BioImpacts. 10(1):45-54.
doi: 10.15171/bi.2020.06
Original Research
Propagation of limbal stem cells on polycaprolactone and polycaprolactone/gelatin fibrous scaffolds and transplantation in animal model
Fatemeh Sanie-Jahromi 1  , Masoomeh Eghtedari 1, Esmaeil Mirzaei 2, Mohammad Hassan Jalalpour 1, Zahra Asvar 2, Mahmood Nejabat 1, *
, Masoomeh Eghtedari 1, Esmaeil Mirzaei 2, Mohammad Hassan Jalalpour 1, Zahra Asvar 2, Mahmood Nejabat 1, *  , Fahimeh Javidi-Azad 3
, Fahimeh Javidi-Azad 3
Author information:
1Poostchi Ophthalmology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
2Department of Medical Nanotechnology, School of Advanced Medical Sciences and Technologies, Shiraz University of Medical Sciences, Shiraz, Iran
3National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran
Abstract
Introduction:
This study was conducted to compare the effect of nanofibrous polycaprolactone (PCL) and PCL/gelatin (PCL/Gel) on limbal epithelial stem cell (LESC) and its efficiency for transplantation in animal model.
Methods:
PCL and PCL/Gel with a mass ratio of 70:30 and 50:50 was fabricated by electrospinning method. Human LESCs were cultured on PCL and PCL/Gel scaffolds and the effect of each scaffold on LESC proliferation, attachment and corneal epithelial regeneration in an animal model was evaluated, considering ease of use of scaffold and final transparency of the cornea.
Results:
Our data showed that PCL was more suitable than PCL/Gel for LESCs adherence, induction of epithelial morphology and proliferation. Histopathologic analysis of corneal sections from transplanted animals showed that epithelium was regenerated almost similar in PCL and PCL/Gel groups; however, vascularization and inflammation were significantly lower in the group receiving PCL.
Conclusion:
The represented data indicated the priority of PCL to PCL/Gel for the LESC attachment, proliferation and final outcome in an animal model of alkaline injury. This finding might be promising for cell therapy of corneal diseases.
Keywords: Fibrous scaffold, Gelatin, limbal epithelial stem cell, Limbal stem cell deficiency, Polycaprolactone, Transplantation
Copyright and License Information
© 2020 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Limbal stem cell deficiency (LSCD) is a corneal abnormality, in which limbal epithelial stem cells (LESCs) are injured or become dysfunctional. Stem cells of the limbal region play a pivotal role in corneal epithelium renewal, and LSCD might lead to chronic epithelial defects, corneal neovascularization, pain, and diminished vision.
1,2
Several hypothetical molecular markers are reported for the LESCs definition. Cytokeratin 19 and vimentin, which are intermediate filaments co-localized in limbal basal cells, are commonly examined for the LESCs definition.
3
Another marker of the LESCs is CD44, a homing-associated cell adhesion molecule (H-CAM) and according to previous studies is one of the powerful tools for molecular characterization of LESCs.
4
P63 transcription factor is also recognized as a prominent and new marker expressed in the nucleus of the LESCs that are located in the basal layer of limbal epithelium.
5
ABCG2 transporter protein is another identifying character of LESCs, however, its expression in a small proportion of limbal basal epithelial cells has been reported.
6
Limbal tissue transplantation is the chief LSCD treatment. However, lack of a suitable donor is the main limitation of this technique.
7
Transplantation of cultivated LESCs can resolve this issue and has been shown to be an optimal strategy for treatment of LSCD.
8
Different surface modifications and scaffolds have been used to improve adherence and facilitate cells transfer to damaged cornea.
9,10
Although natural scaffolds such as amniotic membrane (AM) can provide a suitable extracellular matrix for cell adherence, there are some restrictions associated with their application, including their availability, possible transfer of unknown viral infections and poor surgical stability.
11,12
Electrospun polycaprolactone (PCL) is a synthetic biodegradable scaffold, extensively used to cultivate different cell types.
13
It mimics the natural extracellular matrix in terms of the surface to volume ratio and high porosity and can be handled easily.
9,14
In another study, PCL biocompatibility and absence of immunological response after degradation has been reported.
15
PCL blending with gelatin (Gel) has been attractive to researchers as Gel is a natural component and might be suitable for improving PCL biological properties. Gel is derived from collagen type I, which is part of the extracellular matrix.
16
Gel is a readily available and inexpensive natural polymer characterized by low toxicity and inflammatory response.
17
However, there are controversial results on the effect of PCL/Gel blend on different cell types. In some studies, it was reported that PCL blending with Gel may enhance cell adherence.
18,19
There are also other reports indicating that Gel could not lead to a significant change in the biological properties of the PCL.
20
In this study, the effect of PCL and PCL/Gel on LESC was evaluated. To the best of the authors' knowledge, this is the first study on the PCL/Gel application in the LESCs culture. We compared PCL and PCL/Gel in terms of the LESCs adhesion and proliferation, as well as biocompatibility on the eye and ease of transfer during surgery hoping to introduce a better scaffold for the LESCs culture.
Materials and Methods
Scaffold preparation and analysis
The scaffolds were prepared by the electrospinning method according to previous reports with slight modification, to obtain a stable electrospinning process.
21,22
According to our preliminary study, the PCL and PCL/Gel solution with 6 wt. % concentration resulted in a stable electrospinning process. PCL (PURASORB PC 12, 1.2 dL/g inherent viscosity, Corbion) and Gel (type B from bovine skin, Sigma) were dissolved separately in hexafluoroisopropanol (HFIP) for 1 hour at 50°C under magnetic stirring to obtain homogenous and clear solutions with 6 wt % concentration. PCL solution (6 wt %) was mixed with Gel solution (6 wt %) under magnetic stirring to produce PCL/Gel solution with a ratio of PCL to Gel 70:30 and 50:50. The electrospinning was done using an electrospinning apparatus (ANSTCO RN-X, Asian Nanostructures Co, Tehran, Iran). The solutions were filled in a 10 mL syringe and it was connected to a 21-G metallic cut-ended needle as a nozzle. The syringe was placed in a pump and the needle was connected to a high voltage source. An aluminum foil wrapped around a rotating grounded drum (10-cm diameter and 8 cm width) was used as a collector. The applied voltage, nozzle to collector distance and feed rate were set as 14 kV, 13 cm, and 1.5 mL/h, respectively. The drum speed was 500 rpm and the electrospinning temperature was 30°C.
The prepared scaffolds were dried at 40°C for at least 24 hours before any further investigations. In order to disinfect the prepared scaffolds, UV exposure was applied for 30 minutes. The substrates were incubated in complete growth medium for 45 minutes at 37°C before cell culture.
The scanning electron microscopy (SEM) was utilized to evaluate the morphology of electrospun scaffolds using TESCAN-Vega 3 (Czech Republic) after the sputter coating with gold-palladium. The fiber diameter and pore size were measured by using the Image J software on SEM micrographs at 30 random locations.
23
The contact angle measurement was done to investigate the wettability of scaffolds. Drops of ultra-pure water were dropped on each sample and the contact of the droplet with the sample surface was measured using an optical contact angle system measurement system (CA-500A, IRASOL, Iran). The tensile strength of samples was measured according to a previous study.
13
Briefly, the samples were cut to rectangular sheets (3×1 cm) using a plastic frame with the same dimensions. The thicknesses of samples were measured using a digital micrometer (Beta 1658DGT/25). The prepared specimens were put between the clamps of a universal testing machine (Zwick/Roell, Z020, Germany) and were tensioned at a rate of 10 mm.min-1 until failure.
Fourier transform infra-red (FTIR) spectroscopy was done to investigate the presence of both polymers and possible interactions between PCL and gelatin in PCL/Gel scaffolds. The FTIR spectra were obtained using FTIR spectroscopy (PerkinElmer, Spectrum RXI). Samples were scanned from 400 cm−1 to 4000 cm−1 wavenumber range for 32 times and data was collected with a 2 cm−1 spectral resolution.
Cell culture
LESCs were isolated from corneoscleral rims that were left after transplantation. Surgeons were asked to cut the cornea on another sterile tray before transferring it to the surgical field. Then, the ring-shaped rest of the button was returned to the media. Samples were immediately transferred to the lab under the sterile condition and washed with 3 mL of PBS. The surrounding sclera and conjunctiva were removed and the inner rim surface was scratched. The anterior part of the limbus was cut into 2×2 mm2 pieces and transferred to a 6-well culture plate (Orange) in a way that the epithelial side was faced up. After 15 minutes of incubation at room temperature, DMEM/F12 (BioIdea) supplemented with 10% fetal bovine serum (Gibco), 100 U/mL penicillin (Shellmax) and 100 µg/mL streptomycin (Shellmax), was added to the plate and incubated at 37°C with 5% CO2. Fresh medium was added to the plate every 3 days and the whole medium was replaced once a week. A total of 80% confluence cultures were used for further analysis.
