Bioimpacts. 12(4):295-299.
doi: 10.34172/bi.2021.22179
Perspective
Optogenetics: A new tool for cancer investigation and treatment
Siamak Alizadeh 1, 2  , Abolghasem Esmaeili 1, Jaleh Barar 2
, Abolghasem Esmaeili 1, Jaleh Barar 2  , Yadollah Omidi 3, *
, Yadollah Omidi 3, * 
Author information:
1Department of Cell and Molecular Biology and Microbiology, Faculty of Biological Science and Technology, University of Isfahan, Isfahan, Iran
2Research Center for Pharmaceutical Nanotechnology, Biomedicine Institute, Tabriz University of Medical Sciences, Tabriz, Iran
3Department of Pharmaceutical Sciences, College of Pharmacy, Nova Southeastern University, Fort Lauderdale, Florida 33328, USA
Abstract
Despite the progress made in the diagnosis and treatment of cancer, it has remained the second cause of death in industrial countries. Cancer is a complex multifaceted disease with unique genomic and proteomic hallmarks. Optogenetics is a biological approach, in which the light-sensitive protein modules in combination with effector proteins that trigger reversibly fundamental cell functions without producing a long-term effect. The technology was first used to address some key issues in neurology. Later on, it was also used for other diseases such as cancer. In the case of cancer, there exist several signaling pathways with key proteins that are involved in the initiation and/or progression of cancer. Such aberrantly expressed proteins and the related signaling pathways need to be carefully investigated in terms of cancer diagnosis and treatment, which can be managed with optogenetic tools. Notably, optogenetics systems offer some advantages compared to the traditional methods, including spatial-temporal control of protein or gene expression, cost-effective and fewer off-target side effects, and reversibility potential. Such noticeable features make this technology a unique drug-free approach for diagnosis and treatment of cancer. It can be used to control tumor cells, which is a favorable technique to investigate the heterogeneous and complex features of cancerous cells. Remarkably, optogenetics approaches can provide us with outstanding tool to extend our understanding of how cells perceive, respond, and behave in meeting with complex signals, particularly in terms of cancer evasion from the anticancer immune system functions.
Keywords: Cancer therapy, Optogenetic, Solid tumor, Targeted therapy
Copyright and License Information
© 2022 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
There are many treatment methods for cancer, including chemotherapy, surgery, radiotherapy, hormone therapy, and biological therapy. However most of them associate with harmful impacts on the healthy cells/tissue of the body, imposing a collective undesirable side effects in the patient under such treatment courses.
1-3
In the verge of emerging new cancer treatment techniques era, optogenetics provided exciting opportunities for scientists with the ability of (i) spatial-temporal control of cell function and behavior, (ii) fewer off-target cytotoxicity, and (iii) simple application with no/trivial undesired impacts.
4
The optogenetics concept is based on the combination of genetic and optical techniques for the rapid and reversible control of precise events in specific cells or tissues.
5
Given that light is a low-cost entity and nearly harmless, if used correctly, it can be delivered to the cells or organs with different wavelengths and in a controlled manner.
6
Photosensitive modules can reversibly activate or deactivate gene expression or effector proteins without causing long-term adverse effects in contrary to the traditional genetic perturbation approaches (knockout/knockdown or mutagenesis) that disturb the spatiotemporal features of the signaling network forever.
7
Light-controlled gene expression systems have been recognized for several cell types such as mammalian cells, yeast, and bacteria.
8
Optogenetics approaches are used more than a decade in neuroscience. However, over the last few years, optogenetics lines have been expanded dramatically.
9
Cells can be considered analog robots, with a complex array of sensors and actuators (e.g., cell signaling and biomolecular circuitry) that function among the cell’s exterior and interior.
10
Therefore, various numbers of signaling pathway effectors, which are crucial for cancer expansion and progression and play an important role in cell fate and functions, became traceable for the management by light.
11
Considering this fact, a range of variants of optically activated signaling pathway initiators (e.g., Rho A, Raf 1, and Rac) have been established and used to study the cellular events with extraordinary precision. Table 1 lists some slected signaling pathways established with photoactivatable proteins.
12
Table 1.
