Bioimpacts. 11(4):237-244.
doi: 10.34172/bi.2021.34
Original Research
Efficient conjugation of anti-HBsAg antibody to modified core-shell magnetic nanoparticles (Fe3O4@SiO2/NH2)
Shahram Parvaneh 1, 2, 3  , Fatemeh Khademi 2, Gisya Abdi 4, Abdolhamid Alizadeh 4, 5, Ali Mostafaie 1, 2, *
, Fatemeh Khademi 2, Gisya Abdi 4, Abdolhamid Alizadeh 4, 5, Ali Mostafaie 1, 2, * 
Author information:
1Department of Immunology, Faculty of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran
2Medical Biology Research Center (MBRC), Health Technology Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran
3Department of Dermatology and Allergology, Faculty of Medicine, University of Szeged, Szeged, Hungary
4Academic Centre for Materials and Nanotechnology, AGH University of Science and Technology, al. Adama Mickiewicza 30, 30-059 Krakow, Poland
5Department of Organic Chemistry, Faculty of Physics and Chemistry, Alzahra University, Tehran, 1993893973, Iran
Abstract
Introduction:
Further development of magnetic-based detection techniques could be of significant use in increasing the sensitivity of detection and quantification of hepatitis B virus (HBV) infection. The present work addresses the fabrication and characterization of a new bio-nano composite based on the immobilization of goat anti-HBsAg antibody on modified core-shell magnetic nanoparticles (NPs) by (3-aminopropyl) triethoxysilane (APTES), named Fe3O4@SiO2/NH2, and magnetic NPs modified by chitosan (Fe3O4@CS).
Methods:
At the first step, Fe3O4 was modified with the silica and APTES (Fe3O4@SiO2/NH2) and chitosan (Fe3O4@CS) separately. The goat anti-HBsAg antibody was activated by two different protocols: Sodium periodate and EDC-NHS. Then the resulted composites were conjugated with activated goat anti-HBsAg IgG. An external magnet collected Bio-super magnetic NPs (BSMNPs) and the remained solution was analyzed by the Bradford method to check the amount of attached antibody to the surface of BSMNPs.
Results:
The findings indicated that activation of antibodies by sodium periodate method 15-17 µg antibody immobilized on 1 mg of super magnetic nanoparticles (SMNPs). However, in the EDC-NHS method, 8-10 µg of antibody was conjugated with 1 mg of SMNPs. The resulting bio-magnetic NPs were applied for interaction with the HBsAg target using enzyme-linked immunosorbent assay (ELISA). About 1 µg antigen attached to 1 mg SMNPs, which demonstrated that the fabricated materials are applicable in the detection scope of HBsAg.
Conclusion:
In the present study, we developed new antibody-conjugated magnetic NPs for the detection of HBsAg using an efficient conjugation strategy. The results demonstrated that the binding capacity of Fe3O4@SiO2/NH2 was comparable with commercially available products. Our designed method for conjugating anti-HBsAg antibody to a magnetic nanoparticle opens the way to produce a high capacity of magnetic NPs.
Keywords: Antibody conjugation, HBsAg, Detectiion, Magnetic nanoparticles
Copyright and License Information
© 2021 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Hepatitis B virus (HBV) is a contagious virus resulting in chronic hepatitis, cirrhosis of the liver, and primary liver cancer. HBV is mainly transmitted by transfusing blood or blood products, and however inactive HBV carriers often have no symptoms but still can transmit the virus to others. Thus, investigating validated analytical methods with high specificity, low-cost detection, and relatively simple procedures for rapid detection of the hepatitis B virus surface antigen (HBsAg) is essential.
1
Many techniques, such as enzyme-linked immunosorbent assay (ELISA), chemiluminescence immunoassay (CLIA), radioimmunoassay (RIA), and Polymerase chain reaction (PCR) have been proposed for the quantitative determination of HBsAg.
2-4
In recent years, the immunosensor technique and electrochemical immunoassay have attracted considerable interest from researchers for the detection of protein biomarkers.
2
The immobilization of substrate materials for immunosensors has been investigated by many researchers recently.
3-5
For biomolecule immobilization, modification of the substrate materials such as electrodes, microtiter plates (MTPs), nanoparticles (NPs), nanotubes, graphene, etc., is essential for the introduction of the functional groups binding to biomolecules with high bonding strength and stability, high activity, and proper orientation.
6,7
For bio-sensing applications, materials with functional groups such as hydroxyl, aldehyde, amine, epoxy, carboxylic acid groups are required for conjugation of biomolecules. Silicon and silicon derivatives, metals, and polymers have been frequently used for serving as solid support in bioanalytical platforms.
8
Recent years have witnessed the extensive application of 3-aminopropyltriethoxysilane (APTES) for functionalization of bioanalytical platforms since its involvement in surface modification has been intensively explored and well understood.
9
The successful utilization of magnetic NPs in the immobilization of biomolecules has also been reported.
10,11
Modified magnetic NPs have been applied in many fields such as catalysis, bio-sensing, separation, and drug delivery scopes.
12-16
Magnetic NPs, as special carriers for the fixation of biomolecules, present an alternative as a solid phase surface for the fabrication of the immunosensors and the development of magnetic immunoassay (MIA) methods.
17
Amine groups immobilized on the surface of magnetic NPs can give plenty of anchor sites to form covalent bonds with the activated carboxyl groups or oligosaccharide chains of the CH2 domain on the fragment crystallizable region (Fc region) of antibody molecules. The resulting materials are applicable in detection science for attachment of the antibodies/antigens onto the surface of core-shell magnetic NPs.
18
Magnetic NPs, as special biomolecule immobilizing carriers, offer a promising alternative to the conventional coating methodology for immunosensors. Thus, searching for applicable and straightforward immobilization methods of antibodies (antigens) on the surfaces of magnetic NPs is of practical interest.
Herein, the Fe3O4 magnetic NPs were prepared and coated with SiO2 to form core-shell (Fe3O4@SiO2); then, they were modified with APTES, named as Fe3O4@SiO2/NH2, leaving primary amine groups on the surface. Fe3O4@SiO2/NH2 was conjugated to goat anti-HBsAg antibody (immunoglobulin γ (IgG)) via activation of IgG molecules by two separate protocols: sodium periodate and EDC-NHS. Finally, the bio-supermagnetic NPs (BSMNPs) were applied for the detection of HBsAg molecules.
Materials and Methods
Materials
Sodium periodate, (hydroxymethyl) aminomethane (Tris), hepatitis B surface antigen (HBsAg), (3-aminopropyl) triethoxysilane (APTES), glycine, 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS),sodium dodecyl sulfate (SDS), ferric chloride hexahydrate (FeCl3.6H2O) and ferrous chloride tetrahydrate (FeCl2.4H2O) were purchased from Sigma Aldrich. Methanol, hydrochloric acid, sodium phosphate, potassium phosphate, calcium chloride, sodium chloride orthophosphoric acid, sodium hydroxide, sodium bicarbonate, ammonia, and ethanol. All reagents applied in this paper were of analytical grade and were procured from Merck, and distilled or double-distilled water was used in the preparation of all solutions.
Characterization methods
Recording of Fourier transform infrared (Bruker FTIR) spectra was carried out in KBr pellets in the range of 400-4000 cm-1. The application of transmission electron microscopy (TEM) was done by JEOL JEM-1010n. A vibrating sample magnetometer (VSM, BHV-55, Riken, Japan) at room temperature was applied for the magnetic measurements.
Synthesis of magnetite core (Fe3O4)
Iron oxide (Fe3O4),magnetic NPs were prepared according to a reported procedure by Yang et al.
18
Typically, FeCl3.6H2O (2.70 g, 10 mM) and FeCl2.4H2O (1 g, 5 mM) were dissolved in distilled water (130 mL) in an argon atmosphere. Then, 10 mL ammonium hydroxide 35% was quickly added into the solution under rapid mechanical stirring (900 rpm) to increase the pH value to 11.0 at 60°C. The mixture was stirred for 1 hour under argon. Finally, after cooling at room temperature, the resulting NPs were collected by an external magnet and washed two times with acetone.
