BioImpacts. 9(4):211-217.
doi: 10.15171/bi.2019.26
Original Research
Mode of photoexcited C60 fullerene involvement in potentiating cisplatin toxicity against drug-resistant L1210 cells
Daria Franskevych 1  , Svitlana Prylutska 1, *
, Svitlana Prylutska 1, *  , Iryna Grynyuk 1
, Iryna Grynyuk 1  , Ganna Pasichnyk 2
, Ganna Pasichnyk 2  , Liudmyla Drobot 2
, Liudmyla Drobot 2  , Olga Matyshevska 1, 2
, Olga Matyshevska 1, 2  , Uwe Ritter 3
, Uwe Ritter 3 
Author information:
1Taras Shevchenko National University of Kyiv, Volodymyrska Str., 64, 01601 Kyiv, Ukraine
2Palladin Institute of Biochemistry of the National Academy of Sciences of Ukraine, Leontovicha Str, 9, Kyiv 01030, Ukraine
3Technical University Ilmenau, Institute of Chemistry and Biotechnology, Weimarer Str., 25, 98693 Ilmenau, Germany
Abstract
Introduction:
C60 fullerene has received great attention as a candidate for biomedical applications. Due to unique structure and properties, C60 fullerene nanoparticles are supposed to be useful in drug delivery, photodynamic therapy (PDT) of cancer, and reversion of tumor cells’ multidrug resistance. The aim of this study was to elucidate the possible molecular mechanisms involved in photoexcited C60 fullerene-dependent enhancement of cisplatin toxicity against leukemic cells resistant to cisplatin.
Methods:
Stable homogeneous pristine C60 fullerene aqueous colloid solution (10-4 М, purity 99.5%) was used in the study. The photoactivation of C60 fullerene accumulated by L1210R cells was done by irradiation in microplates with light-emitting diode lamp (420-700 nm light, 100 mW·cm-2). Cells were further incubated with the addition of Cis-Pt to a final concentration of 1 μg/mL. Activation of p38 MAPK was visualized by Western blot analysis. Flow cytometry was used for the estimation of cells distribution on cell cycle. Mitochondrial membrane potential (Δψm) was estimated with the use of fluorescent potential-sensitive probe TMRE (Tetramethylrhodamine Ethyl Ester).
Results:
Cis-Pt applied alone at 1 μg/mL concentration failed to affect mitochondrial membrane potential in L1210R cells or cell cycle distribution as compared with untreated cells. Activation of ROS-sensitive proapoptotic p38 kinase and enhanced content of cells in subG1 phase were detected after irradiation of L1210R cells treated with 10-5M C60 fullerene. Combined treatment with photoexcited C60 fullerene and Cis-Pt was followed by the dissipation of Δψm at early-term period, blockage of cell transition into S phase, and considerable accumulation of cells in proapoptotic subG1 phase at prolonged incubation.
Conclusion:
The effect of the synergic cytotoxic activity of both agents allowed to suppose that photoexcited C60 fullerene promoted Cis-Pt accumulation in leukemic cells resistant to Cis-Pt. The data obtained could be useful for the development of new approaches to overcome drug-resistance of leukemic cells.
Keywords:
C60 fullerene
, Leukemic cisplatin resistant L1210 cells, Photoexcitation, Cell cycle, p38 mitogen-activated protein kinase
Copyright and License Information
© 2019 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Multidrug resistance (MDR) is a major problem in anticancer therapy and approximately 70-90% of patients do not respond to initial chemotherapy.
1
Mechanisms of MDR including reduced drug uptake, active drug efflux by transporters of ATP-binding cassette (ABC) superfamily, decreased intracellular drug concentration, altered cell cycle checkpoints, and induced expression of genes for impairing apoptotic pathways of cell death are well studied.
2, 3
Nevertheless the problem of MDR reversion in cancer still remains. Thus, the chemical development of ABC transporter inhibitors was found to increase the toxicity associated with chemotherapy.
4
Unique properties of C60 fullerene nanoparticles with the size of 10-100 nm distinguish them from other cancer therapeutics; they are non-toxic for normal cells,
5, 6
can bypass traditional drug resistance mechanisms,
7, 8
have therapeutic properties themselves as photosensitizers in photodynamic therapy (PDT),
9-11
and enable combinatory treatment with anticancer drugs.
12, 13
Due to the extended π-conjugated system of molecular orbitals, C60 fullerene is able to generate toxic reactive oxygen species (ROS) in polar solvents after UV/Vis light absorption. Photoexcited C60 molecule is reduced from a long-lived triplet state (3C60*) to radical anion C60-•0 which subsequently reduces O2 to O2-•0; thereby initiating radical chain reactions with the generation of hydroxyl radical and hydrogen peroxide.
9, 14
Photoexcited C60 fullerene or its derivatives have been shown to evoke oxidative stress and to induce apoptosis in cancer cells of different origins.
14-18
In this study, we used the photodynamic potential of C60 to enhance the cytotoxic effect of chemotherapeutic drug cisplatin towards murine leucosis cell line resistant to cisplatin. Cisplatin (cis-[Pt(NH3)2Cl2], Cis-Pt) belongs to the first-line highly efficient cytotoxic agents in current cancer therapy. It is generally accepted, in that the main Cis-Pt target is nuclear DNA, but recent studies have demonstrated that activation of apoptosis signaling pathways in the cytoplasm is the mechanism of Cis-Pt toxicity alternative to DNA damage.
