Bioimpacts. 13(2):159-179.
doi: 10.34172/bi.2023.23839
Review
Functionality of immune cells in COVID-19 infection: development of cell-based therapeutics
Maryam Sayahinouri Investigation, Resources, Writing – original draft, 1, 2, # 
Sahar Mashayekhi Firouz Investigation, Resources, Writing – original draft, 1, # 
Amin Ebrahimi Sadrabadi Investigation, Resources, 3, 4, # 
Mina Masoudnia Investigation, Resources, 5 
Mahnaz Abdolahi Investigation, Resources, 5 
Fatemeh Jafarzadeh Investigation, Resources, 6 
Meshkat Nouripour Investigation, Resources, 7 
Sana Mirzazadeh Investigation, Resources, 8 
Nazanin Zangeneh Investigation, Resources, 9 
Arsalan Jalili Conceptualization, Methodology, Writing – review & editing, 3, 2, 10, * 
Nasser Aghdami Conceptualization, Methodology, Writing – review & editing, 3, * 
Author information:
1Department of Immunology, Afzalipour Faculty of Medicine, Kerman University of Medical Sciences, Kerman, Iran
2Parvaz Research Ideas Supporter Institute, Tehran, Iran
3Department of Stem Cells and Developmental Biology, Royan Institute for Stem Cell Biology and Technology, ACECR, Tehran, Iran
4Cytotech & Bioinformatics Research Group, Tehran, Iran
5Department of Immunology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
6Department of Genetic, Faculty of Modern Sciences, Tehran Medical Branch, Islamic Azad University, Tehran, Iran
7Department of Microbiology, Faculty of Basic Sciences, Karaj Branch, Islamic Azad University, Tehran, Iran
8Department of Biology, Faculty of Biological Sciences, Tehran North Branch, Islamic Azad University, Tehran, Iran
9Department of Biology, Roudehen Branch, Islamic Azad University, Tehran, Iran
10Hematopoietic Stem Cell Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
#These authors contribute equally.
Abstract
Introduction:
In late December 2019, a sudden severe respiratory illness of unknown origin was reported in China. In early January 2020, the cause of COVID-19 infection was announced a new coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Examination of the SARS-CoV-2 genome sequence revealed a close resemblance to the previously reported SARS-CoV and coronavirus Middle East respiratory syndrome (MERS-CoV). However, initial testing of drugs used against SARS-CoV and MERS-CoV has been ineffective in controlling SARS-CoV-2. One of the key strategies to fight the virus is to look at how the immune system works against the virus, which has led to a better understanding of the disease and the development of new therapies and vaccine designs.
Methods: This review discussed the innate and acquired immune system responses and how immune cells function against the virus to shed light on the human body's defense strategies.
Results: Although immune responses have been revealed critical to eradicating infections caused by coronaviruses, dysregulated immune responses can lead to immune pathologies thoroughly investigated. Also, the benefit of mesenchymal stem cells, NK cells, Treg cells, specific T cells, and platelet lysates have been submitted as promising solutions to prevent the effects of infection in patients with COVID-19.
Conclusion: It has been concluded that none of the above has undoubtedly been approved for the treatment or prevention of COVID-19, but clinical trials are underway better to understand the efficacy and safety of these cellular therapies.
Keywords: COVID-19, SARS-CoV-2, Immune responses, Cell therapy, Innate Immune system, Adoptive immune system
Copyright and License Information
© 2023 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
General information about SARS-CoV-2
Coronavirus 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), first diagnosed in December 2019 in Wuhan, China, and since then has globally spread and infected millions.1 Bioinformatics analysis showed that SARS-CoV-2 has the typical characteristics of the coronavirus family.2 Coronaviruses (CoVs) have four main subtypes: alpha, beta, delta, and gamma. Alpha and beta subtypes have been shown to originate from bats and mammals, while gamma and delta often infect birds.3 SARS-CoV-2 belongs to the beta-coronavirus 2B lineage.2 The coronavirus genome (CoVs) (32-27 kb) is a single-stranded positive-sense (ssRNA+) RNA that is larger than any other viral RNA. The nucleocapsid (N) protein forms the capsid outside the genome, and the genome is packaged by a coating linked to three structural proteins: the membrane protein (M), the spike protein (S), and the envelope protein (E). As a member of the coronavirus family, the recently sequenced SARS-CoV-2 genome is approximately 29.9 kb.4 SARS-CoV-2 contains four structural proteins (S, E, M, and N) and sixteen non-structural proteins (nsp1-16). The SARS-CoV-2 genomic RNA consists of two open reading frames (ORFs), ORF1a and ORF1b, covering two-thirds of the genome and translating into polyprotein (pp), pp1a and pp1b proteins. The virus genome encodes two cysteine proteases, a papain-like protease (PLpro) or nsp3 and a 3C-like protease (3CLpro) or nsp5. These proteases divide the pp1a and pp1b polypeptides into 16 unstructured proteins.5 The remaining third of the genome contains overlapping ORFs that encode the four major structural proteins, including S, N, M, E, and some ancillary proteins. S protein of signal peptide (SP), receptor-binding domain (RBD), subdomain1 (SD1), and two subdomains (SD2) in subunit S1 and fusion peptide,6 one heptad repeat (HR1), two heptad repeat (HR2), and transmembrane (TM) is formed in the membrane fusion subunit (S2). Protein E, along with M and N, facilitates virus-like particle formation.7
SARS-CoV-2 enters the host cell by directly integrating the viral coating with the host cell membrane or by integrating the membrane into the endosome after endocytosis. Virus entry begins with the binding of RBD of protein S to human host cell receptors at the cell surface.8 One of the primary receptors for SARS-CoV-2 is the angiotensin-converting enzyme 2 (ACE2), which is widely expressed in the lung, intestine, liver, heart, vascular endothelium, testis, and kidney cells.9 The virus mainly affects the respiratory system, which is the main route of transmission by droplets and respiratory secretions carrying the infectious virus and direct contact with asymptomatic or symptomatic carriers of the virus. Accordingly, the current data indicate a 14-day incubation period.10
The pathogenesis of SARS-CoV-2 pneumonia is described in two stages. The initial phase is characterized by viral replication, resulting in direct tissue-mediated tissue damage, followed by a late phase when infected host cells, using T lymphocytes, monocytes, and neutrophils, stimulate the immune response. It causes the release of cytokines such as tumor necrosis factor-α (TNF-α), granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-1 beta (IL-1β), IL-6, IL-8, IL-12, and interferon-γ (IFN-γ). In the severe form of COVID-19, the overactive immune system leads to a "cytokine storm," characterized by the release of high levels of cytokines, especially IL-6 and TNF-α, into the bloodstream and induces a local and systemic inflammatory response.11 Early in the COVID-19 pandemic, therapeutic management of the disease was limited. Since then, researchers have made significant advances worldwide, leading to a better understanding of the disease and developing new therapies and vaccines. Currently, a variety of treatment options are available, including antiviral drugs, anti-SARS-CoV-2 monoclonal antibodies, ani-inflammatory drugs, and immunomodulatory agents available under FDA-authorized emergency use.12
Innate immune system responses against COVID-19
The immune system mechanism includes innate and adaptive immunity responses. The body's first line of defense against viral infections includes rapid innate immune responses such as activation of complement and toll-like receptors (TLRs), secretion of type Ⅰ IFN, and secretion of inflammatory cytokines, apoptosis, and autophagy. The innate immune system detects the host pathogen-associated molecular patterns13 molecules of the virus with the help of pattern recognition receptor (PRR) receptors. These receptors include TLRs, RIG-like receptors (RLRs), NOD-like receptors (NLRs), C-type lectin-like receptors (CLmin), and free cytoplasmic receptors such as cyclic GMP-AMP synthase (cGAS), interferon-γ-inducible protein 16 (IFI16), Stimulator of interferon genes (STING), and DNA-dependent activator of interferon-regulatory factors.14 When the innate immune system is exposed to microorganisms, the function of this system prepares the adaptive immune system to produce appropriate responses against pathogens.15 This defense barrier is also responsible for causing severe forms of COVID-19 by producing cytokine storms. Therefore, investigating this vital arm of the immune system is crucial for a better understanding of the pathogenicity of this disease. In addition, research on innate immune responses is essential for developing treatment strategies.16
Cells expressing ACE2 are target cells for SARS-CoV-2 infection. In the lung, the virus can infect and damage the mucosal epithelium, alveolar epithelium, bronchial mucosal epithelium, and endothelial cells in the airway. In particular, high levels of ACE2 in type II alveolar epithelial cells in the lungs increase the susceptibility of these cells to SARS-CoV-2 infection. Infection of these cells leads to the pathogenicity of the virus, which plays a role through critical functions. These cells are also involved in immune responses by producing cytokines following alveolar injury. Alveolar injury produces signals which trigger and activate macrophages and other immune cells to elicit an immune response. At the beginning of the infection, local immune responses can successfully maintain and re-establish homeostasis in the airway. One of the essential controllers of viral infection is interferon responses and following events after its activation. Finally, the interaction of innate immune components such as cytokines, chemokines, and cells involved in innate responses activates adaptive immunity and initiates the activity of T lymphocytes.17
Another component of the innate immune response against viruses and bacteria is the complement system, which consists of proteins circulating in the bloodstream in the form of inactive precursors. This system also stimulates pro-inflammatory responses. The complement system functions to activate other immune system components through three pathways, including the classical, alternative, and lectin pathways.16 New evidence has shown that in vivo and in vitro complement activation plays a vital role in the pathogenesis and severity of SARS-CoV-2. Use of virus-infected C3-/- mice and evaluation of complement system activation in SARS-CoV-2 infection indicate that C3 (C: complement component) products (C3a, C3b, inactivated c3 (iC3b), C3c, C3dg) have been observed in the early stages of infection (first day) in the lungs. Complement activation induces pro-inflammatory polypeptides, C3a, C5a, and recruitment of neutrophils and monocytes. Neutrophils produce neutrophil extracellular trap (NET), which contains complement components such as C3, properdin, and factor B, which activate complement through the alternative pathway.18 C5a is a potential cell-signaling protein that activates cytokine storms in the early hours of infection and induces innate immune responses. Excess C5a in the inflammatory environment promotes tissue injury, T lymphocyte exhaustion, and immune paralysis through a plethora of mechanisms.19
The use of complement system inhibitors to treat COVID-19 has begun during the last two years. The first drug used is Eculizumab, a humanized monoclonal antibody that inhibits the breakdown of C5 into C5a and C5b involved in forming the membrane attack complex. This structure (a set of membrane attacks) creates cavities in the plasma membrane that lead to the destruction of the plasma membrane and, ultimately death of the target cell. Inhibition by Eculizumab in four patients with COVID-19 has indicated a reduction in inflammatory markers both in erythrocyte sedimentation rate test (ESR) and C-reactive protein (CRP) test and improvement of patients within the first 48 hours of drug administration. In addition to Eculizumab, patients with COVID-19 used approved AMY-101 as C3 inhibitor of pneumonia. After using this medication, the patient's clinical symptoms improved rapidly after 48 hours, while the patient's leukocytosis and lymphopenia improved at a slower rate. These laboratory markers are associated with improved lung function and reduced oxygen demand.16
Viral infections of mammalian cells elicit rapid innate immune responses. One of these innate antiviral responses is the release of cytokine IFN-I (IFN-α and IFN-β). IFN-γ activates the Janus kinase and signal transducer and activator of transcription (JAK-STAT) signaling pathway, followed by expressing interferon‐stimulated genes (ISG) genes. Finally, the products of these genes prevent the virus from replication and assembly. Genes encoding interferon is regulated by transcription factors nuclear factor kappa-light-chain-enhancer of activated B cells (NF‐κB) and interferon regulatory factor 3 (IRF3). IFN-β activates the synthesis of antiviral, antiproliferative, and immunomodulatory proteins after binding to its receptor at the cell surface. This cytokine also participates in the induction of IFN-α. IFN-α, in turn, enhances antiviral responses.15
PAMPs include proteins, lipids, lipoproteins, and nucleic acids of viral, bacterial, parasitic, and fungal origin detected by TLR receptors. The interaction of these molecules with their respective receptors occurs at the surface of cell membranes, endosomes, lysosomes, and endocytolysosomes. Activation of TLRs induces biological responses following activation of protein adapters such as myeloid differentiation primary response 88 (MyD88), toll/IL-1 receptor adaptor protein (TIRAP), TRAF-interacting protein (TRIP), and TRIF-related adapter molecule (TRAM). These protein adapters are common in the structure of toll/IL-1 receptor1 domains.20 An increase in TLR3 transcription levels after coronavirus infections has been shown in mouse models. In this process, activation of molecules such as TIR-domain-containing adapter-inducing interferon- β21 leads to activating transcription factors such as IRF3 and NF-κB, which ultimately increases producing IFN-I cytokines (IFN-α and IFN-β), inflammatory cytokines (IL-6, TNF) and IFN-γ. Despite the role of TLR3 in producing inflammatory cytokines, the knock-out of the TLR3 gene in mouse models did not reduce the expression of these cytokines. Thus, several alternative pathways associated with TLR3 signaling transduction could produce these cytokines. Two adaptor proteins, TRIF and MyD-88, play an essential role in coronavirus infection during TLR signal transduction pathways. As mentioned above, TRIF is associated with TLR3 signaling transduction and activates IRF3 and NF-κB transcription factors, whereas MyD-88 interacts with TLR4 and several proteins involved in IL-1 function such as IRAK1-2 and IL-1 receptor.21,22 In mouse models, acute pulmonary injury mediated by respiratory virus infections indicates that in infected models with SARS, H1N1, and other pulmonary viruses, this damage is caused by producing oxidized phospholipids. Similar to bacterial LPS, these phospholipids activate TLR4, which activates adaptor proteins and eventually increases the production of inflammatory cytokines. Among these, IL-6 is one of the leading causes of lung damage, so in IL-6-/- mice, the rate of inflammatory infiltration and lung damage have decreased compared to the control group.16 Pro-inflammatory cytokines such as IL-1, IL-6, and TNF-α are the essential cytokines involved in innate immune responses. Tissue macrophages, mast cells, endothelial and epithelial cells are the primary sources of these cytokines during innate immune responses. Cytokine storms are caused by an abrupt increase in various pro-inflammatory cytokines, including IL-1, IL-6, TNF-α, and IFNs. Increasing cytokine levels lead to the infiltration of various immune cells such as macrophages, neutrophils, and T lymphocytes from the bloodstream to the site of infection. The infiltrated cells exert destructive effects on human tissues and result in the instability in cell-cell interactions of endothelial cells, as well as vascular barrier destruction, capillary damage, diffuse alveolar damage, organ failure, and eventually death.23,24 Lung damage is a consequence of cytokine storm that leads to acute lung damage and causes ARDS in severe cases. ARDS reduces saturated oxygen levels, a primary death factor in people with COVID-19 infection. Evidence suggests that several patients with severe COVID-19 infection are affected by a cytokine storm. Detection of cytokines in plasma of 41 patients showed an increase in the following cytokines: IL-1β, IL-7, IL-8, IL-9, IL-10, FGF, granulocyte-colony stimulating factor (G-CSF), GM-CSF, IFN-γ, IP- 10, MCP-1, MIP-1A, MIP1, platelet-derived growth factor (PDGF), TNF-α, vascular endothelial growth factor (VEGF), both in patients in need of intensive care unit (ICU) and those not admitted to ICU compared with healthy people.25 In this review, in addition to representing the innate immune secretory responses, we describe the cellular functions of neutrophils, monocytes/macrophages, dendritic cells, natural killer cells, and mast cells against COVID-19 (Fig. 1).