Immunocytochemistry
Immunocytochemistry was used to identify the cytokeratin 19 and vimentin markers of LESCs expanded from limbal specimens. LESCs were explanted on glass coverslips and cultured in DMEM/F12 supplemented with 10% fetal bovine serum. When LESCs were expanded on the coverslip (usually after 14-21 days) the explanted piece of tissue was removed and coverslips were washed with PBS. LESCs were then fixed and permeabilized with −10°C methanol (Merck) for 5 minutes and then blocked with bovine serum albumin (BSA, Tiba Biotechnology Co.) in PBS (1% w/v) for 20 minutes at room temperature. The cells were incubated with primary antibodies for 1 hour at room temperature. Primary antibodies including cytokeratin 19 and vimentin (both from Santa Cruz, USA) were added in a dilution of 1:50 in 1% BSA in PBST (PBS in Triton X100). The cells were washed 3 times in PBS and incubated with goat anti-mouse IgG-FITC secondary antibody (1:100 in 1.5% BSA in PBST) for 45 min at room temperature in dark. Nuclei were stained with 1 mg/ml of DAPI (Santa Cruz) and mounting media (90% glycerol, 10% PBS) was used to mount the coverslip on the slide. Immunostained samples were observed under a fluorescent microscope (Olympus Bx6) with 420 nm filter for DAPI and 510 nm filter for FITC.
Flow cytometry
The expression of CD44, P63 and ABCG2 markers (as stemness markers of LESCs), was evaluated using flow cytometry. The cultured LESCs were harvested and re-suspended in PBS. Then the cells were rinsed with cold wash buffer (PBs containing 2% FBS and 0.1% sodium azide (Merck). Cells that were to be marked with surface antibodies (CD44 and ABCG2) were incubated with PE Mouse Anti-Human CD44 and APC-conjugated mouse anti-human ABCG2 (BD Pharmingen Inc) for 30 min at room temperature in dark. Cell staining with isotype-matched irrelevant antibodies (BD Pharmingen) was also performed. For intracellular staining cells were fixed with 1% cell fix for 5 minutes at 4°C and washed with 1 mL of ice-cold PBS. Then 0.2% saponin was added and incubated for 10 minutes. Then cells were centrifuged and incubated on ice with rabbit anti-human P63-α primary antibody for 1 h. Afterward, cells were washed twice with PBS and incubated with 10 μL FITC-conjugated goat anti-rabbit (Santa Cruz) secondary antibody for 30 minutes at room temperature in dark. Then cells were rinsed and analyzed with FACS CALIBUR flow cytometer (Becton Dickinson, Franklin Lakes, USA). Final data were analyzed by version 7.6 of FlowJo® software. Unstained controls were used as negative controls in the histograms to detect the percentage of positive cells.
Cell expansion on scaffolds, cytomorphological and SEM analysis
When the cultures reached 80% confluence, LESCs were trypsinized and seeded on PCL, PCL/Gel (70:30) and PCL/Gel (50:50) scaffolds at a density of 10*5 cell cells on circular pieces of each scaffold (17 mm in diameter) and incubated at 37°C. After 48 hours, a scaffold from each group was fixed in ethanol, stained with hematoxylin and eosin and checked under the light microscope for cell adhesion, density, and morphology. For SEM analysis of cell attachment and morphology on the scaffolds, LESCs were cultured on PCL, PCL/Gel (70:30) and PCL/Gel (50:50), after 24 hours, the scaffolds were washed with PBS and transferred to -80°C for another 24 hours. Thenceforward the specimens were dehydrated and freeze-dried utilizing a vacuum pump (Alpha 1-2 LD plus, Christ, Germany) for 48 hours at room temperature. Samples were then coated with gold and analyzed by SEM (TESCAN-Vega 3, Czech Republic).
MTT assay
MTT assay was utilized to analyze the effect of each scaffold on LESCs proliferation. LESCs were cultured at a density of 2.0×104 cells/well in 48 well microplates (Jet Biofil) on PCL, PCL/Gel (70:30) and PCL/Gel (50:50) scaffolds and were incubated at 37°C for 48 hours. LESCs were also cultured in the same way on a well without scaffold and used as a negative control. Next, 10 µL of MTT (GoldBio), 5 mg/mL in phosphate buffer saline) was added to each well and microplates were incubated for an additional 4 hours. Then each well-received 100 µL of 10% SDS (Parstous) in 0.01 M HCL and incubated overnight. The formazan crystals were extracted and solved using SDS. Acidic SDS can penetrate the fibrous scaffold and dissolve all crystals even the deposited ones. After gently shaking the plate, the scaffolds were removed from each well, the supernatant of each well (200 µL) was transferred to a 96 well microplate and the absorption rate (at 545 nm) was obtained using a microplate reader (BMG LABTECH).
24-26
The percentage of LESCs viability on PCL/Gel 70:30 and 50:50 scaffolds was calculated relative to PCL.
Surgical procedure
The prepared limbal stem cell substrates on the PCL and PCL/Gel scaffolds proceeded for use in animal transplantation to identify the ability of cultured LESCs on the scaffolds to regenerate the defect of the corneal surface. In addition, LESCs cultured on the AM was also used to compare the efficacy of prepared scaffolds with AM. Twelve eyes were divided into the following groups: the control, PCL, PCL/Gel (70:30) and PCL/Gel (50:50); each included 3 eyes.
Induction of corneal surface burn
Alkali burn was induced on the ocular surface of the right eye of 6-month-old New Zealand rabbits weighing 2.5-3 kg under general anesthesia induced by ketamine (35 mg/kg) and xylazine (5 mg/kg) and topical use of tetracaine drop. Circle-shaped filter papers (20 mm in diameter) soaked in 0.5 mol/L NaOH were placed on the cornea for about 60 seconds. Then the ocular surface was rinsed with buffered saline until pH value regains its normal value. Animals were under standard care according to the protocol approved by the Ethics Committee of Shiraz University of Medical Sciences. After 3 weeks, the presence of LSCD was evaluated clinically for the presence of surface irregularity and loss of clarity and luster. Eyes who developed infective keratitis or healed completely with remaining clear cornea were excluded and replaced by another case (repeated procedure).
Transplantation of scaffolds and AM
Under general anesthesia, corneal epithelium was removed with a surgical knife, and 180° superior peritomy was performed, then scaffolds were secured on the ocular surface (epithelial side down) by suturing the scaffold lower margin to the cornea and the upper margin to the conjunctiva with interrupted silk 7.0 sutures. Amniotic membrane was placed on one model eye after the same procedure of alkali burn induction to compare the results of scaffolds with AM. Control eyes received no surgical treatment. The examination was done weekly for the evaluation of the transplant and status of the corneas. Eyes were received topical antibiotics three times a day. All eyes were enucleated after 6 weeks and sent for histopathological study.
Histopathological analysis
After enucleation, eyes were fixed in 10% formaldehyde for 48 hours, then anterior caps were removed and the mid corneal cut was performed and stained with hematoxylin and eosin after routine processing. Slides were viewed under the light microscope for surface epithelialization, stromal integrity and vascularization, as well as inflammation. The corneal inflammation was scored as 0: No inflammation; 1: Focal, low number of mixed type inflammatory cells (lymphocyte, neutrophil leukocyte, eosinophil leukocyte); 2: Cases between 1 and 3; and 3: Diffuse, intense, mixed-type inflammatory cells.
27
Statistical analysis
SEM micrographs were analyzed using the ImageJ software at several random locations.
23
FlowJo® software was used to analyze the final data of flow cytometry. Repeated measurement was performed on three samples of in vivo analysis in order to conclude the final results. MTT experiments were done in triplicate and data were shown as means ± standard error (SE). One-way analysis of variance (ANOVA) following by a Duncan post hoc test was used to analyze the significance of the difference. SPSS statistical software package (v. 20, SPSS Inc., Chicago, IL, USA) was utilized to perform the statistical analysis. A probability of P<0.05 was considered statistically significant.
Results
Characterization ofelectrospunnanofibrous scaffolds
The fibrous scaffold of PCL and PCL/Gel blend scaffolds with a mass ratio of 50:50 and 70:30 (PCL/Gel) were successfully prepared by electrospinning using HFIP as a solvent. As illustrated in Fig. 1, The PCL scaffold showed a uniform and smooth worm-like fibers with an average fiber diameter of 1356 ± 200 nm and a pore size of 4.7±1.6 µm (Table 1). Some fusion of traverse and adjacent fibers and formation of point bonding (arrows in Fig. 1) was also observed in the PCL scaffold. Bead-free and smooth fibers with fiber binding were also formed for PCL/Gel scaffolds, but in this case, the uniformity of fibers was less. The presence of large fibers along with small ones was observable in PCL/Gel scaffolds. On the other hand, fibers average diameter was decreased when gelatin was added to PCL. Unlike fiber diameter, the average pore size of scaffolds was increased when gelatin was added (Table 1). Fiber diameter was 910 ± 450 nm and 948 ± 440 nm for PCL/Gel 70:30 and 50:50, respectively. The non-uniformity and wide size distribution of fibers in PCL/Gel scaffolds may be due to phase separation on the electro-spinnability of PCL/Gel/HFIP solutions.
