BioImpacts. 7(4):269-277.
doi: 10.15171/bi.2017.32
Review
Stimuli-responsive chitosan-based nanocarriers for cancer therapy
Marziyeh Fathi 1, Parham Sahandi Zangabad 1, Sima Majidi 2, Jaleh Barar 1, 3, Hamid Erfan-Niya 2, Yadollah Omidi 1, 3, * 
Author information:
1Research Center for Pharmaceutical Nanotechnology, Biomedicine Institute, Tabriz University of Medical Sciences, Tabriz, Iran
2Department of Chemical and Petroleum Engineering, University of Tabriz, Tabriz, Iran
3Department of Pharmaceutics, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
Stimuli-responsive nanocarriers offer unique advantages over the traditional drug delivery systems (DDSs) in terms of targeted drug delivery and on-demand release of cargo drug molecules. Of these, chitosan (CS)-based DDSs offer several advantages such as high compatibility with biological settings.
Methods:
In this study, we surveyed the literature in terms of the stimuli-responsive nanocarriers and discussed the most recent advancements in terms of CS-based nanosystems and their applications in cancer therapy and diagnosis.
Results:
These advanced DDSs are able to release the entrapped drugs in response to a specific endogenous stimulus (e.g., pH, glutathione concentration or certain enzymes) or exogenous stimulus (e.g., temperature, light, ultrasound, and magnetic field) at the desired time and target site. Dual-responsive nanocarriers by the combination of different stimuli have also been developed as efficient and improved DDSs. Among the stimuli-responsive nanocarriers, CS-based DDSs offer several advantages, including biocompatibility and biodegradability, antibacterial activity, ease of modification and functionalization, and non-immunogenicity. They are as one of the most ideal smart multifunction DDSs.
Conclusion:
The CS-based stimuli-responsive multifunctional nanosystems (NSs) offer unique potential for the targeted delivery of anticancer agents and provide great potential for on-demand and controlled-release of anticancer agents in response to diverse external/internal stimuli.
Keywords: Cancer therapy, Chitosan, Smart drug delivery systems, Stimuli-responsive, Nanomedicines, Theranostics
Copyright and License Information
© 2017 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Cancer is a multifaceted disease that begins with a single rebellion cell, in large part because of the induction of hypoxia and/or unprecedented detrimental damages to genes or even at epigenomes and irregular molecular inter-digitation. There exist organ-specific cancer subtypes, while many factors are involved in the intra-tumor heterogeneity.
1
Further, compelling evidence supports a significant association between the lifestyle and cancer.
2
The cancerous cells are able to alter a number of biological features, including shifting oxidative metabolism of glucose form mitochondria to aerobic glycolysis, aberrant metabolism of amino acids such as tryptophan, irregular metabolism of lipids, dysregulation of intracellular and extracellular pH, formation of a permissive milieu known as tumor microenvironment (TME), remodeling of extracellular matrix, irregular exosomal communication with neighboring cells, reforming of microvasculature and resisting the blood hydrostatic pressure with high oncotic pressure through unique interstitial fluid opposing the penetration of chemotherapies into deep regions of solid tumor.
3-11
To recolonize and spread further and farther, cancer harbor a subset of cancer cells with stemness traits, so-called cancer stem cells (CSCs).
12
The currently used strategies to control solid tumors appear to be inefficient, thus, much advancements are needed to fight such formidable disease.
Owing to targeting and controlled delivery and release capability of smart drug delivery systems (DDSs), they provide various benefits in diagnosis and therapy of cancer, including (a) effective targeting of desired biological sites (e.g., various types of cancer cells), (b) overcoming biological barriers, (c) possibility for surface modification and functionalization, (d) responsiveness to endogenous/exogenous stimuli, and (e) inducing maximal pharmacological effects with minimal inevitable side-effects in comparison with the conventional therapeutics.
13-16
Of note, the release of drugs from the smart stimuli-responsive DDSs can be triggered by the external (e.g., light irradiation, ultrasound, mechanical stress, magnetic field) or internal (e.g., intracellular/extracellular variation of pH, temperature, biomolecular activities such as hormones, glutathione, ATP, enzymes, etc).
14,17-20
Of the stimuli-responsive DDSs, intelligent polymeric systems exhibit an abrupt change in their physicochemical properties in response to a small change in environmental stimulus resulting in significant drug release and pharmacological impacts. So far, a number of different stimuli-responsive polymers have been employed for biomedical applications, controlled delivery of drugs and tissue engineering, including poly(N-alkylacrylamide)s, poly(methacrylicacid)s, poly(vinylimidazole)s, cellulose, chitosan, etc. Of these, natural polymers offer excellent biocompatibility and biodegradability, and hence, have widely been used in the development of advanced pharmaceuticals.
21
Among them, much attention has been paid to the natural polymer chitosan (CS) and its derivatives, in large part because of its unique characteristics. It is a biodegradable and mucoadhesive polymer that induces no/trivial toxicity and immunogenicity. Chitosan is produced by a deacetylation process of chitin which is one of the most abundant natural polysaccharides. Chitosan is composed of glucosamine and N-acetyl glucosamine units linked by β(1–4)-glycosidic bonds (Fig. 1).
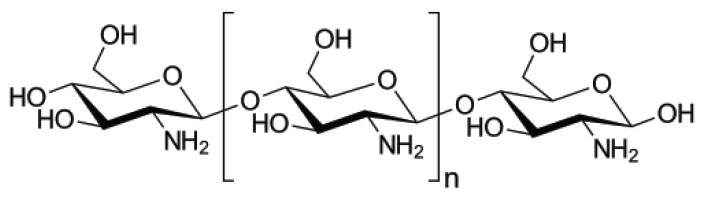
Fig. 1.
The composition of chitosan. Glucosamine and N-acetyl glucosamine units are linked by β(1–4)-glycosidic bonds.
.
The composition of chitosan. Glucosamine and N-acetyl glucosamine units are linked by β(1–4)-glycosidic bonds.
It should be highlighted that the functional groups (amino and hydroxyl moieties) of chitosan provide a great possibility for its modification with various entities resulting in development of smart nanosystems (NSs) to tackle solid tumors.
22-24
In this review, we discuss the most recent advances in the design and development of CS-based smart stimuli-responsive nanocarriers. Scheme 1 represents a CS-based targeted nanocarrier with the capability of controlled-release of cargo drug molecules in response to specific stimulus (e.g., pH, redox gradients, ultrasound, light, temperature, magnetic) or dual stimuli.
