BioImpacts. 9(4):199-209.
doi: 10.15171/bi.2019.25
Original Research
Design, synthesis and evaluation of biological activities of some novel anti-TB agents with bio-reducible functional group
Hossein Khani-Meinagh 1, Hossein Mostafavi 1, *  , Norbert Reiling 2, 3, Majid Mahdavi 4, Gholamreza Zarrini 4
, Norbert Reiling 2, 3, Majid Mahdavi 4, Gholamreza Zarrini 4
Author information:
1Department of Organic and Biochemistry, Faculty of Chemistry, University of Tabriz, Tabriz 5166614766, Iran
2Division of Microbial Interface Biology, Research Center Borstel, Leibniz Lung Center, Parkallee 22, D-23845 Borstel, Germany
3German Center for Infection Research (DZIF), Partner Site Hamburg-Lübeck-Borstel, 23845 Borstel, Germany
4Department of Biology, Faculty of Natural Science, University of Tabriz, Tabriz, Iran
Abstract
Introduction:
With regard to the anti-mycobacterial activity of 2-pyrazinoic acid esters (POEs), recent studies have shown that both pyrazine core and alkyl part of POE interact with the fatty acid synthase type (I) (FAS (I)) precluding a complex formation between NADPH and FAS (I).
Methods:
Considering this interaction at the reductase site of FAS (I) responsible for reduction of β-ketoacyl-CoA to β-hydroxyacyl-CoA, we hypothesized that POE containing a bioreducible center in its alkyl part might show an increased anti-tubercular activity due to the involvement of FAS (I) in extra bio-reduction reaction. Thus, we synthesized novel POEs, confirmed their structures by spectral data, and subsequently evaluated their anti-mycobacterial activity against Mycobacterium tuberculosis (Mtb) (H37Rv) strain at 10 μg/mL concentration.
Results:
Compounds 3c, 3j, and 3m showed higher activity with regard to the inhibition of Mtb growth by 45.4, 45.7, and 51.2% respectively. Unexpectedly, the maltol derived POE 3l having the lowest log p value among the POEs indicated the highest anti-mycobacterial growth activity with 56% prevention. Compounds 3c and 3l showed no remarkable cytotoxicity on human macrophages at 10 μg/mL concentration as analyzed by xCELLigence real-time cell analysis. In further experiments, some of the tested POEs, unlike pyrazinamide (PZA), exhibited significant antibacterial and also anti-fungal activities. POEs showed an enhanced bactericidal activity on gram-positive bacteria as shown for Staphylococcus aureus , e.g. compound 3b with a MIC value of 125 μg/mL but not E. coli as a gram-negative bacteria, except for maltol derived POE (3l) that showed an inverse activity in the susceptibility test. In the anticancer activity test against the human leukemia K562 cell lines using MTT assay, compounds 3e and 3j showed the highest cytotoxic effect with IC50 values of 25±8.0 μΜ and 25±5.0 μΜ, respectively.
Conclusion:
It was found that the majority of POEs containing a bioreducible center showed higher inhibitory activities on Mtb growth when compared to the similar compounds without a bio-reducible functional group.
Keywords: Bioreducible center, Cytotoxicity, Fatty acid biosynthesis, Mycobacterial growth inhibition, 2-Pyrazinoic acid ester
Copyright and License Information
© 2019 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
The World Health Organization (WHO) estimates that Mycobacterium tuberculosis (Mtb) has infected one-third of the globe’s population, the majority of which being latently infected. Recent reports have indicated that 1.4 million people died of this infection in 2016 apart from 0.4 million deaths being associated with HIV infection.
1
A major worry is the nonstop rise of patients who have extensively been infected with multidrug-resistant (MDR) and drug-resistant (XDR) Mtb strains in recent years.
2
TB needs a surge in current efforts as claimed by the WHO.
1
One important strategy is to introduce novel highly potent drugs and effective agents to successfully treat this fatal disease.
3
The thick and complex cell wall having mycolic acids represents a very efficient barrier against many of common antibacterial agents and disinfectants.
4
Pyrazinamide (PZA) represents a key part in current TB chemotherapy due to its unique and strong sterilizing capability that enables this vital drug to kill semi-dormant, non-replicating persistent tubercle bacteria inside the macrophage that other TB drugs fail to kill.
5
Use of PZA in TB combinatorial therapy has been shown to successfully reduce treatment length by 3 months.
5
Having excellent synergy with most anti TB agents such as rifampicin and specially bedaquilline, a recently introduced novel drug, PZA is and will remain an important pillar in current chemotherapy and future multi-drug therapy regimens.
5
The antibacterial activity of PZA is mediated by pyrazinoic acid (POA) which is generated as a result of enzymetically catalyzed conversion in the presence of pyrazinamidase inside Mtb.
6,7
PZA is a selective drug against most isolates of the Mtb complex exceptMycobacteriumbovis . Strong pH dependent efficacy is another peculiar characteristic of PZA against Mtb since a decrease in pH results in an increase of PZA efficacy., The fact that POA still targets PZA-resistant M . tuberculosis , has increased motivation for the development of new POA containing anti–tuberculosis drugs.
5,10
Several target proteins have been identified for PZA of which the ribosomal protein (RpsA) involved in protein translation and fatty acid synthase type (I) (FAS (I)) are the most significant ones.
11-13
It has been demonstrated that 2-pyrazinoic acid esters (POEs) have a greater and broader in vitro activity than PZA and POA against susceptible Mtb bacteria as well as PZA-resistant Mtb isolates and non-tuberculous mycobatcteria.
14-17
Cynamon hypothesized that hydrolysable POEs due to the presence of multiple esterases in Mycobacterium cell could circumvent any need for activation by pyrazinamidase that was inactivated in PZA resistant strains. In addition, conversion of POA to more lipophilic POE could lead to higher penetration of this agent through the Mtb cell wall.
9,16,18
However, Zimhony et al observed that n- propyl pyrazinoate inhibited fatty acid biosynthesis more effectively than PZA at any common pH and its inhibition was not pH-dependent. They ascribed the enhanced inhibition to both increased lipophilicity and intrinsic activity of POEs.
12,19
It has been proven that POE inhibits FAS (I), and that POE inhibited without any need for hydrolysis to POA.
12
The saturation transfer difference (STD) NMR technique clarified that in the FAS (I) binding to propyl ester, the amount of protein contact with the pyrazine core decreased compared to that of PZA, while its connection with alkyl part of POE was intensive.
13,20
This result along with the impact of alkyl chain length on the POE affinity for FAS (I) association, corroborating previous results on the chain length effect on the enzyme inhibition efficacy, demonstrated the instinct inhibitory activity of POEs.
19
POEs disturb Mtb growth by the inhibition of NADPH binding as a bioreductant agent to the eukaryotic FAS (I) responsible for the fatty acid biosynthesis having reductase activity.
19
Β-ketoacyl-CoA is converted to β-hydroxyacyl-CoA at the reductase site of FAS (I) as a single multifunctional enzyme.
21-23
Given the interaction of the alkyl part of POE with FAS (I), introducing POEs containing reducible center may show synergistic effect due to the prevention of NADPH function and involving multi-functional enzyme FAS (I) in another side interaction with reducible center on the POE. As a result, catalytic action of FAS (I) may be limited in the fatty acid biosynthesis besides the inhibition of NADPH function. In addition, the presence of these polar groups may change POEs transport into Mtb due to further interaction with carrier molecules located on the Mtb cell wall. The above mentioned researches on the POEs, motivated us to synthesize new POEs having side bioreducible functional groups and to evaluate their activity against the Mtb growth along with their antibacterial, antifungal, and anticancer efficacy.
Materials and Methods
Materials
All solvents and reagents were purchased from Merck (Merck Co., Germany) without any more purification except eugenol oxide that was synthesized from eugenol using the general procedure reviewed by Fieser and Fieser.
24
EDC.HCL was prepared from Sigma (Sigma-Aldrich Co., Germany). Dimethyl sulfoxide (DMSO) and 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazoliumbromide (MTT) were bought from Sigma (Sigma-Aldrich Co., Germany). The K562 cell line was obtained from the Pasteur Institute (Tehran, Iran). The cell culture medium (RPMI 1640) and penicillin/streptomycin were purchased from Gibco (Life Technologies, Paisley, Scotland). The culture plates were supplied from SPL (Seoul, South Korea). Mtb (H37Rv strain) ATCC (No.27294) was obtained from the National Reference Center (NRC) in Borstel, Germany. 7H9 medium (cat# 271310) was obtained from Becton Dickinson (BD Difco; Becton Dickinson). Rifampicin was obtained from the National Reference Center (NRC) in Borstel, Germany. Staphylococcus aureus (PTCC 1112), Escherichia coli (PTCC 1047), and Candidakefyr (ATCC 38296) were provided by the laboratory of Microbiology (Department of Biology, Faculty of Natural Science, University of Tabriz, Tabriz, Iran).
