Bioimpacts. 12(5):395-404.
doi: 10.34172/bi.2022.23645
Original Research
CFP10–loaded PLGA nanoparticles as a booster vaccine confer protective immunity against Mycobacterium bovis
Zhengmin Liang  , Miaoxuan Li , Jiamin Ni , Tariq Hussain , Jiao Yao , Yinjuan Song , Yiduo Liu , Haoran Wang , Xiangmei Zhou , *
, Miaoxuan Li , Jiamin Ni , Tariq Hussain , Jiao Yao , Yinjuan Song , Yiduo Liu , Haoran Wang , Xiangmei Zhou , * 
Author information:
Key Laboratory of Animal Epidemiology and Zoonosis, Ministry of Agriculture, National Animal Transmissible Spongiform Encephalopathy Laboratory, College of Veterinary Medicine, China Agricultural University, 100193, Beijing, China
Abstract
Introduction:
The limited efficacy of BCG (bacillus Calmette–Guérin) urgently requires new effective vaccination approaches for the control of tuberculosis. Poly lactic-co-glycolic acid (PLGA) is a prevalent drug delivery system. However, the effect of PLGA-based nanoparticles (NPs) against tuberculosis for the induction of mucosal immune response is no fully elucidated. In this study, we hypothesized that intranasal immunization with culture filtrate protein-10 (CFP10)-loaded PLGA NPs (CFP10-NPs) could boost the protective immunity of BCG against Mycobacterium bovis in mice.
Methods:
The recombinant protein CFP10 was encapsulated with PLGA NPs to prepare CFP10-NPs by the classical water–oil-water solvent-evaporation method. Then, the immunoregulatory effects of CFP10-NPs on macrophages in vitro and on BCG-immunized mice in vivo were investigated.
Results:
We used spherical CFP10-NPs with a negatively charged surface (zeta-potential −28.5 ± 1.7 mV) having a particle size of 281.7 ± 28.5 nm in diameter. Notably, CFP10-NPs significantly enhanced the secretion of tumor necrosis factor α (TNF-α) and interleukin (IL)-1β in J774A.1 macrophages. Moreover, mucosal immunization with CFP10-NPs significantly increased TNF-α and IL-1β production in serum, and immunoglobulin A (IgA) secretion in bronchoalveolar lavage fluid (BALF), and promoted the secretion of CFP10-specific interferon-γ (IFN-γ) in splenocytes of mice. Furthermore, CFP10-NPs immunization significantly reduced the inflammatory area and bacterial load in lung tissues at 3-week post-M. bovis challenge.
Conclusion:
CFP10-NPs markedly improve the immunogenicity and protective efficacy of BCG. Our findings explore the potential of the airway mucosal vaccine based on PLGA NPs as a vehicle for targeted lung delivery.
Keywords: PLGA, Nanoparticles, CFP10, Mycobacterium bovis, Mucosal immunization
Copyright and License Information
© 2022 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Tuberculosis (TB) caused by Mycobacterium tuberculosis complex (MTBC) remains a leading cause of death from infection worldwide.
1
As an important member of MTBC, Mycobacterium bovis causes bovine tuberculosis and shares more than 99% nucleotide homologies with M. tuberculosis.
2
It also infects humans, M. bovis accounts for 1.4% to 28% of human TB cases in different countries and regions.
3
The only licensed human TB vaccine BCG (bacillus-Calmette-Guérin) protects children from disseminated TB, however, BCG-elicited efficacy in preventing pulmonary TB in adults varies widely.
4
Despite some progress has been made in the tuberculosis vaccine, attempts to enhance immunity for the control of TB by vaccination have been disappointing.
5
Therefore, the development of improved effective vaccines is a critical step toward controlling tuberculosis.
One important factor that may limit the effectiveness of BCG is the loss of antigenic composition RD1 region which includes the early secretory antigen target 6 kDa protein (ESAT6) and the culture filtrate protein-10 (CFP10), and other ones involved in ESAT6/CFP10 secretion.
6
CFP10 is one of the most immunogenic proteins of M. tuberculosis.
7-9
Therefore, subunit vaccines based upon ESAT6 or CFP10 are commonly used in heterologous BCG-prime-boost strategy for tuberculosis vaccine, some of them have been on the stage of clinical trials.
7-10
The main advantages of subunit vaccination are properties like safety, stability, less toxicity and enhancement of immunogenicity and protection.
11
However, many factors which may interfere with antigen presentation and T cell response affect the protective efficacy of the TB subunit vaccine.
12
Thus, the selection of antigens and efficient antigens delivery vehicles are crucial factors towards the effectiveness of the vaccine.
Increasing evidence suggests that nanoparticles (NPs) have been utilized as vaccine-delivery vehicles to boost vaccine efficacy.
13,14
One of the most commonly used NP materials is poly (lactic-co-glycolic acid) (PLGA).
14
Previous studies have demonstrated that antigen-loaded PLGA NPs could evoke a cell-mediated immune response and boost protection against intracellular bacterium M. tuberculosis and Listeria monocytogenes in mouse models.
15-17
It has been studied that single-dose H1-PLGA NPs containing antigen 85B (Ag85B) and ESAT6 offered long-term protection against M. tuberculosis.
17
Similarly, PLGA NPs improved the immunogenicity of HspX/EsxS (a multistage subunit TB vaccine).
18
Ovalbumin (OVA)-loaded PLGA NPs enhanced the antigen-specific cellular and humoral immune responses.
15,19
M. tuberculosis and M. bovis are typical intracellular pathogens that primarily enter the host through the respiratory route. Respiratory epithelial cells with a covering of mucus on their surface establish an important defense barrier against airborne transmitted mycobacteria.
20
Therefore, vaccines that enhance the function of mucosal immunity should be considered for developing a novel immunization strategy against TB. PLGA has been shown to be an important mucosal adjuvant.
21-24
An early study has shown that delivery of microencapsulated mycobacterial antigen ESAT6 via intranasal immunization can induce robust cell-mediated immune responses against M. tuberculosis.
25
Thus, the mucosal immune strategy of the respiratory tract involving PLGA NPs and appropriate immunogen via the pulmonary route is a promising approach for providing protective immunity against tuberculosis. In the current study, we evaluated the immunogenicity and protective efficacy of CFP10-encapsulated PLGA NPs (CFP10-NPs). CFP10-NPs were first characterized for physicochemical and morphological properties, and their immunomodulatory properties were investigated in a mouse model.
Materials and Methods
Materials
PLGA polymer (the ratio of lactide: glycolide feed was 50:50, Mw: 10 kDa) was purchased from Jinan Daigang Biomaterial Co., Ltd., Shandong, China. The Ni2+ Sepharose High Performance was purchased from GE Healthcare Life Sciences (Piscataway, NJ). The isopropyl-β-D-thiogalactopyranoside (IPTG) was purchased from Solarbio Life Sciences (Beijing, China). The oleic acid-albumin-dextrose-catalase (OADC) enrichment solution was from BD Biosciences (New York, NY, USA), and 7H9 and 7H10 Middlebrook media were from Difco (New York, NY, USA). The mouse monoclonal His-tag antibody was purchased from Proteintech (Wuhan, Hubei, China). Mouse TNF-α, Il-1β, IFN-γ, IgG, and IgA ELISA (enzyme-linked immunosorbent assay) kits were purchased from Neobioscience Technology (Shenzhen, Guangdong, China). The micro-BCA protein assay kit was purchased from Thermo Fisher Scientific (Carlsbad, CA, USA).
Mice
Specific-pathogen-free 6–8-week-old female BALB/c mice were purchased from specific pathogen-free (SPF) Biotechnology (Beijing, China). The mice were kept in the Biosafety Level 3 (BSL3) laboratory of China Agricultural University. The mice were received with food and water ad libitum and were acclimated for 7 days before the experiments. The experiments were carried out in accordance with the Chinese Regulations of Laboratory Animals—The Guidelines for the Care of Laboratory Animals (Ministry of Science and Technology of the People’s Republic of China) and Laboratory Animal Requirements of Environment and Housing Facilities (GB 14925–2010, National Laboratory Animal Standardization Technical Committee).
