BioImpacts. 8(4):281-294.
doi: 10.15171/bi.2018.31
Original Research
Comparative in vitro/theoretical studies on the anti-angiogenic activity of date pollen hydro-alcoholic extract: Highlighting the important roles of its hot polyphenols
Hassan Rasouli 1, Amir Hossein Norooznezhad 1, Tahereh Rashidi 1, Zohreh Hoseinkhani 1, Azadeh Mahnam 1, Mitra Tarlan 1, 2, Narges Moasefi 1, Ali Mostafaei 1, *, Kamran Mansouri 1, 3, *
Author information:
1Medical Biology Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran
2Department of physiology, Faculty of veterinary, Shiraz University, Shiraz, Iran
3Department of Molecular Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran
Abstract
Introduction:
Date palm pollen (DPP) is the male reproductive soft powder from date flowers widely used as the valuable dietary supplement to fortify the size of testis and ovarian to increase the power of sex. This part of date palm significantly exhibited anti-diabetic, anti-inflammation and protective effects against male and female infertility. Though the anticancer activity of date fruits was previously reported, the DPP anti-angiogenic effects were not reported, and as the first study, its inhibitory effects were examined in the current study.
Methods:
The DPP soft powder was collected to prepare its hydro-alcoholic extract to examine its anti-angiogenic activity in an in vitro model. At different concentrations, the cytotoxicity of the prepared extract was examined on human umbilical vein endothelial cells (HUVECs) using lactate dehydrogenase method. Cell proliferation was determined using the MTT assay and cytodex-3D model in collagen gel was used to assay its possible anti-angiogenic activity. The expression of VEGF, MMP-2 and MMP-9 genes was measured using real-time polymerase chain reaction (PCR). Finally, molecular docking simulation was used to highlight the possible role of DPP polyphenols to interact with the associated receptors.
Results:
The prepared hydro-alcoholic extract exhibited significant anti-angiogenic activity in a dose-dependent manner and decreased the endothelial cell proliferation. The calculated IC50 value for the examined extract in angiogenesis model was 260 µg·mL, respectively. Also, the expression of VEGF, MMP-2 and MMP-9 genes were significantly decreased. Docking simulation results unveiled that the isolated DPP polyphenols have the affinity to interact with ctDNA, VEGF and its receptors.
Conclusion:
The DPP is the new source of non-toxic anti-cancer agents to use as a dietary supplement in the pre-treatment of cancer.
Keywords: Angiogenesis, Date palm pollen, Docking simulation, Polyphenols
Copyright and License Information
© 2018 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Cancer is a critical public health problem in the entire world,
1-5
and it is estimated that there exist more than 10 million individuals with cancer throughout the world.
6
For decades, scientists could not find a safe way (or drug) to treat cancer and its related complications,
7,8
Therefore, try to find or search for new effective anti-cancer agents is a critical strategy in any cancer therapy program. Studies indicated that 30% to 50% of cancers are preventable
9
and various factors including healthy diet
10
(especially diet rich in flavonoids),
11
physical activities
12
and avoidance of smoking,
13
alcohol drinking,
14
etc. can reduce the probability of cancer occurrence.
“Let food be thy medicine and thy medicine be thy food” was quoted by Hippocrates more than 2000 years ago,
15
and for centuries oral medicinal plants and their metabolites have widely been applied to alleviate and treat a wide range of human diseases such as inflammation, wounds and infection.
16,17
Thanks to recent advances in biochemical approaches, anti-cancer, anti-diabetic, anti-microbial, anti-inflammatory and antioxidant properties of phenolic acids and flavonoids have been widely reported and researchers considered these bioactive compounds as a valuable source of natural agents to expand their plans to find new drugs.
Date palm, with scientific name Phoenix dactylifera, is a flowering tree widely planted for its sweet fruits. The fruits of dates contain a high rate of carbohydrates (44%-88%), 15 salts and mineral elements, protein (2.3%-5.6%), a considerable amount of roughage (6.4%-11.5%) and vitamins.
18
On the other hand, date palm pollen (DPP) is a white to yellow soft powder among the branches of palm trees which orally consumed for many different proposes including the increases of ovary and testis weight,
19
the strengthen of fertility power
20
and the treatment of ovarian cysts.
21
Though the anticancer activity of date fruits was previously reported,
22
there is not any report about the anti-angiogenic activity of its pollen extract in the literature. So, the current study aims to scrutinize the anti-angiogenic property of DPP hydro-alcoholic extract in vitro.
Material and Methods
Plant material
DPP soft powder was prepared from Agriculture Research Center of Razi University, Kermanshah, Iran in September 2015.
Preparation of DPP hydro-alcoholic extract
To prepare DPP hydro-alcoholic extract, its soft powder was mixed with a 1:1 ratio (w/v) of 50% ethanol and then slowly stirred for 24 hours at 4°C. The extract was then filtered and centrifuged at 12 000 g for 20 minutes at 4°C. Then, the supernatant was collected. In the next step, using a rotary the extract was concentrated. Then it was incubated at 37°C for 72 hours in order to achieve a dry powder.
Cell culture
The human umbilical vein endothelial cells (HUVECs) were used to evaluate the cytotoxic activity, angiogenic-related gene expression, and anti-angiogenic potential of date palm pollen extract (DPPE). To culture the cells, a mixed medium of MCBD-131 was used. The HUVECs cells were obtained from nitrogen flask and after thawing at 37°C, a complete culture medium was added and the prepared mixture was centrifuged for 5 minutes at 1000 g. Then the supernatant was discarded and the fresh culture medium was substituted. Then, the suspension was transferred to the flask and then endothelial cells were passaged. Passages 3 to 5 were applied for further steps.
Cytotoxicity and proliferation assays
The lactate dehydrogenase (LDH) assay was used to measure cell viability. The experimental protocol for LDH assay was performed based on Linford’s method.
23
Cells were cultured in a 96-well culture plate at a density of 1×104 cells/well in MCDB131 supplemented containing 2% FBS incubated for 24 hours. Afterward, different concentrations (25-1000 µg/mL) of the hydro-alcoholic extract of DPP were added to the wells and the plates were incubated for an additional 48 hours. Finally, 100 µL of supernatant was transferred to a 96-wells cultured plate and LDH release was determined by LDH kit at 490 nm with background diminution at 630 nm, respectively.
To assay cell proliferation, after culturing the cells like a previous step (LDH test), the cells were counted after treating with different concentrations (25-1000 µg/mL) of the DPP hydro-alcoholic extract (for 48 hours) by MTT assay. All these steps were repeated in triplicates.
HUVECs capillary tube formation in three-dimensional collagen gel
In this experiment, passages 3-5 of HUVECs were used. Cytodex 3 microcarrier beads (Amersham Pharmacia Biotech) were prepared based on the manufacturer’s instructions. The HUVECs were mixed with the Cytodex 3 microcarrier beads by mildly shaking the cell suspension and microcarriers every 20 minutes in MCDB131 supplemented with 10% FCS for 4 hours at 37°C and 5% CO2. Thereafter, the cell suspension with microcarriers was transferred to a 24-well tissue culture plate and incubated for 12-16 hours under the same conditions. Then in the following day, the cell-incorporated beads were mixed with a collagen solution (collagen type I, 10X MCDB131, 23 mg/mL NaHCO3 and FCS with a ratio of 7.5:1:1:0.5, respectively) on ice, and 50 μL of the solution was added to each well of the 96-well plate and allowed to rigidify in the incubator for 20 minutes at 37°C and 5% CO2. Afterwards, 250 μL of MCDB131 with different concentrations of extract (25-1000 µg/mL) were added. The effects of extract on sprout formation were investigated and the percentage decrease in sprout formation after three days of treatment compared to the control.
Real-time PCR
Total RNA extraction was performed by RNAX-Plus (Cinaclon, Iran) (after 18 hours of incubating HUVECs administered with various doses of the extract [25-600 µg/mL] at 37°C, and 80% humidity) and cDNA synthesis from RNAs was done by EUREX kit according to its instruction. The expression of VEGF, MMP-2 and MMP-9 mRNA levels were assessed with SYBR Green I and amplified with the Rotor-Gene 6000 system (Corbett Research, Australia) by real-time polymerase chain reaction (PCR). Beta-actin (β-actin) was used to normalize the expression results as a reference gene. Analysis of relative gene expression data gained from three sets of experiments was carried out by 2–ΔΔCT method.
Molecular docking
Preparation of ligand and receptor
To perform molecular docking simulation (fully blind docking) the following steps were done. First, the 3D crystallographic structures of vascular endothelial growth factor receptor 1 (VEGFR-1) (PDB ID: 3HNG
24
), VEGFR-2 (PDB ID: 2XIR), VEGF (PDB ID: 2VPF
25
) and circulating tumor DNA (ctDNA) (PDB ID: 1BNA
26
) were obtained from the Protein Data Bank (http://www.rcsb.org/pdb/home/home.do). The 3D structures of DPP secondary metabolites were obtained from PubChem database in “.sdf” format. The OpenBabel tool was used to construct ligands “.pdb
”
files. The AM1 semi-empirical method in HyperChem was used to optimize the chemical structure of the selected ligands (Fig. 1). Molecular docking simulation (blind docking) was performed using the AutoDock 4.2.6 tool .
27
First, the water molecules and other unrelated atoms were eliminated from the initial structures of the selected receptors, and then missing hydrogens and Gasteiger/Kollman charges were added to the receptor and ligand structures. Afterwards, pre-calculation of grid maps was performed using AutoGrid. Next, the docking simulation was performed by inserting a grid map with 60 × 60 × 60 Å points and a grid point spacing of 0.375 Å which was centered on the receptor. The number of independent docking runs performed for each docking simulation was set to 200 with 25 000 000 energy evaluations for each run. For calculation of other parameters, the default values of program were used. Finally, the best conformer with the lowest binding energy was selected for each docking simulation. All generated poses were evaluated using Schrodinger suite 2015 (free trial version) (https://www.schrodinger.com/) and PyMOL, in order to detect the type of interactions including π-π, H-bond and cation-π interactions associated with ligand binding mode.
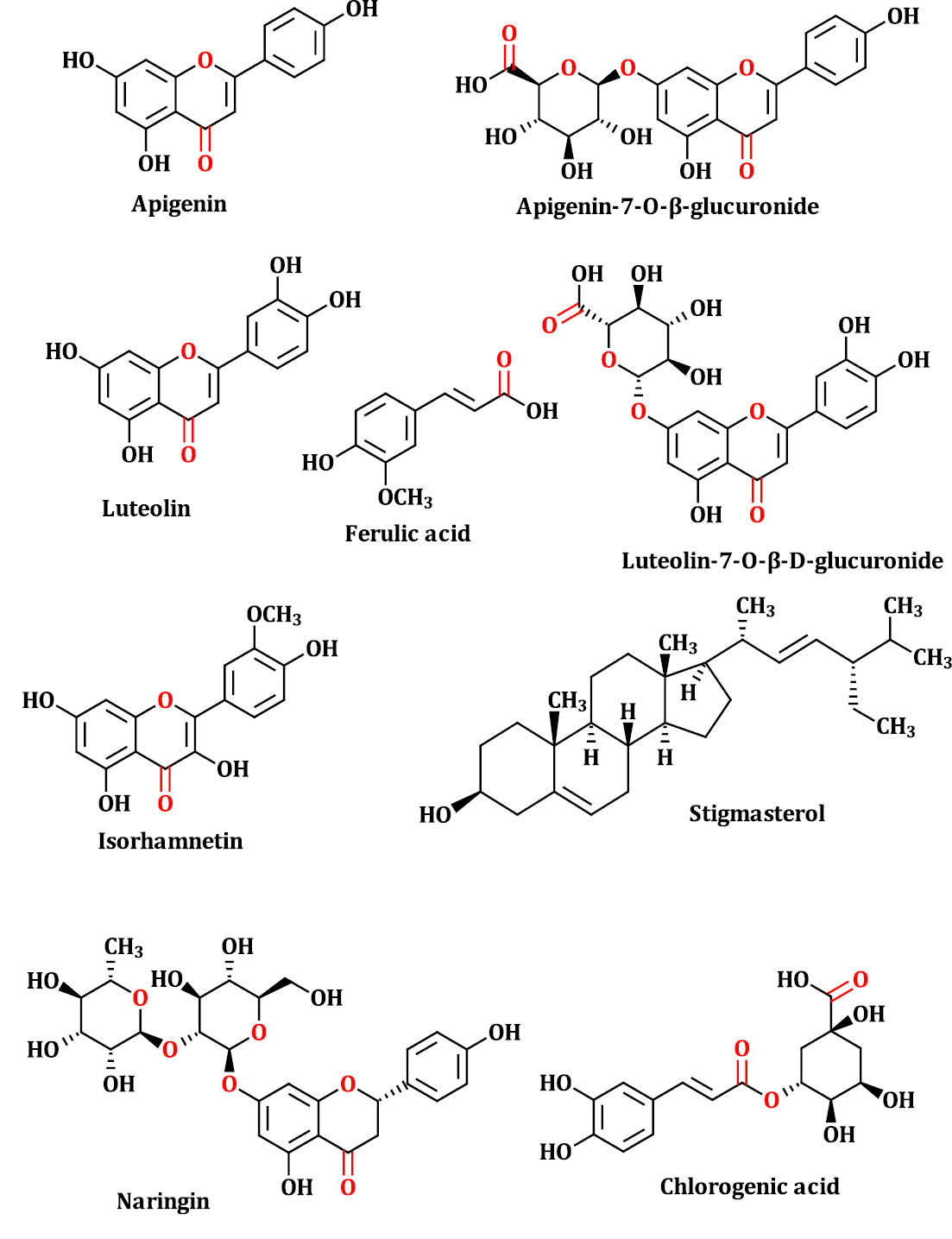
Fig. 1.
