BioImpacts. 8(1):59-75.
doi: 10.15171/bi.2018.08
Review
Combating atherosclerosis with targeted nanomedicines: recent advances and future prospective
Ailar Nakhlband 1, †, Morteza Eskandani 1, †, Yadollah Omidi 1, 2  , Nazli Saeedi 1, Samad Ghaffari 4, Jaleh Barar 1, 2, *
, Nazli Saeedi 1, Samad Ghaffari 4, Jaleh Barar 1, 2, *  , Alireza Garjani 1, 3, *
, Alireza Garjani 1, 3, *
Author information:
1Research Center for Pharmaceutical Nanotechnology, Biomedicine Institute, Tabriz University of Medical Sciences, Tabriz, Iran
2Department of Pharmaceutics, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
3Department of pharmacology, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
4Cardiovascular Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
† These authors are contributed equally to this work and should be considered as co-first authors.
Abstract
Introduction:
Cardiovascular diseases (CVDs) is recognized as the leading cause of mortality worldwide. The increasing prevalence of such disease demands novel therapeutic and diagnostic approaches to overcome associated clinical/social issues. Recent advances in nanotechnology and biological sciences have provided intriguing insights to employ targeted Nanomachines to the desired location as imaging, diagnosis, and therapeutic modalities. Nanomedicines as novel tools for enhanced drug delivery, imaging, and diagnosis strategies have shown great promise to combat cardiovascular diseases.
Methods:
In the current study, we intend to review the most recent studies on the nano-based strategies for improved management of CVDs.
Results:
A cascade of events results in the formation of atheromatous plaque and arterial stenosis. Furthermore, recent studies have shown that nanomedicines have displayed unique functionalities and provided de novo applications in the diagnosis and treatment of atherosclerosis.
Conclusion:
Despite some limitations, nanomedicines hold considerable potential in the prevention, diagnosis, and treatment of various ailments including atherosclerosis. Fewer side effects, amenable physicochemical properties and multi-potential application of such nano-systems are recognized through various investigations. Therefore, it is strongly believed that with targeted drug delivery to atherosclerotic lesions and plaque, management of onset and progression of disease would be more efficient than classical treatment modalities.
Keywords: Atherosclerosis, Cardiovascular diseases, Drug delivery, Nanomedicines, Drug targeting
Copyright and License Information
© 2018 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Cardiovascular diseases (CVDs) are one of the foremost causes of death worldwide, and its incidence is dramatically rapidly growing, in large part due to the lifestyle and the increase of the population of elderly people.
1,2
In spite of global research and advances in the preventive and therapeutic modalities, CVDs will account for more than 23.6 million of deaths worldwide by 2030 according to the World Health Organization(WHO).
2
Although CVDs are complex multifactorial diseases, some important risk factors are considered as the leading causes. Among them, lifestyle-related factors (physical inactivity, poor nutrition/diet, obesity, smoking and passive smoking) play the most significant role in the prevalence of CVDs. Other health-related parameters such as high blood cholesterol levels, high blood pressure, diabetes mellitus, and metabolic syndrome and family history/genetics also correlated with the occurrence of CVDs.
3
Even with the current advancement of medical approaches, the increasing prevalence of CVDs urges early and accurate detection, on the top of more effective therapeutic modalities. Among CVDs, atherosclerosis (the so-called arterial wall chronic inflammation) is considered as the major cause of morbidity and mortality.
4
Atherosclerosis is characterized by the deposition of lipids via infiltration of inflammatory cells (e.g., T cells and circulating monocytes) and further formation of the atheromatous plaque, which mostly affect the tunica intima of the blood vessels (the innermost lining of the vessels).
5
It should be pointed out that a cascade of pathobiological events may result in the formation of atheromatous plaque and arterial stenosis (Fig. 1). However, due to the participation of various cells (e.g., intimal macrophages, monocytes and foam cells) in the atherosclerosis, the recognition of the most influential cellular entities as a treatment targets appears to be a very complicated issue. Recent advances in material science and the emergence of nanotechnology have offered new approaches using nanoscaled pharmaceuticals with the potential of specific targeting to the desired location for simultaneous imaging, diagnosis, and therapy - an approach so-called theranostics/diapeutics. It seems that the application of nanoparticles (NPs) containing active cargo in treatment of atherosclerosis might result in much more convergence in the improvement of human health. Although this field is still in its infancy, nanomedicines seem to provide much more enhanced drug delivery, imaging and diagnostic impacts against CVDs.
6,7
Nanoscaled drug delivery systems (DDSs) can improve the pharmacokinetic (PK) and pharmacodynamic (PD) properties, in large part because of greater accumulation in the diseased sites through passive and active targeting mechanisms. Further, the large ratio of surface area to volume enables NPs surface decoration via conjugation with various targeting, imaging, diagnosing and therapeutic agents.
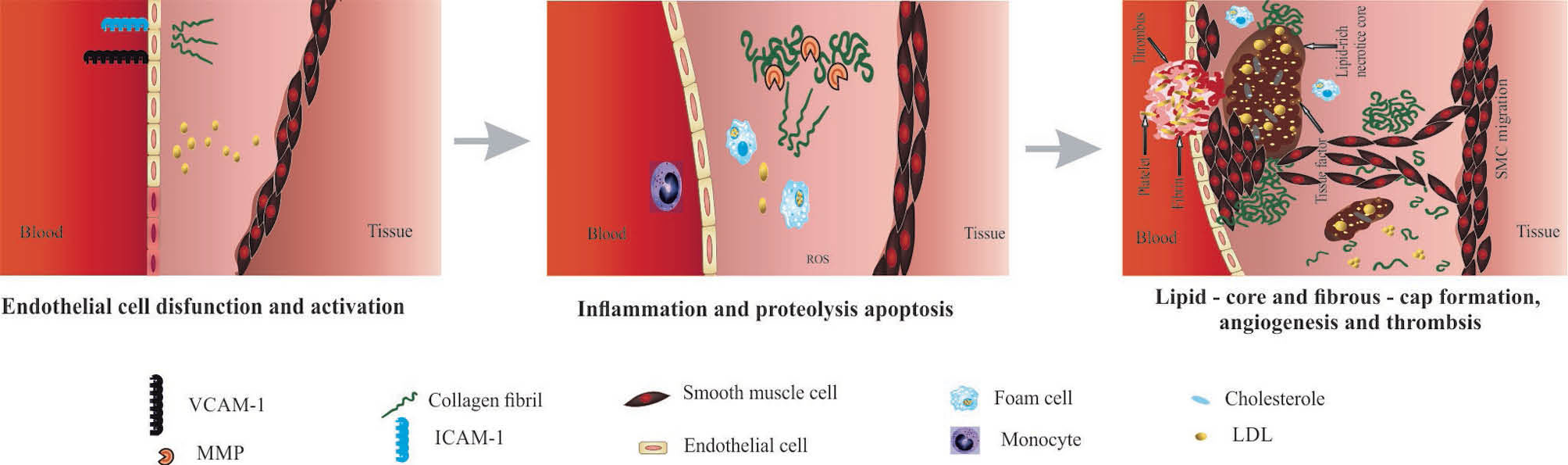
Fig. 1.
Atherosclerosis initiation and progression process.
.
Atherosclerosis initiation and progression process.
In the targeted delivery, specific homing agent such as monoclonal antibodies (mAbs) and their fragments (e.g., scFv, Fv, Fab), aptamers (Aps), peptides and low molecular weight compounds, which may recognize a tissue recognition ligand, is chemically conjugated to the surface of the NPs.
8
In the 1980s, the first attempts were performed to graft a specific Ab to the surface of a liposome to engineer immunoliposome in order to recognize a selected antigen on the target cell. In this context, the conjugation of a targeting agent (e.g., Abs, Aps or ligands) to a delivery system seems to be much more preferable because of their high specificity/affinity towards the overexpressed Ags on target cells. In fact, the active targeting is based on the receptor-mediated internalization of targeted NPs, while the biodistribution of non-targeted NPs depends on passive targeting to tissues is accomplished through the permeable microvasculature of diseased tissue/organ. It is known that the long circulation time of NPs and enhanced permeability and retention (EPR) effect are decisive for efficient passive targeting.
9
The EPR effect may occur in disease such as solid tumors and atherosclerosis. It is noteworthy that fewer side effects and empowered therapeutic impact using targeted drug delivery to treat most of the ailments such as CVDs and diabetes have been approved.
10
Some of the valuable targets for the CVDs include platelet/endothelial cell (EC) adhesion molecule-1 (PECAM-1), intercellular adhesion molecules (ICAMs), vascular cell adhesion molecules (VCAM-1).
11
Herein, we aimed to overview de novo nanomedicines and the nano-based strategies designed to fight the CVDs.
Nanomedicine emergence and drug delivery systems advancements
Delivery of drugs at a controlled rate and targeted manner is very attractive approach that results in maximum therapeutic impacts with minimum undesirable side effects. Nanopharmaceuticals have widely been explored for more than 50 years to overcome some drawbacks of conventional DDSs such as poor PK as well as reduced therapeutic effects with inadvertent induction of undesired side effects.
In this regard, a growing requirement exists for the development of novel DDSs with the potential to target site/cell-specific systems. Long blood-circulating NPs can enhance the residency of drugs in the blood and hence greater extravasation of NPs from vessels and accumulation of drugs in the diseased tissue/organ with leaky microvasculature. Once armed with a homing device, the targeted NPs can specifically/selectively penetrated into the designated tissue resulting in markedly decreased side effects.
12
Nanoscaled DDSs are prepared using various organic, inorganic, lipidic and polymeric biomaterials.
13
Various investigations have shown that NPs’ structural and physicochemical features (e.g., size, shape, surface charge, stability and surface modifications) can influence their in vitro and in vivo performance. For instance, large surface to volume ratio facilitates engineering multifunctional NSs. Furthermore, it is noteworthy that the shape and surface charge of NPs may affect (i) the penetration of NPs throughout the blood-tissue barriers, (ii) organ biodistribution, and (iii) cellular uptake. There are evidence that surface charge of NPs modulates the permeability of the blood-brain barrier (BBB). In addition to the mentioned features, NPs can be surface-tuned to potentially remain in the blood circulation for an extended time and evade the opsonization and immune clearance. This feature may applicable through grafting some compounds onto the surface of NPs such as Polyethylene Glycol (PEG) - a technique so-called PEGylation.
14
NPs are classified based on various properties. Morphologically, they can categorized into nanospheres, nanotubes, dendrimers, and linear, block, and graft structures. Based on the physicochemical properties, they can be classified as stimuli-responsive (e.g,. pH, temperature) NSs, magnetic and stealth NPs. Finally, rooted in NP constituent materials, they can be classified as natural, synthetic, hybrid, or metal NPs.
At the outset, nanotechnology was applied for the targeted delivery of anticancer agents. However, over the years, its applications have been expanded to diagnosis and therapy of other types of diseases. Accordingly, at the beginning of the 21stcentury, the first reports about the use of multifunctional NPs for molecular imaging of CVDs has been reported.
15
It is now clear that nanomedicines tend to have significant impacts on the management of CVDs generally and atherosclerosis particularly. Some of the NSs applied for the diagnosis and therapy of CVDs are listed in Table 1.
Table 1.
