BioImpacts. 8(1):5-12.
doi: 10.15171/bi.2018.02
Original Research
The role of PRP and adipose tissue-derived keratinocytes on burn wound healing in diabetic rats
Navid Hosseini Mansoub 1, *, Mehmet Gürdal 1, Elif Karadadaş 1, Hilal Kabadayi 2, Seda Vatansever 2, 3, Gulinnaz Ercan 1, 4, *
Author information:
1Department of Medical Biochemistry, Faculty of Medicine, Ege University, Izmir, 35100, Turkey
2Department of Histology and Embryology, Faculty of Medicine, Celal Bayar University, Manisa, 45200, Turkey
3Experimental Health Sciences Research Center, Near East University, Mersin, 33010, Turkey
4Department of Stem Cell, Institute of Health Sciences, Ege University, Izmir, 35100, Turkey
Abstract
Introduction:
Diabetic burn wounds and ulcers are significant complications of diabetic patients. The aim of this study is to investigate the use of platelet rich-plasma (PRP) and/or keratinocyte-like cells (KLCs) in diabetic thermal wound rat model and to evaluate EGF, FGF-2, TGF-β1, COL1α2, MCP-1 and VEGF-α as wound healing markers at gene expression level.
Method:
In this study, we used adipose tissue as the source of mesenchymal stem cells (MSCs) and differentiated MSCs into KLCs. KLCs were characterized and transferred to the burn areas on the dorsum of streptozotocine (STZ)-induced diabetic rats. We prepared PRP from rat blood and evaluated its effect alone or in combination with KLCs. On 3rd, 7th, 10th and 14th days after treatment, wound areas were measured and biopsy samples were excised from the wound areas of the KLCs and/or PRP-treated and untreated diabetic rats to analyze gene expression levels of wound healing markers by qPCR.
Results:
We observed that, wound contraction started earlier in the PRP and/or KLCs-treated groups in comparison to the control group. However, PRP and KLCs when applied in combination showed additive affect in wound healing. In all groups treated with KLCs and/or PRP, the gene expression levels of evaluated growth factors and COL1α2 increased, while MCP-1 levels decreased when compared to the untreated diabetic rats. In addition, the most prominent difference in qPCR results belongs to combined PRP and KLCs-treated group.
Conclusion:
We demonstrated that applying PRP and KLCs in combination has a greater potential for treatment of diabetic burn wounds.
Keywords: Burn, Keratinocyte, Diabetes mellitus, Mesenchymal stem cells, PRP, Wound
Copyright and License Information
© 2018 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
The impaired wound healing process in diabetic patients can result in ulcer development, especially in lower extremities. The diabetic foot ulcer is a significant disabling condition that frequently leads to amputation.
1
Normal wound healing is a complicated process which is stimulated by tissue injuries. The healing process consists of four overlapping phases, beginning with hemostasis and inflammation and continuing with proliferation, and then maturation.
2
In diabetes, the healing process is disrupted at different stages by several intrinsic factors such as neurovascular complications or extrinsic factors like infection and callus formation.
3
The chronic interruption of wound healing process in diabetic patients with thermal burns and subsequent complications seen in these patients give rise to conduct intensive research in order to find new strategies to accelerate the healing process and to reduce the scar formation.
Platelets are one of the essential components of the hemostatic system and known to function as a potent mediator of the wound healing with high levels of growth factors found in their granules ready to be secreted. Since one of the crucial roles of platelets as fibrinogen activators to support the use of platelet-rich plasma (PRP) in different clinical applications, they are called “tissue sealants”. Hence, several studies reported a favorable effect of PRP on wound healing process.
4
Additionally, the role of platelets not only in chemotaxis but also in cellular proliferation of fibroblasts, endothelial cells and progenitor cells, make these cells crucial mediators of the wound healing process.
5
Meanwhile, in wound healing process, keratinocytes have also important roles since they come immediately to the wound area to show their functions. This activation process leads to alterations in gene expression profile and keratinocytes become capable of producing signaling molecules that act both in an autocrine and paracrine fashion resulting in pleiotropic effects on multiple cell types. The affected cells respond to keratinocytes by producing several signaling molecules which also regulate keratinocyte activation during wound closure
6
e.g. cytokines and growth factors released from keratinocytes stimulate fibroblasts which also, in turn, activate keratinocytes differentiation. In addition, keratinocytes also stimulate a myofibroblast-like cell generation which is crucial for the wound contraction.
7
The exact mechanisms of the defective repair of wounds in diabetes have not been elucidated yet; however, a decrease in the amount of growth factors is involved in the defective wound healing process.
8
Some of the growth factors have already been investigated for their beneficial effects on keratinocyte functions, since they are the key players in both repairing skin through covering wounds and secreting growth factors during wound healing process.
9,10
In this study, the effect of PRP and/or KLCs, as the crucial sources of growth factors, cytokines and chemokines, was evaluated in diabetic burn wound model for their contribution in wound healing process. We also tested whether PRP has any contribution in the improvement of the effect of KLCs differentiated from adipose tissue-derived mesenchymal stem cells (AD-MSCs) when used in combination for the first time in the literature. In order to prepare KLCs to apply on burn wounds, we preferred using AD-MSCs since adipose tissue can be obtained easily from voluntary donors who underwent to liposuction/lipectomy procedures by getting ethical permission to use this waste material
11
Also, it is known as a good source of MSCs, even better than bone marrow, where the applied aspiration procedure is more invasive (not voluntary). Moreover, adipose tissue contains more MSCs when compared with other sources, particularly bone marrow, which is widely used as MSCs source.
12
MSCs are obtained and used autologously in clinical human studies as well as they could be used without carrying ethical concerns which lead to major problems in the use of embryonic stem cells.
13,14
Materials and Methods
Materials
Streptozotocin provided by Sigma (St Louis, MO, USA), Custom RT2 Profiler™ PCR Array, RT² HT First Strand Kit and RT² SYBR Green qPCR Mastermix were obtained from Qiagen (Hilden, Germany). FBS provided by Biochrom (Berlin, Germany), cytokeratin-8 and cytokeratin-14 were obtained by Santa Cruz Biotechnology (Heidelberg, Germany).
