BioImpacts. 9(4):227-237.
doi: 10.15171/bi.2019.28
Original Research
Water-soluble pristine C60 fullerene attenuates acetaminophen-induced liver injury
Halyna Kuznietsova 1, *  , Oksana Lynchak 1, Natalia Dziubenko 1, Tetyana Herheliuk 1, Yuriy Prylutskyy 1, Volodymyr Rybalchenko 1, Uwe Ritter 2
, Oksana Lynchak 1, Natalia Dziubenko 1, Tetyana Herheliuk 1, Yuriy Prylutskyy 1, Volodymyr Rybalchenko 1, Uwe Ritter 2
Author information:
1Taras Shevchenko National University of Kyiv, Institute of Biology and Medicine, 64 Volodymyrska Str., 01601 Kyiv, Ukraine
2Technical University of Ilmenau, Institute of Chemistry and Biotechnology, 25 Weimarer Str., 98693 Ilmenau, Germany
Abstract
Introduction:
Oxidative stress has been suggested as the main trigger and pathological mechanism of toxic liver injury. Effects of powerful free radical scavenger С60 fullerene on rat liver injury and liver cells (HepG2 line) were aimed to be discovered.
Methods:
Acute liver injury (ALI) was simulated by single acetaminophen (APAP, 1000 mg/kg) administration, on a chronic CLI, by 4 weekly APAP administrations. Pristine C60 fullerene aqueous colloid solution (C60FAS; initial concentration 0.15 mg/mL) was administered per os or intraperitoneally at a dose of 0.5 mg/kg (ALI) or 0.25 mg/kg (CLI) daily for 2 or 28 days, respectively, after first APAP dose. Animals were sacrificed at 24th hour after the last dose. Biochemical markers of blood serum and liver autopsies were analyzed. EGFR expression in HepG2 cells after 48-hour incubation with C60FAS was assessed.
Results:
Increase of serum conjugated and unconjugated bilirubin (up to 1.4-3.7 times), ALT (by 31-37%), and AST (by 18%) in non-treated ALI and CLI rats were observed, suggesting the hepatitis (confirmed by histological analysis). Liver morphological state (ALI, CLI), ALT (ALI and CLI), bilirubin (CLI), α-amylase, and creatinine (ALI) were normalized with C60FAS administration in both ways, which may indicate its protective impact on liver. However, unconjugated bilirubin sharply increased in ALI animals receiving C60FAS (up to 12 times compared to control), suggesting the augmentation of bilirubin metabolism. Furthermore, C60FAS inhibited EGFR expression in HepG2 cells in a dose-dependent manner.
Conclusion:
C60FAS could partially correct acute and chronic toxic liver injury, however, it could not normalize bilirubin metabolism after acute exposure.
Keywords:
C60 fullerene
, Acetaminophen-induced liver injury, EGFR, HepG2 cells
Copyright and License Information
© 2019 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Toxic liver injury includes a wide range of diseases associated with hepatotoxic effects of substances of various origins, which cause morphological changes in liver tissue and associated metabolic disorders. Hepatotoxic effects are inherent in some domestic and industrial chemicals, pesticides, alcohol, a number of medications, nutritional supplements, herbal products, and the like. Toxic hepatitis is an inflammatory disease of liver caused by above agents. It may develop within hours or days after exposure to toxicant. In other cases, the development of symptoms may last for months of regular exposure. The incidence of toxic hepatitis is 10-15 per 10 000 in general population.
1
Drug-induced liver injury (DILI) contributes the most to this category of diseases because of leading role of the liver in drug metabolism and biotransformation. The ability to cause liver damage is implicated in more than 900 medications and is the most common reason for drug withdrawal from the pharmaceutical market.
2
DILI accounts for approximately 10% of all cases of acute hepatitis and approximately 2% of chronic liver disease and cirrhosis. It is responsible for over 50% of all cases of acute liver failure (ALF).
2-4
Symptoms of drug-induced damage encompass almost all known liver lesions - both acute and chronic. Acute lesions may be cytotoxic (hepatocellular) or cholestatic. Chronic ones include hepatocellular, cholestatic, vascular, or neoplastic manifestations.
The production of free radicals such as reactive oxygen and nitrogen species has been considered as an early event of drug hepatotoxicity and an indicator of hepatotoxic potential. A lot of drugs can cause oxidative stress in the liver, in particular the increase in the number of cellular prooxidants, lipid peroxidation, and the depletion of a pool of antioxidants. The oxidative stress induces irretrievable alteration of lipids, proteins, and DNA and therefore triggers hepatic damage. Moreover, it also modulates the signal pathways controlling normal biological functions. Since these pathways regulate genes transcription, protein expression, cell apoptosis, and hepatic stellate cell activation, oxidative stress is considered to be one of the pathological mechanisms leading to the initiation and progression of various liver diseases (chronic hepatitis, alcoholic liver disease, and non-alcoholic steatohepatitis (NASH) etc.). Moreover, systemic oxidative stress arising during liver disease can also cause damage of extra-hepatic organs, such as pancreatic and renal failure and even brain impairment.
1,5,6
A lot of studies demonstrate that agents having antioxidant activity also exhibit hepatoprotective properties.
6,7
Some natural and nutritional antioxidants such as vitamins C and E, zinc, selenium, coenzyme Q10, methionine, alpha lipoic acid, N-acetylcysteine, and silymarin are undergoing the clinical trials in phases II/III and even IV.
8-14
Some of them have been approved for treating patients with fatty liver disease and NASH in some countries.
15,16
Thus, antioxidant therapy could be an effective approach for the prevention and treatment of liver disease.
An important area of research in modern medicine concerns nanomaterials as promising agents with a wide range of mediсal applications. Nanomaterials are currently used as carriers to deliver drugs and other substances to specific types of cells.
17
Nanoparticles as part of chips with immobilized bioactive molecules are used in diagnostic techniques.
18
Carbon, metal and synthetic nanomaterials are utilized in tissue engineering including organ transplantation and tissue regeneration.
19
Carbon-based nanomaterials attract the attention as the most “biocompatible” ones. Among them fullerenes take one of the leading places.
20
C60 fullerene (C60) is almost a spherical molecule whose surface consists of 60 carbon atoms. The C60 spherical surface contains 20 hexagonal and 12 pentagonal structures. Bonds between adjacent hexagons are double, and those between hexagons and pentagons are single. Thus, hexa- and pentagon units are connected by a conjugated π-electron system.
21
The diameter of the C60 molecule is 0.72 nm, which is close to that of polypeptides’ α-helix and the steroid molecules. Thus, the steric compatibility of C60 with biological structures such as receptor recognizing sites or enzyme active centers may be suggested. Non-modified (pristine) C60 is hydrophobic molecule and is able to be embedded into biological membranes and thus to penetrate into the cell.
22
Therefore, C60 is discovered as a promising drug carrier due to its ability to be functionalized with different molecules and therapeutics on the one hand, and to penetrate the cell membrane on the other hand.
23,24
It also possesses antiviral activity because of steric compatibility with hydrophobic cavity of viral enzymes.
25,26
C60 also can be used as potential photosensitizer in photodynamic therapy due to its ability to generate reactive oxygen species (ROS) after photoirradiation.
20,27
It should be noted that double bonds in the C60 framework are electron deficient, which determines the electron-acceptor properties of the molecule and its ability to easily attach reagents containing unpaired electrons (i.e. free radicals).
28
Due to this ability, C60 could act in biological systems as scavenger of free radicals, in particular hydroxyl and superoxide anions, which results in its anti-tumor, anti-inflammatory and hepatoprotective properties.
22,29-34
In an aqueous solution, pristine C60 forms nanosize clusters.
35,36
Additionally, water-soluble pristine C60 is non-toxic in in vitro and in vivo systems at least at low concentrations
37,38
and can be accumulated in liver.
39
Therefore, it is suggested to be a potential treatment of this organ.
In our previous studies we demonstrated that C60 realized anti-inflammatory and hepatoprotective effects on a model of acute colonic inflammation.
32
We also showed the ability of C60 to improve liver biochemical parameters and histological state, particularly to diminish liver inflammation and fibrotic degeneration, under α-naphthylisothiocyanate-induced acute cholangitis.
40
In addition, Halenova et al
30
revealed that C60 could prevent CCl4-induced acute liver injury (ALI). However, they used the dose of C60 exceeded than that in the current experiment in 3 times. Some studies demonstrated the genotoxic and prooxidative effect of C60 realized in a dose-dependent manner,
41,42
whereas another ones – at least no prooxidative and rather antioxidant effects.
43
Thus, the possible effect of lower dose of C60 deserves to be investigated. Furthermore, all mentioned works described the impact of C60 under acute liver pathology. As the processes and mechanisms of development of chronic liver injury and liver adaptation differ from those of acute disease,
44
the chronic action of the substance might differ from acute one. Moreover, if any substance was applied for a prolonged period, possible cumulative effect also should be taken into consideration.