Instrumental Measurement
Melting points were recorded using the melting- point meter (Electro thermal 9100, Staffordshire, UK). IR spectra were performed on FT-IR spectrometer (FT IR-8101M, SHIMADZU, Kyoto, Japan). 1H NMR and 13CNMR spectra were recorded with a Bruker SpectrospinAvance 400 spectrometer operating at 400 MHz (Bucker GmbH, Ettlingen, Germany) and chemical shifts were measured in ppm relative tetramethylsilane. Elemental analyses were conducted with Vario EL ΙΙΙ apparatus (Elementar Co, Langenselbold, Germany). The reactions and purities of the compounds were monitored by TLC on silica gel 60 F254 aluminum sheets purchased from Merck (Darmstadt, Germany). The TLC plates visualized using UV light (254 and 366nm, CAMAG, Muttenz, Switzerland). Column chromatography was carried out on silica gel 60 F254 glass plates (Merck, Darmstadt, Germany, 60A, 70-230 mesh). Continuously live cell proliferation, morphology and viability with label free assay were performed using xCELLigence Real Time cell analysis instrument (ACEA Bioscience, San Diego, CA, USA). The absorbance was measured for MTT assay by using a Bio-Tek Cytation 3 Cell Imaging Multi-Mode Reader (Elx 800 Microplate Reader, Quant Bio-tek Instruments, Winooski, VT, 195 USA).
Optimized procedure for preparation of 2-pyrazinoicacid esters
DMAP (0.3 mmol) was added to a swirling mixture of 2-pyrazin carboxylic acid (1 mmol) in dichloromethane (2 mL) and stirred for 10 minutes, then alcohol compound (0.7 mmol) was added and resulting solution was cooled to 0oC, after that EDC.HCl (0.9 mmol) was added gradually to cool the mixture. After 30 minutes, cooling source was removed and the reaction mixture was stirred for further 4 hours at room temperature, and the proceeding of the reaction was followed by thin layer chromatography (TLC) (Ethyl acetate (ETAC)/n-hexane 1:3). Finally, the mixture was rinsed with saturated sodium bicarbonate and 0.05 N HCl solution, respectively, dried over MgSO4, and the solvent was evaporated to achieve the POE compounds. Some of the obtained solid esters including 3a, 3b, 3c, 3f, and 3i were more purified by recrystallization from appropriate solvents mentioned below,
25
and two other compounds (3e, 3g) were purified on silica gel column chromatography. The resultant products are described below(for more details see supplementary section):
2-methoxyphenyl pyrazine-2-carboxylate (3a):Recrystalized from (ETAC/n-hexane; 1:3). Transparent crystal (80%), mp 65-68°C. 1HNMR(400 MHz, CDCl3): 3.86 (3H, s), 7.02
(2H, m), 7.21 (1H, dd, j= 8, 1.6 Hz), 7.27 (1H, td, j= 8, 1.6 Hz), 8.81 (1H, m), 8.83 (1H, d, j= 2.2 Hz), 9.47 (1H, d, j= 0.65 Hz). 13CNMR(400 MHz, CDCl3): 54.76, 111.39, 119.72, 121.51, 126.40, 138.29, 141.82, 143.56, 145.73, 146.93, 149.81, 160.89. FT-IR(KBr, cm-1): 3068, 2940, 1747, 1611, 1508, 1303, 1264, 1205, 1051, 1025, 777, 760. C12H10N2O3 requires; C, 62.60; N, 12.17; H, 4.38. Found: C, 62.72; N, 12.29; H, 4.25.
4-formyl-2-methoxyphenyl pyrazine-2-carboxylate (3b):Recrystalized from (ETAC/n-hexane; 1:3) White solid (75%), mp 111-112°C. 1HNMR(400 MHz, CDCl3): 3.91 (3H, S) ,7.42 (1H, d, J=8Hz), 7.54-7.57 (2H, m), 8.83-8.84 (1H, m), 8.87 (1H, d, J=2.4 Hz), 9.48 (1H, d, J=1.2 Hz). 13CNMR (400 MHz, CDCl3): 55, 109.9, 122.2, 123.5, 134.6, 141.3, 143.3, 143.6, 145.8, 147.2, 150.7, 160.3, 189.8. FT-IR (KBr, cm-1): 3047, 1772, 1756, 1705, 1611, 1299, 1277.C1 3H10N2O4 requires: C, 60.47; N, 10.85; H, 3.90. Found: C, 60.53; N, 10.63; H, 3.97.
4-formylphenyl pyrazine-2-carboxylate (3c):Recrystalized from (ETAC/n-hexane; 1:3) White solid (80%), mp 134-135°C. 1HNMR(400 MHz, CDCl3): 7.48 (2H, d, J=8.4 Hz), 8.01 (2H, m), 8.84 (1H, m), 8.89 (1H, d, J=2.4 Hz), 9.49 (1H, d, J=1.2 Hz). 13CNMR (400 MHz, CDCl3): 121.34, 130.39, 133.55, 141.45, 143.72, 145.89, 147.45, 153.96, 160.95, 189,74. FT-IR (KBr, cm-1): 3068, 2854, 2769, 1760, 1705, 1611, 1316, 1281, 1222, 1166, 1123, 1029, 884, 863, 824, 773. C12H8N2O3 requires: C, 63.16; N, 12.28; H, 3.53. Found: C, 63.26; N, 12.35; H, 338.
5-allyl-2-methoxyphenyl pyrazine-2-carboxylate (3d): White solid (95%), mp 97-99°C.
1
HNMR(400 MHz, CDCl3): 3.42 (2H, d, J= 6.8 Hz), 3.81 (3H, s), 5.13 (2H, m), 5.99 (1H, m), 6.84 (2H, m), 7.12 (1H, d, 8 Hz), 8.81 (1H, m), 8.83 (1H, d, J= 2.4 Hz), 8.47 (1H, s). 13CNMR(400 MHz, CDCl3): 39.09, 45.80, 111.79, 115.28, 119.21, 121.27, 135.88, 136.63, 138.65, 142, 143.58, 145.81, 146.93, 149.67, 161.08. FT-IR(KBr, cm-1): 3089, 2940, 1764, 1650, 1615, 1517, 1307, 1286, 1277, 1205, 1132, 1038, 1025,927. C15H14N2O3 Requires: C, 66.66; N, 10.36; H, 5.22. Found: 66.49; N, 10.14; H, 5.29.
2-methoxy-5-(oxiran-2-ylmethyl)phenylpyrazine-2-carboxylate (3e): Purified with column chromatography (ETAC/n-hexane, 1:3) low melting tan solid (65%).1HNMR(400 MHz, CDCl3): 2.58 (1H, dd, J= 4.8, 2.8-2.6 Hz), 2.84 (1H, dd, J= 5.8, 4.8 Hz), 2.88-2.90 (2H, m), 3.18-3.20 (1H, m), 3.83 (3H, s), 6.88-6.91 (1H, dd, J= 8, 1.6 Hz), 6.94 (1H, d, J=1.6 Hz), 7.15 (1H, d, J=8 Hz), 8.81 (1H, dd, J= 2.4, 1.6 Hz), 8.84 (1H, d, J= 2.4 Hz), 9.47 (1H, d, J= 1.2 Hz). 13CNMR (400 MHz, CDCl3): 37.68, 45.81, 51.26, 54.87, 112.24, 120.13, 121.46, 135.91, 137.11, 141.92, 143.61, 145.83, 146,99, 149.76, 161.04. FT-IR(KBr, cm-1): 3068, 2940, 1764, 1615, 1521, 1303, 1277, 1205, 1106, 1038, 1025, 978, 957, 940, 871, 841, 798, 779, 777, 760, 734. C15H14N2O4 requires: C, 62.93; N, 9.79; H, 4.93. Found: C, 62.88; N, 9.71; H, 4.99.
