Bioimpacts. 10(4):251-257.
doi: 10.34172/bi.2020.32
Original Research
The role of Hippo signaling pathway in physiological cardiac hypertrophy
Majid Gholipour *  , Arezoo Tabrizi
, Arezoo Tabrizi 
Author information:
Faculty Member of Physical Education Department, Sharif University of Technology, Tehran, Islamic Republic of Iran
Abstract
Introduction:
The role of Hippo signaling pathway, which was identified by genetic studies as a key regulator for tissue growth and organ size, in promoting physiological cardiac hypertrophy has not been investigated.
Methods: Fourteen male Wistar rats were randomly assigned to the exercise and control groups. The exercise group ran 1 hour per day, 5 days/week, at about 65%-75% VO2max on the motor-driven treadmill with 15º slope, and the control group ran 15 min/d, 2 days/week at 9 m/min (0º inclination), throughout the eight-week experimental period. Forty-eight hours after the last session, hearts were dissected and left ventricles were weighed and stored for subsequent RT-PCR analysis.
Results: Despite a significant increase in the MAP4k1 expression levels in the exercise group (P = 0.001), the Mst1 expression was inhibited compared to the control group (P < 0.001) which was followed by suppression of Lats1 expression (P = 0.001). Compared with the control group, significant increases were observed in heart weight/body weight (P = 0.024) and left ventricular weight/body weight (P = 0.034) ratios in the exercise group. The H&E staining confirmed the cardiac hypertrophy that may be partly due to a significant increase in Yap1 expression level compared with the control group (P <0.001), which was confirmed by Western blot analysis.
Conclusion: Increased MAP4K1 expression did not influence Lats1 activation. The exercise training protocol suppressed Mst1 and Lats1 (Hippo pathway) and caused an increase in Yap1 expression level, which led to physiological cardiac hypertrophy in healthy rats. Further studies are suggested to apply this exercise protocol for the prevention and/or rehabilitation of cardiovascular disease and health promotion.
Keywords: Molecular signaling, Cardiomyocyte adaptation, Left ventricular hypertrophy, Uphill-treadmill running, Wistar rat
Copyright and License Information
© 2020 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
The heart is a muscle that pumps blood throughout the circulatory system to deliver adequate oxygen and nutrients to tissues such as skeletal muscles and itself which may lead to improved athletic performance and health promotion.1,2 Most of the heart mass is composed of the cardiomyocytes. Despite, mammalian cardiomyocytes are terminally differentiated soon after birth, characteristics of the cardiac tissue lead to an increase in the heart mass through the hypertrophy of individual cardiomyocytes.3 Cardiomyocyte hypertrophy is characterized by enhanced protein synthesis and an increase in cell size, especially the left ventricle. There are two different classes of cardiac hypertrophy. In the pathological hypertrophy (cardiac dysfunction), which is associated with conditions like hypertension or injuries, inadequate capillaries to supply nutrients and oxygen leads to cardiac hypoxia and remodeling. In contrast, physiological hypertrophy (normal cardiac function) occurs during normal growth of children, as well as in response to exercise training that provokes the physiological cardiac hypertrophy, enhances its function, and it is not considered as a cardiac disease risk factor due to an expanded capillary network to supply adequate blood flow and nourishment,2,4 however, those beneficial effects depend on duration and intensity of exercise. Physiological cardiac hypertrophy is regulated by both organ-extrinsic stimuli such as circulating growth factors, hormonal, and nutritional status and also organ-intrinsic mechanisms that control the heart size via genetic signaling pathways such as PI3K-Akt and mTOR with their downstream targets, p70S6 and eEF2 which are not fully understood.5,6 The earlier studies demonstrated the critical and important role of the Hippo signaling pathway in cardiomyocyte proliferation and hypertrophy, cardiac regeneration, and controlling the heart size. The mammalian genome of the Hippo contains Ste20 family kinases (Mst1 and Mst2), an adaptor protein Salvador, Salv/WW45, two proteins MOBKL1A and MOBKL1B, often collectively referred to as Mob1, and two large tumor suppressor kinases (Lats1 and Lats2),7-9 that phosphorylates and inactivates yes-associated protein (Yap) and transcriptional coactivator with PDZ-binding motif (Taz), the downstream transcriptional regulators of the signaling pathway.10 In contrast, in the inactive state of Hippo pathway, accumulation of Yap and Taz in the nucleus enhances gene expression for organ growth by interacting with other proteins such as SMAD and OCT4.11 The versatile interactions between Akt, MAP4Ks, and Hippo kinase network including Mst1, Lats1, Yap and Taz have been documented,12-14 but none of them included the exercise training protocols. Several studies have been conducted on rodents to evaluate the positive effect of treadmill running on ventricular mass and cardiomyocyte dimensions, and also to investigate the effect of endurance exercise on heart weight and its ratio to body weight,15,16 however, recently it has been shown that eight weeks of endurance treadmill running has no impact on heart weight/body weight ratio in mice.17 To date, the molecular mechanism of adaptive cardiac hypertrophy, especially Hippo pathway, in response to exercise training has not been investigated and the contribution of the Hippo signaling pathway to physiological hypertrophy is unclear. Given that the duration and intensity of exercise have a positive impact on the cardiovascular system and athletic performance, the purpose of the present study was to investigate the effects of an eight-week endurance treadmill running at 15º inclination on cardiac hypertrophy and alteration in Mst1, Lats1, and Yap1 expression levels. In addition, because MAP4Ks had been identified as a direct Lats 1/2-activating kinase,14 assessment of MAP4k1 expression levels was included in this study.
