Bioimpacts. 13(3):219-228.
doi: 10.34172/bi.2023.23585
Original Article
Artesunate reduces sepsis-mediated acute lung injury in a SIRT1-dependent manner
Zhaohui Liu Conceptualization, Resources, Supervision, 1, * 
Yanli Meng Data curation, Methodology, Project administration, 2
Yu Miao Funding acquisition, Writing – original draft, 3
Lili Yu Formal analysis, Writing – review & editing, 1
Qiannan Yu Investigation, Visualization, 1
Author information:
1Department of Anesthesiology, Cangzhou Central Hospital, Cangzhou, Hebei, China
2Department of Gastroenterology, Cangzhou Central Hospital, Cangzhou, Hebei, China
3Department of Neurosurgery, Cangzhou Central Hospital, Cangzhou, Hebei, China
Abstract
Introduction:
Sepsis-mediated acute lung injury (ALI) is a critical clinical condition. Artesunate (AS) is a sesquiterpene lactone endoperoxide that was discovered in Artemisia annua, which is a traditional Chinese herb. AS has a broad set of biological and pharmacological actions; however, its protective effect on lipopolysaccharide (LPS)-induced ALI remains unclear.
Methods:
LPS-mediated ALI was induced in rats through bronchial LPS inhalation. Then NR8383 cells were treated with LPS to establish an in vitro model. Further, we administered different AS doses in vivo and in vitro.
Results:
AS administration significantly decreased LPS-mediated pulmonary cell death and inhibited pulmonary neutrophil infiltration. Additionally, AS administration increased SIRT1 expression in pulmonary sections. Administration of a biological antagonist or shRNA-induced reduction of SIRT1 expression significantly inhibited the protective effect of AS against LPS-induced cellular injury, pulmonary dysfunction, neutrophil infiltration, and apoptosis. This demonstrates that enhanced SIRT1 expression is crucially involved in the observed protective effects.
Conclusion:
Our findings could suggest the use of AS for treating lung disorders through a mechanism involving SIRT1 expression.
Keywords: Artesunate, SIRT1, Lipopolysaccharide, Acute lung injury
Copyright and License Information
© 2023 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Acute lung injury (ALI) is a common sepsis-related occurrence that is associated with a high mortality rate.
1,2
Lipopolysaccharide (LPS)-induced ALI can progress to multi-organ dysfunction in various conditions, including pneumonia, sepsis, acute pancreatitis, trauma, near-drowning, and aspiration of gastric contents.
1,3
Although there are several risk factors for LPS-mediated ALI, the underlying pathogenesis remains unclear. Studies have identified numerous drugs for treating LPS-induced ALI, including phencyclidine hydrochloride, 3-amino-2-hydroxy-4-phenyl-valyl-isoleucine, and propofol
3-5
; however, the evidence remains inadequate. Moreover, few therapeutic strategies have applied native pulmonary defenses. Therefore, there is a need to identify non-toxic treatment regimens for LPS-mediated ALI.
SIRT1 is the most extensively studied member of the sirtuin family that are mammalian class III histone deacetylases. SIRT1 exerts anti-inflammatory, anti-oxidative, and anti-apoptotic effects, as well as modulating mitochondrial autophagy.
6-9
SIRT1 can control several biological processes, including glucose homeostasis, DNA transcription, immune reactions, and cellular stress reactions, by deacetylating several elements.
10-13
The target proteins of SIRT1-induced deacetylation affect cellular signals, critically involved in biological and pathological mechanisms,
8,10,12
SIRT1 has been shown to exert pulmonary protective effects in several lung disorders. SIRT1 protects against lung fibrosis by regulating P300 that is an important regulator of FOXO3a acetylation.
14,15
Wu and Wang reported that SIRT1 protects the lungs from barrier function impairment induced by oxidative stress by controlling inflammation.
16
In one study, SIRT1 inhibited oxidative stress-mediated apoptosis of human alveolar epithelial cells by decreasing the expression of nuclear factor (NF)-κB.
17
SIRT1 suppresses LPS-mediated apoptosis in human alveolar epithelial cells by activating AMP-activated protein kinase (AMPK) pathways.
18
Furthermore, it protects against LPS-mediated ALI by decreasing apoptosis and enhancing autophagy.
18,19
SIRT1 activation protects against LPS-mediated ALI by decreasing endothelial tight junction permeability, as well as suppressing NLRP3 and NF-κB signaling pathways.
20,21
Artesunate (AS) is a water-soluble artemisinin derivative with anti-inflammatory, anti-tumor, anti-apoptotic, and immunomodulatory effects.
22-24
AS represses the growth of prostate cancer cells by inhibiting androgen receptors and exerts potent antitumor effects on B-cell lymphoma.
24,25
Moreover, it can protect against LPS-mediated inflammatory reactions in microglia and exerts protective effects against hepatic fibrosis.
26,27
Zhao et al showed that AS administration protected against LPS-mediated ALI in rats by inhibiting TLR4 expression and activating the Nrf2 pathways.
28
Notably, the biological action of AS is closely associated with SIRT1 expression.
29,30
AS has several advantages, including low toxicity, which increases its utility in therapeutic regimens. However, the mechanisms underlying the treatment of LPS-mediated ALI using AS remain unclear.
We aimed to analyze the treatment effects of AS on LPS-mediated ALI and elucidate the pathogenesis underlying LPS-mediated ALI therapy. Our findings could inform therapeutic strategies for LPS-mediated ALI.
Materials and Methods
Materials
LPS (0111: B4, L2630) was obtained from Sigma (St. Louis, MO, USA). AS was purchased from Aladdin (Shanghai, China). Antibodies against myeloperoxidase (MPO, AF7494), SIRT1 (AF5300), Caspase-3 (AF1213), NLRP3 (AF2155), ASC (AF6234), and Caspase-1 (AF1681) were purchased from Beyotime (Shanghai, China). Horseradish peroxidase-conjugated secondary antibody (sc-2030), fluorescein-conjugated secondary antibody (sc-2012), and rhodamine-conjugated secondary antibody (sc-2091) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). A one-step TUNEL apoptosis analysis kit was obtained from Beyotime (Shanghai, China).
Study design
Sprague Dawley rats (250–300 g) were obtained from the Hebei Medical University of Laboratory Animal Center and kept in an air-habituated room with 12-hour dark/light cycles. All experiments were performed following the National Institute of Health Guidelines for the Care and Use of Research Animals. The rats were fed with normal rodent food, housed in disinfected cages, and allowed access to water ad libitum. The experimental protocol was approved by the Medicine Animal Care and Use Committee of Cangzhou Central Hospital (20200304). The abilities of the rats to ambulate, feed, and drink water, as well as their overall appearance, were assessed after LPS administration. Moreover, they were examined thrice at 24-hour after AS administration.
We established a previously described model of LPS-mediated ALI.
4,28
Pentobarbital in phosphate-buffered saline (PBS) (1.5%, 40 mg/kg) was intraperitoneally injected into the rats. Subsequently, LPS (5 mg/kg; treatment groups) or PBS (control group) was injected into the trachea using a microsyringe, with their distribution in the lungs being ensured by vertically placing the rats for 1 minute. The rats were intraoperatively hydrated using warm saline; further, the rats were placed on a heating pad that maintained the body temperature at 37°C until they became conscious. Subsequently, the rats were sacrificed 24 hours after LPS injection.
One hour after LPS administration, the rats were intraperitoneally injected with AS (7.5, 15, 25 mg/kg). To inhibit SIRT1 expression, some rats were intravenously injected with Sirtinol at 2 hours before LPS administration, while the remaining rats received equal PBS volumes. For SIRT1 silencing using lentivirus (LV), we used a 31-gauge needle to inject 100 µL of ultracentrifugation-purified LV (LV-control or LV-shSIRT1 ≈ 3×107 TU) into the lower pole of the left lung that corresponded to the long axis, followed by careful detachment after 72 hours. Subsequently, the rats received LPS injections.
To evaluate the dosage-dependent effects of AS on LPS-mediated ALI, the rats were assigned to five groups: (1) Sham group; (2) LPS group; (3) LPS + AS7.5 group; (4) LPS + AS15 group; and (5) LPS + AS25 group. The Sham group received an equal volume of vehicle without LPS; the LPS group received an equal volume of vehicle with LPS; the LPS + AS7.5 group received AS injections (7.5 mg/kg) after LPS administration; the LPS + AS15 group received AS injections (15 mg/kg) after LPS administration; and the LPS + AS25 group received AS injections (25 mg/kg). At 24 hours after LPS injection, the rats were decapitated; moreover, serum samples were obtained for future analysis. The lungs were also obtained and washed using ice-cold saline for further examination.
