BioImpacts. 9(3):161-172.
doi: 10.15171/bi.2019.20
Original Research
A short-term plastic adherence incubation of the stromal vascular fraction leads to a predictable GMP-compliant cell-product
Stephan Born 1, *, Max Johannes Dörfel 1, Philip Hartjen 2, Seyed Ali Haschemi Yekani 1, Julia Luecke 1, Juliane Katharina Meutsch 1, Julie Katharina Westphal 1, Moritz Birkelbach 2, Robert Köhnke 2, Ralf Smeets 2, 3, Michael Krueger 1
Author information:
1 OXACELL® AG, Potsdam, Germany
2 Department of Oral and Maxillofacial Surgery, University Hospital Hamburg-Eppendorf, Hamburg, Germany
3 Division, Regenerative Orofacial Medicine, University Hospital Hamburg-Eppendorf, Hamburg, Germany
Abstract
Introduction:
Mesenchymal stromal/stem cells (MSCs) derived from fat tissue are an encouraging tool for regenerative medicine. They share properties similar to the bone marrow-derived MSCs, but the amount of MSCs per gram of fat tissue is 500x higher. The fat tissue can easily be digested by collagenase, releasing a heterogeneous cell fraction called stromal vascular fraction (SVF) which contains a variable amount of stromal/stem cells. In Europe, cell products like the SVF derived from fat tissue are considered advanced therapy medicinal product (ATMPs). As a consequence, the manufacturing process has to be approved via GMP-compliant process validation. The problem of the process validation for SVF is the heterogeneity of this fraction.
Methods:
Here, we modified existing purification strategies by adding an additional plastic adherence incubation of maximal 20 hours after SVF isolation. The resulting cell fraction was characterized and compared to SVF as well as cultivated adipose-derived stromal/stem cells (ASCs) with respect to viability and cell yield, the expression of surface markers, differentiation potential and cytokine expression.
Results:
Short-term incubation significantly reduced the heterogeneity of the resulting cell fraction compared to SVF. The cells were able to differentiate into adipocytes, chondrocytes, and osteoblasts. More importantly, they expressed trophic proteins which have been previously associated with the beneficial effects of MSCs. Furthermore, GMP compliance of the production process described herein was acknowledged by the national regulatory agencies (DE_BB_01_GMP_2017_1018).
Conclusion:
Addition of a short purification-step after the SVF isolation is a cheap and fast strategy to isolate a homogeneous uncultivated GMP-compliant cell fraction of ASCs.
Keywords: Adipose derived stromal/stem cells, Stromal vascular fraction, Mesenchymal stromal/stem cells, Cell therapy, Regenerative medicine, Good manufacturing practice
Copyright and License Information
© 2019 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Mesenchymal stromal/stem cells (MSCs) derived from different sources are encouraging tools for regenerative medicine because of their manifold immunomodulatory and regenerative properties.
1,2
One of these sources is adipose tissue, which can easily be digested by collagenase, releasing a heterogeneous cell fraction called stromal vascular fraction (SVF).
3
Besides granulocytes, monocytes, lymphocytes, endothelial cells, pericytes, erythrocytes, and other cells, this fraction contains a huge number of MSCs, called adipose-derived stromal/stem cells (ASCs).
4
Even the crude SVF, containing only a limited number of ASCs, has a high therapeutic potential.
5-17
These ASCs have similar properties as bone marrow-derived MSCs (BM-MSCs),
18-20
however, the amount of MSCs per gram of fat tissue is 500x higher.
21,22
Additionally, according to the literature, ASCs secrete higher quantities of cytokines with regenerative properties
23,24
and possibly possess a higher angiogenic potential.
25
In Europe, cell products derived by collagenase digestion of tissues are classified as advanced therapy medicinal product (ATMPs).
26,27
As a consequence, the manufacturing process has to be authorized by the corresponding national authorities. Furthermore, a good manufacturing practice (GMP)-compliant quality management has to be established to ensure the production of a consistent and well-characterized product.
26
This, however, is a major problem since SVF by itself is a very heterogeneous cell fraction with a highly varying stromal/stem cell content of 2%-30%.
4
Hence, standardized therapy is hard to establish. To surmount this obstacle, SVF can be cultivated resulting in a more homogenous cell fraction.
28
However, this cultivation process induces phenotypical changes of the cell population including a change of the immunophenotype.
28
Additionally, there is evidence that the cultivation step may affect the functional characteristics of the ASCs. In this regard, a reduction of the homing potential to the site of tissue damage, changes in the expression of adhesion molecules, or changes in the regenerative potential have been described.
5,29,30
In addition to these potentially negative effects on the therapeutic activity of the stromal/stem cells, there are regulatory and economic problems associated with the cultivation process. Although there is no evidence that the changes of the karyotype which could be observed sometimes during cultivation,
31,32
lead to a malign transformation,
33
the EMA is concerned about possible risks for patients.
26,34
As a result, the remaining risk of a possible malign transformation has to be considered carefully, especially for non-life-threatening indications, for example, osteoarthritis or cosmetic rejuvenation. Accordingly, genomic stability has to be demonstrated during the cultivation process.
34
Furthermore, the cultivation process itself is time-consuming and resource-intensive. Nevertheless, this technique is state of the art for the treatment of many diseases with limited therapeutic options.
35
An alternative enrichment approach is based on cell sorting with antibodies against specific surface markers. This method is well established for blood-based products like CAR-T cells.
36
However, for the enrichment of MSCs from SVF only experimental and non-GMP-compliant protocols are available.
37-39
Furthermore, the high costs of a cell sorter (purchase and maintenance) in combination with the need for special trained personnel render this technique less-than-ideal.
Herein we presented an inexpensive and fast (~24 hours) approach to isolate a homogeneous uncultivated GMP-compliant cell fraction of ASCs by applying a short purification step after the SVF isolation.
Materials and Methods
SVF isolation, short-term incubation, and ASC cultivation
Samples of subcutaneous lipoaspirate (300–3300 mL) were obtained from patients undergoing cosmetic liposuction by power-assisted liposuction (PAL) as described before by Barzelay et al.
40
Briefly, 3-7.5 L tumescent solution containing 840 mg/L sodium hydrogen carbonate (B.Braun, Melsungen, Germany), 100 mg/L triamcinolone (Hexal, Holzkirchen, Germany), 200 mg/L procaine hydrochloride (AstraZeneca, Wedel, Germany), 200 mg/L lidocaine hydrochloride (AstraZeneca, Wedel, Germany) and 1.2 mg/L ephedrine hydrochloride (Sanofi, Paris, France) was applied for the superwet technique. A 3.0-mm diameter blunt hollow cannula with a Micro Aire PAL 600 system power-assisted lipoplasty device (MicroAire Surgical Instruments, Charlottesville, USA) and an Ardo Master 45 pump (Ardo medical GmbH, Oberpfaffenhofen, Germany) were used to perform PAL. Fat was decanted, and the fat fraction was aspirated into an empty sterile container. Liposuctions were performed in the Department of Plastic Surgery at the Klinik Sanssouci (Potsdam, Germany) and at the MEOCLINIC (Berlin, Germany). Isolation of the SVF was done by OXACELL®AG (Potsdam, Germany) as described by Zuk et al. (2001)
3
and Zhu et al
41
with some modifications to achieve GMP-compliance. Briefly, lipoaspirate was transferred into closed Flexboy Bags (Satorius Stedim Biotech, Göttingen, Germany) and washed three times with DMEM-F12 (ThermoFisher Scientific, Waltham, USA) containing 2% CTS™ KnockOut™ SR XenoFree Medium (ThermoFisher Scientific, Waltham, USA). The washed lipoaspirate was digested with Collagenase NB 6 GMP Grade (Nordmark, Uetersen, Germany) for 30-60 minutes at 37°C according to the manufacturers’ recommendations. Depending on the volume of the lipoaspirate, the digestion time for < 900 mL lipoaspirate was 30 minutes, for 900-2250 mL 35 minutes, and for > 2250 mL 45-60 minutes. The digestion was stopped with EDTA (Corning Inc., New York , USA) as recommended by Nordmark for Collagenase digestion. This solution was centrifuged for 10 minutes (400 g, 21°C) and the supernatant was discarded. The cell pellet was resuspended in DMEM-F12, 2% KnockOut™ SR XenoFree Medium (ThermoFisher Scientific, Waltham, USA) and filtered through different strainers (Steriflip®, Merck Millipore, Darmstadt, Germany). Afterward, the cells were further separated by density gradient centrifugation with Ficoll-Paque PremiumTM (400 g, 24°C, 30 minutes, GE Healthcare Bio-Sciences, Buckinghamshire, UK) instead of red blood cell (RBC) lysis. The resulting cells were washed twice with PBS w/o Mg2+ Ca2+ (Biochrom, Berlin, Germany), 2% KnockOut™ (ThermoFisher Scientific, Waltham, USA). For short-term incubation and purification, the cells were seeded on cell culture dishes (Greiner Bio-One) at high density (≥ 1.0 x 105 cells/cm2) in DMEM-F12, 2% KnockOut™ SR XenoFree Medium (ThermoFisher Scientific, Waltham, USA) and incubated for 16-20 hours (37°C, 6% CO2, 95% RH). After that time, the cells were trypsinized (Trypsin-EDTA solution, Sigma-Aldrich, Munich, Germany) and suspended in PBS w/o Mg2+ Ca2+ (Biochrom, Berlin, Germany), 2% KnockOut™ (ThermoFisher Scientific, Waltham, USA). After one washing step, the cells were filtered through a cell strainer (Steriflip®, Merck Millipore, Darmstadt, Germany). Thereafter, the cells were washed two more times with 0.9% sodium chloride (B.Braun, Melsungen, Germany) and suspended in a buffered solution for further analysis. The resulting short-term incubated cell fraction was called Oxacells HP (OXACELL® AG, Potsdam, Germany). The whole procedure described above took about 24 hours from the lipoaspirate to the finished cell product, and hands-on time was about 6 hours. The manufacturing protocol was GMP certified (DE_BB_01_GMP_2017_1018) in accordance with Art. 13 and 15 of Directive 2001/20/EC. It should be noted that Oxacells HP is classified as an ATMP for use in specialized health care establishments. The resulting cells were randomly divided into two groups. One group was used directly for further experiments. The cells of the second group were seeded again on cell culture dishes (2000–4000 cells /cm2) and expanded with DMEM-F12 (ThermoFisher Scientific, Waltham, USA) containing 10% (w/v) pooled human serum (GMP-grade, Zentrum für Klinische Transfusionsmedizin, Tübingen, Germany) until the confluency and criteria for ASCs
4
were reached (8-14 days, corresponding to passage 0). Afterward, the cells were washed twice with PBS w/o Mg2+ Ca2+ (Biochrom, Berlin, Germany) and trypsinized (Trypsin-EDTA solution, Sigma-Aldrich, Munich, Germany). Finally, the ASCs were suspended in PBS w/o Mg2+ Ca2+ (Biochrom, Berlin, Germany), 2% KnockOut™ (ThermoFisher Scientific, Waltham, USA) and used for further analysis.