3, 19, 20
Therapeutic efficiencies of Cis-Pt are substantially limited by the development of cancer cells’ MDR.
In the previous studies, we confirmed fullerene C60 nanoparticles penetration into leukemic L1210 cells and demonstrated photoinduced cytotoxicity of accumulated C60 determined by ROS production.
21
The possibility to decrease substantially the viability of cisplatin-resistant L1210R cells by combined treatment with photoexcited C60 fullerene and cisplatin was also shown,
22
but the mechanisms of this phenomenon still need further investigation.
The aim of this study was to elucidate the possible molecular mechanism of photoexcited C60 fullerene-dependent enhancement of cisplatin toxicity against leukemic cells resistant to cisplatin.
Materials and Methods
Materials
The materials used included: RPMI 1640 liquid medium (Sigma-Aldrich Co, Ltd, USA), fetal bovine serum (FBS) (Sigma-Aldrich Co, Ltd, USA), penicillin/streptomycin and L-glutamine (Merck KGaA (Darmstadt, Germany)), propidium iodide (Sigma-Aldrich Co, Ltd, USA), RNase A (Sigma, USA), tetramethylrhodamine ethyl ester perchlorate (TMRE)(Sigma, USA), antibodies against β-actin (1:2000 dilution) (Sigma, USA), anti-phospho-p38 kinase antibodies (1:1000 dilution) (Cell Signaling, USA), DC Protein Assay kit (Bio-Rad, USA), cisplatin (cis-Pt, Sigma-Aldrich Co, Ltd, USA).
Preparation and characterization of pristine C60fullereneaqueous colloid solution
C60 fullerene aqueous colloid solution was synthesized and characterized in the Ilmenau Technical University (Germany) as described by Scharff et al.
23
In brief, the toluene extract was obtained after graphite combustion and after toluene evaporation, C60 fullerene was transferred to the water phase followed by prolonged ultrasound sonication. An aqueous colloid solution of C60 fullerene (concentration 10-4 М, purity 99.5%) was highly stable for 12 months when stored at room temperature.
24
The average hydrodynamic diameter of C60 fullerene nanoparticles was 50 nm, and no changes in their size were detected in RPMI-1640 medium containing 5% FBS.
25
Cell culture
The murine cancer cell line of leukemic origin resistant to cisplatin L1210R was obtained from the Bank of Cell Lines from Human and Animal Tissues, R. E. Kavetsky Institute of Experimental Pathology, Oncology and Radiobiology, NAS of Ukraine (Kyiv, Ukraine). Cells were incubated in RPMI 1640 medium supplemented with 10% FBS, 50 μg·mL-1 penicillin and 100 μg·mL-1 streptomycin at 37°C in a humidified atmosphere with 5% CO2.
Photodynamic treatment
Cells were incubated for 2 hours with or without 10-5 М C60 fullerene in a medium described above. Photoactivation of accumulated C60 fullerene was done by probes irradiation in microplates with light-emitting diode lamp (420-700 nm light, irradiance 100mW·cm-2). Cells were further incubated for an indicated time period without or with the addition of cisplatin to a final concentration of 1 μg/mL in the incubation medium.
Immunoblot analysis
Cells were washed with PBS and lysed with ice-cold lysis buffer containing protease inhibitor. Cell lysates were centrifuged (14 000 g, 15 minutes) and 30 μg of cell lysate protein was loaded onto a gradient 8%-15% SDS-polyacrylamide gel. After electrophoresis, the proteins were transferred onto polyvinylidene difluoride membrane
26
and incubated overnight at 4°C with monoclonal antibody against phospho-p38 kinase (dilution 1:1000). The membranes were washed and incubated for 1 hour with anti-rabbit peroxidase-linked secondary antibody. Immunoreactive bands were visualized by enhanced chemiluminescence plus western blotting detection system (Amersham, USA). Then, the membranes were incubated with antibodies against β-actin to provide the loading control. Finally, protein concentration was determined using DC Protein Assay kit (Bio-Rad, USA).
Cell cycle analysis
For cell cycle analysis, cells (1x106) were resuspended in 0.1 mL PBS (pH 7.4), fixed by adding 0.9 mL of 90% ethanol at -20°C overnight and centrifuged at 13000 g for 1 minute. The fixed cells were rinsed twice with PBS and resuspended in propidium iodide solution (10 µg/mL) containing RNase A (100 µg/mL) in PBS. The stained cells were analyzed by a COULTER EPICS XLTM (Beckman Coulter, USA) and FCS Express 3 Flow Cytometry Software (DeNovo Software, USA).
Mitochondrial transmembrane potential assay
Mitochondrial membrane potential (Δψm) was estimated with the use of fluorescent potential-sensitive probe TMRE. Cells (107/mL) were suspended in buffer A containing (mM): KCl – 5, NaCl – 120, CaCl2 – 1, glucose – 10, MgCl2 – 1, NaHCO3 – 4, HEPES – 10, pH 7.4, incubated with 100 nM TMRE for 40 minutes at 25°C with the addition of 0.05% Pluronic F-127 and washed from the excess of probe. TMRE fluorescence was registered with Shimadzu RF-1501 spectrofluorometer (Japan), λexc=540 nm, λem=595 nm. Relative values of mitochondrial potential were determined as changes in probe fluorescence after the addition of protonophore FCCP (carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone) (1 μM).