Some signaling pathways established with photoactivatable proteins
|
Signaling pathway
|
Light-activatable protein
|
Activation/deactivation wavelength (nm)
|
Signaling protein
|
References
|
| MAPK |
LOV |
Blue light |
Ste5 |
13
|
| Ras/ERK |
PhyB–PIF6 |
650-750 nm s/s |
SOScat |
14
|
| Raf/MEK/ERK |
CRY2–CIBN |
Blue light (~450–480 nm) |
Raf1 |
15
|
| Opto-RTKs |
LOV |
Blue light(~450–480 nm) |
FGFR |
16
|
| Apoptosis |
LOV |
Blue light(~450–480 nm) |
Caspase-7 |
17
|
| PI3K |
CRY2–CIB1 |
Blue light(~450–480 nm) |
SH2 of p85a |
18
|
On the other hand, there are various numbers of protein modules such as cell surface receptors, extracellular proteins, and intracellular kinases that have been detected to spatially assemble into higher-order complexes. All these proteins can be managed by different inducible elements even though such phenomena are still poorly understood.
10,19
Although chemical stimuli have been extensively used in cancer research, their applications have been limited by some drawbacks, including difficulties in eliminating the inducer and diffusion-based transportation.
20
Hence, replacing light with chemical inducers offers an ideal gene expression system due to its extraordinary tunability and spatiotemporal resolution and received considerable attention.
21,22
Different types of light-inducible gene expression systems have been developed recently in mammalian cells.
23
By the development of gene editing technologies, optogenetics can be used in combination with the light-sensitive systems for cancer research and therapy.
20
In view of this, an optimized CRISPR-Cas9-based light-inducible gene expression system was used in bladder cancer cell models using the tumor suppressor p53 gene. Their result indicated effective inhibition of bladder cancer cell 5637 and UMUC-3 proliferation in vitro.
20
As well, a light-controlled caspase (the main regulator of programmed cell death) has been created by combining a photoactivatable protein, light/ oxygen/voltage (LOV) domain to the apoptosis-executing domain of caspase-7. Under blue light induction, LOV domain undergoes conformational change and releases caspase-7 domain, triggering apoptosis within 1 hour.
24
Recently, a light-controllable variant of receptor tyrosine kinases (Opto-RTKs) family, which is fundamental for cancer evolution and angiogenesis, has been reported by different research groups. Considering the clinical significance of RTK inhibitors, Opto-RTKs offer unlimited potential in the oncology field.
16,25
To this end, Opto-RTK has been used successfully to study the epithelial-mesenchymal transition which is one of the principal mechanisms in the cancer cell metastasis initiation and promotion.
2
Furthermore, a light-activatable form of G protein-coupled receptor (GPCR) family that plays the most important role in cell survival and apoptosis and is often dysregulated in most cancerous cells has been developed and used successfully by scientists.
26
In addition, optogenetics tools have been recently extended to remotely control immune responses by modulating dendritic cell (DC) maturation, inflammasome activation, lymphocyte trafficking, and photoactivation of calcium channels and engineered chemokine receptors for antitumor immunity.
27
Design of light-switchable immune cells (opto-immunoengineering) allows selective activation of these cells only at the desired time and location (e.g., a tumor tissue), thereby reducing off-target special effects.
28
For instance, an optogenetic controllable T cell system has been developed based on melanopsin for hepatocellular carcinoma immunotherapy which accurately regulates the functions of T cells via a calcium-NFAT pathway in response to blue light illumination.
29
Aside from the mammalian cells, optogenetics tools can be used in cancer therapy in combination with beneficial probiotic or engineered bacterial species.
30,31
Indeed bacterial cells are intelligent biological entities, which can sense various environmental changes (e.g., glucose, pH, oxygen, light, temperature) and respond properly.
30,32
Recently light responsible plasmids such as pDawn and pMars have been developed and used successfully by scientists. These constructs can express and produce anticancer genes or agents in the appropriate host bacterial species under the blue and red light illumination respectively.
33-35
Likewise, optogenetics tools have extended cancer drug discovery and delivery approaches.
36
Different kinds of screens are applied to drug candidates before subjecting to the clinical studies. These processes include in vitro and cell-based assays, tissues and primary cells screenings, and in vivo organism scale trials.
37
Currently used biochemical and cell-based assays are not specific, cost-effective, and rapid as they require testing a large number of small molecules.
37
Moreover, the employment of invasive measurement devices (for single-cell electrical measurements) or chemical addition may interfere and alter cellular activity in many cellular assays.
14,38
Hence, the development of optogenetics tools that address appropriate targets with reduced cost and ‘contactless’ activation or inhibition of cellular activity promise to disclose new therapeutic codes.
