Dr. Mitra Dolatkhah (Pharm.D.) is a Ph.D. candidate of Pharmaceutics at the Faculty of Pharmacy, Tabriz University of Medical Sciences. Her research activities are focused on targeted cancer drug delivery.
Professor Yadollah Omidi (Pharm.D., Ph.D.) is a Professor of Pharmaceutical and Biomedical Sciences at TUOMS Faculty of Pharmacy, working on the development of advanced multifunctional drug delivery systems, biosensing, and tissue engineering. He has worked at Cardiff University and the University of Pennsylvania. Since 2004, Prof. Omidi has published over 210 papers and 18 book chapters, supervised and trained over 100 graduate/postgraduate students/researchers.
Abstract
Summary
The highly proliferating cancerous cells can form permissive accommodating milieu – the so-called tumor microenvironment (TME). During the initiation of solid tumors, hypoxia plays a key role in glycolysis, which can trigger the anomalous overexpression of several enzymes and transporters involved in the metabolism of glucose. Of these, carbonic anhydrases (CAs), especially CAIX, together with other molecular machinery involved in the production/trafficking of acidic byproducts, play key roles in the regulation of intracellular and extracellular pH. CAIX, along with other molecular machinery of cancer cells such as Na+/H+ exchanger 1 (NHE1) and V-type H+-ATPase (V-ATPase), alkalinizes the tumor cells and maintains the acidic pH condition within the extracellular fluid of the TME. It facilitates the progression and metastasis of cancer and intensifies the migration and invasion of cancer cells. Thus, inhibition of CAIX can be considered a highly effective and promising therapeutic strategy in the treatment of aggressive tumors.
Keywords: Cancer therapy, Carbonic anhydrase IX, Hypoxia, Solid tumor, Tumor microenvironment
Copyright and License Information
© 2019 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Drug resistance mechanisms developed by cancer cells have been a major challenge of cancer therapy in the clinic. More recently, it has been recognized that tumor microenvironment (TME) can greatly influence the currently used anticancer therapies. As a result, it was proposed that the inhibition of extracellular ligand-receptor interactions and downstream pathways involved in the TME may be a solution to overcome drug resistance and improve therapeutic outcomes.
1-3
It is well known that TME has unique physiological characteristics such as irregular acidic pH and hypoxia, which can cause the up- and/or down-regulation of certain biomolecules such as enzymes.
2,4-6
Because of abnormal proliferation of cancer cells and irregular vascular network in not being able to supply sufficient blood to all cancer cells, cancer cells experience hypoxia, and as a consequence, they show an increased dependency on glycolysis for the energy provision instead of oxidative phosphorylation that is defined as Warburg effect.
5,7,8
This glycolysis pathway leads to the production of a large amount of lactate and H+ ions. One of the adaptive mechanisms of cancer cells to adverse conditions is the activation of hypoxia-inducible factor-1 (HIF-1), which may result in the overexpression of the pH-regulating proteins, including isoforms II, IX, and XII of carbonic anhydrases (CAs), Na+/H+ exchanger 1 (NHE1), and V-type H+-ATPase (V-ATPase). The expression levels of these molecular machinery pieces seem to be strongly enhanced under the hypoxic conditions via HIF-1 pathway concurrent with the glycolysis phenomenon.
6,9
Fig. 1 represents the molecular machinery involved in the pH dysregulation phenomenon in the TME. All these events result in the outward transportation of accumulated acidic byproducts into the extracellular fluid (ECF) in the TME, which makes the extracellular pH of solid tumors become more acidic compared to the normal tissues. Such an acidic condition of the TME can influence the integrity of the extracellular matrix (ECM), and hence, remodel the TME by the degradation of the ECM in favor of further metastasis and progression of cancer.
10
The acidic condition in the serial extracellular fluid (ECF) of solid tumors may provoke the exocytosis in cancer cells. All these incidences create a complex dynamic situation within TME, which result in capricious cacophonous functions in cancer cells along with other stromal cells such as the immune system cells. Under such circumstances, the rebellious cancer cells develop defense mechanisms against anticancer agents and circumvent the immunosurveillance functionalities. The emergence of such complexity makes the treatment of solid tumors very problematic.