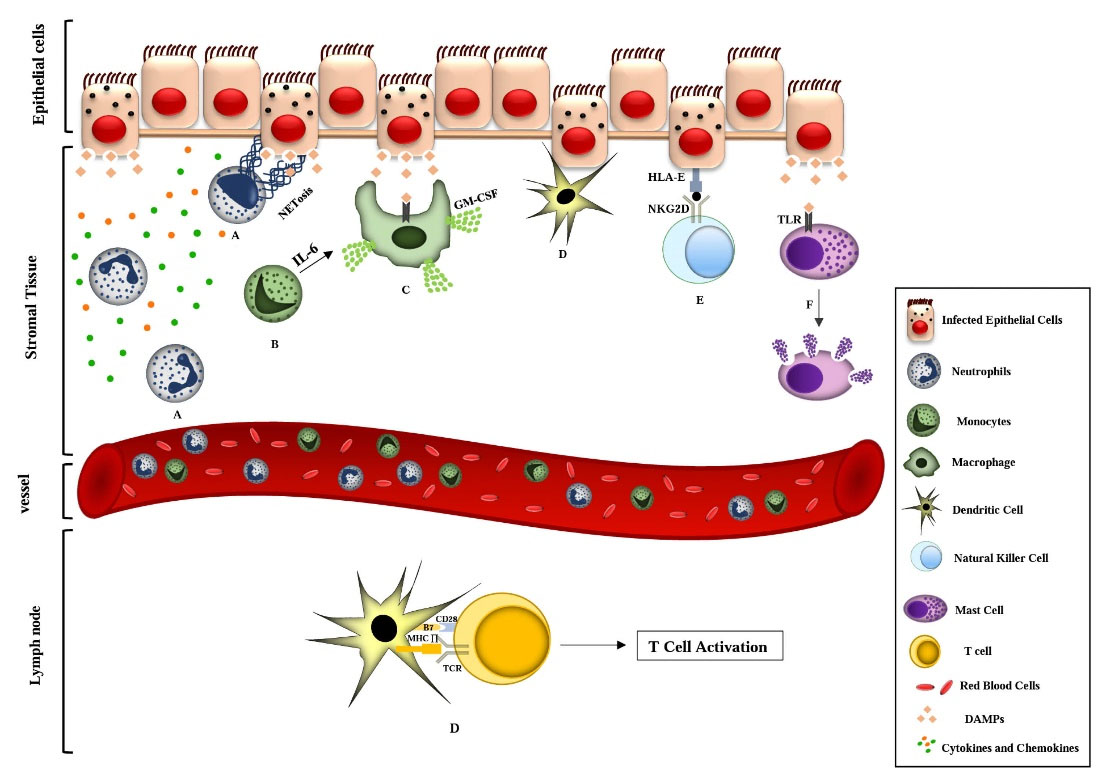

Fig. 1.
Schematic representation of the interaction of innate immune cell responses in COVID-19 in lung tissue. A) After SARS-CoV-2 enters the lung tissue, viral proteins and DAMPs increase inflammatory cytokines, followed by the recruitment of inflammatory cells, including neutrophils. Neutrophilic responses such as increased production of ROS and NETosis lead to the elimination of infection. Also, pro-inflammatory cytokines and chemokines are secreted from this cell because of the recruitment of other inflammatory cells to damaged tissue. B) During the inflammatory process, peripheral blood circulating monocytes enter peripheral tissues and differentiate into macrophage cells (mediated by IL-6) and dendritic cells. C) SARS-CoV-2 -activated macrophages via interaction of DAMPs with PRR receptors phagocytoses the dead tissue and cell debris, resulting in the release of inflammatory factors. One of the cytokines produced by these cells is GM-CSF, which acts as a chemical attractant for the migration of monocytes and neutrophils from the blood to the tissue. D) Virus-infected apoptotic epithelial cells are phagocytosed by dendritic cells, and then these cells migrate to the lymph nodes. After presenting viral antigens to T lymphocytes, they trigger adaptive immune responses against the virus. E) Increased NKG2D expression in NK cells is mediated by virus spike protein. In NK cells, NKG2D expression reduces the expression of IFN-γ, IL-2, TNF-α, and Granzyme B. Expression of HLA-E on the surface of lung epithelial cells and its interaction with NKG2D causes exhaustion in NK cells, which result in an inhibition of the virus-clearing process and ultimately severe damage to lung tissue. F) SARS-CoV-2 virus in the early stages of infection activate airway mast cells and leads to producing pro-inflammatory molecules such as IL-1β, CCL2, IL-6, TNF-α from mast cells. Mast cells detect viral DAMPs through their TLR receptors and cause the inflammation of the lungs and fibrosis by the release of inflammatory mediators. COVID-19, coronavirus Disease of 2019; DAMP, damage-associated molecular patterns; ROS, reactive oxygen species; NET, neutrophil extracellular trap; IL-6, interleukin 6; PRR, pattern recognition receptor; GM-CSF, granulocyte-macrophage colony stimulating factor; NKG2D, natural killer cell group 2 D; NK cells, natural killer cells; IFN-γ, interferon- γ; IL-2, interleukin 2; TNF-α, tumor necrosis factor- α; HLA-E, human leukocyte antigen; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; IL-1β, interleukin 1β; CCL2, CC-chemokine ligand 2; TLR, toll like receptor; MHC, major histocompatibility complex; TCR, T-cell receptor.
.
Schematic representation of the interaction of innate immune cell responses in COVID-19 in lung tissue. A) After SARS-CoV-2 enters the lung tissue, viral proteins and DAMPs increase inflammatory cytokines, followed by the recruitment of inflammatory cells, including neutrophils. Neutrophilic responses such as increased production of ROS and NETosis lead to the elimination of infection. Also, pro-inflammatory cytokines and chemokines are secreted from this cell because of the recruitment of other inflammatory cells to damaged tissue. B) During the inflammatory process, peripheral blood circulating monocytes enter peripheral tissues and differentiate into macrophage cells (mediated by IL-6) and dendritic cells. C) SARS-CoV-2 -activated macrophages via interaction of DAMPs with PRR receptors phagocytoses the dead tissue and cell debris, resulting in the release of inflammatory factors. One of the cytokines produced by these cells is GM-CSF, which acts as a chemical attractant for the migration of monocytes and neutrophils from the blood to the tissue. D) Virus-infected apoptotic epithelial cells are phagocytosed by dendritic cells, and then these cells migrate to the lymph nodes. After presenting viral antigens to T lymphocytes, they trigger adaptive immune responses against the virus. E) Increased NKG2D expression in NK cells is mediated by virus spike protein. In NK cells, NKG2D expression reduces the expression of IFN-γ, IL-2, TNF-α, and Granzyme B. Expression of HLA-E on the surface of lung epithelial cells and its interaction with NKG2D causes exhaustion in NK cells, which result in an inhibition of the virus-clearing process and ultimately severe damage to lung tissue. F) SARS-CoV-2 virus in the early stages of infection activate airway mast cells and leads to producing pro-inflammatory molecules such as IL-1β, CCL2, IL-6, TNF-α from mast cells. Mast cells detect viral DAMPs through their TLR receptors and cause the inflammation of the lungs and fibrosis by the release of inflammatory mediators. COVID-19, coronavirus Disease of 2019; DAMP, damage-associated molecular patterns; ROS, reactive oxygen species; NET, neutrophil extracellular trap; IL-6, interleukin 6; PRR, pattern recognition receptor; GM-CSF, granulocyte-macrophage colony stimulating factor; NKG2D, natural killer cell group 2 D; NK cells, natural killer cells; IFN-γ, interferon- γ; IL-2, interleukin 2; TNF-α, tumor necrosis factor- α; HLA-E, human leukocyte antigen; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; IL-1β, interleukin 1β; CCL2, CC-chemokine ligand 2; TLR, toll like receptor; MHC, major histocompatibility complex; TCR, T-cell receptor.
Neutrophils
Neutrophils are cells that are quickly recruited to the site of infection and exert their immune responses through respiratory bursts and phagocytosis of microorganisms. These cells also cause pathogens' death through forming NET.26 The involvement of these cells in various diseases has made them exciting targets for therapeutic interventions. Various methods have been developed to target neutrophils, including strategies to enhancing, inhibiting, or restoring neutrophil function. Enhancement of neutrophil function is possible by adding G-CSF or inhibiting the CXCR4 receptor and signal-regulating protein (SIRPα). On the other hand, in order to inhibit the function of these cells from selecting antagonists, Anti-integrin antibodies, CXCR (CXC-chemokine receptor) 1, CXCR2, BLT1 (leukotriene B4 receptor 1), and C5aR (C5a receptor) receptor inhibitors, neutralization of neutrophil-derived molecules, and inhibition of NETR inhibitor and N inhibitor Signal transmission molecules are used. There are some diseases in which neutrophils show a marked change from a protective phenotype to a harmful phenotype. In these cases, therapeutic modification of the neutrophil phenotype is considered rather than activating or inhibiting neutrophils. Cancer is a condition in which neutrophils gradually change from a primary anti-tumor phenotype to a pre-tumor phenotype. Myeloid-derived suppressor cells are formed by FATP2 (Fatty acid transport protein 2), resorting to normal neutrophil function by inhibiting lipofermata.27
In a viral infection, expressing viral proteins and local damage to epithelial cells leads to polymorphonuclear (PMN) cell infiltration. The infiltration of inflammatory PMN cells in mice can lead to hemorrhagic wounds. These cells take part in the repair process through virus clearance, producing growth factors, and the re-epithelialization of damaged areas. PMN cells are involved in producing local cytokines and chemokines, which in particular enhance the interaction of these cells with lung cells. In vitro, associating PMN cells with type alveolar epithelial cells infected by the coronavirus, increased mRNA expression of pro-inflammatory cytokines (IL-18, IL-1a, IL-1b, and TNF-α), CXC chemokines (CXC-chemokine ligand-2 (CXCL-2), interferon-inducible protein 10 (IP-10), CXCL-1) and CC-chemokine ligands (CCLs) (CCL- 2, CCL-4, CCL-7, CCL-9, CCL-12, CCL-22). Thus, neutrophils play a role in the early recruitment of inflammatory cells by participating in tissue damage.16
NET is a network-like structure composed of DNA and proteins that trap pathogens and eventually kill the target cell. The formation of NET is a regulatory process. Accordingly, the essential enzymes participate in its formation, including the NE enzyme, causing the breakdown of intracellular proteins and cell fragmentation. Moreover, peptidyl arginine deiminase type-4 enzyme participates in the citrullination of histones to facilitate remodeling chromatin strands and the release of chromosomal DNA, gasdermin D, on the other hand, can facilitate pore forming in the membrane of neutrophils and consequently, the disintegration of cellular membrane for the extrusion of DNA and associated molecules. Excess NET generation can trigger the cascade of inflammatory reactions, and consequently, cancer cell metastasis and destruction of surrounding tissues, microthrombosis, and finally, permanent damage to the organs of the pulmonary, cardiovascular, and renal systems occurs. In patients with severe COVID-19, these organs are affected by this process. NET in the airway mucosal secretions of patients with COVID-19 has a similar role in patients with cystic fibrosis and causes interference in gas exchanges and secondary infection.26
The clinical biochemical data and pathological findings in SARS-CoV-2 infection appear to be associated with NET induced by lung epithelial damage and disease severity. Previous findings on viral lung injury, as well as data on elevated serum NET levels in COVID-19 patients and secreted cytokine profiles, suggested the role of NETs in severe pneumonia associated with SARS-CoV-2 infection. In studies, the rate of NETs in COVID-19 patients increased compared with the healthy control group. Different factors may be related to this mechanism. Initially, the virus’s rapid spread increases the viral load in the lung tissue. Viral proteins are also considered to be potent activators of NETosis. In addition, damaged tissues and neutrophils increase neutrophil activity by producing cytokines. Cytokine secretion occurs after stimulation with SARS-CoV-2. The role of these molecules is the chemotactic recruitment of neutrophils, increased reactive oxygen species (ROS), and the formation of NETosis. Other secretory molecules also induce NETosis from damaged tissues, such as damage-associated molecular patterns.28
Monocytes/Macrophages
Monocytes are innate immune cells involved in inflammatory responses, phagocytosis, antigen presentation, and other functional immune processes. Peripheral circulating monocytes enter the peripheral tissues and differentiate into macrophage and dendritic cells during the inflammatory process. In humans based on the expression of CD14 and CD16 markers, monocytes are divided into three subtypes including (CD14+ CD16-) Classical, Intermediate (CD14+ CD16+) and (CD16+ CD14dim) non-classical.29
Macrophages are a significant component of the mononuclear phagocytic cell system, which plays a significant role in maintaining the body's homeostasis and defense against foreign pathogens. Besides, these cells are abundant in the tumor’s microenvironment and are referred to as tumor-associated macrophages (TAMs). Also, these cells support the survival of tumor cells. Several studies have shown that reducing the effectiveness of these cells has anti-tumor effects on the tumor microenvironment, so they have become targets for immunotherapy. New therapeutic strategies aim to reduce these cells’ population, have targeted their return to M1 macrophages, regulation of phagocytosis signals, and cell engineering. Numerous studies have shown that blocking the CSF-1/CSF-1R and CC-chemokine receptor-2 (CCR2)/CCL2 axis in monocyte cells reduces the population of these cells. The use of NF-κB activators, STAT3 inhibitors, CD40 agonists, and TLRs causes TAMs to return to M1 macrophages. The blockage of MHCⅠ-LIRB1, SIRPa-CD47, and CD24-Siglec-10 axis in the interaction between TAMs and tumor cells also regulates phagocytosis signals. Monocytes/macrophages engineered with chimeric antigen receptor (CAR) also provide immunity against tumor cells. The use of bisphosphonates, Clondlip, Trabectedin, and monoclonal antibodies in the tumor microenvironment reduces the tumorigenic effects of TAMs.30,31
