Bioimpacts. 10(4):235-242.
doi: 10.34172/bi.2020.30
Original Research
Classification and identification of human papillomavirus based on its prevalence and development of cervical lesion among Iranian women
Mitra Moeinzadeh 1  , Babak Kheirkhah 2, *
, Babak Kheirkhah 2, *  , Kumarss Amini 3
, Kumarss Amini 3  , Ali Pouryasin 4
, Ali Pouryasin 4 
Author information:
1Department of Biology, Sirjan Branch, Islamic Azad University, Sirjan, Iran
2Department of Microbiology, Kerman Branch, Islamic Azad University, Kerman, Iran
3Department of Microbiology, Saveh Branch, Islamic Azad University, Saveh, Iran
4Armin Pathobiology Laboratory, Tehran, Iran
Abstract
Introduction:
Cervical cancer is the most common female cancer in large areas of the developing world, and almost half of these cases (54%) arises in Asia, where cervical cancer is still threatening women’s health and survival, which makes it a considerable public problem. Human papillomavirus (HPV) is one of the most powerful human carcinogens. Today, it has been proven that all cervical cancers and primary precancerous lesions are caused by carcinogenic types of HPV infections. HPV genotyping can therefore evaluate the screening programs.
Methods: Five hundred fifty women referring to the gynecological centers were subjected to Pap smear cell samples. The cytopathological diagnosis of obtained cervical samples was based on the Bethesda system. HPV genotyping was carried out using the INNO-LiPA HPV Genotyping Extra II Amp assay.
Results: In a total of 244 HPV positive cases, single‑type HPV infection was observed in 49.6%, while multi‑type HPV infections (including ≥ 2 types) were found in 45.5% of cases. Among the 110 cases with abnormal cytology results, going-over analyses led to the identification of atypical squamous cell of unknown significance (ASCUS) in 73 cases, low‑grade squamous intraepithelial lesions (LSIL) in 24 cases, and high‑grade squamous intraepithelial lesion (HSIL) in 12 cases. In these groups, the infection rate of high-risk HPV (HR-HPV) was 89%, 82%, and 100%, respectively.
Conclusion: In this study, the total population of women suffering from different cervical lesions and malignancy was found to be infected with various HPV genotypes. High prevalence of HPV- 53 and HPV- 16 detected among participants with normal cytology can be considered as a tip-off development of cervical cancer among Iranian women.
Keywords: Human papillomavirus, Screening program, HPV genotyping, Cervical cancer
Copyright and License Information
© 2020 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Among all cancers threatening women’s health and survival all around the world, invasive cervical cancer (ICC) takes the second place1-4 and even the most common female cancer in large areas of the developing world where an estimated 80% of new cases arise.4,5 Based on the International Agency for Research on Cancer (IARC) estimation, each year almost half a million new cases and 274,000 deaths related to cervical cancer occur among women all around the world.3,6-9 Near half of these cases (54%) occur in Asia, where cervical cancer is considered as a warning toward women health and makes it a substantial public problem.3
The population of women over 15 years in Iran is almost over 25 million who are exposed to numerous risks of cervical malignancy types.10 With an estimated incidence rate of 2.2 out of every 100 000 women a year, cervical malignancies are the second major threat to women’s genital and reproductive organ in Iran.10,11
Human papillomavirus (HPV) is one of the most powerful carcinogens and has been entailed in human cancers in different sites.10 Almost 610 000 new cancers per year (5% of all cancers) have been assigned to HPV infection.12 HPV infections are the most common of all sexually transmitted diseases.2-4 More than 40 different types of HPV genotypes specifically infect the anogenital tract.4,13,14 These types can be subsequently classified based on their carcinogenic potential.2,11,14,15 There are three groups on the basis of their epidemiological association with cervical cancer: high-risk HPV (HR-HPV) genotypes (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82), putative high-risk HPV (pHR-HPV) genotypes (types 26, 53, and 66), and low-risk HPV (LR-HPV) genotypes (types 6, 11, 40, 42, 43, 44, and 70).16-18 However, there is no agreed-upon category for many of HPV genotypes with low prevalence given their oncogenicity.17,19
The spread of type-specific HPV varies widely among different populations by age, region, and breed.10 HPV- 16 and 18 are considered as the cause of 70% of all cervical cancers worldwide.2,14,20,21 Despite that, the evaluated contribution of HPV-16 /18 fraction is slenderly higher in developed countries (72%–77%) compared to developing countries (65%–72%).2,22
Therefore, more precise criteria for classifying HPV genotypes in categorizing low-risk and high-risk groups are needed. This criterion should be based on detailed molecular epidemiologic studies that can provide accurate information on the risk scale and carcinogenic potential of the various HPV types.17
HPV infections of the cervix and the epidemiology of HPV related cervical cancer are being investigated using the viral DNA presence in cellular scrapes or biopsy tissue.19
HPV prophylactic vaccines are responsible for reducing the global incidence of cervical cancer. Therefore, identifying and inhibiting of carcinogenic HPV genotypes have become one of the most important and effective goals for cervical cancer prevention control.3,4,23 In developed countries, systematic cervical screening with Pap smear and HPV detection methods have resulted in the prevention of ICC.24
Therefore, it can be claimed that the specific HPV genotype determination has a huge impact on improving the performance of screening programs and reducing of additional treatments.23 In addition, since all HPV vaccines such as available ones (HPV16/HPV18) and the next generation candidates are eventually particular type-specific, the perception of the type-specific HPV distribution in screen diagnosed lesions can also well evaluate the impact of vaccination on the reduction of abnormal findings in cervical screening programs. This research aims to provide information on HPV type-specific distribution and prevalence among Iranian women.
Materials and Methods
Sample collection and study design
550 women with the age range of 15 to 50 years who were sexually active referring to Armin Pathobiology Laboratory with the gynecological clinician’s recommendation for analysis of their routine Pap smear sample and welfare testing from March to August 2017 were subjected with their permission to this cross-sectional study. Three categories of referrals were excluded from the study, including pregnant women, women over 50 years, and all women who had any history of genital organ surgery containing Genitoplasty (Vaginoplasty/Labiaplasty), Hysterectomy and Myomectomy. Samples were taken by gynecologists or nurses and sent to the specialized laboratory (Armin Pathobiology Laboratory) for initial analysis. After the initial examination, the relevant pap smears were considered for designing of the present cross-sectional study as three distinct and separate groups: (1) The first group (group I) of women who did not show any viral infections and cytological cervix damage in the Pap smear test (as a negative control group, a total of 302 persons). (2) The second group (group II) of women who showed viral infection but did not show any cytological and histological malignancies (as a positive control group, a total of 147 patients) 3.The third group (Group III) of women as the last group who also showed signs of viral infections and symptoms of cervical intraepithelial neoplasia (CIN) (a total of 110 cases). Cervical biopsies of these patients were also obtained.