Drug delivery systems for therapy and imaging of atherosclerosis
|
Nanocarrier
|
Cargo
|
Targeting moiety
|
Ligand
|
Purpose
|
Ref.
|
| Immunoliposomes |
Adult bone marrow Stem cells |
Anti-ICAM1 antibody |
ICAM1 |
In vitro stem cell delivery |
16
|
| Liposome |
iohexol |
Anti-ICAM1 antibody |
ICAM1 |
CT imaging |
17
|
| Liposome |
Gadolinium |
Anti-ICAM1 antibody |
ICAM1 |
MRI imaging |
18
|
| Calcium condensed LABL-TAT complexes |
- |
Peptide (cLABL) |
ICAM1 |
Gene delivery |
19
|
| PLGA-PEG |
- |
Peptide (cLABL ) |
ICAM1 |
In vitro targeted delivery |
20
|
| Polymer nanocarriers |
- |
Peptide [binding sequence of fibrinogen (γ3)] |
ICAM1 |
Targeted delivery |
21
|
| Monocrystalline magnetic NP |
- |
VHPKQHR peptide |
VCAM1 |
In vivo, MRI and optical imaging in apolipoprotein E-deficient mice |
22
|
| Liposomes |
siRNA |
Antibody(SAINT-O-Somes) |
VCAM1, E-selectin |
Drug delivery system |
23
|
| Impermeable polymer nano-carriers (PNC) |
Catalase, peroxidase, xanthine oxidase |
Anti-PECAM antibody |
Platelet- EC adhesion molecule-1 |
Enzymes delivery |
24
|
| Liposome |
Gadolinium |
|
|
Molecular imaging |
25
|
| PEG-liposomes |
NMRI relevant contrast agents |
Recombinant interleukin-10 |
Unknown |
Imaging |
26
|
| Micelles |
Anticoagulant drug (hirulog) |
Peptide CREKA (pentapeptide cysteine-arginine-glutamic acid-lysine-alanine) |
Clotted plasma proteins |
Targeted delivery |
27
|
Liposomes and proticles
(protamine-oligonucleotide NPs)
|
Gadolinium |
C-terminal globular domain of adiponectin |
Unknown |
Imaging |
28
|
| Paramagnetic NPs |
Rapamycin |
Peptidomimetic vitronectin antagonist |
αvβ3 integrin |
Targeted delivery |
29
|
| NPs |
Anti-inflammatory peptide Ac2-26, an annexin A1/lipocortin 1-mimetic peptide |
PLGA-PEG-polymer |
Targeting collagen-IV) |
Drug delivery |
30
|
| Cyclodextrin-based nanosponges |
Multi-effective heterocyclic compound, DB103(remodeller of vessels wall) |
- |
- |
L |
31
|
| RGD modified and PEGylated solid lipid NPs |
Puerarin |
RGD |
αvβ3 integrin |
|
32
|
| Poly DL-lactide-co-glycolide NP |
miRNA-126
|
|
Intracellular inhibitor of vascular endothelial growth factor signaling |
Prevention of restenosis after angioplasty |
33
|
| Acetalated β-cyclodextrin |
Rapamycin |
|
|
Atherosclerotic therapy |
33
|
| Mn-doped ZnSe quantum dots |
siRNA against Histone deacetylase 1 |
|
Peroxisome proliferator–activated receptor-γ |
Differentiation of human MSCs into cardiomyocytes |
34
|
Promising targets in targeted therapy of atherosclerosis
Atherosclerotic molecular markers (AMMs) are the major targets, which can be exploited for the development of targeted NPs conjugated with specific ligands in order to deliver imaging/therapeutic agents into the lesions by targeting designated AMM.
35,36
In the atherosclerotic plaques, cellular components are exposed to the circulation due to the high expression levels of certain molecules, by which they can be targeted by the NPs. Additionally, the intra-plaque components are accessible by NPs through the cellular gaps of the endothelial cells. In this regard, up regulatory cell receptors such as VCAM-1, ICAM-1, P-selectin, E-selectin, avb3-integrin over-expressed on the activated endothelia of the luminal wall of microvasculature are some of the promising targets.
37
High-density lipoprotein (HDL) is responsible for the modulation of inflammation. It can also be considered as a reverse cholesterol transporter, and hence as another potential target. It plays an important role in the transportation of cholesterol especially fat-laden macrophages from the peripheral cells to the liver. Hence, the functional presence of HDL is a vital factor in the targeted therapy of atherosclerosis.
38
In this line, PEGylating of HDL, which increases its plasma half-life and favors its anti-atherogenic functions, seems to be a promising strategy.
39
Junctional adhesion molecules (JAMs) are a family of glycoproteins localized on the intercellular junctions of polarized endothelial and epithelial cells, also are expressed by the circulating leukocytes and platelets. JAMs regulate the permeability and leukocyte extravasation; JAM-A is an important factor which may incorporate atherogenic conditions in the body for directing the inflammatory cells to the sites of atherosclerosis.
40
Altogether, JAM-A can be considered as a specific molecular marker for the fabrication of targeted NPs to combat CVDs. On the other hand, the non-cellular components of plaques such as extracellular matrix, and clotted plasma proteins seem to be more favorable targets for the targeted therapy of CVDs.
Nanoparticles as atherosclerosis prevention and treatment devices
Various types of nanocarriers have been developed to combat the atherosclerosis, some of which are discussed in the following sections.
Lipid-based nanoparticles
Various synthetic/natural lipid-based delivery systems such as solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs), and nanoliposomes have received great attention as nanoscaled DDSs.
41
Some potential advantages of lipid NPs are their simple large-scale production, relatively low toxicity and accessibility of the composition. These advantages have made lipid NPs industrially favorable. Different lipid components lead to the production of NPs with various properties and potentials both in vitro and in vivo.
42
In this section, we comprehensively overview different lipidic nanocarriers and their applications in combating the CVDs.
Liposomes
The liposomal formulation of doxorubicin, Doxil, is the first clinically approved PEGylated nanoliposome for the treatment of cancer. Doxil was approved by the US Food and Drug Administration in 1995 for the treatment of Kaposi’s sarcoma and later for some other types of cancers.
43
Liposomes, the most studied NPs among lipid-based NPs, are spherical vesicles fabricated using one or various types of phospholipids. Based on the lamellarity, liposomes are classified as uni-, oligo-, and multilamellar vesicles. With respect to their size, they are divided into 3 groups of small, intermediate, and large liposomes.
44
Liposomes provide an environment for the incorporation of both hydrophobic and hydrophilic agents in the lipid layer or the core, respectively. Moreover, they favor loading of polyanions, such as nucleic acids (DNA and RNA) due to the presence of cationic lipids.
45
The natural composition of liposomes grant them some favorable characteristics such as high biocompatibility, low immunogenicity and efficient drug protection from enzymatic degradation.
46
However, they possess low biological/physical half-life due to the phagocytosis by macrophages which is considered as the main drawback of these carriers as DDSs.
47
To tackle such issue, modifications with various natural and semi-synthetic polymers, peptides, or antibodies can be conjugated to the surface of liposomes. The most extensively applied manipulation is the conjugation of biodegradable PEG, a hydrophilic polymer, to the outer surface of liposomes to produce stealth carriers. The modified hydrophilic surface may prevent carriers from the opsonization and further immune clearance, resulting in 8–10 times prolongation of the plasma biological half-life.
48,49
Attachment of homing devices (e.g., Ab, Ap, etc.) to the free end of PEG chain may result in the formation of long blood circulation along with high target specificity and binding capability. Such drug-laden liposomes become target specific by the attachment of suitable proteins.
Joner et al reported the application of encapsulated prednisolone phosphate in PEGylated 3, 5-dipentadecyloxybenzamidine hydrochloride liposomes in atherosclerotic rabbits showing major suppression of in-stent neointimal growth. These liposomes were directed to bind to chondroitin sulfate proteoglycans by the attachment of GAGs. Chondroitin sulfate is expressed in the sub-endothelial matrix but not on the vascular ECs. Their results demonstrated that the fabricated liposomes possess less systemic side effects.
50
In another interesting study, Calin et al fabricated a VCAM-1 directed target-sensitive liposomes (TSL) containing Teijin, a CCR2 antagonist, to target initial inflammatory processes with decreasing adhesion and transmigration of monocytes as common critical events in atherosclerosis.
51
PEG-stabilized TSLs were prepared by a mixture of DOPE and DOPA, and a functionalized phospholipid anchor (Mal-PEG-DSPE) was conjugated to the surface of the liposome to couple with the VCAM-1 binding peptide. The prepared targeted liposomes were characterized by means of size, release patterns and serum-stability. Its functionality was validated based on binding to the target. The binding efficiency of TSL was quantified by flow cytometry using activated EC by a competitive binding assay. Results indicated substantial specific binding of targeted liposomes to the surface of the cells.
Moreover, the liposomal binding to VCAM-1 was investigated by the mass-sensitive technology of surface acoustic wave sensors using a SAM blue® device. Moreover, measurements of TSL interaction with the target using electrochemical biosensors revealed the existence of an efficient interface. They showed that the adhesion of monocytes to activated EC in the presence of targeted TSL consisting Teijin more enhanced compared to the non-targeted TSL and intact Teijin. In addition, the effect of Teijin-TSL on the adhesion of monocytes to the EC was evaluated in situ by pre-incubation of RAW 264.7 cells with a CCR2 antagonist to determine their adhesion and accumulation in the aortas of ApoE -/- mice. Their results revealed a higher binding to the aorta of ApoE -/- mice for TSL as compared to the non-targeted TSL.
Homem de Bittencourt et al introduced an endothelium-directed cyclopentenone (CP)- prostaglandins (PG)-based liposome (EDCPL) formulations termed as LipoCardium. They aimed at investigating its effects on the inflammation process and subsequently atherosclerosis.
52
They found that the negatively charged liposomes containing anti-VCAM-1 antibodies and PGA2 showed more anti-inflammatory effects on the male LDL receptor knockout (ldlr−/−) mice. It was confirmed in the control non-treated ldlr−/− mice that LipoCardium could impair death by myocardium infarction or stroke. Moreover, LipoCardium led to remission of vascular lesions and the sickness state of the animals and consequently extend their life to the elderly age, even under high-lipid diet. Some evidences suggest that these cellular effects result in a marked decrease in arterial wall thickness, neointimal hyperplasia, and lipid accumulation. Conclusively, the introduced LipoCardium was proved to be a well-established means for the cardioprotection due to its anti-inflammatory, anti-proliferative (and pro-apoptotic only to foam cells), anti-lipogenic and cytoprotection (via heat-shock protein induction) impacts.
Hosseini et al developed phosphatidylserine liposomes (PSLs) and evaluated their atheroprotective potentials as compared to apoptotic cells (ACs) on male apolipoprotein E-knockout (ApoE -/-) hypercholesterolemic mice.
53
Their findings revealed that PSLs lessened the progresion of atherosclerotic lesion in mice by 42% and the accumulation of macrophage by 47%, besides more than 50% attenuation occurred in the atherosclerosis markers such as CD4+, CD8+ and T-cell numbers in atherosclerotic lesions. In addition, the expression of inflammatory and adhesion molecules (MCP-1 and VCAM-1) and proinflammatory cytokines (IFN-γ, IL-17, and IL-18) were decreased in the same manner. In contrast, the expression of anti-inflammatory cytokines including TGF-β and IL-10, and plasma levels of IL-5 were increased. Data indicated that PSLs resembled apoptotic cells in the activation of atheroprotective peritoneal B1a lymphocytes by increasing the polyreactive IgM levels during atherosclerosis development and subsequently reduced local inflammation.
Valk et al pioneered clinical study of the prednisolone liposomal (LN-PLP) formulation in atherosclerosis.
54
To this end, they encapsulated prednisolone phosphate in a liposome and coated it with polyethylene glycol. After the establishment of the pharmacokinetic profile of LN-PLP in humans, they administered the formulations to patients with iliofemoral atherosclerosis. Presence of LN-PLP was confirmed by the isolation of plaque tissue macrophages for and stained with DAPI (cell nuclei), CD68 (macrophages) and PEG (LN-PLP coating) (Fig. 2). Moreover, therapeutic efficiency of LN-PLP in patients with CVDs was assessed via
18
fluorodeoxyglucose positron emission tomography (PET)/computed tomography (FDG-PET/CT) and dynamic contrast enhanced-magnetic resonance imaging (DCE-MRI) scans of their carotid arteries, and arterial wall inflammation of patients was recognized.
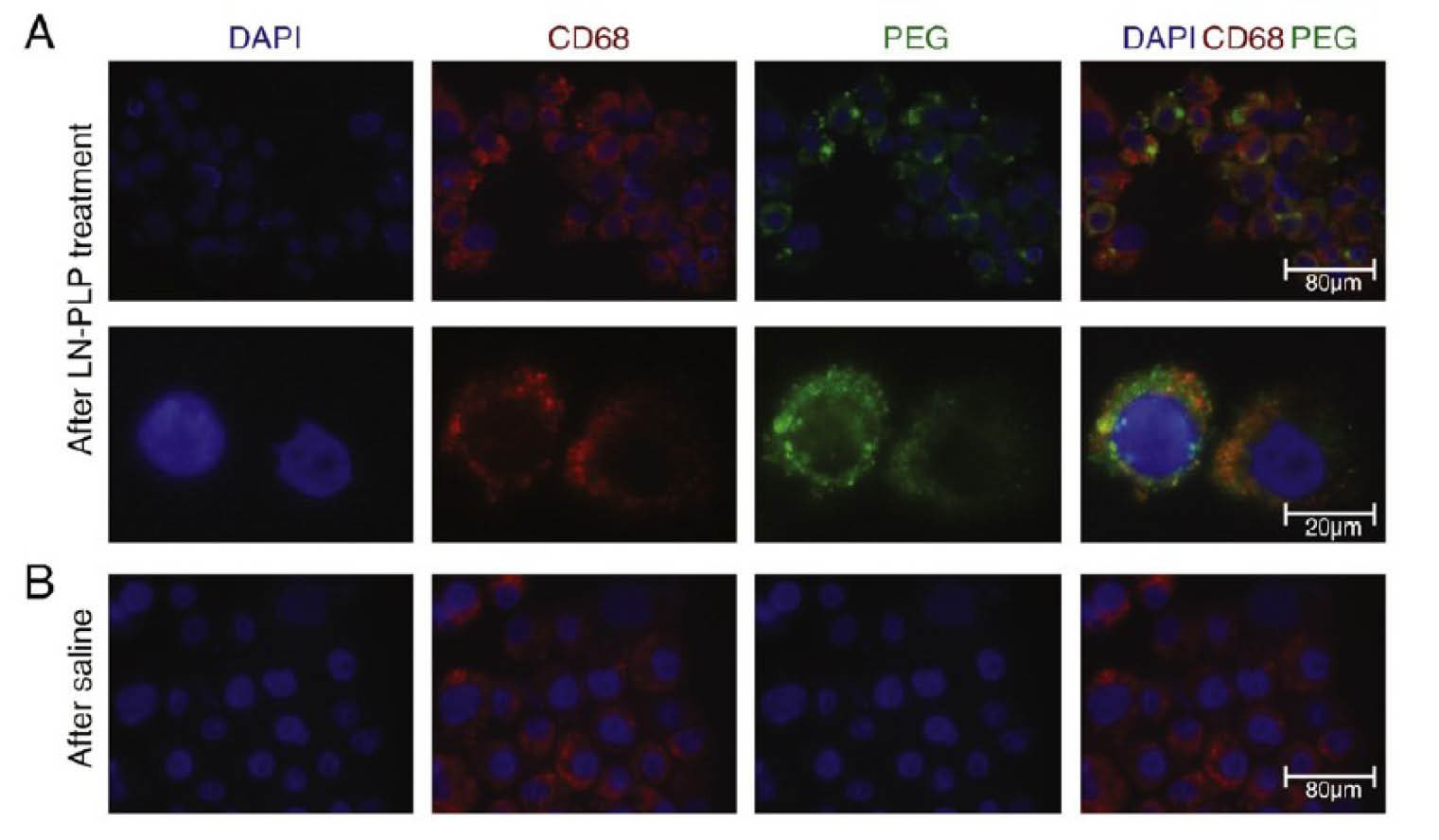
Fig. 2.