2,3,11
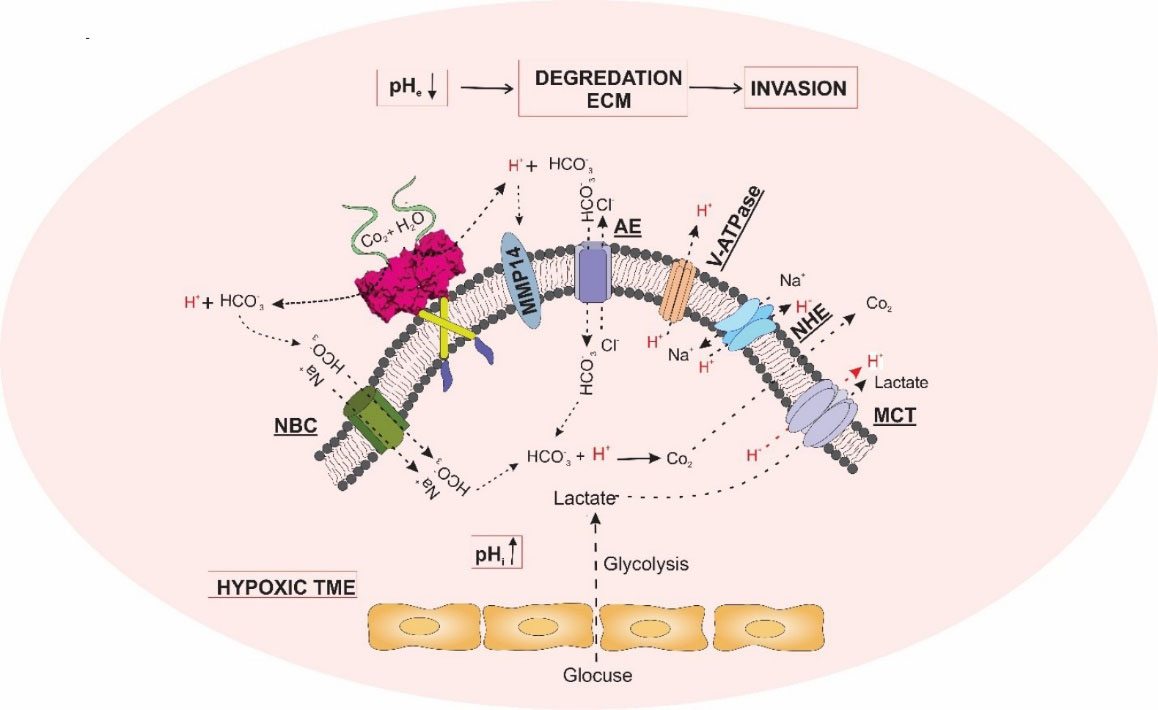
Fig. 1.
Molecular machinery involved in the pH dysregulation phenomenon in the TME. Monocarboxylate transporter (MCT), V-type H+-ATPase (V-ATPase), Na+/H+ exchanger (NHE), bicarbonate co-transport (NBC), anion exchanger (AE), and carbonic anhydrase IX (CA IX) are involved in pH regulation in the TME. The degradation of ECM is triggered by the dysregulation of pH and activation of MMP14 by CAIX, facilitating invasion and metastasis of solid tumors. MMP14: Matrix metallopeptidase 14; HCO3−: bicarbonate; CO2: carbon dioxide; H+: proton; ECM: extracellular matrix. MCT: monocarboxylate transporter; AE: anion exchanger; NBC: electrogenic Na+/HCO- cotransporter; TME: tumor microenvironment.
.
Molecular machinery involved in the pH dysregulation phenomenon in the TME. Monocarboxylate transporter (MCT), V-type H+-ATPase (V-ATPase), Na+/H+ exchanger (NHE), bicarbonate co-transport (NBC), anion exchanger (AE), and carbonic anhydrase IX (CA IX) are involved in pH regulation in the TME. The degradation of ECM is triggered by the dysregulation of pH and activation of MMP14 by CAIX, facilitating invasion and metastasis of solid tumors. MMP14: Matrix metallopeptidase 14; HCO3−: bicarbonate; CO2: carbon dioxide; H+: proton; ECM: extracellular matrix. MCT: monocarboxylate transporter; AE: anion exchanger; NBC: electrogenic Na+/HCO- cotransporter; TME: tumor microenvironment.
