Bioimpacts. 12(4):359-370.
doi: 10.34172/bi.2022.23769
Original Research
Designing multi-epitope based peptide vaccine candidates against SARS-CoV-2 using immunoinformatics approach
Ysrafil Ysrafil 1, *  , Zulfiayu Sapiun 2, 3
, Zulfiayu Sapiun 2, 3  , Indwiani Astuti 4
, Indwiani Astuti 4  , Mohammad Anas Anasiru 5, Nangsih Sulastri Slamet 2
, Mohammad Anas Anasiru 5, Nangsih Sulastri Slamet 2  , Hartati Hartati 2
, Hartati Hartati 2  , Fadli Husain 2, Sukmawati Ahmad Damiti 6
, Fadli Husain 2, Sukmawati Ahmad Damiti 6 
Author information:
1Faculty of Medicine, Universitas Palangka Raya, Palangka Raya 73111, Indonesia
2Department of Pharmacy, Health Polytechnic of Gorontalo, Gorontalo 96135, Indonesia
3Department of Pharmacy, Faculty of Pharmacy, Universitas Hasanuddin, Makassar 90245, Indonesia
4Department of Pharmacology and Therapy, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta 55281, Indonesia
5Department of Nutrition, Health Polytechnic of Gorontalo, Gorontalo 96135, Indonesia
6Department of Midwifery, Health Polytechnic of Palangka Raya, Palangka Raya 73111, Indonesia
Abstract
Introduction:
The current incidence of the novel coronavirus disease has shown only small reductions of cases and has become a major public health challenge. Development of effective vaccines against the virus is still being encouraged such as multi-epitope vaccines designed from the components of SARS-CoV-2 including its spike, nucleocapsid and ORF1a proteins. Since the addition of adjuvants including HABA protein and L7/L12 ribosomal are considered helpful to increase the effectiveness of the designed vaccine, we proposed to design multiepitope vaccines by two different adjuvants.
Methods:
We used the IEDB server to predict BCL and TCL epitopes that were characterized using online tools including VaxiJen, AllPred and IL-10 Prediction. The selected epitopes were further constructed into multiepitope vaccines. We also added two different adjuvants to the vaccine components in order to increase the effectiveness of the vaccines. The 3D-structured vaccines were built using trRosetta. They were further docked with different Toll-like-receptors (TLR 3, 4 and 8) and the entry receptor of SARS-CoV-2, ACE2 using ClusPro, PatchDock and refined by FireDock. All structures were visualized by USCF Chimera and PyMOL.
Results:
In this study, we succeeded in designing two different candidate vaccines by the addition of HABA protein and L7/L12 ribosomal as adjuvants. The two vaccines were almost equally good in terms of their physicochemical properties and characteristics. Likewise, their strong interactions with TLR3 4, 8 and ACE2 show the lowest energy level of both was estimated at more than -1,000. Interactions of vaccines with ACE2 and TLRs are essential for activation of immune responses and production of antibodies.
Conclusion:
The two designed and constructed multiepitope vaccine have good characteristics and may have the potential to activate humoral and cellular immune responses against SARS-CoV-2. Further research is worth considering to confirm the findings of this study.
Keywords: SARS-COV-2, Immunoinformatics, Multi-epitope vaccine candidate, ACE2
Copyright and License Information
© 2022 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
The emergence of the virus that causes novel pneumonia, SARS-CoV-2 in Wuhan, China at the end of 2019 has had major impacts on the health and economic sectors worldwide.
1,2
Since its emergence, the virus has spread rapidly and to date has infected over 190 million people and caused over 4.02 million deaths worldwide.
3
Currently, the drugs used in the treatment of this disease are in accordance with the World Health Organization (WHO) recommendations including lopinavir/ritonavir, remdesivir, hydroxychloroquine and chloroquine as well as other additional drugs such as ribavirin, oseltamivir, favipiravir, broad spectrum antibiotics, anti-inflammatory steroids and monoclonal antibodies. Several therapies are often used with complementary medicine approaches such as traditional Chinese medicine, which in fact are not proven effective to reduce the patient mortality rate. For example, the effectiveness of plasma convalescent therapy in curing patients, which initially had promising results, is now being questioned.
4,5
Various attempts have been made to break the chain of the rapid spreading of this virus. Social distancing, quarantine, and self-isolation appear to only effectively prevent the transmission in the short term. Therefore, the development of an effective therapy for the eradication of this virus is needed. In addition, an increase in immunity in the population through vaccine development may help combat the SARS-CoV-2 infection and halt the transmission.
5,6
The use of vaccines is predicted to involve the priming of SARS-CoV-2 for the body to produce long-term immunity such as the production of neutralizing antibodies and memory B and T cells, which are expected to neutralize and block the fusion and entry of the virus.
6,7
However, until now, there is no vaccine that can effectively eradicate this virus. Along with technological developments, methods in vaccine design and development are becoming more advanced. For example, the use of virus-like particles in human papilloma virus (HPV) vaccines have currently been established, and also the multi-epitope-based vaccine peptide design using the immunoinformatics approach.
8
SARS-CoV-2 is a non-segmented and positive sense RNA virus which is 29.9 kb in length and built by four main structural proteins, namely the spike glycoprotein (S), small envelope protein (E), membrane glycoprotein (M), and nucleocapsid protein (N).
9
These four proteins play crucial roles in the effectiveness of this virus. The S protein of SAS-CoV-2 is responsible for CoV entering into the host cells by binding to its receptor, angiotensin converting enzyme 2 (ACE2), which is on the surface of host cells. This S protein is the main immunogenic component of the virus so it is often used in vaccine development.
2,10,11
Another important and immunogenic protein is N which is a structural protein that functions to bind genetic material of the virus and viral replication. In addition, the non-structural protein (NSP) encoded in ORF1a is another part that plays an important role in the life cycle and viral replication machinery of this virus. Although this gene encodes NSPs, ORF1a remains one of the important SARS-CoV-2 vaccine target proteins that serve as vaccine precursors.
12
Qamar et al demonstrated that designing multiantigen and epitope vaccines using ORF1a and other components could result in good candidate vaccines.
13
These spike, nucleocapsid and ORF1a proteins are the main existing components of SARS-CoV-2 involved in the priming, entering and infectivity of the virus.
9,14
In this study, we aimed to design multiepitope vaccines against SARS-CoV-2 from the three peptides described above using computer-based immunoinformatics analysis.
Materials and Methods
Protein retrieval
Protein selection is the first step in vaccine design using the immunoinformatics approach (Fig. 1). We retrieved the proteins from the National Central Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/protein/) database in December 2020 with the codes: Spike protein (Accession no: QHR63260.2), Nucleocapsid protein (Accession no: QHO60601.1) and Open reading frame 1a (ORF1a) (Accession no: QLI51900.1). All sequences of the proteins were developed in the FASTA format. Three protein selections were further determined for their antigenicity and physicochemical characteristics.
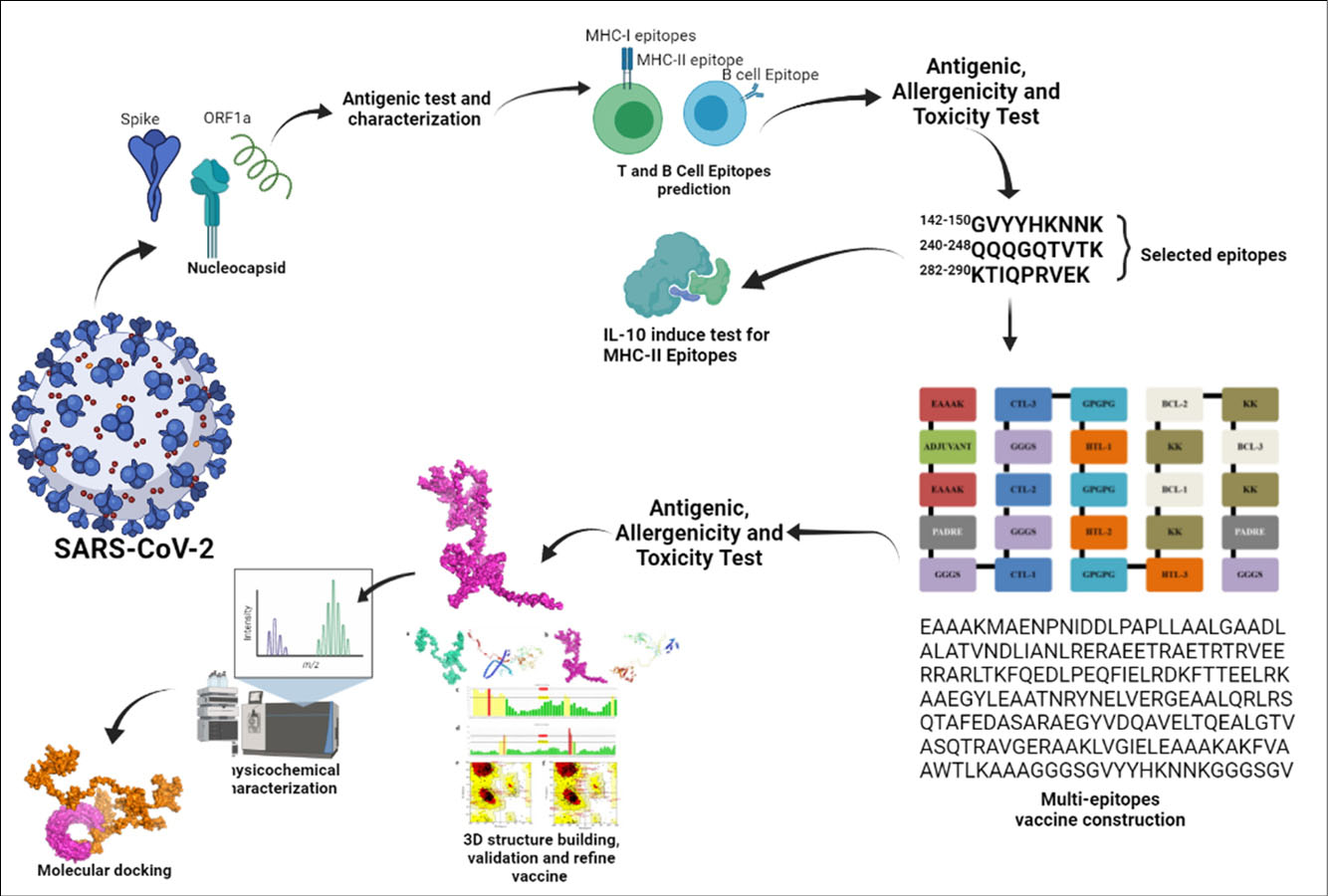
Fig. 1.