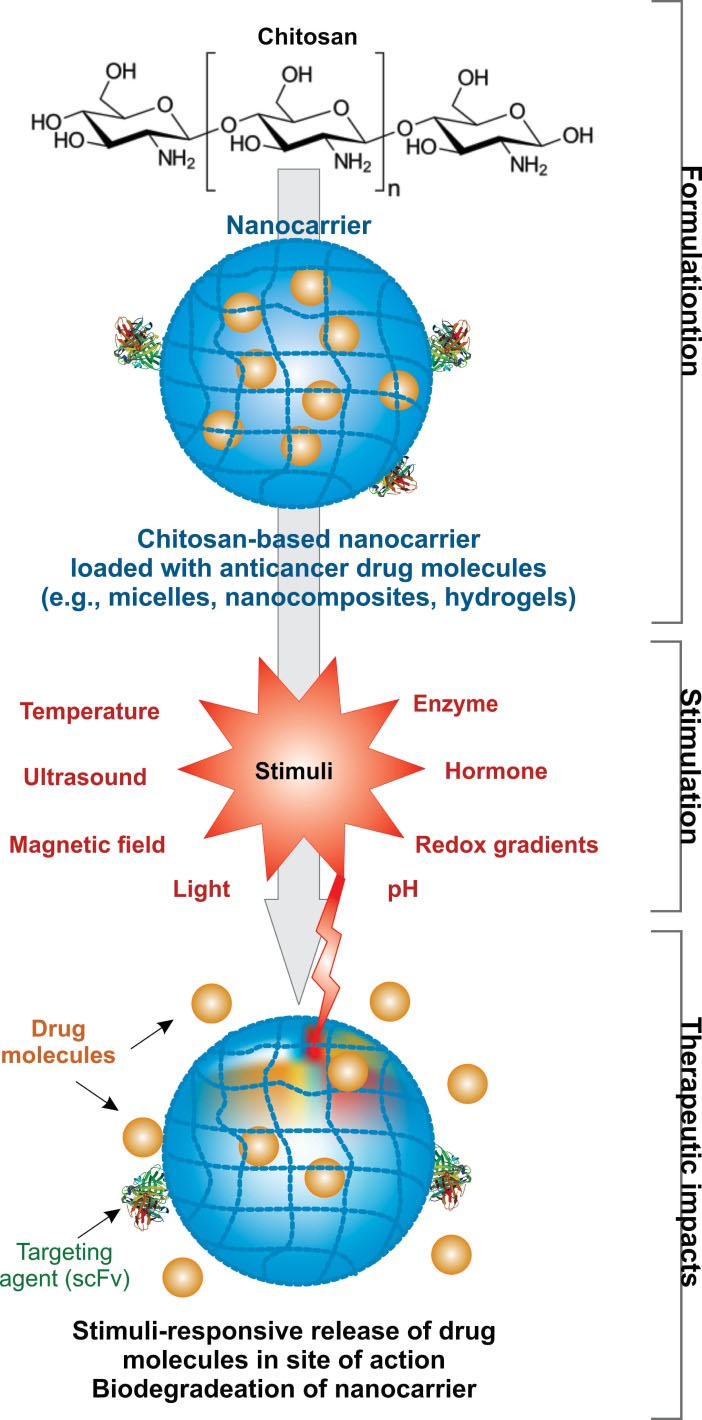
Scheme 1.
The illustration of stimuli-responsive chitosan-based nanocarriers. The release of drug molecules from the carrier is triggered by the internal/external stimulus (e.g., pH, redox gradients, ultrasound, light, temperature, magnetic) or dual stimuli.
.
The illustration of stimuli-responsive chitosan-based nanocarriers. The release of drug molecules from the carrier is triggered by the internal/external stimulus (e.g., pH, redox gradients, ultrasound, light, temperature, magnetic) or dual stimuli.
pH-responsive nanocarriers
Of the endogenous stimuli, pH alteration in biological environments are of specific interest in particular in solid tumors.
3,5
In fact, in the solid tumors, cancer cells metabolize glucose aberrantly producing lactic acids that are transported out of the cells into the extracellular fluid of the TME acidifying its milieu.
3-5
Further, intracellular endosomal/lysosomal compartments (pH at a range of 5-6) of cancer cells possess high local acidities abnormally in comparison with the healthy cells/tissues. The targeted pH-sensitive nanocarriers offer valuable capability for carrying therapeutic agents into the TME and the vesicular trafficking machineries of the cancer cells, in which the release of cargo drug molecules can be triggered by the alterations of pH.
14,20
In a recent study, Unsoy et al developed doxorubicin (DOX) loaded pH-sensitive CS-coated magnetic nanoparticles (MNPs) for the in vivo targeting of tumor sites using magnetic field.
25
The nanocarriers were found to be highly taken up by MCF-7 breast cancer cells through endocytosis. The nanocarriers, despite showing a high stability at the physiological pH 7.4, showed increased swelling rate at the lower pH values resulting in a slow intracellular release of DOX. Such behavior of the nanocarriers could induce an increased drug efficiency in TME maximizing the therapeutic capacity and overcoming the cancer cell resistance to DOX. In another study, a CS-based nanocarrier modified with a pH-cleavable linker was designed to target the hepatocyte mitochondrial targeting. The nanocarrier resulted in multistage pH response, lysosomal escape, controlled-release of cargo drug, and high antitumor activity.
26
Cheng et al synthesized DOX-loaded endosomal-pH-activated nanoscaled self-assembly prodrugs utilizing acidic-cleavable hydrazine bond for the conjugation of DOX.
27
The results revealed no burst release of DOX at the neutral pH, high cellular uptake and enhanced accumulation of the nanocarriers in tumor cells. The in vitro drug release investigation resulted in a fast drug release, change of drug release mechanism from anomalous transport (at pH 7.0) to combination pattern of diffusion/erosion-release (at pH around 5.0-6.0), and high cytotoxicity against HeLa cells with fairly low IC50. Hu et al reported on the synthesis of DOX-encapsulated pH-labile CS-capped mesoporous silica (MSN) nanocapsules, for the formulation of which (3-glycidyloxypropyl)trimethoxysilane was used as the linker between chitosan (i.e., as a gatekeeper) and the MSN. It should be stated that, due to the pKa 6.3 of chitosan, its molecular chains can be protonated in acidic values resulting in swelling of the chitosan chain, and consequently releasing of DOX.
28
Lim et al studied the development of CS-based intelligent theranostic nanocomposites, which were found to cause a pH-sensitive drug release with magnetic resonance-guided images.
29
This nanocomposite was engineered using N-naphthyl-O-dimethymaleoyl (N-nap-O-Mal) chitosan which is a pH-sensitive amphiphilic copolymer. Magnetic nanocrystals (MNCs) and DOX were then encapsulated into the engineered N-nap-O-Mal chitosan. This system displayed a rapid release of DOX upon the increase of the acidity. However, markedly high stability was seen under high pH conditions, which might provide sufficient capacity for diagnosing and monitoring therapeutics. The results demonstrated the effectiveness of this CS-based theranostic platform for the cancer therapy. Table 1 presents several CS-based DDSs that can induce pH-triggered drug release inside the biological milieus.
Table 1.
Several pH-sensitive chitosan based multifunctional nanocarriers and their characteristics
|
pH-Sensitive Nanocarrier
|
Cargo
|
Loading/release efficiency
|
Important results
|
Ref
|
| Chitosan/O-Carboxymethyl chitosan NPs |
DOX |
Loading efficiency: 21.4-72.87%
Release efficiency:
44.99%, 38.36% and 79.36% at pH 1.2, pH 6.0 and pH 7.0, respectively.
|
High entrapment efficiency.
Favorable pH-responsive stability in gastrointestinal tract
Lower drug release in acidic pH
Increased absorption of DOX by small intestine
Improved oral bioavailability (~42%)
Reduced cardiac and renal toxicity
|
30
|
| Chitosan-g-D-α-Tocopheryl polyethylene glycol 1000 (TPGS-g-CT) |
DOX |
Loading efficiency : 40%
Release efficiency: 5.71%, 19.3%, and 23.9 at pH 7.4, pH 6.8, and pH 5.5, respectively.
|
Fast release under lower pH values
High cytotoxicity against human hepatocarcinoma cells (HepG2 and BEL-7402) and human breast adenocarcinoma cells (MCF-7), along with enhanced cancer cell apoptosis
More effectiveness of the DOX loaded nanocarriers compared to adriamycin
Improved pharmacokinetic properties and antitumor activity of DOX loaded nanocarriers compared to adriamycin
|
31
|
| Carboxymethyl chitosan capped AuNPs |
DOX |
Loading efficiency: 83.3%
Release efficiency: 96.6%, 88.82%, and 10.46% in acetate and phosphate buffer, respectively.
|
CS was used as both capping agent and reducing agent
High stability of CS reduced AuNPs in various pH values (With and without DOX)
Enhanced cell uptake of nanocarriers in acidic pH values
Drug release at acidic pH due to ionizing carboxylic groups of CMCs
Enhanced absorbing of DOX loaded AuNPs by cervical cancer cells in comparison with free DOX
|
32
|
|
Low molecular-weight chitosan-conjugated poly(lactic-co-glycolic acid)
|
PTX |
Loading efficiency:
6.3% for PTX-PLGA NPs, and 12.6% for PTX-PLGA-LMWC NPs
Release efficiency:
11-12% and 76-83% by 3 and 24h in pH 7.4, and relatively low values in pH 6.2
|
LMWCs as a pH-sensitive coating and a hydrophilic layer
pH-sensitive cell-interaction of LMWC-PLGA, along with pH-triggered delivery in weakly acidic milieus with enhanced cellular uptake
Decreased opsonization and phagocytic uptake by hydrophilic LMWCs.
|
33
|
Redox-responsive nanocarriers
The redox responsiveness, which is resulted from the alterations in the redox activity between extra-and intra-cellular milieus, has been also made a progress in the design of the CS-based nanocarriers with controlled-release capability. It should be noted that desired nanocarriers can be prepared with selective sensitivity to the cancerous redox environment.
