BioImpacts. 10(2):105-115.
doi: 10.34172/bi.2020.13
Original Research
Conditions for combining gene therapy with bone marrow transplantation in murine Krabbe disease
Mohammad A. Rafi  , Paola Luzi , David A. Wenger *
, Paola Luzi , David A. Wenger * 
Author information:
Department of Neurology, Sidney Kimmel College of Medicine, Thomas Jefferson University, Philadelphia, PA 19107, USA
Abstract
Introduction:
Krabbe disease (KD) is an autosomal recessive lysosomal disorder caused by mutations in the galactocerebrosidase (GALC) gene. This results in defective myelination in the peripheral and central nervous systems due to low GALC activity. Treatment at this time is limited to hematopoietic stem cell transplantation (HSCT) in pre-symptomatic individuals. While this treatment extends the lives of treated individuals, most have difficulty walking by the end of the first decade due to peripheral neuropathy. Studies in the murine model of KD, twitcher (twi) combining bone marrow transplantation (BMT) with AAVrh10-mGALC showed a great extension of life from 40 days to about 400 days, with some living a full life time.
Methods:
In order to find the optimum conditions for dosing and timing of this combined treatment, twi mice were injected with five doses of AAVrh10-mGALC at different times after BMT. Survival, as well as GALC expression were monitored along with studies of sciatic nerve myelination and possible liver pathology.
Results:
Dosing had a pronounced effect on survival and measured GALC activity. There was window of time after BMT to inject the viral vector and see similar results, however delaying both the BMT and the viral injection shortened the lifespans of the treated mice. Lowering the viral dose too much decreased the correction of the sciatic nerve myelination. There was no evidence for hepatic neoplasia.
Conclusion:
These studies provide the conditions optimum for successfully treating the murine model of KD. There is some flexibility in dosing and timing to obtain a satisfactory outcome. These studies are critical to the planning of a human trial combining the "standard of care", HSCT, with a single iv injection of AAVrh10-GALC.
Keywords: Krabbe disease, AAVrh10 gene therapy, Twitcher mice, Bone marrow transplantation, Combined therapy, Myelination
Copyright and License Information
© 2020 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Krabbe disease (KD) or globoid cell leukodystrophy (GLD; OMIM 245200) is an autosomal recessive disorder affecting myelination in the human patients. It is caused by the deficiency of galactocerebrosidase (GALC; EC 3.2.1.46) activity resulting from mutations in the GALC gene.
1
The incidence is about one in 100 000 births in the United States. While most human patients present in infancy or late infancy, later onset patients are also diagnosed. In addition to human patients, genetic mutations in the GALC gene have resulted in GLD in several animal species, including mice, dogs and rhesus monkeys.
2
Affected animals also have very low GALC activity resulting in pathology similar to human patients. Colonies of these species have been established at various locations to use for treatment trials. These include bone marrow transplantation (BMT), enzyme replacement therapy, gene therapy via different routes of administration, substrate reduction therapy, chemical chaperone therapy, small molecule therapy including anti-inflammatory drugs and combinations of these approaches.
3-21
These different approaches have resulted in variable degrees of success. Some of these trials only resulted in minimal extension of life, and significant side effects and pathological events were noted. Other treatments involving multiple injections into the brain, BMT without myeloablation of the recipient and use of toxic chemicals for substrate reduction that probably will not be used in human patients. Obviously, because of the cost and ease of breeding, most of the therapy trials have been done using the twitcher (twi) mouse model. As early as 1984, Yeager et al
3
demonstrated that transplantation of BM cells from wild type mice into twi mice resulted in an extension of life from about 40 days when untreated to about 80 days. Early studies were done using irradiation for myeloablation to prepare the mice for BMT. However, for several reasons busulfan is now more commonly used for myeloablation.
22
The initial purification of human GALC and cloning of the human GALC cDNA by this laboratory in 1993
23,24
was followed by characterization of the gene in 1995.
25
This opened the way to additional studies including mutational analysis of patients. Over 250 disease-causing mutations have been identified in the GALC gene (http://www.hgmd.cf.ac.uk). The identification of the disease-causing mutations in the animal species has facilitated the establishment of colonies for research on the pathology and treatment of this disease. Having GALC clones has also led to in vitro and in vivo gene therapy studies. Initial studies showed that the GALC cDNA in a retroviral vector could transduce GALC-deficient cells resulting in supra-normal GALC activity with no ill effects, and that GALC activity could be excreted by these cells and taken up by neighboring cells.
26
The idea of cross correction is the basis for studies showing that transplanted or virally transduced cells can supply a missing enzyme to neighboring cells, especially in lysosomal disorders.
27
Since that time several groups have placed the species-specific GALC cDNA into different viral vectors for in vivo gene therapy trials alone and in combination with other approaches. It appears that adeno-associated viral vectors are being used most often in animal studies for KD and other genetic disorders.
28,29
In this laboratory AAVrh10 containing the mouse GALC cDNA is being used for gene therapy studies in twi mice.
14,16,17
Initial studies using this vector involved intra-cerebroventricular, intra-cerebellar and intra-venous (iv) injections on PND2.
14
There was a significant extension of life when all three routes of administration were used; the median survival was about 100 days compared to 40 days in untreated mice. There was clear evidence for GALC expression throughout the brain and improved myelination. Also, there was retention of walking, strength, and mobility until the end of life. While the results were positive they clearly were not good enough. Further studies using a single iv injection of AAVrh10-mGALC on PND10 showed dramatic improvement in central and peripheral nerve system myelination, an area significantly affected in human patients and animal models.
16
Starting iv treatment on PND10 instead of PND2 resulted in a longer lifespan for the treated mice, although it was still less than 100 days. PND10 is when mice start to rapidly myelinate, they are bigger and a larger dose of vector can be given. This may also reflect more the delay in treating newborn infants before receiving a definitive diagnosis of KD after birth.
The standard of care in pre-symptomatic infants and mildly affected later-onset patients with KD is HSCT.
30,31
While initial studies were done using bone marrow cells, more recently HSCs isolated from umbilical cord blood are being used. This greatly improves the chances of finding a suitable match for a given patient. While this treatment has been shown to increase the lifespan of treated patients, almost all treated patients present with difficulty walking by the end of the first decade of life and have problems with expressive language.
32
The walking difficulties appear to be due to a failure of HSCT to adequately correct the peripheral nervous system. Our studies in twi mice using a single iv injection of AAVrh10 containing mouse GALC cDNA alone at about PND10 showed near normal myelination in sciatic nerves. Moreover, studies combining BMT with a single iv injection of AAVrh10-mGALC on PND10-11 showed great synergy with many treated mice still alive at 350 days at the time of publication.
17
This compares to the median survival of 75-80 days for BMT or AAVrh10-mGALC when given alone. In order to evaluate the dose of viral vector that would be effective and to determine the timing of the combined treatment and the time from the BMT to viral injection, additional studies were undertaken. Results presented here show that the dose of vector is critical to survival and that there is some flexibility in the timing to perform viral injection treatment after BMT. These studies are very important since the combination of HSCT, the standard of care in humans, with a single iv injection of AAVrh10-hGALC is being considered by the FDA for a human trial.
Materials and Methods
Study design
This study is a follow up of our previous studies on gene therapy in combination with BMT for the mouse model of the KD. While we and others have shown a synergistic effect of AAV gene therapy in combination with BMT,
9,12,14,17,18,20,21
the focus of this study was to investigate treatment conditions for safe and more effective therapy. Experimental variables examined include viral dosage, timing for both viral injection and BMT and use of bone marrow cells from heterozygous donors.
Generation of AAVrh10-mGALC vector
Construction of the viral vector used in this study was previously reported.
16
Briefly, pCB7plasmid, an enhanced construction of AAV2 vector, was received from the Institute for Human Gene Therapy at the University of Pennsylvania. Mouse GALC cDNA was cloned into EcoRI site of this construct, downstream from the human CMV-enhancer/chicken β-actin hybrid promoter. The construct was sequenced and the integrity of the ITRs was confirmed by restriction enzyme analysis using SmaI and NcoI. The functionality of the construct was verified by in vitro cell transfection and measurement of GALC enzyme activity. The recombinant genome was cross-packaged into AAVrh10 capsid by utilizing a chimeric AAV2-Rep/AAV1-Cap and helper plasmids during a triple-transfection procedure.
33,34
Viral packaging and purification were accomplished by the Institute for Human Gene Therapy and the vector was called AAVrh10-mGALC. Viral titer, was determined by PCR of the simian virus 40 poly(A) sequence.
34,35
The viral titer of the vector batch of AAVrh10-mGALC in use is 6×1013 genomic equivalents/mL.
Animal procedures
Studies in mice were performed in accordance with approved protocols from the Animal Care and Use Committee at Jefferson Medical College. Twi mice used in the study were originally obtained from the Jackson Laboratory. These mice with a W339X mutation in the GALC gene are in the C57BL/6 background. Genotyping of the mice was performed on PND1 using polymerase chain reaction (PCR) as previously described.
14,36
Toe clips were used for mouse identification and DNA extraction. PCR fragments were digested with EcoRV and analyzed by electrophoresis on 2.5% MetaPhor agarose gel (Lonza Inc. Allendale, NJ, USA). Male and female mice were used arbitrarily, even before their sex could be determined. No sex-related differences in outcomes following treatment were noted. Transplanted animals were monitored daily for the first week for any signs of problem related to the procedure. They were monitored two to three times a week for the rest of their lives. Body weight was recorded weekly throughout their lives. Treated mice were allowed to survive as long as humanely possible or sacrificed at different time points for analysis. If deemed moribund (inactive and with unexpected weight loss) the mice were killed by carbon dioxide euthanasia and the age of death was recorded.