Cell count and viability
For cell number and viability measurements, the cells were diluted in Guava ViaCount Reagent (Merck Millipore, Darmstadt, Germany) and measured in a Flow cytometer (Guava® easyCyte 6-2L, Merck Millipore, Darmstadt, Germany) according to the instruction manual.
Flow cytometry
A total of 1.5 × 104 cells were suspended in 100 µL PBS w/o Mg2+ Ca2+ (Biochrom, Berlin, Germany), 2% KnockOut™ (ThermoFisher Scientific, Waltham, USA) and incubated with fluorescence-labeled antibodies. For the cell staining, the following antibodies coupled to fluorescein isothiocyanate (FITC), phycoerythrin (PE), or allophycocyanin (APC) were used: Anti-CD-13, Anti-CD31, Anti-CD34, Anti-CD44, Anti-CD45, Anti-CD73, Anti-CD90, Anti-CD105-FITC, Anti-CD235a and Anti-HLA-DR, DP, and DQ. All antibodies were purchased from Miltenyi Biotec, Bergisch Gladbach, Germany. The samples were incubated for 15 minutes in the dark at 2-8°C and washed afterward with PBS w/o Mg2+ Ca2+ (Biochrom, Berlin, Germany), 2% KnockOut™ (ThermoFisher Scientific, Waltham, USA). The samples were suspended in 150 µL PBS w/o Mg2+ Ca2+ (Biochrom, Berlin, Germany), 2% KnockOut™ (ThermoFisher Scientific, Waltham, USA). Before every measurement, the integrity of all channels was verified with an EasyCheck-Kit (Merck Millipore, Darmstadt, Germany). At least 5000 events were analyzed and compared with appropriate isotype controls. All measurements were done with fixed gates and protocols according to the GMP guideline. The final results were calculated by subtracting the background (isotype controls).
Multilineage differentiation protocols
The differentiation experiments were done according to Zhu et al.
41
Briefly, for adipogenic and osteogenic differentiation, 3.0 × 104 cells/chamber were seeded on chamber slides (Sigma-Aldrich, Munich, Germany). For chondrogenic “micromass culture”, 1.0 × 105 cells/chamber were seeded. For all experiments, human serum from pooled human male AB plasma (Sigma-Aldrich, Munich, Germany) was used instead of animal-derived FBS. All differentiation experiments were compared to undifferentiated controls.
Adipogenesis
The adipogenic differentiation medium consisted of DMEM (4.5 g/mL Glucose, ThermoFisher Scientific, Waltham, USA), 10% human Serum (Sigma-Aldrich, Munich, Germany), 1 % penicillin/streptomycin (10 000 I.U/mL and 10 000 mg/mL (Sigma-Aldrich, Munich, Germany), 1.0 µM Dexamethasone (Sigma-Aldrich, Munich, Germany), 0.5 mM 3-isobutyl-1-methylxanthine (Sigma-Aldrich, Munich, Germany), 0.2 mM indomethacin (Sigma-Aldrich, Munich, Germany) and 10.0 µM insulin (Sigma-Aldrich, Munich, Germany). The differentiation was induced at 80% confluency. The cells were cultured for 18-21 days with medium changes every 3-4 days. The adipogenic differentiation was confirmed by Oil Red O histologic staining.
Osteogenesis
For osteogenic differentiation, we used DMEM (4.5 g/mL Glucose, ThermoFisher Scientific, Waltham, USA) containing 5% human serum, 1% penicillin/streptomycin (10 000 I.U/mL and 10 000 mg/mL), 0.1 µM Dexamethasone (Sigma-Aldrich, Munich, Germany), 50 µM L-Ascorbic acid 2-phosphate (Sigma-Aldrich, Munich, Germany), and 10 mM β-glycerophosphate (Sigma-Aldrich, Munich, Germany). The differentiation was induced at a confluency of 50%. The cells were cultured for 18-21 days with medium changes every 3-4 days. The osteogenic differentiation was confirmed by Kossa histologic staining for calcium phosphate.
Chondrogenesis
The chondrogenic medium consisted of DMEM (4.5 g/mL Glucose, ThermoFisher Scientific, Waltham, USA) with 1% human serum, 1% penicillin/streptomycin (10 000 I.U/mL and 10 000 mg/mL), 50 µg/mL L-ascorbic-2-phosphate (Sigma-Aldrich, Munich, Germany), 6.25 µg/mL insulin (Sigma-Aldrich, Munich, Germany), 6.25 mg/mL transferrin (Sigma-Aldrich, Munich, Germany) and 10 ng/mL TGF-β1 (Sigma-Aldrich, Munich, Germany). Micromass pellets were prepared as described by Zhu at al
41
but the cell density was reduced to 1.0 × 105 cells/chamber. The cells were cultured for 18-21 days with medium changes every 3-4 days. The successful differentiation was confirmed by Alcian Blue histologic stain.
Cytokine and growth factor estimation and quantification
Harvest of conditioned media and cells
For the estimation and quantification of cytokine and growth factor, the supernatants of Oxacells HP and ASCs were collected. Briefly, for Oxacells HP, 50 000 nucleated cells/well were seeded in 24-well-plates and attached for 24 hours in DMEM-F12 (ThermoFisher Scientific, Waltham, USA) containing 10% human serum and 1% penicillin/streptomycin (10 000 I.U/mL and 10 000 mg/mL (Sigma-Aldrich, Munich, Germany). The human serum has to be added to the medium at this stage to ensure sufficient attachment of the cells. Afterward, the cells were washed three times with PBS (w/o Ca2+, Mg2+) and cultivated in 0.5 mL media without serum for 72 hours. The supernatant was collected, centrifuged (5 minutes, 300 g, 4°C) and frozen at -20°C. The cells were harvested, the cell number was counted, and the amount of CD90 positive cells was determined. For ASCs, the conditioned media was harvested at the end of the cultivation to passage 0. The supernatant was collected, centrifuged (5 minutes, 300 g, 4°C) and frozen at -20°C. The cells were harvested, the cell number was counted, and the amount of CD90 positive cells was determined.
Measurement of cytokines and growth factors
The estimation and quantification of cytokines and growth factors for Oxacells HP was performed by the Natural and Medical Sciences Institute at the University of Tübingen (NMI, Reutlingen, Germany) as a Multi-Analyte Profile (MAP) platform analysis according to their protocols. The estimation and quantification of cytokines and growth factors for ASCs were performed by OXACELL® AG according to the protocols provided by Bio-Rad (Bio-Rad Laboratories Inc., Hercules, USA) for multiplex measurements of cytokines and growth factors. Fifteen analytes were measured: hepatocyte growth/scatter factor (HGF), nerve growth factor beta (b-NGF), interleukin-1 beta (IL-1 b), interleukin-1 receptor antagonist (IL-1ra), interleukin-5 (IL-5), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-10 (IL-10), interleukin-17 (IL-17), eotaxin-1, granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage-colony-stimulating factor (GM-CSF), monocyte chemotactic protein 1 (MCP-1), tumor necrosis factor alpha (TNF-a), and vascular endothelial growth factor (VEGF). The multiplex kits were provided by NMI and Bio-Rad Laboratories Inc. (Hercules, USA). The samples were successively incubated with the capture microspheres, a multiplexed cocktail of biotinylated reporter antibodies, and a streptavidin-phycoerythrin solution. The analysis was performed at NMI and OXACELL® AG on Luminex 100/200 (Austin, USA) instruments in duplicates or triplicates. The resulting data stream was interpreted using proprietary data analysis software developed at Myriad RBM (Austin, USA) or Bio-Plex Manager™ 6.1 (Bio-Rad Laboratories Inc., Hercules, USA). Unknown values for each of the analytes were determined using 4 and 5 parameter, weighted and non-weighted curve fitting algorithms included in the data analysis package. Afterwards the amount of the factors in the corresponding media alone was subtracted from the measured concentration values.
Analysis strategy of results
Cell number is commonly considered an appropriate normalizer for a variety of assay types.
42
However, the cell composition differs between freshly isolated human adipose tissue‐derived SVF and serial‐passaged ASCs.
28
For this reason, the cytokine and growth factor concentrations were normalized to the cell number of MSCs in each cell fraction which approximately corresponds to the content of CD90+ cells.
43-47
All concentrations are presented as [pg / 106 cells (CD90+)].
Data analysis and statistics
Statistical analysis was performed using GraphPad 7 (GraphPad software, La Jolla, USA). Values were expressed as mean ±SD. Multiple comparisons were made using a one-way ANOVA and a Bonferroni's multiple comparison test or two-way ANOVA and Sidak's multiple comparison tests. Statistical significance was defined as P < 0.05, and all reported statistical tests were two-tailed.
