Professor Mir Reza Majidi has a Ph.D. degree in conducting polymer (1996, University of Wollongong, Australia). He is now working as a full professor of chemistry at the University of Tabriz. His research has mainly been focused on the analytical chemistry. He has published over 100 articles in the field of analytical chemistry.
Professor Yadollah Omidi has a Ph.D. degree in pharmaceutical Sciences (2003, Cardiff University, UK) and completed a postdoctoral program (2004) at Cardiff University. He is the founder of the Research Center for Pharmaceutical Nanotechnology (RCPN), the School of Advanced Biomedical Sciences at Tabriz University of Medical Sciences (TUOMS), the international peer-review journal “BioImpacts", and the National Institute of Medical Sciences. Prof. Omidi’s researches in advanced targeted diagnosis and therapy of diseases have resulted in over 180 published papers in international journals, 18 book chapters, and a few patents. During 20 years of experiences in different institutes (UK; Iran; and USA), his scientific life has been endowed with pharmaceutical sciences.
Abstract
Summary
Through the development of analytical techniques, microscaled devices have displayed attractive advantages, including ultrasensitive detection and analysis, cost-effectiveness, portability, process integrity, multi-process functionality, and in-situ analysis. In the last decade, a new generation of analytical devices has emerged based on the cellulose materials – so-called microfluidic paper-based analytical devices (µPADs) – a field that will change the face of the diagnosis of different diseases and sensing of a wide range of biological/chemical/biochemical phenomena. The main aim of the current editorial is to highlight the importance of the µPADs in the research and development of diagnostic devices and pharmaceuticals.
Keywords: Analytical techniques, Paper-based microfluidics, µPADs, Sensing, Monitoring of diseases, Molecular diagnosis
Copyright and License Information
© 2018 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Paper has been used over the centuries for various experimental purposes such as litmus paper as pH indicator, while the first microfluidic paper-based analytical device (µPAD) was introduced by Whitesides and colleagues in 2007.
1
The field of µPADs has continued to develop at an exponential rate with notable impacts on the academic and industrial communities. These devices use cellulose as substrate to serve as paper-based analytical devices (PADs) for the point-of-care diagnosis, biosensing, environmental monitoring, biomedical and pharmaceutical analysis, clinical diagnosis, and forensic investigations.
2
Furthermore, paper has played an important role in chemical/biochemical analysis, including home pregnancy tests,
3
paper chromatography,
4
paper-based colorimetry, paper-based filtration and purification, pH test, etc. The popularity of PADs is based on several advantages, including (i) very low-cost, (ii) power-free due to cellulose fiber networks,
5, 6
(iii) compatibility with small volume of samples, (iv) the ability to store reagents, (v) easy operation and construction, (vi) portability and disposability. Combination of the microfabrication techniques with the paper has resulted in the generation of de novo cost-effective analytical devices with robust easy and fast applications in different fields of sciences and technologies. Thus, some key insights of various types of papers are discussed, which are used as the substrate, methods for the construction of detection systems, and µPADs for the detection and sensing of biomarkers.
The selection of a paper is largely dependent on the application and construction method. In the last years, Whatman®grade 1 filter, which is one of the standard grade filters, has widely been used in the construction of sensors and microfluidics, in large part because of their suitable flow rate, porosity, and particle retention.
7-9
Some researchers have also used Whatman®grade 4 filters,
10
Whatman® chromatography paper 1,
11
polyester–cellulose blended-paper,
12
and glass microfiber filters.
13
There are various construction methods for the development of µPADs. The first reported method has been based on the photolithography that provides a high-resolution structure between the hydrophilic and hydrophobic areas.
1
The wax screen-printing method is a low-cost and simple approach for the construction of the hydrophobic barriers.
14-16
Polymeric organosilicon compounds have also been used for devising the µPADs. In fact, polydimethylsiloxane (PDMS) plotting is deemed to be an excellent approach for the construction of µPADs.