14,39
A major challenge in the light-based activation process especially for in vivo applications has been the safety and penetration ability of the light deep into the tissue since visible lights are not able to penetrate more than several millimeters in tissue.
40
By the emerging advanced optogenetics toolkits, using implanted internal light sources such as fiber-light emitting diodes and optic light sources, this problem could be solved frequently.
41-43
Moreover, by using lanthanide-doped upconversion nanoparticles (UCNPs) which are capable to convert near-infrared (NIR) light into visible and ultraviolet emission, remote stimulation, and deep penetration into tissue are achievable.
44
These nanosacle transducers can up-convert several lower-energy photons into one high-energy photon which can activate nearly all current optogenetic constructs (e.g., CRY2, LOV2, and ChR2).
27,45
In this line, the NIR-stimulable optogenetic platform (named 'Opto-CRAC') in conjugation with NIR-to-blue emitting UCNPs, has been used effectively to improve the antitumor response with external NIR light in living animals.
46
Another strategy is the use of bioluminescence from luciferases as an alternative light source for the photoactivation of blue/green photosensitive proteins in a procedure that is named bioluminescence resonance energy transfer (BRET). In this method, luciferases are fused to microbial rhodopsin, giving rise to luminopsin which can convert high energy substrate coelenterazine into the low energy product coelenteramide. The light generated in this process is transferred simultaneously to the neighboring chromophore in a photosensitive protein (Fig. 1).
28,29
Likewise, utilizing photosensitive proteins (PhyB–PIF and BphP1–PpsR2) that are excited by longer wavelength (red and NIR) and are able to penetrate tissue simply, could be an alternative approach.
28
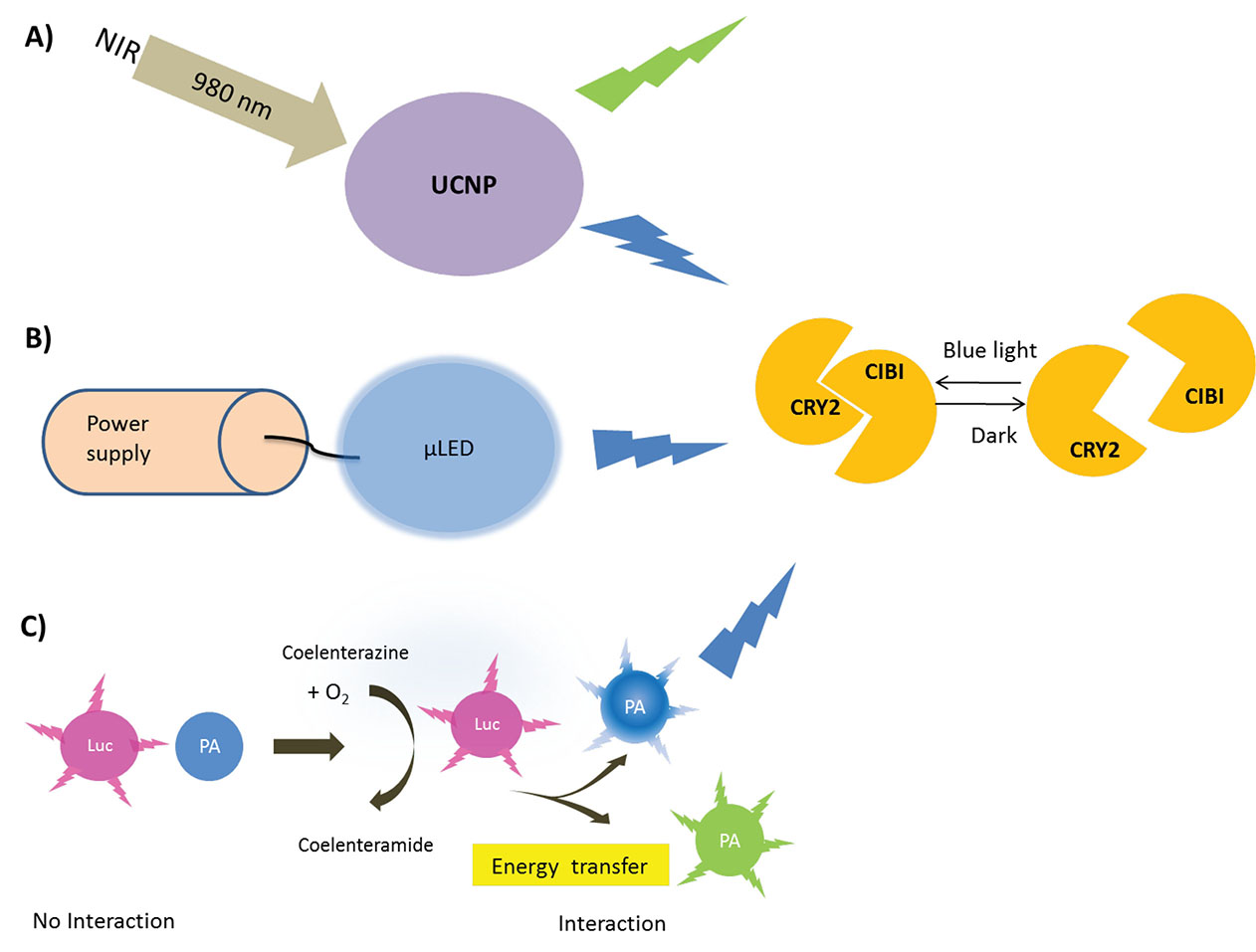
Fig. 1.
