Bioimpacts. 12(5):431-438.
doi: 10.34172/bi.2022.23422
Original Research
Correlation between coenzyme Q10 content and the nutrient sensors in AKI induced by Hemiscorpius lepturus envenomation
Rana Dizaji 1, 2  , Ali Sharafi 2, 3, Jalal Pourahmad 4, Saba Vatanpour 5, Hossein Dinmohammadi 6, Hossein Vatanpour 4, Mir-Jamal Hosseini 2, 7, *
, Ali Sharafi 2, 3, Jalal Pourahmad 4, Saba Vatanpour 5, Hossein Dinmohammadi 6, Hossein Vatanpour 4, Mir-Jamal Hosseini 2, 7, * 
Author information:
1Department of Food Safety and Hygiene, School of Public Health, Zanjan University of Medical Sciences, Zanjan, Iran
2Zanjan Applied Pharmacology Research Center, Zanjan University of Medical sciences, Zanjan, Iran
3Zanjan Pharmaceutical Biotechnology Research Center, Zanjan University of Medical Sciences, Zanjan, Iran
4Departments of Pharmacology and Toxicology, Faculty of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran
5Department of Biology, University of British Columbia, Vancouver, Canada
6Department of Genetics and Molecular Medicine, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran
7Department of Pharmacology and Toxicology, School of Pharmacy, Zanjan University of Medical Sciences, Zanjan, Iran
Abstract
Introduction:
Acute kidney injury (AKI) may have a negative effect on mitochondrial hemostasis and bioenergetics as well as coenzyme Q10 (CoQ10) content. PGC-1α, AMPK, sirtuin 1 (Sirt1), and Sirt3, as the key metabolic regulators under nutritional stress, stimulate energy production via mitochondrial biogenesis during AKI. However, no report is available on the relationship between CoQ10 level and nutrient sensors in the pathophysiology of AKI caused by Hemiscorpius lepturus scorpion envenomation.
Methods:
Three doses of venoms (1, 5, and 10 mg/kg) were administered by subcutaneous (SC) injection to male albino mice. The animals were sacrificed 1 day or 7 days after administration of venom and their kidneys were collected to analyze gene expression involved in AKI, nutrient sensors, and apoptosis signaling activation by real-time polymerase chain reaction (PCR) and the measurement of CoQ10 level using the High-performance liquid chromatography (HPLC) method.
Results:
The data indicated a significant decrease in CoQ10 level after the administration of venom in 5 and 10 mg/kg. In addition, 1 day after the treatment, a significant over-expression of Sirt1 (5 and 10 mg/kg) was observed compared with normal mice. Overexpression of Sirt3 occurred 1 day and 7 days after treatment only at the dose of 5.0 mg/kg of venom. Furthermore, over-expression of AMPK as an important mitochondrial energetic sensor happened 1 day and 7 days after the injection of venom (5 mg/kg) (P < 0.01). The significant increase in the gene expression of caspase-9 and 3 after the injection of venom (5 and 10 mg/kg) confirmed the role of cell death signaling.
Conclusion:
The venom-induced energy-sensing pathways have a key role in gene expression of PGC-1α, AMPK, Sirt3, and CoQ10 content after venom-induced AKI.
Keywords: Acute kidney injury, Coenzyme Q10, Sirtuin 3, Sirtuin 1, 5' AMP-activated protein kinase
Copyright and License Information
© 2022 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Acute kidney injury (AKI) is caused by exposure to ischemic or toxic conditions. Hemiscorpius lepturus scorpion sting in both children and adults in Khuzestan and other south-western areas of Iran during the hot climate has created anxiety and expenses.
1
Previous clinical and practical studies indicated that H. lepturus envenomation could cause nephrotoxicity through proteinuria and due to the presence of intact red blood cells in the urine.
1-7
The clinical data show that the venom of the thin-tailed scorpion is highly toxic, especially in children.
2
However, the precise mechanistic pathways of H. lepturus scorpion toxicity are still unclear. AKI adversely affects mitochondrial homeostasis and ATP level, decreases the antioxidant defenses, leads to oxidative stress due to increased reactive oxygen species (ROS) level, diminishes ATP level, and finally, results in mitochondrial susceptibility to injury or slow recovery of tubular epithelial cells.
8-11
In addition, an increase in cellular ROS level under oxidative stress condition enhances metabolic activity and consequently, provides extra ATP for synthesizing new proteins and repairing the damaged ones.
12,13
Deficiency in coenzyme Q10 (CoQ10) as one of the main electron carriers in the mitochondrial respiratory chain decreases the activities of mitochondrial complexes and declines the ATP generation needed for the modulation of the cellular redox state.
14
The similar oxidative stress condition, results in an over-expression of genes involved in mitochondrial biogenesis genes, which increases the energy inquiry and reach to normal level in cells.
14
Therefore, up-regulation of the main metabolic sensors such as AMPK can compensational regulate the energy status in the kidney.
15
High intracellular AMP/ATP ratio activates AMPK under metabolic stress conditions in order to maintain the energetic homeostasis through beta-oxidation of the fatty acid pathway, protein synthesis, ion transport, and phosphorylated peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α).
16-20
Among the sirtuins family, Sirt3 which is localized into the inner mitochondrial membrane plays an important role as the downstream regulator of mitochondrial biogenesis and ROS regulator.
15,21
Over-expression of uncoupling protein 2 (UCP2) in kidney depends on the Sirt3 level and the decreased level of superoxide in kidneys. Furthermore, the expression of Sirt3 is controlled by AMPK to inhibit renal injury.
22
Based on previous studies, AMPK can be regulated by PGC-1α/Sirt signaling in mitochondrial homeostasis and biosynthesis.
23,24
AMPK also can phosphorylate or deacetylate PGC-1α to regulate ROS-detoxifying enzyme and increase mitochondrial biogenesis.