28,29
Table 1.
Fiber diameter, pore size and contact angles of scaffolds
|
Sample
|
Fiber diameter (nm)
|
Pore size (µm)
|
Contact angle (°)
|
| PCL |
1356±200 |
4.7±1.6 |
130 |
| PCL/Gel (70:30) |
910±450 |
5.3±1.4 |
60 |
| PCL/Gel (50:50) |
948±440 |
5.1±1.4 |
0 |
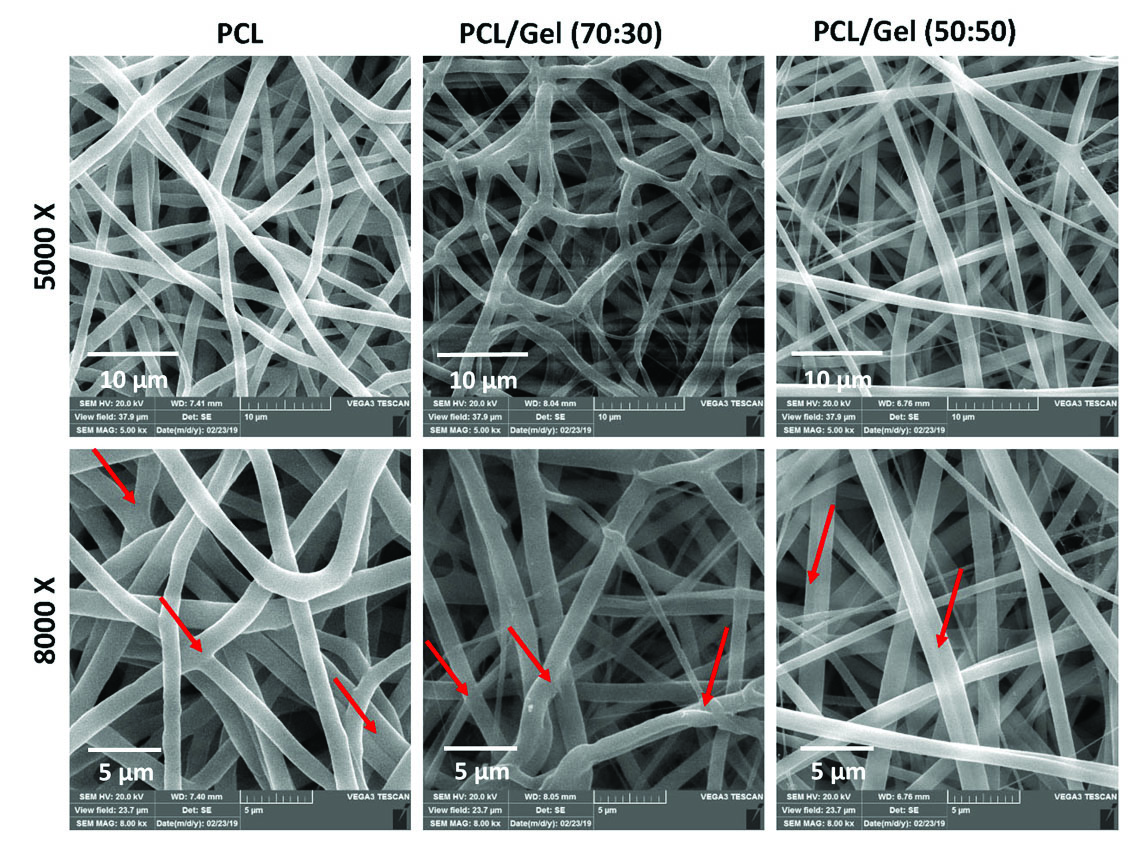
Fig. 1.
Characterization of the engineered AuNPs-PEG-RNase A nanobiosystem.(A)FT-IR spectra of SH-PEG-COOH. (B)DLS measurement of AuNPs-PEG, and (C) AuNPs-PEG-RNase A nanobiosystem. (D) SEM image of AuNPs-PEG-RNase A. (E) UV absorption spectra of AuNPs, AuNPs-PEG, and AuNPs-PEG-RNase A nanobiosystem.
.
Characterization of the engineered AuNPs-PEG-RNase A nanobiosystem.(A)FT-IR spectra of SH-PEG-COOH. (B)DLS measurement of AuNPs-PEG, and (C) AuNPs-PEG-RNase A nanobiosystem. (D) SEM image of AuNPs-PEG-RNase A. (E) UV absorption spectra of AuNPs, AuNPs-PEG, and AuNPs-PEG-RNase A nanobiosystem.
The wettability of scaffolds was investigated using contact angle measurement. The contact angles for pure PCL and PCL/Gel scaffolds are represented in Table 1. The pure PCL showed a contact angle of 130° which indicates a hydrophobic surface. Adding 30% and 50% gelatin to PCL resulted in a remarkable decrease in contact angle. The contact angle for PCL/Gel (70:30) and PCL/Gel (50:50) was 60° and 0°, respectively. The lower contact angle for PCL/Gel scaffolds indicates higher wettability of these scaffolds. PCL is inherently a hydrophobic polymer, while gelatin is a very hydrophilic polymer. Adding gelatin to PCL scaffolds increases the hydrophilicity of scaffold.
Fig. 2 shows the FTIR spectra of PCL and PCL/Gel nanofibrous scaffolds. PCL scaffold showed PCL characteristic bands at 1721 cM-1 (carbonyl stretching), 1293 cM-1 (C–O and C–C stretching), 1240 cM-1 (asymmetric C–O–C stretching) and 1165 cM-1 (symmetric C–O–C stretching).
30
All of these PCL characteristic bands were also observed in PCL/Gel scaffolds without any shift or change expect decrease in the peaks intensity. The PCL/Gel scaffolds also showed common bands of protein at approximately 1650 cM-1 (amide I) and 1537 cM-1 (amide II), corresponding to the stretching vibrations of the C=O bond, and coupling of bending of N–H bond and stretching of C–N bonds, respectively. The amide I band at 1650 cM-1 was attributable to both a random coil and a-helix conformation of gelatin.
21
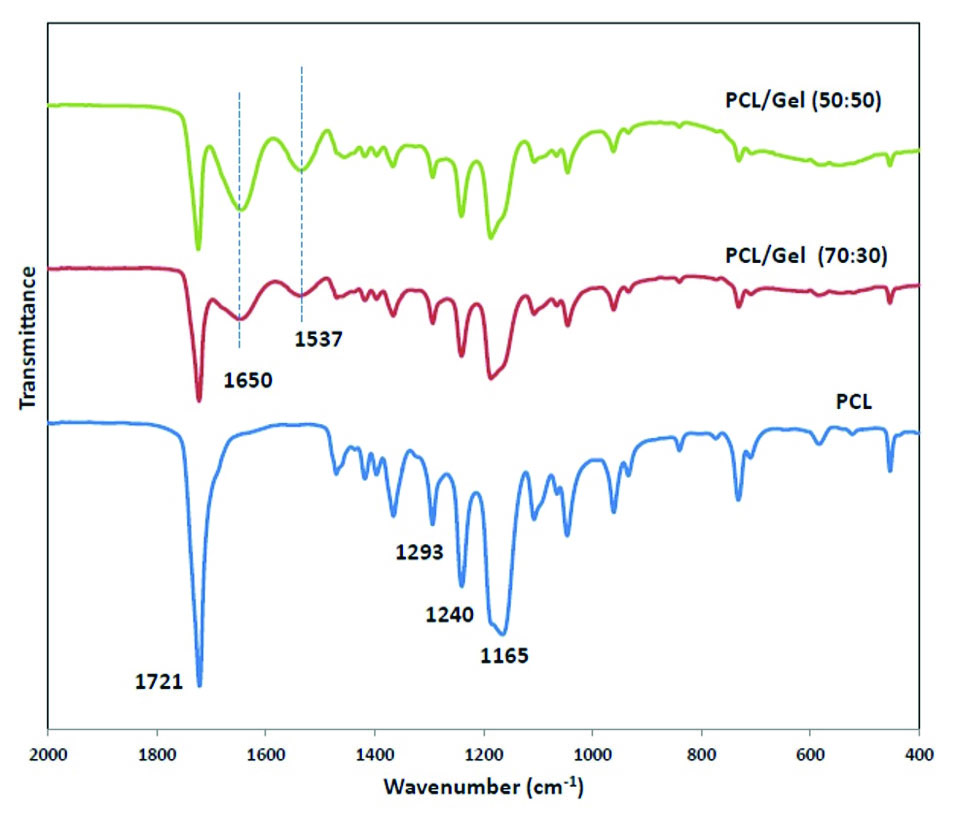
Fig. 2.