Different strategies for the in vivo application of optogenetics tools. (A) UCNPs which can convert near-infrared (NIR) light into visible and ultraviolet emission. (B) µLED implants that can provide wireless light power in deep layers of tissue by means of radio frequencies. (C) Luminopsin which can be created by fusing luciferases with the photoactivable protein can utilize exogenously provided chemical energy existed in small molecules to promote light-powered reactions. Luminopsin oxidases the high energy coelenterazine into the low energy product coelenteramide while transferring the light-energy to the photosensitive protein. UCNPs, upconverting nanoparticles; µLED, Micro LED; NIR, near-infrared; Luc, luciferases; PA, photoactivable protein; CRY2, cryptochrome 2.
.
Different strategies for the in vivo application of optogenetics tools. (A) UCNPs which can convert near-infrared (NIR) light into visible and ultraviolet emission. (B) µLED implants that can provide wireless light power in deep layers of tissue by means of radio frequencies. (C) Luminopsin which can be created by fusing luciferases with the photoactivable protein can utilize exogenously provided chemical energy existed in small molecules to promote light-powered reactions. Luminopsin oxidases the high energy coelenterazine into the low energy product coelenteramide while transferring the light-energy to the photosensitive protein. UCNPs, upconverting nanoparticles; µLED, Micro LED; NIR, near-infrared; Luc, luciferases; PA, photoactivable protein; CRY2, cryptochrome 2.
Nevertheless, some important parameters should be exploited experimentally, including (i) the reversibility and dynamics of the optogenetic tools, (ii) the endogenous availability of chromophores, and (iii) the precise degree of light with the particular wavelength and duration time.
6,47
For instance, a shorter wavelength (e.g., blue and UV lights) is appropriate for the surface of the skin, cell culture, and tissue explants whereas the red light (620–750 nm) and a part of the NIR light (750–1100 nm) are suitable for therapeutic application.
8
It should be noted that the long time exposure of the cell or tissue to the light might raise the temperature and cause tissue damage by severing alteration of cellular nucleic acids and proteins.
48
As well, optogenetic modules that need long exposure time might cause cell death by sustenance or over-activation of signaling pathways.
6
However, the development of more sensitive optogenetics modules with better kinetics could resolve this problem.
49
The optogenetic application could be extended by combining newly emerged genome-editing techniques with the photoactivatable domains for the precise editing of genome sequences.
50
Acknowledgments
The authors would like to thank the Research Center for Pharmaceutical Nanotechnology (RCPN) at Tabriz University of Medical Sciences (Tabriz, Iran) and the University of Isfahan.
Funding sources
None to be declared.
Ethical statement
There is none to be stated.
Competing interests
YO and JB act as the editors of the journal. Based on the journal editorial policy, the peer-review process of this article has been conducted in double-blind manner.
Authors’ contribution
SA, AB, JB, and YO gathered the data and drafted the manuscript. YO finalized the manuscript.
