Bioimpacts. 12(3):211-218.
doi: 10.34172/bi.2021.23401
Original Research
Penehyclidine hydrochloride ameliorates renal ischemia reperfusion-stimulated lung injury in mice by activating Nrf2 signaling
Qiang Yang 1, #  , Lei Li 2, #
, Lei Li 2, #  , Zhaohui Liu 1, *
, Zhaohui Liu 1, *  , Chunlei Li 1
, Chunlei Li 1  , Lili Yu 1
, Lili Yu 1  , Yulin Chang 1
, Yulin Chang 1 
Author information:
1Department of Anesthesiology, Cangzhou Central Hospital, Teaching Hospital of Tianjin Medical University, Cangzhou 061000, Hebei, China
2Physical Examination Center, Cangzhou Central Hospital, Teaching Hospital of Tianjin Medical University, Cangzhou 061000, Hebei, China
#These authors contributed equally to this work.
Abstract
Introduction:
Penehyclidine hydrochloride (PHC) is an anticholinergic with anti-inflammatory and anti-oxidation activities. PHC displayed protectivity against renal ischemia reperfusion (RIR) injury. Nevertheless, the precise protectivity of PHC on RIR-induced lung injury remains unknown.
Methods:
We examined the effects of PHC on RIR-induced lung injury and investigated the underlying mechanism. We induced RIR in mice and administrated PHC to RIR mice. Kidney function was monitored by measuring the blood urea nitrogen (BUN) and creatinine level in serum. We evaluated the lung injury, myeloperoxidase (MPO) activity in lung, pro-inflammatory cytokine level, and oxidative markers in serum and lung tissues. We tested the expression level of nuclear factor erythroid 2-related factor 2 (Nrf-2) and heme oxygenase 1 (HO-1) in lung of RIR mice after PHC treatment. Finally, we evaluated the effects of PHC in RIR Nrf2-/- mice.
Results:
PHC greatly downregulated the serum levels of BUN, creatinine, IL-6, NO, malondialdehyde (MDA), and matrix metalloproteinase-2. PHC also ameliorated the lung injury, decreased the MPO activity, and suppressed production of IL-6, TNF-α, IFN-γ, MDA, and O2-, while it promoted production of superoxide dismutase (SOD) and catalase (CAT) in lung. PHC improved the production of Nrf2 and HO-1.
Conclusion:
The protectivity of PHC was absent in Nrf2-/- mice. PHC ameliorated RIR-induced lung injury through Nrf2 pathway.
Keywords: Penehyclidine hydrochloride, Renal ischemia reperfusion, Lung injury, Nrf2
Introduction
Ischemia/reperfusion injury (IR), also called perfusion injury, is the tissue damage caused by restricted blood flow to an organ after ischemia or hypoxemia.
1
Renal ischemia reperfusion injury (RIR) is a kidney inflammatory disease with high morality which exceeds 80%.
2
Increasing pieces of evidence have proven that kidneys interact with distant organs and RIR has been shown to result in remote organ injury.
3-6
It is well-described that there is interaction between the kidneys and lungs. Lung failure is frequently associated with acute kidney injury. Therefore, experimental RIR model has been utilized to develop therapeutic strategy or exploit drugs to treat lung inflammation.
Inflammation is the key factor in the pathophysiology of RIR. RIR leads to the increase of systemic IL-1β, IL-6, and TNF-α cytokines, causing lung alterations. Increased microvascular permeability and neutrophil infiltration are associated with acute lung injury.
7,8
Reactive oxygen species (ROS) and oxidative stress also result in the activation of inflammation and dampen lung function. Suppression of inflammation by inhibiting NF-κB and inhibition of oxidative stress have been shown to ameliorate IR-stimulated lung injury, suggesting that preventing inflammation and oxidation may be a useful approach in ameliorating IR-induced lung injury.
9,10
Penehyclidine hydrochloride (PHC) is an anti-cholinergic, anti-muscarinic, and anti-nicotinic drug.
11
PHC is widely used in anesthetic premedication and cases of organophosphorus poisoning.
11
It has been shown that PHC inhibits inflammation through reducing MAPK and NF-κB activation.
12
Animal studies demonstrate PHC attenuates pulmonary inflammation by suppressing both inflammation and oxidation.
13
Using RIR rat model, Wang et al reported that PHC protects kidney from RIR damage by inhibiting oxidation, inflammation, and apoptosis.
14
However, whether PHC could protect against RIR-induced lung injury is not described yet. Here we appraised the effects of PHC on lung injury in RIR mice and further explored principal mechanisms.
Materials and Methods
Mice model of RIR
The mice model of RIR was established following a protocol described previously.
15
Male C57BL/6 mice with the age of 8-12 weeks and weight of 20–25 g were purchased from Shanghai Laboratory Animal Center (Shanghai, China). Nuclear factor erythroid 2-related factor 2 (Nrf2) knockout mice were generated by cross breeding of C57BL/6 Nrf2+/- mice as described previously.
16
To anesthetize, 50 mg/kg sodium pentobarbital was intraperitoneally injected into the mice. The kidneys were exposed to midline laparotomy and then renal pedicle closure was performed for 30 minutes using nontraumatic microaneurysm clamps (Shanghai Medical Instruments, Shanghai, China). The microaneurysm clamps were released and the abdomen was sealed when reperfusion was detected. Sham group mice were subjected to the same surgery without renal pedicle closure. After surgery, 0.9% sodium chloride solution was injected intraperitoneally. One hour prior to RIR stimulation, PHC (List Pharmaceutical, Chengdu, China) was intravenously injected into mouse tail veins. Five groups of mice including Sham group, RIR mice group, RIR mice treated with 0.1 mg/kg PHC, RIR mice treated with 0.5 mg/kg PHC, and RIR mice treated with 1 mg/kg PHC were used in this study, with 8 mice in each group. Samples were collected at multiple time points after RIR stimulation for analysis. For certain experiments, 24 mice were used in each group of Sham group, RIR mice group, and RIR mice treated 1 mg/kg PHC. Twenty-four hours after surgery, 6 mice from each group were selected and the lung tissues were harvested for H&E staining, and lung tissues of another 6 mice from each group were harvested for measuring lung wet/dry weight. The lung tissues of remaining 12 mice were homogenized for related test. Six out of 24 mice were randomly chosen for blood collection. For Nrf2 -/- mice, total 18 mice were used in each group. In each group, the lung tissues of 6 Nrf2 -/- mice were harvested for H&E staining and the lung tissues of the left 12 Nrf2 -/- mice were homogenized for related test. All animal studies were reviewed and approved by Cangzhou Central Hospital.