Experimental diabetes model
All animals were maintained at 22 ± 2°C with a standard 12 hours light-dark cycle and given food and water ad libitum. The animals were injected with a single dose of streptozotocin (STZ dissolved in 3.8% citrate Buffer, pH = 4.5 60 mg/kg body weight) intraperitoneally (I.P.) to induce diabetes. Fasting blood glucose (FBG) levels were measured with a glucometer (Accu-CHECK) in blood samples obtained from tail veins 48 hours after the STZ injection. The STZ-injected rats having FBG levels ≥280 mg/dL. were considered as diabetic and were monitored by measuring FBG levels and body weight weekly for 4 weeks.
Thermal wound creation
After removal of hairs on the back of the animals, seconddegree burn wound models were created as spots on the dorsum of the rats under ketamine (80 mg/kg I.P., Sigma-Aldrich) and xylazine (10 mg/kg I.P., Sigma-Aldrich) anesthesia by touching a 1 cm in diameter copper rod as shown in Fig. 1A, heated to 100°C via inserting in boiling water. The heated copper rod was touched on the skin of the rats for 20 seconds without pressure in order to create 4 symmetrical burn spots, 1 cm in diameter on each animal as shown in Fig. 1B, modified from previous studies where the brass probe has been used.
15,16
Rats were then randomly divided into 4 groups each consisting of 6 animals as: (1) Non-treated diabetic rats (control), (2) KLCs-treated diabetic rats (KT), (3) Local PRP-treated diabetic rats (LP), (4) Local PRP + KLCs-treated diabetic rats (LP + KT). PRP (0.3 mL) and/or KLCs (4×105 cells in 0.3 mL culture medium) were injected subcutaneously (SC) into multiple injection points in each wound area to obtain a diffuse distribution of the PRP and/or KLCs.
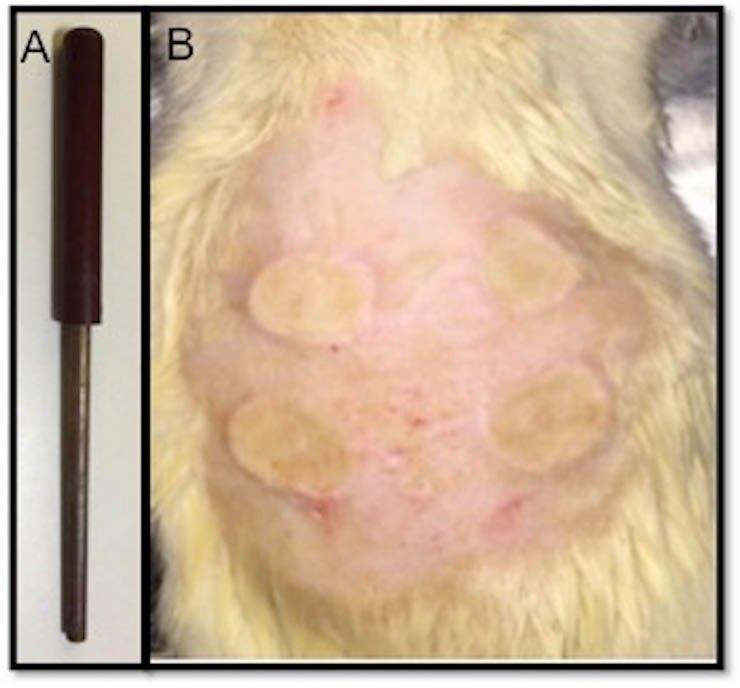
Fig. 1.
Copper rod used in creation of thermal burn wounds on skin of the rats (A) Symmetrical burn spots created on the dorsum of the animals (B).
.
Copper rod used in creation of thermal burn wounds on skin of the rats (A) Symmetrical burn spots created on the dorsum of the animals (B).
Isolation and characterization of rat adipose tissue-derived mesenchymal stem cells
Peritoneal and inguinal adipose tissue samples were obtained from rats (n = 5) under ketamine and xylazine anesthesia under aseptic conditions. Collected tissue samples were washed initially with phosphate buffered saline (PBS) at least three times to remove blood cells. After a washing step with PBS containing 1% penicillin-streptomycin (P/S) solution, the samples were incubated in a sterile tissue culture plate including 0.075 % collagenase type I in alpha-Minimum Essential Medium (α-MEM) culture medium supplemented with 15% fetal calf serum (FCS), 1% L-glutamine, 1% P/S, 1% gentamycin, 0.1% amphotericin B and minced with scalpel, then incubated for 1 hour at 37°C, 5% CO2. After an hour of incubation, the collagenase-digested samples were transferred to the falcon tubes and centrifuged at 1000 rpm for 5 minutes and then the supernatant was discarded. The cells which were resuspended in α-MEM culture medium prepared as above without collagenase type I addition and were incubated at 37°C, 5% CO2 until reaching to 80%-90% confluency and then differentiation protocol was performed using the second passage of AD-MSCs.
17
For characterization of AD-MSCs, they were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton-X 100 after washing with PBS. The cells were incubated with blocking solution and then incubated with anti-CD90 (as a positive marker) and anti-CD44 (as a negative marker) at 4°C overnight. They were washed again with PBS and incubated with the secondary antibody for 2 hours. The immunoreactivity for imaging was achieved using 3,3'-Diaminobenzidine (DAB) chromogen for 5 minutes. The samples were covered with mounting media and then examined under light microscopy (Olympus BX40).