Confirmation of AuNPs-PEG-RNase A conjugates and enzyme activity. (A) SDS-PAGE analysis of AuNPs-PEG-RNase A conjugates. (B) RNase A activity assay in the presence of different concentrations of RNA in free and conjugated forms using Uv-vis. Ez: RNase A.
.
Confirmation of AuNPs-PEG-RNase A conjugates and enzyme activity. (A) SDS-PAGE analysis of AuNPs-PEG-RNase A conjugates. (B) RNase A activity assay in the presence of different concentrations of RNA in free and conjugated forms using Uv-vis. Ez: RNase A.
The resemblance of PCL characteristic bands in PCL/Gel scaffolds compared with PCL scaffold indicate no interaction between PCL and gelatin, which might be due to phase separation on the PCL/Gel/HFIP solutions.
28,29
Tensile strength and maximum elongation for pure PCL scaffold were 1.7 MPa, and 55 %, respectively. Gel addition to PCL leads to an increase in tensile strength of scaffolds. However, the maximum elongation was decreased following gelatin addition (Fig. 3). A similar trend was also reported in a study by Qian et al.
31
The reduction in fiber diameter could be a reason for enhancement in tensile strength of scaffolds after gelatin addition.
31
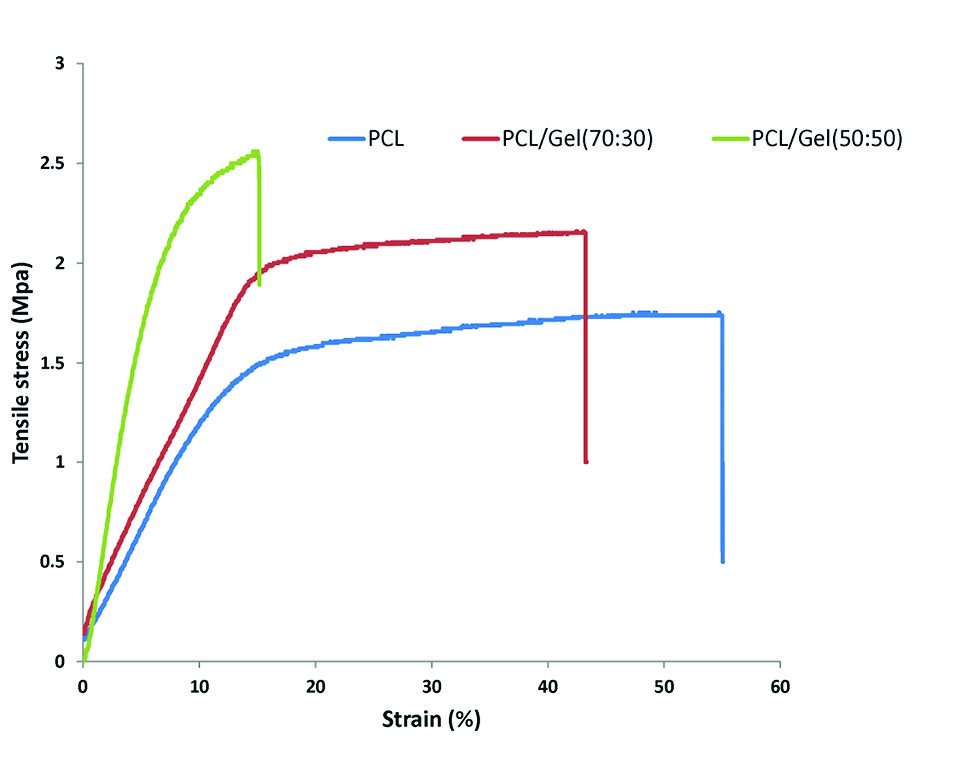
Fig. 3.
Cell viability assay of the SW-480 cell line in different concentrations (0-300 µg/mL) of (A) RNase A, (B) AuNPs, (C) AuNPs-PEG, and (D) AuNPs-PEG-RNase A nanobiosystem after 24, 48, and 72 hours. The data present means ±SD of three separate experiments. Quantification is given from three independent experiments. UT: untreated groups. *P< 0.05, and **P < 0.01.
.
Cell viability assay of the SW-480 cell line in different concentrations (0-300 µg/mL) of (A) RNase A, (B) AuNPs, (C) AuNPs-PEG, and (D) AuNPs-PEG-RNase A nanobiosystem after 24, 48, and 72 hours. The data present means ±SD of three separate experiments. Quantification is given from three independent experiments. UT: untreated groups. *P< 0.05, and **P < 0.01.
Cell culture and limbal stem cell marker expression
Explant cell culture was performed and LESCs migrated from the explant and formed a characteristic monolayer on the plate surface approximately within the second week of the culture period. Microscopic image of the representative cobblestone morphology of LESCs is shown in Fig. 4A. Flow cytometry and ICC analysis of LESCs markers were done in LESCs cultured on plate surface to be sure that the cells which are to be transferred on the scaffolds are expressing limbal stem cell markers. Our data indicated that LESCs expressed stem cell markers. Flow cytometry test confirmed the CD44 (17.2%), P63 (71.5%) and ABCG2 (10.2%) expression in LESCs (Fig. 4B). Immunocytochemistry analysis also confirmed the cytokeratin 19 and vimentin expression as LESCs markers (> 95%, Fig. 4C-H).
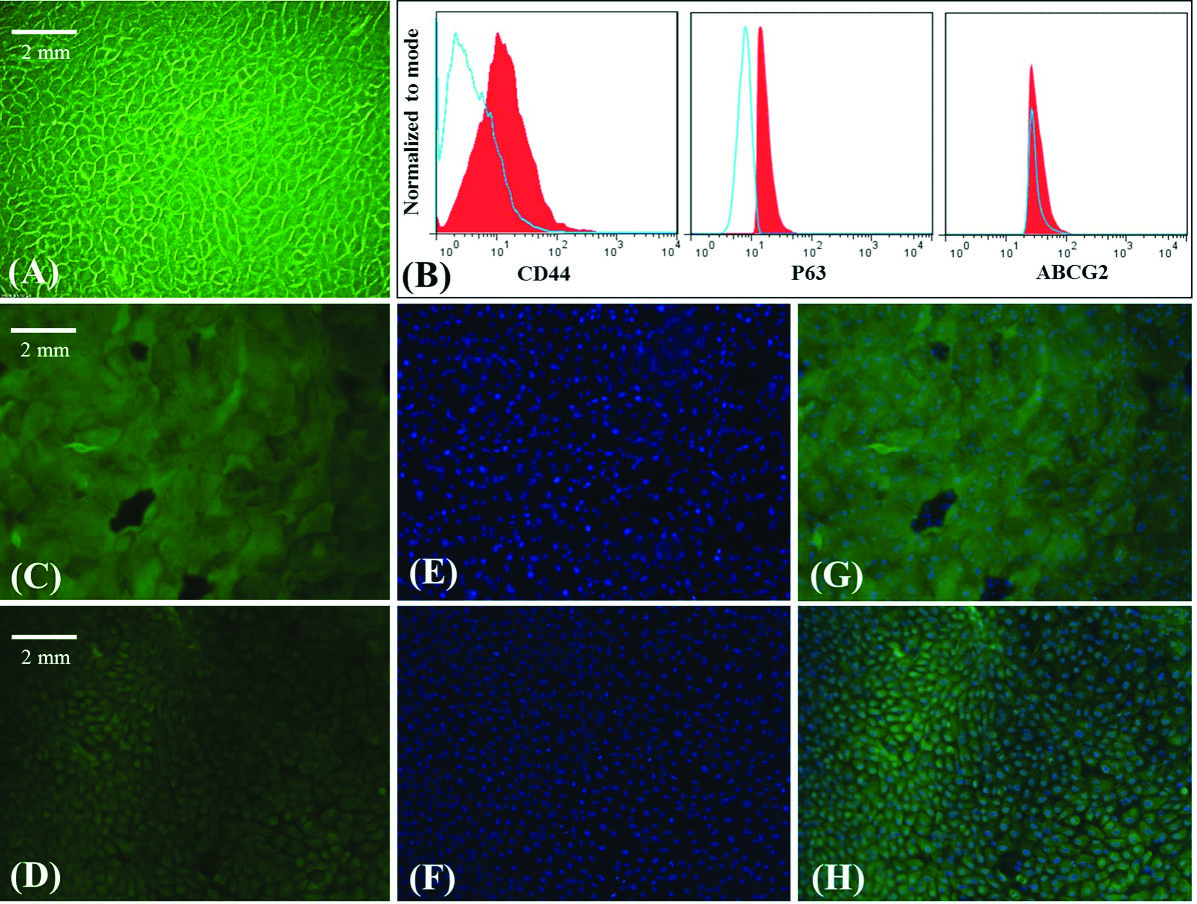
Fig. 4.
Apoptosis assay by DAPI staining of SW-480 nucleus. (A) control group, (B) treated with AuNPs, (C) AuNPs-PEG, (D), free RNase A, and (E, F) AuNPs-PEG-RNase A nanobiosystem. Red arrows depict chromatin condensation and fragmented nuclei.