Renal function assessment
To assess renal function, concentration of blood urea nitrogen (BUN) and serum level of creatinine were detected by commercial urea assay kit (Abcam, Shanghai, China) and creatinine assay kit (Abcam) following manufacture’s protocols.
NO, MDA, MPO, SOD, CAT, O2-, and MMP-2 measurement
Twenty-four hours after RIR, mice serum and lung tissues were collected. Concentrations of plasma or lung nitric oxide (NO), malondialdehyde (MDA), matrix metalloproteinase-2 (MMP-2), myeloperoxidase (MPO), superoxide dismutase (SOD), and catalase (CAT) were measured using Nitric Oxide Assay Kit (Abcam), MDA Assay Kit (Abcam), mouse MMP-2 ELISA Kit (R&D Systems, Minneapolis, MN), MPO Activity Assay Kit (Abcam, China), Superoxide Dismutase Activity Assay Kit (R&D Systems), Catalase Activity Assay Kit (Abcam), and O2- Assay Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) respectively following manufacture’s protocols.
Cytokine measurement
IL-6, TNF-α, and IFN-γ levels in serum or lung tissue homogenates were measured using ELISA kit (Abcam) following the manufacture’s protocols.
Real time polymerase chain reaction
Lung RNAs were extracted by NucleoSpin® RNA Plus kit (Takara, Dalian, China). The PrimeScriptTM II 1st Strand cDNA Synthesis Kit (Takara) was utilized to obtain cDNA. The real time PCR was performed using TB Green® Advantage® qPCR Premix (Takara). The primers used in the present study were:
IL-6 Forward: 5’-TCCAGTTGCCTTC TTGGGAC -3’,
Reverse: 5’- GTGTAATTAAGCCTCCGACTTG-3’
17
TNF-α Forward: 5’-CAT CTT CTC AAA ATT CGA GTG ACA A-3’,
Reverse: 5’-TGGGAGTAGACAAGGTACAAC CC-3’
17
IFN-γ Forward: 5’-GCCACGGCACAGTCATTGA-3’,
Reverse: 5’-TGCTGATGGCCTGATTGTCTT-3’
Nrf2 Forward: 5’-CTTTAGTCAGCGACAGAAGGAC -3’,
Reverse: 5’-AGGCATCTTGTTTGGGAATGTG-3’
18
HO-1 Forward: 5’-ATGACACCAAGGACCAGAGC-3’,
Reverse: 5’-GTGTAAGGACCCATCGGAGA-3’
19
GAPDH Forward: 5’-TTCACCACCATGGAGAAGGC-3’,
Reverse: 5’-GGCATGGACTGTGGTCATGA-3’.
19
Western blot
Lung tissues were lysed in RIPA buffer (Invitrogen) for protein extraction. Western blot procedures were performed using standard protocols. Primary antibodies including anti-Nrf2 (Abcam), anti-lamin B1 (Abcam), anti-heme oxygenase 1 (HO-1), and anti-GAPDH (Abcam) were used. PierceTM ECL Western Blotting Substrate (Thermo Fisher, Waltham, MA) was used for detection of immune-reactive bands. Band intensity was quantitated and analyzed using ImageJ.
Pulmonary wet/dry (W/D) ratio
The pulmonary edema was assessed by W/D weight ratio. After weighing, the new lung was dried at 80°C for 24 hours.
Histology
Lung samples were fixed in 10% formalin solution (Sigma) and embedded in paraffin. The sections were stained with hematoxylin-eosin (H&E) for lung injury evaluation. Two independent investigators scored the slide and the injury score was calculated using following formula: [(alveolar bleeding points/no. of fields) + 2 × (alveolar infiltration points/no. of fields) + 3 × (hemaleucin points/no. of fields) + (hyperemia of alveolar septum/no. of fields)]/the total count of alveolar as described previously.
20
Statistical Analysis
Data were expressed as mean ± SD. One-way or two-way analysis of variance (ANOVA) together with a post hoc test was used to determine statistical difference. When P value was less than 0.05, the difference was considered as statistically significant.
Results
PHC ameliorated RIR-induced injury in mice
We established the RIR mice model and administrated different amounts of PHC in RIR mice. Serum levels of BUN and creatinine were measured at different time points. As presented in Fig. 1A, RIR mice had increased serum level of BUN with time increase. In contrast, serum BUN level did not change in normal mice (Sham group). Twenty-four hours after RIR, RIR mice had significantly higher serum BUN than normal mice. Administration of PHC decreased the serum BUN of RIR mice and the decreasing was positively correlated to the PHC dose. RIR mice treated with 1 mg/kg PHC had the lowest BUN level in serum at each time point. Twenty-four hours after RIR, 0.1, 0.5, and 1 mg/kg PHC treatment significantly reduced serum BUN level. Similarly, RIR mice had increased serum level of creatinine, which became significant at 24 hours when compared to normal mice (Fig. 1B). Administration of PHC decreased serum level of creatinine and the decreasing was positively correlated to the PHC dose. Together, these results demonstrated that PHC decreased serum level of both BUN and creatinine, indicating PHC ameliorated injury in RIR mice. As 1 mg/kg PHC provided the best protection, we adopt this dose in other experiments.