27
Statistics
The data were represented as Mean (M) ± standard deviation (SD) of more than four independent experiments. Mean and SD were calculated for each group. Statistical analysis was performed using two-way ANOVA followed by post Bonferroni test. A value of P < 0.05 was considered statistically significant. Data processing and plotting were performed by IBM PC using specialized applications GraphPad Prism 7 (GraphPad Software Inc., USA) and Gel-Pro Analyzer 6.3 (Media Cybernetics Inc., USA).
Results
Activation of p38 MAPK in L1210R cells after C60 fullerene photoexcitation
Mitogen-activated p38 kinase (MAPK) is one of the important redox-sensitive and stress-activated targets involved in apoptosis induction by phosphorylation of proapoptotic proteins р53 and Вaх.
28, 29
We examined p38 MAPK activity in L1210R cells by estimation of the level of its active phosphorylated form (pp38) using Western blot analysis. As shown in Fig. 1, no statistically valid changes in the level of active p38 kinase were detected at 2-hour incubation of cells loaded with C60 fullerene or irradiated with 420-700 nm light alone, though photoexcitation of C60 fullerene accumulated with L1210R cells was followed by an increase of p38 MAPK level which was found to be 3 times higher than that in the control at 1 hour of incubation and remained at enhanced level at 2 hours.
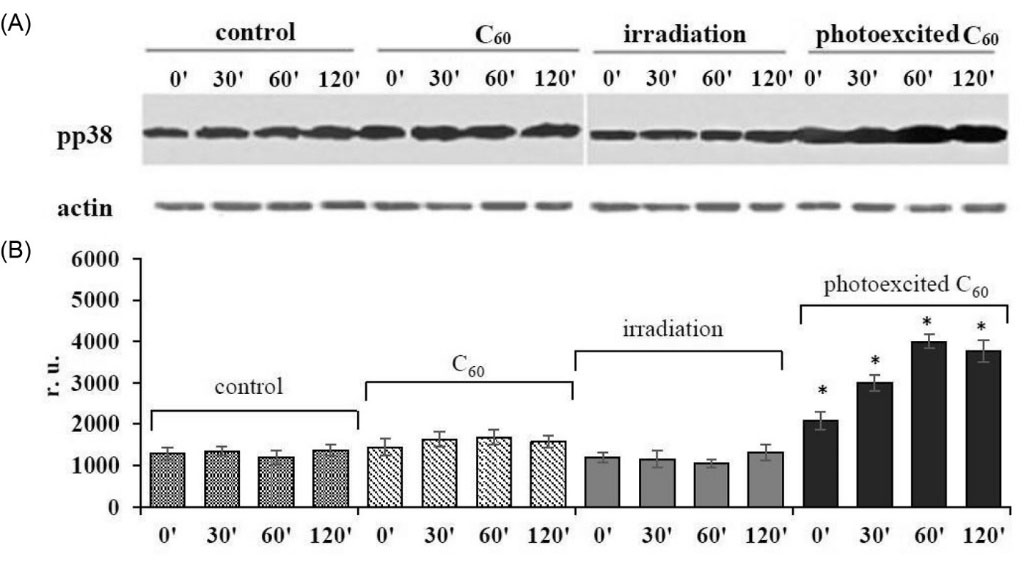
Fig. 1.
Activation of p38 MAP kinase in L1210R cells treated with 10-5 M C60 fullerene and irradiated with 420-700 nm light: (A) Western blot analysis of pp38 MAPK level (typical blotogram); (B) quantitative analysis of the fold increase of pp38 MAPK level. (M±m, n=3); *Р <0.05 in comparison to control.
.
Activation of p38 MAP kinase in L1210R cells treated with 10-5 M C60 fullerene and irradiated with 420-700 nm light: (A) Western blot analysis of pp38 MAPK level (typical blotogram); (B) quantitative analysis of the fold increase of pp38 MAPK level. (M±m, n=3); *Р <0.05 in comparison to control.
This finding is in agreement with data presented by Li et al,
30
where substantial activation of p38 MAP kinase was detected after light irradiation of MCF-7 cells loaded with C60 derivatives С60-phe or C60-gly. This increase was prevented by antioxidant N-acetyl-L-cystein and thus proved to be ROS dependent. Activation of p38 MAP kinase as a result of H2O2-induced oxidation of its thiol groups was also shown by Olson and Hallahan.
29
A growing body of evidence suggests that p38 MAPK is able to control the p53-mediated response to several genotoxic stimuli and could be specific to cancer therapy.
31, 32
The data on increase in the viability of HaCaT cells pretreated with p38 MAPK specific inhibitors before incubation with cisplatin as well as the data obtained in experimental head and neck cancer model indicating that lower activation or lack of activation of p38 MAPK correlates with a more resistant phenotype
33
suggested that the inhibition of p38 MAPK is a potential mechanism of resistance and that activation of this pathway could help to overcome cancer cells drug resistance.