34,35
In a study, for example, a CS-based glycolipid-like micellar nanocarrier (CSO-ss-SA) with high sensitivity to the tumor cell reductive activity was developed. In this work, the biodistribution was shown in the liver, spleen, and tumor, while only the exposure to the intracellular glutathione (GSH) of tumor cells could induce a fast degradation of the nanocarrier followed by an intracellular drug release. This resulted in effective tumor growth inhibition.
35
Ultrasound-responsive nanocarriers
The ultrasound (US) energy have been used to trigger controlled-release of the therapeutic agents.
36-38
Recently, the synthesis of CS-based nanobubble containing a gas core, was reported by Cavalli et al.
36
This nanostructure was suggested as an appropriate dual magnetic resonance imaging (MRI)-US detectable nanoplatform, and used as a theranostic agent for the MRI-guided therapy. The nanosystem was used for the co-delivery of prednisolone phosphate (PLP) as therapeutic agent electrostatically bound to the nanobubbles’ shell and gadolinium (Ga) III complex as MRI-diagnostics agent. In this study, by exposure of the theranostic nanobubbles to the ultrasound stimulation at 37°C, an enhanced release of PLP was observed with no burst release effect (Fig. 2a). Further, changes in relaxivity of the MRI probe and good in vitro echogenicity were also reported. In other studies, ultrasound triggered the release of insulin,
38
and oxygen
37
from CS-coated PLGA NPs (Fig. 2b), and decafluoropentane/chitosan core-shell nanobubbles have been shown, respectively.
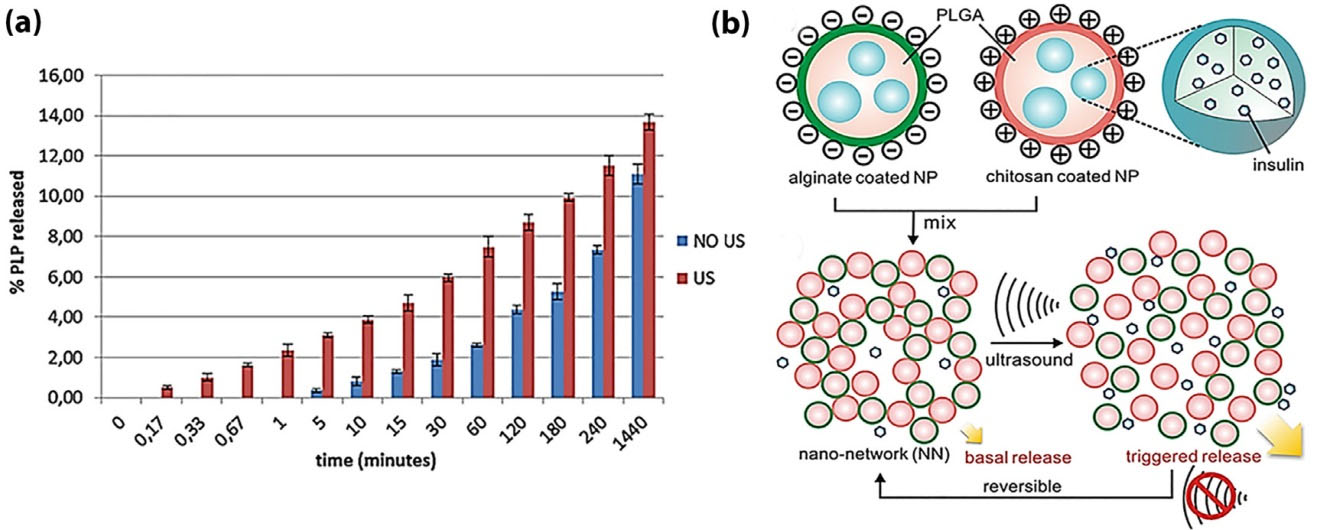
Figure 2.
Schematic illustration of chitosan (CS)-based nanosystem. (a) Prednisolone phosphate (PLP) release profile from the theranostic nanocarriers with or without use of ultrasound (sonication for 1 min).
36
(b) Schematic illustration of focused ultrasound system (FUS)-equipped delivery of insulin employing nano-network, which is acquired via mixing together the oppositely charged NPs, comprised of chitosan/alginate coated poly(lactic-co-glycolic) acid (PLGA) NPs. The FUS induced nano-network dissociation, enhancing the insulin release.
37
Data were adapted with permission from the cited references.
.
Schematic illustration of chitosan (CS)-based nanosystem. (a) Prednisolone phosphate (PLP) release profile from the theranostic nanocarriers with or without use of ultrasound (sonication for 1 min).
36
(b) Schematic illustration of focused ultrasound system (FUS)-equipped delivery of insulin employing nano-network, which is acquired via mixing together the oppositely charged NPs, comprised of chitosan/alginate coated poly(lactic-co-glycolic) acid (PLGA) NPs. The FUS induced nano-network dissociation, enhancing the insulin release.
37
Data were adapted with permission from the cited references.
Light-responsive nanocarriers
Light irradiation is a promising source for the activation of drug release in light-responsive DDSs. The drug release mechanisms include photo-induced-thermal effect, photo-isomerization or photo-cleavage of the chemical bonds in the nanocarrier structure.
14,39
The light-responsive offer a number of advantages, including on-demand stimuli-triggered drug release, simultaneous cancer imaging and drug release,
38
and efficient tumor growth inhibition.
40
In a recent study, Srinivasan et al reported on the production and characterization of IR820-chitosan conjugates and showed its potential as a multifunctional imaging-hyperthermia NS in the diagnosis and therapy.
41
The IR820-chitosan conjugates were shown to produce heat upon the revelation to 808 nm laser resulting in the cell growth prevention through hyperthermia in cancer cells such as MES-SA, SKOV-3, and Dx5 cell lines. These researchers suggested that the localized image-guided hyperthermia could be done using IR820-chitosan conjugates, whose removal for the body was shown to occur via the hepatobiliary system and hence detected in the feces that was different from the free IR820.
Temperature-responsive nanocarriers
Temperature-sensitive materials have also been utilized for the production of smart DDSs using various polymers. Of the thermosensitive polymers, poly(N-isopropylacrylamide) (PNIPAAm) and its derivatives are considered as one of the vastly used polymers in development of the temperature-responsive DDSs.
42,43
In this regard, various CS-based carriers equipped with thermo-responsive moieties have been reported, including nanoaggregates,
44
NPs,
45
core/shell microspheres,
46
micelles
47
and hydrogels.
48-50
The mechanism pertaining to the temperature responsiveness of these polymers is based on the changes in the hydrophobicity in the vicinity of the transition temperature of the polymers, so-called low critical solution temperature (LCST). Below the LCST, these polymers are water-soluble and hydrophilic, and hence are swollen, in large part due to the hydrogen bonding between their functional moieties and water molecules. However, above the LCST, the hydrogen bonding collapses, resulting in the formation of insoluble hydrophobic polymers, which then shrink. Such behavior could serve as an on-demand and triggered the release of cargo drug molecules.