Results presenting that LN-PLP were accumulated in macrophages of iliofemoral plaques. DAPI positive cells isolated from plaques stained also positive for the macrophage marker CD68 and liposomal fluorescent PEG. For the detailed information please refer to the valuable article published by Valk et al.
54
.
Results presenting that LN-PLP were accumulated in macrophages of iliofemoral plaques. DAPI positive cells isolated from plaques stained also positive for the macrophage marker CD68 and liposomal fluorescent PEG. For the detailed information please refer to the valuable article published by Valk et al.
54
Analysis of LN-PLP efficacy in atherosclerotic patients demonstrated that its circulation time is long enough for delivery into atherosclerotic lesions with adequate accumulation in atherosclerotic plaques’ macrophages. However, in patients with atherosclerosis, short-term administration of LN-PLP did not affect arterial wall permeability or its inflammation. Conclusively, regardless of the lack of significant clinical impact, data emphasize the potential of such vehicle for drug delivery to atherosclerotic lesions in CVD.
Additionally, there are also reports of successful delivery of liposome-based formulations of cyclopentenone prostaglandins (CP), a powerful anti-inflammatory agent, and serum amyloid A (SAA) peptide fragments which have displayed significant anti-atherogenic effects in vivo.
55
Other lipidic nanoparticles
SLNs are biocompatible colloidal DDSs that are developed as a suitable candidate to improve bioavailability and bioactivity of lipophilic bioactive compounds.
56
They are routinely fabricated by three main methods, including (i) homogenization, (ii) solvent emulsification/evaporation, and (iii) microemulsion. Non-obligatory use of organic solvent during formulation makes SLNs more preferable to other lipidic carriers. Moreover, loading drugs into the matrix of solid lipid allows sustained release pattern of the loaded compounds. On the other hand, passive drug targeting and protection of entrapped compound against chemical degradation are the other advantages of the SLNs.
57
Kulandaivelu et al encapsulated tea polyphenols (TPPs) in solid lipid NPs (TPP-SLNs) to improve its stability and biological efficacy in CVDs.
58
TPP-SLNs showed prolonged free radical scavenging activity and more efficient overexpression of caspases-9 and -3 in vitro. Plasma hemoglobin, glucose, superoxide dismutase and catalase of Ehrlich's ascites carcinoma-bearing mice increased following oral administration of TPP-SLNs relative to free TPP. Furthermore, oral administration of TPP-SLNs led to significant decrease of other biochemical parameters such as cholesterol, bilirubin, triglyceride, urea, total protein, alanine aminotransferase, alkaline phosphatase and aspartate transaminase. All these reports indicate that the encapsulation of such unstable cardioprotective remedies in SLNs protects the agents and improves its biological efficiency.
Additionally, it is well established that the encapsulation of cardioprotective agents in SLNs protects them from an oxidation, enabling to be consumed as food additives. In this regard, our experiments revealed that when marrubiin was loaded into SLNs, its protective effects against tumor necrosis factors-α (TNF-α) induced oxidative stress and apoptosis in the human umbilical vein endothelial cells (HUVECs) were significantly higher than that of the intact compound.
59
In order to overcome the drawbacks of the oral administration of low molecular weight heparin (LMWH) in the treatment of vascular disorders like deep vein thrombosis (DVT) and pulmonary embolism (PE), Paliwal et al fabricated LMWH-lipid conjugates and encapsulated in phosphatidylcholine stabilized biomimetic SLNs.
60
Hematoxylin and eosin staining and microscopic studies validated the cytocompatibility of the formulated DDSs. They proposed the fabricated NPs as a safe formulation for an oral administration that increased the bioavailability of LMWH.
Gao et al also developed daidzein isoflavonoid SLNs to enhance its oral absorption and bioavailability.
61
The synthesized NSs with the size of 126 ± 14 nm and encapsulation efficiency of 82.5 ± 3.7% with sustain release pattern in vitro. It was observed that the circulation time for daidzein loaded SLNs increased in comparison with plain daidzein. Furthermore, daidzein SLNs could reduce the myocardial oxygen consumption and the coronary resistance more efficiently as compared to the plain drug in anesthetic dogs. A similar impact was observed in the cerebrovascular system as well. From the obtained data, it is evident that SLNs provide an effective drug delivery tool to improve the CVDs’ drug therapy limitations and bring new insights for the treatment of cardio-cerebrovascular diseases.
Lipid nanoemulsions (LNE) are another type of lipidic NPs, wherein liquid lipids are harbored in the composition.
62
They are considered as suitable alternative DDSs with good potentials such as sustained release, targeted delivery, and reduced toxicity. Therapeutic agents can be accommodated in the interior oil phase or in the oil-water interface of the particles. Altogether, LNEs provide an effective means of delivery for unstable drugs with poor aqueous solubility.
63
Tavares et al evaluated the ability of cholesterol-rich LNE associated etoposide on the reduction of lesions and inflammatory processes in atherosclerotic rabbits.
64
Based on their findings, the lesion areas of cholesterol-fed animals reduced by 85% and the intima width reduced by 50% upon treatment with LNE associated etoposide. Furthermore, this therapeutic modality decreased lipoprotein receptors, pro-inflammatory factors, and proliferation markers. Therefore, LDE associated etoposide offers the potential for the treatment of atherosclerotic CVDs.
Following to Tavares’s report, Bulgarelli et al evaluated the effect of LNE nanoemulsions of didodecyl-methotrexate (ddMTX) on atherosclerotic lesions and expression of pro-inflammatory and anti-inflammatory factors.
65
It was observed that the size of the lesions decreased by 65% and the intima-media ratio decreased by 2-fold following LNE-ddMTX treatment. Moreover, LNE-ddMTX reduced the intimal macrophage (67%) and apoptotic cells (88%). No effects on smooth muscle cells migration into the intima were observed. Moreover, LNE-ddMTX downregulated 6 pro-inflammatory genes, including TNF-α, MCP-1, IL-1β, IL-18, MMP-9, and MMP-12 and upregulated the anti-inflammatory IL-10 gene in vivo, and TNF-α IL1-β VAP-1, TLR2 and CXCL2 in vitro. It is deduced that the association of anti-blastic agents with LNE offers an effective strategy to reduce the undesired cytotoxicity and increases the therapeutic efficiency on fighting CVDs.
Furthermore, Leite et al applied a combination therapy of methotrexate and etoposide associated LNE to improve their anti-atherosclerosis effects in comparison with a single agent.
66
This strategy presents a great potential for the clinical application in patients with CVDs.
The NLCs are another lipidic nanocarriers that attract the attention of investigators and have been used as CVDs drug delivery. They are formulated similarly to SLNs with the substitution of solid lipid with liquid forms. They also offer extensive applications as DDSs with improved drug loading capacity, protection and also smaller size in comparison with their solid counterparts.
67
Zhang et al developed Tanshinone IIA-loaded HDL-like NLC (TA-NLC) by using a nanoprecipitation/solvent diffusion method.
68
Spherical TA-NLC incorporated drug in lipid core and formed a shell-core structure. In vitro studies by agarose gel electrophoresis and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) showed that TA-NLC could bind to apolipoprotein A-I (apoA-I) specifically. Following phagocytosis studies documented that TA-NLC cannot trigger immunological responses and could escape from the opsonization, and hence can serve as an effective tool for CVDs. In an interesting study, two physically distinct forms of NLCs, i.e., discoidal and spherical recombinant HDL loaded with tanshinone IIA (TA) (TA-d-rHDL and TA-s-rHDL) were fabricated.
69
The PK studies showed that both TA-d-rHDL and TA-s-rHDL could distinctly improve the PK patterns of TA in vivo. Ex vivo imaging showed that both d-rHDL and s-rHDL have greater tendencies to atherosclerotic lesions than normal vessel walls, in which s-rHDL showed more precise impacts. Additionally, both TA-d-rHDL and TA-s-rHDL revealed much stronger anti-atherogenic efficacy than usual TA-NLC, TA liposomes (TA-L) and commercially available preparation sulfotanshinone sodium injection (SSI), while TA-s-rHDL presenting greater impact. Therefore, recombinant HDL could be designed as a potent NS for delivery of tanshinone IIA, targeting the atherosclerotic lesions, and finally improving the CVDs.
Polymeric nanocarriers
Polymeric nanocarriers, as another type of drug delivery NSs, are consist of various synthetic or natural macromolecules forming different structures, including polymeric micelles and polymeric solid NPs.
70
Similar to the other nanocarriers, different moieties can be conjugated to the surface of NP through active functional groups of polymers. A large number of biocompatible synthetic polymers such as poly(lactic-co-glycolic acid), poly(vinylpyrrolidone), poly(L-lysine), poly(L-glutamic acid), poly(malic acid), poly(aspartamides), and natural polymers including cellulose, alginate, gelatin, chitosan are specifically have been used in the fabrication of polymeric NPs.
71
Owing to the existence of various types of functional groups on the structure of polymers, the physicochemical features of polymeric NPs can be tailor-made based on the requirement of DDSs for efficient delivery and higher drug loading capacity, and physical stability. Moreover, smart and stimuli-responsive polymeric NPs can be engineered following a surface modification. To provide an instance, various pH-sensitive polymeric NPs have been fabricated to deliver and release their cargo in a sire specific manner due to the acidic tumor microenvironment.
71,72
Alike the other types of nanocarriers, polymeric NPs could also be PEGylated to improve their physical and biological performances. In the recent decades, considerable efforts have been devoted to the effective therapy of atherosclerosis via polymer-based targeted nanomedicines. Herein, some of the advancements in this field are summarized as follows.
Poly(lactic-co-glycolic acid)
Poly(lactic-co-glycolic acid) (PLGA) is an extensively used biodegradable and biocompatible polymer. It is applied to the fabrication of DDSs, incorporating a wide range of molecules -hydrophilic or hydrophobic small molecules or macromolecules- with potential for controlling the release of drug contents. Moreover, functional groups on the surface of PLGA-NPs could assist the surface modification of the particles for more efficient and targeted drug delivery. Above all, the FDA approval is the foremost reason that it has become the focus of increasing research in various drug delivery investigations, including cardiovascular drug delivery researches.
73
Until now, different investigations have been focused on delivering of the cargo to the atherosclerosis lesions in the spatial and temporal manner using PLGA NPs, whose some of applications are concisely discussed.
An investigation conducted by Feng et al aimed to introduce a novel device for effective/sustained delivery of paclitaxel to treat cardiovascular restenosis, and hence, to overcome the shortcomings of a free drug such as poor solubility and inadvertent side effects.
74
Paclitaxel as an antiproliferative agent may have potential application in many diseases associated with excessive cell proliferation including cardiovascular restenosis. They fabricated paclitaxel-loaded PLGA NPs using D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) and poly (vinyl alcohol) (PVA) as emulsifiers by solvent extraction/evaporation method. The NPs size and surface morphology were respectively determined by laser light scattering and field-emission scanning electron microscopy (FESEM). They evaluated cellular uptake of NPs by laser scanning spectroscopy in vitro and in vivo. It was shown that cellular uptake after 6 h was higher in the case of TPGS-emulsified NPs compared to PVA-emulsified. All data approved that, NPs formulated with TPGS emulsifier showed higher drug encapsulation, cellular uptake and cytotoxicity as compared to the PVA emulsified NPs. Moreover, MTS assay results conducted to study the cytotoxicity of treatments showed that TPGS emulsified NPs containing paclitaxel presented higher toxicity as compared to the plain drug and PVA emulsified NPs. Therefore, it is proposed that NPs formulated using TPGA can be utilized in developing cardiovascular stents for the local drug delivery and prevention of restenosis.