To evaluate sirtinol-induced inhibition of SIRT1 expression, the rats were assigned to four groups: (1) LPS + Vehicle group; (2) LPS + Sirtinol group; (3) LPS + AS25 group; and (4) LPS + Sirtinol + AS25 group. All the groups received LPS injections, followed by injection of an equal vehicle volume (LPS + Vehicle group), 1 mg/kg sirtinol (LPS + Sirtinol group), 25 mg/kg AS (LPS + AS25 group), and 25 mg/kg AS with 1 mg/kg sirtinol (LPS + Sirtinol + AS25 group). Sirtinol and AS were administered as previously described. The rats were decapitated at 24 hours after LPS administration, followed by the collection of serum samples and the lungs for further analysis as aforementioned.
To evaluate SIRT1 silencing through shRNA-carrying LV, the rats were assigned to four groups: (1) LPS + LV-control group; (2) LPS + LV-shSIRT1 group; (3) LPS + AS25 group; and (4) LPS + LV-shSIRT1 + AS25 group. All groups received LPS injections. The LPS + LV-control group was pre-injected with an equal volume of vehicle. The LPS + LV-shSIRT1 group was treated with LV-carrying SIRT1 shRNA. The LPS + AS25 group was treated with LV-carrying scrambled shRNA and pre-injected with 25 mg/kg AS. The LPS + AS25 + LV-shSIRT1 group was treated with LV-carrying SIRT1 shRNA and post-injected with 25 mg/kg AS. LV and AS treatments were performed as previously described. The rats were decapitated at 24 hours after LPS administration, followed by the collection of serum samples and the lungs for further analysis as aforementioned.
Cell treatment
NR8383 cells were obtained from the Cell Bank of Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). NR8383 cells were grown in Dulbecco's Modified Eagle Medium and 10% fetal bovine serum at 37°C in a humidified incubator (5% CO2), followed by 24-hour incubation with LPS (500 ng/mL) with or without AS (5, 10, 20 μg/mL).
Wet/Dry ratio of the lungs
The lung wet/dry weight ratio, which is an indicator of pulmonary edema, was used to determine lung injury and pulmonary permeability. Immediately after removal, lung sections were weighed (wet weight); subsequently, they were dried using a 60°C oven for 48 hours and reweighed (dry weight). We measured the ratio of the lung weight before and after drying.
ELISA analysis
At 24 hours after LPS administration, lung homogenates were used to measure lung myeloperoxidase through an enzyme-linked immunosorbent assay (ELISA, Beyotime Institute of Biotechnology, China).
Lung cell morphology
For pathological examinations, lung sections were fixed using paraformaldehyde (4 %), embedded in paraffin, and stained using hematoxylin and eosin (H&E). Pulmonary damage was measured on a 0–4 scale based on the lung tissue proportion with alveolar and interstitial edema, inflammatory infiltration, and hemorrhage as follows: 0, no injury; 1, 25% injury; 2, 50% injury; 3, 75% injury; and 4, diffuse injury.
20
These measurements were performed by a blinded pathologist.
Immunohistochemistry and immunofluorescence analysis
For immunohistochemistry (IHC) analysis, lung tissue sections (4-µm thickness) were microwaved in 0.01 mol/L sodium citrate (pH 6.0) thrice for 5 minutes each, followed by incubation with the primary antibody (anti-MPO, 1:50). Next, the sections were washed and nurtured with a biotin-labeled secondary antibody at 37°C for 60 minutes. The color reaction was developed by incubation with 3,3′-diaminobenzidine tetrahydrochloride. The slides were counterstained with hematoxylin and covered with a coverslip. For immunofluorescence (IF) analysis, lung tissue sections were incubated with 0.2% Triton X-100 in PBS for 10 minutes, followed by 5% bovine serum albumin for 1 hour at room temperature. Next, the slides were incubated with anti-SIRT1 (1:100) and anti-caspase-3 (1:400) antibodies at 4°C overnight. After washing, the slides were incubated with FITC-labeled secondary antibody at 37°C for 60 minutes, followed by counterstaining with DAPI. Pictures (at 400× magnification) were taken using a digital camera (Olympus, Tokyo, Japan) and evaluated using Image-Pro Plus 6.0 (Media Cybernetics, Rockville, MD, USA). Findings were described as the number of positive cells/total number of cells.
TUNEL analysis
Apoptosis was evaluated using terminal deoxynucleotidyl transferase-mediated digoxigenin deoxyuridine nick-end labeling (TUNEL) analysis (Beyotime, Shanghai, China) as per the manufacturer’s instructions. Briefly, lung sections were treated in equilibration buffer for 5 minutes, followed by treatment with known response drugs at 37°C for 1 hour and examination for DNA damage in apoptotic cells. We evaluated positive cellular staining using five randomly designated fields (at 400× magnification); additionally, we used the proportion of positive cells in high-power fields as the apoptosis indicator.
Real-time polymerase chain reaction analysis
Regarding RNA separation and real-time polymerase chain reaction (PCR) assays, we separated total RNA from rat tissues using TRIzol (Sangon Biotech, Shanghai, China). We produced high-fidelity cDNA from each RNA sample using the Superscript III cDNA amplification system (Promega, USA). We prepared real-time PCR samples as a mixture using the Quantitect SYBR Green PCR kit (Toyobo, Osaka, Japan), following the manufacturer’s instructions. Reactions were completed using an Applied Biosystems Prism 9700 PCR machine. We used the following PCR conditions: 95°C for 30 seconds followed by 45 cycles of 95°C for 15 seconds, 55°C for 30 seconds, and 72°C for 30 seconds. We applied the following real-time PCR primers: SIRT1 5′-TGTGTCATAGGTTAGGTGGTGA-3′ (forward), SIRT1 5′-AGCCAATTCTTTTTGTGTTCGTG-3′ (reverse). The primer and cDNA concentrations for PCR were 0.5 μM and 20 ng/µL, respectively.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 8.0 software (San Diego, CA, USA). The results are expressed as mean ± standard deviation (SD). Pairwise evaluations were performed using Student’s t-test (two-tailed). Among-group comparisons were performed using a one-way analysis of variance with Bonferroni’s post hoc test. Statistical significance was set at P < 0.05.
Results
The role of AS, sirtinol, and SIRT1 knockdown on pulmonary function and histology after LPS-induced ALI
To explore the protective effects of AS against LPS-mediated ALI, lung tissues underwent H&E staining and IHC analysis. The LPS group showed the disappearance of normal alveolar structure, alveolar septal thickening, alveolar hemorrhage, and inflammatory cell infiltration. Post-injection with AS significantly decreased the lung injury score in both lungs compared with that in the LPS groups (Fig. 1A, P < 0.05). Pulmonary LPS damage increased MPO activity and W/D weight ratio in the LPS group (Fig. 1B, C, P < 0.05), which were significantly decreased by AS post-injection in a dose-dependent manner. Compared with LPS + Vehicle rats, sirtinol-injected rats showed a non-significant increase in MPO activity, W/D weight ratio, and lung injury scores (Fig. 1D-F, P < 0.05). Further, sirtinol injection inhibited the AS-induced amelioration of MPO activity, W/D weight ratio, and lung injury scores (Fig. 1D-F, P < 0.05). Specifically, rats in the LPS + AS25 + sirtinol showed severe and extensive lung injury (Fig. 1D-F, P < 0.05). There were significant differences in MPO activity, W/D weight ratio, and lung injury scores between the LPS + LV-Control and LPS + AS25 groups by H&E staining and IHC analysis (Fig. 1G-I, P < 0.05). Notably, compared with the LPS+ AS25 group, the LPS + AS25+ LV-shSIRT1 groups significantly increased MPO activity, W/D weight ratio, and lung injury scores (Fig. 1G-I, P < 0.05). This suggests that the protective effects of AS against LPS-induced ALI are dependent on SIRT1 expression.
