling, isolation and enrichment of VOCs as biomarkers of lung cancer in EB. This method is based on co-liquefaction of VOCs with an organic solvent. The new method overcomes many of the limitations of the existing gaseous samples preparation methodologies for VOCs separation. The device comprises a portable system, capable of sampling up to 1000 mL of human EB without the need for mechanical impulsion. The simple and sorbent free extraction design benefits from the ultrasonic cold vaporization of small volume of a suitable extraction solvent and then co-liquefaction with trace of VOCs present in gaseous samples. Condensed extract was analyzed by a gas chromatograph with flame ionization detector (GC-FID) and/or mass spectrometry (GC-MS).
Materials and Methods
Chemicals and reagents
Octanal, acetone, hexane, benzene and all other chemicals of analytical grade were purchased from E. Merck (Darmstadt, Germany, www.merck-chemicals.com). Ultrapure nitrogen (99.999%) was purchased from Gulf Cryo (Dubai, United Arabic Emirates, www.gulfcryo.com).
Stock and standard solution
Stock solution of octanal and benzene with a concentration of 8000 μg/mL was prepared separately in acetone on a weekly basis. Standard solutions (8-800 μg/mL) were prepared by diluting the stock solution in acetone on a daily basis. All these solutions were stored in a refrigerator. Model gaseous samples containing 4-400 ng/mL of octanal and benzene was prepared prior to each experiment. For this purpose, 0.5 mL of each standard sample solutions were introduced into 1000 mL extraction device and vaporized by sonification.
Apparatus
A 1000 mL home-made spherical glass with a 15 × 2 cm cooling tube containing salt-ice with a collecting micro tube on the end was fabricated. This device was used for rapid sampling of human breath samples and isolation and enrichment of VOCs on the basis of co-liquefaction with organic solvent.
Monitoring of the analytes was performed using a gas chromatograph (Agilent 7890 A, Agilent technology, Inc, USA) equipped with a flame ionization detector (FID) and a split-splitless injector. Analytes were separated on a 30 m × 0.32 mm HP-5 capillary column (Agilent J&W GC Columns, Agilent technology, Inc, USA) with a film thickness of 0.25 μm. The column temperature was programmed to be held at 40°C for 2 minutes and then increase up to 250°C at a rate of 10°C/min. Flow rate of carrier gas was kept constant at 1.2 mL/min and the make-up gas (nitrogen) flow was 25 mL/min. The detector temperature was maintained at 300°C. Injection was performed in a pulse split mode. Injection port temperature was set at 250°C.
Recognition of volatiles in the real studied gaseous samples was performed by an Agilent GC-MS model 7890A/5975C (Agilent technology, Inc, USA) coupled with a quadrupole mass spectrometer equipped with NIST software. The chromatographic conditions were similar to GC-FID operation conditions. The aux. heater (transfer line), mass source and mass quadruple temperature were set at 310, 230 and 150°C, respectively. Absolute voltage was 1035 V.
Hydrogen gas generator model Claind HG-2200 was purchased from Lenn Co (Italy). Ultrasonic Humidifier model JSS-37501 (Xuyao, Zhejing, China) was used for transferring of extraction solvent in the vapor or aerosol forms. Behdad incubator model 70 (Behdad, Tehran, Iran) was used for thermal vaporization of extraction solvent.
A 1000 mL home-made round bottom glass extraction device with two valves at the sides and solvent introducing port with septum at the top and a cold condensation tube introduced into flask (Fig. 1) was used for sampling of EB and also isolation and enrichment of VOCs.
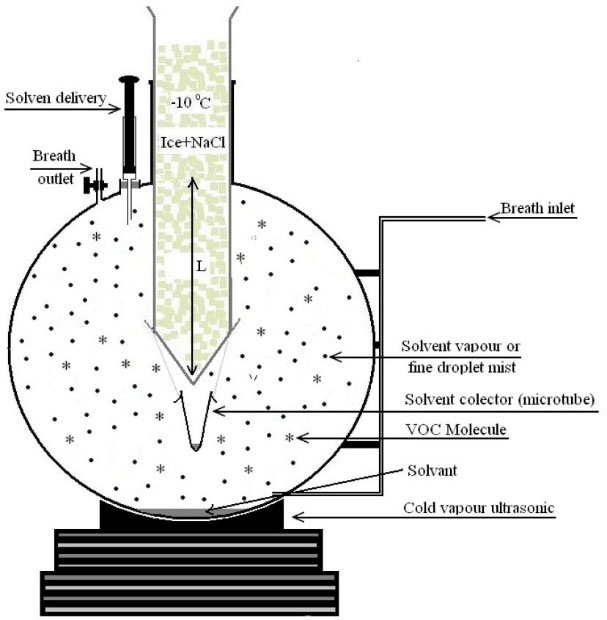
Fig. 1.
Schematic presentation of designed and home-made
sampling and co-liquefaction device.
.
Schematic presentation of designed and home-made
sampling and co-liquefaction device.
Exhaled breath sampling
Twelve breathing samples were delivered from volunteers selected by a physician from radiotherapy and oncology department of Imam Reza hospital. Five healthy controls, 3 lung cancer patients before beginning of any treatment and the rest of patients after beginning of radiotherapy were selected. All patients had a tumor stage of T4 according to (Tumor size, Lymph Node involvement, Metastasis) TNM-classification.
53
1000 mL of EB were taken in the morning after teeth washing with water and before breakfast by simple insufflating into the device via opened valves. The valves were closed and the samples were transferred to laboratory for extraction and monitoring of VOCs. All breath sample donors signed a written consent form approved by the Ethics Committee of Tabriz University of Medical Sciences.
Extraction procedures
0.5 mL of extraction solvent (acetone) was introduced into the extraction device filled by EB. The device was exposed to ultrasonic irradiation for cold vaporization of solvent for 2 min. Cold condensation tube was filled with NaCl /ice bath (-10°C) for co-liquefaction of analytes with solvent molecules. The condensed liquid were derived and collected in the micro tube. This procedure was carried out in 12 minutes. Two microliters of the condensed liquid (extract) was injected into GC injector port.
