Bioimpacts. 12(5):405-414.
doi: 10.34172/bi.2021.25
Original Research
The oncolytic activity of Clostridium novyi nontoxic spores in breast cancer
Fatemeh Abedi Jafari 1  , Asghar Abdoli 2, *
, Asghar Abdoli 2, *  , Reza Pilehchian 3, Neda Soleimani 1, Seyed Masoud Hosseini 1
, Reza Pilehchian 3, Neda Soleimani 1, Seyed Masoud Hosseini 1
Author information:
1Department of Microbiology and Microbial Biotechnology, Faculty of Life Sciences and Biotechnology, Shahid Beheshti University, Tehran, Iran
2Department of Hepatitis and HIV, Pasteur Institute of Iran (IPI), Tehran, Iran
3Specialized Clostridia Research Laboratory, Department of Anaerobic Vaccine Research and Production, Razi Vaccine and Serum Research Institute, Agricultural Research Education and Extension Organization (AREEO), Karaj, Alborz, Iran
Abstract
Introduction:
Hypoxia context is highly specific for tumors and represents a unique niche which is not found elsewhere in the body. Clostridium novyi is an obligate anaerobic bacterium. It has a potential to treat tumors. The aim of this study was to produce the C. novyi nontoxic spores and to investigate its oncolytic effect on breast cancer in mice model.
Methods:
Primarily, the lethal toxin gene in C. novyi type B was removed. Colonies were isolated using PCR testing. To assure the removal of alpha-toxin, plasmid extraction and in vivo assay were conducted. Next, to treat breast cancer model in different sizes of tumors, a single dose of spores of C. novyi nontoxic was tested.
Results:
The results denoted that C. novyi nontoxic lost lethal toxin and appeared to be safe. For smaller than 1000 mm3 tumors, a single dose of C. novyi nontoxic was able to cure 100% of mice bearing breast tumors. Hence the mice remained free of tumor relapse. Tumors larger than 1000 mm3 were not cured by a single dose of C. novyi nontoxic treatment.
Conclusion:
The experiment concluded that the C. novyi nontoxic might be a suitable and safe candidate, a novel therapeutic approach to encounter such hypoxic regions in the center of tumors. Research also showed that bacteriolytic therapy by C. novyi nontoxic could lead to regression in small tumor.
Keywords: Clostridium novyi, Nontoxic, Alpha toxin, Spore, Breast cancer, Hypoxia
Copyright and License Information
© 2022 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Breast cancer is the most often diagnosed form of cancer. Most of the time, breast cancer recurrence after treatment is associated with hypoxia.
2
Although several approaches have been suggested to treat different cancer types including surgery, radiation therapy, chemotherapy, hormone therapy, immunotherapy, and targeted therapy,
3
the current therapeutic strategies remain insufficient. Progress in these approaches and combinational therapeutic regimens depend on the nature of the cancer, and the immune and metabolic profile within the tumor microenvironment. In fact, the critical problem in current treatment methods is the hypoxia in a tumor. Most of the solid tumors contain hypoxia zone.
4
Hypoxia affects tumor cell properties such as neovascularization, cell growth rate, sensibility to treatment and metastasis.
2,5
Triple-negative breast cancer (TNBC) is a tumor that tests negative for estrogen receptors, progesterone receptors, and excess HER2 protein.
6,7
For researchers, there is ardent interest in finding a new method that can cure this kind of breast cancer. TNBC is considered to be more aggressive and has a defective prognosis than other types of breast cancers.
5,8
In recent years, the use of whole live, genetically modified bacteria, bacterial products like endotoxins (lipopolysaccharides), vectors for gene therapy, delivery agents for anticancer drugs, and spores of anaerobic bacteria are the examples of bacteria and their components in the treatment of cancer.
9
Anaerobic bacteria of genus Clostridium are known for their ability to precisely germinate in and eradicate treatment-resistant hypoxic tumors.
10
The hypoxic zone of tumors provides a perfect ecological niche for the growth of anaerobic bacteria.
1,11
Clostridium novyi type B produces beta, alpha, and zeta toxins. The alpha toxin effects are similar to that of C. novyi type A, while beta and zeta toxins cause hemolytic necrosis. This study concentrated on alpha toxin, which can be found in C. novyi types A and B.
12
Alpha toxin is a member of the class of large Clostridia toxins. Alpha toxin is carried by bacteriophage located on p2Cn9691 plasmids.
13
Extra chromosomal DNA plays a major role in diseases developed by C. novyi type B. C. novyi type B DNA contains four or five plasmids, while type A holds only one plasmid.
13
Hence the purpose of this study was to develop C. novi nontoxic spores and to investigate its non-toxigenic and anticancer effects on breast cancer in mice models.
Materials and Methods
Bacterial strains and growth conditions
Clostridium novyi vaccine strain was obtained from Razi Vaccine and Serum Research Institute and was cultured in sterilized liver extraction medium and was incubated under the anaerobic conditions at 37ºC for 18 hours.
Growth on sporulation media
Active suspension of C. novyi was cultured in the sporulation medium for 2 weeks to ensure a maximum yield of mature spores.
14
Endospore purification
In order to increase the chance of obtaining C. novyi nontoxic, the spores were purified by a method previously described.
15
Briefly, the suspension was centrifuged at 12850 × g for 10 minutes at 4°C. The spore pellet was washed once by 1/4 volume of deionized water, then centrifuged as mentioned above, and resuspended in phosphate buffered saline (PBS) (1/4 volume) containing 500 μg/mL lysozyme. The suspended spores were sonicated for 5 minutes and incubated at 37ºCfor 2 hours. In the last step, they were centrifuged at 2050 ×g, 10-14 times for 20 minutes to remove cell debris.
Removing the lethal toxin gene from the wild-type C. novyi
To remove the lethal toxin gene from the wild-type C. novyi strain, two methods were considered. In the first method, C. novyi spores were heated at 70°C for 15 minutes to deactivate the phage carrying the toxin. Then, the spores were placed on F medium agar and incubated anaerobically at 37°C for 48-72 hours. The isolated colonies were harvested in liquid liver extract medium for another 18 hours.
In the second method, C. novyi vegetative cells were cultured in F medium containing 10 µg of acridine orange per mL at pH 7.4.
16
To confirm the absence of the lethal alpha toxin gene, PCR procedure, plasmid extraction, and immunological surveys were performed.
17
Primers and PCR procedure
Clostridium novyi vegetative cells were tested for the presence of the lethal toxin gene by PCR. PCR was performed in a reaction volume of 25 mL containing 12.5 µL of PCR buffer, 0.75 µL of MgCl2, 0.5 µL of the dNTPs mixture, 0.5 µL of Taq DNA polymerase, 0.5 µL of each primer, and 0.5 µL of each DNA template. The forward primer sequence was 5-′CGCTCCTAGC AGTCCCGAAAT-3′ while the reverse primer sequence was 5′-GGTGCGATTCAAGAGGCCACA-3′.