Bacterial culture
BCG (Pasteur strain) and virulent M. bovis Beijing strain C68004 were cultured in 7H9 Middlebrook broth supplemented with 2 g/L sodium pyruvate, 0.05% Tween-80, and 10% OADC enrichment solution at 37oC in BSL3 laboratory. The cultured BCG was frozen at ~3 × 108 CFU/mL (colony-forming unit per milliliter) and stored at −80 °C. BCG was thawed and diluted in cold PBS before injection. Sterile glycerol (10%) was added to the M. bovis culture in the log growing phase, and frozen at ~3 × 108 CFU/mL, and stored at −80 °C until needed for infection.
Preparation of CFP10-NPs
CFP10 was produced in and purified from Escherichia coli host BL21 (DE3). The recombinant E. coli strain pET30 (a)-CFP10-BL21 in log growing phase (OD 0.6– 0.8) were cultured in Luria-Bertani (LB) medium with kanamycin (50 μg/mL) and induced by 1mM IPTG at 37°C for 4 hours. The cells were harvested and broken by probe sonication at 80 W for 20 minutes in an ice bath and soluble CFP10 was purified by Ni2+ chromatography. Finally, we measured protein concentrations using a BCA protein assay kit (Beyotime, Nanjing, China), analyzed the purity by scanning stained gels, and confirmed CFP10 by western blot using an anti-His antibody. Briefly, the purified CFP10 was separated by SDS-PAGE, and the gels were stained with Coomassie blue or transferred to the nitrocellulose (NC) membrane. The membrane was incubated with the mouse anti-His antibody, followed by the goat anti-mouse horseradish peroxidase (HRP) - conjugated secondary antibody.
PLGA NPs were prepared using a previously reported water-oil-water emulsion technique.
17
Briefly, CFP10 protein solution in PBS as excipient was homogenized with 3.2% w/v PLGA in dichloromethane. The water-in-oil emulsion was then added to a 1% w/v solution of polyvinyl alcohol (PVA) for the second emulsion step, and the water-in-oil-in-water double emulsion was added dropwise to 0.5% w/v PVA allowed to evaporate at room temperature. The size and zeta-potential of NPs were measured by a Zetasizer Nano ZS system (Malvern Instruments Ltd., UK). The surface morphology of the NPs was studied using scanning electron microscopy (EVO40; Carl Zeiss, Jena, Germany). The encapsulation efficiency (EE) and loading efficiency (LE) were measured using a micro-BCA protein assay kit.
17
Cell culture and stimulation
J774A.1 cells were obtained from the Cell Culture Center, Peking Union Medical College (Beijing, China) and cultured in DMEM containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37oC with 5% CO2 in a humidified incubator. Then, the cells were transferred to 24-well cell culture plates (5×106 cells in each well) and cultured for 12 hours. The cells were stimulated with CFP10 (10 μg/mL), CFP10-NPs (100 µg/mL) or PBS-NPs (100 µg/mL) for 24 hours. Untreated cells were used as a negative control group. The cell culture supernatants were collected to measure the levels of cytokines.
Vaccination and infection of mice
The mice were randomly divided into six groups (10 mice/group): (1) BCG group; (2) BCG +CFP10 group; (3) BCG +CFP10-NP group; (4) BCG +PBS-NP group; (5) M. bovis group (infection control); and (6) PBS group (normal control). Mice in BCG-vaccinated groups were received subcutaneously (s.c.) 106 CFU of BCG in 100 μL of PBS. Four weeks after immunization, the BCG-vaccinated mice were immunized intranasally (i.n.) with 50 μL of PBS containing 0.5 mg CFP10-NPs (25 mg/kg) or PBS-NPs (25 mg/kg), or 50 µg CFP10 (2.5 mg/kg) for three times with an interval of two weeks. Mice in M. bovis group and PBS group mice received intranasally 50 µL of PBS. Four weeks after the last immunization, mice were infected intranasally with ~1000 CFU of M. bovis in 50 µL of PBS, and mice in the PBS group received intranasally 50 µL of PBS. The immunization/infection schedule was shown in Fig. 1.
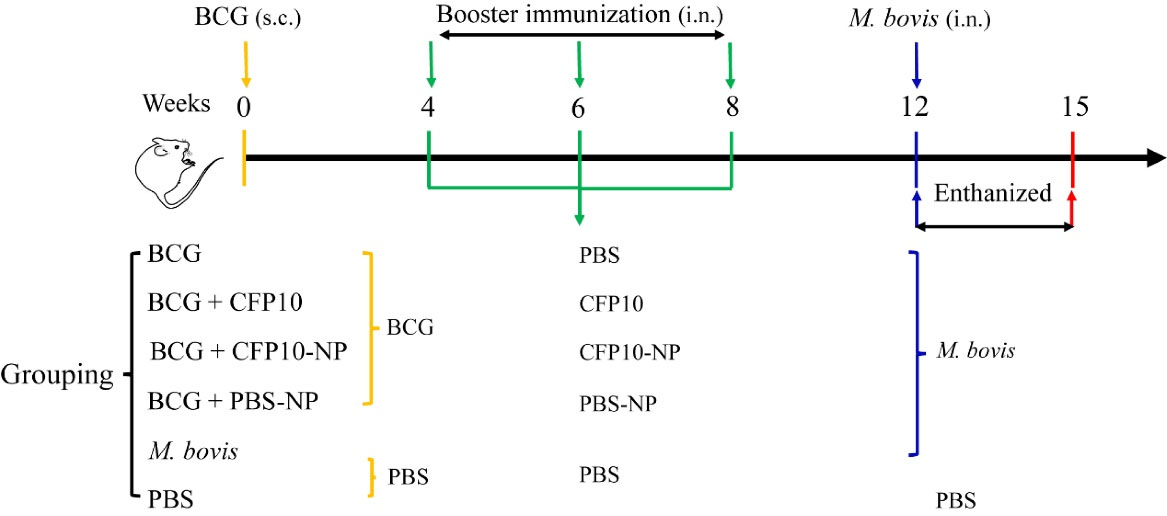
Fig. 1.
Schematic representation of mice immunization / infection model.
BALB/c mice were immunized subcutaneously (s.c.) with 10
6
CFU of BCG. Three weeks before M. bovis infection, mice were immunized intranasally (i.n.) with CFP10 (50 µg/mouse), or PBS-NPs (500 µg/mouse) or CFP10-NPs (500 µg/mouse) three times every two weeks. Four weeks after the last vaccination, three mice were euthanized to evaluate the immunogenicity of CFP10-NPs. The remaining mice except for PBS group mice were infected intranasally with M. bovis. CFU assay was performed to enumerate bacterial load in lungs and spleen to evaluate the efficacy of CFP10-NPs after three weeks of infection.
.
Schematic representation of mice immunization / infection model.
BALB/c mice were immunized subcutaneously (s.c.) with 10
6
CFU of BCG. Three weeks before M. bovis infection, mice were immunized intranasally (i.n.) with CFP10 (50 µg/mouse), or PBS-NPs (500 µg/mouse) or CFP10-NPs (500 µg/mouse) three times every two weeks. Four weeks after the last vaccination, three mice were euthanized to evaluate the immunogenicity of CFP10-NPs. The remaining mice except for PBS group mice were infected intranasally with M. bovis. CFU assay was performed to enumerate bacterial load in lungs and spleen to evaluate the efficacy of CFP10-NPs after three weeks of infection.
Collection of serum and bronchoalveolar lavage fluid (BALF)
The mice were euthanized four weeks after the last vaccination. Serum was obtained by centrifuging blood at 1000 g for 10 minutes at 4°C, and was stored at −80°C prior to the TNF-α, IL-1β, and IgG measurements. BALF samples were collected to measure IgA secretion. To collect BALF, 0.5 mL of ice-cold PBS was flushed into the lungs through a tracheal cannula. This was repeated three times to harvest ~1.3 mL of fluid. The fluid recovered from each sample was centrifuged (4℃, 1000 g, 10 minutes) to obtain supernatants, and the supernatants were kept at -80℃ to measure IgA secretion.