Differentiation of adipose tissue-derived mesenchymal stem cells to keratinocytes
In order to differentiate AD-MSCs into KLCs, AD-MSCs were cultured in plates coated with Matrigel containing α-MEM culture medium supplemented with 0.5nM BMP-4 for 2 weeks. Some of the cells were fixed for 30 minutes with 4% paraformaldehyde for morphological and histological characterization of KLCs with anti-cytokeratin-8 and 14 by an indirect immunoperoxidase technique using anti-cytokeratin-8 and anti-cytokeratin-14 antibodies. Following permeabilization of cells with 0.1% Triton-X 100, cells were washed with PBS for three times and incubated overnight with the antibodies at 4°C. Then the cells were washed with PBS for three times before incubating with the secondary antibody for 2 hours. Applying DAB chromogen for 5 minutes, the immunoreactivity for imaging was achieved. After covering the samples with mounting media, AD-MSCs-derived KLCs were identified. The remaining AD-MSCs-derived KLCs which were not used for characterization analysis were trypsinized and collected to be transferred to the thermal burn wounds created on the back of the rats.
Preparation of PRP
To execute the experimental procedures, intracardiac blood samples of 5 Wistar Albino rats under I.P. pentobarbital (50 mg/kg) anesthesia were withdrawn into anticoagulant (3.8% sodium citrate) containing tubes and then centrifuged at 300 g for 10 minutes. The platelet rich plasma fraction was collected and then centrifuged at 3000 g for 15 minutes. The supernatant consisting of platelet poor fraction was separated and discarded. One milliliter of original plasma and the newly prepared PRP samples were compared via determining platelet concentration. Platelets were found 4-times higher in PRP than the baseline value of the original plasma. For platelet activation of PRP 100 U/mL thrombin in 10% CaCl2 in a 1/0.15 ratio was used as previously described.
18
Data of wound area measurements
Wound areas of the rats were photographed from the firstweek until the end of the fourth week. The wound contraction ratio was calculated based on the decrease in the wound size. The extent of the contraction is calculated with the formula; Wound Contraction (%) = (A1- Atd) / A1 ×100: Where A1 represents the surface area calculated at day 0 while Atd represents the surface area calculated at the day of biopsy sampling of wounds on the 3rd, 7th, 10th and 14th days after PRP and/or KLCs treatment.
Analysis of gene expression by qPCR
Total RNA was isolated from biopsy samples using MagnaPure Compact RNA Isolation Kit. RNA isolation was performed using MagnaPure Compact System. RNA concentrations and RNA purities were determined by Nanodrop® based on the assessment of A260/A280 and A260/A230 ratios. Total RNA (1 μg) of each sample was used for cDNA synthesis (RT2 HT First Strand kit). For RT-PCR, a custom-designed RT2 Profiler PCR array was used. Expression levels of the genes EGF, FGF-2, TGF-β1, collagen-1alpha-2 (COL1α2), MCP-1 and VEGF-α, were analyzed. As housekeeping genes, TATA box binding protein (TBP), beta-2 microglobulin (β2M) and actin beta (ACTB) genes were used. RT2 SYBR Green ROX FAST Mastermix was used with the specific primers designed for each gene. The relative expression level of each target gene was calculated by using the formula; fold change = 2−ΔΔCtwhere ΔCt represents the Ct (treated sample) - Ct (control) while non-treated diabetic rats were used as the control group.
Statistical analysis
All values are expressed as mean ± SD and P value <0.05 was considered as statistically significant. For gene expression analysis Mann-Whitney U test was used while for multiple group comparisons ANOVA was used followed by Tukey post hoc test.
Results
Identification of AD-MSCs and KLCs
AD-MSCs were characterized by the analysis of CD-90 and CD-44 distribution with indirect immunoperoxidase technique. While the cells were positively stained with CD90, CD44 was negative as expected (Figs. 2A and B). For immunohistochemical evaluation of AD-MSCs-derived KLCs, cytokeratin-8 level was evaluated as an early-stage keratinocyte differentiation marker, and in cells that started to differentiate, its expression was found positive (Figs. 3A and B, and Fig. 4A). Cytokeratin-14 was evaluated as a late-stage keratinocyte differentiation marker, and in differentiated cells it was found to be positive. Meanwhile, epithelioid cells were found to be positive for both differentiation markers (Fig. 4B).
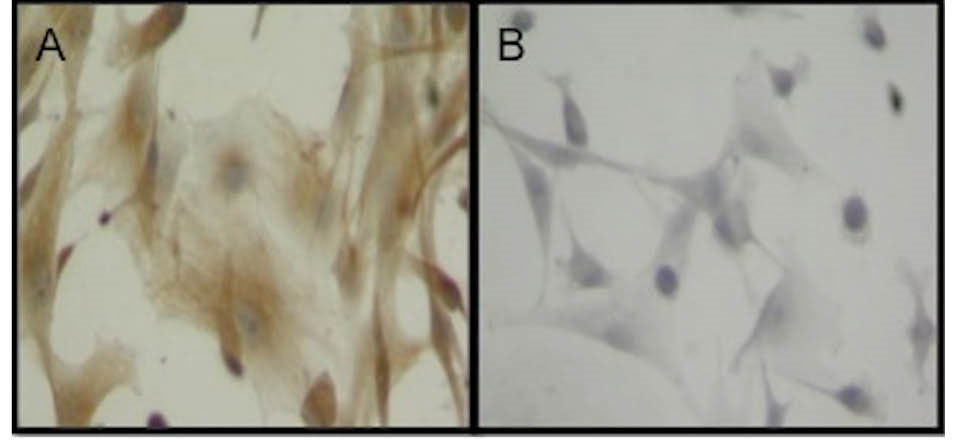
Fig. 2.
Adipogenic mesenchymal stem cells were characterized after immunohistochemical distribution of CD90 (A) and CD44 (B). Original magnification: x400.
.
Adipogenic mesenchymal stem cells were characterized after immunohistochemical distribution of CD90 (A) and CD44 (B). Original magnification: x400.