Recent developments in cancer therapy have generated renewed interest in targeting molecular machinery (genes and proteins), involved in the pH dysregulation within the TME, as platforms for the development of anti-tumor regimens. Of these molecular machinery pieces, CAs have gained much attention in tumor pH regulatory studies as a valuable therapeutic target, in large part because of their cardinal role in maintaining the intracellular pH homeostasis. The CAs are transmembrane zinc-binding enzymes that catalyze the reversible conversion of CO2 molecules. This function can impede the pH homeostasis between the intracellular fluid (ICF) and ECF, resulting in the acidification of ECF by protons. Further, bicarbonate molecules are directly transported to the cytoplasm by bicarbonate transporters, which favor the alkalization of the ICF.
To date, fifteen CA isoforms have been characterized in human.
12,13
Recent studies offer some evidence of increased expression of CAIX in a variety of solid cancers, including breast cancer.
14
CAIX, as a pH-regulating enzyme, plays a key role in maintaining an acidic extracellular pH under hypoxic conditions. Hypoxia is a major trigger for CAIX expression in cancer cells. Moreover, CAIX can be induced in normoxia by high cell-density-mediated pseudohypoxia and by hypoxia-independent mechanisms such as lactate and redox-mediated stabilization of HIF-1. The induction of CAIX might associate with the cell adhesion, migration, and invasion proteins (e.g., MMP14). Such phenomena indicate an intricate role of CAIX in tumor invasiveness, degradation of ECM, distant metastasis, and poor survival in patients. It should be noted that the overexpression of CAIX is associated with poor prognosis.
12,15
Several pre-clinical studies have clearly highlighted the useful effect of addition of CAIX inhibitors to standard cancer-therapy regimens. A number of researches have suggested that CAIX inhibitors, as co-treatments, may increase the effects of chemotherapy agents and/or anti-angiogenic drugs, and also enhance the response of tumors to radiotherapy.
16-18
Compelling data suggest that the inhibition of CAIX might restrict cancer cell proliferation, migration, and invasion. Furthermore, some in vivo studies exhibited that metastatic growth could be limited by such treatment modality.
19
A number of studies indicate that the anti-tumor effects of the inhibition of CAIX may occur through (i) the intrusion in the pH regulation in cancer cells, (ii) the interaction with other pH-independent mechanisms, and (iii) the association with some signaling pathways involved in cancer cells progression and metastasis. Thus, all these biological events may influence the response of cancer cells to the treatment modalities administered.
11
In fact, some CAIX inhibitors are already under consideration in different clinical studies. Small interfering RNAs, therapeutic antibodies, or small molecule inhibitors (e.g., sulfonamide, sulfamate, sulfamide, and coumarin types substances) can be used to investigate the tumor therapeutic potential of CAIX.
20
Taken all, the up-regulation of CAIX appears to play several key roles in the progression and invasion of cancer cells, and therefore, it could potentially be targeted in cancer therapy. Further, the hallmarks of cancer must be taken into consideration for the treatment of solid tumors such as breast cancer.
Funding sources
None to be declared.
Ethical statement
There is none to be stated.
Acknowledgments
The authors like to acknowledge the financial and technical support provided by the Research Center for Pharmaceutical Nanotechnology at Tabriz University of Medical Sciences.
Competing interests
No competing interests to be disclosed.
Authors’ contribution
MD and YO gathered the data and drafted the manuscript. YO finalized the manuscript.
References
- Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett 2017; 387:61-8. doi: 10.1016/j.canlet.2016.01.043 [Crossref] [ Google Scholar]
- Barar J, Omidi Y. Dysregulated pH in Tumor Microenvironment Checkmates Cancer Therapy. Bioimpacts 2013; 3:149-62. doi: 10.5681/bi.2013.036 [Crossref] [ Google Scholar]
- Omidi Y, Barar J. Targeting tumor microenvironment: crossing tumor interstitial fluid by multifunctional nanomedicines. Bioimpacts 2014; 4:55-67. doi: 10.5681/bi.2014.021 [Crossref] [ Google Scholar]
- Xie H-Y, Shao Z-M, Li D-Q. Tumor microenvironment: driving forces and potential therapeutic targets for breast cancer metastasis. Chin J Cancer 2017; 36:36. doi: 10.1186/s40880-017-0202-y [Crossref] [ Google Scholar]
- Eskandani M, Vandghanooni S, Barar J, Nazemiyeh H, Omidi Y. Cell physiology regulation by hypoxia inducible factor-1: Targeting oxygen-related nanomachineries of hypoxic cells. Int J Biol Macromol 2017; 99:46-62. doi: 10.1016/j.ijbiomac.2016.10.113 [Crossref] [ Google Scholar]
- Asgharzadeh MR, Barar J, Pourseif MM, Eskandani M, Jafari Niya M, Mashayekhi MR, Omidi Y. Molecular machineries of pH dysregulation in tumor microenvironment: potential targets for cancer therapy. Bioimpacts 2017; 7:115-33. doi: 10.15171/bi.2017.15 [Crossref] [ Google Scholar]
- Fong MY, Zhou W, Liu L, Alontaga AY, Chandra M, Ashby J. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol 2015; 17:183. doi: 10.1038/ncb3094 [Crossref] [ Google Scholar]
- Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 2006; 441:437. doi: 10.1038/nature04871 [Crossref] [ Google Scholar]
- Parks SK, Pouysségur J. Targeting pH regulating proteins for cancer therapy–Progress and limitations. Semin Cancer Biol 2017; 43:66-73. doi: 10.1016/j.semcancer.2017.01.007 [Crossref] [ Google Scholar]
-
Benej M, Pastorekova S, Pastorek J. Carbonic anhydrase IX: regulation and role in cancer. In: Frost SC, R McKenna, editors. Carbonic Anhydrase: Mechanism, Regulation, Links to Disease, and Industrial Applications. New York: Springer; 2014. p. 199-219.