.
Apoptosis assay by DAPI staining of SW-480 nucleus. (A) control group, (B) treated with AuNPs, (C) AuNPs-PEG, (D), free RNase A, and (E, F) AuNPs-PEG-RNase A nanobiosystem. Red arrows depict chromatin condensation and fragmented nuclei.
SEM and cytomorphological assessment of LSCs cultured on the scaffolds
Fig. 5 shows the SEM photomicrographs of LSCs cultured on the PCL, PCL/Gel (70:30) and (50:50). LSCs attached to the PCL scaffold are more numerous and larger in size compared with PCL/Gel scaffolds (Fig. 5A). However, in PCL/Gel scaffolds (70:30 and 50:50) only small sphere-shaped single cells were observed according to SEM micrographs (Fig. 5B and C). The cytomorphological study of limbal stem cell attachment using H&E staining showed that cell attachment occurred on PCL and PCL/Gel scaffolds; however, PCL seemed to be more appropriate for supplying cell adherence and LESCs induction of epithelial morphology (Fig. 6A-F).
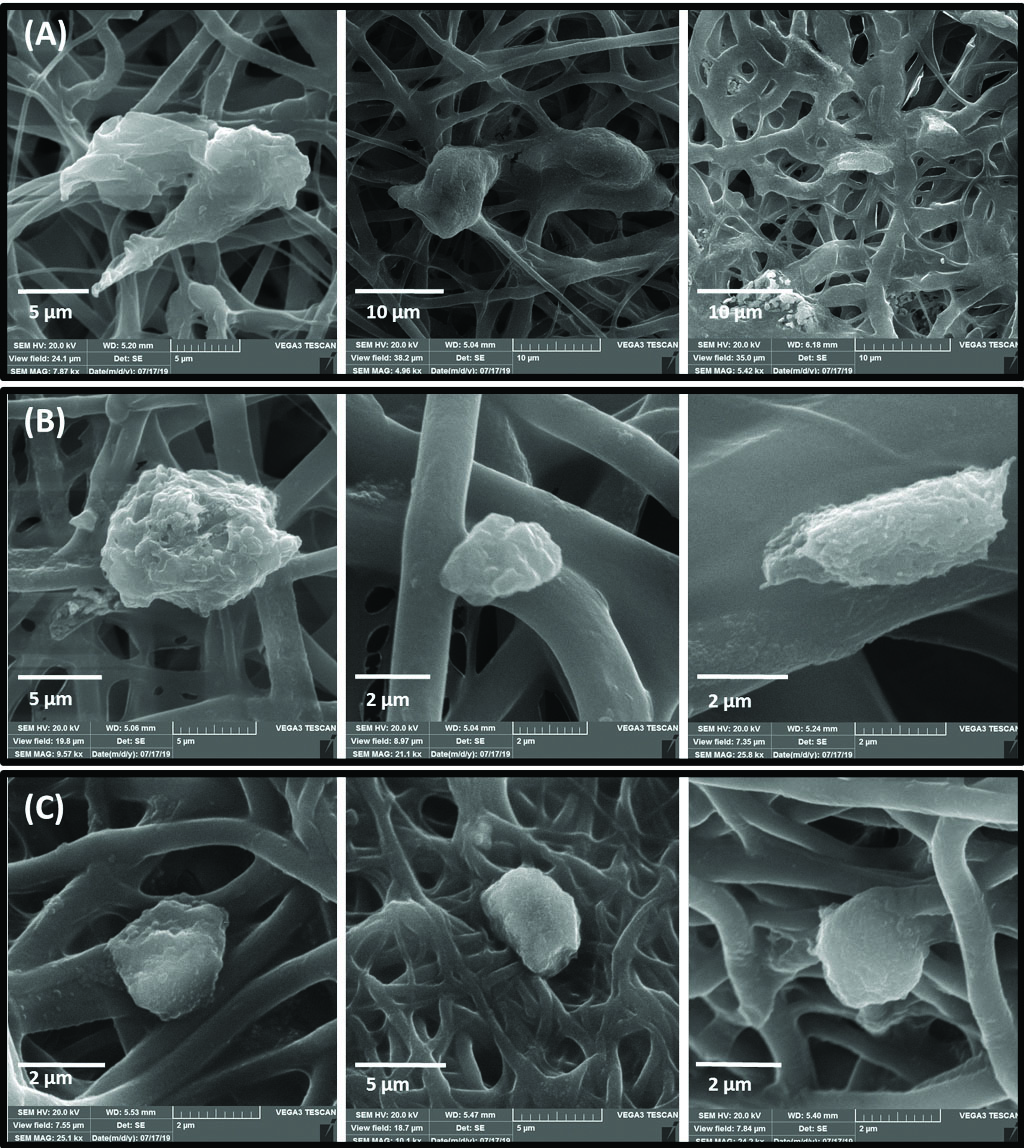
Fig. 5.
SEM micrographs of LESCs cultured on PCL (A panel), PCL/Gel 70:30 (B panel) and PCL/Gel 50:50 (C panel)
.
SEM micrographs of LESCs cultured on PCL (A panel), PCL/Gel 70:30 (B panel) and PCL/Gel 50:50 (C panel)
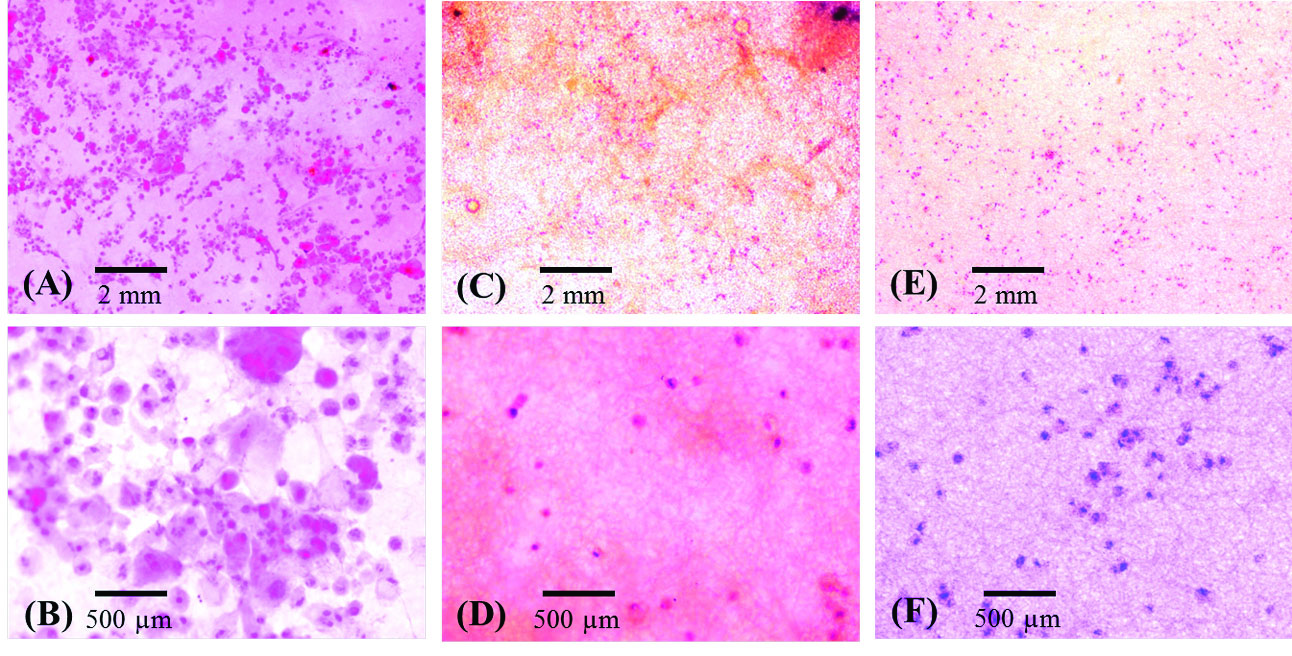
Fig. 6.
Cytomorphological study of limbal stem cells cultured on PCL (A & B), 70:30 PCL/Gel (C & D) and 50:50 PCL/Gel (E & F). Note the epithelial
morphology in B, lack of desirable attachment and proliferation observed in D. (A-F) H&E staining (×100 in A, C and E; ×400 in B, D and F).
.
Cytomorphological study of limbal stem cells cultured on PCL (A & B), 70:30 PCL/Gel (C & D) and 50:50 PCL/Gel (E & F). Note the epithelial
morphology in B, lack of desirable attachment and proliferation observed in D. (A-F) H&E staining (×100 in A, C and E; ×400 in B, D and F).
LESCs viability on the scaffolds
MTT assay showed that PCL provided a more suitable scaffold in comparison with PCL/Gel (70:30) and PCL/Gel (50:50) for cell proliferation. Limbal cell viability percentage on scaffolds was decreased in 70:30 PCL/Gel (56 ± 4%) and 50:50 PCL/Gel (29 ± 6%) in comparison with PCL (100 ± 3%) (Fig. 7).