18
DNA amplification procedure was optimized and performed using Master cycler programmed for a preliminary step of 5 minutes at 95°C, followed by 30 cycles of 60 seconds at 94°C, 60 seconds at 52°C, and 3 minutes at 72°C, with a final extension of 15 minutes at 72°C.
Of the 72 isolates tested, two strains had lost bacteriophage. These strains were designated as colony 33 and colony 55. For further confirmation, colonies 33 and 55 were repeatedly cultured and examined, but again the inconspicuous band was detected. Hence, the second method for the vegetative cell was investigated. PCR was performed again but at this time the inconspicuous band was faded. The isolated bacteria were examined by culturing in solid media with acridine orange to remove the lethal toxin gene.
Plasmid extraction
Alpha toxin gene was located on a plasmid (p2Cn9691)
13
and encoded by bacteriophages. Colonies obtained from the first and second methods were examined for alpha-toxin production via the plasmid extraction procedure. In the next step, PCR was conducted using the above-mentioned primers to show if the extracted plasmids contained alpha toxin gene or not.
In vivo assay
In this procedure, 18-20 g laboratory mice were used to confirm whether isolated colonies had lost their phage gene, where the colonies were examined for alpha-toxin production by the in-vivoassay. Different doses of toxins were injected into 28, six-to-eight-week-old male and female laboratory mice. The mice were divided into four groups with each mouse in every group receiving 0.5 mL of the desired material (IV) as below. Each mouse in the first group was injected with the supernatant of centrifuged active suspension of C. novyi type B vaccine strain. In the second group, the mice were injected with the supernatant of centrifuged active suspension of C. novyinontoxic vaccine strain. For the third and fourth groups, the suspension was centrifuged and the spore pellet was washed and centrifuged again. Then, it was resuspended in PBS as mentioned above via the endospore purification method. C. novyi type B spore suspension was used to inject mice in the third group. The fourth group was injected with the spore suspension of C. novyi nontoxic. One more group was selected as control with each mouse in this group receiving 0.5 mL of PBS.
Macroscopic and microscopic survey
Three dead mice in different groups were dissected. Then, macroscopic observation, as well as histologic examinations of samples from the liver, small intestine, and large intestine collected was done microscopically. Moreover, hematoxylin and eosin (H&E) slide was obtained according to standard procedure guidelines.
Animals and tumor cell line
Animals and tumor cell line experiments were performed on female 6–8-week-old BALB/C mice, weighing 18-20 g. All animals were fed standard laboratory. For administration, the breast tumor model, 4T1 cell was used. The cells were cultivated in RPMI medium, supplemented with 10% FBS, antibiotics, and incubated at 37°Cin an atmosphere of 5% CO2. One million cells were injected s.c. into the right flank of each mouse. Tumor sizes were determined from caliper measurement using the standard formula (length x width2/2).
Injections
Three groups with different tumor volumes were used (smaller than 500 mm3, n = 5, between 500-1000 mm3, n = 5, and larger than 1000 mm3; n = 5). After reaching the predetermined tumor volumes, 107 spores were injected subcutaneously. As controls, mice received PBS alone.
Tumor response
Tumor response was assessed via tumor volume at 12 weeks after spore administration. Tumor measurements were performed with digital calipers.
Side effects of C. novyi nontoxic
Fourteen days after treatment, blood samples were collected from the eyes of mice, using EDTA (ethylenediamine tetra-acetic acid) 10% as an anticoagulant. Approximately, 0.5 mL of blood were collected from each animal. Count of blood cells was done.
Statistical analysis
Statistical analyses were conducted using GraphPad Prism software version 8.0. Statistical evaluation of differences between means of experimental and control groups was performed by analysis of t test. A value of <0.05 was considered to be significant.
Results
Of the 72 isolate colonies tested, two strains had lost bacteriophage. These strains were designated as colony 33 and colony 55. PCR method was conducted three times and it lost the alpha toxin phage (Fig. 1).
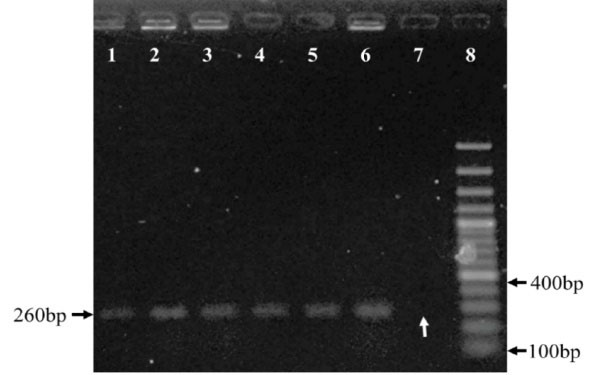
Fig. 1.
Agarose gel 1% electrophoresis of PCR products from C. novyialpha toxin to show C. novyi -NT strains. Lanes 1-6 C. novyi type B after treatment which continued to produce lethal toxin. Lane 7 colony, which lost alpha toxin, called C. novyi-nontoxic, and designated as colony 33. Lane 8, 100bp plus DNA size marker. The white arrow represents the absence of alpha toxin gene.
.
Agarose gel 1% electrophoresis of PCR products from C. novyialpha toxin to show C. novyi -NT strains. Lanes 1-6 C. novyi type B after treatment which continued to produce lethal toxin. Lane 7 colony, which lost alpha toxin, called C. novyi-nontoxic, and designated as colony 33. Lane 8, 100bp plus DNA size marker. The white arrow represents the absence of alpha toxin gene.
Safety assay: plasmid extraction and mouse assay
The obtained colonies of the two methods were examined for alpha-toxin production by plasmid extraction (Fig. 2A). In the next step, PCR was performed to evaluate alpha toxin gene in the extracted plasmids (Fig. 2B).
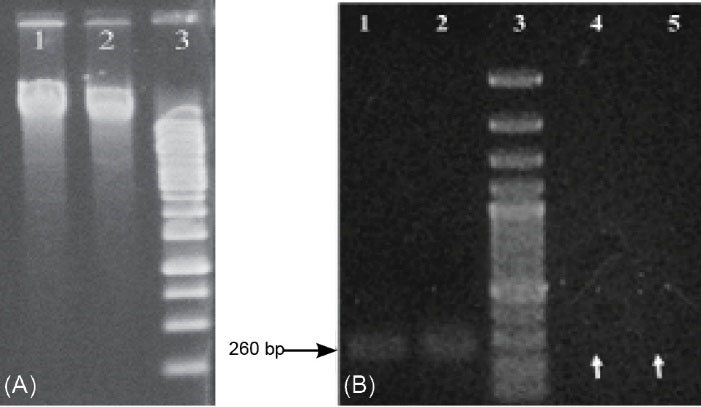
Fig. 2.