Interference into cell cycle transition is suggested to be one of the mechanisms of p38 MAP kinase involvement into apoptosis induction. To elucidate if ROS dependent effects of photoexcited C60 fullerene could disturb cell cycle checkpoints in cisplatin-resistant L1210R cells, we further studied the cells cycle distribution after combined treatment with photoexcited C60 and cisplatin.
Cell cycle distribution of L1210R cells after the combined action of cisplatin and photoexcited C60fullerene
Flow cytometric analysis showed that at 48 hour incubation of L1210R cells in control, the most significant content of cells (47.6±4.6%) was detected in the G0/G1 phase (Figs. 2A, B). Accumulation of cisplatin-resistant cancer cells of different origins in the G0/G1 phase of cell cycle is believed to ensure the transition from G1 to S phase, DNA doubling, and mitosis.
34, 35
No effect on cell cycle profile was detected after L1210R cells light irradiation (data not shown) or treatment with cisplatin alone, while treatment with C60 fullerene was followed by an increase of cells content in the subG1 phase (8.7±3.5% vs. 2.7±1.5% in control) (Figs. 2A, B). After C60 fullerene photoactivation, this effect was enhanced (14.4±2.9% vs. 2.7±1.5% in control) with simultaneous decrease of cells content in the G0/G1 phase. After combined treatment with photoexcited C60 fullerene and cisplatin, a decrease of cells content in the G2/M phase was shown, while the number of cells accumulated in subG1 phase was found to be substantially higher than that after C60 fullerene photoexcitation alone (28±4% vs. 14.4±3% respectively) (Fig. 2B). Cells accumulation in the subG1 phase is considered to be the marker of the blockage of cell transition into S phase and transition to apoptotic pathway.
8
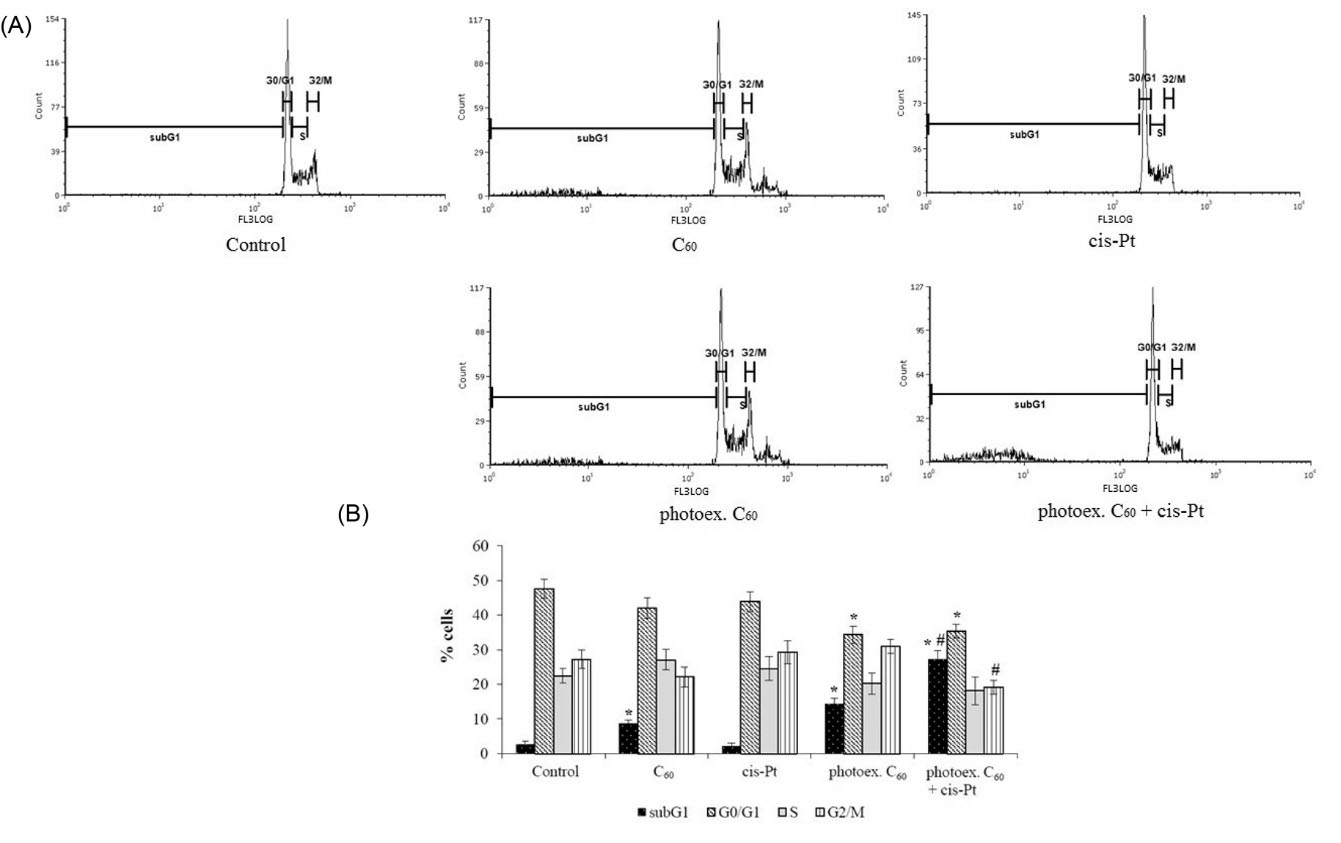
Fig. 2.