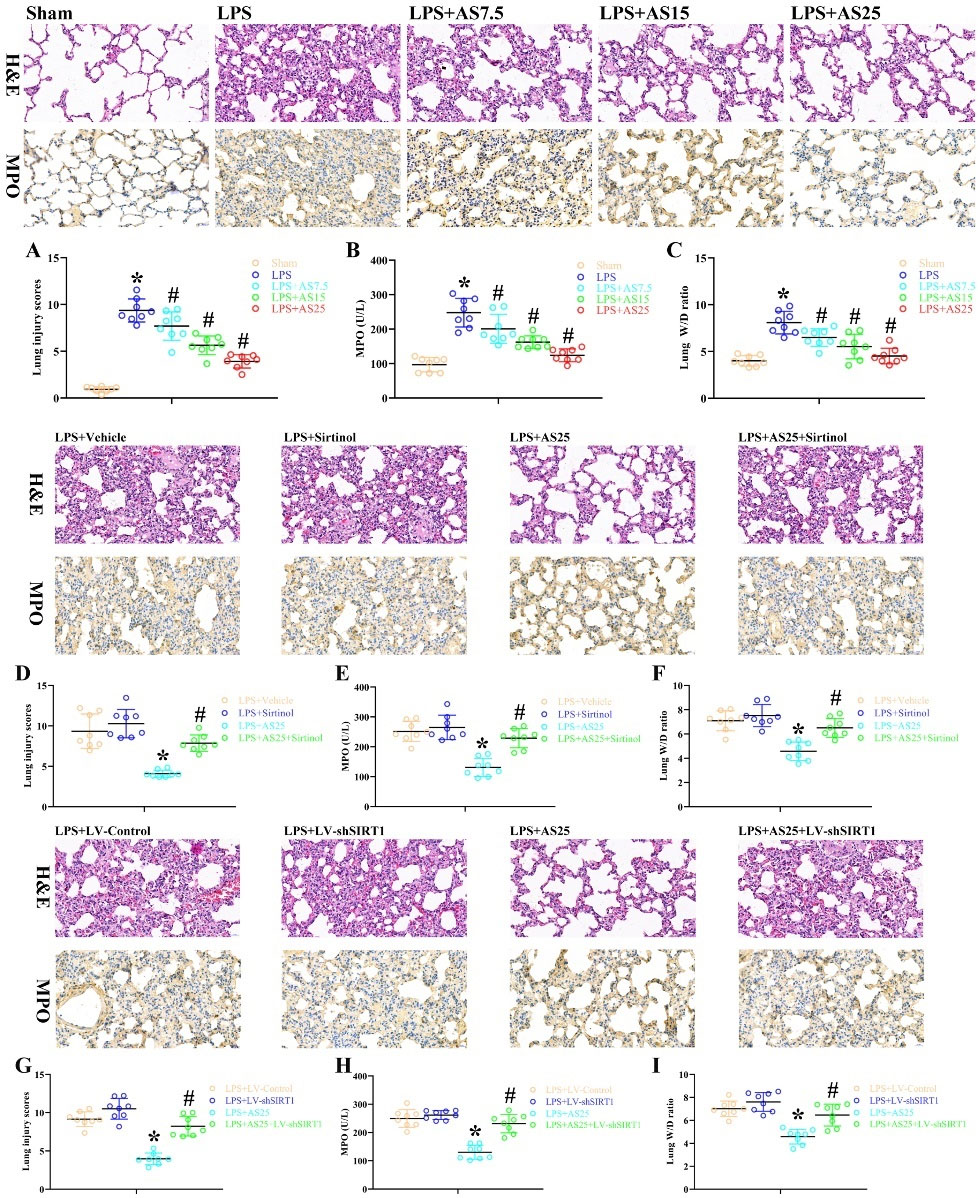

Fig. 1.
The role of AS, sirtinol, and SIRT1 knockdown on pulmonary function and histology after LPS-induced ALI.
Rats were treated with equal volumes of PBS or LPS (5 mg/kg). Subsequently, the rats were post-injected with 7.5, 15, and 25 mg/kg AS at 1 hour after LPS. The LPS + Sirtinol group was pre-injected with 1 mg/kg sirtinol, the LPS + AS25 group was pre-injected with 25 mg/kg AS, and the LPS + AS25 + Sirtinol group was injected with 25 mg/kg AS and 1 mg/kg sirtinol. In the LPS + LV-shSIRT1 group, the rats were treated using LV carrying SIRT1 shRNA. In the LPS + AS25 + LV-shSIRT1 group, the rats were treated using LV carrying SIRT1 shRNA and were pre-injected with 25 mg/kg AS. The rats were sacrificed at 24 hours after LPS administration. Example images of lung tissues stained with H&E and IHC for MPO (original magnification, 400×). A blinded pathologist evaluated the lesion based on the lung injury scores (A, D, G). MPO activity (B, E, H) and the W/D weight ratio (C, F, I) were examined 24 hours after operation. The results are presented as the mean ± SD; n = 8; *P < 0.05 vs. Sham/LPS+Vehicle/LPS+LV-Control; #P < 0.05 vs. LPS/LPS+AS25.
.
The role of AS, sirtinol, and SIRT1 knockdown on pulmonary function and histology after LPS-induced ALI.
Rats were treated with equal volumes of PBS or LPS (5 mg/kg). Subsequently, the rats were post-injected with 7.5, 15, and 25 mg/kg AS at 1 hour after LPS. The LPS + Sirtinol group was pre-injected with 1 mg/kg sirtinol, the LPS + AS25 group was pre-injected with 25 mg/kg AS, and the LPS + AS25 + Sirtinol group was injected with 25 mg/kg AS and 1 mg/kg sirtinol. In the LPS + LV-shSIRT1 group, the rats were treated using LV carrying SIRT1 shRNA. In the LPS + AS25 + LV-shSIRT1 group, the rats were treated using LV carrying SIRT1 shRNA and were pre-injected with 25 mg/kg AS. The rats were sacrificed at 24 hours after LPS administration. Example images of lung tissues stained with H&E and IHC for MPO (original magnification, 400×). A blinded pathologist evaluated the lesion based on the lung injury scores (A, D, G). MPO activity (B, E, H) and the W/D weight ratio (C, F, I) were examined 24 hours after operation. The results are presented as the mean ± SD; n = 8; *P < 0.05 vs. Sham/LPS+Vehicle/LPS+LV-Control; #P < 0.05 vs. LPS/LPS+AS25.
The role of AS and SIRT1 depletion on SIRT1 expression after LPS-induced ALI
There were no significant differences in SIRT1 protein and gene expression between the LPS and Sham groups by IF analysis and RT-PCR (Fig. 2A, B). Notably, compared with the LPS group, the LPS + AS15 and LPS + AS25 groups significantly increased SIRT1 protein and gene expression (Fig. 2A, B, P < 0.05). These findings suggest that SIRT1 may promote the protective effects of AS on LPS-induced ALI. There were significant differences in SIRT1 protein between the LPS + LV-Control and LPS + AS25 groups by IF analysis (Fig. 2C, P < 0.05). Notably, compared with the LPS+ AS25 group, the LPS + AS25+ LV-shSIRT1 groups significantly decreased SIRT1 protein (Fig. 2C, P < 0.05). This suggests that the protective effects of AS against LPS-induced ALI are dependent on SIRT1 expression.
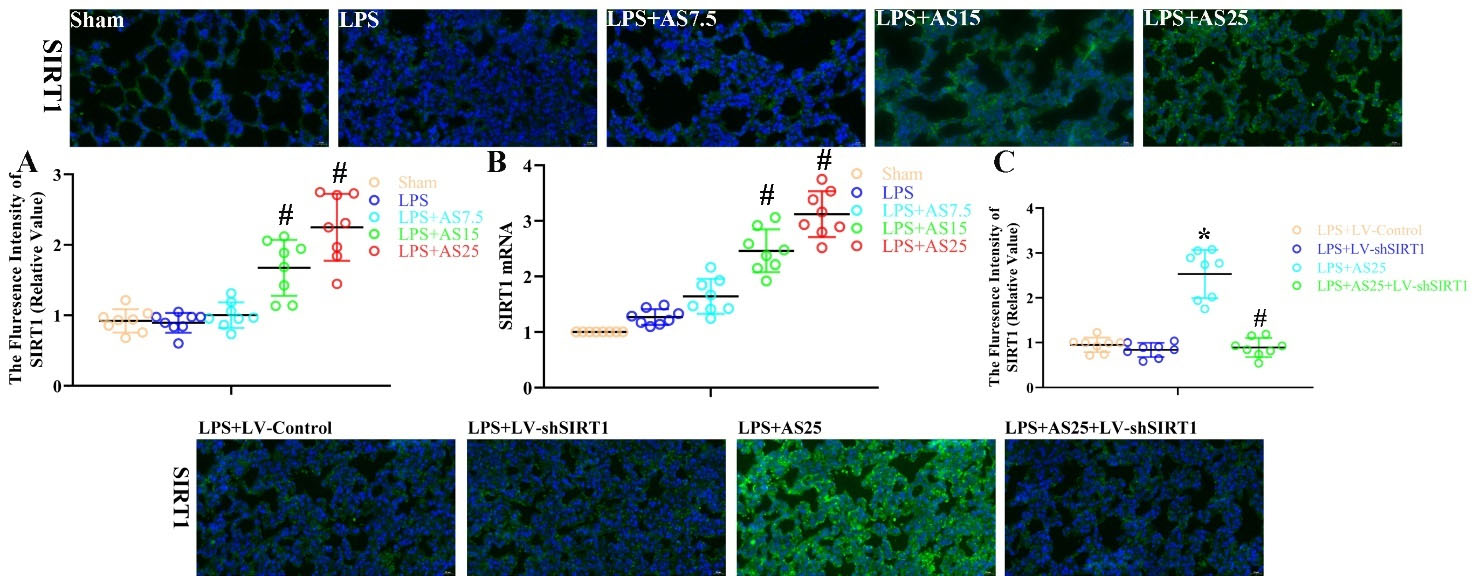
Fig. 2.
