Bioimpacts. 13(3):229-240.
doi: 10.34172/bi.2022.23960
Original Article
Differentiation of human endometrial stem cells encapsulated in alginate hydrogel into oocyte-like cells
Diba Ghasemi Conceptualization, Investigation, Methodology, Project administration, 1 
Somayeh Ebrahimi-Barough Conceptualization, Data curation, Methodology, Project administration, Writing – review & editing, 1
Mohammad Hossein Nekoofar Visualization, 2, 3
Abdolreza Mohamadnia Formal analysis, 4, 5
Nasrin Lotfibakhshaiesh Resources, 1
Naghmeh Bahrami Formal analysis, 1, 6
Roya Karimi Validation, 1
Vajihe Taghdiri Nooshabadi Writing – original draft, 7
Mahmoud Azami Resources, 1
Elham Hasanzadeh Validation, 8
Jafar Ai Conceptualization, Funding acquisition, Project administration, Supervision, 1, * 
Author information:
1Department of Tissue Engineering and Applied Cell Sciences, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran
2Department of Endodontics, School of Dentistry, Tehran University of Medical Sciences, Tehran, Iran
3School of Dentistry, College of Biomedical and Life Sciences, Cardiff University, Cardiff, UK
4Chronic Respiratory Diseases Research Center, National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran
5Department of Biotechnology, School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
6Craniomaxillofacial Research Center, Tehran University of Medical Sciences, Tehran, Iran
7Department of Tissue Engineering and Applied Cell Sciences, School of Medicine, Semnan University of Medical Sciences, Semnan, Iran
8Department of Tissue Engineering and Applied Cell Sciences, School of Advanced Technologies in Medicine, Mazandaran University of Medical Sciences, Sari, Iran
Abstract
Introduction:
Human endometrial mesenchymal stem cells (hEnMSCs) are a rich source of mesenchymal stem cells (MSCs) with multi-lineage differentiation potential, making them an intriguing tool in regenerative medicine, particularly for the treatment of reproductive and infertility issues. The specific process of germline cell-derived stem cell differentiation remains unknown, the aim is to study novel ways to achieve an effective differentiation method that produces adequate and functioning human gamete cells.
Methods:
We adjusted the optimum retinoic acid (RA) concentration for enhancement of germ cell-derived hEnSCs generation in 2D cell culture after 7 days in this study. Subsequently, we developed a suitable oocyte-like cell induction media including RA and bone morphogenetic protein 4 (BMP4), and studied their effects on oocyte-like cell differentiation in 2D and 3D cell culture media utilizing cells encapsulated in alginate hydrogel.
Results:
Our results from microscopy analysis, real-time PCR, and immunofluorescence tests revealed that 10 µM RA concentration was the optimal dose for inducing germ-like cells after 7 days. We examined the alginate hydrogel structural characteristics and integrity by rheology analysis and SEM microscope. We also demonstrated encapsulated cell viability and adhesion in the manufactured hydrogel. We propose that in 3D cell cultures in alginate hydrogel, an induction medium containing 10 µM RA and 50 ng/mL BMP4 can enhance hEnSC differentiation into oocyte-like cells.
Conclusion:
The production of oocyte-like cells using 3D alginate hydrogel may be viable in vitro approach for replacing gonad tissues and cells.
Keywords: BMP4, Differentiation, hEnSCs, Oocyte-like cells differentiation, Retinoic acid, Stem cell
Copyright and License Information
© 2023 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Infertility as a failure to conceive after 12 months of frequent intercourses,
1
affected 7.4% of married women.
2
Currently, health care systems utilize different strategies for female infertility treatment with special challenges such as breast cancer.
3,4
The most exciting method of restoring fertility is stem cell-based therapy. These potentially curative cells are undifferentiated cells with long-term self-renewal and differentiation abilities, as well as the capacity to generate a wide range of specialized cell types.
5,6
Some in vitro studies have demonstrated that germ cells may be successfully produced from induced pluripotent stem cells or embryonic stem cells (iPSCs/ESCs) and can develop into spermatocytes in vivo.
7
In contrast to spermatocytes, oocyte induction from human germ cells has not been fully developed and the scientists were unable to differentiate mouse iPSCs/ESCs to oocytes in vitro.
8,9
Consequently, there is a growing interest in mesenchymal stem cells (MSCs) due to their acceptable safety without causing teratomas and ethical concerns in therapeutic applications. MSCs may be extracted from different tissues as human endometrium.
10
Human endometrial stem cells (hEnSCs) display MSC-specific markers and may differentiate in vitro to a variety of germ cells.
11-13
hEnSCs show favorable in vivo regenerative effects in restoring tissue or cell function in injured tissues.
4,14
The use of signaling molecules, growth factors, and/or hydrogels are efficient approaches in stem cell differentiation. It has been shown that retinoic acid (RA- a vitamin A metabolite) binding to its nuclear receptor in the central area of the mesonephros induces meiosis in genital crest cells.
4
Meiosis division, as a critical process in the reproduction system, creates new haploid gamete cells and enhances genetic diversity. It is believed that studying germ cell sexual differentiation pathways can help us learn more about reproduction diseases and therapies.
6
Furthermore, several studies have demonstrated that RA promotes embryonic stem cell proliferation and differentiation into germ cells.
5
Bone morphogenetic protein 4 (BMP4), is thought to be a crucial signaling mechanism in epiblast primordial germ cell (PGC) formation, survival, and migration to the genital ridge.
5,9
Several studies have found that the knocking down of the BMP4 gene resulted in a lack of PGC development. Many experimental studies have examined the role of RA and BMP4 in germ cell and gamete cell regeneration via stem cell differentiation.
10,15,16
Hydrogel offers major benefits in the generation, cryopreservation, and conserving of stem cell-derived germ cells due to its unique biocompatible, three-dimensional network architecture, transparency, and ability to maintain cell viability effect. It offers as a viable 3D scaffold for different applications in the tissue engineering era.
17
Human germ cell development is the first stage of embryogenesis that begins after implantation, but study into it is too complicated. As a result, germline cells, particularly oocyte development from human stem cells, provide an experimental platform for studying the mechanisms of gametogenesis, oocyte cells, and oocyte-like cell creation.
3,18
Previously published research has reported that germline cells could be produced from different stem cells.
19-21
We previously demonstrated that hEnSCs can differentiate to germ cells in the presence of RA and identified 10M concentration as the optimum dosage.
3
This study looked at the role of RA and BMP4 therapies on cell and tissue growth and development in both 2D and 3D cell culture systems.
In this study, we optimized the dosage of RA for hEnSCs differentiation to oocyte-like cells. Before and after incorporation into alginate hydrogel, the expression levels of SCP3, GDF9, and GDF9B markers associated with oocyte-like cell development were evaluated in both 2D and 3D cell culture systems.
Materials and Methods
hEnSCs isolation and characterization
Separation and characterization of hEnSCs were performed using previously reported methods.
22,23
Biopsies of endometrial tissue were isolated either after hysterectomy or by biopsy from healthy women's endometrium in Imam Khomeini hospital in Tehran. The endometrial tissue specimens were washed with pre-warmed (37°C) phosphate-buffered saline (PBS) (Sigma, USA) containing 2-3% (v/v) Pen/Strep (Sigma, USA) and amphotericin B. Subsequently, tissue digestion was carried out by collagenase type 1 (1 mg/mL; Sigma, USA) in Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12) for 60 min at 37°C, and 5% CO2. To neutralize the enzymatic digestion reaction, we used an equal volume of complete culture media (DMEM-F12, 15% FBS, 1% Pen/Strep) and in the next step, the cells suspension was passed through 70- and 40-μm cell strainer to remove undigested tissues and centrifuged at 1200 rpm 5 min. Ultimately, the cells pellet was dispersed in pre-warmed (37°C) culture media (DMEM-F12, 15% FBS, 1% Pen/Strep) and transferred to T25 culture flasks then incubated at 37°C in a humidified incubator (5% CO2).