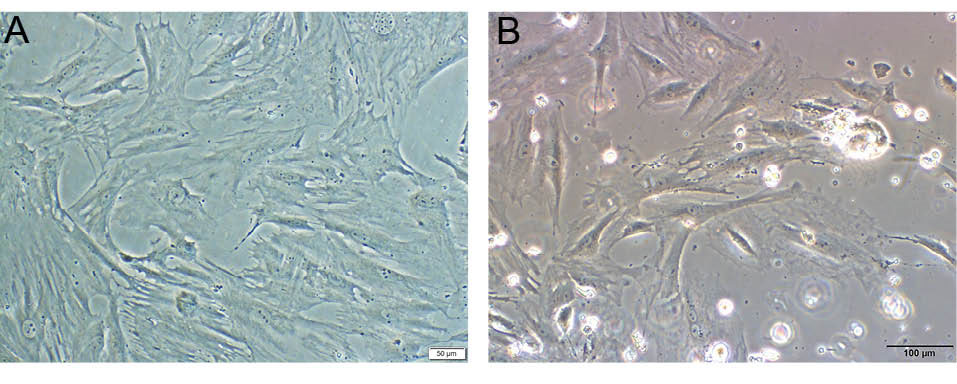
Fig. 3.
Adipogenic stem cell culture, Scale bar = 50 µm (A). Differentiated KLCs, Scale bar = 100 µm (B).
.
Adipogenic stem cell culture, Scale bar = 50 µm (A). Differentiated KLCs, Scale bar = 100 µm (B).
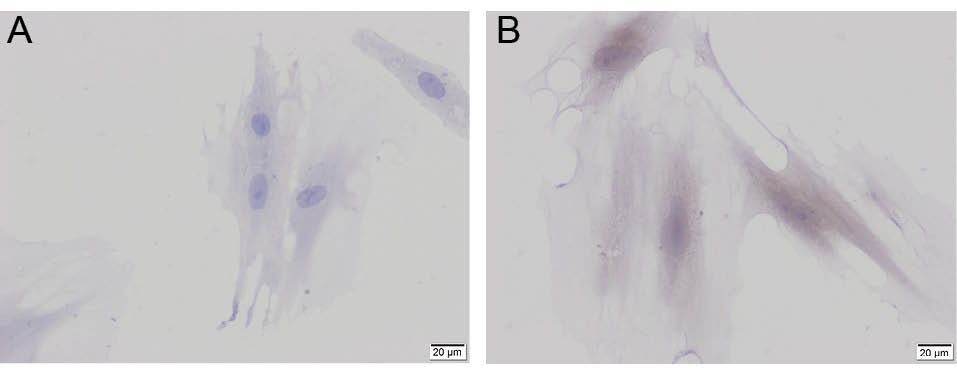
Fig. 4.
Cytokeratin-8 (A) and cytokeratin-14 (B) in differentiated KLCs. Scale bars = 20 µm.
.
Cytokeratin-8 (A) and cytokeratin-14 (B) in differentiated KLCs. Scale bars = 20 µm.
Wound contraction
During observation of wound contraction ratios of all treated and untreated groups for 2 weeks, time-dependent and treatment-dependent differences in the rate of wound contractions were determined as shown in Figs. 5 and 6. The decrease in the size of the wounded area in comparison to its original size due to the wound contraction was 76% in KT group, 78% in LP group, and was 88% in LP + KT group while it was only 67.5% for the control group at the 14thday. At the end of the second week, the wound contraction ratio was significantly higher in LP + KT group in comparison to controls and KT group or LP group (Figs. 5 and 6).
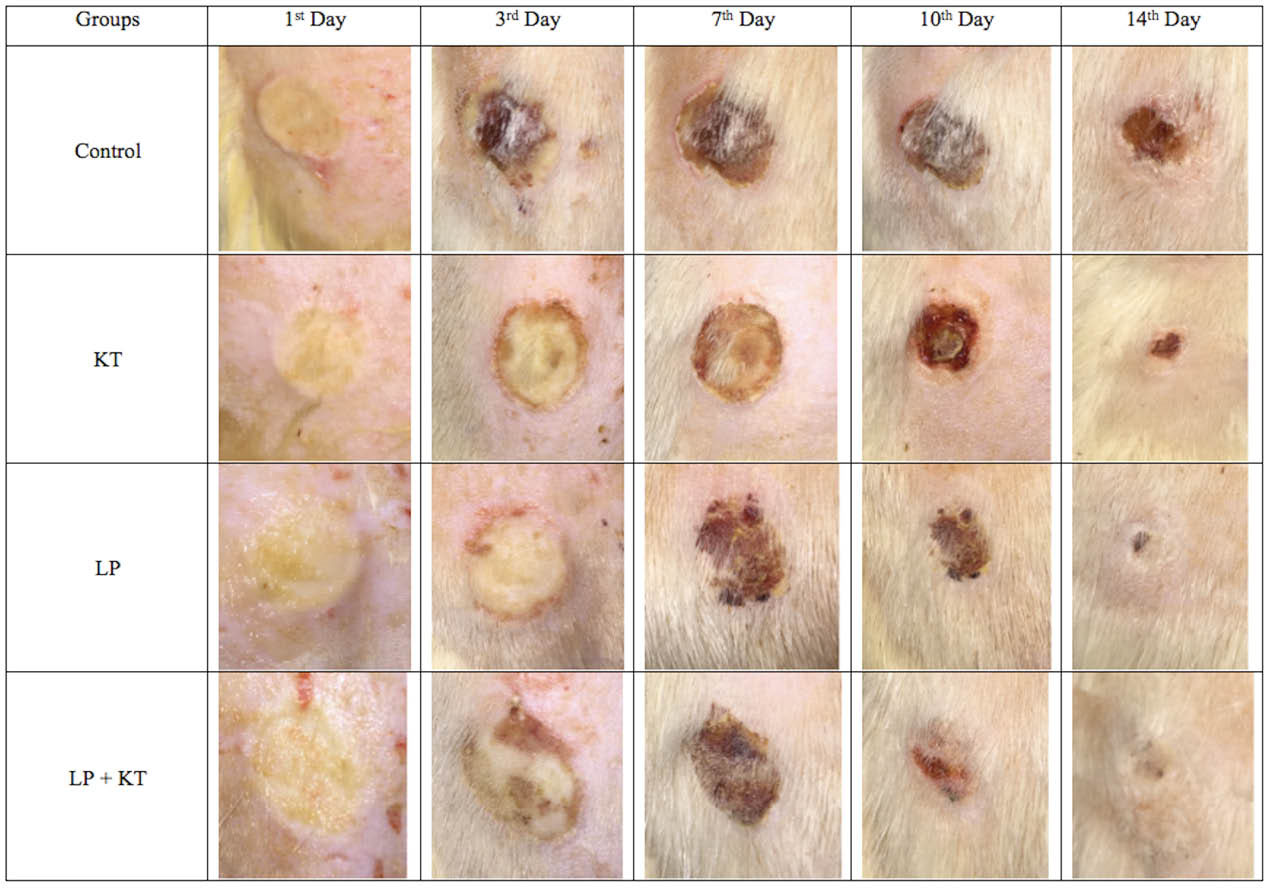
Fig. 5.