The Cytopathological diagnosis of the cervical isolated cells was assessed by the Bethesda system (TBS).20
The second and third groups were consequently isolated for further HPV DNA analysis and subsequently, cervical biopsies of the third group whose participants had abnormal cervical cytology were subjected to histological examination.
Cytology testing
Cervical liquid-based Pap samples were collected in ThinPrep method by using an endocervical plastic spatula combination device (Hologic, 250 Campus Drive, Marlborough, Massachusetts, 01752, USA), immersed and rinsed in a vial filled with 20 ml of PreserveCyt® solution(Hologic, 250 Campus Drive, Marlborough, Massachusetts, 01752, USA). The capped, labeled ThinPrep sample vials were then placed into a ThinPrep® 2000 processor (Hologic, 250 Campus Drive, Marlborough, Massachusetts, 01752, USA) which transferred a thin layer of cells from each sample to a 20 mm diameter circle on a glass slide and followed up by fixation with an alcoholic base solution. Slides were then stained by pap stain and then the slides were pictured by microscope. Cytopathologic characterization was categorized by experienced cytology experts at the laboratory by the Bethesda 2014, a global system for the reporting Cervical/Vaginal Cytologic findings, including atypical squamous cell of unknown significance (ASCUS), low-grade squamous intraepithelial lesions (LSIL), high-grade squamous intraepithelial lesion (HSIL), squamous cell carci-noma (SCC) and adenocarcinoma (ACA). In the present study, cytology results of ASCUS were considered abnormally.19
Biopsies specimens that were collected in 0.1 M PBS (phosphate-buffered saline) solution, subjected to further histological analysis to determine the grade of cervical lesion and malignancy under the supervision of a specialized pathologist.
HPV DNA detection and genotyping
The presence of viral DNA in biopsy specimens and scraped cells collected from the cervix are being used as biological markers in the investigation of cervical HPV infections and HPV associated cervical cancer epidemiology.25
Genomic DNA from cervicovaginal liquid-based ThinPrep samples was extracted using the DNA Extraction Kit DNP, EX6071 (Sinaclon, Tehran, Iran) according to the manufacturer’s protocol. The purity of the extracted DNA was evaluated by NanoDrop spectrophotometer (Quantous Fluorimeter, Promega, Wisconsin, USA). Polymerase chain reaction (PCR) assays were used for the detection of HPV DNA in the cervical exfoliated cell (Thermal cycler T100, Bio-Rad, California, USA). The PCR assay for the β-globin gene was carried out as an internal control for each sample.6,24 Samples with positive results of β-globin were then amplified in PCR assay with general consensus GP5+/GP6+ primers, Forward GP5+ [5'-TTTGTTACTGTGGTAGATACTAC-3'] and Reverse GP6+ [5'-GAAAAATAAACTGTAAATCATATTC-3'] (Marcogen, Seoul 08511, Rep. of Korea).16,18
Subsequently, genotyping analysis of different HPV types was performed using INNO-LiPA HPV Genotyping Extra II Amp assay (Fujirebio Europe N.V, 9052 Gent, Belgium) according to the manufacturer’s instructions. This reverse-hybridization-based SPF10-INNO-LiPA assay is designed to be able to amplify almost 43 distinct genotypes. The short PCR product (65 base pair) has greatly improved the accuracy, sensitivity, and specificity of this assay26 which provides the ability to simultaneously identify 32 different distinct and even multiple genotypes in a single sample.15,16,27 In addition, the result of HPV genotyping with this technique depicted the high rate of similarity to the linear array.16
Statistical analysis
IBM Spss statics 23 software was used for comparative and descriptive analysis. The distribution and prevalence of HPV type-specific infection among cytologically normal samples and also along with cervical dysplasia levels were analyzed (descriptive analysis). Chi-square test was used to show the comparison between the prevalence of different groups and also the comparison of single and multi-type HPV infection risk and severity of cervical lesions according to their category. The significance value was considered as P < 0.05. The correlation between the frequency of HPV and the occurrence of ASCUS, LSIL, and HSIL and also increased number of multiple HPV infections and severity of cervical lesion were performed using the Spearman bivariate correlation test.15
Results
HPV DNA was detected in all patients with different grades of cervical malignancy (110 cases) and 244 of all 546 subjected women. All samples in groups II and III demonstrated the positive result in the β-globin gene PCR. As a result, to detect HPV DNA by PCR, all samples were determined appropriately.
Epidemiological distribution of HPV infection
31 out of overall 32 different genotypes except for HPV- 42 in which the INNO-LiPA HPV Genotyping Extra II Amp assay kit was able to detect, were found in the studied population. All 19 HR-HPV genotypes that the kit covered were observed. The infection rate of HR-HPV was 78% (346/ 445).
Prevalence of type-specific HPV
The ten most frequent HPV genotypes among all studied patients were: HPV- 53, 16, 31, 52, 6, 66, 39, 62, 89 and 58 respectively (Fig. 1).
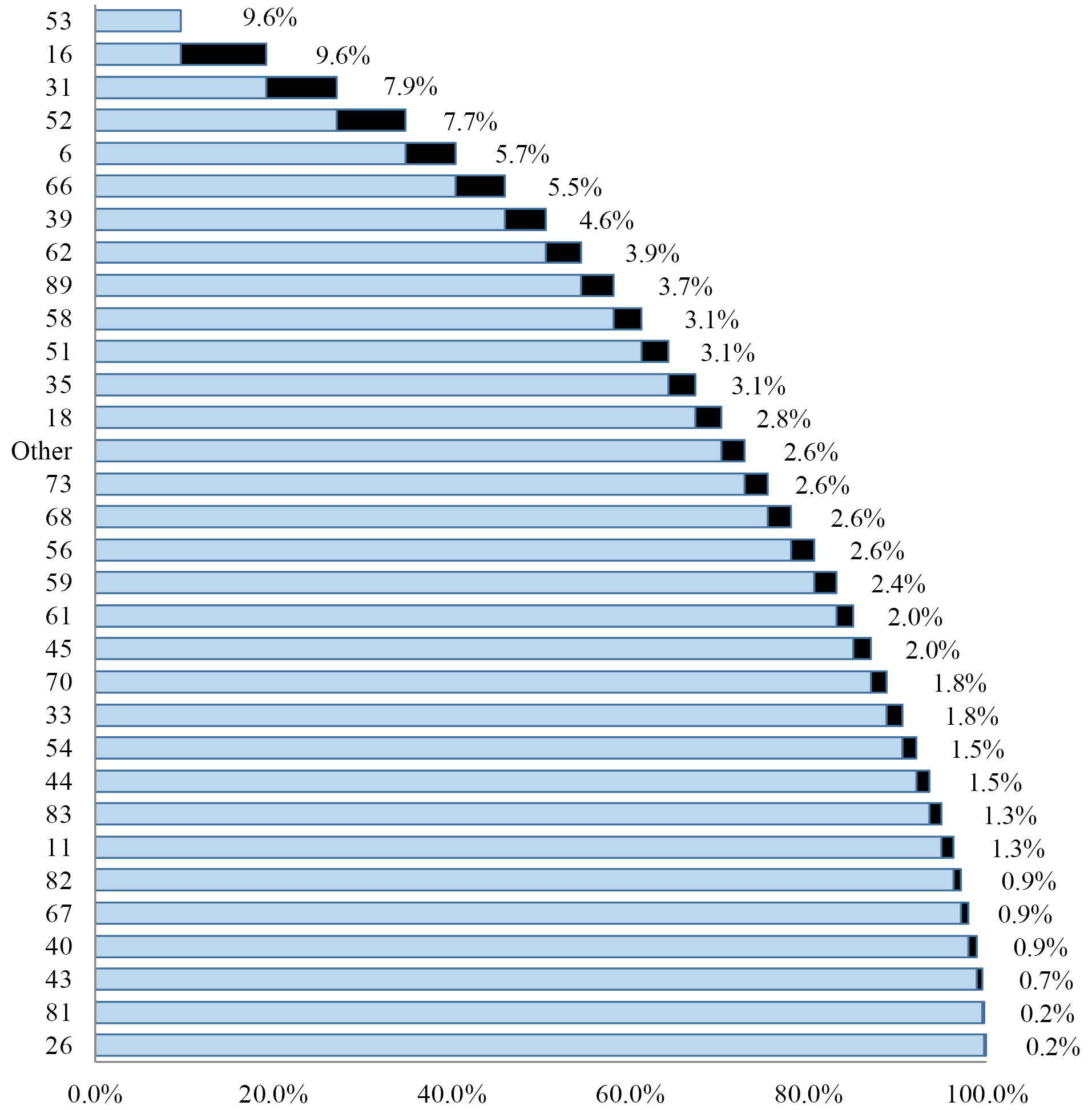
Fig. 1.