The chemical 2D structure of DPPCs. The mentioned compounds in this figure were selected based on previous reports.
28,29
.
The chemical 2D structure of DPPCs. The mentioned compounds in this figure were selected based on previous reports.
28,29
Statistical analysis
Data analysis and diagram drawing for toxicity and inhibition of the HUVECs’ growth were performed by GraphPad Prism® software.
30
The CC50 and IC50 values were computed using charts and obtained linear equation. The statistical significance of experimental data was determined using the one-way or two-way analysis of variance (ANOVA) followed by the Tukey and Bonferroni test respectively. A P value <0.05 was selected to be statistically significant. Each point or column represents the mean ± SEM (n = 3); P < 0.05 (*), P < 0.01 (**), P < 0.001 (***).
Results
Effect of hydro-alcoholic extract on growth and viability of HUVECs
The LDH bioassay was used to measure the viability of HUVECs. The calculated CC50 value for DPPE was 679.74 µg/mL, respectively. The mentioned extract was not toxic at 400 µg/mL concentration. On the other hand, 60% viability was observed at a concentration of 600 µg/mL and toxicity effect was increasing at higher doses. Also, the toxicity of 75% and 100% were observed at 800 and 1000 µg/mL concentrations, respectively (Fig. 2A).
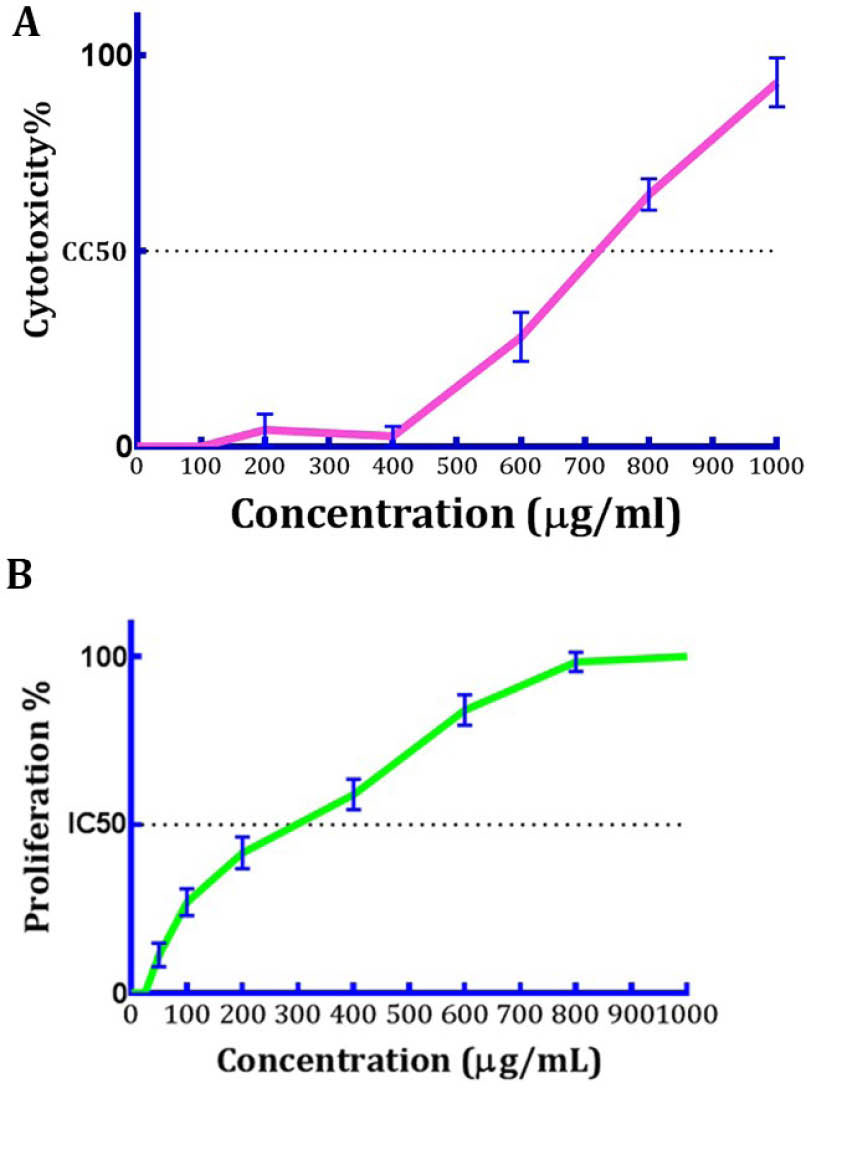
Fig. 2.
(A) The cytotoxicity effect of DPPE on HUVEC cells. At 500- 1000 μg/mL, DPPE decreased cell viability (B) Anti-proliferative effect of DPPE on HUVEC cells. Each point or column represents the mean ± SEM (n=3).
.
(A) The cytotoxicity effect of DPPE on HUVEC cells. At 500- 1000 μg/mL, DPPE decreased cell viability (B) Anti-proliferative effect of DPPE on HUVEC cells. Each point or column represents the mean ± SEM (n=3).
In a dose-dependent behavior, the DPPE showed inhibitory properties on the proliferation of HUVECs and the calculated proliferative inhibitions at doses 50, 100, 200, 400, 600, 800 and 1000 µg/mL were 10%, 15%, 35%, 65%, 83%, 100% and 100%, respectively. From these results, the computed IC50 value for DPPE was 328.23 µg/mL (Fig. 2B).
Anti-angiogenic activity of the hydro-alcoholic extract
Three-dimensional culture of HUVECs on cytodex-3D micro-carriers was used as an in vitro model to find the possible anti-angiogenic effect of DPPE. After 3 days of treatment, untreated controls showed a branching pattern of tube-like vessels. At the concentration of 100 µg/mL, 30% inhibition of angiogenesis was observed in comparison to controls. At higher doses, the inhibition rate was increased as it was predicted. Therefore, at concentrations of 200 and 400 µg/mL, the observed inhibitory ratios were 45% and 70% (Fig. 3), and the calculated IC50 value in this model was 260 µg/mL, respectively.
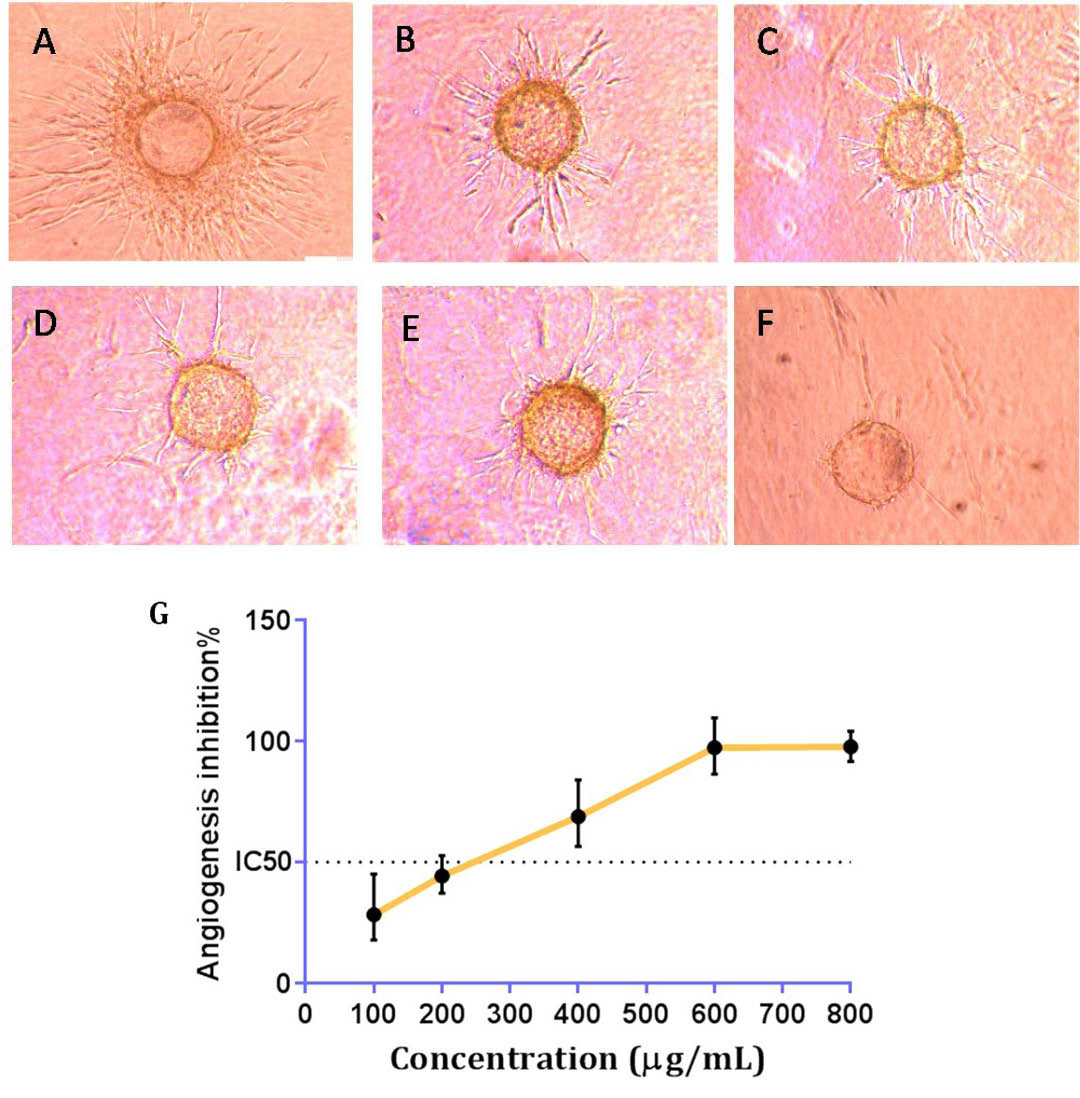
Fig. 3.
The inverted microscopy image of cultured HUVEC cells after 3 days in cytodex collagen gel. (A): Control; (B - F): Test wells treated with 100, 200, 400 and 800 μg/ml of DPPE, respectively. (G): Each point represents the mean ± SEM (n=3).
.
The inverted microscopy image of cultured HUVEC cells after 3 days in cytodex collagen gel. (A): Control; (B - F): Test wells treated with 100, 200, 400 and 800 μg/ml of DPPE, respectively. (G): Each point represents the mean ± SEM (n=3).