Enzyme-linked immunosorbent assay
The concentrations of TNF-α, IL-1β and IgG in serum, IgA in BALF, and IFN-γ in a culture supernatant of splenocytes were measured by ELISA kits. The level of IFN-γ in a culture supernatant of splenocytes stimulated with CFP10 (10 μg/mL) for 24 hours was determined with an ELISA kit. Briefly, 100 µL samples or standards were added to ELISA plates and incubated at 37℃ for 90 minutes. After that, the plates were incubated with 100 µL of the detection antibody at 37°C for 60 minutes and 100 µL of HRP-conjugated antibody at 37°C for 30 minutes. The plates were washed with a washing buffer for 4 times after each incubation step above. Finally, 100 µL of the TMB substrate was added and incubated for 15 min at 37°C in a dark environment. Subsequently, 100 µL of the stop solution was added and OD450 nm was measured by reading the plates with an ELISA plate reader (Thermo Scientific Multiskan FC, Shanghai, China).
CFU assay
CFU assay was performed by plating homogenized lung and spleen tissues on a solid medium to enumerate viable M. bovis. Tissues were homogenized in 1 mL of 7H9 Middlebrook broth using a tissue homogenizer apparatus (Omni International, Bedford, USA). Tissue homogenates were diluted with PBS supplemented with 0.05% Tween-80, and serial 10-fold dilutions were plated on 7H10 agar plates containing 10% OADC, 20 μg/mL amphotericin B, and 20 μg/mL polymyxin B sulfate. An equal volume of sample from each dilution was inoculated on plates in triplicate and cultured at 37°C. After incubation for 3-4 weeks, colonies of M. bovis in each culture plate were counted.
Lung histopathology
Three weeks after M. bovis infection, left lung, liver, and spleen tissues were fixed in 10% normal buffered formalin. The fixed tissues were embedded in paraffin, cut into thin sections (5 µm), and stained with Hematoxylin and eosin (H&E) or acid-fast staining. Inflammatory areas of the lungs were quantitatively analyzed under low magnification by Image-Pro plus 6.0 software (National Institutes of Health, Bethesda, MA, USA).
Statistical analysis
All data are expressed as mean ± standard deviation (SD). Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by the Dunnett’s multiple comparison test by GraphPad Prism version 7.0 software (GraphPad Software Inc., San Diego, CA, USA). Results of the flow cytometry were analyzed by the FlowJo software version 10 (Treestar, CA, USA). A p-value less than 0.05 reflected the findings are statistically significant.
Results
Physicochemical and morphological characterization of CFP10-NPs
The purified CFP10 proteins were identified with Coomassie blue staining and western blot. As shown in Fig. 2, there was a single major band (>95% purity) at the expected molecular size of ~17 kDa on the gel (Fig. 2A) and on the NC membrane probed using an anti-His-tag monoclonal antibody (Fig. 2B). Next, CFP10-NPs were prepared by a double-emulsion solvent evaporation method. Here, we obtained CFP10 protein EE and LE of 86.5% ±2.9% and 1.8% ± 0.06% (Table 1), respectively. Next, we used a micro-BCA kit to estimate the rate of CFP10 release from CFP10-NPs at regular intervals under in vitro conditions. As shown in Fig. 2C, by 24 hours, ~36.25% of CFP10 protein had been released into the supernatant. Notably, a slow and sustained release of the total CFP10 protein was observed on day 14, suggesting that CFP10-NPs could slowly release CFP10 over a longer duration to stimulate the immune system in vivo. The dynamic light scattering of CFP10-NPs suspension on the Zetasizer Nano ZS indicated narrow size distribution and greater colloidal stability of CFP10-NPs, the mean particle diameter was found as 281.7 ± 28.5 nm in PBS, and the zeta-potential of these particles was measured as −28.5 ± 1.7 mV (Table 1, Fig. 2D-2E). Scanning electron microscopy of CFP10-loaded PLGA NPs revealed a smooth appearance, globular molecules with no cavities (Fig. 2F-2G).
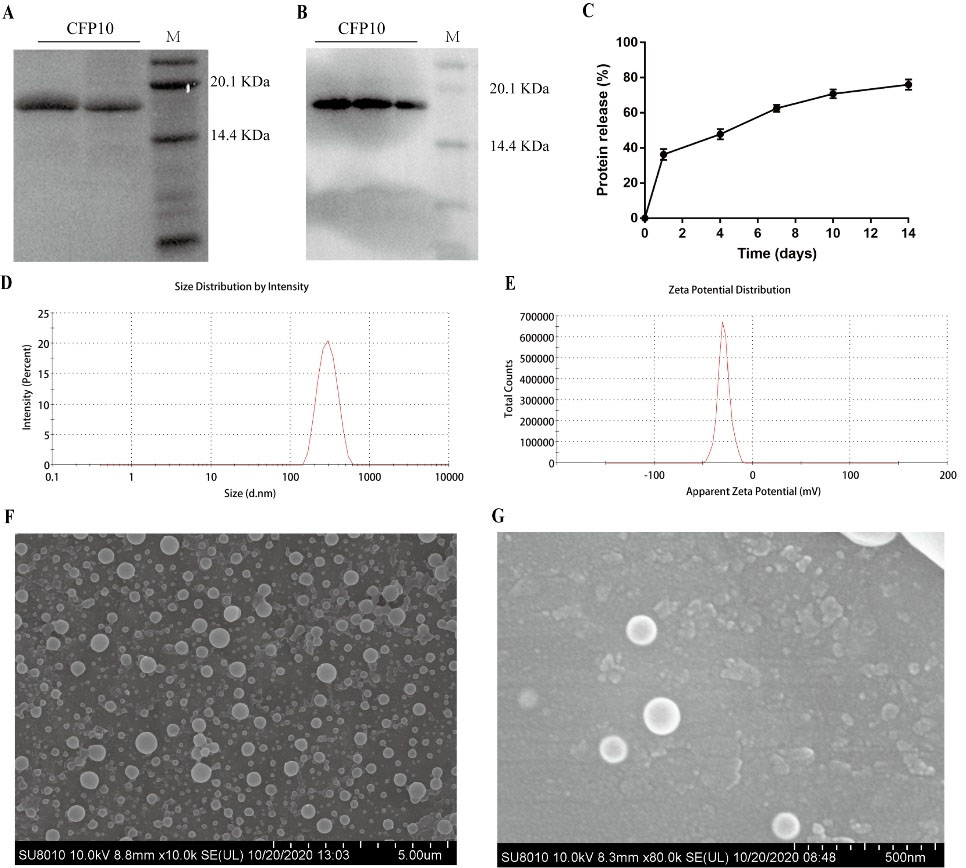
Fig. 2.
Physicochemical and morphological characterization of CFP10-NPs.
(A) The purified CFP10 was analyzed using 12% SDS-PAGE followed with Coomassie blue staining. The first two line represent samples having purified CFP10 and M represents the protein marker. (B) Western blot was performed to identify CFP10 using anti-His-tag antibody. Line M: protein marker. (C) A suspension of CFP10-NPs was prepared in PBS (50 mg/mL) followed by incubation at 37 °C for indicated time periods, and then the release profile of CFP10 protein was estimated by micro-BCA assay. (D-E) The physicochemical characterization of CFP10-NPs was determined by dynamic light scattering (D) and zeta-potential analysis (E). (F-G) Scanning electron microscopy was performed to determine the morphological characterization of CFP10-NPs (F, scale bar: 5 μm; G, scale bar: 500 nm).
.
Physicochemical and morphological characterization of CFP10-NPs.
(A) The purified CFP10 was analyzed using 12% SDS-PAGE followed with Coomassie blue staining. The first two line represent samples having purified CFP10 and M represents the protein marker. (B) Western blot was performed to identify CFP10 using anti-His-tag antibody. Line M: protein marker. (C) A suspension of CFP10-NPs was prepared in PBS (50 mg/mL) followed by incubation at 37 °C for indicated time periods, and then the release profile of CFP10 protein was estimated by micro-BCA assay. (D-E) The physicochemical characterization of CFP10-NPs was determined by dynamic light scattering (D) and zeta-potential analysis (E). (F-G) Scanning electron microscopy was performed to determine the morphological characterization of CFP10-NPs (F, scale bar: 5 μm; G, scale bar: 500 nm).