Macroscopic evaluation of wound closure in a day-dependent and treatment–dependent perspectives. Wound contraction is the best with the combinational approach at the end of the second week of the treatment.
.
Macroscopic evaluation of wound closure in a day-dependent and treatment–dependent perspectives. Wound contraction is the best with the combinational approach at the end of the second week of the treatment.
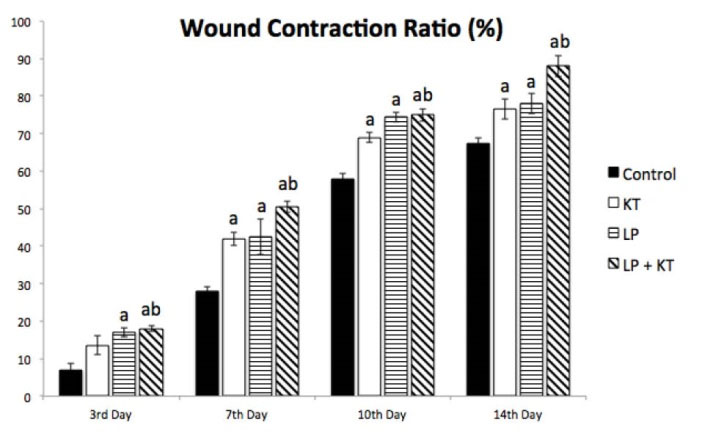
Fig. 6.
Percentage of wound contraction in comparison to the original wound area in all groups until the end of the second week after treatment protocols. The accelerated trend of wound contraction in treatment groups compared to the untreated control group is evident at all days of sampling from the 3rd to 14th days. n = 6; aP < 0.05 vs Control and bP < 0.05 vs KT.
.
Percentage of wound contraction in comparison to the original wound area in all groups until the end of the second week after treatment protocols. The accelerated trend of wound contraction in treatment groups compared to the untreated control group is evident at all days of sampling from the 3rd to 14th days. n = 6; aP < 0.05 vs Control and bP < 0.05 vs KT.
Analysis of gene expression profiles in wound healing
In order to elucidate the effectiveness of our treatment protocols on wound healing process, the changes in mRNA levels of wound healing markers e.g. EGF, FGF-2, TGF-β1, COL1α2, MCP-1 and VEGF-α were evaluated in samples obtained at different time points (the 3rd, 7th, 10th and 14th days). As shown in Fig. 7, the expression levels of EGF, FGF-2, TGF-β1, COL1α2 and VEGF-α were found to be significantly elevated in all treatment groups at the end of the first week (day 7). However, an earlier and significant change in expressions of cytokines except TGF-β1 indicating an earlier response in wound healing process was observed in the LP group and LP + KT group.
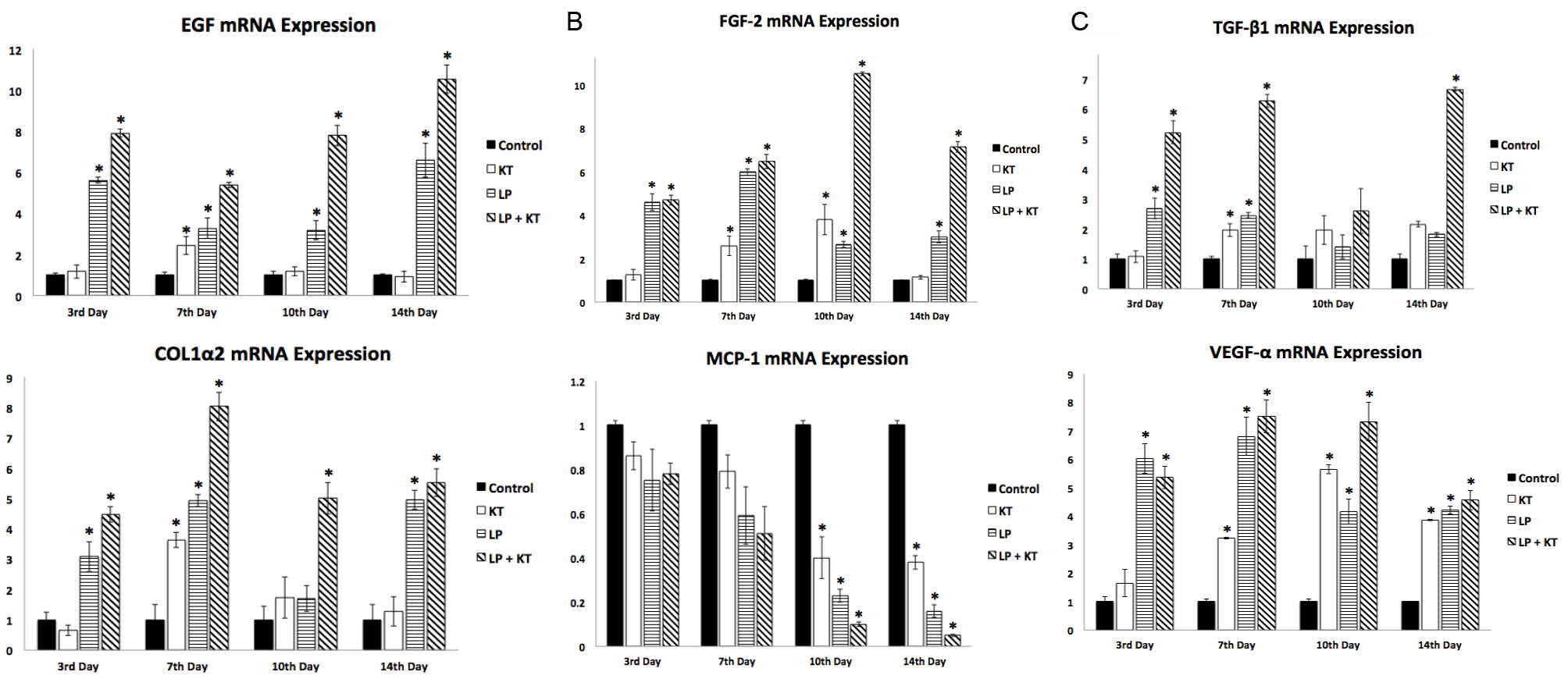
Fig. 7.