2-acetylphenyl pyrazine-2-carboxylate (3f):Recrystalized from (ETAC/n-hexane; 1:4) Off-White solid (70%), mp 62-63°C. 1HNMR (400 MHz, CDCl3): 2.58 (3H, S), 7.3 (1H, dd, J= 8.4, 1.2 Hz), 7.4 (1H, td, J=8, 1.2 Hz), 7.64 (1H, td, J= 8, 1,6 Hz),7.92 (1H, dd, J=8, 1.6 Hz), 8.82 (1H, dd, J=2.4, 1.6 Hz), 8.86 (1H, d, J=2.4 Hz), 9.48 (1H, d, J=1.2 Hz). 13CNMR(400 MHz, CDCl3): 28.13, 122.87, 125,72, 128.85, 129.82, 132.89, 141.87, 143.61, 145.96, 147.12, 147.87, 161.67, 196.15. FT-IR (KBr, cm-1): 3089, 2940, 2876, 1756, 1699, 1611, 1290, 1264, 1145, 1120, 1055, 1029, 769, 751. C21H18N2O5 requires: C, 66.66; H, 4.79; N, 7.40. Found: C, 66.73; H, 4.84; N, 7.29.
Cinnamyl pyrazine-2-carboxylate (3g):Purified with column chromatography (ETAC/n-hexane; 1:3), yellow solid (75%), mp 49-52°C. 1HNMR(400 MHz, CDCl3): 5.10 (2H, dd, j= 6.73, 1.1 Hz), 6.44 (1H, dt, j= 15.85, 6.73 Hz), 6.79 (1H, d, j= 15.85 Hz), 7.26-7.43 (5H, m), 8.78 (1H, d, j= 2.4), 8.74 (1H, m), 9.36 (1H, d, j= 1.4 Hz).13CNMR(400 MHz, CDCl3): 65.87, 121.01, 125.72, 127.31, 127.61, 134.79, 134.88, 142.42, 143.42, 145.35, 146.70, 162.74. FT-IR(KBr, cm-1): 3068, 3047, 2980, 1726, 1307, 1286, 1140, 1055, 1025, 974, 948, 781, 756, 700. C14H12N2O2 requires; C, 69.99; N, 11.66; H, 5.03. Found: C, 69.92; N, 11.50; H, 5.11.
2-oxo-2H-chromen-6-yl pyrazine-2-carboxylate (3h):White precipitate (87%), mp 192-194°C.1HNMR(400 MHz, CDCl3): 6.46 (1H, d, J= 9.56 Hz), 7.23-7.27 (2H, m), 7.59 (1H, d, J= 8.8 Hz), 7.75 (1H, d, J= 9.6 Hz), 8.84 (1H, m), 8.89 (1H, d, J= 2.4 Hz), 9.48 (1H, d, J= 1.2 Hz). 13CNMR (400 MHz, CDCl3): 09.54, 115.54, 116.24, 117.23, 127, 141.30, 141.73, 143.73, 145,95, 147.53, 151.73, 153.70, 159.12, 161. FT-IR(KBr, cm-1): 3068, 3008, 1735, 1709, 1620, 1405, 1307, 1300, 1273, 1234, 1157, 1132, 1098, 1042, 1021, 991, 888, 845, 769, 615. C14H8N2O4 requires: C, 62.69; N, 10.44; H, 3.01. Found: C, 62.79; N, 10.28; H, 2.91.
Benzhydryl pyrazine-2-carboxylate (3i): Recrystalized from (ETAC/n-hexane; 1: 3) White solid (70%), mp 89-90°C. 1HNMR(400 MHz, CDCl3): 7.235 (1H, m), 7.31 (2H, m), 7.37 (4H, m), 7.46 (4H, m), 8.76 (2H, m), 9.38 (1H, s). 13CNMR (400 MHz, CDCl3): 77.63, 126.26, 127.25, 127.65, 138.39, 142.65, 143,65, 145,32, 146.64, 161,99. FT-IR(KBr, cm-1): 3089, 2961, 1735, 1330, 1303, 1273, 1136, 1055, 1025, 969. C18H14N2O2 requires: C, 74.47; N, 9.65; H, 4.86. Found: C, 74.40; N, 9.58; H, 4.89.
2-oxo-1,2-diphenylethyl pyrazine-2-carboxylate (3j):White solid (95%), mp 108°C. 1HNMR(400 MHz, CDCl3): 7.20 (1H, S), 7.37 (5H, m), 7.52 (1H, m), 7.59 (2H, m), 7.97 (2H, m), 8.75 (2H, m), 9.38 (1H, s). 13CNMR(400 MHz, CDCl3): 78, 127.62, 127.77, 127.84, 128.24, 128.6, 131.83, 132.6, 133.15, 141.88, 143.58, 145.44, 146.74, 162.19, 191.52. FT-IR (KBr, cm-1): 3089, 1752, 1730, 1696, 1602, 1465, 1307, 1294, 1157, 1046, 1025, 961, 858, 764, 709, 704, 602. C19H14N2O3 requires: C, 71.69; N, 8.80; H, 4.43. Found: C, 71.63; N, 8.65; H, 4.49.
(5-(benzyloxy)-4-oxo-4H-pyran-2-yl)methylpyrazine-2-carboxylate (3k):White crystalline powder, Yield (74%), mp: 133-135 ºC. 1HNMR (400 MHz, CDCl3): 5.07 (2H, s), 5.23 (2H, S), 6.57 (1H, s), 7.35 (5H, m), 7.56 (1H, s), 8.76 (1H, m), 8.829 (1H, d, j= 2.4 Hz), 9.34 (1H, d, j= 1.2 Hz); 13CNMR (400 MHz, CDCl3): 61.42, 70.6, 113.89, 126.69, 127.46, 127.71, 134.40, 140.29, 140.40, 143.6, 145.51, 146.42, 147.31, 159.23, 162.35, 173.15.IR (KBr, cm-1): 3111, 3068, 1735 (C = Oester), 1658 (C = Opyrone), 1632 (C = Cpyrone), 1611, 1320, 1273, 1317, 1166, 1140, 1055, 1025, 965, 863. C18H14N2O5 requires: C, 63.90; N, 8.28; H, 4.17. Found: C, 64.05; N, 8.40; H, 4.07.
6-methyl-4-oxo-4H-pyran-3-yl pyrazine-2-carboxylate (3l): White solid (80%), mp 119-120 0C. 1HNMR (400 MHz, CDCl3): 2.29 (3H, s), 6.39 (1H, d, J= 5.8 Hz), 7.7 (1H, d, J= 5.8Hz), 8.735 (1H, m), 8.78 (1H, d, J= 2.4HZ), 9.37 (1H, d, J= 1.2Hz); 13CNMR (400 MHz, CDCl3): 13.99, 115.68, 137.57, 140.85, 143.55, 145.87, 147.26, 153.40, 158.20, 159.59, 170.02.(KBr, cm−1): 3111, 3068, 1752 (C = Oester), 1662 (C = Opyrone), 1640 (C = Cpyrone), 1585, 1435, 1303, 1260, 1175, 1102, 1042, 1025, 931, 863, 841, 777. C11H8N2O4 requires: C, 56.90; N, 12.06; H, 3.47. Found: C, 56.83; N, 12.13; H, 3.40.
Prop-2-ynyl pyrazine-2-carboxylate (3m): White solid (85%), mp 79-80°C. 1HNMR(400 MHz, CDCl3): 2.58 (1H, t, J= 2.4 Hz), 5.05 (2H, d, J= 2.4Hz), 8.76 (1H, m), 8.81 (1H, d, J= 2.4Hz), 9.36 (1H, d, J= 1.2 Hz). 13CNMR(400 MHz, CDCl3): 52.50, 74.92, 141.76, 143.50, 145.40, 146.97, 162.12. (One of the propargyl carbons eclipsed by that of CDCl3). FT-IR(KBr, cm-1): 3065, 2918, 2854, 2106, 1730, 1303, 1136, 1051, 1025, 919, 880, 777, 726, 563, 448. C8H6N2O2 requires: C, 59.26; N, 17.28; H, 3.73. Found: C, 59.19; N, 17.12; H, 3.78.
2-methoxyethyl pyrazine-2-carboxylate (3n): Colorless oil (86%). 1HNMR(400 MHz, CDCl3): 3.49 (3H, s), 3.78 (2H, d, J= 4.8), 4.61 (2H, d, J= 4.8), 8.75 (1H, m), 8.78 (1H, d, J= 2.4), 9.34 (1H, s). 13CNMR(400 MHz, CDCl3): 57.94, 63.96, 69.02, 142.24, 143.40, 145.27, 146.60, 162.81. FT-IR(KBr, cm-1): 3082, 2284, 1735, 1311, 1290, 1153, 1122, 1059, 1025, 781. C8H10N2O3 requires: C, 52.74; N, 15.38; H, 5.53. Found: C, 52.64; N, 15.15; H, 5.65.