Results
Comparison of the cell yield and viability for different isolation, incubation, and cultivation steps
Stromal/stem cell of the SVF was isolated from 57 patients undergoing liposuction. The lipoaspirates were taken from 4 male and 53 female donors. The age of the donors ranged from 19-64, with a mean of 39 years. The volume of the processed lipoaspirates varied from 300 to 3300 mL. Immediately after crude purification (pre-Ficoll), the cells were subjected to density gradient centrifugation (Post-Ficoll/SVF) to enrich for stromal/stem cells and reduce erythrocytes. Subsequently, cells were seeded on culture dishes for a short-term incubation (Oxacells HP). Obtained cells were re-cultivated for additional 8-14 days (ASCs). Samples for cell number and viability measurement were taken at each step during the isolation process. The results are summarized in Fig. 1. The yield of the nucleated cells successively decreased with every additional purification step, from 4.2 × 105 ± 1.7 × 105 cells per mL of lipoaspirate before density gradient centrifugation to 1.5 × 105 ± 8.6 × 104 cells/mL, after the centrifugation to 3.1 × 104 ± 9.9 × 104 cells/mL, after the short-term incubation to 9.6 × 104 ± 1.5 × 105 cells/mL at P0. All differences were significant except for post-Ficoll/SVF and HP for cultivated ASCs.
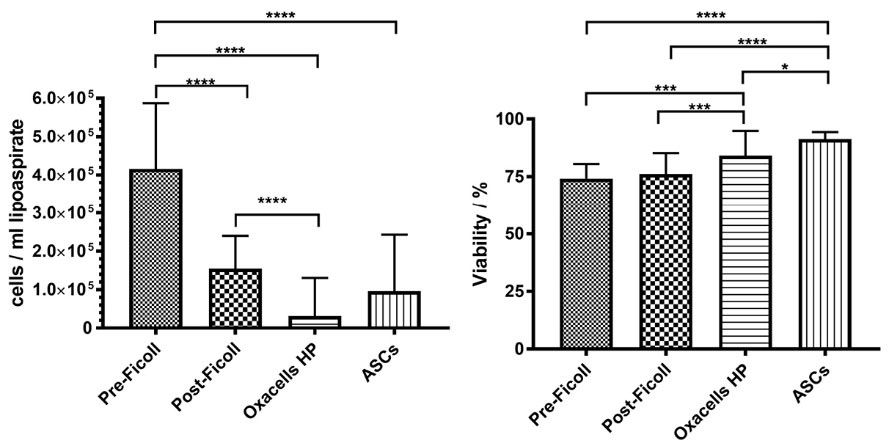
Fig. 1.
Nucleated cell yield and viability of the different cell fractions Yield of nucleated cells per volume of lipoaspirate (A) and viability of the cells (B) of the stromal vascular fraction before Ficoll density centrifugation, after density centrifugation (SVF), after short-term incubation (Oxacells HP) and after cultivation at passage 0. Significant differences are marked (* P < 0.05, ** P < 0.01, *** P < 0.001, **** P ≤ 0.0001). Data represents the mean ± SD of all donors.
.
Nucleated cell yield and viability of the different cell fractions Yield of nucleated cells per volume of lipoaspirate (A) and viability of the cells (B) of the stromal vascular fraction before Ficoll density centrifugation, after density centrifugation (SVF), after short-term incubation (Oxacells HP) and after cultivation at passage 0. Significant differences are marked (* P < 0.05, ** P < 0.01, *** P < 0.001, **** P ≤ 0.0001). Data represents the mean ± SD of all donors.
Inversely, cell viability increased from pre-Ficoll (74 ± 6%) through post-Ficoll/SVF (76 ± 9%) to Oxacells HP (84 ± 11%) with the highest value observed in the cultivated ASC fraction (91 ± 3%). Except for the difference between the pre- and post-Ficoll purification, all other values differed significantly.
Immunophenotypic characterization of short-term incubated cells (Oxacells HP) in comparison to SVF and ASCs
As previously mentioned, samples for immunophenotypic characterization were taken at each step of the purification process and the expression of characteristic ASCs-antigens was measured (Figs. 2 and 3). Positive surface markers for ASCs selected according to Bourin et al
4
were CD13 (Alanine aminopeptidase), CD44, CD73 (5'-ribonucleotide phosphohydrolase), CD90 (Thy-1), and CD105 (Endoglin). Additionally, the unstable positive marker CD34
4
was measured. The ASC negative markers CD31 (PECAM-1), CD45 (leukocyte common antigen), and CD235a (glycophorin A) were selected as suggested previously.
4
Furthermore, the histocompatibility antigens class II, HLA-DR-DP-DQ were determined, representing the immunogenicity of the resulting cell fraction (negative marker).
48
Dot plots of the different antibody combinations are shown in Fig. 2. During the course of the purification, an increasing amount of cells positive for ASCs surface marker (CD34+/CD90+ in Fig. 2A and CD44+/CD73+ in Fig. 2B) could be detected. The cultivation process resulted in a decreased CD34 expression and the CD105 expression started (Fig. 2A). CD34+/CD45+ cells could be detected in the SVF (~5%) but the content dropped during the course of the purification up to zero at passage 0 (Fig. 2A). The presence of other cells, then, ASC (CD45+ and HLA II+ cells in Fig. 2A as well as CD235a+ and CD31+ cells in Fig. 2B) also decreased during the purification steps. The statistical analysis showed that all stable positive markers (CD13, CD44, CD73, CD90, and CD105) increased significantly from the first purification to Oxacell HP and further to the cultivated ASCs (Fig. 3A). As expected, CD105+ cells could only be detected after cultivation at passage 0 whereas CD34+ expression decreased during this process (Fig. 3A). Interestingly, the stromal/stem cell content (CD13+, CD44+, CD73+, and CD90+) was significantly higher in the Oxacells HP fraction than in the purifications steps before. Simultaneously, the number of other cells then ASCs (CD31+, CD45+, CD235a+ and HLA II+ in Fig. 3B) was significantly reduced in the Oxacells HP. This indicates that the Oxacells HP population is significantly less heterogeneous than the upstream intermediate products. After cultivation until P0, no considerable expression (≤2%) of CD31+, CD34+ CD235a+ or HLA II+ cells was detected.
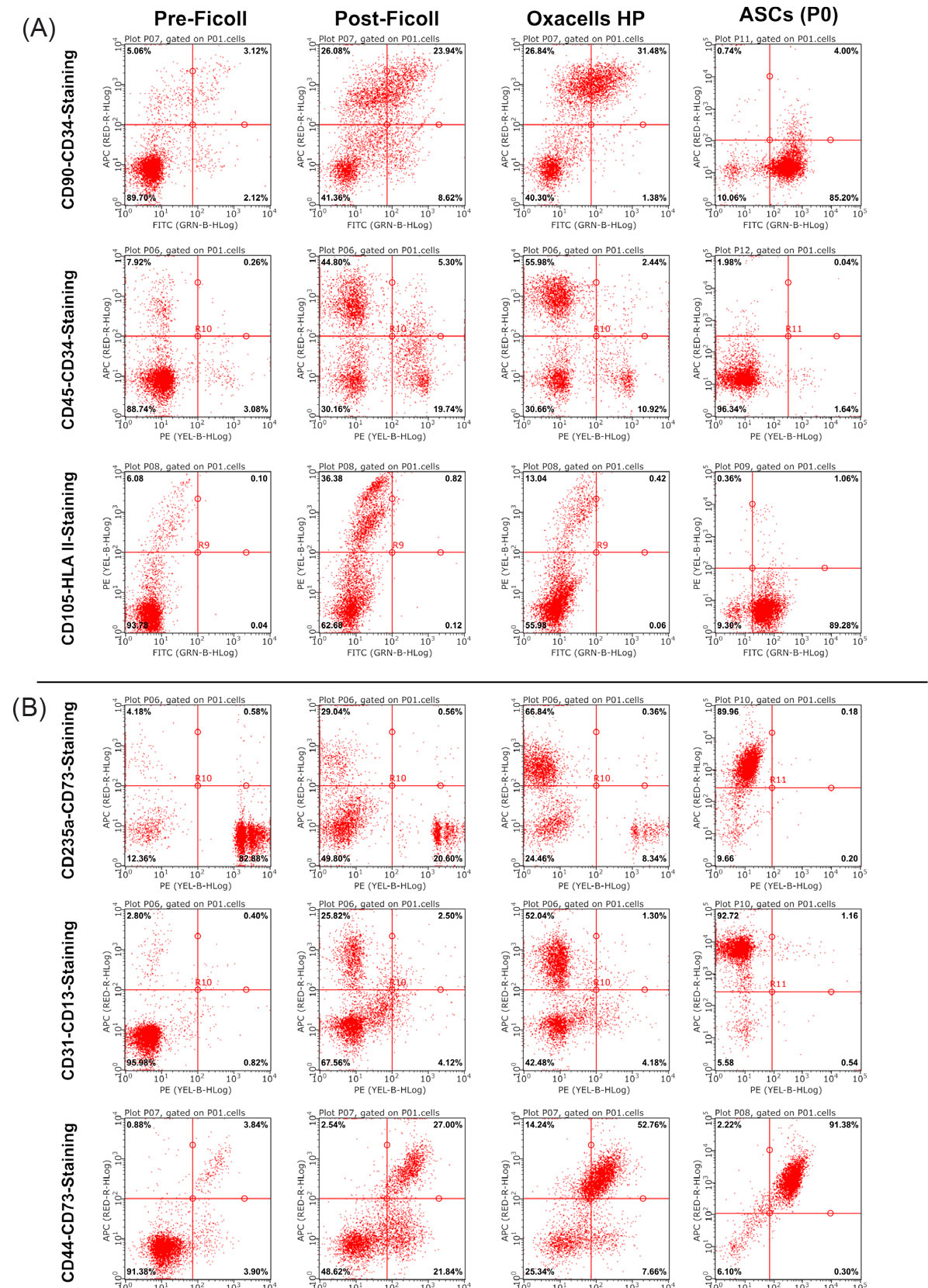
Fig. 2.