Effect of DPPE on VEGF, MMP-2, 9 expressions in HUVECs
From real-time PCR results, the VEGF, MMP-2, and MMP-9 mRNA levels decreased upon treatment with extract in comparison with the control (β-actin mRNA expression). Our results unveiled that the mentioned extract significantly decreased the expression of the selected candidate genes in a dose-dependent manner (Fig. 4A-C). The results indicated that the VEGF gene expression was decreased by 2.3, 2.6 and 2.73 folds in 200 µg/mL, 400 µg/mL, and 600 µg/mL, respectively. The results for MMP-2 gene expression showed a decrease of 0.7 and 1.94 folds in 400 µg/mL and 600 µg/mL, respectively. Also, the results for MMP-9 gene expression showed a decrease of 1.38 and 2.44 folds in 400 µg/mL and 600 µg/mL, respectively.
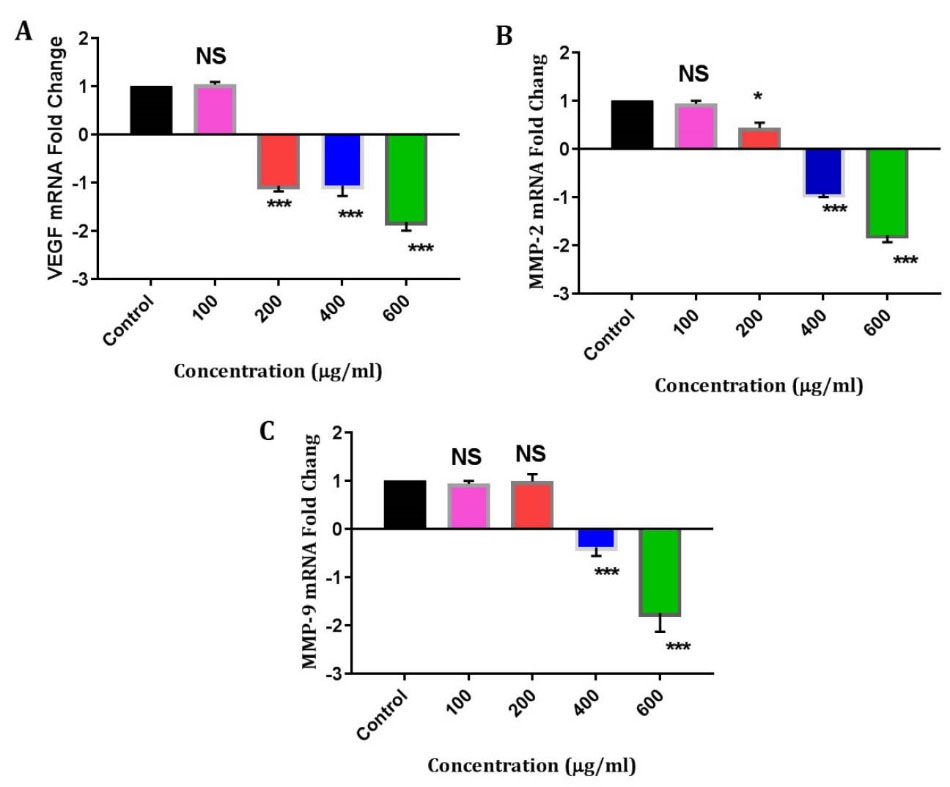
Fig. 4.
Analysis of VEGF, MMP-2 and MMP-9 mRNA levels in HUVECs. The expression of these genes was decreased at different doses of DPPE (100, 200, 400 and 600 μg/ml concentrations respectively). P < 0.05 (*); P < 0.01 (**); P < 0.001(***) and NS: not significant.
.
Analysis of VEGF, MMP-2 and MMP-9 mRNA levels in HUVECs. The expression of these genes was decreased at different doses of DPPE (100, 200, 400 and 600 μg/ml concentrations respectively). P < 0.05 (*); P < 0.01 (**); P < 0.001(***) and NS: not significant.
Computational results
Molecular docking is an interesting tool among bioinformatics approaches to examine or predict the interaction between ligand-receptor complexes.
16,31-33
At the molecular level, the binding mode of small ligands to large protein targets plays an important role at numerous biological processes.
33,34
Though the accurate prediction of interaction between ligand and receptor is a major problem in experimental assays, however, molecular docking simulation (especially fully blind docking approach) can give useful information about the behavior of ligands towards the suspected peripheral, inner/outer and catalytic residues of protein active sites. In this regard, to interpret the results obtained from experimental assays molecular docking can be used as a valuable method to decide about the observed interactions.
Studies reported that DPP grain has comprised from different classes of secondary metabolites including phenolic acids (i.e. ferulic acid and chlorogenic acid), flavonoids (i.e. apigenin, luteolin, isorhamnetin, apigenin-7-O-β-glucuronide, luteolin-7-O-glucuronide and naringin) and phytosterols (i.e. stigmasterol)
28,29
(Fig. 1). We used these compounds as input ligands for molecular docking simulation to predict their binding modes into the selected receptors. Our results indicated that these compounds have the potential to interact with the studied receptors and due to their presence in the hydro-alcoholic extract they can exhibit the observed features such as anti-angiogenesis property.
Interaction with ctDNA
Many studies reported that DNA minor groove binding agents are potent inhibitors to target DNA molecules in many different types of human chronic diseases,
35,36
which form non-covalent and covalent complexes with DNA.
37
These classes of inhibitors have high level of sequence specificity and exhibited a wide range of biological activities from antibacterial to anticancer properties.
37
However, studies indicated that phenolic acids and flavonoids can interact with the DNA minor groove.
38,39
Circulating tumor DNA (ctDNA) is a small fragment of DNA that found in the bloodstream of cancerous patients.
40
Studies suggested that this tumor-based DNA fragment can act as a leader for the migration of tumor cells to other parts of the body.
40
Recently, this small fragment of DNA was considered as detectable biomarker in early- and late-Stage human malignancies.
40
Also, evidence suggests that ctDNA minor groove binding compounds may be useful to inhibit the tumor migration.
41
Herein, to identify the possible binding mode of isolated compounds from DPP into ctDNA molecular docking was performed.
Our results demonstrated that apigenin, luteolin-7-O-β-glucuronide, apigenin-7-O-β-glucuronide and chlorogenic acid were the best interacting compounds (Fig. 5). The calculated docking energies for these compounds were -10.1, -9.38, -8.43 and -8.29 kcal·mol-1, respectively.
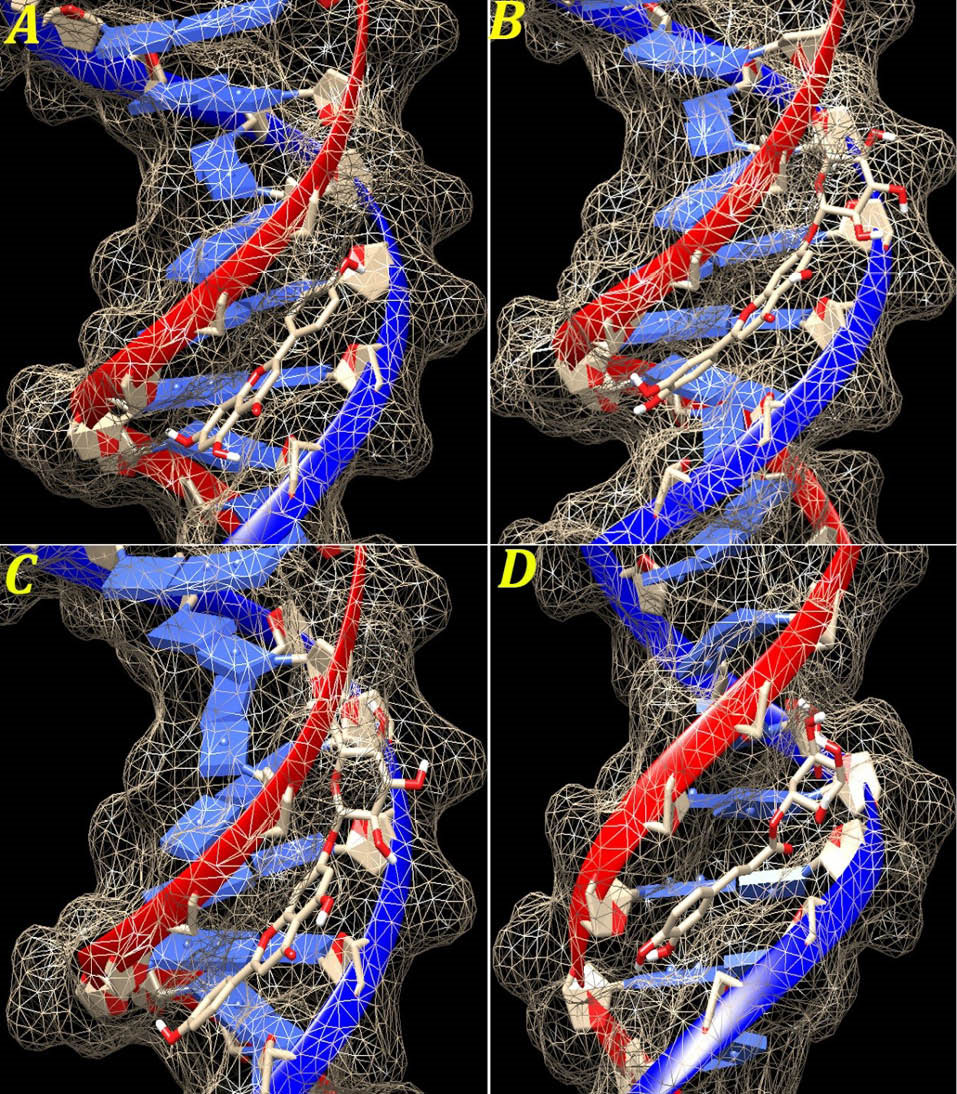
Fig. 5.
The graphical illustration of interaction between the four top docked compounds (A) apigenin; (B) luteolin-7-O-β-glucuronide; (C) apigenin-7-O-β-glucuronide and (D) chlorogenic acid with ctDNA.
.
The graphical illustration of interaction between the four top docked compounds (A) apigenin; (B) luteolin-7-O-β-glucuronide; (C) apigenin-7-O-β-glucuronide and (D) chlorogenic acid with ctDNA.
Except for naringin, other compounds are located in the ctDNA minor groove. The docking simulation displayed that naringin existed in the major groove region. The calculated docking energy for this compound was -2.93 kcal·mol-1, respectively (Table 1). On the other hand, stigmasterol was located in ctDNA minor groove but did not make H-bond with this region. The calculated docking energy for this compound was -6.58 kcal·mol-1, respectively.
Table 1.
The docking energies of ctDNA inhibitors
|
Compound
|
DE* (kcal/mol)
|
SE (kT)
|
VDW (kcal/mol)
|
EIE (Kcal/mol)
|
| Apigenin |
-10.1 |
-7925.22 |
-10.67 |
-8.39 |
| Luteolin-7-O-β-glucuronide |
-9.38 |
-7976.01 |
-1.54 |
-7.09 |
| Apigenin-7-O-β-glucuronide |
-8.43 |
-7963.45 |
-6.54 |
-6.34 |
| Cholorogenic acid |
-8.29 |
-7956.11 |
-3.71 |
-5.11 |
| Isorahamentin |
-8.0 |
-7962.45 |
-5.69 |
-5.23 |
| Ferulic acid |
-7.69 |
-7845.02 |
-3.55 |
-6.68 |
| Stigmasterol |
-6.58 |
-6345.70 |
-0.14 |
-4.45 |
| Luteolin |
-5.78 |
-7749.12 |
-1.07 |
-4.11 |
| Naringin |
-2.93 |
-6348.23 |
-3.08 |
-0.02 |
* DE: docking energy; SE: solvation energy; VDW: Van der Waals; EIE: Electrostatic Interaction Energy.