Expansion of CD14+ CD16+ monocytes secreting IL-6 in the peripheral blood of patients with severe COVID-19 admitted to ICU compared to the patients not admitted to ICU was observed, which was confirmed by single-cell RNA-sequencing (scRNA-seq) analysis of peripheral blood mononuclear cells.32 Circulating monocytes and tissue-resident macrophages are involved in all stages of SARS-CoV-19 disease. Numerous studies have demonstrated targeting of pulmonary macrophages with COVID-19. Human monocytes and macrophages express the molecules ACE2, transmembrane protease, serin2 (TMPRSS2), and furin, which act as targets for spreading SARSCoV-2 infection. Lung macrophages also express alpha-7 nicotinic acetylcholine receptors (nAChRs α7) whose signal transduction is through JAK-STAT3, which cause the inhibition of inflammatory signals by blocking the transmission of p65/p50 NF-κB to the nucleus and degrading this transcription factor inhibitor (IκBα). Recent evidence has suggested that infection of spleen and lymph node macrophages with SARS-CoV-2 is associated with severe apoptosis of lymphocyte cells. Inflammatory macrophages resident in the upper respiratory tract release many pro-inflammatory chemokines and cytokines such as IL-1B, IL-8, IL-18, and TNF-α. Lower airway macrophages also show more robust inflammatory phenotypes, and in general, there is a strong correlation between the activation status of non-resident macrophages and the severity of COVID-19. Other immune cells such as mast cells also work synergistically with macrophages to destroy lung tissues.33
In patients with severe COVID-19, monocytes and monocyte-derived macrophages in lung tissue play a primary and critical role in disease progression through increased cytokine storm and destruction of peripheral tissues. In these patients, bronchoalveolar fluid is rich in two chemokines, CCL2 and CCL7, which increase the recruitment of CCR2+ monocytes.21 SARS-CoV-2-activated macrophages release inflammatory factors once they have phagocytized dead tissue and cell debris and play a vital role in the pathogenesis of fibrosis. These reactions are associated with the interaction of DAMPs with PRR receptors. Activating TLR4, TLR2, TLR3 by SARS-CoV-2 leads to releasing inflammatory cytokines such as IL-1β. So, inhibiting this cytokine has been reported to be effective in many inflammatory diseases such as rheumatoid arthritis.26
Farshi et al reported that monocyte-derived macrophages (infiltrating type) and, to some extent, lung tissue macrophages were involved in removing COVID-19-infected lung cells in the presence of anti-COVID-19 antibodies. It has been reported that neutralizing antibodies have an essential role in the early infection phase of COVID-19. In humans, these antibodies (mostly IgG and IgM isotypes) are produced by B lymphocytes. Participation of phagocytic cells (infiltrating macrophages derived from monocytes and part of macrophages residing in lung tissue) with neutralizing antibodies leads to eliminating COVID-19 infection in humans and mouse models, and thus, these host defense responses control the infection.34
IL-6 and GM-CSF are cytokines that activate monocytes and differentiate them into macrophages. These cytokines increase during the cytokine storm in ICU patients infected with SARS-CoV-2 and are involved in inflammation and immunopathology of autoimmune diseases. In addition, they have been identified as therapeutic targets (blockers) during the cytokine storm of inflammatory responses. IL-6 is secreted by cells such as macrophages, fibroblasts, T lymphocytes, and endothelial cells in response to infection.35 Expansion of CD14+ CD16+ monocytes secreting IL-6 in the peripheral blood of patients with severe COVID-19 admitted to ICU compared to the patients who were not admitted to ICU was observed, which was confirmed by scRNA-seq analysis of peripheral blood mononuclear cells.32
Another cytokine associated with monocyte/macrophage cells is GM-CSF, which plays a crucial role in inflammatory conditions and causes an increase in neutrophils, migration of monocytes, proliferation, and maturation of these cells.29 During inflammation, macrophages, T lymphocytes, endothelial cells, mesenchymal cells, and other immune cells produce GM-CSF. GM-CSF acts as a chemical attractant for the migration of monocytes and neutrophils from the blood to the tissue and can alter neutrophil receptors. Signal transduction of GM-CSF increases the pro-inflammatory phenotype of M1 macrophages and produces several inflammatory cytokines and chemokines through tissue macrophages or monocyte-derived macrophages. The effect of SARS-CoV-2 on the macrophage phenotype has not yet been defined. However, inhibition of IFNs signal transduction in these cells has been reported.33
Dendritic cells
T and B lymphocytes are mediator cells of the immune system, but the function of these cells is controlled by dendritic cells. After receiving antigens and processing them, these cells express lymphocyte-stimulating molecules on their surface and migrate to the lymph nodes, secreting cytokines to trigger adaptive immune responses. In addition to activating lymphocytes, DCs cause them not to activate or respond to autoantigens, thereby reducing autoimmune reactions.36
One of the most potent immunotherapy strategies against tumors is DC vaccines. The purpose of these vaccines is to activate the immune response to remove tumor cells and build long-term immunity. These vaccines are made from DC precursors that differentiate into DC cells, loaded with tumor antigens, and injected into a patient's body. These cells are formed by ex vivo methods from monocytes and hematopoietic precursors of CD34+ or by the proliferation of circulating in vivo DCs, then tumor antigens by transfection of mRNA, DNA, viral vectors, tumor lysates, proteins, and Antigen peptides are loaded in DCs. The DC maturation stage is vital for the effectiveness of the vaccine produced. Maturation stimulants such as pro-inflammatory cytokines, CD40 ligand (CD40 L or CD154), and TLR agonists were used in this stage. After reaching adult DCs, DC vaccines are injected intra-nodal, intradermal, subcutaneous, intravenous, or intra-lymphatic.37
A study by Magro et al showed that in patients with severe COVID-19, non-classical monocytes migrated from the blood to the lungs, and subsequently, CD1c+ conventional dendritic cells follow the same pattern. CD141+ conventional and CD123high plasmacytoid DCs are not present in peripheral blood and lung.38 In innate immune responses to tissue infection or injury, IL-6 is produced by myeloid cells such as DCs after detecting antigens with the help of their TLRs. This cytokine acts as a mediator for the production of antibodies by B lymphocytes.39
ACE2 is a potential diagnostic and prognostic biomarker for chronic inflammatory lung diseases. Structural and functional analyses have revealed that spike protein can bind to this receptor. ACE2 is highly expressed on the surface of epithelial cells in the lung, heart, ileum, kidney, and bladder. Because expressing this receptor is high in the epithelial cells of the alveolar space of the lung, the virus easily enters these cells and destroys the target host cell. Epithelial cells, macrophages, and dendritic cells are three essential components of innate immunity in the airway. Virus-infected apoptotic epithelial cells are phagocytosed by dendritic cells and macrophages, and viral antigens are presented to T lymphocytes, which trigger adaptive immune responses against COVID-19. In addition to ACE2, the virus binds to DC-SIGN and its related proteins. Dendritic-cell specific intercellular adhesion molecule-3-grabbing nonintegrin "DC-SIGN" expression is high in macrophage and dendritic cells, and this molecule is used as a target for direct viral infection on the surface of these cells. Antigen-presenting cells migrate to the lymph nodes. After presenting viral antigens to T lymphocytes, TCD4+ cells increase, producing virus-specific antibodies with the help of B lymphocytes. TCD8+ cells, which play an essential role in the fight against viral infections, kill virus-infected cells.40
In acute SARS-CoV-2 infection, a decrease in immune cells, including T lymphocytes, natural killer cells, and monocytes, has been observed. Also, there has been a significant decrease in dendritic cells with functional impairment, and the ratio of conventional dendritic cells to plasmacytoid dendritic cells has increased in acute severe patients. Although neutralizing antibodies are abundantly produced in patients with lymphocytopenia, the responses of the RBD and nucleocapsid protein (NP) T-specific lymphocytes are delayed in the first three weeks after the onset of symptomatic symptoms. Acute responses of RBD and NP-specific T lymphocytes primarily include TCD4+ lymphocytes. Evidence has shown that dendritic cells with impaired function accompanied by early antibody production and poor responses of TCD8+ lymphocytes can participate in the pathogenesis of acute COVID-19 disease and be helpful in the vaccine production process.41
Natural killer cells
Natural killer cells are vital components of the innate immune system that provide immediate and sufficient responses against pathogens and tumor cells. These cells are present in mucosal and lymphoid tissues and rush to the site of infection. Immature CD56bright NK cells secrete pro-inflammatory cytokines, and mature CD56dim CD16high NK cells have cell killing function. These cells are used for therapeutic and vaccination purposes due to their potential function.42
Therefore, these cells are manipulated in adaptive transmission and drug targeting in vivo. Natural killer cells are essential in modern therapies because they are regulated by a set of inhibitory and activating receptors, enabling them to kill tumor cells while preserving normal cells. In Adaptive NK cell transfer, NK cells are first purified from the peripheral blood of healthy individuals and activated with IL-12 or IL-15 cytokines before injecting the patient in vitro.43
Decreased CD107a, IFN-γ, TNF-α in TCD8+, and NK cells have been observed in patients with COVID-19. Increased natural killer cell group 2 D (NKG2D) expression in T and NK cells indicates cellular exhaustion in patients improved after treatment. The expression of human leukocyte antigen-E (HLA-E) on the surface of lung epithelial cells and its interaction with NKG2D causes exhaustion in NK cells. The spike protein mediates increased expression of NKG2D in NK cells. In vitro, co-culture of peripheral blood NKs with lung epithelial cells, transfected with the gene of SARS-CoV-2 spike protein, showed increased expression of NKG2D and decreased degranulation of them.44
Healthy NK cells in low-risk individuals detect SARS-CoV-2-infected cells through viral proteins on the surface of virus-infected cells as well as cytokines and chemokines produced in response to infection. These cells can directly induce apoptosis through antibody-dependent cell-mediated cytotoxicity in the target cell and indirectly target virus-infected cells by producing cytokines to modulate immune responses. Such effective innate immune responses can clear SARS-CoV-2 infection without causing damage to the lungs. In high-risk people with NK cell dysfunction, these cells cannot detect and respond safely to infection; this process is due to the escaping strategy that the virus uses against the immune system. Accumulation of infected epithelial cells, immune cells, monocytes/macrophages, and neutrophils cause the secretion of chemokines and cytokines, which trigger the recruitment of immune cells such as NK cells in response to IFN-γ to the lungs. This inflammatory condition can act as a catalyst for acute lung injury and ARDS and contribute to the prevalence and death of COVID-19.45
Studies in patients with severe COVID-19 compared with mild and healthy people have reported that their peripheral blood NK levels have dropped sharply. In these cells, the expression level of NKG2D inhibitory marker increased in COVID-19 patients compared to healthy individuals while the percentage of NK cells expressing activation markers such as CD107a, IFN-γ, IL-2, and TNF-α has decreased. In NK cells, NKG2D expression reduces the expression of IFN-γ, IL-2, TNF-α, and granzyme B. Also, Single-cell RNAseq analyzes on peripheral blood mononuclear cell46 of COVID-19 patients indicate an increase in transcripts of cell exhaustion markers such as lymphocyte-activation gene 3 (LAG3), program cell death protein 1, and hepatitis a virus cellular receptor 2 compared to the healthy control group. The studies indicate functional exhaustion of peripheral blood NK cells in SARS-CoV-2 patients, which inhibits the virus clearance processes and ultimately causes severe damage to lung tissue.47,48