The role of AS and SIRT1 depletion on SIRT1 expression after LPS-induced ALI.
Rats were treated with equal volumes of PBS or LPS (5 mg/kg). Subsequently, the rats were post-injected with 7.5, 15, and 25 mg/kg AS at 1 hour after LPS. In the LPS + LV-shSIRT1 group, the rats were treated using LV carrying SIRT1 shRNA. In the LPS + AS25 + LV-shSIRT1 group, the rats were treated using LV carrying SIRT1 shRNA and were pre-injected with 25 mg/kg AS. Next, the rats were sacrificed at 24 hours after LPS administration. Example pictures of SIRT1 expression (A, C) in the lungs as evaluated by IF (original magnification, 400×). Real-time PCR for SIRT1 mRNA expression (B) in the lungs. SIRT1 gene expression for definite mRNA was standardized to β-actin levels. Results are presented as the mean ± SD; n = 8; *P < 0.05 vs. LPS+LV-Control; #P < 0.05 vs. LPS/LPS+AS25.
.
The role of AS and SIRT1 depletion on SIRT1 expression after LPS-induced ALI.
Rats were treated with equal volumes of PBS or LPS (5 mg/kg). Subsequently, the rats were post-injected with 7.5, 15, and 25 mg/kg AS at 1 hour after LPS. In the LPS + LV-shSIRT1 group, the rats were treated using LV carrying SIRT1 shRNA. In the LPS + AS25 + LV-shSIRT1 group, the rats were treated using LV carrying SIRT1 shRNA and were pre-injected with 25 mg/kg AS. Next, the rats were sacrificed at 24 hours after LPS administration. Example pictures of SIRT1 expression (A, C) in the lungs as evaluated by IF (original magnification, 400×). Real-time PCR for SIRT1 mRNA expression (B) in the lungs. SIRT1 gene expression for definite mRNA was standardized to β-actin levels. Results are presented as the mean ± SD; n = 8; *P < 0.05 vs. LPS+LV-Control; #P < 0.05 vs. LPS/LPS+AS25.
The role of AS, sirtinol, and SIRT1 knockdown in apoptosis after LPS-induced ALI
Apoptosis is crucially involved in the mechanism underlying LPS-mediated ALI. As shown in Fig. 3A, B, rats with LPS-induced ALI showed an increased number of TUNEL-positive cells and cleaved caspase-3 levels in the lungs. Compared with the LPS group, the LPS + AS7.5, LPS + AS15, and LPS + AS25 groups showed a significantly decreased number of apoptotic cells and cleaved caspase-3 levels (Fig. 3A, B, P < 0.05). Compared with LPS + Vehicle rats, sirtinol-injected rats showed a non-significant increase in the number of apoptotic cells and cleaved caspase-3 levels (Fig. 3C, D, P < 0.05). Further, sirtinol injection inhibited the AS-induced amelioration of the number of apoptotic cells and cleaved caspase-3 levels (Fig. 3C, D, P < 0.05). There were significant differences in the number of apoptotic cells and cleaved caspase-3 levels between the LPS + LV-Control and LPS + AS25 groups by IF analysis (Fig. 3E, F, P < 0.05). Notably, compared with the LPS+ AS25 group, the LPS + AS25+ LV-shSIRT1 groups significantly increased the number of apoptotic cells and cleaved caspase-3 levels (Fig. 3E, F, P < 0.05). This suggests that the protective effects of AS against LPSinduced ALI are dependent on SIRT1 expression. Taken together, AS and SIRT1 have extensive effects on several biological mechanisms and may promote amelioration of LPS damage (Fig. 4).
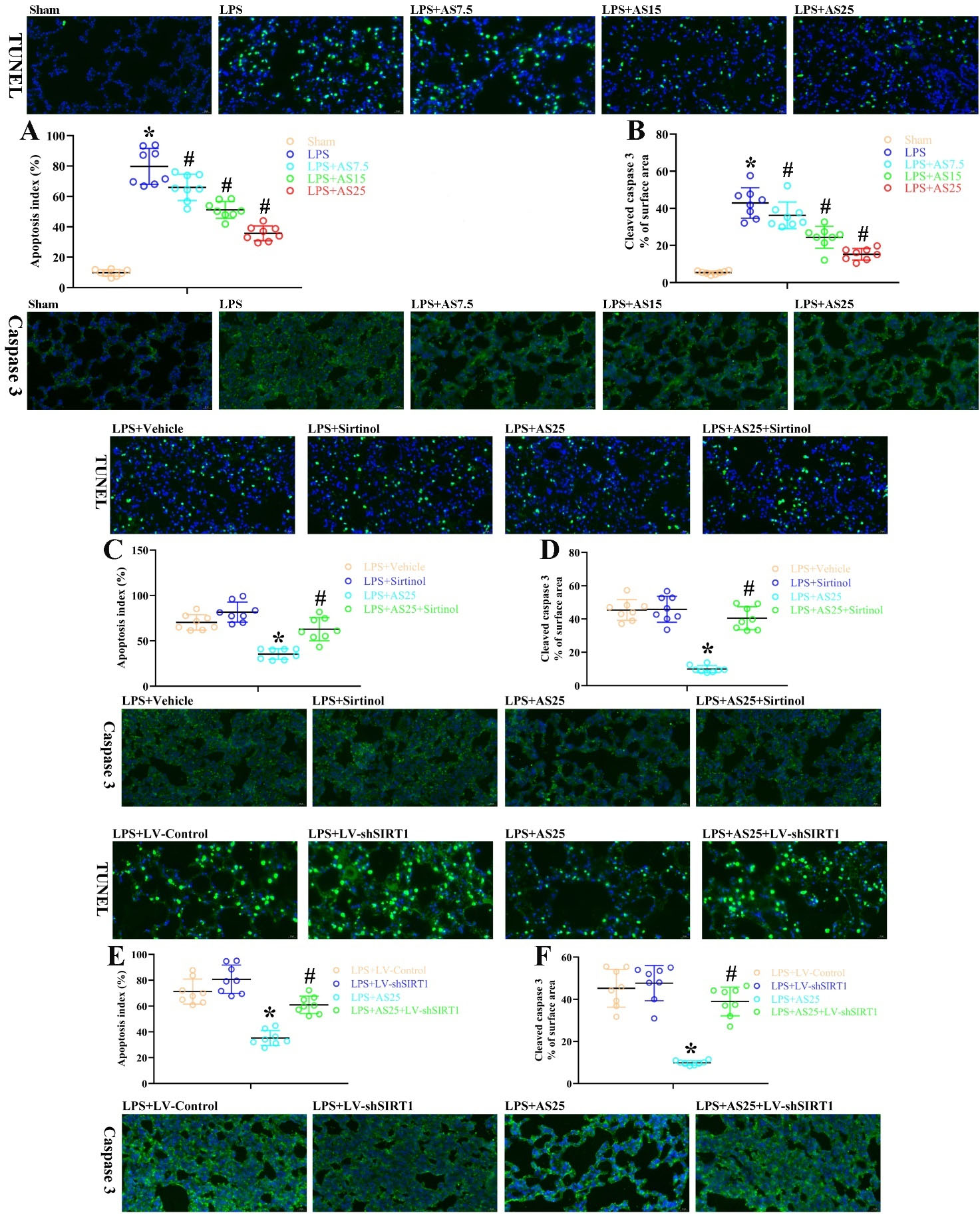

Fig. 3.
The role of AS, sirtinol, and SIRT1 knockdown in apoptosis after LPS-induced ALI.
Rats were treated with equal volumes of PBS or LPS (5 mg/kg). Subsequently, the rats were post-injected with 7.5, 15, and 25 mg/kg AS at 1 hour after LPS. The LPS + Sirtinol group was pre-injected with 1 mg/kg Sirtinol, the LPS + AS25 group was pre-injected with 25 mg/kg AS, and the LPS + AS25 + Sirtinol group was injected with 25 mg/kg AS and 1 mg/kg Sirtinol. In the LPS + LV-shSIRT1 group, the rats were treated using LV carrying SIRT1 shRNA. In the LPS + AS25 + LV-shSIRT1 group, the rats were treated using LV carrying SIRT1 shRNA and were pre-injected with 25 mg/kg AS. The rats were sacrificed at 24 hours after LPS. (A, C, E) An example photomicrograph of TUNEL-positive cells obtained from all groups as well as the proportions of positive cells. (B, D, F) An example photomicrograph of Caspase-3-stained lung sections obtained from all groups as well as the proportion of positive cells. Original magnification, 400×. The results are presented as the mean ± SD; n = 8; *P < 0.05 vs. Sham/LPS+Vehicle/LPS+LV-Control; #P < 0.05 vs. LPS/LPS+AS25.