25,26
As the data indicate, AKI is associated with ROS overproduction, cytochrome c release, and the subsequent apoptotic caspase cascade activation through intrinsic death pathways.
8,25
In the intrinsic pathway, mitochondrial membrane permeability is regulated by the balance between pro-apoptotic Bcl-2-associated X (BAX) protein and the anti-apoptotic protein BCL-2 family proteins as the apoptotic indices.
27,28
After AKI conditioning, the amount of IL-18 as an IFN-γ- prompting element increases in the kidneys, leading to the adjustment of IL-18 precursor matured by caspase-1. Therefore, the source of IL-18 which is a biomarker of kidney injury can be damaged in the proximal tubular cell.
29
As renal cell repair relies on the capability of mitochondria for ATP production, reinstatement of mitochondrial utility can lessen cellular damage and repair renal function, mostly in AKI.
8
Therefore, renal dysfunction in AKI is strongly associated with the decreased Sirt3 gene expression and CoQ10 level. The present study aims to measure the CoQ10 level using the HPLC method and evaluate the gene expression of Il-18, Sirt3, Sirt1 and AMPK, caspase-9, 3 in order to determine selective rescue mechanisms and finally, introduce a new therapeutic target for AKI treatment.
Materials and Methods
Scorpion venom
Hemiscorpius lepturus scorpion venom was provided by the RAZI vaccine and serum research institute of Iran (Karaj, Iran). The obtained venom was stored under refrigeration conditions (-20°C) until use.
Materials
All chemicals and analytical grade solvents used for HPLC analysis were purchased from Sigma-Aldrich CoQ10 and TriPure reagents were prepared from nature’s Bounty Inc., USA, and Rosche company, respectively. SYBR Premix Ex Taq II kit and PrimeScriptTM RT Master Mix (Perfect Real Time) were obtained from Takara Bio Co. (Japan). Primers were designed by primer III software (NCBI) and synthesized by Sinaclon co (Tehran, Iran).
Animals
Forty-two mice (Balb/c mice; 26 ± 2 g) were obtained from Pasteur Institute in Tehran, Iran, and kept in 12 hours of darkness/12 hours of light in a constant-temperature room in the animals' facility of Zanjan University Animal Medical Center. All procedures were conducted on the animal in accordance with the National Institutes of Health guidelines (NIH Publications No. 8023, revised 1978) confirmed by the animal ethics committee of Zanjan University of Medical Sciences.
Experimental design
The mice were watched for 1 day or 7 days after subcutaneous (SC) injection of H. lepturus venom. To determine the toxicity mechanism, higher doses of venom were needed to be used in limited animal groups to make an animal model comparable to the human population.
30
Based on the previous study, LD50 of H. lepturus was determined to be 10 mg/kg.
6
Therefore, three doses of 1, 5, and 10 mg/kg of H. lepturus venom were selected for the evaluation of target gene expression after treatment.
The mice were randomly classified into the following seven groups: groups 1, 2: control groups that received normal saline and were decapitated after 1 day and 7 days; groups 3, 4 and 5 that received a single doses of 1, 5, and 10 mg/kg of H. lepturus venom and were decapitated after 1 day; group 6,7 which received a single dose of 1 or 5 mg/kg of venom and were decapitated after 7 days; group 8 which received the single dose of 10 mg/kg of venom. As all animals which received 10 mg/kg of H. lepturus venom died after less than 7 days, only the effects of 1 and 5 mg/kg of H. lepturus venom after 7 days were evaluated.
Coenzyme Q10 extraction and HPLC analysis
Following the administration of vehicle or venom in all treated groups, the kidneys were quickly dissected on ice after decapitation and kept at -80°C until their being used in the analysis. The method proposed by Andalib et al with a minor modification in flow rate (1.2 mL/min) was implemented to isolate coenzyme Q from the obtained tissues.
31
Finally, after evaporation of the organic layer and reconstitution, 50 µL of the sample was injected into the HPLC instrument. CoQ10 level was measured by a KNAUER HPLC system (Berlin, Germany) with a C18 column (ODS 250*4.6 mm) and isocratic elution pump (ConstaMetric 4100 pumps) coupled with a UV detector (KNAUER, Smartline 2500) at 275 nm, and D-2500 calculating integrator software
Estimation of intracellular ROS by using the DCFH-DA fluorescence method
The mice were sacrificed 1 day or 7 days after treatment with venom and their kidneys were quickly removed, immediately rinsed in saline ice, homogenized, and centrifuged at 8000 g for 10 minutes. Then, ROS formation was evaluated using supernatants. The suspensions were incubated in respiration buffer containing 0.32 mM sucrose, 10 mM Tris-HCl, 20 mM MOPS, 50 μM EGTA, 0.5 mM MgCl2, 0.1 mM KH2PO4, and 5 mM sodium succinate. Then, DCFH-DA (10 μM) was added to the supernatant suspension and incubated for 5 minutes. Finally, the ROS level was measured after 5-10 minutes by using a fluorescence spectrophotometer at excitation and emission wavelengths of 495 and 520 nm, respectively.
32
Real-time PCR analysis
After decapitation of animals, kidneys were collected and transferred to -80°C. According to the manufactures information, total mRNA was purified from mice kidneys using the easy Blue-Kit (Intron Biotechnology, South Korea). The high-capacity cDNA reverse transcription Kit (PrimeScriptTM RT Master Mix, Takara Bio) was employed for cDNA synthesis. Then, cDNA samples were analyzed by real-time polymerase chain reaction (PCR) using the SYBR Green. Gene expression was evaluated using a 2-ΔΔCt method and the Ct values of β-actin gene expression were applied for normalization. The following primers were also utilized to measure the gene expression of IL-18, Sirt1, Sirt3, AMPK, PGC-1α, caspase-9, and caspase-3 (Table 1).
Table 1.