Cumulative frequency of all detected HPV genotypes.
.
Cumulative frequency of all detected HPV genotypes.
Among all 244 women who were tested positive for HPV infections, 191 cases had HR-HPV genotypes (78%), and 41 cases had LR genotypes (14%), the 12 left cases had other genotypes referred as unknown-risk HPVs (8%), there were also some kinds of genotypes in our sample population that could not be identified which were named as untypable.
Generally HPV-53 and HPV-16 (n= 44) followed by HPV-31 (n= 36) was the most prevalent genotypes among all subjects and also the most frequent HR genotypes. HPV-6 was the most prevalent LR genotype (n= 26) with a large difference in comparison with followed HPV-61 (n= 9) and HPV-54 (n= 7).
Out of 244 HPV-positive women, 111 cases (45.5%) were detected with multiple HPV genotypes infection, which of these, 55 cases (44.4%) had normal cervical cytology and 56 cases (51.9%) were associated with deferent cervical lesion grades. Of total 244 HPV-positive women, most subjects with normal cytology (69/134 cases) and LSIL (14/24 cases) showed single type HPV infections, on the other hand, most patients with ASCUS (40/74 cases) and HSIL (8/12 cases) had indicated multi-type HPV infections (Table 1).
Table 1.
Distribution of eachHPV Genotype regarding cervical lesion grade
|
HPV
|
|
NORMAL
|
ASCUS
|
LSIL
|
HSIL
|
Total
|
Std. Deviation
|
|
Single
|
Multiple
|
Single
|
Multiple
|
Single
|
Multiple
|
Single
|
Multiple
|
| HR |
HPV53 |
3 |
17 |
4 |
12 |
2 |
4 |
0 |
2 |
44 |
8.41 |
| HPV16 |
8 |
10 |
10 |
8 |
0 |
8 |
0 |
0 |
44 |
8.72 |
| HPV31 |
2 |
12 |
2 |
14 |
0 |
0 |
0 |
6 |
36 |
7.39 |
| HPV52 |
5 |
11 |
2 |
12 |
0 |
2 |
0 |
3 |
35 |
7.27 |
| HPV66 |
1 |
9 |
2 |
9 |
2 |
2 |
0 |
0 |
25 |
5.19 |
| HPV39 |
7 |
8 |
0 |
2 |
2 |
0 |
0 |
2 |
21 |
6.50 |
| HPV62 |
3 |
5 |
2 |
8 |
0 |
0 |
0 |
0 |
18 |
5.26 |
| HPV35 |
4 |
6 |
2 |
2 |
0 |
0 |
0 |
0 |
14 |
4.73 |
| HPV51 |
3 |
4 |
0 |
5 |
0 |
0 |
2 |
0 |
14 |
3.11 |
| HPV58 |
0 |
3 |
0 |
7 |
2 |
2 |
0 |
0 |
14 |
2.89 |
| HPV18 |
1 |
3 |
0 |
4 |
0 |
1 |
0 |
4 |
13 |
1.50 |
| HPV56 |
3 |
4 |
2 |
1 |
2 |
0 |
0 |
0 |
12 |
2.94 |
| HPV68 |
4 |
4 |
0 |
2 |
0 |
2 |
0 |
0 |
12 |
3.46 |
| HPV73 |
0 |
4 |
0 |
6 |
0 |
0 |
0 |
2 |
12 |
2.58 |
| HPV59 |
0 |
7 |
0 |
4 |
0 |
0 |
0 |
0 |
11 |
3.40 |
| HPV45 |
0 |
3 |
2 |
4 |
0 |
0 |
0 |
0 |
9 |
2.87 |
| HPV33 |
1 |
3 |
0 |
4 |
0 |
0 |
0 |
0 |
8 |
2.31 |
| HPV82 |
0 |
0 |
0 |
2 |
0 |
0 |
2 |
0 |
4 |
1.15 |
| LR |
HPV6 |
5 |
12 |
2 |
4 |
2 |
1 |
0 |
0 |
26 |
7.42 |
| HPV61 |
3 |
4 |
0 |
2 |
0 |
0 |
0 |
0 |
9 |
3.30 |
| HPV44 |
3 |
3 |
0 |
1 |
0 |
0 |
0 |
0 |
7 |
2.87 |
| HPV54 |
2 |
5 |
0 |
0 |
0 |
0 |
0 |
0 |
7 |
3.50 |
| HPV11 |
2 |
2 |
0 |
0 |
2 |
0 |
0 |
0 |
6 |
1.91 |
| HPV40 |
1 |
1 |
0 |
2 |
0 |
0 |
0 |
0 |
4 |
1.15 |
| HPV43 |
1 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
1.50 |
| HPV81 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
1 |
0.50 |
| UR |
HPV89 |
4 |
6 |
2 |
3 |
0 |
0 |
0 |
2 |
17 |
4.35 |
| HPV70 |
1 |
3 |
0 |
4 |
0 |
0 |
0 |
0 |
8 |
2.31 |
| HPV83 |
3 |
1 |
2 |
0 |
0 |
0 |
0 |
0 |
6 |
1.91 |
| HPV67 |
1 |
3 |
0 |
0 |
0 |
0 |
0 |
0 |
4 |
2.00 |
| HPV26 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0.50 |
| Total |
|
71 |
156 |
34 |
123 |
14 |
22 |
4 |
21 |
445 |
97.63 |
| Un Type |
|
10 |
0 |
2 |
0 |
12 |
4.76 |
| Total |
|
237 |
157 |
38 |
25 |
457 |
101.1 |
| Mean |
|
7.41 |
4.91 |
1.19 |
0.78 |
14.3 |
|
| Std. Deviation |
|
5.35 |
5.09 |
1.97 |
1.45 |
11.8 |
|
Cases with abnormal cytology results
Among all the 110 cases of the third group which had abnormal cytology results, repeated examination demonstrated ASCUS in 73 cases, LSIL in 24 cases, and HSIL in 12 cases. HR-HPV infection rate in that group was 89%, 82%, and 100%, respectively (Fig. 2). The analysis of the correlation between the frequency of HPV and the occurrence of ASCUS, LSIL, and HSIL showed a significant relation with P -value = 0.000 in all these three separate abnormal cytological reports.