Schematic of immunoinformatics multi-epitope vaccine SARS-CoV-2 design.
.
Schematic of immunoinformatics multi-epitope vaccine SARS-CoV-2 design.
Evaluation of antigenicity and physicochemical characteristics of protein selection
Determination of antigenicity of the selected proteins was performed using VaxiJen v2.0 with threshold value 0.40 and tumor models.
15
The immunogenic proteins (had antigenic score more than 0.40) were further processed to obtain small peptides that fit with epitope T and B cells. Meanwhile, for the physicochemical properties characterization of the three selected proteins, we used the online characterization tool, ProtParam
16
as described previously by Ullah, Sarkar and Islam.
7
T and B cell epitope prediction
Prediction of T andB cell epitopes was conducted by using the Immune Epitopes Database (IEDB)
17
based on the immunoinformatics guide previously published by Ullah et al.
7,18
Whole protein S, N and/or ORF1a protein were dropped in the server to obtain small peptides that fit with epitope T and B cells. Antigenic peptides for T cell activation are generally administered via MHC-I and MHC-II, so prediction of T cell epitopes is typically conducted by prediction of MHC epitopes. MHC-Class I was presented as CD8+ cytotoxic T cell (CTL) epitope which was predicted by NetMHCpan EL 4.0 prediction method and using HLA-A*11−01 allele with 9 amino acids in length. Meanwhile, the MHC-Class II (Helper T-cell) was developed by IEDB recommendation methods for MHC-II prediction epitope using the HLA DRB1*04−01 allele. The three epitope results were selected based on their percentile scores, antigenicity scores, non-allergenicity, and non-toxicity. B-cell epitopes were predicted by Bepipred Linear Epitope Prediction 2.0. Five of them were selected based on their longer than 10 AA length, antigenicity scores, non-allergenicity, and non-toxicity.
19
Allergenicity, antigenicity and toxicity prediction of epitopes
All epitope predictions were tested for their allergenicity, antigenicity and toxicity prediction. The allergenicity predictions were measured by an online tool named AllergenFP, for an allergenicity prediction by descriptor fingerprints, which have 87.9% accuracy of prediction.
20
Antigenicity predictions of all epitopes were done by VaxiJen v2.0
15
as the most reliable server for prediction of antigenic properties.
21
The measuring of antigenicity of epitopes was done on the tumor model, which is known to generate excellent results using a model with threshold 0.40.
7
Meanwhile, the toxicity of all epitopes was analyzed by the ToxinPred server with the default setting of support-vector machine (SVM) (Swiss-Prot).
22
Prediction of IL-10 specific epitope response
The selected MHC-II epitopes (the three epitopes that had good percentile scores, antigenic, nonallergenic, and non-toxic) were tested for their ability to induce interleukin-10 (IL-10) responses. The IL-10 prediction server was employed to determine the ability of all predicted MHC-II epitopes to induce IL-10 releasing.
23
We conducted these tests with the prediction tool following the previously published study by Khairkhah et al
24
that made the predictions based on the SVM-based model with default value setting at SVM threshold left.
Building the 3D structure and molecular docking of selected epitopes
All selected MHC-I and II epitopes (had the best properties including good antigenic score, being non-allergenic, non-toxic and had the best percentile score) were developed into their 3D structures using the online 3D structure generating server, PEP-FOLD3.
25
The best models were retrieved and further docked with their representative alleles including HLA-A*11-01 allele (PDB ID: 5WJL) for the MHC-I epitope and HLA DRB1*04-01 (PDB ID: 5JLZ) for all MHC-II epitopes.
26
The online server, PatchDock was used for molecular docking.
27
The results of epitopes/MCH allele docking were refined and rescored by the FireDock server.
28
All epitopes/MCH allele docking results were visualized by UCSF Chimera.
29
Vaccine construction
All best CTL, HTL and BCL epitopes from three different proteins: spike, nucleocapsid and ORF1a were constructed to become multiepitope vaccines.
7,30
The epitopes were built with the following sequences: adjuvant, PADRE (Pan HLA-DR reactive epitope) sequence, CTL epitopes, HTL epitopes and BCL epitopes and vaccines built according to these templates are presented in Fig. 2. Two different adjuvants including L7/L12 ribosomal protein and HABA protein were used in the vaccine construction. The PADRE is a vaccine component that can induce CTL in the vaccine. Besides adjuvants and PADRE, some linkers were employed to link vaccine components including: EAAAK to link the adjuvant and PADRE, GGGS linkers for CTL epitopes, GPGPG linkers for HTL epitopes, and KK linkers for BCL epitopes.
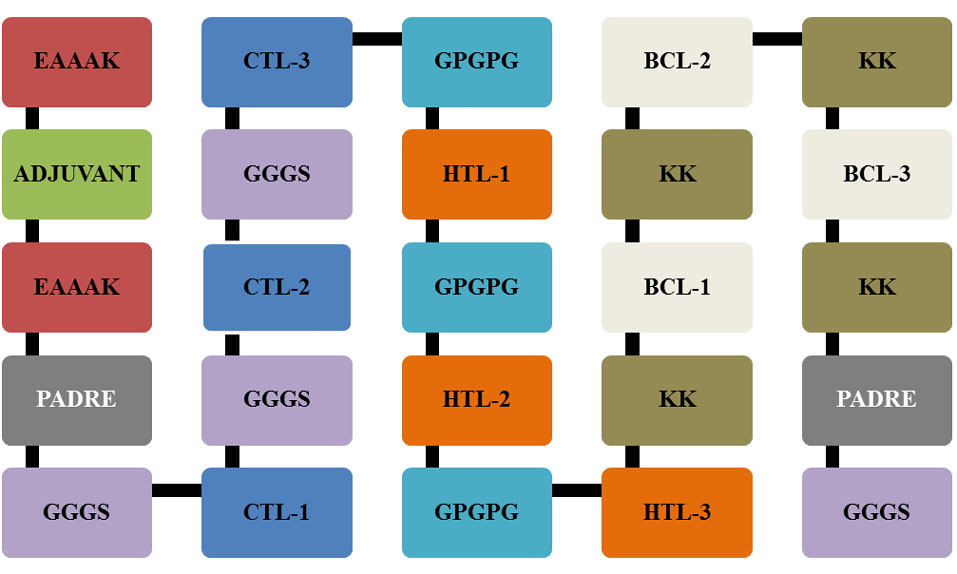
Fig. 2.
Schematic template of multi-epitope vaccine constructs with linkers, PADRE and adjuvants.
.
Schematic template of multi-epitope vaccine constructs with linkers, PADRE and adjuvants.
Evaluation of allergenicity, antigenicity and physicochemical characteristics of vaccine
Determination of allergenicity of the constructed vaccine was performed using VaxiJen v2.0 with threshold value 0.40 and tumor models.
15
Meanwhile, to determine vaccine allergenicity, we used the online allergenicity prediction tools, AllerTop v2.0
31
and AllergenFP servers.
20
Physicochemical properties are important to know, so an online server with characterization tools, ProtParam was used to determine the physicochemical properties of the constructed vaccines.
16
Building of tertiary structure and validation of vaccine constructs
Vaccine constructs were further developed in 3D structures using the trRosetta server,
32
known for fast and accurate protein structure prediction. Tertiary structures of the vaccines were further validated by ERRAT
33
and Ramachandran plots
34
using the online validation tools, PROCHECK.
35
All tertiary vaccine constructs were visualized by PyMOL and USCF Chimera.
Docking vaccine constructs with toll-like receptors (TLRs)
The two different constructed vaccines were docked with TLRs as the sensor pattern of viruses including coronavirus to initiate immune responses. The TLR PDB files including TLR3 (PDB ID:1ziw), TLR4 (4g8a) TLR8 (PDB ID:3w3g) and ACE2 (1r42) were obtained from the data bank, RCSB PDB (https://www.rcsb.org). The proteins were further docked by two online docking tools, including PatchDock
27
and ClusPro server.
36
The results of the vaccine/TLR docking were refined and rescored by the FireDock server.
28
All vaccine/TLR docking results were visualized by UCSF Chimera and PyMOL.
Results
Protein sequence retrieval, antigenic evaluation and determination of their characteristics
The proteins for precursors of the designed vaccines were retrieved from NCBI including Spike (S) protein (Accession code QHR63260.2), Nucleocapsid (N) protein (Accession code QHO60601.1) and Open reading frame 1a (ORF1a) (Accession code QLI51900.1). All of them were downloaded in amino acid FASTA form for further analysis. The results of the analysis of the antigenic potential using VaxiJen 2.0 on the sequence of peptides included (S, N and ORF1a) in this study shown in Table 1 indicate that all peptides have antigenic potential as indicated in their scores which passed the threshold (>0.40). Meanwhile, the initial characterization results showed that ORF1a protein had the highest molecular weight with 489988, and nucleocapsid had the smallest with 45625. This is because ORF1a has a higher number of amino acids (4405 AA) than the other two proteins included in this study (Spike 1273 AA and Nucleocapsid 419 AA).
7
Table 1.