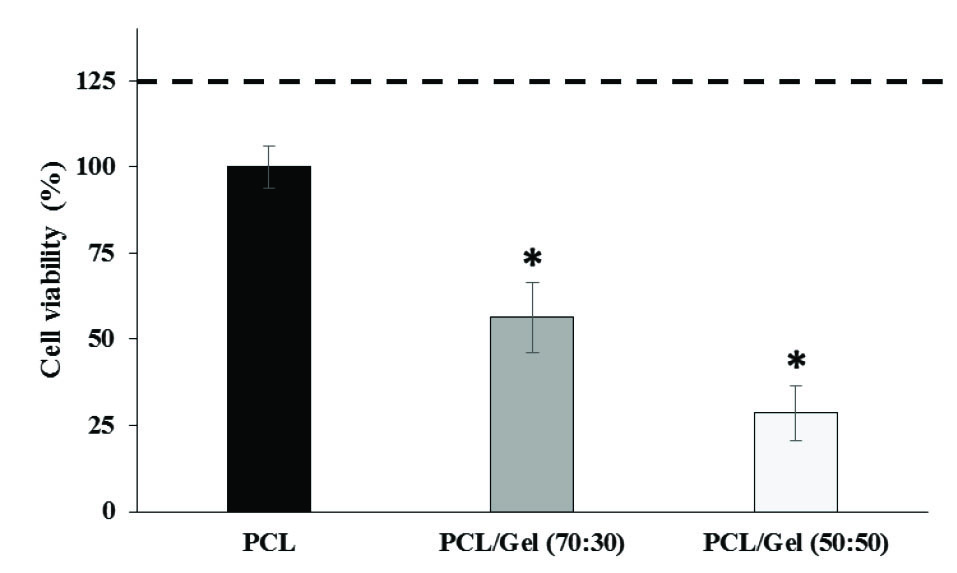
Fig. 7.
Limbal epithelial stem cell viability 48 hours after cultivation on PCL,
PCL/Gel (70:30) or PCL/Gel (50:50) (n=3, mean ± SE). The dashed line
represents the negative control or viability percentage of the limbal cell
cultured on the plate surface with no scaffold. *P<0.05 all values compared
with cultures on PCL using Duncan post hoc test.
.
Limbal epithelial stem cell viability 48 hours after cultivation on PCL,
PCL/Gel (70:30) or PCL/Gel (50:50) (n=3, mean ± SE). The dashed line
represents the negative control or viability percentage of the limbal cell
cultured on the plate surface with no scaffold. *P<0.05 all values compared
with cultures on PCL using Duncan post hoc test.
Transplantation in the animal model
All animals were examined weekly with slit lamps and searched for corneal neovascularization, remaining epithelial defects and corneal opacification. In AM and control groups, delay in corneal epithelialization was observed compared to PCL, PCL/Gel (70:30) and PCL/Gel (50:50). It should be noted that since in this method we used the scaffold as a bandage on the surface of the cornea, transparency of scaffolds is not as important as in amniotic membrane. All scaffolds were sloughed off about 3 weeks after implantation and residual sutures (if any) were removed under the slit lamp. Fig. 8 shows the gross examination of a cornea with secured PCL (Fig. 8A) and AM (Fig. 8B) on the surface 3 weeks after the procedure. Note that in PCL, the cornea at the uncovered area is almost clear, in AM transplanted cornea, visible parts of cornea are opaque, although the epithelium is healed.
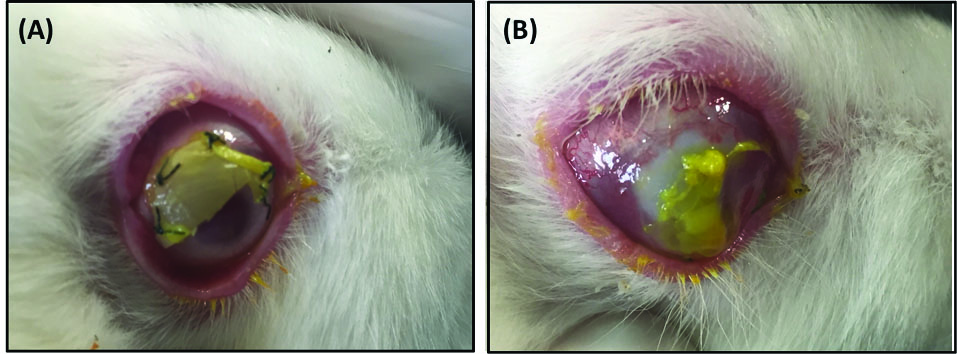
Fig. 8.
Gross examination of a cornea with secured PCL (A) and AM (B)
on surface 3 weeks after the procedure. Note that in (A) the cornea at the
uncovered area is almost clear. (B): Heaped up borders and the retracted
membrane is noted. Visible parts of cornea are opaque. The epithelium is
healed.
.
Gross examination of a cornea with secured PCL (A) and AM (B)
on surface 3 weeks after the procedure. Note that in (A) the cornea at the
uncovered area is almost clear. (B): Heaped up borders and the retracted
membrane is noted. Visible parts of cornea are opaque. The epithelium is
healed.
Regarding stromal neovascularization, neovascularization in 4 quadrants of the cornea and advancement toward the corneal center was more significant in the control and amniotic membrane transplanted groups compared to PCL/Gel (7:30), PCL/Gel (50:50). There was no difference between PCL/Gel (70:30) and PCL/Gel (50:50) in this regard. The corneal opacification was greater in the control group than that of the transplanted groups. Meanwhile, in our experience, it was much easier to secure PCL on the ocular surface rather than PCL/Gel mixture scaffolds.
Histopathological analysis
Histologic sections show that epithelialization occurred almost equally in all treated and control eyes. In the stroma, model eyes who received PCL/Gel (50:50 or 70:30) had more inflammatory cell infiltration than the group receiving PCL (Fig. 9A, B, C, and F). Control groups, including no treatment and AM transplanted group, showed significant stromal vascularization (Fig. 9D and E). Histologic sections were in accordance with the clinical findings. Corneal sections from PCL group versus PCL/Gel (70:30) and PCL/Gel (50:50) shows that even though the epithelium was morphologically similar in all of them, vascularization and inflammation was significantly less in PCL group. In all three groups, stromal integrity was preserved much better than control groups (Fig. 9).
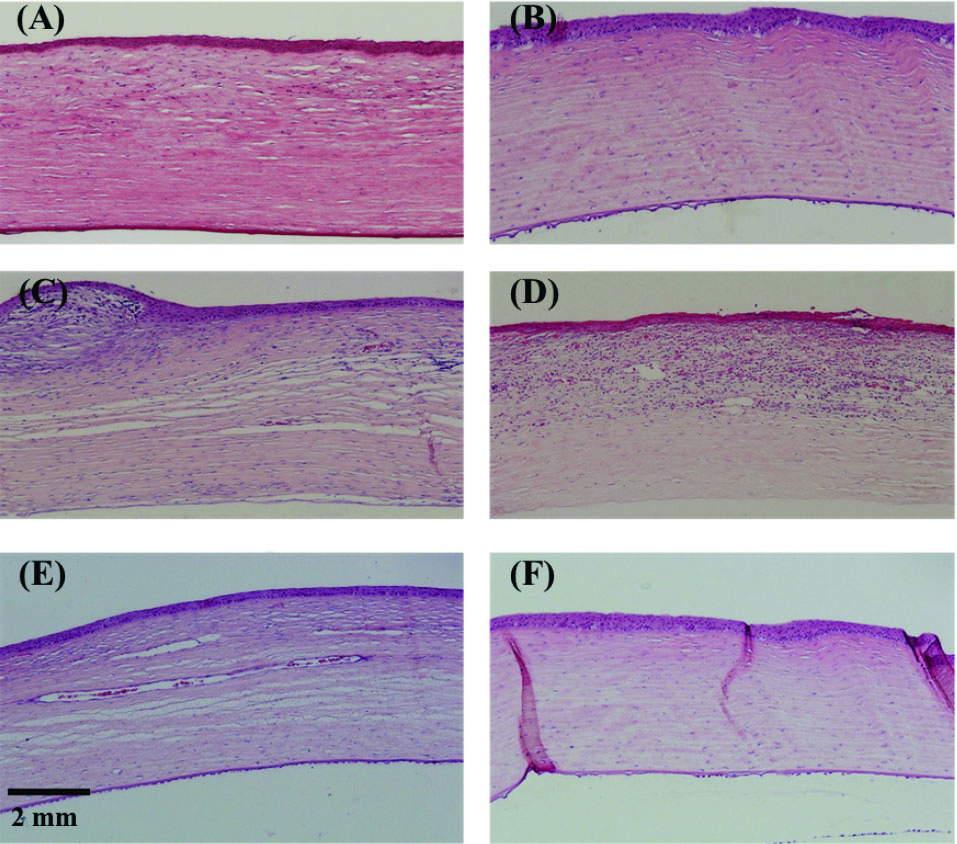
Fig. 9.
