BioImpacts. 7(4):227-239.
doi: 10.15171/bi.2017.27
Original Research
Development and evaluation of exemestane-loaded lyotropic liquid crystalline gel formulations
Muhammad Nuh Musa 1, Sheba Rani David 1, *  , Ihsan Nazurah Zulkipli 1
, Ihsan Nazurah Zulkipli 1  , Abdul Hanif Mahadi 2, Srikumar Chakravarthi 3, Rajan Rajabalaya 1, *
, Abdul Hanif Mahadi 2, Srikumar Chakravarthi 3, Rajan Rajabalaya 1, * 
Author information:
1PAPRSB Institute of Health Sciences, Universiti Brunei Darussalam, Bandar Seri Begawan BE 1410, Brunei Darussalam
2Centre for Advanced Material and Energy Sciences (CAMES), Universiti Brunei Darussalam, Bandar Seri Begawan BE 1410, Brunei Darussalam
3School of Medicine, Perdana University, Jalan MAEPS Perdana, 43400 Serdang, Selangor, Malaysia
Abstract
Introduction:
The use of liquid crystalline (LC) gel formulations for drug delivery has considerably improved the current delivery methods in terms of bioavailability and efficacy. The purpose of this study was to develop and evaluate LC gel formulations to deliver the anti-cancer drug exemestane through transdermal route.
Methods:
Two LC gel formulations were prepared by phase separation coacervation method using glyceryl monooleate (GMO), Tween 80 and Pluronic® F127 (F127). The formulations were characterized with regard to encapsulation efficiency (EE), vesicle size, Fourier transform infrared (FTIR) spectroscopy, surface morphology (using light and fluorescence microscopy), in vitro release, ex vivo permeation, in vitro effectiveness test on MDA-MB231 cancer cell lines and histopathological analysis.
Results:
Results exhibited that the EE was 85%-92%, vesicle size was 119.9-466.2 nm while morphology showed spherical vesicles after hydration. An FTIR result also revealed that there was no significant shift in peaks corresponding to Exemestane and excipients. LC formulations release the drug from cellulose acetate and Strat-MTM membrane from 15%-88.95%, whereas ex vivo permeation ranges from 37.09-63%. The in vitro effectiveness study indicated that even at low exemestane concentrations (12.5 and 25 μg/mL) the formulations were able to induce cancer cell death, regardless of the surfactant used. Histopathological analysis thinning of the epidermis as the formulations penetrate into the intercellular regions of squamous cells.
Conclusion:
The results conjectured that exemestane could be incorporated into LC gels for the transdermal delivery system and further preclinical studies such as pharmacokinetic and pharmacodynamic studies will be carried out with suitable animal models.
Keywords: Breast cancer, Exemestane, Lyotropic liquid crystals, Surfactants, Transdermal delivery, Tween 80
Copyright and License Information
© 2017 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Exemestane, an aromatase inhibitor, is used to treat breast cancer in high-risk post-menopausal women.
1
It works by blocking the binding of androgen to aromatase, thus preventing the formation of estrogen.
2
However the current treatment method of consuming the Exemestane tablets has some undesired effects, including osteoporosis.
2,3
One way to minimize this is to deliver exemestane directly via the transdermal route with the aid of an LC gel formulation. One type of liquid crystalline (LC) gel, the lyotropic LC (LLC) gel has garnered unprecedented attention in recent years as it can incorporate hydrophobic, hydrophilic or amphiphilic drugs, which makes them preferential choice as a drug delivery vehicle.
4–7
The LLC gels can be categorized into three phases, namely; lamellar, cubic and hexagonal. These phases are achieved by varying the amount of water, lipids or surfactants within the formulations during the preparation stage.
8
Formulations based on LLC gel can be prepared based on the coacervation phase separation method, that incorporates an aqueous phase to an oil phase.
9-11
Upon cooling, the LLC gel formulation is formed. The lipids are mostly non-toxic and biodegradable; therefore the use of these formulations will not engender harmful effects to the body.
12
Among the main advantages of using LLC, gel formulations are their capability to exhibit a sustained drug release particularly for the cubic and hexagonal phases, which was observed to follow the Higuchi release kinetic model.
13–15
The drug release from the LLC gel formulations can also be triggered or controlled when the external conditions are changed, such as the pH or temperature. One such study involved the use of phloroglucinol which was incorporated in an LLC that responds to changes in pH.
16
In the study, linoleic acid was incorporated into the formulation. The cubic phase was changed in the LLC, with the help of linoleic acid to the hexagonal phase. This change in phase ensured that phloroglucinol is released into the intended organ. Another advantage is that LLC gel formulations provide an increased drug bioavailability, which was observed in a study where cinnarizine was incorporated in formulations consisted of phytantriol, Pluronic® F127 (F127) and water and a sustained release was achieved.
17
Meanwhile, another study which used amphotericin B encapsulated within cubosomes or the cubic phase has produced a relative bioavailability of 285% compared to Fungizone®.
18
The main challenge for delivering drugs through the transdermal route is that they have to cross the stratum corneum (SC) layer. By incorporating the drugs within the LLC gel formulations, it is possible to overcome this barrier. This was demonstrated in a study where vitamin K was delivered with the help of glyceryl monooleate (GMO) and water-based formulations. The study exhibited that not only the vitamin K was localized within the SC layer, but also some managed to permeate through the skin.
19
Therefore, based on the issue concerning the side effects of administering Exemestane orally, the primary aims of this study are to develop Exemestane-loaded LLC gel formulations using various surfactants and to characterize these formulations for their in vitro release kinetics, ex vivo permeation, as well as to evaluate the efficacy through in vitro and histopathology studies.
Materials and Methods
Materials
The drug exemestane was acquired from PI Chemicals Ltd, Shanghai, China. GMO was procured from Croda International Plc, East Yorkshire, UK. The surfactant Tween 80 was acquired from EMD Millipore Corporation, Billerica, MA, USA. Glycerol, Pluronic® F127 (F127), cholesterol, phosphate buffer saline (PBS) tablets and Dulbecco’s modified Eagle’s medium (DMEM) were acquired from Sigma-Aldrich, St Louis, MO, USA. All other chemicals used were of analytical grade.
Exemestane-loaded LLC gel preparation
Preparation of the LLC gel formulations was carried out by varying the amounts of GMO, distilled water, and either surfactants; Tween 80 and F127. The melted GMO was incorporated to surfactant to prepare the oil phase. Exemestane was then mixed into the oil phase so that the formulations would contain 200 μg/mL of the drug. 1% of cholesterol w/v was added subsequently to stabilize the formulations. Separately, an aqueous phase was prepared by mixing together distilled water and glycerol. Both the phases were maintained at 60°C for about 10 minutes. The aqueous phase was then incorporated into the oil phase followed by thorough mixing by the coacervation phase separation method with the help of a disperser (T 10 basic ULTRA-TURRAX®, IKA® - Werke GmbH & Co.KG, Staufen, Germany).
9
The formulations were then allowed to set overnight at room temperature. Once the formulations have set, they were evaluated visually for opacity and viscosity so that the suitable formulations could be identified.
Encapsulation efficiency
The formulations were centrifuged at 10 000 rpm at a temperature of 4°C for an hour in two cycles (Eppendorf® Model 5810R, Eppendorf, Hamburg, Germany) to separate the drug-containing vesicles from the unentrapped drug. The supernatant and sediment were then recovered and the latter was lysed with methanol. This was then followed by filtering the sediment through a 0.45 µm nylon disk filter. Measurement of the free drug concentration in the supernatant was carried out using high-performance liquid chromatography (1200 HPLC series, Agilent Technologies, Santa Clara, CA, USA).
20
The percentage encapsulation efficiency was determined based on the following formula:
Particle size, zeta potential and polydispersity index (PDI)
Particle size, zeta potential, and PDI were measured using a nanoparticle analyzer based on dynamic light scattering (Horiba ‘nano partica’ SZ-100, Horiba Instruments Incorporated, Irvine, CA, USA). For particle size determination and zeta potential calculations, the diluted formulations were prepared with double distilled water for sonication in an ice bath for 30 seconds. Analysis of the sample was carried out at 173° scattering angle at 250°C. For PDI, the vesicular suspension was diluted with water prior to measurements.
pH measurement, spreadability, and drug stability studies
The pH of the formulations was measured by weighing accurately 0.1 g of the gel mixed with 50 mL of distilled water.
21–23
A disperser (T10 basic ULTRA-TURRAX®, IKA® Werke GmbH & Co.KG, Staufen, Germany) was used to disperse the gel uniformly throughout the solution. A digital pH meter (FiveEasy FE20, Mettler-Toledo AG Analytical, Schwerzenbach, Switzerland) was used to measure the pH value and was fulfilled in triplicate. The mean value was then taken.
The spreadability of the formulations was assessed by spreading the gel between two glass plates, which was done by placing 0.1 g of gel within a pre-marked circle with a diameter of 1 cm on the bottom plate. The upper plate was then placed carefully on top followed by a weight of 500 g. This setup was left for 5 minutes and the increase in diameter of spread was recorded in triplicate.
23
The mean value of triplicate was then taken.
The assessment of the stability of formulations were done by storing the formulations for 90 days in three distinct temperatures, namely fridge range (2–8°C), room range (25 ± 2°C) and high range (45 ± 2°C).
9
EE was evaluated for the samples which were sealed in aluminium foil, after 3 months.
