Bioimpacts. 10(3):187-193.
doi: 10.34172/bi.2020.23
Original Research
Designing a light-activated recombinant alpha hemolysin for colorectal cancer targeting
Siamak Alizadeh 1, 2, Abolfazl Barzegari 2, Abolghasem Esmaeili 1, *, Yadollah Omidi 2, 3, * 
Author information:
1Department of Cell and Molecular Biology & Microbiology, Faculty of Biological Science and Technology, University of Isfahan, Isfahan, Iran
2Research Center for Pharmaceutical Nanotechnology, Biomedicine Institute, Tabriz University of Medical Sciences, Tabriz, Iran
3Department of Pharmaceutics, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
Colorectal cancer (CRC) is one of the main health burden worldwide, which can cause major economic and physiological problems along with relatively high rate of mortality. It is important to develop new methods for the localized delivery of recombinant protein therapeutics, in large part due to the failure of conventional therapies in most cases. Since E. coli Nissle 1917 (EcN) does not produce any virulence factors, here we used these bacteria with the light-activated promoter system to deliver therapeutic agents in the desired location and time.
Methods:
In this study, Staphylococcus aureus alpha hemolysin (SAH), after codon usage optimization, was cloned into blue light activating vector (pDawn) and transferred to EcN strain. Then, the functionality and cytotoxicity of secreted alpha hemolysin was evaluated in the SW480 colon cancer cell line by using different experiments, including blood agar test, flow cytometry analysis, and DAPI staining.
Results:
Our findings revealed that EcN can produce functional SAH under the blue light irradiation against SW480 cancer cells. Moreover, cytotoxicity assays confirmed the dose- and time-dependent toxicity of this payload (SAH) against SW480 cancer cells.
Conclusion:
Based on our results, EcN is proposed as an appropriate light-activated vehicle for delivery of anticancer agents to the target cancer cells/tissues.
Keywords: Alpha hemolysin, Colon cancer, E. coli Nissle 1917, Light-activated vector, Staphylococcus aureus
Copyright and License Information
© 2020 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide after lung and breast cancers, and the fourth-highest cause of oncological deaths, therefore representing a major public health problem.
1,2
Most of conventional treatments such as chemotherapy, radiotherapy, and surgery usually affect only proliferating cells, and often cannot penetrate the deepest part of the tissue. These limitations might enhance the chance of recurrence, metastasis, and mortality.
3
Bacteria can be exploited to deliver and/or produce therapeutic molecules in situ in the desired time and location. Based on such features, the use of bacterial therapy might improve the treatment efficacy significantly.
4,5
Of note, the use genetically-modified bacteria to deliver therapeutic macromolecules under a controllable manner can offer some advantages over the classic treatment methods. As a result, various promoter systems have been established for the controlled expression of genes in bacterial systems by different chemical inducers such as light.
5,6
The main advantage of light, in comparison with chemical inducers, is the ability of light to turn off the expression by eliminating the light source.
7
Over the past years , several types of natural and engineered bacterial species have been developed and used in cancer treatment, including Clostridium, Bifidobacterium, Salmonella, Bacillus, Listeria and Escherichia coli.
8-10
The E. coli Nissle 1917 (EcN) bacteria has widely been used to treat gastrointestinal (GI) disorders such as acute diarrhea, inflammatory bowel disease (IBD), and others,
11
in large part because of its highest tumor-targeting ability among the E. coli strains.
12
Ideally, protein payload should efficiently be released on-demand, penetrate tumor tissue, and effectively kill cancer cells.
13
The secreted protein must be functional after the secretion at the target site. In the case of solid tumors, it should also be noted that many bacterial species can aggregate in the extracellular space of tumors.
14
Various types of payloads have recently been used in bacterial therapy of tumors, including prodrug cleaving enzymes, cytokines, antigens and bacterial toxins.
15-18
Of these, the toxin, SAH, is a 34 kDa water-soluble and pore-forming hemolytic exotoxin that can cause membrane damage to many types of mammalian cells and induce apoptosis in cells.
19,20
Given that the majority of pore-forming proteins acts on the cells externally and do not require endocytosis, they are attractive candidate for the targeted therapy of tumor.
21
Based on these properties, in this study, EcN was selected as the carrier to deliver SAH to colon cancer SW480 cells. Thus, in this study, we hypothesized that the use of safe probiotic bacteria such as EcN, as a robust drug delivery system (DDS) with a triggereable drug release potential, might result in an improved cancer treatment.
Materials and Methods
Materials
SW480 cells were acquired from the National Cell Bank of Iran (Pasteur Institute, Tehran, Iran). RPMI-1640 media, fetal bovine serum (FBS) were purchased from Invitrogen (Paisley, UK). Restriction enzymes (Bam H 1 and Xho 1) and T4 DNA ligase were purchased from Thermo Fisher Scientific, (Waltham, MA, USA). Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit was obtained from eBioscience (Waltham, MA, USA). Kanamycin and DAPI (4, 6-diamidino- 2-phenylindole) were attained from Sigma-Aldrich Co. (Poole, UK). Plasmid DNA extraction kit and DNA gel extraction kit were obtained from Qiagen (Hilden, Germany). The pDawn plasmid was purchased from Addgene (Watertown, MA, USA).
Plasmid and strain
The pDawn plasmid was used for the expression of recombinant proteins in response to the blue light induction.
22
Kanamycin with the concentration of 50 μg/mL was used to maintain the strains transformed with the pDawn-SAH. The E. coli Nissle 1917 (serotype O6:K5:H1) was obtained from Mutaflor® (Herdecke, Germany) and used after the modification. The SW480 cells were normally cultured with a seeding density of 2.0×104 cells/cm2 and kept at 37°C and 5% CO2 in a humidified atmosphere.
Cloning of alpha hemolysin
Plasmid DNA extraction was carried out using Qiagen Mini Kit (Hilden, Germany). The alpha hemolysin ( hia gene) from S. aureus strain MW2 was codon optimized and synthesized with the appropriate restriction site for BamH I and Xho I restriction enzymes on the synthesized gene (GenScript, Singapore) for the expression in E. coli. Then, synthesized sequence was subcloned into pDawn plasmid (pDawn-SAH) using BamH I and Xho I restriction enzymes according to the protocols in published literature.
23
Afterward, the recombinant vector was transformed into the competent EcN using the heat shock method.
24
Briefly, 5 μL of DNA was added to 50 μL of the competent cells and gently mixed for a few times. Afterward, the competent cell/DNA mixture was incubated on ice for 20-30 minutes and located at 42°C in water bath for 45 seconds and placed back in ice for 2 minutes. The resultant bacteria were grown in LB media without antibiotic for 30 minutes in 37°C shaking incubator. Finally, some of the transformant bacteria were plated into LB agar media containing appropriate antibiotic. Consequently, the purified products were sequenced by Macrogene Company (Macrogene, Seoul, Korea).