In another study, Golub et al designed an injectable controlled release system of PLGA NPs encapsulating VEGF by a modified double W/O/W emulsion and used them to induce angiogenesis and arteriogenesis.
75
The NPs’ size was about 400 nm with an effective loading of 5.3%. Hind-limb ischemia surgery and injection of treatments (VEGF-NPs, VEGF, NPs, saline) into ischemic thigh adductor muscles were performed. Subsequently, micro-computed tomography (Micro-CT) system was applied to scan hind-limb. The results demonstrated that total vessel volume and connectivity of VEGF-NPs treated mice increased significantly in comparison with the mice received the same amount of VEGF alone or saline. Finally, immunohistochemistry analysis showed that VEGF-NPs injected hind-limbs have greater vessel numbers than the saline-treated hind-limbs. Taken all, the study suggested that sustained release delivery strategies are more beneficial in the treatment of atherosclerosis at lower overall doses in comparison with the delivery of pure vasculogenic proteins.
Sanchez-Gaytan et al applied hybrid polymer/HDL NPs to target atherosclerosis plaque macrophages.
76
They encapsulated PLGA in lipid/apolipoprotein coating and fabricated HDL-like (PLGA−HDL) NPs by microfluidics technology and stabilized with the attachment of ApoA-I. Moreover, phospholipid corona was applied to construct multifunctional PLGA−HDL NPs. The size of NPs was determined by dynamic light scattering (DLS) ranging from 88 to 156 nm and the charge was about −7.1 mV. The morphology evaluation of such PLGA−HDL NPs with transmission electron microscopy showed a spherical structure similar to that of mature HDL. Sustained release property of the NSs was approved, in which only around 60% of the Nile red dye was released after 24 hours, and 90% of release occurred after 5 days at 37°C. The MTT assay results showed no particle related cytotoxicity after 24 hours incubation of PLGA−HDL NPs or the corresponding amount of PEG−PLGA NPs. In addition, targeting with rhodamine-labeled PLGA−HDL showed selective uptake of PLGA−HDLNPs by macrophages. The level of cholesterol efflux suggested that PLGA−HDL NPs are able to serve as cholesterol acceptors and show biomimetic HDL features. Ex vivo near-infrared (NIR) fluorescence imaging demonstrated liver and spleen clearance of PLGA−HDL in ApoE knockout mice, and also showed the heterogeneous uptake of PLGA−HDL NPs along the aorta. Moreover, localization of PLGA−HDL NPs with CD68+staining revealed the specific interaction of NPs with macrophages. Conclusively, it was proved that the synthetic PLGA−HDL NPs could favorably interact with macrophages and monocytes within the aorta. The applied method introduced these multifunctional NPs with diagnostic, therapeutic, and atherosclerosis-targeting properties.
Chitosan
Chitosan, as a natural biocompatible polymer, has widely been used for drug delivery purposes. Its biodegradability, biocompatibility, and ease of modification have attracted increasing attention for its wide applications. Hydration of chitin and elimination of its acetate moiety results in the formation of chitosan. Its valuable capacities (e.g., slow/controlled drug release, enhanced solubility, stability and reduced toxicity) make it an attractive candidate for drug delivery applications. Furthermore, its nanosized formulations are able to cross through biological barriers. Further, surface modifications of this polymer can lead to the production of multifunctional NSs and improved drug targeting. Moreover, complexation of the amino and carboxyl groups of the chitosan molecule with the glycoprotein of the mucus forms a hydrogen bond that contributes to the adhesive property.
77
On the other hand, chitin and chitosan possess well-established antioxidant activity, which makes them attractive candidate as a drug carrier in CVDs.
78
In this context, Yu et al led a research on evaluating the effects of chitosan oligosaccharides (COS) on atherosclerosis’ plaque stability in apolipoprotein E deficient mice (apoE-/-).
79
After administration of COS to apo E-/- mice, aortas were subjected to photography by which COS anti-atherosclerotic properties were confirmed. Lipid (Oil red O) staining, Masson’s staining, or hematoxylin and eosin staining were applied for plaque morphological/histomorphometric evaluation. The results further approved that COS treatment favors the plaque stability. Immunostaining of macrophages showed no changes in the collagen content and matrix metalloproteinase-9 (MMP-9) levels in the plaques of COS-L (250 mg/kg/d of COS) group and in the COS-H (1000 mg/kg/d) treated groups that demonstrated a beneficial effect on the plaque stability. Lipid profile assessment and western blot analysis of blood samples in COS treated mice revealed decreased plasma cholesterol and triglyceride, ApoB 100 and ApoB48, respectively. Furthermore, the plasma level of pro-inflammatory cytokines, i.e. TNF-α and interleukin 6 (IL-6), did not show any changes upon the administration of COS, which may show no anti-inflammatory effects for this treatment modality. Further, the real-time PCR and western blot analyses of tissue or cultured cells (HepG2 or RAW264.7 cells) showed overexpression of hepatic lipoprotein receptor and scavenger receptor BI (SR-BI). In addition, increased expression of ABCA1 and SR-BI in peripheral macrophages of apoE-/- mice fed with HF diet were reported. As a result, it was claimed that ABCG5 and ABCG8 genes in the small intestine could be overexpressed, which illustrated that the COS could stimulate the elimination of cholesterol from the erythrocytes by ABCG5/8 pathway. In conclusion, it was demanded that the COS treatment could inhibit the atherosclerosis induced by a high-fat diet and further increases the plaque stability in apo E-/- mice.
In another interesting study, Xiying et al used chitosan NPs for developing DNA vaccine against atherosclerosis.
80
They loaded cholesteryl ester transfer protein (pCETP) in chitosan nanocarriers for maximum vaccine protection and attenuation of atherosclerosis. The physicochemical characterization of the synthesized NPs revealed that the average size of NPs was 340.2 ± 14.6 nm with a low polydispersity index (0.22 ± 0.03) showing congruent particles. DNase I protection analysis showed that chitosan nanocarrier could effectively protect pCETP from the degradation at a final concentration of 100 unit/mL. Male New Zealand white rabbits (cholesterol-fed atherosclerosis model) were immunized with chitosan/pCETP NPs intranasally (i.n.) or intramuscularly (i.m.). Serum anti-CETP Ab was measured by ELISA using anti-CETP Ab, and also evaluated by western blot analysis. It was demonstrated that the anti-CETP IgG levels of the i.n. immunized group were higher than those of i.m. group, though it was not significant statistically. Further, they showed that the i.n immunization using chitosan/pCETP NPs can stimulate more specific Abs without affecting its immunogenicity. The analysis of plasma lipids and lipoproteins (i.e., total cholesterol, HDL-C and LDL-C) showed no significant differences between the i.n. and the i.m. groups. Afterwards, harvested aortas and coronary arteries of the rabbits were subjected to atherosclerotic lesion histopathological analysis. Histopathological analysis of aortic arch and coronary artery showed that intimal thickening in coronary artery occurred in all three groups (i.e., i.n., i.m. and saline control) of rabbits. However, intimal thickening in the coronary artery of the saline control group was more noticeable. Moreover, the formation of foam cell and inflammatory cell infiltration were observed in the coronary artery from the saline control group, while the coronary artery of i.n. and i.m. groups underwent little intimal thickening and pathological change. Conclusively, chitosan/pCETP NPs were found to be effectively able to provoke anti-CETP Abs, control the plasma lipoprotein profile and slow down the process of atherosclerotic plaques formation in rabbits, and hence, may provide a novel platform as nasal drug/vaccine delivery system.
In another study, Hong et al encapsulated epigallocatechin gallate (EGCG) (the major bioactive compound in green tea with known beneficial antioxidant effect) into self-assembled NPs of chitosan (CS) and poly aspartic acid (PAA). Their main aim was to protect EGCG from the harsh environment of the gastrointestinal tract.
81
The CS-PAA NPs were fabricated by mixing a positively charged CS/acetic acid solution with a negatively charged PAA solution at room temperature with the CS/PAA weight ratio of 1:1. Further, the synthesis of EGCG-CS-PAA NPs was carried out by mixing a positively charged CS acetic acid solution containing EGCG and a negatively charged PAA solution at room temperature. Physicochemical characterization revealed that NPs are spherical in shape and their size is directly proportional to CS and PAA molecular weight. This pattern was also observed in the values of polydispersity index (PDI). Additionally, the stability of EGCG-CS-PAA NPs at different pHs showed that the small NPs aggregated or reassembled into the larger ones with an increase of pH. The release of EGCG from NPs was measured in different pHs (i.e., 2.5, 4.0, 6.6, 7.0 and 7.4). The stability of EGCG-CS-PAA NPs in the simulated gastric and intestinal media revealed that pH increase could lead to NPs instability and EGCG release. The EGCG-CS-PAA NPs administered to male New Zealand white rabbits orally after high-fat diet and induced atherosclerosis. Animal studies proved that toxic reactions were not triggered by the nanoformulation of EGCG in the rabbits. Furthermore, EGCG NPs efficiently decreased the blood lipid levels in comparison with the free EGCG. Seemingly, they have established a new pH-responsive formulation of EGCG to improve its stability and efficiency. The engineered NPs seem to hold a great promise for treatment of the atherosclerosis.
Cellulose
Among nature gifted materials; cellulose has been shown to offer promising potential as a drug nanocarrier. Cellulose is obtained from various sources, including wood, cotton, hemp, flax, wheat straw, sugar beet, potato tuber, mulberry, bark, ramie, and algae. Different types of nanocelluloses include cellulose nanocrystals (CNCs), cellulose (nano) whiskers, cellulose nanofibrils (CNFs)also called nanofibrillated cellulose (NFC), cellulose nanofibers, and bacterial cellulose nanocomposites (BCNs).
82
It is clear that this diversity in cellulose nanostructures is related to its source material, and the extraction process. This natural nanoscaled material possesses some exclusive characteristics, including special morphology and geometrical dimensions, crystalline structure, high specific surface area, rheological properties, liquid crystalline behavior, alignment and orientation, mechanical reinforcement feature, barrier properties, surface chemical reactivity, biocompatibility, biodegradability and no/little toxicity.
83
A number of investigations have been carried out to fabricate cellulose based nanomedicines for the treatment of CVDs. Li et al applied poly (L-lactic acid) (PLLA) and cellulose acetate butyrate (CAB) carriers for borneol (a natural compound for the treatment of cerebrovascular diseases) to overcome its restrictions such as easy sublimation and, low water solubility. Three types of the spinning solution with various concentrations of the CAB (30%, 50%, and 70%) were prepared.
84
They prepared CAB/borneol composite film (thickness of 200 µm) and also pure PLLA nanofibers. Subsequently, borneol/acetone solution was sprayed on PLLA/CAB fibers to load borneol into a PLLA/CAB composite membrane with an electrospinning process. Morphology characteristics of electrospun PLLA/ CAB fibrous membranes and borneol-loaded samples were evaluated by a FESEM. Drug release analysis showed that more than 80% of borneol was left in the CAB/borneol film; hydrogen bonds and also the solid film may prevent the diffusion of borneol molecules. However, in the case of pure PLLA, more than 70% of the borneol was released in the first hour due to the weaker interaction between PLLA and borneol. For PLLA/CAB (30%) membrane, the release of borneol decreased in comparison with pure PLLA. In the case of PLLA/CAB (70%), more than half of the borneol was left in the membrane owing to the low porosity. In conclusion, it was suggested that adjustable drug release properties of PLLA/CAB nano-fibrous composite nonwoven membranes make it a promising candidate and novel drug vehicle for CVDs.
Gelatin
Gelatin is derived from collagen and widely used in pharmaceutical and medical applications, in large part due to its biodegradability and biocompatibility. Moreover, the ease of modification and crosslinking make gelatin a favorable candidate of drug delivery with improved stability and physicochemical characteristics.
85
Zhang et al applied gelatin–siloxane (GS) NPs to deliver nitric oxide (NO) to vascular cells in order to regulate the vascular cell behavior and prevent restenosis.