Immunophenotyping analyses of hEnSCs by flow cytometry
The immunophenotype characterization of 1×106 isolated cells at passage 3 was performed using specific monoclonal antibodies labeled with Fluorescein isothiocyanate and directed against MSC CD markers (CD105, CD90, CD146, CD73), and as well as endothelial stem cell CD marker (CD31) for 1 hour at room temperature. Afterward, the stained hEnSCs were rinsed with PBS (pH: 7.4) and fixed with a formaldehyde solution. Finally, the cells were studied by BD FACSCaliburTM (BD bioscience San Jose, CA), and acquired data were evaluated by FlowJo version 7 software.
In vitro evaluation of multilineage differentiation ability of hEnSCs
To investigate the potential differentiation of harvested cells into the osteogenic lineage, 2 × 105 hEnSCs at passage 3 were seeded in a 24-well cell culture plate with osteogenic media containing 0.1 µM dexamethasone (Sigma-Aldrich, St Louis, MO), 10 mM β-glycerol phosphate (Sigma-Aldrich), and 0.2 mM ascorbic acid (ASA; Sigma-Aldrich, St Louis, MO). The medium was changed every 3-4 days. To visualize the mineralized matrix in differentiated cells, the fixed cells were stained with 1% alizarin red S (Sigma-Aldrich, St Louis, MO) solution for 15 minutes. Besides, the adipogenic differentiation capacity of hEnSCs was explored for 21 days in an adipogenic medium that contained Dulbecco's Modified Eagle Medium (DMEM) with 0.5 mM 3-isobutyl-1- methylxanthine (IBMX; Sigma-Aldrich), 1 µM hydrocortisone (Sigma-Aldrich, St Louis, MO) and 0.1 mM indomethacin (Sigma-Aldrich, St Louis, MO). The cell culture medium was changed in 3–4-day intervals. The oil-red O staining was used to evaluate the lipid droplets in differentiated cells' cytoplasm. After staining, both samples were examined under a LABOMED TCM 400 microscope.
Differentiation of hEnSCs -derived germ cells into oocyte-like cells
In this study, we established a two-step differentiation method. In the first stage, hEnSCs were cultured and differentiated into germ cells in the third passage, and the germ cells were subsequently transformed into oocyte-like cells by encapsulation in alginate hydrogel in 2D and 3D cell culture systems. To reach the germ cell, the differentiation of hEnSCs was studied in a 24-well cell culture plate. For this purpose, 2×105 hEnSCs at passage 3 were seeded utilizing a complete medium for 24 hours at 37°C and 5% CO2. For each group, we considered three wells. To differentiate hEnSCs into germ cells in vitro, they were treated with differentiating medium (DMEM/F12+ 10%FBS+ 1%pen/strep+ 10µM RA (Sigma, USA)) for 5 days. The wells that were not exposed to RA were considered a negative control. Analysis and quantification of a specific marker of germ cells including DDX4 and DAZL with respect to each control (undifferentiated hEnSCs)/ and treated group (10µM RA) were studied using immunofluorescence and Quantitative/Real-Time PCR analyses. In the next step for hEnSCs derived oocyte-like cells, the 2×105 hEnSCs were cultured in a 24-well cell culture plate for 5 days at 37°C and 5% CO2 to form germ cells, as previously described. Then, to differentiate hEnSCs-derived germ cells into oocyte-like cells in vitro, they were treated complete medium (DMEM/F12+10% FBS+1% pen/strep) with different concentration of BMP4 (30, 50, 100 ng/mL) (Sigma, USA) plus 10µM RA at day5. The well that was not treated with RA was considered as a negative control and the undifferentiated hEnSCs served as control. Based on the results, the 50ng/mL concentration group was chosen as a suitable concentration. Finally, the various cell groups were investigated using immunofluorescence and quantitative/real-time PCR under the LABOMED TCM 400 microscope.
Immunofluorescence staining
We used 4% paraformaldehyde/PBS at room temperature for 45 minutes to fix cells and permeabilization of cells was done by 0.2% Triton X-100 in PBS. The cells were blocked overnight with a blocking solution (5% milk and 0.05% Tween-20 in PBS) and then incubated with primary antibodies by using 1:100 dilution of monoclonal anti-DDX4 (ab13840, Abcam, USA), monoclonal anti-DAZL (ab34139, Abcam, USA), as a germ cell markers and monoclonal anti-SCP3 (ab15093, Abcam, Cambridge, UK), monoclonal anti-GDF9(ab93892, Abcam, USA) and monoclonal anti-GDF9B (ab108413, Abcam, Cambridge, UK) as oocyte like cells markers for 2.5 hrs. Afterward, cells were washed in PBS/Tween 20 (0.1%) (Sigma) three times and incubated with Alexa fluor 488 and 594 secondary antibodies (Sigma, USA) for 1hrs in a dark place at room temperature. Cells were again washed with PBS/Tween 20 (0.1%) three times. Finally, the cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich, D8417) and studied by Olympus DP73 digital camera associated with a fluorescence microscopy IX81 (U-MW-IB3). ImageJ software was used to assess the proportion of DDX4, DAZL, SCP3, GDF9, and GDF9B proteins in the resultant microphotographs.
Quantitative/real-time PCR
RNA extraction of different groups of hEnSCs was harvested by using RiboExTM LS total RNA solution (GeneAll Biotechnology, Seoul, Korea) according to the instructions of the supplier. By using a PrimeScript TM RT reagent Kit (Takara, Tokyo, Japan), cDNA synthesis of each sample was performed. The qPCR assessment was done to quantify the expression levels of the following genes: DDX4, DAZL, SCP3, GDF9, and GDF9B. The NCBI data set was used to determine the complete sequence of the indicated genes, and Primer3 online software was used to help us design the appropriate primers. The primer blast was carried out to detect the homology percent. Table 1 presents the characteristics of primers of germ cells and oocyte-like cell genes. RealQ Plus 2x Master Mix Green (Ampliqon, Herlev, Denmark) was applied to detect the gene expression levels in control and differentiated cells by rotor gene 6000 Corbett real-time PCR System. The housekeeping glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene expression was used as a normalizer. The relative expression of specific genes related to germ cells (DDX4 and DAZL) and oocyte-like cells (SCP3, GDF9, and GDF9B) were measured by qRT-PCR.
Table 1.
Specific forward and reverse genes primers for RT-PCR analysis
|
Gene
|
Primer sequence
|
Tm
|
| DAZL |
FAAATGGCCCACAAAAGAAATCTG
RAAGTGATGCACTCTTTTATCCTTGAAG
|
60
60
|
| DDX4 |
FAATTCTGCGAAACATAGGGGATG
RGTTCCCGATCACCATGAATACTTG
|
60
60
|
| GDF9 |
FTGGAGACCAGGTAACAGGAATCC
RAGTTGTTGATATTGTACAGCAAGACTGAC
|
60
59.5
|
| GDF9B |
FCTCACAGAGGTACCTGGCATATACAG
RAGATTGAAGCGAGTTAGTTAGTTGGAGATG
|
59.5
60
|
| SCP3 |
FAGCAGTTCATAAAGAGTATGGAAGAGTTG
RTTGCTATCTCTTGCTGCTGAGTTTC
|
60
60
|
| GAPDH |
FAGCATTCCGTATTTCCAGCAG
RGCCAGTTGGGGTCTCATACAAA
|
58
60
|
Alginate hydrogel preparation
We prepared alginate hydrogel based on our previous studies. Alginate hydrogel used in this study was prepared by dropping aqueous alginate into a calcium chloride solution.
24,25
In brief, we utilized sodium alginate with a high guluronic acid concentration and a molecular weight of 50 000 (60% in guluronic acid residues, Sigma-Aldrich). In the first step, 200 μL of sterilized sodium-Alginate 2.5% (w/v) dissolved in buffer and containing 2 × 105 cells/mL of differentiated germ-like cells or hEnSCs were dissolved in HEPES buffered solution (pH 7.4). The solution was dropped into the crosslinking solution (as described above) containing a 1% solution of calcium chloride (CaCl2) (w/v). The final Alginate hydrogel concentration was 3% (w/v).
