Bioimpacts. 11(4):281-287.
doi: 10.34172/bi.2021.10
Short Communication
Evaluation of in-situ gel-forming eye drop containing bacteriophage against Pseudomonas aeruginosa keratoconjunctivitis in vivo
Golnar Rahimzadeh 1  , Majid Saeedi 2, Ali Nokhodchi 3, *
, Majid Saeedi 2, Ali Nokhodchi 3, *  , Mahmood Moosazadeh 4, Maryam Ghasemi 5, Seyyed Sohrab Rostamkalaei 6, Parham Mortazavi 7, Mohammad Eghbali 7, Reza Pourbakhshian 7, Mohammad Sadegh Rezai 1, *
, Mahmood Moosazadeh 4, Maryam Ghasemi 5, Seyyed Sohrab Rostamkalaei 6, Parham Mortazavi 7, Mohammad Eghbali 7, Reza Pourbakhshian 7, Mohammad Sadegh Rezai 1, *  , Ebrahim Nemati Hevelaee 8
, Ebrahim Nemati Hevelaee 8
Author information:
1Pediatric Infectious Diseases Research Center, Communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran
2Department of Pharmaceutics, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran
3Pharmaceutics Research Lab, School of Life Sciences, University of Sussex, Brighton, UK
4Gastrointestinal Cancer Research Center, Non-communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran
5Immunogenetics Research Center, Mazandaran University of Medical Sciences, Sari, Iran
6Department of Pharmaceutics, Faculty of Pharmacy, Islamic Azad University, Ayatollah Amoli Branch, Amol, Iran
7Student Research Committee, School of pharmacy, Mazandaran University of Medical Sciences, Sari, Iran
8Laboratory of Microbiology, Bouali Sina Hospital, Mazandaran University of Medical Sciences, Sari, Iran
Abstract
Introduction:
Eradication of Pseudomonas aeruginosa has become increasingly difficult due to its remarkable capacity to resist antibiotics. Bacteriophages have been suggested as an alternative treatment for bacterial infections.
Methods: In-situ
gel-forming eye drop containing phage against P. aeruginosa keratoconjunctivitis was prepared. The Cystoviridae phage was formulated as in-situ gel-forming formulation which is a solution formulation but turns into gel when it contacts the eye. Therapeutic effectiveness of the in-situ gel forming formulation was evaluated by histological examination on day 12 post-infection.
Results:
The viscosity of selected formulation increased when it was instilled into the eye. The histological results showed edema, abscesses, and destruction of the stromal structure of cornea in groups where no in-situ gel-forming formulation was used. In the group where in-situ gel forming formulation was used, re-epithelialization and normal corneal structure were observed.
Conclusion: In-situ
gel-forming ophthalmic formulation containing phage can be effective in the treatment of P. aeruginosa keratoconjunctivitis.
Keywords: Bacteriophage, In-situ gel-forming system, Pseudomonas aeruginosa, Keratitis, Viscosity
Copyright and License Information
© 2021 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Pseudomonas aeruginosa is associated with many types of ocular infections such as conjunctivitis, keratitis, and eyelid disorders. Keratitis is the most serious eye infection and the main cause of corneal blindness. Keratitis can be caused by mechanical trauma, scratches, medical contact lenses, cosmetic contact lenses, and contact with contaminated water. About 30 000 cases of microbial keratitis are reported in the United States annually.
1,2
Pseudomonas aeruginosa possesses a high level of intrinsic resistance to most antibiotics due to its restricted outer membrane permeability, efflux systems that pump antibiotics out of the cell, and production of antibiotic-inactivating enzymes such as β-lactamases.
3-8
Bacteriophages offer an alternative treatment for bacterial infections. Bacteriophages are a type of virus that can infect bacteria. They can specifically destroy the host bacteria without affecting the mammalian cells and the microbiota. Furthermore, bacteriophages are self-replicating and replicate at the site of infection, therefore, it is simple and inexpensive to produce.
9-13
It has been shown that phage therapy can successfully be employed for the treatment of eye infections.
9-11
In a study, eye drops containing bacteriophage have been used three times a day on 28 conjunctivitis patients where the patients have shown signs of recovery. The US-based Phage Biotech Ltd has announced the production of topical phages as a treatment for keratitis caused by contact lenses.
10
Although drug delivery to ocular mucosa to create the local effect is an excellent idea, it is also associated with many challenges and hurdles. Generally, conventional topical ophthalmic products are not suitable for treating eye infections. This is due to poor bioavailability of drugs
14
when it is used through the eye and also due to a shorter residence time of drop solution on the eyes. Typically, the bioavailability of eye drops is less than 5% but it depends on the physicochemical properties of the drug.
15
It seems increasing the viscosity of eye drop solutions may increase the residence time of the formulation and enhance the bioavailability which has been discussed in a review article.
16
In addition, the presence of barriers in the eye can limit drug absorption via these drug delivery systems.
17
In another study, the in-situ gel-forming estradiol eye drops have been used for the development of safe, sterile, and stable formulations for cataract prevention and helped elucidate the mechanisms by which estrogen protects lens transparency.
18
In the current study, an in-situgel-forming system was suggested for the effectiveness of phage therapy in the eye infection. Inthis novel drug delivery system, the drug was mixed with various types of polymers where under certain conditions such as pH, temperature, solvent, and ions the polymers form a gel. In the current research, pH was the only factor used to convert the solution to gel. The in-situ gel-forming system reduces the frequency of dosing, increases the contact time of the drug in the eyes, and increases patient compliance.
19
Based on the above descriptions, the purpose of this study was to produce an in-situ gel-forming eye drop formulation containing phage with new features such as the long-term release of bacteriophage and increasing the durability of bacteriophage in the eye to provide an improved bioavailability for the eye product, and subsequently lower the dose of active phage ingredient. The in-situ gel-forming eye drop formulation containing phage can be used for the subsequent treatment of P. aeruginosa keratoconjunctivitis.