Determination of the anti-TB activity of small-molecules using GFP-expressing M. tuberculosis bacteria
Compounds preparation and dilution : 7H9 (45 mL) (without glycerol and without Tween 80) was supplemented with 5 mL OADC. Starting from a stock solution of 10 mg/mL, 1 μL of each compound was diluted with 800 μL 7H9 test medium in a 1.5 mL Eppendorf Cap. Then, 80 μL of each concentration was added in triplicates to a black 96-well plate with a clear bottom (Corning) and the plates were transferred into the BSL3 facility.
Bacteria : The numbers of aliquots of Mtb bacteria needed were thawed in a heating block at 37°C. The caps were centrifuged for 10 minutes at 4500 rpm (Heraeus, Swingout rotor). The supernatant was discarded and the bacteria were resuspended in 7H9 test medium to reach a concentration of 6 x 10e6/20 μL. Afterward, 20 μL of the bacterial suspension was added to the wells containing 80 μL well in the absence (Ctrl) or presence of the compounds and sealed with an air-permeable membrane (Porvair Sciences) at culture under mild agitation (Heidolph) at 37°C in an incubator. Plates were not stacked. The plate was measured at days 0, 3, and 7. Each plate was prepared with rifampicin as reference compound (dilutions in water) which has known inhibitory activity against M. tuberculosis . Test compounds were diluted in 100% DMSO at a constant concentration of 10 μg/mL. Rifampicin was tested in dose-response for quality control purposes at 1 μg/mL and 0.1 μg/mL. The assay was carried out in 96-well flat bottom microplates in a final volume of 100 μL. Next, 20 μL of the prepared bacterial working solution (s. above) was added to 80 mL of the compound test plate containing 10 μg/mL of the compound to be tested. The plates were incubated at 37°C for 7 days. Finally, bacterial growth was determined by measuring the relative fluorescence intensity using the plate reader Synergy2 (Biotek).
Measurement of in vitro cytotoxic activity on human macrophage cells usingxCELLigenceRTCA
The in vitro cytotoxic effect of the POE compounds was evaluated on the human macrophages using the xCELLigence RTCA system as previously described.
27
Antimicrobial activity assay
The MIC (minimum inhibitory concentration) values of the POEs were determined by the two-fold microdilution method. The test POEs (10 mg) were dissolved in DMSO (0.1 mL) to get a stock solution of 100 μg/μL of each compound. Further progressive double dilution with Muller- Hinton broth for bacteria and Sabouraud dextrose broth for fungi were performed to obtain the required concentrations of 1000, 500, 250, 125, 62.5, 32, 16, 8, 4, and 2 μg/mL. To ensure that the solvent had no effect on bacterial growth, a control test was performed with test medium supplemented with DMSO at the same dilutions as used in the experiment. Gentamicin and nystatin were also diluted in the same manner as control for antibacterial and antifungal tests, respectively. Each microwell was inoculated with overnight cultures of test microorganisms to get inoculums with the size of 5 × 105 colony-forming unit(CFU)/mL. After 18-24 hours incubation at 35˚C, the growth was surveyed by turbidity in microwells. The first concentrations of compounds, which inhibited the growth of microorganisms, were considered as MIC.
MTT assay
MTT assay was used to investigate the cytotoxicity of the POE compounds. The K562 cells (2 × 104 cells/well) were cultivated in 96-well plates in the presence of different concentrations of the POEs (25-150 µM) for 24, 48, and 72 hours. The ultimate volume was 200 μL. After that, 20 μL of MTT (5 mg/mL in PBS) was poured into every well and then further incubated for 4 hours at 37°C. The precipitated MTT formazan was dissolved in DMSO (200 μL). Ultimately, the absorbance values were measured at 570 nm on a multi-well plate reader (Quant Bio-tek Instruments, Winooski, VT, 195 USA). Untreated cells and DMSO treated cells (also Doxorubicin) were used as negative and positive controls, respectively.
Results
Chemistry
Cynamon et al synthesized some POEs via acyl intermediate. The necessity for purification of sensitive pyrazinoyl chloride due to side reactions between thionyl chloride and POA was the most drawback of this method.
16
Later synthesis of POEs was reported by using dicyclohexylcarbodiimide (DCC).
28
Despite the effectiveness of this coupling reagent, it is hard to remove the by-product dicyclohexylurea (DCU) as well as rearrangement product N -acylurea.
29
This obstacle inspired us to use a more effective reagent for the synthesis of new POEs having sensitive functional groups using EDC.HCl (N-(3-Dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride)/DMAP as a nontoxic, water-soluble coupling reagent that prevailed over formerly mentioned disadvantages (Table 1).
Table 1.
Reaction of POA with various alcohols and structures of resultant POEs
|
|
|
|
|
|
|
3a
|
|
|
|
3b |
|
|
|
3c |
|
|
|
3d |
|
|
|
3e
|
|
|
|
3f
|
|
|
|
3g
|
|
|
|
3h
|
|
|
|
3i |
|
|
|
3j |
|
|
|
3k |
|
|
|
|
|
|
|
3m |
|
|
|
3n |
Reaction initiates with the nucleophilic attack of pyrazinoate2, on the diimide carbon of EDC.HCl to form O -acylisourea intermediate 4as a highly reactive intermediate. In the next step, reaction proceeds viaO -acylisourea resulting from the activation of POA by EDC through nucleophilic attack of alcohol on this intermediate (path a) that competes with intramolecular acyl rearrangement resulting in the formation of inert N -acylurea 5 as a byproduct (Path b). Furthermore, O -acylisourea may be attacked by POA to produce anhydride derivative of POA (Scheme 1).
29
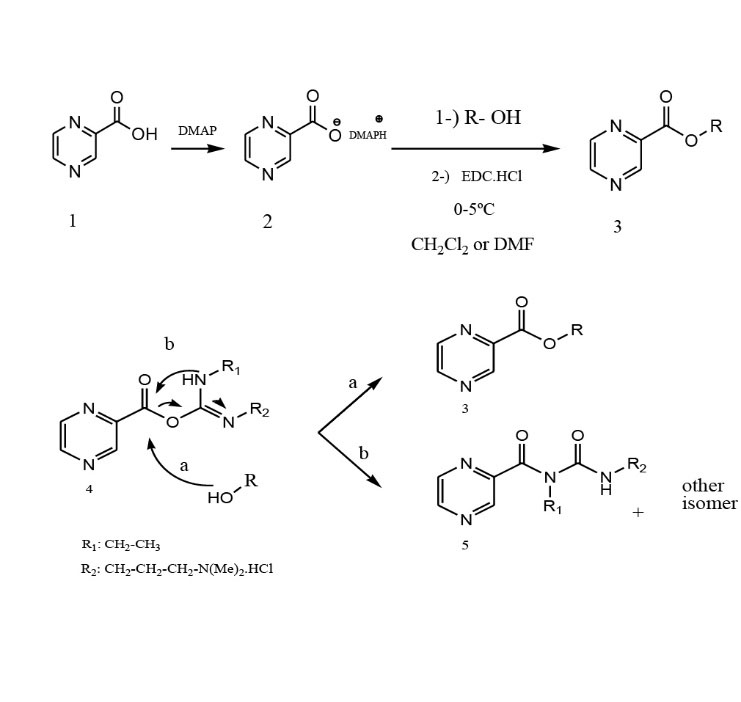
Scheme 1.
Formation pathways of POE andN -acylurea resulted from the reaction of O -acylisourea intermediate.
.
Formation pathways of POE andN -acylurea resulted from the reaction of O -acylisourea intermediate.
Anti-mycobacterial activity
Cell-based assays are key tools in the finding and optimization of new chemical entities against Mtb. The availability of a robust in vitro assay for testing the anti-mycobacterial activity of a new chemical entity is an absolute requirement for the success of a program. The microplate broth dilution assay using a M. tuberculosis strain expressing the green-fluorescent protein (GFP) was selected because of delivering highly reproducible results and allowing screening of a large number of compounds.