Primer sequences used in Real-time PCR assay
|
Name
|
Sequence (5′ → 3′)
|
GenBank Accession No.
|
| PGC-1α |
ACATAGAGTGTGCTGCTCTGG (F)
TTCGCAGGCTCATTGTTGTAC (R)
|
NM-008904.2 |
| AMPK |
ACCTGAGAACGTCCTGCTTG (F)
GGCCTGCGTACAATCTTCCT (R)
|
NM-001013367.3 |
| SIRT1 |
CGGCTACCGAGGTCCATATAC (F)
ACAATCTGCCACAGCGTCAT (R)
|
NM-001159589.2 |
| SIRT3 |
TCTATACACAGAACATCGACGGG (F)
AGACCGTGCATGTAGCTGTTA (R)
|
NM-022433.2 |
| IL-18 |
CAGGCCTGACATCTTCTGCAA (F)
TCTGACATGGCAGCCATTGT (R)
|
NM-008360.2 |
| Caspase-3 |
CTGACTGGAAAGCCGAAACTC (F)
GACTGGATGAACCACGACCC (R)
|
NM-009810.3 |
| Caspase-9 |
GCGGTGGTGAGCAGAAAGA (F)
CCTGGGAAGGTGGAGTAGGA (R)
|
NM-015733.5 |
F: forward primer; R: reverse primer.
Statistical analysis
All statistical analyses were performed using the SPSS software (Window version 18) and Excel 2007 software. The groups were compared by one-way analysis of variance (ANOVA) followed by Tukey's post hoc tests using the GraphPad Prism software (version 6). P ˂ 0.05 was considered statistically significant.
Results
The effects of venom-induced AKI on IL-18 expression
As the statistical analysis shows, IL-18 expression as the AKI biomarker significantly increased in mice receiving 5 and 10 mg/kg of venom after 1 day of envenomation (P < 0.01; Fig. 1). After 7 days, a significant difference was observed between IL-18 expression of the group treated with 5 mg/kg of venom and that of the control group (P < 0.01; Fig. 1).
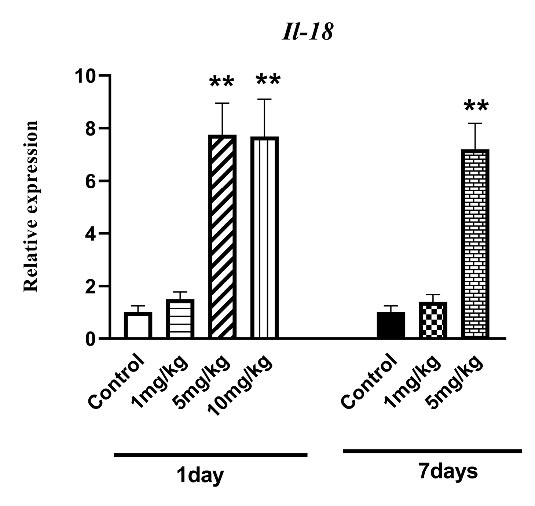
Fig. 1.
The effect of H. lepturus envenomation on IL-18 mRNA expression by RT- PCR in kidneys showed a significant rise in IL-18 expression following receiving 5 and 10 mg/kg of venom after 1 day and only 5 mg/kg of venom compared to the control group after 7 days (n=3). **P < 0.01 indicates the significant difference between the control group.
.
The effect of H. lepturus envenomation on IL-18 mRNA expression by RT- PCR in kidneys showed a significant rise in IL-18 expression following receiving 5 and 10 mg/kg of venom after 1 day and only 5 mg/kg of venom compared to the control group after 7 days (n=3). **P < 0.01 indicates the significant difference between the control group.
The effect of venom-induced AKI on CoQ10 level
One-way ANOVA analysis revealed a remarkable difference in CoQ10 level between animals treated with H. lepturus after 1 day and those treated after 7 days (P < 0.05; Fig. 2). The CoQ10 level of the H. lepturus-treated groups (5 and 10 mg/kg) was also found to be considerably different from normal mice 1 day after envenomation (P < 0.05; Fig. 2). Moreover, the H. lepturus-treated (1 mg/kg) and control (P > 0.05) groups were not significantly different at the CoQ10 level. However, 7 days after envenomation, the CoQ10 level of the H. lepturus-treated groups (5 mg/kg) had a significant effect on the CoQ10 level compared with the control group (P < 0.05; Fig. 2).
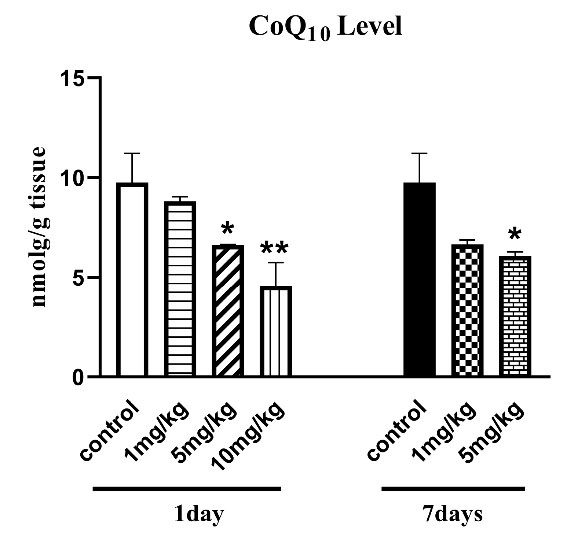
Fig. 2.
The effect of H. lepturus envenomation on CoQ10 level in the kidney confirmed a significant decrease in CoQ10 level following H. lepturus-treated groups (5 and 10 mg/kg) after 1 day and only 5 mg/kg of venom compared to the control group after 7 days (n=3). *P < 0.05, **P < 0.01 indicates the significant difference with the control group.
.
The effect of H. lepturus envenomation on CoQ10 level in the kidney confirmed a significant decrease in CoQ10 level following H. lepturus-treated groups (5 and 10 mg/kg) after 1 day and only 5 mg/kg of venom compared to the control group after 7 days (n=3). *P < 0.05, **P < 0.01 indicates the significant difference with the control group.