Results and Discussion
A new method based on the co-liquefaction phenomena for the isolation and enrichment of VOCs existing in EB prior to their analysis is used in this work. Small volume of extraction solvent must be introduced into the device containing EB and be exposed to the vaporization source so that the extraction solvent is transformed to the gaseous or mist forms. Gaseous molecules of solvent fill the device and mix with VOC molecules. Once the device is cooled down, the gaseous molecules (solvent and VOCs) are co-liquefied and collected at the micro-tube (collector). Suitable volume of the collected solution can be injected and analyzed by GC-FID or GC-MS. Considering the extraction and enrichment mechanism, shape and volume of extraction device, nature and volume of extraction solvent can play important roles on the quantitative parameters. The effect of these parameters on the extraction procedure should be investigated and optimized. Considering the significant increase of some aldehydes, e.g. octanal concentrations in EB of lung cancer patients,
28,29
all of the optimization procedures were performed using octanal as a model compound.
Selection of extraction solvent
Solvent must have some essential properties including rapid and complete evaporation under ultrasonic irradiation as well as complete and easy condensation (liquefaction) at a not very low temperature. As described in the literature,
34,35
solvents with low boiling point and viscosity can atomize rapidly under ultra-sonic irradiation. Considering some essential parameters such as the cost, environmental and health problems, condensation (liquefaction) percent and finally to avoid chromatographic interference with most of VOCs, acetone with boiling point below 60°C and viscosity of 0.295 cP (at 25°C) is selected as the most suitable extraction solvent for the further experiments.
Design of sampling and extraction device
Devices with various shape (spherical and elliptical) and volumes (250, 500 and 1000 mL) were designed and fabricated from glassware. The extraction ability of each device was evaluated using standard gaseous sample containing octanal (b.p.=171°C) and benzene (b.p.=80°C). Preliminary studies revealed that using 1000 mL round bottom flask with two valves at the sides and solvent introducing port with septum at the top and cold condensation tube introduced into flask as shown in (Fig. 1), results in higher concentration factor and higher extraction efficiency regardless of analytes (VOCs) boiling point.
Optimization of cold condensation tube length
Cold condensation tube containing ice/salt, positioned inside the glass device, play an important role on the liquefaction procedure of solvent mist or vapor. To have the optimum length of condensation tube, 0.5 mL acetone was introduced into extraction device and exposed to ultrasonic irradiation for 2 minutes. Liquefaction procedure was performed at -10°C for 15 minutes. For this purpose, the condensation tubes with different length were filled with ice/salt mixture. Variation of recovered volume of acetone on the micro-tube collector versus the length of condensation tube was presented in (Fig. 2). Based on these results no significant variation of recovered solvent volume was observed, and 11.5 cm length was used in further analyses.
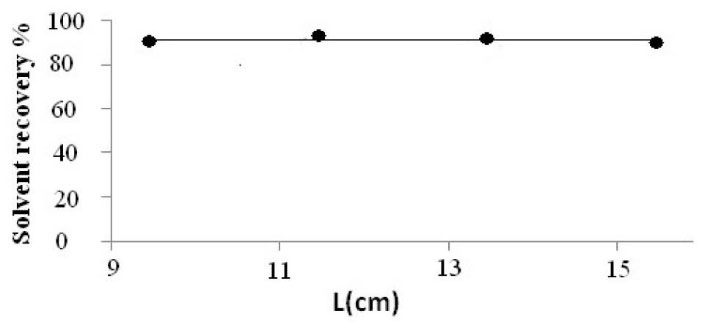
Fig. 2.
Variation of solvent recovery percent with the length (L) of
condensation tube inside 1000 mL extraction device. The volume
of acetone 0.5 mL, vaporization time 2 min. Liquefaction time at
-10ºC 10 min
.
Variation of solvent recovery percent with the length (L) of
condensation tube inside 1000 mL extraction device. The volume
of acetone 0.5 mL, vaporization time 2 min. Liquefaction time at
-10ºC 10 min
Optimization of vaporization time
0.5 mL acetone containing octanal (80 μg/mL) was introduced into 1000 mL extraction device and exposed to ultrasonic irradiation for 1, 2, 5, 10, 15 and 20 minutes. Co-liquefaction procedure was carried out at -10°C for 15 minutes. The extraction efficiency of octanal was performed by GC analyzing of 2 μL of liquefied acetone. The results are illustrated in (Fig. 3). From these results, the extraction efficiency of the studied compound increases by increasing of vaporization time and remains constant after 2 minutes exposure of the device to ultrasonic irradiation. To investigate the effect of the nature of VOCs on the vaporization time, the same experiments have been repeated for benzene with significantly lower polarity and boiling point. The results obtained (Fig. 3) prove that the vaporization time do not depend on the nature of volatile compounds. These are reasonable results because this step is for total transformation of acetone in vapor or mist forms and mixing with gaseous analytes.
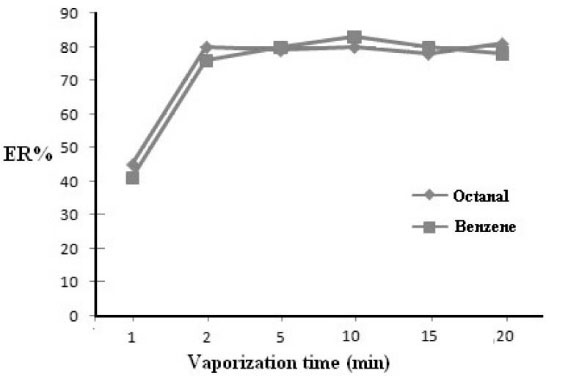
Fig. 3.
Variation of extraction efficiency of octanal versus
vaporization time. Volume of acetone 0.5 mL, octanal concentration
in gaseous media 40 µg/L, liquefaction time at -10ºC 10 min. 2 µL
of extract was injected in GC with split less mode.
.
Variation of extraction efficiency of octanal versus
vaporization time. Volume of acetone 0.5 mL, octanal concentration
in gaseous media 40 µg/L, liquefaction time at -10ºC 10 min. 2 µL
of extract was injected in GC with split less mode.
Optimization of liquefaction time
The same experiment was repeated. After 2 minutes exposure of the extraction vessel to ultrasonic irradiation, the liquefaction procedure was performed at -10°C for different time durations. The extraction efficiency of octanal and benzene was performed by GC analyzing of 2 μL liquefied acetone. From the results obtained (Fig. 4) the extraction efficiency of the studied compounds increases by increasing of cooling time and reaches a maximum at 10 and 15 minutes for octanal (b.p. =171°C) and benzene (b.p.=80°C) respectively and remains quasi constant thereafter. These results reveal that the nature of VOCs has only a little influence on the liquefaction time and 15 minutes liquefaction at -10°C was therefore selected for further experiments.