From these results, it should be noted that several factors including the variability of ligand structures and ability to construct covalent and/or non-covalent bonds could affect their binding affinity towards the ctDNA minor groove. Studies showed that the distribution of electrostatic charges along with a DNA, that it depends on its sequence, is another factor that can change the binding affinity of a ligand into a DNA clef. Mainly, A:T base pairs having the highest negative charge, at the bottom of DNA minor groove, while the C:G base pairs having the highest positive charges in this region.
42
On the other hand, studies suggested that minor groove binding ligands carry a cationic charge, complementing the potential in A:T regions.
42
Herein, our results are fully in accordance with previous reports and the studied compounds have interacted with A:T base pairs in the cleft of ctDNA minor groove (Fig. 6).
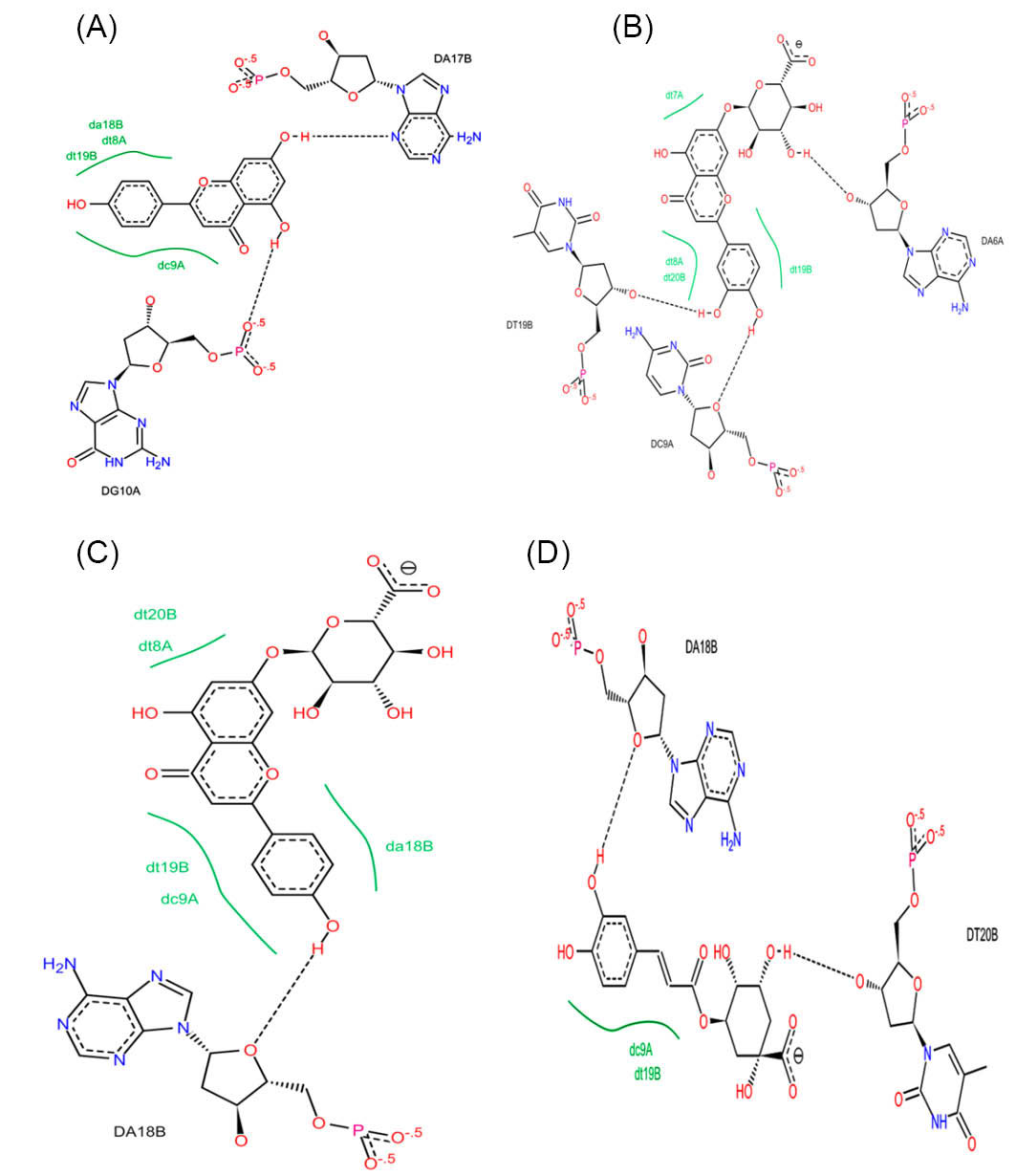
Fig. 6.
The 2D plots of interaction between (A) apigenin; (B) luteolin-7-O-β-glucuronide; (C) apigenin-7-O-β-glucuronide and (D) chlorogenic acid with ctDNA. As shown in this Figure, the mentioned compounds strongly bound to the ctDNA minor groove via detectable H-bonds.
.
The 2D plots of interaction between (A) apigenin; (B) luteolin-7-O-β-glucuronide; (C) apigenin-7-O-β-glucuronide and (D) chlorogenic acid with ctDNA. As shown in this Figure, the mentioned compounds strongly bound to the ctDNA minor groove via detectable H-bonds.
Interaction with VEGF/VEGFRs
At the molecular level, the members of VEGF proteins including VEGF-A (known as VEGF), VEGF-B, VEGF-C, VEGF-D and PIGF (placenta growth factor) play critical roles in vascular biology.
43
Among VEGFs, VEGFA is the most widely studied tumor angiogenic factor. Many studies displayed that the VEGF gene is a key mediator of angiogenesis in cancer.
44
The VEGF protein is homodimeric glycoprotein with a 45 kDa molecular weight.
44
This protein is secreted by a numerous of tumors which plays a central role in the expansion of cancerous cells to other healthy tissues.
Many studies indicated that VEGF inhibitors impaired the interaction of this protein with VEGFR-1 and 2.
45,46
On the other hand, natural compounds (especially phenolic acids and flavonoids) exhibited anti-angiogenesis activity via inhibition of VEGF signaling pathway.
47
According to our results, the evaluated hydro-alcoholic extract significantly decreased the expression of VEGF gene. Therefore, to find the possible mode of action associated with DPP compounds the VEGF protein was selected as input receptor for molecular docking simulation.
Molecular docking results indicated that apigenin-7-O-β-glucuronide, apigenin, isorhamnetin and chlorogenic acid were the best interacting compounds with the suspected binding residues at the VEGF active site (Fig. 7). The calculated docking energies for these compounds were -9.6, -7.8, -6.7 and -6.3 kcal·mol, respectively. Ferulic acid was the weakest interacted compound with this receptor. The computed docking energy for this compound was -4.3 kcal·mol-1, respectively.
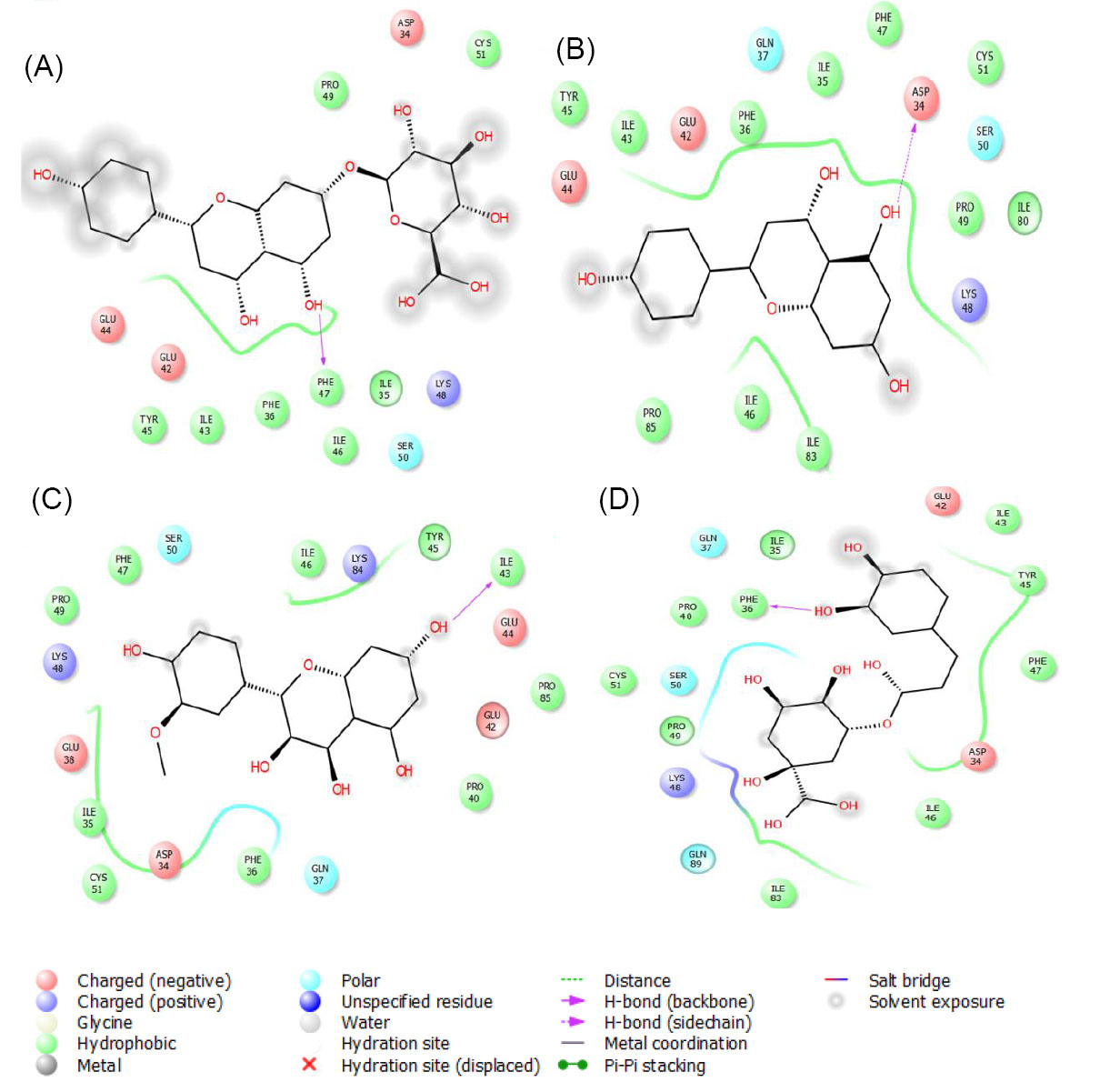
Fig. 7.
The graphical illustration of interaction between (A) apigenin-7-O-β-glucuronide; (B) apigenin; (C) isorahamentin and (D) chlorogenic acid with VEGF protein. As shown in this figure, the docked compounds are located in the nearest of VEGF binding residues. This type of interaction impairs its affinity to bind VEGFR-1 and 2.
.
The graphical illustration of interaction between (A) apigenin-7-O-β-glucuronide; (B) apigenin; (C) isorahamentin and (D) chlorogenic acid with VEGF protein. As shown in this figure, the docked compounds are located in the nearest of VEGF binding residues. This type of interaction impairs its affinity to bind VEGFR-1 and 2.
Also, among docked compounds, chlorogenic acid, isorhamnetin, luteolin-7-O-β-glucuronide and ferulic acid were the best interacting compounds with VEGFR-1 (Fig. 8). The computed docking energies for these compounds were -8.9, -8.3, -7.6 and -7.1 kcal·mol-1 respectively. Stigmasterol was the weakest compound with this receptor. The calculated docking energy for this compound was -3.9 kcal·mol-1.