In contrast to the senescence-related role of T-cell immunoglobulin and mucin domain (Tim-3) in T lymphocytes, it is a functional marker in NK cells and increases IFN-γ expression. However, this marker has also been reported to be associated with inhibition of NK cell killing activity. COVID-19 patients have high levels of inflammatory cytokines and chemokines, including IL-1 α/β, IP-10, and monocyte chemoattractant protein-1 (MCP-1). In severe cases, an increase in TNFα, IL-1, IL-6, IL-8, IL-10, IL-18, MCP-1and macrophage inflammatory protein (MIP-1α) leads to severe destruction of lung tissue. NK cells are likely to play a significant role in this cytokine-induced destruction. Initially, MCP-1 and IP-10 chemokines trigger the recruitment of NK cells to the inflammation sites, especially to the lungs. The IFN-γ and TNF- α produced by these cells may be associated with cellular functional processes such as cell lysis increased expression of intercellular adhesion molecule 1 by NF-κB in target cells involved in this process. Decreased NK cell count in combination with decreased IFN-γ and TNF- α levels leads to natural killer cell group 2 A (NKG2A) expression, reducing the cellular function of NK cells in SARS-CoV-2 patients. IL-6 and IL-10 reduce the cytotoxic function of NK cells. IL-6 directly reduces the expression of perforin and granzyme B while IL-10 is negatively related to the cytotoxicity of NK cells by reducing the expression of IFN-γ and IL-2.48
Mast cells
Mast cells are derived from bone marrow CD34+ myeloid precursors that circulate in the blood and migrate to peripheral tissues, where they are affected by tissue-specific chemokines and cytokines such as SCF (stem cell factor), IL-4, extracellular matrix proteins, and adhesion molecules, and differentiate into adult mast cells. Mast cells are strategically located throughout the body near blood vessels, lymph, and mucosal surfaces such as the skin and gastrointestinal tract to communicate with the external environment. Mast cells are innate immune cells that are involved in adaptive immune mechanisms. These cells play an essential role in the body's first line of defense against viruses and bacteria. These cells have been targeted for therapeutic strategies in cancer immunotherapy in several ways, including reducing the number of cells by inhibiting c-KIT and modulating the activation of these cells with the help of active cell stabilizers.49 FcεR1 signaling pathway inhibitors, anti-inhibitory antibodies and ligands, and TLR agonists.50
Recent studies have shown that CoVs activate the innate immune system cells (natural killer cells, monocytes/macrophages, neutrophils, and mast cells), tissue-resident endothelial and epithelial cells, and induce cytokine storms in the lungs. The SARS-CoV-2 virus activates respiratory mast cells in the early stages of infection. Mast cells lead to fatal inflammatory responses and pulmonary complications during COVID-19 infection by producing pro-inflammatory molecules such as IL-1β, CCL2, IL-6, TNF-α, and Broncho constrictor molecules such as histamine, prostaglandin-D2, and leukotriene-C4. Mast cells detect viral DAMPs through TLR receptors or by inducing IgE-FcεRI crosslinking. They release inflammatory mediators, which in turn cause pneumonia and fibrosis.51 Abnormal production of inflammatory mediators caused by SARS-CoV-2 infection can exacerbate inflammation in the respiratory system resulting in pulmonary complications. Therefore, mast cell stabilizers as supportive therapies can be beneficial in reducing inflammatory responses and pulmonary complications. In other words, it helps reduce deaths due to COVID-19 infection. Thus, endogenous and exogenous stabilizers of mast cells reduce inflammatory responses and pulmonary complications by suppressing the activation of these cells in SARS-CoV-2 infection. The primary inflammatory mediators are the pro-inflammatory cytokines, including IL-1, IL-6, TNF-α, and IL-8. Viral infections lead to the release of IL-1, which in turn leads to inflammation of lung tissue, fever, and fibrosis.49
Adaptive immune system responses against COVID-19
T lymphocyte is considered a pivotal cell in adaptive immunity, which plays an essential role in constraining a viral infection such as SARS-CoV-2. Some proposed factors play a small part in determining disease resulting in viral infections, including available features like T cell responses and the number of activated or effector T cells. This review is going to survey the role of different types of T cells such as memory T cell, TCD8+, and TCD4+ lymphocyte in the control of COVID-19 infection in the human.52
T lymphocytes subpopulation in COVID-19
The role of T lymphocytes in the improvement or exacerbation of COVID-19 infection and long-term protection against re-infection is still unclear. However, recent studies on different aspects of the T cells responses in COVID-19 infection can effectively remove some ambiguities.53 A prominent feature of COVID-19 infection is the decrease in lymphocytes, which return to normal after the disease, has recovery. This decrease is evident not only in TCD4+ and TCD8+ lymphocyte counts but also in the number of B and NK lymphocytes, so studies showed a significant reduction in the number of TCD8+ lymphocytes.54,55
Lymphopenia is a common feature of respiratory tract infections, including influenza,56 which occurs 2-4 days after the onset of symptoms, and along with or by disappearing off the symptoms, the number of lymphocytes returns to normal,56 However, lymphopenia seems to be more lasting during the infection with SARS-CoV-2.57 It is also observed in peripheral blood T cells, which is maybe due to their migration towards inflamed respiratory endothelial cells. However, autopsy and scRNA seq of the alveolar lavage of the lung do not confirm infiltration and accumulation of lymphocytes.58,59 Also, scRNA seq data indicates a more significant decrease in TCD8+ lymphocytes in patients with a severe COVID-19 infection than in milder form.60 The lymphopenia in patients with more severe disease may be associated with higher levels of TNF as well as IL-6 and IL-10,55,61,62 which has directly the potential effect on the T lymphocyte populations63,64 and indirectly influence the number of dendritic cells and neutrophils as important defense cells.65,66 Other factors that reduce the number of T lymphocytes include overexpression of apoptotic molecules such as FAS or CD9513 and tumor necrosis factor-related apoptosis-inducing ligand or CASPASE-3.57
Like other viral infections, the infection with SARS-CoV-2 is suspected of eliciting responses similar to those of T helper-1 lymphocytes. TCD8+ lymphocyte counts show a decrease during COVID-19 infection,53 and in severe form, the number of memory TCD4+ and T regulatory lymphocytes is significantly reduced (Fig. 2). These findings were associated with decreased TCD4+ and TCD8+ lymphocytes in lymph nodes. Spleen and lymph nodes in these patients have developed atrophy, indicating the reduction of these organs' cells.66 The activity of TCD8+ lymphocytes reduces, and expression of a molecular pattern such as NKG2D, a programmed cell death protein 1 (PD1), and Tim1, which are inhibitory molecules, can be confirmed. The decreases of NKG2D expression in lymphocytes from patients who received antiviral therapy seem t normal.67 Lymphocyte counts in patients with a mild form of COVID-19 are lower than in severe cases. This count has been performed in both TCD3+ and TDC8+ lymphocyte groups, and in both mild and severe forms of COVID-19 infection, TDC8+ lymphocyte counts decrease compared with healthy people.68 In addition, TDC8+ lymphocytes are less able to degranulation in COVID-19 infection, and they also secrete lower levels of IL-2, IFN-γ, and granzyme than healthy people. In peripheral blood lymphocytes isolated from patients admitted to ICU, the expression level of PD1 molecule was significantly reduced compared to peripheral blood T lymphocytes of patients with milder form and healthy individuals. These findings suggest that the SARS-CoV-2 can inhibit the adaptive immune system. Like other viruses in the coronavirus family, SARS-CoV-2 inhibits antigen presentation by MHC Ⅰ, II molecules, thereby reducing T lymphocyte immune responses.69
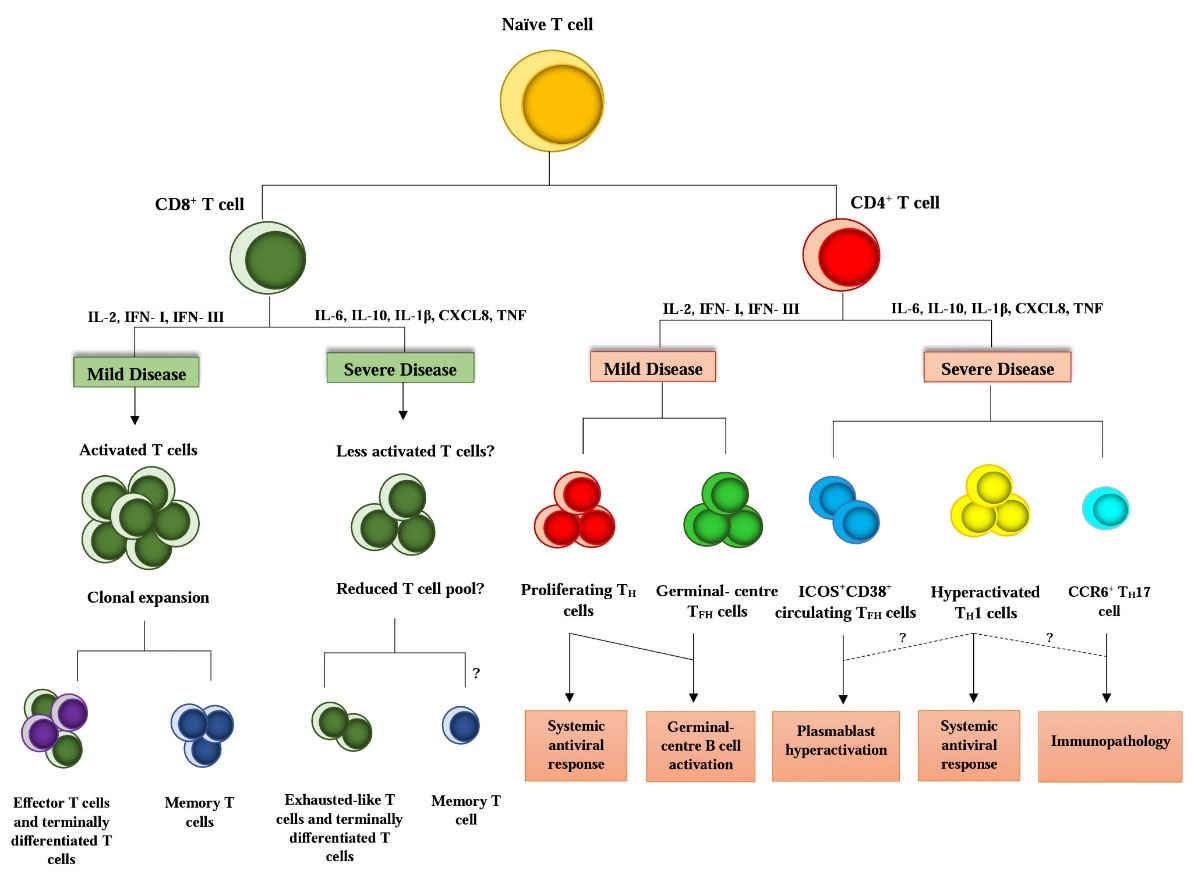
Fig. 2.
Responsive lymphocytes in infection with COVID-19. The phenotype of responsive lymphocytes TCD4+ and TCD8+ and different environmental factors such as cytokines can play a crucial role in promoting or inhibiting immune responses in infection with COVID-19 and determining its mild and severe form. IL, interleukin; IFN-I/III, interferon I/III; CXCL8, CXC-chemokine ligand 8; TNF, tumor necrosis factor; TH cell, T helper cell; TFH cell, T follicular helper cell; TH1 cell, T helper 1 cell; TH17 cell, T helper 17 cell; ICOS, inducible co-stimulator; CCR6, CC-chemokine receptor 6.
.
Responsive lymphocytes in infection with COVID-19. The phenotype of responsive lymphocytes TCD4+ and TCD8+ and different environmental factors such as cytokines can play a crucial role in promoting or inhibiting immune responses in infection with COVID-19 and determining its mild and severe form. IL, interleukin; IFN-I/III, interferon I/III; CXCL8, CXC-chemokine ligand 8; TNF, tumor necrosis factor; TH cell, T helper cell; TFH cell, T follicular helper cell; TH1 cell, T helper 1 cell; TH17 cell, T helper 17 cell; ICOS, inducible co-stimulator; CCR6, CC-chemokine receptor 6.
Memory T lymphocytes
An appropriate and mediate immune response requires employment and activation of naïve CD4+ and CD8+ T cells, rapid expansion, and specification into the proper effector cell type.70 Memory cells, as well as TCD4+ and TCD8+ cells, were detected in 100% and 70% of recovered patients with COVID-19, respectively.71 In addition, memory T lymphocyte responses have been detected for several SARS-CoV-2 proteins, including spike protein, nucleoprotein, and membrane protein.72 To develop protective responses by the adaptive immune system against various new antigens, this system needs to create a variety of receptors ready to detect new pathogens, such as SARS-CoV-2. Therefore, the size and diversity of TCR is a crucial factor in identifying antigens. TCRs can be divided into pristine forms that do not detect antigens and memory receptors.73 Recent studies have revealed that in the peripheral blood of people who have not yet been exposed to the virus, there are lymphocytes with receptors detecting SARS-CoV-2, which may indicate a cross-reaction between lymphocytes that distinguish between coronavirus and common cold viruses.74 Middle-aged people and those with chronic diseases had defects in the extent and quality of immune responses with moderate but persistent inflammation, which is associated with changes in the immune responses of lymph node lymphocytes.68,75,76 This defect also occurred in B lymphocytes, which includes a decrease in their ability to respond to viral infections and a decline in the capacity of antibodies to bind viruses.77 TCD4+ lymphocytes were also impaired, and their differentiation into TH-1 lymphocytes was increased. TCD8+ lymphocytes exhibit defective activity and decreased expression of CD28, which is a stimulatory marker. On the other hand, these changes were accompanied by an increase in memory B-cells constantly homing in tissues leading to inducing inflammatory responses, which ultimately decreases the activity of the immune system in the elderly.77,78 Besides, various factors influence the quality of immune responses in viral infection, especially other types of T cell explained in follow.
TCD8+ lymphocytes
Preliminary studies of several acute patients indicated changing activity and differentiation of TCD8+ lymphocytes. As shown in Table 1, some results of these studies show the decrease in the count of these cells and their final differentiation and an increase in expressing inhibitory receptors such as PD1, LAG3, Tim3, CTLA4, NKG2A, and CD39.13,54,57,61,67,79 Studies have shown that after inoculation of the attenuated live vaccine, the number of HLA-DR+ or Ki67 TCD8+ cells increase in the blood, indicating the presence of specific T lymphocytes in COVID-19 infection. In addition, these cells were not seen in all COVID-19 patients due to the diverse pattern of TCD8+ lymphocyte responses in these patients.80 In viral infections, T-effector lymphocytes play a crucial role in eliminating virus-infected cells, and TCD4+ lymphocytes have a supporting function for these cells and B lymphocytes. B lymphocytes that secrete neutralizing antibodies are essential for virus clearance and promoting long-term immunity.66
Table 1.