The compounds were assayed against slowly growing M. tuberculosis (H37Rv strain) at 10 μg/mL concentration. Compound 3ashowed no inhibitory effect on mycobacterial growth, but compound 3b as a vanillin analog reduced mycobacterial growth by 21.7%. Compound3c having side aldehyde group showed higher activity with 45.42% inhibition. In the presence of compound3dand its epoxidized derivative3e,mycobacterial growth was interrupted by 27.9% and 33.5%, respectively. It was notable that replacing double bond with more active epoxide ring in eugenol pyrazinoate3d made it stronger against Mtb. Compound3f as a ketone compound showed no effect on mycobacterial growth. Between compounds 3g and 3h as cynamyl alcohol and coumarin derivatives, the coumarin-derived compound having internal ester moiety showed to be much more active reagent than the cynamyl alcohol-derived one against Mtb as shown by growth inhibition of 9.83% and 22.5%, respectively. Compound 3jcontaining an alpha-hydroxy carbonyl group was able to limit mycobacterial growth by 45.7% which showed a higher activity against mycobacteria than the compound 3i- the analog without possessing carbonyl group, which showed 41% inhibition. Between hydroxypyrone derivatives, due to having α,β-unsaturated carbonyl (enone) moiety, compound 3lshowed higher anti-mycobacterial activity, reducing Mtb growth value by 56% while compound 3k led to an inhibition of 22.4%. Between compounds 3m and 3n, propargyl-derived pyrazinoate 3mshowed greater anti-mycobaterial activity, preventing mycobacterial growth by 51.2% than compound 3n with 31.7% inhibitory effect (Fig. 1, Table 2).
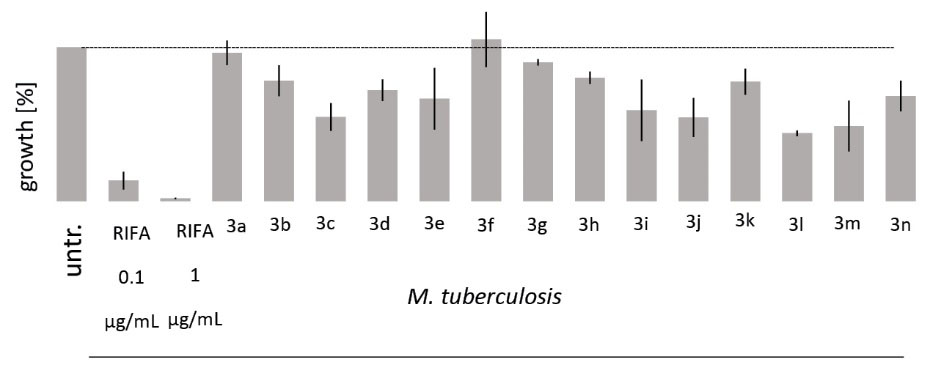
Fig. 1.
Mtb growth (H37Rv) subjected to POEs (10 μg/mL) after 7 days using 7H9 (without glycerol/Tween 80) supplemented with 10% OADC.
Note. Untr.: untreated, RIFA: rifampicin.
.
Mtb growth (H37Rv) subjected to POEs (10 μg/mL) after 7 days using 7H9 (without glycerol/Tween 80) supplemented with 10% OADC.
Note. Untr.: untreated, RIFA: rifampicin.
Table 2.
Anti-TB activity of POE derivatives at 10 μg/mL concentration along with log P values
|
Compound
|
Concentration
μg/mL/μM
|
Inhibition (%)
|
Log
p
b
|
|
Controla
|
|
0 |
|
| RIFA |
0.10 /0.12 |
88.70 |
|
| RIFA |
1.0 /1.2 |
98.30 |
|
| 3a |
10/ 43 |
3.60 |
1.14 |
| 3b |
10/ 39 |
21.70 |
0.89 |
| 3c |
10/ 44 |
45.42 |
1.01 |
| 3d |
10/ 37 |
27.93 |
2.19 |
| 3e |
10/ 35 |
33.50 |
0.94 |
| 3f |
10/ 41 |
3.42 |
0.58 |
| 3g |
10/ 41 |
9.83 |
1.85 |
| 3h |
10/ 37 |
19.89 |
1.06 |
| 3i |
10/ 34 |
41.06 |
3.05 |
| 3j |
10/ 31 |
45.70 |
2.41 |
| 3k |
10/ 29 |
22.44 |
-0.48 |
| 3l |
10/ 43 |
56.00 |
-1.72 |
| 3m |
10/62 |
51.23 |
-0.18 |
| 3n |
10/55 |
31.70 |
-0.55 |
a As negative control, bacteria were incubated at the same conditions without the presence of POE.
b The log P values (hydrophobic indicator) were calculated using the program CS ChemOffice Ultra ver. 11.0 (CambridgeSoft, Cambridge, MA, USA).
In vitro cytotoxic activity
The xCELLigence real-time cell analysis (RTCA) was utilized to evaluate the cytotoxicity of the compounds on macrophages. The xCELLigence system is able to monitor the cultured cell viability in a safe way without any invasion, employing unique gold microelectrodes. Electrical impedance output as cell index (CI) value is evaluated to gain notable information about viability, cell number, cell proliferation status, and morphology.
27
In Fig. 2, the analysis of putative cytotoxic effects of the compounds 3cand 3l on human macrophages is shown. Prolonged incubation of macrophages with these two compounds for 47 hours, in contrast to staurosporine as a positive control, did not lead to a substantial decrease in normalized cell index values as an indicator of macrophage cell attachment. These data suggest that the tested POEs do not show a remarkable cytotoxic effect on macrophage cell adhesion and survival if present in a concentration range up to 10 μg.
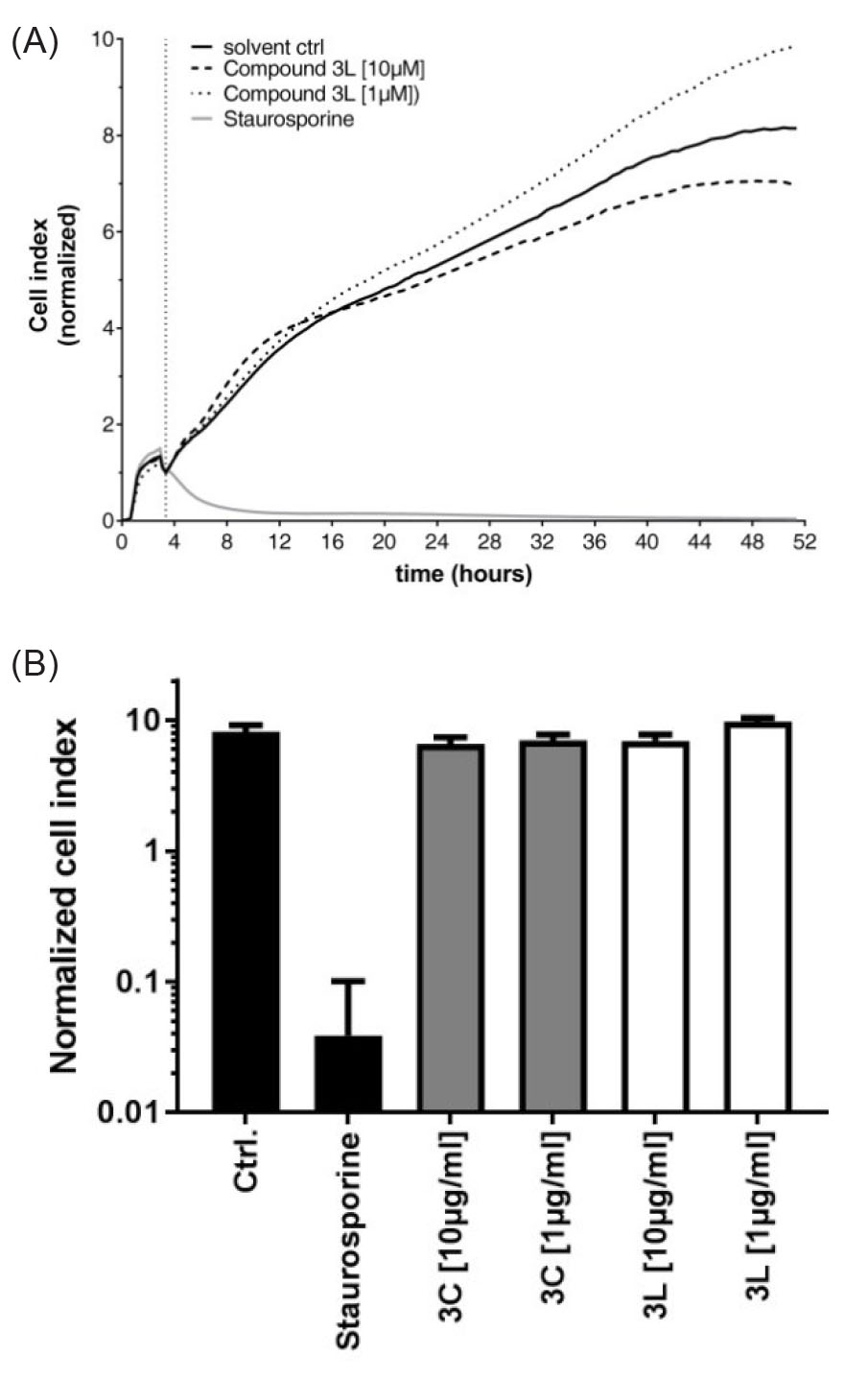
Fig. 2.