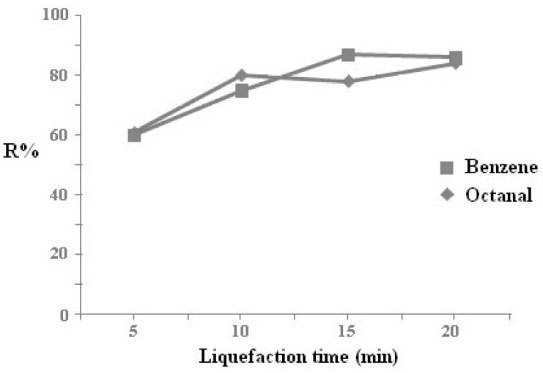
Fig. 4.
Variation of extraction efficiency of octanal versus
liquefaction time. The vaporization time was 2 min and other
experimental conditions were as Fig. 3.
.
Variation of extraction efficiency of octanal versus
liquefaction time. The vaporization time was 2 min and other
experimental conditions were as Fig. 3.
Extraction efficiency and enrichment factor
Extraction efficiency of octanal was calculated by using Eq. 1:
ER% = 100 (qE/q0) (Eq. 1)
Where q0 is the initial amount of the octanal in 1000 mL model gaseous sample (40 µg), qE is the extracted amount in extract (0.45 mL). qE was calculated according to Eq. 2:
qE= q1 (VR/V1) (Eq. 2)
q1 represents the amount (µg) of octanal from recovered volume of extraction solvent (extract). VR and V1 are the recovered volume of extract (mL) and the injected volume of extract (mL) respectively. q1 was calculated using Eq. 3:
q1/q2 = S1/S2 (Eq. 3)
Where q2 is the amount of analyte injected from standard solution (C0=80 µg mL-1). S1 and S2 are the chromatographic peak area of q1 and q2 microgram of analyte, respectively.
q2 was calculated using Eq. 4:
q2= C0× V2 (Eq. 4)
V2 is injected volume of extract (mL).
Enrichment factor of the proposed method was calculated according to Eq. 5:
EF = (ER/100) (Vg/VR) (Eq. 5)
Vg and VR represent volume of model gaseous sample (500 mL) and the volume of extract (0.45 mL), respectively. Experimental measured and calculated data are presented in (Table 1).
Table 1.
Enrichment factor (EF) and extraction sufficiency (EF%) of the proposed extraction method
|
Experimental data (measured)
|
C
0
(µg mL
-1
)
|
q
0
(µg)
|
S
1
|
S
2
|
V
1
|
V
2
(mL)
|
Vg (mL)
|
|
80.0
|
40.0
|
207.2
|
199.9
|
2 (µL) or 0.002 mL
|
2 (µL) or 0.002 mL
|
1000
|
| Calculated data |
q2 (Eq. 4)
|
q1(Eq. 3)
|
qE(Eq. 2)
|
ER% (Eq. 1) |
EF (Eq. 5) |
|
|
| 0.160 (µg) |
0.15 (µg) |
34.73 (µg) |
86.83% |
1929.55 |
|
|
Analytical approach
Characteristics quantification data including repeatability, limits of detection, linear dynamic range, limit of quantification, coefficient of determination for the calibration graph and relative standard deviation obtained by using the proposed method are presented in (Table 2). The wide linear range of calibration curve, good coefficient of determination, repeatability, low LODs and LOQs were achieved.
Table 2.
Characteristics calibration graph and analytical data for the studied compound using co-liquefaction extraction and capillary GC-FID
|
Calibration graph equation
|
R
2a
|
LOD
b
(ng mL-1)
|
LOQ
c
(ng mL-1)
|
LDR
d
(ng mL-1)
|
RSD%
f
(n = 3)
|
EF
g
|
| y = 36.517x-33.653 |
0.999 |
0.008 |
0.026 |
0.026-400 |
3% |
> 1900 |
a Correlation coefficient; b Limit of detection; cLimit of quantification; d Linear dynamic range; f Relative standard deviation; g Enrichment factor.
Comparison of the proposed method with other methods
Table 3 summarizes the characteristics of some other analytical methods with those of the proposed co-liquefaction method for the extraction and determination of VOCs in EB. Most of the characteristics of the proposed method are good and comparable with other corresponding methods some of them which are very expensive and time consuming.
Table 3.
Comparison of some methods for the sampling and enrichment of VOCs in exhaled breath
|
Method
|
LDR
a
(µg L-1)
|
LOD
b
(µg L-1)
|
RSD
c
%
|
EF
d
|
Cost
|
Remarks
|
References
|
| SPME-GC-MS-Chemometry |
2 order of magnitude |
0.31 |
<10% |
- |
Expensive |
- Universal (All VOCs)
- Limited extraction capacity
- Real profile of VOCs is not always attainable
|
18
|
| On-fiber SPME-GC-MS |
2 order of magnitude |
0.0001 |
7-15% |
- |
Expensive |
- Selective (C1-C10 Aldehydes)
- Limited extraction capacity
- Realprofile of VOCs is not always attainable
|
10,20
|
| Co-liquefaction-GC |
5 order of magnitude |
0.008 |
3% |
> 1900
|
Non-expensive |
- Universal (All VOCs)
-Unlimited extraction capacity
-Real profile of VOCs is always attainable
|
This work |
a Linear dynamic range; b Limit of detection; c Relative standard deviation; d Enrichment factor.