.
The role of AS, sirtinol, and SIRT1 knockdown in apoptosis after LPS-induced ALI.
Rats were treated with equal volumes of PBS or LPS (5 mg/kg). Subsequently, the rats were post-injected with 7.5, 15, and 25 mg/kg AS at 1 hour after LPS. The LPS + Sirtinol group was pre-injected with 1 mg/kg Sirtinol, the LPS + AS25 group was pre-injected with 25 mg/kg AS, and the LPS + AS25 + Sirtinol group was injected with 25 mg/kg AS and 1 mg/kg Sirtinol. In the LPS + LV-shSIRT1 group, the rats were treated using LV carrying SIRT1 shRNA. In the LPS + AS25 + LV-shSIRT1 group, the rats were treated using LV carrying SIRT1 shRNA and were pre-injected with 25 mg/kg AS. The rats were sacrificed at 24 hours after LPS. (A, C, E) An example photomicrograph of TUNEL-positive cells obtained from all groups as well as the proportions of positive cells. (B, D, F) An example photomicrograph of Caspase-3-stained lung sections obtained from all groups as well as the proportion of positive cells. Original magnification, 400×. The results are presented as the mean ± SD; n = 8; *P < 0.05 vs. Sham/LPS+Vehicle/LPS+LV-Control; #P < 0.05 vs. LPS/LPS+AS25.
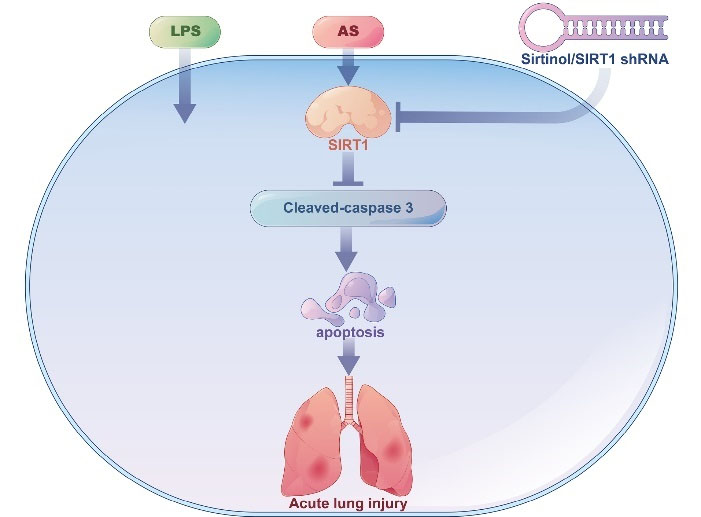
Fig. 4.
Anticipated outline of SIRT1 and other mediators of the protective effects of artesunate (AS) against LPS-mediated ALI. AS administration
significantly attenuated LPS-mediated cellular injury, lung dysfunction, and apoptosis. Consistent with the protective effects of AS on LPS-mediated ALI, AS treatment increased SIRT1 expression and diminished Caspase-3 activity. Biological antagonism or shRNA-induced SIRT1 inhibition significantly abolished the protective effects of AS on LPS-induced cellular injury, lung dysfunction, and apoptosis.
.
Anticipated outline of SIRT1 and other mediators of the protective effects of artesunate (AS) against LPS-mediated ALI. AS administration
significantly attenuated LPS-mediated cellular injury, lung dysfunction, and apoptosis. Consistent with the protective effects of AS on LPS-mediated ALI, AS treatment increased SIRT1 expression and diminished Caspase-3 activity. Biological antagonism or shRNA-induced SIRT1 inhibition significantly abolished the protective effects of AS on LPS-induced cellular injury, lung dysfunction, and apoptosis.
AS ameliorates LPS-mediated release of pyroptosis-related proteins and inflammatory cytokines in rats
We examined the effects of AS on LPS-mediated release of pyroptosis-related proteins by examining the protein levels of cleaved caspase-1, ASC, and NLRP3 in rats. IF analysis revealed that LPS increased the fluorescence intensity of NLRP3, ASC, and caspase-1, which was decreased by AS in a dose-dependent manner (P < 0.05, Fig. 5A–C). AS-induced inactivation of pyroptosis-related inflammatory cytokines inhibited IL-18 and IL-1β secretion. At 24 hours after LPS injection, there were increased inactivated IL-1β levels, which were significantly decreased by the three AS doses in a dose-dependent manner (P < 0.05, Fig. 5D and 5E). These findings demonstrate that the AS-induced inactivation of pyroptosis-related proteins and inflammatory cytokines results in effective anti-inflammatory effects.
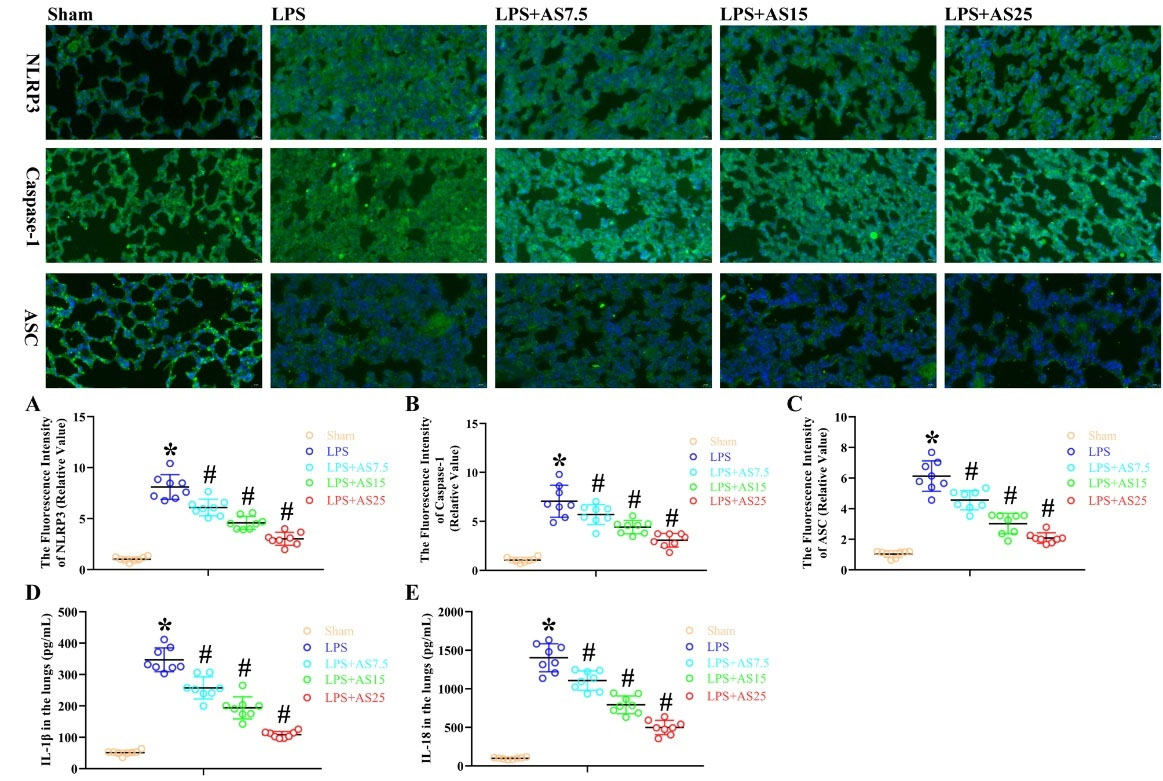
Fig. 5.
AS ameliorates LPS-mediated release of pyroptosis-related proteins and inflammatory cytokines in rats.
Rats were administered with LPS (5 mg/kg), followed by the administration of AS (7.5, 15, 25 mg/kg) after 24 hours. (A-C) Immunofluorescence analysis was used to measure the fluorescence intensity of NLRP3, Caspase-1, and ASC; (D, E) We used ELISA to measure IL-1β and IL-18 protein levels (*P < 0.05 vs. Sham; #P < 0.05 vs. LPS).
.
AS ameliorates LPS-mediated release of pyroptosis-related proteins and inflammatory cytokines in rats.
Rats were administered with LPS (5 mg/kg), followed by the administration of AS (7.5, 15, 25 mg/kg) after 24 hours. (A-C) Immunofluorescence analysis was used to measure the fluorescence intensity of NLRP3, Caspase-1, and ASC; (D, E) We used ELISA to measure IL-1β and IL-18 protein levels (*P < 0.05 vs. Sham; #P < 0.05 vs. LPS).