Thus, the purpose of this work was to study the effect of water-soluble biocompatible pristine С60 on the rat liver function under its acute and chronic toxic injury and on liver cells in vitro as well as to evaluate the ability of these nanoparticles to prevent extrahepatic complications.
Materials and Methods
Chemical reagents
Acetaminophen (Merck, Darmstadt, Germany) was used for the simulation of liver injury. Reagent kits for detection of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total and direct (conjugated) bilirubin, creatinine, urea (Filisit diagnosis, Dnipro, Ukraine), alkaline phosphatase (ALP), α-amylase, triglycerides and total protein (Diagnosticum Zrt, Budapest, Hungary) were used in biochemical assays. Ethanol, acetic acid (Henan Bright Commercial Co., Zhengzhou, China), picric acid, formalin (Biopharma, Kyiv, Ukraine), paraffin, hematoxylin, eosin, orange G (Merck, Darmstadt, Germany) were used in histological assays. Dulbecco’s Modified Eagle Medium (DMEM), fetal bovine serum (FBS), L-glutamine (Merck, Darmstadt, Germany), and gentamicin (Biopharma, Kyiv, Ukraine) were used for cell culturing. Epidermal growth factor receptor (EGFR) monoclonal antibodies and reagent kit for immunohistochemical visualization (Dako, Santa Clara, CA, USA) were used in immunohistochemical assay. Au(111) films (SPI Supplies, West Chester, PA, USA) were used for scanning tunneling microscopy (STM). We used following equipment: light microscope Olympus BX-41 (Olympus Europa GmbH, Munich, Germany), spectrophotometer ULAB 101 (Ulab, Kyiv Ukraine), CO2 incubator (Memmert GmbH, Schwabach, Germany), STM (NT-MDT Spectrum instruments, Moscow, Russia), and Zetasizer Nano-ZS90 (Malvern, Worcestershire, UK).
Preparation and characterization of C60fullerene aqueous colloid solution
A highly stable C60 fullerene aqueous colloid solution (C60FAS) was prepared according to the protocols described before.
36
The initial concentration of C60 was 0.15 mg/mL.
Dynamic light scattering (DLS) and zeta potential measurements (ζ) were used for ascertaining the hydrodynamic size and electrokinetic potential of C60 particles in a prepared C60FAS. Measurements were conducted on Zetasizer Nano-ZS90 at 25°C. Obtained results were evaluated using the Smoluchowski approximation, which is known to be rigorously valid only for spherical-like particles.
Samples for STM analysis were obtained after deposition of C60 on an Au(111) surface by precipitation from the droplet of corresponding stock solutions. STM measurements were performed after complete evaporation of the solvents. The morphology of C60 particles in deposited films was monitored using STM technique. Typical values of the tunneling current and voltage were chosen within the ranges of 0.01–0.1 nA and 0.1–0.8 V, respectively.
Animals
Studies were conducted using 64 Wistar male rats with the initial body weight of 200±10 g (acute trial) and 130±10 g (chronic one), which were kept in vivarium of Taras Shevchenko National University of Kyiv at natural light on a standard diet with free access to drinking water. All experiments were conducted in compliance with bioethic principles, legislative norms, and provisions of the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (Strasbourg, 1986), General Ethical Principles for Experiments on Animals, adopted by the First National Bioethics Congress (Kyiv, 2001), and approved by an institutional review committee.
Design of the studies
ALI was simulated by a single oral administration of APAP at a dose of 1000 mg/kg
45
dissolved in sunflower oil (total volume 0.5 mL). In 5 and 24 hours after APAP dose, animals were administered with C60FAS in a volume corresponding to the amount of С60 0.5 mg/kg body weight (total received dose equal to 1 mg/kg) either intraperitoneally (i.p.) or per os (p.o.) (Table 1).
Table 1.
Experimental groups
|
Experimental groups
|
Study, duration
|
|
Acute, 48 h
|
Chronic, 28 days
|
|
APAP
|
C
60
FAS, corresponded to dose of C
60
|
APAP
|
C
60
FAS, corresponded to dose of C
60
|
| Control, n=8 |
- |
- |
- |
- |
| APAP, n=8 |
1000 mg/kg once |
- |
1000 mg/kg 4 times weekly |
- |
|
APAP+C60FAS i.p., n=8
|
1000 mg/kg once |
0.5 mg/kg 2 times (in 5 and 24 h after APAP) i.p. |
1000 mg/kg 4 times weekly |
0.25 mg/kg daily during 28 days i.p. |
|
APAP+C60FAS p.o., n=8
|
1000 mg/kg once |
0.5 mg/kg 2 times (in 5 and 24 h after APAP) p.o. |
1000 mg/kg 4 times weekly |
0.25 mg/kg daily during 28 days p.o. |
Note . APAP: acetaminophen, C60FAS: C60 fullerene aqueous colloid solution, i.p.: intraperitoneally, p.o.: per os.
To induce chronic liver injury (CLI), animals were ingested with APAP weekly for 28 days at a dose corresponding to that in acute study. In 24 hours, animals were administered with C60FAS in a volume corresponding to the amount of С60 0.25 mg/kg body weight daily during 28 days (total received dose equal to 5 mg/kg) (Table 1). All manipulations with animals in comparison groups were conducted similarly to animals of experimental ones, including the administration of solvents.
As acute and chronic effects of C60FAS on healthy animals were previously investigated and described,
32,39
we did not include the description of these groups in the manuscript. In 24 hours after the last dose, animals were euthanized by inhalation of CO2 and subsequent cervical dislocation.
Biochemical assays
The blood for biochemical analysis was collected immediately after the sacrifice from the femoral vein, left for 20 minutes to form a clot and then centrifuged for 10 minutes at 1000 g. ALT, AST, ALP and α-amylase enzymes, total and direct (conjugated) bilirubin, triglycerides, creatinine, urea and total protein as markers of liver, kidneys and pancreas functional states were determined in blood serum according to the protocols provided by the manufacturers.
Histological assay
The liver fragments were harvested immediately after the sacrifice and fixed in Bouin’s mixture for 14 days for histological assay. Then, they were embedded into paraffin, sliced into 5 𝜇m sections, stained with hematoxylin-eosin-orange, and examined under the light microscope. States of liver centrilobular and periportal areas were estimated separately.
In vitro immunohistochemical assay
HepG2 cells were cultured under standard conditions (37°C, 5% CO2, 95% humidity), in DMEM containing 10% FBS, 2 mM L-glutamine, and 40 mg/mL gentamicin. To evaluate the EGFR expression, the cells were seeded on coated glass in 6-well plates with 2×104 cells/cm2 densitу and used in 48 hours of cultivation. Cells were incubated in medium containing 10 or 100 μg of C60FAS per mL for 48 hours. Immunohistochemical assay was performed using primary EGFR monoclonal antibodies according to the protocol provided by the manufacturer. In addition, specimens were stained with hematoxylin for nuclei visualization. The number of immunopositive cells was counted in 10 random microscopic fields of view for each sample using a standard scale of measurement (object-micrometer) at the same magnification and calculated as a percentage of the total cell number taken for 100%. At least 200 cells were counted.
Statistical analysis
The Gaussian distribution of the data was estimated using Shapiro-Wilk test. As the distribution was normal, the statistical analysis of data was performed using one-way analysis of variance (ANOVA) with the Tukey post hoc test. The difference was considered statistically significant at P <0.05.
Results
DLS and STM studies
To fully understand the bioactivity of prepared water-soluble C60 particles, we examined their size distribution profile and electrokinetic potential (ζ potential) value with DLS technique. In C60FAS, C60 have the hydrodynamic diameter of 65 nm. Tested C60 nanoparticles demonstrated a slightly negative average surface charge (ζ= -13.70 mV), indicating a further tendency for their aggregation. Finally, a studied nanoparticle system with polydispersity index value 0.25 was indicated that has moderately disperse distribution.
STM image of particles deposited on Au(111) surface from C60FAS is presented in Fig. 1. The height of particles was estimated along the cross-section line (Z-profile) marked by yellow (Fig. 1 top). As one can see, C60FAS in addition to single C60 molecules (~0.72 nm) also contains nanoparticles (C60 aggregates) with a height more than 1.2 nm (Fig. 1 bottom).
