Abstract
Summary
Aptamers ( Aps ) are short single-strand nucleic acids exhibiting unique 3D structure which facilitate their targeting potential against various cancer molecular markers ( CMMs ). Such features of Aps not only make them as suitable homing agents in targeted drug delivery systems (DDSs) but also candidate them as macromolecules that inhibit the interaction of the target ligand with other proteins. On the other hand, the conjugation of Aps with another therapeutic molecule such as antisense oligonucleotides (ASOs), siRNAs/miRNAs, Aps , toxins, chemotherapeutic agents, DNAzymes/Ribozymes provides hopeful strategy to eradicate the malignancies and overcome the off-target unwanted side effects. Such prominent features of Aps make them a promising treatment modality to overcome the tumor complexity and heterogeneity, which can be consequently applied for personalized therapy of cancer by using bispecific Ap-based therapeutics.
Keywords: Aptamer, Aptamedicine, Nanomedicine, Drug delivery systems, Personalized medicine, Cancer therapy
Copyright and License Information
© 2019 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Aptamers (Aps), as short single-strand nucleic acids (DNA or RNA), can bind to their specific molecular targets with the high affinity and specificity through their unique three or two dimensional (3D/2D) structures. Aps are considered as great alternatives to the antibodies, in large part because of offering flexible conformations, high stability, ease of modifications, low/or no immunogenicity, and cost-effective synthesis.
1,2
Aps can act as the therapeutic agents per se, or as the targeting agents. Nowadays, a number of Aps with the ability of binding to the specific cancer molecular markers (CMMs) have been selected using the systematic evolution of ligands by exponential enrichment (SELEX) process and synthesized artificially. Aps with specificity to the tumor-associated proteins have widely been applied in the molecular diagnosis and therapy of cancer.
3
Further, the exclusive features of Aps make them robust tools for enhancing the effects of various remedies and establishment of different therapeutic systems, including Ap chimera, bispecific Aps and Ap-decorated multimodal nanosystems as shown in Fig. 1.
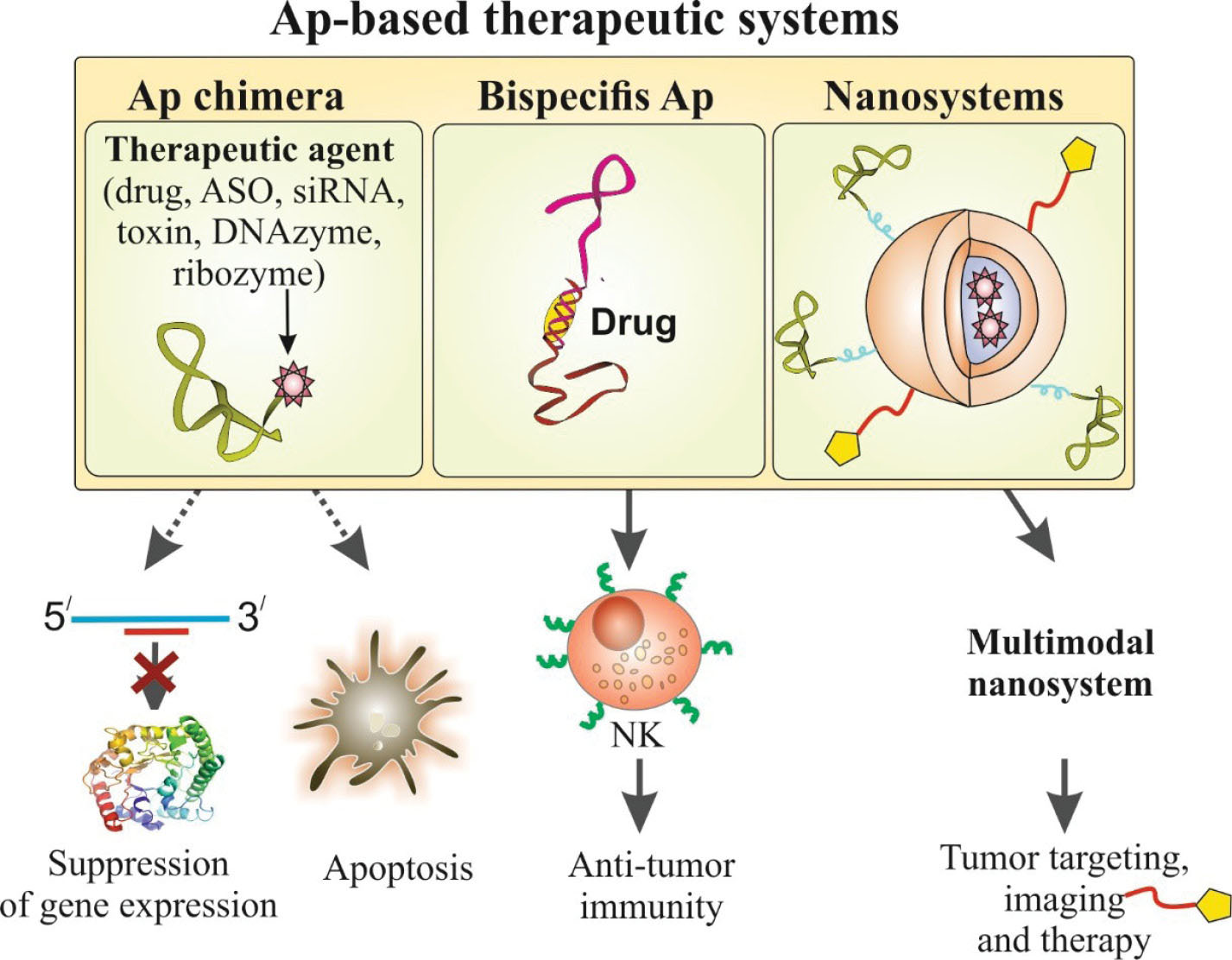
Fig. 1.
Schematic representation of various aptamer (Ap)-based therapeutic systems.
.
Schematic representation of various aptamer (Ap)-based therapeutic systems.