Applications
The proposed method was validated by isolation and enrichment of VOCs from twelve breathing samples. The extraction and enrichment were performed at the optimum conditions and the monitoring of VOCs in extract (condensed liquid) was performed by GC-FID and GC-MS. More than one hundred compounds classified into 15 distinct chemical classes such as hydrocarbons, halogenated hydrocarbons, organic acids, esters, alcohols, aldehydes, ketones, amines, amides, oximes, phthalates and other oxygenated compounds are identified in EB samples (Table 4). Only few compounds such as 1-dodecene; cycloheptane; 2-tetradecene, (E)-tetradecane; butylated hydroxytoluene; 1,2-benzenedicarboxylic acid and 1,1,2-trimethyl-cycloundecane are commonly found in all human’s EB but the presence and concentration of the majority of VOCs varies between each person. From these results, however, octanal is found only in EB of lung cancer before beginning of any treatment. Due to the high enrichment factor of the proposed method (EF >1900 for octanal), quantitative analysis of octanal in extract can be performed by GC-FID. The determined octanal amount in the studied EB and representative GC-FID chromatograms of EB extract of patients with lung cancer are shown in (Table 5) and (Fig. 5). From these results, the concentration of octanal in EB of patient before any treatment is about
1 ng/mL, whereas its concentration in EB of patient after beginning of treatment and healthy controls is lower than method LOD. These results reveal the high efficiency of the proposed extraction method for easy and effective collection and enrichment of VOCs from gaseous samples (EB) which allows quantitative analysis by relatively easy and inexpensive GC-FID method instead of more sophistic and expensive GC-MS method. It must be noted that the numbers of patients in this study were very limited to properly evaluate the test performance with respect to early recognition of lung cancer. Exhaled aldehydes concentrations should have been determined in a larger number of healthy controls, lung cancer in different stages before and after radiotherapy or chemotherapy. However, the main objective of this study was to develop a new, rapid, simple, inexpensive, universal and integrative gas sampling, isolation and enrichment of exhaled VOCs for their analysis with GC-FID instead of more sophistic and expensive GC-MS method.
Table 4.
Volatile organic compounds found in the studied exhaled breath samples
|
No
|
Name of compounds
|
No
|
Name of compounds
|
| Hydrocarbons |
39 |
2-Tetradecene, (E)- |
| 1 |
2-Dodecene, (Z)- |
40 |
4-Tetradecene, (Z)- |
| 2 |
3-Dodecene, (E)- |
41 |
1-Hexadecene |
| 3 |
3-Decene |
42 |
7-Hexadecene, (Z)- |
| 4 |
Cyclopentane, pentyl- |
43 |
Heptadecene |
| 5 |
1-Decene |
44 |
5-Octadecene, (E)- |
| 6 |
1-Nonene |
45 |
17-Pentatriacontene |
| 7 |
6-Dodecene, (E)- |
46 |
1-Octadecene |
| 8 |
1-Tetradecene |
47 |
1-Nonadecene |
| 9 |
Nonane, 5-(1-methylpropyl)- |
48 |
6,11-Dimethyl-2,6,10-dodecatrien… |
| 10 |
1-Undecene, 5-methyl- |
49 |
4,8,12-Tetradecatrienal, 5,9,13-… |
| 11 |
5-Tetradecene, (E)- |
50 |
Docosa-2,6,10,14,18-pentaen-22-a… |
| 12 |
4-Undecene, 5-methyl-, (Z)- |
51 |
Squalene |
| 13 |
1-Tridecene |
52 |
Z-8-Hexadecene |
| 14 |
Cyclopropane, 1-hexyl-2-propyl-,… |
Halogenated Hydrocarbons |
| 15 |
1-Dodecene |
53 |
Acetic acid, trifluoro-, decyl e… |
| 16 |
Decane |
54 |
Acetic acid, trifluoro-, octyl e… |
| 17 |
3-Undecene, 9-methyl-, (E)- |
55 |
1-Fluorononane |
| 18 |
Hexadecane, 1,1-bis(dodecyloxy)- |
56 |
Dodecane, 1-fluoro- |
| 19 |
cis-1-Butyl-2-methylcyclopropane |
57 |
Pentafluoropropionic acid, hexad... |
| 20 |
Tritetracontane |
58 |
Pentafluoropropionic acid, octad... |