The effects of venom-induced AKI on ROS level
As shown in Fig. 3, one-way ANOVA analysis demonstrated significant effects of H. lepturus after 1 day and 7 days on the ROS production levels in the kidney (P < 0.05; Fig. 3). The post-hoc analysis also indicated the increased ROS level after H. lepturus envenomation compared to control mice (P < 0.001, Fig. 3). It is worth mentioning that the ROS level decreased in mice that received treatment 7 days after treatment in comparison with that treated one day after envenomation (Fig. 3).
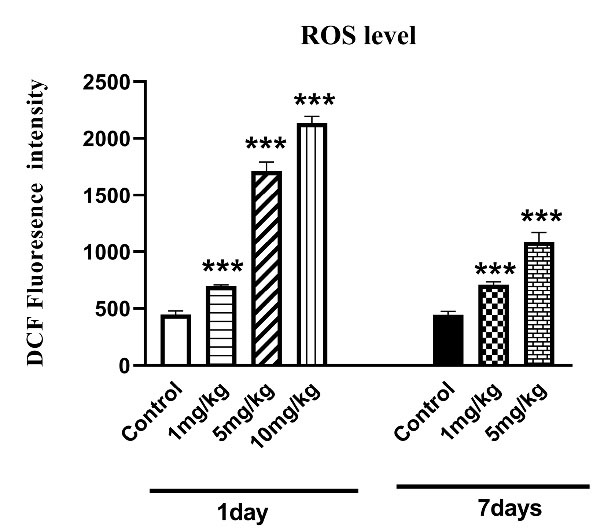
Fig. 3.
The effect of H. lepturus envenomation on ROS levels in kidneys revealed that increase in ROS levels after H. lepturus envenomation following 1 and 7 days of envenomation compared to control mice (n=3). *P < 0.05, **P < 0.01 indicates the significant difference with the control group.
.
The effect of H. lepturus envenomation on ROS levels in kidneys revealed that increase in ROS levels after H. lepturus envenomation following 1 and 7 days of envenomation compared to control mice (n=3). *P < 0.05, **P < 0.01 indicates the significant difference with the control group.
The effects of venom-induced AKI on cell death signaling
Changes in the gene expression of cell death signaling including caspase-9 and caspase-3 was assessed by the real time-PCR after H. lepturus envenomation.
Caspase-9 gene expression
One-way ANOVA in Fig. 4A indicates a significant rise in caspase-9 gene expression as a potential initiator caspase of apoptosis in mice that received only 10 mg/kg and 5 mg/kg of venom 1 day and 7 days after inoculation, respectively.
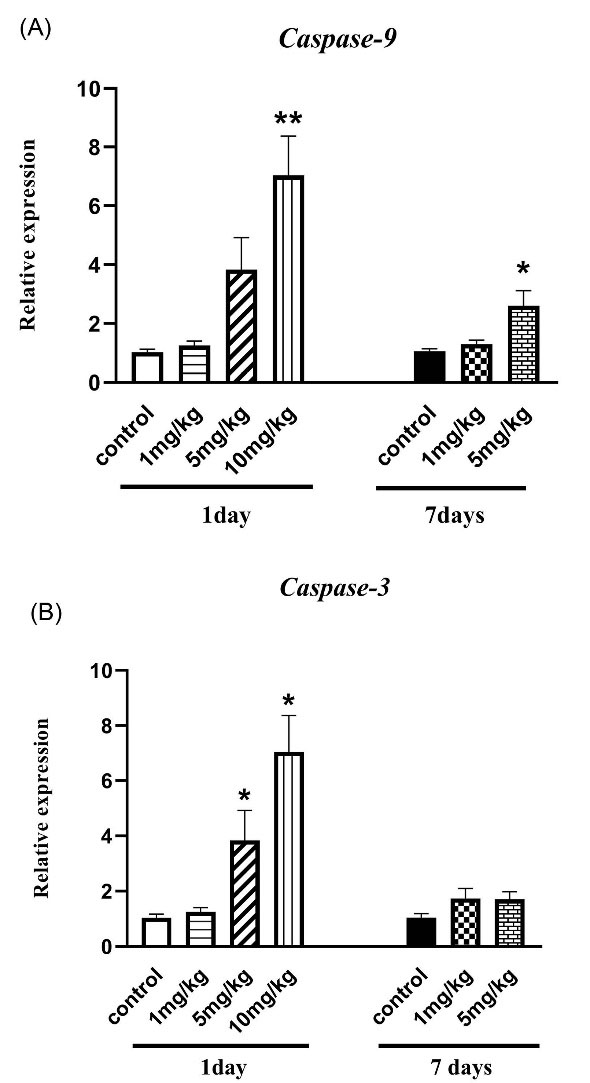
Fig. 4.
The effect of H. lepturus envenomation on (a) Caspase-3 and (b) Caspase-9 mRNA expression by RT- PCR in kidneys showed that a significant rise in caspase-9 gene expression in mice that received only 10 mg/kg and 5mg/kg of venom 1 day and 7 days after inoculation, respectively. Also, an increase in expression of caspase-3 was observed in 5 and 10 mg/kg of venom after 1-day envenomation without no significant difference after 7 days (n=3). *P < 0.05, ***P < 0.001 indicates the significant difference between the control group.
.
The effect of H. lepturus envenomation on (a) Caspase-3 and (b) Caspase-9 mRNA expression by RT- PCR in kidneys showed that a significant rise in caspase-9 gene expression in mice that received only 10 mg/kg and 5mg/kg of venom 1 day and 7 days after inoculation, respectively. Also, an increase in expression of caspase-3 was observed in 5 and 10 mg/kg of venom after 1-day envenomation without no significant difference after 7 days (n=3). *P < 0.05, ***P < 0.001 indicates the significant difference between the control group.