Retrieval proteins for design multi-epitope vaccine candidate and their antigenicity prediction
|
Protein
|
ID Number
|
Length
|
Molecular Weight
|
Antigenic Score
|
Antigenicity
|
| Spike Protein |
QHR63260.2 |
1273 |
141178 |
0.5346 |
Antigenic |
| Nucleocapsid |
QHO60601.1 |
419 |
45625 |
0.7094 |
Antigenic |
| ORF1a |
QLI51900.1 |
4405 |
489988 |
0.4955 |
Antigenic |
T and B cell epitope prediction and their characteristics
T cell epitope prediction is done to assess the ability of the spike protein to induce CD8+ cytotoxic T lymphocyte (CTL) and helper T lymphocyte cell (HTL) responses. CTL epitopes were predicted by screening the epitopes which can bind to MHC class I, which is a macromolecule that has the function of presenting foreign antigens to CTL. Meanwhile, the HTL epitopes were predicted by the ability of foreign antigens to bind with MHC-II. Three of the twenty best epitope results were selected based on the percentile score and further tested for their antigenicity, immunogenicity, non-toxicity and non-allergenicity (Table 2). On protein spikes, those selected in the epitope CTL were S142-150(GVYYHKNNK), S550-558(GVLTESNKK), S529-537(KSTNLVKNK). In Nucleocapsid and ORF1a, the selected epitopes were N240-248 (QQQGQTVTK), N53-61(FTALTQHGK), N291-299(LIRQGTDYK), and ORF1a2192-2200 (ASMPTTIAK), ORF1a282-290(KTIQPRVEK), and ORF1a3260-3268(AVLQSGFRK), respectively (Table 2). Additionally, for HTL, there were: S53-64DLFLPFFSNVTW, S115-126QSLLIVNNATNV, S52-63QDLFLPFFSNVT, N344-355PNFKDQVILLNK, N305-316AQFAPSA SAFFG, N304-315IAQFAPSASAFF ORF1a2519-2530SHFVNLDNLRAN, ORF1a1188-1199SSFLEMK SEKQV, and ORF1a2520-2531HFVNLDNLRANN. The selected HTL epitopes were further tested for their ability to induce the cytokine IL-10. The prediction results showed that most of the selected epitopes could induce IL-10 (Table 3).
Table 2.
Selected MHC Class I (CTL) Epitopes of spike protein, nucleocapsids and ORF1a based on their antigenicity, non-allergenicity, non-toxicity and percentile score
|
Protein
|
Epitope
|
Position
|
Antigenicity score
|
Antigenicity
|
Allergenicity
|
Toxicity
|
Percentile
|
| Spike |
GVYYHKNNK |
142-150 |
0.4563 |
Antigenic |
Nonallergen |
Nontoxic |
0.13 |
| GVLTESNKK |
550-558 |
1.2654 |
Antigenic |
Nonallergen |
Nontoxic |
0.14 |
| KSTNLVKNK |
529-537 |
0.709 |
Antigenic |
Nonallergen |
Nontoxic |
0.21 |
| Nucleocapsid |
QQQGQTVTK |
240-248 |
1.2568 |
Antigenic |
Nonallergen |
Nontoxic |
0.19 |
| FTALTQHGK |
53-61 |
1.0498 |
Antigenic |
Nonallergen |
Nontoxic |
0.22 |
| LIRQGTDYK |
291-299 |
1.1264 |
Antigenic |
Nonallergen |
Nontoxic |
0.76 |
| ORF1a |
ASMPTTIAK |
2192-2200 |
0.5358 |
Antigenic |
Nonallergen |
Nontoxic |
0.01 |
| KTIQPRVEK |
282-290 |
1.0814 |
Antigenic |
Nonallergen |
Nontoxic |
0.01 |
| AVLQSGFRK |
3260-3268 |
0.666 |
Antigenic |
Nonallergen |
Nontoxic |
0.01 |
Table 3.
Selected MHC Class II (HTL) Epitopes of spike protein, nucleocapsids and ORF1a base on their antigenicity, non-allergenicity, non-toxicity, percentile score and cytokine IL-10 prediction
|
Protein
|
Epitope
|
Position
|
Antigenicity score
|
Antigenicity
|
Allergenicity
|
Toxicity
|
Percentile
|
IL-10 prediction
|
| Spike |
DLFLPFFSNVTW |
53-64 |
0.8885 |
Antigenic |
Nonallergen |
Nontoxic |
0.62 |
Inducer |
| QSLLIVNNATNV |
115-126 |
0.6194 |
Antigenic |
Nonallergen |
Nontoxic |
0.75 |
Non Inducer |
| QDLFLPFFSNVT |
52-63 |
0.9430 |
Antigenic |
Nonallergen |
Nontoxic |
0.95 |
Inducer |
| Nucleocapsid |
PNFKDQVILLNK |
344-355 |
0.4967 |
Antigenic |
Nonallergen |
Nontoxic |
1.80 |
Inducer |
| AQFAPSASAFFG |
305-316 |
0.1321 |
nonantigenic |
Nonallergen |
Nontoxic |
3.20 |
Inducer |
| IAQFAPSASAFF |
304-315 |
0.4432 |
Antigenic |
Nonallergen |
Nontoxic |
5.10 |
Non Inducer |
| ORF1a |
SHFVNLDNLRAN |
2519-2530 |
0.6316 |
Antigenic |
Nonallergen |
Nontoxic |
0.16 |
Non Inducer |
| SSFLEMKSEKQV |
1188-1199 |
0.6326 |
Antigenic |
Nonallergen |
Nontoxic |
0.20 |
Inducer |
| HFVNLDNLRANN |
2520-2531 |
0.5754 |
Antigenic |
Nonallergen |
Nontoxic |
0.23 |
Non Inducer |
Meanwhile, B-cell epitopes were predicted to recognize and present small peptides that fit with B cell epitopes, which are some of the antigen presenting cells that could recognize and present peptide antigens to stimulate immune responses. The five best epitopes were then selected to be included in the constructed vaccines based on their longer than 10 AA length, antigenicity scores, non-allergenicity, and non-toxicity (Table 4).
Table 4.
Selected B-Cell (LBL) epitopes of spike protein, nucleocapsids and ORF1a base on their antigenicity, non-allergenicity, non-toxicity, percentile score and cytokine IL-10 prediction
|
Protein
|
Epitope
|
Position
|
Antigenicity score
|
Antigenic prediction
|
Allergenicity
|
Toxicity
|
| Spike |
SQCVNLTTRTQLPPAYTNSFTRGVY |
13-37 |
0.51 |
Antigenic |
Nonallergen |
Nontoxic |
| MDLEGKQGNFKNL |
177-189 |
1.4189 |
Antigenic |
Nonallergen |
Nontoxic |
| KHTPINLVRDLPQGFS |
206-221 |
0.9646 |
Antigenic |
Nonallergen |
Nontoxic |
| KSFTVEKGIYQTSNFRVQP |
304-322 |
0.5392 |
Antigenic |
Nonallergen |
Nontoxic |
| FPNITNLCPFGEVFNATRFASVYAWNRKRISNCVA |
329-363 |
0.7126 |
Antigenic |
Nonallergen |
Nontoxic |
| Nucleocapsid |
NGPQNQRNAPRI |
4-15 |
0.4945 |
Antigenic |
Nonallergen |
Nontoxic |
| FGGPSDSTGSNQNGERSGARSKQRRPQGLPNN |
17-48 |
1.1986 |
Antigenic |
Nonallergen |
Nontoxic |
| HGKEDLKFPRGQGVPINTNSSPDDQIGYYRRATRRIRGGDGKMKDLS |
59-105 |
0.6915 |
Antigenic |
Nonallergen |
Nontoxic |
| GALNTPKDHIGTRNPANNAAIVLQLPQ |
137-163 |
0.4605 |
Antigenic |
Nonallergen |
Nontoxic |
| RLNQLESKMSGKGQQQQGQTVTKKSAAEASKKPRQKRTATKA |
226-267 |
1.2401 |
Antigenic |
Nonallergen |
Nontoxic |
| ORF1a |
HNESGLKTILRKGGR |
388-402 |
1.1024 |
Antigenic |
Nonallergen |
Nontoxic |
| CGETSWQTGDFVKAT |
326-340 |
0.6505 |
Antigenic |
Nonallergen |
Nontoxic |
| KKFDTFNGECPNFVFPLNSIIKTIQPRVEKKKLDGFMG |
261-298 |
0.8059 |
Antigenic |
Nonallergen |
Nontoxic |
| PLECIKDLLARAGKASCTLS |
197-216 |
1.0063 |
Antigenic |
Nonallergen |
Nontoxic |
| KNGNKGAGGHSYGADLKSFDLGDELGTDPYEDFQENWNTKHSSGV |
125-169 |
0.5987 |
Antigenic |
Nonallergen |
Nontoxic |
Development 3D structures and molecular docking of selected epitopes
Activation of T cells can occur if the antigen is successfully presented by MHC-I and Class II to T cells. To achieve this goal, the presented peptides must of course fit with the MHC epitopes. To find this out, we used docking of the selected small peptides with MHC. Selected epitopes of MHC-I and II were developed to build their tertiary structures and docked with their representative MHC allele. As shown in Table S1, Supplementary file 1, MHC-I or CTL epitopes were docked with HLA-A*11-01 allele, while HTL epitopes were docked with HLA DRB1*04-01 allele (shown in Table S1, Supplementary file 1). In CTL epitopes, S142-150(GVYYHKNNK) was the best epitope of the S protein and had the lowest global energy -50.28 Kcal/mol. Meanwhile, the best docked epitopes of nucleocapsid and ORF1a were N53-61(FTALTQHGK) ORF1a3260-3268(AVLQSGFRK) with lowest global energy -39.02 Kcal/mol and -58.65 Kcal/mol, respectively (Fig. 3A).