Immune phenotypic characterization of the purified cell populations. Representative dot plots of double-stained cells from the 4 different purification steps (before Ficoll density centrifugation, after density centrifugation, after short-term incubation (Oxacells HP) and after cultivation to passage 0). Shown is data from two donors (A and B). For donor A, the antibody combinations CD90-FITC / CD34-APC, CD45-PE / CD34-APC and CD105-FITC / HLA II (HLA-DR, DP, DQ)-PE were selected. For the second subset the combinations of anti-CD235a-PE / -CD73-APC, anti-CD-31-PE / -CD13-APC and anti-CD44-FITC / -CD73-APC were taken. The percentage of the positive single-stained cells is shown in the lower right and upper left corner, the percentage of double positive cells is shown in the upper right corner. The cells in the lower left corner are negative for the corresponding marker combination.
.
Immune phenotypic characterization of the purified cell populations. Representative dot plots of double-stained cells from the 4 different purification steps (before Ficoll density centrifugation, after density centrifugation, after short-term incubation (Oxacells HP) and after cultivation to passage 0). Shown is data from two donors (A and B). For donor A, the antibody combinations CD90-FITC / CD34-APC, CD45-PE / CD34-APC and CD105-FITC / HLA II (HLA-DR, DP, DQ)-PE were selected. For the second subset the combinations of anti-CD235a-PE / -CD73-APC, anti-CD-31-PE / -CD13-APC and anti-CD44-FITC / -CD73-APC were taken. The percentage of the positive single-stained cells is shown in the lower right and upper left corner, the percentage of double positive cells is shown in the upper right corner. The cells in the lower left corner are negative for the corresponding marker combination.
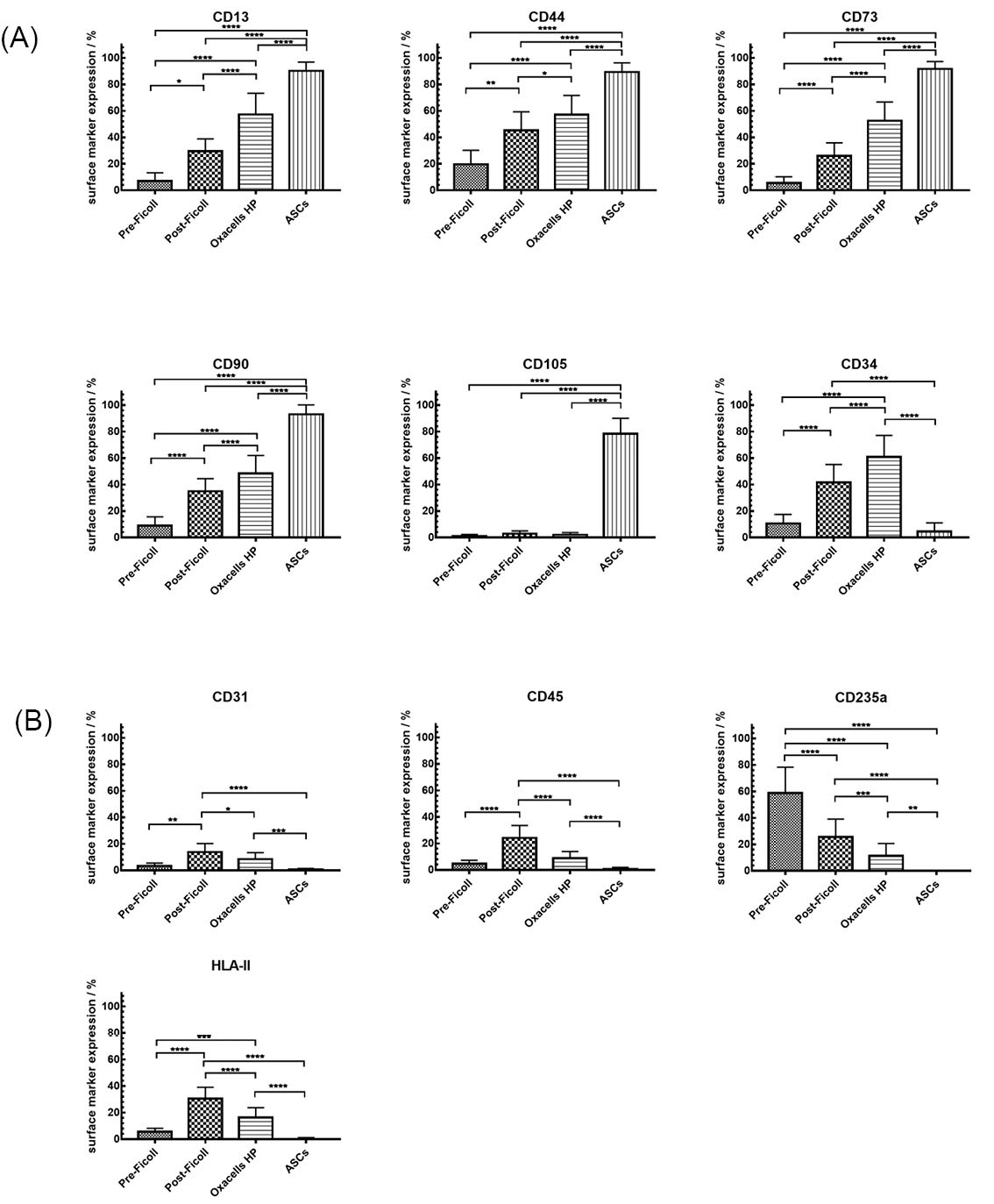
Fig. 3.
summary of the surface marker expression in the different cell fractions. Expression of characteristic ASCs-antigens measured in the stromal vascular fraction before Ficoll density centrifugation, after density centrifugation (SVF), after short-term incubation (Oxacells HP) and after cultivation at passage 0. The staining of the positive marker (A) CD13 CD44, CD73, CD90, CD105, he unstable progenitor marker CD34 (A) and the negative marker (B) CD31, CD45, CD235a and HLA II (HLA-DR, DP, DQ) were performed as single and multiple staining. Significant differences in marker expression between the different groups are marked (* P < 0.05, ** P < 0.01, *** P < 0.001, **** P ≤ 0.0001). Data represents mean expression (percentage) ± SD of all donors.
.
summary of the surface marker expression in the different cell fractions. Expression of characteristic ASCs-antigens measured in the stromal vascular fraction before Ficoll density centrifugation, after density centrifugation (SVF), after short-term incubation (Oxacells HP) and after cultivation at passage 0. The staining of the positive marker (A) CD13 CD44, CD73, CD90, CD105, he unstable progenitor marker CD34 (A) and the negative marker (B) CD31, CD45, CD235a and HLA II (HLA-DR, DP, DQ) were performed as single and multiple staining. Significant differences in marker expression between the different groups are marked (* P < 0.05, ** P < 0.01, *** P < 0.001, **** P ≤ 0.0001). Data represents mean expression (percentage) ± SD of all donors.
Efficiency of different purification procedures
The fraction of CD90+ cells is roughly equivalent to the MSC content.
43-47
Accordingly, the mean stromal/stem cell yield was calculated for every purification step based on the cell number per mL lipoaspirate (Fig. 1) and CD90 expression (Fig. 3). As shown in Fig. 4 , the highest yield of ASCs per mL lipoaspirate was achieved by cultivation (9.0 x 104 ± 1.4 × 105 CD90+ cells/mL), followed by Ficoll purification (5.4 × 104 ± 3.0 × 104 CD90+ cells/mL), Pre-Ficoll purification (3.7 × 104 ± 1.5 × 104 CD90+ cells/mL), and short-term incubation (1.5 × 104 ± 4.8 × 104 CD90+ cells/mL). The differences between the values, however, were only significant between Oxacells HP and cultivated ASC, as well as between post-Ficoll and Oxacells HP.
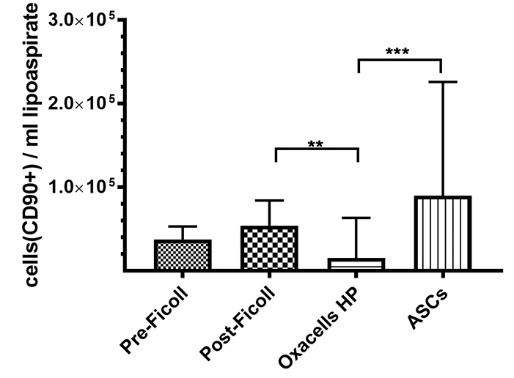
Fig. 4.
Adipose-derived stem cell yield in the different cell fractions. The stem cell yield was calculated for every purification step based on he number of purified CD90+cells per volume of processed lipoaspirate. Significant differences for each group are marked (* P < 0.05, ** P < 0.01, *** P < 0.001, **** P ≤ 0.0001). All graphs represent the mean yield for all donors ± SD.
.
Adipose-derived stem cell yield in the different cell fractions. The stem cell yield was calculated for every purification step based on he number of purified CD90+cells per volume of processed lipoaspirate. Significant differences for each group are marked (* P < 0.05, ** P < 0.01, *** P < 0.001, **** P ≤ 0.0001). All graphs represent the mean yield for all donors ± SD.
Multilineage differentiation of short-term incubated cells (Oxacells HP)
To check the stromal/stem cell characteristics of the ASCs in the short-term incubated cell fraction, the cells were seeded on chamber slides and differentiated into adipogenic, chondrogenic, and osteogenic lineages (Fig. 5A, C and E). The differentiated cells were stained with the appropriate dyes according to their lineage (Oil Red O, Alcian Blue, and Kossa) and compared to undifferentiated controls (Fig. 5B, D and E). As shown in Fig. 5A, C and E, the treated cells could be stained according to their lineage, indicating a successful differentiation.