17
In this method, a computer-controlled plotter is routinely used for plotting of the PDMS on the paper for the flow delivery in the microfluidics chips. Further, the development of bio-microelectromechanical systems (bio-MEMS) is extensively dependent on the soft lithography for imprinting of the microfluidic channels in both organic and inorganic settings. This technique offers several advantages, including (i) the lower cost than that of the traditional photolithography in terms of large-scale production, (ii) better pattern-transferring methods compared to the traditional lithography techniques and (iii) unnecessity of having a photo-reactive surface devising micro-/nano-design. Some other techniques have also been reported, including inkjet etching/printing,
5
plasma etching,
10
flexographic printing,
18
and cutting
19
as well as electron-beam lithography (EBL). Of these, EBL is believed to be one of the tools of choice for the imprinting micro- and/or nano-designs on the surface of a wide variety of materials, in large part due to its capability of imprinting the nano-sized structures on the tiny surface (up to mm2) with great details.
So far, we have developed and applied a number of different biosensors for the monitoring and sensing of various inorganic/organic materials using differently advanced nanobiomaterials.
20-26
The colorimetric detection is a cost-effective simplest method for the detection of various biological entities.
27-29
This latter approach is largely dependent on the implementation of µPADs. As a matter of fact, the integration of µPADs with the electrochemical detection approaches were shown to provide a very useful, portable, accurate and robust detection systems – applicable for the detection in various types of settings.
30,31
Other detection techniques like chemiluminescence,
7,32
and fluorescence
33
can also be implemented using µPAD technologies. The µPADs are used in various fields, including environmental monitoring they of contaminations,
34
food safety,
35
health diagnostics,
36,37
biodefence (micro-organisms sensing),
38,39
and drug discovery
31
as well as a biomarker and single cell detections. Fig. 1 represents a schematic illustration of the µPAD technology.
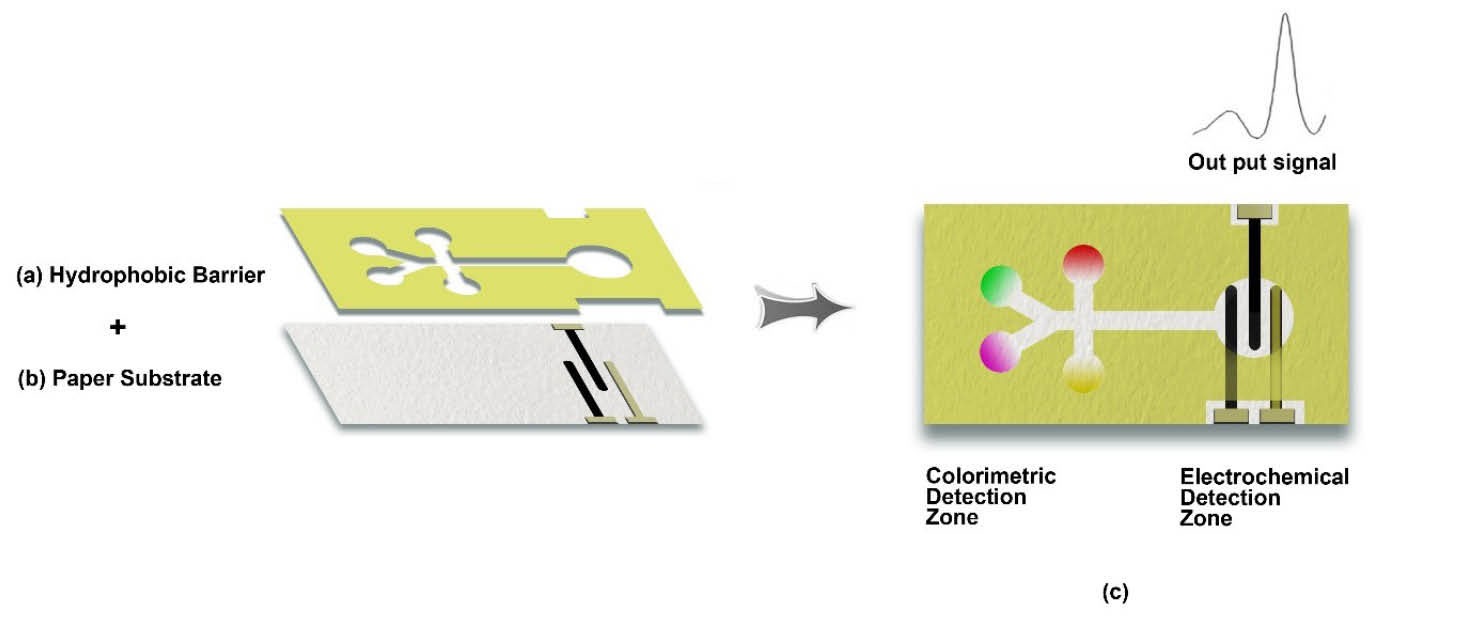
Fig. 1.