Blood agar test
Overnight cultures of EcN transformed with pDawn-SAH were diluted 1:10
7
in LB and plated on blood base agar plates mixed with 5% sheep erythrocytes. After 12 hours of growth in the dark condition at 37°C for colony formation, plates were transferred to either light (blue∼480 nm, 37.1 μW/cm2) or dark conditions at 25°C for 24 hours prior to imaging.
25
Anticancer potential of SAH
Overnight cultures of EcN transformed with pDawn-SAH were diluted and allowed to grow to an OD600 of ∼1.0 and then induced with blue light (∼480 nm and 37.1 μW/ cm2) or dark conditions at 25°C for 24 hours. Then supernatant of induced and non-induced cultures were sterilized and subjected to next experimental procedures. Cytotoxicity assay was evaluated using different methods, including trypan blue assay, flow cytometry analysis,and DAPI staining.
Cell viability test
The cell viability assay was carried out using trypan blue exclusion test. Briefly, SW480 cells were seeded in a 24 well plate and incubated for 24 hours at 37°C with 5% CO2. Subsequently, the cells were treated with two different concentrations (0.5% and 1% (v/v)) of various supernatants and incubated up to 24 hours. Treated cells were washed with PBS (3x) and detached with trypsin. Then, 100 µL trypan blue was added to the cell suspension and maintained for 5 minutes at room temperature. Finally, the viable and dead cells were counted.
26
Flow cytometry analysis
The flow cytometry analysis was used to detect apoptotic and necrotic population of the treated SW480 cells. Manufacturer’s protocol was used to prepare samples. Shortly, cells were treated with supernatant of induced and non-induced cultures for 6 hours (OD600: 1.0) and centrifuged at 111 ×g for 5 minutes and cell pellets were washed with PBS (3x). Subsequently, the cells were stained with annexin V-FITC for 15 minutes, and incubated at room temperature in the dark. Finally, the cells were transferred to the PI binding buffer and prepared for the measurement of apoptosis. The Cell Quest software (Becton Dickinson, San Jose, USA) was used for the analysis of data.
DAPI staining
The SW480 cells were seeded in 6-well plates, allowed to reach 70% confluency, and treated with sterile supernatant of 6-hour induced and non-induced cultures. Then, 4% paraformaldehyde was used for the fixation of the cells for 10 minutes and washed with PBS (3x), then permeabilized with 0.1% Triton X-100 for 10 minutes. Finally, 4′, 6-diamidino-2- phenylindole (DAPI) was used to stain the treated cells for 5 minutes. The IX81 inverted fluorescent microscope (Olympus Corp., Tokyo, Japan) equipped with an Olympus DP72 digital camera was used to evaluate the morphology of the cells.
27
Results
Sub-cloning of alpha hemolysin
The pUC57 plasmid containing synthesized SAH and also pDawn plasmid were simultaneously subjected to BamH I and Xho I restriction enzymes and digested products were run in 2% gel electrophoresis. The digested product of hia gene was purified using DNA gel extraction kit and ligated with the restricted pDawn vector using T4 DNA ligase enzyme as shown in Fig. 1.
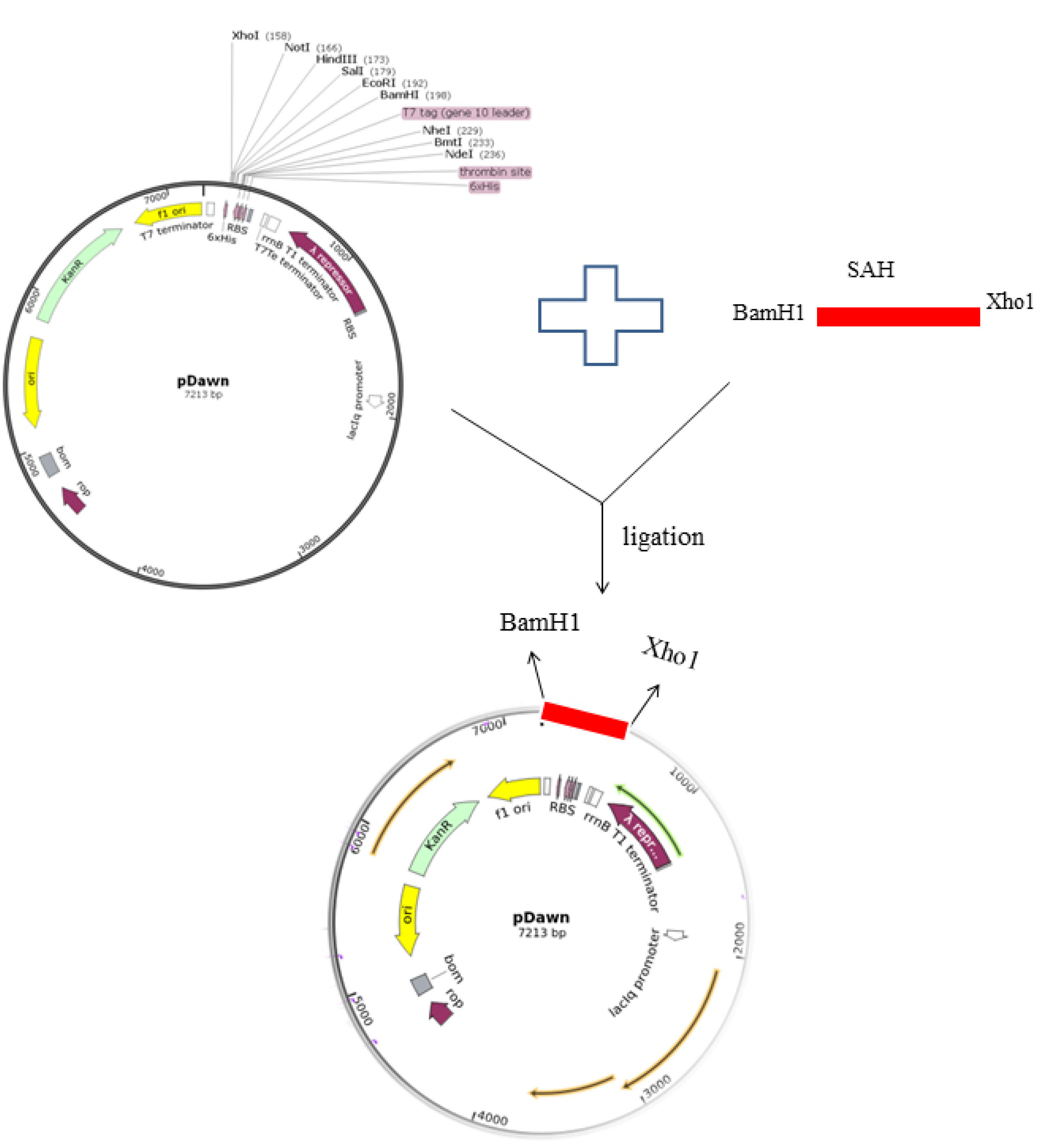
Fig. 1.