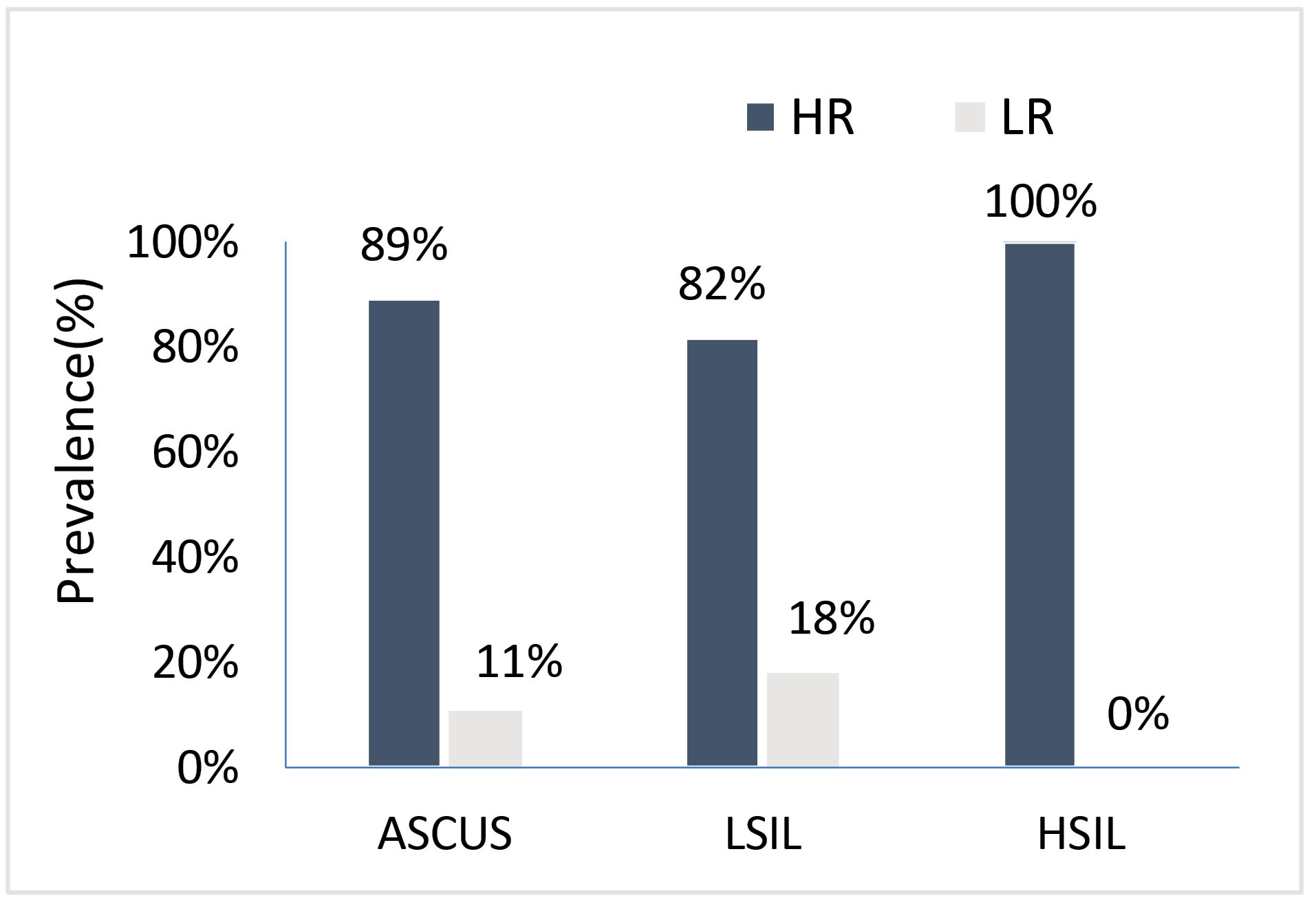
Fig. 2.
Prevalence of the infective rate of HR-HPV among all 110 cases with abnormal cervical cytology result.
.
Prevalence of the infective rate of HR-HPV among all 110 cases with abnormal cervical cytology result.
Among all 244 HPV positive cases, Single-type HPV infection was observed in 49.6%, while multi-type HPV infections (including ≥2 types) were found in 45.5% cases. Of these, 47 cases with two-type infection (42%), 40 cases with three-type infection (36%), 16 cases with four-type infection (14%), 4 cases with five-type infection (4%), and 2 cases with six and seven-type infection were detected (2%) (Fig. 3).