Materials and Methods
Experimental design
Fourteen male Wistar rats, eight-week-old (180-200 g), were randomly assigned to either exercise (n = 7) or control (n = 7) groups. Three-four rats were housed per standard cage and maintained in a light-dark cycle of 12 hours, 22 ± 2°C, 50 ± 5% relative humidity, and had access to pelleted rodent diet and water ad libitum. Throughout the study, the same person handled the animals in each group and all experimental protocols were conducted during the dark cycle. The body weight was measured every week.
Exercise training
All rats completed a two-week familiarization schedule, 5 days/week, which consisted of running on the motor-driven treadmill with stainless steel grids at the end of the lines to provide an electrical stimulus (0.5 mA, 1 stimulus of 200-ms duration every second). For the exercise group, the intensity of running gradually increased in speeds and grades to achieve the initial condition of the main exercise training protocol. To determine exercise intensity, before starting the main protocol and at the every other weekend, VO2max (maximum oxygen consumption as an endurance capacity index) test was performed with uphill-running on the treadmill (15º inclination) as previously described.18 Briefly, each rat warmed up for 10 minutes at 40-50% VO2max, and then treadmill velocity was increased by 0.03 m/s every 2 minutes until the rat was unable to run further. During the eight-week experimental period, all rats in the exercise group ran 1 hour per day, 5 days/week, at about 65%-75% VO2max on the treadmill with 15º slope.19 Each session included 10 minutes warm-up and cool-down periods at 50%-60% VO2max with 0º slopes. Given that 15 minutes running at 0º slope at 9 m/min does not yield any training response, the treadmill running skill was maintained in control rats for 2 days/week throughout the study (Fig. 1).18
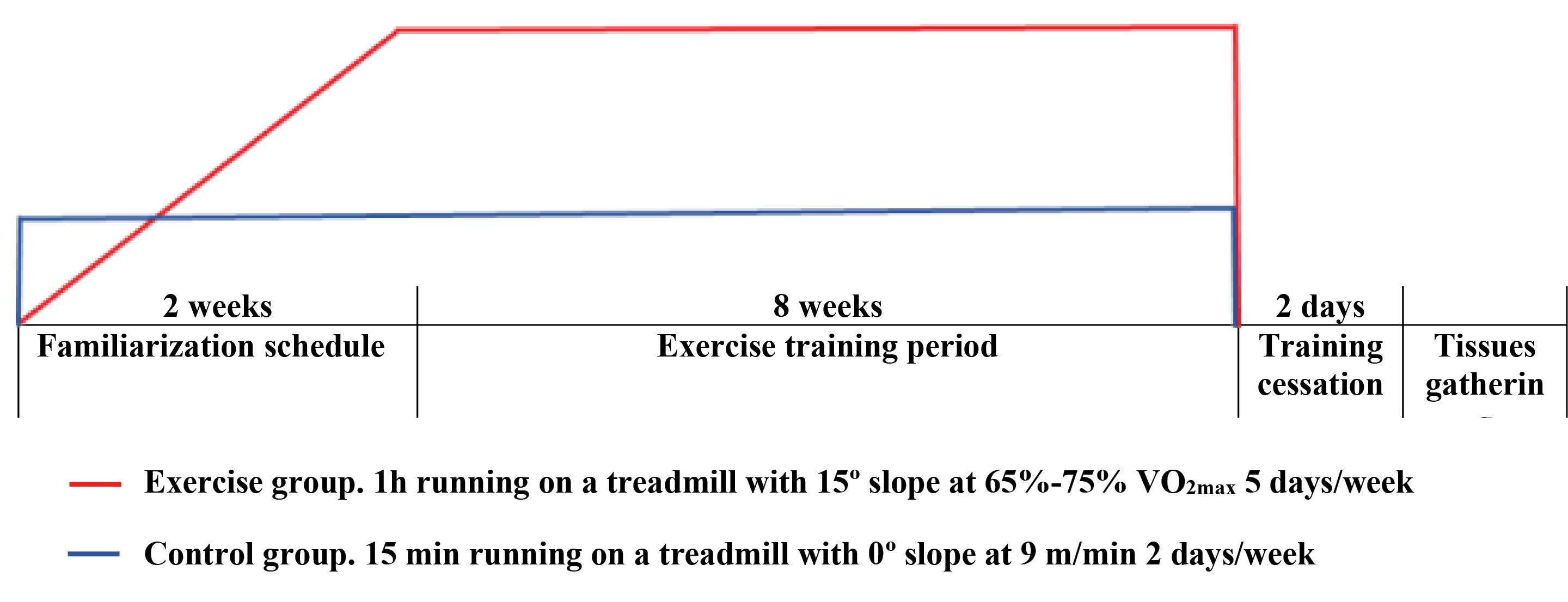
Fig. 1.
Illustration of familiarization period and exercise training protocol including duration and intensity.
.
Illustration of familiarization period and exercise training protocol including duration and intensity.