Fig. 1.
Penehyclidine hydrochloride ameliorated renal ischemia reperfusion-induced injury in mice. The levels of blood urea nitrogen (A) and serum creatinine (B) were measured 0, 4, 12, and 24 hours after surgery. n = 8 for each group. Data are presented as mean ± SD. ***P < 0.001 compared to sham group, #P < 0.05, ##P < 0.01, and ###P < 0.001 compared to RIR group.
.
Penehyclidine hydrochloride ameliorated renal ischemia reperfusion-induced injury in mice. The levels of blood urea nitrogen (A) and serum creatinine (B) were measured 0, 4, 12, and 24 hours after surgery. n = 8 for each group. Data are presented as mean ± SD. ***P < 0.001 compared to sham group, #P < 0.05, ##P < 0.01, and ###P < 0.001 compared to RIR group.
PHC inhibited systemic inflammation in RIR-mice
Next, we evaluated the effects of PHC on inflammation in RIR mice by measuring serum NO, MDA, IL-6, and MMP-2. RIR mice had significantly increased plasma NO (Fig. 2A), MDA (Fig. 2B), IL-6 (Fig. 2C), and MMP-2 (Fig. 2D). Administration of 1 mg/kg PHC significantly decreased plasma NO (Fig. 2A), MDA (Fig. 2B), IL-6 (Fig. 2C), and MMP-2 (Fig. 2D). Collectively, our data demonstrated that PHC inhibited the systemic inflammation in RIR mice.
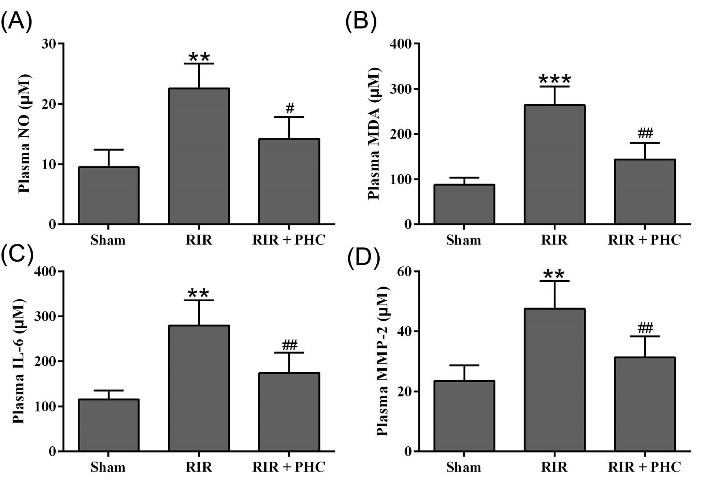
Fig. 2.
Effects of penehyclidine hydrochloride on serum inflammatory molecule levels after renal ischemia reperfusion injury (RIR) in mice. Serum levels of NO (A), MDA (B), IL-6 (C), MMP-2 (D) were measured 24 hours after surgery. n = 6. Data are presented as mean ± SD. **P < 0.01, ***P < 0.001 compared to sham group, #P < 0.05, ##P < 0.01 compared to RIR group.
.
Effects of penehyclidine hydrochloride on serum inflammatory molecule levels after renal ischemia reperfusion injury (RIR) in mice. Serum levels of NO (A), MDA (B), IL-6 (C), MMP-2 (D) were measured 24 hours after surgery. n = 6. Data are presented as mean ± SD. **P < 0.01, ***P < 0.001 compared to sham group, #P < 0.05, ##P < 0.01 compared to RIR group.
PHC ameliorated pulmonary injury in RIR mice
When compared to normal mice, RIR mice had obvious pulmonary inflammation (Fig. 3A) and significantly increased injury score (Fig. 3B). In contrast, PHC-treated RIR mice had decreased pulmonary inflammation and injury score. Correspondingly, RIR mice had significantly increased lung wet/dry weight ratio (Fig. 3C) and pulmonary MPO activity (Fig. 3D). PHC treatment greatly reduced the lung wet/dry weight ratio and suppressed MPO activity in RIR mice. Taken together, our data demonstrated that PHC ameliorated lung inflammation in RIR mice.
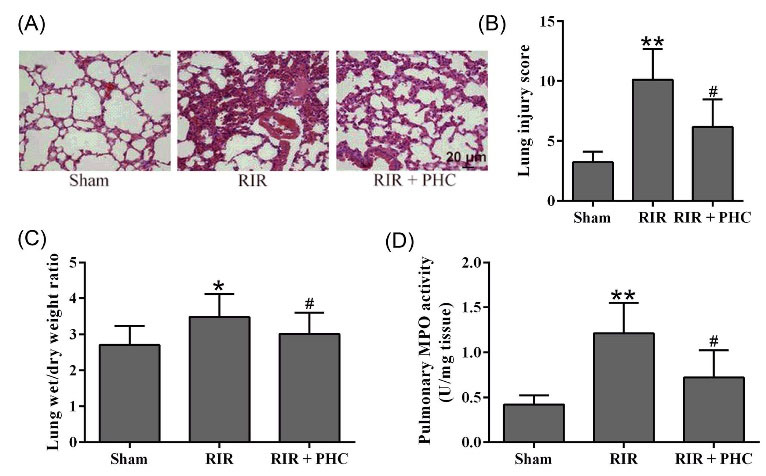
Fig. 3.
Penehyclidine hydrochloride ameliorated pulmonary injury in renal ischemia reperfusion (RIR) mice. A, Representative photographs of lung samples stained with H&E at 24 hours after surgery and semi-quantitative score was used to evaluate the lung injury (B). The lung levels of wet/dry weight ratio (C) and MPO activity (D) were also measured. n = 6. Data are presented as mean ± SD. *P < 0.05, **P < 0.01 compared to sham group, #P < 0.05 compared to RIR group.