AS ameliorates LPS-mediated release of pyroptosis- and apoptosis-related proteins in NR8383 cells
We examined the effects of AS on LPS-mediated activation of pyroptosis- and apoptosis-related proteins by measuring the protein levels of NLRP3, Caspase-1, ASC, and caspase-3 in NR8383 cells. LPS increased the fluorescence intensity of NLRP3, caspase-1, ASC, and caspase-3 protein in NR8383 cells, which were significantly decreased by the three AS doses in a dose-dependent manner (P < 0.05, Figs. 6–9). Taken together, these findings suggested that AS-induced inactivation of pyroptosis- and apoptosis-related proteins could exert anti-inflammatory effects in NR8383 cells.
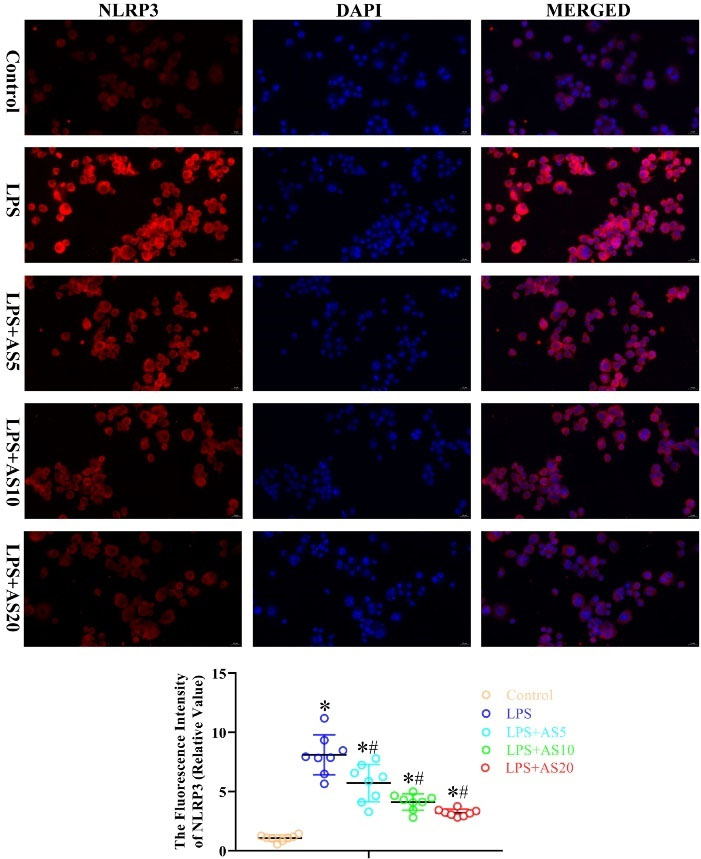
Fig. 6.
AS ameliorates LPS-mediated production of pyroptosis-related protein NLRP3 in NR8383 cells.
NR8383 cells were incubated with LPS (500 ng/mL) with or without AS (5, 10, and 20 μg/mL) for 24 hours. Immunofluorescence analysis was used to measure the fluorescence intensity of NLRP3 (*P < 0.05 vs. Control; #P < 0.05 vs. LPS).
.
AS ameliorates LPS-mediated production of pyroptosis-related protein NLRP3 in NR8383 cells.
NR8383 cells were incubated with LPS (500 ng/mL) with or without AS (5, 10, and 20 μg/mL) for 24 hours. Immunofluorescence analysis was used to measure the fluorescence intensity of NLRP3 (*P < 0.05 vs. Control; #P < 0.05 vs. LPS).
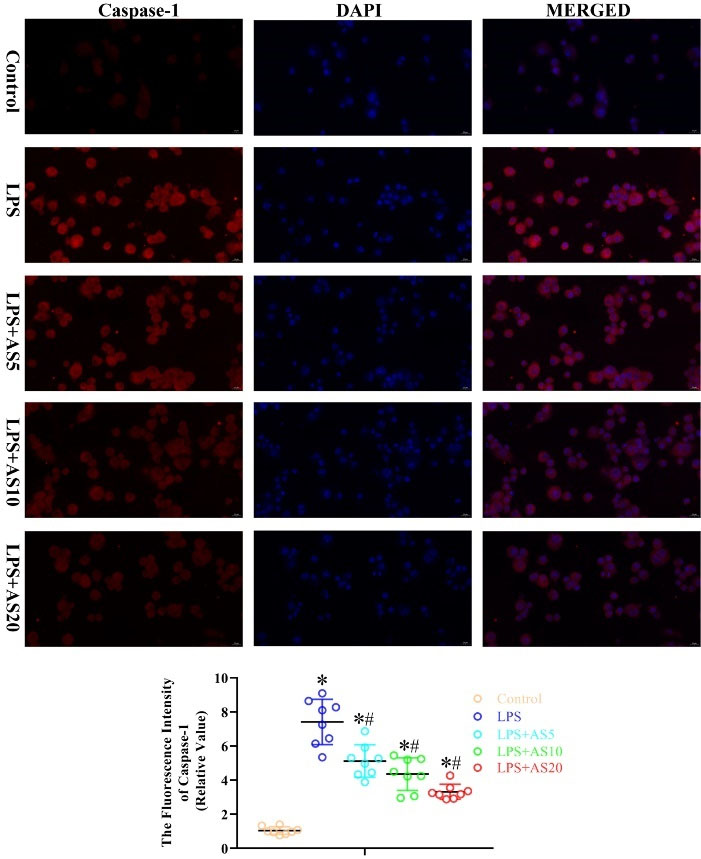
Fig. 7.
AS ameliorates LPS-mediated production of pyroptosis-related protein caspase-1 in NR8383 cells.
NR8383 cells were incubated with LPS (500 ng/mL) with or without AS (5, 10, and 20 μg/mL) for 24 hours. Immunofluorescence analysis was used to measure the fluorescence intensity of Caspase-1 (*P < 0.05 vs. Control; #P < 0.05 vs. LPS).
.
AS ameliorates LPS-mediated production of pyroptosis-related protein caspase-1 in NR8383 cells.
NR8383 cells were incubated with LPS (500 ng/mL) with or without AS (5, 10, and 20 μg/mL) for 24 hours. Immunofluorescence analysis was used to measure the fluorescence intensity of Caspase-1 (*P < 0.05 vs. Control; #P < 0.05 vs. LPS).
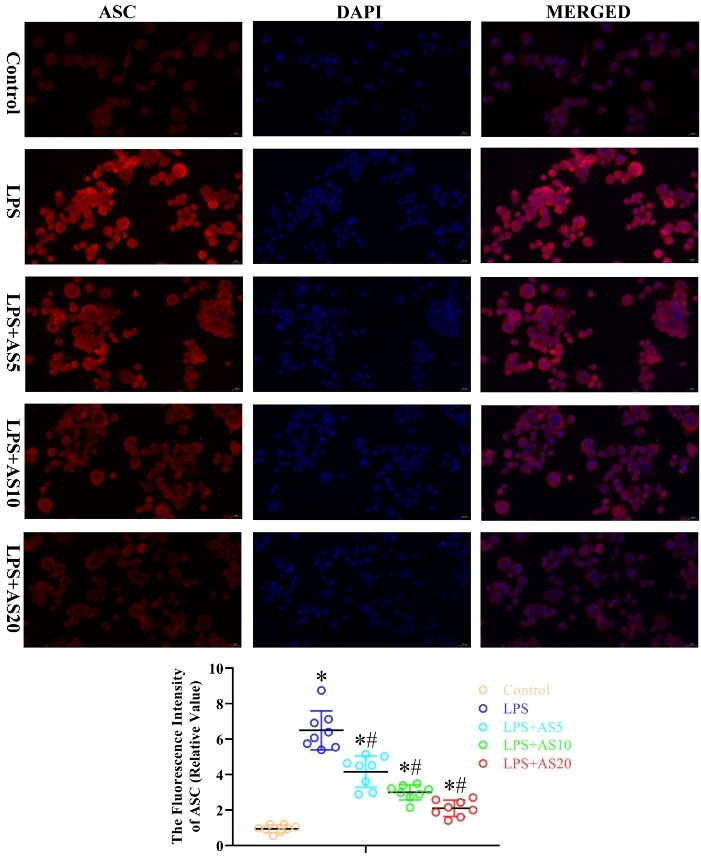
Fig. 8.
AS ameliorates LPS-mediated production of pyroptosis-related protein ASC in NR8383 cells.
NR8383 cells were incubated with LPS (500 ng/mL) with or without AS (5, 10, and 20 μg/mL) for 24 hours. Immunofluorescence analysis was used to measure the fluorescence intensity of ASC (*P < 0.05 vs. Control; #P < 0.05 vs. LPS).