Cell cycle distribution of L1210R cells at 48 hour time point after combined treatment with photoexcited C60 fullerene and cisplatin. (A) Representative histograms of a typical experiment. (B) % of total cell population in phases. (M±m, n=3); *Р <0.05 in comparison with control, #Р <0.05 in comparison with photoexcited С60 fullerene.
.
Cell cycle distribution of L1210R cells at 48 hour time point after combined treatment with photoexcited C60 fullerene and cisplatin. (A) Representative histograms of a typical experiment. (B) % of total cell population in phases. (M±m, n=3); *Р <0.05 in comparison with control, #Р <0.05 in comparison with photoexcited С60 fullerene.
Activation of proapoptotic MAP kinases in L1210R cells after C60 fullerene photoexcitation could be the initial step of the cell death program, but its realization needs reinforcement of apoptotic signals particularly at the level of mitochondria. Since acute apoptosis induced by cisplatin is shown to be associated with mitochondrial ROS response,
20, 36
we tested whether combined treatment of L1210R cells with photoexcited C60 fullerene and cisplatin had an impact on mitochondrial redox status.
Mitochondrial membrane potential in L1210R cells after the combined action of cisplatin and photoexcited C60 fullerene
The relative value of mitochondrial membrane potential (Δψm) in L1210R cells incubated for 2 hours after the treatment with either cisplatin or photoexcited C60 fullerene alone or their combination was estimated with the use of fluorescent probe TMRE.
No effect of light irradiation (data not shown) or 1 µg/mL cisplatin alone on the Δψm value in L1210R cells was detected, while the tendency to its decrease after cells treatment with C60 fullerene was revealed (Fig. 3).
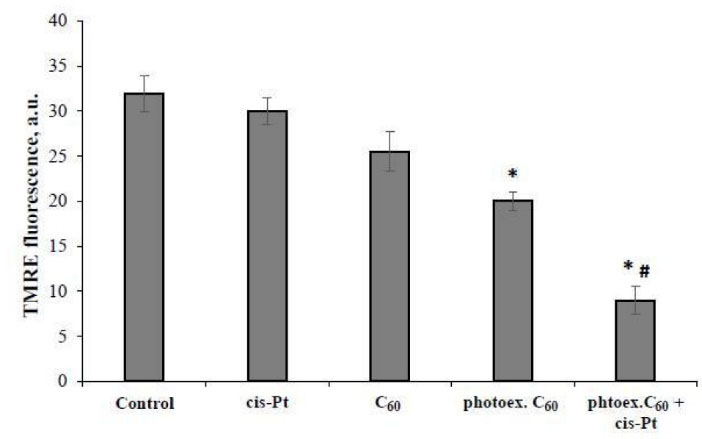
Fig. 3.
Relative value of mitochondrial membrane potential in L1210R cells at 2 hour time point after combined treatment with photoexcited C60 fullerene and cisplatin. (M±m, n=5); *Р <0.05 in comparison with control, #Р <0.05 in comparison with photoexcited С60 fullerene.
.
Relative value of mitochondrial membrane potential in L1210R cells at 2 hour time point after combined treatment with photoexcited C60 fullerene and cisplatin. (M±m, n=5); *Р <0.05 in comparison with control, #Р <0.05 in comparison with photoexcited С60 fullerene.
C60 fullerene photoexcitation was followed by 1.7 fold decrease of Δψ relative value in L1210R cells as compared with control. Combined treatment with photoexcited C60 fullerene and cisplatin was followed by further substantial decrease of TMRE fluorescent signal, the Δψ relative value was decreased 3.9 folds as compared with the control and 2 folds as compared with the effect of photoexcited C60 fullerene alone, indicating the cisplatin involvement in mitochondria redox status disturbance.
Discussion
The ability of photoexcited C60 fullerene to induce apoptosis in human leukemic cells was confirmed in our previous studies, where the depletion of mitochondrial Ca2+-pool, cytochrome c release from mitochondria to the cytosol, caspase - 3 activation and DNA fragmentation with the formation of the “ladder pattern” after UV/Vis irradiation (320-600nm) of cells treated with 10-5 M C60 fullerene were demonstrated.
37, 38
As we have shown earlier, the treatment of cisplatin resistant L1210R leukemic cells with cisplatin in a range of 0.1-10 µg/mL had no effect on cell viability, while substantial C60-mediated photodamaging effect was detected with 50% decrease of cell viability at 48 hour time point after photoexcitation (420-700 nm) of accumulated carbon nanostructure.
21
The intense ROS production detected at 3 hour time point after irradiation of L1210R cells loaded with C60 fullerene confirmed the ability of photoactivated C60 fullerene to generate O2-• in intracellular space.
22
In this study we demonstrated the activation of p38 MAP kinase in L1210R cells after treatment with C60 fullerene and light irradiation. These data indicate that p38 MAPK could be the target of ROS produced by photoexcited C60 and thus be involved in the molecular mechanisms of photoexcited C60 toxic effect against leukemic cells resistant to cisplatin. This suggestion was confirmed by the data indicating the ability of photoexcited C60 to evoke L1210R cells accumulation in proapoptotic subG1 phase of cell cycle.