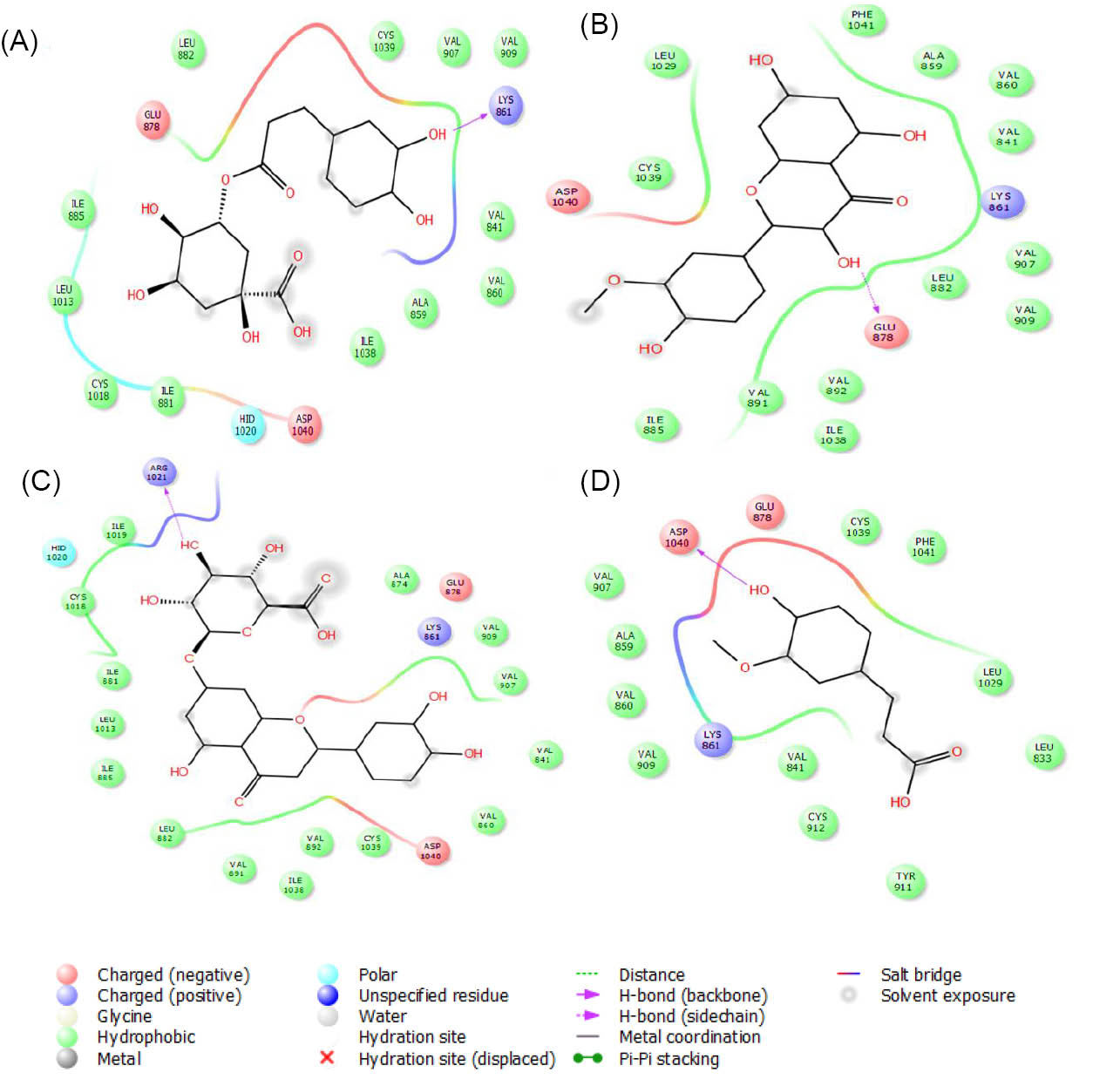
Fig. 8.
The graphical illustration of interaction between (A) chlorogenic acid; (B) isorahamentin; (C) luteolin-7-O-β-glucuronide and (D) ferulic acid with VEGFR-1.
.
The graphical illustration of interaction between (A) chlorogenic acid; (B) isorahamentin; (C) luteolin-7-O-β-glucuronide and (D) ferulic acid with VEGFR-1.
According to our molecular docking results, apigenin, naringin, chlorogenic acid and luteolin were the best interacting compounds with VEGFR-2 (Fig. 9). The calculated docking energies for these compounds were -9.4, -8.9, -7.6 and -7.3 kcal·mol-1, respectively. Except for stigmasterol, other compounds were located in the VEGFR-2 active site. The observed docking energy for this compound was -3.5 kcal·mol-1 which did not make H-bond with the existed residues in this region.
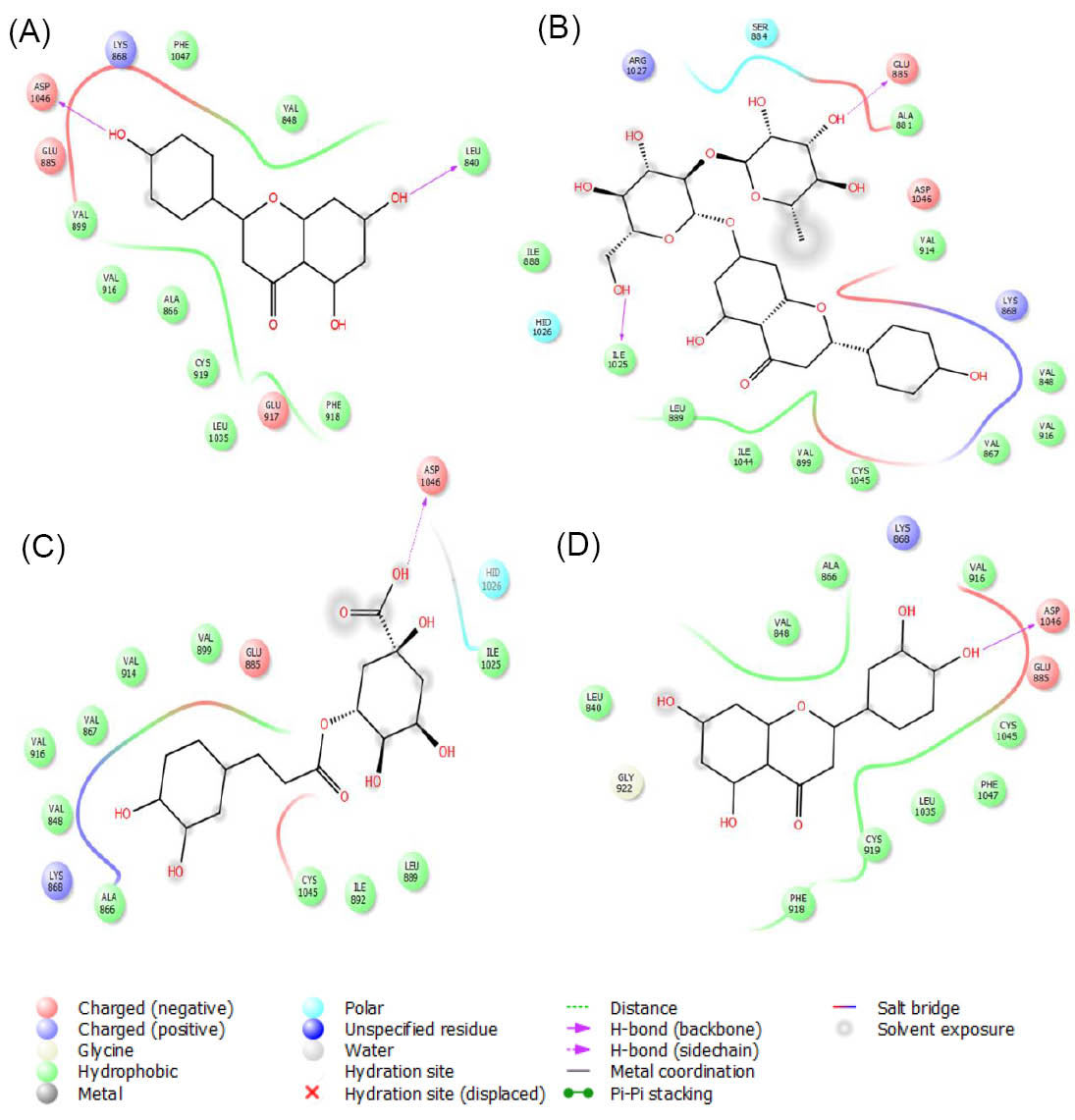
Fig. 9.
The graphical illustration of interaction between (A) apigenin; (B) naringin; (C) chlorogenic acid and (D) luteolin with VEGFR-2.
.
The graphical illustration of interaction between (A) apigenin; (B) naringin; (C) chlorogenic acid and (D) luteolin with VEGFR-2.
The detailed interaction energies for all docked compounds are listed in Table 2.
Table 2.
The docking energies of VEGF/VEGFR inhibitors
|
Compound
|
Receptor
|
DE (kcal/mol)
|
SE (kT)
|
VDW (kcal/mol)
|
EIE (kcal/mol)
|
| Stigmasterol |
VEGF |
-5.6 |
-2068.628 |
-1.2979 |
-0.07277 |
|
|
VEGFR-1 |
-3.9 |
-8359.984 |
-1.1157 |
-0.31115 |
|
|
VEGFR-2 |
-3.5 |
-5435.427 |
-0.0564 |
-0.29491 |
| Apigenin |
VEGF |
-7.8 |
-2096.281 |
-3.64048 |
-9.65219 |
|
|
VEGFR-1 |
-6.1 |
-8387.301 |
-5.10059 |
-4.2378 |
|
|
VEGFR-2 |
-9.4 |
-5494.698 |
-2.74363 |
-5.12448 |
| Apigenin-7-O-β-glucuronide |
VEGF |
-9.6 |
-2080.67 |
-7.73188 |
-13.7057 |
|
|
VEGFR-1 |
-5.7 |
-8352.803 |
-9.56820 |
-10.9582 |
|
|
VEGFR-2 |
-6.8 |
-5488.201 |
-10.7391 |
-7.47193 |
| Chlorogenic acid |
VEGF |
-6.3 |
-2090.167 |
-7.0811 |
-5.61221 |
|
|
VEGFR-1 |
-8.9 |
-8367.643 |
-3.0391 |
-7.09720 |
|
|
VEGFR-2 |
-7.6 |
-5465.598 |
-6.0912 |
-9.079410 |
| Naringin |
VEGF |
-4.5 |
-2057.588 |
-3.88656 |
-1.193673 |
|
|
VEGFR-1 |
-5.6 |
-8321.324 |
-1.37514 |
-2.062841 |
|
|
VEGFR-2 |
-8.9 |
-5451.272 |
-1.89640 |
- 1.170682 |
| Luteolin |
VEGF |
-5.9 |
-2095.77 |
-10.7339 |
-6.130221 |
|
|
VEGFR-1 |
-5.1 |
-8358.241 |
-6.7795 |
-7.786643 |
|
|
VEGFR-2 |
-7.3 |
-5494.300 |
-8.7790 |
- 5.377954 |
| Luteolin-7-O-β-glucuronide |
VEGF |
-5.7 |
-2070.673 |
-8.90761 |
-4.3109123 |
|
|
VEGFR-1 |
-7.6 |
-83456.129 |
-6.27990 |
-8.7433921 |
|
|
VEGFR-2 |
-5.2 |
-5487.870 |
-8.84682 |
-2.0008144 |
| Isorahamentin |
VEGF |
-6.7 |
-2095.413 |
-1.29281 |
-3.7180134 |
|
|
VEGR-1 |
-8.3 |
-8367.210 |
-5.96710 |
-5.2834756 |
|
|
VEGFR-2 |
-5.8 |
-5495.932 |
-4.83243 |
-2.210868 |
| Ferulic acid |
VEGF |
-4.3 |
-2101.615 |
-4.85457 |
-3.572598 |
|
|
VEGFR-1 |
-7.1 |
-8329.320 |
-5.03915 |
-4.097232 |
|
|
VEGFR-2 |
-7.2 |
-5499.175 |
-3.77985 |
-3.775500 |
DE: docking energy; SE: solvation energy; VDW: Van der Waals; EIE: Electrostatic Interaction Energy.
We observed that chlorogenic acid showed binding affinity to interact with ctDNA, VEGF and VEGF receptors. Apigenin interacted with ctDNA, VEGF and VEGFR-2. Also, apigenin-7-O-β-glucuronide has affinity to interact with ctDNA and VEGF. On the other hand, luteolin-7-O-β-glucuronide interacted with ctDNA and VEGFR-1. Except stigmasterol, other polyphenols interacted with critical residues in the structure of VEGF and VEGFRs with different binding energies. However, due to the critical roles of VEGF and VEGFRs in metastasis and angiogenesis,
31,48
among receptor tyrosine kinases (RTKs) their mode of action have widely studied over the past decades.