20,24
Fourier transform infrared spectroscopy
FTIR spectra comparison of each of the components used namely, exemestane, GMO, Tween 80 and F127 and also formulation mixtures GMO/Tween 80/Exemestane and GMO/F127/exemestane were performed to determine changes in the spectrum. The FTIR was performed using an ATR-FTIR spectrometer (Agilent Cary 630 ATR-FTIR Spectrometer, Santa Clara, CA, USA) and the spectra were determined between 650 – 4000 cm-1 frequency with 4 cm-1 resolution.
Optical and fluorescence microscopy
The LC gel formulation was spread as a thin layer on a glass slide, after 1% dilution with PBS solution, and the slide was investigated under a light microscope to determine the presence of vesicles or drug precipitation. The aforementioned method was used for the fluorescence microscopy observation upon confirmation of the presence of vesicles. For the fluorescence microscopy, a fluorescein sodium salt (FSS) was added to both Tween 80 and F127-based LC gel formulations individually. The FSS is highly soluble in aqueous fluids but insoluble in non-aqueous medium. An optimized concentration of 0.5% FSS fluid was prepared for the direct visualization of the LLC vesicles. The LLC gel formulations were diluted with pH 7.4 PBS solution (0.5 mL) then FSS was mixed. A very thin layer of the LLC gel sample was smeared on the glass slide and was immediately observed for fluorescence with a CCD camera (Nikon Eclipse 90i, Nikon Corporation, Tokyo, Japan) using a TRITC filter.
In vitro release studies
Franz diffusion cells were used for carrying out the in vitro release studies for the formulations. A circular piece with a diameter of 2.5 cm either Strat-MTM or cellulose acetate membrane was placed on top of the donor side. The formulation (1 g) to be studied was weighed and placed on top of either Strat-MTM or cellulose acetate membrane. Meanwhile, 18 mL of freshly prepared PBS solution (pH of 7.4) was used in the receptor compartment, which was stirred between 40-50 rpm by a magnetic stirrer. Water was circulated continuously, to maintain 32 ± 0.5°C temperature, through the jacket surrounding the receptor compartment in order to mimic the temperature in the human skin.
The volume of the receptor liquid was fixed such the synthetic membrane touches the surface of the receptor liquid horizontally for the release of drug molecules. An aliquot of 1.0 mL was taken every hour from the receptor liquid throughout the 8 hour period and replaced immediately with fresh PBS solution. Then the absorbance value of the sample was determined with UV spectrophotometer at 290 nm after a necessary dilution. The average of triplicate was noted.
20,25
The in vitro release data from both Strat-MTM and cellulose membranes were then analyzed with available kinetic models to establish the mechanism of Exemestane release.
Ex vivo permeation studies
Ex vivo studies followed the same methodology as the in vitro release studies using the Strat-MTM and cellulose acetate membranes, but the membrane was replaced with the abdominal skin of a female Sprague-Dawley rat. Each rat was sacrificed by cervical dislocation method. The skin was initially treated with an isotonic solution then attached to the donor compartment without any air gap. The dermal portion horizontally touched the PBS solution to allow drug permeation. The temperature of the receptor compartment, the volume of sample collection and method of analysis followed the same method as in the in vitro release studies.
20,26
In vitro effectiveness studies
The MDA-MB231 cancer cell line was maintained in a complete culture medium containing Dulbecco’s Modified Eagle Medium (DMEM) that was augmented with 10% w/v fetal bovine serum (FBS) and 1% w/v Penicillin-Streptomycin. Prior to the study, cell plating was carried out after MDA-MB231 cells have 80%–90% confluence. A 96-well plate was used to plate the cells, then incubated in 5% CO2 incubator at 37˚C in a humidified environment overnight for cell adhesion to the wells. Then, the selected exemestane-loaded formulations were prepared as suspensions by diluting the formulations with the culture medium. From these, serial dilutions were provided to produce five different concentrations of exemestane (12.5, 25, 50, 100 and 200 μg/mL). following the overnight incubation, the media in the wells were emptied and the formulation suspensions (100 μL) were mixed. The plate with 96 wells was then incubated in the same incubator at similar conditions for a day. Once the incubation period was completed, the formulation suspension was removed and then 30 μL of MTT working solution was added and incubated for 4 additional hours. The plate was placed in a dark room overnight, after 100 μL isopropanol addition, to dissolve the formazan crystals. The absorbance was then measured at 570 nm, to assess cell viability, using the microplate reader (BioTek Epoch 2 Microplate Reader, BioTek Instruments, Inc. Winooski, VT, USA).
27
Histopathological studies
The formulations which exhibited the highest cumulative permeation percentage from both surfactants were studied, where the formulations were applied to a full thick hairless rat skin and observed under a light microscope with the help of hematoxylin and eosin (H & E) stain. Two formulations were selected for histopathological studies. In addition, low-substituted hydroxypropyl cellulose (L-HPC) gel formulations represented commercially available formulations was used as the negative control (i.e., without exemestane) and the positive control (i.e., with exemestane) for the studies. The setup was similar to the ex vivo skin permeation studies. The sample was smeared on the SC. After 4 hours of application, the excess LC gel on the skin was wiped off and the skin tissue was immediately dipped in neutral buffered formalin solution (10%). Subsequently, the tissue was processed by the following process, embedded and sectioned into 5 mm cuts and dipped in H & E stains.
20
Results
Exemestane-loaded LLC gel preparation
Based on the compositions of the aqueous and non-aqueous phases, the formulations varied in opacity and viscosity. Exemestane was distinguished to be fully dissolved in the oil phase. Amongst many prepared formulations, only four were deemed suitable for further characteristic studies and the composition and appearance are tabulated in Table 1.
Table 1.
Composition of the selected formulations
|
Formulation code
|
Surfactants (mL)
|
Water (mL)
|
Appearance
|
|
GMO (mL)
|
Tween 80
|
F127
|
| A1 |
1.0 |
2.0 |
- |
1.5 |
Translucent, viscous* |
| A2 |
1.0 |
1.5 |
- |
2.0 |
Translucent, viscous* |
| B1 |
1.0 |
- |
1.5 |
2.0 |
Opaque, viscous** |
| B2 |
1.5 |
- |
1.0 |
2.0 |
Opaque, viscous** |
GMO: glyceryl monooleate and F127: Pluronic® F127.
*Viscous; ** more viscous.
In terms of opacity, the Tween 80-based formulations were translucent while F127-based formulations were opaque. Moreover, in terms of viscosity, formulations made using Tween 80 were viscous, unlike the F127-based formulations which were high viscous intrinsically.
Encapsulation efficiency
The encapsulation efficiency (EE) values obtained, are presented in Table 2. The formulations exhibited EE in the range of 85 to 92%. Formulation A2 made from Tween 80 was demonstrated to have the highest EE, with 92%. In addition, most of the formulations have achieved EE above 80%.
Table 2.
Characterization studies of the LC formulations
|
Formulation code
|
Encapsulation efficiency (%)
|
Particle size (nm)
|
Zeta potential (mV)
|
PDI
|
pH
|
Spreadability (cm)
|
Drug stability studies (%)
|
|
2 – 8°C
|
25 ± 2°C
|
45 ± 2°C
|
|
A1
|
87 ± 2.36 |
139.8
± 8.01
|
- 25.5
± 2.46
|
0.333 |
4.75
± 0.08
|
3.53 ± 0.42 |
85 ± 1.35 |
84 ± 2.19 |
72 ± 2.47 |
|
A2
|
92 ± 4.57 |
119.9
± 11.34
|
- 24.8
± 1.93
|
0.392 |
5.33
± 0.03
|
3.47 ± 0.06 |
90 ± 2.36 |
88 ± 4.59 |
75 ± 2.81 |
|
B1
|
89 ± 1.63 |
451.1
± 21.31
|
- 17.0
± 2.52
|
0.440 |
4.02
± 0.03
|
0.93 ± 0.12 |
86 ± 2.78 |
84 ± 2.64 |
77 ± 1.92 |
|
B2
|
85 ± 2.30 |
466.2
± 16.38
|
- 23.7
± 2.05
|
0.428 |
5.12
± 0.02
|
1.03 ± 0.06 |
83 ± 3.05 |
82 ± 3.61 |
76 ± 2.32 |
PDI: polydispersity index. Values are given as mean ± SD.
Particle size, zeta potential and polydispersity index
The particle size, zeta potential and PDI values of the vesicles are tabulated in Table 2. The range of the vesicle particle sizes was from 119.9 to 466.2 nm. Tween 80-based formulations have produced smaller particles; A1 with 139.8 nm and A2 with 119.9 nm. F127-based formulations exhibited larger particle sizes than the Tween 80 formulations with B1 and B2 having particle sizes of 451.1 and 466.2 nm, respectively. The zeta potential values for formulations made from Tween 80 were of higher negative value, –25.5 ± 2.46 mV for A1 and –24.8 ± 1.93 mV for A2. In contrast, formulations made from F127 produced a lower negative value where B1 was –17.0 ± 2.52 mV and B2 was –23.7 ± 2.05 mV. The PDI values of the formulations did not differ much, as the values were relatively similar for both Tween 80 and F127-based formulations. The lowest PDI value was obtained from A1 while the highest was from B1, with values of 0.333 and 0.440 respectively.
pH measurement, spreadability, and drug stability studies
The pH of the preparations is presented in Table 2. All of the preparations were observed to be mildly acidic with pH values in 4.02 to 5.12 range, in which two formulations; A2 and B2 were shown to have a pH value of more than 5.00., i.e. pH 4.02 to 5.33. The highest pH 5.33 was obtained from A2, whereas the lowest pH value 4.02 was obtained from B1. The spreadability of formulations made from Tween 80 ranges from 3.47 to 3.53 cm, whereas the formulations made using F127 ranged from 0.93 to 1.03 cm. The spreadability values presented in Table 2 exhibits that the incorporated surfactants enabled the formulations to spread only by a small amount of shear. Variations in drug content and mean vesicle diameter of the vesicles in stability studies are exhibited in Table 2. Most of the formulations were shown to be stable at temperatures between 2–8˚C as the EE was above the 80%. For stability studies, an increase in temperature led to the gradual decline of the EE value from 10% to 15%.