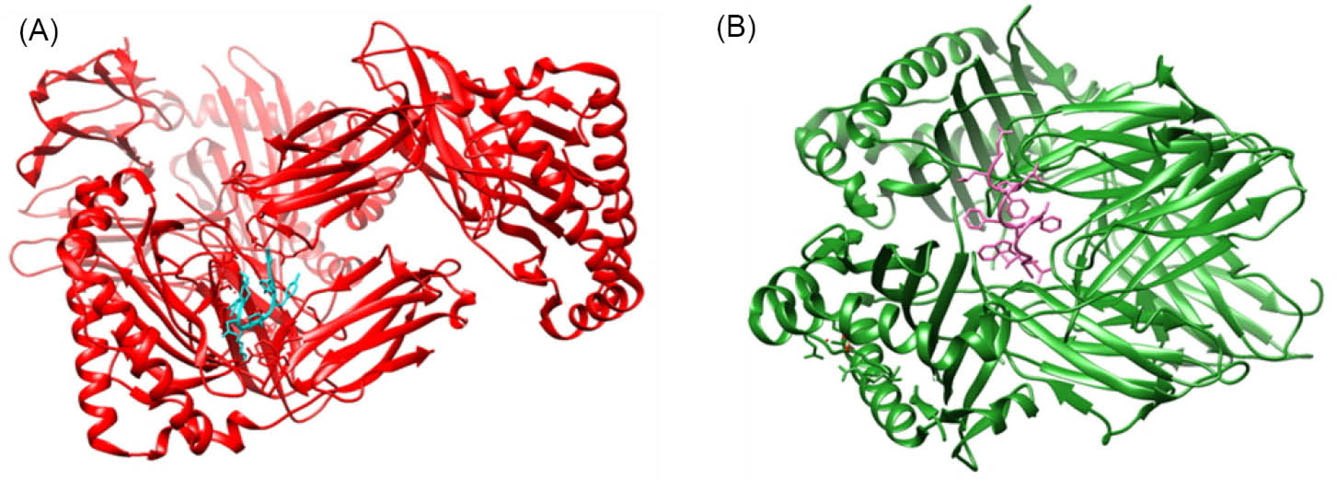
Fig. 3.
Representative figures of docking of MHC-I epitope (cyan) with HLA-A*11-01 (red) (A), and MHC-II Epitope (magenta) with HLA DRB1*04-01 (green) (B).
.
Representative figures of docking of MHC-I epitope (cyan) with HLA-A*11-01 (red) (A), and MHC-II Epitope (magenta) with HLA DRB1*04-01 (green) (B).
Meanwhile, docking between the selected MHC-II or HTL epitopes also generated good interaction of both epitopes and HLA DRB1* 04-01 allele. The best docking of these were namely, S115-126QS LLIVNNATNV, N305-316AQFAPSASAFFG, and ORF1a1188-1199SSFLEMKSEKQV which had global energy -10.79 Kcal/mol, -19.47 Kcal/mol and -1.80 Kcal/mol, respectively (Fig. 3B). The results indicated that the selected epitopes had strong interactions with both MHC alleles.
Constructed vaccines
All selected peptide epitopes including CLT, HTL and BCL were constructed to become multiepitope vaccines using some additional components including adjuvants, PADRE and linkers to link one epitope with another epitope. PADRE is a vaccine component that could increase CTL response. Meanwhile, adjuvants were used to enhance the response of the vaccines, antibody production, and reduce the quantity of antigen input of the constructed vaccines. In this study, we used two different adjuvants which were L7/L12 ribosomal protein and HABA protein.
37
The two constructed vaccines were named CVCoV1 for the multiepitope vaccine with HABA adjuvant and CVCoV2 as the vaccine using the L7/L12 ribosomal protein as adjuvant (Table S3, Supplementary file 1).
The constructed vaccines were further characterized for their physicochemical properties, allergenicity, and the antigenicity which are shown in Table S4, Supplementary file 1. CVCoV1 was the longer and wider constructed vaccine, and the characterization results showed that both of the vaccines were antigenic, non-allergenic and stable. The tertiary structures of the vaccines were built by trRosetta which provides fast and accurate protein structure prediction. The 3D structures of both vaccines are shown in Figs. 4A (CVCoV1) and 5A (CVCoV2). The built structures were further refined and validated using ERRAT and Ramachandran plots. Both of the constructed vaccines showed almost the same validation results. Results showed that the vaccines were closely similar in the Ramachandran plot results of both vaccines and but CVCoV1 had a higher quality factor value than CVCoV2 (Table S5, Supplementary file 1) (Figs. 4B, 4C, 5B and 5C).
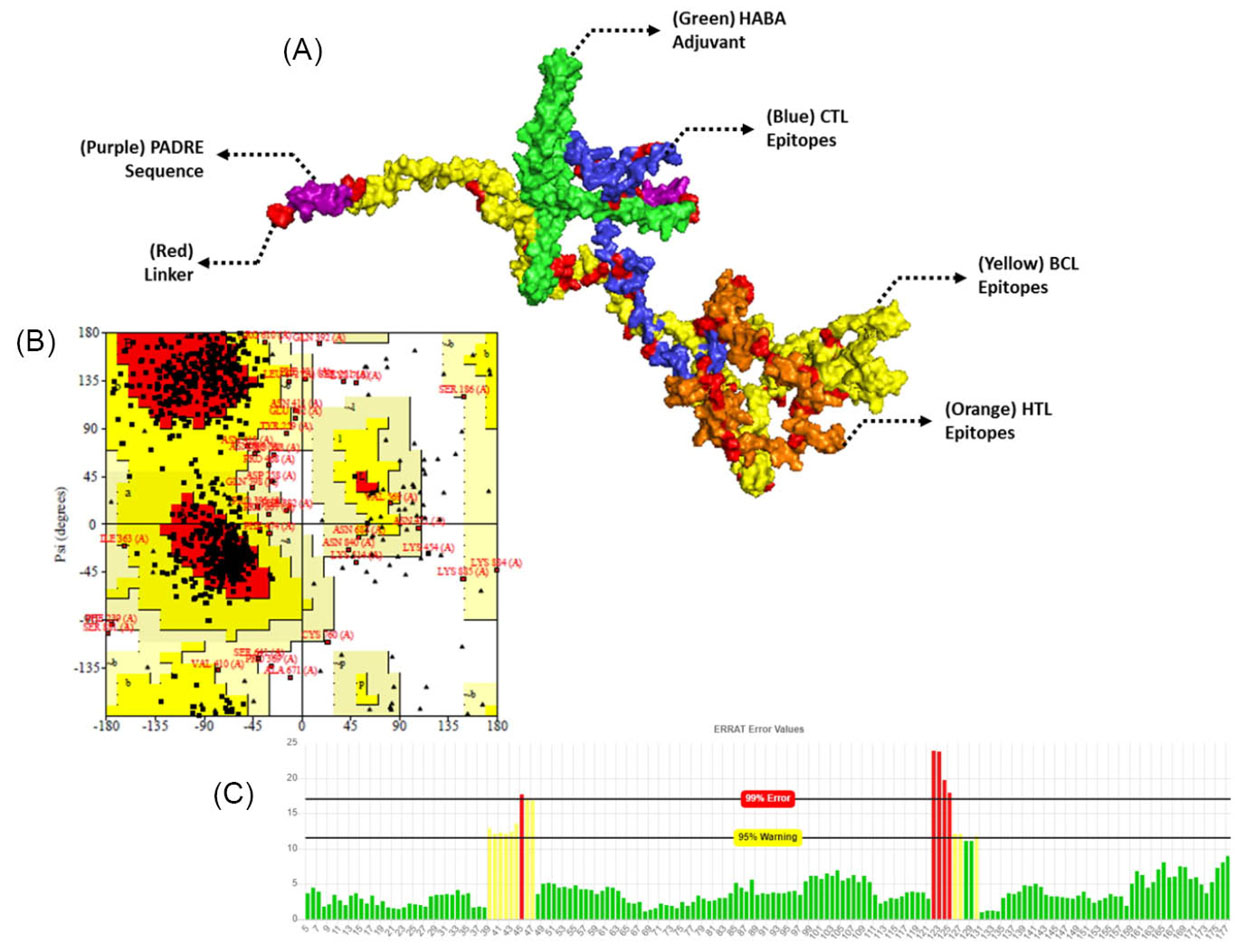
Fig. 4.
Tertiary structure of the constructed vaccine with HABA adjuvant (A), validation and refinement by Ramachandran plot of 3D structure vaccines (B), and ERRAT (C).
.
Tertiary structure of the constructed vaccine with HABA adjuvant (A), validation and refinement by Ramachandran plot of 3D structure vaccines (B), and ERRAT (C).