.
Penehyclidine hydrochloride ameliorated pulmonary injury in renal ischemia reperfusion (RIR) mice. A, Representative photographs of lung samples stained with H&E at 24 hours after surgery and semi-quantitative score was used to evaluate the lung injury (B). The lung levels of wet/dry weight ratio (C) and MPO activity (D) were also measured. n = 6. Data are presented as mean ± SD. *P < 0.05, **P < 0.01 compared to sham group, #P < 0.05 compared to RIR group.
PHC attenuated pulmonary oxidative stress in RIR mice
Next the effects of PHC on oxidation in lung of RIR mice were explored. RIR mice had significantly decreased pulmonary SOD (Fig. 4A) and CAT (Fig. 4C), while they had significantly increased pulmonary MDA (Fig. 4B) and O2- (Fig. 4D) when compared to normal mice, indicating strong oxidative stress in lung of RIR mice. In contrast, administration of PHC in RIR mice resulted in significantly increased pulmonary SOD (Fig. 4A) and CAT (Fig. 4C) and decreased pulmonary MDA (Fig. 4B) and O2- (Fig. 4D), suggesting that PHC attenuated the oxidative stress in lung of RIR mice.

Fig. 4.
Penehyclidine hydrochloride ameliorated pulmonary oxidative stress in renal ischemia reperfusion (RIR) mice. The concentrations of SOD (A), MDA (B), CAT (C) and O2- (D) in lung tissues were measured by ELISA. n = 6. Data are presented as mean ± SD. **P < 0.01, ***P < 0.001 compared to sham group, ##P < 0.01 compared to RIR group.
.
Penehyclidine hydrochloride ameliorated pulmonary oxidative stress in renal ischemia reperfusion (RIR) mice. The concentrations of SOD (A), MDA (B), CAT (C) and O2- (D) in lung tissues were measured by ELISA. n = 6. Data are presented as mean ± SD. **P < 0.01, ***P < 0.001 compared to sham group, ##P < 0.01 compared to RIR group.
PHC ameliorated pulmonary inflammation in RIR mice
We further investigated the effects of PHC on inflammation in lung of RIR mice by measuring the IL-6, TNF-α, and IFN-γ levels. When compared to normal mice, RIR mice had significantly increased protein levels of IL-6 (Fig. 5A), TNF-α (Fig. 5B), and IFN-γ (Fig. 5C) in lung. We also identified remarkably increased mRNA levels of IL-6 (Fig. 5D), TNF-α (Fig. 5E), and IFN-γ (Fig. 5F) in lung of RIR mice. Administration of PHC to RIR mice resulted in significantly decreased protein and mRNA levels of IL-6 (Fig. 5A&D), TNF-α (Fig. 5B&E), and IFN-γ (Fig. 5C&F). Collectively, our data showed that PHC ameliorated pulmonary inflammation in RIR mice.
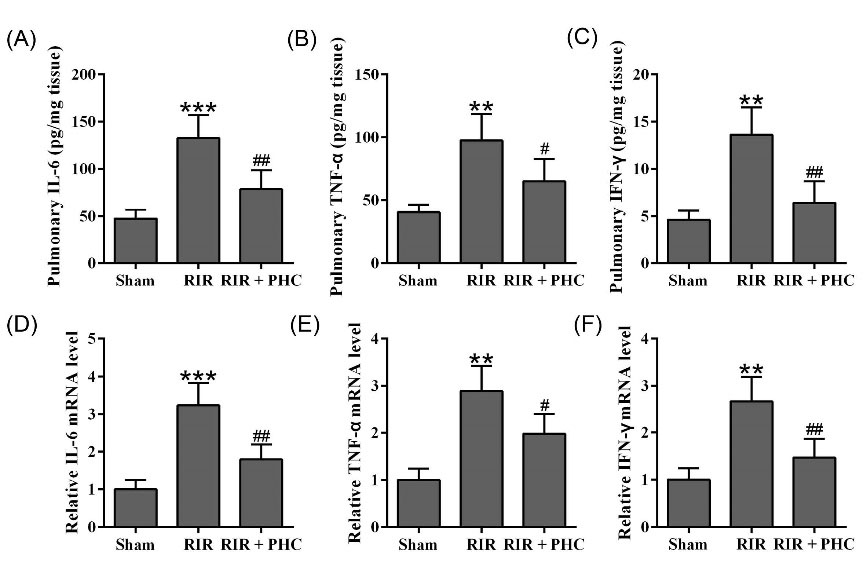
Fig. 5.
Penehyclidine hydrochloride ameliorated pulmonary inflammation in renal ischemia reperfusion (RIR) mice. The concentrations of IL-6 (A), TNF-α (B), IFN-γ (C) in lung tissue were measured by ELISA. qRT-PCR was used to analyze the mRNA levels of IL-6 (D), TNF-α (E), and IFN-γ (F) in lung tissue. GAPDH was set as a loading control and the relative expressions were normalized to sham group. n = 6. Data are presented as mean ± SD. **P < 0.01, ***P < 0.001 compared to sham group, #P < 0.05, ##P < 0.01 compared to RIR group.
.
Penehyclidine hydrochloride ameliorated pulmonary inflammation in renal ischemia reperfusion (RIR) mice. The concentrations of IL-6 (A), TNF-α (B), IFN-γ (C) in lung tissue were measured by ELISA. qRT-PCR was used to analyze the mRNA levels of IL-6 (D), TNF-α (E), and IFN-γ (F) in lung tissue. GAPDH was set as a loading control and the relative expressions were normalized to sham group. n = 6. Data are presented as mean ± SD. **P < 0.01, ***P < 0.001 compared to sham group, #P < 0.05, ##P < 0.01 compared to RIR group.
PHC activated Nrf2/HO-1 pathway
Nrf2/HO-1 pathway regulated inflammation and oxidative stress.