Fourier transform infrared spectroscopy
FTIR spectra of Exemestane, GMO, Tween 80, GMO/Tween 80/Exemestane and GMO/F127/Exemestane are shown in Fig. 1. The characteristics of Exemestane peaks were observed at 2943 cm-1, 1732 cm-1, 1658 cm-1, 690-900 cm-1 which correspond to the C-H stretching, C=O, C=C and C-H bending, respectively. GMO and F127 show their C-H stretching peaks at 2861 cm-1 and 2882 cm-1 respectively and the C=C bond peaks at 1463 cm-1. The special characteristic of Tween 80 peaks of asymmetric and symmetric CH2 were observed at 2938 cm-1 and 2869 cm-1, respectively. The corresponding C=O bond peaks were clearly shown at 1730 cm-1.
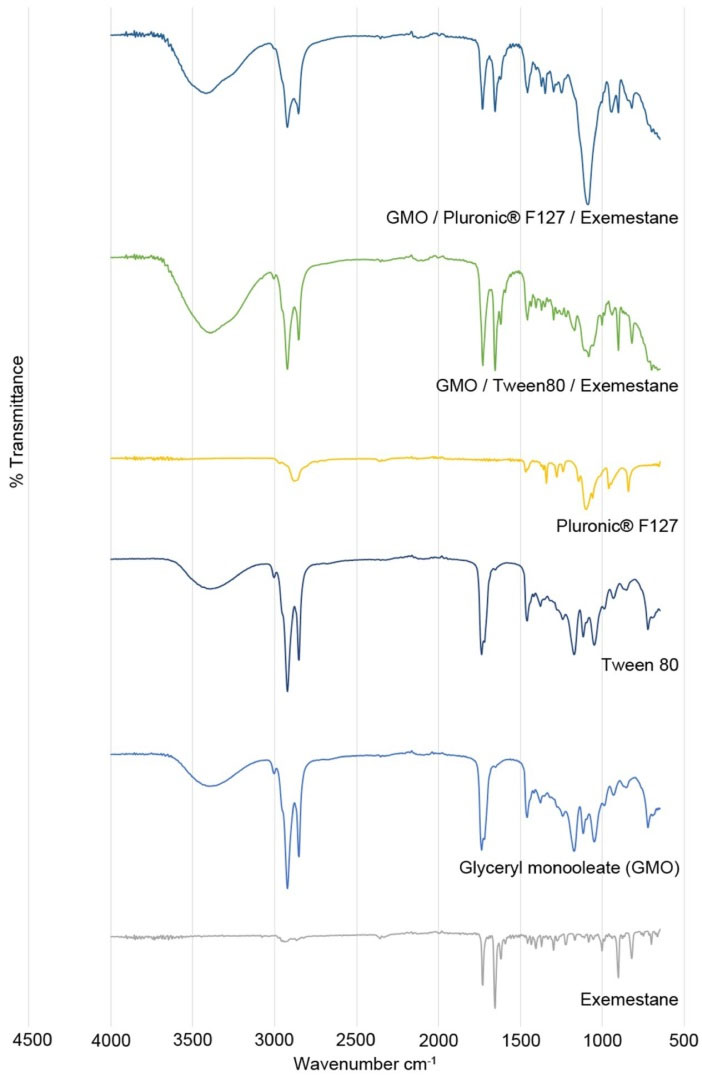
Fig. 1.
FTIR spectra obtained for the individual components and formulations.
.
FTIR spectra obtained for the individual components and formulations.
Optical and fluorescence microscopy
Optical microscope images of A2 and B2 are presented in the Figs. 2A and 2B, respectively. It is clear from the figure that the formation of liquid crystals either normal or reversed hexagonal in shape. The fluorescence excitation and emission of LC vesicle of formulations A2 and B2 in PBS solutions were studied and also shown in Fig. 2.
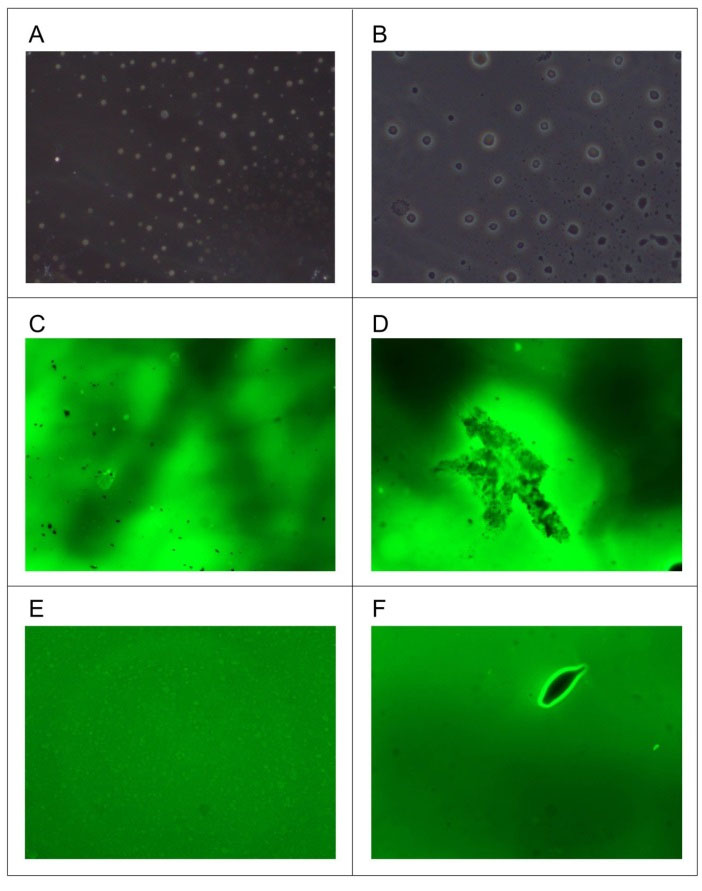
Fig. 2.
Optical and Fluorescence microscopy studies of formulations A2 and B2.
.
Optical and Fluorescence microscopy studies of formulations A2 and B2.
Minuscule black colored micellar vesicles were recognized in formulation A2 as shown in Fig. 2A, and after 3 to 5 minutes observation, the surfactant heads contributed to a change in the micelle structure from spherical to rod-like, presented in Fig. 2B. The measured micelle sizes of the B2 formulation were distinguished to be well within the range of the resolution of the measurements; images were observed as fluorescence dots indicating the micelles of the formulation as shown in Fig. 2C. During the observation, shown in Fig. 2D, the spherical vesicle changed its phase into a rod-like shape due to the PBS solution as well as the lamp heat.
In vitro release studies
Two different synthetic membranes, Strat-MTM membrane and cellulose acetate membrane, were utilized for the in vitro release studies. The cumulative percentage release from each formulation using the Strat-MTM membrane for an 8 hour period is shown in Table 3. From the table, it can be noticed that as the amount of surfactant in the formulations decreases with respect to GMO, the percentage release increases; however, this was not the case for F127-based formulations as the percentage release increases with increased surfactant amount. In addition, the cumulative percentage release from the F127-based formulations was higher (52.08% and 66.42%) compared to the Tween 80-based formulations (26.24% and 31.12%).
Table 3.
Cumulative percentage release, release rate, r2 and KH after 8 hours by using Strat-MTM membrane and cellulose acetate membrane
|
Formulation code
|
|
Cumulative % after 8 h
|
Release rate at 8th hour (ug/cm
2
/h)
|
Zero-order
|
First order
|
Higuchi
|
|
r
2
|
K
0
(h
-1
)
|
r
2
|
K
1
(h
-1
)
|
r
2
|
K
H
|
| Strat-MTM membrane |
A1 |
26.24 |
6.61 |
0.942 |
2.367 |
0.889 |
1.678 |
0.962 |
11.54 |
|
|
A2 |
31.12 |
7.83 |
0.921 |
2.591 |
0.873 |
1.540 |
0.979 |
13.61 |
|
|
B1 |
52.08 |
13.12 |
0.910 |
6.642 |
0.806 |
2.026 |
0.968 |
21.55 |
|
|
B2 |
66.42 |
16.73 |
0.937 |
4.698 |
0.837 |
1.354 |
0.990 |
25.27 |
| Cellulose acetate membrane |
A1 |
15.09 |
3.80 |
0.914 |
2.097 |
0.851 |
2.451 |
0.942 |
5.70 |
|
|
A2 |
55.91 |
14.08 |
0.935 |
2.949 |
0.836 |
1.671 |
0.969 |
19.41 |
|
|
B1 |
80.87 |
20.37 |
0.875 |
3.148 |
0.817 |
1.067 |
0.936 |
28.36 |
|
|
B2 |
88.95 |
22.41 |
0.861 |
3.590 |
0.838 |
1.972 |
0.985 |
34.32 |
K0: zero order constant, K1: first order constant and KH: Higuchi dissolution constant.