Cardiac harvesting
Forty-eight hours after the last exercise session, the rats in the two groups were anesthetized with 50 mg/kg ketamine hydrochloride and 10 mg/kg xylazine intraperitoneally. Then the animals were euthanized by draining the blood directly from their hearts and the hearts were excised and flushed free of blood in saline and were weighed after absorbing the excess water by tissue paper. Thereafter, all dissected left ventricles were weighed and stored at -80°C for qRT-PCR, Western blot, and Histological analyses.
Gene expression
Quantitative real-time PCR
Approximately 40–50 mg of each left ventricle was homogenized and according to manufacturer’s instruction, TRizol reagent (Qiazol, cat. no. 79306, USA) was used to isolate total RNA. Purity and concentration of RNA were determined spectrophotometrically by NanoDrop 2000 (Thermo Scientific, Rockford, USA), and 1% agarose gel stained with Nancy-520 was used to determine RNA integrity electrophoretically (Sigma-Aldrich, Sao Paulo, SP, Brazil). cDNA was synthesized from 2 mg of total RNA using a cDNA synthesis kit (Fermentas, Glen Burnie, MD, USA). The quantitative real-time polymerase chain reaction (qRT-PCR) was run to assess the messenger RNA (mRNA) levels of the target genes and reference gene (GAPDH) separately. Amplifications were performed with an ABI Prism 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) by using SYBR Green/High ROX qPCR Master Mix (Ampliqon, Denmark) and purity of the amplification products was confirmed using melting point dissociation curves. According to the manufacturer method, compared cycle threshold (Ct) values were expressed. GAPDH was considered as housekeeping gene and fold-change was determined using 2-∆∆Ct.
The primer sequences used in this study are shown in Table 1.
Table 1.
The primer sequences of genes
|
Gene name |
Gene ID
|
Forward Sequence
|
Reverse Sequence
|
| Mst1 |
24566 |
5՛-GCAATAGCTCAGTCCTTC-3՛ |
5՛-CTCGGTGTATATCTCACTCT-3՛ |
| Lats1 |
308265 |
5՛-TGTGATTGGTGGAGTGTTGGTG-3՛ |
5՛-TGAGGTGGGATGTGAAGAGAAG-3՛ |
| Yap1 |
363014 |
5՛-ACTGGAGGAGGATGGAGGGA-3՛ |
5՛-AGGGTGTTCTGGCTGATGGTGT-3՛ |
| MAP4K1 |
292763 |
5՛-GAGGCTGTGGGAATGGTTGGA-3՛ |
5՛-GTAGACGGAAGGTGAGGGTGG-3՛ |
| GAPDH |
108351137 |
5՛-AAGTTCAACGGCACAGTCAAGG-3՛ |
5՛-CATACTCAGCACCAGCATCACC-3՛ |
Western blot analysis
Homogenized left ventricles in RIPA buffer (cytomatingene) with a protease inhibitor cocktail (Sigma) centrifuged at 15 000 rpm for 10 minutes at 4°C and supernatants were collected to assess protein content by the Lowry method. Polyacrylamide gel electrophoresis (Bio-Rad) via 4%–20% gradient polyacrylamide gels containing 0.1% sodium dodecyl sulfate for 2 hours at 95 V was applied for electrophoresis and separated proteins were transferred to PVDF membranes (Roth) for 80 minutes at 80 V (Bio-Rad). Nonspecific sites were blocked overnight at 4°C in TBS containing Tween and 5% non-fat milk (Sigma). Membranes were incubated 2 hours with primary antibodies directed against the proteins of interest at room temperature. The protein abundance of Yap1 and Gapdh were determined in left ventricle samples. Following incubation, membranes were washed extensively with PBS-Tween and then incubated with secondary antibodies 1 hour at room temperature. Thereafter, membranes were developed using DAB and related images were captured and analyzed using the ImageJ software.
Histological analysis
All left ventricle tissues were fixed with formalin (10%), embedded in paraffin, and then, the samples were cut into sections (3 μm thick). To evaluate histological changes, the paraffinized sections were stained using hematoxylin and eosin (H&E). Images of the stained sections were taken (×400 magnification).
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM). Between-group differences were evaluated using independent Student’s t test and Mann-Whitney U test for normally and non-normally distributed data, respectively, using SPSS version 24. Statistical significance was accepted at P < 0.05.
Results
Findings of the present study indicate that compared with the control group, the eight-week endurance treadmill running at 15º slope had a positive effect on the left ventricular hypertrophy of rats in the exercise group which could be due to Mst1, Lats1, and Yap1 genes alteration.
The effect of exercise training on Hippo signaling-related genes
Left ventricular expression levels of Mst1, Lats1, and Yap1 genes were evaluated using qRT-PCR analysis to reveal the molecular phenotype of physiological cardiac hypertrophy related to Hippo signaling. The eight-week endurance training protocol caused a significant reduction in expression levels of Mst1 (P < 0.001) in the left ventricles of the exercise group compared with the control group (Fig. 2A). It has been demonstrated that MAP4Ks, as components of the expanded Hippo pathway, in parallel to Mst1/2 regulate the Lats1/2 and Yap/Taz. The results showed that there was a significant increase in MAP4k1 expression level compared with the control group (P = 0.001) after eight weeks of endurance exercise training (Fig. 2B). Despite the increased MAP4K1 expression, according to the Hippo signaling pathway and due to reduced Mst1 level, the Lats1 expression was significantly suppressed in the exercise group (Fig. 2C) compared with the control group (P = 0.001). It is noteworthy that the increased MAP4K1 expression level alone was not able to inhibit the Yap1 expression in the exercise group. As shown in Fig. 2D, following the suppression of Lats1, the expression levels of Yap1 increased significantly in the exercise group compared with the control group (P < 0.001).