Caspase-3 gene expression
Based on Fig. 4B, the expression of caspase-3 as an execution caspase in cell death signaling activation (5 and 10 mg/kg) significantly increased 1 day after the treatment (P < 0.05). Furthermore, no significant difference was observed between treatment and control groups after 7 days (P > 0.05).
The effects of venom-induced AKI on mitochondrial biogenesis
Changes in the gene expression of mitochondrial biogenesis such as AMPK, PGC-1α, Sirt1 and Sirt3, were assessed by the real time-PCR after H. lepturus envenomation.
AMPK gene expression
AMPK as a cellular energy controller promotes mitochondrial biogenesis through the transcriptional regulation of some nuclear genes. Fig. 5A represents the over-expression of AMPK one day after treatments with 5 and 10 mg/kg of venom and seven days after administration of only 5 mg/kg 7 days, compared to control or normal animals (P < 0.05).
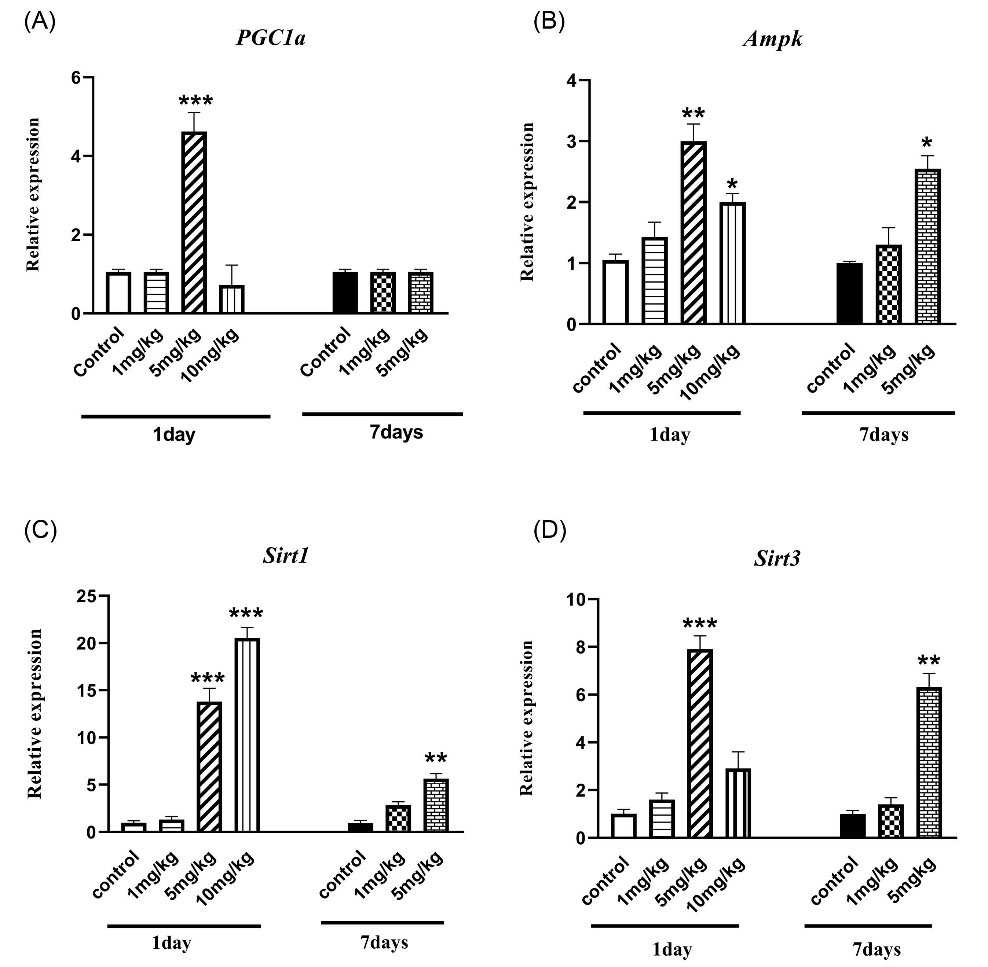
Fig. 5.
The effect of H. lepturus envenomation on mitochondrial biogenesis gene expression by RT- PCR in kidneys showed a different pattern. (a)PGC-1α: Up-regulation of PGC-1α significantly occurred in mice treated with only 5 mg/kg of venom only 1 day after inoculation;(b) AMPK: over-expression of AMPK one day after treatments with 5 and 10 mg/kg of venom and seven days after administration of only 5 mg/kg 7 days;(c) Sirt 1: It revealed a similar over-expression pattern with AMPK; (d)Sirt 3 over-expression was observed only in the group receiving 5 mg/kg of venom after 1 and 7 days (n=3).*P < 0.05, **P < 0.01, ***P < 0.001 indicates the significant difference between the control group.
.
The effect of H. lepturus envenomation on mitochondrial biogenesis gene expression by RT- PCR in kidneys showed a different pattern. (a)PGC-1α: Up-regulation of PGC-1α significantly occurred in mice treated with only 5 mg/kg of venom only 1 day after inoculation;(b) AMPK: over-expression of AMPK one day after treatments with 5 and 10 mg/kg of venom and seven days after administration of only 5 mg/kg 7 days;(c) Sirt 1: It revealed a similar over-expression pattern with AMPK; (d)Sirt 3 over-expression was observed only in the group receiving 5 mg/kg of venom after 1 and 7 days (n=3).*P < 0.05, **P < 0.01, ***P < 0.001 indicates the significant difference between the control group.
PGC-1α expression
One-way ANOVA analysis (Fig. 5B) revealed that up-regulation of PGC-1α, as the main mitochondriogenesis co-activator, significantly occurred in mice treated with only 5 mg/kg of venom(P < 0.001). However, PGC-1α gene expressions in 10 mg/kg administration were suppressed in comparison with the control group. Also, PGC-1α expression after 7 days of venom inoculation can reverse to normal condition (P > 0.05).