(A) Compounds 3c and 3l do not affect human macrophage viability. Over a time period of 47 hours the cellular viability of human
macrophage (40.000 cells/well) expressed as cell index was determined
by real-time impedance measurement using the xCELLigence system
(RTCA, SPACEA). After 3.5 hours of equilibration, the compounds
were added at the indicated concentrations (normalization time point),
(Staurosporine, 1 μg/mL). (B) Normalized cell index depicted as area
under the curve was calculated using RTCA 2.0 software.
.
(A) Compounds 3c and 3l do not affect human macrophage viability. Over a time period of 47 hours the cellular viability of human
macrophage (40.000 cells/well) expressed as cell index was determined
by real-time impedance measurement using the xCELLigence system
(RTCA, SPACEA). After 3.5 hours of equilibration, the compounds
were added at the indicated concentrations (normalization time point),
(Staurosporine, 1 μg/mL). (B) Normalized cell index depicted as area
under the curve was calculated using RTCA 2.0 software.
Antibacterial assay
PZA possessing a narrow spectrum of activity against Mtb bacteria has no notable bactericidal activity against non-tuberculous as well as gram-positive and gram-negative bacteria. In contrast, the evaluation of antimicrobial activity of the POE compounds showed that these compounds had antimicrobial activity against gram-positive bacteria as well as Candidakefyr . The MIC test results showed that the tested compounds showed an improved activity against gram-positive bacteria and remarkable fungicidal activity on C . kefyr . The POEs 3b,3d, 3e, 3i, and 3m including vanillin, eugenol, eugenol epoxide, diphenylcarbinol, and propargyl alcohol in the alkyl part showed a very good antifungal activity against C.kefyr with MIC values of 250±20, 250±20, 250±20, 62.5±5, and 125±10 μg/mL, respectively. This increased fungicidal activity resulted from either POE`s or alkyl part`s activity. It seems the most efficacy of the compound 3i is more likely related to the presence of benzhydryl moiety in its structure, as this structure is an important part in the antifungal medicine clotrimazole. The compounds3a,guaiacol-derived ester, and 3e, the oxidized-eugenol one,had a remarkable activity against S . aureus with a MIC value of 500±50 μg/mL. The compound 3b exhibited very good bactericidal activity with MIC value of 125±10 μg/mL, respectively. Yeast and gram-positive bacteria are surrounded by a thick layer of polysaccharides but without outer membrane containing lipopolysaccharide found in the gram-negative bacterial cell wall. Therefore, the compounds can be more easily absorbed through S. aureus and C.kefyr cell walls, but cannot penetrate the lipid layer of E. coli envelope. The eugenol epoxide-derived POE (3e) presented a moderate antibacterial activity on E . coli as gram-negative bacteria with MIC value of 2000±200 μg/mL, although the compound 3d, simple analog without epoxide moiety, showed a weak bactericidal activity with MIC value of 4000±400 μg/mL. The rest of the compounds exhibited no great activity against E . coli except for compound 3l that showed intermediate activity with MIC value of 1000±100 μg/mL. This observation is notable because this is the only POE that shows greater bactericidal activity on gram-negative than gram-positive bacteria with a MIC value of 2000 μg/mL. This peculiar activity is probably related to the maltol ability to bind divalent cations located on the E . coli ’s outer membrane that results in the increasing permeability of E . coli to the ester (Table 3).
30
Table 3.
MIC values for POEs (μg/mL)
|
Compound
|
S. aureus
MIC (μg/mL)
|
E. coli
|
C.
kefyr
|
| 3a |
500±50 |
3500±350 |
1000±100 |
| 3b |
125±10 |
3500±350 |
250±20 |
| 3c |
2000±200 |
3500±350 |
1000±100 |
| 3d |
2000±200 |
4000±400 |
250±20 |
| 3e |
500±50 |
2000±200 |
250±20 |
| 3f |
2000±200 |
3500±350 |
1000±100 |
| 3g |
4000±400 |
4000±400 |
2000±200 |
| 3h |
2500±250 |
4000±400 |
1000±100 |
| 3i |
2000±200 |
4000±400 |
62.5±5 |
| 3j |
1500±75 |
4000±400 |
4000±400 |
| 3k |
2500±250 |
4000±400 |
2000±200 |
| 3l |
2000±200 |
1000±100 |
1000±100 |
| 3m |
4000±400 |
>4000±500 |
125±10 |
| 3n |
2000±200 |
>4000±500 |
1000±100 |
|
Controla
|
8±0.4 |
8±0.4 |
2±0.1 |
a Gentamicin and nystatin were used as control in antibacterial and antifungal tests, respectively.
Anticancer potency
One of the prevalent ways to assess the in vitro anticancer potency of the synthesized compounds is to evaluate the cytotoxicity in terms of viability in cancer cell by using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. The K562 cells were cultured as supplemented with various concentrations (25-150 µM) of the POEs for 3 days to measure the value of each POE`s inhibitory effect on the viability of the cells by MTT assay. The percentages of viability and IC50 (inhibition of concentration 50) values for all compounds at various concentrations are depicted in Table 4. As shown in Table 4, cell viability was reduced in a time and dose dependent manner for all compounds. Among tested POEs,3e and 3j as eugenol oxide and benzoin pyrazinoate, showed favorable cytotoxicity on the K562 cell lines having IC50 values of 25±5.0 and 25±8.0 µM after 72 hours. As understood from Table 4, substitution of double bond located on eugenol ester3d by epoxide motif sharply affected the cytotoxic efficacy and decreased IC50 value by 75 µM. The rest of the compounds had IC50 values about 100 µM or more.
Table 4.