Materials and Methods
Materials
In the microbial tests, chocolate agar and blood agar were purchased from Merck, Germany. Kirby–Bauer technique was performed by antibiotic discs and all discs including meropenem (10 µg), cefotaxime (30 µg), gentamycin (10 µg), and ciprofloxacin discs (10 µg) were purchased from Padtan Teb, Iran. Luria-Bertani (Quelab, USA), agarose (ACROS, Belgium), ammonium acetate (ACROS, Belgium), uranyl acetate (ACROS, Belgium) were used for the isolation and characterization of bacteriophage. For the preparation of the in-situ gel-forming system, sodium phosphate, sodium chloride, methylparaben, propylparaben, sodium carboxymethyl cellulose (NaCMC), sodium bicarbonate (NaHCO3), calcium chloride-2 H2O, KCl, hematoxylin and Eosin were obtained from Merck, Germany.
Preparation of bacterial strains
Pseudomonas aeruginosa was provided by Pasteur Institute of Iran, Tehran. It was cultured on the chocolate agar and blood agar, followed by overnight incubation at 37°C. To determine antibiotic susceptibility test, Kirby–Bauer technique was performed by antibiotic discs namely meropenem (10 µg), cefotaxime (30 µg), gentamycin (10 µg), and ciprofloxacin (10 µg).
20,21
Isolation and purification of bacteriophage
Sewage sample (100 mL) was incubated for 24 hours at 37°C in a shaker incubator. The sample was centrifuged at 11 000 × g for 15 minutes followed by filtration of the supernatant through a 0.22 µm syringe filter at the sterile conditions and room temperature.
22,23
Spot test
The overnight cultured P. aeruginosa (100 µL) was inoculated in a top agar and was poured into the bottom agar. Then the supernatant of bacteriophage (10 µL) was poured over the solidified agar followed by incubation at 37°C overnight. The formation of the inhibition zone was checked.
22,23
After 24 hours, the formation of the inhibition zone showed the isolation of phage and its lytic activity against bacteria.
Double-layer plaque assay (DLA assay)
Luria-Bertani (Quelab, USA), 900 μL, was added to 8 sterile tubes. Then 100 μL of phage was added to tube no. 1 and was vortexed. After vortexing the tube, 100 μL from tube no. 1 was taken and added to the tube no. 2. This procedure was repeated until tube no. 8.
Tube no. 9 (900 μL of P. aeruginosa) and tube no. 10 (900 μL of Luria-Bertani) were selected as the positive and negative controls, respectively. Then, 200 µL from each of the diluted phage was added to 200 µL P. aeruginosa (1.5× 108 CFU/mL). The mixture was added to the top agar followed by adding the top agar to the bottom agar. The plates were incubated overnight at 37°C.
22,23
To calculate the plaque-forming unit (PFU), the following equation was used:
Plaque forming units (PFU) = the number of plaques×10×the inverse of the dilution factor
Transmission electron microscopy (TEM) study
The phage was centrifuged at 25 000×g for 60 minutes. To concentrate the bacteriophage, the phage was washed in 0.1 M neutral ammonium acetate. The phage was deposited on carbon-coated copper grids and was stained by 2% uranyl acetate (pH 4-4.5). The phage was observed on a Zeiss EM 900 TEM at 150 kV.
Preparation of in-situ gelling system
Sterile isotonic phosphate buffer (pH 7.4) was prepared using 0.160 g monobasic sodium phosphate solution, 0.758 g dibasic sodium phosphate solution, and 0.44 g sodium chloride in 100 mL distilled water. To prepare the in-situ gel-forming system of bacteriophage, 0.05263 g methylparaben and 0.01052 g propylparaben were dissolved in 95 mL of hot phosphate buffer followed by the addition of 5 mL of bacteriophage (1010PFU/mL) at 25°C under stirring conditions using a magnetic stirrer. In the end, 3 g of pectin and 0.5 g of sodium carboxymethyl cellulose (NaCMC) were added to the solution and allowed the polymers to hydrate fully overnight. The solution was mixed with a mechanical homogenizer after 24 hours. The procedure was performed under a laminar flow hood. The placebo was made using the same procedure without bacteriophage.
24
Viscosity measurement and rheological behavior study
The viscosity of the prepared formulation was measured by Brookfield viscometer (Brookfield, DV-II +, USA) using spindle S5 at pH 7.4 before and after adding simulated tear fluid (STF) to the solution (2:5 ratio). The viscosity of the formulation was determined at different speeds (5, 10, 20, 50, 100 rpm). The temperatures of STF and the solution were 37°C and 25°C respectively. The appearance and clarity of the formulation before and after gelation was also checked visually under the light.
Gelling capacity studies
The gelling capacity was determined by adding 100 µL of in-situ gel-forming system in a test tube containing 2 mL of STF. The test tube was equilibrated at 37 ± 1℃ and the formation of the gel was visually assessed. The time needed for the formation of gel and also dissolving the gel was recorded.
In vitro gelation study
The strength of the gels was evaluated by placing a drop of polymeric solution in a beaker containing 50 mL of freshly prepared STF equilibrated at 37℃. The composition of STF contained NaCl 0.680 g, sodium bicarbonate (NaHCO3) 0.220 g, calcium chloride dihydrate 0.008 g, KCl 0.14 g, and purified water q.s. 100.0 mL.