Lymphocyte Enumeration and responses reports in some patients with COVID-19
|
Study group
|
Sample type
|
Applied Techniques
|
Lymphocyte count results
|
Ref.
|
|
|
Peripheral blood |
Flow cytometry |
TCD4+ and TCD8+ lymphocytes reacted to COVID-19 antigens, and T lymphocytes cross-reacted with common cold antigens. |
84
|
|
|
Peripheral blood |
Flow cytometry |
TCD4+ and TCD8+ lymphocytes in these people responded to COVID-19 epitopes. |
85
|
|
|
Peripheral blood |
Flow cytometry |
T lymphocytes in people with mild and severe form of disease reacted with epitopes of COVID-19 during recovery |
86
|
|
|
Peripheral blood |
Flow cytometry |
Lymphopenia in COVID-19 patients compared with healthy group and increased activated phenotype. |
84
|
|
|
Peripheral blood |
Flow cytometry |
TCD4+ lymphocytes in the patients in ICU, secrete more GM-CSF and IL6 cytokines than the non-ICU group. |
87
|
|
|
Peripheral blood |
Flow cytometry |
Severe decrease in T lymphocytes in severe form compared whit control group. |
88
|
|
|
Peripheral blood |
High performance Flow cytometry |
Both groups had lymphopenia but in the female group lymphocytes was more active. In men with severe form, decrease in lymphocyte activity was obvious compared with the group with constant symptoms. |
89
|
|
|
Nasopharyngeal and alveolar fluid |
10x Genomics
scRNA- seq |
Fewer cytotoxic lymphocytes but more active in mild form which reactive with immune and epithelial cells. |
90
|
|
|
Bronchoalveolar lavage |
10xGenomics scRNA- seq, 10×Genomics scTCR- seq |
Higher clonal proliferation of lymphocyte in mild form of disease were seen that were more persistent in tissues. |
59
|
-
5 healthy people
-
5 early recovery people
-
5 late recovery people
|
Peripheral blood |
10xGenomics scRNA- seq, 10xGenomics scTCR- seq |
Higher colonal proliferation of T lymphocytes in late cured people were seen, Lower number of TCD8+ lymphocytes but with more cytotoxicity in early cured people were seen. |
91
|
|
|
Peripheral blood |
Seq-Well- scRNA- seq |
Multiple immune responses, Over activated T lymphocytes in patients were seen which they required a ventilator. |
92
|
|
|
Peripheral blood |
Seq-Well- scRNA- seq |
Severe decrease in T lymphocytes, changes in lymphocyte differentiation and over activation of T lymphocytes in the severe form of disease were seen |
69
|
|
|
Peripheral blood |
10x Genomics scRNA- seq (influenza and COVID-19), Flow cytometry |
Total and activated lymphocyte counts were identical in both groups |
93
|
Both TCD4+ and TCD8+ lymphocytes secrete IFN-γ, which had antiviral effects.21 Another group of regulatory T lymphocytes belonging to CD4+ and CD8+ cells, expressing forkhead box p3 (FOXP3) factor, interacting with these cells to suppress their activity if needed.81 IL-10 released by these cells inhibits the immune response and leads to anergy of lymphocytes. It also prevented inflammation induced by the innate immune response via inhibiting the production of IL-6 by neutrophils, which increases the secretion of IL-10, tumor growth factor-β (TGF-β), and IDO.82 While the expansion of Tregs, TGF-β seems to have a regulatory impact on lung damage caused by inflammation as it can increase the apoptosis mediates by neutrophils and reduces the infiltration of neutrophils to damaged tissues. Therefore, it can be concluded that Tregs play a role in tissue repairing83; thus, Regulatory T lymphocytes are also involved in regenerating damaged tissues.6 Some information about lymphocyte enumeration and responses in COVID-19 patients has been presented in Table 1.
TCD4+ lymphocytes
Similar to TCD8+ responses, there is evidence of dysfunction, increasing or decreasing TCD4+ lymphocytes in COVID-19 patients.55,61 The results show that TCD8+ lymphocyte activity is higher than TCD4+ lymphocyte activity due to the presence of CD38 and HLA-DR.83,94,95 Nevertheless, some other studies suggest that TCD4+ activity is higher in patients with more severe forms of COVID-19. In some patients with a milder form, the increase in IFN-γ secretion by TH-1 lymphocytes is more severe than in those with a more severe form. TCD4+ lymphocytes specific for spike COVID-19 protein have also been identified in the acute phase of infection.84 The role of T helper lymphocytes in the severe form of COVID-19 is well known, and TH-2 lymphocyte response is normal in individuals with moderate severity of this infection. Patients with an acute form of COVID-19 were reported to have a strong presence of CCR6+ TCD4+ lymphocytes,14,94 indicating the potential role of TH-17 lymphocytes that balances the immune response. Many studies have indicated the possibility of an increase in several TGF-β-secreting TCD4+ lymphocytes,96 as well as a population of GM-CSF-secreting TCD4+ lymphocytes in patients with COVID-19.95 Activated circulating effector T helper ICOS+ CD38+ and circulating T follicular lymphocytes, may be altered in patients with COVID-19, which may be associated with an increase in circulating immunoblasts.13 Some studies have shown that Tregs can play a role in tissue healing by inducing the releasing IL-18 and IL-33.97 Simultaneous presence of TGF-β and IL-6 reduces the expressing of FOXP3 and T regulatory lymphocytes.98 It should be noted that the effect of lymphopenia on TCD4+ population reduction is more significant than on TCD8+ lymphocytes57,99 and it should be determined what is related to the activity or dysfunction of TCD4+ lymphocytes and TCD8+ memory lymphocytes. In patients recovering from COVID-19, TCD4+ memory cells have been identified,70,71,86,100 which may indicate the role of memory cells in protection against COVID-19. Most viral infections induce the activity and proliferation of both TCD4+ and TCD8+ lymphocytes; however, in some patients with severe COVID-19, several factors, including increased secretion of IL-6, CXCL8, CXCL10, and CXCL9, as well as incomplete differentiation of TH-17 lymphocytes, impede the proper functioning of the cells.54,93,101
The immune response to SARS-CoV-2 is mediated through the adaptive immune system and the presentation of viral antigens by antigen-presenting cells to TH lymphocytes, which stimulates the production of neutralizing antibodies from B lymphocytes forming memory cells to produce antibodies during re-exposure to the virus. When TH-1 lymphocytes are activated, they activate cytotoxic lymphocyte precursors due to the secretion of cytokines IL-2 and IL-12; on the other hand, cytotoxic lymphocytes can secrete granzyme and proteinase to suppress infected cells in mild cases of infection. In severe cases of infection, cytotoxic lymphocytes express inhibitory receptors PD1, T cell immunoreceptor with immunoglobulin and ITIM domains (TIGIT), and cytotoxic t-lymphocyte-associated protein 4 (CTLA4). In these individuals, the lysis of virus-infected cells will be decreased due to the presence of cytotoxic T lymphocyte102 lymphocytes whose CD28 activating receptor is reduced.103
T cell therapy is an immunotherapy used to treat some cancers. The two main types of T cell therapy include treatment with Tumor-infiltrating lymphocytes (TIL) and treatment with genetically modified T cells (TCRs or CARs). TILs are a heterogeneous cell population found in neoplastic lesions and are composed mainly of T cells. A fraction of TILs express TCRs against unique or common tumor-associated antigens and exert cytotoxic effects against malignant cells. These TILs can be isolated from resected tumors, selected, and expanded under environmental conditions. TTCRs are T cells cloned with TCRs in which the variable region of the α and β chains is specific to a specific tumor antigen (from a patient or human mice immunized with tumor antigens). Such T cells detect processed peptide antigens expressed in the MHC region. One of the limitations of using TTCRs is that this treatment can only be recommended for MHC-compliant patients. In addition, tumors often lose antigen expression through reduced MHC. CAR technology has been developed to overcome these limitations. CAR T cells are made by integrating an antibody-derived single-strand variable fragment (scFv) into T cell intracellular signaling domains. Such T cells detect cell surface antigens in a non-MHC-restricted manner and are not dependent on antigen processing and delivery. First-generation CARs contained the scFv associated with the CD3x intracellular signaling domain. Second-and third-generation CARs were developed to improve the longevity and proliferation of injected T cells, containing the intracellular domains of one or more excitatory molecules such as CD28, OX40, and 4-1BB, which produce the second signaling.104
Relying on old knowledge about T-cell therapy for various pathological conditions, including viral infections, the use of virus-specific T cells against SARS-CoV-2 seems to be a rational therapeutic approach for treating COVID-19. Autologous or allogeneic viral-specific T cells can be expanded in vitro and injected to restore effective antiviral immunity. ARS-CoV-2-specific T cells can be isolated from the bloodstream of recovering donors and expanded using SARS-CoV-2-derived peptides and used to treat severe cases of COVID-19. However, the efficacy, treatment-related toxicities, and challenges associated with using T cell therapy have limited its use in COVID-19. It is important to note that using allogeneic T cells is impossible due to genetic limitations,65 and in vitro T cells show cellular exhaustion with prolonged stimulation to achieve the required cellular efficiency. Transfected T cells, in turn, can contribute to the cytokine storm that leads eventually to complications of COVID-19.105
Cell therapy for COVID-19
Cell therapy is a method that uses the patient's modified living cells or donor's cells to combat the disease.104 Several cell-based therapies using different cells, including mesenchymal stem cells, NK cells, Treg cells, specific T cells, and platelet lysate, are undergoing clinical trials to achieve COVID-19 therapy, described in this review.
Mesenchymal stem cells
Mesenchymal stem cells‚ also called mesenchymal stromal cells are a subset of adult non-hematopoietic stem cells that originate in the mesoderm. Mesenchymal stem cells can be obtained from various sources, including adipose tissue, dental pulp, bone marrow, umbilical cord, menstrual blood, fetal liver, and their rapid proliferation in cell culture also allows the production and freezing of large cell banks for repeated therapeutic applications. In addition, non-immunogenic mesenchymal stem cells are distinguished with low MHC I expression and no MHC II expression, and this property makes them an ideal tool for allogeneic cell therapy.105
So far, the efficacy and safety of mesenchymal stem cells have been well established in several clinical trials and the treatment of immune and non-immune diseases. Mesenchymal stem cells can modulate the immune system,106,107 making them potential therapeutic tools for repairing damaged tissue and inflammation in immune disorders (Table 2).108 The main feature of mesenchymal stem cells (MSCs) is their ability to interact with the innate and adaptive immune system (Fig. 3) by identifying the site of inflammation and detecting the presence of microbes by stimulating TLRs on their surface. Mesenchymal stem cells secrete pro-inflammatory signals (such as CXCL10 and IL-6) to utilize NK cells and active T cells in the absence of inflammatory signals (such as low levels of TNF-α and IFN-γ) through stimulation of TLR4 receptors by bacterial lipopolysaccharides. Conversely, in the presence of an inflammatory environment (high levels of TNF-α and IFN-γ) or stimulation of TLR3 by viral RNA, mesenchymal stem cells secrete indoleamin2,3-deoxygenase (IDO1), prostaglandin E2 (PEG2), and TGF-β as non-inflammatory signals, which leads to the emergence of regulatory dendritic cells as well as regulatory T cells. This balance is controlled by a subtle interaction between the MSC and tissue-resident macrophages to maintain tissue homeostasis.109
Table 2.
Performance of immune modulation by mesenchymal stem cells in COVID-19
|
Effects of immunomodulation following MSCs injection
|
Pathological changes in COVID-19
|
| Improve lung function and pulmonary fibrosis by lung accumulation and protection of the alveolar epithelium |
Inflammatory pulmonary lesions |
| Promoting endogenous repair in tissue/cellular organization by improving conditions in the microenvironment of the organization |
Increased levels of aspartic aminotransferase and creatine kinase in serum enzymes due to multiple organ failure |
| Rearrange the functions of immune cell subsets |
Decreased and hyperactive TCD4+ and TCD8+ cells as a result of depletion of immune cells. |
| Regulation of inflammatory cytokines and inhibition of B and T lymphocytes |
Induction of cytokine storm that leads to increased levels of IL-7, IL-2, IP-10, MCP-1, G-CSF, MIP-1α, TNF-α |

Fig. 3.
Immune modulating properties of active mesenchymal stem cells against overactive immune cells during COVID-19. MSCs effectively suppress the immune response through the secretion of soluble molecules or cell-to-cell contact. PEG2, prostaglandin E2; IL, interleukin; TSG6, tumor necrosis factor-stimulated gene-6; IDO, indoleamin2,3-deoxygenase; HLA-G, human leukocyte antigen-G; NO, nitric oxide; TGF-β, tumor growth factor-β; FASL, FAS ligand; programmed death-ligand 1 (PDL1); G-SCF,G-CSF; IP-10,interferon-inducible protein 10; MIP-1α, macrophage inflammatory protein-1 α; TNF- α, tumor necrosis factor- α; CXC-chemokine receptor (CXCR), NK cells, natural killer cells; TH-2, T helper 2; SARS-Cov-2, severe acute respiratory syndrome coronavirus 2.
.
Immune modulating properties of active mesenchymal stem cells against overactive immune cells during COVID-19. MSCs effectively suppress the immune response through the secretion of soluble molecules or cell-to-cell contact. PEG2, prostaglandin E2; IL, interleukin; TSG6, tumor necrosis factor-stimulated gene-6; IDO, indoleamin2,3-deoxygenase; HLA-G, human leukocyte antigen-G; NO, nitric oxide; TGF-β, tumor growth factor-β; FASL, FAS ligand; programmed death-ligand 1 (PDL1); G-SCF,G-CSF; IP-10,interferon-inducible protein 10; MIP-1α, macrophage inflammatory protein-1 α; TNF- α, tumor necrosis factor- α; CXC-chemokine receptor (CXCR), NK cells, natural killer cells; TH-2, T helper 2; SARS-Cov-2, severe acute respiratory syndrome coronavirus 2.
By producing significant amounts of inflammatory agents, including several cytokines, chemokines, and immune response cells, SARS-CoV-2 causes an exaggerated immune reaction in the body, which causes edema, air exchange disorders, acute heart damage, secondary infections, and eventually death. It can be hypothesized that mesenchymal stem cell therapy prevents cytokine storm mediated by the activated immune system. Immune modulation and differentiation are two main mechanisms of mesenchymal stem cells. Moreover, coronavirus enters the host cell by binding the spike protein of the virus surface to ACE2 on the host cell surface, which is not expressed in mesenchymal stem cells.110 Currently, more than 100 clinical trials in which MSCs could potentially be used as a therapeutic agent for COVID 19 have been established (Table 3).106
Table 3.