Viability values of K562 cells treated with POE compounds at different concentrations and times along with IC50 values at 72 hours
| Compound name |
Concentration (µM) |
Viability (% of Control) |
IC50 (µM) at 72 h |
| 24 h |
48 h |
72 h |
| 3a |
0 |
100 |
100 |
100 |
100±10.0 |
| 25 |
82±4.5 |
77±4.0 |
70±2.0 |
| 50 |
70±5.0 |
68±0.5 |
62±2.5 |
| 100 |
65±5.5 |
62±4.5 |
58±2.5 |
| 125 |
55±3.0 |
50±4.0 |
47±3.0 |
| 150 |
42±5.0 |
40±4.5 |
36±2.0 |
| 3b |
0 |
100 |
100 |
100 |
100±4.0 |
| 25 |
85±2.5 |
75±4.2 |
67±4.0 |
| 50 |
72±4.0 |
65±1.5 |
60±3.0 |
| 100 |
68±3.5 |
60±2.5 |
55±3.5 |
| 125 |
60±3.5 |
50±5.5 |
45±4.0 |
| 150 |
55±4.0 |
42±4.0 |
32±3.0 |
| 3c |
0 |
100 |
100 |
100 |
>150 |
| 25 |
98±2.0 |
95±1.0 |
93±2.5 |
| 50 |
95±2.5 |
94±2.0 |
91±3.0 |
| 100 |
92±2.0 |
91±3.0 |
88±1.0 |
| 125 |
86±2.0 |
80±5.0 |
75±2.5 |
| 150 |
80±5.0 |
74±4.0 |
67±3.0 |
| 3d |
0 |
100 |
100 |
100 |
100±2.0 |
| 25 |
93±2.0 |
85±0.5 |
68±2.5 |
| 50 |
80±0.5 |
62±1.5 |
57±3.5 |
| 100 |
75±3.5 |
55±2.5 |
50±4.0 |
| 125 |
63±4.0 |
50±3.0 |
45±2.0 |
| 150 |
52±5.0 |
40±4.5 |
35±3.0 |
| 3e |
0 |
100 |
100 |
100 |
25±8.0 |
| 25 |
75±4.0 |
55±4.0 |
45±3.5 |
| 50 |
62±2.0 |
40±3.5 |
30±3.0 |
| 100 |
45±3.0 |
27±3.5 |
15±2.5 |
| 125 |
30±4.0 |
20±2.0 |
12±3.0 |
| 150 |
22±4.0 |
17±3.5 |
8.0±2.0 |
| 3f |
0 |
100 |
100 |
100 |
>100 |
| 25 |
93±3.0 |
90±1.0 |
88±3.5 |
| 50 |
90±2.0 |
87±3.0 |
86±3.0 |
| 100 |
88±3.0 |
85±1.0 |
82±2.0 |
| 125 |
80±5.0 |
75±2.0 |
68±3.0 |
| 150 |
72±5.0 |
66±4.0 |
62±4.0 |
| 3g |
0 |
100 |
100 |
100 |
>100 |
| 25 |
92±2.4 |
89±1.2 |
88±2.0 |
| 50 |
90±1.6 |
85±3.3 |
84±2.2 |
| 100 |
87±3.2 |
83±1.3 |
82±2.4 |
| 125 |
82±3.0 |
80±3.0 |
70±5.0 |
| 150 |
75±5.0 |
70±4.5 |
66±4.0 |
| 3h |
0 |
100 |
100 |
100 |
100±3.0 |
| 25 |
83±3.5 |
75±4.2 |
67±4.0 |
| 50 |
73±2.5 |
65±4.5 |
58±5.0 |
| 100 |
67±5.5 |
50±1.5 |
47±2.5 |
| 125 |
60±2.0 |
50±3.5 |
40±2.0 |
| 150 |
55±5.0 |
45±2.0 |
33±4.0 |
| 3i |
0 |
100 |
100 |
100 |
>100 |
| 25 |
95±3.5 |
92±1.5 |
90±1.5 |
| 50 |
93±2.5 |
88±3.5 |
87±2.5 |
| 100 |
90±2.0 |
87±3.0 |
85±1.0 |
| 125 |
82±4.0 |
83±2.0 |
80±2.0 |
| 150 |
78±2.0 |
75±1.5 |
70±1.0 |
| 3j |
0 |
100 |
100 |
100 |
25±5.0 |
| 25 |
70±3.0 |
52±3.0 |
45±3.0 |
| 50 |
60±2.5 |
43±3.0 |
32±2.0 |
| 100 |
42±3.5 |
25±1.5 |
20±2.0 |
| 125 |
33±2.0 |
20±3.0 |
16±2.0 |
| 150 |
25±3.0 |
15±1.5 |
10±1.0 |
| 3k |
|
ND |
ND |
ND |
|
| 3l |
|
ND |
ND |
ND |
|
| 3m |
|
ND |
ND |
ND |
|
| 3n |
0 |
100 |
100 |
100 |
>100
|
| 25 |
97±1.0 |
95±1.0 |
91±1.5 |
| 50 |
95±2.5 |
92±4.0 |
90±1.0 |
| 100 |
93±1.0 |
90±2.0 |
88±2.0 |
| 125 |
90±1.0 |
84±1.0 |
80±1.5 |
| 150 |
85±2.0 |
77±3.5 |
70±5.0 |
| DOX |
0.5 |
50±2.0 |
40±4.0 |
25±5.0 |
|
Discussion
The anti-mycobacterial activity results are somewhat consistent with our surmise that POE containing bio-reducible functional group is more likely to show higher activity against Mtb than its similar compound without reducible center due to probably involving FAS (I) in a side bioreduction process. Formerly Seitz et al ascribed a high inhibitory activity of 4-acetoxybenzyl containing pyrazinoate to self-immolative activity of this substitution that showed increased synergistic effect against Mtb.
28
It was also found that 3-ketohexadecanoic acid inhibited fatty acid biosynthesis in Mycobacterium smegmatis .
31
Considering that the whole ester molecule is responsible for anti-mycobacterial activity according to the results reported in recent studies, it remains unclear whether these results were observed due to the increased inhibitory activity of alkyl part of POEs on FAS (I) or due to an increased uptake and bioactivity because of the presence of more polar functional groups.
The outcome of the antibacterial assay shows the importance of functional group in altering the bactericidal activity of POE. The compound 3e has greater antibacterial activity than 3d in both gram-positive and gram-negative susceptibility tests indicating the importance of epoxide moiety in increasing the antibacterial activity. In the case of fosfomycin, the epoxide-containing antibiotic, it has been clarified that nucleophilic attack of the amino acid systeine on the epoxide center of the drug, results in irreversible inactivation of the enzyme involved in the formation of bacterial cell wall.
32
The MTT assay supports our presumption; also, for example, benzoin-derived POE possessing alpha-hydroxy ketone functional group presents increased cytotoxicity on the K562 cell lines. Another justifying example is the oxirane-containing POE that has higher cytotoxic effect in comparison with its analog without epoxide functional group. Stronger interaction with cellular target, as a result of increased nucleophilic reactivity of the epoxide ring may be the probable reason for this observation.
33,34
Conclusion
In this study, we synthesized the POE compounds and evaluated their inhibitory effect on mycobacterial growth, antibacterial, antifungal, and anticancer activities. We used EDC.HCl as a non-toxic and water-soluble coupling agent to avoid unwanted side reaction happening because of the presence of sensitive functional groups other than hydroxyl group and to remove water-soluble by-products by the simple work-up. The results showed that bioreducible functional group containing POEs showed a greater anti mycobacterial growth activity in comparison with their simple analogs. Some POEs containing bio-reducible center also showed activity in antibacterial and anticancer assay, showing the importance of bio-reducible center in increasing the biological activity of the POEs. The obtained results were in accordance with our surmise that bio-reducible center located on alkyl part of POE might involve FAS (I) in side reaction, besides the inhibitory effect of the pyrazinoate core on binding NADPH to FAS (I). To further prove this concept, tested POEs should be evaluated in further studies for their direct inhibitory activity on FAS (I).
Regarding POA core`s antibacterial inefficacy against non-tuberculous as well as gram-positive and gram-negative bacteria mostly due to having effective efflux system to move POA out of the cytoplasmic membrane, it is concluded from results that improved antifungal and antibacterial activity could be a result of either released bioreducibe-containing alkyl part after hydrolysis in cytoplasm or the intrinsic antibacterial activity of POE due to the presence of bioreducible moiety in the ester structure. Furthermore, the stability of the POE compound may influence the rate of efflux and compound permeability through the bacterial cell wall.
The results showed that the POEs containing bioreducible functional groups have more potential to suppress the viability of the K562 cells.
All kinds of the tests confirmed the significant importance of the epoxide center in improving the biological activity of the eugenole-containing POE.
Funding sources
This work was financially and technically supported by Faculty of Chemistry, University of Tabriz.
Ethical Statement
Not applicable to this study.
Acknowledgments
We gratefully acknowledge financial support from the Research Council of the University of Tabriz. Furthermore, we sincerely acknowledge Ms. Lisa Niwinski (Research Center Borstel) for expert help and technical assistance.
Competing interests
None to be declared.
Authors' contribution
HM devised the project, the main conceptual ideas, proof outline, and involved in planning, and supervised the work. HK meinagh contributed to sample preparation and drafted the manuscript. NR performed the antimycobacterial assay. MM performed the anticancer assay. GZ performed the antimicrobial assay. All authors discussed the results and commented on the manuscript.
Research Highlights
What is the current knowledge?
simple
-
√ It has been clarified that both pyrazine core and alkyl part
of the POE compound interact with the FAS (I) in the Mtb
precluding complex formation between NADPH and FAS (I).
-
√ PZA as an effective anti-TB medicine has no antibacterial
activity against non-tuberculous mycobacteria as well as
gram-positive and gram-negative bacteria.
What is new here?
simple
-
√ Bio-reducible center-containing POEs have a greater antimycobacterial
activity against Mtb (H37Rv) than similar
analogs without a bio-reducible functional group probably
due to involvement of (FAS (I) in side reductase activity.
-
√ he conversion of the eugenol-containing POE to the
epoxidized one increases anti-mycobacterial, anti-bacterial
activity, and cytotoxic effect on the K562 cell lines.
References
-
World Health Organization. Global tuberculosis report 2017. http://apps.who.int/medicinedocs/documents/s23360en/s23360en.pdf.