Bone marrow transplantation
The procedure for BMT was previously described.
17
Briefly, BMT recipient mice were myelo-suppressed using busulfan (Sigma, St. Louis, MO). A 3 mg/ml solution of busulfan was prepared by dissolving it in dimethylsulfoxide (30% of the final volume), and adding the remaining volume of sterile phosphate-buffered saline. Eight or 9-day-old affected mice (this small variability in injection times was due to the timing of births) were weighed, and 30 mg/kg of body weight of the busulfan solution was injected intraperitoneally (ip). BM cells from the syngeneic donor mice were obtained by flashing tibiae and femora using ice-cold Hepes buffered Hanks’ balanced salt solution (Mediatech, Manassas, VA). The cells were counted, centrifuged, and resuspended in Dulbecco's modified Eagle's medium. Twenty-four hours after busulfan injection, mice received an ip injection of 3–4 × 107 BM cells in a total volume of 0.2 mL. For at least two weeks after BMT, the mice were provided with prophylactic Neomycin (final concentration 500 μg/mL) (Sigma) in drinking water. Mice that received BMT and died less than PND30 were not included in these studies. Initially BM cells from non-carrier donors were used, but in some later studies BM cells from carrier mice were used.
Viral delivery
The young mice were cryo-anesthetized on ice before the injections. Injections were carried out on a light box to facilitate visualizing the tail vein. In our original publication a total of 4 × 1013 gc/kg body weight of virus were injected into the tail vein using a 28G insulin syringe.
17
The needle was inserted into the vein, and the solution containing the virus was injected manually. The success of injection was verified by noting blanching of the vein. The time for viral injection was between PND10 and PND40 depending the parameter to be investigated. After the injection, pups were warmed and returned to their cage. Mice that died within a few days of the injection (less than 10%) were not included in this study.
Tissue preparation for GALC assay
Tissues from treated and untreated mice were removed immediately after carbon dioxide euthanasia. The tissues were quickly frozen and stored at −80°C or immediately homogenized in distilled water using a Polytron apparatus (Brinkmann Instruments, Westbury, NY). Protein concentration was determined according to the method of Lowry et al.
37
GALC activity was measured using [3H]galactosylceramide substrate, according to our published method.
38
GALC activity was expressed as nmol substrate hydrolyzed/h/mg protein.
Preparation of semi-thin sections of sciatic nerves and toluidine blue staining
Treated and control mice were sacrificed by carbon dioxide euthanasia. Sciatic nerves were removed and submerged into 2% glutaraldehyde in 0.1M phosphate buffer, pH 7.4, followed by post-fixation in 1% osmium tetroxide in 0.1M phosphate buffer. The tissue fragments were dehydrated in series of graded ethanol penetrated with mixtures of embedding medium (Spurr) and ethanol and polymerized over night at 80°C. Semi-thin (0.5 micron) sections were cut on a UC7 ultramicrotome (Leica Microsystems, Wetzlar, Germany), using glass knives. The sections were transferred to glass microscopic slides, and stained with Toluidine Blue O.
Hematoxylin and eosin stain of liver sections
Livers were removed from untreated and treated mice, fixed in 10% formalin and paraffin-embedded. Four micrometers thick sections were cut on a microtome at room temperature, mounted on Superfrost Plus microscope slides (Thermo-Fisher Scientific), and air-dried. Sections were de-paraffinized in xylene, re-hydrated in a series of graded ethanol, and distilled water. Sections were stained with hematoxylin and eosin (H&E) using the manufacturer’s protocol. After staining and final washes, slides were mounted in ProlongGold (Life Technologies) and covered with a coverslip for examination.
Statistical analysis
Survival analysis was performed using GraphPad Prism 7.0 software (GraphPad software Inc., San Diego, USA). The survival curves were analyzed using the log-rank (Mantel-Cox) test. The survival rates of all experimental groups were compared to our previously published method where the mice received BMT around PND10 and iv viral injection (4 × 1013 gc/kg body weight) on the following day. GALC activity of the CNS and PNS of the mice treated with different viral doses were compared to the standard viral dose using two-tailed, unpaired t test (* P < 0.05, **P < 0.01, *** P < 0.001, **** P < 0.0001).
Results
Update on the previously published method of treatment combining BMT and iv AAVrh10-mGALC
Intra-peritoneal (ip) injection of the affected mice at PND9 with busulfan (30 mg/kg body weight) was followed after 24 hours by an ip injection of 3-4 × 107 bone marrow cells from non-carrier syngeneic mice. The following day mice received an iv injection of 4 × 1013 gc/kg body weight (called 1X dose) of AAVrh10-mGALC [17]. At the time of that publication most of the treated mice were still living, and additional mice were treated in the same manner. On Fig. 1 the survival of untreated twi mice and mice treated with combined therapy are compared to mice receiving BMT alone or AAVrh10 alone. As can be seen on the figure the median age of survival of the mice treated with combined therapy is nearly 400 days with some living over 500 days compared to 40 days if untreated. Due to the known tropism of AAVrh10, the heart was found to have the highest GALC activity followed by skeletal muscle, liver, sciatic nerve, spinal cord and cerebellum using the standard dosing and timing (Fig. 2A and B, black bar). However, while the GALC activities measured in nervous tissues are lower than heart, liver and muscle they are within the range measured in wild type mice and well above the very low activity measured in untreated twi mice. The GALC activities are maintained in all tested tissues for the life of the treated mice (Table 1).
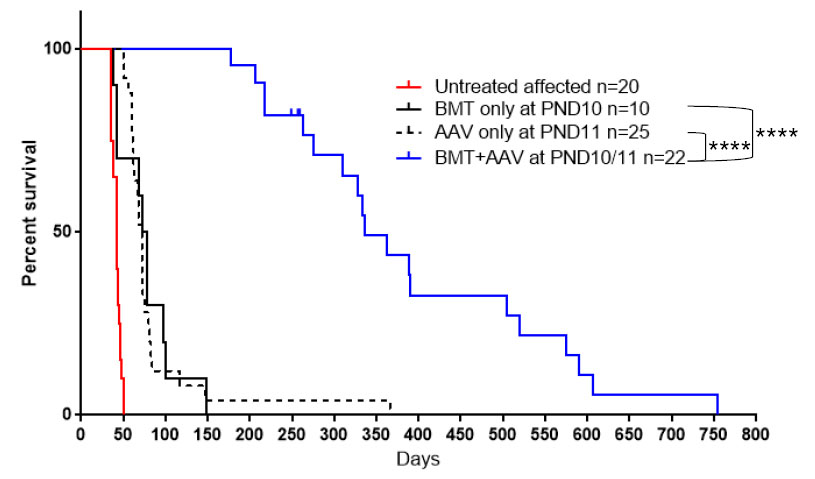
Fig. 1.
Effect of different treatment strategy on twi mice. Survival of twi mice either untreated, treated with BMT only, AAVrh10-mGALC only and combined BMT plus AAVrh10-mGALC on PND10/11 using the standard 1X dose. ****P < 0.0001.
.
Effect of different treatment strategy on twi mice. Survival of twi mice either untreated, treated with BMT only, AAVrh10-mGALC only and combined BMT plus AAVrh10-mGALC on PND10/11 using the standard 1X dose. ****P < 0.0001.
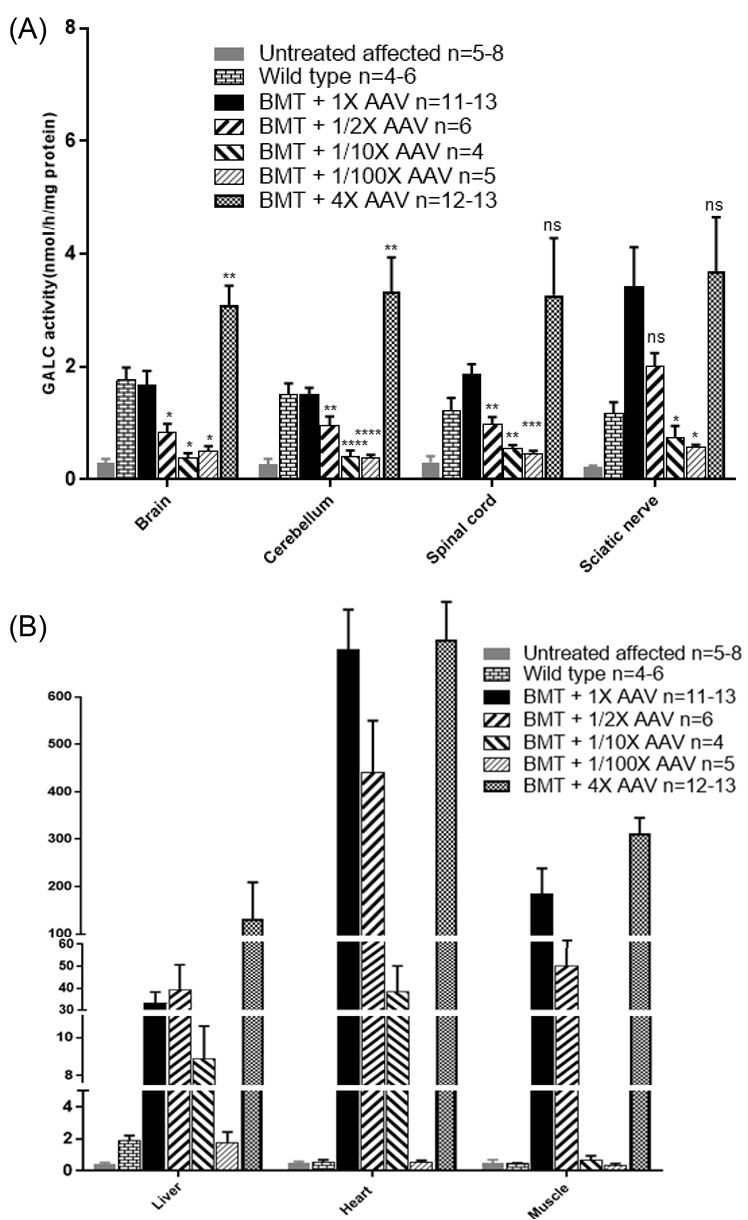
Fig. 2.