Table 1.
Characterization of PLGA-NPs with sizes and surface chemistries
|
PLGA-NPs
|
Size (nm)
|
PDI
|
Zeta Potential (mV)
|
EE (%)
|
LE (%)
|
| PBS-NPs |
213.1 ± 19.5 |
0.15 ± 0.03 |
−25.4 ± 0.8 |
|
|
| CFP10-NPs |
281.7 ± 28.5 |
0.19 ± 0.05 |
−28.5 ± 1.7 |
86.5 ± 2.9 |
1.8 ± 0.06 |
CFP10-NPs induce pro-inflammatory cytokines production in macrophages
Inflammatory cytokines are important for the control of mycobacterial infection.
12
Here, we investigated the role of CFP10-NPs on the expression of cytokines TNF-α and IL-1β in J774A.1 macrophages. J774A.1 macrophages were stimulated with CFP10, CFP10-NPs, or PBS-NPs for 24 hours. As shown in Fig. 3, CFP10-NPs, CFP10, or PBS-NPs markedly promoted the expression of TNF-α and IL-1β compared with untreated cells (Fig. 3A and 3B). Moreover, compared with CFP10 or PBS-NPs, CFP10-NPs significantly increased levels of TNF-α and IL-1β in macrophages. These findings suggest that CFP10-NPs mediate the secretion of pro-inflammatory cytokines in vitro.
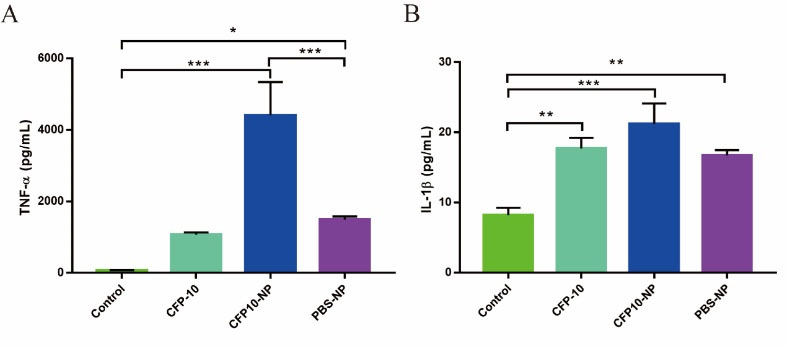
Fig. 3.
CFP10-NPs induce pro-inflammatory cytokine secretion in macrophages.
J774A.1 macrophages were stimulated with CFP10, CFP10-NPs, or PBS-NPs for 24 h. (A-B) ELISA assay was performed to determine levels of TNF-α (A) and IL-1β (B). Data represent the mean ± SD from three independent experiments (* P < 0.05; ** P < 0.01; *** P < 0.001).
.
CFP10-NPs induce pro-inflammatory cytokine secretion in macrophages.
J774A.1 macrophages were stimulated with CFP10, CFP10-NPs, or PBS-NPs for 24 h. (A-B) ELISA assay was performed to determine levels of TNF-α (A) and IL-1β (B). Data represent the mean ± SD from three independent experiments (* P < 0.05; ** P < 0.01; *** P < 0.001).
CFP10-NPs promote cytokine and IgA production in BCG-immunized mice
To further investigate the role of CFP10-NPs on the host immune responses, the BCG-immunized mice were vaccinated with CFP10 or CFP10-NPs as described above (Fig. 1). After four weeks of the last immunization, the levels of cytokine and immunoglobulin in serum, IgA in BALF, and CFP10-specific IFN-γ in splenocytes were measured. As shown in Fig. 4, CFP10 significantly increased the levels of TNF-α and IL-1β in serum. Interestingly, CFP10-NPs-vaccinated mice exhibited higher levels of TNF-α and IL-1β than CFP10 or PBS-NPs-vaccinated mice (Fig. 4A-4B). Furthermore, there was a significant increase in the secretion of CFP10-specific IFN-γ in mice vaccinated with CFP10-NPs, whereas CFP10 failed to significantly promote the secretion of CFP10-specific IFN-γ (Fig. 4C). Moreover, both CFP10 and CFP10-NPs increased the production of IgA in BALF, however, CFP10-NPs induced a higher level of IgA than CFP10. Surprisingly, PBS-NPs also markedly induced IgA secretion (Fig. 4D). However, no significant difference was found in IgG production among all vaccinated groups (Fig. 4E).
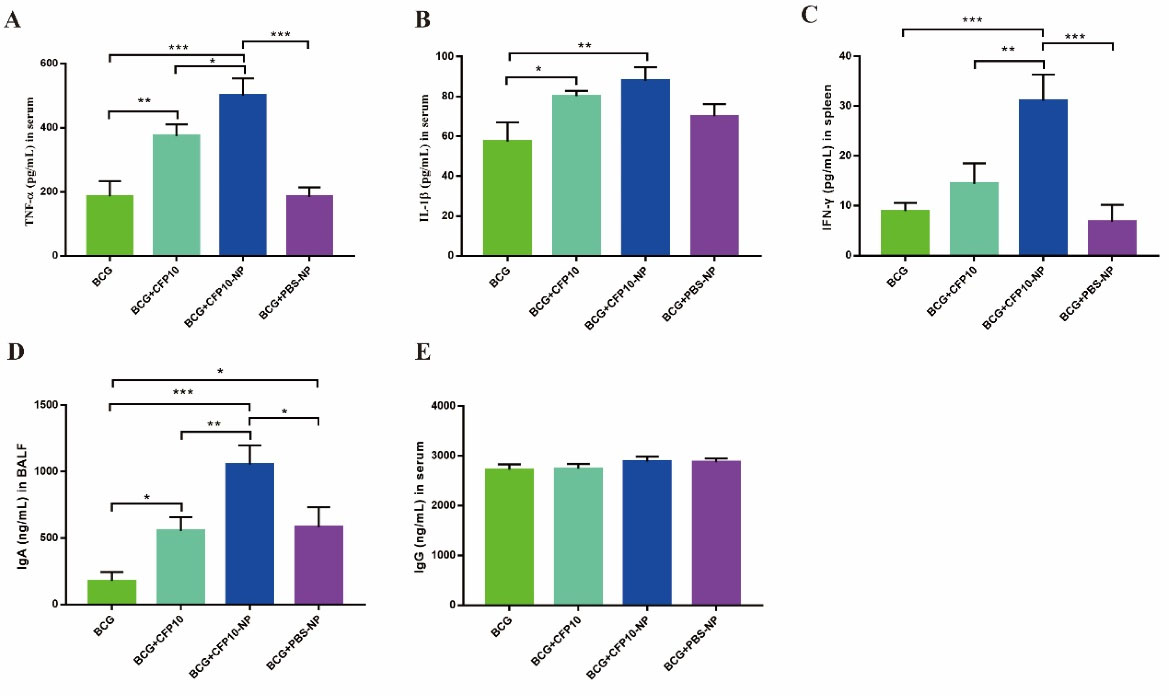
Fig. 4.
CFP10-NPs induce cytokine and antibody production in BCG-immunized mice.
Mice were euthanized after four weeks of the last vaccination for evaluation of cytokine and antibody production. The levels of cytokines and antibodies were determined with ELISA kits (n=4). (A-B) The concentrations of TNF-α (A) and IL-1β (B) in serum. (C) The level of CFP10-specific IFN-γ in culture supernatant of splenocytes induced by CFP10. (D-E) Levels of IgA in BALF (D) and IgG in serum (E). Data represent the mean ± SD of three independent experiments (* P < 0.05; ** P < 0.01; *** P < 0.001).
.
CFP10-NPs induce cytokine and antibody production in BCG-immunized mice.
Mice were euthanized after four weeks of the last vaccination for evaluation of cytokine and antibody production. The levels of cytokines and antibodies were determined with ELISA kits (n=4). (A-B) The concentrations of TNF-α (A) and IL-1β (B) in serum. (C) The level of CFP10-specific IFN-γ in culture supernatant of splenocytes induced by CFP10. (D-E) Levels of IgA in BALF (D) and IgG in serum (E). Data represent the mean ± SD of three independent experiments (* P < 0.05; ** P < 0.01; *** P < 0.001).