15,51
Further, diverse applications regarding thermo-sensitive CS-based platforms have been exploited for anticancer drug delivery,
45,46,50
tissue regenerative applications,
49
nasal drug delivery,
48
and macromolecules (e.g., protein) delivery.
44
In a study conducted by Qi et al, temperature-responsive self-assembled NPs composed of chitosan-g-PNIPAAm and sodium alginate-g-PNIPAAm was synthesized using free radical polymerization method in order to control the release of 5-fluorouracil.
52
At the temperatures above the LCST (e.g., 37°C), the hydrophilic to the hydrophobic transition of PNIPAAm chains occurs leading to an aggregation of NPs. In the study performed by Cheng et al., a PNIPAAm-g-carboxymethyl chitosan with a size range of 100-200 µm was formulated as hydrogels, which displayed a proper hydrophilicity and the LCST near 32°C.While the hydrogels were non-toxic to NIH3T3 cells, cisplatin incorporated hydrogels demonstrated markedly high anticancer activity against A549 cancer cells via a sustained-release pattern.
50
Magnetic-responsive nanocarriers
By the injection of magnetic-responsive nanocarriers and the externally used of the focused magnetic field on target tumor site, these nanocarriers could be directed to the cancerous cells resulting in an efficient effective accumulation and impacts of the drug on the desired location.
16,53
Given that MNPs are generally produced in an organic phase, an appropriate surface modification is necessary for their transformation to the aqueous media. In this line, amphiphilic polymers display much better physicochemical stability, biocompatibility, half-life in the blood. Further, surface functionalization of polymeric backbone is plausible using different functional entities such as imaging agents, targeting/homing agents and stimuli-responsive moieties.
54
Among various polymers, chitosan and its derivatives have been introduced as one of the widely used coating materials for MNPs.
54
Mansouri et al reported the use of palmitoyl chitosan for the co-encapsulation of superparamagnetic iron oxide (SPINOs) and PTX by the nanoprecipitation method.
55
The magnetic-responsive behavior of the engineered NPs was investigated by the Vibrating Sample Magnetometer method. These NPs exhibited about 69% PTX loading potential. The in vitro release examination confirmed that the drug release pattern could be controlled by applying a magnetic field. The encapsulated PTX molecules together with the hyperthermic effect of SPIONs by applying an external magnetic field exhibited an enhanced cell death in the breast cancer MCF-7 cells. Recently, Zhong et al fabricated folic acid (FA)-conjugated magnetic chitosan nanocapsules, which were used for the reduction-responsive release of coumarin 6.
56
The thiolated chitosan was functionalized with FA and immobilized with thiolated MNPs, the drug molecules and fluorescent probes were included via the sonochemical method to produce nanocapsules. The synthesized nanocarrier displayed a spherical morphology and an excellent magnetic-responsiveness. The nanocapsules were shown to be selectively uptake by the Hela cells via folate receptor-mediated endocytosis. Furthermore, the reduction-responsive drug release behavior indicated the potential of the engineered nanocarriers as the dual magnetic- and reduction-responsive DDS. These advanced DDSs are further discussed in the following section.
Dual stimuli-responsive nanocarriers
Combination of different stimuli may result in the formulation of improved DDSs with unprecedented properties.
14,20
In a study, a chitosan-based micellar nanocarrier was developed via the self-assembly of amphiphilic glycol chitosan-o-nitrobenzyl succinate conjugates (GC-NBSCs), from which the release of drug molecules was triggered by the dual pH and light stimuli. The nanocarrier was reported to be efficiently internalized by the MCF-7 cells via endocytosis, releasing the anticancer drug, camptothecin (CPT) into the cancer cells (Fig. 3a). In fact, under simultaneous acidic pH values and UV light irradiation, an enhanced drug release profile was obtained indicating cytotoxicity and high cancer cell proliferation inhibition efficacy (Fig. 3b).
57
In another study, the pH and temperature stimuli were exploited as a dual responsive system, in which chitosan was grafted to N-(2-hydroxyethyl)prop-2-enamide (HEPE) forming a self-assembled core-shell nanostructure. At the lower pH (e.g., pH=5.4) and higher temperatures over LCST (e.g., 37°C), this nanocarrier showed a rapid release profile.
58
Further, other pH/temperature dual-sensitive chitosan-based platforms for the cancer therapy have also been reported in some studies.
59,60
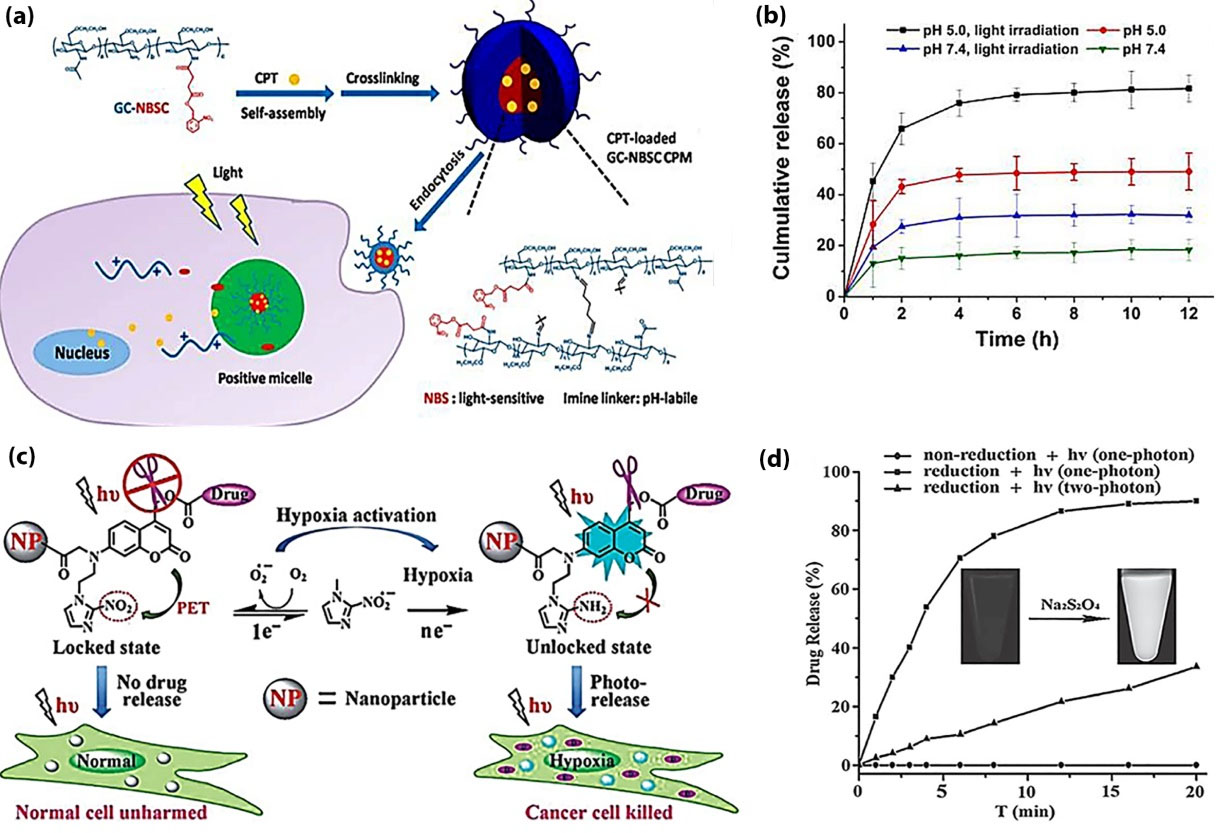
Figure 3.