Subsequently, the acquired hydrogel containing encapsulated differentiated germ-like cells were washed with sterile PBS (Sigma-Aldrich) and then rinsed in holding media kept in a sealed 24-well plate with parafilm in a cooled incubator set. Following 72 hours of hypothermic storage, the gelled beads were immediately used for characterization assays, degradation tests, and cell culture studies. Before proceeding, the gelled beads containing cells were left at room temperature for 15 minutes. Wells containing just gel was exposed to the same cell growth conditions as previously described and served as controls (i.e., no cells).
Alginate hydrogel identification
The in vitro degradation rate of the alginate hydrogel was measured by checking the changes in scaffold weight in PBS (pH 7.4) at 37ºC. PBS solutions were changed every 3 days. The alginate hydrogel was incubated at 37ºC for 1 hour with fully swollen 3 percent (w/v) alginate hydrogel in 10 mL PBS for gelation and formation of a 3D network structure. The hydrogel was taken out and weighted at predefined time intervals. The degradation was characterized by hydrogel fraction based on the initial weight of the totally swollen hydrogel. At each time point (days 1, 2, 3, 4, 6, 8, 11, and 14), three samples were taken out from their molds and their weight changes were measured to evaluate degradation. The percentage of degradation was calculated as the following:
Degradation (%) = [(W0- Wt)/W0] ×100
Where W0 and Wt denote the weights of hydrogel before and after soaking in PBS, respectively. The rheological properties of alginate gel, which is a viscoelastic polymer, were characterized using a programmable rheometer (Anton-Paar RheoCompass, Ashland, USA) operating at a constant stress mode. The alginate gel directly was placed onto the parallel plate. Following that, rheological measurements were performed at 37°C for 1 hour at frequencies ranging from 0.1 to 10 Hz. Finally, the storage (Gʹ) and loss (Gʺ) moduli of the hydrogel were measured. In addition, after 3 days of incubation at 37°C and 5% CO2, we studied and observed the cell encapsulated morphology by SEM examination. The alginate hydrogel was washed with PBS and fixed in 2.5% glutaraldehyde solution at 48ºC. Following a one-hour incubation period, the fixed hydrogel samples were washed twice in PBS, followed by dehydration (with 75% ethanol) and drying procedures. The samples were then placed in a freeze-dryer device for 2 hours. Finally, the samples were coated with a thin coating of gold and examined using an SEM (QUANTA SEM system; FEI Company, Hillsboro, OR, USA) at a voltage of 15 kV.
Cell viability assessment by MTT analysis
The encapsulated differentiated germ-like cell viability was studied in both 2D and 3D media. The vitality of the cells was determined by 3-[4, 5-dimethyl-2-thiazolyl]-2, 5-diphenyl-2H-tetrazolium bromide (MTT) at 1, 3, and 5 days after seeding. This was done to investigate the effect of alginate hydrogel as a 3D scaffold on differentiated cells. In each 96-well plate, 1 × 104 cells were encapsulated in formed hydrogel and then cultured in 100 μL of complete medium. Cells were cultured in triplicate wells for each group. After each time point, the cell culture medium was eliminated and cells were washed with PBS. Subsequently, the cells were incubated for 4 hours with 10 µL/well 3-(4, 5-dimethylthiazolyl-2)-2, 5 diphenyltetrazolium bromide (5 mg/mL MTT in PBS, Sigma, USA). The produced formazan crystals were dissolved in 200 µL of DMSO solution after 30 minutes of incubation at room temperature on a shaker. Their optical density was estimated by an ELISA reader (Expert 96, Asys Hitch, Ec Austria) at 570 nm. The control group consisted of wells containing cells that were not encapsulated in alginate hydrogel.
Statistical analysis
Each experimental condition was performed more than three times. The results were shown as mean ± SD. A statistical software tool was used to analyze our data (SPSS 19, IBM). To determine the statistical significance between our control group (undifferentiated hEnSCs) and all experimental groups, one-way ANOVA analysis was used. Meanwhile, the P value less than 0.5 were considered significant.
Results
Characterization of harvested hEnSCs
Morphology of passage 3 harvested hEnSCs in normal cell culture condition was spindle-shaped fibroblast-like cells. After 21 days, the hEnSCs morphology in osteogenic media resulted in hydroxyapatite crystals formation that was verified by alizarin red staining (Fig. 1). The morphological alterations of stem cells and the intracellular presence of neutral lipid vacuoles (by oil-red staining) of hEnMSCs cultivated in adipogenic inducers for 21 days validated our adipogenic differentiation accuracy (Fig. 1). The immunophenotyping characterization of isolated hEnMSCs was performed by flow cytometric analysis for CD markers including CD105, CD90, CD146, CD73, and CD31. As demonstrated in Fig. 1, the cells harvested using the specified technique were negative for the endothelial cell marker CD31. hEnSCs expressed MSC markers (CD105 (89%), CD90 (93%), CD73 (95%)). Ninety-two percent of hEnSCs expressed CD146 as a specific marker of endometrial stem cells. These findings showed that the cells obtained were endometrial MSCs, with no contamination of endothelial cells.
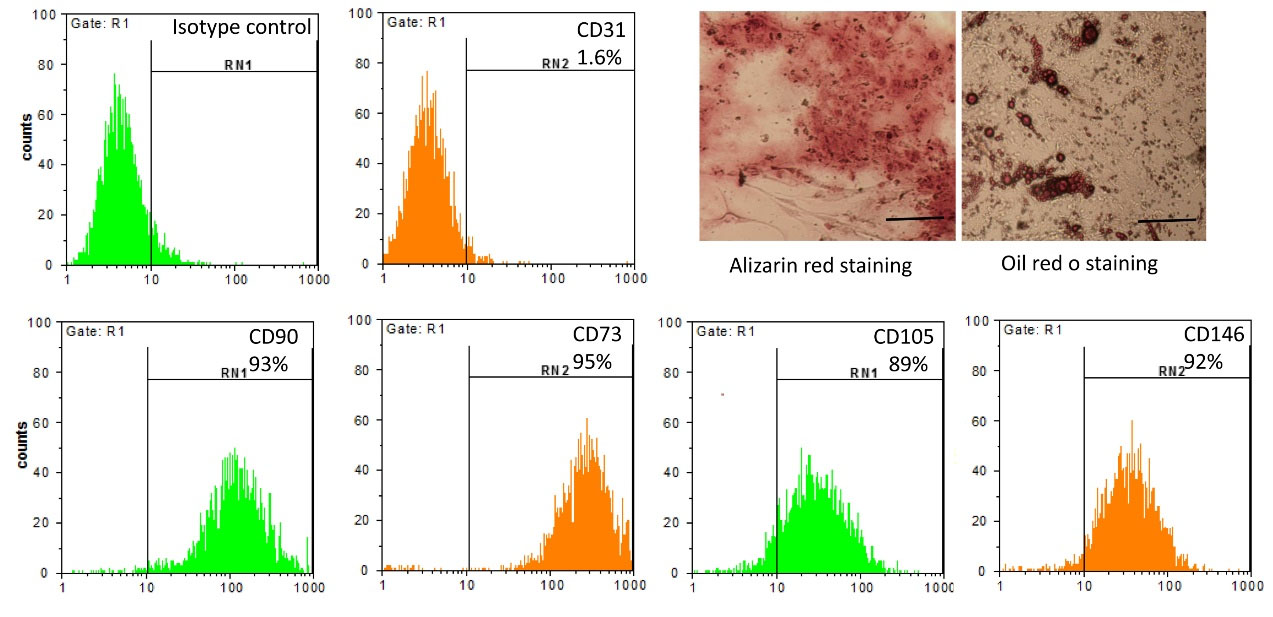
Fig. 1.
Harvested hEnSCs characterization.
Microphotographs of isolated hEnSCs after 21 days culture in osteogenic and adipogenic induction media stained with alizarin red and oil red o staining, respectively. Flow cytometric analysis exhibited positive expression of CD105, CD90, CD73, CD146 (mesenchymal cell markers) and negative expression of CD31 (endothelial cell markers) in cultured hEnSCs. The scale bar is 50 µm.
.
Harvested hEnSCs characterization.