21
To investigate the effects of PHC on Nrf2/HO-1 pathway, we monitored the mRNA and protein levels of Nrf2 and HO-1 in lung tissues of RIR mice after PHC treatment. RIR mice had greatly reduced mRNA level (Fig. 6A) as well as protein level (Fig. 6B&C) of Nrf2 in lung when compared to normal mice. In contrast, RIR mice treated with PHC had markedly elevated mRNA and protein level of Nrf2. Similarly, HO-1 mRNA and protein levels were markedly reduced in lung of RIR mice (Fig. 6D-F) while PHC treatment significantly upregulated mRNA and protein levels of HO-1. Together, these results indicated that PHC upregulated Nrf2 and HO-1 in RIR mice.
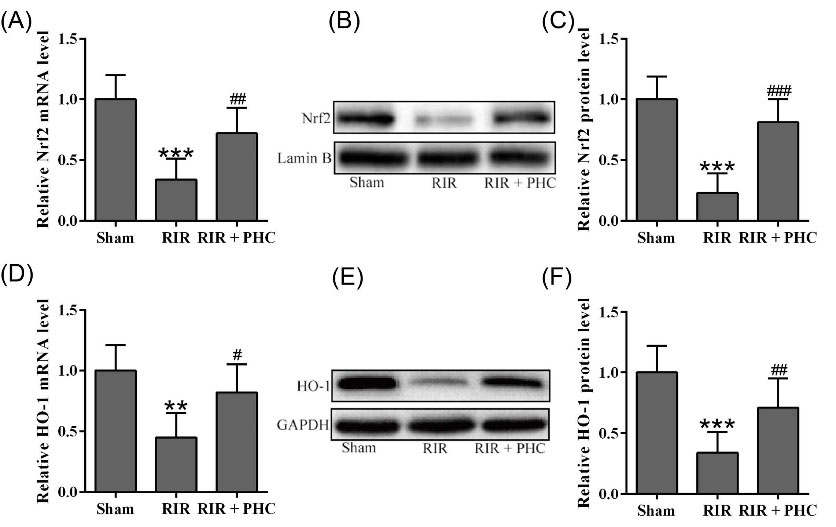
Fig. 6.
Penehyclidine hydrochloride activated Nrf2/HO-1 pathway in lung tissues from renal ischemia reperfusion (RIR) mice. qRT-PCR was used to analyze the mRNA levels of Nrf2 (A) and HO-1 (D) in lung tissue. GAPDH was set as a loading control and the relative expressions were normalized to sham group. Western blotting was used to measure the protein levels of Nrf2 (B) and HO-1 (E) in lung tissue. Lamin B, a nuclear protein and GAPDH were set as a loading control and the relative expressions were normalized to sham group (C and F). n = 6. Data are presented as mean ± SD. **P < 0.01, ***P < 0.001 compared to sham group, #P < 0.05, ##P < 0.01 and ###P < 0.001 compared to RIR group.
.
Penehyclidine hydrochloride activated Nrf2/HO-1 pathway in lung tissues from renal ischemia reperfusion (RIR) mice. qRT-PCR was used to analyze the mRNA levels of Nrf2 (A) and HO-1 (D) in lung tissue. GAPDH was set as a loading control and the relative expressions were normalized to sham group. Western blotting was used to measure the protein levels of Nrf2 (B) and HO-1 (E) in lung tissue. Lamin B, a nuclear protein and GAPDH were set as a loading control and the relative expressions were normalized to sham group (C and F). n = 6. Data are presented as mean ± SD. **P < 0.01, ***P < 0.001 compared to sham group, #P < 0.05, ##P < 0.01 and ###P < 0.001 compared to RIR group.
The protective effects of PHC on lung injury in RIP mice depended on Nrf2
Next, we explored the role of Nrf2 in PHC-mediated protection using Nrf2 -/- mice. As shown in Fig. 7A, RIR caused significant lung damage in Nrf2 -/- mice. Administration of PHC did not change the lung injury score in RIR Nrf2 -/- mice. RIR Nrf2 -/- mice also had significantly increased pulmonary MPO activity (Fig. 7B), MDA (Fig. 7C), IL-6 (Fig. 7E), and TNF-α (Fig. 7F), and significantly decreased SOD level (Fig. 7D). Administration of PHC did not affect level of MPO, MDA, SOD, IL-6, and TNF-α in RIR Nrf2 -/- mice. Collectively, our data demonstrated that PHC did not affect lung injury in RIR Nrf2 -/- mice, indicating that the protective functions of PHC depended on Nrf2.
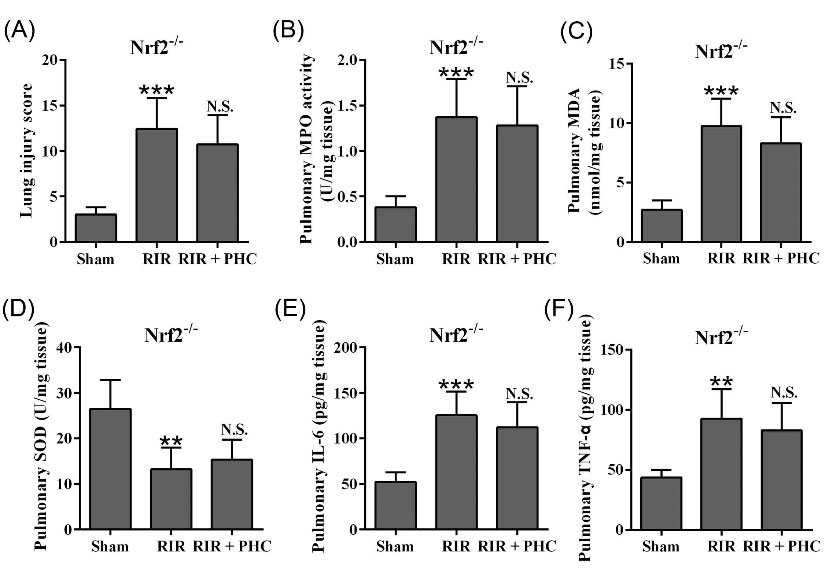
Fig. 7.