Effect of the viral dose on GALC activity of different tissues in twi mice. All BMT and viral injections were performed on PND10/11. (A) GALC activities in brain, cerebellum, spinal cord and sciatic nerve of twi mice receiving BMT and the standard 1X dose of vector are shown by the solid black bar (n=11-13). The following viral doses were used: 1/2X (n=6), 1/10X (n=4), 1/100X (n=5) and 4X (n=12-13). GALC activities were compared to the standard dose. Two-tailed, unpaired t test was used for statistical analysis. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (B) Effect of the viral dose on GALC activities of heart, skeletal muscle and liver is compared with our standard treatment (black bar). As shown in the figure, the GALC activity of the heart was highest compared to skeletal muscle and liver. As the GALC activities were so high in these tissues compared to the untreated twi mice and wild type mice statistical analysis was not performed. The mean and SEM is presented.
.
Effect of the viral dose on GALC activity of different tissues in twi mice. All BMT and viral injections were performed on PND10/11. (A) GALC activities in brain, cerebellum, spinal cord and sciatic nerve of twi mice receiving BMT and the standard 1X dose of vector are shown by the solid black bar (n=11-13). The following viral doses were used: 1/2X (n=6), 1/10X (n=4), 1/100X (n=5) and 4X (n=12-13). GALC activities were compared to the standard dose. Two-tailed, unpaired t test was used for statistical analysis. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (B) Effect of the viral dose on GALC activities of heart, skeletal muscle and liver is compared with our standard treatment (black bar). As shown in the figure, the GALC activity of the heart was highest compared to skeletal muscle and liver. As the GALC activities were so high in these tissues compared to the untreated twi mice and wild type mice statistical analysis was not performed. The mean and SEM is presented.
Table 1.
GALC activitya of different organs at different ages of mice treated with BMT and 1X AAV
|
Mice age (days)
|
Number of mice
|
Tissues
|
|
Brain
|
Sciatic nerve
|
Liver
|
Heart
|
| 100-300 |
n=3 |
0.7-1.9 |
- |
5.6-47.0 |
111.0-625.1 |
| 300-500 |
n=5 |
0.7-2.2 |
1.3-2.3 |
14.5-37.9 |
396.6-712.2 |
| 500-750 |
n=3-5 |
1.1-3.6 |
2.7-6.5 |
31.0-75.0 |
238.8-627.8 |
As shown in the previous publication
17
luxol fast blue/periodic acid Schiff (LFB/PAS) staining showed normal myelination of the CNS and PNS for the lives of the treated mice. Grossly the sciatic nerves of the untreated twi mice are enlarged due to edema (Fig. 3B), while they look thin like normal in twi mice receiving BMT plus AAV (Fig. 3C). The lack of normal myelination plus edema is confirmed on toluidine blue stained semi-thin sections of sciatic nerve from an untreated twi mouse (Fig. 4B) compared to a wild type mouse (Fig. 4A). A 606-day-old twi mouse treated with combined therapy using standard dosing and timing shows normal myelinated axons with no edema (Fig. 4C). Normal myelination in the sciatic nerve is important as this tissue is not corrected by HSCT alone in human patients and twi mice.
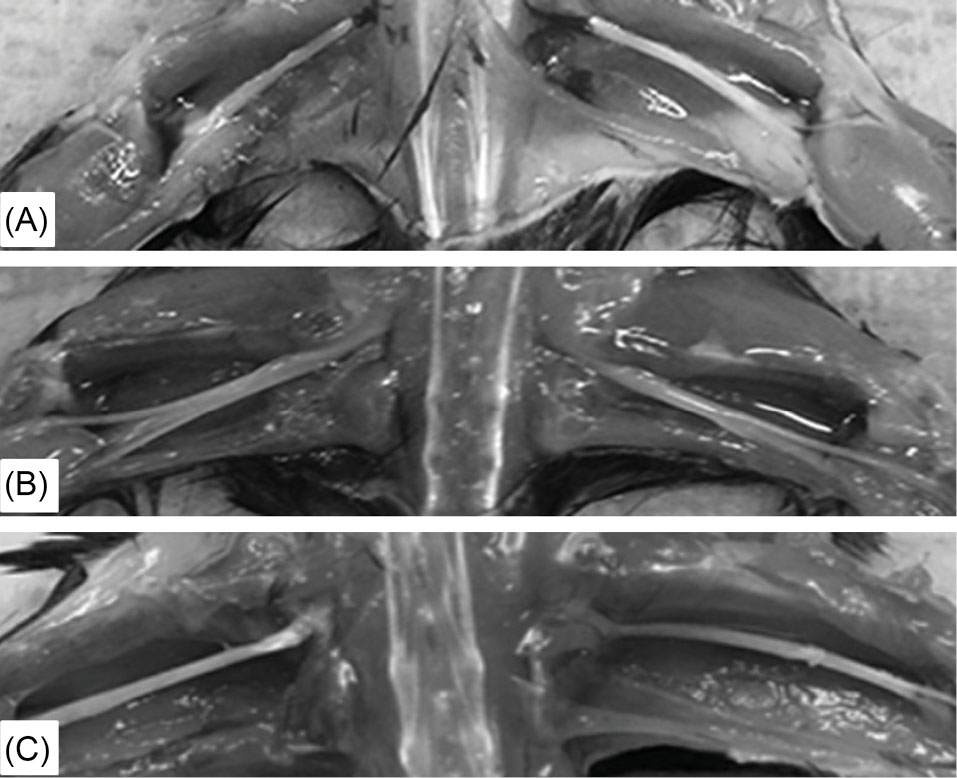
Fig. 3.
Gross appearance of sciatic nerves in wild type and untreated and treated twi mice.
(A) Sciatic nerves from a 60-day-old wild type mouse. (B) 37-day-old untreated twi mouse. (C) 250-day-old mouse treated with both BMT and AAVrh10-mGALC on PND10/11 using the standard 1X dose.
.
Gross appearance of sciatic nerves in wild type and untreated and treated twi mice.
(A) Sciatic nerves from a 60-day-old wild type mouse. (B) 37-day-old untreated twi mouse. (C) 250-day-old mouse treated with both BMT and AAVrh10-mGALC on PND10/11 using the standard 1X dose.
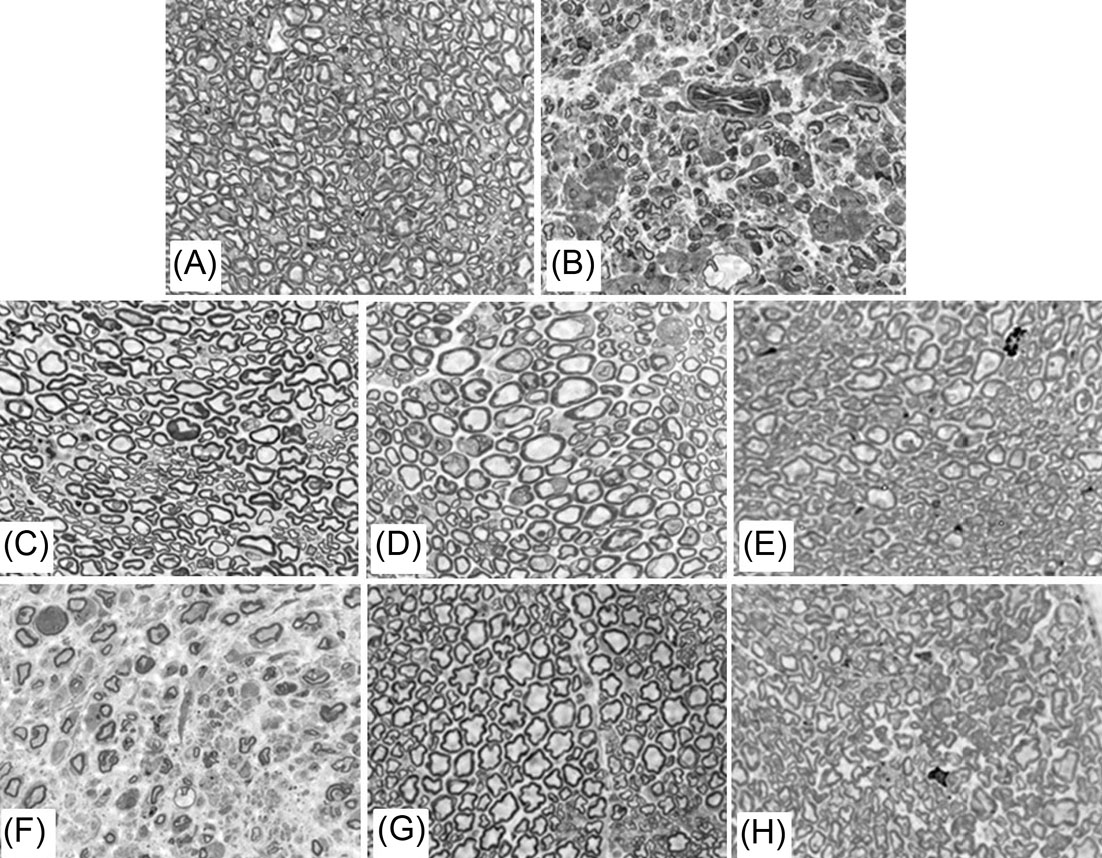
Fig. 4.