List of COVID-19 clinical trials using mesenchymal stem cells (www.clinicaltrials.gov).
|
NCT Number
|
Title
|
Status
|
Conditions
|
Interventions
|
Locations
|
| 1 |
NCT04492501 |
Investigational Treatments for COVID-19 in Tertiary Care Hospital of Pakistan |
Completed |
• COVID-19
• Cytokine Release Syndrome
• Critical Illness
• ARDS |
• Procedure: Therapeutic Plasma exchange
• Biological: Convalescent Plasma, Mesenchymal stem cell therapy
•Drug: Tocilizumab,
Remdesivir |
Pak Emirates Military Hospital, Rawalpindi, Punjab, Pakistan |
| 2 |
NCT04288102 |
Treatment with human umbilical cord-derived mesenchymal stem cells for severe coronavirus disease 2019 (COVID-19) |
Completed |
COVID-19 |
Biological: UC-MSCs,
Saline containing1% Human serum Albumin
(Solution without UC-MSCs) |
• General Hospital of Central Theater Command, Wuhan, Hubei, China
• Maternal and Child Hospital of Hubei Province, Wuhan, Hubei, China
• Wuhan Huoshenshan Hospital, Wuhan, Hubei, China |
| 3 |
NCT04573270 |
Mesenchymal Stem Cells for the Treatment of COVID-19 |
Completed |
• COVID-19
• Prophylaxis |
• Biological: PrimePro
• Other: Placebo |
Southern California Hospital at Culver City /
Southern California Hospital at Hollywood,
Culver City, California, United States |
| 4 |
NCT04276987 |
A pilot clinical study on inhalation of mesenchymal stem cells exosomes treating severe novel coronavirus pneumonia |
Completed |
Coronavirus |
Biological: MSCs-derived exosomes |
Ruijin Hospital Shanghai Jiao Tong University
School of Medicine, Shanghai, Shanghai, China |
| 5 |
NCT04445454 |
Mesenchymal Stromal Cell Therapy for Severe COVID-19 Infection |
Recruiting |
Coronavirus Infection |
Biological: Mesenchymal stromal cells |
CHU de Liège, Liège, Belgium |
| 6 |
NCT04400032 |
Cellular Immuno-Therapy for COVID-19 acute respiratory distress syndrome - Vanguard |
Recruiting |
• Acute Respiratory Distress Syndrome
• COVID-19 |
Biological: Mesenchymal Stromal Cells |
The Ottawa Hospital
Ottawa, Ontario, Canada |
| 7 |
NCT04252118 |
Mesenchymal Stem Cell Treatment for Pneumonia Patients Infected With COVID-19 |
Recruiting |
COVID-19 |
Biological: Mesenchymal Stromal Cells |
Beijing 302 Military Hospital of China
Beijing, China |
| 8 |
NCT04399889 |
hCT-MSCs for COVID-19 ARDS |
Recruiting |
• COVID
• Corona Virus Infection
• COVID19 |
Biological: human cord tissue mesenchymal stromal cells |
Duke Hospital
Durham, North Carolina, United States |
| 9 |
NCT04313322 |
Treatment of COVID-19 Patients Using Wharton's Jelly-Mesenchymal Stem Cells |
Recruiting |
Use of Stem Cells for COVID-19 Treatment |
Biological: WJ-MSCs |
Stem Cells Arabia
Amman, Jordan |
| 10 |
NCT04525378 |
MSC-based Therapy in COVID-19-associated acute respiratory distress syndrome |
Recruiting |
• COVID-19
• ARDS, Human |
Other: Mesenchymal stromal cell-based therapy |
Hospital São Rafael
Salvador, Bahia, Brazil |
| 11 |
NCT04339660 |
Clinical Research of Human Mesenchymal Stem Cells in the Treatment of COVID-19 Pneumonia |
Recruiting |
COVID-19 |
• Biological: UC-MSCs
• Other: Placebo |
Puren Hospital Affiliated to Wuhan University of Science and Technology
Wuhan, Hubei, China |
| 12 |
NCT04366063 |
Mesenchymal Stem Cell Therapy for SARS-CoV-2-related acute respiratory distress syndrome |
Recruiting |
COVID-19 |
Biological: Cell therapy protocol 1
Cell therapy protocol 2 |
Royan Institute
Tehran, Iran |
| 13 |
NCT04537351 |
The Mesenchymal COVID-19 Trial: A Pilot Study to Investigate Early Efficacy of MSCs in Adults With COVID-19 |
Recruiting |
• COVID-19
• Acute Respiratory Distress Syndrome |
Biological: CYP-001 |
Nepean Hospital
Kingswood, New South Wales, Australia
Westmead Hospital
Westmead, New South Wales, Australia |
| 14 |
NCT04457609 |
Administration of Allogenic UC-MSCs as Adjuvant Therapy for Critically-Ill COVID-19 Patients |
Recruiting |
• COVID
• Pulmonary Infection
• SARS-CoV2 |
• Drug: Oseltamivir
Azithromycin
• Biological: Umbilical Cord Mesenchymal Stem Cells |
•Cipto Mangunkusumo General Hospital
Jakarta Pusat, DKI Jakarta, Indonesia
• Persahabatan General Hospital
Jakarta, DKI Jakarta, Indonesia
• Sulianti Saroso Center for Infectious Disease
Jakarta, DKI Jakarta, Indonesia
• Universitas Indonesia Hospital
Depok, West Java, Indonesia |
| 15 |
NCT04392778 |
Clinical Use of Stem Cells for the Treatment of COVID-19 |
Recruiting |
• COVID-19
• Pneumonia
• Multiple Organ Failure
• Corona Virus Infection
|
Biological:
MSC Treatment
Saline Control |
• Istinye University
Istanbul, Turkey
• SBÜ Dr. Sadi Konuk Eğitim ve Araştırma Hastanesi
Istanbul, Turkey |
Natural killer cells
NK cells, one of the prominent members of the innate immune system, play a vital role in responding to viral infections in humans and animal models. Studies have shown that although premature activation of NK cells and the release of IFN-γ by these cells is considered beneficial in the process of combating infections, prolonged and excessive stimulation of NK cells could lead to a reduction in the number of them and induction of exhausted phenotype which is in the close correlation with increased sever systematic inflammatory response syndrome, sepsis and eventually a raise in mortality.45
Studies on patients with SARS-CoV-2 have revealed a reduced number of circulatory NK cells with high levels of inhibitory receptors like NKG2A and producing low levels of INF-γ in these groups. These findings have provided the crucial element of treatment based on NK cells against COVID-19. Treatment products of NK cells can be extracted from peripheral blood mononuclear cells,46 hematopoietic stem cells, or by designing immortal NK cell lines by genetic engineering.69 Specifically, genetically modified NK cells called CAR-NK cells are now being investigated to exert more efficiency when facing COVID-19.106 These cells that contain chimeric receptors are engineered to express each of these receptors and are designed to enhance the capacity of NK cells to eliminate cancer cells. Although the eligibility of CAR-NK cells to control viral infection is yet undergoing clinical trials and has not been evaluated on a large scale, the safety profile of CAR-NK cell therapy in patients with cancer who were also diagnosed with immunodeficiency suggests that CAR-NK cell therapy in patients with mild COVID-19 infection could be tolerable. Notably, CAR-NK cell therapy can be assumed safe to use less likely to lead to cytokine release syndrome, considering a commonly undesirable phenomenon in processing CAR-T cell therapy. However, given that positive results have not been reported so far, it is essential to take necessary precautions of using CAR-NK cells, and the usage of CAR-NK cells in severe cases of COVID-19 (Table 4).45
Table 4.
List of COVID-19 clinical trials using NK cells
|
Category
|
Therapeutic
|
Trial Identifier
|
Phase
|
Location
|
Mechanism of action
|
| Adaptive NK cells |
NK cells |
NCT04280224 |
I |
Henan, China |
Adaptive NK cell therapy |
| CYNK-001 |
NTC04365101 |
I/II |
New Jersey, USA |
From human placental CD34+ cells and culture-expanded |
| CAR-NK cells |
NK cells isolate from healthy donor PBMCs |
NCT04344548 |
I/II |
Bogota, Colombia |
Isolated NK cells ex vivo stimulated with IL-2 and IL-15 |
NKG2D-ACE2
CAR-NK cell therapy from umbilical cord blood |
NCT04324996 |
I/II |
Chongqing, China |
IL-15 prolongs NK cell lifespan, GM-CSF neutralizing scFV reduces recruitment of inflammatory cells, NKG2D-ACE2 CAR-NK cells can target virally infected cells, ACE2 CAR-NK can act as decoy cell |
Regulatory T cells
Disruption of the inflammatory processes induced by SARS-CoV-2 in patients with severe COVID-19 is partly due to the dysfunction of Treg cells, which is responsible for inhibiting inflammation. Given that CD4+ T cell immunotherapy is a promising approach for CD8+ T cell dysfunction in chronic infections and cancers, acquired therapy with Treg cells may be an effective strategy for treating COVID-19 by modulating inflammation in lung tissue (Table S1, Supplementary file 1). Cellenkos company has developed a new allogeneic cell therapy (CK0802) consisting of Treg cells to moderate or suppress immune system dysfunction by relieving chronic inflammation. In addition, the Treg cells express a homing factor in the lung, and the residing of these cells in the lung can lead to disruption of the cytokine storm mediated by SARS-CoV-2. In a clinical study using a damaged lung model, the effects of Treg transplantation included a reduction in inflammatory T cells, inflammatory cytokines such as IL-6 and IL-17, and pulmonary hemorrhage, as well as regeneration of the lung epithelium and alveoli.111
Virus-specific T cells
Acquired transmission of antigen-specific T cells to treat cancers, autoimmune diseases, and viral infections such as cytokine release syndrome (HBV), HCV, and cytomegalovirus has been established. In this procedure, SARS-CoV-2-specific T cells are isolated from the blood of donors who have recovered from the disease. Antiviral-specific T cell clones are in vitro amplified using SARS-CoV-2-derived peptides and are used to treat severe cases of COVID-19. However, the efficacy, treatment-related toxicity, and challenges associated with using acquired T cell therapy have limited its use in COVID-19. The critical point is that using heterogeneous allogeneic T cells is not possible due to genetic limitations (HLA I). On the other hand, long-term stimulation of T cells in vitro to achieve the desired cell efficiency can lead to forming exhausted T cells, which can play a role in the cytokine storm formation and complications COVID-19. However, several clinical trials are currently underway to treat acquired T cells for COVID-19 (Table S2, Supplementary file 1).112
SARS-specific TCD8+ cells have functioned normally and may be a potential therapeutic tool for SARS infection. Recently, the number of TCD8+ cells has dramatically decreased during SARS infection, which causes an increase in the CD4+/CD8+ T ratio. The decrease in the number of TCD8+ cells is associated with the severity of the disease. Also, the number of TCD8+ cells increase after treatment, leading to a decrease in the ratio of the CD4+/CD8+ T cell. Based on these findings, COVID-19-specific TCD8+ transmission may be an effective treatment strategy.113
Platelet lysates
Platelets have been shown to cover a wide range of functions. In addition to their involvement in homeostasis, platelets have immunological functions and therefore participate in the interaction between pathogens and host defense. Platelets have a wide range of receptor molecules that enable them to sense invasive pathogens and inflammation caused by infection. As a result, platelets exert antimicrobial mechanisms; however, they also interact strongly with other innate and adaptive immune arms, including neutrophils, monocytes/macrophages, dendritic cells, B cells, and T cells. There is a fragile balance between advantageous antimicrobial effects and harmful reactions involved in pathogenesis, and many pathogens have developed mechanisms to influence these two outcomes.114
Research has indicated the role of platelets in the defense against viral infections, which requires specific receptor-ligand interactions. Platelets interact directly with viral pathogens by PRRs. This interaction subsequently leads to platelet activation. Activated and virus-filled platelets are then removed from the bloodstream by the reticuloendothelial system, facilitating viral load clearance. Platelet activation leads to degranulation and release of prominent amounts of growth factors and biomolecules, which participate in host defense mechanisms. In this regard, a new biological molecule called kinocidin modulates the immune system, such that platelet factor 4 (PF-4/CXCL4) has been reported as the strongest kinocidin. CXCL4 is generated by tissue damage, inflammation, oxidative stress, and pathogen stimulation due to platelet-virus interaction. Another important chemokine produced during viral infections in host defense is CCL5, which is involved in viral lung infections. Active platelets release β-defensin that effectively neutralizes a large number of viruses. Other platelet-derived peptides, such as thymosin β4, CXCL 7, or the degradation products of antimicrobial peptides (fibrinopeptides A, B, and thrombocidins) do not act directly against viral infections.115 However, they can prevent secondary bacterial infections in the host. During platelet degranulation, the expression of P-selectin is increased, which is part of the inner membrane of α-granules binding P-selectin receptor glycoprotein ligand 1 on the surface of leukocytes. Direct interaction of platelets and leukocytes leads to their activation and increases phagocytosis and the production of oxygen mediators in neutrophils. The interaction of platelets and monocyte augments activation and differentiation, as well as increases the expression of tissue factors.109,116
Platelets in a complex process are transformed into two forms called platelet-rich plasma and platelet lysates. Platelet-rich plasma is a concentrate of platelet-rich proteins widely used in various diseases. The next generation of platelet products is platelet lysate, obtained through the rupture of platelet membranes and the concentration of active biomolecules. Platelet lysates, like platelets themselves, play an essential role in the lysis of the virus. Platelet lysate is a product rich in growth factors that can lead to cell regeneration through increased proliferation and angiogenesis. These properties should be further investigated for using platelet lysates as an adjuvant in COVID-19 patients.117
Review Highlights
What is the current knowledge?
√ The involvement of innate and adaptive immune cells in the pathologic events in COVID-19 patients.
What is new here?
√ The usage of cells with regulatory functions and platelet lysis in COVID-19 treatment.