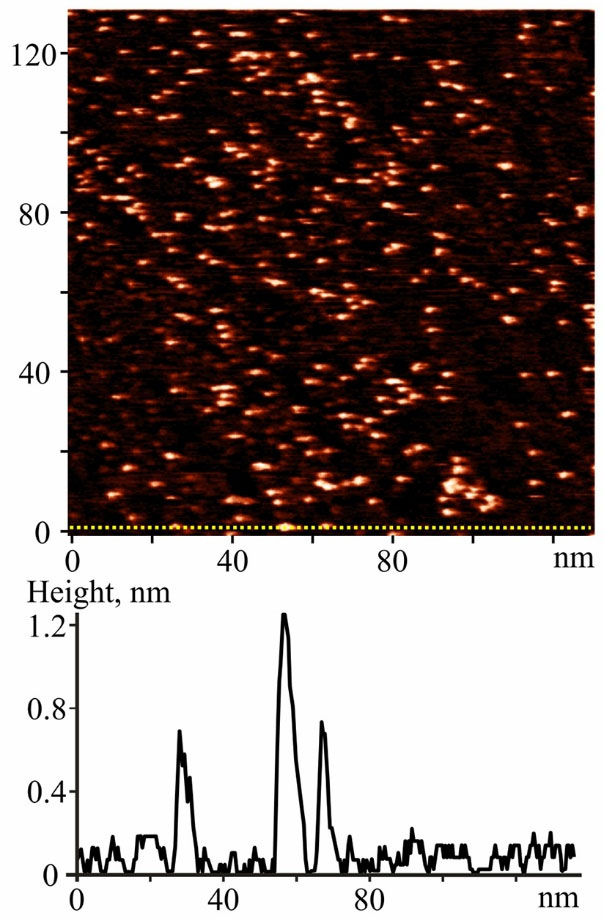
Fig. 1.
STM image of C60 submonolayer film deposited from C60FAS (0.15 mg/mL) on Au(111) surface. Scanning parameters: It=93 pA, Ut=713 mV (top); Z-profile (bottom) along the yellow line marked on the image (top).
.
STM image of C60 submonolayer film deposited from C60FAS (0.15 mg/mL) on Au(111) surface. Scanning parameters: It=93 pA, Ut=713 mV (top); Z-profile (bottom) along the yellow line marked on the image (top).
Acute liver injury
The liver of ALI animals demonstrated no visible lesions. However, the blood vessels overflow and sinusoid hemocapillars elevation was observed at light microscopy level. Signs of hepatocyte necrosis, protein and lipid dystrophy were also detected, as well as the ground-glass hepatocytes occurrence, lymphocytic infiltration and fibrosis of portal tracts (Fig. 2, Table 2). Observed features have been suggested to be typical for the acute hepatitis.
46
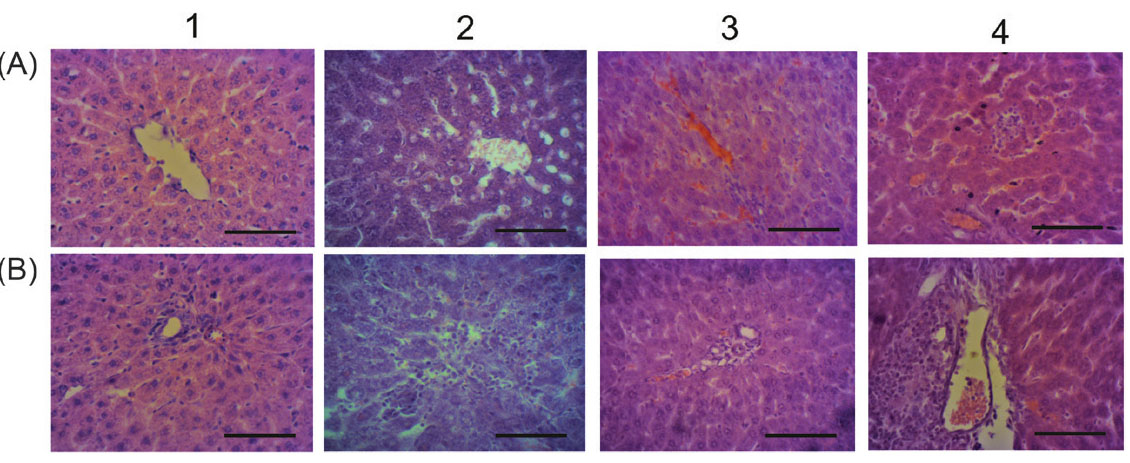
Fig. 2.
Microphotographs of the liver for ALI rats received C60FAS: 1 – control; 2 – APAP; 3 – APAP+C60FAS i.p.; 4 – APAP+C60FAS p.o.; A and B - centrilobular
and periportal areas, respectively. Hematoxylin-eosin-orange staining, ×400, scale 100 μm. Sinusoids and blood vessels dilation (2 A, 3 A, 4 A), lymphocytic
infiltration of portal tracts (2 B, 4 B) and surrounding tissue (2 B), hepatocyte necrosis (2 A).
.
Microphotographs of the liver for ALI rats received C60FAS: 1 – control; 2 – APAP; 3 – APAP+C60FAS i.p.; 4 – APAP+C60FAS p.o.; A and B - centrilobular
and periportal areas, respectively. Hematoxylin-eosin-orange staining, ×400, scale 100 μm. Sinusoids and blood vessels dilation (2 A, 3 A, 4 A), lymphocytic
infiltration of portal tracts (2 B, 4 B) and surrounding tissue (2 B), hepatocyte necrosis (2 A).
Table 2.
Pathological changes of liver of ALI rats receiving C60FAS
|
Pathological changes
|
Experimental groups
|
|
Control
|
APAP
|
APAP+C
60
FASi.p.
|
APAP+C
60
FAS p.o.
|
| Protein dystrophy of hepatocytes |
- |
++ |
+ |
+ |
| Lipid dystrophy of hepatocytes |
- |
++ |
+ |
- |
| Ground-glass hepatocytes |
- |
++ |
+ |
+ |
| Necrotic hepatocytes |
- |
+++ |
+ |
+ |
| Connective tissue accumulation |
- |
++ |
+ |
++ |
| Lymphocytes accumulation |
+ |
+++ |
++ |
+++ |
| Blood vessel dilation |
- |
++ |
++ |
++ |
Note . APAP: acetaminophen, C60FAS: C60 fullerene aqueous colloid solution, i.p.: intraperitoneally, p.o.: per os.; trait intensity: “-“ – not observed, “+”: single or slight, “++”: moderate, “+++”: strong.
Under acute APAP exposure, ALT, AST, and unconjugated (indirect) bilirubin increased ,by 37%, 18% and 185%, respectively, as well as conjugated (direct) bilirubin and ALP which increased by 34% and 14% respectively (P >0.1), suggesting the damage of hepatocytes. The level of creatinine also tended up by 15% (P >0.1), which might indicate renal dysfunction. Additionally, triglycerides level was diminished by 48%, maybe due to the impairment of their absorption from the gastrointestinal tract and/or a violation of their synthesis in liver and, consequently, insufficient entering into the blood. The level of total protein was unchanged. The serum α-amylase was significantly lower (23%) compared to control, which is a typical phenomenon of acute and chronic hepatitis (Fig. 3).
46
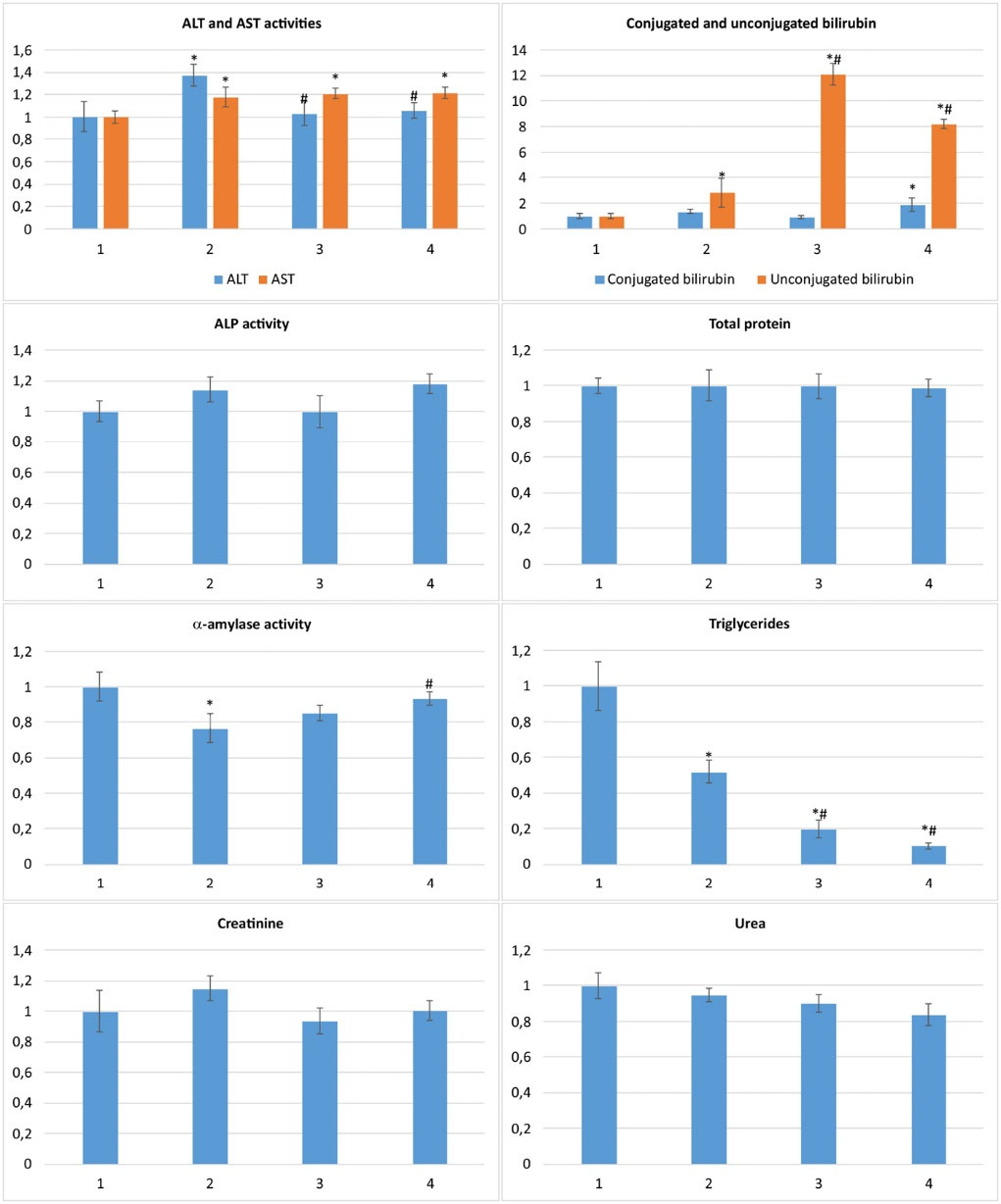
Fig. 3.