Silica coating on the magnetic cores (Fe3O4@SiO2)
Following the modified Stöber method, 1 g magnetic NPs were dispersed in 50 mL distilled water by the ultrasonic treatment for 20 minutes to form a ferrofluid. Subsequently, the resultant dispersion was centrifuged for 30 minutes. 2 mL ferrofluid was first diluted with 40 mL distilled water, and the consequent suspension and 5 mL NH3OH (30% wt.) were poured into 140 mL ethanol with vigorous stirring at 40°C. Finally, under continuous stirring, 1 mL of tetraethyl orthosilicate diluted in 20 mL ethanol was dropwise added to this dispersion. The resulting dispersion was stirred for 14 hours at room temperature. Afterward, Fe3O4@SiO2 was collected by magnetic separation and washed with distilled water and ethanol in sequence.
Synthesis of amino groups modified silica-coated magnetic NPs (Fe3O4@SiO2/NH2)
To set the amine group (-NH2) on the Fe3O4@SiO2 surface for conjugation with antibodies, we applied 3-(triethoxysilyl)-propylamine on the surface of MNPs. For this step, 1 mL APTES (5 mM) was dissolved in 100 mL of dried Toluene. This mixture was added to 1 g Fe3O4@SiO2 and stirred for 18 hours at 60°C. The amine groups modified silica-coated magnetic NPs (Fe3O4@SiO2/NH2) were washed with Toluene, and separated by a magnet, followed by drying in a vacuum.
Synthesis of chitosan-coated magnetic NPs (Fe3O4@CS)
Also, the surface of the (Fe3O4), magnetic NPs was covered by chitosan (CS). Chitosan is a natural polymer derived from chitin, which is consists of N-acetyl D-glucosamine units and has amine groups to conjugate to other biomolecules. In a typical procedure, 0.5 g magnetic NPs were dispersed in a surfactant-containing CTAB (2 g CTAB dissolved in 200 mL deionized water). Then, 150 mL chitosan solution (0.025 g CS powders dissolved in 100 mL 1% wt. Acetic acid solution) was added dropwise. The mixture was continuously stirred for 1 h at room temperature. Then, magnetic NPs coated by CS (Fe3O4@CS) were separated from the solution by an external magnet and continually washed several times with ethanol and deionized water, and put to dry overnight at 60°C.
Goat immunization with HBsAg
A goat was immunized four times at two-week intervals with 1 mg/mL of pure HBsAg (Iranian Pasteur Institute) which was homogenated by complete Freund's adjuvant (Sigma-Aldrich) for the first injection, and incomplete Freund’s adjuvant (Sigma-Aldrich) for the booster injections. The prepared solution was injected subcutaneously on 10 spots on the body of the goat, especially at the shoulders. Two weeks after the third injection, during the fourth injection, 5 mL blood was drawn from the jugular vein and tested for serum anti-HBsAg antibody titer using the ELISA method. For this purpose, pure HBsAg (5 μg/mL) in a carbonate/bicarbonate buffer (0.05 M, pH 9.5) was coated for one hour at room temperature. Wells were washed three times with phosphate buffer saline-tween (PBS-T), and the remaining active sites were blocked with 250 μL of 1% BSA in PBS-T for 30 minutes at room temperature. Then the isolated serum was discharged into the wells at the dilutions from 1/4000 to 1/128 000 and incubated for two hours at room temperature. After incubation time, the plates were washed three times with PBS-T followed by incubation with rabbit anti-goat IgG-HRP (Razi Biotech, Iran) for 30 min at room temperature. After washing the wells, tetramethylbenzidine (TMB) substrate solution was added to develop the color. Then, the reaction stopped by adding 2 M sulfuric acid after 15 minutes. Absorbance was read at 450 nm by ELISA reader (Awareness Technology Stat Fax 2100 Microplate Reader).
Purification of Goat antibody against HBV surface antigen (Goat anti-HBsAg IgG)
After identification of antibody generated against the antigen and appropriate titration (above dilution of 1/64 000), blood sampling was performed. The serum was separated, and the IgG fraction was precipitated with semi-saturated ammonium sulfate (50%). The precipitated fraction was then dialyzed against 50 mM Tris-HCl, pH 8. Then 7 mL of 15 mg/mL concentration of that dialyzed fraction loaded to a diethyl aminoethyl (DEAE)-Sepharose Column (GE Healthcare Life Sciences) at a size of 1.2 × 10 cm equilibrated with the same buffer. The column was washed with 50 mM Tris-HCl (pH 8) and the IgG fraction was removed from the column at the first peak of the chromatogram. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was applied under reduced and non-reduced conditions for the assessment of the purity of the anti-HBsAg antibody; Coomassie Blue R-250 staining was done afterward.
Activation of antibody (Goat anti-HBsAg IgG) by sodium periodate
The overall goal of this study was to optimize antibody conjugation onto the super magnetic nanoparticles (SMNPs) surface. This section and the following sections describe an efficient procedure for the best conjugation of antibodies to SMNPs for use in immunoassays. Goat polyclonal anti-HBsAg IgG antibody, purified in this study, was activated by oxidation with sodium periodate to convert oligosaccharide residues offragment crystallizable region (Fc region) of IgG molecules (IgG-Fc region) into an aldehyde functional group, and subsequently, the aldehydes group was bonded to amines group on the surface of SMNPs. For this step, the purified IgG antibody was dissolved in physiological serum, pH 6, and dialyzed against the same buffer. A solution of 5 mg/mL antibody in the same buffer was prepared in a dark dish. For each 5 mg of purified antibody, 100 μL of 100 mM sodium periodate solution was added drop-wise to a stirring antibody solution. The solution was stirred for two hours at room temperature to allow sodium periodate to oxidize the saccharides ring of the antibody molecules. After two hours, the solution was dialyzed against 10 mM sodium acetate buffer (pH 5.5) and kept in the refrigerator for conjugation with SMNPs in the next step of the protocol.
19
Activation of antibody using EDC and NHS mediators
Activation of carboxyl groups of the amino acid residues of antibody molecules using EDC-NHS was performed as follows. Initially, the antibody was dialyzed against the physiological serum and then the desired concentration of antibody was prepared in a dark container. EDC solution was added at the concentration of 10 mM to the antibody solution. Then, 100 mM NHS solution was added to ultimately reach a 20 mM concentration in the antibody solution. The above solution was stirred for 3 hours at laboratory temperature. Afterward, it was dialyzed against the physiological serum at pH 6 and stored for the next step in the refrigerator.
20
Conjugation of goat anti-HBsAg IgG with SMNPs
100 mg Fe3O4@SiO2/NH2 or (Fe3O4@CS)was dissolved in 10 mL carbonate buffer (0.1 M, pH 9) separately, and 2 mg of the oxidized antibody was added to the suspension. After stirring for 3 hours at room temperature, 1 mL of Tris buffer (1 M, pH 8) was added. Subsequently, 1 mL of 100 mM Sodium borohydride was added and the solution was stored overnight in the refrigerator. After the sedimentation of super-magnetic NPs with an external magnet, the concentration of antibody in the supernatant was tested by the Bradford assay. The amount of attached antibody was determined based on the difference in initial concentration and final concentration.
20
Protein assay
The concentration of protein in the supernatant was measured after exposure to the antibody by the Micro Bradford method. Initially, concentrations of 25, 50, 100, 200 μg/mL of standard protein Bovine serum albumin (BSA) were prepared. 500 μL of the working solution of the Bradford Reagent was added to each tube, and then 50 μL of standards and samples were added, respectively. After ten minutes, the absorption of tubes was read at 595 nm against the blank sample.