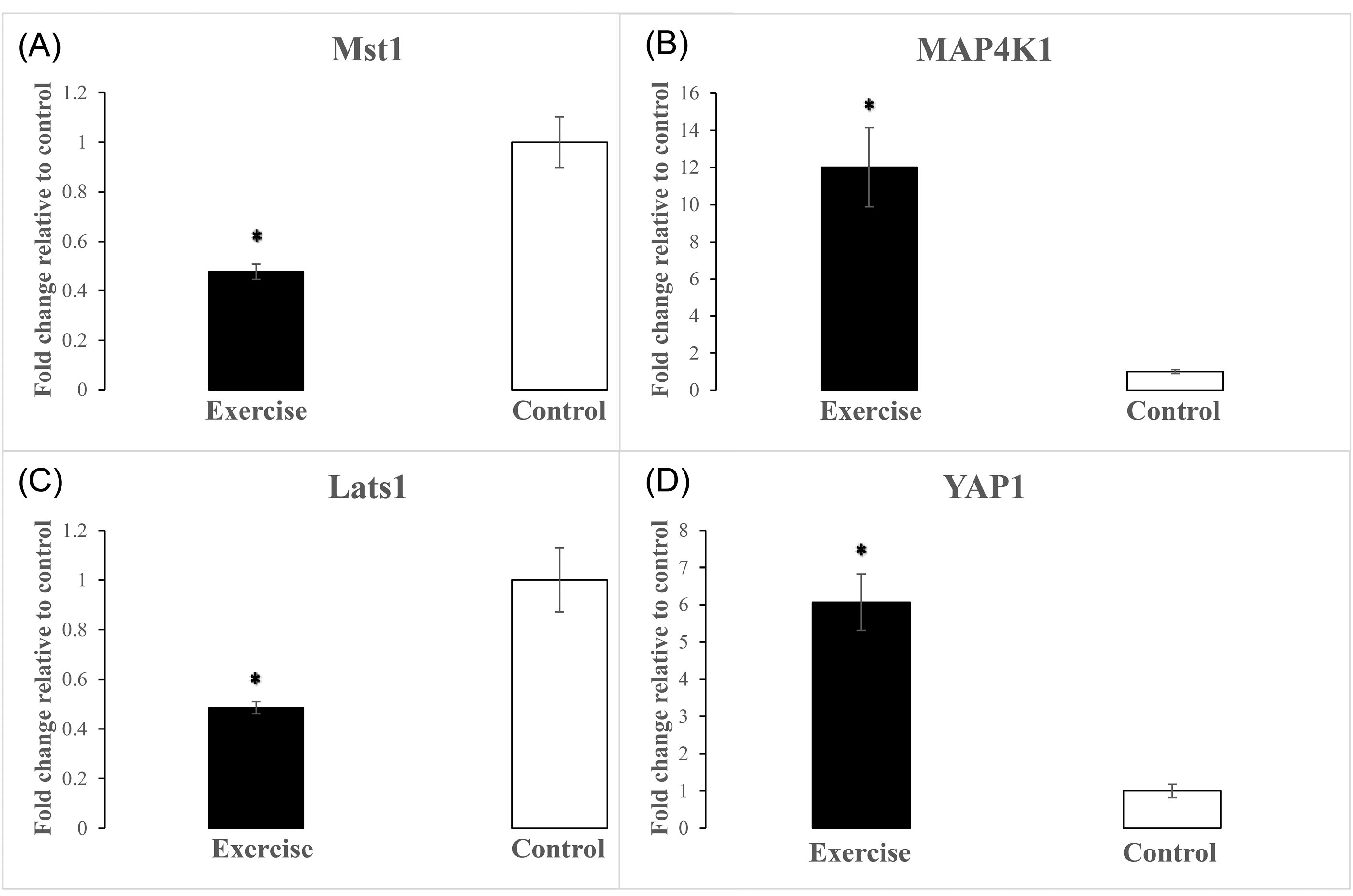
Fig. 2.
The effect of uphill-endurance treadmill running on alteration of left ventricular mRNA expression levels of Hippo signaling components. * Indicates a significant difference of Mst1 (A), MAP4K1 (B), Lats1 (C), and Yap1 (D) gene expression levels from the control group (n=7 per group). P ≤0.001.
.
The effect of uphill-endurance treadmill running on alteration of left ventricular mRNA expression levels of Hippo signaling components. * Indicates a significant difference of Mst1 (A), MAP4K1 (B), Lats1 (C), and Yap1 (D) gene expression levels from the control group (n=7 per group). P ≤0.001.
Also, Western blot analysis was used for more verification of Yap1 expression. The results confirmed a significant increase in Yap1 (P < 0.001) compared with the control group (Fig. 3A-B).