Toluidine blue-stained semi-thin sections of sciatic nerves from wild type and untreated and treated twi mice. (A) 62-day-old wild type mouse. (B) 36-day-old untreated twi mouse. (C) 606-day-old twi mouse treated with AAVrh10 (1X dose) on PND11. (D) 236-day-old twi mouse treated with AAVrh10-GALC (4X dose) on PND11. (E) 235-day-old twi mouse treated with AAVrh10 (1/2X dose) on PND11. (F) 50-day-old twi mouse treated with AAVrh10 (1/100X dose) on PND11. (G) 332-day-old twi mouse treated with AAVrh10 (1X dose) on PND15. (H) 420-day-old twi mouse treated with AAVrh10 (1X dose) on PND20. All treated mice received BMT on PND10 followed by a single iv viral injection. Original magnification is 400X.
.
Toluidine blue-stained semi-thin sections of sciatic nerves from wild type and untreated and treated twi mice. (A) 62-day-old wild type mouse. (B) 36-day-old untreated twi mouse. (C) 606-day-old twi mouse treated with AAVrh10 (1X dose) on PND11. (D) 236-day-old twi mouse treated with AAVrh10-GALC (4X dose) on PND11. (E) 235-day-old twi mouse treated with AAVrh10 (1/2X dose) on PND11. (F) 50-day-old twi mouse treated with AAVrh10 (1/100X dose) on PND11. (G) 332-day-old twi mouse treated with AAVrh10 (1X dose) on PND15. (H) 420-day-old twi mouse treated with AAVrh10 (1X dose) on PND20. All treated mice received BMT on PND10 followed by a single iv viral injection. Original magnification is 400X.
Effect of viral dose following BMT on survival, GALC activity and myelination of twi mice
In order to see the effect of viral dose on survival, different doses of AAVrh10-mGALC were injected iv 24 hours following BMT as described above. Initial studies were done using the following viral doses: 4 × 1013 (1X), 2 × 1013(1/2X), 4 × 1012(1/10X)and 4 × 1011(1/100X) gc/kg body weight. The p values compare the survival of mice receiving different viral doses to the standard dose (1X). As can be seen on Fig. 5A injecting one-half of the published viral dose the median survival dropped to about 180 days (P = 0.01) about half of the median survival when the standard dose was used. When one-tenth of the usual dose was used the median survival was only about 80 days (P = 0.0001), and when one-hundredth of the usual dose was used there was essentially no effect on survival beyond untreated or BMT-treated twi mice showing the dramatic effect of viral dose on survival. When the dose of viral vector was increased to four times the published dose, now 1.6 × 1014(4X), the median survival, while not statistically significant, was over 500 days, again showing the importance of viral dose on survival after BMT in the twi mice.
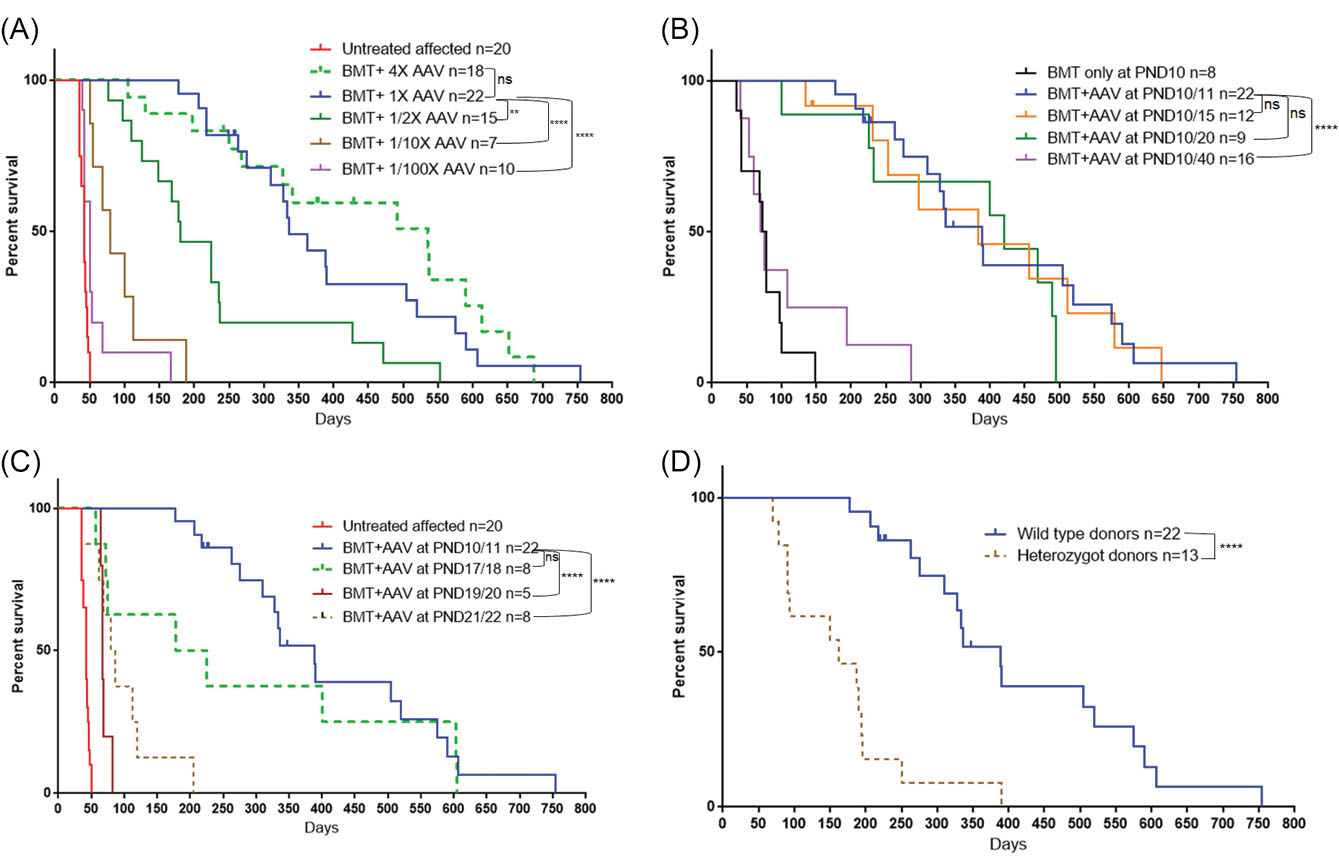
Fig. 5.
Survival of twi mice either untreated or treated under different conditions.
(A) Effect of viral dose on survival. (B) Effect of timing of viral injection (1X dose) after BMT on PND11. (C) Effect of doing both BMT and viral injection (1X dose) at a later time point. (D) Effect of using heterozygote donors for BMT followed by AAVrh10 (1X dose) on PND11. The survival curves were analyzed using the log-rank test. The survival of all experimental groups were compared to the standard method (solid blue color). **P < 0.01, ****P < 0.0001.
.
Survival of twi mice either untreated or treated under different conditions.
(A) Effect of viral dose on survival. (B) Effect of timing of viral injection (1X dose) after BMT on PND11. (C) Effect of doing both BMT and viral injection (1X dose) at a later time point. (D) Effect of using heterozygote donors for BMT followed by AAVrh10 (1X dose) on PND11. The survival curves were analyzed using the log-rank test. The survival of all experimental groups were compared to the standard method (solid blue color). **P < 0.01, ****P < 0.0001.
In addition to the decreased survival with a viral dose lower than the standard dose, there was a decrease in GALC activity in the tissues tested (Fig. 2A, B). P values presented on the figure compare the GALC activities measured with the standard dose (1X) to the activities measured with changes in the viral dose. The effects of viral dose following BMT on GALC activities in nervous tissues are shown on Fig. 2A. There was a statistically significant drop in GALC activity in brain, cerebellum and spinal cord with the 1/2X, 1/10X and 1/100X doses. In sciatic nerve the GALC activities measured were also lower at the smaller vector doses although they only reached statistical significance at the 1/10 and 1/100X doses. When the 4X dose was injected following BMT, GALC activities were increased in all nervous tissues but did not reach statistical significance in the spinal cord and sciatic nerve. However, all doses resulted in much higher GALC activity than untreated twi. These results show the ability of this treatment to bring GALC activity to the needed tissues. As expected, due to the tropism of AAVrh10, high GALC activity was measured in heart, liver and skeletal muscle (Fig. 2B). Very high GALC activity was measured in those tissues using the three highest viral doses.