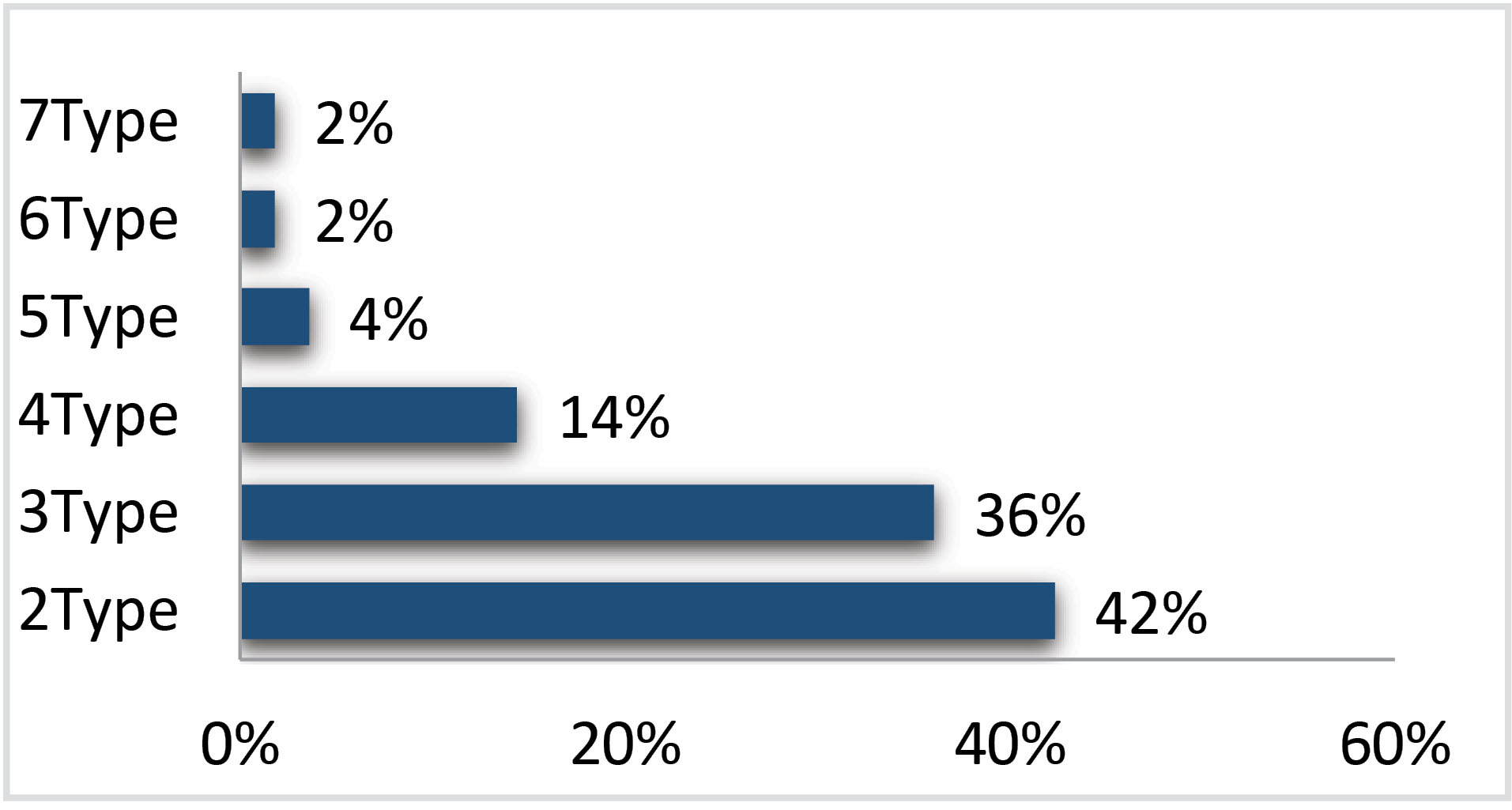
Fig. 3.
Prevalence of each multi-type infection in all HPV-positive cases.
.
Prevalence of each multi-type infection in all HPV-positive cases.
In this research, no significant relationship between single and multi-type HPV infection and intensive severity of cervical lesion was found, and also analyzing data showed no significant relationship between the increasing number of multi-type HPV infection and severity of CIN.
Distribution of HR-HPV genotypes based on cervical cytology findings and grades of the lesion are presented in Fig. 4.
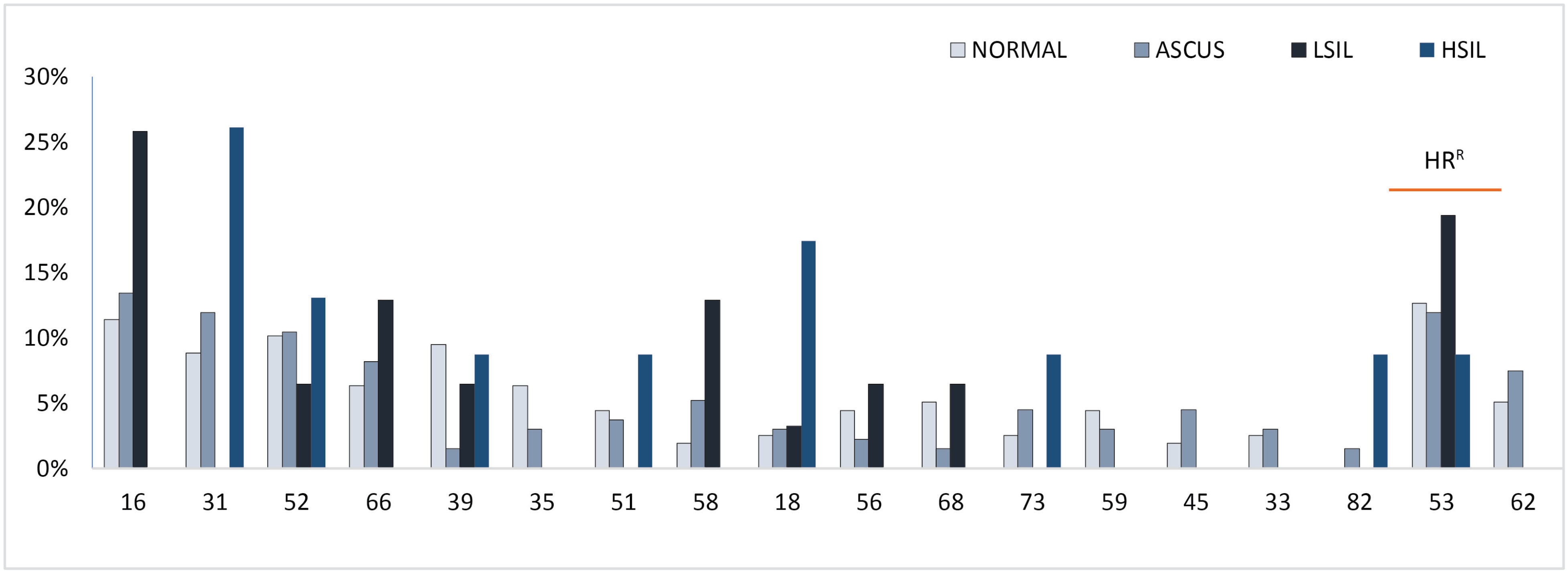
Fig. 4.
The High-risk HPV type distribution stratified by the grade of cervical lesion; HRR, Regional High-risk genotypes according to their prevalence and oncogenicity.
.
The High-risk HPV type distribution stratified by the grade of cervical lesion; HRR, Regional High-risk genotypes according to their prevalence and oncogenicity.
HPV-31 was the most prevailing genotype detected in HSIL findings with occurrence in 6 cases (26%) followed by HPV-18 and HPV-52. Besides, HPV-16 was the dominant genotype in ASCUS patients with 18 cases involved (13%) followed by HPV-53 and HPV-31. HPV-16 with the incidence in 8 patients with LSIL lesion (26%) was the most dominant genotype as well, followed by HPV-53 and HPV- 58. Finally, the most common genotype found in cytologically normal cases was HPV-53, which was discovered in 20 cases (13%) followed by HPV-16 and HPV-52 (Fig. 4).