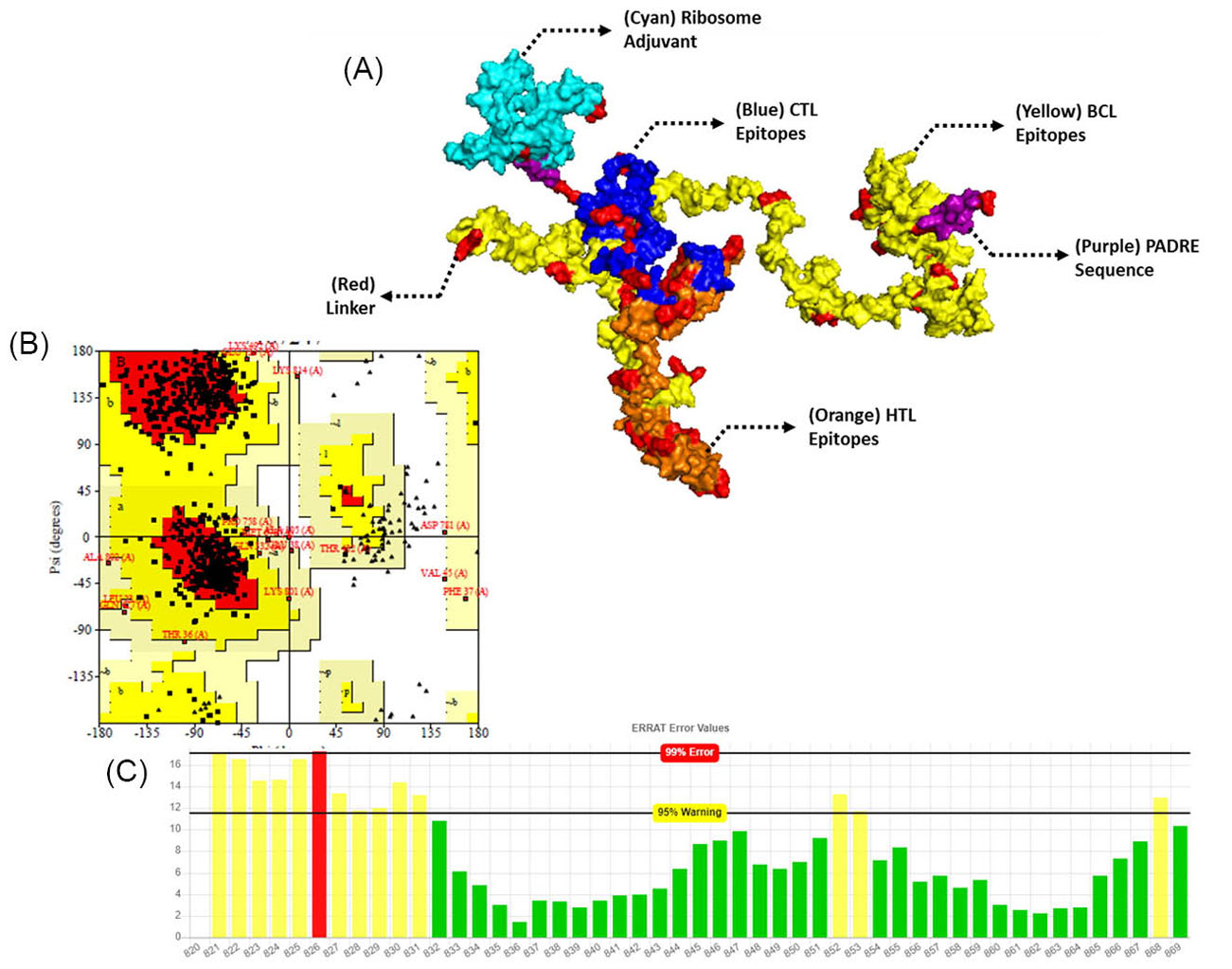
Fig. 5.
Tertiary structure of the constructed vaccine with L7/L12 ribosomal adjuvant (A), validation and refinement by Ramachandran plot of 3D structure vaccines (B), and ERRAT (C).
.
Tertiary structure of the constructed vaccine with L7/L12 ribosomal adjuvant (A), validation and refinement by Ramachandran plot of 3D structure vaccines (B), and ERRAT (C).
Molecular docking of vaccine constructs with TLRs
The constructed vaccines must provide good interaction with body components to stimulate immune system activation. Therefore, the 3D constructed vaccines were further docked with TLR 3 and 8 proteins using ClusPro, PatchDock and further refined by the FireDock server. Docking results of vaccines with TLR 3 and 8 were visualized and are shown in Figs. 6 and 7. Docking results show that both of the constructed vaccines generated strong interactions with both TLR 3 and TLR 8. The vaccine/protein interaction showed the lowest energy level at -1324.9 and -1431.5, for both CVCoV1-TLR3 and TLR8 complex, respectively (shown in Figs. 6A and 6B). Meanwhile, the CVCoV2-TLR3 and TLR8 complex profile had the lowest energy levels at -1206.2 and -1256.3, respectively (shown in Figs. 7A and 7B). The results of docking using the PatchDock and FireDock servers are presented in Table S6, Supplementary Data.
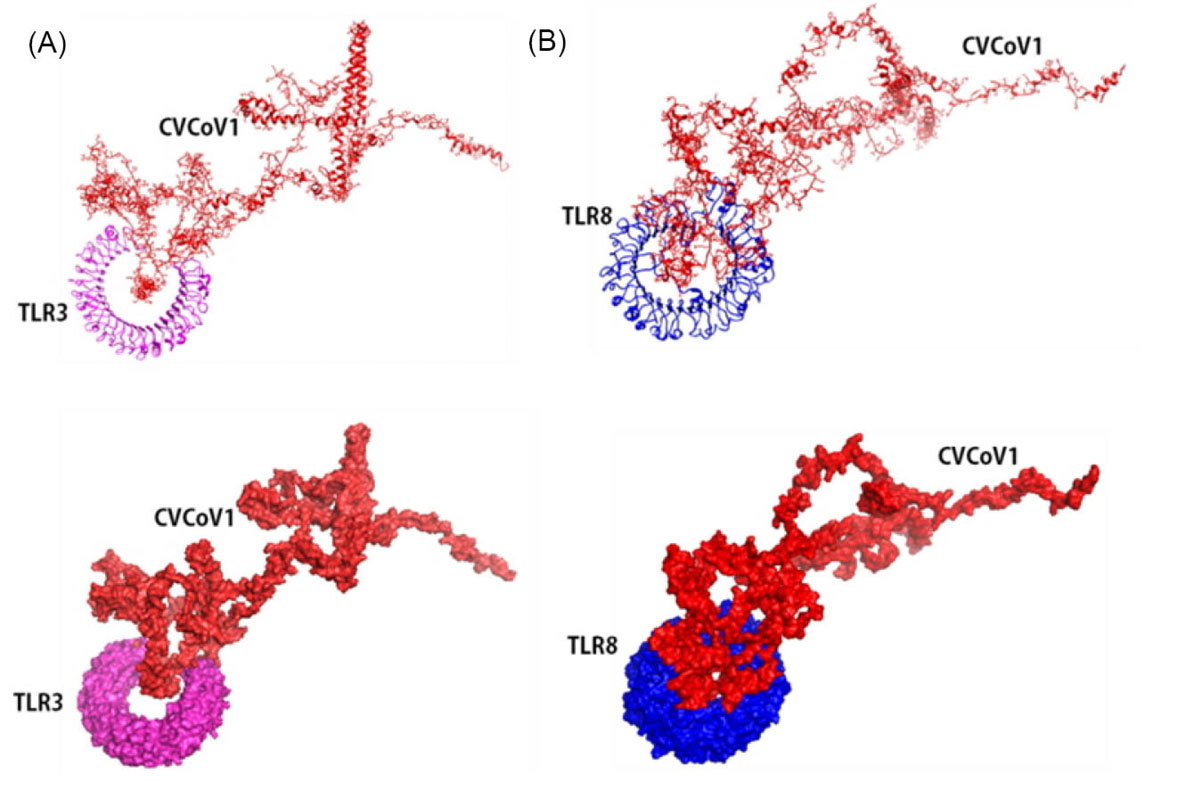
Fig. 6.
Molecular docking of CVCoV1 with Toll Like Receptor 3 and 8. Vaccine docked complex of CVCoV1/TLR3 (A) and CVCoV1/TLR8 (B).
.
Molecular docking of CVCoV1 with Toll Like Receptor 3 and 8. Vaccine docked complex of CVCoV1/TLR3 (A) and CVCoV1/TLR8 (B).
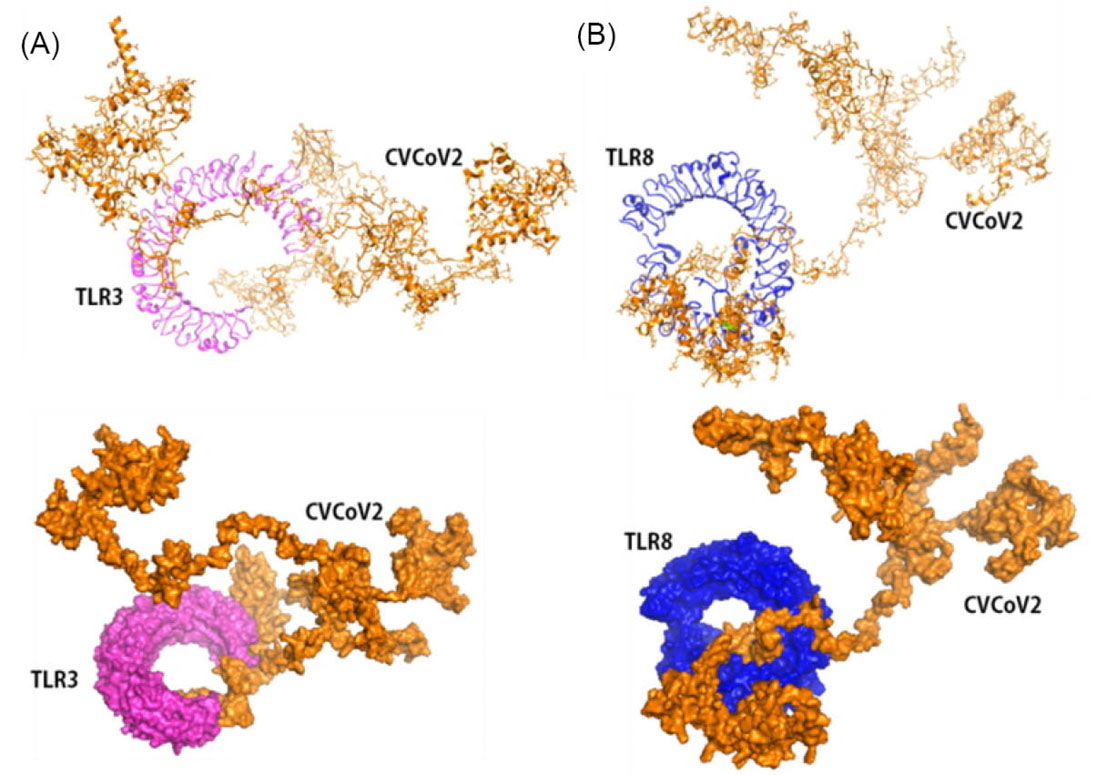
Fig. 7.
Molecular docking of CVCoV2 with Toll-like receptor 3 and 8. Vaccine docked complex of CVCoV2/TLR3 (A) and CVCoV2/TLR8 (C).
.
Molecular docking of CVCoV2 with Toll-like receptor 3 and 8. Vaccine docked complex of CVCoV2/TLR3 (A) and CVCoV2/TLR8 (C).