Noteworthy features of Ap-based multifunctional systems (the so-called aptamedicine) have been the driving force of many researches, resulting in the development of various therapeutic modalities against various malignancies. It is envisioned that Ap-based advanced pharmaceuticals to be translated into potential clinical applications against various detrimental diseases, including different types of cancers. In fact, the specific conformation of Aps allows them to act as the protein antagonist, which may inhibit the interaction of the target protein with other molecular entities. Some Aps were shown to possess the anticancer ability and reduce the proliferation of cancer cells by the suppression of specific target proteins. To date, several Aps have been developed against important clinical targets, including platelet-derived growth factor (PDGF)
4
and vascular endothelial growth factor (VEGF)
5
(Table 1). Of these, pegaptanib (Macugen®), which is an RNA Ap specific to VEGF, is the first nucleic acid Ap approved by the U.S. FDA in 2004 and used for the neovascular age-related macular degeneration (nAMD) disease.
6
Further, other Aps have been examined in different phases of clinical trials for diagnosis and treatment of cancer. For examples, it has recently been demonstrated that the Sgc8 single-stranded DNA Ap has a high accumulation in the PTK7-positive tumors and is currently in the early-phase I clinical trial (ID: NCT03385148) to evaluate its diagnostic value in colorectal cancer.
7
Also, PEGylated NOX-A12 (Olaptesed pegol), specifically bind to the chemokine (C-X-C motif) ligand 12 (CXCL12). It is in the phase IIa in multiple myeloma which validates its safety profile without any additional toxicity.
8
Table 1.
Aptamers for cancer therapy in clinical trials
|
Aptamer drug
|
Target
|
Disease
|
Type of study
(ClinicalTrials.gov Identifier)
|
| Pegaptanib (Macugen®) |
VEGF |
Age-related macular degeneration
Retinal vein occlusion Diabetic macular edema
|
Phase IV, recruiting
(NCT01573572)
|
| 68Ga-Sgc8 |
PTK7 |
Colorectal cancer |
Early-phase I (NCT03385148) |
| AS1411 (AGRO-100) |
NF-κB, BCL-2 |
AML
Advanced solid tumors
Metastatic renal cell carcinoma
|
Phase II completed (NCT01034410)
Phase 1, completed (NCT00881244)
Phase 2, unknown (NCT00740441)
|
| NOX-A12 (Speigelmer®) |
CXCL12 |
Multiple myelomas
Chronic lymphocytic
Leukemia metastatic colorectal cancer
|
Phase II, completed (NCT01521533)
Phase II, completed (NCT01486797)
Phase I (NCT03168139)
|
| ARC1779 |
C5 |
Intracranial embolism cerebral Thromboembolism carotid stenosis |
Phase II, terminated (NCT00742612) |
| NOX-E36 |
Chemokine (C-C motif) ligand 2 |
Type 2 diabetes mellitus
Mellitus albuminuria
|
Phase II, completed (NCT01547897) |
| E10030 |
PDGF |
Macular degeneration |
Phase I, completed (NCT005691400 |
| ARC19499 |
TFPI |
Hemophilia |
Phase 1, terminated (NCT01191372) |
| REG1 |
|
Coronary artery disease |
Phase 2, completed (NCT00715455) |
VEGF: Vascular endothelial growth factor, PTK7: Protein tyrosine kinase-7, NF-κB: Nuclear factor kappa light chain enhancer of activated B cells, CXCL12: Chemokine (C-X-C motif) ligand 12, C5: Complement component 5, PDGF: Platelet-derived growth factor, TFPI: Tissue factor pathway inhibitor.