21
Antigen binding to magnetic NPs coated with antibodies
The antigen-binding to BSMNPs coated with antibodies was evaluated as follows. The solution containing HBsAg was added to 100 μL of BSMNPs coated with antibodies and without antibodies separately. After 30 minutes stirring, an external magnet was applied to collect the NPs. BSMNPs were repeatedly washed with PBS buffer. Then, 0.5 mL of glycine solution (0.1 M, pH 2.8) was added to isolate the antigen from the antibodies on the BSMNPs. After five minutes, a magnet was used to collect the NPs, and the supernatant was removed. Tris buffer (1 M, pH 8) was added to the supernatant to neutralize the pH. The antigen-binding to BSMNPs coated with antibodies was evaluated using an ELISA kit (Enzygnost HBsAg 6.0, Siemens, Germany). For this step, 100 μL separated supernatant (dilution of 1/1000), as well as the standards were introduced into the wells. Then, 25 μL of the biotin-conjugated anti-HBsAg was added and incubated for 1 hour at 37°C. After washing four times, 100 μL of the peroxidase-streptavidin solution was added to each well and incubated for 30 minutes at 37°C. The plate was washed five times and then 100 μL of TMB solution was added to the wells. After 15 minutes, the reaction was stopped by adding 100 μL of 1 M sulfuric acid. Finally, the absorbance of the wells was measured at 450 nm.
Results
Production and purification of antibodies against HBsAg
One week after the third injection, a goat immunized with HBsAg revealed a noticeable elevation in the anti-HBsAg antibody level. Based on the ELISA, the anti-HBsAg antibody titers of 128 000 were achieved after immunization. Titers were demarcated as the highest dilution of antiserum to reach an OD of 1 at 450 nm. A week later, the fourth injection was done. Two weeks after the fourth injection of the cervical spine, about 100 mL of blood was drawn to purify the antibody. After separating the serum and dilution with PBS buffer, the antibody was purified using ammonium sulfate fractionation and ion-exchange chromatography (DEAE-Sepharose column). The IgG fraction was removed from the column at the first peek of the chromatogram. The purity of antibodies was verified by SDS-PAGE followed by Coomassie Blue staining. Two bands at the anticipated positions for the antibody light chain (25–27 kDa) and heavy chain (50–52 kDa) under reduced conditions were observed (Fig. 1).
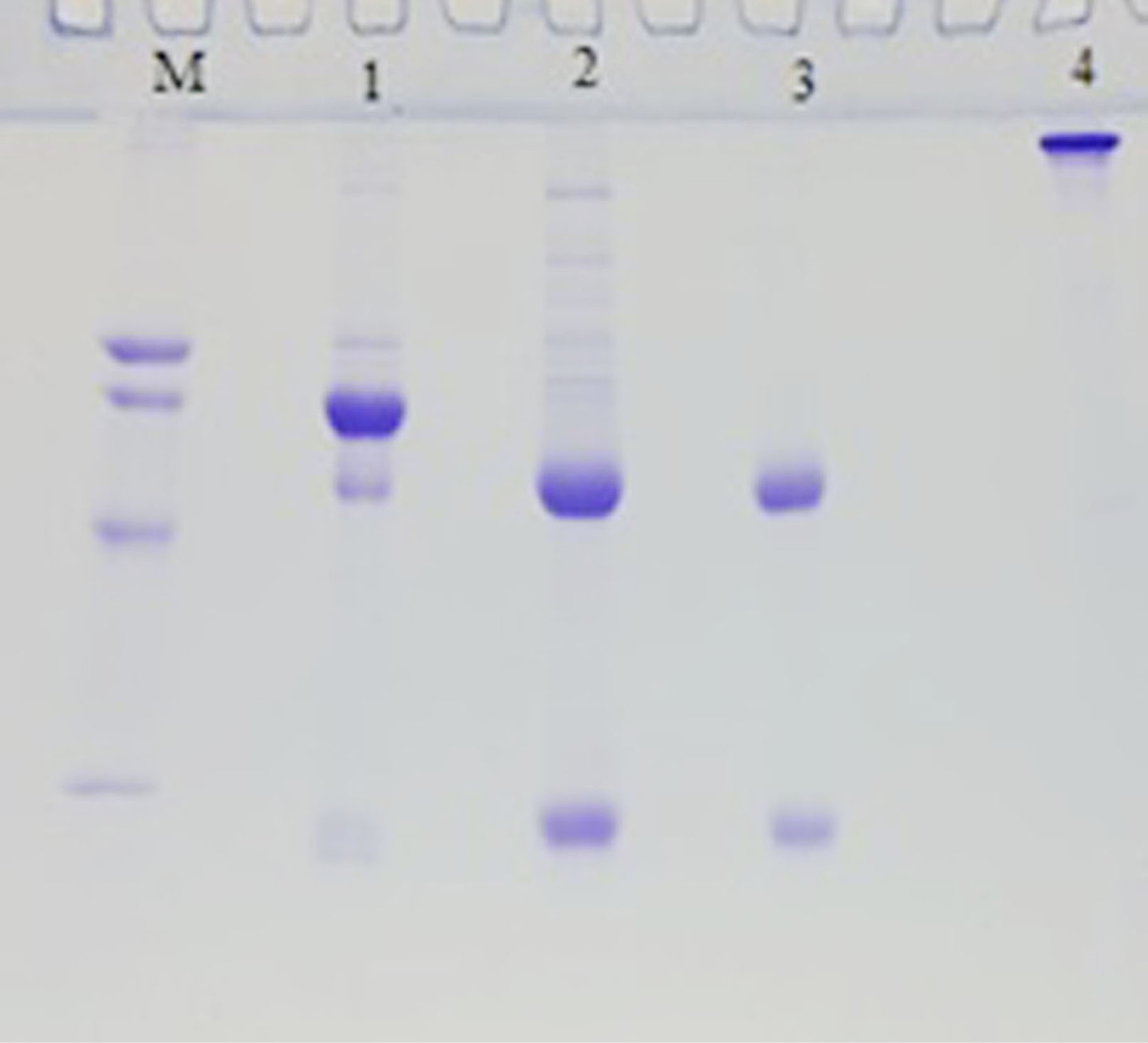
Fig. 1.
SDS-PAGE pattern of purification steps of goat anti-HBsAg IgG. Lane M; marker protein from up to down 78, 66, 45 and 29 kDa, lane1; goat serum, lane2; Ammonium sulfate precipitate fraction of goat serum, lane3; purified goat anti- HBsAg IgG fraction by DEAE-Sepharose, lane 1, 2, and 3 are reduced form, lane4; the non-reduced form of purified IgG.
.
SDS-PAGE pattern of purification steps of goat anti-HBsAg IgG. Lane M; marker protein from up to down 78, 66, 45 and 29 kDa, lane1; goat serum, lane2; Ammonium sulfate precipitate fraction of goat serum, lane3; purified goat anti- HBsAg IgG fraction by DEAE-Sepharose, lane 1, 2, and 3 are reduced form, lane4; the non-reduced form of purified IgG.
Production of super magnetite nanoparticles coated by amine groups
Silica-coated magnetite NPs (Fe3O4@SiO2) were fabricated and analyzed by different techniques. After creating the amine group on the surface Fe3O4@SiO2/NH2, glutaraldehyde was added to the solution of that composite. Aggregation and deposition of particles accrued, followed by the addition of it, because of the linking role of a tow aldehyde functional group of glutaraldehyde revealing abundant amine groups on the surface of NPs. Fig. 2 shows the X-ray diffraction (XRD) pattern of Fe3O4@SiO2/NH2 composite, 2θ peaks at 2°, 30°, 35.6°, 43.3°, 53.4°, 57°, and 62.7° indicates the cubic structure and represents the formation of Fe3O4 NPs. The average size of Fe3O4@SiO2/NH2 was calculated by the Scherrer equation (1), and the particle size was calculated around 25-30 nm from the following formula.