.
AS ameliorates LPS-mediated production of pyroptosis-related protein ASC in NR8383 cells.
NR8383 cells were incubated with LPS (500 ng/mL) with or without AS (5, 10, and 20 μg/mL) for 24 hours. Immunofluorescence analysis was used to measure the fluorescence intensity of ASC (*P < 0.05 vs. Control; #P < 0.05 vs. LPS).
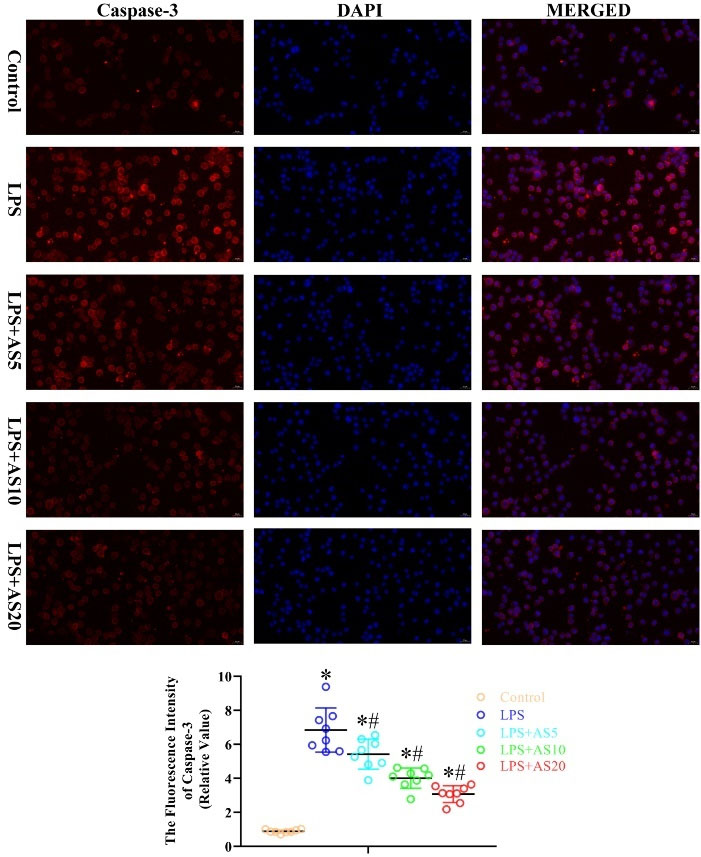
Fig. 9.
AS ameliorates LPS-mediated production of apoptosis-related protein caspase-3 in NR8383 cells.
NR8383 cells were incubated with LPS (500 ng/mL) with or without AS (5, 10, and 20 μg/mL) for 24 hours. Immunofluorescence analysis was used to measure the fluorescence intensity of Caspase-3 (*P < 0.05 vs. Control; #P < 0.05 vs. LPS).
.
AS ameliorates LPS-mediated production of apoptosis-related protein caspase-3 in NR8383 cells.
NR8383 cells were incubated with LPS (500 ng/mL) with or without AS (5, 10, and 20 μg/mL) for 24 hours. Immunofluorescence analysis was used to measure the fluorescence intensity of Caspase-3 (*P < 0.05 vs. Control; #P < 0.05 vs. LPS).
Discussion
Sepsis is a common cause of ALI, which is characterized by reduced respiratory function.
31
Although there are various therapeutic strategies, including lung replacement treatment, ALI is associated with a very high mortality rate.
32
Therefore, LPS-induced lung injury may have multifactorial pathogenesis, including free radical injury, inflammatory reactions, and hypoxia. There remains no effective clinical therapy for LPS-mediated ALI and further research is warranted. Suppressing cell death and the apoptotic pathway is a therapeutic strategy for rats with LPS-induced injury. AS is a traditional Chinese herb derived from Artemisia annua. It is a sesquiterpene lactone endoperoxide whose molecular activity has been extensively studied in tumors, inflammatory responses, and apoptosis.
25,29
Recent studies have shown that AS exerts neuroprotective effects against sepsis.
26
SIRT1 is associated with extensive cellular processes. Furthermore, SIRT1 stimulation shows age-related protective effects against LPS-mediated ALI.
20
Contrastingly, SIRT1 mRNA depletion significantly increases the vulnerability to LPS-mediated ALI. Additionally, lung-specific SIRT1 upregulation could protect against ALI. We found that AS administration (7.5, 15, and 25 mg/kg) significantly improved pulmonary function and pathology after LPS-induced ALI. Additionally, preconditioning with 25 mg/kg AS significantly improved pulmonary function and pathology at 24 hours after LPS-induced ALI. Our findings strongly demonstrated the treatment effects of AS against LPS-mediated ALI. Additionally, our findings demonstrated that AS-mediated antiapoptosis activity is crucially involved in the protection against sepsis. Taken together, AS and SIRT1 have extensive effects on several biological mechanisms and may promote amelioration of LPS damage (Fig. 4).
AS increases SIRT1 expression; however, the underlying mechanisms remain unclear. Furthermore, AS increases AMPK levels, which could in turn increase SIRT1 levels. However, AS inhibits SIRT1 expression in cancer cells. AS-mediated regulation of inherent mRNA levels could result in cellular changes. Notably, we observed that AS administration significantly increased SIRT1 expression at 24 hours after LPS damage; moreover, the suppression of SIRT1 expression inhibited the protective effects of AS against LPS-mediated ALI. AS administration significantly decreased apoptosis and increased the cellular proliferation rate, which further confirms the protective properties of SIRT1. Taken together, we found that AS may exert its protective effects against LPS-induced ALI by increasing SIRT1 levels. SIRT1 levels could be modulated in vivo and in vitro. Therefore, there is a need to elucidate the molecular pathogenesis underlying AS-mediated modulation of SIRT1 levels in pulmonary cells.
Furthermore, we found that AS protected against LPS-mediated ALI through a mechanism involving decreased apoptosis. Notably, increased SIRT1 expression was strongly associated with increased pulmonary function in AS-treated rats after LPS-induced ALI. Inhibition of SIRT1 expression abolished the pulmonary-protective effects of AS against LPS-induced ALI. Moreover, increased SIRT1 levels resulted in decreased apoptosis, which suggested that AS may enhance cell survival through the SIRT1 pathway to exert pulmonary-protective effects in LPS-mediated ALI (Fig. 2). AS has been shown to protect against metabolic disorders in a SIRT1-dependent manner and to modulate SIRT1 expression in pulmonary cells.
Research Highlights
What is the current knowledge?
√ AS is a sesquiterpene lactone endoperoxide with a broad set of biological and pharmacological properties.
What is new here?
√ AS reduces sepsis-mediated ALI in a SIRT1-dependent manner.
Conclusion
Collectively, our study established that AS exerts protective effects on LPS-induced ALI and that the protective role is largely dependent on apoptosis amelioration and regeneration elevation along with the enhanced expression of SIRT1, while amelioration of SIRT1 expression or activity reversed the protective role. It is likely that AS ameliorates LPS-induced ALI in a SIRT-reliant manner, indicating that AS is a drug for the treatment of lung disorders in the presence of SIRT1 expression. This requires further confirmation using in vitro and in vivo studies.
Competing Interests
The authors declare no competing interests related to the manuscript.
Ethical Statement
All animal experiments were reviewed and approved by the Ethics Committee of Cangzhou Central Hospital.
Funding
This study had no sources of funding.
References
- Mokra D, Kosutova P. Biomarkers in acute lung injury. Respir Physiol Neurobiol 2015; 209:52-8. doi: 10.1016/j.resp.2014.10.006 [Crossref] [ Google Scholar]
- Weng J, Chen M, Lin Q, Chen J, Wang S, Fang D. Penehyclidine hydrochloride protects against LPS-induced ALI in rats by mitigating endoplasmic reticulum stress and promoting the Hes1/Notch1 pathway. Gene 2019; 721:144095. doi: 10.1016/j.gene.2019.144095 [Crossref] [ Google Scholar]
- Ye S, Yang X, Wang Q, Chen Q, Ma Y. Penehyclidine hydrochloride alleviates lipopolysaccharide-induced acute lung injury by ameliorating apoptosis and endoplasmic reticulum stress. J Surg Res 2020; 245:344-53. doi: 10.1016/j.jss.2019.07.080 [Crossref] [ Google Scholar]
-
Wang B, Wang J, Lu D, Qi N, Liu Q. The protective action of LYRM03 on LPS-induced acute lung injury by NF-kappaB/TLR4/NLRP3 signals. J Invest Surg 2019: 1-13. 10.1080/08941939.2019.1634165.