qPCR analysis of wound healing markers, EGF, FGF-2, TGF-β1, COL1α2, MCP-1 and VEGF-α are shown as 6-1 to 6-6 respectively. Note that there is a general trend of higher/significantly higher mRNA expressions for all cytokines having a prominent role in wound healing process in the treated groups when compared to the control group in all sampling days while a day-dependent significant decrease is particularly observed in the mRNA expression for the chemokine MCP-1 at the 10th and 14th days. n = 3; *P <0.05.
.
qPCR analysis of wound healing markers, EGF, FGF-2, TGF-β1, COL1α2, MCP-1 and VEGF-α are shown as 6-1 to 6-6 respectively. Note that there is a general trend of higher/significantly higher mRNA expressions for all cytokines having a prominent role in wound healing process in the treated groups when compared to the control group in all sampling days while a day-dependent significant decrease is particularly observed in the mRNA expression for the chemokine MCP-1 at the 10th and 14th days. n = 3; *P <0.05.
Overall, an increase in gene expression results of cytokines indicating a better response to therapies was observed in LP group in comparison to KT and control groups while the highest increase in gene expression results of cytokines/growth factors indicating the best response to therapies was achieved in LP + KT group in nearly all sampling days until the end of the second week.
Collagen-1 is an important extracellular matrix constituent, also associated with diverse biological processes such as skin morphogenesis, platelet activation, and extracellular matrix organization. COL1α2 gene expression was significantly increased in LP + KT group in biopsy samples collected at all different time points. A similar pattern was also observed for LP group. However, KT group showed an increase in COL1α2 gene expression only at the end of the first week that may suggest that treatment with KLCs is relatively weak in stimulation of wound healing-related signaling. MCP-1 is a chemokine, an inflammation marker that showed a trend of time-dependent decrease gradually in mRNA levels at all biopsy sampling days which is particularly statistically significant on the 10th day and 14th day in all treated groups. The decrease is more prominent in LP + KT group in comparison to the other treated groups.
Discussion
In this study, we demonstrated that the application of PRP and/or KLCs in thermal wounds of diabetic rats leads to enhanced expression profiles of the wound healing markers, where the enhancement is more prominent and statistically significant when PRP and KLCs are combined together. The overexpression of the growth factors e.g. EGF, VEGF-α, FGF-2, TGF-β1 and COL1α2 and the decrease in expression of the chemokine MCP-1 which is an inflammation marker indicates an acceleration in the wound closure of diabetic rats with treatment protocols which is more prominent in PRP-applied groups. However, the highest difference in mRNA expression levels of wound healing markers was determined in the combined PRP + KLCs-applied group. According to our results as shown in Figs. 5 and 6, re-epithelization and wound contraction started earlier and progressed faster in all treated groups in comparison to the untreated diabetic control group. The most accelerated wound healing process was observed on the dorsum of the animals who were treated with combined PRP and KLCs as shown in Fig. 5. Similarly, the time-dependent exaggerated changes in mRNA expression of wound healing markers represent an additive effect of PRP and KLCs when combined together during the impaired wound healing process in diabetes as previously shown.
19
Since the decrease in expression of growth factors has been shown to be associated with delayed wound healing, these are so crucial in this process. Among these, VEGF-α is a well-characterized cytokine responsible particularly for the induction of angiogenesis. The current data suggests that, it has an important role especially in collagen production, collagen deposition and epithelization which are also necessary for re-epithelization phase of the wound healing process.
20
Previously, EGF and FGF-2 were also shown as the necessary cytokines for a better re-epithelization process in experimental wound models.
21
Besides, FGF-2 knock-out mice were shown as having a defective re-epithelization phase in wound healing process which confirms this situation.
22
Meanwhile, an extensively studied factor, TGF-β1, is one of the major regulators of the wound healing process. In skin tissue homeostasis, the TGF-β1-activated pathway is responsible for the cell cycle arrest that is crucial in preventing dermal hyperplasia. The pathway is activated in response to the wound healing, where all isoforms of this protein actively participate. Specifically, the mechanism works via integrin proteins that enhance the activation of keratinocytes in wounds.
23
Previous in vitro studies demonstrated that the latent form of TGF-β1 is activated and the mature protein is released during re-epithelization that leads to an elevation in phosphorylated form of Smad2 in acute wound models. In accordance with this situation, the deletion of Smad proteins was shown to cause a delayed wound closure, which indicates that TGF-β-mediated signaling pathway is critical for effective and successful epithelization of the wounded area.
23
Type I collagen is expressed ubiquitously in the dermal matrix and migrating keratinocytes possibly interact with this protein. Keratinocytes migrate through the newly formed granulation tissue formed in the wounded site by which re-epithelization is achieved.
24
For this reason, in our experimental design, we analyzed VEGF-α, FGF-2, EGF, TGF-β1 and type I collagen (COL1α2) gene expressions. There are differences in the gene expression profiles of the cytokines changing according to the sampling times. We noticed a common increase in the expression levels of the examined cytokines and type I collagen on the seventh day of sampling in all treatment protocols applied in comparison to control. However, an earlier and significant change in the expressions of cytokines except TGF-β1 indicating an earlier response in wound healing process was observed mainly in the only PRP-applied and combined PRP and KLCs-applied treatment groups while the most prominent increase belongs to the combined treatment group.