Microphotographs of isolated hEnSCs after 21 days culture in osteogenic and adipogenic induction media stained with alizarin red and oil red o staining, respectively. Flow cytometric analysis exhibited positive expression of CD105, CD90, CD73, CD146 (mesenchymal cell markers) and negative expression of CD31 (endothelial cell markers) in cultured hEnSCs. The scale bar is 50 µm.
Immunofluorescence staining analysis for germ cell differentiated cells from EnSCs
To induce hEnSCs into germ cells, at passage 3, the hEnSCs were treated with 10μM RA concentration in a complete cell culture medium, and cell morphology was investigated every day under a phase contrast microscope. After 7 days of induction, the resulting cells typically seemed to be long spindles cells that formed adequate interaction with each other with proper growth and proliferation capacity, while the control group of untreated hEnSCs with flat morphology were 100% confluent on day 7 (Fig. 2A). To characterize and establish germ-like cell differentiation, we utilized immunofluorescence (IF) staining to study the protein expression of germ cells marker and real-time RT-PCR for molecular analysis in our experimental groups (control group cells and treated cells with RA). DAZL and DDX4-positive cells were used to determine the rate of differentiation as a percentage of the total number of DAPI-stained cells in one experimental group, but there was no expression in the control group (undifferentiated hEnSCs). The acquired images showed a high expression of these markers in the treated cells with RA (Fig. 2B, 2C, Table 1).

Fig. 2.
Differentiation analysis of isolated hEnSCs into germ cell-like cells treated with 10μM RA in 2D cell culture. A:
Microphotographs of hEnSCs derived germ cell-like cells after 7 days/RA 10μM and untreated hEnSCs cells after 7 days/ without RA. B and C: Immunofluorescence staining of hEnSCs derived germ cell-like cells with anti-DAZL and DDX4 with DAPI fluorophore on day 7 post-differentiation. D) DAZL and DDX4 gene expression levels in hEnSCs-derived germ cell-like cells treated with 10μM RA and non-treated cells. GAPDH was considered as housekeeping gene control.The scale bar is 50 µm.
.
Differentiation analysis of isolated hEnSCs into germ cell-like cells treated with 10μM RA in 2D cell culture. A:
Microphotographs of hEnSCs derived germ cell-like cells after 7 days/RA 10μM and untreated hEnSCs cells after 7 days/ without RA. B and C: Immunofluorescence staining of hEnSCs derived germ cell-like cells with anti-DAZL and DDX4 with DAPI fluorophore on day 7 post-differentiation. D) DAZL and DDX4 gene expression levels in hEnSCs-derived germ cell-like cells treated with 10μM RA and non-treated cells. GAPDH was considered as housekeeping gene control.The scale bar is 50 µm.
Molecular analysis by real-time-PCR assay for germ cells derived from EnSCs
After induction of germ-like cells, their molecular gene expression profile for DAZL and DDX4 were examined by real-time PCR for 7 days in 2D cell culture conditions. The GAPDH gene was considered the housekeeping gene control. As demonstrated in Fig. 2D, the expression of DAZL and DDX4 markers increased significantly in germ-like cells (P < 0.05), with DAZL being more expressed than the DDX4 gene (Table 1).
Analysis of hEnSCs -derived germ-like cells differentiation into oocyte-like cells
HEnSCs were cultured in oocyte-like cells induction medium supplemented with RA and different concentrations of BMP4 in both 2D and 3D cell culture conditions. These cells were kept in cell culture condition for 1 week and they were studied at three different time points under a phase contrast microscope. As shown in Fig. 3, hEnSCs were first observed as fibroblast-like cells in 2D cell culture conditions, and after 5 days, the hEnSCs spontaneously produced small round cells in complete cell culture media supplemented with 10µM RA, which was then cultured in induction media containing different concentrations of BMP4 (30, 50 and 100 ng/mL) for further maturation. According to the microscopy results, differentiation of hEnSCs into oocyte-like cells after 3 days of BMP4 treatment was insufficient, but hEnSCs-derived oocyte-like cells began to modify their differentiated morphology after 7 days of induction. The findings indicate that proper development and ordered morphology of hEnSCs-driven oocyte-like cells occurred after 5 days of induction media treatment. We showed that after 7 days of post-induction with RA10+BMP4 100ng/mL, the cells has died and this concentration of BMP4 was not used for this study.

Fig. 3.
Evaluation of hEnSCs-derived germ cells-like differentiation into oocyte-like cells in 2D culture.
Light microscope images of hEnSCs treated with 10μM RA differentiation into oocyte-like cells in four different concentrations of BMP4 (30, 50,100 ng/mL) after 5 days.
.
Evaluation of hEnSCs-derived germ cells-like differentiation into oocyte-like cells in 2D culture.
Light microscope images of hEnSCs treated with 10μM RA differentiation into oocyte-like cells in four different concentrations of BMP4 (30, 50,100 ng/mL) after 5 days.
Measurement of SCP3, GDF9B, and GDF9 protein expression in encapsulated cells
We probed the RA and BMP4 (30, 50, and 100ng/mL) on the expression of meiotic (SCP3) and post-meiotic (GDF9, GDF9B) specific proteins in oocyte-like cells derived from hEnSCs after 5 days of induction. immunofluorescence study of encapsulated cells in alginate hydrogel using immunofluorescence staining revealed that hEnSCs exposed to RA and BMP4 exhibited the SCP3, GDF9, and GDF9B markers on differentiated cells (female germ cells). As shown in Fig. 4A, we detected that the protein expression level of meiotic and post-meiotic related markers increased after treatment with an induction medium, and changes in protein expression were observed differently. At 5 days, hEnSCs cultivated in RA+ 50 ng/mL BMP4 expressed more SCP3, GDF9, and GDF9B proteins than the cultured control group in RA+ BMP4. We examined the expression of these markers associated with oocyte-like cell differentiation between treatment groups and found that they were most abundant in hEnSCs-derived oocyte-like cells treated with 50 ng/mL BMP4 on day 5 of induction.
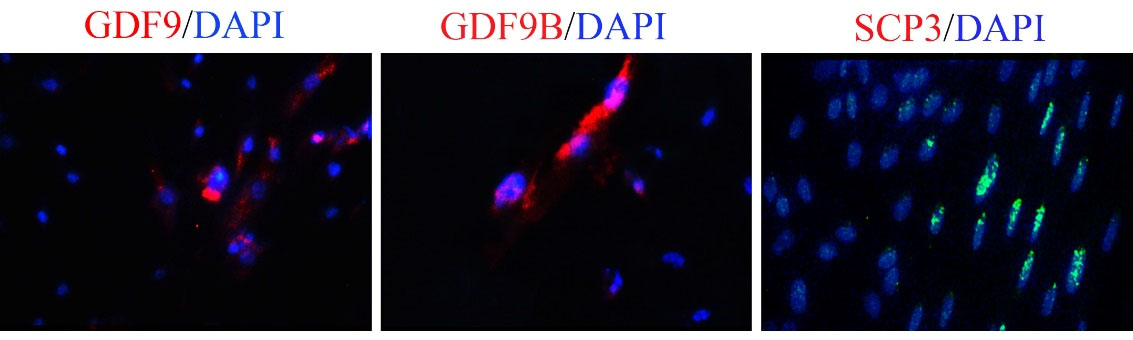
Fig. 4.
Differentiation of encapsulated hEnSCs into oocyte-like cells in 3D alginate hydrogel.
Immunofluorescence staining microphotographs of hEnSCs derived oocyte-like cells with anti GDF9, GDF9B and SCP3 with DAPI fluorophore on day 5 post-differentiation in 3D alginate hydrogel. The scale bar is 20 µm.
.
Differentiation of encapsulated hEnSCs into oocyte-like cells in 3D alginate hydrogel.
Immunofluorescence staining microphotographs of hEnSCs derived oocyte-like cells with anti GDF9, GDF9B and SCP3 with DAPI fluorophore on day 5 post-differentiation in 3D alginate hydrogel. The scale bar is 20 µm.