For the cellulose acetate membrane, the cumulative percentage release from each formulation for the same 8 hour period is also shown in Table 3. As the amount of surfactant increases with respect to GMO, the percentage release increases. However, this was not observed in F127-based formulations. The cumulative percentage release from F127-based formulations was again higher (80.87% and 88.95%) compared to Tween 80-based formulations (15.09% and 55.91%).
The percentage cumulative release of Exemestane formulations exhibited a linear correlation when plotted with respect to the square root of time, with Strat-MTM membrane as demonstrated in Fig. 3A. Furthermore, the r2 correlation coefficient value, was greater than 0.9, indicating diffusion-controlled release of Exemestane. In terms of release rate at the eighth hour, the formulations made with F127 were demonstrated to be higher than that of Tween 80, where the rates were nearly twice compared to Tween 80-based formulations, with 6.61 μg/cm2/h and 7.83 μg/cm2/h for A1 and A2 respectively and 13.12 μg/cm2/h and 16.73 μg/cm2/h for B1 and B2 respectively.
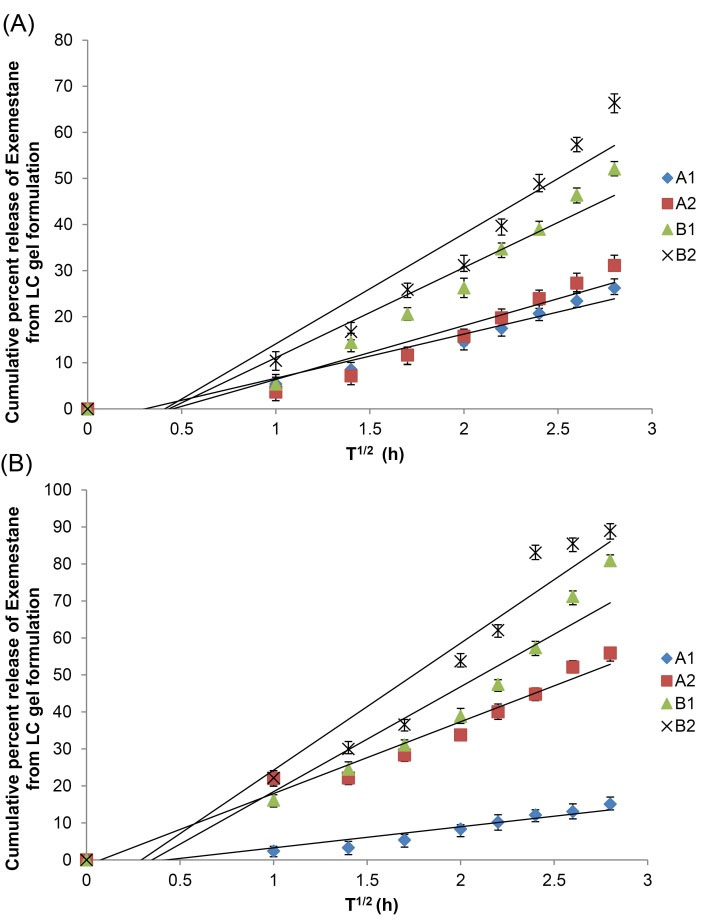
Fig. 3.
Percentage release of exemestane from LC gel formulations. A. Release with Strat-MTM membrane and B. Release with cellulose acetate membrane. Error bars represent the standard deviation.
.
Percentage release of exemestane from LC gel formulations. A. Release with Strat-MTM membrane and B. Release with cellulose acetate membrane. Error bars represent the standard deviation.
Meanwhile, the percentage cumulative release of Exemestane from the formulations with cellulose acetate membrane was plotted with respect to the square root of time as demonstrated in Fig. 3B, and a linear correlation was also obtained. In addition, the correlation coefficient value, r2, was also greater than 0.9. For the release rates at the 8th hour, F127-based formulations were also demonstrated to be higher, with 3.80 μg/cm2/h and 14.08 μg/cm2/h for A1 and A2 respectively and 20.37 μg/cm2/h and 22.41 μg/cm2/h for B1 and B2 respectively.
Ex vivo permeation studies
The cumulative percentage permeation for 8 hours by the formulations through a biological membrane (Sprague-Dawley rat skin) was plotted with respect to time and is shown in Fig. 4. Formulations A2 and B2 produced the highest percentage permeation with 61.90% and 63.01%, respectively. All of the formulations were observed to produce an initial burst or release of Exemestane, shown by the sharp increase in Fig. 4. The initial permeation rate values were higher in the F127-based formulations, with 105.40 μg/cm2/h and 105.06 μg/cm2/h for B1 and B2, respectively. The values produced by the Tween 80-based formulations were about half of the F127-based formulations with 52.20 μg/cm2/h for A1 and 53.87 μg/cm2/h for A2. Each plot was gradual and the permeation rate after a few hours was fairly uniform. The drug accumulation on the surface of the skin may be the reason for the direct proportional increase of the rate of drug permeation with a concentration of the drug. As the concentration of Exemestane increases in the formulations the permeation graph exhibits the first-order kinetics which is concentration dependant.
20
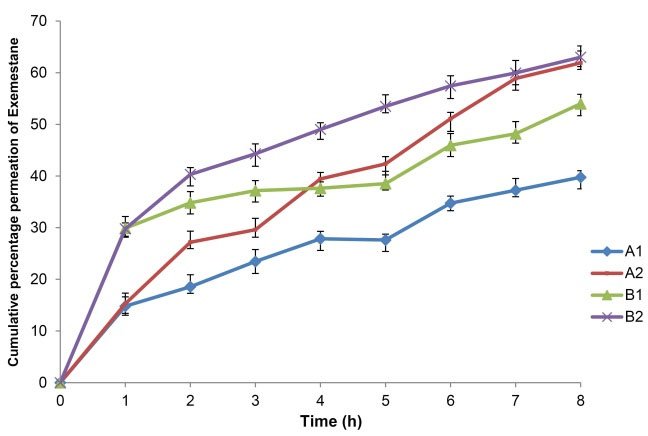
Fig. 4.
Percentage cumulative permeation by the LC gel formulations through rat skin in vitro. Error bars represent the standard deviation.
.
Percentage cumulative permeation by the LC gel formulations through rat skin in vitro. Error bars represent the standard deviation.
In vitro effectiveness studies
Formulations A2 and B2 were selected for this study as they produced the highest cumulative percentage of Exemestane permeated in the ex vivo studies. It was observed that both formulations have exhibited a similar pattern which is shown in Fig. 5. However, The increase in cell viability as the concentration of Exemestane increases was unexpected. Further increase in Exemestane concentration caused the cell viability to decrease as expected. Formulations loaded with 12.5 μg/mL Exemestane produced the least viability profile which was observed in both formulations A2 and B2 with 24.00% and 19.50%, respectively.
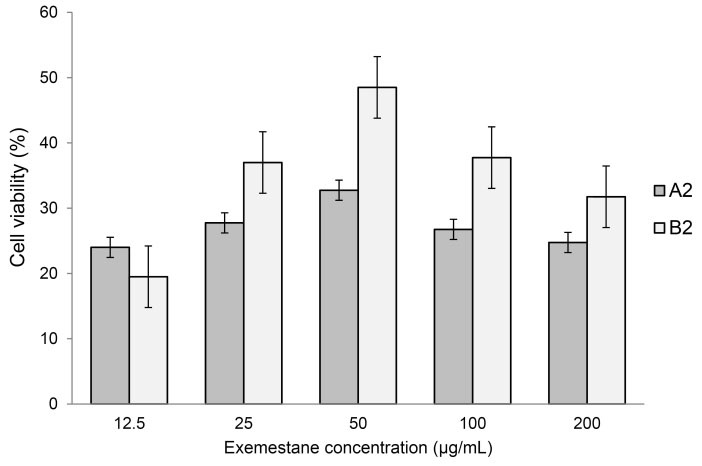
Fig. 5.
The in vitro effectiveness of exemestane-loaded formulations in terms of percentage cell viability. MDA-MB231 cancer cell line was used in this study and was incubated with the LC gel formulations for a period of 24 hours. Error bars represent standard deviation.
.
The in vitro effectiveness of exemestane-loaded formulations in terms of percentage cell viability. MDA-MB231 cancer cell line was used in this study and was incubated with the LC gel formulations for a period of 24 hours. Error bars represent standard deviation.
Histopathological studies
This study used low-substituted hydroxypropyl cellulose (L-HPC) gel formulations to mimic commercially available formulations. Figs. 6A and 6B show the picture of an L-HPC control formulation, without the drug, applied to the rat skin. The epidermis was demonstrated to be intact and appears to be of normal morphology and thickness. Fibrous connective tissue with adequate vascularization and scattered appendages such as hair follicles and sweat glands were distinguished in the dermis. Figs. 6C and 6D show another L-HPC-based formulation loaded with Exemestane as a positive control. The epidermis appeared slightly thickened and exhibited features of mild hyperkeratosis and mild acanthosis. Prominent SC was observed without any parakeratosis and the dermis showed adequate normal connective tissue.