Effects of penehyclidine hydrochloride on renal ischemia reperfusion-stimulated lung injury in Nrf2-/- mice. A, Semi-quantitative score was used to evaluate the lung injury from H&E staining. B, MPO activity in lung was measured. The concentrations of MDA (C), SOD (D), IL-6 (E) and TNF-α (F) in lung tissues were measured by ELISA 24 hours after surgery. n = 6. Data are presented as mean ± SD. **P < 0.01, ***P < 0.001 compared to sham group. n.s. means no significant difference compared to RIR group.
.
Effects of penehyclidine hydrochloride on renal ischemia reperfusion-stimulated lung injury in Nrf2-/- mice. A, Semi-quantitative score was used to evaluate the lung injury from H&E staining. B, MPO activity in lung was measured. The concentrations of MDA (C), SOD (D), IL-6 (E) and TNF-α (F) in lung tissues were measured by ELISA 24 hours after surgery. n = 6. Data are presented as mean ± SD. **P < 0.01, ***P < 0.001 compared to sham group. n.s. means no significant difference compared to RIR group.
Discussion
We evaluated the effects of PHC on RIR-induced lung injury. We described that PHC treatment decreased RIR-induced pulmonary injury by suppressing both inflammation and oxidative stress. RIR caused significant renal dysfunction as we detected remarkably increased serum levels of BUN and creatinine in our RIR mice. Consistent to a previous study, PHC treatment significantly decreased serum concentrations of BUN and creatinine, indicating protective roles of PHC on RIR-induced renal dysfunction.
14
We demonstrated that PHC attenuated RIR-induced inflammation by decreasing the concentration of inflammatory cytokines including TNF-α, IL-6 in both serum and lung. We also showed PHC prevented RIR-induced oxidative stress by inhibiting the production of MPO, MDA, NO, MMP-2, and O2-, and promoting the production of SOD and CAT. More importantly, we demonstrated that the PHC protected against lung injury through Nrf2/HO-1.
Increased inflammatory cytokine concentration has been implicated in the pathophysiology of RIR.
22
In RIR rat, increased TNF-α was found in heart after renal ischemia. TNF-α is capable of upregulating its own expression and expression of other inflammatory genes. Exposure of tissue to TNF-α results in tissue damage and dysfunction.
23
Blocking TNF-α expression by knocking down TNF-α alleviated severity of lung injury in intestinal ischemia and reperfusion rat.
24
The anti-inflammatory activity of PHC has been well-described. Guo et al showed that PHC decreased TNF-α and IL-6 production in lung and attenuated LPS-induced lung injury in rats.
25
In the present study, we detected significantly increased pro-inflammatory cytokine level in both serum and lung of RIR mice while PHC treatment significantly suppressed the pro-inflammatory cytokines production in RIR mice. MPO is an indirect marker of neutrophil infiltration. Increased MPO activity was detected in lung after RIR, indicating leukocyte infiltration into lung.
26
We detected elevated MPO activity in lung after RIR while PHC treatment reduced MPO activity, suggesting PHC treatment reduced neutrophil infiltration. All these results suggested PHC protected lung from injury after RIR.
Oxidative stress is another contributor to lung injury. During RIR, damaged tissue-produced ROS caused oxidative stress. We detected decreased activity of SOD and CAT in lung of RIR mice while the level of oxidative stress indictors including MDA, NO, and O2 - was increased, suggesting an imbalance between oxidant and anti-oxidant. Our finding was consistent with previous findings for which decreased activities of SOD and CAT were detected in liver of RIR mice,
27
implicating oxidative stress in RIR. Our data revealed that PHC enhanced the SOD and CAT and suppressed the production of MDA, NO, and O2 -, the indicators of oxidative stress. Using a RIR rat model, Wang and colleagues reported that PHC ameliorated renal injury by attenuating oxidative stress.
14
Wang et al also demonstrated that PHC decreased lung tissue content of ROS, MDA, and MPO after lung ischemia-reperfusion.
28
Our and their findings strongly suggested PHC could be used as a novel therapeutic strategy to treat RIR injury.
Nrf2 is a transcriptional factor that regulates the expression of proteins participating in oxidation and inflammation, and balances cellular redox.
21
Nrf2 has been shown to regulate multiple antioxidant proteins including SOD, glutathione peroxidase 1, CAT, glutathione-S-transferase, and HO-1.
29
The inhibition of inflammation by activation of Nrf2 pathway has been described.
30
HO-1 is a protein with anti-inflammatory and anti-oxidant function. Since Nrf2/HO-1 pathway is a key factor in inflammation, activation of Nrf2/HO-1 has been suggested as a potential strategy to treat inflammatory disorders.
31
Our previous study demonstrated that artesunate activated HO-1 pathway and suppressed RIR-induced lung inflammation.
32
Here we demonstrated that PHC enhanced the expression of both Nrf-2 and HO-1, and activated Nrf-2/HO-1 pathway. PHC activates Nrf2/O-1 pathway in other disease models. Using a rhabdomyolysis model, Zhao and colleagues described that PHC activated Nrf2/HO-1 pathway by promoting Nrf2 and HO-1 expression and ameliorated rhabdomyolysis-induced acute kidney injury.
33
These findings strongly suggested that reagents which can activate Nrf2/HO-1 pathway could be good therapeutic candidates to treat inflammatory disease.
There are still several limitations in the present study. Nrf2 functions as a transcription factor to activate HO-1 expression. In the current study, we demonstrated that PHC up-regulated Nrf2 expression. However, we did not fractionate the cells for western blot. Although HO-1 expression reflected the Nrf2 nuclear translocation, it is still useful to provide direct evidence to demonstrate the nuclear translocation of Nrf2 after PHC treatment. To evaluate the kidney injury, we only measured the serum BUN and creatinine levels, which are not precise indicators of the extent and intensity of the damage. It is better to measure the level of kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL), which are new biomarkers of kidney injury.