Schematic illustration of SAH sub-cloning in pDawn plasmid with restriction enzymes.
.
Schematic illustration of SAH sub-cloning in pDawn plasmid with restriction enzymes.
Blood agar assessment
The blood agar test was performed for the verification of the functional α-hemolysin production by the transformed EcN. For this purpose, the kanamycin-resistant transformants were cultured onto blood agar plates. Clear zones of lysis were observed around the colonies that were transformed with pDawn-SAH under the blue-light induction, while no clear zones of lysis were achieved in dark condition (Fig. 2).
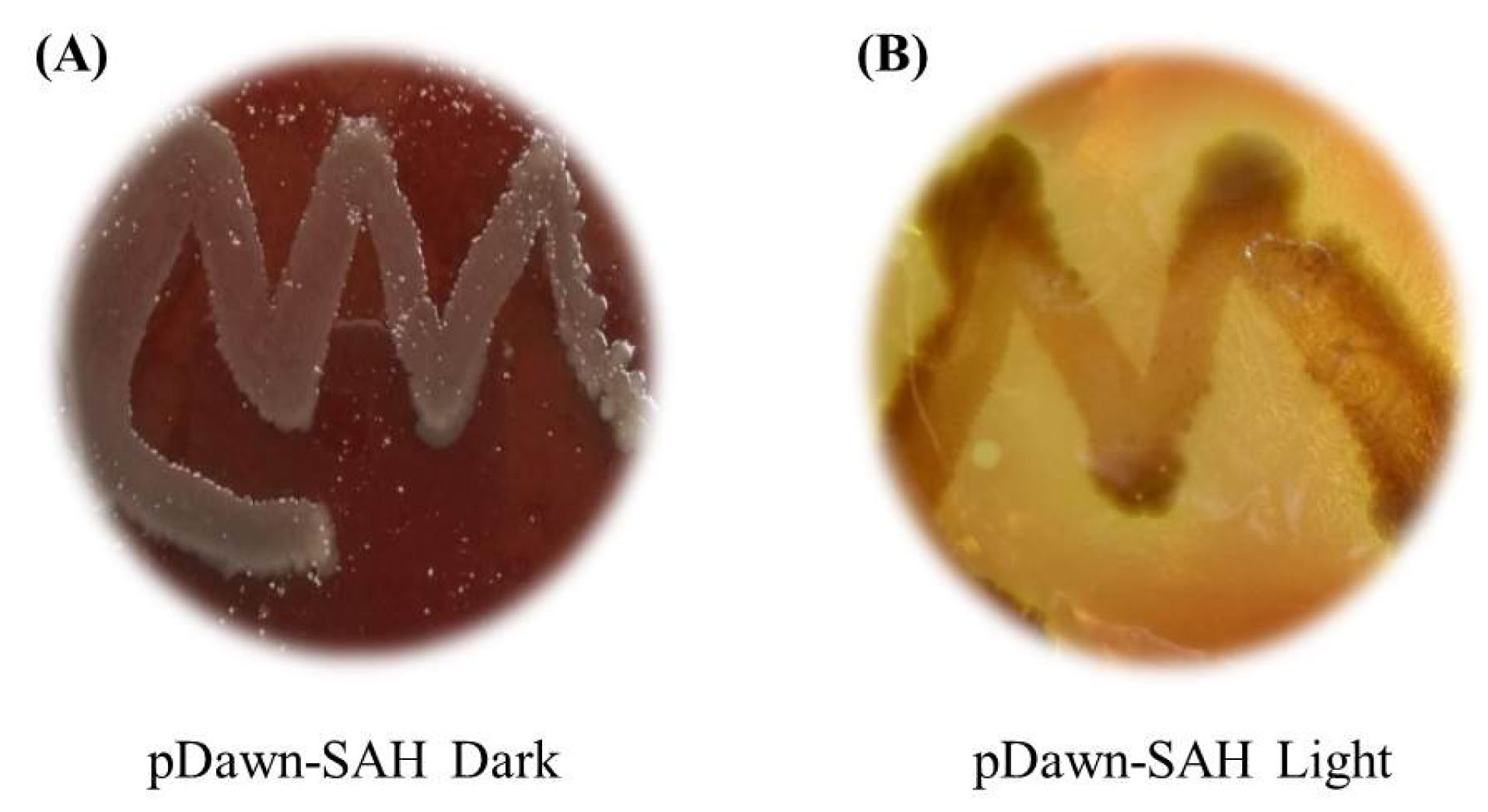
Fig. 2.
Blood agar tests with the pDawn-SAH transformed in EcN. (A) No clear zone in non-induced culture in dark. (B) Clear zone around the colonies indicating the blue light-dependent expression of α-hemolysin.
.
Blood agar tests with the pDawn-SAH transformed in EcN. (A) No clear zone in non-induced culture in dark. (B) Clear zone around the colonies indicating the blue light-dependent expression of α-hemolysin.
Cell proliferation assay
The trypan blue staining is a rapid and convenient assay that can analyze the number of live (unstained) and dead (blue) cells by means of light microscope.
26
In this study, fresh supernatants of EcN, EcN-dark and EcN-blue light were subjected to SW480 cells in various concentrations of 0.5% and 1 % (v/v). As shown in Fig. 3, the rate of dead cells treated with 0.5% (v/v) of supernatant were more than 50% in the EcN-blue light and about 20% in EcN and EcN-dark in 10 hours, while in the cells treated with 1% (v/v) these ratios were more than 80% for EcN-blue light and about 30% for EcN and EcN-dark in 10 hours. Therefore, these results indicated that the rate of dead cells including apoptotic and necrotic cells were increased by increasing the time and concentration of toxin.