49
These proteins are responsible for inducing angiogenesis and activating of PLCγ-PKC-MAPK signaling pathway.
48
The VEGF signaling pathway is triggered with the interaction between VEGF and its specific receptor tyrosine kinases and activating the receptors through auto-phosphorylation of intrinsic tyrosine kinase domain.
50
As shown in Fig. 10, the expression of VEGF-A can regulate the expression of other upstream or downstream proteins. Therefore, the inhibition of the interaction between VEGF and VEGFRs is an important way to prevent angiogenesis and the development of malignancy to other tissues. Various types of VEGF signaling cascade inhibitors including antagonistic VEGF mutants, soluble receptors, receptor antagonists, monoclonal antibodies, and deterrents of VEGF receptor function are used as anti-cancer drugs.
51
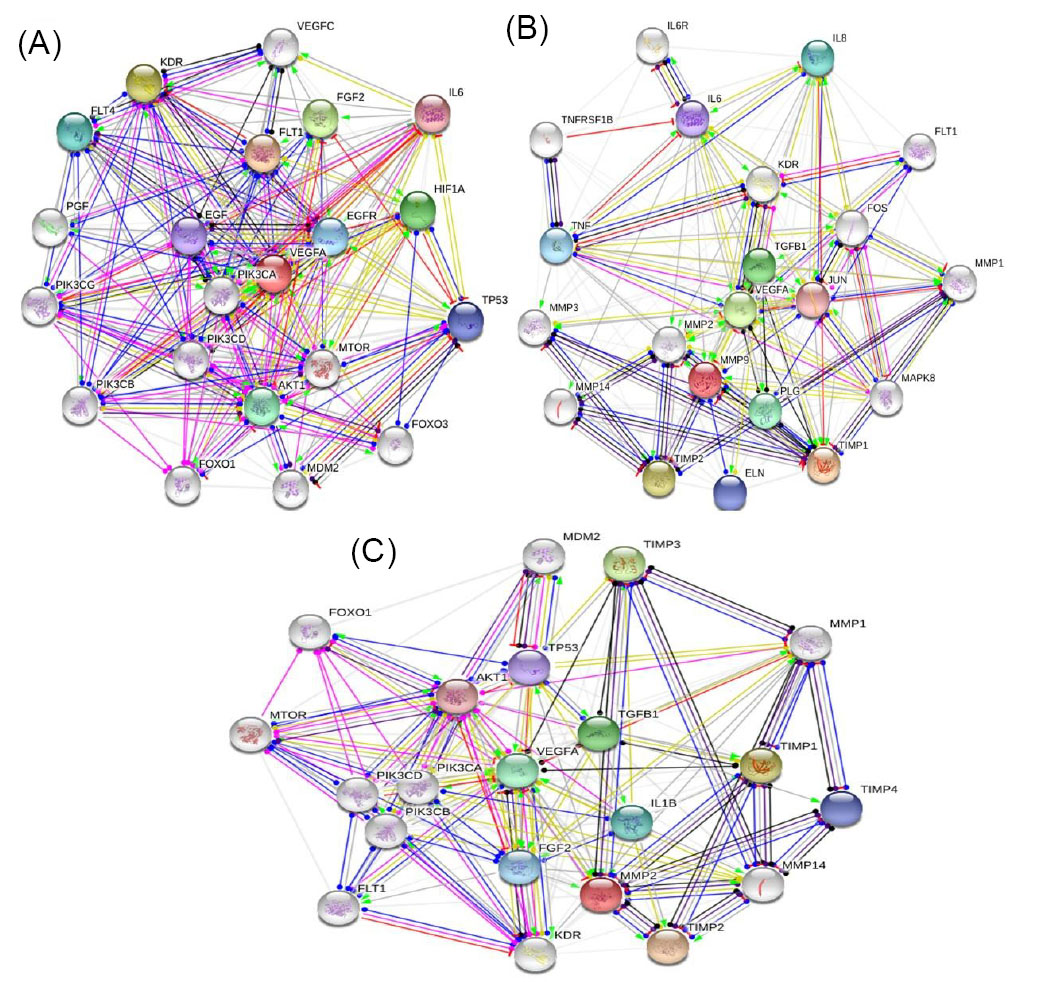
Fig. 10
.
The associated signaling cascades for (A) VEGF-A; (B) MMP-9 and (C) MPP-2. In this figure, action types were showed with different colors including green (activation); blue (binding), red (inhibition); black (reaction); pink (posttranslational modifications); dark blue (catalysis) and yellow (transcriptional regulation) lines.
.
The associated signaling cascades for (A) VEGF-A; (B) MMP-9 and (C) MPP-2. In this figure, action types were showed with different colors including green (activation); blue (binding), red (inhibition); black (reaction); pink (posttranslational modifications); dark blue (catalysis) and yellow (transcriptional regulation) lines.
The MMP-2 and MMP-9 genes also participate in the angiogenesis process. Studies indicated that MMP-2/9 genes play a central role in the high invasion ability of angiogenic cells.
52
The signaling pathways for these genes suggested that they are directly involved in the expression of VEGF-A to induce the inflammation and angiogenesis.
53
Different factors including cytokines, growth factors and mechanical stress can regulate or activate MMPs.
54,55
VEGF is a potent enhancer to release the MMP-2 and MMP-9 by endothelial cells and induces the vascular permeability to allow the permeation of plasma proteins (e.g. fibronectin).
55,56
In the activated endothelial cells integrin receptors such as α5β1 and αvβ3 are suitable targets to interact with fibronectin and fibrin.
57,58
Studies showed that MMP-2 can bind to αvβ3 integrin
59
to trigger the invading potential of endothelial cells.
55
Therefore, the inhibition of VEGF and MMPs expression can delay the angiogenesis and cell malignancy.
It should be noted that the edible medicinal plants' hydro-alcoholic extracts exhibited anti-angiogenesis property via the inhibition of cell proliferation and suppression the VEGF secretion. Hydro-alcoholic extracts comprised many different anti-angiogenic constitutes, which showed their inhibitory potential mainly via down-regulation of essential mediators such as VEGF and MMPs.
60,61
The anti-angiogenic activity of medicinal plants hydro-alcoholic extracts significantly depends on the administrated doses in the in vitro and in vivo models.
62,63
Studies reported that the inhibitory properties of hydro-alcoholic extracts are directly associated with the presence of plant secondary metabolites from polyphenols to phytosterols.
64-66
, Therefore, each plant hydro-alcoholic extract has mild to strong anti-angiogenic activity due to the variability of its secondary metabolites.
Discussion
Date palm is one of the most important planted trees for humans since ancient times for both medicinal uses and religious ceremonies.
67
Its sweet fruits and pollens (the male reproductive cells of palm flowers) widely consumed throughout the world,
68
and it is estimated that about 1000 tons of date pollens are produced every year in the Arabic regions.
19
As mentioned in above sections, studies reported that DPP comprised different classes of natural products including non-flavonoids (especially phenolic acids), flavonoids,
29
amino acids including aspartic, proline, threonine, phenylalanine, alanine, glycine, arginine, methionine, isoleucine, leucine, tyrosine, glutamine, lysine, histidine, valine, and serine,
69
vitamins (B1, B2, B12, A, D, C, E),
68
fatty acids, phytosterols,
68
and mineral and trace elements (e.g. potassium, calcium, sodium, manganese, phosphorous, zinc, iron, selenium).
68-70
Studies reported that the consumption of DPP can fortify the size and weight of female sex organs.
19
Also, it can change or enhance the secretion of sexual hormones such as testosterone,
71
estrogen and progesterone.
72
The role of DPP grains in the improvement of testicular dysfunction induced by cadmium chloride
73
and thyroid disorders in male rats
74
is well documented.
The administration of DPP to male animals (rats and rabbits) displayed spermatogenic property and increased the count of sperms, sperms quality, sperms motility and the size of the testis.
75,76
The DPPs also showed antioxidant activity
67
and decreased the activity of catalase, glutathione peroxidase
77
and superoxide dismutase.
67,73
Though the protective roles of DPP grains against male infertility were previously reported,
68,78
however, its anti-angiogenic activity was not reported and as the first study, our investigation showed that in addition to sexual effects this natural product has a significant anti-cancer property. We showed that this date palm derived product has significant anti-angiogenesis activity. Of course, it should be noted that the behavior of plant extracts in real phase (i.e. Clinical studies) may show different pattern due to the complexity of human body and the involved signaling pathways. Therefore, to gain the best results from clinical investigations various factors including the optimum doses of plant extract, the age of patients and the time of administration should be considered in order to approve the efficacy of the examined plant extract.
Flavonoids are abundant groups of bioactive secondary metabolites widely distributed in fruits and vegetables.
15,16,79
Phenolic acids and flavonoids exhibited a wide range of biological activities from anti-diabetic to anti-cancer properties.
80
Thanks to their anti-cancer activities, thousands of papers have published over the past decade to highlight this fact that how these compounds are effective agents in the prevention of cancer and the associated disorders. These natural compounds have low or no adverse effects for the human body and the regular consumption of foods rich in flavonoids and phenolic acids can improve the quality of life. Recently, Al-Samarrai et al isolated different classes of natural products from Iraqi DPP grains. Polyphenols including isorhamnetin (122.251 µg/g), chlorogenic acid (71.146 µg/g), ferulic acid (99.188 µg/g), naringin (64.574 µg/g), apigenin (109.117 µg/g), apigenin-7-O-β glycopyranoside (48.391 µg/g), luteolin (28.883 µg/g) and luteolin-7-O-β-glucuronide (18.291 µg/g) were the most abundant compounds isolated from DPPs.
29
Generally, food products containing high levels of natural products including flavonoids and phenolic acids, vitamins, minerals and trace elements have considered as “functional foods” to provide many health benefits for humans.
81,82
Many studies reported that the presence of essential bioactive compounds in plant-based foods can improve the physiological and biochemical condition of human health,
83
and therefore the regular consumption of these products improve the quality of life.
84
The consumption of foods originated from edible plants decreased the incidences of certain chronic diseases and improved lifestyle habits and life expectancies.
85
On the other hand, the anticancer activities of functional foods
86,7
have been investigated in years and the correlation between healthy diet and cancer prevention is well documented.
88
As previously reported, the DPP grains included different classes of bioactive compounds which can be considered as new functional food to use as a dietary supplement in the treatment of different diseases. The use of “functional food” term for DPP is relatively depending on the applicability of this product in the human diet. For years, this part of date palm has wasted in local regions due to the lacking of food conversion industries to prepare it for commercial uses. Beyond the biological and pharmacological activities of DPP grains, the trading of this product can improve the economic condition of producers to sell DPP as a valuable food product like other agricultural products. However, we selected the presented polyphenols in DPP to examine their possible interaction with proteins involved in angiogenesis.
Our results unveiled that the titled compounds showed different affinity to interact with the studied receptors. The observed variable binding energies for docked ligands may be due to the structural variation of these compounds. Many studies suggested that the chemical structure of polyphenols has a pivotal role in the interaction of these compounds with molecular targets. The number of attached –OH groups, sugar units, methylation, the position of B-ring, C2-C3 double bond and the molecular weight of polyphenols are other important features affected the binding affinity of these compounds.
15,16
However, studied showed that flavonoids have strong anti-angiogenic properties
89,90
and researchers suggested that these compounds are useful targets to consider their structure backbones to design new effective supplementary agents in the pre-treatment of cancer. On the other hand, each angiogenesis study aims to achieve an acceptable agent for further experimental and clinical studies. As a general consensus, our results for evaluating the anti-angiogenic activity of DPP hydro-alcoholic extract is fully in line with previous studies to identify the inhibitory properties of medicinal plant hydro-alcoholic extracts.
Conclusion
DPP is orally consumed as functional food which possesses numerous health benefits as anti-diabetic, antioxidant, anti-inflammation and protective effect against male infertility. As the first study, we evaluated its potential to inhibit angiogenesis and the results successfully confirmed our hypothesis. Further in vivo and clinical studies regarding DPP grains to use as a useful supplementary agent in the pre-treatment of cancer are highly recommended.