The condition of the sciatic nerve myelin was examined using toluidine blue staining. Using the standard dose of viral vector the sciatic nerve myelin looks normal in a 606-day-old mouse (Fig. 4C) and a 239-day-old mouse that received 4X the standard dose (Fig. 4D). The myelin looks slightly less normal when 1/2X dose was used (Fig. 4E) and very abnormal when only 1/100X dose was injected (Fig. 4F). The results clearly show that the dose of AAVrh10-mGALC after BMT is critical to overall survival as well as the health of the myelin in sciatic nerve.
Effect of the timing of the viral injection following BMT on PND10
Initially twi mice were given busulfan on PND 9-10 and BMT the next day followed 24 hours later by an iv injection of the viral vector.
17
However, in this study the standard dose of virus was injected five days, ten days or 30 days after the BMT. As can be seen in Fig. 5B, there was no significant difference in survival when the viral vector was injected five and 10 days after the BMT compared to one day after BMT. The sciatic nerve myelin looked completely normal when the viral vector was injected five days after BMT (Fig. 4G) but less than completely normal when injected 10 days after BMT (Fig. 4H). However, the lifespans of the treated mice at these two time points were very similar (Fig. 5B). Injecting the virus 30 days after the BMT (PND40) resulted in a poor outcome, with the median survival near that of mice receiving BMT alone with only a few mice living more than 100 days (Fig. 5B). The GALC activities in the tissues of the mice in the timing study are presented on Table 2. At all-time points of viral injection there is high GALC activity in heart and liver and less, but significant, activity in brain and sciatic nerve. These studies indicate that damage to the nervous system must have occurred before the increase in GALC activity was provided by the injections of viral vector.
Table 2.
Effect of viral injection time on GALC activitya of the different tissues
|
BMT & Viral injection time
B
|
Brain
|
Sciatic nerve
|
Liver
|
Heart
|
Muscle
|
Age (days)
|
| Untreated affected, n = 6 |
0.24 (0.0-0.6) |
0.2 (0.1-0.3) |
0.5 (0.1-0.8) |
0.5 (0.2-1.1) |
0.5 (0.0-1.3) |
30-45 |
| Wild type, n = 4-6 |
1.8 (1.0-2.5) |
1.2 (0.7-2.0) |
1.9 (1.2-2.6) |
0.6 (0.3-0.8) |
0.4 (0.4-0.6) |
32-90 |
| AAV @ PND11-12, n=11-13 |
1.7 (0.5-3.6) |
3.4 (1.3-8.6) |
33.1 (13.0-75.0) |
516.0 (140.0-758.0) |
161.7 (51.0-433.2) |
263-754 |
| AAV @ PND15, n=5 |
2.0 (1.3-2.6) |
7.9 (2.3-22.9) |
73.0 (35.7-159.0) |
550.1 (218.9-764.0) |
124.7 (22.9-247.5) |
90-647 |
| AAV @ PND20, n=7 |
1.8 (0.8-3.4) |
3.5 (1.5-5.4) |
60.0 (18.4-100.7) |
421.0 (95.9-657.6) |
94.4 (9.5-222.2) |
233-495 |
| AAV @ PND40, =4 |
1.1 (0.7-1.7) |
3.5 (1.2-3.6) |
135.0 (70.5-255.7) |
218.0 (115.4-324.2) |
9.1 (2.7-12.7) |
53-287 |
a nmol/h/mg protein expressed as mean and range.
b All treated mice received BMT on PND10 followed by 1X viral dose at different times.
The question of how long does it take after vector injection to measure GALC expression in different tissues was answered by iv injection of 4 × 1013 gc/kg virus on PND11 with or without BMT into twi mice. Samples of heart, liver, brain and sciatic nerve were taken from sacrificed mice and assayed for GALC activity 24, 48, 72 and 96 hours after viral injection. As can be seen on Table 3 higher than normal GALC activity was measured after only 48 hours in heart and liver. Normal GALC activity could be measured in sciatic nerve after 48 hours, and supranormal GALC activity could be measured after 72 hours. Busulfan treatment and BMT had no effect on the rapid increase in GALC activity in the tissues analyzed.
Table 3.
Time of GALC expressiona after iv injection of AAVrh10-mGALC on PND11
|
Organ
|
24 h
|
48 h
|
72 h
|
96 h
|
Untreated twi mice
|
Wild type
|
| Brain |
0.3, 0.3 |
0.4, 0.2, 0.4b
|
0.6, 0.7 |
1.1b, 1.5b
|
0-0.1 |
1.0-3.6 |
| Sciatic nerve |
0, 0.2 |
2.2, 1.9, 1.9b
|
5.5, 7.9 |
17.2b, 41.1b
|
0-0.2 |
0.7, 2.0 |
| Liver |
0.8, 0.9 |
14.2, 20.6, 20.7b
|
18.0, 36.9 |
58.8b, 71.8b
|
0.1-0.3 |
1.7, 4.4 |
| Heart |
0.4, 0.4 |
13.1, 11.2, 12.3b
|
23.8, 28.8 |
70.4b, 65.3b
|
0.3-0.6 |
0.4-2.1 |
a nmol/h/mg protein; b These mice also received BMT on PND10.
Effect on survival when both BMT and viral injection are performed later
When treatment with busulfan and BMT were delayed until about PND17 and AAVrh10-GALC was injected the following day there appeared to be a drop in the median age of survival compared to when BMT plus AAVrh10 injection was performed about PND10-11, although it did not reach statistical significance (Fig. 5C). If the start of treatment was delayed until PND19-20 or PND21-22 there was a very significant drop in survival to less than 100 days. Since the tremor in untreated twi mice begins on about PND20 it appears that starting treatment too close to that age is not as effective as starting treatment at a younger age. This highlights the importance of starting BMT at a young age. Even, as shown above, GALC activity can be measured in critical tissues very rapidly after iv injection, it may be too late to correct damage that has already occurred.
Effect when bone marrow cells from heterozygous donors are used for transplantation before viral injection
Studies in humans show that HSCT alone has a positive effect on lifespan and partial correction or stabilization of some neurodiagnostic parameters. The effect of HSCT alone is more successful in human patients with survival into the second decade possible following HSCT if the individual is treated when pre-symptomatic or very early symptomatic.
32
BMT treatment alone in twi mice extends their lives from about 40 days to about 80 days, which is not as great of an effect as seen in human patients. In most human patients the HSC donor is a closely human leukocyte antigen (HLA) matched allogeneic donor obtained from umbilical cord blood. In some cases, HSC from a related donor is used. In either case, it is ideal for the donor to have good GALC activity so that engrafted cells can donate GALC activity to neighboring cells. In addition to these donor HSCs providing a source of GALC activity it is thought that they also have an anti-inflammatory component. However, it is possible with the increasingly diverse human population in the United States that a suitable allogeneic donor may not be available. A relative who is a suitable HLA match but is a carrier of KD may be the best option. Since AAVrh10-mGALC has a strong synergistic effect when combined with BMT in the mice, and the AAVrh10-GALC provides more GALC activity than the BMT, it was thought that using heterozygous donor mice as a source of BM cells would be equally as good as non-carrier donors. As shown on Fig. 5D, the use of BM cells from carriers lowered the median survival to less than 200 days. The survival was similar to the survival observed when half of the usual dose of AAVrh10 was used in combination with BMT (Fig. 5A). However, the GALC activities in the tissues from the twi mice treated using BM cells from heterozygous donors and gene therapy are similar to values measured in tissues from twi mice treated using non-carrier donors (data not shown). While this is an interesting finding in the twi mice, its relevance to the use of carrier donors in humans is still open to question. It is also well known that there are common polymorphisms in the GALC gene that lower the GALC activity measured in leukocytes to values measured in obligate carriers. It is probable that some human patients receiving HSCT were transplanted with HSC from a donor having one or more of these enzyme-lowering polymorphisms. What effect this has on overall outcome is not known.
Pathological examination of liver sections from treated twi mice
In twi mice treated with combined BMT and iv gene therapy that died or were sacrificed less than 300 days of age all organs appeared normal on gross examination, including the heart and liver with high GALC activity. Liver samples from mice treated by different methods were taken, sliced and stained with H&E for pathological examination. While most regions of the liver samples from long-living treated mice appeared normal (Fig. 6A), pathological changes were noted in the livers of a few animals, notably dilated sinusoids (Fig. 6B), hepatocellular hyperplasia (Fig. 6C) and presence of vacuolar cytoplasmic aggregates (Fig. 6D). Rare foci of extra-medullary hematopoiesis were also noted. Similar changes have been reported in some patients receiving blood stem cell transplantation and with other hematological disorders.
39
There have been reports of hepatocellular carcinoma in livers of mice treated with AAV vectors.
40-43
However, the examination of liver tissues from over 50 treated mice showed no evidence of neoplastic changes.