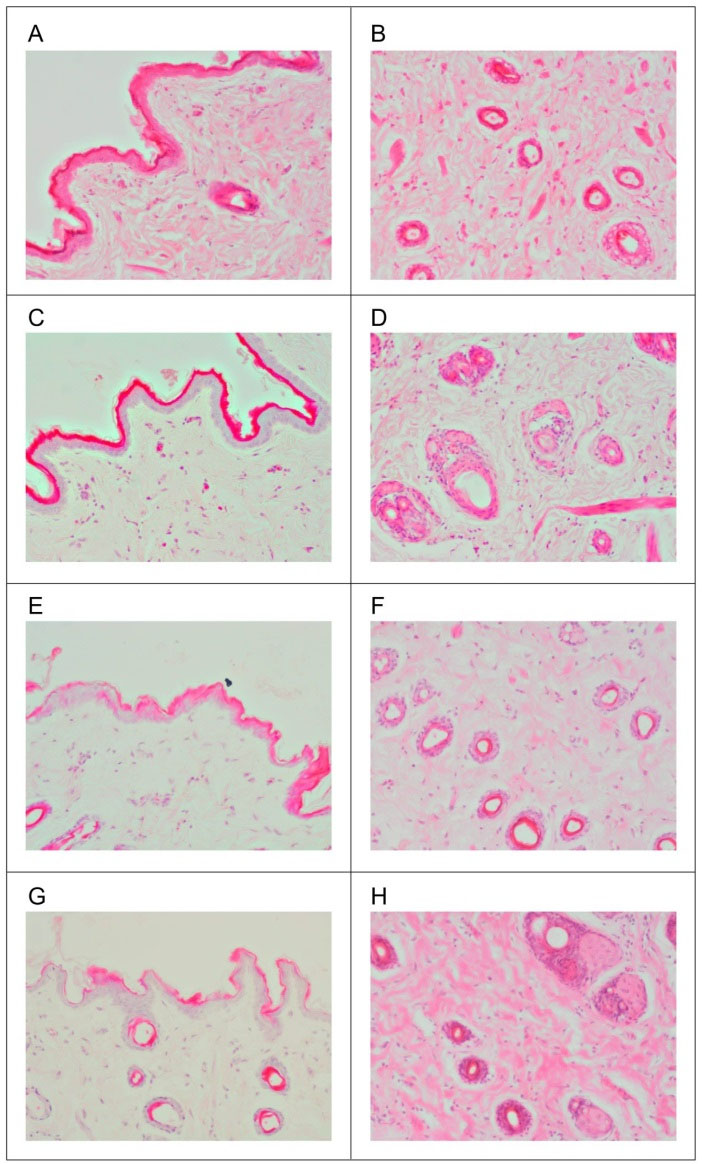
Fig. 6.
Histopathology of hematoxylin and eosin (H&E) stained skin sections. A. Longitudinal section for L-HPC control formulation, B. Skin surface for L-HPC control formulation, C. Longitudinal section for L-HPC positive control formulation, D. Skin surface for L-HPC positive control formulation, E. Longitudinal section for formulation A2, F. Skin surface for formulation A2, G. Longitudinal section for formulation B2, H. Skin surface for formulation B2. L-HPC: low-substituted hydroxypropyl cellulose.
.
Histopathology of hematoxylin and eosin (H&E) stained skin sections. A. Longitudinal section for L-HPC control formulation, B. Skin surface for L-HPC control formulation, C. Longitudinal section for L-HPC positive control formulation, D. Skin surface for L-HPC positive control formulation, E. Longitudinal section for formulation A2, F. Skin surface for formulation A2, G. Longitudinal section for formulation B2, H. Skin surface for formulation B2. L-HPC: low-substituted hydroxypropyl cellulose.
Analogous to the in vitro effectiveness study, formulations A2 and B2 were again selected. Figs. 6E and 6F show formulation A2, where the epidermis remained intact, but thinned out in focal areas. The SC was observed to have a varied thickness; least on the left lateral side and the thickness gradually but irregularly increases towards the right. The dermis showed fibrous connective tissue with adequate vascularization and normally scattered appendages. Formulation B2, shown in Figs. 6G and 6H, was observed to have similar features as in formulation A2 but with less intensity. The epidermis was intact without any discontinuity or ulceration or necrosis but thinned out in focal areas. The thickness of SC was variable but distinctly thinned out when compared to the normal and positive controls due to the penetration of Exemestane into the intercellular regions of squamous cells in a similar fashion to formulation A2. In addition, the dermis showed loose myxoid connective tissue with mild edematous change.
Discussion
Exemestane-loaded LLC gel preparation
Exemestane, which is a hydrophobic drug, was able to dissolve within the oil phase that consisted of GMO and surfactants. It is proposed that exemestane was encapsulated and incorporated successfully in the four formulations. This is in agreement with several studies pointed out that the method could be used to incorporate the lipophilic or hydrophobic drug into the LLC gels.
28–31 The viscosity of the formulations depends on the type of surfactant used as well as the amount within the formulation. The use of GMO makes the formulations to be mechanically stable as mentioned in few studies.
32–34
Tween 80 has been used as part of a mixture to produce nanoparticles and self-microemulsifying drug delivery system.
35,36
In addition, Tween 80 has also been reported to have been used in the preparation of nanoparticles and nanoemulsions.
37–39
Meanwhile, the use of F127 in producing LLC gel formulations has been widely established to prepare nanoparticles for drug deliveries.
40–43
Encapsulation efficiency
EE is an important crucial factor for stability. Hence, EE studies were conducted on all of the formulations. The concentrations of surfactants are crucial as they tend to affect the vesicle morphology as well as drug loading. This leads to the interference of the structure of the vesicles, which in turn promotes drug leakage before they diffuse into the skin.
44
EE also determines a critical positive correlation with oil to the concentrations of surfactants and water. The encapsulation of Exemestane is primarily due to its ability to dissolve in the molten non-aqueous phase and its partition between the hydrophilic phase and other surfactants.
The high EE values in all four formulations may be due to reduced bilayer permeability as well as a higher lipophilic bilayer. This would lead to the effective intercalation of the lipophilic drug within the core of the bilayer which is hydrophobic.
45
However, the EE values decreased with increasing the amount of surfactant content. The higher amount of surfactant with GMO may contribute to the hydrophilic phase viscosity to increase which then leads to the controlled drug release from the vesicles. This is attributed to the high solubilization of the drug in the hydrophobic surfactant bilayers.
46
In other words, the lesser amount of aqueous phase leads to higher viscosity formation of LC vesicles with a regular structure bilayer formation and more accommodation of drug in the surfactant hydrophobic chains.
20,45
This would help to the formation of suitable viscosity and phase of LC gels phase, that may bring about the capability to deliver a controlled release of the drug.
Particle size, zeta potential and polydispersity index
The size of the vesicle and its distribution in the formulations are of paramount importance for transdermal drug delivery system.
44
The PDI value of less than 0.1 denotes a population which is homogenous, while greater than 0.3 indicates an increased heterogeneity.
47
In this study, all the four formulations were demonstrated to have PDI values higher than 0.3. The vesicle size distributions were heterogeneous and were within the acceptable limits from both Tween 80 and F127-based formulations.
The zeta potential of the formulations was higher in Tween 80 compared to F127-based formulations which may be due to Tween 80 lower molecular weight than F127. It is clear from this study that the higher negative values of Tween 80-based formulations tend to repel each other and does not result in particle-particle aggregation,
48,49
whereas, the F127-based formulations have low zeta potential values that result in particle-particle aggregation. This is evidenced by the lower particle size of Tween 80 than F127-based formulations.
pH measurement, spreadability, and drug stability studies
The formulations contain very limited quantities of the aqueous phase; hence the pH measurements were undertaken for quality control and to assess the compatibility with skin pH. The formulations containing Tween 80 exhibited a slightly higher pH than F127 formulation, however, both formulations are deemed suitable for the skin and would avoid any risk of skin irritation due to pH incompatibility.
50
The therapeutic efficacy of an LLC gel formulation depends on its ability to spread uniformly. This property is also essential to predict the formulations’ rheological behavior such as the flow of formulations from the tube by pressure.
51
It was observed that the spreadability was lower in F127-based formulations than Tween 80-based formulations. This may be due to higher stress required to spread F127 due to its higher molecular weight than Tween 80. Meanwhile, formulations made with higher concentration of Tween 80 yielded higher spreadability values compared to those made with a lower concentration, which may be due to the increase in viscosity.
The formulations undergoing 90 days stability study at various temperatures (2–8°C/25°C/45°C) had high leakage of drug from the vesicles at the highest temperature which may have been due to the loosening of vesicles and higher fluidity of the lipid bilayers.
20
Meanwhile, at lowest temperatures 2-8°C, acceptable stability values were achieved.
Fourier transform infrared spectroscopy
The individual components and formulation mixtures GMO/Tween 80/exemestane and GMO/F127/exemestane demonstrated the compatibility of the surfactants with the drug in the formulation as the spectra were without significant peak shifts. There were few characteristic peaks of the drug which were overlapping in the region as that of the surfactants possibly due to the encapsulation of the drug between the layers.
Optical and fluorescence microscopy
Optical microscope clearly shows that tween 80 formulations formed very small spherical shape compared to F127, which was strongly supported by particle size data. The direct visualization of the FSS-LC micelles in PBS solution was distinguished by Fluorescence microscopy. From the results, it is clear that the micellar structural changes occurred during the direct visualization of the LLC gel formulations. It was also shown that the spherical micelles, in F127-based formulations, were initially merged into the larger rod-like micelles. However, in Tween 80-based formulations, the vesicles have lower fluorescence strength compared to F127-based formulations, which could be due to (i) lower size of the particles and (ii) insolubility of FSS in the hydrophobic chain in the surfactants. The repulsion between the surfactant head groups leads to increased curvature, in GMO/F127 micelles, forming spherical vesicles.