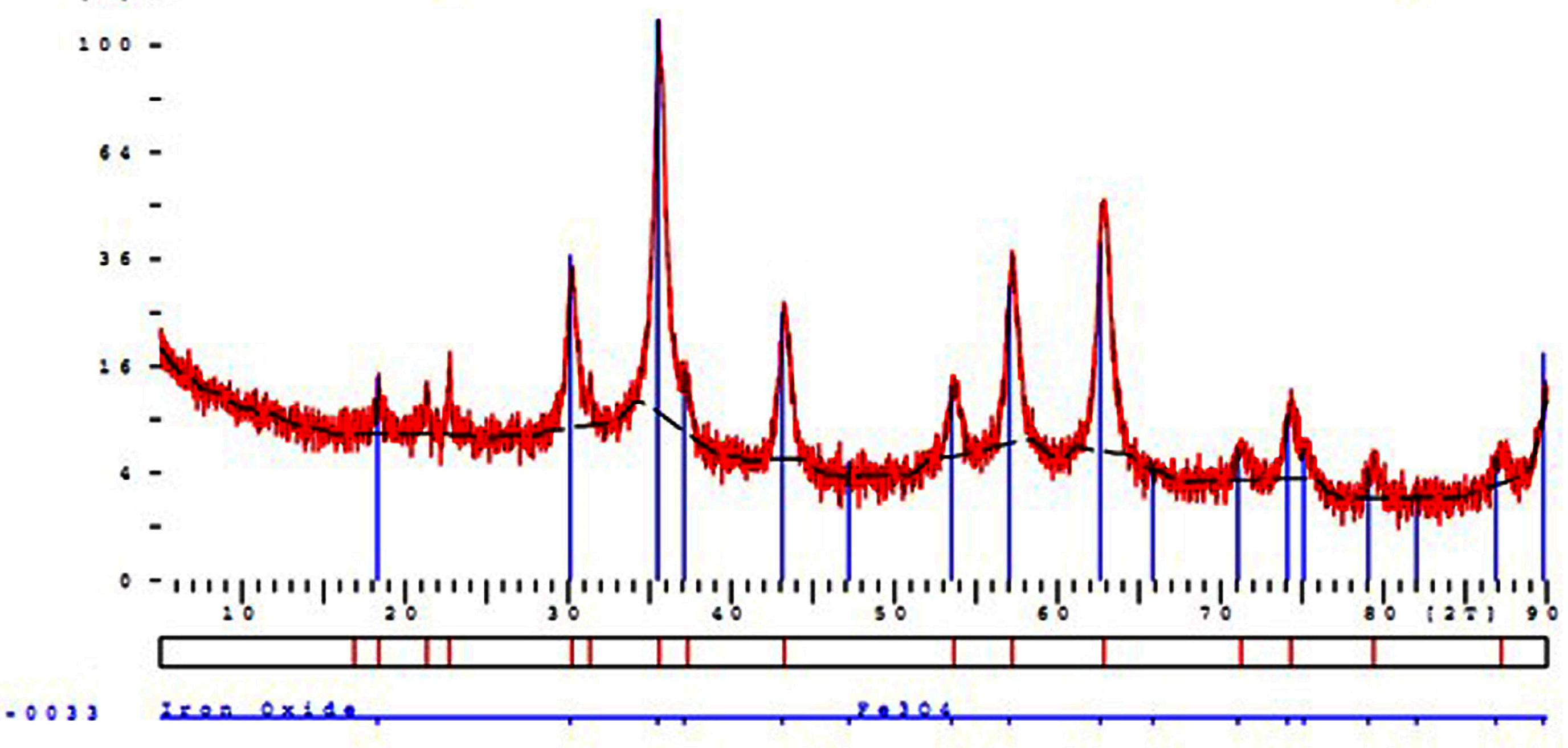
Fig. 2.
XRD Characterization of Fe3O4 nanoparticles.
.
XRD Characterization of Fe3O4 nanoparticles.
D= Kλ /βcos θ(1)
K, λ, β, and Ɵ refer to Scherrer constant (0.94), X-Ray wavelength (1.54 Å), the peak width in half maximum (FWHM), and Bragg diffraction angle, respectively. The fabricated Fe3O4@SiO2/NH2 composite was analyzed in terms of size and shape using the TEM technique, demonstrated in Fig. 3. After the silica coating (Fig. 2B), the Fe3O4@SiO2 with a diameter of about 10-15 nm shows an approximately homogeneous silica shell which increases the diameter of the composite.
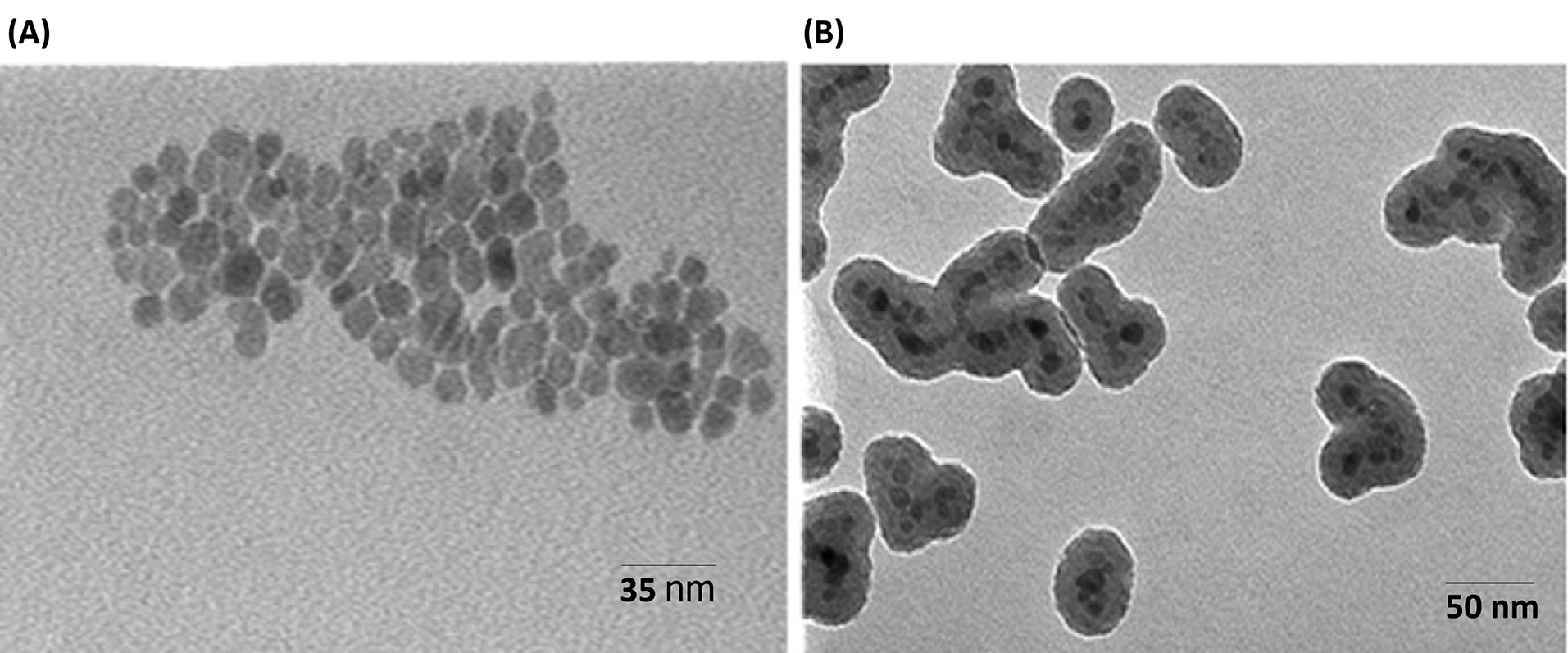
Fig. 3.
Transmission electron microscopy (TEM) image of: (A) Fe3O4; (B) Fe3O4@SiO2/NH2 composite.
.