Sterilization and microbial test
The finished formulations were sterilized by autoclaving at 121℃ and 15 Pa for 20 minutes. The sterilized formulations were stored in a refrigerator until use. In addition, 2 µL of the sterilized formulation was aseptically transferred to the fluid thioglycolate medium (20 mL) and soya bean-casein digest medium (20 mL), separately. The inoculated media were incubated for not less than 14 days at 30–35℃ in the case of fluid thioglycolate medium and 20– 25℃ in the case of soya bean-casein digest medium.
Experimental model of P. aeruginosa keratitis
Thirty-five New Zealand male rabbits each weighing around 2 kg were purchased from Pasteur Institute, Karaj, Iran. The rabbits were caged under controlled conditions of light, room temperature, and humidity for a week before the investigation.
The rabbits were anaesthetized with an intramuscular injection of ketamine: xylazine (30 mg/kg). The left eye of rabbits was scratched by a 25-gauge sterile needle. Bacterial corneal infection was induced by P. aeruginosa suspension (1.5 ×108CFU/mL) 30 minutes after scratching the eye. The Rabbits were divided into five groups with 7 rabbits in each group (the total number of rabbits was 35). These groups were designated as group A (treatment; an in-situ gel-forming eye drops containing 1010 PFU/mL phage), group B (placebo), group C (positive control; povidone-iodine 5%), group D (negative control; the left eye was scratched and infected), and group E (non-treatment; the left eye was scratched and non-infected).
Histological examination
To evaluate the effect of the formulations on the treatment of the infected eye, histological examination was performed after 12 days. The rabbits were sacrificed by chloroform. The eyes were harvested and fixed in 4% formalin. The eyes were embedded in paraffin and the sections were stained with hematoxylin and eosin. The samples were investigated in terms of inflammation, angiogenesis, corneal destruction, infection, re-epithelialization, and conjunctivitis.
20,21
Statistical analysis
Fisher exact test was used to show the significance of the data obtained. To this end, SPSS 23 software was used.
Results and Discussion
Characterization of bacteriophage
In our study, P. aeruginosa was confirmed as a host cell by conventional microbial methods shown in Table 1. The Kirby-Bauer technique showed that P. aeruginosa is resistant to meropenem (10 µg), cefotaxime (30 µg), gentamycin (10 µg), but sensitive to ciprofloxacin (10 µg).
Table 1.
Specifications of Pseudomonas aeruginosa
|
Test
|
Results
|
| Gram- staining |
Gram-negative |
| Simmons’ citrate agar |
Positive |
| Catalase test |
Positive |
| Oxidase test |
Positive |
| TSI agar test |
k/k |
| MR-VP tests |
Negative |
To determine the phage host and the lytic activity of phage, the spot test was performed. The formation of the inhibition zone was observed after 24 hours (Fig. 1). The phage titerwas calculated by DLA assay (1010 PFU/mL).
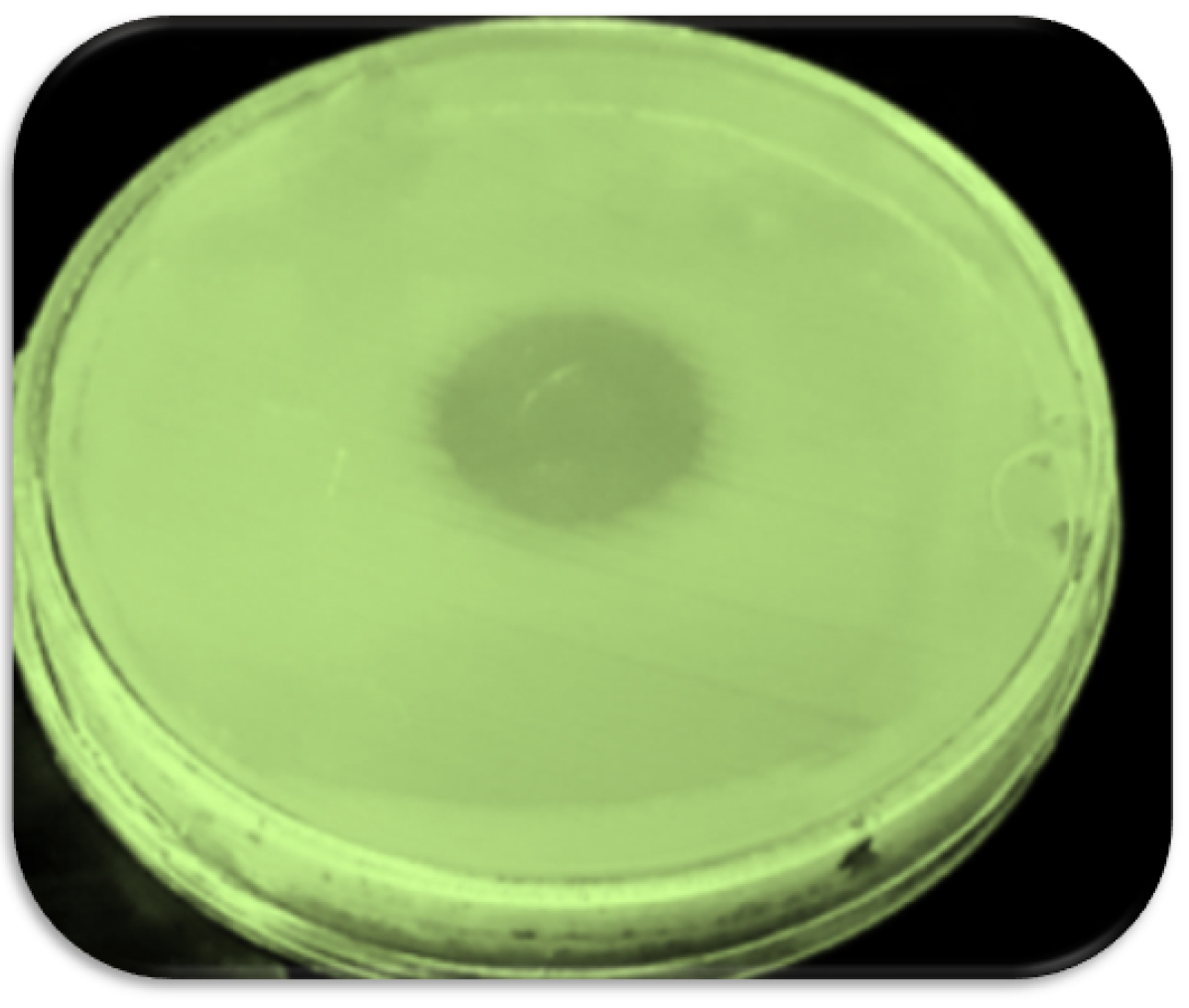
Fig. 1.