We showed that under combined treatment with photoexcited C60 and cisplatin in a low 1 μg/mL concentration, the synergic effect of both agents became apparent both in dropping the mitochondrial membrane potential and inducing L1210R cells accumulation in subG1 phase of cell cycle.
The synergic cytotoxic activity of photoexcited C60 and cisplatin could be realized on condition that cisplatin enters L1210R cells and is accumulated in intracellular space. The ability of C60 fullerene derivatives to reactivate cisplatin endocytosis in cancer cells and thus to circumvent tumor resistance to cisplatin
8
as well as incapability of P-gp type of ABC transporters to recognize pristine C60 fullerene nanoparticles and to prevent their accumulation in drug-resistant K562R leukemic cells
7
confirm this assumption. Additionally, the expression of ABC family transporters responsible for drug efflux from cancer cells is shown to be ROS-regulated. Thus, ROS induced down-regulation of both P-glycoprotein in prostate tumor cells
39
and MDR-associated protein (MRP1) expression in urinary bladder cells
40
were demonstrated. The results of our study allow suggesting that photoexcitation of C60 fullerene may affect the components of the system controlling cisplatin influx and accumulation in L1210R cancer cells, thereby promoting overcoming of drug resistance.
Conclusion
Series of genetic and metabolic rearrangements allow cancer cells to prevent the cytotoxic effects of anticancer drugs. The phenomenon of cancer cells MDR substantially reduces the effect of anticancer therapy and the efficiency of cisplatin as the commonly used drug. In this respect, application of C60 fullerene nanoparticles seems to be perspective as they penetrate into cancer cells, avoid efflux by transporters of ABC family and facilitate drug delivery, produce toxic ROS after photoexcitation and enable combinatory treatment with anticancer drugs.
In this study the activation of p38 MAP kinase and the decrease of Δψm value as the inducing markers of ROS-dependent apoptotic pathways in resistance to cisplatin leukemic L1210R cells treated with 10-5 M C60 fullerene and irradiated with visible light was shown. The data obtained indicated that combined treatment of L1210R cells with photoexcited C60 fullerene and cisplatin in a low 1 µg/mL dose was followed by more intense proapoptotic effect as compared with treatment with photoexcited C60 fullerene alone. Dissipation of Δψm at early term period, the blockage of cell transition into S phase and mitosis with accumulation in the proapoptotic subG1 phase of the cell cycle at long-term period after combined treatment of L1210R cells were detected. The effect of synergic cytotoxic activity allowed us to suppose that photoexcited C60 fullerene promoted cisplatin accumulation in L1210R cells. The data obtained could be useful for the development of approaches to overcome drug-resistance of leukemic cells and to extend the methods of PDT.
Funding sources
None to be declared.
Ethical Statement
Not applicable.
Acknowledgments
SP is grateful to DAAD for support.
Competing interests
The authors declare that they have no competing interests.
Authors' contribution
OM, LD, UR: the conceptual idea and design of the experiments; SP, IG, GP, DF: realization of experiments, data handling, and data analysis; OM, DF, SP: writing the manuscript.
Research Highlights
What is the current knowledge?
simple
-
√ C60 fullerene is potential for PDT and phenomenon of
cancer cells MDR.
What is new here?
simple
-
√ Activation of ROS-sensitive proapoptotic p38 kinase and
enhanced content of cells in proapoptotic subG1 phase were
detected when leukemic cell line L1210 resistant to Cis-Pt
was treated with 10-5M C60 fullerene and irradiated with
visible light.
-
√ Combined treatment of L1210R cells with photoexcited
C60 fullerene and Cis-Pt in low concentration was followed
by the intensification of proapoptotic effects.
-
√ The effect of the synergic cytotoxic activity of both agents
allowed us to suppose that photoexcited C60 fullerene
promoted Cis-Pt accumulation in leukemic cells resistant to
Cis-Pt.