Dual stimuli-responsive nanocarriers. (a) The synthesis of CPT-loaded GC-NBSC CPMs, and pH and light responsive drug release inside the cancer cells. (b) The cumulative drug release from the CPT-loaded nanocarrier triggered by the stimuli.
57
(c) The hypoxia-anitiated light-responsive drug release inside the cancer cells. (d) Drug release analysis by HPLC under visible light irradiation ( λ > 400 nm, 120 mW/cm2) or two-photon NIR irradiation by a femtosecond (FS) laser (800 nm) in the presence or absence of the reducing agent (Na2S2O4); inset image: TAP fluorescence via an excitation at 365 nm before and after the activation via using Na2S2O4 as reducing agent.
62
Data were adapted with permission from the cited references. CPT: Camptothecin. GC-NBSC: Glycol chitosan-o-nitrobenzyl succinate conjugate. HPLC: High performance liquid chromatography. NIR: Near infrared. TAP: Thioacetyl aminophthalimide.
.
Dual stimuli-responsive nanocarriers. (a) The synthesis of CPT-loaded GC-NBSC CPMs, and pH and light responsive drug release inside the cancer cells. (b) The cumulative drug release from the CPT-loaded nanocarrier triggered by the stimuli.
57
(c) The hypoxia-anitiated light-responsive drug release inside the cancer cells. (d) Drug release analysis by HPLC under visible light irradiation ( λ > 400 nm, 120 mW/cm2) or two-photon NIR irradiation by a femtosecond (FS) laser (800 nm) in the presence or absence of the reducing agent (Na2S2O4); inset image: TAP fluorescence via an excitation at 365 nm before and after the activation via using Na2S2O4 as reducing agent.
62
Data were adapted with permission from the cited references. CPT: Camptothecin. GC-NBSC: Glycol chitosan-o-nitrobenzyl succinate conjugate. HPLC: High performance liquid chromatography. NIR: Near infrared. TAP: Thioacetyl aminophthalimide.
Yadavalli et al capitalized on the synthesis of a multimodal imaging and dual stimuli-responsive chitosan-based nanocarrier tagged with fluorescein and FA, which was used for the targeted delivery of curcumin.
61
In this work, PNIPAAm and gadolinium-doped nickel ferrite MNPs were employed respectively as the thermo-sensitive moiety and the enhanced T1 MRI contrast agent. The NS was able to perform the contrast function via the superparamagnetic feature, and the hyperthermia and controlled-release of cargo molecules via applying an alternating current magnetic field. This NS indicated intrinsic biocompatibility and non-toxicity, and consequently resulted in thermo-responsive drug release and cancer cell apoptosis.
Smart dual-sensitive DDSs have shown great potential for the co-delivery of drugs. Recently, in order to overcome anti-cancer drug resistance and inhibition of cancer metastasis mainly occurred by CSCs, a nanocarrier was prepared for the co-delivery of hydrophobic DOX and hydrophilic CPT by an active targeting and dual pH/temperature-responsive drug release. Hyaluronic acid (HA) was used for the active targeting of the CD44 receptor, commonly overexpressed on various CSCs. The results demonstrated a high efficiency in the delivery and fast release of drug molecules, which provided an enhanced synergistic anticancer impact.
63
It should be also noted that hypoxia/light dual responsive chitosan nanocarriers have been considered as a novel DDS. Lin et al synthesized CS-based micellar with selective photorelease behavior. Once exposed to the specific physiological condition of tumor-hypoxia, the engineered locked-phototrigger NS could be unlocked by the biological reduction resulting in the liberation of the caged drug molecules by means of the visible light/ two-photon NIR excitation. The CS-based nanostructure was proposed to provide a markedly high discriminating photorelease of anticancer drug based on the hypoxia in the tumor microenvironment impacting the hypoxic tumor cells. Such phenomenon was achieved through photo-cleavage of nitroimidazole-locked coumarin chromophore as shown in Fig. 3c.
62
In this study, a significant photo-triggered release of anticancer drug molecules was attained in the hypoxic cells, while the healthy oxygenated cells remained untouched, indicating selective eradication of tumor cells solely (Fig. 3d).
Final remarks
Despite the recent development in the targeted DDSs with a controlled-release profile, some limitations such as the premature and low release of chemotherapeutics agents to the target cancer cells may limit the in vivo impacts of these modalities. As a result, several advanced smart DDSs have been developed to provide a stimulus-triggered on/off drug release in response to various extra/intra stimulus in an on-demand manner. Of various polymers used for the development of such smart DDSs, chitosan and its derivatives possess unique characteristics. As a natural polymer, it can be functionalized with targeting and imaging agents, becoming a multifunctional DDS. Such capacity is due to the presence of functional groups in the polymeric matrix of chitosan, which makes it as a promising candidate for the formulation of smart nanocarriers. Compared with various synthetic polymers that impose cytotoxicity and even genotoxicity,
64-73
chitosan and its derivatives offer great biocompatibility with excellent biodegradability. It is a mucoadhesive polymer, and hence, can be developed as a suitable DDS for the topical uses in the mucosal cancers.
74-76
Smart chitosan-based NSs are envisioned to be translated into the clinical applications for the delivery of drug molecules with an on-demand release of cargo drugs into the target site. Once modified with homing and imaging agents such as quantum dots,
77
the CS-based multimodal stimuli-responsive NSs can be used as a theranostics. The CS-based targeting NSs can be designed in a way to get benefits from both passive and active targeting mechanisms and to respond simultaneously to the endogenous and exogenous stimuli.
Competing interests
As the contributing authors to this study, JB, and YO act as the Editors of the journal. It is hereby declared that their associations with the journal have neither influenced the review process nor affected the acceptance of the study.
Ethical approval
Not applicable.
Acknowledgment
Authors would like to acknowledge the Iranian National Science Foundation (INSF) for the financial support (Grant No: 93030668), the Research Center for Pharmaceutical Nanotechnology at Tabriz University of Medical Sciences and the Department of Chemical and Petroleum Engineering at the University of Tabriz.
Review Highlights
What is current knowledge?
simple
-
√ Stimuli responsive nanocarriers as advanced drug delivery
system (DDS) are able to release the entrapped drugs
efficiently in response to specific stimuli (e.g., pH, enzymes,
temperature, light, ultrasound, magnetic field, etc.).
-
√ As a natural polymer, chitosan is highly biocompatible and
biodegradable.
-
√ Chitosan can be modified with different agents becoming a
smart multifunctional DDS.
What is new here?
simple
-
√ Dual stimuli-responsive DDSs consisting of two stimuli
provide an improved drug delivery platform for an enhanced
cancer therapy.
-
√ Chitosan-based multimodal stimuli-responsive
nanoplatforms can be used as a theranostic for simultaneous
imaging and therapy of cancer.
-
√ Chitosan-based stimuli-responsive nanoplatforms can be
designed to benefit from both passive and active targeting
mechanisms.
-
√ Chitosan-based multimodal stimuli-responsive nanoplatforms
can be used as an alternative to the conventional chemotherapies.