Formation inhibition zone in spot test.
.
Formation inhibition zone in spot test.
The morphology of phage was investigated by TEM and is shown in Fig. 2. As the phagewas spherical (80-85 nm) in shape with a lipid membrane around the capsomere, thusthe phage can beclassified into the Cystoviridae family (Fig. 2). The Cystoviridaephage (1010 PFU/mL) was incorporated to in-situ gel-forming eye formulation. In 2016, Furusawa et al isolated ɸR12, ɸR 12-1, ɸR 12-3, ɸR 26, ɸR 50, and ɸR 18 phages from sewage treatment plants at Sapporo and Ebetsu, Japan. The Myoviridaeand Podoviridaephages (109 PFU/mL) were formulated as an eye drop.
25
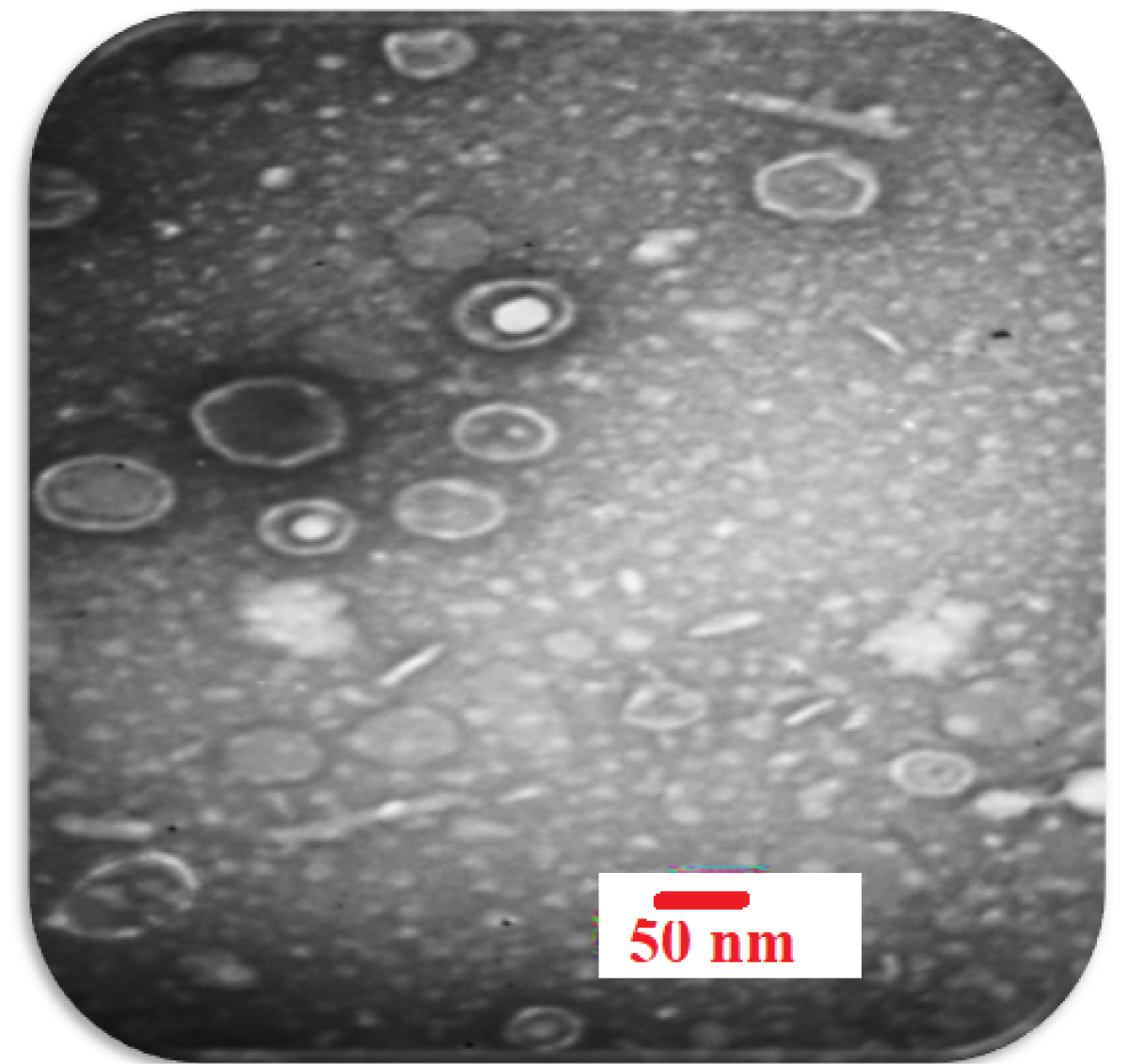
Fig. 2.
Electron micrographs of P. aeruginosa phage belonging to the Cystoviredae family. With 2% uranyl acetate (pH = 4-4.5) was stained. (voltage 150 kV, the scale bar 50 nm).
.