Serum biochemical markers of liver, pancreas and kidney functional states for ALI rats received C60FAS normalized against control:
1 – control; 2 – APAP; 3 – APAP+C60FAS i.p.; 4 – APAP+C60FAS p.o.; *P<0.05 compared to control, #P<0.05 compared to APAP.
.
Serum biochemical markers of liver, pancreas and kidney functional states for ALI rats received C60FAS normalized against control:
1 – control; 2 – APAP; 3 – APAP+C60FAS i.p.; 4 – APAP+C60FAS p.o.; *P<0.05 compared to control, #P<0.05 compared to APAP.
Blood vessels and hemocapillars dilation, signs of hemostasis, and thrombosis were observed in ALI rats receiving C60FAS by both ways. However, hepatocytes protein dystrophy was weaker compared to APAP group and lipid dystrophy was not detected. Moreover, less number of necrotic cells was found. In addition, areas of fibrosis and lymphocytic infiltration surrounding portal tracts were diminished under C60FAS i.p. (Fig. 2, Table 2).
In C60FAS-received ALI rats, either i.p. and p.o. ALT rates closed to control values, indicating reduction of hepatocyte cytolysis. At the same time, AST rates remained elevated (by 21% compared to control), the unconjugated bilirubin even more increased (up to 12 and 8 times compared to control and up to 4 and 3 times compared to APAP group for C60FAS i.p. and p.o., respectively), which might indicate a bilirubin metabolism disorder. An increase of unconjugated bilirubin fraction without significant changes in conjugated one may be the consequence of increased bilirubin production (due to hemolysis) or a violation of its hepatic absorption and/or conjugation. C60 has been shown to have weak hemolytic properties,
47,48
which might be the reason of observed changes. On the other hand, it has been established that the increase of unconjugated bilirubin may also occur due to liver function disorders.
49
Hyperbilirubinemia may follow as increased bilirubin production, as abnormalities of any stage of its metabolism, including capture from the blood, intracellular conjugation and biliary excretion. Unconjugated bilirubin elevation is often caused by drugs affecting any stage of its metabolism. Usually, the effect of those on the uridine 5'-diphospho-glucuronosyltransferase (UDP-glucuronosyltransferase) catalyzing the conversion of unconjugated bilirubin into conjugated one by conjugation with glucuronic acid
50
is taken into account. Although, according to Shipelin et al,
51
C60 does not affect the activity of this enzyme, it can affect another bilirubin metabolic pathways, in particular, its transport within the hepatocyte via glutathione-S-transferase. Recently, we have shown that C60 could inhibit its activity.
32
C60FAS when administered by both ways also caused reduction of serum triglycerides and normalized α-amylase and creatinine, indicating the pancreatic and renal function restore (Fig. 3). Serum triglycerides decrease might be an indirect consequence of C60 antioxidant properties; Halim et al.
52
showed that the therapeutic use of antioxidants leads to triglycerides accumulation in the liver and, consequently, to decline their levels in blood serum.
Chronic liver injury
The liver of CLI rats was spotty, had dilated large blood vessels, suggesting the macroscopic features of hepatitis. Pathological changes were aggravated compared to acute ones; blood vessels and sinusoid hemocapillars dilation, thrombosis and hemostasis, hepatocytes protein and lipid dystrophy, necrotic and apoptotic cells, a lot of ground-glass hepatocytes, portal tracts’ fibrosis and lymphocytic infiltration were observed (Fig. 4, Table 3). ALT activity increased (by 31%), as well as conjugated and unconjugated bilirubin (up to 2 and 3.7 times, respectively), indicating chronic hepatitis development.
46
Creatinine and α-amylase tended up (by 23% and 25%, respectively, P >0.1), suggesting the kidney and pancreas function impairment. At the same time, urea decreased by 22% (Fig. 5), while other parameters were unchanged.
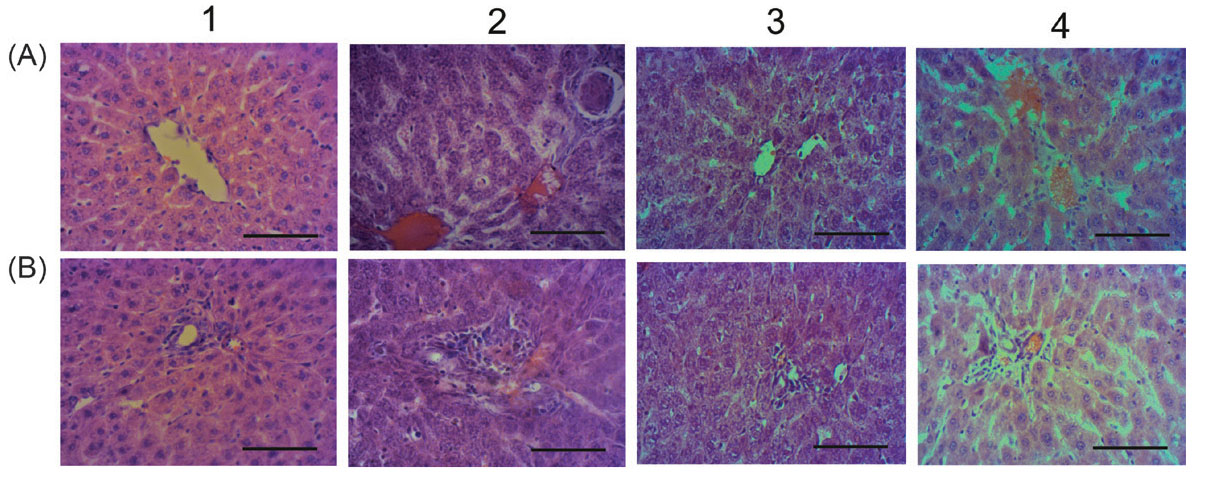
Fig. 4.
Microphotographs of the liver for CLI rats received C60FAS: 1 – control; 2 – APAP; 3 – APAP+C60FAS i.p.; 4 – APAP+C60FAS p.o.;
A and B - centrilobular and periportal areas, respectively. Hematoxylin-eosin-orange staining, ×400, scale 100 μm. Sinusoids and blood
vessels dilatation and overflow (2 A, 4 A), lymphocytic infiltration of portal tracts and surrounding tissue (2 B), hepatocyte necrosis (2 A)
.