The expression profile of female germ cell-specific genes in differentiated hEnSCs-derived germ cells
Real-time PCR was used on day 5 of treatment in both cell culture conditions, including monolayer cell culture and encapsulated in alginate hydrogel, to examine the relative expression profile of the genes during the differentiation of hEnSC germ cells (Fig. 5). SCP3 is a frequent meiotic marker that has been shown to be expressed in oocyte-like cells in vitro. In addition, the post-meiotic GDF9 and GDF9B markers participated in the primary follicle development from the primordial follicle cells. Expression of these genes increased in the 30 and 50ng/mL BMP4 groups in 3D group in comparison with the 30 and 50 ng/mL BMP4 concentrations in 2D cell culture groups. The expression of these genes in RA+BMP4 100 ng/mL was not high and this difference was not statistically significant between 2D and 3D groups. On the other hand, the gene expression profile of these oocyte differentiation markers showed the highest expression level of SCP3, GDF9, and GDF9B genes in 50 ng/mL BMP4 group on day 5. The outcomes of the study indicated a synergistic relationship between RA and 50 ng/mL BMP4 in increasing genes associated with oocyte development. The qPCR and immunofluorescence results established an increase in the expression of SCP3, GDF9, and GDF9B at day 5 in RA plus 50 ng/mL BMP4 group (Fig. 4B).
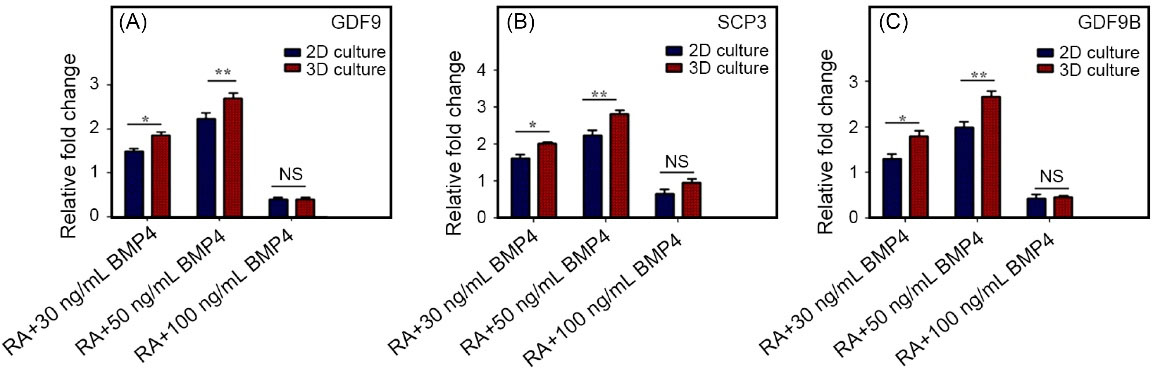
Fig. 5.
Real-time PCR analysis for the expression level of oocyte-like cells makers.
The expression of GDF9, SCP3, and GDF9B genes in oocyte-like cells in 3D scaffold compared to 2D cell culture. The expression of all genes was significantly decreased compared with undifferentiated cells as a control (P< 0.001). GAPDH was considered as housekeeping gene control. Error bars show ± SD, n =3 samples.
.
Real-time PCR analysis for the expression level of oocyte-like cells makers.
The expression of GDF9, SCP3, and GDF9B genes in oocyte-like cells in 3D scaffold compared to 2D cell culture. The expression of all genes was significantly decreased compared with undifferentiated cells as a control (P< 0.001). GAPDH was considered as housekeeping gene control. Error bars show ± SD, n =3 samples.
Alginate hydrogel features
The degradation rate is an important metric for biomaterial scaffold applications in tissue engineering. Biocompatible biodegradable hydrogels will disintegrate at the same rates as cell proliferation and extracellular matrix synthesis in injured tissues. We measured the Alginate hydrogel degradation rate after 22 days of soaking in a cell culture medium, as shown in figure 6A. After 24 hours of incubation, the alginate hydrogel degradation rate profile showed gradual degradation, which occurred at a faster rate as incubation time increased. This finding revealed that alginate hydrogel may provide a reliable, long-lasting biodegradable scaffold for a variety of tissue engineering applications. The result of SEM images showed the existence of encapsulated cells in alginate hydrogel (Fig. 6B). Furthermore, cell culture assays were carried out to study the biocompatibility of alginate-hydrogel on encapsulation of hEnSCs-derived germ-cell-like cells into the hydrogel. The figure shows the form and morphology of alginate hydrogel with or without seed cells during a 5-day period. On the other hand, the viability and proliferation rate of encapsulated cells were detected via the MTT test (Fig. 6C). In fact, cell viability in 3D alginate hydrogel was investigated after 1, 3 and 5 days of 3D and 2D cell culture by MTT assay. The most interesting feature of the findings in figure 6C is a steady increase in hEnSC-derived germ-like cell viability under 2D and 3D cell culture conditions. Although, the viability of cultured cells in 3D condition was higher than that of a 2D culture. Furthermore, detailed rheological measurements were tested for the mechanical properties of the alginate hydrogel. Fig. 6D shows the storage (Gʹ) and loss (Gʺ) moduli of the hydrogel, which indicate their elastic and viscous properties, respectively. According to Fig. 6D, the Gʹ and Gʺ curves of alginate hydrogel first increased. Based on the results, Gʹ levels of hydrogel were higher than Gʺ values, indicating the dominant elastic properties of this hydrogel and showing improved alginate gel stiffness.
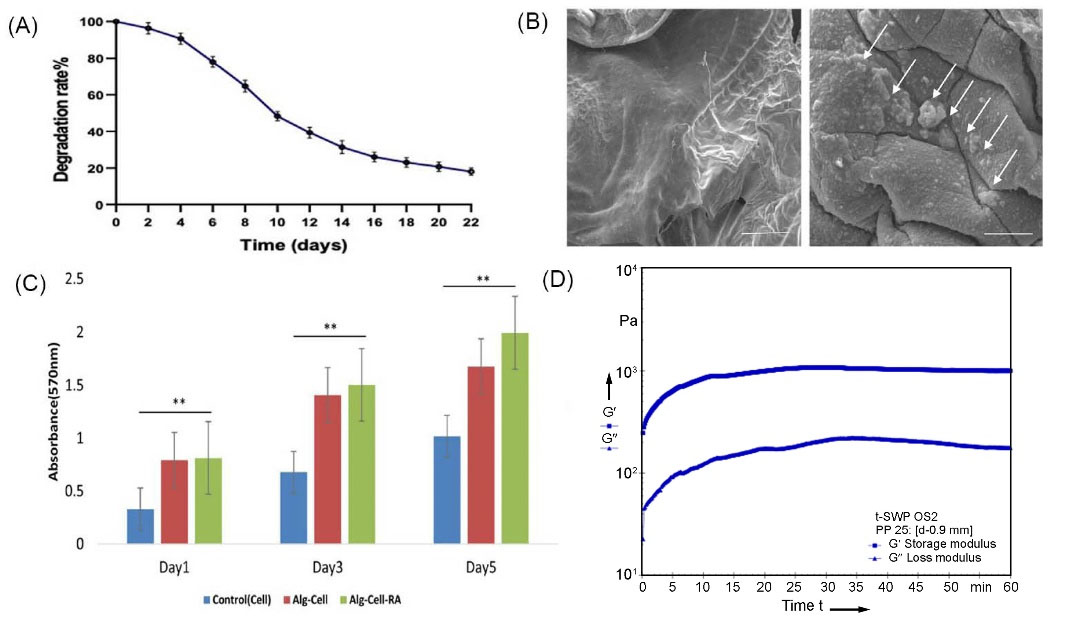
Fig. 6.
Fabricated Alginate hydrogel analysis. A:
In vitro degradability of 3D alginate hydrogel. B: SEM analysis of 3D alginate hydrogel without and with hEnSCs cultured for 5 days within the 3D alginate hydrogel. C: Cell viability of hEnSCs cultured in alginate hydrogel, alginate hydrogel with RA and polystyrene plate after 1, 3 and 5 days. D: Rheological examination with frequency sweep between 0.1 and 10 Hz.