Electron micrographs of P. aeruginosa phage belonging to the Cystoviredae family. With 2% uranyl acetate (pH = 4-4.5) was stained. (voltage 150 kV, the scale bar 50 nm).
Characteristics of in-situ gel-forming system
In this study, an in-situ gel system was used. The selected formulation was a light yellow and clear solution with a pH of 7.4. The rheological study showed that the eye solution followed pseudoplastic behavior that is the reduction in viscosity with an increase in shear rate (Table 2). For example, when the solution was stirred at 5 rpm the viscosity was 80 cP, whereas this viscosity reduced to 24 cP when the stirring rate increased to 100 rpm (Table 2). The same pattern was obtained for the final formulation when the eye solution was mixed with the phage formulation. It was interesting to note that when the eye solution was added to the phage formulation, the viscosity of the final formulation increased (Table 2). For instance, at 5 rpm the viscosity of eye solution was 80 cP and this viscosity increased to 160 cP when the eye solution was added to the phage formulation. The same pattern was obtained for all other stirring rates (Table 2).
Table 2.
Viscosity of in-situgelling solution at different speeds
|
Spindle number
|
Speed (RPM)
|
In-situ gelling solution
|
In-situ gelling solution after adding STF
|
|
Torque (%)
|
Viscosity (cP)
|
Torque (%)
|
Viscosity (cP)
|
| 5 |
5 |
0.1 |
80 |
0.2 |
160 |
| 5 |
10 |
0.2 |
60 |
0.2 |
80 |
| 5 |
20 |
0.2 |
40 |
0.2 |
50 |
| 5 |
50 |
0.4 |
32 |
0.4 |
35 |
| 5 |
100 |
0.6 |
24 |
0.7 |
28 |
Because of the in-situ gel-forming system over the administration of the eye drop, it is expected that the effectiveness of bacteriophage and its contact time in the eyes should increase. When the eye drop is instilled into the eye, it becomes a gel within a couple of seconds and remains in gel state for around 10 minutes. The main factor for the formation of the gel is the presence of pectin in the formulation and calcium ions in the eye secretion. Pectines are from a polysaccharide family, where the polymer backbone mostly consists of α-(1,4)-D-galacturonic acid residues. Low methoxy pectines which are linked to the degree of esterification <50% can form a gel in aqueous solution in the presence of free calcium ions, that crosslink with galacturonic acid chains and enhances the viscosity.
The water solubility of pectin is one of the important advantages of the in-situ gel-forming formulation as there is no need to use organic solvents in the formulations. The in-situ gel-forming of pectin induced by calcium ions existing in lacrimal fluid has been reported in a US patent.
24
In addition, pectin-based in-situ gel-forming formulation can prolong drug release from the formulations.
The microbial growth test ruled out any microbial growth in the sterilized ophthalmic in-situ gel-forming formulations.
Histopathological examination
The results of eye histopathology showed the highest destruction of the stromal structure of the cornea, edema of the corneal stroma, and large neutrophilic abscesses in the groups B, C, D, E on the 12th day. However, when the in-situgel-forming eye drop formulation containing bacteriophage was used, the corneal structure was observed to be normal (Fig. 3).
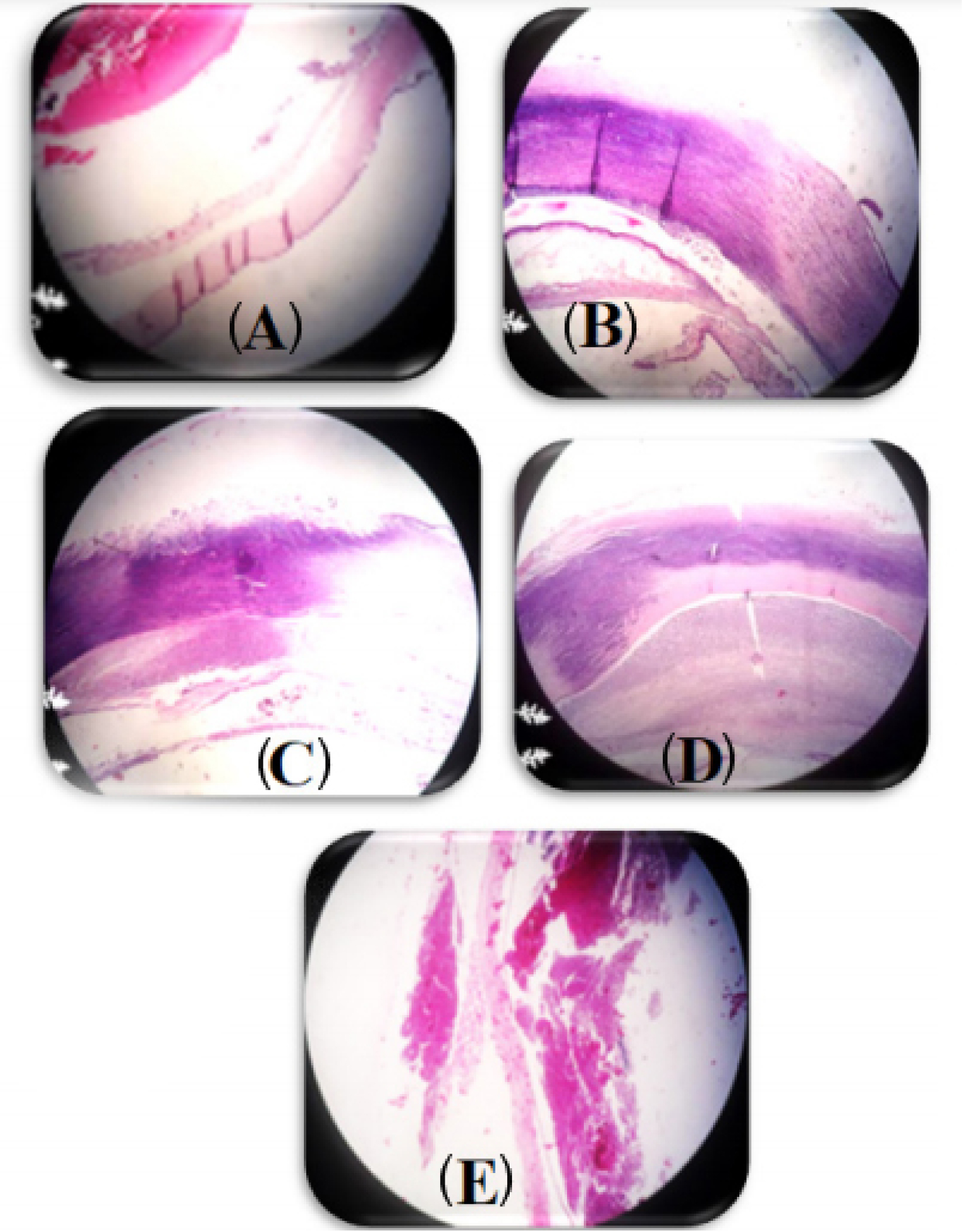
Fig. 3.