(A)
Agarose gel 1% electrophoresis of the extracted plasmid. Lane 1, colony 33 plasmids; Lane 2, C. novyi type B plasmid; Lane 3, 1 kb DNA size marker. (B) Agarose gel electrophoresis of the PCR products. Lanes 1 and 2, C. novyi type B wild strain; Lane 3, 100 bp DNA size marker, Lanes 4 and 5, two samples of C. novyi colony 33 (C. novyi nontoxic) representing lost alpha toxin gene (white arrows).
.
(A)
Agarose gel 1% electrophoresis of the extracted plasmid. Lane 1, colony 33 plasmids; Lane 2, C. novyi type B plasmid; Lane 3, 1 kb DNA size marker. (B) Agarose gel electrophoresis of the PCR products. Lanes 1 and 2, C. novyi type B wild strain; Lane 3, 100 bp DNA size marker, Lanes 4 and 5, two samples of C. novyi colony 33 (C. novyi nontoxic) representing lost alpha toxin gene (white arrows).
The colonies were examined for alpha-toxin production by the in-vivoassay. In order to ensure that the isolated colony is not toxic, alpha-toxin production was tested by the mouse assay. The active bacterial culture was centrifuged. Then, the pellet and supernatant were IV injected separately into white female laboratory mice, in each group, and the number of deaths was recorded (Tables 1 and 2 and Fig. 3).
Table 1.
The results of supernatant injections of bacterial culture of C. novyi nontoxic and C. novyi
Groups
duration of culture
|
Injected material
|
MLD
|
Volume (mL)
|
Number of mice
|
Result
|
|
18h
|
72h
|
| Experiments |
24h |
Type B supernatant* |
1500 |
0.5 |
6 |
D-D
D-D
D-D
|
D-D
D-D
D-D
|
| Nontoxic supernatant** |
0 |
0.5 |
6 |
A-A
A-A
A-A
|
A-A
A-A
A-A
|
| Phosphate buffered saline |
NE |
0.5 |
6 |
A-A
A-A
A-A
|
A-A
A-A
A-A
|
| 48 h |
Type B supernatant |
1500 |
0.5 |
6 |
D-D
D-D
D-D
|
D-D
D-D
D-D
|
| Nontoxic supernatant |
0 |
0.5 |
6 |
A-A
A-A
A-A
|
A-A
A-A
A-A
|
| Control |
48 h |
Phosphate buffered saline |
NE |
0.5 |
6 |
A-A
A-A
A-A
|
A-A
A-A
A-A
|
* Supernatant of centrifuged C. novyi type B active suspension.
** Supernatant of centrifuged C. novyi NT (colony 33) active suspension.
D, Dead; A, Alive; NE, Not examined; MLD, minimum lethal dose.
Table 2.
The results of injecting pellet of centrifuged C. novyi type B and pellet of centrifuged C. novyiNT (colony 33)
Groups
duration of culture
|
Injected material
|
Number of bacteria/mL
|
Volume (mL)
|
Number of mice
|
Result
|
|
18h
|
72h
|
| Experiments |
24h |
Type B pellet* |
4×107
5×107
|
0.5 |
6
6
|
§D-D
D-D
D-D
|
D-D
D-D
D-D
|
| Nontoxic pellet** |
4×107
Upper than 4 ×10 7
|
0.5 |
6
6
|
§§A-A
A-A
A-A
|
A-A
A-A
A-A
|
| 48h |
Type B pellet |
Upper than 5 ×10 7
|
0.5 |
6 |
D-D
D-D
D-D
|
D-D
D-D
D-D
|
| Nontoxic pellet |
Upper than 5 ×10 7
|
0.5 |
6 |
A-A
A-A
A-D
|
A-A
A-A
A-D
|
| Control |
- |
Phosphate buffered saline |
NE# |
0.5 |
6 |
A-A
A-A
A-A
|
A-A
A-A
A-A
|
* Pellet of centrifuged C. novyi type B.
** Pellet of centrifuged C. novyi nontoxic (colony 33).
D, Dead; A, Alive; NE, Not examined.
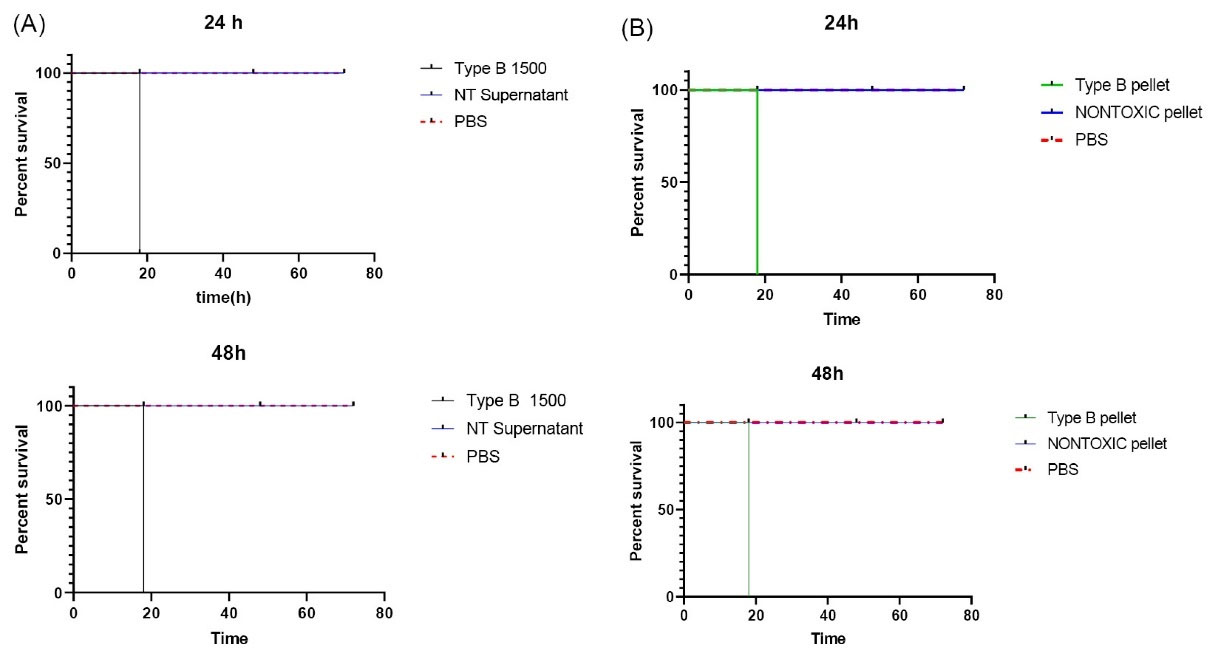
Fig. 3.
(A) Survival chart obtained from single-dose injections of supernatant of Clostridium novyi nontoxic against supernatant of C. novyi Type B after 24 h or 48 h culture. All were monitored for 72 h. (B) Survival chart obtained from single-dose injections of pellet of C. novyi - nontoxic supernatant and pellet of C. novyi Type B after 24 h or 48 h culture. All were monitored for 72 h.