References
- Arruebo M, Vilaboa N, Sáez-Gutierrez B, Lambea J, Tres A, Valladares M. Assessment of the evolution of cancer treatment therapies. Cancers (Basel) 2011; 3:3279-330. doi: 10.3390/cancers3033279 [Crossref] [ Google Scholar]
-
Nakhjavani SA, Afsharan H, Khalilzadeh B, Ghahremani MH, Carrara S, Omidi Y. Gold and silver bio/nano-hybrids-based electrochemical immunosensor for ultrasensitive detection of carcinoembryonic antigen. Biosens Bioelectron 2019; 111439. 10.1016/j.bios.2019.111439
- Maleki-Kakelar H, Dehghani J, Barzegari A, Barar J, Shirmohamadi M, Sadeghi J. Lactobacillus plantarum induces apoptosis in gastric cancer cells via modulation of signaling pathways in Helicobacter pylori. BioImpacts: 2020; 10:65. doi: 10.34172/bi.2020.09 [Crossref] [ Google Scholar]
- Zhang K, Cui B. Optogenetic control of intracellular signaling pathways. Trends Biotechnol 2015; 33:92-100. doi: 10.1016/j.tibtech.2014.11.007 [Crossref] [ Google Scholar]
- Towne C, Thompson KR. Overview on Research and Clinical Applications of Optogenetics. Curr Protoc Pharmacol 2016; 75:11 9 1-9 21. doi: 10.1002/cpph.13 [Crossref] [ Google Scholar]
- Kielbus M, Czapinski J, Odrzywolski A, Stasiak G, Szymanska K, Kalafut J. Optogenetics in cancer drug discovery. Expert Opin Drug Discov 2018; 13:459-72. doi: 10.1080/17460441.2018.1437138 [Crossref] [ Google Scholar]
-
Tan P, He L, Han G, Zhou Y. Optogenetic immunomodulation: shedding light on antitumor immunity. Trends Biotechnol 2017; 35: 215-26. 10.1016/j.tibtech.2016.09.002
- Müller K, Weber W. Optogenetic tools for mammalian systems. Mol Biosyst 2013; 9:596-608. doi: 10.1039/c3mb25590e [Crossref] [ Google Scholar]
- Ingles-Prieto A, Reichhart E, Schelch K, Janovjak H, Grusch M. The optogenetic promise for oncology: Episode I. Mol Cell Oncol 2014; 1:e964045. doi: 10.4161/23723548.2014.964045 [Crossref] [ Google Scholar]
- Goglia AG, Toettcher JE. A bright future: optogenetics to dissect the spatiotemporal control of cell behavior. Curr Opin Chem Biol 2019; 48:106-13. doi: 10.1016/j.cbpa.2018.11.010 [Crossref] [ Google Scholar]
- Tischer D, Weiner OD. Illuminating cell signalling with optogenetic tools. Nat Rev Mol Cell Biol 2014; 15:551-8. doi: 10.1038/nrm3837 [Crossref] [ Google Scholar]
- Tischer D, Weiner OD. Illuminating cell signalling with optogenetic tools. Nat Rev Mol Cell Biol 2014; 15:551. doi: 10.1038/nrm3837 [Crossref] [ Google Scholar]
- Strickland D, Lin Y, Wagner E, Hope CM, Zayner J, Antoniou C. TULIPs: tunable, light-controlled interacting protein tags for cell biology. Nat Methods 2012; 9:379. doi: 10.1038/nmeth.1904 [Crossref] [ Google Scholar]
- Toettcher JE, Weiner OD, Lim WA. Using optogenetics to interrogate the dynamic control of signal transmission by the Ras/Erk module. Cell 2013; 155:1422-34. doi: 10.1016/j.cell.2013.11.004 [Crossref] [ Google Scholar]
- Zhang K, Duan L, Ong Q, Lin Z, Varman PM, Sung K. Light-mediated kinetic control reveals the temporal effect of the Raf/MEK/ERK pathway in PC12 cell neurite outgrowth. PloS One 2014; 9:e92917. doi: 10.1371/journal.pone.0092917 [Crossref] [ Google Scholar]
- Grusch M, Schelch K, Riedler R, Reichhart E, Differ C, Berger W. Spatio‐temporally precise activation of engineered receptor tyrosine kinases by light. EMBO J 2014; 33:1713-26. doi: 10.15252/embj.201387695 [Crossref] [ Google Scholar]
- Mills E, Chen X, Pham E, Wong S, Truong K. Engineering a photoactivated caspase-7 for rapid induction of apoptosis. ACS Synth Biol 2011; 1:75-82. doi: 10.1021/sb200008j [Crossref] [ Google Scholar]