References
- Persidis A. Cancer multidrug resistance. Nat Biotechnol 1999; 17:94-5. doi: 10.1038/5289 [Crossref] [ Google Scholar]
- Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med 2002; 53:615-27. doi: 10.1146/annurev.med.53.082901.103929 [Crossref] [ Google Scholar]
- Florea AM, Busselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) 2011; 3:1351-71. doi: 10.3390/cancers3011351 [Crossref] [ Google Scholar]
- Binkhathlan Z, Lavasanifar A. P-glycoprotein inhibition as a therapeutic approach for overcoming multidrug resistance in cancer: current status and future perspectives. Curr Cancer Drug Targets 2013; 13:326-46. doi: 10.2174/15680096113139990076 [Crossref] [ Google Scholar]
- Johnston HJ, Hutchison GR, Christensen FM, Aschberger K, Stone V. The biological mechanisms and physicochemical characteristics responsible for driving fullerene toxicity. Toxicol Sci 2010; 114:162-82. doi: 10.1093/toxsci/kfp265 [Crossref] [ Google Scholar]
- Prylutska SV, Grynyuk II, Grebinyk SM, Matyshevska OP, Prylutskyy YI, Ritter U. Comparative study of biological action of fullerenes C60 and carbon nanotubes in thymus cells. Materwiss Werksttech 2009; 40:238-41. doi: 10.1002/mawe.200900433 [Crossref] [ Google Scholar]
- Xu X, Li R, Ma M, Wang X, Wang Y, Zou H. Multidrug resistance protein P-glycoprotein does not recognize nanoparticle C60: experiment and modeling. Soft Matter 2012; 8:2915-23. doi: 10.1039/C2SM06811G [Crossref] [ Google Scholar]
- Liang XJ, Meng H, Wang Y, He H, Meng J, Lu J. Metallofullerene nanoparticles circumvent tumor resistance to cisplatin by reactivating endocytosis. Proc Natl Acad Sci U S A 2010; 107:7449-54. doi: 10.1073/pnas.0909707107 [Crossref] [ Google Scholar]
- Yamakoshi Y, Umezawa N, Ryu A, Arakane K, Miyata N, Goda Y. Active oxygen species generated from photoexcited fullerene (C60) as potential medicines: O2-* versus 1O2. J Am Chem Soc 2003; 125:12803-9. doi: 10.1021/ja0355574 [Crossref] [ Google Scholar]
- Huang YY, Sharma SK, Yin R, Agrawal T, Chiang LY, Hamblin MR. Functionalized fullerenes in photodynamic therapy. J Biomed Nanotechnol 2014; 10:1918-36. doi: 10.1166/jbn.2014.1963 [Crossref] [ Google Scholar]
- Wang Y, Liu J, Ma X, Liang X-J. Nanomaterial-assisted sensitization of oncotherapy. Nano Research 2018; 11:2932-50. doi: 10.1007/s12274-017-1961-0 [Crossref] [ Google Scholar]
- Niu Y, Yan C. The effect of fullerenol combined with cisplatin on the proliferation of cervical cancer HeLa cells. J Cancer Ther 2016; 7:232-8. doi: 10.4236/jct.2016.73024 [Crossref] [ Google Scholar]
- Prylutska SV, Skivka LM, Didenko GV, Prylutskyy YI, Evstigneev MP, Potebnya GP. Complex of C60 Fullerene with Doxorubicin as a Promising Agent in Antitumor Therapy. Nanoscale Res Lett 2015; 10:499. doi: 10.1186/s11671-015-1206-7 [Crossref] [ Google Scholar]
- Mroz P, Pawlak A, Satti M, Lee H, Wharton T, Gali H. Functionalized fullerenes mediate photodynamic killing of cancer cells: Type I versus Type II photochemical mechanism. Free Radic Biol Med 2007; 43:711-9. doi: 10.1016/j.freeradbiomed.2007.05.005 [Crossref] [ Google Scholar]
- Prylutska SV, Grynyuk Grynyuk, II II, Palyvoda KO, Matyshevska OP. Photoinduced cytotoxic effect of fullerenes C60 on transformed T-lymphocytes. Exp Oncol 2010; 32:29-32. [ Google Scholar]
- Hu Z, Zhang C, Huang Y, Sun S, Guan W, Yao Y. Photodynamic anticancer activities of water-soluble C(60) derivatives and their biological consequences in a HeLa cell line. Chem Biol Interact 2012; 195:86-94. doi: 10.1016/j.cbi.2011.11.003 [Crossref] [ Google Scholar]
- Metanawin T, Tang T, Chen R, Vernon D, Wang X. Cytotoxicity and photocytotoxicity of structure-defined water-soluble C60/micelle supramolecular nanoparticles. Nanotechnology 2011; 22:235604. doi: 10.1088/0957-4484/22/23/235604 [Crossref] [ Google Scholar]
- Asada R, Liao F, Saitoh Y, Miwa N. Photodynamic anti-cancer effects of fullerene [C(6)(0)]-PEG complex on fibrosarcomas preferentially over normal fibroblasts in terms of fullerene uptake and cytotoxicity. Mol Cell Biochem 2014; 390:175-84. doi: 10.1007/s11010-014-1968-8 [Crossref] [ Google Scholar]
- Brozovic A, Ambriovic-Ristov A, Osmak M. The relationship between cisplatin-induced reactive oxygen species, glutathione, and BCL-2 and resistance to cisplatin. Crit Rev Toxicol 2010; 40:347-59. doi: 10.3109/10408441003601836 [Crossref] [ Google Scholar]
- Berndtsson M, Hagg M, Panaretakis T, Havelka AM, Shoshan MC, Linder S. Acute apoptosis by cisplatin requires induction of reactive oxygen species but is not associated with damage to nuclear DNA. Int J Cancer 2007; 120:175-80. doi: 10.1002/ijc.22132 [Crossref] [ Google Scholar]