| 21 |
5-Dodecene, (E)- |
59 |
Pentafluoropropionic acid, undec... |
| 22 |
4-Dodecene |
60 |
Pentafluoropropionic acid, octad... |
| 23 |
Cyclopropane, 1-ethyl-2-heptyl- |
61 |
Pentafluoropropionic acid, octad… |
| 24 |
Tetracontane, 3,5,24-trimethyl- |
62 |
Heptafluorobutyryloxydecane |
| 25 |
1-Decanol, 2-hexyl- |
63 |
Acetic acid, trifluoro-, decyl e... |
| 26 |
Cycloheptane |
64 |
Pentafluoropropionic acid, nonyl |
| 27 |
Cyclopentane, butyl- |
65 |
Heptafluorobutanoic acid, heptad… |
| 28 |
6-Tetradecene, (E)- |
66 |
Heptafluorobutyric acid, n-octad... |
| 29 |
1R,2c,3t,4t-Tetramethylcyclo-hexane |
67 |
Octane, 2-chloro- |
| 30 |
3-Octene, 4-ethyl- |
68 |
Heptacosane, 1-chloro- |
| 31 |
1-Hexene, 3-methyl- |
69 |
Hexadecane, 1-chloro- |
| 32 |
3-Tetradecene, (E)- |
70 |
Trichloroacetic acid, dodecyl ester |
| 33 |
Tetradecane |
71 |
Trichloroacetic acid, tetradecyl... |
| 34 |
Cycloundecane, 1,1,2-trimethyl- |
72 |
Trichloroacetic acid, pentadecyl… |
| 35 |
7-Tetradecene, (E)- |
73 |
Dichloroacetic acid, heptadecyl ... |
| 36 |
3-Undecene, (Z)- |
74 |
2- Chloropropionic acid, octadec… |
| 37 |
Cyclododecane |
75 |
Hexadecane, 1-bromo- |
| 38 |
Cycloundecane, 1,1,2 |
76 |
Trichloroacetic acid, 2-octyl ester |
| 77 |
Tridecane, 1-bromo- |
107 |
1-Nonadecanol |
| 78 |
Tetrapentacontane, 1,54-dibromo- |
108 |
Ethanol, 2-(tetradecyloxy)- |
|
|
siloxanes
|
109 |
Estra-1,3,5(10)-trien-17.beta.-ol |
| 79 |
Cyclotrisiloxane, hexamethyl- |
110 |
Ethanol, 2-(hexadecyloxy)- |
| 80 |
Cyclotetrasiloxane, octamethyl- |
|
Aldehydes
|
|
|
Citrates
|
111 |
Benzaldehyde, 2-nitro-4-trimethy… |
| 81 |
Butyl citrate |
112 |
Octanal |
| 82 |
Tributylacetylcitrate |
113 |
Butanal, 3-methyl- |
|
|
Organic acids
|
114 |
Hexanal |
| 83 |
Nonahexacontanoic acid |
115 |
2-Decenal, (E)- |
| 84 |
Tetradecanoic acid |
116 |
Heptanal |
| 85 |
9-Hexadecenoic acid |
117 |
Decanal |
| 86 |
Octadec-9-enoic acid |
|
Ketones
|
|
|
Esters
|
118 |
2-Pentanone, 4-hydroxy-4-methyl- |
| 87 |
Oxalic acid, decyl propyl ester |
|
Amines
|
| 88 |
Oxalic acid, isobutyl nonyl ester |
119 |
3,3’-Iminobispropylamine |
| 89 |
Oxalic acid, allylhexadecyl ester |
120 |
Benzenamine, N,N-diethyl-4-nitroso- |
| 90 |
Oxalic acid, allyl dodecyl ester |
121 |
1-Dodecanamine |
| 91 |
Oxalic acid, cyclobutyltetradec. |
122 |
Piperazine, 2-methyl- |
| 92 |
Oxalic acid, allylundecyl ester |
|
Amides
|
| 93 |
Oxalic acid, allyldecyl ester |
123 |
1,4-Benzenedicarboxamide, 2-nitro- |
| 94 |
Oxalic acid, allyltridecyl ester |
124 |
Ergoline-8-carboxamide, 9,10-did… |
| 95 |
Carbonic acid, hexadecyl 2,2,2-t… |
125 |
Oximes
|
| 96 |
Hexadecanoic acid, methyl ester |
126 |
Oxime-, methoxy-phenyl- |
| 97 |
1,2-Benzenedicarboxylic acid, bu… |
|
Phthalates
|
| 98 |
Heptadecanoic acid, heptadecyl e |
127 |
Didodecyl phthalate |
| 99 |
Sulfurous acid, 2-propyl tetrade… |
128 |
Ethylene brassylate |
| 100 |
Octadecanoic acid, methyl ester |
|
Other oxygenated compounds
|
| 101 |
Carbonic acid, octadecyl 2,2,2-t… |
129 |
Oxirane, [[(2-ethylhexyl)oxy]met... |
| 102 |
1,2-Benzenedicarboxylic acid, di… |
130 |
4-Propionyloxytridecane |
|
|
Alcohols
|
131 |
Octadecane, 1-(ethenyloxy)- |
| 103 |
1-Pentanol, 2-methyl- |
132 |
Octadecane, 1-(ethenyloxy)- |
| 104 |
1-Octanol, 2-butyl- |
133 |
ButylatedHydroxytoluene |
| 105 |
1-Octyn-3-ol |
134 |
Aspidospermidin-17-ol, 1-acetyl-… |
| 106 |
1-Heptadecanol |
|
|
Table 5.
Co-liquefaction extraction and GC-FID analysis of octanal in exhaled breath
|
Exhaled breath sample
|
Concentration (ng/mL)
|
| Healthy volunteers |
ND |
| Patient with lung cancer after beginning of treatment |
ND |
| Patient with lung cancer before beginning of treatment |
1-1.5 |
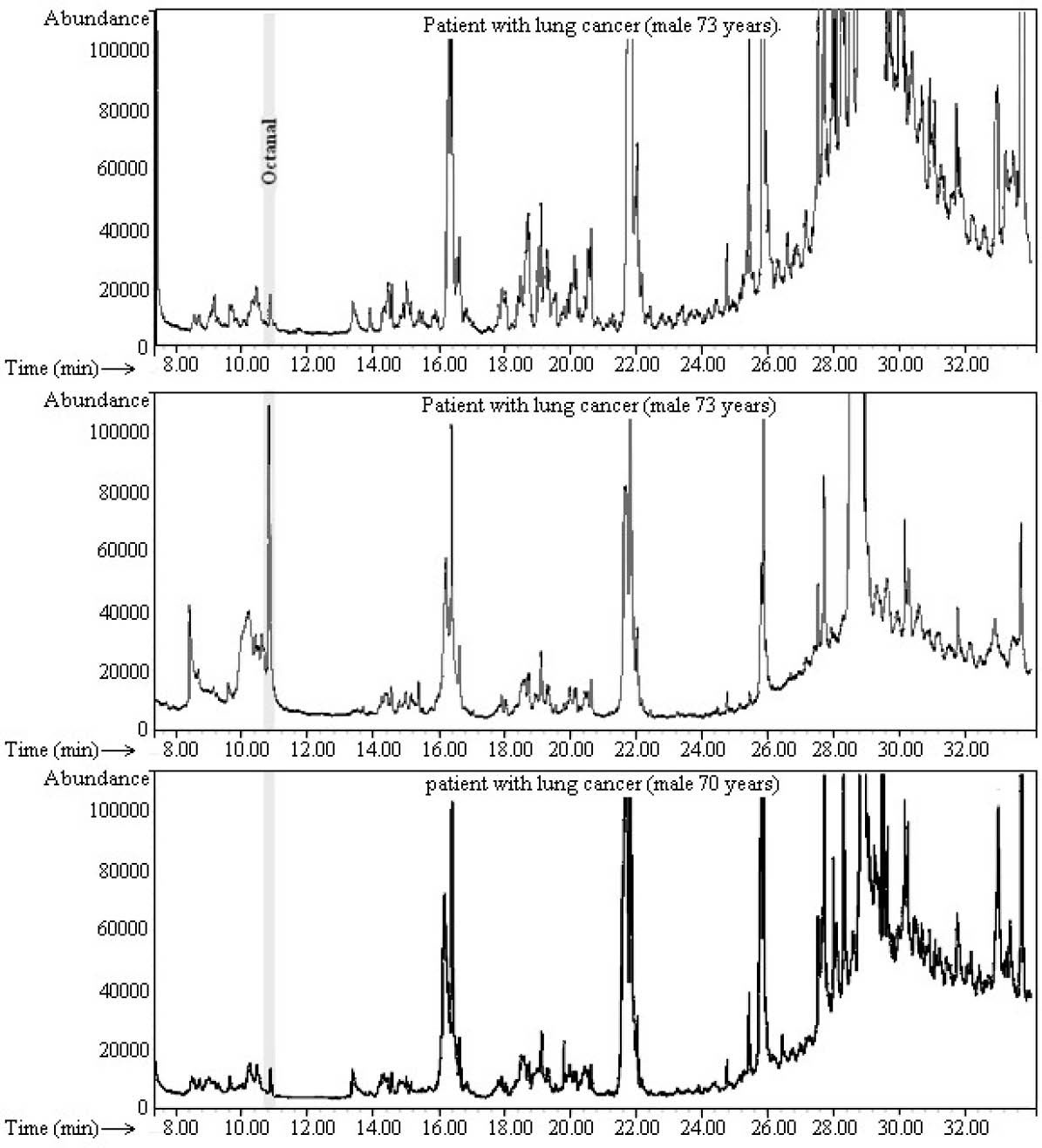
Fig. 5.