Acknowledgments
The authors would like thank the editor in chief and the anonymous reviewers for providing effective, instructive and invaluable comments to fortify the quality of the current paper.
Funding sources
None to be declared.
Ethical statement
This work was approved by the affiliated medical research center and their reviewer board.
Competing interests
Authors certify that no actual or potential conflict of interest in relation to this article exists.
Authors contribution
Conception and design: KM, AM, HR, Development of methodology: KM, ZH, AHN, HR, AM, NM, TR and FO Acquisition of data: KM, AM, MT analysis and interpretation of data: KM, HR, AM and AHN Writing and revision of the manuscript.
Research Highlights
What is the current knowledge?
simple
-
√ Date palm pollen (DPP) is a valuable source of natural
products (polyphenols to phytosterols) to treat human
diseases.
What is new here?
simple
-
√ As the first report, the study discuss the probable
mechanism for anti-angiogenesis activity of DPP
-
√ This study provides useful information about this food
product to use as supplementary agent in cancer therapy
strategies.
References
- Rasouli H, Parvaneh S, Mahnam A, Rastegari-Pouyani M, Hoseinkhani Z, Mansouri K. Anti-angiogenic potential of trypsin inhibitor purified from Cucumis melo seeds: Homology modeling and molecular docking perspective. Int J Biol Macromol 2017; 96:118-28. doi: 10.1016/j.ijbiomac.2016.12.027 [Crossref] [ Google Scholar]
- Rasouli H, Mansouri K, Mahamed-Khosroushahi L. Anti-angiogenic/inflammatory behavior of mushroom ganoderma lucidum extract could be effective for treatment of corneal neovascularization: A hypothesis. J Rep Pharm Sci 2014; 3:14-8. [ Google Scholar]
- Bagnardi V, Blangiardo M, La Vecchia C, Corrao G. A meta-analysis of alcohol drinking and cancer risk. British J Cancer 2001; 85:1700-5. doi: 10.1054/bjoc.2001.2140 [Crossref] [ Google Scholar]
- Mehrabi M, Mahdiuni H, Rasouli H, Mansouri K, Shahlaie M, Khodarahmi R. Comparative experimental/theoretical studies on the EGFR dimerization under the effect of EGF/EGF analogues binding: Highlighting the importance of EGF/EGFR interactions at site III interface. Int J Biol Macromol 2018; 115:1-42. doi: 10.1016/j.ijbiomac.2018.04.066 [Crossref] [ Google Scholar]
- Mehrabi M, Mansouri K, Soleymani B, Hoseinkhani Z, Shahlaie M, Khodarahmi R. Development of a human epidermal growth factor derivative with EGFR-blocking and depleted biological activities: A comparative in vitro study using EGFR-positive breast cancer cells. Int J Biol Macromol 2017; 103:275-85. doi: 10.1016/j.ijbiomac.2017.05.035 [Crossref] [ Google Scholar]
-
Anonymous. WHO cancer fact sheet. World health organiation; 2018; Available from: http://www.who.int/mediacentre/factsheets/fs297/en/.
- Chakraborty S, Rahman T. The difficulties in cancer treatment. Ecancermedicalscience 2012; 6:1-5. doi: 10.3332/ecancer.2012.ed16 [Crossref] [ Google Scholar]
- Mehrabi M, Khodarahmi R, Shahlaei M. Critical effects on binding of epidermal growth factor produced by amino acid substitutions. J Biomol Struct Dyn 2017; 35:1085-101. doi: 10.1080/07391102.2016.1171799 [Crossref] [ Google Scholar]
- Anand P, Kunnumakara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res 2008; 25:2097-116. doi: 10.1007/s11095-008-9661-9 [Crossref] [ Google Scholar]
- Key TJ, Allen NE, Spencer EA, Travis RC. The effect of diet on risk of cancer. Lancet 2002; 360:861-8. doi: 10.1016/S0140-6736(02)09958-0 [Crossref] [ Google Scholar]
- Batra P, Sharma AK. Anti-cancer potential of flavonoids: recent trends and future perspectives. 3 Biotech 2013; 3:439-59. doi: 10.1007/s13205-013-0117-5 [Crossref] [ Google Scholar]
- Winzer BM, Whiteman DC, Reeves MM, Paratz JD. Physical activity and cancer prevention: a systematic review of clinical trials. Cancer Causes Control 2011; 22:811-26. doi: 10.1007/s10552-011-9761-4 [Crossref] [ Google Scholar]
- Graham AL, Burke MV, Jacobs MA, Cha S, Croghan IT, Schroeder DR. An integrated digital/clinical approach to smoking cessation in lung cancer screening: study protocol for a randomized controlled trial. Trials 2017; 18:568-82. doi: 10.1186/s13063-017-2312-x [Crossref] [ Google Scholar]
- Ma K, Baloch Z, He T-T, Xia X. Alcohol consumption and gastric cancer risk: A meta-analysis. Med Sci Monit 2017; 23:238-46. doi: 10.12659/MSM.899423 [Crossref] [ Google Scholar]
- Rasouli H, Farzaei MH, Khodarahmi R. Polyphenols and their benefits: A review. Int J Food Prop 2017; 20:1700-41. doi: 10.1080/10942912.2017.1354017 [Crossref] [ Google Scholar]
- Rasouli H, Hosseini-Ghazvini SM-B, Adibi H, Khodarahmi R. Differential α-amylase/α-glucosidase inhibitory activities of plant-derived phenolic compounds: a virtual screening perspective for the treatment of obesity and diabetes. Food Funct 2017; 8:1942-54. doi: 10.1039/C7FO00220C [Crossref] [ Google Scholar]
- Puccinelli MT, Stan SD. Dietary Bioactive Diallyl Trisulfide in Cancer Prevention and Treatment. Int J Mol Sci 2017; 18:1645-63. doi: 10.3390/ijms18081645 [Crossref] [ Google Scholar]
- Al-Shahib W, Marshall RJ. The fruit of the date palm: its possible use as the best food for the future?. Int J Food Sci Nutr 2003; 54:247-59. doi: 10.1080/09637480120091982 [Crossref] [ Google Scholar]
- Soliman F, Soliman A. The gonad stimulating potency of date palm pollen grains. Cell Mol Life Sci 1958; 14:92-3. [ Google Scholar]
- Abdi F, Roozbeh N, Mortazavian AM. Effects of date palm pollen on fertility: research proposal for a systematic review. BMC Res Notes 2017; 10:363-7. doi: 10.1186/s13104-017-2697-3 [Crossref] [ Google Scholar]
- Karimi Jashni H, Kargar Jahromi H, Bagheri Z. The Effect of Palm Pollen Extract on Polycystic Ovary Syndrome (POS) in Rats. Int J Med Res Health Sci 2016; 5:317-21. [ Google Scholar]
- Ishurd O, Kennedy JF. The anti-cancer activity of polysaccharide prepared from Libyan dates (Phoenix dactylifera L). Carbohydr Polym 2005; 59:531-5. doi: 10.1016/j.carbpol.2004.11.004 [Crossref] [ Google Scholar]
- Linford NJ, Dorsa DM. 17β-Estradiol and the phytoestrogen genistein attenuate neuronal apoptosis induced by the endoplasmic reticulum calcium-ATPase inhibitor thapsigargin. Steroids 2002; 67:1029-40. [ Google Scholar]
-
Tresaugues L, Roos A, Arrowsmith C, Berglund H, Bountra C, Collins R, et al. Crystal structure of VEGFR1 in complex with N-(4-Chlorophenyl)-2-((pyridin-4-ylmethyl) amino) benzamide. The RCSB PDB 2013.
- Muller YA, Christinger HW, Keyt BA, de Vos AM. The crystal structure of vascular endothelial growth factor (VEGF) refined to 193 Å resolution: multiple copy flexibility and receptor binding. Structure 1997; 5:1325-38. doi: 10.1016/S0969-2126(97)00284-0 [Crossref] [ Google Scholar]
- Drew HR, Wing RM, Takano T, Broka C, Tanaka S, Itakura K. Structure of a B-DNA dodecamer: conformation and dynamics. Proc Natl Acad Sci USA 1981; 78:2179-83. doi: 10.2210/pdb1BNA/pdb [Crossref] [ Google Scholar]
- Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem 2009; 30:2785-91. [ Google Scholar]
-
Hadrami AE, Daayf F, Hadrami IE. Secondary metabolites of date palm. Date palm biotechnology2011. p. 653-74.
- Al-Samarrai RR,
Hamad
Al –Samarrai A-M
, Al-Salihi FG. Identification of Flavonoids in Iraqi Date Palm Pollen by HPLC. Orient J Chem 2017; 33:985-8. doi: 10.13005/ojc/330252 [Crossref] [ Google Scholar]
-
Motulsky H. Analyzing data with GraphPad prism: GraphPad Software Incorporated; 1999.