Transmission electron microscopy (TEM) image of: (A) Fe3O4; (B) Fe3O4@SiO2/NH2 composite.
SMNPs were investigated for magnetism using a VSM device (BHV-55, Riken, Japan) at various temperatures. The magnitude of magnetization did not change at different temperatures indicating that the crystals are super magnetic (Fig. 4). The values of saturation magnetization for Fe3O4 and Fe3O4@SiO2/NH2 were about 73.7 emu/g and 46.7 emu/g, respectively. The reduction of magnetization after coating by silica is due to silica layer coating on the surface of Fe3O4. However, the magnetization value of Fe3O4@SiO2/NH2 is high enough for immunoassay applications in the presence of an external field. The Fe3O4 coating by chitosan was investigated using IR spectroscopy (Fig. 5B), represents N-H at 3425 nm, and proves the presence of chitosan on the surface of SMNPs.
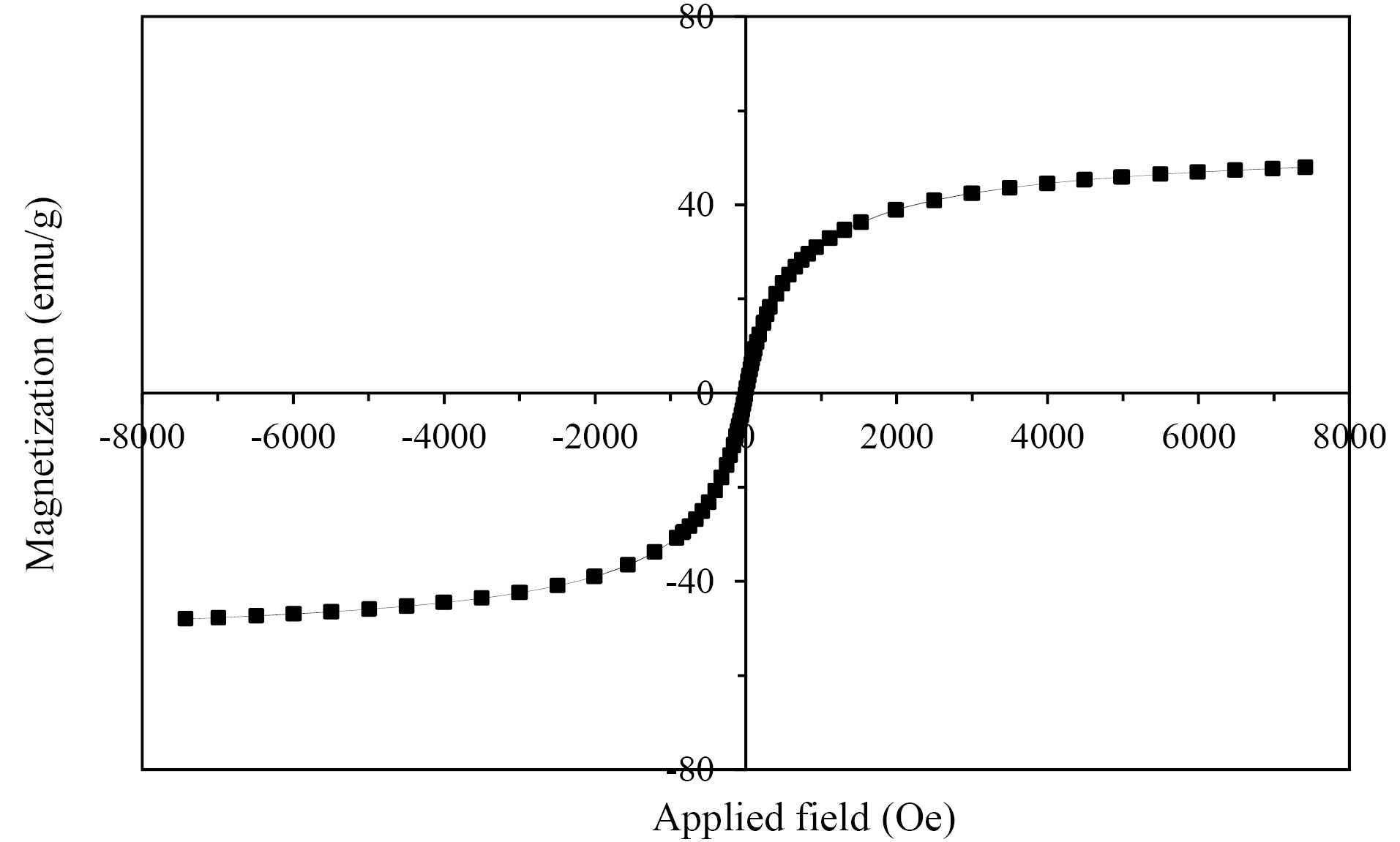
Fig. 4.
Magnetization curves obtained by VSM at room temperature of Fe3O4@SiO2/NH2 composite.
.
Magnetization curves obtained by VSM at room temperature of Fe3O4@SiO2/NH2 composite.
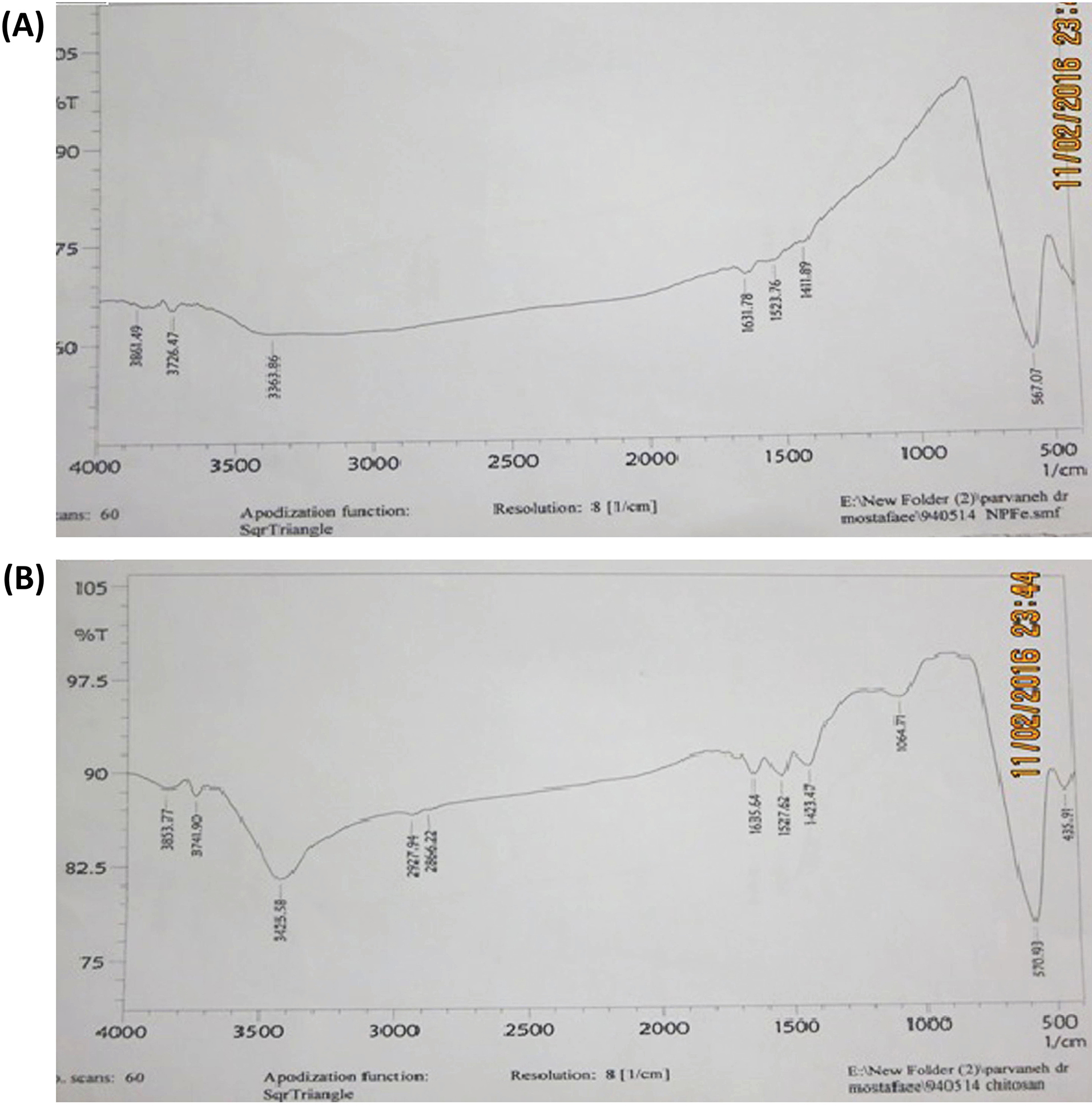
Fig. 5.