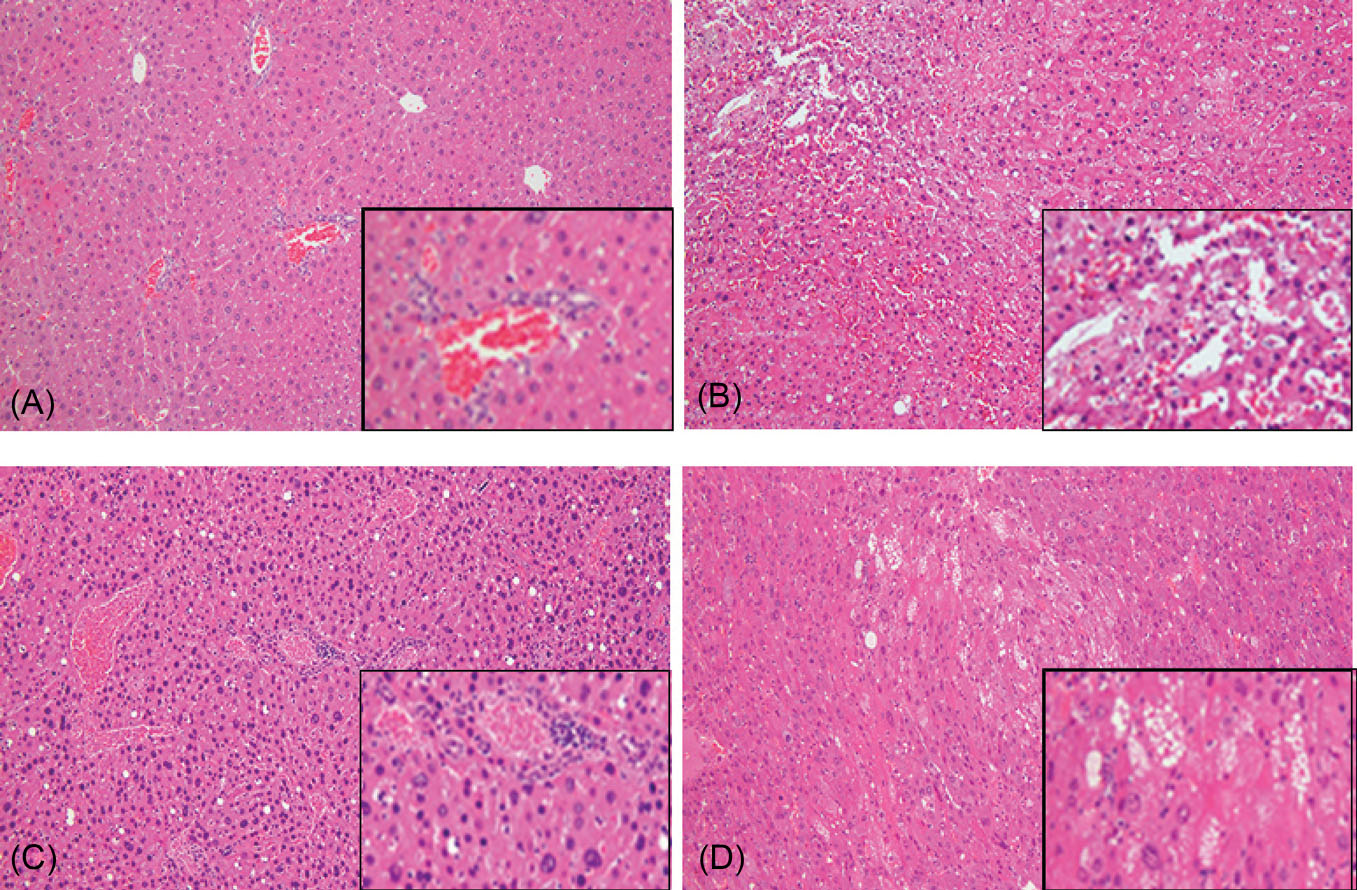
Fig. 6.
Hematoxylin and eosin staining of liver sections. (A) 606-day-old twi mouse treated with BMT plus 1X AAVrh10-mGALC on PND10/11 showing normal morphology. (B) 606-day-old twi treated with BMT and 1X AAVrh10-mGALC on PND10/11 showing dilated sinusoids. (C) 754-day-old twi mouse treated with BMT plus 1X AAVrh10-mGALC on PND10/11 showing hepatocellular hyperplasia. (D) 472-day-old twi mouse treated with BMT plus 1/2X AAVrh10-mGALC on PND10/11 showing vacuolar changes. The original images are 10X and the inserts are 20X.
.
Hematoxylin and eosin staining of liver sections. (A) 606-day-old twi mouse treated with BMT plus 1X AAVrh10-mGALC on PND10/11 showing normal morphology. (B) 606-day-old twi treated with BMT and 1X AAVrh10-mGALC on PND10/11 showing dilated sinusoids. (C) 754-day-old twi mouse treated with BMT plus 1X AAVrh10-mGALC on PND10/11 showing hepatocellular hyperplasia. (D) 472-day-old twi mouse treated with BMT plus 1/2X AAVrh10-mGALC on PND10/11 showing vacuolar changes. The original images are 10X and the inserts are 20X.
Discussion
The naturally occurring twi mouse has proven to be an excellent model to study the pathology and treatment of KD. It has a mutation causing a premature stop codon resulting in little if any GALC protein to be made.
36
Mice homozygous for this mutation die by about 40 days and are not fertile. Since the initial attempt at treating this model with BMT by Yeager and colleagues,
3
many other attempts have been made using a variety of procedures and drugs. Without referencing all of the literature, it appears that some type of combined therapy involving BMT and gene therapy is the best option at this time. In some studies, multiple injections into the brain, additional drugs and irradiation before BMT were used but these methods may not be applicable for a human trial. HSCT in pre-symptomatic infants predicted to develop KD has shown the most promise in slowing the course of the disease while preserving most of cognitive function.
32
However, by the end of the first decade almost all of the treated individuals show significant peripheral neuropathy. Therefore, adding a simple gene therapy treatment to HSCT to help with that aspect would be most advantageous. Studies in twi mice combining BMT with AAV gene therapy show great promise in that regard. A number of viral vectors available for gene therapy trials using different routes of administration are showing promise in treating neurodegenerative disorders.
33-35
The use of a single iv injection of AAVrh10-mGALC following HSCT seems to be the least complicated therapy to implement. Also HSCT is already considered the “standard of care” for pre-symptomatic individuals. As shown herein, there is some flexibility in dosing and timing that would permit adaption to a human trial.
When the mice received a single iv injection of AAVrh10-mGALC in PND10 they showed normal or supranormal GALC activity in all tissues analyzed, including brain, spinal cord and sciatic nerve.
16
LFB/PAS staining showed normal myelination in the cerebrum, spinal cord and sciatic nerve. The median survival of these mice was 72 days with only one living more than 100 days. This was better than twi mice receiving the iv injection at PND2.
14
Clearly twi mice need more than just high GALC activity to live longer lives. Most infantile human patients treated before five weeks of age can live at least into the second decade with only HSCT. That treatment only provides a relatively small amount of GALC activity to the tissues, therefore the HSCs must provide an additional component to the treatment. BMT of twi mice has been shown to slightly lower the psychosine concentration in brain but not sciatic nerves.
44
However, it has been shown to lower many cytokines and chemokines in brain related to the inflammatory component of this disease.
10
Therefore combining BMT with iv injection of AAVrh10-GALC seems to offer the best option for successful treatment of this disease at this time.
When our initial studies combining BMT with a single iv injection of AAVrh10-mGALC were published most of the treated twi mice were still living.
17
Additional mice were treated the same way. Since that time most of those mice given our published dose of 4 ×1013 gc/kg on PND10-11 have died. The median survival is 389 days and with some living over 500 days. The studies reported here show that the survival is related to the dose of AAVrh10 vector; when less vector than used in the original paper was injected after BMT the survival rate dropped significantly as shown on Fig. 5A. In addition, the use of lower doses of vector compared to the standard dose resulted statistically lower levels of GALC activity in brain, cerebellum and spinal cord (Fig. 2A). When 4X vector was injected on PND10 the median survival appeared to increased further although it did not reach statistical significance. The higher vector dose resulted in more GALC activity measured in all nervous tissues although it did not reach statistical significance in spinal cord and sciatic nerve (Fig. 2A). These results clearly show that vector dose has a significant effect on outcome.
The initial studies combining BMT with iv AAVrh10 were done starting at PND9 with busulfan injection, followed by BMT the next day and viral injection the following day. The data presented here show that delaying the viral injection by 5 or 10 days had no significant effect on the median survival of these mice (Fig. 5B). This may not be surprising since it has been shown that BMT alone at PND10 can prolong the lives of twi mice to an average of about 80 days (Fig. 1) which agrees with the study of Yeager et al.
3
It can also be shown that there is a small increase in GALC activity in CNS with BMT treatment alone at least in young mice. However, some earlier studies were done using irradiation for myelo-suppression which may disrupt membrane integrity. Also, there may not be a need for much GALC activity before PND10 in mice as myelin is just being laid on newly formed axons. When the time for viral injection is delayed to 30 days following BMT (PND40), the survival is very similar to BMT alone, although a few mice lived more than 100 days (Fig. 5B). After PND20, when the untreated twi mice start to have tremors, and the sciatic nerves of twi mice treated only with BMT have abnormal pathology, there is a need for more GALC activity provided by the viral vector to correct the myelination in the PNS. Studies injecting the viral vector 20 days after BMT (PND30) are underway but the mice are too young to reach a conclusion regarding the latest time point that can still result in a lifespan near that of mice that received viral injections on PND10-11, PND15 and PND20. While starting the BMT around PND10 followed by viral injection one, five or ten days later was found to be satisfactory, additional studies were done delaying both the BMT and viral injection to PND17-18, PND19-20 and PND21-22. The experiments show that delaying the start of both treatments resulted in overall shortened survival (Fig. 5C). While these studies in mice may have little relevance to the timing of viral injection in human patients following HSCT, they do show that timing should be considered when planning a clinical trial. Our studies show that GALC expression is detected within 48 hours following iv injection (Table 3). This is important to the successful treatment of a severe genetic disease that can have a rapid downhill course.