Expression of cytokines and growth factors
To quantify the expression of cytokines and growth factors, a MAP analysis of the supernatants after short-term incubation and after cultivation of ASCs was performed. Additionally, the cells were harvested to determine the content of CD90+ cells. The measured concentrations were normalized to 1 x 106 CD90+ cells (Fig. 6) as described in the “Materials and Methods”. All 15 analytes could be detected in the supernatant of cultured ASCs in various amounts, however, nerve growth factor beta (b-NGF) was absent in the supernatant of Oxacells HP (Fig. 6). Several soluble factors were found in higher amounts in the supernatant of Oxacells HP compared to ASCs, albeit not significantly: IL-1b (3x higher), IL-1ra (15x higher), IL-6 (2x higher), IL-8 (2x higher), IL-17 (2x higher), Eotaxin (3x higher), and G-CSF (35x higher). The other seven factors seem to be highly expressed by cultured ASCs when compared to Oxacells HP, however, only the change for MCP-1 was significant.
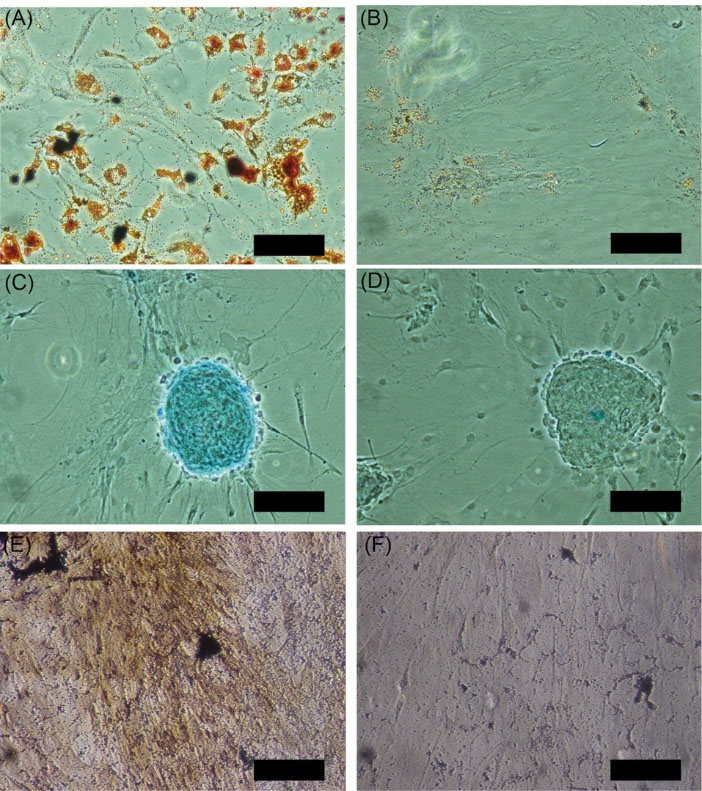
Fig. 5.
Differentiation potential of short term incubated cells. Short-term incubated cells (Oxacells HP) were seeded on chamber slides and differentiated into the adipogenic (A), chondrogenic (C) und osteogenic (E) lineage. The differentiated cells were stained accordingly with Oil Red O (A+B), Alcian Blue (C+D) and Kossa (E+F) and compared to undifferentiated controls (B, D, and F, respectively). Shown are representative pictures of four experiments. Scale bar corresponds to 0.1 mm.
.
Differentiation potential of short term incubated cells. Short-term incubated cells (Oxacells HP) were seeded on chamber slides and differentiated into the adipogenic (A), chondrogenic (C) und osteogenic (E) lineage. The differentiated cells were stained accordingly with Oil Red O (A+B), Alcian Blue (C+D) and Kossa (E+F) and compared to undifferentiated controls (B, D, and F, respectively). Shown are representative pictures of four experiments. Scale bar corresponds to 0.1 mm.
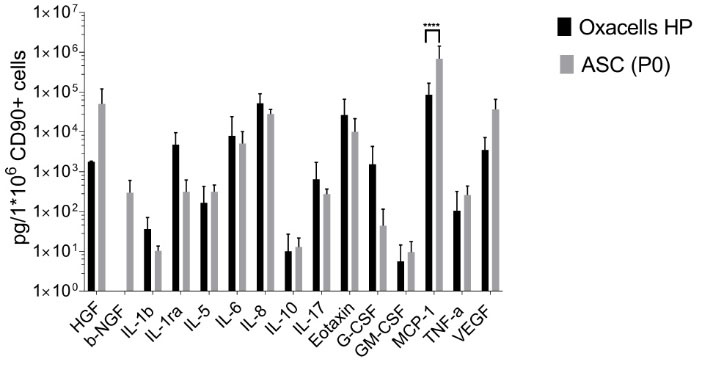

Fig. 6.
Concentrations of cytokines and growth factors in the supernatants of Oxacells HP and adipose-derived stem cells. The concentration of 15 analytes (Hepatocyte Growth/scatter Factor = HGF, Nerve growth factor beta = b-NGF, Interleukin-1 beta = IL-1 b, Interleukin-1 receptor antagonist = IL-1ra, Interleukin-5 = IL-5, Interleukin-6 = IL-6, Interleukin-8 = IL-8, Interleukin-10 = IL-10, Interleukin-17 = IL-17, Eotaxin-1, Granulocyte-Colony Stimulating Factor = G-CSF, Granulocyte-Macrophage Colony-Stimulating Factor = GM-CSF, Monocyte Chemotactic Protein 1 = MCP-1, Tumor Necrosis Factor alpha = TNF-a and Vascular Endothelial Growth Factor = VEGF) was measured in the supernatants of Oxacells HP and adipose-derived stem cells by multiplex technique described in material and methods. All measured concentrations were normalized to 1 x 106 CD90+ cells. Plotted were mean values ±SD of multiple measurements (Oxacells HP n=6, ACSs n=8) on a logarithmic scaled Y axis. Significant differences between Oxacells HP and ASCs (P0) are marked (* P < 0.05, ** P < 0.01, *** P < 0.001, **** P ≤ 0.0001).
.
Concentrations of cytokines and growth factors in the supernatants of Oxacells HP and adipose-derived stem cells. The concentration of 15 analytes (Hepatocyte Growth/scatter Factor = HGF, Nerve growth factor beta = b-NGF, Interleukin-1 beta = IL-1 b, Interleukin-1 receptor antagonist = IL-1ra, Interleukin-5 = IL-5, Interleukin-6 = IL-6, Interleukin-8 = IL-8, Interleukin-10 = IL-10, Interleukin-17 = IL-17, Eotaxin-1, Granulocyte-Colony Stimulating Factor = G-CSF, Granulocyte-Macrophage Colony-Stimulating Factor = GM-CSF, Monocyte Chemotactic Protein 1 = MCP-1, Tumor Necrosis Factor alpha = TNF-a and Vascular Endothelial Growth Factor = VEGF) was measured in the supernatants of Oxacells HP and adipose-derived stem cells by multiplex technique described in material and methods. All measured concentrations were normalized to 1 x 106 CD90+ cells. Plotted were mean values ±SD of multiple measurements (Oxacells HP n=6, ACSs n=8) on a logarithmic scaled Y axis. Significant differences between Oxacells HP and ASCs (P0) are marked (* P < 0.05, ** P < 0.01, *** P < 0.001, **** P ≤ 0.0001).
Discussion
A prerequisite for the approval of a stromal/stem cell therapy by the authorities is a robust manufacturing process that guarantees the production of a well-defined product of consistent quality. For ASCs, however, published purification protocols either result in a non-homogenous cell fraction (SVF) or are time-consuming and result in phenotypical changes of the cell population (cultivated ASCs). Particularly CD34 expression was found to be negatively correlated with cell proliferation rate.
29
Therefore we tested whether a short-term plastic adherence incubation step after SVF isolation was sufficient to enrich the adipose tissue-derived progenitors as a medicinal product without depleting CD34+ cells. Cells were seeded at high density (≥1.0 ×105 cells / cm2) and harvested after 16-20 hours to circumvent cell division. The ASC containing and GMP-compliant cell product (DE_BB_01_GMP_2017_1018) which we named Oxacells HP, was ready-to-use in less than 24 hours.
As shown in Figs. 2 and 3, already this short incubation step significantly altered the cell population composition when compared to SVF. Particularly, the proportion of cells expressing the negative markers CD31+, CD45+, CD235+, and HLA-II+ decreased significantly, whereas the proportion expressing the positive markers CD13+, CD44+, CD73+, CD90+, and CD34+ significantly increased. In contrast to cultivated ASCs and in line with our initial hypothesis, Oxacells HP are still expressing CD34+ cells and not expressing CD105 yet (Fig. 3A, B). Little is known about how CD34 affects the biological features and the functionality of SVF cells and/or ASCs (for a review see Scherberich et al.
49
Some groups suggest that the negativity of CD34 expression is an artifact of culture.
50
However, expanded ASCs retain the proliferative capacity and multipotency even after several passages despite the loss of CD34 expression during in vitro culture.
49
Yet, Maumus and colleagues showed that CD34+ ASCs are the only subpopulation of ASC containing clonogenic cells, and the only one able to differentiate into adipogenic and osteogenic lineages.
29
This finding was confirmed and extended to unexpanded SVF cells by several groups.
51-55
In this context, it is interesting to note that there is some evidence from animal models that treatment with SVF might be preferable over cultured CD34-negative ASCs. The treatment of a murine experimental autoimmune encephalomyelitis model with SVF, for example, mediated more robust improvements to CNS pathology than ASC treatment based on significant modulations of inflammatory factors.
5
Furthermore, Bowles et al
56
recently showed that only SVF led to a partial recovery of motor function in the SVF-treated EAE mice, whereas ASC treatment was unable to counter the inflammatory phase of the disease and did not provide therapeutic benefit. However, a recent study suggests that not the CD34 expression alone but the ratio of CD34+/CD31- cells to HSC-progenitor cells (CD34lowCD45+) in SVF may provide the key for successful cell therapy.