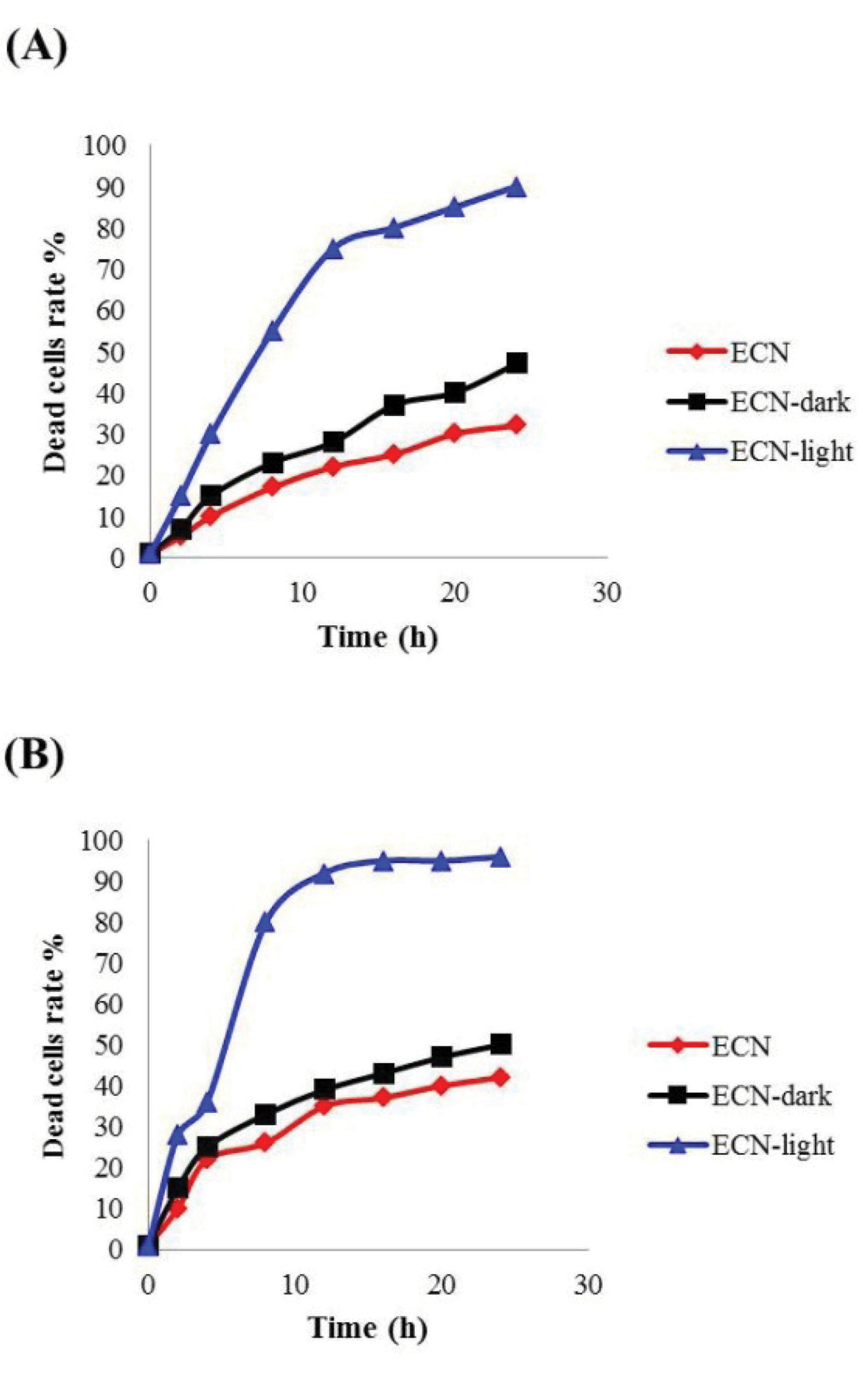
Fig. 3.
Effect of different supernatants on the SW80 cells. Fresh supernatants of EcN (control without vector), EcN-dark (control with vector) and EcN-blue light (EcN including pDawn-SAH induced with blue light) were administered to SW80 cells in a final concentration of (A) 0.5% and (B)1% (v), (n=3).
.
Effect of different supernatants on the SW80 cells. Fresh supernatants of EcN (control without vector), EcN-dark (control with vector) and EcN-blue light (EcN including pDawn-SAH induced with blue light) were administered to SW80 cells in a final concentration of (A) 0.5% and (B)1% (v), (n=3).
Annexin V apoptosis detection by flow cytometry
Annexin V based flow cytometry assay was carried out for the separation of the necrotic and apoptotic cells from the normal cells. Annexin V binds to phosphatidylserine (PS) of the plasma membrane that externalized after apoptosis.
28
Compared to the untreated control cells (Fig. 4A), about 26.34% apoptosis and 1.20% necrosis were observed in the SW480 cells treated with sterile supernatant of non-induced (Fig. 4B), while 36.08% apoptosis and 48.41% necrosis were observed in the SW480 cells treated with the sterile supernatant of induced cells (Fig. 4C).
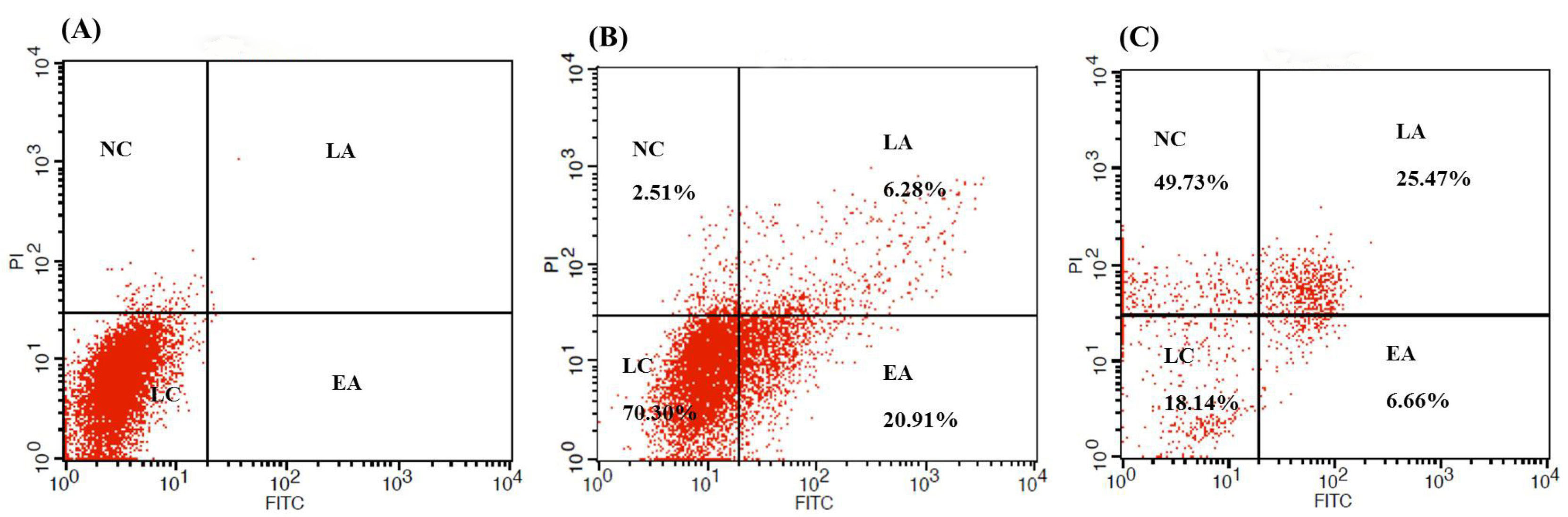
Fig. 4.