The studies utilizing heterozygous mice as BM donors were performed to determine if the outcomes were comparable to those receiving BM from non-carrier BM donors. This was done for several reasons. (1) To determine if the GALC activity in the carrier bone marrow cells, which might be about half of that from non-carrier mice, worked as well in prolonging life when combined with viral gene therapy. (2) To provide some insight as to the question of whether human related donors who are carriers of mutations in the GALC gene would be suitable to be used as an HSC donor. BM cells from heterozygous donors should still provide the anti-inflammatory component to the treatment since carriers of KD have no obvious clinical issues while the viral vector should provide adequate GALC activity. At least in twi mice, it appears that using BM cells from carrier mice plus iv gene therapy did not result in the lifespans observed when BM cells from non-carrier mice were used (Fig. 5D). However, it may not be true for all human families. In some families only a related donor is a good HLA match for the patient and will be used for HSCT regardless of carrier status. Also, since there is a high frequency of GALC activity lowering polymorphisms in the “normal” GALC gene, some recipients of HSCT are receiving bone marrow cells from donors with GALC activity within the wide carrier range.
There is controversy regarding the increase in the incidence of hepatocellular carcinoma in livers of mice treated with AAV vectors.
40-43
However, in our study there were no significant pathological changes in mice living less than 300 days. Most regions of the liver samples from long-living treated mice appeared normal, however, some pathological changes were noted in the livers of a few animals, notably dilated sinusoids, hepatocellular hyperplasia and presence of vacuolar cytoplasmic aggregates. While some rare foci of extra-medullary hematopoiesis were noted, similar changes have been reported in some patients receiving blood stem cell transplantation and with other hematological disorders.
39
However, the examination of liver tissues from over 50 treated mice showed no evidence of neoplastic changes.
Conclusion
It is commonly assumed that earlier is better when it comes to instituting any type of therapy in human patients, especially in those disorders involving significant neurologic involvement. That is done to prevent pathological changes in the CNS and PNS before they become irreversible. For this reason newborn screening (NBS) for some genetic disorders, including KD, has been started in several states.
45
This is being done to identify individuals who will develop a given disease before clinical features appear and before there is significant damage to the developing nervous systems. NBS tests that were developed to identify individuals at a “high risk” for developing infantile disease have been mostly successful. However, there are still issues relating to onset, predicting the severity of the disease, and timing to start treatment to be settled. There may be delays in making a conclusive diagnosis as to the type of disease (infantile, late infantile, or late-onset) and in finding a suitable donor for HSCT. The more we can learn from the animal models related to dosing, timing and donor status, the better the outcomes for treating human patients will be. It appears that combining a single iv injection of AAVrh10-GALC following HSCT, the current standard of care, offers the best hope for the individuals found to be at risk for developing KD.
Acknowledgments
We thank Han Zhi Rao for her technical assistance, and we are grateful to Professor Fred Gorstein, Former Chairman of the Department of Pathology, for examination of liver slices. This research was sponsored in part by a grant from The Legacy of Angels Foundation.
Funding sources
The study was partially funded by Legacy of Angels Foundation.
Ethical statement
The authors declare no ethical issue to be considered.
Competing interests
The authors declare no conflicts of interests.
Authors’ contribution
MAR, PL and DAW planned the studies, assisted in performing the necessary assays and analysis of the data. All contributed to the writing and editing of the manuscript.
Research Highlights
What is the current knowledge?
simple
-
√ Krabbe disease (KD) is an autosomal recessive lysosomal
disorder.
-
√ KD is caused by mutations in the galactocerebrosidase
(GALC) gene.
-
√ Low GALC activity causes defective myelination in the
peripheral and central nervous systems.
-
√ Hematopoietic stem cell transplantation (HSCT) in presymptomatic
individuals is the only effective treatment.
-
√ HSCT extends the lives of treated individuals, while most
have difficulty walking by the end of the first decade due to
peripheral neuropathy.
What is new here?
simple
-
√ Combining BMT with a single iv injection of AAVrh10-
mGALC greatly extends the lives of twitcher (twi) mice, a
murine model of KD.
-
√ The dose of viral vector has a profound effect on survival
and measured GALC activity.
-
√ Delaying both the BMT and the viral injection can shorten
the lifespans of the treated mice.
References
- Wenger DA, Rafi MA, Luzi P. Krabbe disease: One Hundred years from the bedside to the bench to the bedside. J Neurosci Res 2016; 94:982-9. doi: 10.1002/jnr.23743 [Crossref] [ Google Scholar]
- Wenger DA. Murine, canine and non-human primate models of Krabbe disease. Mol Med Today 2000; 6:449-51. [ Google Scholar]
- Yeager AM, Brennan S, Tiffany C, Moser HW, Santos GW. Prolonged survival and remyelination after hematopoietic cell transplantation in the twitcher mouse. Science 1984; 225:1052-4. doi: 10.1126/science.6382609 [Crossref] [ Google Scholar]
- LeVine SM, Pedchenko TV, Bronshteyn IG, Pinson DM. L-cycloserine slows the clinical and pathological course in mice with globoid cell leukodystrophy (twitcher mice). J Neurosci Res 2000; 60:231-6. doi: 10.1002/(SICI)1097-4547(20000415)60:2<231::AIDJNR12>3.0.CO;2-E [Crossref] [ Google Scholar]
- Wu YP, McMahon EJ, Matsuda J, Suzuki K, Matsushima GK, Suzuki K. Expression of immune-related molecules is downregulated in twitcher mice following bone marrow transplantation. J Neuropathol Exp Neurol 2001; 60:1062-74. doi: 10.1093/jnen/60.11.1062 [Crossref] [ Google Scholar]
- Biswas S, LeVine SM. Substrate-reduction therapy enhances the benefits of bone marrow transplantation in young mice with globoid cell leukodystrophy. Pediatr Res 2002; 51:40-7. doi: 10.1203/00006450-200201000-00009 [Crossref] [ Google Scholar]
- Rafi MA, Zhi Rao H, Passini MA, Curtis M, Vanier MT, Zaka M. AAV-mediated expression of galactocerebrosidase in brain results in attenuated symptoms and extended life span in murine models of globoid cell leukodystrophy. Mol Ther 2005; 11:734-44. doi: 10.1016/j.ymthe.2004.12.020 [Crossref] [ Google Scholar]
- Lee WC, Tsoi YK, Troendle FJ, DeLucia MW, Ahmed Z, Dicky CA. Single-dose intracerebroventricular administration of galactocerebrosidase improves survival in a mouse model of globoid cell leukodystrophy. FASEB J 2007; 21:2520-7. doi: 10.1096/fj.06-6169com [Crossref] [ Google Scholar]
- Lin D, Donsante A, Macauley S, Levy B, Vogler C, Sands MS. Central nervous system-directed AAV2/5-mediated gene therapy synergizes with bone marrow transplantation in the murine model of globoid-cell leukodystrophy. Mol Ther 2007; 15:44-52. doi: 10.1038/sj.mt.6300026 [Crossref] [ Google Scholar]
- Luzi P, Abraham RM, Rafi MA, Curtis M, Hooper DC, Wenger DA. Effects of treatments on inflammatory and apoptotic markers in the CNS of mice with globoid cell leukodystrophy. Brain Res 2009; 1300:146-58. doi: 10.1016/j.brainres.2009.09.017 [Crossref] [ Google Scholar]
- Strazza M, Luddi A, Carbone M, Rafi MA, Costantino-Ceccarini E, Wenger DA. Significant correction of pathology in brains of twitcher mice following injection of genetically modified mouse neural progenitor cells. Mol Genet Metab 2009; 97:27-34. doi: 10.1016/j.ymgme.2009.01.005 [Crossref] [ Google Scholar]
- Reddy AS, Kim JH, Hawkins-Salsbury JA, Macauley SL, Tracy ET, Vogler CA. Bone marrow transplantation augments the effect of brain- and spinal cord-directed adeno-associated virus 2/5 gene therapy by altering inflammation in the murine model of globoid-cell leukodystrophy. J Neurosci 2011; 31:9945-57. doi: 10.1523/JNEUROSCI.1802-11.2011 [Crossref] [ Google Scholar]