Microphotographs of the liver for CLI rats received C60FAS: 1 – control; 2 – APAP; 3 – APAP+C60FAS i.p.; 4 – APAP+C60FAS p.o.;
A and B - centrilobular and periportal areas, respectively. Hematoxylin-eosin-orange staining, ×400, scale 100 μm. Sinusoids and blood
vessels dilatation and overflow (2 A, 4 A), lymphocytic infiltration of portal tracts and surrounding tissue (2 B), hepatocyte necrosis (2 A)
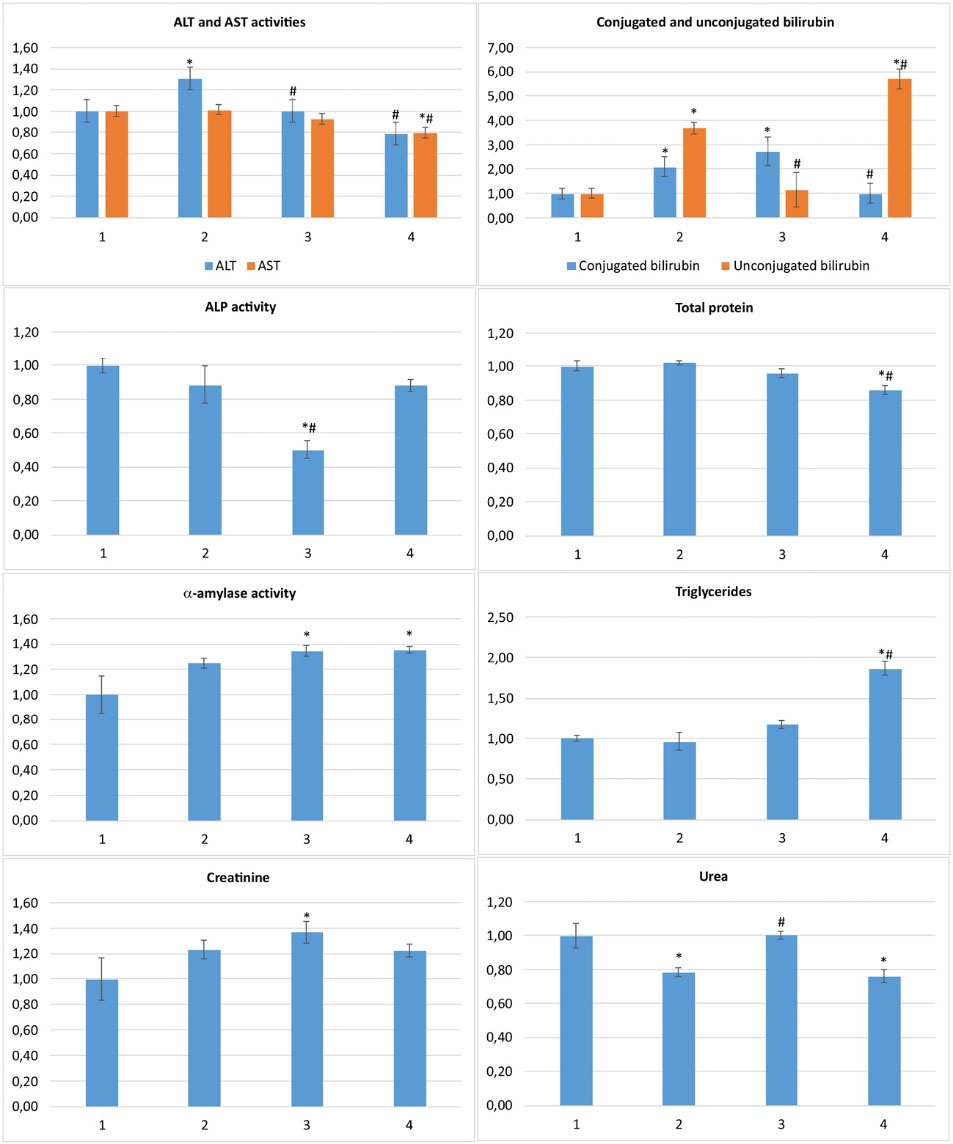
Fig. 5.
Serum biochemical markers of liver, pancreas and kidney functional states for CLI rats received C60FAS normalized against control: 1 – control; 2 – APAP; 3 – APAP+C60FAS i.p.; 4 – APAP+C60FAS p.o.; *P <0.05 compared to control, #P <0.05 compared to APAP.
.
Serum biochemical markers of liver, pancreas and kidney functional states for CLI rats received C60FAS normalized against control: 1 – control; 2 – APAP; 3 – APAP+C60FAS i.p.; 4 – APAP+C60FAS p.o.; *P <0.05 compared to control, #P <0.05 compared to APAP.
Table 3.
Pathological changes of liver of CLI rats receiving C60FAS
|
Pathological changes
|
Experimental groups
|
|
Control
|
APAP
|
APAP+C
60
FASi.p.
|
APAP+C
60
FAS p.o.
|
| Protein dystrophy of hepatocytes |
- |
+++ |
++ |
+ |
| Lipid dystrophy of hepatocytes |
- |
+++ |
++ |
- |
| Ground-glass hepatocytes |
- |
+++ |
+ |
+ |
| Necrotic hepatocytes |
- |
+++ |
+ |
++ |
| Apoptotic hepatocytes |
- |
+++ |
+ |
+ |
| Connective tissue accumulation |
- |
+++ |
+ |
++ |
| Lymphocytes accumulation |
+ |
+++ |
+ |
++ |
| Blood vessel dilation |
- |
+++ |
+ |
++ |
Note . APAP: acetaminophen, C60FAS: C60 fullerene aqueous colloid solution, i.p.: intraperitoneally, p.o.: per os.; trait intensity: “-“ – not observed, “+”: single or slight, “++”: moderate, “+++”: strong.
C60FAS if applied i.p. improved the liver state; the blood vessels and sinusoid hemocapillars were dilated slightly, less overflowed and with no thrombosis compared to non-treated CLI animals. Necrotic and apoptotic foci were local, fibrosis and lymphocytic infiltration of portal tracts decreased significantly. However, signs of hepatocytes dystrophy still occurred (Fig. 4, Table 3). ALT, unconjugated bilirubin, and urea restored to control values, but conjugated bilirubin remained elevated. Moreover, creatinine and α-amylase increased significantly (by 37% and 34%, respectively), which might indicate renal and pancreatic dysfunction deterioration compared to non-treated CLI animals. Serum triglycerides tended up (by 17%, p>0.1) and ALP – declined (by 50%) (Fig. 5).
Under C60FAS p.o. action, hepatic dystrophy was lesser compared to non-treated CLI animals, lymphocytic infiltration and fibrosis of portal tracts also were diminished. However, blood vessels and hemocapillars remained dilated and overflowed, thrombosis features and necrotic foci still occurred (Fig. 4, Table 3). ALT and conjugated bilirubin restored to control values, but unconjugated bilirubin even more increased (up to 6 times compared to control). Creatinine and urea were maintained at the level of APAP group. Serum α-amylase and triglycerides also increased (by 35% and 87%, respectively) in these animals like under C60FAS i.p. application (Fig. 5).
Serum triglycerides elevation might indicate liver dysfunction, in particular, the glutathione system disorders, such as inhibition of glutathione synthesis and/or modification of metabolic pathways responsible for xenobiotics detoxifying.
52
Recently, we have shown that C60 caused depletion of reduced glutathione pool in liver probably through upregulation of glutathione peroxidase which uses reduced glutathione as a substrate.
32
EGFR expression
C60FAS inhibited the expression of EGFR in HepG2 cells after 48 hours incubation in a dose-dependent manner (Fig. 6): there were 41±2% immunopositive cells in control and only 25.3±1.3% and 12.3±0.6% in C60FAS 10 μg/mL and C60FAS 100 μg/mL groups, respectively.
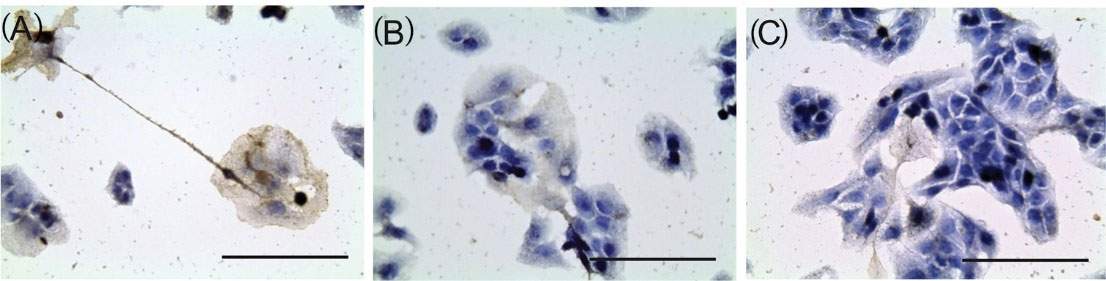
Fig. 6.
EGFR expression in HepG2 cells after 48 h incubation with C60FAS: 1 – control; 2 – C60FAS 10 µg/mL; 3 – C60FAS 100 µg/mL. Antibodies V9 clon (IS630, Dako, USA) and hematoxylin staining, ×400, scale 100 µm
.
EGFR expression in HepG2 cells after 48 h incubation with C60FAS: 1 – control; 2 – C60FAS 10 µg/mL; 3 – C60FAS 100 µg/mL. Antibodies V9 clon (IS630, Dako, USA) and hematoxylin staining, ×400, scale 100 µm
Discussion
APAP is one of the most widely used analgesic and antipyretic medications. It is safe and effective if used at recommended doses, while overdose can lead to hepatotoxicity and ALF. APAP-induced hepatotoxicity is the most common cause of ALF. APAP is metabolized in liver, predominantly via UDP-glucuronosyltransferase and sulfotransferase, to non-toxic urine-excreted compounds. However, 5-9% of APAP is metabolized by cytochromes P450, preferably CYP 2E1, to the highly reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI). Usually, NAPQI rapidly conjugates with glutathione, but APAP overdose depletes hepatic glutathione pool, an excessive amount of NAPQI covalently binds to sulfhydryl groups of cellular proteins, in particular mitochondrial ones, which ultimately result in mitochondrial dysfunction and oxidative stress, and lead to hepatocytes necrosis.