.
(A) Survival chart obtained from single-dose injections of supernatant of Clostridium novyi nontoxic against supernatant of C. novyi Type B after 24 h or 48 h culture. All were monitored for 72 h. (B) Survival chart obtained from single-dose injections of pellet of C. novyi - nontoxic supernatant and pellet of C. novyi Type B after 24 h or 48 h culture. All were monitored for 72 h.
Pathology studies
Clinical observations revealed that the mice treated with C. novyi nontoxic did not show clinical symptoms as lethargy and weakness. However, the mice treated with wild C. novyi were dead as we expected.
The dissected mice treated with wild C. novyi showed hemorrhage and bleeding under the skin, while in the mice treated with C. novyinontoxic, these signs were not observed. Ostensibility changes were also seen in the size and shape of the internal organs (Fig. 4).
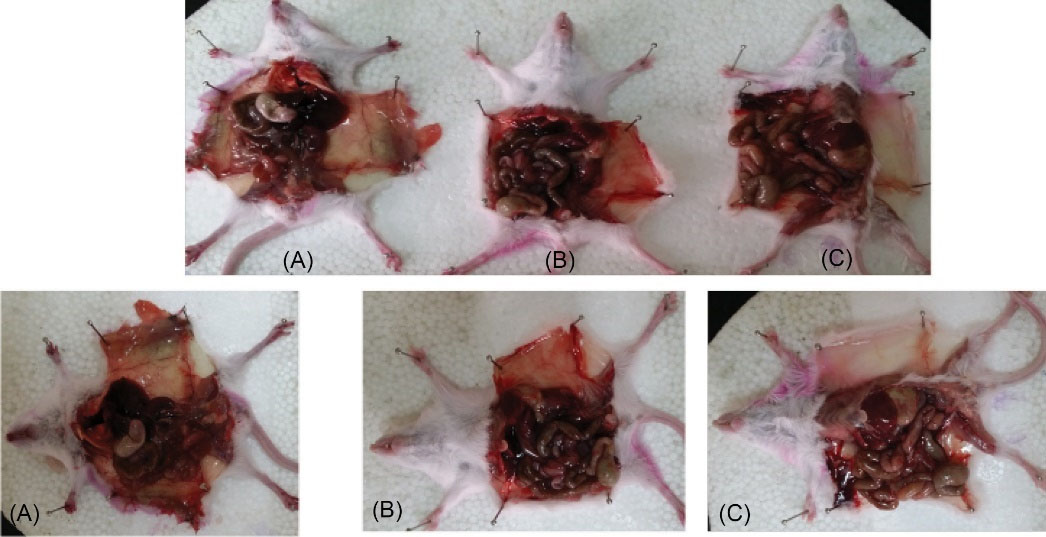
Fig. 4.
The dissected mice treated with wild C. novyi showed hemorrhage and bleeding under the skin, while in the mice treated with C. novyi nontoxic, these signs were not observed. Ostensibility changes were also seen in the size and shape of the internal organs (A): the mice injected with C. novyi type B. (B): the mice injected with C. novyi nontoxic (colony 33). (C): control.
.
The dissected mice treated with wild C. novyi showed hemorrhage and bleeding under the skin, while in the mice treated with C. novyi nontoxic, these signs were not observed. Ostensibility changes were also seen in the size and shape of the internal organs (A): the mice injected with C. novyi type B. (B): the mice injected with C. novyi nontoxic (colony 33). (C): control.
Pathology of the mice treated with Clostridium novyi type B
In the small intestinal tissue, epithelial necrosis and mucosal edema were observed. In the large intestinal tissue, the surface epithelium was removed, and submucosal edema with mucosal epithelial necrosis was observed (Fig. 5A). Interstitial hemorrhage, perinatal centers, intercostals edema, and necrosis centers were observed in the globular center in the liver tissue (Fig. 5B). Severe desensitization of the renal tubules in the cortex and center - edema around the glomeruli - bleeding in the parenchyma of the central kidney (Fig. 5C).
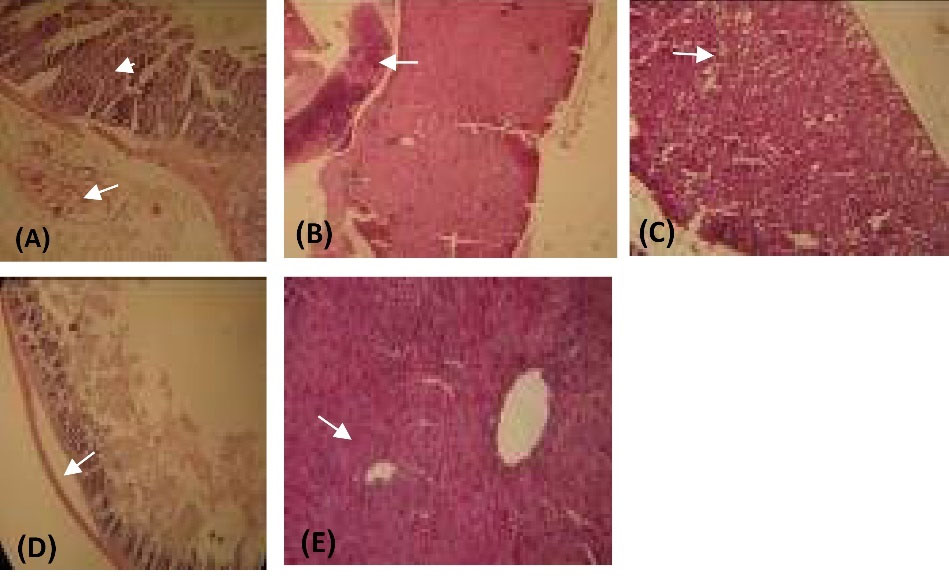
Fig. 5.
Histology results. H&E staining. Line 1: The mice treated with C. novyi type B. A: intestinal. B: liver, and C: kidney. Line 2: The mice treated with C. novyi nontoxic. D: intestinal. E: liver.
.
Histology results. H&E staining. Line 1: The mice treated with C. novyi type B. A: intestinal. B: liver, and C: kidney. Line 2: The mice treated with C. novyi nontoxic. D: intestinal. E: liver.
Pathology of the mice treated with Clostridium novyi nontoxic
The liver tissue was seen in porous and pale skin with numerous coronades of edema and hyperemia. In the intestinal tract, multiple protozoan sections were seen in the lumen duct (parasite entrainment). In the small intestinal tissue, mild edema was observed (Fig. 5D, E).
The mice with no treatment (control)
In the liver tissue, small nodules of necrosis were seen on the lobule margin. In the large intestinal surface, the epithelium was found in the lumen duct. In the small intestinal tissue, mild edema was observed (data not shown).