- Mohammadi-Motlagh H-R, Shokohinia Y, Mojarrab M, Rasouli H, Mostafaie A. 2-Methylpyridine-1-ium-1-sulfonate from Allium hirtifolium: An anti-angiogenic compound which inhibits growth of MCF-7 and MDA-MB-231 cells through cell cycle arrest and apoptosis induction. Biomed Pharmacother 2017; 93:117-29. doi: 10.1016/j.biopha.2017.06.013 [Crossref] [ Google Scholar]
- Sousa SF, Fernandes PA, Ramos MJ. Protein–ligand docking: current status and future challenges. Proteins: Struct, Funct, Bioinf 2006; 65:15-26. doi: 10.1002/prot.21082 [Crossref] [ Google Scholar]
- Rasouli H, Mehrabi M, Arab SS, Khodarahmi R. Are Pro8/Pro18 really critical for functional dynamic behavior of human endostatin N-terminal peptide? A comparative molecular dynamics study. J Iran Chem Soc 2017; 14:2023-39. [ Google Scholar]
- Pagadala NS, Syed K, Tuszynski J. Software for molecular docking: a review. Biophys Rev 2017; 9:91-102. doi: 10.1007/s12551-016-0247-1 [Crossref] [ Google Scholar]
- Khan GS, Shah A, Barker D. Chemistry of DNA minor groove binding agents. J Photochem Photobiol 2012; 115:105-18. [ Google Scholar]
- Saini KS, Ashraf R, Mandalapu D, Das S, Siddiqui MQ, Dwivedi S. New orally active DNA minor groove binding small molecule CT‐1 acts against breast cancer by targeting tumor DNA damage leading to p53‐dependent apoptosis. Mol Carcinog 2017; 56:1266-80. doi: 10.1002/mc.22588 [Crossref] [ Google Scholar]
- Baraldi PG, Bovero A, Fruttarolo F, Preti D, Tabrizi MA, Pavani MG. DNA minor groove binders as potential antitumor and antimicrobial agents. Med Res Rev 2004; 24:475-528. doi: 10.1002/med.20000 [Crossref] [ Google Scholar]
- Bi S, Qiao C, Song D, Tian Y, Gao D, Sun Y. Study of interactions of flavonoids with DNA using acridine orange as a fluorescence probe. Sens Actuators, B 2006; 119:199-208. doi: 10.1016/j.snb.2005.12.014 [Crossref] [ Google Scholar]
- Kanakis C, Tarantilis P, Polissiou M, Diamantoglou S, Tajmir-Riahi H. DNA interaction with naturally occurring antioxidant flavonoids quercetin, kaempferol, and delphinidin. J Biomol Struct Dyn 2005; 22:719-24. doi: 10.1080/07391102.2005.10507038 [Crossref] [ Google Scholar]
- Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N. Detection of circulating tumor DNA in early-and late-stage human malignancies. Sci Transl Med 2014; 6:224ra24-ra24. [ Google Scholar]
- Mati SS, Roy SS, Chall S, Bhattacharya S, Bhattacharya SC. Unveiling the groove binding mechanism of a biocompatible naphthalimide-based organoselenocyanate with calf thymus DNA: an “ex vivo” fluorescence imaging application appended by biophysical experiments and molecular docking simulations. J Phys Chem B 2013; 117:14655-65. [ Google Scholar]
- Neidle S. DNA minor-groove recognition by small molecules. Nat Prod Rep 2001; 18:291-309. [ Google Scholar]
- Stacker SA, Achen MG. The VEGF signaling pathway in cancer: the road ahead. Chin J Cancer 2013; 32:297-302. doi: 10.5732/cjc.012.10319 [Crossref] [ Google Scholar]
- Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005; 69:4-10. doi: 10.1159/000088478 [Crossref] [ Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350:2335-42. doi: 10.1056/NEJMoa032691 [Crossref] [ Google Scholar]
- Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell 2009; 15:167-70. doi: 10.1016/j.ccr.2009.02.007 [Crossref] [ Google Scholar]
- Luo H, Jiang B-H, King SM, Chen YC. Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutr Cancer 2008; 60:800-9. doi: 10.1080/01635580802100851 [Crossref] [ Google Scholar]
- Roskoski R. Vascular endothelial growth factor (VEGF) and VEGF receptor inhibitors in the treatment of renal cell carcinomas. Pharm Res 2017; 120:116-32. [ Google Scholar]
- Carpenter G, Liao H-J. Trafficking of receptor tyrosine kinases to the nucleus. Exp Cell Res 2009; 315:1556-66. [ Google Scholar]
- Eichmann A, Simons M. VEGF signaling inside vascular endothelial cells and beyond. Curr Opin Cell Biol 2012; 24:188-93. [ Google Scholar]
- Sousa Moreira I, Alexandrino Fernandes P, Joao Ramos M. Vascular endothelial growth factor (VEGF) inhibition-a critical review. Anticancer Agents Med Chem 2007; 7:223-45. [ Google Scholar]
- Kanayasu‐Toyoda T, Tanaka T, Ishii‐Watabe A, Kitagawa H, Matsuyama A, Uchida E. Angiogenic Role of MMP‐2/9 Expressed on the Cell Surface of Early Endothelial Progenitor Cells/Myeloid Angiogenic Cells. J Cell Physiol 2015; 230:2763-75. doi: 10.1002/jcp.25002 [Crossref] [ Google Scholar]
- Mohammad G, Siddiquei MM. Role of matrix metalloproteinase-2 and -9 in the development of diabetic retinopathy. J Ocul Biol Dis Infor 2012; 5:1-8. doi: 10.1007/s12177-012-9091-0 [Crossref] [ Google Scholar]
- Overall CM, López-Otín C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer 2002; 2:657-72. doi: 10.1038/nrc884 [Crossref] [ Google Scholar]
- Rundhaug JE. Rundhaug JEMatrix Metalloproteinases, Angiogenesis, and Cancer: Commentary re: AC Lockhart et al, Reduction of Wound Angiogenesis in Patients Treated with BMS-275291, a Broad Spectrum Matrix Metalloproteinase InhibitorClinCancer Res, 9: 00–00, 2003. Clin Cancer Res 2003; 9:551-4. [ Google Scholar]
- Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol 2002; 20:4368-80. [ Google Scholar]
- Laurens N, Engelse MA, Jungerius C, Löwik CW, van Hinsbergh VW, Koolwijk P. Single and combined effects of αvβ3-and α5β1-integrins on capillary tube formation in a human fibrinous matrix. Angiogenesis 2009; 12:275-85. doi: 10.1007/s10456-009-9150-8 [Crossref] [ Google Scholar]
- Eliceiri BP, Cheresh DA. Adhesion events in angiogenesis. Curr Opin Cell Biol 2001; 13:563-8. [ Google Scholar]
- Jiao Y, Feng X, Zhan Y, Wang R, Zheng S, Liu W. Matrix metalloproteinase-2 promotes αvβ3 integrin-mediated adhesion and migration of human melanoma cells by cleaving fibronectin. PloS one 2012; 7:e41591-e603. doi: 10.1371/journal.pone.0041591 [Crossref] [ Google Scholar]
- Yavari N, Emamian F, Yarani R, Reza Mohammadi-Motlagh H, Mansouri K, Mostafaie A. In vitro inhibition of angiogenesis by heat and low pH stable hydroalcoholic extract of Peganum harmala seeds via inhibition of cell proliferation and suppression of VEGF secretion. Pharm Biol 2015; 53:855-61. doi: 10.3109/13880209.2014.946057 [Crossref] [ Google Scholar]
- Yarani R, Mansouri K, Mohammadi-Motlagh HR, Mahnam A, Emami Aleagha MS. In vitro inhibition of angiogenesis by hydroalcoholic extract of oak (Quercus infectoria) acorn shell via suppressing VEGF, MMP-2, and MMP-9 secretion. Pharm Biol 2013; 51:361-8. doi: 10.3109/13880209.2012.729147 [Crossref] [ Google Scholar]
- Badrhadad A, Kh P, Mansouri K. In vitro anti-angiogenic activity fractions from hydroalcoholic extract of Elaeagnus angustifolia L flower and Nepeta crispa L arial part. J Med Plant Res 2012; 6:4633-9. [ Google Scholar]
- Li L-H, Baek IK, Kim JH, Kang KH, Koh YS, Jung YD. Methanol extract of Elaeagnus glabra, a Korean medicinal plant, inhibits HT1080 tumor cell invasion. Oncol Rep 2009; 21:559-63. [ Google Scholar]
- Silva JPB, Peres ARMN, Paixão TP, Silva ASB, Baetas AC, Barbosa WLR. Antifungal activity of hydroalcoholic extract of Chrysobalanus icaco against oral clinical isolates of Candida Species. Pharmacognosy Res 2017; 9:96-100. doi: 10.4103/0974-8490.199772 [Crossref] [ Google Scholar]
- Barbosa WLR, Peres A, Gallori S, Vincieri FF. Determination of myricetin derivatives in Chrysobalanus icaco L(Chrysobalanaceae). Braz J Pharmacogn 2006; 16:333-7. [ Google Scholar]
- Castilho R, Kaplan MA. Phytochemical study and antimicrobial activity of Chrysobalanus icaco. Chem Nat Comp 2011; 47:436-7. [ Google Scholar]
- El‐Neweshy M, El‐Maddawy Z, El‐Sayed Y. Therapeutic effects of date palm (Phoenix dactylifera L) pollen extract on cadmium‐induced testicular toxicity. Andrologia 2013; 45:369-78. doi: 10.1111/and.12025 [Crossref] [ Google Scholar]
- Tatar T, Akdevelioğlu Y. Effect of Pollen, Pit Powder, and Gemmule Extract of Date Palm on Male Infertility: A Systematic Review. J Am Coll Nutr 2018; 37:154-60. doi: 10.1080/07315724.2017.1364183 [Crossref] [ Google Scholar]
- Desoukey SY, Bishr M. Comparative Study of the Nutritional Value of Four Types of Egyptian Palm Pollens. J Pharm Nut Sci 2012; 2:50-6. [ Google Scholar]
- Hassan HM. Chemical composition and nutritional value of palm pollen grains. Global J Biotechnol Biochem 2011; 6:1-7. [ Google Scholar]
- Abedi A, Parviz M, Karimian S, Sadeghipour Rodsari H. The effect of aqueous extract of Phoenix dactylifera pollen grain on sexual behavior of male rats. J Phys Pharm Adv 2012; 2:235-42. [ Google Scholar]
- Mehrabani D. Effect of palm pollen extract on sexual hormone levels and follicle numbers in adult female BALB/c mice. Horizon Med Sci 2014; 20:139-43. [ Google Scholar]
- Hassan WA, El-kashlan AM, Mohamed NA. Egyptian date palm pollen ameliorates testicular dysfunction induced by cadmium chloride in adult male rats. J Am Sci 2012; 8:659-69. [ Google Scholar]
- El-Kashlan AM, Nooh MM, Hassan WA, Rizk SM. Therapeutic potential of date palm pollen for testicular dysfunction induced by thyroid disorders in male rats. PloS one 2015; 10:1-23. doi: 10.1371/journal.pone.0139493 [Crossref] [ Google Scholar]
- Faleh BH, Sawad AA. Effect of palm pollen grains extracts (Phoenix dactylifera L) on spermatogenic activity of male rabbits. Basrah J Date Palm Res 2006; 5:1-10. [ Google Scholar]
- Bahmanpour S, Talaei T, Vojdani Z, Panjehshahin M, Poostpasand A, Zareei S. Effect of Phoenix dactylifera pollen on sperm parameters and reproductive system of adult male rats. Iran J Med Sci 2015; 31:20-212. [ Google Scholar]
- El-Far AH, Shaheen HM, El-Daim MA, Al Jaouni SK, Mousa JSA. Date palm (Phoenix dactylifera): protection and remedy food. Curr Trends Nutraceuticals 2016; 1:1-10. [ Google Scholar]
- Marbeen M, Al-Snafi AE, Marbut M, Allahwerdy I. The probable therapeutic effects of date palm pollen in the treatment of male infertility. Tikrit J Pharm Sci 2005; 1:30-5. [ Google Scholar]
- Valdés L, Cuervo A, Salazar N, Ruas-Madiedo P, Gueimonde M, González S. The relationship between phenolic compounds from diet and microbiota: impact on human health. Food Funct 2015; 6:2424-39. doi: 10.1039/C5FO00322A [Crossref] [ Google Scholar]
- Rasouli H, Farzaei MH, Mansouri K, Mohammadzadeh S, Khodarahmi R. Plant cell cancer: may natural phenolic compounds prevent onset and development of plant cell malignancy? A literature review. Molecules 2016; 21:1104-30. doi: 10.3390/molecules21091104 [Crossref] [ Google Scholar]
- Hasler CM. Functional foods: benefits, concerns and challenges—a position paper from the American Council on Science and Health. J Nutr 2002; 132:3772-81. doi: 10.1093/jn/132.12.3772 [Crossref] [ Google Scholar]
- Luo C, Zou X, Li Y, Sun C, Jiang Y, Wu Z. Determination of flavonoids in propolis-rich functional foods by reversed phase high performance liquid chromatography with diode array detection. Food Chem 2011; 127:314-20. doi: 10.1016/j.foodchem.2011.01.006 [Crossref] [ Google Scholar]
- Ortega R. Importance of functional foods in the Mediterranean diet. Public Health Nutr 2006; 9:1136-40. doi: 10.1017/S1368980007668530 [Crossref] [ Google Scholar]
- David AVA, Arulmoli R, Parasuraman S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn Rev 2016; 10:84. doi: 10.4103/0973-7847.194044 [Crossref] [ Google Scholar]
- Kushi LH, Lenart EB, Willett WC. Health implications of Mediterranean diets in light of contemporary knowledge 1 Plant foods and dairy products. Am J Clin Nutr 1995; 61:1407S-15S. [ Google Scholar]
- Zeng Y-W, Du J, Pu X-Y, Yang J-Z, Yang T, Yang S-M. Coevolution between human’s anticancer activities and functional foods from crop origin center in the world. Asian Pac J Cancer Prev 2015; 16:2119-28. [ Google Scholar]
- Aghajanpour M, Nazer MR, Obeidavi Z, Akbari M, Ezati P, Kor NM. Functional foods and their role in cancer prevention and health promotion: a comprehensive review. Am J Cancer Res 2017; 7:740. [ Google Scholar]
- Mayne ST, Playdon MC, Rock CL. Diet, nutrition, and cancer: past, present and future. Nat Rev Clin Oncol 2016; 13:504. doi: 10.1038/nrclinonc.2016.24 [Crossref] [ Google Scholar]
- Kumazawa S, Kubota S, Yamamoto H, Okamura N, Sugiyamab Y, Kobayashia H. Antiangiogenic activity of flavonoids from Melia azedarach. Nat Prod Commun 2013; 8:1719-20. [ Google Scholar]
- Abbaszadeh H, Ebrahimi SA, Akhavan MM. Antiangiogenic activity of xanthomicrol and calycopterin, two polymethoxylated hydroxyflavones in both in vitro and ex vivo models. Phytother Res 2014; 28:1661-70. doi: 10.1002/ptr.5179 [Crossref] [ Google Scholar]