52
This is supported by the higher value of zeta potential in the ranges between -17 to -25 mV, thus indicating good dispersion of LLC vesicles in the F127-based formulations when diluted with the PBS solution.
53
Rod-like micelles were directly observed in Tween 80-based formulations when diluted with the PBS solution. This may be due to the decrease in repulsion between the surfactant heads of the micelle, which was attributed to the microscope heat sources compacting the electrical double layer adjacent to the anionic heads.
54
This closer packing may convert the spherical micelle to a rod-like structure to convert from a spherical. In an assumption, direct visualization obviously revealed the evolutions of micelle transition from spherical to rod-like structures.
In vitro release studies
Artificial Strat-MTM membrane mimics human skin and was studied against cellulose acetate membrane to compare the effectiveness of both membranes on release behavior of Exemestane. The cumulative percentages of Exemestane released from the four formulations exhibited the trend that the release of Exemestane from F127-based formulations was higher compared to Tween 80-based formulations in the 8 hours period, which unmistakably demonstrates the occurrence of the drug sink condition and membrane permeability. It may be due to interactions of F127 with the other components within the formulation such as glycerol.
55
The particle size of the formulations may also contribute to the difference in results as the Tween 80-based formulations have smaller particle size compared to the F127-based formulations. However, the study has demonstrated that this was not the case as B1 and B2 tend to have a higher release rate or in some cases, about 2.5 times higher than A1 and A2. This may suggest that B1 and B2 can undergo phase changes when there are changes to the external environment like temperature and that this may not apply in formulations made with Tween 80.
42
LC gel absorbs moisture from skin inducing swelling of gel network system thus increasing the drug molecular diffusion increasing drug movement in the networking system.
The in vitro release data studied using mathematical modeling showed that Exemestane release from the LLC gel formulations was diffusion-controlled, which followed zero-order kinetics as evidenced by higher r2values. Moreover, plots shown in Figs. 2A and 2B have also exhibited that the total drug release percentage increases proportionally with increase in the square root of time.
13–15
Higuchi kinetics data also demonstrated that the formulations incorporated of this low water-soluble drug in LC gels exhibited mixed order release kinetics exists. Lara et al
56
hypothesized that LC which exhibit the formulations having drug load concentration independent of the release kinetics was assumed to be of cubic phase. Therefore, these formulations are at least assumed to be of hexagonal or cubic phase.
In addition, the percentage of permeation across the cellulose acetate membrane was higher compared to Strat-MTM membrane; which may be due to the ability of the vesicles of the formulations to diffuse freely through the cellulose acetate membrane pores, which have higher pore radius than its counterpart, which is due to overnight hydration. Otherwise, the release behavior of the formulations was same as to Strat-MTM. Overall, it is suggested that Strat-MTM membrane may be suitable to perform release studies for LC gels system. The Strat-MTM membrane, which mimics the human skin, can be used to predict the effectiveness of the selected formulations. The cellulose membrane, on the other hand, can be used for preliminary drug release studies.
57,58
Based on the data, all formulations showed promising results thus were selected for the ex vivo permeation studies using rat skin.
Ex vivo permeation studies
The results demonstrated that Exemestane successfully permeated through the rat skin. For Tween 80-based formulations, it was shown that the Exemestane in formulation A2 permeates more than that of formulation A1. The particle size may be responsible as formulation A2 had much smaller particle size compared to formulation A1. In addition, Tween 80 surfactant is hydrophilic intrinsically hence hydrates LC gel with skin fluids. Moreover, as the LC gel hydrates the skin layers, the pore size radius is increased; consequently, more exemestane will be permeated due to drug release from the LC gel system through the hydrated skin layers. For the F127-based formulations, the exemestane in formulation B2 permeates more than in formulation B1. In contrast to the Tween 80-based formulations where smaller particle size would produce a higher amount of exemestane permeation, formulation B2 has a larger particle size than formulation B1.
It is clear that time lag was not observed in any of the formulations, it strongly proposes the formulation of LC vesicles which release the drug from the micellar system continuously.
47
During the in vitro release studies, the initial burst was not observed. But during the ex vivo permeation studies, such phenomenon occurred for all the formulations. Such occurrences may be due to two reasons: (i) the diffusion of the PBS solution from the receptor fluid to the skin membrane which enhanced the release of Exemestane from the vesicles, leading to more permeation across the rat skin.
59
Also (ii) it may be due to the presence of surfactants in the formulations, as the permeation was higher since the Exemestane solubility in the bilayer hydrophobic core was enhanced. The surfactants also help to modify the structural and permeability characteristics of the SC, thus increase its fluidity and oppose the barrier function. This results in higher drug permeation from the vesicles into the SC layer.
20,60,61
Generally the rat skin biological membrane bears negative charge; it is anticipated that the predominantly positive charged membrane layer of the vesicles may improve skin interaction that leads to higher skin permeation due to electrostatic attraction in the SC.
62
A mechanism was proposed that the penetration may be due to the hydration gradient, where the vesicle system was able to deform.
63
The mechanism proposed that the water activity on both sides of the skin may be able to drag the vesicles through the skin. This study suggests that formulations with more water content may perform better, although the drug used in this study is hydrophobic. Also, the water may help to allow the formulations to spread easily and evenly on the skin surface, causing drug permeation to be more effective. The remarkable enhancement in the permeation of Exemestane through rat skin certainly suggests that there is intact vesicle transfer through skin and interaction between the skin and LC vesicle, furthermore, these are key factors enhancing the transdermal delivery of the drug.
64
In vitro effectiveness studies
The overall results exhibited that both formulations gave a relatively similar effect due to the similar pattern despite different surfactants being used. Both formulations were shown to be able to induce death on the MDA-MB231 cells. It was also noted that with exemestane concentration of 200 μg/mL, which was the actual concentration of exemestane in the formulations, was sufficient to induce cell death of more than 50%. It was presumed that there would be an inversely proportional correlation between exemestane concentration and cell viability. However, this was not observed; at 12.5 and 25 μg/mL, the cell viability was low and the pattern was observed for both formulations. This may also suggest that low concentration of drugs may be sufficient to induce cell death. In addition, it is suggested that Tween 80 may help to enhance the absorption and accumulation of the Exemestane into the MDA-MB231 cells by altering the cell membrane as demonstrated in a study involving Etoposide and lung adenocarcinoma cells.
65
As the methodology employed in this study was relatively new, more investigations are required to ensure that the results are valid. Repetitions may be carried out using other human cancer cell lines as well as other cell lines such as HeLa cells and fibroblasts.
Histopathological studies
The positive control group shows a mild increase in thickness of the epidermis, which is not significant, as it is not associated with any inflammation or edema. The well-defined squamous keratinocytes in the deeper layers could be due to the increased keratin formation by the SC as a protective response to the applied drug. However, the lack of a prominent inflammatory infiltrates rules out any allergic or toxic reaction.
The irregularity of the SC observed in formulation A2 suggests that Tween 80, which is also used as a drug enhancer, thins out the squamous intercellular regions in SC during its penetration. Moreover, the surfactant which penetrated into the intercellular matrix may interact and bind with the keratin filaments leading to intracellular disruption. In formulation B2, the changes in the dermis suggest that the formulation diffused well through the skin. Both formulations A2 and B2 exhibited thinning of the epidermis at varying degrees, with the impression that the formulations have moved through the SC and these are morphological changes accounting for the above explanation. Both formulations A2 and B2 also exhibit no signs of ulceration, sloughing, discontinuity or necrosis, thereby ruling out any possibility of toxicity or adverse reactions of both formulations, and they are deemed safe to be used per se or in combination with other drugs for transdermal absorption.
Conclusion
The use of LLC gels has improved the current drug delivery methods considerably. In this study, Exemestane LLC gel formulations were successfully prepared, using GMO and surfactants such as Tween 80 and F127, by a simple process, involving coacervation phase separation principle. Results have also demonstrated that the formulations developed in this study were able to produce a sustained release and permeate a full thickness skin without any visible adverse reactions. It is hoped that in the future more studies will be carried out to ensure targeted drug delivery efficiently and safely.
Ethical approval
University of Brunei Darussalam (UBD) Research Ethics Committee (UBD/PNC2/2/RG/1(317)) approved the procedures and animal care for the experiments undertaken in this project.
Competing interests
There is no conflict of interests to be reported.
Acknowledgments
The authors express their gratitude to the University of Brunei Darussalam for their financial support through University Research Grant fund for this study No. UBD/PNC2/2/RG/1(317).
Research Highlights
What is current knowledge?
simple
-
√ Lyotropic LC technology is considered to be an effective
carrier method in enhancing the skin
What is new here?
simple
-
√ Diffusion profile of Exemestane through Strat-MTM/cellulose
acetate membranes as an LC gel is not enhanced by classic
Fick’s law of diffusion in this study.
-
√ Lyotropic LC gels nano size formulations are permeated
through the rat skin and also non-irritant to skin
histopathologically.
-
√ Lyotropic LC drug delivery through transdermal
administration appears to be a promising technique to avoid
oral-related severe side effects such as osteoporosis.