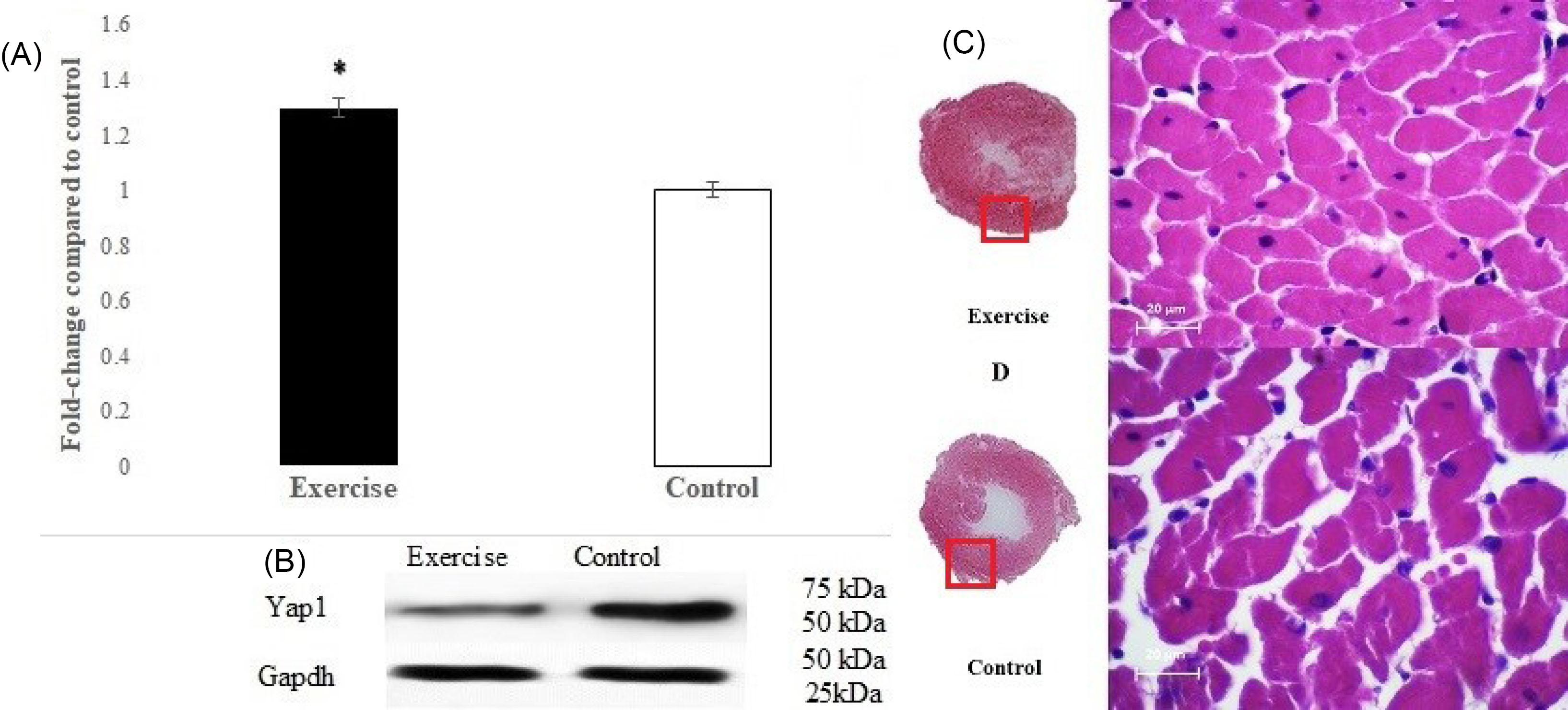
Fig. 3.
(A) Significant difference (P <0.001) from the control group in left ventricular Yap1 protein expression. (B) Protein expression levels of Yap1 in exercise and control groups, assessed by Western blotting, whereas Gapdh was used as a control for loading. (C and D) H&E stained left ventricle sections of rats in exercise and control groups. Compared with the control group, uphill-endurance treadmill running for eight weeks caused increased hypertrophy in the left ventricle that may partly be due to the alterations of Hippo signaling components. (n=7 per group)
.
(A) Significant difference (P <0.001) from the control group in left ventricular Yap1 protein expression. (B) Protein expression levels of Yap1 in exercise and control groups, assessed by Western blotting, whereas Gapdh was used as a control for loading. (C and D) H&E stained left ventricle sections of rats in exercise and control groups. Compared with the control group, uphill-endurance treadmill running for eight weeks caused increased hypertrophy in the left ventricle that may partly be due to the alterations of Hippo signaling components. (n=7 per group)
The effect of endurance exercise training on body, heart, and left ventricular weights and their ratio
The physiological characteristics of the rats are presented in Table 2. There were no significant between-group differences in body, heart, and left ventricular weights. The morphological cardiac changes including increased heart and left ventricular weight (7.37% and 7.90% respectively) and especially the heart weight/body weight and left ventricular weight/body weight ratios in the exercise group were significantly higher than the control group (9.38%; P = 0.024 and 9.64%; P = 0.034 respectively), indicating that the training protocol improved cardiac hypertrophy, which was confirmed by H&E staining. The results revealed that the left ventricular hypertrophy in the exercise group (Fig. 3C) is higher than the control group (Fig. 3D).
Table 2.