Tumor response
When the tumors were near 500 mm3 in size, the animals were injected intratumorally with 107C. novyinontoxicspores (time0), Control groups were given PBS, and each group consisted of five mice. All of the mice treated with a single dose of C. novyinontoxic were cured (Table 3, Fig. 6A-B), the mice with the breast tumor size between 500 mm3 to 1000 mm3 were cured completely without any relapsing after 8 weeks (Fig. 7, Table 3, Fig. 8A-B). But the treatment was not successful in breast tumors in the size larger than 1000 mm3 (Table 3, Fig. 9A-B).
Table 3.
Summary of results obtained from single-dose injections of 107C. novyi nontoxicspores in the breast tumor model
|
<500 mm3
|
Tumor response
|
Between 500 mm3 to 1000 mm3
|
Tumor response
|
>1000 mm3
|
Tumor response
|
| 334 |
Cured |
807 |
Cured |
1553 |
Not cured |
| 316 |
Cured |
661 |
Cured |
1048 |
Not cured |
| 136 |
Cured |
526 |
Cured |
2558 |
Not cured |
| 135 |
Cured |
600 |
Cured |
2000 |
Not cured |
| 250 |
Control |
500 |
Control |
1300 |
Control |
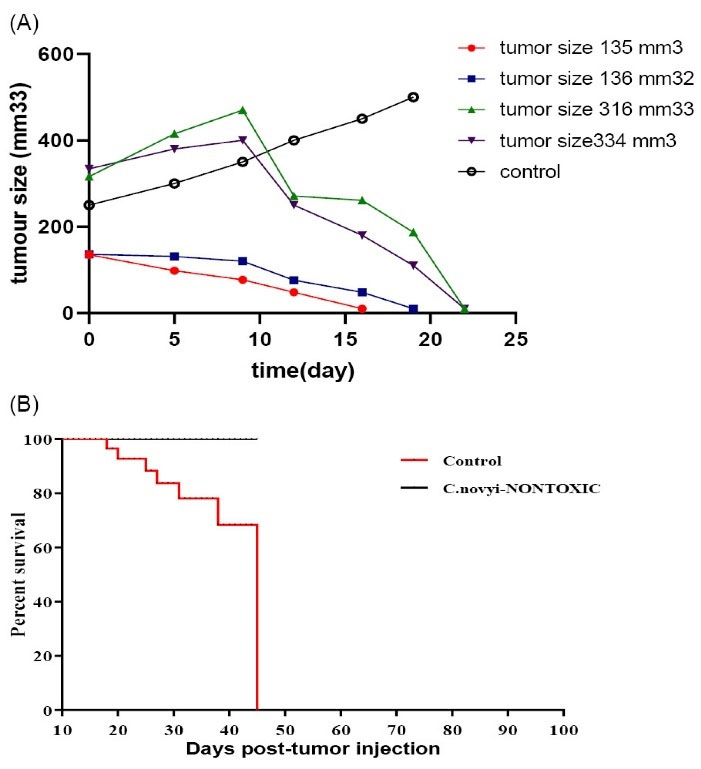
Fig. 6.
(A) The animals were injected intratumorally with 107Clostridium novyinontoxic spores. The sizes of the tumors were smaller than 500 mm3.Controls were cancerous mice that are injected with PBS. (B) Survival chart for Clostridium novyinontoxic to treat breast tumors smaller than 500 mm3 in size.
.
(A) The animals were injected intratumorally with 107Clostridium novyinontoxic spores. The sizes of the tumors were smaller than 500 mm3.Controls were cancerous mice that are injected with PBS. (B) Survival chart for Clostridium novyinontoxic to treat breast tumors smaller than 500 mm3 in size.
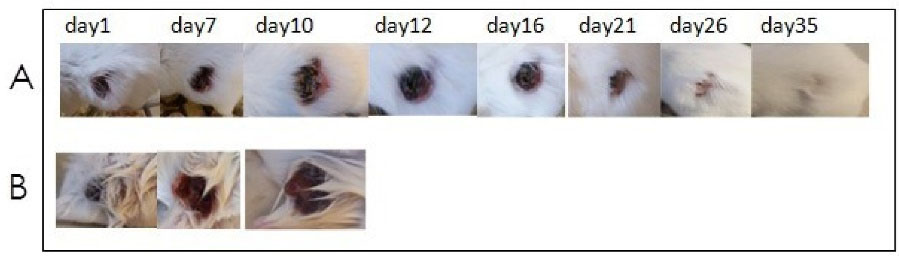
Fig. 7.
(A) Photographs of mice with 4t1 tumor smaller than 1000 mm3, receiving a single dose of C. novyinontoxicspores. Beginning on day 1 up to mice cured completely. (B) Photographs of mice with 4t1 tumor smaller than 1000 mm3, receiving a single dose of PBS as control.
.
(A) Photographs of mice with 4t1 tumor smaller than 1000 mm3, receiving a single dose of C. novyinontoxicspores. Beginning on day 1 up to mice cured completely. (B) Photographs of mice with 4t1 tumor smaller than 1000 mm3, receiving a single dose of PBS as control.
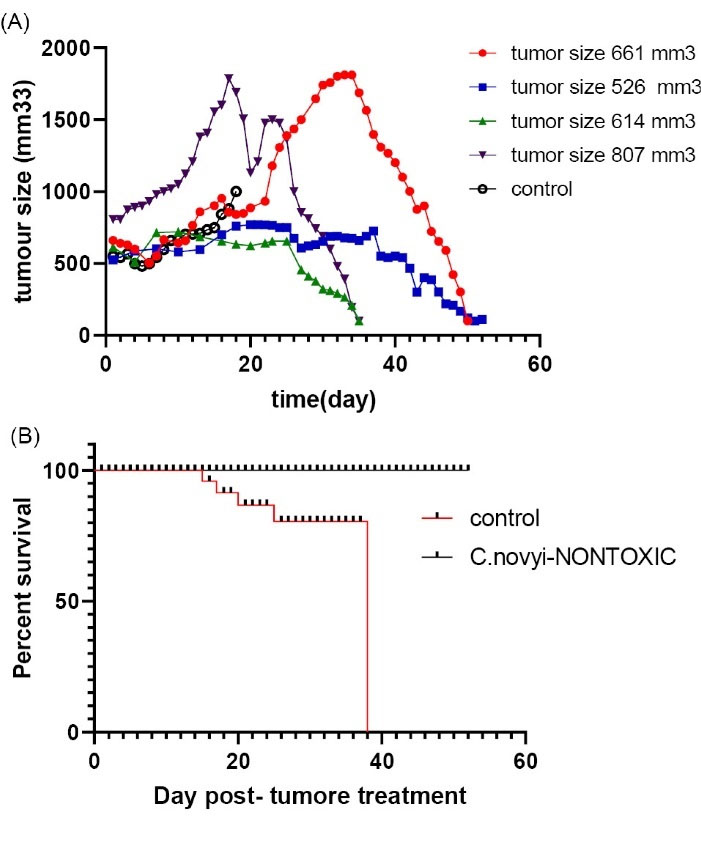
Fig. 8.