CFP10-NPs enhances the efficacy of BCG against M. bovis
The efficacy of CFP10-NPs was subsequently evaluated in mice (Fig. 1). Gross morphology of the spleen appeared lesions with variable degrees of swelling in M. bovis-infected mice, however, the splenomegaly was obviously ameliorated in CFP10-NPs-vaccinated mice (Fig. 5A). In addition, lung tissues showed the appearance of widespread visible grayish-white nodular lesions at the third week post-infection in non-vaccinated mice. However, fewer lesions were found in lung tissues of CFP10 or CFP10-NPs- immunized mice compared with BCG control mice (Fig. 5B). Furthermore, the size of the inflammatory area in the lungs evaluated by H&E staining was shown in Fig. 5C, compared with PBS (normal) control, M. bovis infection resulted in numerous inflammatory cells surrounding the blood vessels and bronchi, these inflammatory cells aggregated and formed the inflammatory and/ or necrosis foci with different sizes. However, immunization significantly decreased the size of the inflammatory area, and as expected, BCG combined with CFP10-NPs significantly reduced the percentage of inflammatory area compared with BCG control (Fig. 5C, 5G). Additionally, numerous acid-fast bacilli (AFB) were observed in the lungs of all immunized mice, however, fewer AFB were seen in the lungs of mice immunized with CFP10-NPs (Fig. 5D). Similarly, CFU enumeration results indicated that BCG combined with CFP10-NPs significantly reduced the number of M. bovis in both lung and spleen tissues compared with BCG control. However, CFP10 or PBS-NPs immunization failed to reduce bacterial load to a significant level (Fig. 5E-5F). In addition, we also performed microscopy of H&E stained sections of the liver and spleen. As shown in Fig. 6A-6B, multiple inflammation foci were present in hepatic lobules after M. bovis infection, however, the number of inflammation foci in BCG- immunized groups was obviously reduced, and CFP10-NPs immunization was beneficial to the reduction of inflammation foci. Moreover, we found an uneven distribution of white pulp and red pulp, and several inflammation foci in the spleen of M. bovis-infected mice. However, a decreased degree of lesions in the spleen was observed in mice vaccinated with BCG and CFP10-NPs (Fig. 6C-D).
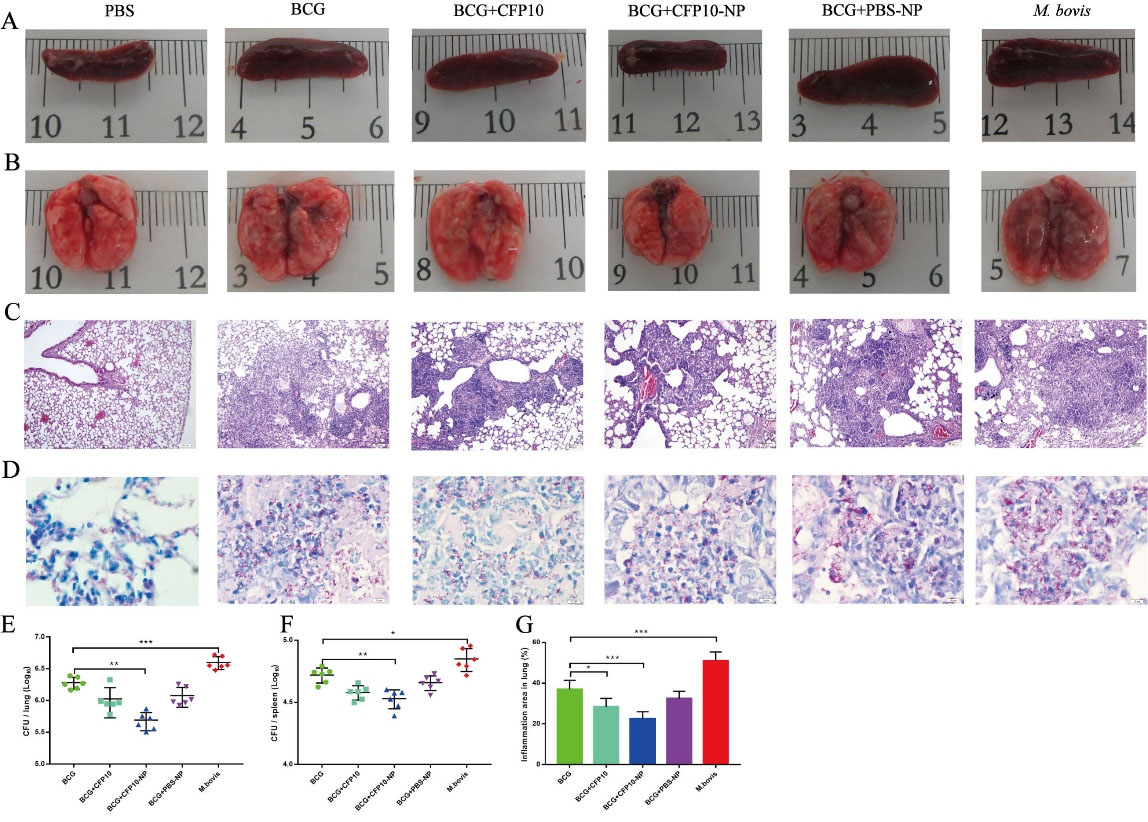

Fig. 5.
CFP10-NPs enhance the efficacy of BCG against M. bovis.
Mice were vaccinated with CFP10 or CFP10-NPs post BCG-immunization. Lung and spleen tissues were collected after three weeks of M. bovis infection (n=6). (A-B) Representative images for gross pathology of spleen (A) and lung (B) tissues. (C) H&E staining was performed to evaluate histopathological changes in lungs (images at ×100 magnification, scale bar: 100 μm). M. bovis infection resulted in the formation of inflammatory and/ or necrosis foci. (D) The dissemination of M. bovis bacilli were observed in lungs using acid-fast staining (images at ×1000 magnification, scale bar: 10 μm). (E-F) CFU in lungs (E) and spleen (F) were determined at three weeks after infection by plating right lung and spleen homogenates onto 7H10 plates. (G) The percentage of lung area covered by inflammatory lesions relative to total lung area was measured using image-j software. Data shown are representative of six mice per group. Data collected are expressed as the mean ± SD. (* P < 0.05; ** P < 0.01; *** P < 0.001).
.
CFP10-NPs enhance the efficacy of BCG against M. bovis.
Mice were vaccinated with CFP10 or CFP10-NPs post BCG-immunization. Lung and spleen tissues were collected after three weeks of M. bovis infection (n=6). (A-B) Representative images for gross pathology of spleen (A) and lung (B) tissues. (C) H&E staining was performed to evaluate histopathological changes in lungs (images at ×100 magnification, scale bar: 100 μm). M. bovis infection resulted in the formation of inflammatory and/ or necrosis foci. (D) The dissemination of M. bovis bacilli were observed in lungs using acid-fast staining (images at ×1000 magnification, scale bar: 10 μm). (E-F) CFU in lungs (E) and spleen (F) were determined at three weeks after infection by plating right lung and spleen homogenates onto 7H10 plates. (G) The percentage of lung area covered by inflammatory lesions relative to total lung area was measured using image-j software. Data shown are representative of six mice per group. Data collected are expressed as the mean ± SD. (* P < 0.05; ** P < 0.01; *** P < 0.001).