Physiological characteristics of the rats
|
Variables
|
Exercise (n=7)
|
Control (n=7)
|
P
value
|
% Change
|
| BW (g) |
321.57 ± 9.67 |
326.29 ± 7.55 |
0.707 |
- 1.45 |
| HW (mg) |
1010.71 ± 38.49 |
941.29 ± 27.25 |
0.167 |
7.37 |
| LVW (mg) |
694.43 ± 24.39 |
643.57 ± 13.18 |
0.092 |
7.90 |
| HW/BW% |
3.15 ± 0.09 * |
2.88 ± 0.04 |
0.024 |
9.38 |
| LVW/BW% |
2.16 ± 0.07 * |
1.97 ± 0.03 |
0.034 |
9.64 |
| LVW/HW% |
0.688 ± 0.012 |
0.685 ± 0.014 |
0.885 |
0.44 |
BW, body weight; HW, heart weight; LVW, left ventricular weight in exercise and control groups (n=7 per group). * indicates significant difference from control group. Data are presented as Mean ± SEM.
Discussion
To the best of our knowledge, this is the first study that examines the effect of exercise training on the Hippo signaling pathway to reveal the cellular signaling responsible for uphill-endurance treadmill running-induced physiological hypertrophy in cardiac tissue. Although no significant changes in HW/BW ratio after eight weeks of endurance treadmill running in mice has been reported,17 the significant increased HW/BW (9,38%, P = 0.024) and LVW/BW (9,64%, P = 0.034) ratios in our exercise group (Table 2) confirmed the physiological hypertrophy effects of endurance exercise on cardiac tissue.20 Protein synthesis and consequently physiological cardiac hypertrophy depend on the activation of systemic growth factors and related signaling pathways such as IGF-1/PI3K/Akt/mTOR.21 Also, Mst1/2, Lats1/1, and Yap/Taz, the mammalian core Hippo signaling components, are considered as important regulators of cell proliferation, differentiation, and organ growth. Given that cardiomyocyte hypertrophy is regulated by both Hippo and Akt-mTOR networks, the cross-talk between Hippo and Akt-mTOR signaling components has been studied, but in non-exercise training models. In this regard, it has been shown that Akt phosphorylates the Hippo signaling components to provoke the signaling induction responsible for cardiomyocyte hypertrophy.12,22 It has been shown that Akt phosphorylates Mst1, well known as an apoptosis component, to promote cell survival by protecting Mst1 from apoptotic cleavage,13 however, evidence gathered from some studies indicate that Mst1/2 are not essential for Lats1/2 activation.14 On the other hand, inactivated WW45 (Salv) which forms an active complex with Mst kinases, promoted cardiomyocyte proliferation and heart size.23 The result of the present study is consistent with those reports and showed that compared with the control group, Mst1 expression levels reduced in response to upstream regulators (Fig. 2A). Considering the Hippo signaling cascade, Mst is activated in response to upstream regulators and can phosphorylate Lats1/2. Phosphorylated Lats1/2, in turn, inhibits the transcriptional cofactors Yap and Taz by phosphorylating multiple serine residues.24 Apart from this, recently, it has been reported that Mst1/2 are not essential for the Lats1/2 activation, and also MAP4Ks are considered as alternative kinases for phosphorylating Lats1/2 and deletion of both MAP4Ks and Mst1/2 is necessary to suppress phosphorylation of Lats1/2 and Yap/Taz. In addition, the deletion of Lats1/2 abolishes Yap/Taz phosphorylation.14 Our results showed that following the reduction of Mst1 levels, Lats1 expression levels reduced compared with the control group (Fig. 2C), whereas, MAP4K1 expression level increase (Fig. 2B). Thus, the increased MAP4K1 expression levels concomitant with reduction of Mst1 confirm the necessity of both MAP4Ks and Mst1/2 deletion to suppresses phosphorylation of Lats1/2 and consequently Yap/Taz.14 There is convincing evidence that the Lats1 tumor suppressor can phosphorylate and inactivates the transcription regulator Yap, the major downstream effector of the mammalian Hippo pathway, in vitro and in vivo. Depending on phosphorylation site, overexpression of Mst/Lats leads to phosphorylation and inactivation of Yap oncogenic function. Phosphorylated Yap is sequestered and localized in the cytoplasm as a transcriptionally inactive form. Conversely, non-phosphorylated Yap exists in the nuclear and regulates gene expression by down-regulating or up-regulating many oncogenes or tumor suppressor genes to promote cardiomyocyte adaptation.25 It has been shown that inactivated lats1/2 and consequently Yap/Taz enhance cardiomyocyte regeneration in adult mice with myocardial infarction.26 Indeed, depending on the condition, Hippo pathway involves in both physiological and pathological hypertrophy. The effect of endurance exercise training on the alterations of Hippo components expression level and consequently cardiomyocyte hypertrophy has not been directly investigated but the role of Yap in muscle hypertrophy has been reviewed and suggested. Indeed, there is an interaction between the Hippo and Akt-mTOR pathways. In fact, overexpression of Yap/Taz, increases the phosphorylation of Akt to prevent cardiomyocyte apoptosis, and inversely, Yap is phosphorylated by Akt, for example in an exercise training condition, and thereby, its transcriptional role is suppressed. Also, Yap induces cardiomyocyte hypertrophy through the upregulation of miR-206.27,28 Recently, It has been shown that endogenous Yap has a critical role in hypertrophic and survival adaptive role to acute overload pressure.29 In this regard, increased HW/BW and LVW/BW ratios in our subjects (Table 2) are consistent with the result of a study that showed inactivated Hippo pathway components in the mouse heart, promoted cardiomyocyte proliferation and heart size23 and Yap has been identified as a major Hippo effector in cardiomyocyte development.30 Moreover, our findings support the view in which physical exercise, such as endurance running, cause adult’s cardiac growth31 and more intense uphill-treadmill running in the present study may be considered as a more powerful stimulator. Additionally, Western blots confirmed that Yap1 protein expression in the exercise group significantly increased compared with the control group (Fig. 3A-B). Increased Yap1 expression can lead to protein synthesis in the heart and consequently, cardiac hypertrophy which was occurred in the exercise group and confirmed by H&E staining (Fig. 3C-D).