Morphological changes of the rabbit’s eye lesion 12th days after scratched induction. The hematoxylin & eosin, ×40. (A) rabbits treated with an in-situ gelling eye drops containing 1010 PFU/mL phage; (B)rabbits treated with an in-situ gelling eye drops without phage; (C) rabbits treated with Betadine 5%; (D) untreated infected rabbits ;(E) untreated and non- infected rabbits.
.
Morphological changes of the rabbit’s eye lesion 12th days after scratched induction. The hematoxylin & eosin, ×40. (A) rabbits treated with an in-situ gelling eye drops containing 1010 PFU/mL phage; (B)rabbits treated with an in-situ gelling eye drops without phage; (C) rabbits treated with Betadine 5%; (D) untreated infected rabbits ;(E) untreated and non- infected rabbits.
The inflammation, angiogenesis, and abscess were significantly at the highest levels in the control group and placebo group, whereas inflammation, angiogenesis, abscess, and conjunctivitis were significantly at the lowest levels in the in-situgel-forming eye drop containing bacteriophage (P < 0.001) (see Fig. 3 and Table 3). In contrast, the inflammation, angiogenesis, and abscess were observed in the placebo group which data indicated that the base gel (plain gel) was not effective in the treatment of keratitis.
The histopathological results showed the highest conjunctivitis in the groups B, C, D, E whereas this was not the case when the in-situgel-forming eye drop containing bacteriophage was used (Table 3). In Table 4, the results showed the highest re-epithelialization whenthe in-situgel-forming eye drop containing bacteriophage was employed(P < 0.001).
The antimicrobial properties of bacteriophages incorporated in in-situgel-forming eye drop may also influence the process of scratch and infection healing.
20,25
These data indicated antimicrobial properties of bacteriophages against bacterial host. Bacteriophages have lytic activity by the production of endolysin, which are responsible for the degradation of peptidoglycan - the main structural component of the bacterial cell wall.
12,13,22,23,26
Table 3.
Conjunctivitis of the cornea on 12 days of treatment in 5 groups (n = 7)
|
Conjunctivitis
|
|
Phage eye drop
|
Betadine
|
Placebo
|
Scratched and infected eye
|
Scratched and non-infected eye
|
P
value
|
| No |
Count |
7 |
0 |
0 |
0 |
0 |
<0.001 |
| % Within group |
100.0% |
0.0% |
0.0% |
0.0% |
0.0% |
| % Of total |
20.0% |
0.0% |
0.0% |
0.0% |
0.0% |
| + |
Count |
0 |
3 |
0 |
0 |
0 |
<0.001 |
| % Within group |
0.0% |
42.9% |
.0% |
0.0% |
0.0% |
| % Of total |
0.0% |
8.6% |
.0% |
0.0% |
0.0% |
| ++ |
Count |
0 |
3 |
2 |
0 |
0 |
<0.001 |
| % Within group |
0.0% |
42.9% |
28.6% |
0.0% |
0.0% |
| % Of total |
0.0% |
8.6% |
5.7% |
0.0% |
0.0% |
| +++ |
Count |
0 |
1 |
5 |
7 |
7 |
<0.001 |
| % Within group |
0.0% |
14.3% |
71.4% |
100.0% |
100.0% |
| % Of total |
0.0% |
2.9% |
14.3% |
20.0% |
20.0% |
Table 4.
Re-epithelization of the cornea on the 12th day of treatment in 5 groups (n = 7)
|
Reepithelization
|
|
Phage eye drop
|
Betadine
|
Placebo
|
Scratched and infected eye
|
Scratched and non-infected eye
|
P
value
|
|
No
|
Count |
0 |
4 |
7 |
7 |
7 |
|
| % Within group |
0.0% |
57.1% |
100.0% |
100.0% |
100.0% |
<0.001 |
| % Of total |
0.0% |
11.4% |
20.0% |
20.0% |
20.0% |
|
|
+
|
Count |
0 |
3 |
0 |
0 |
0 |
|
| % Within group |
0.0% |
42.9% |
0.0% |
0.0% |
0.0% |
<0.001 |
| % Of total |
0.0% |
8.6% |
0.0% |
0.0% |
0.0% |
|
|
+++
|
Count |
7 |
0 |
0 |
0 |
0 |
<0.001 |
| % Within group |
100.0% |
0.0% |
0.0% |
0.0% |
0.0% |
|
| % Of total |
20.0% |
0.0% |
0.0% |
0.0% |
0.0% |
|
It has been shown that the antibacterial activity of phages against eye infection was positive in mouse models.