Molecular docking was also performed to assess the interaction between the constructed vaccines with TLR4 and the host cell receptors of SARS-CoV-2 entry, namely ACE2. The results show strong interactions between the vaccines with TLR4 and ACE2 (Table S6). Docking of CVCoV1-TLR4 and ACE2 complex generated the same results with docking of TLR3 and 8 that had the lowest energy levels, lower than -1000 for both TLR4 (show in Fig. 8A) and ACE2 (shown in Fig. 8B), respectively. Meanwhile, the other docked results in Fig. 6 also generated the expected lowest energy levels in both of the CVCoV2-TLR4 (Fig. 9A) and CVCoV2-ACE2 (Fig. 9B) complexes at -1346.0 and -1296.8, respectively.
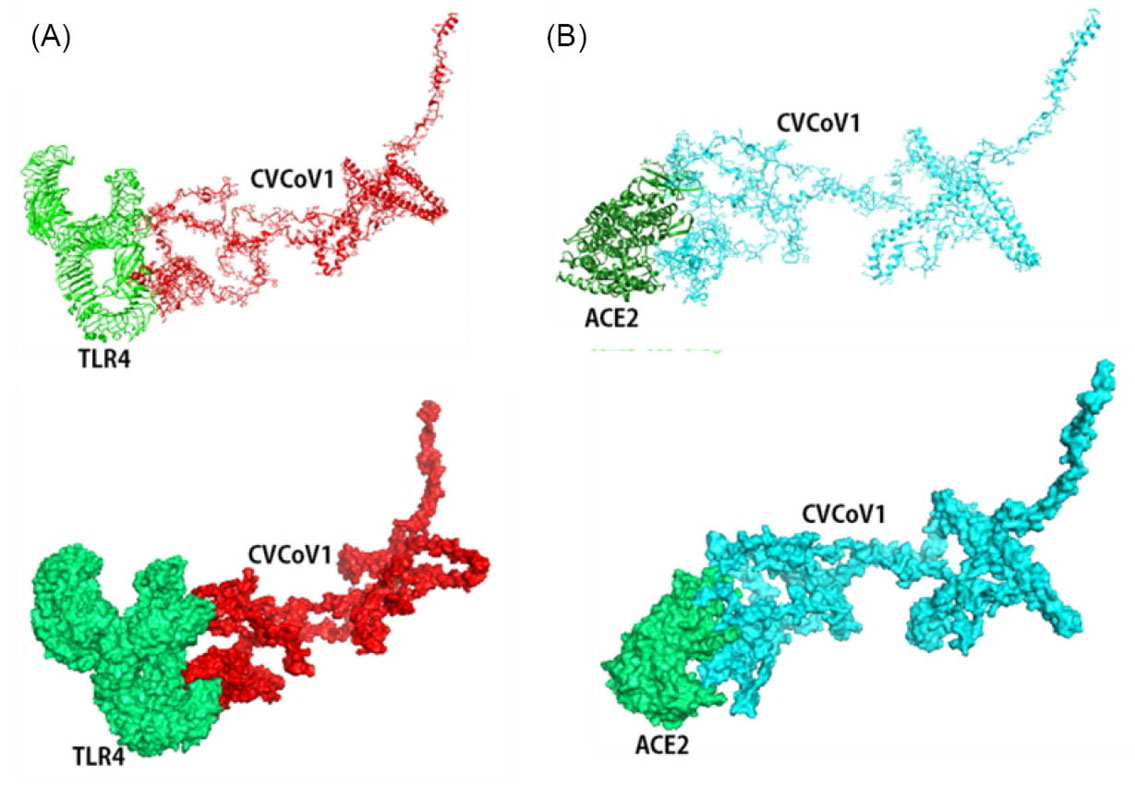
Fig. 8.
Molecular docking of CVCoV1 with Toll-like receptor 4 and angiotensin converting enzyme 2. Vaccine docked complex of CVCoV1/TLR4 (A) and CVCoV1/ACE2 (B).
.
Molecular docking of CVCoV1 with Toll-like receptor 4 and angiotensin converting enzyme 2. Vaccine docked complex of CVCoV1/TLR4 (A) and CVCoV1/ACE2 (B).
Fig. 9.
Molecular docking of CVCoV2 with Toll-like receptor 4 and angiotensin converting enzyme 2. Vaccine docked complex of CVCoV2/TLR4 (A), and CVCoV2/ACE2 (B).
.
Molecular docking of CVCoV2 with Toll-like receptor 4 and angiotensin converting enzyme 2. Vaccine docked complex of CVCoV2/TLR4 (A), and CVCoV2/ACE2 (B).
Discussion
Advances in technology and computerization in the discovery of drugs that effectively treat certain diseases have brought major impact on the development of novel therapies. This also has positively impacted the design and development of vaccines that are effective against SARS-CoV-2, which are currently needed to stop the global spread of the highly infectious disease. The development of the science and method of bioinformatics has had significant impact in the design of an effective vaccine. The approach is used by immunologists in designing vaccines against many viruses and bacteria that cause deadly diseases, including Ebola virus,
7
human immunodeficiency virus (HIV),
38
infectious bronchitis virus (IBV),
39
HPV,
40
SARS-CoV,
41
Streptococcus pneumoniae,
42
and the newly developed Echinococcus granulosusvaccine that was proven effective in vitro and in vivo.
43
The development of vaccines using the bioinformatic method approach or known as immunoinformatics is done by identifying immunodominant T- and B-cell epitopes from several proteins that are considered to play key roles in the primary infection of a virus. They are further constructed into multi-epitope vaccines. In several recently conducted studies, additional sequences of viral epitopes such as adjuvants and PADRE were considered effective to create successful vaccines.
7,30,44,45
In the present study, we designed multi-epitope vaccines against SARS-CoV-2 that were constructed from selected CTL, HTL and B-cell epitopes which were extracted from spike, nucleocapsid, and ORF1a peptide proteins of SARS-CoV-2.
These three proteins are the most important components in viral entry, infectivity and viral replication. Spike protein is a key protein for SARS-CoV-2 entry in human cells by binding to its receptor, ACE2 on the surface of the host cells. Currently, many therapeutic developments have made this protein as the main target of vaccines and drug development. In addition, nucleocapsid and ORF1a are also two other important components of this novel coronavirus. Nucleocapsid or N proteins play a key role in binding and protecting the viral genome.
Vaccination is expected to activate B cells and T cells which are very important components in the body's immunity against SARS-CoV-2 infection. Vaccines containing viral antigens are expected to be recognized by antigenic presenting cells, namely dendritic cells, macrophages and B cells which further present antigens to MHC class I and II.
44,46
Presentation of the antigens to MHC class I will further induce activation of CD8+ T cells, as effector cells that can directly eradicate virus or virus-related infected cells. Meanwhile, presentation of viral peptides by MHC class II will trigger the activation of T helper cells, CD4+ T cells that further trigger activation of B cells. The activated B cells will then be triggered to produce antibodies and memory B cells that will protect the patient in the long-term when a real infection occurs.
7,44,46
The humoral response from memory B-cells can easily overcome the emergence of SARS-CoV-2. One previously conducted in silico study by Khairkhah et al demonstrated that designing vaccines from multi-epitopes based on the S, N and M proteins of SARS CoV-2 could generate promising results. They predicted some epitopes with good characteristics including non-allergenic, high quality proteasomal cleavage could induce IL-10, IFN, IgG and IgA responses. Furthermore, their constructed vaccine showed strong interactions with TLRs 2, 3 and 4.
24
The similar study conducted by Rahman et al showed that their vaccine constructed from multi-epitopes against SARS-CoV-2 had strong interactions with TLR4, TLR7, and TLR8.
30
All selected epitopes of MHC-I, MHC-II alleles, and B-cell that were extracted from spike, nucleocapsid, and ORF1a proteins showed good immunogenic properties and were predicted not to cause allergenic and toxic reactions in host cells. These are important profile characteristics of the effectivity of vaccines to stimulate immune response for further production of neutralizing antibodies, memory B and T cells and their safety.
The immunogenic and nonallergenic MHC class II epitopes were further tested for their ability to induce IL-10. Most of MHC class II epitopes were predicted to induce IL-10 including S53-64DLFLPFFSNVTW, S52-63QDLFLPFFSNVT, N344-355PNFKDQVILLNK N305-316AQFAPSASAFFG, ORF1a1188-1199SSFLEMKSEKQV, since they are expected to induce T helper cell response that subsequently activates B-cells, macrophage and cytotoxic T-cells.
24
Selected epitopes of MHC-I and MHC-II also showed good docking results with representative MHC alleles (HLA-A*11−01 and HLA DRB1*04−01 allele), which indicate that they could react well with their target MHC to induce an immune response.
14
HLA‐A*11:01 is a predominant MHC-I allele in Asia and found in East and Southeast Asia which is associated with SARS-CoV-2 infection. These alleles have been linked to immune responses in upper respiratory tract infections, the influenza vaccination process and the novel coronavirus infection.
47,48
Likewise, the HLA DRB1 *04−01 allele which is found in many Caucasians and Saudi Arabians is also associated with immune responses to COVID-19.
49,50
Both of these alleles have been widely used and are most commonly used as representatives of the MHC-I and MHC-II alleles to assess the possible interaction of COVID-19 vaccine design and vaccine design for other viruses.
7,14,30,44
In activation of the immune response, MHC-II or helper T cell epitopes could also overlap and activate B cells. Apart from overlapping helper T cells, B cells can also act as antigen presenting cells, that could recognize, process and present peptide antigens to effector immune cells. Therefore, the prediction of B cell epitopes is also absolutely necessary.
46
In this study, we found that the small peptides produced in prediction B cell epitopes are antigenic, non-allergenic and non-toxic. This situation allows for the activation of immune cells, and ultimately the B cells themselves. This finding suggested activation of immune system cells could produce the formation of antibodies, memory B cells and memory T cells.
41,44,46,51-53
Constructions of selected epitopes to become vaccines were linked by some linkers such as GPGPG. It is glycine rich linker which is often used to link epitopes in vaccine construction since it could increase binding and enable accessibility of the adjoining domain.