Representative GC-FID chromatograms of exhaled breath extract of patients with lung cancer. Volume of breathing 1000 mL,
volume of extraction solvent (acetone) 0.5 mL, vaporization time 2, liquefaction time at -10 ºC 10 min. 2 µL of extract was injected in GC
with split less mode.
.
Representative GC-FID chromatograms of exhaled breath extract of patients with lung cancer. Volume of breathing 1000 mL,
volume of extraction solvent (acetone) 0.5 mL, vaporization time 2, liquefaction time at -10 ºC 10 min. 2 µL of extract was injected in GC
with split less mode.
Conclusion
A new method for the isolation and concentration of VOCs from exhaled breath samples was investigated. The proposed method is based on co-liquefaction of the trace and ultra trace amounts of analytes existing in human exhaled breath accompanying with small volume of extraction solvent. The advantage of this adsorbent-free method is integrating the sampling and sample preparation steps which reduces the analysis time and simplifies the analysis procedure. Moreover, no adsorbent is required for adsorption and trapping of the analytes which reduces the risk of memory effect and sample pollution. Also high concentration factor can be achieved at a reasonable time without need to any expensive devices (EF >1900). The main drawbacks of the presented method include; 1) using some common chemicals in the procedure, i.e. acetone could be found in the lab air which is used as a solvent.
It could also be originated from breath samples. 2) the method was tested on only two VOC, and should be further investigated using other VOCs. Using the proposed method, high extraction recovery of up to 86% is obtainable for octanal as an important lung cancer marker. Some real samples including exhaled breath of healthy controls, patients with lung cancer before and after beginning of treatment were analyzed to demonstrate the effectiveness of the developed method.
Future perspective
EB and EBC analyses provide non-invasive samples to be analyzed for monitoring endogenous and exogenous analytes. Due to very low concentration of analytes in these samples more sophisticated and expensive analytical methods are usually employed. Early diagnosis of serious diseases like cancer provides great opportunity to better management of the patients and reducing the cost of health services. Low cost and easily conductible screening methods are in demand in the health care systems.
As noticed above and discussed in this work, EB analysis are conducted using high cost and complex methods. The presented method in this work is a simple and low cost screening method to monitor volatile analytes in EB. The applicability of the method was shown using small number of patient and healthy EB samples and the method could be employed in clinical investigations, especially in screening tests on a large populations for providing prospective studies on serious health problems such as cancer, lung and heart diseases.
Competing interests
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Ethical approval
The authors state that they have obtained appropriate institutional review board approval and followed the principles outlined in the Declaration of Helsinki for human investigations. In addition, informed consent has been obtained from the participants involved.
Research Highlights
What is current knowledge?
simple
-
√ Exhaled breath is an alternative sample to invasive blood
samples.
-
√ High cost and sophisticated setups restrict analysis of trace
levels of analytes in exhaed breath.
What is new here?
simple
-
√ An efficient pre-concentration method to enrich trace
levels is developed.
-
√ The method is successfully applied to monitor octanal
levels in lung cancer patients.
References
- Liu H, Dasgupta PK. Analytical chemistry in a drop Solvent extraction in a microdrop. Anal Chem 1996; 68:1817-1821. doi: 10.1021/ac960145h [Crossref] [ Google Scholar]
- Ligor T, Buszewski B. Isolation of trace organic pollutants from aqueous samples by single drop method. Chromatographia 2000; 52:279-282. [ Google Scholar]
- Alpendurada MF. Solid-phase microextraction: a promising technique for sample preparation in environmental analysis. J Chromatogr A 2000; 889:3-14. doi: 10.1016/S0021-9673(00)00453-2 [Crossref] [ Google Scholar]
- Safarova V I, Sapelnikova SV, Djazhenko EV, Teplova GI, Shajdulina GF, Kudasheva FKh. Gas chromatography–mass spectrometry with headspace for the analysis of volatile organic compounds in waste water. J Chromatogr B 2004; 800:325-330. doi: 10.1016/j.jchromb.2003.10.070 [Crossref] [ Google Scholar]
- Djozan Dj, Baheri T, Farshbaf R, Azhari Sh. Investigation of solid-phase microextraction efficiency using pencil lead fiber for in vitro and in vivo sampling of defensive volatiles from insect's scent gland. Anal Chim Acta 2005; 554:197-201. doi: 10.1016/j.aca.2005.08.049 [Crossref] [ Google Scholar]
- Djozan Dj, Movafeghi A, Razeghi J, Baheri T. Solid phase microextraction of volatile organic compounds released from leaves, roots and gum of Astragalus microcephalus Willd, followed by GC and GC/MS analysis. Nat Prod Res 2008; 24:1660-1669. doi: 10.1080/14786410701877930 [Crossref] [ Google Scholar]
- Grosch W. Evaluation of the key odorants of foods by dilution experiments, aroma models and omission. Chem Senses 2001; 26:533-545. [ Google Scholar]