Conclusion
In conclusion, the host immune responses are the crucial factor in destining COVID-19. Moreover, further analysis of these responses has indicated their effectiveness on the severity of the disease in different individuals, as some infected cases show mild symptoms, and others show no symptoms. Therefore, clear comprehension of these reactions in individuals with severe symptoms and carriers can better recognize the involved molecular mechanisms. Accordingly, with the help of which, not only can we pave the way to reach a long-term protective immunity against this virus, but also it might be possible to take preventive and therapeutic measures to overcome the outbreak of this virus and other kinds of CoVs. Cell therapy is a novel therapeutic method that prescribes cellular material for medical purposes. Different kinds of cells, including hematopoietic stem cells, mesenchymal stem cells, lymphocytes, NK cells, and DCs, can be utilized in cell therapy. At present, applying these cells to prevent or cure infected individuals of COVID-19 is undergoing clinical trials to gain full knowledge of their efficiency. It is hoped that this new medicinal approach can successfully treat patients diagnosed with COVID-19.
Acknowledgement
We gratefully thanks Parvaz Research Ideas Supporter Institute and Cytotech & Bioinformatics Research Group for all support and suggestions.
Funding sources
None.
Ethical statement
Not applicable.
Competing interests
Authors declare no conflict of interest in this study.
Supplementary files
Supplementary file 1 contains Tables S1 and S2.
(pdf)
References
- Florindo HF, Kleiner R, Vaskovich-Koubi D, Acúrcio RC, Carreira B, Yeini E. Immune-mediated approaches against COVID-19. Nat Nanotechnol 2020; 15:630-45. doi: 10.1038/s41565-020-0732-3 [Crossref] [ Google Scholar]
- Wang MY, Zhao R, Gao LJ, Gao XF, Wang DP, Cao JM. SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development. Front Cell Infect Microbiol 2020; 10:587269. doi: 10.3389/fcimb.2020.587269 [Crossref] [ Google Scholar]
- Alsobaie S. Understanding the Molecular Biology of SARS-CoV-2 and the COVID-19 Pandemic: A Review. Infect Drug Resist 2021; 14:2259-68. doi: 10.2147/IDR.S306441 [Crossref] [ Google Scholar]
- Naqvi AAT, Fatima K, Mohammad T, Fatima U, Singh IK, Singh A. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. BiochimBiophys Acta Mol Basis Dis 2020; 1866:165878. doi: 10.1016/j.bbadis.2020.165878 [Crossref] [ Google Scholar]
- Wu A, Peng Y, Huang B, Ding X, Wang X, Niu P. Genome Composition and Divergence of the Novel Coronavirus (2019-nCoV) Originating in China. Cell Host Microbe 2020; 27:325-8. doi: 10.1016/j.chom.2020.02.001 [Crossref] [ Google Scholar]
- Gracia-Hernandez M, Sotomayor EM, Villagra A. Targeting Macrophages as a Therapeutic Option in Coronavirus Disease 2019. Front Pharmacol 2020; 11:577571. doi: 10.3389/fphar.2020.577571 [Crossref] [ Google Scholar]
- Siu YL, Teoh KT, Lo J, Chan CM, Kien F, Escriou N. The M, E, and N structural proteins of the severe acute respiratory syndrome coronavirus are required for efficient assembly, trafficking, and release of virus-like particles. J Virol 2008; 82:11318-30. doi: 10.1128/JVI.01052-08 [Crossref] [ Google Scholar]
- Zhang Q, Xiang R, Huo S, Zhou Y, Jiang S, Wang Q. Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. Signal Transduct Target Ther 2021; 6:233. doi: 10.1038/s41392-021-00653-w [Crossref] [ Google Scholar]
- Cheng VC, Lau SK, Woo PC, Yuen KY. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev 2007; 20:660-94. doi: 10.1128/CMR.00023-07 [Crossref] [ Google Scholar]
- Ludwig S, Zarbock A. Coronaviruses and SARS-CoV-2: A Brief Overview. AnesthAnalg 2020; 131:93-6. doi: 10.1213/ANE.0000000000004845 [Crossref] [ Google Scholar]
- Azkur AK, Akdis M, Azkur D, Sokolowska M, van de Veen W, Bruggen MC. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020; 75:1564-81. doi: 10.1111/all.14364 [Crossref] [ Google Scholar]
- Coopersmith CM, Antonelli M, Bauer SR, Deutschman CS, Evans LE, Ferrer R. The Surviving Sepsis Campaign: Research Priorities for Coronavirus Disease 2019 in Critical Illness. Crit Care Med 2021; 49:598-622. doi: 10.1097/ccm.0000000000004895 [Crossref] [ Google Scholar]
- Kuri-Cervantes L, Pampena MB, Meng W, Rosenfeld AM, Ittner CAG, Weisman AR, et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol 2020; 5. 10.1126/sciimmunol.abd7114.
- Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov 2020; 10:783-91. doi: 10.1158/2159-8290.CD-20-0422 [Crossref] [ Google Scholar]
- Shokri S, Mahmoudvand S, Taherkhani R, Farshadpour F. Modulation of the immune response by Middle East respiratory syndrome coronavirus. J Cell Physiol 2019; 234:2143-51. doi: 10.1002/jcp.27155 [Crossref] [ Google Scholar]
- Birra D, Benucci M, Landolfi L, Merchionda A, Loi G, Amato P. COVID 19: a clue from innate immunity. Immunol Res 2020; 68:161-8. doi: 10.1007/s12026-020-09137-5 [Crossref] [ Google Scholar]
- Hall MW, Joshi I, Leal L, Ooi EE. Immune modulation in COVID-19: Strategic considerations for personalized therapeutic intervention. Clin Infect Dis 2020. 10.1093/cid/ciaa904.
- Java A, Apicelli AJ, Liszewski MK, Coler-Reilly A, Atkinson JP, Kim AH, et al. The complement system in COVID-19: friend and foe? JCI Insight 2020; 5. 10.1172/jci.insight.140711.
- Chauhan AJ, Wiffen LJ, Brown TP. COVID-19: A collision of complement, coagulation and inflammatory pathways. J ThrombHaemost 2020; 18:2110-7. doi: 10.1111/jth.14981 [Crossref] [ Google Scholar]
- Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P. Coronavirus infections and immune responses. J Med Virol 2020; 92:424-32. doi: 10.1002/jmv.25685 [Crossref] [ Google Scholar]
- Piras V, Selvarajoo K. Beyond MyD88 and TRIF Pathways in Toll-Like Receptor Signaling. Front Immunol 2014; 5:70. doi: 10.3389/fimmu.2014.00070 [Crossref] [ Google Scholar]
- Zhou S, Wang G, Zhang W. Effect of TLR4/MyD88 signaling pathway on sepsis-associated acute respiratory distress syndrome in rats, via regulation of macrophage activation and inflammatory response. Exp Ther Med 2018; 15:3376-84. doi: 10.3892/etm.2018.5815 [Crossref] [ Google Scholar]
- Chen N ZM, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020 395: 507-13. 10.1016/S0140-6736(20)30211-7.
- Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 Cytokine Storm; What We Know So Far. Front Immunol 2020; 11:1446. doi: 10.3389/fimmu.2020.01446 [Crossref] [ Google Scholar]
- Shimizu M. Clinical Features of Cytokine Storm Syndrome. In: Cron RQ, EM Behrens, editors. Cytokine Storm Syndrome. Cham: Springer International Publishing; 2019. p. 31-41.
- Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med 2020; 217:e20200652. doi: 10.1084/jem.20200652 [Crossref] [ Google Scholar]
- Németh T, Sperandio M, Mócsai A. Neutrophils as emerging therapeutic targets. Nat Rev Drug Discov2020. 19: 253-75.
- Cicco S, Cicco G, Racanelli V, Vacca A. Neutrophil Extracellular Traps (NETs) and Damage-Associated Molecular Patterns (DAMPs): Two Potential Targets for COVID-19 Treatment. Mediators Inflamm 2020; 2020:7527953. doi: 10.1155/2020/7527953 [Crossref] [ Google Scholar]
- Gomez-Rial J, Rivero-Calle I, Salas A, Martinon-Torres F. Role of Monocytes/Macrophages in COVID-19 Pathogenesis: Implications for Therapy. Infect Drug Resist 2020; 13:2485-93. doi: 10.2147/IDR.S258639 [Crossref] [ Google Scholar]
- Shu Y, Cheng P. Targeting tumor-associated macrophages for cancer immunotherapy. BiochimBiophys Acta Rev Cancer 2020; 1874:188434. doi: 10.1016/j.bbcan.2020.188434 [Crossref] [ Google Scholar]
- Tian L, Lei A, Tan T, Zhu M, Zhang L, Mou H. Macrophage-Based Combination Therapies as a New Strategy for Cancer Immunotherapy. Kidney Dis (Basel) 2021; 8:26-43. doi: 10.1159/000518664 [Crossref] [ Google Scholar]
- Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nature Reviews Immunology 2020; 20:355-62. [ Google Scholar]
- Booz GW, Altara R, Eid AH, Wehbe Z, Fares S, Zaraket H. Macrophage responses associated with COVID-19: A pharmacological perspective. Eur J Pharmacol 2020; 887:173547. doi: 10.1016/j.ejphar.2020.173547 [Crossref] [ Google Scholar]
- Farshi E, Kasmapur B, Arad A. Investigation of immune cells on elimination of pulmonary-Infected COVID-19 and important role of innate immunity, phagocytes. Rev Med Virol 2021; 31:e2158. doi: 10.1002/rmv.2158 [Crossref] [ Google Scholar]
- Pence BD. Severe COVID-19 and aging: are monocytes the key?. Geroscience 2020; 42:1051-61. doi: 10.1007/s11357-020-00213-0 [Crossref] [ Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998; 392:245-52. doi: 10.1038/32588 [Crossref] [ Google Scholar]
- Constantino J, Gomes C, Falcão A, Neves BM, Cruz MT. Dendritic cell-based immunotherapy: a basic review and recent advances. Immunol Res 2017; 65:798-810. [ Google Scholar]
- Magro CM, Mulvey JJ, Laurence J, Sanders S, Crowson AN, Grossman M. The differing pathophysiologies that underlie COVID-19-associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol 2021; 184:141-50. doi: 10.1111/bjd.19415 [Crossref] [ Google Scholar]
- Bonaventura A, Vecchie A, Wang TS, Lee E, Cremer PC, Carey B. Targeting GM-CSF in COVID-19 Pneumonia: Rationale and Strategies. Front Immunol 2020; 11:1625. doi: 10.3389/fimmu.2020.01625 [Crossref] [ Google Scholar]
- Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin Immunol 2020; 215:108427. doi: 10.1016/j.clim.2020.108427 [Crossref] [ Google Scholar]
- Zhou R, To KK, Wong YC, Liu L, Zhou B, Li X. Acute SARS-CoV-2 Infection Impairs Dendritic Cell and T Cell Responses. Immunity 2020; 53:864-77e5. doi: 10.1016/j.immuni.2020.07.026 [Crossref] [ Google Scholar]
- Hammer Q, Rückert T, Romagnani C. Natural killer cell specificity for viral infections. Nat Immunol 2018; 19:800-8. [ Google Scholar]
- Chiossone L, Vienne M, Kerdiles YM, Vivier E, editors editors. Natural killer cell immunotherapies against cancer: checkpoint inhibitors and more. Semin Immunol 2017; 31:55-63. doi: 10.1016/j.smim.2017.08.003 [Crossref] [ Google Scholar]
- Manickam C, Sugawara S, Reeves RK. Friends or foes? The knowns and unknowns of natural killer cell biology in COVID-19 and other coronaviruses in July 2020. PLoSPathog 2020; 16:e1008820. doi: 10.1371/journal.ppat.1008820 [Crossref] [ Google Scholar]
- Market M, Angka L, Martel AB, Bastin D, Olanubi O, Tennakoon G. Flattening the COVID-19 Curve With Natural Killer Cell Based Immunotherapies. Front Immunol 2020; 11:1512. doi: 10.3389/fimmu.2020.01512 [Crossref] [ Google Scholar]
- Buttner M, Miao Z, Wolf FA, Teichmann SA, Theis FJ. A test metric for assessing single-cell RNA-seq batch correction. Nat Methods 2019; 16:43-9. doi: 10.1038/s41592-018-0254-1 [Crossref] [ Google Scholar]
- McKechnie JL, Blish CA. The Innate Immune System: Fighting on the Front Lines or Fanning the Flames of COVID-19?. Cell Host Microbe 2020; 27:863-9. doi: 10.1016/j.chom.2020.05.009 [Crossref] [ Google Scholar]
- van Eeden C, Khan L, Osman MS, Cohen Tervaert JW. Natural Killer Cell Dysfunction and Its Role in COVID-19. Int J Mol Sci 2020; 21. 10.3390/ijms21176351.
- Kılınç E, Baranoğlu, Y Mast cell stabilizers as a supportive therapy can contribute to alleviate fatal inflammatory responses and severity of pulmonary complications in COVID-19 infection. Anatolian Clinic the Journal of Medical Sciences 2020; 111-8. 10.21673/anadoluklin.720116.
- Lichterman JN, Reddy SM. Mast Cells: A New Frontier for Cancer Immunotherapy. Cells 2021; 10:1270. [ Google Scholar]
- Kempuraj D, Selvakumar GP, Ahmed ME, Raikwar SP, Thangavel R, Khan A. COVID-19, Mast Cells, Cytokine Storm, Psychological Stress, and Neuroinflammation. Neuroscientist 2020; 26:402-14. doi: 10.1177/1073858420941476 [Crossref] [ Google Scholar]
- Walker LJ, Sewell AK, Klenerman P. T cell sensitivity and the outcome of viral infection. Clin Exp Immunol 2010; 159:245-55. doi: 10.1111/j.1365-2249.2009.04047.x [Crossref] [ Google Scholar]
- Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020; 130:2620-9. doi: 10.1172/JCI137244 [Crossref] [ Google Scholar]
- Martines RB, Ng DL, Greer PW, Rollin PE, Zaki SR. Tissue and cellular tropism, pathology and pathogenesis of Ebola and Marburg viruses. J Pathol 2015; 235:153-74. doi: 10.1002/path.4456 [Crossref] [ Google Scholar]
- Mazzoni A, Salvati L, Maggi L, Capone M, Vanni A, Spinicci M. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J Clin Invest 2020; 130:4694-703. doi: 10.1172/JCI138554 [Crossref] [ Google Scholar]
- McClain MT, Park LP, Nicholson B, Veldman T, Zaas AK, Turner R. Longitudinal analysis of leukocyte differentials in peripheral blood of patients with acute respiratory viral infections. J Clin Virol 2013; 58:689-95. doi: 10.1016/j.jcv.2013.09.015 [Crossref] [ Google Scholar]
- Laing AG LA, Del Barrio IDM, Das A, Fish M, Monin L, et al. A consensus COVID-19 immune signature combines immuno-protection with discrete sepsis-like traits associated with poor prognosis. MedRxiv 2020. https://www.medrxiv.org/content/10.1101/2020.06.08.20125112v1.