7,14,30,51
The constructed vaccines produced in this study had good characteristics. They had molecular weight 96 631 and 92 443 kDa and instability index were more than 31 which is classified as stable vaccines. Additionally, they showed good immunogenic properties and were nonallergenic so that the vaccines were predicted to not cause an allergic reaction in patients. Both of the vaccines are also predicted to have extinction coefficient of 54 235 and 49, 765 M−1cm−1, while the aliphatic index and theoretical pI are almost the same, namely 61.20 vs 62.10 and 10.09 vs 10.04, respectively for both CVCoV-1 and 2. This aliphatic index corresponds to relative volume occupied by aliphatic amino acids in the side chains of the vaccines and also indicates the thermostability of the vaccine constructs.
14,51,54
Meanwhile, the constructed vaccines show that they have hydrophilic properties and high solubility in water. It is indicated by the negativity of GRAVY value, namely -0.732 and -0.639 of both vaccines.
55
The validation of 3D structures of constructed vaccines by ERRAT and Ramachandran plot analysis generated a good quality factor of 90.75 vs 71.43 and high percentage of favored region with 79% vs 83.2%, both for vaccine CVCoV1 and 2, respectively.
The vaccines that are produced should be able to recognize and interact with the receptors in the host cells, for further activation immune responses. To assess it, the constructed vaccines were docked with three important and different TLRs, namely TLR3, TLR4 and TLR8 and ACE2, which are the entry receptors of SARS-CoV-2. TLR3 are endosomal receptors that can recognize presence of RNA or dsRNA genome of coronavirus which further induces immune responses.
9
Meanwhile, TLR8 are receptors that can induce dendritic cell activation and subsequently initiate T helper cell 1 (Th1 cell) and CD8 + T cell responses. TLR8 activation can also induce interferons to stop viral pathogenesis.
10,30
The present study showed strong interactions in both of the constructed vaccines with TLR3 and 8 (Figs. 6 and 7) that generate the lowest energy level (LEL). The LEL of both docked constructed vaccines were estimated to be more than -1000. These results indicate that the vaccine produced is predicted to activate TLR3 and 8 to induce both humoral and cellular immune responses.
Meanwhile, the docking results with TLR4 also showed strong interactions between the vaccines and the receptors as shown in Fig. 8, as well as with the viral entry receptor, namely ACE2 as shown in Fig. 9. These two proteins, TLR4 and ACE2 play an important role in the introduction of viral antigens to trigger the host's immune response. TLR4 are prototypical pattern recognition receptors that have the ability to recognize structural and non-structural components of the virus to stimulate production of inflammatory cytokines.
56
Whereas, ACE2 are cellular receptors of humans which are responsible for SARS-CoV-2 entry into the host cell. The SARS-CoV-2 spike can bind to ACE2 and mediate viral entry into host cells.
57,58
Research Highlights
What is the current knowledge?
√ Globally, there have been more than 190 million people diagnosed and 4.09 million deaths because of COVID-19 infection.
√ Researchers developed several effective vaccines to break the chain of pandemic.
√ Vaccine development needs support from animal trials.
What is new here?
√ We propose a new approach to vaccine development using immunoinformatics that designs vaccines from virus peptides with adjuvants.
√ The constructed multi-epitope vaccines have good characteristics and provide strong interactions with TLR3, 4, 8 and ACE2.
Conclusion
In summary, we concluded spike, nucleocapsid and ORF1a proteins could be used as precursors for designing multiantigen and multi-epitope vaccines using the immunoinformatics approach. Furthermore, the constructed vaccines provide good characteristics. The vaccines were predicted to generate strong interactions with several receptors including TLR3, 4, 8 and ACE2 and further suggested to stimulate production of immune defenses including antibodies, memory T and B cells as the aim of vaccination. However, these results cannot be immediately generalized to indicate that they (both of the constructed vaccines, CVCoV1 and CVCoV2) can induce real immune responses in infected human hosts, so further research is needed to confirm the findings in this study.
Acknowledgements
The authors gratefully thank the Department of Pharmacology and Therapy, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Indonesia for support in conducting this research.
Funding sources
Not applicable.
Ethical statement
No applicable.
Competing interests
None declared.
Authors' contribution
YY designed the project, conducted experiments, analyzed the data and wrote the manuscript. ZS conducted experiments, analyzed the data, performed statistical analyses and wrote manuscript. MAA conducted experiments and wrote manuscript. NSS conducted experiments, wrote manuscript and discussion. IA, HH, SAD and FH: scientific support, critically reviewed the manuscript and revised the grammatical errors. All authors read and approved the final manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary Materials
Supplementary file 1 contains Tables S1-S6.
(pdf)
References
- Zheng J. SARS-CoV-2: an emerging coronavirus that causes a global threat. Int J Biol Sci 2020; 16:1678-85. doi: 10.7150/ijbs.45053 [Crossref] [ Google Scholar]
- Mittal A, Manjunath K, Ranjan RK, Kaushik S, Kumar S, Verma V. COVID-19 pandemic: insights into structure, function, and hACE2 receptor recognition by SARS-CoV-2. PLoSPathog 2020; 16:e1008762. doi: 10.1371/journal.ppat.1008762 [Crossref] [ Google Scholar]
-
Coronavirus (COVID-19) [database on the Internet]. WHO. 2020 [cited November 29, 2020]. Available from: https://who.sprinklr.com/
- Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ 2020; 371:m3939. doi: 10.1136/bmj.m3939 [Crossref] [ Google Scholar]
- Ysrafil Y, Astuti I, Mus R, Gama NI, Rahmaisyah D, Nur’amalia R. A summary of Coronavirus Disease 2019: what we should know?. Pharm Sci 2020; 26:S24-S35. [ Google Scholar]
-
Lozano JM, Salazar LM, Torres Á, Arévalo-Jamaica A, Franco-Muñoz C, Mercado-Reyes M, et al. COVID-19 infection detection and prevention by SARS-CoV-2 active antigens: a synthetic vaccine approach. Vaccines (Basel) 2020; 8; 692. 10.3390/vaccines8040692
- Ullah MA, Sarkar B, Islam SS. Exploiting the reverse vaccinology approach to design novel subunit vaccines against Ebola virus. Immunobiology 2020; 225:151949. doi: 10.1016/j.imbio.2020.151949 [Crossref] [ Google Scholar]
-
Huang L, Rong Y, Pan Q, Yi K, Tang X, Zhang Q, et al. SARS-CoV-2 vaccine research and development: conventional vaccines and biomimetic nanotechnology strategies. Asian J Pharm Sci 2020. 10.1016/j.ajps.2020.08.001
- Astuti I, Ysrafil Ysrafil. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. Diabetes MetabSyndr 2020; 14:407-12. doi: 10.1016/j.dsx.2020.04.020 [Crossref] [ Google Scholar]
- Yazdani Z, Rafiei A, Yazdani M, Valadan R. Design an efficient multi-epitope peptide vaccine candidate against SARS-CoV-2: an in silico analysis. Infect Drug Resist 2020; 13:3007-22. doi: 10.2147/idr.S264573 [Crossref] [ Google Scholar]
-
Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020; 181: 281-92.e6. 10.1016/j.cell.2020.02.058
- Pourseif MM, Parvizpour S, Jafari B, Dehghani J, Naghili B, Omidi Y. A domain-based vaccine construct against SARS-CoV-2, the causative agent of COVID-19 pandemic: development of self-amplifying mRNA and peptide vaccines. Bioimpacts 2021; 11:65-84. doi: 10.34172/bi.2021.11 [Crossref] [ Google Scholar]
- Tahir Ul Qamar M, Rehman A, Tusleem K, Ashfaq UA, Qasim M, Zhu X. Designing of a next generation multiepitope based vaccine (MEV) against SARS-COV-2: immunoinformatics and in silico approaches. PLoS One 2020; 15:e0244176. doi: 10.1371/journal.pone.0244176 [Crossref] [ Google Scholar]
- Sarkar B, Ullah MA, Johora FT, Taniya MA, Araf Y. Immunoinformatics-guided designing of epitope-based subunit vaccines against the SARS Coronavirus-2 (SARS-CoV-2). Immunobiology 2020; 225:151955. doi: 10.1016/j.imbio.2020.151955 [Crossref] [ Google Scholar]
- Doytchinova IA, Flower DR. VaxiJen: a server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinformatics 2007; 8:4. doi: 10.1186/1471-2105-8-4 [Crossref] [ Google Scholar]
- Gasteiger E, Hoogland C, Gattiker A, Wilkins MR, Appel RD, Bairoch A. Protein identification and analysis tools on the ExPASy server. In: The Proteomics Protocols Handbook. Springer; 2005. p. 571-607.