17,86
Acid orange 7 (AO7) assay was applied to quantify amino groups on GS NPs. After preparing NO-releasing S-nitrosothiol (RSNO) modified GS NPs (GS-NO NPs), they were subjected to size determination. The size of NPs was in the range of 130–800 nm and the average diameter was about 300 nm. The morphology of GS and GS-NO NPs was studied by FESEM through which spherical shape with a granular surface of NPs was observed. Additionally, the NPs surface chemistry was determined by Fourier transform infrared (FTIR) analysis. No obvious toxicity was observed using Alamar Blue assay after 1 and 7 days for either GS or GS-NO groups. The nuclear staining and cell counting were conducted to study cellular proliferation in response to GS-NO, and the obtained results demonstrated a concentration-dependent regulation of proliferation. The cellular uptake of FITC-labeled GS-NO NPs revealed that particles could be internalized by the human aortic smooth muscle cells (AoSMCs) after 2h. Conclusively, they have introduced an efficient NO delivery system to prevent post-surgery restenosis. In other words, they showed that the new GS-NO NPs were able to release NO in a controlled-manner and inhibited the main processes of restenosis development (e.g., vascular smooth muscle cells’ over-proliferation).
In a similar study, Vogt et al prepared gelatin-based nanofibrous matrix with a light-responsive NO release property.
87
The matrix surface was functionalized with S-nitroso-N-acetyl-D-penicillamine (SNAP) to induce a light controllable release property. It appeared that the ion elimination simplified the development of a gelatin nanofibrous matrix. Morphological studies showed that none of the cross-linking and SNAP functionalization procedures caused any changes in the shape, resulting in an increase in the size and interstitial space of the fibers. However, the fiber diameters remained less than 1 μm anyway. The chemical structure characterization and effect of SNAP functionalization on the gelatin chemical structure were evaluated with an attenuated total reflectance-infrared spectroscopy (FTIR-ATR), which revealed that SNAP groups were covalently bonded to the gelatin molecules. Inductively coupled plasma (ICP) mass spectroscopy was utilized to define the ion content of the gelatin and the effect of purification process on the concentration of selected metal ions in the gelatin. It was observed that the ion concentrations was reduced significantly by the purification. Light-activated NO release was conducted with a 527 nm wavelength light-emitting diode and a multiple output voltage controller. Also, LED was in series with an additional 138 Ω resistor. Nanofibrous matrices showed a stable, well-regulated NO release profile during light exposure. Furthermore, the long-term NO release under physiological conditions was estimated by the Griess assay, and all of the gelatin matrices showed similar NO release pattern with a rapid NO generation within the first hour and a slower release rate in the remaining time. The antibacterial properties of gelatin nanofibrous matrix were evaluated against Staphylococcus aureus and revealed that the SNAP-gelatin created a zone of inhibition when light activates the NO release. Conclusively, novel nanofibrous gelatin matrix functionalized with NO-releasing molecules, SNAP showed an efficient light controllable release of NO. More to imply is that the removal of divalent metal ions from the gelatin resulted in a finer and more porous structure as well as an enhanced NO preserving capacity.
Kobayashi et al established erythropoietin (EPO)–gelatin hydrogel DDS and studied its effects on MI, left ventricular (LV) remodeling, and function.
88
The gelatin was extracted from pig skin (i.e., type I collagen) via an acid process and then was utilized in the formation of gelatin sheets containing EPO. The drug showed a continuous release from gelatin hydrogel patches for over 14 days. Cardiac tissue erythropoietin content after injection of intact EPO or application of gelatin hydrogel patch with and without EPO to the heart was measured by ELISA. Four groups of rabbit models of MI were subjected to the recombinant human EPO, EPO-DDS, EPO free gelatin hydrogel patch, and saline immediately after infarction. It was observed that LV end-systolic and end-diastolic dimensions were significantly smaller, whereas the LV ejection fraction, fractional shortening, and +dP/dt were significantly larger in the EPO-DDS group than in the saline, DDS, or EPO-systemic group. On the second day after MI, no difference was detected in terms of infarct size as a percentage of the area at risk through the infarct size in the EPO-DDS group is likely to be reduced in comparison with the saline group. A decrease in infarct size in EPO-DDS group observed on 14 days and further on 2 months post-MI. Fibrotic areas were significantly smaller in the EPO-DDS group than other groups on 14 days post-MI. An indirect immunoperoxidase method was applied for the immunohistochemical staining of cardiac sections. Through this, it was observed that on day 14 post-MI, the density of the CD31-positive microvessels (capillary density) was markedly greater in the infarct border zone in hearts of the EPO-DDS group than other groups. Additionally, an increase in expression of proMMP-1 was observed in the border zone and un-infarcted areas in the EPO-DDS group, but not in the EPO-systemic or saline group. Western blot analysis indicated that the myocardial expression of EPOR was significantly higher in hearts in the EPO-DDS group than other groups on day 2 post-MI. In the infarct border zone, significant increases in the levels of Stat3, Akt, ERK, and GSK-3b activation, which were observed as their phosphorylated forms (i.e., p-Akt, p-GSK-3b, p-Stat3, and p-ERK), indicated in hearts of EPO-DDS group as compared to those from the EPO systemic or saline group on day 2 post-MI. Additionally, on the 2 days after MI, the expressions of ProMMP-1, VEGF, and Bcl-2, were upregulated in hearts from the EPO-DDS group. On day 14 post-MI, the expressions of EPO-R, p-Akt, p-ERK, and pro-MMP-1 were increased, but VEGF, Bcl-2, Bcl-xL, p-STAT3, or p-GSK3b did not show any changes. In conclusion, it was appealed in this study that EPO-DDS, the post-MI treatment develops LV remodeling and function, most likely by the activating prosurvival signaling, anti-fibrosis and angiogenesis without causing any side effect.
Alginate
Owing to its physicochemical characteristics, alginate has widely been considered as a suitable drug delivery carrier. This anionic polymer is composed of α-L-guluronic acid (G) and β-D-mannuronic acid (M) residues, linearly linked by 1,4-glycosidic linkages and its properties are directly influenced by the composition and sequence of the G and M residues. Alginate is a non-toxic, cost-effective, highly available, biocompatible, nonimmunogenic polymer that favors the fabrication/encapsulating of various drugs. Furthermore, chemical modification of alginate can lead to more efficient and specific capacities in the fabrication of targeted NPs. It is noteworthy that no organic solvent is requisite for the preparation of alginate NPs, which makes it a valuable candidate for the encapsulation of sensitive materials. Alginate NPs are available in the forms of nanoagregates, nanocapsules, and nanospheres with sizes of 10 to 1000 nm. Alginate-based NSs can either attach to or entrap various compounds.
89
To combat CVDs, Ruvinov et al produced an injectable alginate matrix with heparin-binding potential.
90
To this end, they fabricated affinity-binding alginate containing hepatocyte growth factor (HGF), a promising agent to treat various ischemic CVDs, as microbeads. Constructed microbeads demonstrated sustain release pattern over time. Western blot analysis performed to determine MAPK activation that indicates bioactivity of the released HGF. It was exerted that released HGF induced high levels of ERK1/2 phosphorylation - the foremost downstream target of HGF signaling. In vitro oxidative stress and apoptosis assays (DAPI staining), demonstrated a markedly decline in the cardiac cell death within the samples treated with the released HGF. A murine model of hindlimb ischemia subjected to the injection of HGF in affinity-binding alginate solution and its control treatments. Significantly greater blood vessel density and vessel maturity are the indications of a proficient delivery system, which was found to provide temporal passive support and a microenvironment for more effective tissue repair with efficacious delivery. Taken all together, this study introduced a platform for the controlled and more predictable delivery patterns of therapeutic heparin-binding proteins for CVDs regenerative applications.
Metal nanoparticles
Nanosized metals within the dimension of 1-100 nm have widely been used for the biomedical sciences/applications. They could be modified by adding various chemical functional groups to facilitate their conjugation with various targeting and therapeutic moieties (e.g. antibodies and aptamers).
91
Among several metals, magnetic iron oxide (Fe3O4), gold, and silver NPs have made great impacts on medical sciences. Gold NPs (AuNPs) are colloidal gold or suspension of nanometer-sized particles of gold with reddish color for particles with the size of smaller than 100 nm. However, the properties and applications of AuNPs depend on its morphological shape and other physicochemical features. The AuNPs possess distinctive optical properties which make them an ideal candidate for wide applications in biomedicine, including bioimaging.
92
Roma-Rodrigues et al, designed peptides, which could selectively interact with angiogenesis cellular receptors, and conjugated them onto AuNPs.
93
Accordingly following to the synthesis of gold nanospheres, they were functionalized and stabilized with oligo-ethylene glycol (OEG) then three different peptides (activator, scramble, inhibitor) were anchored to OEG capped AuNPs. Ex vivo chorioallantoic membrane (CAM) assay was carried out to validate their potency in the in vivo angiogenesis. The CAM analyzes images showed in Fig. 3. Comparing the percentage of the newly formed arterioles with the control group revealed that inhibitor peptide-NP induced a clear reduction in the formation of new arterioles with regard to scramble peptide-NP. Conversely, activator peptide-NP markedly stimulated new arterioles formation. The results demonstrated the potency of AuNPs as efficient targeted DDSs which can induce neovascularization and improve the CVDs.
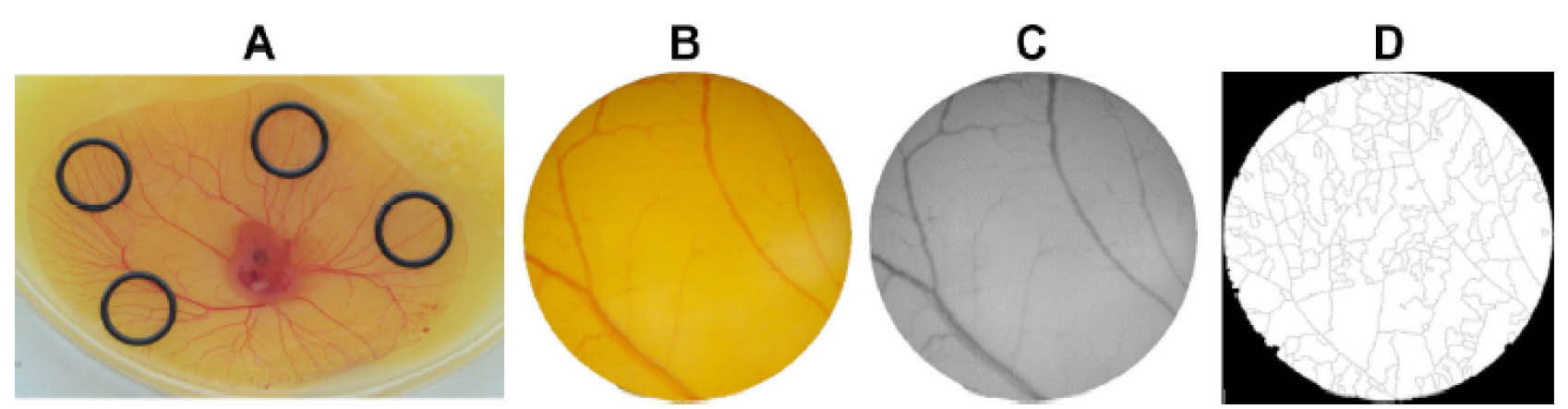
Fig. 3.
Ex vivo chorioallantoic membrane (CAM) assay. Panel A shows the position of the developing blood vessels plexus of the yolk sac membrane. Panel B & C demonstrate the image of O-ring interior used for counting the number of veins. Panel D is the binary of the segmented image utilized to calculate the number of branches. For the detailed information please refer to the valuable article written by Roma-Rodrigues et al.
93
.
Ex vivo chorioallantoic membrane (CAM) assay. Panel A shows the position of the developing blood vessels plexus of the yolk sac membrane. Panel B & C demonstrate the image of O-ring interior used for counting the number of veins. Panel D is the binary of the segmented image utilized to calculate the number of branches. For the detailed information please refer to the valuable article written by Roma-Rodrigues et al.
93
Silver NPs (AgNPs) have found increasing application in the biomedical field. They are comprised of nanosized silver particles (i.e., 1-100 nm). In the recent years, much attention has been paid to the incorporation of AgNPs into various medical devices (e.g., bone cement, surgical instruments, surgical masks, etc.). Moreover, some landmarks of AgNPs as the potent nanomaterials for use in the surface plasmon resonance (SPR) and large effective scattering cross-section make them an ideal candidate for the molecular labeling.
94
AL-Dujaili et al evaluated the effect of AgNPs and rosuvastatin on hyperlipidemic rats and reported that AgNPs declines serum levels of Obestatin and Endothelin even more than rosuvastatin.
95
Shi et al evaluated AgNPs toxicity and effects on ECs injury.
96
They reported that exposure to AgNPs leads to the inhibition of proliferation, damage to the cell membrane and the induction of apoptosis. Furthermore, they claimed that AgNPs increase levels of inflammatory cytokines, adhesion molecules, and chemokines and more importantly ROS production in HUVECs. Altogether, they suggested that AgNPs induce early atherosclerosis by induction of endothelial injury and dysfunction through the activation of IκB kinase (IKK)/NF-κB. On the other hand, some studies showed an adverse effect of AgNPs on angiogenesis,
97
and hence CVDs.
Iron (III) oxide (Fe2O3) as the paramagnetic and Fe3O4 as a superparamagnetic form are both found in nature.