The flow cytometry analysis of the FITC-AnnexinV / PI showing apoptotic and necrotic cells rate in the unstain SW480 cells (A) and cells treated with sterile supernatant of non-induced panel (B) and induced (C) cultures for 6 hours. NC: Necrotic cells; EA: Early apoptosis; LA: Late apoptosis; LC: Living cells.
.
The flow cytometry analysis of the FITC-AnnexinV / PI showing apoptotic and necrotic cells rate in the unstain SW480 cells (A) and cells treated with sterile supernatant of non-induced panel (B) and induced (C) cultures for 6 hours. NC: Necrotic cells; EA: Early apoptosis; LA: Late apoptosis; LC: Living cells.
DAPI staining and cell apoptosis
The analysis of the morphological changes was performed using DAPI staining after 6 hours of the treatment with the sterile supernatant of non-induced and induced cultures (Fig. 5). As compared to the control cells (Fig. 5A), no morphological changes were observable in the treated non-induced cells (Fig. 5B), while a significant fragmentation was observed in the nucleus of the light-induced cells (Fig. 5C) .
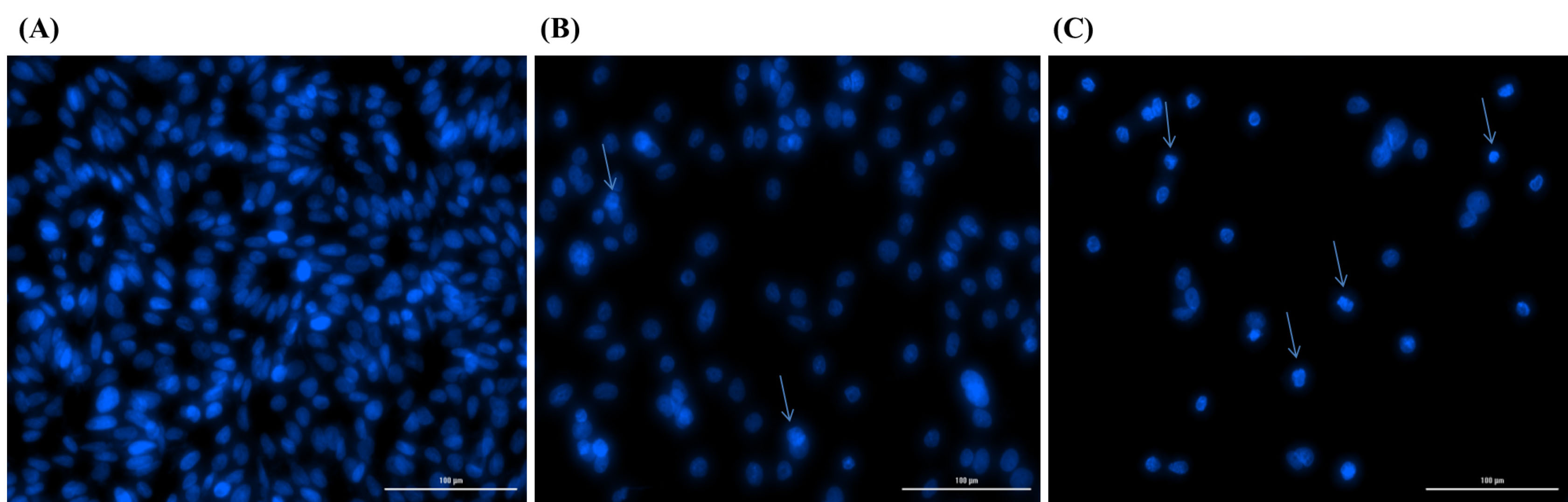
Fig. 5.
Representative photograph showing DAPI staining of untreated control SW480 cells (A) and cells treated with sterile supernatant of non-induced (B) and induced (C) cultures for 6 hours. Morphological changes such as nuclear fragmentation and chromatin condensation reveals apoptosis in blue light induced treated cells (C).
.
Representative photograph showing DAPI staining of untreated control SW480 cells (A) and cells treated with sterile supernatant of non-induced (B) and induced (C) cultures for 6 hours. Morphological changes such as nuclear fragmentation and chromatin condensation reveals apoptosis in blue light induced treated cells (C).
Discussion
In this study, we capitalized on the EcN as a vehicle to deliver biologically active SAH to the CRC cells. SAH is a pore forming protein whose cytolytic and/or cytotoxic activity against many mammalian cell types has been shown in several studies.
20,29
It can cause membrane damage in the multiple cell types without need to endocytosis that makes it an effective treatment in multidrug resistant tumors.
30
The use of live engineered bacteria, as nonpathogenic safe strains with the controllable stimuli, offer the localized and continuous drug delivery potential to prevent malignant cells from restoring their membrane by persistent representation of the toxin.
31
Different light-inducible gene expression strategies have recently been reported in mammalian cells with some advantages such as the tunability potential and the spatiotemporal impacts.
32,33
This study was divided into two parts. First, we cloned and evaluated the functional expression of SAH protein in the presence of blue light (induced) and without light (non-induced) using the pDawn plasmid (Fig. 1) as a light-activated expression system for light regulated expression of recombinant proteins in E. coli. Second, we looked at the anticancer effect of produced SAH against SW480 cells by different techniques. Our results indicated that EcN can successfully express and secrete SAH into the culture under the blue light induction. This finding was in agreement with a recent study that indicated effective expression of cytolysin A by EcN under blue light induction.
34
Of note, the main objective in the construction of gene products is the assessment of the functionality of products. Because some modifications made in gene structure like His-tag addition to the N-terminal or C-terminal may show somewhat conflict with the formation of native and functional protein structure. Blood agar test is a simple and cost-effective experiment that has been done in many studies for the evaluation of cloned hemolysin.
19,25
Blood agar test confirmed that the secreted SAH protein is fully functional and does not need any additional modification(s). We also investigate the cytotoxicity of produced toxin on the SW480 cells. It should be pointed out that rypan blue is used in cell viability test, in which live cells have undamaged plasma membranes that can eliminate different chemicals like trypan blue, while dead cells have broken plasma membranes and cannot exclude trypan blue.
26
Since pore forming toxins cause host cell membrane damage, it is possible that the created pores by toxin may allow trypan blue to enter the host cell.
19,35
The cell viability assay accomplished in this study confirmed that affectivity of SAH is largely dependent on the exposure time of toxin (Fig. 2). Such a finding was in good agreement with other experiments accomplished with bacterial toxins.
35
Likewise, based on the literature, effect of SAH on the cells depends on the amount of toxin and cell types. For example, the high concentration of toxin mostly yields necrosis, while the low concentration of toxin might induce apoptosis or inflammatory response in the target cells.