- Eom IY, Tugulea AM, Pawliszyn J. Development and application of needle trap devices. J Chromatogr A 2008; 3(9):1196-1197. doi: 10.1016/j.chroma.2008.02.090 [Crossref] [ Google Scholar]
- Phillips M, Herrera J, Krishnan S, Zain M, Greenberg J, Cataneo RN. Variation in volatile organic compounds in the breath of normal humans. J Chromatogr B Biomed Sci Appl 1999; 729:75-88. doi: 10.1016/S0378-4347(99)00127-9 [Crossref] [ Google Scholar]
- Phillips M, Cataneo RN, Ditkoff BA, Fisher P, Greenberg J, Gunawardena R, Kwon CS, Rahbari-Oskoui Rahbari-Oskoui, F F, Wong C. Volatile markers of breast cancer in the breath. Breast J 2003; 9:184-191. doi: 10.1046/j.1524-4741.2003.09309.x [Crossref] [ Google Scholar]
- Ras MR, Borrull F, Marce RM. Sampling and preconcentration techniques for determination of volatile organic compounds in air samples. Trends Anal Chem 2009; 28:347-361. doi: 10.1016/j.trac.2008.10.009 [Crossref] [ Google Scholar]
- Horváth I, Lázár Z, Gyulai N, Kollai M, Losonczy G. Exhaled biomarkers in lung cancer. Eur Respir J 2009; 34:261-275. doi: 10.1183/09031936.00142508 [Crossref] [ Google Scholar]
- uszewski B, Kesy M, Ligor T, Amann A. Human exhaled air analytics: biomarkers of diseases. Biomed Chromatogr 2007; 21:553-566. doi: 10.1002/bmc.835 [Crossref] [ Google Scholar]
- Deng C, Zhang J, Yu X, Zhang W, Zhang X. Determination of acetone in human breath by gas chromatography–mass spectrometry and solid-phase microextraction with on-fiber derivatization. J Chromatogr B 2004; 810:269-275. doi: 10.1016/j.jchromb.2004.08.013 [Crossref] [ Google Scholar]
- Rudnicka J, Kowalkowski T, Ligor L, Buszewski B. Determination of volatile organic compounds as biomarkers of lung cancer by SPME–GC–TOF/MS and chemometrics. J Chromatogr B 2011; 879:3360-3366. doi: 10.1016/j.jchromb.2011.09.001 [Crossref] [ Google Scholar]
- Phillips M, Gleeson K, Hughes J, Greenberg J, Cantaneo R, Baker L, McVay W. Volatile organic compounds in breath as markers of lung cancer: a cross-sectional study. Lancet 1999; 353:1930-1933. doi: 10.1016/S0140-6736(98)07552-7 [Crossref] [ Google Scholar]
- Buszewski B, Ulanowska A, Kowalkowski T, Cieśliński K. Investigation of lung cancer biomarkers by hyphenated separation techniques and chemometrics. Clin Chem Lab Med 2012; 50:573-581. doi: 10.1515/cclm.2011.769 [Crossref] [ Google Scholar]
- Wang Y, Hu Y, Wang D, Yu K, Wang L, Zou Y, Zhao C, Zhang X, Wang P, Ying K. The analysis of volatile organic compounds biomarkers for lung cancer in exhaled breath, tissues and cell lines. Cancer Biomark 2012; 11:129-137. doi: 10.3233/CBM-2012-00270 [Crossref] [ Google Scholar]
- Buszewski B, Rudnicka J, Ligor T. Analytical and unconventional methods of cancer detection using odor. Trends Anal Chem 2012; 38:1-12. doi: 10.1016/j.trac.2012.03.019 [Crossref] [ Google Scholar]
- Altomare DF, Di Lena M, Porcelli F, Trizio L, Travaglio E, Tutino M, Dragonieri S, Memeo V, De Gennaro G. Exhaled volatile organic compounds identify patients with colorectal cancer. Br J Surg 2013; 100:144-150. doi: 10.1002/bjs.8942 [Crossref] [ Google Scholar]
- Phillips M, Altorki N, Austin JH, Cameron RB, Cataneo RN, Kloss R, Maxfield RA, Munawar MI, Pass HI, Rashid A, Rom WN, Schmitt P, Wai J. Detection of lung cancer using weighted digital analysis of breath biomarkers. Clin Chim Acta 2008; 393:76-84. doi: 10.1016/j.cca.2008.02.021 [Crossref] [ Google Scholar]
- Phillips M, Cataneo RN, Cummin AR, Gagliardi AJ, Gleeson K, Greenberg J, Maxfield RA, Rom WN. Detection of lung cancer with volatile markers in the breath. Chest 2003; 123:2115-2123. doi: 10.1378/chest.123.6.2115 [Crossref] [ Google Scholar]
- Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens DNA damage and p53 mutations in smoking associated cancers. Oncogene 2002; 21:7435-51. doi: 10.1038/sj.onc.1205803 [Crossref] [ Google Scholar]
- Wahl HG, Hoffmann A, Haring HU, Liebich HM. Identification of plasticizers in medical products by a combined direct thermodesorption-cooled injection system and gas chromatography-mass spectrometry. J Chromatogr A 1999; 847:1-7. doi: 10.1016/S0021-9673(99)00138-7 [Crossref] [ Google Scholar]
- Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res 1991; 51:794-798. [ Google Scholar]
- Toyokuni S. Molecular mechanisms of oxidative stress-induced carcinogenesis: from epidemiology to oxygenomics. IUBMB Life 2008; 60:441-7. doi: 10.1002/iub.61 [Crossref] [ Google Scholar]
- Di Natale C, Paolesse R, Martinelli E, Capuano R. Solid-state gas sensors for breath analysis: A review. Anal Chim Acta 2014; 824:1-17. doi: 10.1016/j.aca.2014.03.014 [Crossref] [ Google Scholar]
- Poli D, Goldoni M, Corradi M, Acampa O, Carbognani P, Internullo E, Casalini A, Mutti A. Determination of aldehydes in exhaled breath of patients with lung cancer by means of on-fiber-derivatisation SPME–GC/MS. J Chromatogr B 2010; 878:2643-2651. doi: 10.1016/j.jchromb.2010.01.022 [Crossref] [ Google Scholar]
- Fuchs P, Loeseken C, Schubert JK, Miekisch W. Breath gas aldehydes as biomarkers of lung cancer. Int J Cancer 2010; 126:2663-2670. doi: 10.1002/ijc.24970 [Crossref] [ Google Scholar]
- Chan HP, Lewis C, Thomas PS. Exhaled breath analysis: novel Novel approach for early detection of lung cancer. Lung Cancer 2009; 63:164-168. doi: 10.1016/j.lungcan.2008.05.020 [Crossref] [ Google Scholar]