98
Some of the superparamagnetic iron oxide NPs (SPIONs) characteristics such as their ultrafine size, magnetic properties, and biocompatibility have made them an ideal candidate for various biomedical applications. They have been applied as the resolution enhancing contrast agents for the magnetic resonance imaging (MRI) and also employed for the targeted drug delivery and imaging, hyperthermia, gene therapy, stem cell tracking, molecular/cellular tracking, magnetic separation technologies (e.g., rapid DNA sequencing). Also, they can be helpful in the early detection of inflammatory, cancer, diabetes, and atherosclerosis. High magnetization values of SPIONS make them favorable for all of the aforementioned biomedical applications. Moreover, the surface of these NPs could simply be modified, which enable them for imminent conjugation with various biomolecules for targeting/imaging purposes. However, some challenges with regard to the toxicity of these magnetic NPs may limit their applications in biomedical sciences. Nemmar et al evaluated the effect of ultra-small superparamagnetic iron oxide NPs (USPIO) on cardiac system and thrombosis.
99
They found out that USPIO stimulates a prothrombotic effect in the arterioles and venules in vivo, increase plasma plasminogen activator inhibitor-1 (PAI-1), and aggregate the platelet in vitro. More to the point, these researchers observed that USPIO shortened activated partial thromboplastin time (PTT) and prothrombin time (PT) while the particles resulted in an increase in the plasma levels of creatine phosphokinase-MB isoenzyme (CK-MB), lactate dehydrogenase (LDH) and troponin-I as well as the cardiac levels of markers of oxidative stress (e.g., lipid peroxidation, reactive oxygen species, and superoxide dismutase activity). Taking all together, the administration of USPIO may be inappropriate as targeting device for the CVDs detection and therapy, in large part due to its adverse effects on thrombosis, cardiac oxidative stress, and DNA integrity. Other parallel studies also reported that iron oxide NPs toxic effects on ECs, which occur due to their ability to induce oxidative stress and apoptosis that may lead to injuries and consequently atherosclerosis, hypertension, and myocardial infarction.
100
Contrarily, Xiong et al reported no significant toxicity of Fe2O3 NPs on the normal cardiomyocytes and introduced Fe2O3 NPs as a potentially useful strategy to treat CVDs.
101
In another study, Zheng et al applied platinum NPs (PtNPs) on a blood vessel-mimicking microfluidic chip as antioxidant drugs.
102
Designed PtNPs displayed superoxide dismutase (SOD)-like roles and scavenged ROS while improving cell-cell junctions under hyperglycemic, hyperlipidemic and proinflammatory conditions. They introduced PtNPs as a potential antioxidant vehicle which might be promising treatment candidate for the vascular diseases such as atherosclerosis.
Applications and limitations of nanomedicine in clinical studies
The unique characteristics of nanomaterials (e.g., shape, size, and charge) make them promising tools for both diagnosis and therapy approaches. Nevertheless, toxicity and ethical issues of some types of NPs such as carbon nanotubes, and cost-effectiveness in large-scale preparation continue to be challenging issues in their applications.
24
However, FDA has approved several nanomedicines for administrations via various routes, such as oral (Gastromark; silicone-coated superparamagnetic iron oxide), local (DepoCyt; cytarabine liposomal), topical (Estrasorb; micellar Estradiol), and systemic (LipoDox;) applications. Inorganic nanomaterials (iron oxide, silica, gold and etc.) have been used in few clinical applications such as in vivo imaging as contrast agents, photothermal therapy of tumors and cancer-associated anemia.
103,104
However, in practice, organic NPs (lipidic, protein-based and polymeric NPs) have shown promising results in clinical settings. They have been developed for various applications, including vaccine design, drug/gene delivery, imaging contrast agent. However, the clinical application of nanomedicines in CVDs is still new field of research and development. Some limitations hold back the efficient translation of nanomedicines from bench-to-bedside for diagnosis/therapy of CVDs that requires careful attention. As an example, the pathophysiology of plaques formation is different between different animal models and human. The atherosclerotic plaque development is much faster in mice and with a different function. In addition, mice plaques differ in terms of neovascularization, rupture and thrombosis. Thus, more and precise interpretation is needed for the preclinical results. It should be also pointed out that these results without further validations do not rationalize the clinical uses of the NSs. In spite of these, some nanomaterials have already been entered into the clinical trials or even approved for the application (e.g., Lipocardium),
55
or introduced in the form of nanotheranostics that lighten the future of clinical utility of nanomedicine against CVDs.
105
Some of these nanomaterials are summarized in Table 2.
Table 2.
Clinical trials
|
Nanostructure
|
Clinical phase
|
Application
|
Source
|
| Nanoparticulate estradiol + progesterone |
Phase 2 |
Evaluation of the effects of micronized and nanoparticulate transdermal hormone therapy on blood pressure, ultra-sensitive C-reactive protein, and cardiovascular risk factors in postmenopausal women |
ClinicalTrials.gov Identifier:
NCT02467673
|
| NANOM-FIM |
Phase 2 |
Demolish and reverse the plaque especially in combination with stem cell technologies promising functional restoration of the vessel wall by nano burning |
ClinicalTrials.gov Identifier:
NCT01270139
|
| NANOM PCI |
Phase 1 |
Plasmonic photothermal and stem cell therapy of atherosclerosis with the use of gold nanoparticles with iron oxide-silica shells versus stenting |
ClinicalTrials.gov Identifier:
NCT01436123
|
| Bio-engineered Sirolimus-eluting Stent (OrbusNeich Combo stent™) |
|
The Combo Stent is composed of the OrbusNeich R stent™, with an abluminal coating of a bioabsorbable polymer matrix formulated with sirolimus for sustained release, and an anti-CD34 antibody cell capture coating on the luminal surface |
|
|
NanoTM Polymer-free Sirolimus-Eluting Coronary Stent System(Nano+ DES)
|
Phase 4 |
Evaluation of Efficacy and Safety of Nano+ Polymer-free Sirolimus-Eluting Stent in the Treatment of Patients With De Novo Lesion |
ClinicalTrials.gov Identifier:
NCT01925027
|
XIENCE Nano™ Everolimus Eluting Coronary Stent
|
|
Evaluation of safety and effectiveness of the |
ClinicalTrials.gov Identifier:
NCT01435031
|
Conclusion and future perspectives
Atherosclerosis is a multifactorial chronic systemic inflammatory disease of arterial walls that leads to arteries stenosis. Pathologically, it originates with endothelium dysfunction and a cascade of events with participation of various cells and molecules that results in the formation and progression of atheromatous plaque and arterial stenosis. Due to the commencement of atherosclerosis at the molecular/cellular level, it seems rational to thwart the progression of the disease at this level effectively using nanomedicines. Several studies showed that different types of DDSs such as lipid-based, polymeric and metal NPs improve different aspects of common therapeutics, including poor PK features and limited specificity or severe side-effects.Although NP-based DDSs possess many advantages over the traditional forms, they display some drawbacks such as limited diffusibility, possible toxicity, immunostimulatory, or immunosuppressive properties. All these issues still need to be addressed prior to any application/translational approaches. The direction of future studies should mostly aim to plaques targeted drug delivery, site-specific targeting, NPs biodistribution and multifunctional NPs fabrication. Conclusively, this review suggests that atherosclerosis combating nanomedicine is in its infancy but early insights in the clinical trials demonstrated their value as an important tool for improving multifunctional theranostic agent against CVDs.
Ethical approval
Not applicable.
Competing interests
There is no conflict of interests to be reported.
Acknowledgment
This work is a part of a Ph.D. thesis supported (grant No: 93014) by the Research Center for Pharmaceutical Nanotechnology, Tabriz University of Medical Sciences.
Review Highlights
What is current knowledge?
simple
-
√ Cardiovascular diseases (CVDs) is recognized as the
leading cause of mortality worldwide.
-
√ Nanomedicines as novel tools for enhanced drug delivery,
imaging, and diagnosis strategies have shown great promise
to combat cardiovascular diseases.
What is new here?
simple
-
√ Promising targets in targeted therapy of atherosclerosis
have been overview.
-
√ Recent studies regarding different drug delivery systems
(DDSs) including lipid-based, polymeric and metal
nanoparticles used for combating atherosclerosis have been
well reviewed.
-
√ Applications and limitations of nanomedicine in clinical
studies were highlighted.
References
- Santulli G. Epidemiology of cardiovascular disease in the 21st century: updated numbers and updated facts. JCvD 2013; 1:1-2. [ Google Scholar]
-
Mendis S, Puska P, Norrving B. Global atlas on cardiovascular disease prevention and control. WHO; 2011.
- Lee JT, Lawson KD, Wan Y, Majeed A, Morris S, Soljak M. Are cardiovascular disease risk assessment and management programmes cost effective? A systematic review of the evidence. Prev Med 2017; 99:49-57. doi: 10.1016/j.ypmed.2017.01.005 [Crossref] [ Google Scholar]
- Viles-Gonzalez JF, Fuster V, Badimon JJ. Atherothrombosis: a widespread disease with unpredictable and life-threatening consequences. Eur Heart J 2004; 25:1197-207. doi: 10.1016/j.ehj.2004.03.011 [Crossref] [ Google Scholar]
- Psarros C, Lee R, Margaritis M, Antoniades C. Nanomedicine for the prevention, treatment and imaging of atherosclerosis. Maturitas 2012; 73:52-60. doi: 10.1016/j.maturitas.2011.12.014 [Crossref] [ Google Scholar]
- Lobatto ME, Fuster V, Fayad ZA, Mulder WJ. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat Rev Drug Discov 2011; 10:835-52. doi: 10.1038/nrd3578 [Crossref] [ Google Scholar]
- Schiener M, Hossann M, Viola JR, Ortega-Gomez A, Weber C, Lauber K. Nanomedicine-based strategies for treatment of atherosclerosis. Trends Mol Med 2014; 20:271-81. doi: 10.1016/j.molmed.2013.12.001 [Crossref] [ Google Scholar]
- Conde-Estévez D. Targeted cancer therapy: interactions with other medicines. Clin Transl Oncol 2017; 19:21-30. doi: 10.1007/s12094-016-1509-x [Crossref] [ Google Scholar]
- Chen WC, Zhang AX, Li SD. Limitations and niches of the active targeting approach for nanoparticle drug delivery. Eur J Nanomedicine 2012; 4:89-93. doi: 10.1515/ejnm-2012-0010 [Crossref] [ Google Scholar]
- Al-Jamal KT.