- Qin EY, Hawkins-Salsbury JA, Jiang X, Reddy AS, Farber NB, Ory DS. Bone marrow transplantation increases efficacy of central nervous system-directed enzyme replacement therapy in the murine model of globoid cell leukodystrophy. Mol Genet Metab 2012; 107:186-96. doi: 10.1016/j.ymgme.2012.05.021 [Crossref] [ Google Scholar]
- Rafi MA, Rao HZ, Luzi P, Curtis MT, Wenger DA. Extended normal life after AAVrh10-mediated gene therapy in the mouse model of Krabbe disease. Mol Ther 2012; 20:2031-42. doi: 10.1038/mt.2012.153 [Crossref] [ Google Scholar]
- Berardi AS, Pannuzzo G, Graziano A, Costantino-Ceccarini E, Piomboni P, Luddi A. Pharmacological chaperones increase residual beta-galactocerebrosidase activity in fibroblasts from Krabbe patients. Mol Genet Metab 2014; 112:294-301. doi: 10.1016/j.ymgme.2014.05.009 [Crossref] [ Google Scholar]
- Rafi MA, Rao HZ, Luzi P, Luddi A, Curtis MT, Wenger DA. Intravenous injection of AAVrh10-GALC after the neonatal period in twitcher mice results in significant expression in the central and peripheral nervous systems and improvement of clinical features. Mol Genet Metab 2015; 114:459-66. doi: 10.1016/j.ymgme.2014.12.300 [Crossref] [ Google Scholar]
- Rafi MA, Rao HZ, Luzi P, Wenger DA. Long-term Improvements in Lifespan and Pathology in CNS and PNS After BMT Plus One Intravenous Injection of AAVrh10-GALC in Twitcher Mice. Mol Ther 2015; 23:1681-90. doi: 10.1038/mt.2015.145 [Crossref] [ Google Scholar]
- Hawkins-Salsbury JA, Shea L, Jiang X, Hunter DA, Guzman AM, Reddy AS. Mechanism-based combination treatment dramatically increases therapeutic efficacy in murine globoid cell leukodystrophy. J Neurosci 2015; 35:6495-505. doi: 10.1523/JNEUROSCI.4199-14.2015 [Crossref] [ Google Scholar]
- Ricca A, Rufo N, Ungari S, Morena F, Martino S, Kulik W. Combined gene/cell therapies provide long-term and pervasive rescue of multiple pathological symptoms in a murine model of globoid cell leukodystrophy. Hum Mol Genet 2015; 24:3372-89. doi: 10.1093/hmg/ddv086 [Crossref] [ Google Scholar]
- Karumuthil-Melethil S, Marshall MS, Heindel C, Jakubauskas B, Bongarzone ER, Gray SJ. Intrathecal administration of AAV/GALC vectors in 10-11-day-old twitcher mice improves survival and is enhanced by bone marrow transplant. J Neurosci Res 2016; 94:1138-51. doi: 10.1002/jnr.23882 [Crossref] [ Google Scholar]
- Marshall MS, Issa Y, Jakubauskas B, Stoskute M, Elackattu V, Marshall JN. Long-Term Improvement of Neurological Signs and Metabolic Dysfunction in a Mouse Model of Krabbe's Disease after Global Gene Therapy. Mol Ther 2018; 26:874-89. doi: 10.1016/j.ymthe.2018.01.009 [Crossref] [ Google Scholar]
- Wilkinson FL, Sergijenko A, Langford-Smith KJ, Malinowska M, Wynn RF, Bigger BW. Busulfan conditioning enhances engraftment of hematopoietic donor-derived cells in the brain compared with irradiation. Mol Ther 2013; 21:868-76. doi: 10.1038/mt.2013.29 [Crossref] [ Google Scholar]
- Chen YQ, Rafi MA, de Gala G, Wenger DA. Cloning and expression of cDNA encoding human galactocerebrosidase, the enzyme deficient in globoid cell leukodystrophy. Hum Mol Genet 1993; 2:1841-5. doi: 10.1093/hmg/2.11.1841 [Crossref] [ Google Scholar]
- Chen YQ, Wenger DA. Galactocerebrosidase from human urine: purification and partial characterization. Biochim Biophys Acta 1993; 1170:53-61. doi: 10.1016/0005-2760(93)90175-9 [Crossref] [ Google Scholar]
- Luzi P, Rafi MA, Wenger DA. Structure and organization of the human galactocerebrosidase (GALC) gene. Genomics 1995; 26:407-9. doi: 10.1016/0888-7543(95)80230-j [Crossref] [ Google Scholar]
- Rafi MA, Fugaro J, Amini S, Luzi P, de Gala G, Victoria T. Retroviral vector-mediated transfer of the galactocerebrosidase (GALC) cDNA leads to overexpression and transfer of GALC activity to neighboring cells. Biochem Mol Med 1996; 58:142-50. doi: 10.1006/bmme.1996.0042 [Crossref] [ Google Scholar]
- Fratantoni JC, Hall CW, Neufeld EF. Hurler and Hunter syndromes: mutual correction of the defect in cultured fibroblasts. Science 1968; 162:570-2. doi: 10.1126/science.162.3853.570 [Crossref] [ Google Scholar]
- Colella P, Ronzitti G, Mingozzi F. Emerging Issues in AAV-Mediated In Vivo Gene Therapy. Mol Ther Methods Clin Dev 2018; 8:87-104. doi: 10.1016/j.omtm.2017.11.007 [Crossref] [ Google Scholar]
- Gessler DJ, Tai PWL, Li J, Gao G. Intravenous Infusion of AAV for Widespread Gene Delivery to the Nervous System. Methods Mol Biol 2019; 1950:143-63. doi: 10.1007/978-1-4939-9139-6_8 [Crossref] [ Google Scholar]
- Escolar ML, Poe MD, Provenzale JM, Richards KC, Allison J, Wood S. Transplantation of umbilical-cord blood in babies with infantile Krabbe's disease. N Engl J Med 2005; 352:2069-81. doi: 10.1056/NEJMoa042604 [Crossref] [ Google Scholar]
- Krivit W, Shapiro EG, Peters C, Wagner JE, Cornu G, Kurtzberg J. Hematopoietic stem-cell transplantation in globoid-cell leukodystrophy. N Engl J Med 1998; 338:1119-26. doi: 10.1056/NEJM199804163381605 [Crossref] [ Google Scholar]
- Wright MD, Poe MD, DeRenzo A, Haldal S, Escolar ML. Developmental outcomes of cord blood transplantation for Krabbe disease: A 15-year study. Neurology 2017; 89:1365-72. doi: 10.1212/WNL.0000000000004418 [Crossref] [ Google Scholar]
- Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM. Gene therapy vectors based on adeno-associated virus type 1. J Virol 1999; 73:3994-4003. [ Google Scholar]
- Hildinger M, Auricchio A, Gao G, Wang L, Chirmule N, Wilson JM. Hybrid vectors based on adeno-associated virus serotypes 2 and 5 for muscle-directed gene transfer. J Virol 2001; 75:6199-203. doi: 10.1128/JVI.75.13.6199-6203.2001 [Crossref] [ Google Scholar]
- Passini MA, Wolfe JH. Widespread gene delivery and structure-specific patterns of expression in the brain after intraventricular injections of neonatal mice with an adeno-associated virus vector. J Virol 2001; 75:12382-92. doi: 10.1128/JVI.75.24.12382-12392.2001 [Crossref] [ Google Scholar]
- Sakai N, Inui K, Tatsumi N, Fukushima H, Nishigaki T, Taniike M. Molecular cloning and expression of cDNA for murine galactocerebrosidase and mutation analysis of the twitcher mouse, a model of Krabbe's disease. J Neurochem 1996; 66:1118-24. doi: 10.1046/j.1471-4159.1996.66031118.x [Crossref] [ Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193:265-75. [ Google Scholar]
-
Wenger DA WC. Screening for lysosomal disorders. In: Hommes FA, editor. Techniques in Diagnostic Human Biochemical Genetics. New York, NY: Wiley-Liss; 1991. p. 587-617.
- Yamamoto K, Miwa Y, Abe-Suzuki S, Abe S, Kirimura S, Onishi I. Extramedullary hematopoiesis: Elucidating the function of the hematopoietic stem cell niche (Review). Mol Med Rep 2016; 13:587-91. doi: 10.3892/mmr.2015.4621 [Crossref] [ Google Scholar]
- Donsante A, Vogler C, Muzyczka N, Crawford JM, Barker J, Flotte T. Observed incidence of tumorigenesis in long-term rodent studies of rAAV vectors. Gene Ther 2001; 8:1343-6. doi: 10.1038/sj.gt.3301541 [Crossref] [ Google Scholar]
- Bell P, Wang L, Lebherz C, Flieder DB, Bove MS, Wu D. No evidence for tumorigenesis of AAV vectors in a large-scale study in mice. Mol Ther 2005; 12:299-306. doi: 10.1016/j.ymthe.2005.03.020 [Crossref] [ Google Scholar]
- Buning H, Schmidt M. Adeno-associated Vector Toxicity-To Be or Not to Be?. Mol Ther 2015; 23:1673-5. doi: 10.1038/mt.2015.182 [Crossref] [ Google Scholar]
- Chandler RJ, LaFave MC, Varshney GK, Burgess SM, Venditti CP. Genotoxicity in Mice Following AAV Gene Delivery: A Safety Concern for Human Gene Therapy?. Mol Ther 2016; 24:198-201. doi: 10.1038/mt.2016.17 [Crossref] [ Google Scholar]
- Ichioka T, Kishimoto Y, Brennan S, Santos GW, Yeager AM. Hematopoietic cell transplantation in murine globoid cell leukodystrophy (the twitcher mouse): effects on levels of galactosylceramidase, psychosine, and galactocerebrosides. Proc Natl Acad Sci U S A 1987; 84:4259-63. doi: 10.1073/pnas.84.12.4259 [Crossref] [ Google Scholar]
- Gelb MH. Newborn Screening for Lysosomal Storage Diseases: Methodologies, Screen Positive Rates, Normalization of Datasets, Second-Tier Tests, and Post-Analysis Tools. Int J Neonatal Screen 2018; 4. doi: 10.3390/ijns4030023 [Crossref]