Aps-based drug delivery systems (DDSs) have recently been developed for the targeted therapy of various malignancies.
9-11
These advanced systems can specifically target the intended cells through specific affinity of Aps to designated oncomarkers, and therefore, reduce the nonspecific cytotoxic impacts of chemotherapy agents that can non-specifically affect both cancerous and healthy cells/tissues.
12
Various Ap-based nanosystems/multifunctional theranostics have successfully been designed for targeted delivery of a wide variety of anticancer drugs/genes owing to the surface functionalization of nanoscaled DDSs with Aps.
13
Additionally, modification of Aps with various chemical groups allows them to be further conjugated with other molecules (Ap-X), including chemotherapy agents, other Aps, siRNAs/miRNAs, antisense oligonucleotides (ASOs), Abs, toxins, ribozymes, DNAzymes, peptides, and imaging agents (so-called Ap chimera).
14
In these hybrid systems (i.e. Ap-X nanoconjugates), the chimerization of an Ap with another Ap produces bispecific Aps, which can be further functionalized to engineer new multifunctional Ap-based structures.
15
In Ap-drug nanoconjugates, some anthracyclines drugs such as doxorubicin (DOX) can be non-covalently intercalated to the GC rich double-strand region of Aps without affecting the Ap conformation.
16
The small size of the Ap-drug nanoconjugates facilitates its penetration into the tumor mass and accumulation in the tumor microenvironment (TME).
17,18
Ap-siRNA/or antisense chimera systems have been developed in order to specifically suppress the overexpressed genes involved in the initiation, progression, and chemoresistance of malignancy.
19-22
Bispecific Aps have been reported to provide enhanced clinical anticancer outcomes.
23
Engineering bispecific Aps with antitumor immunity impacts, through the activation of immune system effector cells (e.g., natural killer cells, dendritic cells, and effector T lymphocytes) is deemed to result in an Ap-mediated antitumor immunity.
24
This can be achieved by recruiting immune system effector cells to the target tumor cells through bispecific Aps, which can concurrently bind to the specific receptors on the surface of tumor and the immune system effector cells.
25
Moreover, designing appropriate bi/multivalent Aps specific to the T cell receptors can lead to the co-stimulation of T cells and enhanced tumor immunogenicity.
26,27
This latter approach may revolutionize the cell therapy strategies.
Bispecific Aps, which can synchronously bind to the different type of CMMs, are deemed to provide promising strategy to overcome the tumor complexity and heterogeneity. It should be noted that the tumor heterogeneity is often referred to as the differences between same types of tumors in different patients and between cancer cells within a particular tumor. Such hallmarks, together with other traits of cancer cells (e.g., genetics and epigenetics changes) might reduce the therapeutic impacts of anticancer agents and hence result in the different responses, even in the same patients.
28
In this context, the coherency of drugs/genes to such structures provides a powerful therapeutic platform which can be further evolved towards "personalized nanomedicines" by means of bispecific Ap-based therapeutics. It appears that the personalized medicine, informed by a molecular understanding of the disease, might provide a great possibility for much more effective preventive and therapeutic interventions. Personalized medicine uses information about a person’s genes, proteins, and environment to prevent, diagnose, and treat disease.
29
With the progress in cancer genomics (in particular oncogenomics), epigenetics and metabolomics using high-throughput sequencing methods, it seems that such specific bi/multi-functional Aps structures targeting different CMMs provide the maximal therapeutic impacts in an individual patient.