The mean age of all cases with HPV positive infection (including women in the groups II and III) and HPV negative cases (women in the group I) was 33.8 and 30.2 years respectively. Moreover, the mean age of cases with normal cytology (all women in the groups I and II) was 30.7 years. ASCUS, LSIL and HSIL cases (i.e., the third group) had the mean age of 35.9, 36.3 and 37.6 years respectively (Fig. 5).
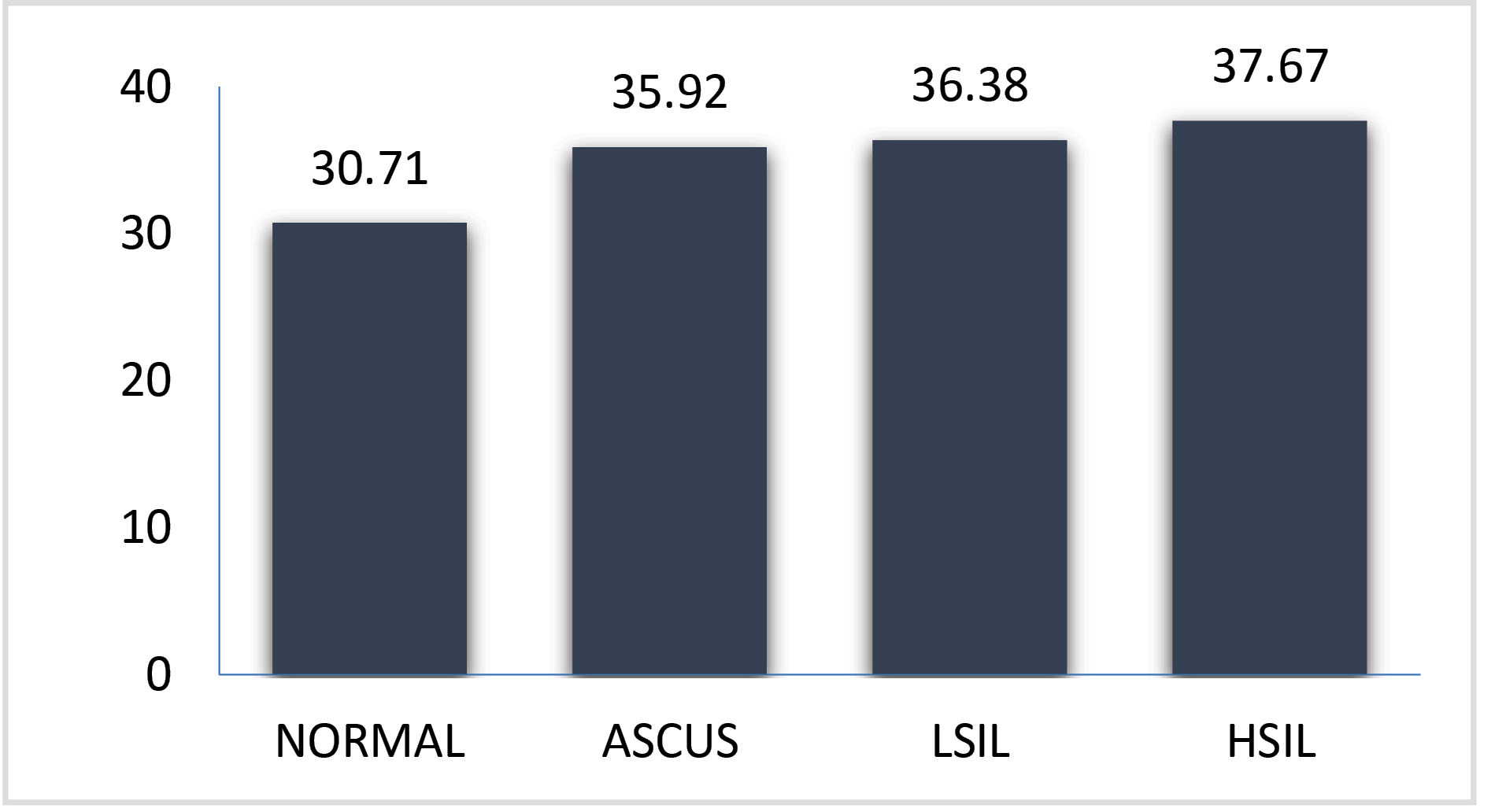
Fig. 5.
Mean Age of cases with normal cytology and cervical lesions.
.
Mean Age of cases with normal cytology and cervical lesions.
Discussion
Extensive studies and researches have proven that all cervical cancers and primary precancerous lesions are caused by infections with approximately 15 carcinogenic anogenital HPV genotypes and this is now widely accepted all around the world.17,27,28 Furthermore, the incidence rates of cervical cancer is far different in various geographical regions.29 Such differences (more than 10 times) are influenced by the interaction between infection distribution and development of effective screening programs.6
Sufficient information about the spread of type-specific HPV infections in different geographical regions is important from two aspects: first, estimation of the HPV vaccines effects in each region and second, its impression on effective screening planning.2
This study’s data presents the prevalence of HPV type-specific among over 540 women in Iran and describes the distribution of HPV genotypes with emphasizing regional HR genotypes in cervical samples of studied Iranian women with normal cytology and subjects with different grades of CIN.
Altogether 31 distinct HPV genotypes were identified in three separate subgroups, comprised 18 HR-HPV, 8 LR-HPV, and 5 UR-HPV genotypes. Among them, six new genotypes were identified (HPV- 61, 62, 70, 81, 83, 89) that have not been reported in the Iranian population.10,15,30 Unlike to previous reports, HPV-53 was the most frequent genotype alongside with HPV-16.23,31
The possibility of replacing the HR HPV DNA testing with conventional screening methods is currently under consideration.3
HPV-53 was the most prevalent genotype among the Iranian women with and without cervical neoplasia. Thus, according to the previous study by Salehi-Vaziri et al that reported HPV-53 as the most second frequent genotype in the Iranian population,15 we can comprehend that the oncogenicity of this genotype in Iran population is probably higher than the worldwide estimation. This opinion should be taken into future women's health programs.
Likely to the previous studies in Iran, the result of this research did not show a significant relationship between multiple HPV infections (in comparison with single infections) and increased risk of CIN. It should be noted that there is no consensus agreement with the entailment of multi-type HPV infection and CIN development yet.15 however, in a number of studies which was conducted in different parts of the world including China, Australia, and the United State, it has been shown a significant correlation between the multiple HPV genotypes infections and the increased risk of CIN and ICC.19
Divergent spread and distribution of HPV genotypes in different populations require a comprehensive review of this carcinogenic virus prevalence in various geographical areas. Erenow, there is no adequate information about the deployment of HPV among the Iranian population. And this determines the importance and necessity of more research in this field to promote immunization against HPV infections and expanding improvement of early screening and diagnostic tests.10
With an acceptable sample size, these findings effectively increase our knowledge of the HPV infection distribution among women with different grades of CIN in Iran. Of course, it should be stated that our study was limited as some other studies using a proportionally insensitive assay that could not identify all isolated genotypes. The predominant presence of multiple HPV infections in specimens with higher levels of cervical intraepithelial dysplasia in quite a larger sample size could be able to indicate that infection with multi-HPV genotypes might cause more lesion persistence and deterioration.