- Franskevych D, Palyvoda K, Petukhov D, Prylutska S, Grynyuk I, Schuetze C. Fullerene C(60) penetration into leukemic cells and its photoinduced cytotoxic effects. Nanoscale Res Lett 2017; 12:40. doi: 10.1186/s11671-016-1819-5 [Crossref] [ Google Scholar]
- Franskevych DV, Prylutska SV, Grynyuk Grynyuk, II II, Grebinyk DM, Matyshevska OP. Enhanced cytotoxicity of photoexcited fullerene C60 and cisplatin combination against drug-resistant leukemic cells. Exp Oncol 2015; 37:187-91. [ Google Scholar]
- Scharff P, Carta-Abelmann L, Siegmund C, Matyshevska OP, Prylutska SV, Koval TV. Effect of X-Ray and UV irradiation of the C60 fullerene aqueous solution on biological samples. Carbon 2004; 42:1199-201. doi: 10.1016/j.carbon.2003.12.055 [Crossref] [ Google Scholar]
- Ritter U, Prylutskyy Y, Evstigneev M, Davidenko NA, Cherepanov V, Senenko A. Structural Features of Highly Stable Reproducible C60 Fullerene Aqueous Colloid Solution Probed by Various Techniques. Fullerenes, Nanotubes and Carbon Nanostructures 2015; 23:530-4. doi: 10.1080/1536383X.2013.870900 [Crossref] [ Google Scholar]
- Grynyuk І, Prylutska S, Slobodyanik N, Chunikhin Yu, Matyshevska О. The aggregate state of C60-fullerene in various media. Biotechnologia Acta 2013; 6:71-6. doi: 10.15407/biotech6.06.071 [Crossref] [ Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A 1979; 76:4350-4. [ Google Scholar]
- Scaduto RC, Grotyohann LW. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophysical journal 1999; 76:469-77. doi: 10.1016/S0006-3495(99)77214-0 [Crossref] [ Google Scholar]
- Obata T, Brown GE, Yaffe MB. MAP kinase pathways activated by stress: the p38 MAPK pathway. Crit Care Med 2000; 28:N67-77. doi: 10.1097/00003246-200004001-00008 [Crossref] [ Google Scholar]
- Olson JM, Hallahan AR. p38 MAP kinase: a convergence point in cancer therapy. Trends Mol Med 2004; 10:125-9. doi: 10.1016/j.molmed.2004.01.007 [Crossref] [ Google Scholar]
- Li Z, Zhang F-l, Wang Z, Pan L-l, Shen Y-y, Zhang Z-z. Fullerene (C60) nanoparticles exert photocytotoxicity through modulation of reactive oxygen species and p38 mitogen-activated protein kinase activation in the MCF-7 cancer cell line. J Nanopart Res 2013; 15:2102. doi: 10.1007/s11051-013-2102-7 [Crossref] [ Google Scholar]
- Bulavin DV, Saito S, Hollander MC, Sakaguchi K, Anderson CW, Appella E. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J 1999; 18:6845-6854. doi: 10.1093/emboj/18.23.6845 [Crossref] [ Google Scholar]
- Sanchez-Prieto R, Rojas JM, Taya Y, Gutkind JS. A role for the p38 mitogen-acitvated protein kinase pathway in the transcriptional activation of p53 on genotoxic stress by chemotherapeutic agents. Cancer Res 2000; 60:2464-72. [ Google Scholar]
- Hernandez Losa J, Parada Cobo C, Guinea Viniegra J, Sanchez-Arevalo Lobo VJ, Ramon y Cajal S, Sanchez-Prieto R. Role of the p38 MAPK pathway in cisplatin-based therapy. Oncogene 2003; 22:3998-4006. doi: 10.1038/sj.onc.1206608 [Crossref] [ Google Scholar]
- Barr MP, Gray SG, Hoffmann AC, Hilger RA, Thomale J, O'Flaherty JD. Generation and characterisation of cisplatin-resistant non-small cell lung cancer cell lines displaying a stem-like signature. PLoS One 2013; 8:e54193. doi: 10.1371/journal.pone.0054193 [Crossref] [ Google Scholar]
- Shen H, Perez RE, Davaadelger B, Maki CG. Two 4N cell-cycle arrests contribute to cisplatin-resistance. PLoS One 2013; 8:e59848-e. doi: 10.1371/journal.pone.0059848 [Crossref] [ Google Scholar]
- Marullo R, Werner E, Degtyareva N, Moore B, Altavilla G, Ramalingam SS. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS One 2013; 8:e81162. doi: 10.1371/journal.pone.0081162 [Crossref] [ Google Scholar]
- Palyvoda KO, Grynyuk Grynyuk, II II, Prylutska SV, Samoylenko AA, Drobot LB, Matyshevska OP. Apoptosis photoinduction by C60 fullerene in human leukemic T cells. Ukr Biokhim Zh (1999)2010; 82:121-7. [ Google Scholar]
- Grebinyk SM, Palyvoda KO, Prylutska SV, Grynyuk Grynyuk, II II, Samoylenko AA, Drobot LB. Photoactivated fullerene C60 induces store-operated Ca2 entry and cytochrome c release in Jurkat cells. Ukr Biokhim Zh (1999) 2012; 84:58-63. [ Google Scholar]
- Wartenberg M, Ling FC, Schallenberg M, Baumer AT, Petrat K, Hescheler J. Down-regulation of intrinsic P-glycoprotein expression in multicellular prostate tumor spheroids by reactive oxygen species. J Biol Chem 2001; 276:17420-8. doi: 10.1074/jbc.M100141200 [Crossref] [ Google Scholar]
- Li X, Wang H, Wang J, Chen Y, Yin X, Shi G. Emodin enhances cisplatin-induced cytotoxicity in human bladder cancer cells through ROS elevation and MRP1 downregulation. BMC Cancer 2016; 16:578. doi: 10.1186/s12885-016-2640-3 [Crossref] [ Google Scholar]