Funding sources
This work is a part of a Ph.D. thesis (No: 94/009/155/2) supported by the Research Center for Pharmaceutical Nanotechnology (grant No: 94009), Biomedicine Institute, Tabriz University of Medical Sciences.
Ethical statement
There is none to be declared.
Competing interests
No competing interests to be disclosed.
Authors contribution
SV, ME, JB and YO conceptualized the study. SV and ME drafted the manuscript. JB reviewed the manuscript. YO finalized the manuscript.
References
- Morita Y, Leslie M, Kameyama H, Volk DE, Tanaka T. Aptamer Therapeutics in Cancer: Current and Future. Cancers (Basel) 2018; 10. doi: 10.3390/cancers10030080 [Crossref]
- Lee JF, Stovall GM, Ellington AD. Aptamer therapeutics advance. Curr Opin Chem Biol 2006; 10:282-9. doi: 10.1016/j.cbpa.2006.03.015 [Crossref] [ Google Scholar]
- Mercier MC, Dontenwill M, Choulier L. Selection of Nucleic Acid Aptamers Targeting Tumor Cell-Surface Protein Biomarkers. Cancers (Basel) 2017; 9. doi: 10.3390/cancers9060069 [Crossref]
- Green LS, Jellinek D, Jenison R, Ostman A, Heldin CH, Janjic N. Inhibitory DNA ligands to platelet-derived growth factor B-chain. Biochemistry 1996; 35:14413-24. doi: 10.1021/bi961544+ [Crossref] [ Google Scholar]
- Green LS, Jellinek D, Bell C, Beebe LA, Feistner BD, Gill SC. Nuclease-resistant nucleic acid ligands to vascular permeability factor/vascular endothelial growth factor. Chem Biol 1995; 2:683-95. [ Google Scholar]
- Zhou B, Wang B. Pegaptanib for the treatment of age-related macular degeneration. Exp Eye Res 2006; 83:615-9. doi: 10.1016/j.exer.2006.02.010 [Crossref] [ Google Scholar]
- Xiao Z, Shangguan D, Cao Z, Fang X, Tan W. Cell-specific internalization study of an aptamer from whole cell selection. Chemistry 2008; 14:1769-75. doi: 10.1002/chem.200701330 [Crossref] [ Google Scholar]
- Ludwig H, Weisel K, Petrucci MT, Leleu X, Cafro AM, Garderet L. Olaptesed pegol, an anti-CXCL12/SDF-1 Spiegelmer, alone and with bortezomib-dexamethasone in relapsed/refractory multiple myeloma: a Phase IIa Study. Leukemia 2017; 31:997-1000. doi: 10.1038/leu.2017.5 [Crossref] [ Google Scholar]
- Guo X, Zhu X, Gao J, Liu D, Dong C, Jin X. PLGA nanoparticles with CD133 aptamers for targeted delivery and sustained release of propranolol to hemangioma. Nanomedicine (Lond) 2017; 12:2611-24. doi: 10.2217/nnm-2017-0130 [Crossref] [ Google Scholar]
- Hanafi-Bojd MY, Moosavian Kalat SA, Taghdisi SM, Ansari L, Abnous K, Malaekeh-Nikouei B. MUC1 aptamer-conjugated mesoporous silica nanoparticles effectively target breast cancer cells. Drug Dev Ind Pharm 2018; 44:13-8. doi: 10.1080/03639045.2017.1371734 [Crossref] [ Google Scholar]
- Jurek PM, Zablocki K, Wasko U, Mazurek MP, Otlewski J, Jelen F. Anti-FGFR1 aptamer-tagged superparamagnetic conjugates for anticancer hyperthermia therapy. Int J Nanomedicine 2017; 12:2941-50. doi: 10.2147/ijn.s125231 [Crossref] [ Google Scholar]
- Vandghanooni S, Eskandani M, Barar J, Omidi Y. AS1411 aptamer-decorated cisplatin-loaded poly (lactic-co-glycolic acid) nanoparticles for targeted therapy of miR-21-inhibited ovarian cancer cells. Nanomedicine (Lond) 2018; 13:2729-58. doi: 10.2217/nnm-2018-0205 [Crossref] [ Google Scholar]