(A) The animals were injected intratumorally with 107Clostridium novyinontoxicspores. Size of the tumors were between 500 mm3up to 1000 mm3. Controls were cancerous mice that were injected with PBS. (B). Survival chart for Clostridium novyinontoxic to treat breast tumors between 500-1000 mm3 in size.
.
(A) The animals were injected intratumorally with 107Clostridium novyinontoxicspores. Size of the tumors were between 500 mm3up to 1000 mm3. Controls were cancerous mice that were injected with PBS. (B). Survival chart for Clostridium novyinontoxic to treat breast tumors between 500-1000 mm3 in size.
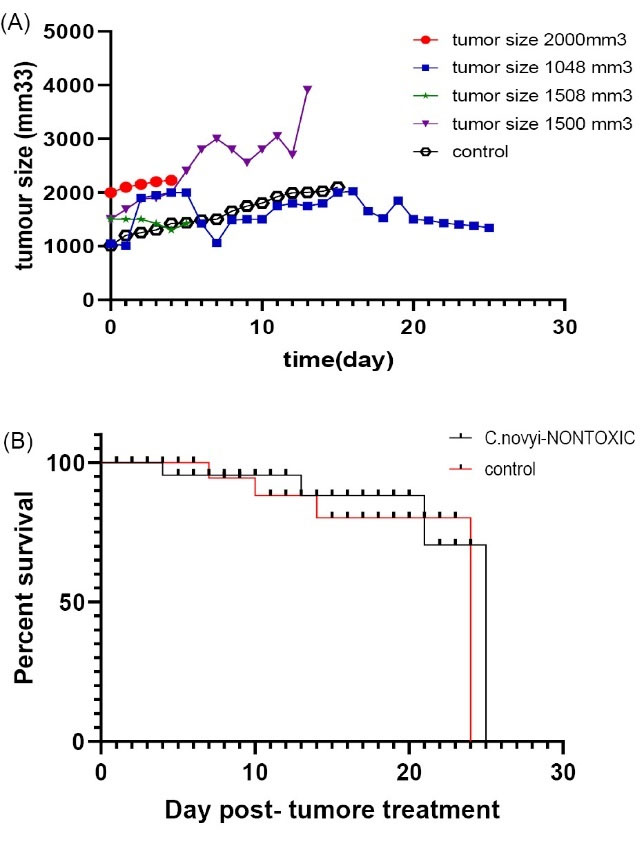
Fig. 9.
(A) Animals injected intratumorally with 107Clostridium novyinontoxic spores. The Size of the tumor was upper than 10 mm3. Controls were cancerous mice that were injected with PBS. (B). Survival chart for Clostridium novyinontoxic to treat breast tumors larger than 1000 mm3 in size.
.
(A) Animals injected intratumorally with 107Clostridium novyinontoxic spores. The Size of the tumor was upper than 10 mm3. Controls were cancerous mice that were injected with PBS. (B). Survival chart for Clostridium novyinontoxic to treat breast tumors larger than 1000 mm3 in size.
Side effects of Clostridium novyi nontoxic
Lower significant WBC counts (8.4× 103/µL) were observed in C. novyi nontoxic treated mice –within normal range- compared with cancerous mice (15.5×103/µL). The granulocyte cell count increased in cancerous mice that were then reduced by treating mice with C. novyinontoxic,
19
while it was increased compared to healthy mice.
No significant changes were observed in MCV, RBC, MCH, MCHC, lymphocytes, and RDW count, among the mice treated with C. novyinontoxic compared to healthy or cancerous mice.
A decrease in hemoglobin and hematocrit levels was observed in mice treated with C. novyi nontoxic compared to healthy and cancerous mice though in normal range. (Fig. 10, Table 4).
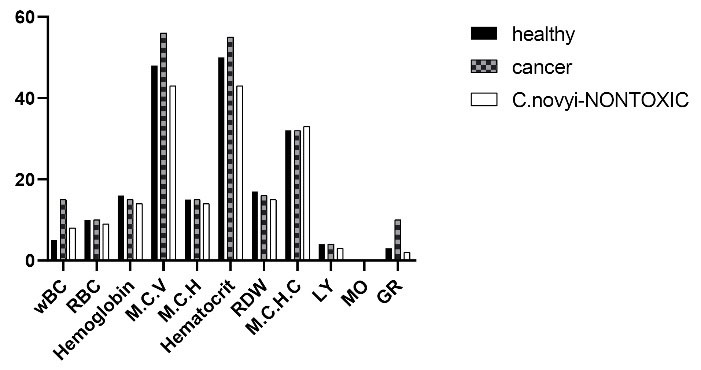
Fig. 10.
Blood parameters count in BALB/c mice, WBC: White blood cell, RBC: Red blood cell, HGB: Hemoglobin, HCT: Hematocrit, MCV: Mean corpuscular volume, MCH: Mean corpuscular hemoglobin, PLT: Platelet, RDW: Red cell distribution width, MCHC: Mean corpuscular hemoglobin concentration, LY: Lymphocytes, MO: Monocytes, GR: Granulocyte.
.
Blood parameters count in BALB/c mice, WBC: White blood cell, RBC: Red blood cell, HGB: Hemoglobin, HCT: Hematocrit, MCV: Mean corpuscular volume, MCH: Mean corpuscular hemoglobin, PLT: Platelet, RDW: Red cell distribution width, MCHC: Mean corpuscular hemoglobin concentration, LY: Lymphocytes, MO: Monocytes, GR: Granulocyte.
Table 4.
Blood parameters count in BALB/c mice
|
Blood parameters
|
Units of measure
|
C. novyi
nontoxic
|
Cancer
|
Healthy
|
Normal range
|
| WBC |
103/µL
|
8.4 |
15.5 |
5.05 |
5.69-14.84 |
| RBC |
106/µL
|
9.44 |
10.4 |
10.4 |
8.16-11.69 |
| HGB |
g/dL |
14 |
15.7 |
16.3 |
12.4-18.9 |
| HCT |
% |
43.5 |
55 |
50.2 |
43.5-67 |
| M.C.V |
fl |
43.5 |
56 |
50.8 |
50.8-64.1 |
| M.C.H |
pg |
14.8 |
15.4 |
15.45 |
13-17.6 |
| M.C.H.C |
g/dL |
32.2 |
32.7 |
33.1 |
23.9-33.1 |
| PLT |
103/µl
|
2182 |
697.5 |
708 |
476-1611 |
| RDW |
% |
15.8 |
16.4 |
17.3 |
16.9-23.5 |
| LY |
103/µL
|
4.8 |
4.40 |
3.7 |
3.6-11.5 |
| MO |
103/µL
|
0.2 |
0.7 |
0.4 |
0.34-1.37 |
| GR |
103/µL
|
3.4 |
10.4 |
2 |
- |
WBC: White blood cell, RBC: Red blood cell, HGB: Hemoglobin, HCT: Hematocrit, MCV: Mean corpuscular volume, MCH: Mean corpuscular hemoglobin, PLT: Platelet, RDW: Red cell distribution width, MCHC: Mean corpuscular hemoglobin concentration, LY: lymphocytes, MO: Monocytes, GR: Granulocyte.