Histologic sections of the corneas in the rabbit model (Hematoxylin
and Eosin, ×100). A & B: PCL: Complete re-epithelialization with moderate
anterior stromal scarring. Minimal inflammation is observed. C: PCL/
Gel (50:50): Surface irregularity with subepithelial pannus formation
and inflammatory cell infiltration in the stroma with a minimal amount of
stromal vascularization. D: amniotic membrane transplanted eye: stromal
inflammatory cell infiltration with a moderate amount of vascularization.
E: No treatment eye showed deep extensive stromal vascularization. F:
PCL/Gel (70:30): moderate stromal inflammation with the preservation of
stromal layers and minimal scarring.
.
Histologic sections of the corneas in the rabbit model (Hematoxylin
and Eosin, ×100). A & B: PCL: Complete re-epithelialization with moderate
anterior stromal scarring. Minimal inflammation is observed. C: PCL/
Gel (50:50): Surface irregularity with subepithelial pannus formation
and inflammatory cell infiltration in the stroma with a minimal amount of
stromal vascularization. D: amniotic membrane transplanted eye: stromal
inflammatory cell infiltration with a moderate amount of vascularization.
E: No treatment eye showed deep extensive stromal vascularization. F:
PCL/Gel (70:30): moderate stromal inflammation with the preservation of
stromal layers and minimal scarring.
Discussion
Many kinds of scaffolds have been tried in recent years to facilitate LESCs transfer while maintaining cellular properties.
32-34
AM is the best known and most common membrane used in promoting cellular regeneration in ocular surface diseases.
35
Despite its unique characteristics for inducing cell proliferation and epithelialization, AM application is associated with a high risk of viral transmission.
11,12
Synthetic nanofibers with controllable properties are promising substitutes for ocular cell therapy. This study surveyed the effect of PCL and PCL/Gel blends on LESCs adherence and proliferation. Our data indicated that PCL could preserve LESCs viability and morphology better than PCL/Gel. This observation might be due to the morphological difference of PCL and PCL/Gel scaffold. The non-uniformity and wide size distribution of fibers in PCL/Gel scaffolds may affect the pore diameter of the PCL/Gel relative to PCL. As previously reported the different morphological characters of scaffolds may induce different cell attachment and proliferation.
29,36
As stated by Mijovic et al “the thicker fibers will result in wider pores inter formations, which will facilitate the cell attachment and further penetration in the inner structure”.
14
So the thicker diameter of PCL fiber compared to PCL/Gel blend may be a beneficial property for LESC adherence and proliferation. In this study, we showed that natural hydrophilicity of Gel improved the wettability of PCL, but it had no significant effect on LESCs cell attachment. In contrast, LESCs were attached and stretched on the PCL scaffold, while maintaining original morphology. In line with our findings, there are some reports indicating that Gel could not lead to a significant change in the biological properties of the PCL.
20
Considering the controversial results on the ability of different cell types to attach and proliferate to PCL/Gel scaffolds, it might be suggested that attachment and proliferation of cells to Gel containing scaffold is a cell-specific response and various cell types may respond differently to Gel containing matrix. This effect can be explained not only by the different morphological characters of PCL and PCL/Gel, but also by the specific matrix need for LESCs activity. In other words this observation might not be as a result of the toxicity or inflammatory response of Gel, but might be due to the fact that limbal stem cells are very sensitive to treatment with trypsin and need a special matrix to adhere and proliferate. Our results showed that Gel could not simulate that special matrix and so the proliferation of LESCs decreased when the Gel was added to the PCL. LESCs require a specialized niche for adherence and proliferation. Collagens are amongst the major proteins present in the basement membrane. Collagen types VII, XV, XVI, XVII, and XVIII are expressed in the limbal region and provide a suitable matrix for corneal epithelial stem cells and later for epithelial progenitor cells.
37
A recent study on S5Y5 neuroblastoma cells showed that Gel within the PCL could not improve S5Y5 attachment and proliferation suggesting that Gel was a non-specific adhesion protein for nerve cells.
20
Recently, a comparison between Gel membrane and collagen shields had shown that the collagen shield was more appropriate for LESCs proliferation.
38
Our data indicated that PCL/Gel blend could not preserve for LESC viability and morphology and in the transplanted animal model. This may suggest that Gel is not a specific matrix protein for LESC attachment and proliferation. Our experience on the animal model was in favor of ease of transfer and superior results in the PCL group in comparison with other scaffolds and control groups in terms of corneal clarity and stromal vascularization; however, further studies are needed in other disease states of the eye.
Conclusion
According to the presented study, PCL blending with Gel could not improve the PCL surface properties in LESC culture, which emphasizes on the suitability of PCL for limbal cell adhesion and proliferation compared to PCL/Gel. Ease of transfer during surgery and histopathologic analysis of corneas after surgery were a good indication for the efficacy of PCL in comparison with the PCL/Gel blends and AM.
Acknowledgments
We would like to thank Mrs. Hakimzadeh and her lab staff for histological preparation and technical support. The authors wish to thank Mr. H. Argasi at the Research Consultation Centre (RCC) at Shiraz University of Medical Sciences for his invaluable assistance in editing this manuscript.
Funding sources
This study was supported by Shiraz University of Medical Sciences grant no. 10446.19.01.94.
Ethical Statement
Animals were treated according to the protocol approved by the Ethics Committee of Shiraz University of Medical Sciences.
Competing interests
No competing interests are declared by any of the authors.
Authors' contribution
FSJ and ME contributed to data handling, experiments’ design, data analysis, data presentation, draft preparation, study consultation, writing and reviewing. EM contributed to data handling, draft preparation and study consultation. ZA, MHJ and FJA contributed to data handling. MN contributed to project administration, conceptualization, provision of study materials and equipment, study validation and supervision.
Research Highlights
What is the current knowledge?
simple
-
√ Transplantation of cultivated limbal stem cells on a suitable
scaffold is an optimal strategy for the treatment of LSCD.
-
√ PCL is a synthetic biodegradable scaffold, extensively used
to cultivate different cell types.
-
√ PCL/Gel blend is attractive to researchers as Gel is a natural
component and seems to be suitable for improving PCL
biological properties.
-
√ There are controversial results on the effect of PCL/Gel
blend on different cell types.
What is new here?
simple
-
√ PCL blending with Gel could not improve the PCL surface
properties in LESC culture
-
√ PCL is more appropriate than PCL/Gel for supplying
LESCs adherence, proliferation and induction of epithelial
morphology.