26
In their study, the number of viable bacteria in the infected corneas which were treated with Myoviridae and Podoviridae phages (109 PFU/mL) was determined and the result has shown that the phage was effective up to 3 hours after infection.
25
In the study carried out by Furusawa et al, the eye of 8-week-old C57BL/6 male mice was infected by 5 µL of P. aeruginosa NE-126 (104 CFU). After initial infection, 109 PFU of phages in 5 µL was applied to the corneal surface at 30 minutes, 1, 3, 6 and 12 hours. The results showed the administration of phage mostly eradicated the pathogen within 3 hours after infection.
25
But the phages did not prevent bacterial growth at 6 and 12 hours post-infection since P. aeruginosa invaded the deep corneal stroma and phages could not infiltrate there.
In another study, the eye of eight-week-old female C57BL/6 mice was infected by 5 µL of P. aeruginosa PA33 (5×106 CFU). After the initial infection, 5×108 PFU of phages in 5 µL was applied to the corneal surface on days 1, 3 and 5. The results showed only slight or focal corneal opacities and the lowest bacterial load on day 5 post-infection. The histopathological results showed that the corneas of non-treated mice exhibited denuded epithelium and central thinning. In addition, edema of the corneal stroma, large neutrophilic abscesses, and the presence of numerous inflammatory cells in the cornea were observed, but in phage-treated mice, a normal corneal structure on day 5 post-infection was observed.
20
Compared to the published articles,
20,25
in our study, the in-situ gel-forming system is one of the promising approaches to improve the retention time of drugs on the ocular surface. The in-situ gel-forming system was developed to increase the ocular residence time, drug penetration across the ocular barriers, and ophthalmic bioavailability.
The potency of bacteriophages has been assessed for the treatment of keratitis in previous studies.
9-12,22,23
Proskurov reported a good clinical outcome in 17 patients with conjunctivitis and blepharitis who were treated with phage eye-drops in 1970.
26
Slopek reported the effect of phage therapy for recovery of patients with conjunctivitis between 1981 and 1986.
27
Kilasonia and Karanadze described the successful treatment of 32 children with acute bacterial conjunctivitis.
28
In this study, the proposed in-situ gel-forming eye drop containing phage showed an excellent outcome for the treatment of P. aeruginosa keratoconjunctivitis. The in-situ gel-forming technique can be used to produce bacteriophage eye products with new attributes such as prolonging the release of bacteriophage, increasing the durability of bacteriophage in the eye, hence lower dose of active phage ingredient. Furthermore, this technique provides a better bioavailability for the products in the treatment of eye infections.
Conclusion
In this study, in-situ gel-forming eye drop formulation containing phage for the treatment of P. aeruginosa keratoconjunctivitis was developed. The results proved that the in-situ gel-forming technique can be used to produce bacteriophage eye products with new features such as prolonging bacteriophage release and increasing the durability of bacteriophage in the eyes. These features can lead to the use of a low dose of active phage ingredient in the formulation. Moreover, this type of formulation can improve the performance of the products for the treatment of eye infections.
Acknowledgments
The authors would like to thank the deputy of Research and Technology of Mazandaran University of Medical Sciences for financial support. Also Pediatric Infectious Diseases Research Center Lab, Bouali Sina Hospital, Sari, Iran.
Funding sources
No funding.
Ethical statement
This study was approved by the Ethics Committee of Mazandaran University of Medical Sciences, Sari, Iran (IR. MAZUMS.REC.1397.1333).
Competing interests
The authors declare that they have no conflict of interests.
Authors’ contribution
GR, MSR: experiment design. MS, SSR, ME: formulation design. GR, AN, MSR, MS: supervision, writing and reviewing. MM: data analysis. M GH: histopathological analysis. GR, ME, PM, RP: experimental performance.
Research Highlights
What is the current knowledge?
simple
-
√ The phage drop can be used for treatment of P. aeruginosakeratoconjunctivitis.
What is new here?
simple
-
√ The in-situ gel-forming eye drop containing bacteriophage with new attributes such as the long-term release of bacteriophage and increased durability of bacteriophage in the eye. These features can lead to the use of a lower dose of active phage ingredient in the formulation. In addition, this technique provides a better bioavailability of the products for the treatment of eye infections.