IR images of (A), Fe3O4 nanoparticles and (B), Fe3O4@CS, N-H peak at 3425 nm represents and proves the presence of amine groups on the surface of nanoparticles.
.
IR images of (A), Fe3O4 nanoparticles and (B), Fe3O4@CS, N-H peak at 3425 nm represents and proves the presence of amine groups on the surface of nanoparticles.
Conjugation of SMNPs to Goat anti-HBsAg antibody
The stability of Fe3O4@CS, Fe3O4@SiO2/NH2, and BSMNPs in various pH was compared. The efficiency of antibody conjugation was examined using spectrophotometry and ELISA analyses. After conjugation of antibodies to NPs, the concentration of proteins in the solution was measured by the Bradford method. The binding capacity was determined based on the difference in initial concentrations and final concentrations after the binding process. Results demonstrated that the binding capacity and stability of Fe3O4@SiO2/NH2 were higher than Fe3O4@CS. The amount of the conjugated anti- HBsAg IgG (Ab) to Fe3O4@CS was about 8-10 μg Ab/mg NPs. With a change in a buffer solution to glycine (pH 2.8, 0.1 M), antibodies were removed from the surface of NPs and parts of the NPs were degraded, especially in the case of Fe3O4@CS. While, in the fact of Fe3O4@SiO2/NH2, the binding ratio was more than 15-17 μg Ab per 1 mg SMNPs. Without a change in buffer solution, they were fully resistant, and there was no change in the performance (Fig. 6). The binding ratio of the antibody that was activated by the EDC-NHS method was about 10-12 μg Ab per 1 mg SMNPs to the Fe3O4@SiO2/NH2. The attachment amount of non-activated antibody to the Fe3O4@SiO2/NH2 (NPs surface absorption) was determined at 2-3 μg Ab per 1 mg SMNPs (Fig. 7).
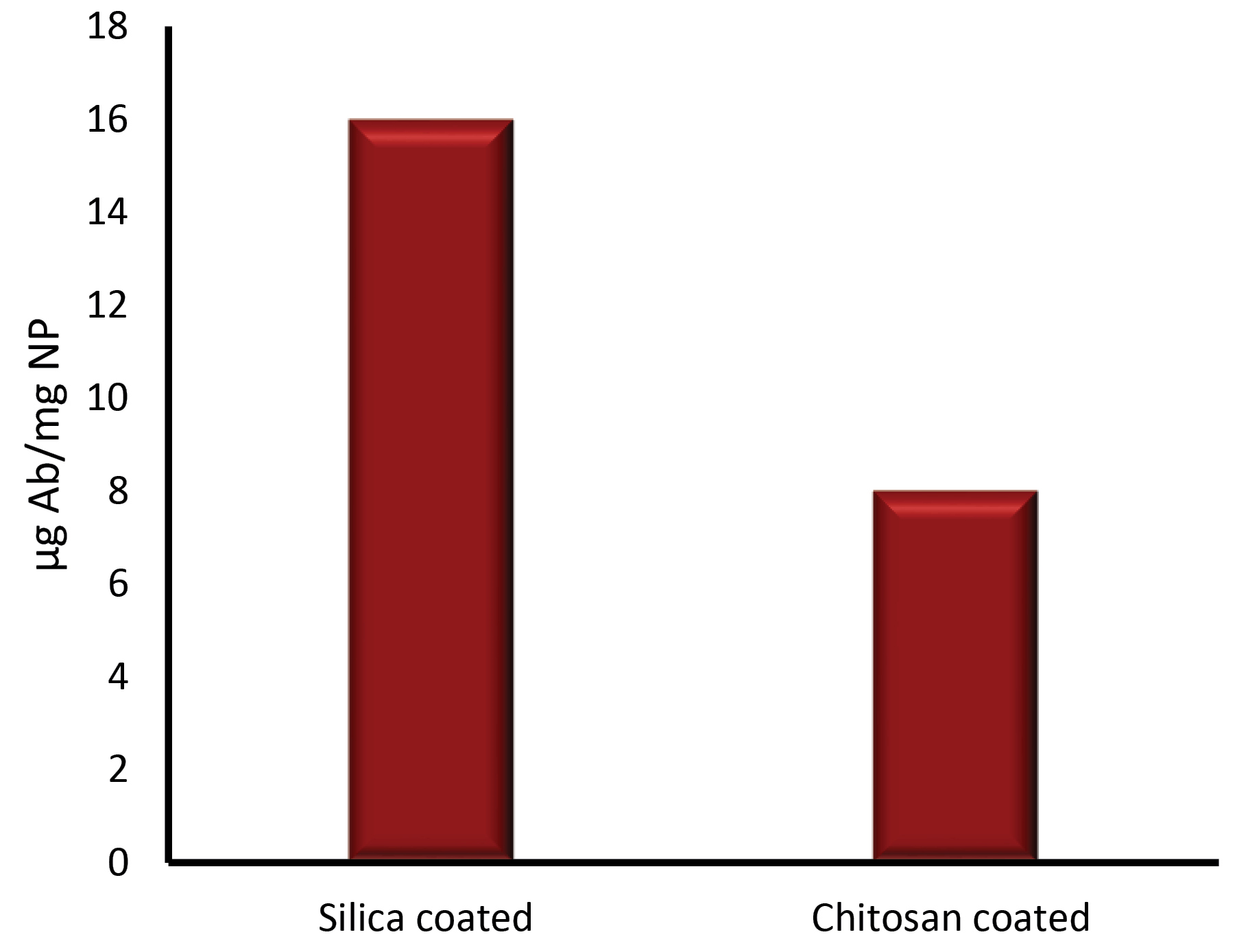
Fig. 6.
Comparison the quantity of activated Ab by NaIO4 binding to 1mg of Fe3O4@SiO2/NH2 and Fe3O4@CS protocols separately.
.
Comparison the quantity of activated Ab by NaIO4 binding to 1mg of Fe3O4@SiO2/NH2 and Fe3O4@CS protocols separately.