References
- De Sousa EMF, Vermeulen L, Fessler E, Medema JP. Cancer heterogeneity--a multifaceted view. EMBO Rep 2013; 14:686-95. doi: 10.1038/embor.2013.92 [Crossref] [ Google Scholar]
- Ruiz-Casado A, Martin-Ruiz A, Perez LM, Provencio M, Fiuza-Luces C, Lucia A. Exercise and the Hallmarks of Cancer. Trends Cancer 2017; 3:423-41. doi: 10.1016/j.trecan.2017.04.007 [Crossref] [ Google Scholar]
- Asgharzadeh MR, Barar J, Pourseif MM, Eskandani M, Jafari Niya M, Mashayekhi MR. Molecular machineries of pH dysregulation in tumor microenvironment: potential targets for cancer therapy. Bioimpacts 2017; 7:115-33. doi: 10.15171/bi.2017.15 [Crossref] [ Google Scholar]
- Omidi Y, Barar J. Targeting tumor microenvironment: crossing tumor interstitial fluid by multifunctional nanomedicines. Bioimpacts 2014; 4:55-67. doi: 10.5681/bi.2014.021 [Crossref] [ Google Scholar]
- Barar J, Omidi Y. Dysregulated pH in Tumor Microenvironment Checkmates Cancer Therapy. Bioimpacts 2013; 3:149-62. doi: 10.5681/bi.2013.036 [Crossref] [ Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74. doi: 10.1016/j.cell.2011.02.013 [Crossref] [ Google Scholar]
- Lazebnik Y. What are the hallmarks of cancer?. Nat Rev Cancer 2010; 10:232-3. doi: 10.1038/nrc2827 [Crossref] [ Google Scholar]
- O'Driscoll L. Expanding on exosomes and ectosomes in cancer. N Engl J Med 2015; 372:2359-62. doi: 10.1056/NEJMcibr1503100 [Crossref] [ Google Scholar]
- Kosaka N, Yoshioka Y, Fujita Y, Ochiya T. Versatile roles of extracellular vesicles in cancer. J Clin Invest 2016; 126:1163-72. doi: 10.1172/JCI81130 [Crossref] [ Google Scholar]
- Rafi MA, Omidi Y. A prospective highlight on exosomal nanoshuttles and cancer immunotherapy and vaccination. Bioimpacts 2015; 5:117-22. doi: 10.15171/bi.2015.22 [Crossref] [ Google Scholar]
- Eskandani M, Vandghanooni S, Barar J, Nazemiyeh H, Omidi Y. Cell physiology regulation by hypoxia inducible factor-1: Targeting oxygen-related nanomachineries of hypoxic cells. Int J Biol Macromol 2017; 99:46-62. doi: 10.1016/j.ijbiomac.2016.10.113 [Crossref] [ Google Scholar]
- Sancho P, Barneda D, Heeschen C. Hallmarks of cancer stem cell metabolism. Br J Cancer 2016; 114:1305-12. doi: 10.1038/bjc.2016.152 [Crossref] [ Google Scholar]
- Fathi M, Entezami AA, Arami S, Rashidi M-R. Preparation of N-Isopropylacrylamide/Itaconic Acid Magnetic Nanohydrogels by Modified Starch as a Crosslinker for Anticancer Drug Carriers. Int J Polym Mater Polym Biomater 2015; 64:541-9. doi: 10.1080/00914037.2014.996703 [Crossref] [ Google Scholar]
- Karimi M, Ghasemi A, Sahandi Zangabad P, Rahighi R, Moosavi Basri SM, Mirshekari H. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem Soc Rev 2016; 45:1457-501. doi: 10.1039/c5cs00798d [Crossref] [ Google Scholar]
- Karimi M, Eslami M, Sahandi-Zangabad P, Mirab F, Farajisafiloo N, Shafaei Z. pH-Sensitive stimulus-responsive nanocarriers for targeted delivery of therapeutic agents. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2016; 8:696-716. doi: 10.1002/wnan.1389 [Crossref] [ Google Scholar]
- Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater 2013; 12:991-1003. doi: 10.1038/nmat3776 [Crossref] [ Google Scholar]
- Azhdarzadeh M, Atyabi F, Saei AA, Varnamkhasti BS, Omidi Y, Fateh M. Theranostic MUC-1 aptamer targeted gold coated superparamagnetic iron oxide nanoparticles for magnetic resonance imaging and photothermal therapy of colon cancer. Colloids Surf B Biointerfaces 2016; 143:224-32. doi: 10.1016/j.colsurfb.2016.02.058 [Crossref] [ Google Scholar]
- Omidi Y. Smart multifunctional theranostics: simultaneous diagnosis and therapy of cancer. Bioimpacts 2011; 1:145-7. doi: 10.5681/bi.2011.019 [Crossref] [ Google Scholar]
- Karimi M, Mirshekari H, Aliakbari M, Sahandi-Zangabad P, Hamblin MR. Smart mesoporous silica nanoparticles for controlled-release drug delivery. Nanotechnol Rev 2016; 5:195-207. [ Google Scholar]
-
Karimi M, Zangabad PS, Ghasemi A, Hamblin MR. Smart Internal Stimulus-Responsive Nanocarriers for Drug and Gene Delivery: Morgan & Claypool Publishers; 2015. Available from: 10.1088/978-1-6817-4257-1.
- Priya James H, John R, Alex A, Anoop KR. Smart polymers for the controlled delivery of drugs - a concise overview. Acta Pharm Sin B 2014; 4:120-7. doi: 10.1016/j.apsb.2014.02.005 [Crossref] [ Google Scholar]
- Babu A, Ramesh R. Multifaceted Applications of Chitosan in Cancer Drug Delivery and Therapy. Mar Drugs 2017; 15. doi: 10.3390/md15040096 [Crossref]
- Fathi M, Zangabad PS, Aghanejad A, Barar J, Erfan-Niya H, Omidi Y. Folate-conjugated thermosensitive O-maleoyl modified chitosan micellar nanoparticles for targeted delivery of erlotinib. Carbohydr Polym 2017; 172:130-41. doi: 10.1016/j.carbpol.2017.05.007 [Crossref] [ Google Scholar]
- Fathi M, Sahandi Zangabad P, Barar J, Aghanejad A, Erfan-Niya H, Omidi Y. Thermo-sensitive chitosan copolymer-gold hybrid nanoparticles as a nanocarrier for delivery of erlotinib. Int J Biol Macromol 2018; 106:266-76. doi: 10.1016/j.ijbiomac.2017.08.020 [Crossref] [ Google Scholar]
- Unsoy G, Khodadust R, Yalcin S, Mutlu P, Gunduz U. Synthesis of Doxorubicin loaded magnetic chitosan nanoparticles for pH responsive targeted drug delivery. Eur J Pharm Sci 2014; 62:243-50. doi: 10.1016/j.ejps.2014.05.021 [Crossref] [ Google Scholar]
- Chen Z, Zhang L, Song Y, He J, Wu L, Zhao C. Hierarchical targeted hepatocyte mitochondrial multifunctional chitosan nanoparticles for anticancer drug delivery. Biomaterials 2015; 52:240-50. doi: 10.1016/j.biomaterials.2015.02.001 [Crossref] [ Google Scholar]
- Chen C, Zhou JL, Han X, Song F, Wang XL, Wang YZ. A prodrug strategy based on chitosan for efficient intracellular anticancer drug delivery. Nanotechnology 2014; 25:255101. doi: 10.1088/0957-4484/25/25/255101 [Crossref] [ Google Scholar]
- Hu X, Wang Y, Peng B. Chitosan-capped mesoporous silica nanoparticles as pH-responsive nanocarriers for controlled drug release. Chem Asian J 2014; 9:319-27. doi: 10.1002/asia.201301105 [Crossref] [ Google Scholar]
- Lim EK, Sajomsang W, Choi Y, Jang E, Lee H, Kang B. Chitosan-based intelligent theragnosis nanocomposites enable pH-sensitive drug release with MR-guided imaging for cancer therapy. Nanoscale Res Lett 2013; 8:467. doi: 10.1186/1556-276x-8-467 [Crossref] [ Google Scholar]