Active drug targeting: Lessons learned and new
things to consider
. Int J Pharm 2013; 454:525-6. doi: 10.1016/j.ijpharm.2013.03.050 [Crossref] [ Google Scholar]
- Nishikimi T. [Molecular target drug for hypertension and cardiovascular disease]. Nihon Rinsho 2010; 68:1911-6. [ Google Scholar]
- Sahoo SK. Applications of nanomedicine. Asia Pac Biotech News 2005; 9:1048-50. [ Google Scholar]
- Mulder WJ, Strijkers GJ, van Tilborg GA, Cormode DP, Fayad ZA, Nicolay K. Nanoparticulate assemblies of amphiphiles and diagnostically active materials for multimodality imaging. Acc Chem Res 2009; 42:904-14. doi: 10.1021/ar800223c [Crossref] [ Google Scholar]
- Omidi Y, Barar J. Impacts of blood-brain barrier in drug delivery and targeting of brain tumors. BI 2012; 2:BI 2012; 2. doi: 10.5681/bi.2012.002 [Crossref] [ Google Scholar]
- Yang X. Nano- and microparticle-based imaging of cardiovascular interventions: Overview. Radiology 2007; 243:340-7. doi: 10.1148/radiol.2432060307 [Crossref] [ Google Scholar]
- Herbst SM, Klegerman ME, Kim H, Qi J, Shelat H, Wassler M. Delivery of stem cells to porcine arterial wall with echogenic liposomes conjugated to antibodies against CD34 and intercellular adhesion molecule-1. Mol Pharm 2010; 7:3-11. doi: 10.1021/mp900116r [Crossref] [ Google Scholar]
- Danila D, Partha R, Elrod DB, Lackey M, Casscells SW, Conyers JL. Antibody-labeled liposomes for CT imaging of atherosclerotic plaques: In vitro investigation of an anti-ICAM antibody-labeled liposome containing iohexol for molecular imaging of atherosclerotic plaques via computed tomography. Tex Heart Inst J 2009; 36:393-403. [ Google Scholar]
- Paulis LEM, Jacobs I, van den Akker NM, Geelen T, Molin DG, Starmans LWE. Targeting of ICAM-1 on vascular endothelium under static and shear stress conditions using a liposomal Gd-based MRI contrast agent. J Nanobiotechnology 2012:10. doi: 10.1186/1477-3155-10-25 [Crossref]
- Khondee S, Baoum A, Siahaan TJ, Berkland C. Calcium condensed LABL-TAT complexes effectively target gene delivery to ICAM-1 expressing cells. MolPharm 2011; 8:788-98. doi: 10.1021/mp100393j [Crossref] [ Google Scholar]
- Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation 2008; 117:379-87. doi: 10.1161/CIRCULATIONAHA.107.741181 [Crossref] [ Google Scholar]
- Garnacho C, Serrano D, Muro S. A fibrinogen-derived peptide provides intercellular adhesion molecule-1-specific targeting and intraendothelial transport of polymer nanocarriers in human cell cultures and mice. J Pharmacol Exp Ther 2012; 340:638-47. doi: 10.1124/jpet.111.185579 [Crossref] [ Google Scholar]
- Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik DE, Aikawa E, Libby P. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation 2006; 114:1504-11. doi: 10.1161/CIRCULATIONAHA.106.646380 [Crossref] [ Google Scholar]
- Kowalski PS, Lintermans LL, Morselt HWM, Leus NGJ, Ruiters MHJ, Molema G. Anti-VCAM-1 and anti-E-selectin SAINT-O-somes for selective delivery of siRNA into inflammation-activated primary endothelial cells. Mol Pharm 2013; 10:3033-44. doi: 10.1021/mp4001124 [Crossref] [ Google Scholar]
- Dziubla TD, Shuvaev VV, Hong NK, Hawkins BJ, Madesh M, Takano H. Endothelial targeting of semi-permeable polymer nanocarriers for enzyme therapies. Biomaterials 2008; 29:215-27. doi: 10.1016/j.biomaterials.2007.09.023 [Crossref] [ Google Scholar]
- Maiseyeu A, Mihai G, Kampfrath T, Simonetti OP, Sen CK, Roy S. Gadolinium-containing phosphatidylserine liposomes for molecular imaging of atherosclerosis. J Lipid Res 2009; 50:2157-63. doi: 10.1194/jlr.M800405-JLR200 [Crossref] [ Google Scholar]
- Almer G, Frascione D, Pali-Schöll I, Vonach C, Lukschal A, Stremnitzer C. Interleukin-10: An anti-inflammatory marker to target atherosclerotic Lesions via PEGylated Liposomes. Mol Pharm 2013; 10:175-86. doi: 10.1021/mp300316n [Crossref] [ Google Scholar]
- Peters D, Kastantin M, Kotamraju VR, Karmali PP, Gujraty K, Tirrell M. Targeting atherosclerosis by using modular, multifunctional micelles. Proc Natl Acad Sci U S A 2009; 106:9815-9. doi: 10.1073/pnas.0903369106 [Crossref] [ Google Scholar]
- Almer G, Wernig K, Saba-Lepek M, Haj-Yahya S, Rattenberger J, Wagner J. Adiponectin-coated nanoparticles for enhanced imaging of atherosclerotic plaques. Int J Nanomedicine 2011; 6:1279-90. [ Google Scholar]
- Cyrus T, Zhang H, Allen JS, Williams TA, Hu G, Caruthers SD. Intramural delivery of rapamycin with αvβ3-targeted paramagnetic nanoparticles inhibits stenosis after balloon injury. Arterioscler Thromb Vasc Biol 2008; 28:820-6. doi: 10.1161/ATVBAHA.107.156281 [Crossref] [ Google Scholar]
- Joner M, Morimoto K, Kasukawa H, Steigerwald K, Merl S, Nakazawa G. Site-specific targeting of nanoparticle prednisolone reduces in-stent restenosis in a rabbit model of established atheroma. Arterioscler Thromb Vasc Biol 2008; 28:1960-6. doi: 10.1161/ATVBAHA.108.170662 [Crossref] [ Google Scholar]
- Coviello V, Sartini S, Quattrini L, Baraldi C, Gamberini MC, La Motta C. Cyclodextrin-based nanosponges for the targeted delivery of the anti-restenotic agent DB103: A novel opportunity for the local therapy of vessels wall subjected to percutaneous intervention. Eur J Pharm Biopharm 2017; 117:276-85. doi: 10.1016/j.ejpb.2017.04.028 [Crossref] [ Google Scholar]
- Dong Z, Guo J, Xing X, Zhang X, Du Y, Lu Q. RGD modified and PEGylated lipid nanoparticles loaded with puerarin: Formulation, characterization and protective effects on acute myocardial ischemia model. Biomed Pharmacother 2017; 89:297-304. doi: 10.1016/j.biopha.2017.02.029 [Crossref] [ Google Scholar]
- Izuhara M, Kuwabara Y, Saito N, Yamamoto E, Hakuno D, Nakashima Y. Prevention of neointimal formation using miRNA-126-containing nanoparticle-conjugated stents in a rabbit model. PLoS ONE 2017:12. doi: 10.1371/journal.pone.0172798 [Crossref]
- Li L, Dong F, Yang CJ, Ye XH, Xu X, Cao JN. Delivery of HDAC1 siRNA by Mn-doped ZnSe quantum dots to induce human mesenchymal stem cells differentiation into cardiomyocytes. J Biomater Tissue Eng 2016; 6:180-9. doi: 10.1166/jbt.2016.1428 [Crossref] [ Google Scholar]
- Almer G, Wernig K, Saba-Lepek M, Haj-Yahya S, Rattenberger J, Wagner J. Adiponectin-coated nanoparticles for enhanced imaging of atherosclerotic plaques. Int J Nanomedicine 2011; 6:1279-90. doi: 10.2147/ijn.s18739 [Crossref] [ Google Scholar]
- Koenig W, Khuseyinova N. Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler Thromb Vasc Biol 2007; 27:15-26. doi: 10.1161/01.ATV.0000251503.35795.4f [Crossref] [ Google Scholar]
- Westein E, Flierl U, Hagemeyer CE, Peter K. Destination Known: Targeted Drug Delivery in Atherosclerosis and Thrombosis. Drug Development Research 2013; 74:460-71. doi: 10.1002/ddr.21103 [Crossref] [ Google Scholar]
- Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fogelman AM. Mechanisms of disease: proatherogenic HDL--an evolving field. Nat Clin Pract Endocrinol Metab 2006; 2:504-11. doi: 10.1038/ncpendmet0245 [Crossref] [ Google Scholar]
- Murphy AJ, Funt S, Gorman D, Tall AR, Wang N. Pegylation of high-density lipoprotein decreases plasma clearance and enhances antiatherogenic activity. Circ Res 2013; 113:e1-9. doi: 10.1161/CIRCRESAHA.113.301112 [Crossref] [ Google Scholar]
- Bazzoni G. The JAM family of junctional adhesion molecules. Curr Opin Cell Biol 2003; 15:525-30. doi: 10.1016/S0955-0674(03)00104-2 [Crossref] [ Google Scholar]
- Buse J, El-Aneed A. Properties, engineering and applications of lipid-based nanoparticle drug-delivery systems: Current research and advances. Nanomedicine 2010; 5:1237-60. doi: 10.2217/nnm.10.107 [Crossref] [ Google Scholar]
-
Tan A, Prestidge C. Improving the performance of lipid formulations: Nanoparticle layers and solid hybrid particles. American Pharmaceutical Review 2013; 16.
- James JS. DOXIL approved for KS. AIDS Treat News 1995:6.
- Lombardo D, Calandra P, Barreca D, Magazù S, Kiselev MA. Soft interaction in liposome nanocarriers for therapeutic drug delivery. Nanomaterials 2016:6. doi: 10.3390/nano6070125 [Crossref]
- Majzoub RN, Ewert KK, Safinya CR. Cationic liposome-nucleic acid nanoparticle assemblies with applications in gene delivery and gene silencing. Philos Trans A Math Phys Eng Sci: Mathematical, Physical and Engineering Sciences 2016:374. doi: 10.1098/rsta.2015.0129 [Crossref]
- Safinya CR, Ewert KK, Majzoub RN, Leal C. Cationic liposome-nucleic acid complexes for gene delivery and gene silencing. New J Chem 2014; 38:5164-72. doi: 10.1039/c4nj01314j [Crossref] [ Google Scholar]
- Zhang YY, Chen JM. Existing problems and strategies in liposome-mediated nucleic acid delivery. Yaoxue Xuebao 2011; 46:261-8. [ Google Scholar]
- Owens Iii DE, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm 2006; 307:93-102. doi: 10.1016/j.ijpharm.2005.10.010 [Crossref] [ Google Scholar]
- Yan X, Scherphof GL, Kamps JAAM. Liposome opsonization. J Liposome Res 2005; 15:109-39. doi: 10.1081/LPR-64971 [Crossref] [ Google Scholar]
- Joner M, Morimoto K, Kasukawa H, Steigerwald K, Merl S, Nakazawa G. Site-specific targeting of nanoparticle prednisolone reduces in-stent restenosis in a rabbit model of established atheroma. Arterioscler Thromb Vasc Biol 2008; 28:1960-6. doi: 10.1161/ATVBAHA.108.170662 [Crossref] [ Google Scholar]
- Calin M, Stan D, Schlesinger M, Simion V, Deleanu M, Constantinescu CA. VCAM-1 directed target-sensitive liposomes carrying CCR2 antagonists bind to activated endothelium and reduce adhesion and transmigration of monocytes. Eur J Pharm Biopharm 2015; 89:18-29. doi: 10.1016/j.ejpb.2014.11.016 [Crossref] [ Google Scholar]
- Homem de Bittencourt Jr PI, Lagranha DJ, Maslinkiewicz A, Senna SM, Tavares AMV, Baldissera LP. LipoCardium: Endothelium-directed cyclopentenone prostaglandin-based liposome formulation that completely reverses atherosclerotic lesions. Atherosclerosis 2007; 193:245-58. doi: 10.1016/j.atherosclerosis.2006.08.049 [Crossref] [ Google Scholar]
- Hosseini H, Li Y, Kanellakis P, Tay C, Cao A, Tipping P. Phosphatidylserine liposomes mimic apoptotic cells to attenuate atherosclerosis by expanding polyreactive IgM producing B1a lymphocytes. Cardiovasc Res 2015; 106:443-52. doi: 10.1093/cvr/cvv037 [Crossref] [ Google Scholar]
- van der Valk FM, van Wijk DF, Lobatto ME, Verberne HJ, Storm G, Willems MCM. Prednisolone-containing liposomes accumulate in human atherosclerotic macrophages upon intravenous administration. Nanomedicine 2015; 11:1039-46. doi: 10.1016/j.nano.2015.02.021 [Crossref] [ Google Scholar]
-
Homem de Bittencourt
PI
, Lagranha DJ, Maslinkiewicz A, Senna SM, Tavares AM, Baldissera LP. LipoCardium: endothelium-directed cyclopentenone prostaglandin-based liposome formulation that completely reverses atherosclerotic lesions. Atherosclerosis 2007; 193:245-58. doi: 10.1016/j.atherosclerosis.2006.08.049 [Crossref] [ Google Scholar]
- Eskandani M, Nazemiyeh H. Self-reporter shikonin-Act-loaded solid lipid nanoparticle: Formulation, physicochemical characterization and geno/cytotoxicity evaluation. Eur J Pharm Sci 2014; 59:49-57. doi: 10.1016/j.ejps.2014.04.009 [Crossref] [ Google Scholar]
- Ezzati Nazhad Dolatabadi J, Omidi Y. Solid lipid-based nanocarriers as efficient targeted drug and gene delivery systems. TrAC - Trends Analyt Chem 2016; 77:100-8. doi: 10.1016/j.trac.2015.12.016 [Crossref] [ Google Scholar]