Schematic presentation for the construction of a dual-detection potential microfluidics paper-based analytical devices (μPADs). (a)
The patterning of appropriate wax on the paper. (b) The paper substrate. (c) The completed μPAD with dual colorimetric and electrochemical
detection methods.
.
Schematic presentation for the construction of a dual-detection potential microfluidics paper-based analytical devices (μPADs). (a)
The patterning of appropriate wax on the paper. (b) The paper substrate. (c) The completed μPAD with dual colorimetric and electrochemical
detection methods.
The emergence of µPADs is envisioned to overcome many barriers and even eliminate the traditional detection systems. In addition to the environmental monitoring (e.g., contaminations of water, soil and air),
40
the µPADs have been utilized for the sensing of toxic agents in the biological samples as well as drug abuse.
41
Although the µPADs have originally been developed for the point-of-care diagnostic applications in developing countries, they are going to change the direction of clinical diagnosis of various types of diseases mechanistically, in large part because of being rapid, cost-effective, portable and reliable devices. Various biological molecules like DNA,
42
proteins,
43
and cancer cells
44
have been detected by these devices. Their impacts on the detection of pathogenic microorganisms such as viruses and bacteria make them robust monitoring tools in the field of biodefense. For instance, Li et al developed a new paper-based device for the detection of avian influenza virus (AIV). Given that the detection of pesticide and residues of fertilizers in foods can improve the global health, their successful applications in tracking residuals have also been reported.
35
We envision that the µPADs are in early development stage and are going to become very popular and user-friendly devices in the near future. Taken all, it should be highlighted that the µPADs will change the path of the pharmaceutical research and development and medical sciences in the very near future, opening a new horizon in drug discovery and diagnosis.
Acknowledgments
The authors like to acknowledge the financial support provided by the Research Center for Pharmaceutical Nanotechnology at Tabriz University of Medical Sciences on the microfluidics project.
Funding sources
None to be declared.
Ethical statement
There is none to be declared.
Competing interests
No competing interests to be disclosed.
Authors contribution
AAA, MRM and YO gathered the data and drafted the manuscript. YO finalized the manuscript.