30
Apart from all that, it should be mentioned that Oxacell HP is still less homogenous than cultivated ASCs and the surface marker expression did not reach the criteria of cultivated ASCs as defined by the International Federation for Adipose Therapeutics (IFATS) and Science and the International Society for Cellular Therapy (ISCT).
4
However, the homogeneity of the cell product was sufficiently qualified as an ATMP and the described manufacturing process was authorized by the national regulatory agencies (DE_BB_01_GMP_2017_1018).
One hallmark of the ASCs is their multipotency and ability to give rise to multiple lineages.
4
As shown in Fig. 5, Oxacells HP could be successfully differentiated to the adipogenic, chondrogenic, and osteogenic lineages, verifying that this cell product contains functional ASCs.
According to Maumus et al,
57
the regenerative potential of MSCs can predominantly be attributed to their paracrine activity. VEGF and IL-6, for instance, exhibit anti-apoptotic effects,
57,58
and IL-1ra and IL-10 have an impact on immunosuppression.
23,59
IL-6, IL-8, and MCP-1 can influence the growth of hepatocytes,
23,60
and Eotaxin is involved in wound healing.
61
Therefore, we examined the expression of 15 cytokines and growth factors in the supernatant of Oxacells HP as well as in the supernatant of cultivated ASCs at passage 0. We did not include SVF in the comparison here since we do not believe that an accurate measurement of the supernatant of SVF is technically possible. Although Blaber et al described a secretion profile of the SVF,
62
others and we have shown that the short cultivation time that is necessary to generate conditioned media leads to the phenotypical changes of the seeded cells
29
which would distort the data. All tested analytes were identified at varying amounts in the supernatants of both cell products except for b-NGF which was absent in the supernatant of Oxacells HP (Fig. 6). Seven soluble factors were found in higher amounts in the supernatant of Oxacells HP compared to cultured ASCs, albeit not significantly. From the other seven factors which seemed to be highly expressed by cultured ASCs, only the change for MCP-1 was significant. MCP-1 together with b-NGF, IL-6, IL-8, and other factors are responsible for immunosuppression, hepatocyte growth, and hematopoiesis.
63-66
However, MCP-1 is also an inflammatory-related factor so it is difficult to predict its actual in vivo activity.
23
Taken together, the observed differences in expression quite possibly affect the therapeutic potential of the two cell fractions. Whether this change, however, is positive or negative and/or depending on the context of the disease, it is pure speculation at this point and has to be examined in detail on a case-by-case basis.
Similar to the immune phenotype, the viability of the nucleated cells in Oxacells HP did range somewhere between the SVF (before and after Ficoll purification) and the ASC population (Fig. 1B). The measured viabilities for SVF and cultivated ASC were both within the normal range of ≥ 75% and ≥ 90%, respectively.
4
As expected, all purification steps were associated with a cell loss: the relative cell yield per volume of lipoaspirate dropped significantly after density gradient centrifugation and again after short-term plastic adherence (Fig. 1A). For SVF, we achieved an average yield of 1.5 × 105 ± 8.6 × 104 nucleated cells/mL lipoaspirate. This is in line with the published range for manual purification methods based on enzymatic digestion (1.0 × 105 – 1.3 × 106 cells/mL)
67
or machine-assisted SVF isolations using collagenase digestion (1.9 × 104 – 1.2 ×106 cells/mL).
68
To calculate the stromal/stem cell yield, CD90+ cells were considered ASCs as described by Iyyanki et al.
47
By analogy with the total cell yield, also the stromal/stem cell yield decreased during short term incubation (only significant when compared to SVF after density centrifugation but not to pre-Ficoll) (Fig. 4). Again, this is probably due to a constant loss of cells during the purification process, including a loss of ASCs. Additionally, as explained before, the plastic adherence step is a purification, not a cultivation step. As a consequence, the loss of cells is not compensated by cell division. Anyway, compared to the obtained yield from lipoaspirate described by Iyyanki et al,
47
the yield of total cells and ASCs measured herein for SVF and Oxacells HP was much higher.
To date, a wide range of doses (1.6 × 106 to 1 × 108 cells) has been used for the treatment of osteoarthritis with SVF and ASCs.
69
However, a recently published single‐arm, open‐label, dose‐escalating clinical trial showed that a single low dosage of autologous ASC (2 × 106 cells) in patients with severe primary knee OA exhibited the best response to ASC.
70
With the purification procedure described herein, such a dose can be produced within 24 hours from around 133 mL lipoaspirate. In comparison to cultured ASCs, this could reduce the suffering of the patients and the costs, for example, for hospitalization of the patients.
Taken together, here we reported a fast, GMP-compliant purification procedure for the extraction of therapeutic stromal/stem cells from adipose tissue. The cell product which we call Oxacells HP contains a high amount of CD34+ cells and expresses several cytokines and growth factors with potential regenerative function.
Conclusion
Here, we added an additional short-term incubation (<20 hours) to the well-established isolation process of the SVF initially described by Zuk et al.
3
The resulting process yielded a stromal/stem cell fraction (with a high content of CD34+ cells) that is able to differentiate and express trophic proteins which have been linked to the therapeutic effects of MSCs as well as ASCs.
71-75
, More importantly, this process modification improved the homogeneity of the cell product, allowing production in accordance with GMP. The manufacturing protocol described here is fast, inexpensive and easy to establish, requiring the minimal infrastructure one can find in every cell culture laboratory.
Acknowledgment
We thank the members of our research group, including Jana Langer and Anke Antonicek for general support of this project. For the abandonment of the surgical site to perform the liposuction, we thank the Klinik Sanssouci - Potsdam and MEOCLINIC - Berlin. Our last but not least thank goes to the NMI-Reutlingen and the group of Thomas Joos for performing the MAP analysis of the cytokines and growth factors.
Funding sources
This work was funded, in part, by the German Federal Ministry for Economic Affairs and Energy (The Central Innovation Programme for SMEs - KF3305101).
Ethical statement
All donors were thoroughly informed about the studies and written consent was obtained. All studies were performed according to the amended Declaration of Helsinki.
Conflict of interests
All authors except PH, MB, RHK, and RS are associated with Oxacell AG.
Authors’ contribution
The conception and the design of the study were performed by SB, RS, and MK; development of the GMP-compliant protocols were carried out by SB, MJD, SAHY, JL, JKM, and JKW; funding was obtained by PH, SAHY, JKM, MB, RK, RS, and MK; study material was provided by SAHY and MK; experiments and data collection were conducted by SB, PH, and JL; assembly and analysis of the data as well as data interpretation were performed by SB, MJD, PH, MB, RK, RS, and MK; preparation of figures was carried out by SB; MJD, JL, and PH; statistical analysis was performed by SB; and the project was administered by MK. All authors participated in manuscript writing, as well as a critical review of the manuscript and approval of the final version.
Research Highlights
What is the current knowledge?
simple
-
√ The manufacturing process of SVF-derived cell products has to be approved via GMP-compliant process validation.
-
√ The heterogeneity of SVF is a major obstacle during process validation.
What is new here?
simple
-
√
A plastic adherence incubation following SVF isolation significantly improves the homogeneity of the cell fraction.
-
√
The isolated cells are able to differentiate and express trophic proteins.
-
√
The presented cell product can be produced according to GMP.