As the crucial role of the HPV infection has been proven in the development of CINs.9 In this study, HPV infection has been detected in all women with cervical lesions. In addition, there is no evidence of unrelated HPV infection cervical lesion persistence as a biological fact associated with the potential of ICC risk so far.32
Knowledge of molecular epidemiologic studies about HPV infections and subsequent cervical neoplasia has led to the development of cervical cancer prevention methods through using effective screening and HPV vaccination timely.33 The accurate and competent HPV genotyping is becoming more important, since (I) the oncogenicity of various HR-HPV genotypes and their association with severity of cervical lesion is so different, (II) development of multivalent vaccines is essential to improve the utility of vaccines and immunization against a greater range of HPVs, and (III) genotyping is very impressive for epidemiological studies to evaluate the incidence of HPV infection and awareness of different genotypes prevalence in various populations.16
The high prevalence of HPV-16 and HPV-18 in almost all cervical cancers worldwide has led to the production and development of HPV vaccines against HPV 6, 11, 16 and 18, which might have the capacity to decrease cervical cancers demonstrations.10
Although prophylactic vaccines against the most common carcinogenic HPV genotypes are available, the demand is still low. Therefore, it should be considered that although the early stage of cervical neoplasia and cancer are curable by surgery or radiotherapy, increasing level of deterioration and metastatic cancers are not treatable and in this regard, new therapeutic approaches would be better to be replaced.5
Conclusion
Our present study indicated that the transmission of HPV infection is highly increasing in Iran and the variety of detected HPV genotypes in studied subjects refers to the severity of this infection prevalence. Since the top three frequent genotypes in both groups of HPV-positive subjects were all HR according to their oncogenicity characterization either in women with normal cervical cytology or women with different grades of cervical malignancies, such a high frequency of this HR HPV types is an important public health concern and it is necessary for health politicians and decision-makers to immediately act effectively in this area. Although in most cases, HPV infections are benign and passing and do not pose a serious threat to human health but it should not be overlooked that persistent infection with HR genotypes can lead to the incidence and development of cervical and other anogenital cancers. Data obtained from participants of the study showed that the severity of cervical lesions increases with age. Hence, due to the relatively long interval between HPV infection and cervical cancer, timely diagnosis by screening and HPV testing can prevent the progression of ICC. Also in addition to previous statements, it should be noted that cervical cancer is preventable by improving initial screening programs and effective public vaccination.
Acknowledgments
The authors are thankful to the director and staff of Pasargad Microbiology Research Group for their professional collaboration.
Funding sources
The cost of this study was entirely borne by the authors and the study was not financially supported by any organization.
Ethical statement
The consent of each participant was declared by filling out the informed consent form and the code of National Committee of Ethics in Biomedicine Researches was approved in Kerman University of Medical Science (ID: IR.KMU.REC.1398.361).
Competing interests
The authors declare no competing of interest.
Authors’ contribution
BK and MM conceived the presented idea, designed the model and the computational framework, also planned the experiments and analyzed the data. MM developed the theory and performed the computations. BK and KA verified the analytical methods. BK encouraged MM to investigate [a specific aspect] and supervised the findings of this work and the whole project. MM, BK and AP contributed to sample preparation and interpretation of the results. MM and BK provides the study materials and equipment and also carried out the experiment. MM performed the analytic calculations and the numerical simulations and wrote the manuscript with support from BK’s supervision and KA helped to consultation of the project. Both MM and BK participated in presenting the last draft. All authors provided critical feedback furtherance the research.
Research Highlights
What is the current knowledge?
simple
-
√ All cervical cancers and primary precancerous lesions are
caused by carcinogenic types of HPV infections.
-
√ Cervical cancer rate differs significantly from one
geographical area to the others.
-
√ Localized HPV type-distribution is essential for designing
HPV vaccines.
What is new here?
simple
-
√ Six new genotypes identified (HPV-61, 62, 70, 81, 83, 89).
-
√ HPV-53 is the most frequent genotype alongside with
HPV-16.
-
√ Multiple HPV infections are dominant in patients with
HSIL and ASCUS.
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global Cancer Statistics , 2002. CA Cancer J Clin 2005; 55:74-108. doi: 10.3322/canjclin.55.2.74 [Crossref] [ Google Scholar]
- Clifford G, Franceschi S, Diaz M, Mu˜noz N, Villa LL. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine 24S3 2006; 3:26-34. doi: 10.1016/j.vaccine.2006.05.026 [Crossref] [ Google Scholar]
- Bao Y, Li N, Smith JS, Qiao Y. Human papillomavirus type distribution in women from Asia: a meta-analysis. Int J Gynecol Cancer 2008; 18:71-79. doi: 10.1111/j.1525-1438.2007.00959.x [Crossref] [ Google Scholar]
- Franceschi S, Plummer M, Mun N. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer 2003:63-73. doi: 10.1038/sj.bjc.6600688 [Crossref]
- Network TCGAR. Integrated genomic and molecular characterization of cervical cancer. Nature 2017; 543:378-384. doi: 10.1038/nature21386.Integrated [Crossref] [ Google Scholar]
- Schiffman M, Doorbar J, Wentzensen N, de Sanjosé S, Fakhry C, Monk BJ. Carcinogenic human papillomavirus infection. Nat Rev | Dis Prim 2016; 2:1-20. doi: 10.1038/nrdp.2016.86 [Crossref] [ Google Scholar]
- Mirabello L, Clarke MA, Nelson CW, Dean M, Wentzensen N, Yeager M. The intersection of HPV epidemiology, genomics and mechanistic studies of HPV-mediated carcinogenesis. Viruses 2018:10. doi: 10.3390/v10020080 [Crossref]