Sirt 1 and 3 gene expression
The gene expression of Sirt1, as a renal protective molecule, was investigated and the results (Fig. 5c) revealed a significant overexpression only 24 hours after administration of 5, 10 mg/kg (P < 0.001). A significant increase was observed in the expression of the Sirt1 gene 7 days after treatment with 5 mg/kg of venom, compared with the control group (Fig. 5C, P < 0.01).
As shown in Fig. 5D, up-regulation of Sirt3 as the mitochondrial-specific protein deacetylase which plays a key role in mitochondrial function and integrity was significantly induced only in the group receiving 5 mg/kg of venom after 1 day (P < 0.001). However, our data revealed no significant rise in Sirt3 gene expression in the mice receiving 10 mg/kg of venom compared control mice (Fig. 5d). Furthermore, a major difference was observed in Sirt3 expression 7 days after the administration of 5 mg/kg of venom (Fig. 5d, P < 0.01).
Discussion
Lack of perfect molecular mechanisms has been found in various studies to be the pathological cause of venom-induced AKI.
2-4,6,33
Numerous clinical and preclinical studies have concluded that nephrotoxicity is one of the serious systemic effects of H. lepturus envenomation. Unfortunately, these toxic effects of scorpion venom are more severe and fatal in younger victims.
2,4
Up-regulation in renal IL-18 gene expression can be observed under stressful conditions such as ischemia-reperfusion injury and cisplatin-induced nephrotoxicity. It shows that IL-18 is a mediator of tubular injury-inducing both neutrophil and monocyte infiltration of the renal parenchyma.
29,34
Based on the present study, venom-induced AKI can introduce IL-18 up-regulation as the main biomarker in the inflammation pathway.
AKI stressful status interrupts the mitochondrial function and hemostasis, resulting in ROS formation, an increase in cytochrome c release from mitochondria to cytosol, a probable decline of mitochondrial essential macromolecules (CoQ10), and finally, a decrease in ATP level.
13
Our previous data revealed that H. lepturus induced AKI is related to hemolysis, and hemoglobinuria followed by a significant increase in ROS production and Fenton reactions which contributed to hemodynamic changes and destabilization of the lysosomal membrane. These proteases and the released hydroxyl radicals could either directly target the mitochondrial outer membranes or indirectly activate Bid or Bax and other lytic enzymes including phospholipase A2 to open the MPT pore and release cytochrome c. So, it can initiate the downstream events that finally trigger apoptotic cell death signaling including caspase-9 and caspase-3 activation which was evaluated in the present study.
6,7
Furthermore, we decided to determine the association between CoQ10 and nutrient sensor gene expression to find out mechanistic approaches involved in venom-induced AKI.
Based on the recently-obtained data, mitochondria can show an adaptive response to stimulators like inflammation and oxidative stress conditions in order to save cellular homeostasis via mitochondrial biogenesis and mitochondrial-selective autophagy. The injured mitochondria affected by fragmentation before mitophagy can result in a significant increase in ROS formation and ATP depletion.
8,17
Decrease in CoQ10 as an antioxidant effector in cellular metabolism disturbs the electron transport chain through mitochondrial membrane potential collapse, increased ROS level, and finally, decreased ATP amount.
14,35
Our data revealed that CoQ10 depletion is associated with venom-induced AKI model so that all mice died within 4 days after envenomation (10 mg/kg), implying that the CoQ10 depletion is a non-redundant player in AKI. Furthermore, CoQ10 depletion and energy hemostasis disruption increase caspase-9 and 3 and activate cell death signaling.
Due to a decrease in CoQ10 level, an increase in ROS production, and consequently, ATP disturbances in cells, AMPK as the main metabolic sensor is over-expressed to regulate the cellular homeostasis through PGC-1α gene expression. However, it plays different roles in up-taking glucose and cell cycle regulation.
12,36
Sirtuins can also regulate energy-sensing pathways through mitochondrial biogenesis. In the sirtuin family, Sirt3 controls mitochondrial ROS formation to maintain the mitochondrial morphology and decrease cellular apoptosis following AKI.
11,37
The biological role of the Sirt1 gene is hypothetically intermediated by the deacetylation of multiple transcription factors, such as PGC-1α.
21
Sirt1 gene expression also increases in the venom-treated mice after 1 day. In addition, Sirt3 gene expression which is essential for the recovery of tubular injury decreases after the administration of 10 mg/kg of venom and increases after receiving 5 mg/kg of venom (Fig. 4). It suggested that the low to moderate dose of venom caused Sirt3 over-expression which can be able to detoxify ROS levels via non-enzymatic antioxidant (e.g. GSH) and enzymatic antioxidant (e.g. SOD, catalase) capacity. Therefore it seems that in high dosage, Sirt3 gene expression cannot decrease ROS formation during AKI. The similar results regarding the upregulation and suppression of PGC-1α gene expression were observed in mice treated with 5 and 10 mg/kg of venom in comparison to control group, respectively. It confirms that PGC-1α as the main mitochondriogenesis co-activator is activated by deacylation if the cells need more energy.
7
Therefore, the expression of multiple key mitochondrial regulators such as Sirt3 and PGC-1α can be considered as biomarkers for mitochondrial hemostasis.
21
Conclusion
The present study showed that a significant increase in Sirt3 and AMPK gene expression can remarkably raise PGC-1α gene expression, supporting the previous study by the authors. Sirt3 as a critical mitochondrial deacetylase controls mitochondrial oxidative phosphorylation and activates enzymes responsible for ROS clearance. The coordinate reduction in Sirt3, AMPK, and CoQ10 can lead to the interaction of energy homeostasis and consequently, the lethality in mice. Decreased CoQ10 level at low doses of venom acts as a motivating force in AMPK upregulation, which is essential in energy homeostasis under oxidative stress and inflammatory stimulus.