References
- Kalidas M, Brown P. Aromatase Inhibitors for the Treatment and Prevention of Breast Cancer. Clin Breast Cancer 2005; 6(1):27-37. doi: 10.3816/CBC.2005.n.006 [Crossref] [ Google Scholar]
- Bundred NJ. Aromatase Inhibitors and Bone Health. Curr Opin Obstet Gynecol 2009; 21(1):60-67. doi: 10.1097/GCO.0b013e32831da80e [Crossref] [ Google Scholar]
- Bundred NJ, Barnes NLP. Potential use of COX-2-Aromatase Inhibitor Combinations in Breast Cancer. Br J Cancer 2005; 93(Suppl I):S10-S15. doi: 10.1038/sj.bjc.6602690 [Crossref] [ Google Scholar]
- Guo C, Wang J, Cao F, Lee RJ, Zhai G. Lyotropic Liquid Crystal Systems in Drug Delivery. Drug Discov Today 2010; 15(23-24):1032-1040. doi: 10.1016/j.drudis.2010.09.006 [Crossref] [ Google Scholar]
- Hitesh J, Rushikesh G, Gauri J, Jagruti M, Nirali T. Liquid Crystal as Accelerant in Drug Absorption from Topical Formulations. Int Res J Pharm 2011; 2(4):86-89. [ Google Scholar]
- Chen Y, Ma P, Gui S. Cubic and Hexagonal Liquid Crystals as Drug Delivery Systems. Biomed Res Int 2014; 2014:1-12. doi: 10.1155/2014/815981 [Crossref] [ Google Scholar]
- Rajabalaya R, Musa MN, Kifli N, David SR. Oral and Transdermal Drug Delivery Systems: Role of Lipid-Based Lyotropic Liquid Crystals. Drug Des Devel Ther 2017; Volume11:393-406. doi: 10.2147/DDDT.S103505 [Crossref] [ Google Scholar]
- Kovářová A, Světlík S, Kozmík V, Svoboda J, Novotná V, Pociecha D. Unusual Polymorphism in New Bent-Shaped Liquid Crystals Based on Biphenyl as a Central Molecular Core. Beilstein J Org Chem 2014; 10:794-807. doi: 10.3762/bjoc.10.75 [Crossref] [ Google Scholar]
- Shamsheer AS, Sabareesh M, Khan PR, Sai Krishna P, Sudheer B. Formulation and Evaluation of Lisinopril Dihydrate Transdermal Proniosomal Gels. J Appl Pharm Sci 2011; 1(8):181-185. [ Google Scholar]
- Wagh VD, Deshmukh OJ. Itraconazole niosomes drug delivery dystem and its antimycotic activity against Candida albicans. ISRN Pharm 2012; 2012:1-7. doi: 10.5402/2012/653465 [Crossref] [ Google Scholar]
- Rajabalaya R, David SR, Chellian J, Xin Yun G, Chakravarthi S. Transdermal Delivery of Oxybutynin Chloride Proniosomal Gels for the Treatment of Overactive Bladder. Drug Deliv 2016; 23(5):1578-1587. doi: 10.3109/10717544.2015.1116027 [Crossref] [ Google Scholar]
- Rangasamy M, Parthiban KG. Recent Advances in Novel Drug Delivery Systems. Int J Res Ayurveda Pharm 2010; 1(2):316-326. [ Google Scholar]
- Higuchi WI. Diffusional Models Useful in Biopharmaceutics. J Pharm Sci 1967; 56(3):315-324. doi: 10.1002/jps.2600560302 [Crossref] [ Google Scholar]
- Huang X, Brazel CS. On the Importance And Mechanisms of Burst Release in Matrix-Controlled Drug Delivery Systems. J Control Release 2001; 73:121-136. doi: 10.1016/S0168-3659(01)00248-6 [Crossref] [ Google Scholar]
-
Siegel RA, Rathbone MJ. Overview of Controlled Release Mechanisms. In: Fundamentals and Applications of Controlled Release Drug Delivery. Boston, MA: Springer; 2012. p. 19-43 10.1021/la200591u.
- Negrini R, Mezzenga R. pH-responsive Lyotropic Liquid Crystals for Controlled Drug Delivery. Langmuir 2011; 27(9):5296-5303. [ Google Scholar]
- Nguyen TH, Hanley T, Porter CJH, Boyd BJ. Nanostructured Reverse Hexagonal Liquid Crystals Sustain Plasma Concentrations for a Poorly Water-Soluble Drug after Oral Administration. Drug Deliv Transl Res 2011; 1(6):429-438. doi: 10.1007/s13346-011-0045-z [Crossref] [ Google Scholar]
- Yang Z, Tan Y, Chen M, Dian L, Shan Z, Peng X. Development of Amphotericin b-loaded cubosomes through the solemuls technology for enhancing the oral bioavailability. AAPS Pharm Sci Tech 2012; 13(4):1483-1491. doi: 10.1208/s12249-012-9876-2 [Crossref] [ Google Scholar]
- Lopes LB, Speretta FFF, Bentley MVLB. Enhancement of Skin Penetration of Vitamin K Using Monoolein-Based Liquid Crystalline Systems. Eur J Pharm Sci 2007; 32(3):209-215. doi: 10.1016/j.ejps.2007.07.006 [Crossref] [ Google Scholar]
- Rajabalaya R, Leen G, Chellian J, Chakravarthi S, David S. Tolterodine Tartrate Proniosomal Gel Transdermal Delivery for Overactive Bladder. Pharmaceutics 2016; 8(3):27. doi: 10.3390/pharmaceutics8030027 [Crossref] [ Google Scholar]
- Helal DA, Abd El-rhman D, Abdel-Halim SA, El-Nabarawi MA. Formulation and Evaluation of Flucazole Topical Gel. Int J Pharm Pharm Sci 2012; 4:176-183. [ Google Scholar]
- Khan PA, Thube R, Rab RA. Formulation Development and Evaluation of Silymarin Gel for Psoriasis Treatment. J Innov Pharm Biol Sci 2014; 1(1):21-26. [ Google Scholar]
- Gaba B, Fazil M, Khan S, Ali A, Baboota S, Ali J. Nanostructured Lipid Carrier System For Topical Delivery of Terbinafine Hydrochloride. Bull Fac Pharmacy, Cairo Univ 2015:147-159.
- Sheba Rani Nakka David, Mah Si Hui, Chong Fui Pin,
Foo Yun Ci Rajabalaya
R
. Formulation and In Vitro Evaluation of Ethosomes as Vesicular Carrier for Enhanced Topical Delivery of Isotretinoin. Int J Drug Deliv 2013; 5(1):28-34. [ Google Scholar]
- Rajabalaya R, Khanam J, Nanda A. Design of a Matrix Patch Formulation for Long-Acting Permeation of Diclofenac Potassium. Asian J Pharm Sci 2008; 3(1):30-39. [ Google Scholar]
- Rajabalaya R, Tor L, David S. Formulation and In Vitro Evaluation of Ondansetron Hydrochloride Matrix Transdermal Systems Using Ethyl Cellulose / Polyvinyl Pyrrolidone Polymer Blends. 2012:1304-1308.