34
Research Highlights
What is the current knowledge?
√ PHC is an anti-cholinergic drug with anti-inflammatory and anti-oxidant activities.
What is new here?
√ PHC significantly decreased the serum levels of BUN, creatinine, IL-6, NO, MDA, and MMP-2.
Conclusion
We identified the protective effects of PHC in RIR by suppressing inflammation and oxidative stress. PHC induced the expression of Nrf2 and HO-1. Interestingly, the protective effects of PHC depended on Nrf2 as these effects were abolished in the absence of Nrf2. Taken together, we showed that PHC ameliorated RIR-stimulated lung injury through activating Nrf2 signaling.
Funding sources
None.
Ethical statement
All animal studies were reviewed and approved by the Ethics Commitment of Cangzhou Central Hospital, China.
Competing interests
None to declare.
Authors’ contributions
QY, ZL, and LL acquired the data, analyzed, and interpreted the data; QY, ZL, LL, CL, LY, and YC conceived, designed the project, and wrote the paper. All authors approved the final submission.
References
- Malek M, Nematbakhsh M. The preventive effects of diminazene aceturate in renal ischemia/reperfusion injury in male and female rats. Adv Prev Med 2014; 2014:740647. doi: 10.1155/2014/740647 [Crossref] [ Google Scholar]
- Chao CT, Hou CC, Wu VC, Lu HM, Wang CY, Chen L. The impact of dialysis-requiring acute kidney injury on long-term prognosis of patients requiring prolonged mechanical ventilation: nationwide population-based study. PLoS One 2012; 7:e50675. doi: 10.1371/journal.pone.0050675 [Crossref] [ Google Scholar]
- Youssef MI, Mahmoud AA, Abdelghany RH. A new combination of sitagliptin and furosemide protects against remote myocardial injury induced by renal ischemia/reperfusion in rats. BiochemPharmacol 2015; 96:20-9. doi: 10.1016/j.bcp.2015.04.010 [Crossref] [ Google Scholar]
- Gu J, Chen J, Xia P, Tao G, Zhao H, Ma D. Dexmedetomidine attenuates remote lung injury induced by renal ischemia-reperfusion in mice. Acta AnaesthesiolScand 2011; 55:1272-8. doi: 10.1111/j.1399-6576.2011.02526.x [Crossref] [ Google Scholar]
- Seifi B, Kadkhodaee M, Najafi A, Mahmoudi A. Protection of liver as a remote organ after renal ischemia-reperfusion injury by renal ischemic postconditioning. Int J Nephrol 2014; 2014:120391. doi: 10.1155/2014/120391 [Crossref] [ Google Scholar]
- Kadkhodaee M, Golab F, Zahmatkesh M, Ghaznavi R, Hedayati M, Arab HA. Effects of different periods of renal ischemia on liver as a remote organ. World J Gastroenterol 2009; 15:1113-8. doi: 10.3748/wjg.15.1113 [Crossref] [ Google Scholar]
- Schmeling DJ, Caty MG, Oldham KT, Guice KS, Hinshaw DB. Evidence for neutrophil-related acute lung injury after intestinal ischemia-reperfusion. Surgery 1989; 106:195-201. [ Google Scholar]
- Foulds S, Mireskandari M, Kalu P, Jackson W, Cheshire NJ, Mansfield AO. Visceral ischemia and neutrophil activation in sepsis and organ dysfunction. J Surg Res 1998; 75:170-6. doi: 10.1006/jsre.1998.5276 [Crossref] [ Google Scholar]
- Matsuda A, Yang WL, Jacob A, Aziz M, Matsuo S, Matsutani T. FK866, a visfatin inhibitor, protects against acute lung injury after intestinal ischemia-reperfusion in mice via NF-kappaB pathway. Ann Surg 2014; 259:1007-17. doi: 10.1097/SLA.0000000000000329 [Crossref] [ Google Scholar]
- Mao YF, Zheng XF, Cai JM, You XM, Deng XM, Zhang JH. Hydrogen-rich saline reduces lung injury induced by intestinal ischemia/reperfusion in rats. BiochemBiophys Res Commun 2009; 381:602-5. doi: 10.1016/j.bbrc.2009.02.105 [Crossref] [ Google Scholar]
- Xiao HT, Liao Z, Tong RS. Penehyclidine hydrochloride: a potential drug for treating COPD by attenuating Toll-like receptors. Drug Des DevelTher 2012; 6:317-22. doi: 10.2147/DDDT.S36555 [Crossref] [ Google Scholar]
- Wu XJ, Liu HM, Song XM, Zhao B, Leng Y, Wang EY. Penehyclidine hydrochloride inhibits TLR4 signaling and inflammation, and attenuates blunt chest trauma and hemorrhagic shock-induced acute lung injury in rats. Mol Med Rep 2018; 17:6327-36. doi: 10.3892/mmr.2018.8644 [Crossref] [ Google Scholar]
- Wu XJ, Xia ZY, Wang LL, Luo T, Zhan LY, Meng QT. Effects of penehyclidine hydrochloride on pulmonary contusion from blunt chest trauma in rats. Injury 2012; 43:232-6. doi: 10.1016/j.injury.2011.10.009 [Crossref] [ Google Scholar]
- Wang YP, Li G, Ma LL, Zheng Y, Zhang SD, Zhang HX. Penehyclidine hydrochloride ameliorates renal ischemia-reperfusion injury in rats. J Surg Res 2014; 186:390-7. doi: 10.1016/j.jss.2013.07.041 [Crossref] [ Google Scholar]