53,54
Generally, biotransformation of certain xenobiotics by the enzymes of CYP family is accompanied by the production of short-lived highly reactive metabolites, such as electrophiles, nucleophiles, free radicals, and redox-active reactants. These chemical species can covalently bind cellular proteins and lipids, and in case of overproduction cause damage of cellular organelles, including mitochondria, and lead to development of oxidative stress, endoplasmic reticulum stress, hepatocytes apoptosis and necrosis.
55
These events have been suggested the background of liver toxic injury.
The assessment of antioxidants effect under toxic liver injury, in particular DILI, viral and non-viral hepatitis, alcoholic liver disease and NASH were aimed to be discovered in numerous in vitro and in vivo studies. The therapeutic efficacy of vitamins C and E, silymarin, N-acetylcysteine, glycyrrhizinic and ursodeoxycholic acids, plant extracts and antioxidant mixtures were demonstrated in combination therapy and separately.
6,56
Nevertheless, their clinical effectiveness still remains unproved. The only drug approved in clinical practice for DILI treatment is N-acetylcysteine - APAP specific antidote. However, this remedy is less effective in case of non-APAP ALF.
14
In the setting of DILI with autoimmune features and hypersensitivity reactions, corticosteroids have been used predominantly.
57
It should be noticed that all the abovementioned non-hormonal agents possess antioxidant properties which contribute to their therapeutic activity.
10,11,14,58-60
These chemicals can affect the signaling pathways associated with the production and/or neutralization of free radicals and proinflammatory cytokines and, moreover, can directly scavenge free radicals.
61,62
Pristine C60 is one of the most powerful free radical scavengers.
33,63,64
One C60 molecule is able to bind up to 34 radicals depending on their size.
65
Therefore, we suggest that therapeutic effects of C60 might be realized through its strong antioxidant properties. Indeed, the ability of C60 to inhibit lipid peroxidation and to stimulate antioxidant defense enzymes under CCl4-induced ALI were shown.
30
We also proved this ability of C60 on model of colonic inflammation.
32
We have shown that C60 could suppress EGFR expression in HepG2 cells. EGFR is known to be involved in liver regeneration. Its upregulation is tightly related to liver fibrogenesis and fibrosis and cirrhosis development, and its downregulation attenuates the fibrotic lesions.
66
Therefore, mitigation of periportal fibrosis under C60FAS chronic exposure might be through inhibition of EGFR expression by that. Additionally, the rapid downregulation of EGFR under acute APAP-induced liver injury diminishes liver damage and inhibits cell necrosis possibly by another mechanism. Hence, it was shown
67
that immediately after APAP application (within 1-4 h) EGFR is overexpressed and upregulated and translocated in mitochondria, and the intensity of these events correlates with the rate of liver necrotic lesions. Moreover, the administration of EGFR inhibitor in this period reduces and even eliminates completely APAP toxic effects on liver. Authors explained this phenomenon by the fact that EGFR activation is involved in apoptosis through its ability to phosphorylate “death receptor” (CD95) and stabilize the death-inducing signaling complex. Accordingly, inhibition of EGFR results in inhibition of apoptosis. Therefore, we might suggest that inhibition of EGFR could contribute to hepatoprotective effect of C60.
Some studies revealed that C60 could realize prooxidative effects in biological systems and cause oxidative DNA damage.
41
However, these effects strongly depend on C60 nanoparticles size and dozing. The C60 system we have used in the current study did not demonstrate any toxic or another effects which could be interpreted as prooxidative ones.
29,32,34,37,40
Nevertheless, possible accumulation of C60 in tissues and organs especially after prolonged exposure
68
should not be neglected. Therefore, local high amount of C60 in some organs, for instance in pancreas, might occur. The last could cause observed negative effects like renal and pancreatic hypofunction.
Conclusion
We concluded that C60FAS application reduces the hepatocytes cytolysis and restores the liver morphological state, but affects the bilirubin metabolism through APAP-induced ALI. C60FAS also contributes to the recovery of renal and pancreatic functions.
Under chronic APAP exposure, C60FAS attenuates the dystrophic processes in hepatocytes and manifestations of apoptosis and necrosis, as well as normalizing the values of most liver functional biochemical markers. However, renal and pancreatic dysfunction features also occur, suggesting the possible cumulative effects of C60. Therapeutic effects of C60 might be realized through its ability to scavenge free radicals and affect the expression of EGFR.
Acknowledgement
The scientific results presented in the manuscript were presented and discussed at the International Research and Practice Conference «Nanotechnology and Nanomaterials» (NANO-2018) that was held in Kyiv, Ukraine, at 27-30 Aug 2018.
Funding sources
This work was supported by fundamental research grant of Ministry of Education and Science of Ukraine for young scientists “Biocompatible water soluble C60 fullerenes as antifibrotic and antineoplastic treatment of liver malignancies” (No. 0118U000244) and Fundamental research grant of Ministry of Education and Science of Ukraine “Novel hybrid antineoplastic and anti-inflammatory nano-complexes based on pyrroles and C60 fullerene: development, characterization, pharmacodynamics and toxicological characteristics” (No. 19BP07-03).
Conflicts of interest
Authors declare no conflicts of interest.
Ethical statement
All experiments with animals use were conducted in compliance with bioethics principles, legislative norms and provisions of the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (Strasbourg, 1986), General Ethical Principles for Experiments on Animals, adopted by the First National Bioethics Congress (Kyiv, 2001), and approved by an institutional review committee.
Authors’ contribution
The work presented here was carried out in collaboration between all authors. YP and UR prepared and characterized C60FAS. HK and ND performed in vivo experiments, biochemical study and statistical analysis. OL performed histological study. TH performed in vitro experiment and immunohistochemical study. YP and VR coordinated the experimental work and analyzed data. HK wrote the manuscript. All authors discussed the results and commented on the manuscript. All authors read and approved the final manuscript.
Research Highlights
What is the current knowledge?
simple
-
√ Toxic liver injury is accompanied by oxidative stress and
has no universal and effective treatment.
What is new here?
simple
-
√ Water-soluble pristine C60 fullerenes as the powerful
free radical scavengers reduce the local and systemic
manifestations of toxic liver injury in rats.
-
√ C60 fullerenes inhibit EGFR expression in HepG2 cells.
References
- Zimmerman HJ. Drug-induced liver disease. Clin Liver Dis 2000; 4(1):73-96. doi: 10.1016/S1089-3261(05)70097-0 [Crossref] [ Google Scholar]
- Pandit А, Sachdeva T, Bafna P. Drug-Induced Hepatotoxicity: A Review. J Appl Pharm Sci 2012; 02(05):233-243. doi: 10.7324/JAPS.2012.2541 [Crossref] [ Google Scholar]
- Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davron JT, Han SHB. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002; 137:947-954. doi: 10.7326/0003-4819-137-12-200212170-00007 [Crossref] [ Google Scholar]
- Yu Y, Mao Y, Chen C, Chen J, Chen J, Cong W. CSH guidelines for the diagnosis and treatment of drug-induced liver injury. Hepatol Int 2017; 11:221-241. doi: 10.1007/s12072-017-9793-2 [Crossref] [ Google Scholar]
- Muriel P. Role of free radicals in liver diseases. Hepatol Int 2009; 3:526-536. doi: 10.1007/s12072-009-9158-6 [Crossref] [ Google Scholar]
- Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, Feng Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int J Mol Sci 2015; 16:26087-26124. doi: 10.3390/ijms161125942 [Crossref] [ Google Scholar]
- Singal AK, Jampana SC, Weinman SA. Antioxidants as Therapeutic Agents for Liver Disease. Liver Int 2011; 31(10):1432-1448. doi: 10.1111/j.1478-3231.2011.02604.x [Crossref] [ Google Scholar]
- Hiraganahalli BD, Chinampudur VC, Dethe S, Mundkinajeddu D, Pandre MK, Balachandran J. Hepatoprotective and antioxidant activity of standardized herbal extracts. Pharmacogn Mag 2012; 8(30):116-23. doi: 10.4103/0973-1296.96553 [Crossref] [ Google Scholar]
- Farias MS, Budni P, Ribeiro CM, Parisotto EB, Santos CE, Dias JF. Antioxidant supplementation attenuates oxidative stress in chronic hepatitis C patients. Gastroenterol Hepatol 2012; 35(6):386-94. doi: 10.1016/j.gastrohep.2012.03.004 [Crossref] [ Google Scholar]
- Sarkhy AA, Al-Hussaini AA, Nobili V. Does vitamin E improve the outcomes of pediatric nonalcoholic fatty liver disease? A systematic review and meta-analysis. Saudi J Gastroenterol 2014; 20(3):143-53. doi: 10.4103/1319-3767.132983 [Crossref] [ Google Scholar]
- Zhang L, Liu XH, Qi HB, Li Z, Fu XD, Chen L. Ursodeoxycholic acid and S-adenosylmethionine in the treatment of intrahepatic cholestasis of pregnancy: a multi-centered randomized controlled trial. Eur Rev Med Pharmacol Sci 2015; 19(19):3770-6. [ Google Scholar]
- Zhang S, Pan H, Peng X, Lu H, Fan H, Zheng X. Preventive use of a hepatoprotectant against anti-tuberculosis drug-induced liver injury: A randomized controlled trial. J Gastroenterol Hepatol 2016; 31(2):409-16. doi: 10.1111/jgh.13070 [Crossref] [ Google Scholar]
- Farsi F, Mohammadshahi M, Alavinejad P, Rezazadeh A, Zarei M, Engali KA. Functions of Coenzyme Q10 Supplementation on Liver Enzymes, Markers of Systemic Inflammation, and Adipokines in Patients Affected by Nonalcoholic Fatty Liver Disease: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. J Am Coll Nutr 2016; 35(4):346-53. doi: 10.1080/07315724.2015.1021057 [Crossref] [ Google Scholar]
- Nabi T, Nabi S, Rafiq N, Shah A. Role of N-acetylcysteine treatment in non-acetaminophen-induced acute liver failure: A prospective study. Saudi J Gastroenterol 2017; 23(3):169-175. doi: 10.4103/1319-3767.207711 [Crossref] [ Google Scholar]
- Lazebnik LB, Radchenko VG, Golovanova EV, Zvenigorodskaya LA, Konev YV, Seliverstov PV. Nonalcoholic fatty liver disease: diagnostic, symptoms, treatment Guidelines were approved by the XV Gastroenterological Scientific Society of Russia in 2015. Eksp Klin Gastroenterol 2015(7):85-96.