References
- Maumus M, Guerit D, Toupet K, Jorgensen C, Noel D. Mesenchymal stem cell-based therapies in regenerative medicine: applications in rheumatology. Stem Cell Res Ther 2011; 2:14. doi: 10.1186/scrt55 [Crossref] [ Google Scholar]
- Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007; 110:3499-506. doi: 10.1182/blood-2007-02-069716 [Crossref] [ Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001; 7:211-28. doi: 10.1089/107632701300062859 [Crossref] [ Google Scholar]
- Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013; 15:641-8. doi: 10.1016/j.jcyt.2013.02.006 [Crossref] [ Google Scholar]
- Bowles AC, Strong AL, Wise RM, Thomas RC, Gerstein BY, Dutreil MF. Adipose Stromal Vascular Fraction-Mediated Improvements at Late-Stage Disease in a Murine Model of Multiple Sclerosis. Stem Cells 2017; 35:532-44. doi: 10.1002/stem.2516 [Crossref] [ Google Scholar]
- Riordan NH, Ichim TE, Min WP, Wang H, Solano F, Lara F. Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J Transl Med 2009; 7:29. doi: 10.1186/1479-5876-7-29 [Crossref] [ Google Scholar]
- Vigani B, Mastracci L, Grillo F, Perteghella S, Preda S, Crivelli B. Local biological effects of adipose stromal vascular fraction delivery systems after subcutaneous implantation in a murine model. Journal of Bioactive and Compatible Polymers 2016; 31:600-12. doi: 10.1177/0883911516635841 [Crossref] [ Google Scholar]
- Zhou L, Song Q, Shen J, Xu L, Xu Z, Wu R. Comparison of human adipose stromal vascular fraction and adipose-derived mesenchymal stem cells for the attenuation of acute renal ischemia/reperfusion injury. Sci Rep 2017; 7:44058. doi: 10.1038/srep44058 [Crossref] [ Google Scholar]
- Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg 2008; 32:48-55; discussion 6. doi: 10.1007/s00266-007-9019-4 [Crossref] [ Google Scholar]
- Van Pham P, Hong-Thien Bui K, Quoc Ngo D, Tan Khuat L, Kim Phan N. Transplantation of Nonexpanded Adipose Stromal Vascular Fraction and Platelet-Rich Plasma for Articular Cartilage Injury Treatment in Mice Model. J Med Eng 2013; 2013:832396. doi: 10.1155/2013/832396 [Crossref] [ Google Scholar]
- Tyrnenopoulou P, Karayannopoulou M, Angelopoulou S, Pyrros A, Mparous E, Koliakos G. Successful management of an equine carpal chip fracture by intra-articularly injected adipose-derived stromal vascular fraction after arthroscopic removal. Iran J Vet Res 2016; 17:59-61. doi: 10.22099/ijvr.2016.3606 [Crossref] [ Google Scholar]
- Siennicka K, Zolocinska A, Stepien K, Lubina-Dabrowska N, Maciagowska M, Zolocinska E. Adipose-Derived Cells (Stromal Vascular Fraction) Transplanted for Orthopedical or Neurological Purposes: Are They Safe Enough?. Stem Cells Int 2016; 2016:5762916. doi: 10.1155/2016/5762916 [Crossref] [ Google Scholar]
- Rodriguez JP, Murphy MP, Hong S, Madrigal M, March KL, Minev B. Autologous stromal vascular fraction therapy for rheumatoid arthritis: rationale and clinical safety. Int Arch Med 2012; 5:5. doi: 10.1186/1755-7682-5-5 [Crossref] [ Google Scholar]
- Kim YS, Lee HJ, Choi YJ, Kim YI, Koh YG. Does an injection of a stromal vascular fraction containing adipose-derived mesenchymal stem cells influence the outcomes of marrow stimulation in osteochondral lesions of the talus? A clinical and magnetic resonance imaging study. Am J Sports Med 2014; 42:2424-34. doi: 10.1177/0363546514541778 [Crossref] [ Google Scholar]
- Granel B, Daumas A, Jouve E, Harle JR, Nguyen PS, Chabannon C. Safety, tolerability and potential efficacy of injection of autologous adipose-derived stromal vascular fraction in the fingers of patients with systemic sclerosis: an open-label phase I trial. Ann Rheum Dis 2015; 74:2175-82. doi: 10.1136/annrheumdis-2014-205681 [Crossref] [ Google Scholar]
- Frisbie DD, Kisiday JD, Kawcak CE, Werpy NM, McIlwraith CW. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J Orthop Res 2009; 27:1675-80. doi: 10.1002/jor.20933 [Crossref] [ Google Scholar]
- Cousin B, Andre M, Arnaud E, Penicaud L, Casteilla L. Reconstitution of lethally irradiated mice by cells isolated from adipose tissue. Biochem Biophys Res Commun 2003; 301:1016-22. doi: 10.1016/S0006-291X(03)00061-5 [Crossref] [ Google Scholar]
- Lee RH, Kim B, Choi I, Kim H, Choi HS, Suh K. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem 2004; 14:311-24. doi: 10.1159/000080341 [Crossref] [ Google Scholar]
- Im GI, Shin YW, Lee KB. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells?. Osteoarthritis Cartilage 2005; 13:845-53. doi: 10.1016/j.joca.2005.05.005 [Crossref] [ Google Scholar]
- Kim SJ, Cho HH, Kim YJ, Seo SY, Kim HN, Lee JB. Human adipose stromal cells expanded in human serum promote engraftment of human peripheral blood hematopoietic stem cells in NOD/SCID mice. Biochem Biophys Res Commun 2005; 329:25-31. doi: 10.1016/j.bbrc.2005.01.092 [Crossref] [ Google Scholar]
- Varma MJ, Breuls RG, Schouten TE, Jurgens WJ, Bontkes HJ, Schuurhuis GJ. Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev 2007; 16:91-104. doi: 10.1089/scd.2006.0026 [Crossref] [ Google Scholar]
- Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol 2006; 24:150-4. doi: 10.1016/j.tibtech.2006.01.010 [Crossref] [ Google Scholar]
- Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Osaki M. IFATS collection: in vivo therapeutic potential of human adipose tissue mesenchymal stem cells after transplantation into mice with liver injury. Stem Cells 2008; 26:2705-12. doi: 10.1634/stemcells.2008-0034 [Crossref] [ Google Scholar]
- Yoo KH, Jang IK, Lee MW, Kim HE, Yang MS, Eom Y. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol 2009; 259:150-6. doi: 10.1016/j.cellimm.2009.06.010 [Crossref] [ Google Scholar]
- Kim Y, Kim H, Cho H, Bae Y, Suh K, Jung J. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol Biochem 2007; 20:867-76. doi: 10.1159/000110447 [Crossref] [ Google Scholar]
- Wuchter P, Bieback K, Schrezenmeier H, Bornhauser M, Muller LP, Bonig H. Standardization of Good Manufacturing Practice-compliant production of bone marrow-derived human mesenchymal stromal cells for immunotherapeutic applications. Cytotherapy 2015; 17:128-39. doi: 10.1016/j.jcyt.2014.04.002 [Crossref] [ Google Scholar]
- Izeta A, Herrera C, Mata R, Astori G, Giordano R, Hernandez C. Cell-based product classification procedure: What can be done differently to improve decisions on borderline products?. Cytotherapy 2016; 18:809-15. doi: 10.1016/j.jcyt.2016.03.292 [Crossref] [ Google Scholar]
- Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 2006; 24:376-85. doi: 10.1634/stemcells.2005-0234 [Crossref] [ Google Scholar]
- Maumus M, Peyrafitte JA, D'Angelo R, Fournier-Wirth C, Bouloumie A, Casteilla L. Native human adipose stromal cells: localization, morphology and phenotype. Int J Obes (Lond) 2011; 35:1141-53. doi: 10.1038/ijo.2010.269 [Crossref] [ Google Scholar]
- Kilinc MO, Santidrian A, Minev I, Toth R, Draganov D, Nguyen D. The ratio of ADSCs to HSC-progenitors in adipose tissue derived SVF may provide the key to predict the outcome of stem-cell therapy. Clin Transl Med 2018; 7:5. doi: 10.1186/s40169-018-0183-8 [Crossref] [ Google Scholar]
- Bellotti C, Stanco D, Ragazzini S, Romagnoli L, Martella E, Lazzati S. Analysis of the karyotype of expanded human adipose-derived stem cells for bone reconstruction of the maxillo-facial region. Int J Immunopathol Pharmacol 2013; 26:3-9. doi: 10.1177/03946320130260S102 [Crossref] [ Google Scholar]
- Borgonovo T, Vaz IM, Senegaglia AC, Rebelatto CL, Brofman PR. Genetic evaluation of mesenchymal stem cells by G-banded karyotyping in a Cell Technology Center. Rev Bras Hematol Hemoter 2014; 36:202-7. doi: 10.1016/j.bjhh.2014.03.006 [Crossref] [ Google Scholar]
- Tarte K, Gaillard J, Lataillade JJ, Fouillard L, Becker M, Mossafa H. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood 2010; 115:1549-53. doi: 10.1182/blood-2009-05-219907 [Crossref] [ Google Scholar]
- Barkholt L, Flory E, Jekerle V, Lucas-Samuel S, Ahnert P, Bisset L. Risk of tumorigenicity in mesenchymal stromal cell-based therapies--bridging scientific observations and regulatory viewpoints. Cytotherapy 2013; 15:753-9. doi: 10.1016/j.jcyt.2013.03.005 [Crossref] [ Google Scholar]
- Frese L, Dijkman PE, Hoerstrup SP. Adipose Tissue-Derived Stem Cells in Regenerative Medicine. Transfusion Medicine and Hemotherapy 2016; 43:268-74. doi: 10.1159/000448180 [Crossref] [ Google Scholar]
- Levine BL, Miskin J, Wonnacott K, Keir C. Global Manufacturing of CAR T Cell Therapy. Mol Ther Methods Clin Dev 2017; 4:92-101. doi: 10.1016/j.omtm.2016.12.006 [Crossref] [ Google Scholar]
- Ishimura D, Yamamoto N, Tajima K, Ohno A, Yamamoto Y, Washimi O. Differentiation of adipose-derived stromal vascular fraction culture cells into chondrocytes using the method of cell sorting with a mesenchymal stem cell marker. Tohoku J Exp Med 2008; 216:149-56. doi: 10.1620/tjem.216.149 [Crossref] [ Google Scholar]
- Lv XJ, Zhou GD, Liu Y, Liu X, Chen JN, Luo XS. In vitro proliferation and differentiation of adipose-derived stem cells isolated using anti-CD105 magnetic beads. Int J Mol Med 2012; 30:826-34. doi: 10.3892/ijmm.2012.1063 [Crossref] [ Google Scholar]
- Indumathi S, Mishra R, Harikrishnan R, Rajkumar JS, Kantawala N, Dhanasekaran M. Lineage depletion of stromal vascular fractions isolated from human adipose tissue: a novel approach towards cell enrichment technology. Cytotechnology 2014; 66:219-28. doi: 10.1007/s10616-013-9556-4 [Crossref] [ Google Scholar]