Conclusion
The results of the present study showed that the increased Yap1 expression level induced by endurance treadmill running at 15º inclination caused cardiomyocyte hypertrophy in healthy rats. In accordance with Hippo signaling cascade, compared with Mst1, MAP4K1 does not affect the activation of Lats1 in response to exercise training. Cardiac hypertrophy and consequently enhanced cardiac contractility contribute to improving cardiac pump capacity that may lead to more blood and oxygen delivery to the heart itself and also other tissues like skeletal muscle32 which may help to promote individual and public health. Also, these findings may help to make a more effective exercise training program for rehabilitation and/or prevention of cardiovascular disease.
Funding sources
The present study was conducted with no external financial support.
Ethical statement
All experiments were reviewed and approved by the research ethics committee of sport sciences research institute of Iran.
Competing interests
There is no conflict of interest to be declared.
Authors’ contribution
MGH and AT have the same contribution in designing, conducting, data gathering, statistical analyzing, and writing the manuscript. MGH also reviewed and prepared the final version of the manuscript.
Research Highlights
What is the current knowledge?
simple
-
√ Heart is a vital organ in health promotion.
-
√ Genetic studies have established the role of Hippo pathway
in organ size regulation.
-
√ Its role in physiological cardiac hypertrophy has not been
investigated.
What is new here?
simple
-
√ Exercise training inhibited Mst1 expression levels, followed
by suppression of Lats1.
-
√ The significantly increased expression of MAP4K1 did not
influence Lats1 activation.
-
√ The exercise protocol increased Yap1 expression which was
confirmed by Western blotting.
-
√ Confirmed cardiac hypertrophy by H&E staining may
partly be due to increased Yap1.
-
√ These findings may help to make a more effective exercise
training program for rehabilitation and/or prevention of
cardiovascular disease.
References
- Olver TD, Ferguson BS, Laughlin MH. Molecular mechanisms for exercise training-induced changes in vascular structure and function: skeletal muscle, cardiac muscle, and the brain. Prog Mol Biol Transl Sci 2015; 135:227-257. doi: 10.1016/bs.pmbts.2015.07.017 [Crossref] [ Google Scholar]
- Shimizu I, Minamino T. Physiological and pathological cardiac hypertrophy. J Mol Cell Cardiol 2016; 97:245-262. doi: 10.1016/j.yjmcc.2016.06.001 [Crossref] [ Google Scholar]
- Maillet M, van Berlo JH, Molkentin JD. Molecular basis of physiological heart growth: fundamental concepts and new players. Nat Rev Mol Cell Biol 2013; 14:38-48. doi: 10.1038/nrm3495 [Crossref] [ Google Scholar]
- Ooi JY, Bernardo BC, McMullen J. The therapeutic potential of miRNAs regulated in settings of physiological cardiac hypertrophy. Future Med Chem 2014; 6:205-22. doi: 10.4155/fmc.13.196 [Crossref] [ Google Scholar]
- Stanger BZ. Organ size determination and the limits of regulation. Cell Cycle 2008; 7:318-24. doi: 10.4161/cc.7.3.5348 [Crossref] [ Google Scholar]
- Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem 2004; 279:32771-9. doi: 10.1074/jbc.M403528200 [Crossref] [ Google Scholar]
- Praskova M, Xia F, Avruch J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol 2008; 18:311-21. doi: 10.1016/j.cub.2008.02.006 [Crossref] [ Google Scholar]
- Callus BA, Verhagen AM, Vaux DL. Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. FEBS J 2006; 273:4264-76. doi: 10.1111/j.1742-4658.2006.05427.x [Crossref] [ Google Scholar]
- Chan EH, Nousiainen M, Chalamalasetty RB, Schäfer A, Nigg EA, Silljé HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene 2005; 24:2076-86. doi: 10.1038/sj.onc.1208445 [Crossref] [ Google Scholar]
- Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol 2008; 28:2426-36. doi: 10.1128/MCB.01874-07 [Crossref] [ Google Scholar]
- Hong W, Guan KL. The YAP and TAZ transcription co-activators: key downstream effectors of the mammalian Hippo pathway. Semin Cell Dev Biol 2012; 23:785-93. doi: 10.1016/j.semcdb.2012.05.004 [Crossref] [ Google Scholar]