- Vandghanooni S, Eskandani M, Barar J, Omidi Y. Recent advances in aptamer-armed multimodal theranostic nanosystems for imaging and targeted therapy of cancer. Eur J Pharm Sci 2018; 117:301-12. doi: 10.1016/j.ejps.2018.02.027 [Crossref] [ Google Scholar]
- Kanwar JR, Roy K, Kanwar RK. Chimeric aptamers in cancer cell-targeted drug delivery. Crit Rev Biochem Mol Biol 2011; 46:459-77. doi: 10.3109/10409238.2011.614592 [Crossref] [ Google Scholar]
- Vandghanooni S, Eskandani M, Barar J, Omidi Y. Bispecific therapeutic aptamers for targeted therapy of cancer: a review on cellular perspective. J Mol Med 2018. doi: 10.1007/s00109-018-1669-y [Crossref]
- Bagalkot V, Farokhzad OC, Langer R, Jon S. An aptamer-doxorubicin physical conjugate as a novel targeted drug-delivery platform. Angew Chem Int Ed Engl 2006; 45:8149-52. doi: 10.1002/anie.200602251 [Crossref] [ Google Scholar]
- Barar J. Bioimpacts of nanoparticle size: why it matters?. Bioimpacts 2015; 5:113-5. doi: 10.15171/bi.2015.23 [Crossref] [ Google Scholar]
- Omidi Y, Barar J. Targeting tumor microenvironment: crossing tumor interstitial fluid by multifunctional nanomedicines. Bioimpacts 2014; 4:55-67. doi: 10.5681/bi.2014.021 [Crossref] [ Google Scholar]
- Subramanian N, Kanwar JR, Akilandeswari B, Kanwar RK, Khetan V, Krishnakumar S. Chimeric nucleolin aptamer with survivin DNAzyme for cancer cell targeted delivery. Chem Commun 2015; 51:6940-3. doi: 10.1039/c5cc00939a [Crossref] [ Google Scholar]
- Iaboni M, Russo V, Fontanella R, Roscigno G, Fiore D, Donnarumma E. Aptamer-miRNA-212 Conjugate Sensitizes NSCLC Cells to TRAIL. Mol Ther Nucleic Acids 2016; 5:e289. doi: 10.1038/mtna.2016.5 [Crossref] [ Google Scholar]
- Dai F, Zhang Y, Zhu X, Shan N, Chen Y. Anticancer role of MUC1 aptamer-miR-29b chimera in epithelial ovarian carcinoma cells through regulation of PTEN methylation. Target Oncol 2012; 7:217-25. doi: 10.1007/s11523-012-0236-7 [Crossref] [ Google Scholar]
- Zhao N, Pei SN, Qi J, Zeng Z, Iyer SP, Lin P. Oligonucleotide aptamer-drug conjugates for targeted therapy of acute myeloid leukemia. Biomaterials 2015; 67:42-51. doi: 10.1016/j.biomaterials.2015.07.025 [Crossref] [ Google Scholar]
- Gilboa E, McNamara J, 2nd 2nd, Pastor F. Use of oligonucleotide aptamer ligands to modulate the function of immune receptors. Clin Cancer Res 2013; 19:1054-62. doi: 10.1158/1078-0432.ccr-12-2067 [Crossref] [ Google Scholar]
- Pastor F. Aptamers: A New Technological Platform in Cancer Immunotherapy. Pharmaceuticals (Basel) 2016; 9. doi: 10.3390/ph9040064 [Crossref]
- Boltz A, Piater B, Toleikis L, Guenther R, Kolmar H, Hock B. Bi-specific aptamers mediating tumor cell lysis. J Biol Chem 2011; 286:21896-905. doi: 10.1074/jbc.M111.238261 [Crossref] [ Google Scholar]
- Schrand B, Berezhnoy A, Brenneman R, Williams A, Levay A, Kong LY. Targeting 4-1BB costimulation to the tumor stroma with bispecific aptamer conjugates enhances the therapeutic index of tumor immunotherapy. Cancer Immunol Res 2014; 2:867-77. doi: 10.1158/2326-6066.cir-14-0007 [Crossref] [ Google Scholar]
- Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 2013; 13:227-42. doi: 10.1038/nri3405 [Crossref] [ Google Scholar]
- Allison KH, Sledge GW. Heterogeneity and cancer. Oncology (Williston Park) 2014; 28:772-8. [ Google Scholar]
-
National Cancer Institute. Personalized medicine 2011; Available from: http://www.cancer.gov/dictionary/?CdrID=561717.