- Gurunathan S, Han JW, Eppakayala V, Jeyaraj M, Kim JH. Cytotoxicity of Biologically Synthesized Silver Nanoparticles In MDA-MB-231 Human Breast Cancer Cells. Biomed Res Int 2013:2013. doi: 10.1155/2013/535796 [Crossref]
- Esposito E, Cortesi R, Drechsler M, Paccamiccio L, Mariani P, Contado C. Cubosome Dispersions as Delivery Systems for Percutaneous Administration of Indomethacin. Pharm Res 2005; 22(12):2163-2173. doi: 10.1007/s11095-005-8176-x [Crossref] [ Google Scholar]
- Lai J, Chen J, Lu Y. Glyceryl Monooleate/Poloxamer 407 Cubic Nanoparticles as Oral Drug Delivery Systems: i In Vitro Evaluation and Enhanced Oral Bioavailability of the Poorly Water-Soluble Drug Simvastatin. AAPS Pharm Sci Tech 2009; 10(3):960-966. doi: 10.1208/s12249-009-9292-4 [Crossref] [ Google Scholar]
- Chen Y, Lu Y, Zhong Y, Wang Q, Wu W, Gao S. Ocular Delivery Of Cyclosporine a Based on Glyceryl Monooleate/Poloxamer 407 Liquid Crystalline Nanoparticles: Preparation, Characterization, In Vitro Corneal Penetration and Ocular Irritation. J Drug Target 2012; 20(10):856-863. doi: 10.3109/1061186X.2012.723214 [Crossref] [ Google Scholar]
- Thapa RK, Baskaran R, Madheswaran T, Kim JO, Yong CS, Yoo BK. In Vitro Release and Skin Permeation of Tacrolimus from Monoolein-Based Liquid Crystalline Nanoparticles. J Drug Deliv Sci Technol 2012; 22(6):479-484. doi: 10.1016/S1773-2247(12)50084-5 [Crossref] [ Google Scholar]
- Han K, Pan X, Chen M. Phytantriol-Based Inverted Type Bicontinuous Cubic Phase for Vascular Embolization and Drug Sustained Release. Eur J Pharm Sci 2010; 41(5):692-699. doi: 10.1016/j.ejps.2010.09.012 [Crossref] [ Google Scholar]
- Nie Y, Ji L, Ding H, Xie L, Li L, He B. Cholesterol derivatives based charged liposomes for doxorubicin delivery: preparation, in vitro and in vivo characterization. Theranostics 2012; 2(11):1092-1103. doi: 10.7150/thno.4949 [Crossref] [ Google Scholar]
- Lee J, Saw PE, Gujrati V, Lee Y, Kim H, Kang S. Mono-arginine cholesterol-based small lipid nanoparticles as a systemic sirna delivery platform for effective cancer therapy. Theranostics 2016; 6(2):192-203. doi: 10.7150/thno.13657 [Crossref] [ Google Scholar]
- Subramanian N, Ray S, Ghosal SK, Bhadra R, Moulik SP. Formulation Design of self-microemulsifying drug delivery systems for improved oral bioavailability of celecoxib. Biol Pharm Bull 2004; 27(12):1993-1999. doi: 10.1248/bpb.27.1993 [Crossref] [ Google Scholar]
- Kanani MH, Vadalia KR. Development and characterization of polymeric nanoparticulate delivery system for exemestane. Int J Pharma Bio Sci 2015; 245:796-809. [ Google Scholar]
- Ma Y, Zheng Y, Zeng X, Jiang L, Chen H, Liu R. Novel docetaxel-loaded nanoparticles based on Pcl-Tween 80 Copolymer for Cancer Treatment. Int J Nanomedicine 2011; 6:2679-2688. doi: 10.2147/IJN.S25251 [Crossref] [ Google Scholar]
- Prabhakar K, Afzal SM, Surender G, Kishan V. Tween 80 containing lipid nanoemulsions for delivery of indinavir to brain. Acta Pharm Sin B 2013; 3(5):345-353. doi: 10.1016/j.apsb.2013.08.001 [Crossref] [ Google Scholar]
- Sheikh Z, Morshed N. Optimizing oral drug delivery using lipid based formulations. Int Res J Pharm 2014; 5(7):514-522. doi: 10.7897/2230-8407.0507105 [Crossref] [ Google Scholar]
- Antunes FE, Gentile L, Oliviero C, Tavano L, Antonio G. Gels of Pluronic F127 and nonionic surfactants from rheological characterization to controlled drug permeation. Colloids Surf B Biointerfaces 2011; 87(1):42-48. doi: 10.1016/j.colsurfb.2011.04.033 [Crossref] [ Google Scholar]
- Abdelrahman FE, Elsayed I, Gad MK, Badr A, Mohamed MI. Investigating the cubosomal ability for transnasal brain targeting: in vitro optimization, ex vivo permeation and in vivo biodistribution. Int J Pharm 2015; 490(1-2):281-291. doi: 10.1016/j.ijpharm.2015.05.064 [Crossref] [ Google Scholar]
- Rarokar NR, Saoji SD, Raut NA, Taksande JB, Khedekar PB, Dave VS. Nanostructured cubosomes in a thermoresponsive depot system: an alternative approach for the controlled delivery of docetaxel. AAPS PharmSciTech 2015; 17:1-10. doi: 10.1208/s12249-015-0369-y [Crossref] [ Google Scholar]
- Mendonça DV, Lage LM, Lage DP, Chávez-Fumagalli MA, Ludolf F, Roatt BM. Poloxamer 407 (Pluronic(®) F127)-based polymeric micelles for amphotericin B: In vitro biological activity, toxicity and in vivo therapeutic efficacy against murine tegumentary leishmaniasis. Exp Parasitol 2016; 169:34-42. doi: 10.1016/j.exppara.2016.07.005 [Crossref] [ Google Scholar]
- Du Plessis J, Ramachandran C, Weiner N, Müller DG. The influence of particle size of liposomes on the deposition of drug into skin. Int J Pharm 1994; 103(3):277-282. doi: 10.1016/0378-5173(94)90178-3 [Crossref] [ Google Scholar]
- El-Samaligy MS, Afifi NN, Mahmoud EA. Increasing bioavailability of silymarin using a buccal liposomal delivery system: preparation and experimental design investigation. Int J Pharm 2006; 308(1-2):140-148. doi: 10.1016/j.ijpharm.2005.11.006 [Crossref] [ Google Scholar]
- David SRN, Hui MS, Pin CF, Ci FY, Rajabalaya R. Formulation and in vitro evaluation of ethosomes as vesicular carrier for enhanced topical delivery of isotretinoin. Int J Drug Deliv 2013; 5(1):28-34. [ Google Scholar]
- Jukanti R, Sheela S, Bandari S, Veerareddy PR. Enhanced bioavailability of exemestane via proliposomes based transdermal delivery. J Pharm Sci 2011; 100(8):3208-3222. doi: 10.1002/jps.22542 [Crossref] [ Google Scholar]
- Scholes PD, Coombes AG, Illum L, Davis SS, Watts JF, Ustariz C. Detection and determination of surface levels of poloxamer and PVA surfactant on biodegradable nanospheres using SSIMS and XPS. J Control Release 1999; 59(3):261-278. doi: 10.1016/S0168-3659(98)00138-2 [Crossref] [ Google Scholar]
- Zhang Y, Tang L, Sun L. A Novel Paclitaxel-Loaded Poly(ε-Caprolactone)/Poloxamer 188 Blend Nanoparticle Overcoming Multidrug Resistance for Cancer Treatment. Acta Biomater 2010; 6(6):2045-2052. doi: 10.1016/j.actbio.2009.11.035 [Crossref] [ Google Scholar]
- Lucero MJ, Vigo J, León MJ. A study of shear and compression deformations on hydrophilic gels of tretinoin. Int J Pharm 1994; 106(2):125-133. doi: 10.1016/0378-5173(94)90310-7 [Crossref] [ Google Scholar]
- Bhalaria MK, Naik S, Misra AN. Ethosomes: a novel delivery system for antifungal drugs in the treatment of topical fungal diseases. Indian J Exp Biol 2009; 47(5):368-375. [ Google Scholar]
-
Krister Holmberg BL, Kronberg B, Holmberg K, Lindman B. Polymers in Solution. In: Surface Chemistry of Surfactants and Polymers. Chichester, UK: John Wiley & Sons, Ltd; 2014. p. 175-195.
- Shustova NB, McCarthy BD, Dincǎ M. Turn-on fluorescence in tetraphenylethylene-based metal-organic frameworks: an alternative to aggregation-induced emission. J Am Chem Soc 2011; 133(50):20126-20129. doi: 10.1021/ja209327q [Crossref] [ Google Scholar]
- Guan W, Zhou W, Lu C, Tang BZ. Synthesis and design of aggregation-induced emission surfactants: direct observation of micelle transitions and microemulsion droplets. Angew Chemie Int Ed 2015; 54(50):15160-15164. doi: 10.1002/anie.201507236 [Crossref] [ Google Scholar]
- Dong Y, Larson I, Hanley T, Boyd BJ. Bulk and Dispersed aqueous phase behavior of phytantriol: effect of vitamin e acetate and F127 Polymer on liquid crystal nanostructure. Langmuir 2006(5):9512-9518. doi: 10.1021/la061706v [Crossref]
- Lara MG, Bentley MVLB, Collett JH. In vitro drug release mechanism and drug loading studies of cubic phase gels. Int J Pharm 2005; 293(1-2):241-250. doi: 10.1016/j.ijpharm.2005.01.008 [Crossref] [ Google Scholar]
- Lehr C-M. Lectin-mediated drug delivery: the second generation of bioadhesives. J Control Release 2000; 65(1-2):19-29. doi: 10.1016/S0168-3659(99)00228-X [Crossref] [ Google Scholar]
- Grabovac V, Guggi D, Bernkop-Schnürch A. Comparison of the mucoadhesive properties of various polymers. Adv Drug Deliv Rev 2005; 57(11):1713-1723. doi: 10.1016/j.addr.2005.07.006 [Crossref] [ Google Scholar]
- Fang J-YY, Yu S-YY, Wu P-CC, Huang Y-B Bin, Tsai Y-HH. In vitro skin permeation of estradiol from various proniosome formulations. Int J Pharm 2001; 215(1-2):91-99. doi: 10.1016/S0378-5173(00)00669-4 [Crossref] [ Google Scholar]
- El Maghraby GM, Williams AC, Barry BW. Skin delivery of 5-fluorouracil from ultradeformable and standard liposomes in-vitro. J Pharm Pharmacol 2001; 53(8):1069-1077. doi: 10.1211/0022357011776450 [Crossref] [ Google Scholar]
- Verma DD, Verma S, Blume G, Fahr A. Liposomes Increase skin penetration of entrapped and non-entrapped hydrophilic substances into human skin: a skin penetration and confocal laser scanning microscopy study. Eur J Pharm Biopharm 2003; 55(3):271-277. doi: 10.1016/S0939-6411(03)00021-3 [Crossref] [ Google Scholar]
- Ogiso T, Niinaka N, Iwaki M. Mechanism for Enhancement effect of lipid disperse system on percutaneous absorption. J Pharm Sci 1996; 85(1):57-64. doi: 10.1021/js950178x [Crossref] [ Google Scholar]
- Cevc G, Gebauer D. Hydration-driven transport of deformable lipid vesicles through fine pores and the skin barrier. Biophys J 2003; 84(2):1010-1024. doi: 10.1016/S0006-3495(03)74917-0 [Crossref] [ Google Scholar]
- Schreier H, Bouwstra J. Liposomes and niosomes as topical drug carriers: dermal and transdermal drug delivery. J Control Release 1994; 30(1):1-15. doi: 10.1016/0168-3659(94)90039-6 [Crossref] [ Google Scholar]
- Tsujino I, Yamazaki T, Masutani M, Sawada U, Horie T. Effect of Tween-80 on Cell killing by etoposide in human lung adenocarcinoma cells. Cancer Chemother Pharmacol 1999; 43(1):29-34. doi: 10.1007/s002800050859 [Crossref] [ Google Scholar]