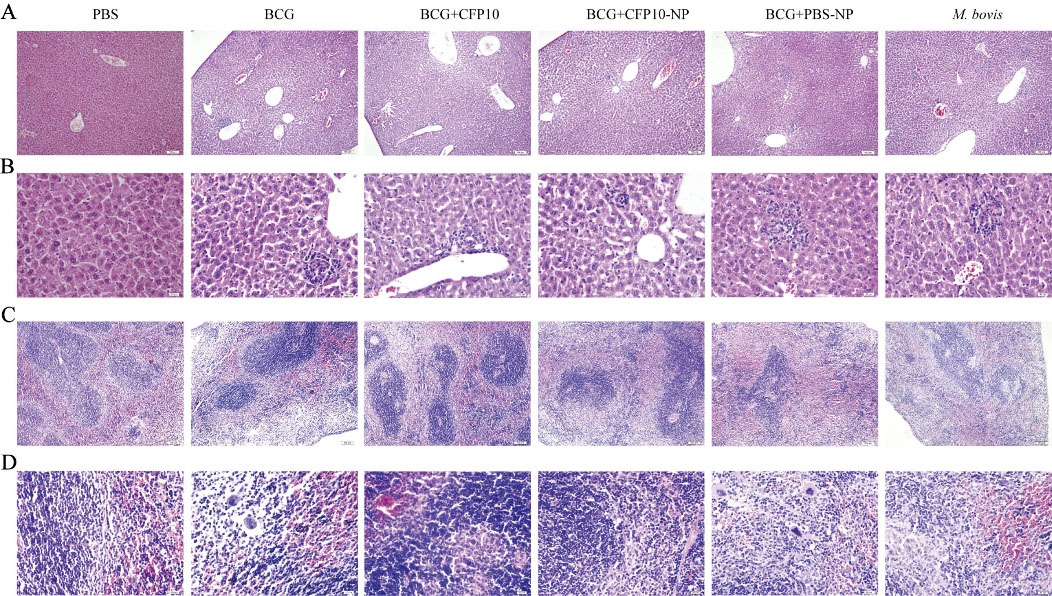
Fig. 6.
CFP10-NPs reduces the pathological damage in liver and spleen.
The immunized BALB/c mice were slaughtered after three weeks of M. bovis infection to perform H&E staining for evaluation of histopathological changes (n=4). (A-D) The histopathological changes of liver (A-B) and spleen (C-D) tissues (A-C, scale bar: 100 μm, images at ×100 magnification; B-D, scale bar: 20 μm, images at ×400 magnification). M. bovis infection resulted in the formation of multiple inflammation foci in liver and spleen tissues. However, the number and size of inflammation foci in hepatic lobules from mice vaccinated with BCG and CFP10-NPs were obviously reduced.
.
CFP10-NPs reduces the pathological damage in liver and spleen.
The immunized BALB/c mice were slaughtered after three weeks of M. bovis infection to perform H&E staining for evaluation of histopathological changes (n=4). (A-D) The histopathological changes of liver (A-B) and spleen (C-D) tissues (A-C, scale bar: 100 μm, images at ×100 magnification; B-D, scale bar: 20 μm, images at ×400 magnification). M. bovis infection resulted in the formation of multiple inflammation foci in liver and spleen tissues. However, the number and size of inflammation foci in hepatic lobules from mice vaccinated with BCG and CFP10-NPs were obviously reduced.
Discussion
Currently, M. bovis-derived BCG vaccine is the only available vaccine against tuberculosis and has high protective efficacy in children, however, it is not effective in reducing the global tuberculosis burden in adults.
26
Therefore, the insufficient efficacy of BCG demands new effective vaccines. Increasing evidence suggests that T-cell-mediated immune response plays a critical role in controlling tuberculosis. There are several adjuvants such as IC31, AS01, and GLA-SE, which induce significant levels of antigen-specific Th1 cytokines such as IL-12, IFN-γ, or TNF-α as a marker of protective immunity against tuberculosis.
10,27
In this milieu, increasing attention has focused on the utility of NP-based delivery system for next-generation vaccination strategies. Earlier attempts were made using mycobacteria immunogenic antigens, such as Ag85B, ESAT6, or bivalent fusion protein (Ag85B-TB10.4), and polymer-based delivery carriers to stimulate host immune response. However, the formulations in these studies were tested only in the micrometer range,
25,28,29
while PLGA particles in the nanometer size induce superior antigen-specific T cell responses compared to microparticles.
30,31
These earlier formulations were only investigated under in vitro conditions that could not fulfill the demands for evaluating the efficacy of vaccine candidate.
28,29
Recently, M. tuberculosis antigens-loaded PLGA NPs were administered by subcutaneous
18
or intraperitoneal
17
routes in a mouse model, and thus it was necessary to compare mucosal route and other parenteral routes. In the current study, we evaluated the vaccine potential of CFP10-NPs after intranasal immunization in a mouse model. Our results showed that boosting with CFP10-NPs enhanced the immune response of BCG against M. bovis in mice by significantly reducing bacterial load in lung and spleen tissues.
The structural integrity of the encapsulated protein is crucial to the competency of the NP-based vaccine-delivery system. PLGA NPs are one of the most stable particles.
13,32
It has been reported that PLGA NPs have negative zeta-potential due to their carboxyl groups.
33
In line with this, we found that CFP10-NPs with a particle size of ~280 nm also carried a negative charge (zeta-potential −28.5 ± 1.7 mV). Moreover, the shape and morphology of NPs are also crucial for their uptake by the immune cells. An early study showed that spherical polystyrene particles (diameters ~200 nm) conjugated to OVA-induced stronger Th1 immune and humoral immune responses compared to rod-shaped particles in vivo.
34
In addition, many processes could influence drug release in PLGA-based drug delivery systems.
35
Ag85B-ESAT6–loaded PLGA NPs
36
released ~75% of the total protein on day 14, which was in line with the release profile of CFP10-NPs. However, OVA-loaded PLGA NPs released at most one-half of their content within one month.
37
In line with the previous studies, we found that CFP10-NPs met the standard demands such as uniform size, shape, and controlled protein release for the induction of stronger cellular and humoral immune responses.
Reduction in the strength of macrophage differentiation and activation is a major strategy of M. tuberculosis immune evasion. Cytokines like TNF-α and IL-1β, secreted by the activated macrophages, play an important role in TB immunity.
12
In our current study, we found significant upregulations of TNF-α and IL-1β in CFP10-NPs-treated macrophages. Similarly, a recent study reported that Alhagi honey polysaccharide-based PLGA NPs induced over secretion of cytokines in macrophages.
38
Next, we vaccinated BCG-immunized mice with three doses of CFP10-NPs to evaluate the efficacy of CFP10-NPs as a novel vaccine candidate. Notably, we found that CFP10-NPs markedly increased the secretion of TNF-α and IL-1β, both in ex vivo and in BCG-immunized mice. Moreover, the contribution of Th1 cytokines especially IFN-γ is highly associated with protective immune responses against tuberculosis.
12
In this study, we found an enhanced CFP10-specific IFN-γ secretion by activated splenocytes from CFP10-NPs-immunized mice. Our current findings were consistent with previous studies that reported that Ag85B-ESAT6–loaded PLGA NPs enhanced protective immunity against tuberculosis in mice
17
and another MOMP (the major outer-membrane protein of Chlamydia trachomatis)–based PLGA NPs exhibited high immunogenicity in mice.
39
M. tuberculosis is an intracellular pathogen, previous studies showed that antibodies have no protective role against TB.
40
However, new evidence indicated that antibody-mediated immunity plays an important role in cleaning M. tuberculosis.
40,41
In addition, it has been reported that IgA plays an important role in protective immunity against TB, high level of IgA in BALF was associated with protection.
42
Similarly, our results revealed a significant increase in the secretion of IgA induced by CFP10 in BALF, and PLGA-NPs further enhanced this effect.Our findings of IgA secretion induced by CFP10-NPs in mice are in line with the previous results observed with CFP10-based Ad5-CEAB vaccination.
43
In our previous study, intranasal infection with a dose of ~1000 CFU could not kill mice three weeks after infection
44
, thus, the data on animal survival was not collected. Inconsistent with reports in most TB vaccine studies,
45,46
we evaluated the protective efficacy of CFP10-NPs by determining bacterial load in tissues. In the current study, we demonstrated that boosting BCG with CFP10-NPs significantly enhanced protection against M. bovis infection.
It is well documented that M. bovis shares more than 99% nucleotide homologies with M. tuberculosis,
2
and the DNA sequences of the CFP10 gene in M. tuberculosis and M. bovis are the same. It suggests that M. bovis infection model can be exploited in the development of new TB vaccine strategies, and CFP10-based vaccines may provide protective immunity against M. tuberculosis and M. bovis. Collectively, these observations suggest that CFP10-NPs might be a novel vaccine candidate for boosting BCG-induced immunity against tuberculosis.