36-38
Our results were found to be in agreements with such findings. As confirmed by the flow cytometry results, supernatant of non-induced cultures could cause apoptosis (Fig. 3B), while supernatant of light induced cultures cause necrosis more than apoptosis in SW480 cells (Fig. 3C). Furthermore, the presence of apoptosis in non-induced cultures can elicit this suggestion that EcN alone can induce apoptosis to a certain degree or EcN express SAH to some extent without the blue light induction. This finding was further confirmed by DAPI staining method. A few number of apoptotic cells were observed in non-induced treatments compared to the control cells (Fig. 5), and clear apoptosis was observed in light-induced treatment (Fig. 5A). Mechanistically, SAH is speculated to directly elicit membrane damage in the cell membrane and perhaps interfere with the function of cell surface transporters too (Fig. 6). Notably, anticancer properties of probiotic bacteria can occur through the regulation of different stages of signaling pathways and hence the induction of apoptosis as reported in many studies.
39-41
Besides, some clinical studies have reported the in situ expression or delivery of tumor-specific proteins, antibodies, cytokines, and prodrugs. It seems they are considered to be a relatively applicable delivey system while the safety and effective tumor-colonization feature of these bacteria need to be fully addressed.
42,43
Taken all, safe non-pathogenic probiotic strains like EcN could offer some benefits, including simplicity in genetic manipulation, and lack of toxicity to the normal cells. Based on these results, EcN could be used as a competent drug delivery vehicle for the localized delivery of anticancer agents.
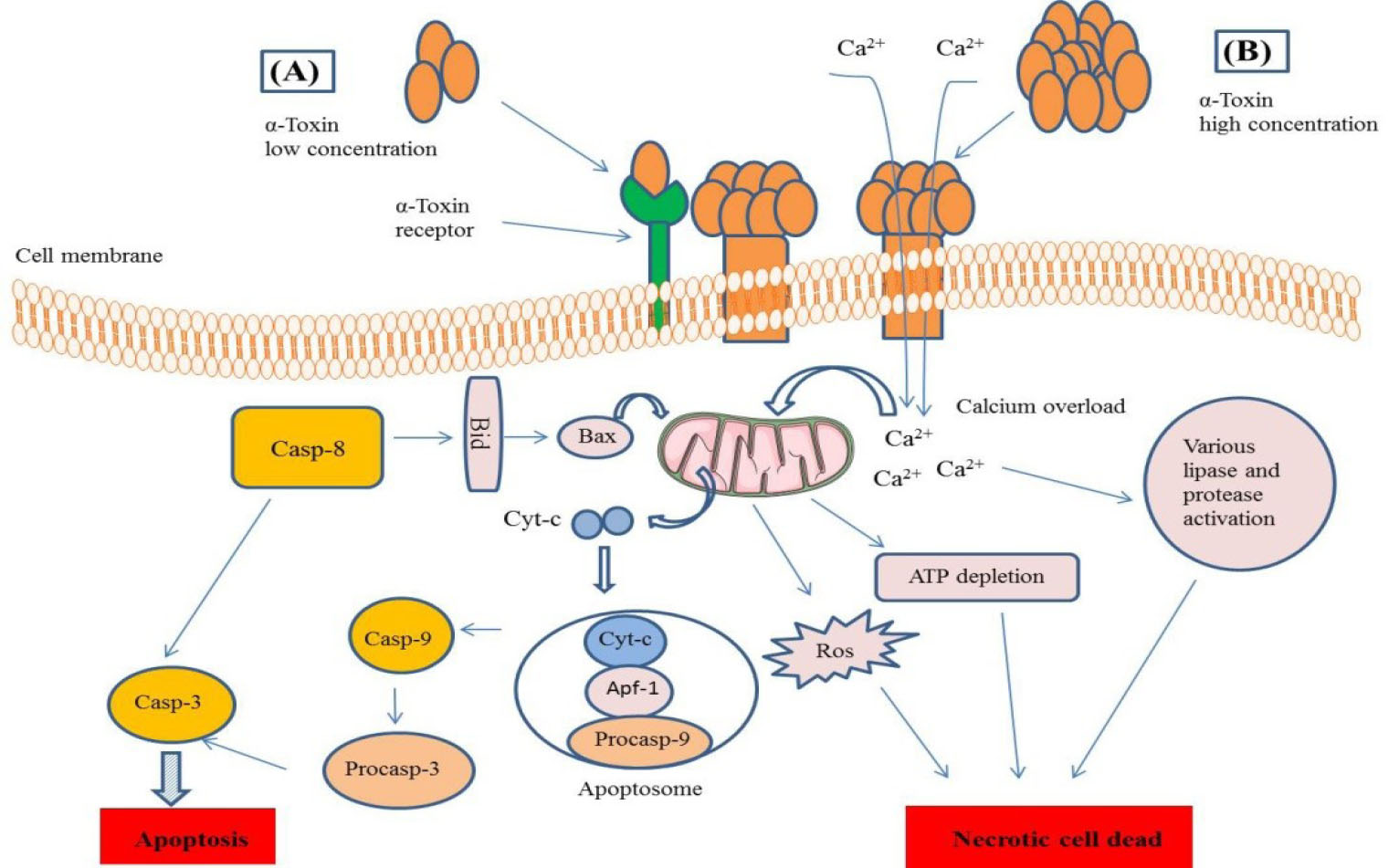
Fig. 6.
Schematic representation of α- toxin pore formation mechanism and cell death pathways. (A) The α- toxin at low concentration usually attaches to specific receptor (ADAM10) to oligomerize and form pore and trigger intrinsic or caspase related apoptotic pathway. (B) At the high concentration, it makes membrane pore without need to the specific receptors and trigger apoptotic or necrotic cell dead signaling pathways through Ca
2+
influx and perturbation of Ca
2+
or other ions hemostasis.
.
Schematic representation of α- toxin pore formation mechanism and cell death pathways. (A) The α- toxin at low concentration usually attaches to specific receptor (ADAM10) to oligomerize and form pore and trigger intrinsic or caspase related apoptotic pathway. (B) At the high concentration, it makes membrane pore without need to the specific receptors and trigger apoptotic or necrotic cell dead signaling pathways through Ca
2+
influx and perturbation of Ca
2+
or other ions hemostasis.
Conclusion
Since the cancer is a complex disease with different mechanisms of creation, chemotherapy or other treatment approaches are not enough to cure all parts of the cancer alone.
44,45
Therefore, combination of conventional therapies with new techniques seems to be more profitable. Bacterial based therapy specially using nonpathogenic or safe strain displays more promises due to possible capability of bacteria to deliver active therapeutics into the cancerous tissue. Therefore we aimed to investigate anticancer properties of SAH delivered in the nonpathogenic EcN by induction of blue light in vitro. Based upon our results, EcN is a good bacterial system to deliver active therapeutic into the tumoral region by light controlled manner as well as SAH which has strong cytotoxicity against SW480 colon cancer cells. However, additional studies with various cell lines and also in vivo examinations are needed for acquiring precise information for the clinical application.