- Wang X, Liu C, Wang G. Propofol protects rats and human alveolar epithelial cells against lipopolysaccharide-induced acute lung injury via inhibiting HMGB1 expression. Inflammation 2016; 39:1004-16. doi: 10.1007/s10753-016-0330-6 [Crossref] [ Google Scholar]
- Tang BL. Sirt1 and the mitochondria. Mol Cells 2016; 39:87-95. doi: 10.14348/molcells.2016.2318 [Crossref] [ Google Scholar]
- Zhang W, Huang Q, Zeng Z, Wu J, Zhang Y, Chen Z. Sirt1 inhibits oxidative stress in vascular endothelial cells. Oxid Med Cell Longev 2017; 2017:7543973. doi: 10.1155/2017/7543973 [Crossref] [ Google Scholar]
- Yang H, Bi Y, Xue L, Wang J, Lu Y, Zhang Z. Multifaceted modulation of SIRT1 in cancer and inflammation. Crit Rev Oncog 2015; 20:49-64. doi: 10.1615/critrevoncog.2014012374 [Crossref] [ Google Scholar]
- Luo G, Jian Z, Zhu Y, Zhu Y, Chen B, Ma R. Sirt1 promotes autophagy and inhibits apoptosis to protect cardiomyocytes from hypoxic stress. Int J Mol Med 2019; 43:2033-43. doi: 10.3892/ijmm.2019.4125 [Crossref] [ Google Scholar]
- García-Vizcaíno EM, Liarte S, Alonso-Romero JL, Nicolás FJ. Sirt1 interaction with active Smad2 modulates transforming growth factor-beta regulated transcription. Cell Commun Signal 2017; 15:50. doi: 10.1186/s12964-017-0205-y [Crossref] [ Google Scholar]
- Nguyen V, Tiemann D, Park E, Salehi A. Alpha-2 agonists. Anesthesiol Clin 2017; 35:233-45. doi: 10.1016/j.anclin.2017.01.009 [Crossref] [ Google Scholar]
- Li Y, Wang P, Yang X, Wang W, Zhang J, He Y. SIRT1 inhibits inflammatory response partly through regulation of NLRP3 inflammasome in vascular endothelial cells. Mol Immunol 2016; 77:148-56. doi: 10.1016/j.molimm.2016.07.018 [Crossref] [ Google Scholar]
- Carnevale I, Pellegrini L, D’Aquila P, Saladini S, Lococo E, Polletta L. SIRT1-SIRT3 axis regulates cellular response to oxidative stress and etoposide. J Cell Physiol 2017; 232:1835-44. doi: 10.1002/jcp.25711 [Crossref] [ Google Scholar]
- Jeung YJ, Kim HG, Ahn J, Lee HJ, Lee SB, Won M. Shikonin induces apoptosis of lung cancer cells via activation of FOXO3a/EGR1/SIRT1 signaling antagonized by p300. Biochim Biophys Acta 2016; 1863:2584-93. doi: 10.1016/j.bbamcr.2016.07.005 [Crossref] [ Google Scholar]
- Zeng Z, Cheng S, Chen H, Li Q, Hu Y, Wang Q. Activation and overexpression of Sirt1 attenuates lung fibrosis via P300. Biochem Biophys Res Commun 2017; 486:1021-6. doi: 10.1016/j.bbrc.2017.03.155 [Crossref] [ Google Scholar]
- Wu NC, Wang JJ. Niacin pretreatment attenuates lung ischemia and reperfusion-induced pulmonary barrier function impairment by reducing oxidative stress and activating SIRT1 in an isolated-perfused rat lung model. Transplant Proc 2018; 50:2834-8. doi: 10.1016/j.transproceed.2018.04.047 [Crossref] [ Google Scholar]
- Liu X, Yang T, Sun T, Shao K. SIRT1mediated regulation of oxidative stress induced by Pseudomonas aeruginosa lipopolysaccharides in human alveolar epithelial cells. Mol Med Rep 2017; 15:813-8. doi: 10.3892/mmr.2016.6045 [Crossref] [ Google Scholar]
- Li X, Jamal M, Guo P, Jin Z, Zheng F, Song X. Irisin alleviates pulmonary epithelial barrier dysfunction in sepsis-induced acute lung injury via activation of AMPK/SIRT1 pathways. Biomed Pharmacother 2019; 118:109363. doi: 10.1016/j.biopha.2019.109363 [Crossref] [ Google Scholar]
- Yang X, Jing T, Li Y, He Y, Zhang W, Wang B. Hydroxytyrosol attenuates LPS-induced acute lung injury in mice by regulating autophagy and sirtuin expression. Curr Mol Med 2017; 17:149-59. doi: 10.2174/1566524017666170421151940 [Crossref] [ Google Scholar]
- Fu C, Hao S, Xu X, Zhou J, Liu Z, Lu H. Activation of SIRT1 ameliorates LPS-induced lung injury in mice via decreasing endothelial tight junction permeability. Acta Pharmacol Sin 2019; 40:630-41. doi: 10.1038/s41401-018-0045-3 [Crossref] [ Google Scholar]
- Xu Z, Wang D, Zhou Z, Chen Q, Zhang D, Chen S. Dexmedetomidine attenuates renal and myocardial ischemia/reperfusion injury in a dose-dependent manner by inhibiting inflammatory response. Ann Clin Lab Sci 2019; 49:31-5. [ Google Scholar]
- Yuan Q, Xiao F, Liu Q, Zheng F, Shen S, He Q. M3 receptor is involved in the effect of penehyclidine hydrochloride reduced endothelial injury in LPS-stimulated human pulmonary microvascular endothelial cell. Pulm Pharmacol Ther 2018; 48:144-50. doi: 10.1016/j.pupt.2017.11.007 [Crossref] [ Google Scholar]
- Liu Z, Zhang J, Li S, Jiang J. Artesunate inhibits renal ischemia reperfusion-stimulated lung inflammation in rats by activating HO-1 pathway. Inflammation 2018; 41:114-21. doi: 10.1007/s10753-017-0669-3 [Crossref] [ Google Scholar]
- Wang Z, Wang C, Wu Z, Xue J, Shen B, Zuo W. Artesunate suppresses the growth of prostatic cancer cells through inhibiting androgen receptor. Biol Pharm Bull 2017; 40:479-85. doi: 10.1248/bpb.b16-00908 [Crossref] [ Google Scholar]
- Våtsveen TK, Myhre MR, Steen CB, Wälchli S, Lingjærde OC, Bai B. Artesunate shows potent anti-tumor activity in B-cell lymphoma. J Hematol Oncol 2018; 11:23. doi: 10.1186/s13045-018-0561-0 [Crossref] [ Google Scholar]
- Lee IS, Ryu DK, Lim J, Cho S, Kang BY, Choi HJ. Artesunate activates Nrf2 pathway-driven anti-inflammatory potential through ERK signaling in microglial BV2 cells. Neurosci Lett 2012; 509:17-21. doi: 10.1016/j.neulet.2011.12.034 [Crossref] [ Google Scholar]
- Lai L, Chen Y, Tian X, Li X, Zhang X, Lei J. Artesunate alleviates hepatic fibrosis induced by multiple pathogenic factors and inflammation through the inhibition of LPS/TLR4/NF-kappaB signaling pathway in rats. Eur J Pharmacol 2015; 765:234-41. doi: 10.1016/j.ejphar.2015.08.040 [Crossref] [ Google Scholar]
- Zhao D, Zhang J, Xu G, Wang Q. Artesunate protects LPS-induced acute lung injury by inhibiting TLR4 expression and inducing Nrf2 activation. Inflammation 2017; 40:798-805. doi: 10.1007/s10753-017-0524-6 [Crossref] [ Google Scholar]
- Chen X, Zhang XL, Zhang GH, Gao YF. Artesunate promotes Th1 differentiation from CD4+ T cells to enhance cell apoptosis in ovarian cancer via miR-142. Braz J Med Biol Res 2019; 52:e7992. doi: 10.1590/1414-431X20197992 [Crossref] [ Google Scholar]
- Guo Z, Yu S, Chen X, Ye R, Zhu W, Liu X. NLRP3 is involved in ischemia/reperfusion injury. CNS Neurol Disord Drug Targets 2016; 15:699-712. doi: 10.2174/1871527315666160321111829 [Crossref] [ Google Scholar]
- Aziz M, Ode Y, Zhou M, Ochani M, Holodick NE, Rothstein TL. B-1a cells protect mice from sepsis-induced acute lung injury. Mol Med 2018; 24:26. doi: 10.1186/s10020-018-0029-2 [Crossref] [ Google Scholar]
- Spadaro S, Park M, Turrini C, Tunstall T, Thwaites R, Mauri T. Biomarkers for acute respiratory distress syndrome and prospects for personalised medicine. J Inflamm (Lond) 2019; 16:1. doi: 10.1186/s12950-018-0202-y [Crossref] [ Google Scholar]