- Leoni S, Tovoli F, Napoli L, Serio I, Ferri S, Bolondi L. Current guidelines for the management of non-alcoholic fatty liver disease: A systematic review with comparative analysis. World J Gastroenterol 2018; 24(30):3361-3373. doi: 10.3748/wjg.v24.i30.3361 [Crossref] [ Google Scholar]
- Nikalje AP. Nanotechnology and its Applications in Medicine. Med Chem 2015; 5:081-089. doi: 10.4172/2161-0444.1000247 [Crossref] [ Google Scholar]
- Gorjikhah F, Davaran S, Salehi R, Bakhtiari M, Hasanzadeh A, Panahi Y. Improving “lab-on-a-chip” techniques using biomedical nanotechnology: a review. Artif Cell Nanomed B 2016; 44(7):1609-1614. doi: 10.3109/21691401.2015.1129619 [Crossref] [ Google Scholar]
- Padmanabhan J, Kyriakides TR. Nanomaterials, inflammation, and tissue engineering. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2015; 7(3):355-370. doi: 10.1002/wnan.1320 [Crossref] [ Google Scholar]
- Bakry R, Vallant RM, Najam-ul-Haq M, Rainer M, Szabo Z, Huck CW. Medicinal applications of fullerenes. Int J Nanomedicine 2007; 2(4):639-49. [ Google Scholar]
- Kroto HW, Heath JR, O'Brien SC, Curl RF, Smalley RE. C60: Buckminsterfullerene. Nature 1985; 318:162-163. doi: 10.1038/318162a0 [Crossref] [ Google Scholar]
- Moussa F. [60]Fullerene and derivatives for biomedical applications. Nanobiomaterials 2018:113-136. doi: 10.1016/B978-0-08-100716-7.00005-2 [Crossref]
- Schuetze C, Ritter U, Scharff P, Bychko A, Prylutska S, Rybalchenko V, Prylutskyy Yu. Interaction of N-fluorescein-5-isothiocyanate pyrrolidine-C60 compound with a model bimolecular lipid membrane. Mater Sci Engineer C 2011; 31(5):1148-1150. doi: 10.1016/j.msec.2011.02.026 [Crossref] [ Google Scholar]
- Prylutska SV, Skivka LM, Didenko GV, Prylutskyy YI, Evstigneev MP, Potebnya GP. Complex of C60 Fullerene with Doxorubicin as a Promising Agent in Antitumor Therapy. Nanoscale Res Lett 2015; 10(1):499. doi: 10.1186/s11671-015-1206-7 [Crossref] [ Google Scholar]
- Shoji M, Takahashi E, Hatakeyama D, Iwai Y, Morita Y, Shirayama R. Anti-influenza activity of C60 fullerene derivatives. PLoSOne 2013; 8(6):e66337. doi: 10.1371/journal.pone.0066337 [Crossref] [ Google Scholar]
- Al Garalleh H, Thamwattana N, Cox BJ, Hill JM. Modeling interactions between C60 antiviral compounds and HIV protease. Bull Math Biol 2015; 77(1):184-201. doi: 10.1007/s11538-014-0056-2 [Crossref] [ Google Scholar]
- Scharff P, Ritter U, Matyshevska OP, Prylutska SV, Grynyuk II, Golub AA. Therapeutic reactive oxygen generation. Tumori 2008; 94(2):278-283. doi: 10.1177/030089160809400221 [Crossref] [ Google Scholar]
- Xie Q, Perez–Cordero E, Echegoyen L. Electrochemical Detection of C60 and C70: Enhanced Stability of Fullerides in Solution. Am Chem Soc 1992; 114:3978-3980. doi: 10.1021/ja00036a056 [Crossref] [ Google Scholar]
- Didenko G, Prylutska S, Kichmarenko Y, Potebnya G, Prylutskyy Y, Slobodyanik N. Evaluation of the antitumor immune response to C60 fullerene. Materwiss Werksttech 2013; 44(2-3):124-128. doi: 10.1002/mawe.201300082 [Crossref] [ Google Scholar]
- Halenova TI, Vareniuk IM, Roslova NM, Dzerzhynsky ME, Savchuk OM, Ostapchenko LI. Hepatoprotective effect of orally applied water-soluble pristine C60 fullerene against CCl4-induced acute liver injury in rats. RSC Adv 2016; 6(102):100046-100055. doi: 10.1039/C6RA20291H [Crossref] [ Google Scholar]
- Lynchak OV, Prylutskyy YuI, Rybalchenko VK, Kyzyma OA, Soloviov D, Kostjukov VV. Comparative analysis of the antineoplastic activity of C60 fullerene with 5-fluorouracil and pyrrole derivative in vivo. Nanoscale Res Lett 2017; 12:8. doi: 10.1186/s11671-016-1775-0 [Crossref] [ Google Scholar]
- Byelinska IV, Kuznietsova HM, Dziubenko NV, Lynchak OV, Rybalchenko TV, Prylutskyy YuI. Effect of С60 fullerenes on the intensity of colon damage and hematological signs of ulcerative colitis in rats. Mat Sci Eng C 2018; 93:505-517. doi: 10.1016/j.msec.2018.08.033 [Crossref] [ Google Scholar]
- Ferreira CA, Ni D, Rosenkrans ZT, Cai W. Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Res 2018; 11(10):4955-4984. doi: 10.1007/s12274-018-2092-y [Crossref] [ Google Scholar]
- Vereshchaka IV, Bulgakova NV, Maznychenko AV, Gonchar OO, Prylutskyy YuI, Ritter U. C60 fullerenes diminish the muscle fatigue in rats comparable to N-acetylcysteine or β-alanine. Front Physiol 2018; 9:517. doi: 10.3389/fphys.2018.00517 [Crossref] [ Google Scholar]
- Scharff P, Carta-Abelmann L, Siegmund C, Matyshevska OP, Prylutska SV, Koval TV. Effect of X-ray and UV irradiation of the C60 fullerene aqueous solution on biological samples. Carbon 2004; 42(5-6):1199-1201. doi: 10.1016/j.carbon.2003.12.055 [Crossref] [ Google Scholar]
- Ritter U, Prylutskyy YuI, Evstigneev MP, Davidenko NA, Cherepanov VV, Senenko AI. Structural features of highly stable reproducible C60 fullerene aqueous colloid solution probed by various techniques. Fullerenes, Nanotubes and Carbon Nanostructures 2015; 23(6):530-534. doi: 10.1080/1536383X.2013.870900 [Crossref] [ Google Scholar]
- Prylutska SV, Grynyuk II, Grebinyk SM, Matyshevska OP, Prylutskyy YuI, Ritter U. Comparative study of biological action of fullerenes C60 and carbon nanotubes in thymus cells. Materwiss Werksttech 2009; 40(4):238-241. doi: 10.1002/mawe.200900433 [Crossref] [ Google Scholar]
- Singla R, Sharma C, Shukla AK, Acharya A. Toxicity Concerns of Therapeutic Nanomaterials. J Nanosci Nanotechnol 2019; 19(4):1889-1907. doi: 10.1166/jnn.2019.16502 [Crossref] [ Google Scholar]
- Gharbi N, Pressac M, Hadchouel M, Szwarc H, Wilson SR, Moussa F. C60 fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett 2005; 5:2578-2585. doi: 10.1021/nl051866b [Crossref] [ Google Scholar]
- Kuznietsova HM, Lynchak OV, Dziubenko NV, Osetskyi VL, Ogloblya OV, Prylutskyy YuI. Water-soluble C60 fullerenes reduce manifestations of acute cholangitis in rats. Appl Nanosci 2018. doi: 10.1007/s13204-018-0700-5 [Crossref]
- Folkmann JK, Risom L, Jacobsen NR, Wallin H, Loft S, Moller P. Oxidatively damaged DNA in rats exposed by oral gavage to C60 fullerenes and single-walled carbon nanotubes. Environ Health Perspect 2008; 117:703-8. doi: 10.1289/ehp.11922 [Crossref] [ Google Scholar]
- Jacobsen NR, Pojana G, White P, Moller P, Cohn CA, Korsholm KS. Genotoxicity, cytotoxicity, and reactive oxygen species induced by single-walled carbon nanotubes and C60 fullerenes in the FE1-Muta™Mouse lung epithelial cells. Environ Mol Mutagen 2008; 49(6):476-487. doi: 10.1002/em.20406 [Crossref] [ Google Scholar]
- Aly FM, Othman A, Haridy MAM. Protective Effects of Fullerene C60 Nanoparticles and Virgin Olive Oil against Genotoxicity Induced by Cyclophosphamide in Rats. Oxid Med CellLongev 2018; 2018. doi: 10.1155/2018/1261356 [Crossref]
- Xing TJ. Clinical Classification of Liver Failure: Consensus, Contradictions and New Recommendations. J Clin Gastroenterol Hepatol 2017; 1:2. doi: 10.21767/2575-7733.1000016 [Crossref] [ Google Scholar]
- Wojnarova L, Kutinova Canova N, Farghali H, Kucera T. Sirtuin 1 Modulation in Rat Model of Acetaminophen-Induced Hepatotoxicity. Physiol Res 2015; 64(Suppl . 4):S477-S487. [ Google Scholar]
-
Suriawinata AA, ThungSN. Liver pathology. An atlas and concise guide. Demos Medical Publishing; 2011. p. 260.