- Vita R, Overton JA, Greenbaum JA, Ponomarenko J, Clark JD, Cantrell JR. The Immune Epitope Database (IEDB) 3.0. Nucleic Acids Res 2015; 43:D405-12. doi: 10.1093/nar/gku938 [Crossref] [ Google Scholar]
- Vita R, Mahajan S, Overton JA, Dhanda SK, Martini S, Cantrell JR. The Immune Epitope Database (IEDB): 2018 update. Nucleic Acids Res 2019; 47:D339-d43. doi: 10.1093/nar/gky1006 [Crossref] [ Google Scholar]
- Larsen JE, Lund O, Nielsen M. Improved method for predicting linear B-cell epitopes. Immunome Res 2006; 2:2. doi: 10.1186/1745-7580-2-2 [Crossref] [ Google Scholar]
- Dimitrov I, Naneva L, Doytchinova I, Bangov I. AllergenFP: allergenicity prediction by descriptor fingerprints. Bioinformatics 2014; 30:846-51. doi: 10.1093/bioinformatics/btt619 [Crossref] [ Google Scholar]
- Solanki V, Tiwari V. Subtractive proteomics to identify novel drug targets and reverse vaccinology for the development of chimeric vaccine against Acinetobacter baumannii. Sci Rep 2018; 8:9044. doi: 10.1038/s41598-018-26689-7 [Crossref] [ Google Scholar]
- Gupta S, Kapoor P, Chaudhary K, Gautam A, Kumar R, Raghava GP. In silico approach for predicting toxicity of peptides and proteins. PLoS One 2013; 8:e73957. doi: 10.1371/journal.pone.0073957 [Crossref] [ Google Scholar]
- Nagpal G, Usmani SS, Dhanda SK, Kaur H, Singh S, Sharma M. Computer-aided designing of immunosuppressive peptides based on IL-10 inducing potential. Sci Rep 2017; 7:42851. doi: 10.1038/srep42851 [Crossref] [ Google Scholar]
- Khairkhah N, Aghasadeghi MR, Namvar A, Bolhassani A. Design of novel multiepitope constructs-based peptide vaccine against the structural S, N and M proteins of human COVID-19 using immunoinformatics analysis. PLoS One 2020; 15:e0240577. doi: 10.1371/journal.pone.0240577 [Crossref] [ Google Scholar]
- Lamiable A, Thévenet P, Rey J, Vavrusa M, Derreumaux P, Tufféry P. PEP-FOLD3: faster de novo structure prediction for linear peptides in solution and in complex. Nucleic Acids Res 2016; 44:W449-54. doi: 10.1093/nar/gkw329 [Crossref] [ Google Scholar]
- María R, Arturo C, Alicia JA, Paulina M, Gerardo AO. The impact of bioinformatics on vaccine design and development. In: Vaccines. Rijeka, Croatia; InTech; 2017.
- Schneidman-Duhovny D, Inbar Y, Nussinov R, Wolfson HJ. PatchDock and SymmDock: servers for rigid and symmetric docking. Nucleic Acids Res 2005; 33:W363-7. doi: 10.1093/nar/gki481 [Crossref] [ Google Scholar]
- Mashiach E, Schneidman-Duhovny D, Andrusier N, Nussinov R, Wolfson HJ. FireDock: a web server for fast interaction refinement in molecular docking. Nucleic Acids Res 2008; 36:W229-32. doi: 10.1093/nar/gkn186 [Crossref] [ Google Scholar]
-
Ysrafil Y, Astuti I. Chitosan nanoparticle antimiRNA-324-5p effect to decrease ovarian cancer cell proliferation by regulation of GLI1 expression. Bioimpacts 2020. 10.34172/bi.2021.22119
- Rahman N, Ali F, Basharat Z, Shehroz M, Khan MK, Jeandet P. Vaccine design from the ensemble of surface glycoprotein epitopes of SARS-CoV-2: an immunoinformatics approach. Vaccines (Basel) 2020; 8:423. doi: 10.3390/vaccines8030423 [Crossref] [ Google Scholar]
- Dimitrov I, Flower DR, Doytchinova I. AllerTOP: a server for in silico prediction of allergens. BMC Bioinformatics 2013; 14 Suppl 6:S4. doi: 10.1186/1471-2105-14-s6-s4 [Crossref] [ Google Scholar]
- Yang J, Anishchenko I, Park H, Peng Z, Ovchinnikov S, Baker D. Improved protein structure prediction using predicted interresidue orientations. Proc Natl Acad Sci U S A 2020; 117:1496-503. doi: 10.1073/pnas.1914677117 [Crossref] [ Google Scholar]
- Colovos C, Yeates TO. Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci 1993; 2:1511-9. doi: 10.1002/pro.5560020916 [Crossref] [ Google Scholar]
- Ramachandran GN, Ramakrishnan C, Sasisekharan V. Stereochemistry of polypeptide chain configurations. J Mol Biol 1963; 7:95-9. doi: 10.1016/s0022-2836(63)80023-6 [Crossref] [ Google Scholar]
- Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Cryst 1993; 26:283-91. doi: 10.1107/S0021889892009944 [Crossref] [ Google Scholar]
- Comeau SR, Gatchell DW, Vajda S, Camacho CJ. ClusPro: a fully automated algorithm for protein-protein docking. Nucleic Acids Res 2004; 32:W96-9. doi: 10.1093/nar/gkh354 [Crossref] [ Google Scholar]
- Wu CY, Monie A, Pang X, Hung CF, Wu TC. Improving therapeutic HPV peptide-based vaccine potency by enhancing CD4+ T help and dendritic cell activation. J Biomed Sci 2010; 17:88. doi: 10.1186/1423-0127-17-88 [Crossref] [ Google Scholar]
- Chuang GY, Acharya P, Schmidt SD, Yang Y, Louder MK, Zhou T. Residue-level prediction of HIV-1 antibody epitopes based on neutralization of diverse viral strains. J Virol 2013; 87:10047-58. doi: 10.1128/jvi.00984-13 [Crossref] [ Google Scholar]
- Awadelkareem EA, Ali SA. Vaccine design of coronavirus spike (S) glycoprotein in chicken: immunoinformatics and computational approaches. Transl Med Commun 2020; 5:13. doi: 10.1186/s41231-020-00063-0 [Crossref] [ Google Scholar]
- Baidya S, Das R, Kabir MG, Arifuzzaman M. Epitope design of L1 protein for vaccine production against Human Papilloma Virus types 16 and 18. Bioinformation 2017; 13:86-93. doi: 10.6026/97320630013086 [Crossref] [ Google Scholar]
- Oany AR, Emran AA, Jyoti TP. Design of an epitope-based peptide vaccine against spike protein of human coronavirus: an in silico approach. Drug Des DevelTher 2014; 8:1139-49. doi: 10.2147/dddt.S67861 [Crossref] [ Google Scholar]
- Dorosti H, Eslami M, Nezafat N, Fadaei F, Ghasemi Y. Designing self-assembled peptide nanovaccine against Streptococcus pneumoniae: an in silico strategy. Mol Cell Probes 2019; 48:101446. doi: 10.1016/j.mcp.2019.101446 [Crossref] [ Google Scholar]
- Pourseif MM, Moghaddam G, Nematollahi A, Khordadmehr M, Naghili B, Dehghani J. Vaccination with rEGVac elicits immunoprotection against different stages of Echinococcus granulosus life cycle: a pilot study. Acta Trop 2021; 218:105883. doi: 10.1016/j.actatropica.2021.105883 [Crossref] [ Google Scholar]
- Naz A, Shahid F, Butt TT, Awan FM, Ali A, Malik A. Designing multi-epitope vaccines to combat emerging Coronavirus Disease 2019 (COVID-19) by employing immuno-informatics approach. Front Immunol 2020; 11:1663. doi: 10.3389/fimmu.2020.01663 [Crossref] [ Google Scholar]
- Waqas M, Haider A, Sufyan M, Siraj S, Sehgal SA. Determine the potential epitope based peptide vaccine against novel SARS-CoV-2 targeting structural proteins using immunoinformatics approaches. Front Mol Biosci 2020; 7:227. doi: 10.3389/fmolb.2020.00227 [Crossref] [ Google Scholar]
- Janeway C, Murphy K, Travers P, Walport M. Janeway's Immunobiology. New York: Garland Science; 2008.
- Liu SD, Su J, Zhang SM, Dong HP, Wang H, Luo W. Identification of HLA-A*11:01-restricted Mycobacterium tuberculosis CD8(+) T cell epitopes. J Cell Mol Med 2016; 20:1718-28. doi: 10.1111/jcmm.12867 [Crossref] [ Google Scholar]
-
Warren RL, Birol I. Retrospective in silico HLA predictions from COVID-19 patients reveal alleles associated with disease prognosis [Preprint]. medRxiv 2020. 10.1101/2020.10.27.20220863
-
Langton DJ, Bourke SC, Lie BA, Reiff G, Natu S, Darlay R, et al. The influence of HLA genotype on susceptibility to, and severity of, COVID-19 infection [Preprint]. medRxiv 2021. 10.1101/2020.12.31.20249081
- Naemi F, Al-adwani S, Al-khattabi H, Al-nazawi A. Frequency of HLA alleles among COVID-19 infected patients: Preliminary data from. Virology 2021; 560:1-7. doi: 10.1016/j.virol.2021.04.011 [Crossref] [ Google Scholar]
- Kar T, Narsaria U, Basak S, Deb D, Castiglione F, Mueller DM. A candidate multi-epitope vaccine against SARS-CoV-2. Sci Rep 2020; 10:10895. doi: 10.1038/s41598-020-67749-1 [Crossref] [ Google Scholar]
- Lim HX, Lim J, Jazayeri SD, Poppema S, Poh CL. Development of multi-epitope peptide-based vaccines against SARS-CoV-2. Biomed J 2021; 44:18-30. doi: 10.1016/j.bj.2020.09.005 [Crossref] [ Google Scholar]
- Lin L, Ting S, Yufei H, Wendong L, Yubo F, Jing Z. Epitope-based peptide vaccines predicted against novel coronavirus disease caused by SARS-CoV-2. Virus Res 2020; 288:198082. doi: 10.1016/j.virusres.2020.198082 [Crossref] [ Google Scholar]
- Ikai A. Thermostability and aliphatic index of globular proteins. J Biochem 1980; 88:1895-8. [ Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol 1982; 157:105-32. doi: 10.1016/0022-2836(82)90515-0 [Crossref] [ Google Scholar]
- Brandão SCS, Ramos JOX, Dompieri LT, Godoi E, Figueiredo JL, Sarinho ESC. Is Toll-like receptor 4 involved in the severity of COVID-19 pathology in patients with cardiometabolic comorbidities?. Cytokine Growth Factor Rev 2020; 58:102-10. doi: 10.1016/j.cytogfr.2020.09.002 [Crossref] [ Google Scholar]
- Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol 2020; 251:228-48. doi: 10.1002/path.5471 [Crossref] [ Google Scholar]
- Yang J, Petitjean SJL, Koehler M, Zhang Q, Dumitru AC, Chen W. Molecular interaction and inhibition of SARS-CoV-2 binding to the ACE2 receptor. Nat Commun 2020; 11:4541. doi: 10.1038/s41467-020-18319-6 [Crossref] [ Google Scholar]