Conclusion
In summary, the prepared CFP10-NPs had ideal physical characterization. Mucosal immunization with CFP10-NPs via the intranasally route promoted Th1 immune response and IgA secretion in mice. This enhanced immune response led to a significant reduction in pulmonary inflammatory lesions and bacterial load in the lungs and spleen in M. bovis-infected mice. Overall, CFP10-NPs could significantly boost the immunogenicity and protective efficacy of BCG against M. bovis to a certain degree in vivo. These results suggested that CFP10 PLGA NPs could be exploited as an effective airway mucosal subunit vaccine candidate against tuberculosis.
Research Highlights
What is the current knowledge?
√ Prepared CFP10-loaded PLGA NPs against M. bovis infection.
√ The CFP10-NPs boosted the immunogenicity and protective efficacy of BCG.
What is new here?
√ The CFP10-NPs could be exploited as an effective airway mucosal subunit vaccine candidate against tuberculosis.
Funding sources
This research was funded by the National Key Research and Development Program (Project No. 2017YFD0500901), the National Natural Science Foundation of China (Project No.31873005); the MoSTRCUK international cooperation project (Project No. 2013DFG32500); and the High-end Foreign Experts Recruitment Program (Project No. GDW20151100036, GDW20161100071).
Ethical statement
This study was supported by The Laboratory Animal Ethical Committee of China Agricultural University and the license number was AW91110202-2.
Competing interests
The authors declare no conflict of interest.
Authors’ contribution
XMZ, ZML, and MXL designed experiments. ZML wrote the manuscript. ZML and MXL performed experiments, data analysis, study validation, supervision, data presentation, and draft preparation. XMZ and TH revised the manuscript. JMN, JY, YJS, YDL, and HRW assisted to complete experiments. ZML and MXL contributed equally to this work.
References
-
World Health Organization (WHO). Global Tuberculosis Report. 2019. Available from: https://www.who.int/health-topics/tuberculosis. Accessed 30 November 2019.
- Waters WR, Palmer MV, Thacker TC, Davis WC, Sreevatsan S, Coussens P. Tuberculosis immunity: opportunities from studies with cattle. Clin Dev Immunol 2011; 2011:768542. doi: 10.1155/2011/768542 [Crossref] [ Google Scholar]
- Olea-Popelka F, Muwonge A, Perera A, Dean AS, Mumford E, Erlacher-Vindel E. Zoonotic tuberculosis in human beings caused by Mycobacterium bovis-a call for action. Lancet Infect Dis 2017; 17:e21-e5. doi: 10.1016/s1473-3099(16)30139-6 [Crossref] [ Google Scholar]
- Li J, Zhan L, Qin C. The double-sided effects of Mycobacterium Bovis bacillus Calmette-Guérin vaccine. NPJ Vaccines 2021; 6:14. doi: 10.1038/s41541-020-00278-0 [Crossref] [ Google Scholar]
- McShane H. Insights and challenges in tuberculosis vaccine development. Lancet Respir Med 2019; 7:810-9. doi: 10.1016/s2213-2600(19)30274-7 [Crossref] [ Google Scholar]
- Brosch R, Gordon SV, Garnier T, Eiglmeier K, Frigui W, Valenti P. Genome plasticity of BCG and impact on vaccine efficacy. Proc Natl Acad Sci USA 2007; 104:5596-601. doi: 10.1073/pnas.0700869104 [Crossref] [ Google Scholar]
- Aguilo N, Gonzalo-Asensio J, Alvarez-Arguedas S, Marinova D, Gomez AB, Uranga S. Reactogenicity to major tuberculosis antigens absent in BCG is linked to improved protection against Mycobacterium tuberculosis. Nat Commun 2017; 8:16085. doi: 10.1038/ncomms16085 [Crossref] [ Google Scholar]
- Wang C, Lu J, Du W, Wang G, Li X, Shen X. Ag85b/ESAT6-CFP10 adjuvanted with aluminum/poly-IC effectively protects guinea pigs from latent mycobacterium tuberculosis infection. Vaccine 2019; 37:4477-84. doi: 10.1016/j.vaccine.2019.06.078 [Crossref] [ Google Scholar]
- Vasina DV, Kleymenov DA, Manuylov VA, Mazunina EP, Koptev EY, Tukhovskaya EA. First-In-Human Trials of GamTBvac, a Recombinant Subunit Tuberculosis Vaccine Candidate: Safety and Immunogenicity Assessment. Vaccines (Basel) 2019; 7(4):166. doi: 10.3390/vaccines7040166 [Crossref] [ Google Scholar]
- Lewis K Schrager JV, Nick Drager, David M Lewinsohn, Ole F Olesen. The status of tuberculosis vaccine development. Lancet Infect Dis 2020; 20:e28-e37. doi: 10.1016/S1473-3099(19)30625-5 [Crossref] [ Google Scholar]
- Dalmia N, Ramsay AJ. Prime-boost approaches to tuberculosis vaccine development. Expert Rev Vaccines 2012; 11:1221-33. doi: 10.1586/erv.12.94 [Crossref] [ Google Scholar]
- Ernst JD. Ernst JDMechanisms of Mtuberculosis Immune Evasion as Challenges to TB Vaccine Design. Cell Host Microbe 2018; 24:34-42. doi: 10.1016/j.chom.2018.06.004 [Crossref] [ Google Scholar]
- Gregory AE, Titball R, Williamson D. Vaccine delivery using nanoparticles. Frontiers in Cellular & Infection Microbiology 2013; 3:13. doi: 10.3389/fcimb.2013.00013 [Crossref] [ Google Scholar]
- Ding D, Zhu Q. Recent advances of PLGA micro/nanoparticles for the delivery of biomacromolecular therapeutics. Mater Sci Eng C Mater Biol Appl 2018; 92:1041-60. doi: 10.1016/j.msec.2017.12.036 [Crossref] [ Google Scholar]
- de Groot AM, Du G, Monkare J, Platteel ACM, Broere F, Bouwstra JA. Hollow microneedle-mediated intradermal delivery of model vaccine antigen-loaded PLGA nanoparticles elicits protective T cell-mediated immunity to an intracellular bacterium. J Control Release 2017; 266:27-35. doi: 10.1016/j.jconrel.2017.09.017 [Crossref] [ Google Scholar]
- Khademi F, Sahebkar A, Fasihi-Ramandi M, Taheri RA. Induction of strong immune response against a multicomponent antigen of Mycobacterium tuberculosis in BALB/c mice using PLGA and DOTAP adjuvant. APMIS 2018; 126:509-14. doi: 10.1111/apm.12851 [Crossref] [ Google Scholar]
- Malik A, Gupta M, Mani R, Bhatnagar R. Single-dose Ag85B-ESAT6-loaded poly(lactic-co-glycolic acid) nanoparticles confer protective immunity against tuberculosis. Int J Nanomedicine 2019; 14:3129-43. doi: 10.2147/IJN.S172391 [Crossref] [ Google Scholar]
- Khademi F, Yousefi A, Derakhshan M, Najafi A, Tafaghodi M. Enhancing immunogenicity of novel multistage subunit vaccine of Mycobacterium tuberculosis using PLGA:DDA hybrid nanoparticles and MPLA: Subcutaneous administration. Iran J Basic Med Sci 2019; 22:893-900. doi: 10.22038/ijbms.2019.33962.8079 [Crossref] [ Google Scholar]
- Wusiman A, Gu P, Liu Z, Xu S, Zhang Y, Hu Y. Cationic polymer modified PLGA nanoparticles encapsulating Alhagi honey polysaccharides as a vaccine delivery system for ovalbumin to improve immune responses. Int J Nanomedicine 2019; 14:3221-34. doi: 10.2147/IJN.S203072 [Crossref] [ Google Scholar]
- Chai Q, Lu Z, Liu CH. Host defense mechanisms against Mycobacterium tuberculosis. Cell Mol Life Sci 2020; 77:1859-78. doi: 10.1007/s00018-019-03353-5 [Crossref] [ Google Scholar]