- Barzelay A, Levy R, Kohn E, Sella M, Shani N, Meilik B. Power-Assisted Liposuction Versus Tissue Resection for the Isolation of Adipose Tissue-Derived Mesenchymal Stem Cells: Phenotype, Senescence, and Multipotency at Advanced Passages. Aesthet Surg J 2015; 35:NP230-40. doi: 10.1093/asj/sjv055 [Crossref] [ Google Scholar]
- Zhu M, Heydarkhan-Hagvall S, Hedrick M, Benhaim P, Zuk P. Manual isolation of adipose-derived stem cells from human lipoaspirates. J Vis Exp 2013:e50585. doi: 10.3791/50585 [Crossref]
- Silva LP, Lorenzi PL, Purwaha P, Yong V, Hawke DH, Weinstein JN. Measurement of DNA concentration as a normalization strategy for metabolomic data from adherent cell lines. Anal Chem 2013; 85:9536-42. doi: 10.1021/ac401559v [Crossref] [ Google Scholar]
- Sibov TT, Severino P, Marti LC, Pavon LF, Oliveira DM, Tobo PR. Mesenchymal stem cells from umbilical cord blood: parameters for isolation, characterization and adipogenic differentiation. Cytotechnology 2012; 64:511-21. doi: 10.1007/s10616-012-9428-3 [Crossref] [ Google Scholar]
- Moraes DA, Sibov TT, Pavon LF, Alvim PQ, Bonadio RS, Da Silva JR. A reduction in CD90 (THY-1) expression results in increased differentiation of mesenchymal stromal cells. Stem Cell Res Ther 2016; 7:97. doi: 10.1186/s13287-016-0359-3 [Crossref] [ Google Scholar]
- Davies OG, Cooper PR, Shelton RM, Smith AJ, Scheven BA. Isolation of adipose and bone marrow mesenchymal stem cells using CD29 and CD90 modifies their capacity for osteogenic and adipogenic differentiation. J Tissue Eng 2015; 6:2041731415592356. doi: 10.1177/2041731415592356 [Crossref] [ Google Scholar]
- Yamamoto M, Nakata H, Hao J, Chou J, Kasugai S, Kuroda S. Osteogenic Potential of Mouse Adipose-Derived Stem Cells Sorted for CD90 and CD105 In Vitro. Stem Cells Int 2014; 2014:576358. doi: 10.1155/2014/576358 [Crossref] [ Google Scholar]
- Iyyanki T, Hubenak J, Liu J, Chang EI, Beahm EK, Zhang Q. Harvesting technique affects adipose-derived stem cell yield. Aesthet Surg J 2015; 35:467-76. doi: 10.1093/asj/sju055 [Crossref] [ Google Scholar]
- McIntosh K, Zvonic S, Garrett S, Mitchell JB, Floyd ZE, Hammill L. The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells 2006; 24:1246-53. doi: 10.1634/stemcells.2005-0235 [Crossref] [ Google Scholar]
- Scherberich A, Di Maggio ND, McNagny KM. A familiar stranger: CD34 expression and putative functions in SVF cells of adipose tissue. World J Stem Cells 2013; 5:1-8. doi: 10.4252/wjsc.v5.i1.1 [Crossref] [ Google Scholar]
- Lin CS, Ning H, Lin G, Lue TF. Is CD34 truly a negative marker for mesenchymal stromal cells?. Cytotherapy 2012; 14:1159-63. doi: 10.3109/14653249.2012.729817 [Crossref] [ Google Scholar]
- Ferraro GA, De Francesco F, Nicoletti G, Paino F, Desiderio V, Tirino V. Human adipose CD34+ CD90+ stem cells and collagen scaffold constructs grafted in vivo fabricate loose connective and adipose tissues. J Cell Biochem 2013; 114:1039-49. doi: 10.1002/jcb.24443 [Crossref] [ Google Scholar]
- Suga H, Matsumoto D, Eto H, Inoue K, Aoi N, Kato H. Functional implications of CD34 expression in human adipose-derived stem/progenitor cells. Stem Cells Dev 2009; 18:1201-10. doi: 10.1089/scd.2009.0003 [Crossref] [ Google Scholar]
- De Francesco F, Tirino V, Desiderio V, Ferraro G, D'Andrea F, Giuliano M. Human CD34/CD90 ASCs are capable of growing as sphere clusters, producing high levels of VEGF and forming capillaries. PLoS One 2009; 4:e6537. doi: 10.1371/journal.pone.0006537 [Crossref] [ Google Scholar]
- Guven S, Mehrkens A, Saxer F, Schaefer DJ, Martinetti R, Martin I. Engineering of large osteogenic grafts with rapid engraftment capacity using mesenchymal and endothelial progenitors from human adipose tissue. Biomaterials 2011; 32:5801-9. doi: 10.1016/j.biomaterials.2011.04.064 [Crossref] [ Google Scholar]
- Busser H, Najar M, Raicevic G, Pieters K, Velez Pombo R, Philippart P. Isolation and Characterization of Human Mesenchymal Stromal Cell Subpopulations: Comparison of Bone Marrow and Adipose Tissue. Stem Cells Dev 2015; 24:2142-57. doi: 10.1089/scd.2015.0172 [Crossref] [ Google Scholar]
- Bowles AC, Wise RM, Gerstein BY, Thomas RC, Ogelman R, Manayan RC. Adipose stromal vascular fraction attenuates TH1 cell-mediated pathology in a model of multiple sclerosis. J Neuroinflammation 2018; 15:77. doi: 10.1186/s12974-018-1099-3 [Crossref] [ Google Scholar]
- Maumus M, Jorgensen C, Noel D. Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. Biochimie 2013; 95:2229-34. doi: 10.1016/j.biochi.2013.04.017 [Crossref] [ Google Scholar]
- Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev 2009; 20:419-27. doi: 10.1016/j.cytogfr.2009.10.002 [Crossref] [ Google Scholar]
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105:1815-22. doi: 10.1182/blood-2004-04-1559 [Crossref] [ Google Scholar]
- Berardis S, Dwisthi Sattwika P, Najimi M, Sokal EM. Use of mesenchymal stem cells to treat liver fibrosis: current situation and future prospects. World J Gastroenterol 2015; 21:742-58. doi: 10.3748/wjg.v21.i3.742 [Crossref] [ Google Scholar]
- Ma HC, Wang X, Wu MN, Zhao X, Yuan XW, Shi XL. Interleukin-10 Contributes to Therapeutic Effect of Mesenchymal Stem Cells for Acute Liver Failure via Signal Transducer and Activator of Transcription 3 Signaling Pathway. Chin Med J (Engl) 2016; 129:967-75. doi: 10.4103/0366-6999.179794 [Crossref] [ Google Scholar]
- Blaber SP, Webster RA, Hill CJ, Breen EJ, Kuah D, Vesey G. Analysis of in vitro secretion profiles from adipose-derived cell populations. J Transl Med 2012; 10:172. doi: 10.1186/1479-5876-10-172 [Crossref] [ Google Scholar]
- Yokoyama T, Komori A, Nakamura M, Takii Y, Kamihira T, Shimoda S. Human intrahepatic biliary epithelial cells function in innate immunity by producing IL-6 and IL-8 via the TLR4-NF-kappaB and -MAPK signaling pathways. Liver Int 2006; 26:467-76. doi: 10.1111/j.1478-3231.2006.01254.x [Crossref] [ Google Scholar]
- Komori A, Nakamura M, Fujiwara S, Yano K, Fujioka H, Migita K. Human intrahepatic biliary epithelial cell as a possible modulator of hepatic regeneration: Potential role of biliary epithelial cell for hepatic remodeling in vivo. Hepatol Res 2007; 37 Suppl 3:S438-43. doi: 10.1111/j.1872-034X.2007.00237.x [Crossref] [ Google Scholar]
- Rasi G, Serafino A, Bellis L, Lonardo MT, Andreola F, Zonfrillo M. Nerve growth factor involvement in liver cirrhosis and hepatocellular carcinoma. World J Gastroenterol 2007; 13:4986-95. doi: 10.3748/wjg.v13.i37.4986 [Crossref] [ Google Scholar]
- Oakley F, Trim N, Constandinou CM, Ye W, Gray AM, Frantz G. Hepatocytes express nerve growth factor during liver injury: evidence for paracrine regulation of hepatic stellate cell apoptosis. Am J Pathol 2003; 163:1849-58. doi: 10.1016/s0002-9440(10)63544-4 [Crossref] [ Google Scholar]
- Aronowitz JA, Lockhart RA, Hakakian CS. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus 2015; 4:713. doi: 10.1186/s40064-015-1509-2 [Crossref] [ Google Scholar]
- van Dongen JA, Tuin AJ, Spiekman M, Jansma J, van der Lei B, Harmsen MC. Comparison of intraoperative procedures for isolation of clinical grade stromal vascular fraction for regenerative purposes: a systematic review. J Tissue Eng Regen Med 2018; 12:e261-e74. doi: 10.1002/term.2407 [Crossref] [ Google Scholar]
- Iijima H, Isho T, Kuroki H, Takahashi M, Aoyama T. Effectiveness of mesenchymal stem cells for treating patients with knee osteoarthritis: a meta-analysis toward the establishment of effective regenerative rehabilitation. NPJ Regen Med 2018; 3:15. doi: 10.1038/s41536-018-0041-8 [Crossref] [ Google Scholar]
- Pers YM, Rackwitz L, Ferreira R, Pullig O, Delfour C, Barry F. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl Med 2016; 5:847-56. doi: 10.5966/sctm.2015-0245 [Crossref] [ Google Scholar]
- Doorn J, Moll G, Le Blanc K, van Blitterswijk C, de Boer J. Therapeutic applications of mesenchymal stromal cells: paracrine effects and potential improvements. Tissue Eng Part B Rev 2012; 18:101-15. doi: 10.1089/ten.TEB.2011.0488 [Crossref] [ Google Scholar]
- Winkler S, Hempel M, Bruckner S, Tautenhahn HM, Kaufmann R, Christ B. Identification of Pathways in Liver Repair Potentially Targeted by Secretory Proteins from Human Mesenchymal Stem Cells. Int J Mol Sci 2016:17. doi: 10.3390/ijms17071099 [Crossref]
- Thomas H, Jager M, Mauel K, Brandau S, Lask S, Flohe SB. Interaction with mesenchymal stem cells provokes natural killer cells for enhanced IL-12/IL-18-induced interferon-gamma secretion. Mediators Inflamm 2014; 2014:143463. doi: 10.1155/2014/143463 [Crossref] [ Google Scholar]
- Jing W, Chen Y, Lu L, Hu X, Shao C, Zhang Y. Human umbilical cord blood-derived mesenchymal stem cells producing IL15 eradicate established pancreatic tumor in syngeneic mice. Mol Cancer Ther 2014; 13:2127-37. doi: 10.1158/1535-7163.MCT-14-0175 [Crossref] [ Google Scholar]
- Nedeau AE, Bauer RJ, Gallagher K, Chen H, Liu ZJ, Velazquez OC. A CXCL5- and bFGF-dependent effect of PDGF-B-activated fibroblasts in promoting trafficking and differentiation of bone marrow-derived mesenchymal stem cells. Exp Cell Res 2008; 314:2176-86. doi: 10.1016/j.yexcr.2008.04.00 [Crossref] [ Google Scholar]