- Dewey C HS, Goelz E, Linzer M. Supporting Clinicians During the COVID-19 Pandemic. Ann Intern Med 2020; 172:752-3. doi: 10.7326/M20-1033 [Crossref] [ Google Scholar]
- Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med 2020; 26:842-4. doi: 10.1038/s41591-020-0901-9 [Crossref] [ Google Scholar]
- Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang YQ. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther 2020; 5:33. doi: 10.1038/s41392-020-0148-4 [Crossref] [ Google Scholar]
- Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front Immunol 2020; 11:827. doi: 10.3389/fimmu.2020.00827 [Crossref] [ Google Scholar]
- Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020; 27:992-1000 e3. doi: 10.1016/j.chom.2020.04.009 [Crossref] [ Google Scholar]
- Bottcher JP, Schanz O, Garbers C, Zaremba A, Hegenbarth S, Kurts C. IL-6 trans-signaling-dependent rapid development of cytotoxic CD8+ T cell function. Cell Rep 2014; 8:1318-27. doi: 10.1016/j.celrep.2014.07.008 [Crossref] [ Google Scholar]
- Zamora V, Rodero M, Ibanez-Escribano A, Andreu-Ballester JC, Mendez S, Cuellar C. Expansion of T regulatory lymphocytes by murine bone marrow dendritic cells previously stimulated with Anisakis simplex larval antigens. Mem Inst Oswaldo Cruz 2021; 116:e200560. doi: 10.1590/0074-02760200560 [Crossref] [ Google Scholar]
- de Waal Malefyt R, Haanen J, Spits H, Roncarolo MG, te Velde A, Figdor C. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med 1991; 174:915-24. doi: 10.1084/jem.174.4.915 [Crossref] [ Google Scholar]
- Lu Q, Wang Z, Yin Y, Zhao Y, Tao P, Zhong P. Association of Peripheral Lymphocyte and the Subset Levels With the Progression and Mortality of COVID-19: A Systematic Review and Meta-Analysis. Front Med (Lausanne) 2020; 7:558545. doi: 10.3389/fmed.2020.558545 [Crossref] [ Google Scholar]
- Zheng HY, Zhang M, Yang CX, Zhang N, Wang XC, Yang XP. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol 2020; 17:541-3. doi: 10.1038/s41423-020-0401-3 [Crossref] [ Google Scholar]
- Azevedo FR, Ikeoka D, Caramelli B. Effects of intermittent fasting on metabolism in men. Rev Assoc Med Bras (1992) 2013; 59:167-73. doi: 10.1016/j.ramb.2012.09.003 [Crossref] [ Google Scholar]
- Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol 2020; 17:533-5. doi: 10.1038/s41423-020-0402-2 [Crossref] [ Google Scholar]
- Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020; 181:1489-501 e15. doi: 10.1016/j.cell.2020.05.015 [Crossref] [ Google Scholar]
- Ni L, Ye F, Cheng ML, Feng Y, Deng YQ, Zhao H. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity 2020; 52:971-7 e3. doi: 10.1016/j.immuni.2020.04.023 [Crossref] [ Google Scholar]
- Shrotri M, van Schalkwyk MCI, Post N, Eddy D, Huntley C, Leeman D. T cell response to SARS-CoV-2 infection in humans: A systematic review. PLoS One 2021; 16:e0245532. doi: 10.1371/journal.pone.0245532 [Crossref] [ Google Scholar]
- Shao Y, Forster SC, Tsaliki E, Vervier K, Strang A, Simpson N. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019; 574:117-21. doi: 10.1038/s41586-019-1560-1 [Crossref] [ Google Scholar]
- Yang X, Xie L, Li Y, Wei C. More than 9,000,000 unique genes in human gut bacterial community: estimating gene numbers inside a human body. PLoS One 2009; 4:e6074. doi: 10.1371/journal.pone.0006074 [Crossref] [ Google Scholar]
- Akash MS, Rehman K, Chen S. Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus. J Cell Biochem 2013; 114:525-31. doi: 10.1002/jcb.24402 [Crossref] [ Google Scholar]
- Chigbu DI, Loonawat R, Sehgal M, Patel D, Jain P. Hepatitis C virus infection: host–virus interaction and mechanisms of viral persistence. Cells 2019; 8:376. doi: 10.3390/cells8040376 [Crossref] [ Google Scholar]
- Hubbard RE, O'Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med 2009; 13:3103-9. doi: 10.1111/j.1582-4934.2009.00733.x [Crossref] [ Google Scholar]
- Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc 2007; 55:864-71. doi: 10.1111/j.1532-5415.2007.01186.x [Crossref] [ Google Scholar]
- Ikeoka D, Mader JK, Pieber TR. Adipose tissue, inflammation and cardiovascular disease. Rev Assoc Med Bras (1992) 2010; 56:116-21. doi: 10.1590/s0104-42302010000100026 [Crossref] [ Google Scholar]
- Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity 2008; 28:710-22. doi: 10.1016/j.immuni.2008.02.020 [Crossref] [ Google Scholar]
- Lechien JR, Chiesa-Estomba CM, Place S, Van Laethem Y, Cabaraux P, Mat Q. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med 2020; 288:335-44. doi: 10.1111/joim.13089 [Crossref] [ Google Scholar]
- Okeke EB, Uzonna JE. The Pivotal Role of Regulatory T Cells in the Regulation of Innate Immune Cells. Front Immunol 2019; 10:680. doi: 10.3389/fimmu.2019.00680 [Crossref] [ Google Scholar]
- Thevarajan I, Nguyen THO, Koutsakos M, Druce J, Caly L, van de Sandt CE. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat Med 2020; 26:453-5. doi: 10.1038/s41591-020-0819-2 [Crossref] [ Google Scholar]
- Weiskopf D, Schmitz KS, Raadsen MP, Grifoni A, Okba NMA, Endeman H. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol 2020; 5:eabd2071. doi: 10.1126/sciimmunol.abd2071 [Crossref] [ Google Scholar]
- Altmann DM, Boyton RJ. SARS-CoV-2 T cell immunity: Specificity, function, durability, and role in protection. Sci Immunol 2020; 5:eabd6160. doi: 10.1126/sciimmunol.abd6160 [Crossref] [ Google Scholar]
- Peng Y, Mentzer AJ, Liu G, Yao X, Yin Z, Dong D, et al. Broad and strong memory CD4 (+) and CD8 (+) T cells induced by SARS-CoV-2 in UK convalescent COVID-19 patients. bioRxiv 2020. 10.1101/2020.06.05.134551.
- Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev 2020; 7:998-1002. doi: 10.1093/nsr/nwaa041 [Crossref] [ Google Scholar]
- Wang F, Nie J, Wang H, Zhao Q, Xiong Y, Deng L. Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. J Infect Dis 2020; 221:1762-9. doi: 10.1093/infdis/jiaa150 [Crossref] [ Google Scholar]
- Takahashi T, Ellingson MK, Wong P, Israelow B, Lucas C, Klein J. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020; 588:315-20. doi: 10.1038/s41586-020-2700-3 [Crossref] [ Google Scholar]
- Chua RL, Lukassen S, Trump S, Hennig BP, Wendisch D, Pott F. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat Biotechnol 2020; 38:970-9. doi: 10.1038/s41587-020-0602-4 [Crossref] [ Google Scholar]
- Wen W, Su W, Tang H, Le W, Zhang X, Zheng Y. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov 2020; 6:31. doi: 10.1038/s41421-020-0168-9 [Crossref] [ Google Scholar]
- Wilk AJ, Rustagi A, Zhao NQ, Roque J, Martinez-Colon GJ, McKechnie JL. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med 2020; 26:1070-6. doi: 10.1038/s41591-020-0944-y [Crossref] [ Google Scholar]
- Mudd PA, Crawford JC, Turner JS, Souquette A, Reynolds D, Bender D, et al. Targeted Immunosuppression Distinguishes COVID-19 from Influenza in Moderate and Severe Disease. medRxiv 2020. 10.1101/2020.05.28.20115667.
- Chen R, Sang L, Jiang M, Yang Z, Jia N, Fu W. Longitudinal hematologic and immunologic variations associated with the progression of COVID-19 patients in China. J Allergy Clin Immunol 2020; 146:89-100. doi: 10.1016/j.jaci.2020.05.003 [Crossref] [ Google Scholar]
- Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M. Immunology of COVID-19: Current State of the Science. Immunity 2020; 52:910-41. doi: 10.1016/j.immuni.2020.05.002 [Crossref] [ Google Scholar]
- Oja AE, Saris A, Ghandour CA, Kragten NAM, Hogema BM, Nossent EJ. Divergent SARS-CoV-2-specific T- and B-cell responses in severe but not mild COVID-19 patients. Eur J Immunol 2020; 50:1998-2012. doi: 10.1002/eji.202048908 [Crossref] [ Google Scholar]
- Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell 2015; 162:1078-89. doi: 10.1016/j.cell.2015.08.021 [Crossref] [ Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006; 441:235-8. doi: 10.1038/nature04753 [Crossref] [ Google Scholar]
- Taga K TG. IL-10 inhibits human T cell proliferation and IL-2 production. J Immunol 1992; 148:1143-8. [ Google Scholar]
- Neidleman J, Luo X, Frouard J, Xie G, Gill G, Stein ES. SARS-CoV-2-Specific T Cells Exhibit Phenotypic Features of Helper Function, Lack of Terminal Differentiation, and High Proliferation Potential. Cell Rep Med 2020; 1:100081. doi: 10.1016/j.xcrm.2020.100081 [Crossref] [ Google Scholar]
- Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol 2003; 33:2706-16. doi: 10.1002/eji.200324228 [Crossref] [ Google Scholar]
- Haghverdi L, Lun ATL, Morgan MD, Marioni JC. Batch effects in single-cell RNA-sequencing data are corrected by matching mutual nearest neighbors. Nat Biotechnol 2018; 36:421-7. doi: 10.1038/nbt.4091 [Crossref] [ Google Scholar]
- Holuka C, Merz MP, Fernandes SB, Charalambous EG, Seal SV, Grova N, et al. The COVID-19 Pandemic: Does Our Early Life Environment, Life Trajectory and Socioeconomic Status Determine Disease Susceptibility and Severity? Int J Mol Sci 2020; 21. 10.3390/ijms21145094.
- Rajarshi K, Chatterjee A, Ray S. Combating COVID-19 with mesenchymal stem cell therapy. Biotechnol Rep (Amst) 2020; 26:e00467. doi: 10.1016/j.btre.2020.e00467 [Crossref] [ Google Scholar]
- Thanunchai M, Hongeng S, Thitithanyanont A. Mesenchymal Stromal Cells and Viral Infection. Stem Cells Int 2015; 2015:860950. doi: 10.1155/2015/860950 [Crossref] [ Google Scholar]
- Li X, Wang K, Lyu Y, Pan H, Zhang J, Stambolian D. Deep learning enables accurate clustering with batch effect removal in single-cell RNA-seq analysis. Nat Commun 2020; 11:2338. doi: 10.1038/s41467-020-15851-3 [Crossref] [ Google Scholar]
- Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q. Transplantation of ACE2(-) Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis 2020; 11:216-28. doi: 10.14336/AD.2020.0228 [Crossref] [ Google Scholar]
- Cancio M, Ciccocioppo R, Rocco PRM, Levine BL, Bronte V, Bollard CM. Emerging trends in COVID-19 treatment: learning from inflammatory conditions associated with cellular therapies. Cytotherapy 2020; 22:474-81. doi: 10.1016/j.jcyt.2020.04.100 [Crossref] [ Google Scholar]
- Canham MA, Campbell JDM, Mountford JC. The use of mesenchymal stromal cells in the treatment of coronavirus disease 2019. J Transl Med 2020; 18:359. doi: 10.1186/s12967-020-02532-4 [Crossref] [ Google Scholar]
- Glenn JD, Whartenby KA. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J Stem Cells 2014; 6:526-39. doi: 10.4252/wjsc.v6.i5.526 [Crossref] [ Google Scholar]
- Yang L, Liu S, Liu J, Zhang Z, Wan X, Huang B. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduct Target Ther 2020; 5:128. doi: 10.1038/s41392-020-00243-2 [Crossref] [ Google Scholar]
- Toor SM, Saleh R, Sasidharan Nair V, Taha RZ, Elkord E. T-cell responses and therapies against SARS-CoV-2 infection. Immunology 2021; 162:30-43. doi: 10.1111/imm.13262 [Crossref] [ Google Scholar]
- Hossein-Khannazer N, Shokoohian B, Shpichka A, Aghdaei HA, Timashev P, Vosough M. Novel therapeutic approaches for treatment of COVID-19. J Mol Med (Berl) 2020; 98:789-803. doi: 10.1007/s00109-020-01927-6 [Crossref] [ Google Scholar]
- Grigorian M, Hartenstein V. Hematopoiesis and hematopoietic organs in arthropods. Dev Genes Evol 2013; 223:103-15. doi: 10.1007/s00427-012-0428-2 [Crossref] [ Google Scholar]
- Yeaman MR. Platelets in defense against bacterial pathogens. Cell Mol Life Sci 2010; 67:525-44. doi: 10.1007/s00018-009-0210-4 [Crossref] [ Google Scholar]
- Assinger A. Platelets and infection - an emerging role of platelets in viral infection. Front Immunol 2014; 5:649. doi: 10.3389/fimmu.2014.00649 [Crossref] [ Google Scholar]
- Jeyaraman M, Ranjan R, Kumar R, Arora A, Chaudhary D, Ajay SS. Cellular Therapy: Shafts of Light Emerging for COVID-19. Stem Cell Investig 2020; 7:11. doi: 10.21037/sci-2020-022 [Crossref] [ Google Scholar]