- Zhao K, Zhang Y, Zhang X, Shi C, Wang X, Wang X. Chitosan-coated poly(lactic-co-glycolic) acid nanoparticles as an efficient delivery system for Newcastle disease virus DNA vaccine. Int J Nanomedicine 2014; 9:4609-19. doi: 10.2147/ijn.s70633 [Crossref] [ Google Scholar]
- Kabiri M, Sankian M, Sadri K, Tafaghodi M. Robust mucosal and systemic responses against HTLV-1 by delivery of multi-epitope vaccine in PLGA nanoparticles. Eur J Pharm Biopharm 2018; 133:321-30. doi: 10.1016/j.ejpb.2018.11.003 [Crossref] [ Google Scholar]
- Singh SM, Alkie TN, Nagy É, Kulkarni RR, Hodgins DC, Sharif S. Delivery of an inactivated avian influenza virus vaccine adjuvanted with poly(D,L-lactic-co-glycolic acid) encapsulated CpG ODN induces protective immune responses in chickens. Vaccine 2016; 34:4807-13. doi: 10.1016/j.vaccine.2016.08.009 [Crossref] [ Google Scholar]
- Leya T, Ahmad I, Sharma R, Tripathi G, Kurcheti PP, Rajendran KV. Bicistronic DNA vaccine macromolecule complexed with poly lactic-co-glycolic acid-chitosan nanoparticles enhanced the mucosal immunity of Labeo rohita against Edwardsiella tarda infection. Int J Biol Macromol 2020; 156:928-37. doi: 10.1016/j.ijbiomac.2020.04.048 [Crossref] [ Google Scholar]
- Carpenter ZK, Williamson ED, Eyles JE. Mucosal delivery of microparticle encapsulated ESAT-6 induces robust cell-mediated responses in the lung milieu. J Control Release 2005; 104:67-77. doi: 10.1016/j.jconrel.2005.01.014 [Crossref] [ Google Scholar]
- Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PE. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis 2014; 58:470-80. doi: 10.1093/cid/cit790 [Crossref] [ Google Scholar]
- Tait DR, Hatherill M, Van Der Meeren O, Ginsberg AM, Van Brakel E, Salaun B. Final Analysis of a Trial of M72/AS01E Vaccine to Prevent Tuberculosis. N Engl J Med 2019; 381:2429-39. doi: 10.1056/NEJMoa1909953 [Crossref] [ Google Scholar]
- Shi S, Hickey AJ. PLGA microparticles in respirable sizes enhance an in vitro T cell response to recombinant Mycobacterium tuberculosis antigen TB104-Ag85B. Pharm Res 2010; 27:350-60. doi: 10.1007/s11095-009-0028-7 [Crossref] [ Google Scholar]
- Lu D, Garcia-Contreras L, Xu D, Kurtz SL, Liu J, Braunstein M. Poly (lactide-co-glycolide) microspheres in respirable sizes enhance an in vitro T cell response to recombinant Mycobacterium tuberculosis antigen 85B. Pharm Res 2007; 24:1834-43. doi: 10.1007/s11095-007-9302-8 [Crossref] [ Google Scholar]
- Joshi VB, Geary SM, Salem AK. Biodegradable particles as vaccine delivery systems: size matters. AAPS J 2013; 15:85-94. doi: 10.1208/s12248-012-9418-6 [Crossref] [ Google Scholar]
- Jin Z, Gao S, Cui X, Sun D, Zhao K. Adjuvants and delivery systems based on polymeric nanoparticles for mucosal vaccines. Int J Pharm 2019; 572:118731. doi: 10.1016/j.ijpharm.2019.118731 [Crossref] [ Google Scholar]
- Mir M, Ahmed N, Rehman AU. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf B Biointerfaces 2017; 159:217-31. doi: 10.1016/j.colsurfb.2017.07.038 [Crossref] [ Google Scholar]
- Wang Y, Li P, Kong L. Chitosan-modified PLGA nanoparticles with versatile surface for improved drug delivery. AAPS PharmSciTech 2013; 14:585-92. doi: 10.1208/s12249-013-9943-3 [Crossref] [ Google Scholar]
- Kumar S, Anselmo AC, Banerjee A, Zakrewsky M, Mitragotri S. Shape and size-dependent immune response to antigen-carrying nanoparticles. J Control Release 2015; 220:141-8. doi: 10.1016/j.jconrel.2015.09.069 [Crossref] [ Google Scholar]
- Fredenberg S, Wahlgren M, Reslow M, Axelsson A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems--a review. Int J Pharm 2011; 415:34-52. doi: 10.1016/j.ijpharm.2011.05.049 [Crossref] [ Google Scholar]
- Malik A, Gupta M, Mani R, Bhatnagar R. Single-dose Ag85B-ESAT6-loaded poly(lactic-co-glycolic acid) nanoparticles confer protective immunity against tuberculosis. Int J Nanomedicine 2019; 14:3129-43. doi: 10.2147/IJN.S172391 [Crossref] [ Google Scholar]
- de Groot AM, Du G, Monkare J, Platteel ACM, Broere F, Bouwstra JA. Hollow microneedle-mediated intradermal delivery of model vaccine antigen-loaded PLGA nanoparticles elicits protective T cell-mediated immunity to an intracellular bacterium. J Control Release 2017; 266:27-35. doi: 10.1016/j.jconrel.2017.09.017 [Crossref] [ Google Scholar]
- Wusiman A, He J, Zhu T, Liu Z, Gu P, Hu Y. Macrophage immunomodulatory activity of the cationic polymer modified PLGA nanoparticles encapsulating Alhagi honey polysaccharide. Int J Biol Macromol 2019; 134:730-9. doi: 10.1016/j.ijbiomac.2019.05.038 [Crossref] [ Google Scholar]
- Rose F, Wern JE, Ingvarsson PT, van de Weert M, Andersen P, Follmann F. Engineering of a novel adjuvant based on lipid-polymer hybrid nanoparticles: A quality-by-design approach. J Control Release 2015; 210:48-57. doi: 10.1016/j.jconrel.2015.05.004 [Crossref] [ Google Scholar]
- Achkar JM, Casadevall A. Antibody-mediated immunity against tuberculosis: implications for vaccine development. Cell Host Microbe 2013; 13:250-62. doi: 10.1016/j.chom.2013.02.009 [Crossref] [ Google Scholar]
- Li H, Wang XX, Wang B, Fu L, Javid B. Latently and uninfected healthcare workers exposed to TB make protective antibodies against Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 2017; 114:5023-8. doi: 10.1073/pnas.1611776114 [Crossref] [ Google Scholar]
- Dijkman K, Sombroek CC, Vervenne RAW, Hofman SO, Boot C, Remarque EJ. Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat Med 2019; 25:255-62. doi: 10.1038/s41591-018-0319-9 [Crossref] [ Google Scholar]
- Li W, Li M, Deng G, Zhao L, Liu X, Wang Y. Prime-boost vaccination with Bacillus Calmette Guerin and a recombinant adenovirus co-expressing CFP10, ESAT6, Ag85A and Ag85B of Mycobacterium tuberculosis induces robust antigen-specific immune responses in mice. Mol Med Rep 2015; 12:3073-80. doi: 10.3892/mmr.2015.3770 [Crossref] [ Google Scholar]
- Cheng G, Hussain T, Sabir N, Ni J, Li M, Zhao D. Comparative Study of the Molecular Basis of Pathogenicity of M bovis Strains in a Mouse Model. Int J Mol Sci 2018; 20(1):5. doi: 10.3390/ijms20010005 [Crossref] [ Google Scholar]
- Kaufmann E, Sanz J, Dunn JL, Khan N, Mendonca LE, Pacis A. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell 2018; 172:176-90. doi: 10.1016/j.cell.2017.12.031 [Crossref] [ Google Scholar]
- Counoupas C, Ferrell KC, Ashhurst A, Bhattacharyya ND, Nagalingam G, Stewart EL. Mucosal delivery of a multistage subunit vaccine promotes development of lung-resident memory T cells and affords interleukin-17-dependent protection against pulmonary tuberculosis. NPJ Vaccines 2020; 5:105. doi: 10.1038/s41541-020-00255-7 [Crossref] [ Google Scholar]