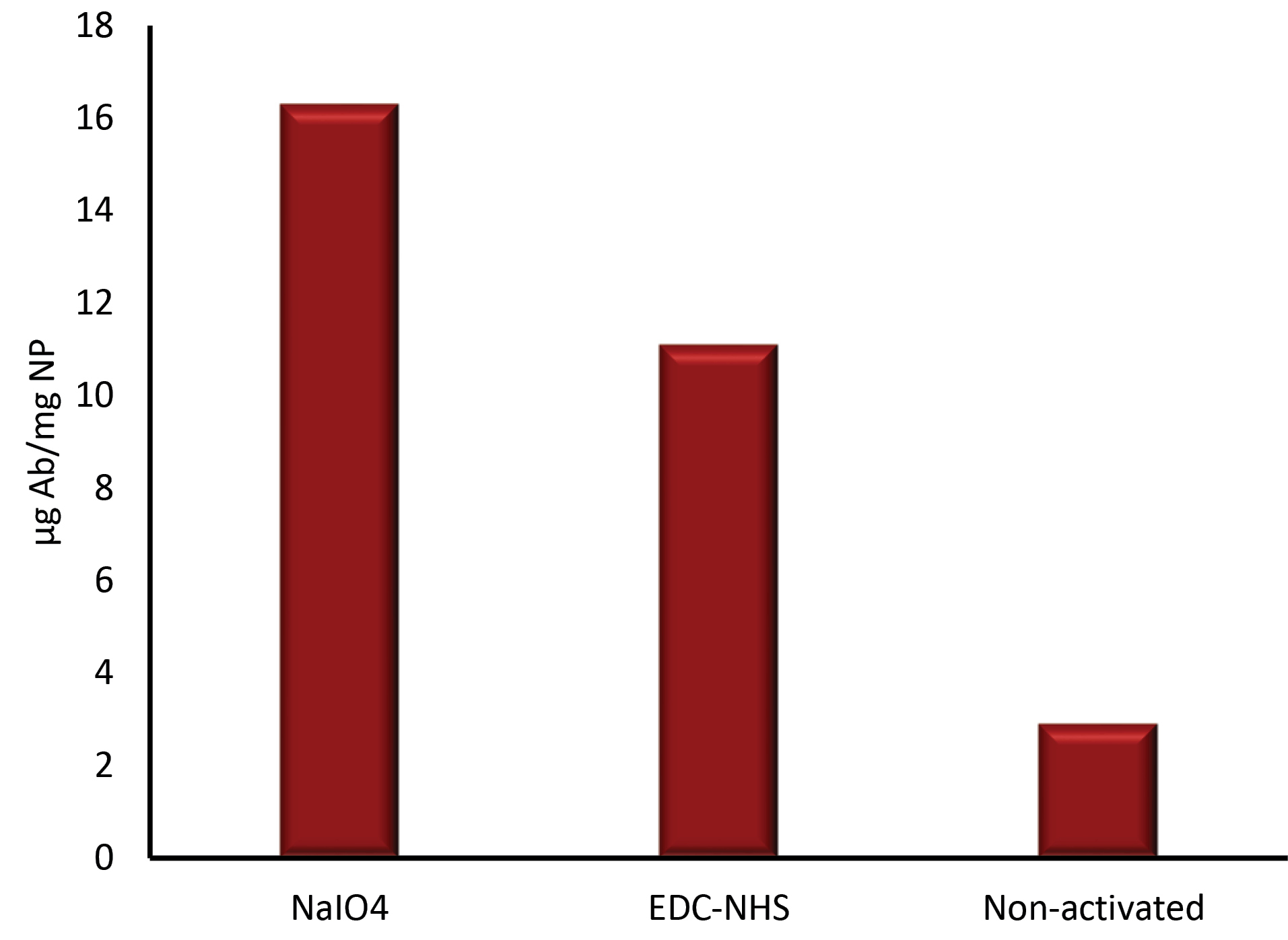
Fig. 7.
Comparison of antibody binding to Fe3O4@SiO2/NH2 nanoparticles in different activation methods. NaIO4: Sodium periodate, EDC: 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide, NHS: N-Hydroxysuccinimide.
.
Comparison of antibody binding to Fe3O4@SiO2/NH2 nanoparticles in different activation methods. NaIO4: Sodium periodate, EDC: 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide, NHS: N-Hydroxysuccinimide.
We also used the ELISA method to measure the separation efficiency. For this purpose, after collection of the magnetic NPs by an external magnet, the concentration of separated antigen (HBsAg) eluted by acidic glycine buffer was tested. The level of HBsAg was determined at 1 μg/mL.
Discussion
Several methods such as ELISA, CLIA, RIA, and PCR have been developed to diagnose and quantify HBV infection.
4-8
Despite reasonable specificity, PCR and ELISA-based methods are not always sensitive enough to detect HBV antigens in biological samples. Many studies have shown that the utilization of MIA can remarkably improve the sensitivity of diagnostic methods. The possibility of detecting NPs associated with HBV antigens by surface plasmon resonance, nuclear magnetic resonance, and electrochemistry has been shown. Further development of magnetic NP-based detection techniques could be of significant use in increasing the sensitivity of detection and quantification of HBV infection and decreasing the time and costs of testing. In the present study, we developed antibody-conjugated super magnetic NPs (BSMPNs) for the detection of HBsAg using an efficient conjugation strategy. For this purpose, we first developed a specific polyclonal antibody against HBsAg by the immunization of a goat with pure HBsAg obtained from the Iranian Pasteur Institute. It was found that the serum of immunized goat has high antibody titers (1/128 000), as detected by ELISA. After purification, the produced antibody was conjugated to SMNPs.
We prepared Fe3O4 covered with a silica layer, then amine ligands (-NH2) were coupled to the surface of the Fe3O4@SiO2, to form Fe3O4@SiO2/NH2. With the XRD pattern, we approved a crystal structure and calculated the average size of the magnetic crystal around 25-30 nm. Analysis of the magnetic behavior of Fe3O4@SiO2/NH2 by VSM demonstrated super-magnetic property of 46.7 emu/g. The binding capacity and stability of Fe3O4@SiO2/NH2 were higher than Fe3O4@CS. Nowadays, super-magnetic NPs with various types of shells and surface functional groups with different binding capacities are being produced commercially by big companies. The microbeads produced by Invitrogen Company (Dynabeads, M-270 Epoxy, Dynal/Invitrogen) has a binding capacity of 7-8 µg Ab per 1 mg SMNP. According to our findings in the present study, the binding capacity of Fe3O4/SiO2/NH2 was about 15-17 µg Ab per 1 mg SMNP, which is comparable with commercially available products. The fabricated monodisperse BSMNPs were subsequently applied to capture HBsAg in biological solutions and verified the great application prospects for bioseparation technology and developing the solid phase magnetic-based assay in the research and diagnostic arena. Our designed methods to synthesis BSMNPs for the detection of HBsAg opens the way to produce a high capacity of SMNPs.
Several conjugation linkers have been used extensively for the conjugation of biomolecules to SMNPs, for example, EDC and NHS are carboxylate linkers.
22
The random conjugation of the carboxylic group of amino acid residues (such as glutamic acid and aspartic acid) is the limitation of these conjugation methods which affects the orientation of antigen-binding domains. This characteristic can reduce binding potency and thus affect diagnostic or other applications. In this work, activation of antibodies for conjugation to NPs was performed using two protocols: periodate oxidation, and EDC-NHS protocols. Periodate oxidation of oligosaccharide residues on the CH2 domain of antibody molecules achieved Fc-directed conjugation of Fe3O4@SiO2/NH2; the binding ratio of antibody was obtained at 15-17 µg Ab per 1 mg SMNP that was better than EDC-NHS method (10-12 Ab per 1 mg SMNP).
Conclusion
Bio-magnetic hybrids consisting of Fe3O4@SiO2/NH2 and antibody molecules were successfully prepared. Stable and high active SMNPs were fabricated, and antibodies were conjugated to the surface of them from the Fc region, causing a higher binding capacity with no alteration or inhibition in the paratope area of the antibody molecule. The findings showed 15-17 µg of antibody conjugated to 1 mg of SMNPs by activation of antibodies through sodium periodate procedure. These materials exhibited the remarkable detection capacity of HBsAg molecules. The results indicated that about 1 µg antigen was attached to 1 mg BSMNPs, and it could have high potential applications in detection scopes.
Acknowledgments
Vice-Chancellor for Research and Technology, Kermanshah University of Medical Sciences (94465) supported this paper.
The authors are grateful to Kermanshah University of Medical Sciences for the completion of the work and provision of required services. The authors also thankNanoscience and Nanotechnology Research Center (NNRC), Razi University, Kermanshah, Iran, for their partial support of this project.
Funding sources
Kermanshah University of Medical Sciences under grant number 94465.
Ethical statement
The authors declare no ethical issues to be considered.
Competing interests
The authors declare no conflicts of interest.