Therefore, the results indicate that the use of PRP and KLCs induces re-epithelialization earlier and faster. Our results are also in concordance with Xian et al’s data who reported 20% PRP addition in co-culture medium of keratinocytes and fibroblasts significantly enhanced granulocyte-macrophage colony-stimulating factor which has a role in chemotaxis of neutrophils, monocytes and macrophages to the wounded area to support re-epithelization and angiogenesis.
25
Similarly, other researchers also reported that PRP supplementation in co-culture medium plays a significant role not only in promoting cellular migration of keratinocytes and fibroblasts but also in releasing various growth factors related to the wound healing process.
26,27
Contrary to some of the reports, where keratinocytes localized in the wounded area are shown as producing MCP-1 and starting the re-epithelization process, in our study, we found a significant decrease in MCP-1 levels in KLCs-applied groups.
28
Similarly, in our study, PRP application also led to a decreased level of MCP-1 expression which is in concordance with some earlier reports where PRP was indicated as a suppressive factor for MCP-1.
29
Therefore, the elucidation of the working mechanism for the role of MCP-1 in the re-epithelization process are still obscured and needs further studies. Since in accordance with our results, an inverse correlation between PRP concentration and secreted MCP-1 levels was shown, previously, we can conclude that PRP may support acceleration of the healing process via leading to a reduction in monocyte-mediated inflammatory response.
25
Wound repair mechanism is negatively affected in diabetes. The decrease of cytokines was suggested to be responsible for this impaired wound healing process in diabetes.
8
Until now, many growth factors have been tried specifically in order to detect their beneficial effects on functions of keratinocytes which are the key players in repairing skin through covering wounds.
9,10
Since applying growth factors in combination may be more effective in stimulating wound repair in vivo than the application of each growth factor alone locally to the wound area, by applying PRP and KLCs in combination, we managed to accelerate wound healing process as represented in our data.
9
Our previous data demonstrating immunohistochemical analysis of wound healing markers were also in concordance with our gene expression results in the present study where the increase in FGF-2, EGF, VEGF-α and COL1α2 and the decrease in MCP-1 protein expression levels were similar while the most prominent alteration is observed in combined PRP and KLCs-treated group.
30
Since a pool of PRP prepared from blood samples of 5 different rats for topical application on wounded areas and PRP-applied groups had better results for a better wound healing process, the allogeneic use of PRP is proven to be useful in this study. Therefore, the use of the allogeneic PRP could be recommended for clinical applications in diabetic patients who suffer from lethal, deep burns and wounds while autologous application could be only recommended for preventing or decreasing scar formation of lesions which are not lethal. Previously, the use of allogeneic PRP was shown in different applications other than burn wounds of regenerative treatments.
31
In our study, the effectiveness of allogeneic PRP as a treatment agent was also confirmed in vivo in the burn wound healing process.
Conclusion
In conclusion, our study showed that the application of PRP and KLCs in combination leads to the more accelerated wound healing process when compared with other therapies alone. From a different perspective, the results of the current study showed that the use of adjuvant biomaterials, e.g, PRP and KLCs derived from AD-MSCs could be promising in the treatment of diabetic wounds.
Ethical approval
This current work was approved by the Ethics Committee of Ege University.
Competing interests
There is no conflict of interests to be reported.
Research Highlights
What is current knowledge?
simple
-
√ Platelets are as known a potent mediator of wound healing
process due to the high levels of growth factors found in
their granules ready to be secreted in need and keratinocytes
release some cytokines and growth factors stimulating
fibroblasts. Thus, these events lead to an accelerated wound
healing process.
What is new here?
simple
-
√ PRP increases the effect of the keratinocyte-like cells
(KLCs), which are differentiated from adipose tissueoriginated
stem cells when it is applied in combination.
Acknowledgements
This work is supported by scientific research foundation of Ege University, Faculty of Medicine (15-TIP-014 and 13-TIP-053). The 15-TIP-014 project is the Doctorate Thesis of Navid Hosseini Mansoub.
References
-
International Diabetes Federation e-atlas of diabetes. 2015; Available from: http://www.idf.org.
- Medina A, Scott PG, Ghahary A, Tredget EE. Pathophysiology of chronic nonhealing wounds. J Burn Care Rehabil 2005; 26:306-19. doi: 10.1097/01.BCR.0000169887.04973.3A [Crossref] [ Google Scholar]
- Kwon DS, Gao X, Liu YB, Dulchavsky DS, Danyluk AL, Bansal M. Treatment with bone marrow‐derived stromal cells accelerates wound healing in diabetic rats. Int Wound J 2008; 5:453-63. doi: 10.1111/j.1742-481X.2007.00408.x [Crossref] [ Google Scholar]
- Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg 2004; 114:1502-8. doi: 10.1097/01.PRS.0000138251.07040.51 [Crossref] [ Google Scholar]