- Fakhraei F, Haghshenas MR. Human papillomaviruses and cancer. J Maz Univ Med Sci 2013; 23:458-480. doi: 10.1016/j.radonc.2013.06.004 [Crossref] [ Google Scholar]
- Aggarwal R, Gupta S, Nijhawan R, Suri V, Kaur A, Bhasin V. Prevalence of high - risk human papillomavirus infections in women with benign cervical cytology: A hospital based study from North India. Indian J Cancer 2006; 43:110-116. doi: 10.4103/0019-509X.27932 [Crossref] [ Google Scholar]
- Yousefzadeh A, Mostafavizadeh SM, Jarollahi A, Raeisi M, Garshasbi M, Siavashvahabi S. Human papillomavirus (HPV) prevalence and types among women attending regular gynecological visit in Tehran, Iran. Clin Lab 2014; 60(2):267-273. doi: 10.7754/clin.lab.2013.130221 [Crossref] [ Google Scholar]
- Abedini A, Karimi A, Shamsy S, Mansour Ghanaie R, Gholinejad Z. Seroepidemiological Evaluation of High-Risk Human Papillomavirus Types Among Married and Unmarried Iranian Women in Tehran , Iran. Arch Pediatr Infect Dis 2016; 4:4-8. doi: 10.5812/pedinfect.37766.Research [Crossref] [ Google Scholar]
- Thenarasu V, Gurunathan D. Human papilloma virus and cervical cancer. Biomed 2018; 38:453-455. doi: 10.1016/S0140-6736(13)60022-7 [Crossref] [ Google Scholar]
- Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV. Epidemiologic Classification of Human Papillomavirus Types Associated with Cervical Cancer. New Engl J Med Orig 2003; 348:518-527. doi: 10.1056/NEJMoa021641 [Crossref] [ Google Scholar]
- Erickson BK, Alvarez RD, Huh WK. Human Papillomavirus: What Every Provider Should Know. Am J Obs Gynecol 2014; 208:169-175. doi: 10.1016/j.ajog.2012.09.007.Human [Crossref] [ Google Scholar]
- Salehi-vaziri M, Sadeghi F, Hashemi FS, Haeri H, Bokharaei-Salim F, Monavari SH. Distribution of Human Papillomavirus Genotypes in Iranian Women According to the Severity of the Cervical Lesion. Iran Red Crescent Med J 2016; 18:16-21. doi: 10.5812/ircmj.24458 [Crossref] [ Google Scholar]
- Hamont DV, Ham MAPC, Bakkers JMJE, Massuger L, Melchers W. Evaluation of the SPF 10 -INNO LiPA Human Papillomavirus (HPV) Genotyping Test and the Roche Linear Array HPV Genotyping Test. J Clin Microbiol 2006; 44:3122-3129. doi: 10.1128/JCM.00517-06 [Crossref] [ Google Scholar]
- Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV. Epidemiologic Classification of Human Papillomavirus Types Associated with Cervical Cancer. N Engl J Med 2003; 348:518-527. doi: 10.1056/NEJMoa021641 [Crossref] [ Google Scholar]
- Schmitt M, Dondog B, Waterboer T, Pawlita M, Tommasino M, Gheit T. Abundance of Multiple High-Risk Human Papillomavirus (HPV) Infections Found in Cervical Cells Analyzed by Use of an Ultrasensitive HPV Genotyping Assay. J Clin Microbiol 2010; 48:143-149. doi: 10.1128/JCM.00991-09 [Crossref] [ Google Scholar]
- You W, Li S, Du R, Zheng J, Shen A. Epidemiological study of high-risk human papillomavirus infection in subjects with abnormal cytological findings in cervical cancer screening. Exp Ther Med 2018; 15:412-418. doi: 10.3892/etm.2017.5357 [Crossref] [ Google Scholar]
- Davey DD. Cervical cytology classification and the Bethesda System. Cancer J 2003; 9:327-334. doi: 10.1097/00130404-20030900000002 [Crossref] [ Google Scholar]
- Sotlar K, Stubner A, Diemer D, Menton S, Menton M, Dietz K. Detection of high-risk human papillomavirus E6 and E7 oncogene transcripts in cervical scrapes by nested RT-polymerase chain reaction. J Med Virol 2004; 74:107-116. doi: 10.1002/jmv.20153 [Crossref] [ Google Scholar]
- Castle PE, Solomon D, Schiffman M, Wheeler CM. Human Papillomavirus Type 16 Infections and 2-Year Absolute Risk of Cervical Precancer in Women With Equivocal or Mild Cytologic Abnormalities. J Natl Cancer Inst 2005; 97:14-19. doi: 10.1093/jnci/dji186 [Crossref] [ Google Scholar]
- Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta GM. Human Papillomavirus Genotype Distribution in Low-Grade Cervical Lesions: Comparison by Geographic Region and with Cervical Cancer. Cancer Epidemiol Biomarkers Prev 2005; 14:1157-1165. doi: 10.1158/1055-9965.EPI-04-0812 [Crossref] [ Google Scholar]
- Majidi A, Ghiasvand R, Hadji M, Nahvijou A, Mousavi AS, Pakgohar M. Original Article Priority Setting for Improvement of Cervical Cancer Prevention in Iran. Int J Health Policy Manag 2016; 5:225-232. doi: 10.15171/ijhpm.2015.201 [Crossref] [ Google Scholar]
- Bosch FX, Sanjos S De. The epidemiology of human papillomavirus infection and cervical cancer. IOS Press 2007; 23:213-227. doi: 10.1155/2007/914823 [Crossref] [ Google Scholar]
- Nilyanimit P, Chansaenroj J, Poomipak W, Praianantathavorn K, Payungporn S, Poovorawan Y. Comparison of Four Human Papillomavirus Genotyping Methods: Next-generation Sequencing, INNO-LiPA, Electrochemical DNA Chip, and Nested-PCR. Ann Lab Med 2018; 38:139-146. doi: 10.3343/alm.2018.38.2.139 [Crossref] [ Google Scholar]
- Castle PE, Porras C, Quint WG, Rodriguez AC, Schiffman M, Gravitt PE. Comparison of Two PCR-Based Human Papillomavirus Genotyping Methods. J Clin Microbiol 2008; 46:3437-3445. doi: 10.1128/JCM.00620-08 [Crossref] [ Google Scholar]
- Zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology 2009; 384:260-265. doi: 10.1016/j.virol.2008.11.046 [Crossref] [ Google Scholar]
- Clifford GM, Gallus S, Herrero R, Muñoz N, Snijders PJ, Vaccarella S. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet 2005; 366(9490):991-998. doi: 10.1016/S0140-6736(05)67069-9 [Crossref] [ Google Scholar]
- Jalilian S, Izadi B, Madani SH, Mohajeri P. The Prevalence and Genotype Distribution of Human Papillomavirus Types in the General Female Population in West of Iran. Jundishapur J Microbiol 2017; 10:e40855. doi: 10.5812/jjm.40855 [Crossref] [ Google Scholar]
- Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine 2008; 26:K1-K16. doi: 10.1016/j.vaccine.2008.05.064 [Crossref] [ Google Scholar]
- Zuna RE, Wang SS, Rosenthal DL, Jeronimo J, Schiffman M, Solomon D; ALTS Group. Determinants of human papillomavirus-negative, low-grade squamous intraepithelial lesions in the atypical squamous cells of undetermined significance/low-grade squamous intraepithelial lesions triage study (ALTS). CA Cancer J Clin 2005; 105:253-262. doi: 10.1002/cncr.21232 [Crossref] [ Google Scholar]
- Bosch FX, Broker TR, Forman D, Moscicki AB, Gillison ML, Doorbar J. Comprehensive Control of Human Papillomavirus Infections and Related Diseases. Vaccine 2013; 31:H1-H31. doi: 10.1016/j.vaccine.2013.10.003 [Crossref] [ Google Scholar]