- Idevall-Hagren O, Dickson EJ, Hille B, Toomre DK, De Camilli P. Optogenetic control of phosphoinositide metabolism. Proc Natl Acad Sci U S A 2012; 109:E2316-E23. doi: 10.1073/pnas.1211305109 [Crossref] [ Google Scholar]
- Rai AK, Chen J-X, Selbach M, Pelkmans L. Kinase-controlled phase transition of membraneless organelles in mitosis. Nature 2018; 559:211. doi: 10.1038/s41586-018-0279-8 [Crossref] [ Google Scholar]
- Lin F, Dong L, Wang W, Liu Y, Huang W, Cai Z. An efficient light-inducible P53 expression system for inhibiting proliferation of bladder cancer cell. Int J Biol Sci 2016; 12:1273. doi: 10.7150/ijbs.16162 [Crossref] [ Google Scholar]
- Möglich A, Hegemann P. Biotechnology: programming genomes with light. Nature 2013; 500:406. doi: 10.1038/500406a [Crossref] [ Google Scholar]
- Toettcher JE, Voigt CA, Weiner OD, Lim WA. The promise of optogenetics in cell biology: interrogating molecular circuits in space and time. Nat Methods 2011; 8:35. doi: 10.1038/nmeth.f.326 [Crossref] [ Google Scholar]
- Müller K, Naumann S, Weber W, Zurbriggen MD. Optogenetics for gene expression in mammalian cells. Biol Chem 2015; 396:145-52. doi: 10.1515/hsz-2014-0199 [Crossref] [ Google Scholar]
- Nagaraj S, Mills E, Wong SS, Truong K. Programming membrane fusion and subsequent apoptosis into mammalian cells. ACS Synth Biol 2012; 2:173-9. doi: 10.1021/sb3000468 [Crossref] [ Google Scholar]
- Chang K-Y, Woo D, Jung H, Lee S, Kim S, Won J. Light-inducible receptor tyrosine kinases that regulate neurotrophin signalling. Nat Commun 2014; 5:4057. doi: 10.1038/ncomms5057 [Crossref] [ Google Scholar]
- Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature 2009; 458:1025. doi: 10.1038/nature07926 [Crossref] [ Google Scholar]
- Khiavi MA, Safary A, Somi MH. Recent advances in targeted therapy of colorectal cancer: impacts of monoclonal antibodies nanoconjugates. BioImpacts 2019; 9:123. doi: 10.15171/bi.2019.16 [Crossref] [ Google Scholar]
- Mansouri M, Strittmatter T, Fussenegger M. Light‐Controlled Mammalian Cells and Their Therapeutic Applications in Synthetic Biology. Adv Sci (Weinh) 2019; 6:1800952. doi: 10.1002/advs.201800952 [Crossref] [ Google Scholar]
- Berglund K, Clissold K, Li HE, Wen L, Park SY, Gleixner J. Luminopsins integrate opto-and chemogenetics by using physical and biological light sources for opsin activation. Proc Natl Acad Sci U S A 2016; 113:E358-E67. doi: 10.1073/pnas.1510899113 [Crossref] [ Google Scholar]
- Hosseinidoust Z, Mostaghaci B, Yasa O, Park B-W, Singh AV, Sitti M. Bioengineered and biohybrid bacteria-based systems for drug delivery. Adv Drug Deliv Rev 2016; 106:27-44. doi: 10.1016/j.addr.2016.09.007 [Crossref] [ Google Scholar]
- Kakelar HM, Barzegari A, Hanifian S, Barar J, Omidi Y. Isolation and molecular identification of Lactobacillus with probiotic potential from abomasums driven rennet. Food Chem 2019; 272:709-14. doi: 10.1016/j.foodchem.2018.08.081 [Crossref] [ Google Scholar]
-
Alizadeh S, Esmaeili A, Barzegari A, Rafi MA, Omidi Y. Bioengineered smart bacterial carriers for combinational targeted therapy of solid tumours. J Drug Target 2020; 1-14. 10.1080/1061186X.2020.1737087
- Ohlendorf R, Vidavski RR, Eldar A, Moffat K, Möglich A. From dusk till dawn: one-plasmid systems for light-regulated gene expression. J Mol Biol 2012; 416:534-42. doi: 10.1016/j.jmb.2012.01.001 [Crossref] [ Google Scholar]