- Pierce GF, Mustoe TA, Altrock BW, Deuel TF, Thomason A. Role of platelet‐derived growth factor in wound healing. J Cell Biochem 1991; 45:319-26. doi: 10.1002/jcb.240450403 [Crossref] [ Google Scholar]
- Komine M, Rao LS, Kaneko T, Tomic-Canic M, Tamaki K, Freedberg IM. Inflammatory Versus Proliferative Processes in Epidermis Tumor Necrosis Factor Α Induces K6b Keratin Synthesis Through A Transcriptional Complex Containing NFκB AND C/EBPβ. J Biol Chem 2000; 275:32077-88. doi: 10.1074/jbc.M001253200 [Crossref] [ Google Scholar]
- Werner S, Krieg T, Smola H. Keratinocyte–fibroblast interactions in wound healing. J Invest Dermatol 2007; 127:998-1008. doi: 10.1038/sj.jid.5700786 [Crossref] [ Google Scholar]
- Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003; 83:835-70. doi: 10.1152/physrev.00031.2002 [Crossref] [ Google Scholar]
- Peplow PV, Chatterjee MP. A review of the influence of growth factors and cytokines in in vitro human keratinocyte migration. Cytokine 2013; 62:1-21. doi: 10.1016/j.cyto.2013.02.015 [Crossref] [ Google Scholar]
- Pastore S, Mascia F, Mariani V, Girolomoni G. The epidermal growth factor receptor system in skin repair and inflammation. J Invest Dermatol 2008; 128:1365-74. doi: 10.1038/sj.jid.5701184 [Crossref] [ Google Scholar]
- Malhotra S, Hu MS, Marshall CD, Leavitt T, Cheung ATM, Gonzalez JG. Mesenchymal Stromal Cells as Cell-Based Therapeutics for Wound Healing. Stem Cells Int 2016; 2016:4157934. doi: 10.1155/2016/4157934 [Crossref] [ Google Scholar]
- Schäffler A, Büchler C. Concise review: adipose tissue-derived stromal cells--basic and clinical implications for novel cell-based therapies. Stem Cells 2007; 25(4):818-27. doi: 10.1634/stemcells.2006-0589 [Crossref] [ Google Scholar]
- Lo B, Parham L. Ethical Issues in Stem Cell Research. Endocr Rev 2009; 30(3):204-213. doi: 10.1210/er.2008-0031 [Crossref] [ Google Scholar]
- King NMP, Perrin J. Ethical issues in stem cell research and therapy. Stem Cell Res Ther 2014; 5(4):85. doi: 10.1186/scrt474 [Crossref] [ Google Scholar]
- Han M, Durmus A, Karabulut E, Yaman I. Effects of Turkish propolis and silver sulfadiazine on burn wound healing in rats. Revue de médecine vétérinaire 2005; 156:624. [ Google Scholar]
- Durmus A, Han MC, Yaman I. Comperative evaluation of collagenase and silver sulfadiazine on burned wound healing in rats. Firat Universitesi Saglik Bilimleri Veteriner Dergisi 2009; 23:135-9. [ Google Scholar]
- Balducci L, Alessandri G. Isolation, Expansion, and Immortalization of Human Adipose-Derived Mesenchymal Stromal Cells from Biopsies and Liposuction Specimens. Methods Mol Biol 2016; 1416:259-74. doi: 10.1007/978-1-4939-3584-0_15 [Crossref] [ Google Scholar]
- Simman R, Hoffmann A, Bohinc RJ, Peterson WC, Russ AJ. Role of platelet-rich plasma in acceleration of bone fracture healing. Ann Plast Surg 2008; 61:337-44. doi: 10.1097/SAP.0b013e318157a185 [Crossref] [ Google Scholar]
- Albertson S, Hummel 3rd RP, Breeden M, Greenhalgh DG. PDGF and FGF reverse the healing impairment in protein-malnourished diabetic mice. Surgery 1993; 114:368-72. [ Google Scholar]
- Stojadinovic O, Kodra A, Golinko M, Tomic-Canic M, Brem H. A Novel, Non-angiogenic, Mechanism Of Vegf: Stimulation of Keratinocyte and Fibroblast Migration. Wound Repair Regen 2007; 15:A30. [ Google Scholar]
- Demidova-Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: biology, causes, and approaches to care. Adv Skin Wound Care 2012; 25:304-14. doi: 10.1097/01.ASW.0000416006.55218.d0 [Crossref] [ Google Scholar]
- Ortega S, Ittmann M, Tsang SH, Ehrlich M, Basilico C. Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proc Natl Acad Sci U S A 1998; 95:5672-7. doi: 10.1073/pnas.95.10.5672 [Crossref] [ Google Scholar]
- Ramirez H, Patel SB, Pastar I. The role of TGFβ signaling in wound epithelialization. Adv Wound Care (New Rochelle) 2014; 3:482-91. doi: 10.1089/wound.2013.0466 [Crossref] [ Google Scholar]
- Brett D. A Review of Collagen and Collagen-based Wound Dressings. Wounds 2008; 20:347-56. [ Google Scholar]
- Xian LJ, Chowdhury SR, Saim AB, Idrus RBH. Concentration-dependent effect of platelet-rich plasma on keratinocyte and fibroblast wound healing. Cytotherapy 2015; 17:293-300. doi: 10.1016/j.jcyt.2014.10.005 [Crossref] [ Google Scholar]
- Roubelakis MG, Trohatou O, Roubelakis A, Mili E, Kalaitzopoulos I, Papazoglou G. Platelet-rich plasma (PRP) promotes fetal mesenchymal stem/stromal cell migration and wound healing process. Stem Cell Rev 2014; 10:417-28. doi: 10.1007/s12015-013-9494-8 [Crossref] [ Google Scholar]
- Ranzato E, Patrone M, Mazzucco L, Burlando B. Platelet lysate stimulates wound repair of HaCaT keratinocytes. Br J Dermatol 2008; 159:537-45. doi: 10.1111/j.1365-2133.2008.08699.x [Crossref] [ Google Scholar]
- Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol 2001; 69:513-21. doi: 10.1189/jlb.69.4.513 [Crossref] [ Google Scholar]
- El-Sharkawy H, Kantarci A, Deady J, Hasturk H, Liu H, Alshahat M. Platelet-rich plasma: growth factors and pro-and anti-inflammatory properties. J Periodontol 2007; 78:661-9. doi: 10.1902/jop.2007.060302 [Crossref] [ Google Scholar]
- Mansoub NH, Kabadayi H, Ercan G, Vatansever HS. The use of keratinocytes and PRP in experimental burn wound model of diabetic rat. Turk J Biochem 2016; 41:S4. [ Google Scholar]
-
Textor J. Platelet-Rich Plasma (PRP) as a Therapeutic Agent: Platelet Biology, Growth Factors and a Review of the Literature. In: Lana J, Andrade Santana M, Dias Belangero W, Malheiros Luzo A, eds. Platelet-Rich Plasma. Lecture Notes in Bioengineering. Berlin, Heidelberg: Springer; 2014. doi: 10.1007/978-3-642-40117-6_2.