The most significant side effect observed in the study of blood factors in mice receiving C. novyinontoxic was high levels of platelet (Fig. 11, Table 4).
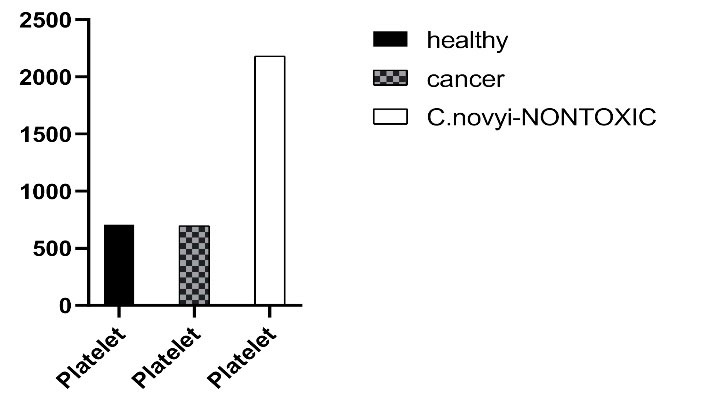
Fig. 11.
Comparing blood platelet count in BALB/c mice in three groups: healthy mice, cancerous mice, and mice injected with C. novyinontoxic.
.
Comparing blood platelet count in BALB/c mice in three groups: healthy mice, cancerous mice, and mice injected with C. novyinontoxic.
Blood platelet count is the factor that should be considered in the treatment with C. novyinontoxic. On the other hand, we had the platelet downturn in chemotherapy treatment. Combining these two treatments could be a good option to adjust the blood platelet level (Fig. 10, Table 4).
Statistical analyses
A P value of less than 0.05 was required for significance. The test was significant for first and second groups: P value = 0.0244 and <0.0001. This result allowed the acceptance of the hypothesis, C. novyi nontoxic could be the candidate oncolytic agent, depending on tumor size.
Discussion
In this study, we tried to obtain a C. novyinontoxic spore- no alpha toxin gene. In contrast, Eklund et al stated that the phage removal by heating C. novyi type B bacteria was not possible.
20
Nevertheless, the data presented in our study suggested that in most cases it was rare, but still possible. This could be due to the differences in primers or bacterial growth conditions. Using an agar-containing acridine orange medium, the probability of choosing the correct bacteria increased.
13
While C. novyi type B caused disease and death in mice, the same concentration of bacteria, without lethal toxin gene did not lead to death. This death was due to the lethal toxin gene of C. novyi. It was also confirmed that C. novyitype B. lost its phage containing the alpha toxin. It was observed that the risk of treating mice with C. novyi nontoxic is minimal. Notably, in this study, vegetative strain of C. novyi bacteria was used and injected systemically, yielding the maximum toxicity in contrast to local injection of spore.
Alpha toxin is an exotoxin.
21
Another encouraging feature associated with the C. novyi nontoxic was the inability of the supernatant of C. novyi nontoxic media. All the mice remained alive without any special clinical symptoms even at the highest dosage of injection. However, the supernatant from the liquid media of C. novyi type B caused death in all of the mice.
Another interesting point was that several times frozen and cultured in different media, notably, it did not lose its properties. To make sure, C. novyi nontoxic was checked by PCR amplification again. C. novyi nontoxic was safe and could be used for treating cancer. Therefore, use of this bacterium as an anti-cancer agent commenced.
In 2001, Vogelstein group stated that spores of C. novyinontoxic are very competent to root out established solid tumors.
22
The results of this research showed that C. novyi nontoxic could regress the experimental tumors in mice, though it depended on tumor size. Although it was expected that C. novyi nontoxic just could destroy the hypoxia zone, the aerobic part of the breast tumor was regressed completely. While Dang et al in 2001 reported that C. novyi nontoxic had only 30% cure rate in the athymic nu/numice with colon cancer,
17
our findings denoted that it is the immune system that destroyed the oxygenated zone.
Our research also showed that the tumors with the size smaller than 1000 mm3 responded to C. novyinontoxic completely. But large tumors could not be treated successfully with C. novyi nontoxic alone. In 2010, Maletzki et al demonstrated in pancreatic carcinoma that the animals carrying tumors of about 250 mm3 were optimal for treating with C. novyinontoxic.
14
Our findings suggested that for breast cancer tumor: 1. the optimal size was smaller than 1000 mm3, 2. while all tumors smaller than 1000 mm3 responded completely, yet the variation in curation times were important; that means, the larger the tumor, the longer time to cure.
Theoretically, larger tumors expected to have larger anaerobic zone
23
and expected to have a better response. But in this study, we took a 100% response from breast tumors smaller than 1000 mm3 but not larger than 1000 mm3.
Like all other anaerobic bacteria, C. novyi nontoxic could not leave hypoxia zone, although it could destroy the oxygenated zone. This result could be due to two reasons: first: C. novyi nontoxic triggers the immune system
24
, second: C. novyi nontoxic could release exotoxins not sensitive to oxygen. As chemotherapy drugs used for different tumors, bacteriotherapy should also be used for different tumors.
In this study, we took a 100% response from breast tumors smaller than 1000 mm3, while the effect of this bacterium varied from one kind of cancer to another. The result from the use of these bacteria differed for other cancers like colon cancer, melanoma, pancreatic carcinoma, and so on. The effect of this bacterium on blood factors should also be considered.
Whereas the tumors larger than 1000 mm3 had larger oxygenated zone, they required combination therapy, which would be considered in future studies.
Conclusion
In conclusion, we showed that bacteriolytic therapy with C. novyinontoxic could be a promising treatment for solid tumors. These results suggested that C. novyi nontoxic should be investigated further for its ability to affect larger tumors, as well as its potential for clinical treatment.
Research Highlights
What is the current knowledge?
√ The presence of a hypoxia zone inside the tumor is a good niche for the growth of safe anaerobic bacteria such as C. novyi-nontoxic.
What is new here?
√ This bacterium can treat the whole tumor in small tumors (hypoxia and oxygenated zone).
Acknowledgments
This study was carried out in Razi Vaccine and Serum Research Institute and Pasteur Institute of Iran. The authors wish to express their special thanks to Dr. Alireza Pardis and all of the scientific members and staff of Anaerobic Vaccine Production Department of Razi Institute and Department of Hepatitis and HIV, Pasteur Institute of Iran (IPI), for their support and assistance.