.
Fabricated Alginate hydrogel analysis. A:
In vitro degradability of 3D alginate hydrogel. B: SEM analysis of 3D alginate hydrogel without and with hEnSCs cultured for 5 days within the 3D alginate hydrogel. C: Cell viability of hEnSCs cultured in alginate hydrogel, alginate hydrogel with RA and polystyrene plate after 1, 3 and 5 days. D: Rheological examination with frequency sweep between 0.1 and 10 Hz.
Discussion
Our study showed that, after 5 days of encapsulation in alginate hydrogel, hEnSCs were shown to be capable of differentiating to oocyte-like cells at an adjusted dose of 10µM RA and 50 ng/mL BMP4. The biocompatibility and safety of the produced alginate hydrogel on differentiated cells were validated by our research. For the first time, we developed a 3D scaffold made of alginate composite to enhance hEnSC development in oocyte-like cells.
Infertility is a frequent problem in the lives of couples, with psychological, social, economic, and medical consequences.
26
While the rate of infertility remains steady, there has recently been an increase in demand for infertility therapies.
27,28
This necessitated a more sophisticated approach to the diagnosis and treatment of reproductive capacity disorders. Recently, the area of tissue engineering has emerged as a novel field of medical research for the treatment of infertility by three main components, including different forms of stem cells, biodegradable scaffolding, and growth factors.
Because of the infinite differentiation in regenerative medicine, stem cell research has recently become a popular area of research in medical research.
29,30
Previous research has shown that stem cell-based treatment employing embryonic stem cells, induced pluripotent stem cells
31
and multipotent stem cells
28
can restore germ line cells in in vitro and in vivo studies. Although stem cell technology is well established and quickly expanding in infertility treatment research, there are numerous problems in medical applications, including differentiation capacity limitation, tumor development, and difficulty to produce in vitro gametes.
7,32
As a result, numerous types of research have indicated that hEnSCs would be an attractive alternative source of germ cell generation in in vitro investigations due to their pluripotent characteristics. In 2D and 3D culture conditions, our group differentiated hEnSCs into germ-like cells for the first time.
3
In this study, we presented a novel approach for hEnSCs encapsulated into alginate hydrogel differentiation to oocyte-like cells in vitro by utilizing RA and BMP4. A naïve microenvironment is crucial for stem cells to differentiate into oocytes. There are only a few growth factors and regulators known to promote germ cell differentiation and gamete maturation, including BMP4 and RA.
33
In vitro mammalian gamete differentiation relies on these recognized factors as key regulators. BMP4 plays a critical role in mitosis triggering and oocyte differentiation.
7
In addition to controlling the sex-specific timing of meiosis entry, RA induces meiosis-specific markers, including Stra8, synaptonemal complex proteins (SCP), Dmc1, and Rec8. Studies have shown that RA plays an integral role in determining the fate of male and female embryonic gonads by regulating migration and postmigration of germ cells.
34
Other investigations discovered a cocktail of soluble growth factors, including RA, KL, LIF, BMP4, SDF-1, and bFGF, as well as chemicals, are able to sustain the survival and self-renewal of mouse germ cells in the absence of somatic cell support.
35
Growth factors and compounds were able to prevent significant levels of apoptosis in germ cells, stimulate their proliferation, and allow them to progress through meiotic prophase I after adhering to an acellular substrate.
36,37
Furthermore, RA is known to promote the development of germ cells and it was used to direct functional gametogenesis in vitro.
38
RA makes the meiosis of ovary PGCs about 13.5 days post coitum (dpc) while at the same time in testis, RA reduces and inhibits PGCs' meiosis entry. RA was displayed as the stimulator of differentiation and proliferation of different types of MSCs to germ cell induction. Also, it can control the induction of meiosis cell cycle entry and the prevention of apoptosis in the first step of germ cell development.
34
The active derivative of vitamin A, RA, influences the differentiation of germ cells and is required to transition into meiosis in both male and female germ cells. In addition to Sertoli cells, germ cells express RA receptors, which are stimulated by RA. Researchers observed differentiation of human umbilical cord Wharton's jelly-derived MSCs into germ-like cells in vitro after treating ESCs and bone marrow cells with RA.
39
As indicated in Fig.1, isolated hEnSCs exhibited MSC markers such as CD105, CD90, CD44, and Oct4 as a pluripotency marker, as well as CD146. These harvested hEnSCs were negative for hematopoietic cells and endothelial cell markers expression such as markers CD45, CD34, CD133, and CD31. The multi-lineage differentiation assays demonstrated the adipogenic and osteogenic capacity of hEnSCsexpression (Fig. 1B, 1C). Then we differentiated hEnSCs to germ-like cells with induction media supplemented with 10 µM RA. As shown in Figs. 2A and 2C, morphological inspection, immunofluorescence and real-time PCR assays verified that hEnSCs characterized a more prominent germ-like cell marker (DAZL and DDX4) compared to undifferentiated hEnSCs for 7 days with induction media.
Recently, tissue engineering applied a powerful tool to regenerate injured tissues by 3D cell culture technique, which mimics the cell-cell contacts and the robust microenvironment. The first pioneering investigation explored the 3D scaffold effects on mouse follicle growth improvement and in vitro spermatogenesis in the murine model.
40
In the current study, we assessed whether BMP4 and RA cooperatively promote the differentiation of the oocyte-like cells from hEnSCs encapsulated into alginate hydrogel in in vitro study.
We prepared Alginate hydrogel through the ionic crosslinking mechanism. The alginate-based hydrogel could form a three-dimensional network via anionic crosslinking reactions with divalent cations, such as Ca2+, as well as chemical crosslinking, comprising enzymatic or photo-initiation.
25
The Alginate hydrogel is regarded as a suitable functional biomaterial in regenerative medicine and one of the most widely used biomaterials for microencapsulation, due to several advantages such as high biocompatibility, proper stability, easy entrapment of live cell, high affinity to water, facilitates the transport of nutrients and waste products through the gel and ability to form hydrogels under very mild conditions. Results showed that this hydrogel applied had enough properties as a relevant and useful 3D hydrogel for in vitro and in vivo biomedical applications.
41-43
The structure and architecture of the fabricated scaffold and cell attachments were observed by SEM analysis. SEM assessment revealed high porosity of the scaffold as well as perfect spreading and attachment of encapsulated cells. The morphology and size of hEnSCs derived germ-like cells were the same as the germ cells. Moreover, the resulting images showed a fine cell structure, integrity and right interaction with other cells and the alginate scaffold. The SEM results demonstrated that the alginate scaffold had no effect on cell structure or morphology (Fig. 6B). SEM analysis revealed a porous 3D network structure allowing cells to penetrate and diffuse nutrients for survival.
43
Fine cell distribution and cell-cell contact inside alginate hydrogel were observed without any side effects on cell microstructural morphology. Pores were interconnected and almost spherical in shape. An interconnected porous appearance might facilitate cell attachment, proliferation, and migration in tissue engineering. The porous structure of fabricated scaffolds may also facilitate the delivery of oxygen, nutrients, and metabolites throughout the system. The lack of cell adhesion moieties in produced hydrogels might explain why cells stuck to the surface of hydrogels and displayed a good spheroid shape. The cells were physically entrapped on hydrogel scaffolds rather than biochemically.
25
SEM assay illustrated a proper porous 3D network structure for allowing cell penetration and diffusion of nutrient substances for cell survival. It revealed the fine cell distribution and cell-cell contact inside alginate hydrogel without any side effects on cell microstructural morphology. MTT analysis results were also satisfactory for hEnSC-derived germ-like cells and confirmed higher cell viability within 5 days of differentiation in 3D cell culture compared to 2D cell culture.