- Poli D, Goldoni M, Corradi M, Acampa O, Carbognani P, Internullo E, Casalini A, Mutti A. Determination of aldehydes in exhaled breath of patients with lung cancer by means of on-fiber-derivatisation SPME–GC/MS. J Chromatogr B 2010; 878:2643-2651. doi: 10.1016/j.jchromb.2010.01.022 [Crossref] [ Google Scholar]
- Poli D, Carbognani P, Corradi M, Goldoni M, Acampa O, Balbi B, Bianchi L, Rusca M, Mutti A. Exhaled volatile organic compounds in patients with non-small cell lung cancer: cross sectional and nested short-term follow-up study. Respir Res 2005; 6:71. doi: 10.1186/1465-9921-6-71 [Crossref] [ Google Scholar]
- Johnson GR, Morawska L. The mechanism of breath aerosol formation. J Aerosol Med Pulm Drug Deliv 2009; 22:229-237. doi: 10.1088/1752-7155/3/1/016005 [Crossref] [ Google Scholar]
- Kuban P, Foret F. Exhaled breath condensate: Determination of non-volatile compounds and their potential for clinical diagnosis and monitoring: A review. Anal Chim Acta 2013; 805:1-18. doi: 10.1016/j.aca.2013.07.049 [Crossref] [ Google Scholar]
- Natale CD, Paolesse R, Martinelli E, Capuano R. Solid-state gas sensors for breath analysis: A review. Anal Chim Acta 2014; 824:1-17. doi: 10.1016/j.aca.2014.03.014 [Crossref] [ Google Scholar]
- Dodig S, Cepelak I. Exhaled breath condensate – from an analytical point of view. Biochem Med 2013; 23:281-295. doi: 10.11613/BM.2013.034 [Crossref] [ Google Scholar]
- Amann A, Costello BDL, Miekisch W, Schubert J, Buszewski B, Pleil J, Ratcliffe N, Risby T. The human volatilome: Volatile organic compounds (VOCs) in exlahed breath, skin emanations, urine, feces and saliva. J Breath Res 2014; 8:034001. doi: 10.1088/1752-7155/8/3/034001 [Crossref] [ Google Scholar]
- Khoubnasabjafari M, Ansarin K, Jouyban A. Review of exhaled biomarkers in different pulmonary diseases Med J Tabriz Uni Med Sc. Heath Serv 2013; 35:96-105. [ Google Scholar]
-
Jouyban A, Khoubnasabjafari M, Ansarin K, Jouyban-Gharamaleki V. Breath sampling setup. Iranian Patent, 2013; 81363.
- Zamani-Kalajahi M, Hasanzadeh M, Shadjou N, Khoubnasabjafari M, Ansarin K, Jouyban-Gharamaleki V, Jouyban A. Electrodeposition of taurine on gold surface and electro-oxidation of malondialdehyde. Surf Eng 2015; 31(3):194-201. doi: 10.1179/1743294414Y.0000000349 [Crossref] [ Google Scholar]
- Casimirri E, Stendardo M, Bonci M, Andreoli R, Bottazzi B, Leone R, Schito M, Vaccari A, Papi A, Contoli M, Corradi M, Boschetto P. Biomarkers of oxidative-stress and inflammation in exhaled breath condensate from hospital cleaners. Biomarkers 2016; 21(2):115-122. doi: 10.3109/1354750X.2015.1118541 [Crossref] [ Google Scholar]
- Khoubnasabjafari M, Ansarin K, Jouyban-Gharamaleki V, Panahi-Azar V, Shayanfar A, Mohammadzadeh L, Jouyban A. Extraction and analysis of methadone in exhaled breath condensate using a validated HPLC-UV method. J Pharm Pharmaceut Sci 2015; 18:207-219. [ Google Scholar]
- Meyer MR, Rosenborg S, Stenberg M, Beck O. First report on the pharmacokinetics of tramadol and O-desmethyltramadol in exhaled breath compared to plasma and oral fluid after a single oral dose
. Biochem Pharmacol 2015l; 98:502-510. doi: 10.1016/j.bcp.2015.09.008 [Crossref] [ Google Scholar]
- Beck O, Stephanson N, Sandqvist S, Franck J. Determination of amphetamine and methylphenidate in exhaled breath of patients undergoing attention-deficit/hyperactivity disorder treatment. Ther Drug Monit 2014; 36(4):528-534. doi: 10.1097/FTD.0000000000000046 [Crossref] [ Google Scholar]
- Demeestere K, Dewulf J, De Witte B, Van Langenhove H. Sample preparation for the analysis of volatile organic compounds in air and water matrices. J Chromatogr A 2007; 1153:130-144. doi: 10.1016/j.chroma.2007.01.012 [Crossref] [ Google Scholar]
- Money CD, Gray CN. Exhaled breath analysis as a measure of workplace exposure to benzene ppm. Ann Occup Hyg 1989; 33:257-262. doi: 10.1093/annhyg/33.2.257 [Crossref] [ Google Scholar]
- Lee K, Yanagisawa Y. Sampler for measurement of alveolar carbon monoxide. Environ Sci Technol 1995; 29:104-107. doi: 10.1021/es00001a013 [Crossref] [ Google Scholar]
- Ljungkvist GM, Nordlinder RG. A field method for sampling benzene in end-exhaled air. Am Ind Hyg Assoc J 1995; 56:693-697. doi: 10.1080/15428119591016719 [Crossref] [ Google Scholar]
- Song G, Qin T, Liu H, Xu GB, Pan YY, Xiong FX, Gu KS, Sun GP, Chen ZD. Quantitative breath analysis of volatile organic compounds of lung cancer patients. Lung Cancer 2010; 67:227-231. doi: 10.1016/j.lungcan.2009.03.029 [Crossref] [ Google Scholar]
- Li J, Hou C, Huo D, Yang M, Fa HB, Yang P. Development of a colorimetric sensor Array for the discrimination of aldehydes. Sens Actuators B 2014; 196:10-17. doi: 10.1016/j.snb.2014.01.054 [Crossref] [ Google Scholar]
- Gaspar EM, Lucena AF, Duro da Costa J, Chaves das Neves H. Organic metabolites in exhaled human breath—A multivariate approach for identification of biomarkers in lung disorders. J Chromatogr A 2009; 1216:2749-2756. doi: 10.1016/j.chroma.2008.10.125 [Crossref] [ Google Scholar]
- Buszewski B, Ulanowska A, Ligor T, Denderz N, Amann A. Analysis of exhaled breath from smokers, passive smokers and non-smokers by solid-phase microextraction gas chromatography/mass spectrometry. Biomed Chromatogr 2009; 23:551-556. doi: 10.1002/bmc.1141 [Crossref] [ Google Scholar]
-
Edge SB, Byrd DR, Compton CC, Fritz AG, Green FL, Trotti A. AJCC cancer staging Manual. 7th ed. New York: Springer; 2010.