- Feng C, Wang Z, Jiang C, Kong M, Zhou X, Li Y. Chitosan/o-carboxymethyl chitosan nanoparticles for efficient and safe oral anticancer drug delivery: in vitro and in vivo evaluation. Int J Pharm 2013; 457:158-67. doi: 10.1016/j.ijpharm.2013.07.079 [Crossref] [ Google Scholar]
- Guo Y, Chu M, Tan S, Zhao S, Liu H, Otieno BO. Chitosan-g-TPGS nanoparticles for anticancer drug delivery and overcoming multidrug resistance. Mol Pharm 2014; 11:59-70. doi: 10.1021/mp400514t [Crossref] [ Google Scholar]
- Madhusudhan A, Reddy GB, Venkatesham M, Veerabhadram G, Kumar DA, Natarajan S. Efficient pH dependent drug delivery to target cancer cells by gold nanoparticles capped with carboxymethyl chitosan. Int J Mol Sci 2014; 15:8216-34. doi: 10.3390/ijms15058216 [Crossref] [ Google Scholar]
- Amoozgar Z, Park J, Lin Q, Yeo Y. Low molecular-weight chitosan as a pH-sensitive stealth coating for tumor-specific drug delivery. Mol Pharm 2012; 9:1262-70. doi: 10.1021/mp2005615 [Crossref] [ Google Scholar]
- Guerry A, Cottaz S, Fleury E, Bernard J, Halila S. Redox-stimuli responsive micelles from DOX-encapsulating polycaprolactone-g-chitosan oligosaccharide. Carbohydr Polym 2014; 112:746-52. doi: 10.1016/j.carbpol.2014.06.052 [Crossref] [ Google Scholar]
- Hu YW, Du YZ, Liu N, Liu X, Meng TT, Cheng BL. Selective redox-responsive drug release in tumor cells mediated by chitosan based glycolipid-like nanocarrier. J Control Release 2015; 206:91-100. doi: 10.1016/j.jconrel.2015.03.018 [Crossref] [ Google Scholar]
- Cavalli R, Argenziano M, Vigna E, Giustetto P, Torres E, Aime S. Preparation and in vitro characterization of chitosan nanobubbles as theranostic agents. Colloids Surf B Biointerfaces 2015; 129:39-46. doi: 10.1016/j.colsurfb.2015.03.023 [Crossref] [ Google Scholar]
- Magnetto C, Prato M, Khadjavi A, Giribaldi G, Fenoglio I, Jose J. Ultrasound-activated decafluoropentane-cored and chitosan-shelled nanodroplets for oxygen delivery to hypoxic cutaneous tissues. RSC Advances 2014; 4:38433-41. doi: 10.1039/C4RA03524K [Crossref] [ Google Scholar]
- Di J, Price J, Gu X, Jiang X, Jing Y, Gu Z. Ultrasound-triggered regulation of blood glucose levels using injectable nano-network. Adv Healthc Mater 2014; 3:811-6. doi: 10.1002/adhm.201300490 [Crossref] [ Google Scholar]
-
Karimi M, Zangabad PS, Ghasemi A, Hamblin MR. Smart External Stimulus-Responsive Nanocarriers for Drug and Gene Delivery: Morgan & Claypool Publishers; 2015. Available from: 10.1088/978-1-6817-4202-1.
- Fu G, Zhu L, Yang K, Zhuang R, Xie J, Zhang F. Diffusion-Weighted Magnetic Resonance Imaging for Therapy Response Monitoring and Early Treatment Prediction of Photothermal Therapy. ACS Appl Mater Interfaces 2016; 8:5137-47. doi: 10.1021/acsami.5b11936 [Crossref] [ Google Scholar]
- Srinivasan S, Manchanda R, Fernandez-Fernandez A, Lei T, McGoron AJ. Near-infrared fluorescing IR820-chitosan conjugate for multifunctional cancer theranostic applications. J Photochem Photobiol B 2013; 119:52-9. doi: 10.1016/j.jphotobiol.2012.12.008 [Crossref] [ Google Scholar]
- Karimi M, Sahandi Zangabad P, Ghasemi A, Amiri M, Bahrami M, Malekzad H. Temperature-Responsive Smart Nanocarriers for Delivery Of Therapeutic Agents: Applications and Recent Advances. ACS Appl Mater Interfaces 2016; 8:21107-33. doi: 10.1021/acsami.6b00371 [Crossref] [ Google Scholar]
- Massoumi B, Abdollahi M, Fathi M, Entezami AA, Hamidi S. Synthesis of novel thermoresponsive micelles by graft copolymerization of N-isopropylacrylamide on poly(ε-caprolactone-co-α-bromo-ε-caprolactone) as macroinitiator via ATRP. J Polym Res 2013; 20:47. doi: 10.1007/s10965-012-0047-7 [Crossref] [ Google Scholar]
- Park KM, Choi JH, Bae JW, Joung YK, Park KD. Nano-aggregates using thermosensitive chitosan copolymers as a nanocarrier for protein delivery. J Exp Nanosci 2009; 4:269-75. doi: 10.1080/17458080802516909 [Crossref] [ Google Scholar]
- Rejinold NS, Sreerekha PR, Chennazhi KP, Nair SV, Jayakumar R. Biocompatible, biodegradable and thermo-sensitive chitosan-g-poly (N-isopropylacrylamide) nanocarrier for curcumin drug delivery. Int J Biol Macromol 2011; 49:161-72. doi: 10.1016/j.ijbiomac.2011.04.008 [Crossref] [ Google Scholar]
- Gui R, Wang Y, Sun J. Encapsulating magnetic and fluorescent mesoporous silica into thermosensitive chitosan microspheres for cell imaging and controlled drug release in vitro. Colloids Surf B Biointerfaces 2014; 113:1-9. doi: 10.1016/j.colsurfb.2013.08.015 [Crossref] [ Google Scholar]
- Fathi M, Zangabad PS, Aghanejad A, Barar J, Erfan-Niya H, Omidi Y. Folate-conjugated thermosensitive O-maleoyl modified chitosan micellar nanoparticles for targeted delivery of erlotinib. Carbohydr Polym 2017; 172:130-41. doi: 10.1016/j.carbpol.2017.05.007 [Crossref] [ Google Scholar]
- Nazar H, Fatouros DG, van der Merwe SM, Bouropoulos N, Avgouropoulos G, Tsibouklis J. Thermosensitive hydrogels for nasal drug delivery: the formulation and characterisation of systems based on N-trimethyl chitosan chloride. Eur J Pharm Biopharm 2011; 77:225-32. doi: 10.1016/j.ejpb.2010.11.022 [Crossref] [ Google Scholar]
- Walker KJ, Madihally SV. Anisotropic temperature sensitive chitosan-based injectable hydrogels mimicking cartilage matrix. J Biomed Mater Res B Appl Biomater 2015; 103:1149-60. doi: 10.1002/jbm.b.33293 [Crossref] [ Google Scholar]
- Cheng C, Xia D, Zhang X, Chen L, Zhang Q. Biocompatible poly(N-isopropylacrylamide)-g-carboxymethyl chitosan hydrogels as carriers for sustained release of cisplatin. J Mater Sci 2015; 50:4914-25. doi: 10.1007/s10853-015-9036-7 [Crossref] [ Google Scholar]
- Cheng X, Jin Y, Sun T, Qi R, Fan B, Li H. Oxidation- and thermo-responsive poly(N-isopropylacrylamide-co-2-hydroxyethyl acrylate) hydrogels cross-linked via diselenides for controlled drug delivery. RSC Advances 2015; 5:4162-70. doi: 10.1039/C4RA13500H [Crossref] [ Google Scholar]
- Qi M, Li G, Yu N, Meng Y, Liu X. Synthesis of thermo-sensitive polyelectrolyte complex nanoparticles from CS-g-PNIPAM and SA-g-PNIPAM for controlled drug release. Macromol Res 2014; 22:1004-11. doi: 10.1007/s13233-014-2134-6 [Crossref] [ Google Scholar]