Following that, we examined the impact of combining RA with different concentrations of BMP4 (30, 50, and 100 ng/mL) on the ability of hEnSCs to differentiate into oocyte-like cells in 2D and 3D culture systems after 3, 5, and 7 days of incubation. Ultimately, immunofluorescence and gene expression profiling methods were used to determine the expression level of meiotic (SCP3) and post-meiotic (GDF9, GDF9B) specific oocyte-like cell markers in differentiated and undifferentiated cells. The resulting data from immunofluorescence and real-time PCR analysis indicated that hEnSCs derived oocyte-like cells kept their nature and showed more expression of specific proteins related to oocyte-like cells after 5 days in 3D cell culture with culture media supplemented with 10µM RA and 50 ng/mL BMP4. For the first time, we demonstrated the generation of oocyte-like cells from hEnSCs encapsulated in a 3D alginate hydrogel with the incorporation of key inductive factors, such as RA and BMP4, in culture media.
Recently, studies described the role of RA and BMP4 as key regulators of the meiosis cell cycle and germ cell induction. Previous investigations have shown that oocyte-like cell generation was induced with the incorporation of RA and BMP4 in a culture medium in 2D and 3D in vitro conditions. Moreover, based on our acquired results, Alginate hydrogel containing hEnSCs with RA and BMP4 substances considerably improved the proliferation and differentiation of hEnSCs to oocyte-like cells.
10,33
Research Highlights
What is the current knowledge?
√ RA and BMP4 can differentiate endometrial stem cells into oocyte cells oocyte-like cells derived EnSCs express GDF9.
What is new here?
√ Promoted oocyte-like cell differentiation of EnSCs as a promising source of stem cells, in a hydrogel scaffold and three-dimensional cell culture.
√ After 5 days of encapsulation in alginate hydrogel, hEnSC was shown to be capable of differentiating to oocyte-like cells at an adjusted dose of 10µM RA and 50 ng/mL BMP4.
Conclusion
Our findings showed that after 5 days of encapsulation in alginate hydrogel, hEnSCs may develop into oocyte-like cells at an adjusted dose of 10µM RA and 50 ng/mL BMP4. Our results confirmed the suitable biocompatibility and safety of fabricated Alginate hydrogel on the differentiated cells. Furthermore, we established for the first time a 3D scaffold by alginate composite for improvement of hEnSCs differentiation to oocyte-like cells. Also, hEnSCs differentiation accuracy into oocyte-like cells was proved by analysis of specific meiotic and post-meiotic oocyte-like cell proteins utilizing immunofluorescence staining and real-time PCR for SCP3, GDF9 and GDF9B.
Funding sources
This study is financially supported by the Tehran University of Medical Sciences with grant number 95-03-87-32964.
Ethical statement
Tehran University of Medical Sciences (TUMS) under Ethical No approved this work: IR.TUMS.VCR.REC.1397.592.
Competing Interests
The authors declare no conflict of interest.
References
- Zhu JL, Basso O, Obel C, Bille C, Olsen J. Infertility, infertility treatment, and congenital malformations: Danish national birth cohort. BMJ 2006; 333:679. doi: 10.1136/bmj.38919.495718.AE [Crossref] [ Google Scholar]
-
Taghdiri Nooshabadi V. Endometrial Mesenchymal Stem Cell-Derived Exosome Promote Endothelial Cell Angiogenesis in a Dose Dependent Manner: A New Perspective on Regenerative Medicine and Cell-Free Therapy. Arch Neurosci2019. 6: e94041. 10.5812/ans.94041.
- Ramzgouyan MR, Ai J. Differentiation of human endometrial stem cells into germ cell – Like cell in fibrin scaffold. J Med Hypotheses Ideas 2015; 9:90-93. doi: 10.1016/j.jmhi.2015.09.001 [Crossref] [ Google Scholar]
- Fathi A, Khanmohammadi M, Goodarzi A, Foroutani L, Taherian-Mobarakeh Z, Saremi J. Fabrication of Chitosan-Polyvinyl Alcohol and Silk Electrospun Fiber Seeded with Differentiated Keratinocyte for Skin Tissue Regeneration in Animal Wound Model. J Biol Eng 2020; 14:1-14. doi: 10.21203/rs.3.rs-38854/v3 [Crossref] [ Google Scholar]
- Eskandari N, Hassani Moghaddam M, Atlasi MA, Amini Mahabadi J, Taherian A, Nikzad H. The combination of retinoic acid and estrogen can increase germ cells genes expression in mouse embryonic stem cells derived primordial germ cells. Biologicals 2018; 56:39-44. doi: 10.1016/j.biologicals.2018.10.001 [Crossref] [ Google Scholar]
- Arbel‐Eden A, Simchen G. Elevated Mutagenicity in Meiosis and Its Mechanism. BioEssays 2019; 41:1800235. doi: 10.1002/bies.201800235 [Crossref] [ Google Scholar]
- Kee K, Gonsalves JM, Clark AT, Pera RAR. Bone Morphogenetic Proteins Induce Germ Cell Differentiation from Human Embryonic Stem Cells. Stem Cells Dev 2006; 15:831-837. doi: 10.1089/scd.2006.15.831 [Crossref] [ Google Scholar]
- De Angelis MT, Parrotta EI, Santamaria G, Cuda G. Short-term retinoic acid treatment sustains pluripotency and suppresses differentiation of human induced pluripotent stem cells. Cell Death Dis 2018; 9:6. doi: 10.1038/s41419-017-0028-1 [Crossref] [ Google Scholar]
- Dyce PW, Tenn N, Kidder GM. Retinoic acid enhances germ cell differentiation of mouse skin-derived stem cells. J Ovarian Res 2018; 11:19. doi: 10.1186/s13048-018-0390-3 [Crossref] [ Google Scholar]
- Dudley B, Palumbo C, Nalepka J, Molyneaux K. BMP signaling controls formation of a primordial germ cell niche within the early genital ridges. Dev Biol 2010; 343:84-93. doi: 10.1016/j.ydbio.2010.04.011 [Crossref] [ Google Scholar]
- Zhu H, Pan Y, Jiang Y, Li J, Zhang Y, Zhang S. Activation of the Hippo/TAZ pathway is required for menstrual stem cells to suppress myofibroblast and inhibit transforming growth factor β signaling in human endometrial stromal cells. Hum Reprod 2019; 34(4):635-645. doi: 10.1093/humrep/dez001 [Crossref] [ Google Scholar]
- Hasanzadeh E, Ebrahimi‐Barough S, Mahmoodi N, Mellati A, Nekounam H, Basiri A. Defining the role of 17β‐estradiol in human endometrial stem cells differentiation into neuron‐like cells. Cell Biol Int 2021; 45(1):140-153. doi: 10.1002/cbin.11478 [Crossref] [ Google Scholar]
- Taghdiri Nooshabadi V, Khanmohammadi M, Shafei S, Banafshe HR, Veisi-Malekshahi Z, Ebrahimi-Barough S. Impact of atorvastatin loaded exosome as an anti-glioblastoma carrier to induce apoptosis of U87 cancer cells in 3D culture model. BiochemBiophys Rep 2020; 23:100792. doi: 10.1016/j.bbrep.2020.100792 [Crossref] [ Google Scholar]
- Arabpour Z, Baradaran‐Rafii A, Lotfi-Bakhshaiesh N, Ai J, Ebrahimi-Barough S, Esmaeili Malekabadi H. Design and characterization of biodegradable multi layered electrospun nanofibers for corneal tissue engineering applications. J Biomed Mater Res A 2019; 107:2340-2349. doi: 10.1002/jbm.a.36742 [Crossref] [ Google Scholar]
- Tremblay KD, Dunn NR, Robertson EJ. Mouse embryos lacking Smad1 signals display defects in extra-embryonic tissues and germ cell formation. Development 2001; 128:3609-21. doi: 10.1242/dev.128.18.3609 [Crossref] [ Google Scholar]
- West FD, Roche-Rios MI, Abraham S, Rao RR, Natrajan MS, Bacanamwo M. KIT ligand and bone morphogenetic protein signaling enhances human embryonic stem cell to germ-like cell differentiation. Hum Reprod 2010; 25:168-178. doi: 10.1093/humrep/dep338 [Crossref] [ Google Scholar]
-
Arabpour Z, Youseffi M, Soon CP, Sultana N, Bazgeir MR, Mozafari M, et al. Designing Biomaterials for Regenerative Medicine: State-of-the-Art and Future Perspectives. In: Tissue Engineering Strategies for Organ Regeneration. CRC Press,Taylor & Francis Group. 2020. p. 1-9. 10.4324/9780429422652-1.