- Magaraci MS, Veerakumar A, Qiao P, Amurthur A, Lee JY, Miller JS. Engineering Escherichia coli for light-activated cytolysis of mammalian cells. ACS Synth Biol 2014; 3:944-8. doi: 10.1021/sb400174s [Crossref] [ Google Scholar]
- Alizadeh S, Esmaeili A, Omidi Y. Anti-cancer properties of Escherichia coli Nissle 1917 against HT-29 colon cancer cells through regulation of Bax/Bcl-xL and AKT/PTEN signaling pathways. Iran J Basic Med Sci 2020; 23:886-93. doi: 10.22038/ijbms.2020.43016.10115 [Crossref] [ Google Scholar]
- Alizadeh S, Barzegari A, Esmaeili A, Omidi Y. Designing a light-activated recombinant alpha hemolysin for colorectal cancer targeting. BioImpacts: 2020; 10:187. doi: 10.34172/bi.2020.23 [Crossref] [ Google Scholar]
- Agus V, Janovjak H. Optogenetic methods in drug screening: technologies and applications. Curr Opin Biotechnol 2017; 48:8-14. doi: 10.1016/j.copbio.2017.02.006 [Crossref] [ Google Scholar]
- Grosenick L, Marshel JH, Deisseroth K. Closed-loop and activity-guided optogenetic control. Neuron 2015; 86:106-39. doi: 10.1016/j.neuron.2015.03.034 [Crossref] [ Google Scholar]
- Szobota S, Isacoff EY. Optical control of neuronal activity. Annu Rev Biophys 2010; 39:329-48. doi: 10.1146/annurev.biophys.093008.131400 [Crossref] [ Google Scholar]
- Kim YR, Kim S, Choi JW, Choi SY, Lee SH, Kim H. Bioluminescence-activated deep-tissue photodynamic therapy of cancer. Theranostics 2015; 5:805-17. doi: 10.7150/thno.11520 [Crossref] [ Google Scholar]
- Choi M, Choi JW, Kim S, Nizamoglu S, Hahn SK, Yun SH. Light-guiding hydrogels for cell-based sensing and optogenetic synthesis in vivo. Nat Photonics 2013; 7:987-94. doi: 10.1038/nphoton.2013.278 [Crossref] [ Google Scholar]
- Kim TI, McCall JG, Jung YH, Huang X, Siuda ER, Li Y. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 2013; 340:211-6. doi: 10.1126/science.1232437 [Crossref] [ Google Scholar]
- Bagley AF, Hill S, Rogers GS, Bhatia SN. Plasmonic photothermal heating of intraperitoneal tumors through the use of an implanted near-infrared source. ACS Nano 2013; 7:8089-97. doi: 10.1021/nn4033757 [Crossref] [ Google Scholar]
- Zhu Y, Luo TM, Jobin C, Young HA. Gut microbiota and probiotics in colon tumorigenesis. Cancer Lett 2011; 309:119-27. doi: 10.1016/j.canlet.2011.06.004 [Crossref] [ Google Scholar]
- Iyer C, Kosters A, Sethi G, Kunnumakkara AB, Aggarwal BB, Versalovic J. Probiotic Lactobacillus reuteri promotes TNF‐induced apoptosis in human myeloid leukemia‐derived cells by modulation of NF‐κB and MAPK signalling. Cell Microbiol 2008; 10:1442-52. doi: 10.1111/j.1462-5822.2008.01137.x [Crossref] [ Google Scholar]
-
Akbarzadeh KM, Safary A, Barar J, Ajoolabady A, Somi M, Omidi Y. Multifunctional nanomedicines for targeting epidermal growth factor receptor in colorectal cancer. Cell Mol Life Sci 2019. 10.1007/s00018-019-03305-z
- Xiang Y, Yuan Q, Vogt N, Looger LL, Jan LY, Jan YN. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature 2010; 468:921-6. doi: 10.1038/nature09576 [Crossref] [ Google Scholar]
- Fodor L, Elman M, Ullmann Y. Aesthetic applications of intense pulsed light. Springer Science & Business Media; 2010.
- Allen BD, Singer AC, Boyden ES. Principles of designing interpretable optogenetic behavior experiments. Learn Mem 2015; 22:232-8. doi: 10.1101/lm.038026.114 [Crossref] [ Google Scholar]
- Konermann S, Brigham MD, Trevino AE, Hsu PD, Heidenreich M, Cong L. Optical control of mammalian endogenous transcription and epigenetic states. Nature 2013; 500:472. doi: 10.1038/nature12466 [Crossref] [ Google Scholar]