Funding sources
This study was financially supported by grant No: 96/3079 of the Biotechnology Development Council of the Islamic Republic of Iran.
Ethical statement
All procedures performed in studies involving animals were in accordance with the ethical standards of Shahid Beheshti University (Code of ethics:1398.027).
Competing interests
The authors have disclosed no potential conflict of interests.
Authors’ contribution
SMH, NS, and FAJ wrote the concept of the study and the experiments. FAJ designed and performed the experiments. AA and RP analyzed and interpreted the data. All authors drafted and revised the manuscript. The whole study was performed under the supervision of RP and AA.
References
- Janku F, Zhang HH, Pezeshki A, Goel S, Murthy R, Wang-Gillam A. Intratumoral Injection of Clostridium novyi-NT Spores in Patients with Treatment-refractory Advanced Solid Tumors. Clin Cancer Res 2021; 27:96-106. doi: 10.1158/1078-0432.Ccr-20-2065 [Crossref] [ Google Scholar]
- Wigerup C, Påhlman S, Bexell D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacology & Therapeutics 2016; 164:152-69. doi: 10.1016/j.pharmthera.2016.04.009 [Crossref] [ Google Scholar]
- Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: current advances and future directions. Int J Med Sci 2012; 9:193-9. doi: 10.7150/ijms.3635 [Crossref] [ Google Scholar]
- Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckland, NZ) 2015; 3:83-92. doi: 10.2147/HP.S93413 [Crossref] [ Google Scholar]
-
Park SY, Choi JH, Nam JS. Targeting Cancer Stem Cells in Triple-Negative Breast Cancer. Cancers (Basel) 2019; 11. 10.3390/cancers11070965.
- Sachdev JC, Sandoval AC, Jahanzeb M. Update on Precision Medicine in Breast Cancer. Cancer Treat Res 2019; 178:45-80. doi: 10.1007/978-3-030-16391-4_2 [Crossref] [ Google Scholar]
- Yin L, Duan J-J, Bian X-W, Yu S-c. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Research 2020; 22:61. doi: 10.1186/s13058-020-01296-5 [Crossref] [ Google Scholar]
- Liu S, Xu X, Zeng X, Li L, Chen Q, Li J. Tumor-targeting bacterial therapy: A potential treatment for oral cancer (Review). Oncol Lett 2014; 8:2359-66. doi: 10.3892/ol.2014.2525 [Crossref] [ Google Scholar]
- Sedighi M, Zahedi Bialvaei A, Hamblin MR, Ohadi E, Asadi A, Halajzadeh M. Therapeutic bacteria to combat cancer; current advances, challenges, and opportunities. Cancer medicine 2019; 8:3167-81. doi: 10.1002/cam4.2148 [Crossref] [ Google Scholar]
- Staedtke V, Roberts NJ, Bai RY, Zhou S. Clostridium novyi-NT in cancer therapy. Genes Dis 2016; 3:144-52. doi: 10.1016/j.gendis.2016.01.003 [Crossref] [ Google Scholar]
- Forbes NS, Coffin RS, Deng L, Evgin L, Fiering S, Giacalone M. White paper on microbial anti-cancer therapy and prevention. J Immunother Cancer 2018; 6:78. doi: 10.1186/s40425-018-0381-3 [Crossref] [ Google Scholar]
- C Nyaoke A, Navarro M, Beingesser J, A Uzal F. Infectious necrotic hepatitis caused by Clostridium novyi type B in a horse: case report and review of the literature. J Vet Diagn Invest 2017; 30:104063871773712. doi: 10.1177/1040638717737125 [Crossref] [ Google Scholar]
- Skarin H, Segerman B. Plasmidome interchange between Clostridium botulinum, Clostridium novyi and Clostridium haemolyticum converts strains of independent lineages into distinctly different pathogens. PLoS One 2014; 9:e107777. doi: 10.1371/journal.pone.0107777 [Crossref] [ Google Scholar]
- Maletzki C, Gock M, Klier U, Klar E, Linnebacher M. Bacteriolytic therapy of experimental pancreatic carcinoma. World J Gastroenterol 2010; 16: 3546-52. 10.3748/wjg.v16.i28.3546.
- Yang WW, Crow-Willard EN, Ponce A. Production and characterization of pure Clostridium spore suspensions. J Appl Microbiol 2009; 106:27-33. doi: 10.1111/j.1365-2672.2008.03931.x [Crossref] [ Google Scholar]
- Eklund MW, Poysky FT, Peterson ME, Meyers JA. Relationship of bacteriophages to alpha toxin production in Clostridium novyi types A and B. Infect Immun 1976; 14:793-803. [ Google Scholar]
- Dang LH, Bettegowda C, Huso DL, Kinzler KW, Vogelstein B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc Natl Acad Sci U S A 2001; 98:15155-60. doi: 10.1073/pnas.251543698 [Crossref] [ Google Scholar]
- Heffron A, Poxton I. A PCR approach to determine the distribution of toxin genes in closely related Clostridium species: Clostridium botulinum type C and D neurotoxins and C2 toxin, and Clostridium novyi toxin. J Med Microbiol 2007; 56:196-201. doi: 10.1099/jmm.0.46802-0 [Crossref] [ Google Scholar]
- Agrawal N, Bettegowda C, Cheong I, Geschwind J-F, Drake CG, Hipkiss EL. Bacteriolytic therapy can generate a potent immune response against experimental tumors. Proc Natl Acad Sci U S A 2004; 101:15172-7. doi: 10.1073/pnas.0406242101 [Crossref] [ Google Scholar]
- Eklund MW, Poysky FT, Peterson ME, Meyers JA. Relationship of bacteriophages to alpha toxin production in Clostridium novyi types A and B. Infect Immun 1976; 14:793-803. [ Google Scholar]
- Selzer J, Hofmann F, Rex G, Wilm M, Mann M, Just I. Clostridium novyi alpha-toxin-catalyzed incorporation of GlcNAc into Rho subfamily proteins. J Biol Chem 1996; 271:25173-7. doi: 10.1074/jbc.271.41.25173 [Crossref] [ Google Scholar]
- Dang LH, Bettegowda C, Huso DL, Kinzler KW, Vogelstein B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc Natl Acad Sci U S A 2001; 98:15155-60. doi: 10.1073/pnas.251543698 [Crossref] [ Google Scholar]
- Li X-F, O'Donoghue JA. Hypoxia in microscopic tumors. Cancer Lett 2008; 264:172-80. doi: 10.1016/j.canlet.2008.02.037 [Crossref] [ Google Scholar]
- Wang L, Wang Q, Tian X, Shi X. Learning from Clostridium novyi-NT: How to defeat cancer. J Cancer Res Ther 2018; 14:S1-S6. doi: 10.4103/0973-1482.204841 [Crossref] [ Google Scholar]