Acknowledgement
The authors would like to thank the Research Center for Pharmaceutical Nanotechnology (RCPN) at Tabriz University of Medical Sciences (Tabriz, Iran) and Isfahan University (Isfahan, Iran) for the financial and technical support of this project.
Funding Sources
This study is part of a PhD thesis funded by RCPN of Tabriz University of Medical Sciences.
Ethical statement
None to be declared.
Competing interests
The authors declare that YO is the EIC of the journal and had no influnce on the peer-review process, which was conducted carefully based on COPE guideline.
References
- Favoriti P, Carbone G, Greco M, Pirozzi F, Pirozzi REM, Corcione F. Worldwide burden of colorectal cancer: a review. Updates surg 2016; 68:7-11. doi: 10.1007/s13304-016-0359-y [Crossref] [ Google Scholar]
- Khiavi MA, Safary A, Aghanejad A, Barar J, Rasta SH, Golchin A. Enzyme-conjugated gold nanoparticles for combined enzyme and photothermal therapy of colon cancer cells. Colloid Surf a Physicochem Eng Asp 2019; 572:333-44. doi: 10.1016/j.colsurfa.2019.04.019 [Crossref] [ Google Scholar]
- Jean ATS, Swofford CA, Panteli JT, Brentzel ZJ, Forbes NS. Bacterial delivery of Staphylococcus aureus α-hemolysin causes regression and necrosis in murine tumors. Mol Ther 2014; 22:1266-74. doi: 10.1038/mt.2014.36 [Crossref] [ Google Scholar]
- Forbes NS. Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer 2010; 10:785-94. doi: 10.1038/nrc2934 [Crossref] [ Google Scholar]
- Hartman AH, Liu H, Melville SB. Construction and characterization of a lactose-inducible promoter system for controlled gene expression in Clostridium perfringens. Appl Environ Microbiol 2011; 77:471-8. doi: 10.1128/AEM.01536-10 [Crossref] [ Google Scholar]
- Terpe K. Overview of bacterial expression systems for heterologous protein production: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol 2006; 72:211. doi: 10.1007/s00253-006-0465-8 [Crossref] [ Google Scholar]
- Polstein LR, Gersbach CA. Light-inducible spatiotemporal control of gene activation by customizable zinc finger transcription factors. J Am Chem Soc 2012; 134:16480-3. doi: 10.1021/ja3065667 [Crossref] [ Google Scholar]
- Tangney M, Gahan CG. Listeria monocytogenes as a vector for anti-cancer therapies. Curr Gene Ther 2010; 10:46-55. doi: 10.2174/156652310790945539 [Crossref] [ Google Scholar]
- Cronin M, Morrissey D, Rajendran S, El Mashad SM, Van Sinderen D, O'sullivan GC. Orally administered bifidobacteria as vehicles for delivery of agents to systemic tumors. Mol Ther 2010; 18:1397-407. doi: 10.1038/mt.2010.59 [Crossref] [ Google Scholar]
- Pawelek JM, Low KB, Bermudes D. Bacteria as tumour-targeting vectors. Lancet Oncol 2003; 4:548-56. doi: 10.1016/S1470-2045(03)01194-X [Crossref] [ Google Scholar]
- Schultz M. Clinical use of E coli Nissle 1917 in inflammatory bowel disease. Inflamm Bowel Dis 2008; 14:1012-8. doi: 10.1002/ibd.20377 [Crossref] [ Google Scholar]
- Stritzker J, Weibel S, Hill PJ, Oelschlaeger TA, Goebel W, Szalay AA. Tumor-specific colonization, tissue distribution, and gene induction by probiotic Escherichia coli Nissle 1917 in live mice. Int J Med Microbiol 2007; 297:151-62. doi: 10.1016/j.ijmm.2007.01.008 [Crossref] [ Google Scholar]
- St Jean AT, Swofford CA, Panteli JT, Brentzel ZJ, Forbes NS. Bacterial delivery of Staphylococcus aureus alpha-hemolysin causes regression and necrosis in murine tumors. Mol Ther 2014; 22:1266-74. doi: 10.1038/mt.2014.36 [Crossref] [ Google Scholar]
- Kasinskas RW, Forbes NS. Salmonella typhimurium lacking ribose chemoreceptors localize in tumor quiescence and induce apoptosis. Cancer Res 2007; 67:3201-9. doi: 10.1158/0008-5472.can-06-2618 [Crossref] [ Google Scholar]
- Theys J, Pennington O, Dubois L, Anlezark G, Vaughan T, Mengesha A. Repeated cycles of Clostridium-directed enzyme prodrug therapy result in sustained antitumour effects in vivo. Br J Cancer 2006; 95:1212-9. doi: 10.1038/sj.bjc.6603367 [Crossref] [ Google Scholar]
- Gentschev I, Fensterle J, Schmidt A, Potapenko T, Troppmair J, Goebel W. Use of a recombinant Salmonella enterica serovar Typhimurium strain expressing C-Raf for protection against C-Raf induced lung adenoma in mice. BMC Cancer 2005; 5:15. doi: 10.1186/1471-2407-5-15 [Crossref] [ Google Scholar]
- al-Ramadi BK, Fernandez-Cabezudo MJ, El-Hasasna H, Al-Salam S, Bashir G, Chouaib S. Potent anti-tumor activity of systemically-administered IL2-expressing Salmonella correlates with decreased angiogenesis and enhanced tumor apoptosis. Clin Immunol 2009; 130:89-97. doi: 10.1016/j.clim.2008.08.021 [Crossref] [ Google Scholar]
- Jiang SN, Phan TX, Nam TK, Nguyen VH, Kim HS, Bom HS. Inhibition of tumor growth and metastasis by a combination of Escherichia coli-mediated cytolytic therapy and radiotherapy. Mol Ther 2010; 18:635-42. doi: 10.1038/mt.2009.295 [Crossref] [ Google Scholar]
- Abdel-Fattah GM, Hafez EE, Zaki ME, Darwesh NM. Cloning and Expression of Alpha Hemolysin Toxin Gene of Staphylococcus aureus Against Human Cancer Tissue. Int J Appl Sci Biotechnol 2017; 5:22-9. [ Google Scholar]
- Jonas D, Walev I, Berger T, Liebetrau M, Palmer M, Bhakdi S. Novel path to apoptosis: small transmembrane pores created by staphylococcal alpha-toxin in T lymphocytes evoke internucleosomal DNA degradation. Infect Immun 1994; 62:1304-12. [ Google Scholar]