- Cinar B, Fang PK, Lutchman M, Di Vizio D, Adam RM, Pavlova N. The pro-apoptotic kinase Mst1 and its caspase cleavage products are direct inhibitors of Akt1. EMBO J 2007; 26:4523-34. doi: 10.1038/sj.emboj.7601872 [Crossref] [ Google Scholar]
- Jang SW, Yang SJ, Srinivasan S, Ye K. Akt phosphorylates MstI and prevents its proteolytic activation, blocking FOXO3 phosphorylation and nuclear translocation. J Biol Chem 2007; 282:30836-44. doi: 10.1074/jbc.M704542200 [Crossref] [ Google Scholar]
- Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, Hansen CG, Hong AW. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat Commun 2015; 6:8357. doi: 10.1038/ncomms9357 [Crossref] [ Google Scholar]
- Jin H, Yang R, Li W, Lu H, Ryan AM, Ogasawara AK. Effects of exercise training on cardiac function, gene expression, and apoptosis in rats. AM J Physiol Circ Physiol 2000; 279:H2994-3002. doi: 10.1152/ajpheart.2000.279.6.H2994 [Crossref] [ Google Scholar]
- Kemi OJ, Loennechen JP, Wisløff U, Ellingsen Ø. Intensity-controlled treadmill running in mice: cardiac and skeletal muscle hypertrophy. J Appl Phyisol 2002; 93:1301-9. doi: 10.1152/japplphysiol.00231.2002 [Crossref] [ Google Scholar]
- Han GS. Endurance exercise effects on cardiac hypertrophy in mice. J Phys Ther Sci 2013; 25:1525-7. doi: 10.1589/jpts.25.1525 [Crossref] [ Google Scholar]
- Wisløff U, Helgerud J, Kemi OJ, Ellingsen Ø. Intensity-controlled treadmill running in rats: Vo2max and cardiac hypertrophy. Am J Physiol Heart Circ Physiol 2001; 280:H1301-10. doi: 10.1152/ajpheart.2001.280.3.H1301 [Crossref] [ Google Scholar]
- Kemi OJ, Haram PM, Loennechen JP, Osnes JB, Skomedal T, Wisløff U. Moderate vs high exercise intensity: differential effects on aerobic fitness, cardiomyocyte contractility, and endothelial function. Cardiovasc Res 2005; 67:161-72. doi: 10.1016/j.cardiores.2005.03.010 [Crossref] [ Google Scholar]
- Schaible TF, Scheuer J. Cardiac function in hypertrophied hearts from chronically exercised female rats. J Appl Physiol Respir Environ Exerc Physiol 1981; 50:1140-5. doi: 10.1152/jappl.1981.50.6.1140 [Crossref] [ Google Scholar]
- Kemi OJ, Ceci M, Wisloff U, Grimaldi S, Gallo P, Smith GL. Activation or inactivation of cardiac Akt/mTOR signaling diverges physiological from pathological hypertrophy. J Cell Physiol 2008; 214:316-21. doi: 10.1002/jcp.21197 [Crossref] [ Google Scholar]
- Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell 2003; 11:11-23. doi: 10.1016/S1097-2765(02)00776-1 [Crossref] [ Google Scholar]
- Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 2011; 332:458-61. doi: 10.1126/science.1199010 [Crossref] [ Google Scholar]
- Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF (beta-TRCP). Genes Dev 2010; 24(1):72-85. doi: 10.1101/gad.1843810 [Crossref] [ Google Scholar]
- Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem 2008; 283:5496-09. doi: 10.1074/jbc.M709037200 [Crossref] [ Google Scholar]
- Heallen T, Morikawa Y, Leach J, Tao G, Willerson JT, Johnson RL. Hippo signaling impedes adult heart regeneration. Development 2013; 140:4683-90. doi: 10.1242/dev.102798 [Crossref] [ Google Scholar]
- Gnimassou O, Francaux M, Deldicque L. Hippo pathway and skeletal muscle mass regulation in mammals: a controversial relationship. Front Physiol 2017; 8:190. doi: 10.3389/fphys.2017.00190 [Crossref] [ Google Scholar]
- Zhou W, Zhao M. How Hippo signaling pathway modulates cardiovascular development and diseases. J Immunol Res 2018; 2018:3696914. doi: 10.1155/2018/3696914 [Crossref] [ Google Scholar]
- Byun J, Del Re DP, Zhai P, Ikeda S, Shirakabe A, Mizushima W. Yes-associated protein (YAP) mediates adaptive cardiac hypertrophy in response to pressure overload. J Biol Chem 2019; 294:3603-17. doi: 10.1074/jbc.RA118.006123 [Crossref] [ Google Scholar]
- Von Gise A, Lin Z, Schlegelmilch K, Honor LB, Pan GM, Buck JN. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc Natl Acad Sci USA 2012; 109:2394-9. doi: 10.1073/pnas.1116136109 [Crossref] [ Google Scholar]
- Ellison GM, Waring CD, Vicinanza C, Torella D. Physiological cardiac remodelling in response to endurance exercise training: cellular and molecular mechanisms. Heart 2012; 98:5-10. doi: 10.1136/heartjnl-2011-300639 [Crossref] [ Google Scholar]
- Richardson RS. Oxygen transport: Air to muscle cell. Med Sci Sports Exerc 1998; 30:53-9. doi: 10.1097/00005768-199801000-00008 [Crossref] [ Google Scholar]