- Shpakova NM, Nipot OS, Ishchenko IO, Prylutska SV, Bohutska KI, Cherepanov VV. Effect of C60 fullerene on viscoelastic properties of human erythrocytes membrane. Fiziol Zh 2014; 60(5):82-88. [ Google Scholar]
- Tishevskaya NV, Golubotovsky E. V, Pharizova KO, Omarova DM Effects of fullerenol C60(OH)24 on physiological and compensatory erythropoiesis. Nanotechnol Russ 2015; 10(7):645-650. doi: 10.1134/S1995078015040199 [Crossref] [ Google Scholar]
- Ye J, Cui L, Zhou Y, Huang Y, Banafa O, Hou X. "Gilbert's-like" syndrome as part of a spectrum of persistent unconjugated hyperbilirubinemia in post-chronic hepatitis patients. Sci Rep 2018; 8(1):2008. doi: 10.1038/s41598-018-19847-4 [Crossref] [ Google Scholar]
- Roy-Chowdhury J, Roy-Chowdhury N, Listowsky I, Wolkoff AW. Drug- and drug abuse-associated hyperbilirubinemia: experience with atazanavir. Clin Pharmacol Drug Dev 2017; 6(2):140-146. doi: 10.1002/cpdd.314 [Crossref] [ Google Scholar]
- Shipelin VA, Arianova EA, Trushina EN, Avren'eva LI, Batishcheva SIu, Cherkashin AV. Toxicological and sanitary characteristics of fullerene C60 administered to the rat gastrointestinal tract. Gig Sanit 2012(2):90-4.
- Halim AB, el-Ahmady O, Hassab-Allah S, Abdel-Galil F, Hafez Y, Darwish A. Biochemical effect of antioxidants on lipids and liver function in experimentally-induced liver damage. Ann Clin Biochem 1997; 34(Pt 6):656-63. doi: 10.1177/000456329703400610 [Crossref] [ Google Scholar]
- Parman T, Bunin DI, Ng HH, McDunn JE, Wulff JE, Wang A. Toxicogenomics and metabolomics of pentamethylchromanol (PMCol)-induced hepatotoxicity. Toxicol Sci 2011; 124(2):487-501. doi: 10.1093/toxsci/kfr238 [Crossref] [ Google Scholar]
- Du K, Ramachandran A, Jaeschke H. Oxidative stress during acetaminophen hepatotoxicity: sources, pathophysiological role and therapeutic potential. Redox Biol 2016; 10:148-156. doi: 10.1016/j.redox.2016.10.001 [Crossref] [ Google Scholar]
- Yan M, Huo Y, Yin S, Hu H. Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol 2018; 17:274-283. doi: 10.1016/j.redox.2018.04.019 [Crossref] [ Google Scholar]
- Gu X, Manautou JE. Molecular mechanisms underlying chemical liver injury. Expert Rev Mol Med 2012; 14:e4. doi: 10.1017/S1462399411002110 [Crossref] [ Google Scholar]
- Stine JG, Lewis JH. Current and future directions in the treatment and prevention of drug-induced liver injury: a systematic review. Expert Rev Gastroenterol Hepatol 2016; 10(4):517-36. doi: 10.1586/17474124.2016.1127756 [Crossref] [ Google Scholar]
- Gong G, Xiang L, Yuan L, Hu L, Wu W, Cai L. Protective effect of glycyrrhizin, a direct HMGB1 inhibitor, on focal cerebral ischemia/reperfusion-induced inflammation, oxidative stress, and apoptosis in rats. PLoS One 2014; 9(3):e89450. doi: 10.1371/journal.pone.0089450 [Crossref] [ Google Scholar]
- Zhu GQ, Shi KQ, Huang GQ, Wang LR, Lin YQ, Braddock M. A network meta-analysis of the efficacy and side effects of UDCA-based therapies for primary sclerosing cholangitis. Oncotarget 2015; 6(29):26757-26769. doi: 10.18632/oncotarget.5610 [Crossref] [ Google Scholar]
- Federico A, Dallio M, Loguercio C. Silymarin Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules 2017; 22(2):191. doi: 10.3390/molecules22020191 [Crossref] [ Google Scholar]
- Halasi M, Wang M, Chavan TS, Gaponenko V, Hay N, Gartel AL. ROS inhibitor N-acetyl-L-cysteine antagonizes the activity of proteasome inhibitors. Biochem J 2013; 454(2):201-8. doi: 10.1042/BJ20130282 [Crossref] [ Google Scholar]
- Surai PF. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants (Basel) 2015; 4(1):204-247. doi: 10.3390/antiox4010204 [Crossref] [ Google Scholar]
- Eswaran SV. Water soluble nanocarbon materials: a panacea for all?. Curr Sci 2018; 114(9):1846-1850. doi: 10.18520/cs/v114/i09/1846-1850 [Crossref] [ Google Scholar]
- Gonchar OO, Maznychenko AV, Bulgakova NV, Vereshchaka IV, Tomiak T, Ritter U. C60 fullerene prevents restraint stress-induced oxidative disorders in rat tissues: possible involvement of the Nrf2/ARE-antioxidant pathway. Oxid Med Cell Longev 2018; 2018:2518676. doi: 10.1155/2018/2518676 [Crossref] [ Google Scholar]
- Krusic PJ, Wasserman E, Keizer PN, Morton JR, Preston KF. Radical reactions of C60. Science 1991; 254(5035):1183-5. doi: 10.1126/science.254.5035.1183 [Crossref] [ Google Scholar]
- Liang D, Chen H, Zhao L, Zhang W, Hu J, Liu Z. Inhibition of EGFR attenuates fibrosis and stellate cell activation in diet-induced model of nonalcoholic fatty liver disease. Biochim Biophys Acta Mol Basis Dis 2018; 1864(1):133-142. doi: 10.1016/j.bbadis.2017.10.016 [Crossref] [ Google Scholar]
- Bhushan B, Chavan H, Borude P, Xie Y, Du K, McGill MR. Dual Role of Epidermal Growth Factor Receptor in Liver Injury and Regeneration after Acetaminophen Overdose in Mice. Toxicol Sci 2017; 155(2):363-378. doi: 10.1093/toxsci/kfw213 [Crossref] [ Google Scholar]
- Hendrickson OD, Morozova OV, Zherdev AV, Yaropolov AI, Klochkov SG, Bachurin SO. Study of Distribution and Biological Effects of Fullerene C60 after Single and Multiple Intragastrical Administrations to Rats. Fuller Nanotub Car N 2015; 23(7):658-668. doi: 10.1080/1536383X.2014.949695 [Crossref] [ Google Scholar]