- Panchal RG, Smart ML, Bowser DN, Williams DA, Petrou S. Pore-forming proteins and their application in biotechnology. Curr Pharm Biotechnol 2002; 3:99-115. doi: 10.2174/1389201023378418 [Crossref] [ Google Scholar]
- Ohlendorf R, Vidavski RR, Eldar A, Moffat K, Möglich A. From dusk till dawn: one-plasmid systems for light-regulated gene expression. J Mol Biol 2012; 416:534-42. doi: 10.1016/j.jmb.2012.01.001 [Crossref] [ Google Scholar]
- Shanehbandi D, Saei AA, Zarredar H, Barzegari A. Vibration and glycerol-mediated plasmid DNA transformation for Escherichia coli. FEMS Microbiol Lett 2013; 348:74-8. doi: 10.1111/1574-6968.12247 [Crossref] [ Google Scholar]
- Froger A, Hall JE. Transformation of plasmid DNA into E coli using the heat shock method. J Vis Exp 2007:e253. doi: 10.3791/253 [Crossref]
- Magaraci MS, Veerakumar A, Qiao P, Amurthur A, Lee JY, Miller JS. Engineering Escherichia coli for light-activated cytolysis of mammalian cells. ACS Publications 2014. doi: 10.1021/sb400174s [Crossref]
-
Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol 2015; 111: A3. B. 1-A3. B. 10.1002/0471142735.ima03bs111
- Saadat YR, Saeidi N, Vahed SZ, Barzegari A, Barar J. An update to DNA ladder assay for apoptosis detection. BioImpacts 2015; 5:25. doi: 10.15171/bi.2015.01 [Crossref] [ Google Scholar]
- Asoudeh-Fard A, Barzegari A, Dehnad A, Bastani S, Golchin A, Omidi Y. Lactobacillus plantarum induces apoptosis in oral cancer KB cells through upregulation of PTEN and downregulation of MAPK signalling pathways. BioImpacts 2017; 7:193. doi: 10.15171/bi.2017.22 [Crossref] [ Google Scholar]
- Walev I, Martin E, Jonas D, Mohamadzadeh M, Müller-Klieser W, Kunz L. Staphylococcal alpha-toxin kills human keratinocytes by permeabilizing the plasma membrane for monovalent ions. Infect Immun 1993; 61:4972-9. [ Google Scholar]
- Berube B, Wardenburg J. Staphylococcus aureus α-toxin: nearly a century of intrigue. Toxins 2013; 5:1140-66. doi: 10.3390/toxins5061140 [Crossref] [ Google Scholar]
- Ganai S, Arenas R, Forbes N. Tumour-targeted delivery of TRAIL using Salmonella typhimurium enhances breast cancer survival in mice. Br J Cancer 2009; 101:1683. doi: 10.1038/sj.bjc.6605403 [Crossref] [ Google Scholar]
- Muller K, Naumann S, Weber W, Zurbriggen MD. Optogenetics for gene expression in mammalian cells. Biol Chem 2015; 396:145-52. doi: 10.1515/hsz-2014-0199 [Crossref] [ Google Scholar]
- Lin F, Dong L, Wang W, Liu Y, Huang W, Cai Z. An Efficient Light-Inducible P53 Expression System for Inhibiting Proliferation of Bladder Cancer Cell. Int J Biol Sci 2016; 12:1273-8. doi: 10.7150/ijbs.16162 [Crossref] [ Google Scholar]
- Magaraci MS, Veerakumar A, Qiao P, Amurthur A, Lee JY, Miller JS. Engineering Escherichia coli for light-activated cytolysis of mammalian cells. ACS Synth Biol 2014; 3:944-8. doi: 10.1021/sb400174s [Crossref] [ Google Scholar]
- Tran S-L, Puhar A, Ngo-Camus M, Ramarao N. Trypan blue dye enters viable cells incubated with the pore-forming toxin HlyII of Bacillus cereus. PloS One 2011; 6:e22876. doi: 10.1371/journal.pone.0022876 [Crossref] [ Google Scholar]
- Haslinger B, Strangfeld K, Peters G, Schulze‐Osthoff K, Sinha B. Staphylococcus aureusα‐toxin induces apoptosis in peripheral blood mononuclear cells: role of endogenous tumour necrosis factor‐α and the mitochondrial death pathway. Cell Microbiol 2003; 5:729-41. doi: 10.1046/j.1462-5822.2003.00317.x [Crossref] [ Google Scholar]
- Menzies BE, Kourteva I. Staphylococcus aureus α-toxin induces apoptosis in endothelial cells. FEMS Immunol Med Mic 2000; 29:39-45. doi: 10.1111/j.1574-695X.2000.tb01503.x [Crossref] [ Google Scholar]
- Dragneva Y, Anuradha C, Valeva A, Hoffmann A, Bhakdi S, Husmann M. Subcytocidal attack by staphylococcal alpha-toxin activates NF-κB and induces interleukin-8 production. Infect Immun 2001; 69:2630-5. doi: 10.1128/IAI.69.4.2630-2635.2001 [Crossref] [ Google Scholar]
- Ambalam P, Raman M, Purama RK, Doble M. Probiotics, prebiotics and colorectal cancer prevention. Best Pract Res Clin Gastroenterol 2016; 30:119-31. doi: 10.1016/j.bpg.2016.02.009 [Crossref] [ Google Scholar]
- Uccello M, Malaguarnera G, Basile F, D'Agata V, Malaguarnera M, Bertino G. Potential role of probiotics on colorectal cancer prevention. BMC Surg 2012; 12 Suppl 1:S35. doi: 10.1186/1471-2482-12-s1-s35 [Crossref] [ Google Scholar]
- Becker HM, Apladas A, Scharl M, Fried M, Rogler G. Probiotic Escherichia coli Nissle 1917 and commensal E coli K12 differentially affect the inflammasome in intestinal epithelial cells. Digestion 2014; 89:110-8. doi: 10.1159/000357521 [Crossref] [ Google Scholar]
- Li R, Helbig L, Fu J, Bian X, Herrmann J, Baumann M. Expressing cytotoxic compounds in Escherichia coli Nissle 1917 for tumor-targeting therapy. Res Microbiol 2019; 170:74-9. doi: 10.1016/j.resmic.2018.11.001 [Crossref] [ Google Scholar]
- Cheng CM, Lu YL, Chuang KH, Hung WC, Shiea J, Su YC. Tumor-targeting prodrug-activating bacteria for cancer therapy. Cancer Gene Ther 2008; 15:393-401. doi: 10.1038/cgt.2008.10 [Crossref] [ Google Scholar]
- Alizadeh S, Esmaeili A, Omidi Y. Anti-cancer properties of Escherichia coli Nissle 1917 against HT-29 colon cancer cells through regulation of Bax/Bcl-xL and AKT/PTEN signaling pathways. Iran J Basic Med Sci 2020. doi: 10.22038/ijbms.2020.43016.10115 [Crossref]
- Alizadeh S, Esmaeili A, Barzegari A, Rafi MA, Omidi Y. Bioengineered smart bacterial carriers for combinational targeted therapy of solid tumours. J Drug Target 2020. doi: 10.1080/1061186X.2020.1737087 [Crossref]