References
- Martinez AW, Phillips ST, Butte MJ, Whitesides GM. Patterned paper as a platform for inexpensive, low‐volume, portable bioassays. Angew Chem Int Ed 2007; 46:1318-20. doi: 10.1002/anie.200603817 [Crossref] [ Google Scholar]
- Lisowski P, Zarzycki PK. Microfluidic paper-based analytical devices (μPADs) and micro total analysis systems (μTAS): development, applications and future trends. Chromatographia 2013; 76:1201-14. doi: 10.1007/s10337-013-2413-y [Crossref] [ Google Scholar]
- Gong MM, Sinton D. Turning the page: advancing paper-based microfluidics for broad diagnostic application. Chem Rev 2017; 117:8447-80. doi: 10.1021/acs.chemrev.7b00024 [Crossref] [ Google Scholar]
- Meredith NA, Quinn C, Cate DM, Reilly TH, Volckens J, Henry CS. based analytical devices for environmental analysis. Analyst 2016; 141:1874-87. doi: 10.1039/C5AN02572A [Crossref] [ Google Scholar]
- Abe K, Suzuki K, Citterio D. Inkjet-printed microfluidic multianalyte chemical sensing paper. Anal Chem 2008; 80:6928-34. doi: 10.1021/ac800604v [Crossref] [ Google Scholar]
- Delaney JL, Hogan CF, Tian J, Shen W. Electrogenerated chemiluminescence detection in paper-based microfluidic sensors. Anal Chem 2011; 83:1300-6. doi: 10.1021/ac102392t [Crossref] [ Google Scholar]
- Yu J, Ge L, Huang J, Wang S, Ge S. Microfluidic paper-based chemiluminescence biosensor for simultaneous determination of glucose and uric acid. Lab Chip 2011; 11:1286-91. doi: 10.1039/c0lc00524j [Crossref] [ Google Scholar]
- Carvalhal RF, Kfouri SoM, Piazetta MH, Gobbi AL, Kubota LT. Electrochemical detection in a paper-based separation device. Anal Chem 2010; 82:1162-5. doi: 10.1021/ac902647r [Crossref] [ Google Scholar]
- Ellerbee AK, Phillips ST, Siegel AC, Mirica KA, Martinez AW, Striehl P. Quantifying colorimetric assays in paper-based microfluidic devices by measuring the transmission of light through paper. Anal Chem 2009; 81:8447-52. doi: 10.1021/ac901307q [Crossref] [ Google Scholar]
- Li X, Tian J, Garnier G, Shen W. Fabrication of paper-based microfluidic sensors by printing. Colloids Surf B Biointerfaces 2010; 76:564-70. doi: 10.1016/j.colsurfb.2009.12.023 [Crossref] [ Google Scholar]
- Martinez AW, Phillips ST, Carrilho E, Thomas III SW, Sindi H, Whitesides GM. Simple telemedicine for developing regions: camera phones and paper-based microfluidic devices for real-time, off-site diagnosis. Anal Chem 2008; 80:3699-707. doi: 10.1021/ac800112r [Crossref] [ Google Scholar]
- Nie Z, Nijhuis CA, Gong J, Chen X, Kumachev A, Martinez AW. Electrochemical sensing in paper-based microfluidic devices. Lab Chip 2010; 10:477-83. doi: 10.1039/b917150a [Crossref] [ Google Scholar]
- Bandara GC, Heist CA, Remcho VT. Patterned polycaprolactone-filled glass microfiber microfluidic devices for total protein content analysis. Talanta 2018; 176:589-94. doi: 10.1016/j.talanta.2017.08.031 [Crossref] [ Google Scholar]
- Dungchai W, Chailapakul O, Henry CS. A low-cost, simple, and rapid fabrication method for paper-based microfluidics using wax screen-printing. Analyst 2011; 136:77-82. doi: 10.1039/C0AN00406E [Crossref] [ Google Scholar]
- Jahanshahi-Anbuhi S, Pennings K, Leung V, Kannan B, Brennan JD, Filipe CDM. Design Rules for Fluorocarbon-Free Omniphobic Solvent Barriers in Paper-Based Devices. ACS Appl Mater Interfaces 2015; 7:25434-40. doi: 10.1021/acsami.5b08301 [Crossref] [ Google Scholar]