- Törün T, Güngör G, Özmen I, Bölükbaşı Y, Maden E, Bıçakçı B. Side effects associated with the treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2005; 9:1373-7. [ Google Scholar]
- Zumla AI, Gillespie SH, Hoelscher M, Philips PP, Cole ST, Abubakar I. New antituberculosis drugs, regimens, and adjunct therapies: needs, advances, and future prospects. Lancet Infect Dis 2014; 14:327-40. doi: 10.1016/S1473-3099(13)70328-1 [Crossref] [ Google Scholar]
- Brennan PJ, Nikaido H. The envelope of mycobacteria. Annu Rev Biochem 1995; 64:29-63. doi: 10.1146/annurev.bi.64.070195.000333 [Crossref] [ Google Scholar]
- Zhang Y, Mitchison D. The curious characteristics of pyrazinamide: a review. Int J Tuberc Lung Dis 2003; 7:6-21. [ Google Scholar]
- Konno K, Feldmann FM, McDermott W. Pyrazinamide susceptibility and amidase activity of tubercle bacilli. Am Rev Respir Dis 1967; 95:461-9. doi: 10.1164/arrd.1967.95.3.461 [Crossref] [ Google Scholar]
- Zhang Y, Scorpio A, Nikaido H, Sun Z. Role of acid pH and deficient efflux of pyrazinoic acid in unique susceptibility of Mycobacterium tuberculosis to pyrazinamide. J Bacteriol 1999; 181:2044-9. [ PubMed] [ Google Scholar]
- Mitchison D. The action of antituberculosis drugs in short-course chemotherapy. Tubercle 1985; 66:219-25. doi: 10.1016/0041-3879(85)90040-6 [Crossref] [ Google Scholar]
- Basso L, Santos D. Drugs that inhibit mycolic acid biosynthesis in Mycobacterium tuberculosis-an update. Med Chem Rev Online 2005; 2:393-413. doi: 10.2174/156720305774330458 [Crossref] [ Google Scholar]
- Liu J, Barry CE, Besra GS, Nikaido H. Mycolic acid structure determines the fluidity of the mycobacterial cell wall. J Biol Chem 1996; 271:29545-51. doi: 10.1074/jbc.271.47.29545 [Crossref] [ Google Scholar]
- Shi W, Zhang X, Jiang X, Yuan H, Lee JS, Barry CE. Pyrazinamide inhibits trans-translation in Mycobacterium tuberculosis. Science 2011; 333:1630-2. doi: 10.1126/science.1208813 [Crossref] [ Google Scholar]
- Zimhony O, Vilchèze C, Arai M, Welch JT, Jacobs WR. Pyrazinoic acid and its n-propyl ester inhibit fatty acid synthase type I in replicating tubercle bacilli. Antimicrob Agents Chemother 2007; 51:752-4. doi: 10.1128/AAC.01369-06 [Crossref] [ Google Scholar]
- Sayahi H, Zimhony O, Jacobs Jr WR, Shekhtman A, Welch JT. Pyrazinamide, but not pyrazinoic acid, is a competitive inhibitor of NADPH binding to Mycobacterium tuberculosis fatty acid synthase I. Bioorg Med Chem Lett 2011; 21:4804-7. doi: 10.1016/j.bmcl.2011.06.055 [Crossref] [ Google Scholar]
- Simões MF, Valente E, Gómez MJR, Anes E, Constantino L. Lipophilic pyrazinoic acid amide and ester prodrugs: stability, activation and activity against M tuberculosis. Eur J Pharm Sci 2009; 37:257-63. doi: 10.1016/j.ejps.2009.02.012 [Crossref] [ Google Scholar]
- Cynamon MH, Gimi R, Gyenes F, Sharpe CA, Bergmann KE, Han HJ. Pyrazinoic acid esters with broad spectrum in vitro antimycobacterial activity. J Med Chem 1995; 38:3902-7. doi: 10.1021/jm00020a003 [Crossref] [ Google Scholar]
- Cynamon MH, Klemens SP, Chou TS, Gimi RH, Welch JT. Antimycobacterial activity of a series of pyrazinoic acid esters. J Med Chem 1992; 35:1212-5. doi: 10.1021/jm00085a007 [Crossref] [ Google Scholar]
- Speirs R, Welch J, Cynamon M. Activity of n-propyl pyrazinoate against pyrazinamide-resistant Mycobacterium tuberculosis: investigations into mechanism of action of and mechanism of resistance to pyrazinamide. Antimicrob Agents Chemother 1995; 39:1269-71. doi: 10.1128/AAC.39.6.1269 [Crossref] [ Google Scholar]
- Bergmann KE, Cynamon MH, Welch JT. Quantitative Structure− Activity Relationships for the in Vitro Antimycobacterial Activity of Pyrazinoic Acid Esters. J Med Chem 1996; 39:3394-400. doi: 10.1021/jm950538t [Crossref] [ Google Scholar]
- Ngo SC, Zimhony O, Chung WJ, Sayahi H, Jacobs WR, Welch JT. Inhibition of isolated Mycobacterium tuberculosis fatty acid synthase I by pyrazinamide analogs. Antimicrob Agents Chemother 2007; 51:2430-5. doi: 10.1128/AAC.01458-06 [Crossref] [ Google Scholar]
- Sayahi H, Pugliese KM, Zimhony O, Jacobs WR, Shekhtman A, Welch JT. Analogs of the antituberculous agent pyrazinamide are competitive inhibitors of NADPH binding to M tuberculosis fatty acid synthase I. Chem Biodiversity 2012; 9:2582-96. doi: 10.1002/cbdv.201200291 [Crossref] [ Google Scholar]
- Bloch K. Control mechanisms for fatty acid synthesis in Mycobacterium smegmatis. Adv Enzymol Relar Areas Mol Biol 1977; 45:1-84. [ Google Scholar]
- Kikuchi S, Rainwater D, Kolattukudy P. Purification and characterization of an unusually large fatty acid synthase from Mycobacterium tuberculosis var bovis BCG. Arch Biochem Biophys 1992; 295:318-26. doi: 10.1016/0003-9861(92)90524-Z [Crossref] [ Google Scholar]
- Peterson D, Bloch K. Mycobacterium smegmatis fatty acid synthetase Long chain transacylase chain length specificity. J Biol Chem 1977; 252:5735-9. [ Google Scholar]
-
Fieser LF, Fieser M. Reagents for organic synthesis: Wiley Inter Science, New York; 1967.
-
Vogel AI. A text-book of practical organic chemistry including qualitative organic analysis, London: Longmans Green And Co; 2013.
- Kolbe K, Möckl L, Sohst V, Brandenburg J, Engel R, Malm S. Azido Pentoses: A New Tool To Efficiently Label Mycobacterium tuberculosis Clinical Isolates. Chembiochem 2017; 18:1172-6. doi: 10.1002/cbic.201600706 [Crossref] [ Google Scholar]
-
Ke N, Wang X, Xu X, Abassi YA. The xCELLigence system for real-time and label-free monitoring of cell viability. Mammalian Cell Viability. Humana Press; 2011. p. 33-43.
- Seitz LE, Suling WJ, Reynolds RC. Synthesis and antimycobacterial activity of pyrazine and quinoxaline derivatives. J Med Chem 2002; 45:5604-6. doi: 10.1021/jm020310n [Crossref] [ Google Scholar]
-
Smith MB, March J. March's advanced organic chemistry: reactions, mechanisms, and structure: John Wiley & Sons; 2007.
- Schved F, Pierson M, Juven B. Sensitization of Escherichia coli to nisin by maltol and ethyl maltol. Lett Appl Microbiol 1996; 22:189-91. doi: 10.1111/j.1472-765X.1996.tb01139.x [Crossref] [ Google Scholar]
- Morbidoni HR, Vilchèze C, Kremer L, Bittman R, Sacchettini JC, Jacobs Jr WR. Dual inhibition of mycobacterial fatty acid biosynthesis and degradation by 2-alkynoic acids. Chem Biol 2006; 13:297-307. doi: 10.1016/j.chembiol.2006.01.005 [Crossref] [ Google Scholar]
- Zhu J-Y, Yang Y, Han H, Betzi S, Olesen S, Marsilio F. Functional consequence of the covalent reaction of phosphoenolpyruvate with UDP-N-acetylglucosamine 1-carboxyvinyltransferase (MurA). J Biol Chem 2012; 13; 287(16):12657-67. doi: 10.1074/jbc.M112.342725 [Crossref] [ Google Scholar]
- Joshi P, Misra L, Siddique AA, Srivastava M, Kumar S, Darokar MP. Epoxide group relationship with cytotoxicity in withanolide derivatives from Withania somnifera. Steroids 2014; 79:19-27. doi: 10.1016/j.steroids.2013.10.008 [Crossref] [ Google Scholar]
- Anderson WK, Dewey RH. Synthesis and structure-activity relation studies of cytotoxic epoxide derivatives of 7-oxabicyclo [22 1] heptane. J Med Chem 1977; 20:306-8. doi: 10.1021/jm00212a025 [Crossref] [ Google Scholar]