Authors’ contribution
SHP: performing and designing experiments; FKH: conceptualization, writing, and reviewing; GA: interpretation of data, drafting the manuscript; AA: data analysis; AM: supervision, provision of study equipment and materials.
Research Highlights
What is the current knowledge?
simple
-
√ SMNPs have a wide range of biomedical applications in research, diagnosis, and even treatment.
-
√ SMNPs conjugated with specific Ab molecules are used in the MIA procedure leading to the selectivity and sensitivity against target molecules and the ease of use in the sensing systems.
-
√ Bio-magnetic hybrids were consisting of modified core-shell (Fe3O4@SiO2/NH2) and goat anti-HBsAg antibody exhibit remarkable detection capacity of HBsAg molecules.
What is new here?
simple
-
√ Stable and high active BSMNPs were fabricated and conjugated with antibodies from the Fc region, which showed a higher binding capacity with no alteration or inhibition in the paratope area of the antibody molecule.
-
√ The binding capacity of Fe3O4@SiO2/NH2 was about 15-17 µg Ab per 1mg SMNPs, which is comparable with commercially available products.
-
√ The designed method for conjugating of anti-HBsAg antibody to a magnetic nanoparticle for detection of HBsAg opens the way to produce a high capacity of magnetic NPs.
References
- Alizadeh N, Hallaj R, Salimi A. Dual Amplified Electrochemical Immunosensor for Hepatitis B Virus Surface Antigen Detection Using Hemin/G-Quadruplex Immobilized onto Fe3O4-AuNPs or (Hemin-Amino-rGO-Au) Nanohybrid. Electroanalysis 2018; 30:402-414. doi: 10.1002/elan.201700727 [Crossref] [ Google Scholar]
- Novack L, Sarov B, Goldman-Levi R, Yahalom V, Safi J, Soliman H. Impact of pooling on accuracy of hepatitis B virus surface antigen screening of blood donations. Trans R Soc Trop Med Hyg 2008; 102:787-792. doi: 10.1016/j.trstmh.2008.04.005 [Crossref] [ Google Scholar]
- Zhang P, Lu H, Chen J, Han H, Ma W. Simple and sensitive detection of HBsAg by using a quantum dots nanobeads based dot-blot immunoassay. Theranostics 2014; 4:307-315. doi: 10.7150/thno.8007 [Crossref] [ Google Scholar]
- Shen Y, Shen G, Zhang Y, Zhang C, Li H. A novel label-free electrochemical immunosensor based on aldehyde-terminated ionic liquid. Talanta 2017; 175:347-351. doi: 10.1016/j.talanta.2017.07.023 [Crossref] [ Google Scholar]
- Tang D, Li H, Liao J. Ionic liquid and nanogold-modified immunosensing interface for electrochemical immunoassay of hepatitis B surface antigen in human serum. Microfluid Nanofluidics 2008; 6:403-409. doi: 10.1007/s10404-008-0385-2 [Crossref] [ Google Scholar]
- Fahnrich KA, Pravda M, Guilbault GG. Disposable amperometric immunosensor for the detection of polycyclic aromatic hydrocarbons (PAHs) using screen-printed electrodes. Biosens Bioelectron 2003; 18:73-82. [ Google Scholar]
- Mohamed SA, Al-Harbi MH, Almulaiky YQ, Ibrahim IH, El-Shishtawy RM. Immobilization of horseradish peroxidase on Fe3O4 magnetic nanoparticles. Electron J Biotechnol 2017; 27:84-90. doi: 10.1016/j.ejbt.2017.03.010 [Crossref] [ Google Scholar]
- Lee JH, Choi HK, Lee SY, Lim M-W, Chang JH. Enhancing immunoassay detection of antigens with multimeric protein Gs. Biosens Bioelectron 2011; 28:146-51. doi: 10.1016/j.bios.2011.07.011 [Crossref] [ Google Scholar]
- Datta S, Christena LR, Rajaram YRS. Enzyme immobilization: an overview on techniques and support materials. Biotech 2013; 3:1-9. doi: 10.1007/s13205-012-0071-7 [Crossref] [ Google Scholar]
- Cheng T-L, Chuang K-H, Chen B-M, Roffler SR. Analytical measurement of PEGylated molecules. Bioconjug Chem 2012; 23:881-899. doi: 10.1021/bc200478w [Crossref] [ Google Scholar]
- Kyeong S, Jeong C, Kang H, Cho H-J, Park S-J, Yang J-K. Double-Layer Magnetic Nanoparticle-Embedded Silica Particles for Efficient Bio-Separation. PLoS One 2015; 10:e0143727. doi: 10.1371/journal.pone.0143727 [Crossref] [ Google Scholar]
- Vashist SK, Lam E, Hrapovic S, Male KB, Luong JHT. Immobilization of Antibodies and Enzymes on 3-Aminopropyltriethoxysilane-Functionalized Bioanalytical Platforms for Biosensors and Diagnostics. Chem Rev 2014; 114:11083-130. doi: 10.1021/cr5000943 [Crossref] [ Google Scholar]
- Sonti S V, Bose A. Cell Separation Using Protein-A-Coated Magnetic Nanoclusters. J Colloid Interface Sci 1995; 170:575-585. doi: 10.1006/jcis.1995.1137 [Crossref] [ Google Scholar]
- Liu Z-M, Yang H-F, Li Y-F, Liu Y-L, Shen G-L, Yu R-Q. Core–shell magnetic nanoparticles applied for immobilization of antibody on carbon paste electrode and amperometric immunosensing. Sensors Actuators B Chem 2006; 113:956-962. doi: 10.1016/J.SNB.2005.04.002 [Crossref] [ Google Scholar]
- Deng YH, Yang WL, Wang CC, Fu SK. A Novel Approach for Preparation of Thermoresponsive Polymer Magnetic Microspheres with Core–Shell Structure. Adv Mater 2003; 15:1729-32. doi: 10.1002/adma.200305459 [Crossref] [ Google Scholar]
-
Sauzedde F, Elaissari A, Pichot C. Thermosensitive magnetic particles as solid phase support in an immunoassay. Macromol Symp 2000; 151: 617–23. 10.1002/1521-3900(200002)151:1<617::AID-MASY617>3.0.CO;2-T.
- Chen D-H, Liao M-H. Preparation and characterization of YADH-bound magnetic nanoparticles. J Mol Catal B Enzym 2002; 16:283-291. doi: 10.1016/S1381-1177(01)00074-1 [Crossref] [ Google Scholar]
- Abdi G, Alizadeh A, Zinadini S, Moradi G. Removal of dye and heavy metal ion using a novel synthetic polyethersulfone nanofiltration membrane modified by magnetic graphene oxide/metformin hybrid. J Memb Sci 2018; 552:326-335. doi: 10.1016/J.MEMSCI.2018.02.018 [Crossref] [ Google Scholar]
- Arruebo M, Fernández-Pacheco R, Ibarra MR, Santamaría J. Magnetic nanoparticles for drug delivery. Nano Today 2007; 2:22-32. doi: 10.1016/S1748-0132(07)70084-1 [Crossref] [ Google Scholar]
-
Šafařík I, Šafaříková M. Magnetic Nanoparticles and Biosciences BT - Nanostructured Materials. In: Hofmann H, Rahman Z, Schubert U, editors. Vienna: Springer Vienna; 2002. p. 1–23. 10.1007/978-3-7091-6740-3_1
- Neuberger T, Schöpf B, Hofmann H, Hofmann M, von Rechenberg B. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J Magn Magn Mater 2005; 293:483-496. doi: 10.1016/J.JMMM.2005.01.064 [Crossref] [ Google Scholar]
- Tseng SH, Chou MY, Chu IM. Cetuximab-conjugated iron oxide nanoparticles for cancer imaging and therapy. Int J Nanomedicine 2015; 10:3663-3685. doi: 10.2147/IJN.S80134 [Crossref] [ Google Scholar]