- Jiang Y, Hao Z, He Q, Chen H. A simple method for fabrication of microfluidic paper-based analytical devices and on-device fluid control with a portable corona generator. Rsc Adv 2016; 6:2888-94. doi: 10.1039/C5RA23470K [Crossref] [ Google Scholar]
- Bruzewicz DA, Reches M, Whitesides GM. Low-cost printing of poly (dimethylsiloxane) barriers to define microchannels in paper. Anal Chem 2008; 80:3387-92. doi: 10.1021/ac702605a [Crossref] [ Google Scholar]
- Olkkonen J, Lehtinen K, Erho T. Flexographically printed fluidic structures in paper. Anal Chem 2010; 82:10246-50. doi: 10.1021/ac1027066 [Crossref] [ Google Scholar]
- Fenton EM, Mascarenas MR, López GP, Sibbett SS. Multiplex lateral-flow test strips fabricated by two-dimensional shaping. ACS Appl Mater Interfaces 2008; 1:124-9. doi: 10.1021/am800043z [Crossref] [ Google Scholar]
- Barar J, Omidi Y. Surface modified multifunctional nanomedicines for simultaneous imaging and therapy of cancer. Bioimpacts 2014; 4:3-14. doi: 10.5681/bi.2014.011 [Crossref] [ Google Scholar]
- Mashinchian O, Johari-Ahar M, Ghaemi B, Rashidi M, Barar J, Omidi Y. Impacts of quantum dots in molecular detection and bioimaging of cancer. Bioimpacts 2014; 4:149-66. doi: 10.15171/bi.2014.008 [Crossref] [ Google Scholar]
- Ebrahimi M, Johari-Ahar M, Hamzeiy H, Barar J, Mashinchian O, Omidi Y. Electrochemical impedance spectroscopic sensing of methamphetamine by a specific aptamer. Bioimpacts 2012; 2:91-5. doi: 10.5681/bi.2012.013 [Crossref] [ Google Scholar]
- Johari-Ahar M, Rashidi MR, Barar J, Aghaie M, Mohammadnejad D, Ramazani A. An ultra-sensitive impedimetric immunosensor for detection of the serum oncomarker CA-125 in ovarian cancer patients. Nanoscale 2015; 7:3768-79. doi: 10.1039/c4nr06687a [Crossref] [ Google Scholar]
- Saberian-Borujeni M, Johari-Ahar M, Hamzeiy H, Barar J, Omidi Y. Nanoscaled aptasensors for multi-analyte sensing. Bioimpacts 2014; 4:205-15. doi: 10.15171/bi.2014.015 [Crossref] [ Google Scholar]
- Majidi MR, Omidi Y, Karami P, Johari-Ahar M. Reusable potentiometric screen-printed sensor and label-free aptasensor with pseudo-reference electrode for determination of tryptophan in the presence of tyrosine. Talanta 2016; 150:425-33. doi: 10.1016/j.talanta.2015.12.064 [Crossref] [ Google Scholar]
- Karami P, Majidi MR, Johari-Ahar M, Barar J, Omidi Y. Development of screen-printed tryptophan-kynurenine immunosensor for in vitro assay of kynurenine-mediated immunosuppression effect of cancer cells on activated T-cells. Biosens Bioelectron 2017; 92:287-93. doi: 10.1016/j.bios.2016.11.010 [Crossref] [ Google Scholar]
- Zhu W-J, Feng D-Q, Chen M, Chen Z-D, Zhu R, Fang H-L. Bienzyme colorimetric detection of glucose with self-calibration based on tree-shaped paper strip. Sens Actuators B Chem 2014; 190:414-8. doi: 10.1016/j.snb.2013.09.007 [Crossref] [ Google Scholar]
- Sechi D, Greer B, Johnson J, Hashemi N. Three-dimensional paper-based microfluidic device for assays of protein and glucose in urine. Anal Chem 2013; 85:10733-7. doi: 10.1021/ac4014868 [Crossref] [ Google Scholar]
- Demirel G, Babur E. Vapor-phase deposition of polymers as a simple and versatile technique to generate paper-based microfluidic platforms for bioassay applications. Analyst 2014; 139:2326-31. doi: 10.1039/c4an00022f [Crossref] [ Google Scholar]