- Ward C, Meehan J, Gray M, Kunkler IH, Langdon SP, Argyle DJ. Carbonic Anhydrase IX (CAIX), Cancer, and Radiation Responsiveness. Metabolites 2018; 8:13. doi: 10.3390/metabo8010013 [Crossref] [ Google Scholar]
- Singh S, Lomelino CL, Mboge MY, Frost SC, McKenna R, Supuran CT. Cancer Drug Development of Carbonic Anhydrase Inhibitors beyond the Active Site. Molecules 2018; 23:E1045. doi: 10.3390/molecules23051045 [Crossref] [ Google Scholar]
- Pastorek J, Pastorekova S. Hypoxia-induced carbonic anhydrase IX as a target for cancer therapy: from biology to clinical use. Semin Cancer Biol 2015; 31:52-64. doi: 10.1016/j.semcancer.2014.08.002 [Crossref] [ Google Scholar]
- Zandberga E, Zayakin P, Ābols A, Pūpola D, Trapencieris P, Linē A. Depletion of carbonic anhydrase IX abrogates hypoxia-induced overexpression of stanniocalcin-1 in triple negative breast cancer cells. Cancer Biol Ther 2017; 18:596-605. doi: 10.1080/15384047.2017.1345390 [Crossref] [ Google Scholar]
- McDonald PC, Swayampakula M, Dedhar S. Coordinated Regulation of Metabolic Transporters and Migration/Invasion by Carbonic Anhydrase IX. Metabolites 2018; 8:20. doi: 10.3390/metabo8010020 [Crossref] [ Google Scholar]
- Meehan J, Ward C, Turnbull A, Bukowski-Wills J, Finch AJ, Jarman EJ. Inhibition of pH regulation as a therapeutic strategy in hypoxic human breast cancer cells. Oncotarget 2017; 8:42857. doi: 10.18632/oncotarget.17143 [Crossref] [ Google Scholar]
- Supuran CT, Alterio V, Di Fiore A, D’Ambrosio K, Carta F, Monti SM, De Simone G. Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: Three for the price of one. Med Res Rev 2018. doi: 10.1002/med.21497 [Crossref]
- van Kuijk SJ, Parvathaneni NK, Niemans R, van Gisbergen MW, Carta F, Vullo D. New approach of delivering cytotoxic drugs towards CAIX expressing cells: A concept of dual-target drugs. Eur J Med Chem 2017; 127:691-702. doi: 10.1016/j.ejmech.2016.10.037 [Crossref] [ Google Scholar]
- Swayampakula M, McDonald P, Vallejo M, Coyaud E, Chafe S, Westerback A. The interactome of metabolic enzyme carbonic anhydrase IX reveals novel roles in tumor cell migration and invadopodia/MMP14-mediated invasion. Oncogene 2017; 36:6244. doi: 10.1038/onc.2017.219 [Crossref] [ Google Scholar]
- Winum J-Y, Colinas PA, Supuran CT. Glycosidic carbonic anhydrase IX inhibitors: a sweet approach against cancer. Bioorg Med Chem 2013; 21:1419-26. doi: 10.1016/j.bmc.2012.10.043 [Crossref] [ Google Scholar]