References
- Gracner B, Gracner T, Falez M, Pahor D. Pseudomonas-keratoconjunctivitis in a contact lens wearer, the therapy and keratoplasty a chaud. Klin Monatsble Augenh 2006; 223:699-702. doi: 10.1055/s-2006-926879 [Crossref] [ Google Scholar]
- Taher EE, Mahmoud NF, Negm S, Abdallah I. Severe, sight threatening microbial keratitis: coinfection of acanthamoeba and Pseudomonas in contact lens associated keratitis. Adv Environ Biol 2016; 10:231-40. doi: 10.1097/OPX.0b013e31827f15b4 [Crossref] [ Google Scholar]
- Feltman H, Schulert G, Khan S, Jain M, Peterson L, Hauser AR. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology 2001; 147:2659-69. doi: 10.1099/00221287-147-10-2659 [Crossref] [ Google Scholar]
- Hancock RE, Speert DP. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and impact on treatment. Drug Resist Updat 2000; 3:247-55. doi: 10.1054/drup.2000.0152 [Crossref] [ Google Scholar]
- Lambert P. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa. J Roy Soc Med 2002; 95:22-6. [ Google Scholar]
- Mah T-F, Pitts B, Pellock B, Walker GC, Stewart PS, O'toole GA. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 2003; 426:306-10. doi: 10.1038/nature02122 [Crossref] [ Google Scholar]
- Pokharel K, Dawadi BR, Bhatt CP, Gupte S. Prevalence of pseudomonas aeruginosa and its antibiotic sensitivity pattern. J Nepal Health Res Counc 2019; 17:109-13. doi: 10.33314/jnhrc.1877 [Crossref] [ Google Scholar]
- Poole K, Krebes K, McNally C, Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J Bacteriol 1993; 175:7363-72. doi: 10.1128/jb.175.22.7363-7372.1993 [Crossref] [ Google Scholar]
- Górski A, Targońska M, Borysowski J, Weber-Dąbrowska B. The potential of phage therapy in bacterial infections of the eye. Ophthalmologica 2009; 223:162-5. doi: 10.1159/000193293 [Crossref] [ Google Scholar]
- Pirnay J-P, De Vos D, Verbeken G, Merabishvili M, Chanishvili N, Vaneechoutte M. The phage therapy paradigm: pret-a-porter or sur-mesure?. Pharm Res 2011; 28:934-7. doi: 10.1007/s11095-010-0313-5 [Crossref] [ Google Scholar]
- Principi N, Silvestri E, Esposito S. Advantages and limitations of bacteriophages for the treatment of bacterial infections. Front Pharmacol 2019; 10:513. doi: 10.3389/fphar.2019.00513 [Crossref] [ Google Scholar]
- Rahimzadeh G, Gill P, Rezai MS. Ultra structural characteristics of methicillin resistant Staphylococcus aureus cell wall after affecting with lytic bacteriophages using atomic force microscopy. Iran J Basic Med Sci 2019; 22:290-5. doi: 10.22038/ijbms.2019.31226.7521 [Crossref] [ Google Scholar]
- Rahimzadeh G, Saeedi M, Farshidi F, Rezai MS. Phage therapy in treatment of gram-negative bacterial infections: a systematic review. J Mazandaran Univ Med Sci 2018; 28:203-12. [ Google Scholar]
- Davies NM. Biopharmaceutical considerations in topical ocular drug delivery. Clin Exp Pharmacol Physiol 2000; 27:558-62. doi: 10.1046/j.1440-1681.2000.03288.x [Crossref] [ Google Scholar]
- Nanjawade BK, Manvi F, Manjappa A. RETRACTED: In situ-forming hydrogels for sustained ophthalmic drug delivery. J Control Release 2007; 122:119-34. doi: 10.1016/j.jconrel.2007.07.009 [Crossref] [ Google Scholar]
- Fathi M, Barar J, Aghanejad A, Omidi Y. Hydrogels for ocular drug delivery and tissue engineering. BioImpacts 2015; 5:159-64. doi: 10.15171/bi.2015.31 [Crossref] [ Google Scholar]
-
Addo E, Bamiro OA, Siwale R. Anatomy of the eye and common diseases affecting the eye. In: Addo R, eds. Ocular drug delivery: Advances, challenges and applications. Cham: Springer; 2016. p. 11-25. 10.1007/978-3-319-47691-9_2
- Kotreka UK, Davis VL, Adeyeye MC. Development of topical ophthalmic in situ gel-forming estradiol delivery system intended for the prevention of age-related cataracts. PloS one 2017; 12:e0172306. doi: 10.1371/journal.pone.0172306 [Crossref] [ Google Scholar]
- Wu Y, Liu Y, Li X, Kebebe D, Zhang B, Ren J. Research progress of in-situ gelling ophthalmic drug delivery system. Asian J Pharm Sci 2019; 14:1-15. doi: 10.1016/j.ajps.2018.04.008 [Crossref] [ Google Scholar]
- Fukuda K, Ishida W, Uchiyama J, Morita T, Harada Y, Sumi T. Therapeutic Effects of Topical Bacteriophage KPP12 Administration on Pseudomonas aeruginosa Keratitis in Mice. Invest Ophthalmol Vis Sci 2012; 53:6201. [ Google Scholar]
- Saussereau E, Debarbieux L. Bacteriophages in the experimental treatment of Pseudomonas aeruginosa infections in mice. Adv Virus Res 2012; 83:123-41. doi: 10.1016/B978-0-12-394438-2.00004-9 [Crossref] [ Google Scholar]
- Rahimzadeh G, Gill P, Rezai MS. Characterization and lytic activity of methicillin-resistant Staphylococcus aureus (MRSA) phages isolated from NICU. Australas Med J 2016; 9:169. doi: 10.21767/AMJ.2016.2642 [Crossref] [ Google Scholar]
- Rahimzadeh G, Gill P, Rezai MS. Characterization of methicillin-resistant Staphylococcus aureus (MRSA) phages from sewage at a tertiary pediatric hospital. Arch Pediatr Infect Dis 2017; 5:e39615. doi: 10.5812/pedinfect.39615 [Crossref] [ Google Scholar]
-
Dawson CR, Bowman LM, Insite Vision Inc, assignee. Topical treatment or prevention of ocular infections. United States patent US 6,569,443. 2003 May 27.
- Furusawa T, Iwano H, Hiyashimizu Y, Matsubara K, Higuchi H, Nagahata H. Phage therapy is effective in a mouse model of bacterial equine keratitis. Appl Environ Microbiol 2016; 82:5332-9. doi: 10.1128/AEM.01166-16 [Crossref] [ Google Scholar]
- Proskurov VA. Treatment of staphylococcal eye infections. Vet Ophthalmol 1970; 6:82-3. [ Google Scholar]
- Slopek S, Weber-Dabrowska B, Dabrowski M, Kucharewicz-Krukowska A. Results of bacteriophage treatment of suppurative bacterial infections in the years 1981–1986. Arch Immunol Ther Exp (Warsz) 1987; 35:569-83. [ Google Scholar]
- Abedon ST, Kuhl SJ, Blasdel BG, Kutter EM. Phage treatment of human infections. Bacteriophage 2011; 1:66-85. doi: 10.4161/bact.1.2.15845 [Crossref] [ Google Scholar]