- Rattanarat P, Dungchai W, Siangproh W, Chailapakul O, Henry CS. Sodium dodecyl sulfate-modified electrochemical paper-based analytical device for determination of dopamine levels in biological samples. Anal Chim Acta 2012; 744:1-7. doi: 10.1016/j.aca.2012.07.003 [Crossref] [ Google Scholar]
- Shiroma LY, Santhiago M, Gobbi AL, Kubota LT. Separation and electrochemical detection of paracetamol and 4-aminophenol in a paper-based microfluidic device. Anal Chim Acta 2012; 725:44-50. doi: 10.1016/j.aca.2012.03.011 [Crossref] [ Google Scholar]
- Wang S, Ge L, Song X, Yu J, Ge S, Huang J. based chemiluminescence ELISA: lab-on-paper based on chitosan modified paper device and wax-screen-printing. Biosens Bioelectron 2012; 31:212-8. doi: 10.1016/j.bios.2011.10.019 [Crossref] [ Google Scholar]
- Allen PB, Arshad SA, Li B, Chen X, Ellington AD. DNA circuits as amplifiers for the detection of nucleic acids on a paperfluidic platform. Lab Chip 2012; 12:2951-8. doi: 10.1039/c2lc40373k [Crossref] [ Google Scholar]
- Wang L, Chen W, Xu D, Shim BS, Zhu Y, Sun F. Simple, rapid, sensitive, and versatile SWNT− paper sensor for environmental toxin detection competitive with ELISA. Nano Lett 2009; 9:4147-52. doi: 10.1021/nl902368r [Crossref] [ Google Scholar]
- Hossain SZ, Luckham RE, McFadden MJ, Brennan JD. Reagentless bidirectional lateral flow bioactive paper sensors for detection of pesticides in beverage and food samples. Anal Chem 2009; 81:9055-64. doi: 10.1021/ac901714h [Crossref] [ Google Scholar]
- Nie Z, Deiss F, Liu X, Akbulut O, Whitesides GM. Integration of paper-based microfluidic devices with commercial electrochemical readers. Lab Chip 2010; 10:3163-9. doi: 10.1039/C0LC00237B [Crossref] [ Google Scholar]
- Martinez AW, Phillips ST, Nie Z, Cheng C-M, Carrilho E, Wiley BJ. Programmable diagnostic devices made from paper and tape. Lab Chip 2010; 10:2499-504. doi: 10.1039/c0lc00021c [Crossref] [ Google Scholar]
- Li C-z, Vandenberg K, Prabhulkar S, Zhu X, Schneper L, Methee K. Paper based point-of-care testing disc for multiplex whole cell bacteria analysis. Biosens Bioelectron 2011; 26:4342-8. doi: 10.1016/j.bios.2011.04.035 [Crossref] [ Google Scholar]
- Jokerst JC, Adkins JA, Bisha B, Mentele MM, Goodridge LD, Henry CS. Development of a paper-based analytical device for colorimetric detection of select foodborne pathogens. Anal Chem 2012; 84:2900-7. doi: 10.1021/ac203466y [Crossref] [ Google Scholar]
- Lisak G, Cui J, Bobacka J. Paper-based microfluidic sampling for potentiometric determination of ions. Sens Actuators B Chem 2015; 207:933-9. doi: 10.1016/j.snb.2014.07.044 [Crossref] [ Google Scholar]
- He M, Li Z, Ge Y, Liu Z. Portable upconversion nanoparticles-based paper device for field testing of drug abuse. Anal Chem 2016; 88:1530-4. doi: 10.1021/acs.analchem.5b04863 [Crossref] [ Google Scholar]
- Lu J, Ge S, Ge L, Yan M, Yu J. Electrochemical DNA sensor based on three-dimensional folding paper device for specific and sensitive point-of-care testing. Electrochim Acta 2012; 80:334-41. doi: 10.1016/j.electacta.2012.07.024 [Crossref] [ Google Scholar]
- Tang C, Vaze A, Rusling J. Paper-based electrochemical immunoassay for rapid, inexpensive cancer biomarker protein detection. Anal Methods 2014; 6:8878-81. doi: 10.1039/C4AY01962H [Crossref] [ Google Scholar]
- Su M, Ge L, Ge S, Li N, Yu J, Yan M. Paper-based electrochemical cyto-device for sensitive detection of cancer cells and in situ anticancer drug screening. Anal Chim Acta 2014; 847:1-9. doi: 10.1016/j.aca.2014.08.013 [Crossref] [ Google Scholar]