- Makar K, Sasaki K. Roadmap of germline development and in vitro gametogenesis from pluripotent stem cells. Andrology 2020; 8:842-51. doi: 10.1111/andr.12726 [Crossref] [ Google Scholar]
- Volarevic V, Bojic S, Nurkovic J, Volarevic A, Ljujic B, Arsenijevic N. Stem cells as new agents for the treatment of infertility: current and future perspectives and challenges. BioMed Res Int 2014; 2014:507234. doi: 10.1155/2014/507234 [Crossref] [ Google Scholar]
- Moreno I, Míguez-Forjan JM, Simón C. Artificial gametes from stem cells. Clin Exp Reprod Med 2015; 42:33. doi: 10.5653/cerm.2015.42.2.33 [Crossref] [ Google Scholar]
- Zhang P-Y, Fan Y, Tan T, Yu Y. Generation of artificial gamete and embryo from stem cells in reproductive medicine. Front BioengBiotechnol 2020; 8:781. doi: 10.3389/fbioe.2020.00781 [Crossref] [ Google Scholar]
- Heidari-Keshel S, Rezaei TM, Ai J, Soleimani M, Ghanbari Z, Baradaran-Rafii Baradaran-Rafii. Isolation and characterization of endometrial mesenchymal stem cells and the evaluation of surface markers in comparison to bone marrow mesenchymal stem cells. Sci J Iran Blood Transfus Organ 2015; 11:295-305. [ Google Scholar]
- Panda B, Sharma Y, Gupta S, Mohanty S. Mesenchymal Stem Cell-Derived Exosomes as an Emerging Paradigm for Regenerative Therapy and Nano-Medicine: A Comprehensive Review. Life (Basel) 2021; 11:784. doi: 10.3390/life11080784 [Crossref] [ Google Scholar]
- Shafei S, Khanmohammadi M, Heidari R. Exosome loaded alginate hydrogel promotes tissue regeneration in full‐thickness skin wounds: An in vivo study. J Biomed Mater Res A 2020; 108:545-556. doi: 10.1002/jbm.a.36835 [Crossref] [ Google Scholar]
- Goodarzi A, Khanmohammadi M, Ebrahimi-Barough S, Azami M, Amani A, Baradaran-Rafii AR. Alginate-Based Hydrogel containing taurine-loaded chitosan nanoparticles in biomedical application. Arch Neurosci 2019; 6:e86349. doi: 10.5812/ans.86349 [Crossref] [ Google Scholar]
- Abou Neel EA, Cheema U, Knowles JC, Brown RA, Nazhat SN. Use of multiple unconfined compression for control of collagen gel scaffold density and mechanical properties. Soft Matter 2006; 2:986. doi: 10.1039/b609784g [Crossref] [ Google Scholar]
- Lorzadeh N, Kazemirad N. Application of stem cells to infertility treatment with emphasis on mesenchymal stem cells and ovarian stem cells. Am J Perinatol 2018; 35:1142-1147. doi: 10.1055/s-0038-1646948 [Crossref] [ Google Scholar]
- Fazeli Z, Abedindo A, Omrani MD, Ghaderian SMH. Mesenchymal stem cells (MSCs) therapy for recovery of fertility: a systematic review. Stem Cell Rev Rep 2018; 14:1-12. doi: 10.1007/s12015-017-9765-x [Crossref] [ Google Scholar]
- Einabadi M, Ai J, Kargar M, Kafilzadeh F, Taghdiri Nooshabadi V, Jamali H. Mesenchymal cell-derived exosomes as novel useful candidates for drug delivery. Arch Neurosci 2020; 7:e98722. doi: 10.5812/ans.98722 [Crossref] [ Google Scholar]
- Taghdiri-Nooshabadi V, Khanmohamadi M, Valipour E, Mahdipour S, Salati A, Veisi-Malekshahi Z. Impact of exosome‐loaded chitosan hydrogel in wound repair and layered dermal reconstitution in mice animal model. J Biomed Mater Res A 2020; 108:2138-49. doi: 10.1002/jbm.a.36959 [Crossref] [ Google Scholar]
- Li Y, Wang X, Feng X, Liao S, Zhang D, Cui X. Generation of male germ cells from mouse induced pluripotent stem cells. in vitro Stem Cell Res 2014; 12:517-30. doi: 10.1016/j.scr.2013.12.007 [Crossref] [ Google Scholar]
- Ko K, Tapia N, Wu G, Kim J, Marcos J, Bravo A. Induction of Pluripotency in Adult Unipotent Germline Stem Cells. Cell Stem Cell 2009; 5:87-96. doi: 10.1016/j.stem.2009.05.025 [Crossref] [ Google Scholar]
- Zuo Q, Jin J, Jin K, Sun C, Song J, Zhang Y. Distinct roles of retinoic acid and BMP4 pathways in the formation of chicken primordial germ cells and spermatogonial stem cells. Food Funct 2019; 10:7152-63. doi: 10.1039/C9FO01485C [Crossref] [ Google Scholar]
- Teletin M, Vernet N, Ghyselinck NB, Mark M. Roles of Retinoic Acid in Germ Cell Differentiation. Current Topics in Developmental Biology 2017; 125:191-225. doi: 10.1016/bs.ctdb.2016.11.013 [Crossref] [ Google Scholar]
- Nayernia K, Nolte J, Michelmann HW, Lee JH, Rathsack K, Drusenheimer N. In vitro differentiated embryonic stem cells give rise to male gametes that can generate offspring mice. Dev Cell 2006; 11:125-132. doi: 10.1016/j.devcel.2006.05.010 [Crossref] [ Google Scholar]
- Farini D, Scaldaferri ML, Iona S, La Sala G, De Felici M. Growth factors sustain primordial germ cell survival, proliferation and entering into meiosis in the absence of somatic cells. Dev Biol 2005; 285:49-56. doi: 10.1016/j.ydbio.2005.06.036 [Crossref] [ Google Scholar]
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S. Retinoid signaling determines germ cell fate in mice. Science 2006; 312:596-600. doi: 10.1126/science.1125691 [Crossref] [ Google Scholar]
- Latifpour M, Shakiba Y, Amidi F, Mazaheri Z, Sobhani A. Differentiation of Human Umbilical Cord Matrix-Derived Mesenchymal Stem Cells into Germ-Like Cells. Avicenna J Med Biotechnol 2014; 6(4):218-27. [ Google Scholar]
- Huang P, Min Lin L, Ying Wu X, Ling Tang Q, Yong Feng X, Yu Lin G. Differentiation of human umbilical cord Wharton's jelly-derived mesenchymal stem cells into germ-like cells in vitro. J Cell Biochem 2010; 109:747-54. doi: 10.1002/jcb.22453 [Crossref] [ Google Scholar]
- Baert Y, Dvorakova-Hortova K, Margaryan H, Goossens E. Mouse in vitro spermatogenesis on alginate-based 3D bioprinted scaffolds. Biofabrication 2019; 11:035011. doi: 10.1088/1758-5090/ab1452 [Crossref] [ Google Scholar]
- Amorim C, Langendonckt A, David A, Dolmans M, Donnez J. Survival of human pre-antral follicles after cryopreservation of ovarian tissue, follicular isolation and in vitro culture in a calcium alginate matrix. Hum Rep 2009; 24:92-9. doi: 10.1093/humrep/den343 [Crossref] [ Google Scholar]
- Silva GM, Rossetto R, Chaves RN, Duarte ABG, Araújo VR, Feltrin C. In vitro development of secondary follicles from pre-pubertal and adult goats cultured in two-dimensional or three dimensional systems. Zygote 2015; 23(4):475-84. doi: 10.1017/S0967199414000070 [Crossref] [ Google Scholar]
- Bhat A, Hoch AI, Decaris ML, Leach JK. Alginate hydrogels containing cell-interactive beads for bone formation. FASEB J 2013; 27:1-9. doi: 10.1096/fj.12-213611 [Crossref] [ Google Scholar]