- Baek S, Singh RK, Khanal D, Patel KD, Lee EJ, Leong KW. Smart multifunctional drug delivery towards anticancer therapy harmonized in mesoporous nanoparticles. Nanoscale 2015; 7:14191-216. doi: 10.1039/c5nr02730f [Crossref] [ Google Scholar]
- Kang T, Li F, Baik S, Shao W, Ling D, Hyeon T. Surface design of magnetic nanoparticles for stimuli-responsive cancer imaging and therapy. Biomaterials 2017; 136:98-114. [ Google Scholar]
- Mansouri M, Nazarpak MH, Solouk A, Akbari S, Hasani-Sadrabadi MM. Magnetic responsive of paclitaxel delivery system based on SPION and palmitoyl chitosan. J Magn Magn Mater 2017; 421:316-25. [ Google Scholar]
- Zhong S, Zhang H, Liu Y, Wang G, Shi C, Li Z. Folic acid functionalized reduction-responsive magnetic chitosan nanocapsules for targeted delivery and triggered release of drugs. Carbohydr Polym 2017; 168:282-9. doi: 10.1016/j.carbpol.2017.03.083 [Crossref] [ Google Scholar]
- Meng L, Huang W, Wang D, Huang X, Zhu X, Yan D. Chitosan-based nanocarriers with pH and light dual response for anticancer drug delivery. Biomacromolecules 2013; 14:2601-10. doi: 10.1021/bm400451v [Crossref] [ Google Scholar]
- Huang W, Wang Y, Zhang S, Huang L, Hua D, Zhu X. A Facile Approach for Controlled Modification of Chitosan under γ-Ray Irradiation for Drug Delivery. Macromolecules 2013; 46:814-8. doi: 10.1021/ma302434c [Crossref] [ Google Scholar]
- Temtem M, Barroso T, Casimiro T, Mano JF, Aguiar-Ricardo A. Dual stimuli responsive poly(N-isopropylacrylamide) coated chitosan scaffolds for controlled release prepared from a non residue technology. J Supercrit Fluid 2012; 66:398-404. doi: 10.1016/j.supflu.2011.10.015 [Crossref] [ Google Scholar]
- Chen JP, Cheng TH. Thermo-responsive chitosan-graft-poly(N-isopropylacrylamide) injectable hydrogel for cultivation of chondrocytes and meniscus cells. Macromol Biosci 2006; 6:1026-39. doi: 10.1002/mabi.200600142 [Crossref] [ Google Scholar]
- Yadavalli T, Ramasamy S, Chandrasekaran G, Michael I, Therese HA, Chennakesavulu R. Dual responsive PNIPAM–chitosan targeted magnetic nanopolymers for targeted drug delivery. J Magn Magn Mater 2015; 380:315-20. doi: 10.1016/j.jmmm.2014.09.035 [Crossref] [ Google Scholar]
- Lin Q, Bao C, Yang Y, Liang Q, Zhang D, Cheng S. Highly discriminating photorelease of anticancer drugs based on hypoxia activatable phototrigger conjugated chitosan nanoparticles. Adv Mater 2013; 25:1981-6. doi: 10.1002/adma.201204455 [Crossref] [ Google Scholar]
- Wang H, Agarwal P, Zhao S, Xu RX, Yu J, Lu X. Hyaluronic acid-decorated dual responsive nanoparticles of Pluronic F127, PLGA, and chitosan for targeted co-delivery of doxorubicin and irinotecan to eliminate cancer stem-like cells. Biomaterials 2015; 72:74-89. doi: 10.1016/j.biomaterials.2015.08.048 [Crossref] [ Google Scholar]
- Hollins AJ, Benboubetra M, Omidi Y, Zinselmeyer BH, Schatzlein AG, Uchegbu IF. Evaluation of generation 2 and 3 poly(propylenimine) dendrimers for the potential cellular delivery of antisense oligonucleotides targeting the epidermal growth factor receptor. Pharm Res 2004; 21:458-66. doi: 10.1023/B:PHAM.0000019300.04836.51 [Crossref] [ Google Scholar]
- Omidi Y, Hollins AJ, Drayton RM, Akhtar S. Polypropylenimine dendrimer-induced gene expression changes: the effect of complexation with DNA, dendrimer generation and cell type. J Drug Target 2005; 13:431-43. doi: 10.1080/10611860500418881 [Crossref] [ Google Scholar]
- Hollins AJ, Omidi Y, Benter IF, Akhtar S. Toxicogenomics of drug delivery systems: Exploiting delivery system-induced changes in target gene expression to enhance siRNA activity. J Drug Target 2007; 15:83-8. doi: 10.1080/10611860601151860 [Crossref] [ Google Scholar]
- Ahmadian S, Barar J, Saei AA, Fakhree MA, Omidi Y. Cellular toxicity of nanogenomedicine in MCF-7 cell line: MTT assay. J Vis Exp 2009; ?. doi: 10.3791/1191 [Crossref]
- Nakhlband A, Barar J, Bidmeshkipour A, Heidari HR, Omidi Y. Bioimpacts of anti epidermal growth factor receptor antisense complexed with polyamidoamine dendrimers in human lung epithelial adenocarcinoma cells. J Biomed Nanotechnol 2010; 6:360-9. [ Google Scholar]
- Kafil V, Omidi Y. Cytotoxic impacts of linear and branched polyethylenimine nanostructures in A431 cells. Bioimpacts 2011; 1:23-30. doi: 10.5681/bi.2011.004 [Crossref] [ Google Scholar]
- Barar J, Omidi Y. Translational Approaches towards Cancer Gene Therapy: Hurdles and Hopes. Bioimpacts 2012; 2:127-43. doi: 10.5681/bi.2012.025 [Crossref] [ Google Scholar]
- Hamidi A, Sharifi S, Davaran S, Ghasemi S, Omidi Y, Rashidi MR. Novel aldehyde-terminated dendrimers; synthesis and cytotoxicity assay. Bioimpacts 2012; 2:97-103. doi: 10.5681/bi.2012.014 [Crossref] [ Google Scholar]
- Nourazarian AR, Najar AG, Farajnia S, Khosroushahi AY, Pashaei-Asl R, Omidi Y. Combined EGFR and c-Src antisense oligodeoxynucleotides encapsulated with PAMAM Denderimers inhibit HT-29 colon cancer cell proliferation. Asian Pac J Cancer Prev 2012; 13:4751-6. [ Google Scholar]
- Barar J, Omidi Y. Intrinsic bio-signature of gene delivery nanocarriers may impair gene therapy goals. Bioimpacts 2013; 3:105-9. doi: 10.5681/bi.2013.028 [Crossref] [ Google Scholar]
- Chuah LH, Billa N, Roberts CJ, Burley JC, Manickam S. Curcumin-containing chitosan nanoparticles as a potential mucoadhesive delivery system to the colon. Pharm Dev Technol 2013; 18:591-9. doi: 10.3109/10837450.2011.640688 [Crossref] [ Google Scholar]
- Sosnik A, Menaker Raskin M. Polymeric micelles in mucosal drug delivery: Challenges towards clinical translation. Biotechnol Adv 2015; 33:1380-92. doi: 10.1016/j.biotechadv.2015.01.003 [Crossref] [ Google Scholar]
- Goldberg M, Manzi A, Aydin E, Singh G, Khoshkenar P, Birdi A. Development of a Nanoparticle-Embedded Chitosan Sponge for Topical and Local Administration of Chemotherapeutic Agents. J Nanotechnol Eng Med 2014; 5:0409051-4090511. doi: 10.1115/1.4030899 [Crossref] [ Google Scholar]
- Mashinchian O, Johari-Ahar M, Ghaemi B, Rashidi M, Barar J, Omidi Y. Impacts of quantum dots in molecular detection and bioimaging of cancer. Bioimpacts 2014; 4:149-66. doi: 10.15171/bi.2014.008 [Crossref] [ Google Scholar]