Designing innovative therapies and determining possible key mediators of damage in pharmacological studies can accelerate renal repair. The data show the role of AMPK, Sirt 3, and Sirt1 in venom-induced AKI which is accompanied by CoQ10 depletion. Therefore, Sirt3/AMPK signaling and CoQ10 content are expected to act as feature mechanisms in venom-induced AKI. Furthermore, CoQ10 which can be an important regulator of AKI needs to be more investigated in similar studies.
Research Highlights
What is the current knowledge?
√ Our data revealed that Sirt3/AMPK signaling pathway and CoQ10 content are involved in AKI condition.
What is new here?
√ The coordinate reduction in Sirt3 and AMPK leads to the interaction of energy homeostasis and lethality in venom-induced AKI. Besides, a decrease in CoQ10 level at low doses of venom acts as a boosting factor in AMPK upregulation, which is essential in energy homeostasis under ROS production.
Funding sources
This work was supported by the Deputy of Research of Zanjan University of Medical Sciences (Grant NO: A-12-769-34).
Ethical statement
The study was approved by the Ethics Committee of the Zanjan University of Medical Sciences, Zanjan, Iran (IR.ZUMS.REC.1398.267).
Competing interests
The authors declare no conflict of interest in publishing this paper.
Authors’ contribution
RD performed most of the experiments. RD, AS, JP, SV, HD, HV, and MJH helped to draft and edited the manuscript. MJH and RD proposed the idea of the current study. MJH, HV, HD, JP, and AS analyzed the data and provided the results. All of the authors approved the final version to be published and agreed to be accountable for the accuracy or integrity of any part of the work.
References
- Pipelzadeh MH, Dezfulian A-R, Jalali MT, Mansouri A-K. In vitro and in vivo studies on some toxic effects of the venom from Hemiscorpious lepturus scorpion. Toxicon 2006; 48:93-103. doi: 10.1016/j.toxicon.2006.04.017 [Crossref] [ Google Scholar]
- Jalali A, Pipelzadeh MH, Sayedian R, Rowan E. A review of epidemiological, clinical and in vitro physiological studies of envenomation by the scorpion Hemiscorpius lepturus (Hemiscorpiidae) in Iran. Toxicon 2010; 55:173-9. doi: 10.1016/j.toxicon.2009.09.012 [Crossref] [ Google Scholar]
- Jalali A, Pipelzadeh M, Seyedian R, Rahmani A, Omidian N. In vivo pharmacological study on the effectiveness of available polyclonal antivenom against Hemiscorpius lepturus venom. J Venom Anim Toxins Incl Trop Dis 2011; 17:142-9. doi: 10.1590/S1678-91992011000200004 [Crossref] [ Google Scholar]
- Pipelzadeh MH, Jalali A, Taraz M, Pourabbas R, Zaremirakabadi A. An epidemiological and a clinical study on scorpionism by the Iranian scorpion Hemiscorpius lepturus. Toxicon 2007; 50:984-92. doi: 10.1016/j.toxicon.2007.07.018 [Crossref] [ Google Scholar]
- Seyedian R, Pipelzadeh MH, Jalali A, Kim E, Lee H, Kang C. Enzymatic analysis of Hemiscorpius lepturus scorpion venom using zymography and venom-specific antivenin. Toxicon 2010; 56:521-5. doi: 10.1016/j.toxicon.2010.05.008 [Crossref] [ Google Scholar]
-
Dizaji R, Sharafi A, Pourahmad J, Abdollahifar M-A, Vatanpour H, Hosseini M-J. Induction of two independent immunological cell death signaling following hemoglobinuria-induced acute kidney injury: In vivo study 2019; 163: 23-31. 10.1016/j.toxicon.2019.03.011.
- Dizaji R, Sharafi A, Pourahmad J, Vatanpour S, Hosseini M-J, Vatanpour H. The effects of Hemiscorpius lepturus induced-acute kidney injury on PGC-1α gene expression: From induction to suppression in mice. Toxicon 2020; 174:57-63. doi: 10.1016/j.toxicon.2019.12.154 [Crossref] [ Google Scholar]
- Bhargava P, Schnellmann RG. Mitochondrial energetics in the kidney. Nat Rev Nephrol 2017; 13:629. doi: 10.1038/nrneph.2017.107 [Crossref] [ Google Scholar]
- Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 2011; 121:4210-21. doi: 10.1172/JCI45161 [Crossref] [ Google Scholar]
- Morigi M, Perico L, Benigni A. Sirtuins in renal health and disease. J Am Soc Nephrol 2018; 29:1799-809. doi: 10.1681/ASN.2017111218 [Crossref] [ Google Scholar]
- Morigi M, Perico L, Rota C, Longaretti L, Conti S, Rottoli D. Sirtuin 3–dependent mitochondrial dynamic improvements protect against acute kidney injury. J Clin Invest 2015; 125:715-26. doi: 10.1172/JCI77632 [Crossref] [ Google Scholar]
- Rabinovitch RC, Samborska B, Faubert B, Ma EH, Gravel S-P, Andrzejewski S. AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell Rep 2017; 21:1-9. doi: 10.1016/j.celrep.2017.09.026 [Crossref] [ Google Scholar]
- Lee H-C, Wei Y-H. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int J Biochem Cell Biol 2005; 37:822-34. doi: 10.1016/j.biocel.2004.09.010 [Crossref] [ Google Scholar]
- Morris G, Anderson G, Berk M, Maes M. Coenzyme Q10 depletion in medical and neuropsychiatric disorders: potential repercussions and therapeutic implications. Mol Neurobiol 2013; 48:883-903. doi: 10.1007/s12035-013-8477-8 [Crossref] [ Google Scholar]