References
- Haagdorens M, Van Acker SI, Van Gerwen V, Ní Dhubhghaill S, Koppen C, Tassignon M-J. Limbal stem cell deficiency: current treatment options and emerging therapies. Stem Cells Int 2016; 2016. doi: 10.1155/2016/9798374 [Crossref]
- Dua HS, Saini JS, Azuara-Blanco A, Gupta P. Limbal stem cell deficiency: concept, aetiology, clinical presentation, diagnosis and management. Indian J Ophthalmol 2000; 48:83. doi: 10.1136/bjo.83.4.414 [Crossref] [ Google Scholar]
- Schlötzer-Schrehardt U, Kruse FE. Identification and characterization of limbal stem cells. Exp Eye Res 2005; 81:247-64. doi: 10.1016/j.exer.2005.02.016 [Crossref] [ Google Scholar]
- Vascotto SG, Griffith M. Localization of candidate stem and progenitor cell markers within the human cornea, limbus, and bulbar conjunctiva in vivo and in cell culture. Anat Rec A Discov Mol Cell Evol Biol 2006; 288:921-31. doi: 10.1002/ar.a.20346 [Crossref] [ Google Scholar]
- Ebrahimi M T-AE, Baharvand H. Limbal Stem Cells in Review. J Ophthalmic Vis Res 2009; 4:40-58. [ Google Scholar]
- Joe AW, Yeung SN. Concise review: identifying limbal stem cells: classical concepts and new challenges. Stem Cells Transl Med 2014; 3:318-22. doi: 10.5966/sctm.2013-0137 [Crossref] [ Google Scholar]
- Nishida K. Tissue engineering of the cornea. Cornea 2003; 22:S28-S34. doi: 10.1097/00003226-200310001-00005 [Crossref] [ Google Scholar]
- Grueterich M, Espana EM, Touhami A, Ti S-E, Tseng SC. Phenotypic study of a case with successful transplantation of ex vivo expanded human limbal epithelium for unilateral total limbal stem cell deficiency. Ophthalmology 2002; 109:1547-52. doi: 10.1016/S0161-6420(02)01105-3 [Crossref] [ Google Scholar]
- Baradaran-Rafii A, Biazar E, Heidari-Keshel S. Cellular response of limbal stem cells on polycaprolactone nanofibrous scaffolds for ocular epithelial regeneration. Curr Eye Res 2016; 41:326-33. doi: 10.3109/02713683.2015.1019004 [Crossref] [ Google Scholar]
- Hassan E, Deshpande P, Claeyssens F, Rimmer S, MacNeil S. Amine functional hydrogels as selective substrates for corneal epithelialization. Acta Biomate 2014; 10:3029-37. doi: 10.1016/j.actbio [Crossref] [ Google Scholar]
- Gomes JA, Romano A, Santos MS, Dua HS. Amniotic membrane use in ophthalmology. Curr Opin Ophthalmol 2005; 16:233-40. doi: 10.1097/01.icu.0000172827.31985.3a [Crossref] [ Google Scholar]
- Rahman I, Said D, Maharajan V, Dua H. Amniotic membrane in ophthalmology: indications and limitations. Eye 2009; 23:1954. doi: 10.1038/eye.2008.410 [Crossref] [ Google Scholar]
- Asvar Z, Mirzaei E, Azarpira N, Geramizadeh B, Fadaie M. Evaluation of electrospinning parameters on the tensile strength and suture retention strength of polycaprolactone nanofibrous scaffolds through surface response methodology. J Mech Behav Biomed Mater 2017; 75:369-78. doi: 10.1016/j.jmbbm.2017.08.004 [Crossref] [ Google Scholar]
- Mijovic B, Trcinc MT, Agic A, Zdraveva E, Bujic M, Spoljaric I. Study on cell adhesion detection onto biodegradable electrospun PCL scaffolds. Journal of Fiber Bioengineering and Informatics 2012; 5:33-40. doi: 10.3993/jfbi03201202 [Crossref] [ Google Scholar]
- Sharma S, Mohanty S, Gupta D, Jassal M, Agrawal AK, Tandon R. Cellular response of limbal epithelial cells on electrospun poly-ε-caprolactone nanofibrous scaffolds for ocular surface bioengineering: a preliminary in vitro study. Mol Vis 2011; 17:2898. [ Google Scholar]
- de la Mata A, Nieto-Miguel T, López-Paniagua M, Galindo S, Aguilar MR, García-Fernández L. Chitosan–gelatin biopolymers as carrier substrata for limbal epithelial stem cells. J Mater Sci Mater Med 2013; 24:2819-29. doi: 10.1007/s10856-013-5013-3 [Crossref] [ Google Scholar]
- Tominac Trcin M, Dekaris I, Mijović B, Bujić M, Zdraveva E, Dolenec T. Synthetic vs natural scaffolds for human limbal stem cells. Croat Med J 2015; 56:246-56. doi: 10.3325/cmj.2015.56.246 [Crossref] [ Google Scholar]
- Kuppan P, Sethuraman S, Krishnan UM. Interaction of human smooth muscle cells with nanofibrous scaffolds: effect of fiber orientation on cell adhesion, proliferation, and functional gene expression. J Biomed Mater Res A 2015; 103:2236-50. doi: 10.1002/jbm.a.35360 [Crossref] [ Google Scholar]
- Mota A, Lotfi AS, Barzin J, Hatam M, Adibi B, Khalaj Z. Human bone marrow mesenchymal stem cell behaviors on PCL/gelatin nanofibrous scaffolds modified with a collagen IV-derived RGD-containing peptide. Cell J 2014; 16:1. [ Google Scholar]
- Chiono V, Ciardelli G, Vozzi G, Cortez J, Barbani N, Gentile P. Enzymatically‐modified melt‐extruded guides for peripheral nerve repair. Engineering in Life Sciences 2008; 8:226-37. doi: 10.1007/s10544-009-9321-9 [Crossref] [ Google Scholar]
- Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani M-H, Ramakrishna S. Electrospun poly (ɛ-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008; 29:4532-9. doi: 10.1016/j.biomaterials.2008.08.007 [Crossref] [ Google Scholar]
- Dulnik J, Denis P, Sajkiewicz P, Kołbuk D, Choińska E. Biodegradation of bicomponent PCL/gelatin and PCL/collagen nanofibers electrospun from alternative solvent system. Polymer Degradation and Stability 2016; 130:10-21. doi: 10.1016/j.polymdegradstab.2016.05.022 [Crossref] [ Google Scholar]
- Xue J, He M, Liu H, Niu Y, Crawford A, Coates PD. Drug loaded homogeneous electrospun PCL/gelatin hybrid nanofiber structures for anti-infective tissue regeneration membranes. Biomaterials 2014; 35:9395-405. doi: 10.1016/j.biomaterials.2014.07.060 [Crossref] [ Google Scholar]
- Suntornnond R, An J, Chua CK. Effect of gas plasma on polycaprolactone (PCL) membrane wettability and collagen type I immobilized for enhancing cell proliferation. Materials Letters 2016; 171:293-6. doi: 10.1016/j.matlet.2016.02.059 [Crossref] [ Google Scholar]
- Pereira IH, Ayres E, Averous L, Schlatter G, Hebraud A, de Paula ACC. Differentiation of human adipose-derived stem cells seeded on mineralized electrospun co-axial poly (ε-caprolactone)(PCL)/gelatin nanofibers. J Mater Sci Mater Med 2014; 25:1137-48. doi: 10.1007/s10856-013-5133-9 [Crossref] [ Google Scholar]
- Jahani H, Kaviani S, Hassanpour-Ezatti M, Soleimani M, Kaviani Z, Zonoubi Z. The effect of aligned and random electrospun fibrous scaffolds on rat mesenchymal stem cell proliferation. Cell J 2012; 14:31. [ Google Scholar]
- Sari ES, Yazıcı A, Aksit H, Yay A, Sahin G, Yildiz O. Inhibitory effect of sub-conjunctival tocilizumab on alkali burn induced corneal neovascularization in rats. Curr Eye Res 2015; 40:48-55. doi: 10.3109/02713683.2014.914541 [Crossref] [ Google Scholar]
- Dutta RC, Dey M, Dutta AK, Basu B. Competent processing techniques for scaffolds in tissue engineering. Biotechnol Adv 2017; 35:240-50. doi: 10.1016/j.biotechadv.2017.01.001 [Crossref] [ Google Scholar]
- Feng B, Duan H, Fu W, Cao Y, Jie Zhang W, Zhang Y. Effect of inhomogeneity of the electrospun fibrous scaffolds of gelatin/polycaprolactone hybrid on cell proliferation. J Biomed Mater Res A 2015; 103:431-8. doi: 10.1002/jbm.a.35184 [Crossref] [ Google Scholar]
- Gautam S, Dinda AK, Mishra NC. Fabrication and characterization of PCL/gelatin composite nanofibrous scaffold for tissue engineering applications by electrospinning method. Mater Sci Eng C 2013; 33:1228-35. doi: 10.1016/j.msec.2012.12.015 [Crossref] [ Google Scholar]
- Qian Y, Zhang Z, Zheng L, Song R, Zhao Y. Fabrication and characterization of electrospun polycaprolactone blended with chitosan-gelatin complex nanofibrous mats. Journal of Nanomaterials 2014; 2014:1. doi: 10.1155/2014/964621 [Crossref] [ Google Scholar]
- Ceccarelli G, Presta R, Benedetti L, Cusella De Angelis MG, Lupi SM, Rodriguez y Baena R. Emerging perspectives in scaffold for tissue engineering in oral surgery. Stem Cells Int 2017; 2017. doi: 10.1155/2017/4585401 [Crossref]
- Chan B, Leong K. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J 2008; 17:467-79. doi: 10.1007/s00586-008-0745-3 [Crossref] [ Google Scholar]
- Wei J, Han J, Zhao Y, Cui Y, Wang B, Xiao Z. The importance of three-dimensional scaffold structure on stemness maintenance of mouse embryonic stem cells. Biomaterials 2014; 35:7724-33. doi: 10.1016/j.biomaterials.2014.05.060 [Crossref] [ Google Scholar]
- Sharma A, Yadav K. Amniotic membrane-A Novel material for the root coverage: A case series. J Indian Soc Periodontol 2015; 19:444. doi: 10.4103/0972-124X.154166 [Crossref] [ Google Scholar]
- Bružauskaitė I, Bironaitė D, Bagdonas E, Bernotienė E. Scaffolds and cells for tissue regeneration: different scaffold pore sizes—different cell effects. Cytotechnology 2016; 68:355-69. doi: 10.1007/s10616-015-9895-4 [Crossref] [ Google Scholar]
- Schlötzer-Schrehardt U, Dietrich T, Saito K, Sorokin L, Sasaki T, Paulsson M. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp Eye Res 2007; 85:845-60. doi: 10.1016/j.exer.2007.08.020 [Crossref] [ Google Scholar]
- Willoughby C, Batterbury M, Kaye S. Collagen corneal shields. Surv Ophthalmol 2002; 47:174-82. doi: 10.1016/S0039-6257(01)00304-6 [Crossref] [ Google Scholar]