- Liu Z, Yang Q, Wei Q, Chang Y, Qu M, Yu L. The protective effect of miR-377 inhibitor against renal ischemia-reperfusion injury through inhibition of inflammation and oxidative stress via a VEGF-dependent mechanism in mice. Mol Immunol 2019; 106:153-8. doi: 10.1016/j.molimm.2018.12.028 [Crossref] [ Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. BiochemBiophys Res Commun 1997; 236:313-22. doi: 10.1006/bbrc.1997.6943 [Crossref] [ Google Scholar]
- Zhu H, Lu X, Ling L, Li H, Ou Y, Shi X. Houttuynia cordata polysaccharides ameliorate pneumonia severity and intestinal injury in mice with influenza virus infection. J Ethnopharmacol 2018; 218:90-9. doi: 10.1016/j.jep.2018.02.016 [Crossref] [ Google Scholar]
- Zhang Y, Rong S, Feng Y, Zhao L, Hong J, Wang R. Simvastatin attenuates renal ischemia/reperfusion injury from oxidative stress via targeting Nrf2/HO-1 pathway. Exp Ther Med 2017; 14:4460-6. doi: 10.3892/etm.2017.5023 [Crossref] [ Google Scholar]
- Xu Z, Cho H, Hartsock MJ, Mitchell KL, Gong J, Wu L. Neuroprotective role of Nrf2 for retinal ganglion cells in ischemia-reperfusion. J Neurochem 2015; 133:233-41. doi: 10.1111/jnc.13064 [Crossref] [ Google Scholar]
- Liu Z, Qu M, Yu L, Song P, Chang Y. Artesunate Inhibits Renal Ischemia-Reperfusion-Mediated Remote Lung Inflammation Through Attenuating ROS-Induced Activation of NLRP3 Inflammasome. Inflammation 2018; 41:1546-56. doi: 10.1007/s10753-018-0801-z [Crossref] [ Google Scholar]
- Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci 2016; 73:3221-47. doi: 10.1007/s00018-016-2223-0 [Crossref] [ Google Scholar]
- Faubel S. Pulmonary complications after acute kidney injury. Adv Chronic Kidney Dis 2008; 15:284-96. doi: 10.1053/j.ackd.2008.04.008 [Crossref] [ Google Scholar]
- Donnahoo KK, Shames BD, Harken AH, Meldrum DR. Review article: the role of tumor necrosis factor in renal ischemia-reperfusion injury. J Urol 1999; 162:196-203. doi: 10.1097/00005392-199907000-00068 [Crossref] [ Google Scholar]
- Yang Z, Zhang XR, Zhao Q, Wang SL, Xiong LL, Zhang P. Knockdown of TNFalpha alleviates acute lung injury in rats with intestinal ischemia and reperfusion injury by upregulating IL10 expression. Int J Mol Med 2018; 42:926-34. doi: 10.3892/ijmm.2018.3674 [Crossref] [ Google Scholar]
- Guo Y, Wei M, Yan Z, Wang G. [Penehyclidine hydrochloride attenuates LPS-induced acute lung injury in rats]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2017; 33:1486-90. [ Google Scholar]
- Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol 2003; 14:1549-58. doi: 10.1097/01.asn.0000064946.94590.46 [Crossref] [ Google Scholar]
- Serteser M, Koken T, Kahraman A, Yilmaz K, Akbulut G, Dilek ON. Changes in hepatic TNF-alpha levels, antioxidant status, and oxidation products after renal ischemia/reperfusion injury in mice. J Surg Res 2002; 107:234-40. doi: 10.1006/jsre.2002.6513 [Crossref] [ Google Scholar]
- Wang Y, Lin D, Tan H, Gao Y, Ma J. Penehyclidine hydrochloride preconditioning provides pulmonary and systemic protection in a rat model of lung ischaemia reperfusion injury. Eur J Pharmacol 2018; 839:1-11. doi: 10.1016/j.ejphar.2018.09.012 [Crossref] [ Google Scholar]
- Habtemariam S. The Nrf2/HO-1 Axis as Targets for Flavanones: Neuroprotection by Pinocembrin, Naringenin, and Eriodictyol. Oxid Med Cell Longev 2019; 2019:4724920. doi: 10.1155/2019/4724920 [Crossref] [ Google Scholar]
- Luo JF, Shen XY, Lio CK, Dai Y, Cheng CS, Liu JX. Activation of Nrf2/HO-1 Pathway by Nardochinoid C Inhibits Inflammation and Oxidative Stress in Lipopolysaccharide-Stimulated Macrophages. Front Pharmacol 2018; 9:911. doi: 10.3389/fphar.2018.00911 [Crossref] [ Google Scholar]
- Kim J, Cha YN, Surh YJ. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res 2010; 690:12-23. doi: 10.1016/j.mrfmmm.2009.09.007 [Crossref] [ Google Scholar]
- Liu Z, Zhang J, Li S, Jiang J. Artesunate Inhibits Renal Ischemia Reperfusion-Stimulated Lung Inflammation in Rats by Activating HO-1 Pathway. Inflammation 2018; 41:114-21. doi: 10.1007/s10753-017-0669-3 [Crossref] [ Google Scholar]
- Zhao W, Huang X, Zhang L, Yang X, Wang L, Chen Y. Penehyclidine Hydrochloride Pretreatment Ameliorates Rhabdomyolysis-Induced AKI by Activating the Nrf2/HO-1 Pathway and Alleviating [corrected] Endoplasmic Reticulum Stress in Rats. The. PLoS One 2016; 11:e0151158. doi: 10.1371/journal.pone.0151158 [Crossref] [ Google Scholar]
- Wasilewska A, Taranta-Janusz K, Debek W, Zoch-Zwierz W, Kuroczycka-Saniutycz E. KIM-1 and NGAL: new markers of obstructive nephropathy. Pediatr Nephrol 2011; 26:579-86. doi: 10.1007/s00467-011-1773-5 [Crossref] [ Google Scholar]