- Kulandaivelu K, Mandal AKA. Positive regulation of biochemical parameters by tea polyphenol encapsulated solid lipid nanoparticles at in vitro and in vivo conditions. IET Nanobiotechnology 2016; 10:419-24. doi: 10.1049/iet-nbt.2015.0113 [Crossref] [ Google Scholar]
- Nakhlband A, Eskandani M, Saeedi N, Ghafari S, Omidi Y, Barar J. Marrubiin-loaded solid lipid nanoparticles’ impact on TNF-α treated umbilical vein endothelial cells: A study for cardioprotective effect. Colloids Surf B Biointerfaces 2018; 164:299-307. doi: 10.1016/j.colsurfb.2018.01.046 [Crossref] [ Google Scholar]
- Paliwal R, Paliwal SR, Agrawal GP, Vyas SP. Biomimetic solid lipid nanoparticles for oral bioavailability enhancement of low molecular weight heparin and its lipid conjugates: In vitro and in vivo evaluation. Mol Pharm 2011; 8:1314-21. doi: 10.1021/mp200109m [Crossref] [ Google Scholar]
- Gao Y, Gu W, Chen L, Xu Z, Li Y. The role of daidzein-loaded sterically stabilized solid lipid nanoparticles in therapy for cardio-cerebrovascular diseases. Biomaterials 2008; 29:4129-36. doi: 10.1016/j.biomaterials.2008.07.008 [Crossref] [ Google Scholar]
- Hörmann K, Zimmer A. Drug delivery and drug targeting with parenteral lipid nanoemulsions - A review. J Control Release 2016; 223:85-98. doi: 10.1016/j.jconrel.2015.12.016 [Crossref] [ Google Scholar]
- Souto EB, Nayak AP, Murthy RSR. Lipid nanoemulsions for anti-cancer drug therapy. Pharmazie 2011; 66:473-8. doi: 10.1691/ph.2011.0392 [Crossref] [ Google Scholar]
- Tavares ER, Freitas FR, Diament J, Maranhão RC. Reduction of atherosclerotic lesions in rabbits treated with etoposide associated with cholesterol-rich nanoemulsions. Int J Nanomedicine 2011; 6:2297-304. doi: 10.2147/IJN.S24048 [Crossref] [ Google Scholar]
- Bulgarelli A, Leite Jr ACA, Dias AAM, Maranhão RC. Anti-atherogenic effects of methotrexate carried by a lipid nanoemulsion that binds to ldl receptors in cholesterol-fed rabbits. Cardiovasc Drugs Ther 2013; 27:531-9. doi: 10.1007/s10557-013-6488-3 [Crossref] [ Google Scholar]
- Leite ACA, Solano TV, Tavares ER, Maranhão RC. Use of Combined Chemotherapy with Etoposide and Methotrexate, both Associated to Lipid Nanoemulsions for Atherosclerosis Treatment in Cholesterol-fed Rabbits. Cardiovasc Drugs Ther 2015; 29:15-22. doi: 10.1007/s10557-014-6566-1 [Crossref] [ Google Scholar]
- Beloqui A, Solinís MÁ, Rodríguez-Gascón A, Almeida AJ, Préat V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine 2016; 12:143-61. doi: 10.1016/j.nano.2015.09.004 [Crossref] [ Google Scholar]
- Zhang WL, Gu X, Bai H, Yang RH, Dong CD, Liu JP. Nanostructured lipid carriers constituted from high-density lipoprotein components for delivery of a lipophilic cardiovascular drug. Int J Pharm 2010; 391:313-21. doi: 10.1016/j.ijpharm.2010.03.011 [Crossref] [ Google Scholar]
- Zhang W, He H, Liu J, Wang J, Zhang S, Zhang S. Pharmacokinetics and atherosclerotic lesions targeting effects of tanshinone IIA discoidal and spherical biomimetic high density lipoproteins. Biomaterials 2013; 34:306-19. doi: 10.1016/j.biomaterials.2012.09.058 [Crossref] [ Google Scholar]
- Banik BL, Fattahi P, Brown JL. Polymeric nanoparticles: The future of nanomedicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2016; 8:271-99. doi: 10.1002/wnan.1364 [Crossref] [ Google Scholar]
- Lim EK, Chung BH, Chung SJ. Recent advances in ph-sensitive polymeric nanoparticles for smart drug delivery in cancer therapy. Curr Drug Targets 2016:17. doi: 10.2174/1389450117666160602202339 [Crossref]
- Karagkiozaki V. Nanomedicine highlights in atherosclerosis. J Nanopar Res 2013; 15:1-17. doi: 10.1007/s11051-013-1529-1 [Crossref] [ Google Scholar]
- Kapoor DN, Bhatia A, Kaur R, Sharma R, Kaur G, Dhawan S. PLGA: A unique polymer for drug delivery. Ther Deliv 2015; 6:41-58. doi: 10.4155/tde.14.91 [Crossref] [ Google Scholar]
- Feng SS, Zeng W, Lim YT, Zhao L, Win KY, Oakley R. Vitamin E TPGS-emulsified poly(lactic-co-glycolic acid) nanoparticles for cardiovascular restenosis treatment. Nanomedicine 2007; 2:333-44. doi: 10.2217/17435889.2.3.333 [Crossref] [ Google Scholar]
- Golub JS, Kim YT, Duvall CL, Bellamkonda RV, Gupta D, Lin AS. Sustained VEGF delivery via PLGA nanoparticles promotes vascular growth. Am J Physiol Heart Circ Physiol 2010:298. doi: 10.1152/ajpheart.00199.2009 [Crossref]
- Sanchez-Gaytan BL, Fay F, Lobatto ME, Tang J, Ouimet M, Kim Y. HDL-Mimetic PLGA Nanoparticle To Target Atherosclerosis Plaque Macrophages. Bioconjug Chem 2015; 26:443-51. doi: 10.1021/bc500517k [Crossref] [ Google Scholar]
- Prabaharan M. Chitosan-based nanoparticles for tumor-targeted drug delivery. Int J Biol Macromol 2015; 72:1313-22. doi: 10.1016/j.ijbiomac.2014.10.052 [Crossref] [ Google Scholar]
- Masotti A, Ortaggi G. Chitosan micro- and nanospheres: Fabrication and applications for drug and DNA delivery. Mini Rev Med Chem 2009; 9:463-9. doi: 10.2174/138955709787847976 [Crossref] [ Google Scholar]
- Yu Y, Luo T, Liu S, Song G, Han J, Wang Y. Chitosan oligosaccharides attenuate atherosclerosis and decrease Non-HDL in ApoE-/-mice. J Atheroscler Thromb 2015; 22:926-41. doi: 10.5551/jat.22939 [Crossref] [ Google Scholar]
- Yuan X, Yang X, Cai D, Mao D, Wu J, Zong L. Intranasal immunization with chitosan/pCETP nanoparticles inhibits atherosclerosis in a rabbit model of atherosclerosis. Vaccine 2008; 26:3727-34. doi: 10.1016/j.vaccine.2008.04.065 [Crossref] [ Google Scholar]
- Hong Z, Xu Y, Yin JF, Jin J, Jiang Y, Du Q. Improving the effectiveness of (-)-epigallocatechin gallate (EGCG) against rabbit atherosclerosis by EGCG-loaded nanoparticles prepared from chitosan and polyaspartic acid. J Agric Food Chem 2014; 62:12603-9. doi: 10.1021/jf504603n [Crossref] [ Google Scholar]
- Trache D, Hussin MH, Haafiz MKM, Thakur VK. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017; 9:1763-86. doi: 10.1039/c6nr09494e [Crossref] [ Google Scholar]
- Wang S, Lu A, Zhang L. Recent advances in regenerated cellulose materials. Prog Polym Sci 2016; 53:169-206. doi: 10.1016/j.progpolymsci.2015.07.003 [Crossref] [ Google Scholar]
- Li L, Luo X, Leung PHM, Law HKW. Controlled release of borneol from nano-fibrous poly(L-lactic acid)/ cellulose acetate butyrate membrane. Textile Res J 2016; 86:1202-9. doi: 10.1177/0040517515603812 [Crossref] [ Google Scholar]
- Yasmin R, Shah M, Khan SA, Ali R. Gelatin nanoparticles: A potential candidate for medical applications. Nanotech Rev 2017; 6:191-207. doi: 10.1515/ntrev-2016-0009 [Crossref] [ Google Scholar]
- Zhang QY, Wang ZY, Wen F, Ren L, Li J, Teoh SH. Gelatin-siloxane nanoparticles to deliver nitric oxide for vascular cell regulation: Synthesis, cytocompatibility, and cellular responses. J Biomed Mater Res A 2015; 103:929-38. doi: 10.1002/jbm.a.35239 [Crossref] [ Google Scholar]
- Vogt C, Xing Q, He W, Li B, Frost MC, Zhao F. Fabrication and characterization of a nitric oxide-releasing nanofibrous gelatin matrix. Biomacromolecules 2013; 14:2521-30. doi: 10.1021/bm301984w [Crossref] [ Google Scholar]
- Kobayashi H, Minatoguchi S, Yasuda S, Bao N, Kawamura I, Iwasa M. Post-infarct treatment with an erythropoietin-gelatin hydrogel drug delivery system for cardiac repair. Cardiovasc Res 2008; 79:611-20. doi: 10.1093/cvr/cvn154 [Crossref] [ Google Scholar]
- Ruvinov E, Cohen S. Alginate biomaterial for the treatment of myocardial infarction: Progress, translational strategies, and clinical outlook From ocean algae to patient bedside. Adv Drug Deliv Rev 2016; 96:54-76. doi: 10.1016/j.addr.2015.04.021 [Crossref] [ Google Scholar]
- Ruvinov E, Leor J, Cohen S. The promotion of myocardial repair by the sequential delivery of IGF-1 and HGF from an injectable alginate biomaterial in a model of acute myocardial infarction. Biomaterials 2011; 32:565-78. doi: 10.1016/j.biomaterials.2010.08.097 [Crossref] [ Google Scholar]
- Manojkumar K, Sivaramakrishna A, Vijayakrishna K. A short review on stable metal nanoparticles using ionic liquids, supported ionic liquids, and poly(ionic liquids). J Nanopart Res 2016:18. doi: 10.1007/s11051-016-3409-y [Crossref]
- Cao-Milán R, Liz-Marzán LM. Gold nanoparticle conjugates: Recent advances toward clinical applications. Expert Opin Drug Deliv 2014; 11:741-52. doi: 10.1517/17425247.2014.891582 [Crossref] [ Google Scholar]
- Roma-Rodrigues C, Heuer-Jungemann A, Fernandes AR, Kanaras AG, Baptista PV. Peptide-coated gold nanoparticles for modulation of angiogenesis in vivo. Int J Nanomedicine 2016; 11:2633-9. doi: 10.2147/IJN.S108661 [Crossref] [ Google Scholar]
- Abbasi E, Milani M, Aval SF, Kouhi M, Akbarzadeh A, Nasrabadi HT. Silver nanoparticles: Synthesis methods, bio-applications and properties. Crit RevMicrobiol 2016; 42:173-80. doi: 10.3109/1040841X.2014.912200 [Crossref] [ Google Scholar]
- Al-Dujaili ANG, Al-Shemeri MK. Effect of silver nanoparticles and rosuvastatin on endothelin and Obestatin in rats induced by high fat-diet. Res J Pharm Biol Chem Sci 2016; 7:1022-30. [ Google Scholar]
- Shi J, Sun X, Lin Y, Zou X, Li Z, Liao Y. Endothelial cell injury and dysfunction induced by silver nanoparticles through oxidative stress via IKK/NF-κB pathways. Biomaterials 2014; 35:6657-66. doi: 10.1016/j.biomaterials.2014.04.093 [Crossref] [ Google Scholar]
- Gurunathan S, Lee KJ, Kalishwaralal K, Sheikpranbabu S, Vaidyanathan R, Eom SH. Antiangiogenic properties of silver nanoparticles. Biomaterials 2009; 30:6341-50. doi: 10.1016/j.biomaterials.2009.08.008 [Crossref] [ Google Scholar]
- El-Sherbiny IM, Elbaz NM, Sedki M, Elgammal A, Yacoub MH. Magnetic nanoparticles-based drug and gene delivery systems for the treatment of pulmonary diseases. Nanomedicine 2017; 12:387-402. doi: 10.2217/nnm-2016-0341 [Crossref] [ Google Scholar]
-
Nemmar A, Beegam S, Yuvaraju P, Yasin J, Tariq S, Attoub S, et al. Ultrasmall superparamagnetic iron oxide nanoparticles acutely promote thrombosis and cardiac oxidative stress and DNA damage in mice. Part Fibre Toxicol 2016; 13.
- Zhu MT, Wang Y, Feng WY, Wang B, Wang M, Ouyang H. Oxidative stress and apoptosis induced by iron oxide nanoparticles in cultured human umbilical endothelial cells. J Nanosci Nanotechnol 2010; 10:8584-90. doi: 10.1166/jnn.2010.2488 [Crossref] [ Google Scholar]
-
Xiong F, Wang H, Feng Y, Li Y, Hua X, Pang X, et al. Cardioprotective activity of iron oxide nanoparticles. Sci Rep 2015; 5.
- Zheng W, Jiang B, Hao Y, Zhao Y, Zhang W, Jiang X. Screening reactive oxygen species scavenging properties of platinum nanoparticles on a microfluidic chip. Biofabrication 2014:6. doi: 10.1088/1758-5082/6/4/045004 [Crossref]
- Huang HC, Barua S, Sharma G, Dey SK, Rege K. Inorganic nanoparticles for cancer imaging and therapy. J Control Release 2011; 155:344-57. doi: 10.1016/j.jconrel.2011.06.004 [Crossref] [ Google Scholar]
- Anselmo AC, Mitragotri S. A Review of Clinical Translation of Inorganic Nanoparticles. AAPS J 2015; 17:1041-54. doi: 10.1208/s12248-015-9780-2 [Crossref] [ Google Scholar]
- Tang J, Lobatto ME, Read JC, Mieszawska AJ, Fayad ZA, Mulder WJM. Nanomedical Theranostics in Cardiovascular Disease. Curr Cardiovasc Imaging Rep 2012; 5:19-25. doi: 10.1007/s12410-011-9120-6 [Crossref] [ Google Scholar]