- Gounden S, Phulukdaree A, Moodley D, Chuturgoon A. Increased SIRT3 expression and antioxidant defense under hyperglycemic conditions in HepG2 cells. Metab Syndr Relat Disord 2015; 13:255-63. doi: 10.1089/met.2014.0140 [Crossref] [ Google Scholar]
- Hardie DG. AMPK: positive and negative regulation, and its role in whole-body energy homeostasis. Curr Opin Cell Biol 2015; 33:1-7. doi: 10.1016/j.ceb.2014.09.004 [Crossref] [ Google Scholar]
- Zhang C-S, Lin S-C. AMPK promotes autophagy by facilitating mitochondrial fission. Cell Metab 2016; 23:399-401. doi: 10.1016/j.cmet.2016.02.017 [Crossref] [ Google Scholar]
- Hardie DG. AMPK—sensing energy while talking to other signaling pathways. Cell Metab 2014; 20:939-52. doi: 10.1016/j.cmet.2014.09.013 [Crossref] [ Google Scholar]
- Oakhill JS, Scott JW, Kemp BE. AMPK functions as an adenylate charge-regulated protein kinase. Trends Endocrinol Metab 2012; 23:125-32. doi: 10.1016/j.tem.2011.12.006 [Crossref] [ Google Scholar]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 2007; 8:774. doi: 10.1038/nrm2249 [Crossref] [ Google Scholar]
- Chuang PY, Cai W, Li X, Fang L, Xu J, Yacoub R. Reduction in podocyte SIRT1 accelerates kidney injury in aging mice. Am J Physiol Renal Physiol 2017; 313:F621-F8. doi: 10.1152/ajprenal.00255.2017 [Crossref] [ Google Scholar]
- Pan JS-C, Huang L, Belousova T, Lu L, Yang Y, Reddel R. Stanniocalcin-1 inhibits renal ischemia/reperfusion injury via an AMP-activated protein kinase-dependent pathway. J Am Soc Nephrol 2015; 26:364-78. doi: 10.1681/ASN.2013070703 [Crossref] [ Google Scholar]
- Chen L, Li W, Qi D, Lu L, Zhang Z, Wang D. Honokiol protects pulmonary microvascular endothelial barrier against lipopolysaccharide-induced ARDS partially via the Sirt3/AMPK signaling axis. Life Sci 2018; 210:86-95. doi: 10.1016/j.lfs.2018.08.064 [Crossref] [ Google Scholar]
- Duan W-J, Li Y-F, Liu F-L, Deng J, Wu Y-P, Yuan W-L. A SIRT3/AMPK/autophagy network orchestrates the protective effects of trans-resveratrol in stressed peritoneal macrophages and RAW 2647 macrophages. Free Radic Biol Med 2016; 95:230-42. doi: 10.1016/j.freeradbiomed.2016.03.022 [Crossref] [ Google Scholar]
- Wang H, Guan Y, Karamercan MA, Ye L, Bhatti T, Becker LB. Resveratrol rescues kidney mitochondrial function following hemorrhagic shock. Shock 2015; 44:173. [ Google Scholar]
- Cantó C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol 2009; 20:98. doi: 10.1097/MOL.0b013e328328d0a4 [Crossref] [ Google Scholar]
- Trachootham D, Lu W, Ogasawara MA, Valle NR-D, Huang P. Redox regulation of cell survival. Antioxid Redox Signal 2008; 10:1343-74. doi: 10.1089/ars.2007.1957 [Crossref] [ Google Scholar]
- Mei S, Li L, Wei Q, Hao J, Su Y, Mei C. Double knockout of Bax and Bak from kidney proximal tubules reduces unilateral urethral obstruction associated apoptosis and renal interstitial fibrosis. Sci Rep 2017; 7:44892. doi: 10.1038/srep44892 [Crossref] [ Google Scholar]
- Charlton JR, Portilla D, Okusa MD. A basic science view of acute kidney injury biomarkers. Nephrology Dialysis Transplantation 2014; 29:1301-11. doi: 10.1093/ndt/gft510 [Crossref] [ Google Scholar]
-
Klaassen CD, Amdur MO. Casarett and Doull's Toxicology: The Basic Science of Poisons. New York: McGraw-Hill; 2013. 10.1036/0071470514.
- Andalib S, Mashhadi-Mousapour M, Bijani S, Hosseini M-J. Coenzyme Q 10 Alleviated Behavioral Dysfunction and Bioenergetic Function in an Animal Model of Depression. Neurochem Res 2019; 44:1182-91. doi: 10.1007/s11064-019-02761-0 [Crossref] [ Google Scholar]
- Hosseini M, Jafarian I, Farahani S, Khodadadi R, Tagavi S, Naserzadeh P. New mechanistic approach of inorganic palladium toxicity: impairment in mitochondrial electron transfer. Metallomics 2016; 8:252-9. doi: 10.1039/c5mt00249d [Crossref] [ Google Scholar]
- Dehghani R, Khamechian T, Vazirianzadeh B, Vatandoost H, Moravvej S. Toxic effects of scorpion, Hemiscorpius lepturus (Hemiscorpiidae) venom on mice. J Anim Plant Sci 2012; 22:593-6. doi: 10.1186/s40409-018-0145-z [Crossref] [ Google Scholar]
- Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol 2008; 48:463-93. doi: 10.1146/annurev.pharmtox.48.113006.094615 [Crossref] [ Google Scholar]
- Rodríguez-Hernández Á, Cordero MD, Salviati L, Artuch R, Pineda M, Briones P. Coenzyme Q deficiency triggers mitochondria degradation by mitophagy. Autophagy 2009; 5:19-32. doi: 10.4161/auto.5.1.7174 [Crossref] [ Google Scholar]
- Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med 2016; 48:e245. doi: 10.1038/emm.2016.81 [Crossref] [ Google Scholar]
- Li Y, Ye Z, Lai W, Rao J, Huang W, Zhang X. Activation of sirtuin 3 by silybin attenuates mitochondrial dysfunction in cisplatin-induced acute kidney injury. Front Pharmacol 2017; 8:178. doi: 10.3389/fphar.2017.00178 [Crossref] [ Google Scholar]