Bioimpacts. 12(2):171-174.
doi: 10.34172/bi.2022.23980
Spotlight
Cholinergic anti-inflammatory pathway and COVID-19
Danial Mehranfard 1  , Robert C. Speth 1, 2, *
, Robert C. Speth 1, 2, * 
Author information:
1College of Pharmacy, Nova Southeastern University, Fort Lauderdale, FL, USA
2Department of Pharmacology and Physiology, School of Medicine, Georgetown University, Washington, DC, USA
Abstract
The cholinergic anti-inflammatory pathway (CAP) first described by Wang et al, 2003 has contemporary interest arising from the COVID-19 pandemic. While tobacco smoking has been considered an aggravating factor in the severity of COVID-19 infections, it has been suggested by some that the nicotine derived from tobacco could lessen the severity of COVID-19 infections. This spotlight briefly describes the CAP and its potential role as a therapeutic target for the treatment of COVID-19 infections using vagus nerve stimulation or selective alpha7 nicotinic acetylcholine receptor agonists.
Keywords: Cholinergic anti-inflammatory pathway, Vagus nerve stimulation, Alpha 7 nicotinic acetylcholine receptor, COVID-19
Copyright and License Information
© 2022 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Cholinergic system
The cholinergic system (CS) is composed primarily of organized nerve cells that use or respond to the neurotransmitter acetylcholine (ACh) to communicate with other neurons and cells,
1,2
most notably, the activation of skeletal muscle contraction by the voluntary cholinergic neuronal stimulation of nicotinic ACh receptors. The CS can be subdivided into neuronal, in which ACh acts as a neurotransmitter, and non-neuronal in which ACh, in a paracrine manner, acts as a local cellular signaling molecule, involved in the regulation of the cellular functions.
3
Cholinergic anti-inflammatory pathway
The vagus nerve which is the major parasympathetic nerve, is the body’s longest nerve which innervates several major organs including the lungs, the heart, and the gastrointestinal tract.
4
The parasympathetic nervous system via the vagus nerve, plays an important role in mediating inflammatory responses.
5-7
The afferent vagus nerve can detect inflammation in peripheral tissues, sending this information to the brain. The dorsal motor nucleus of the vagus in the brainstem, through the efferent vagus nerve, can exert anti-inflammatory effects. This is known as the cholinergic anti-inflammatory pathway (CAP) in which ACh, is the key anti-inflammatory mediator.
7,8
Alpha 7 nicotinic acetylcholine receptor
As shown in Fig. 1, ACh exerts its anti-inflammatory effects via the alpha 7 nicotinic acetylcholine receptor (α7nAChR) subtype on macrophages via a circuitous pathway from the ganglia of the celiac-superior mesenteric plexus, traveling along the splenic nerve
9-11
resulting in noradrenergic stimulation of ACh secreting T-cells.
12
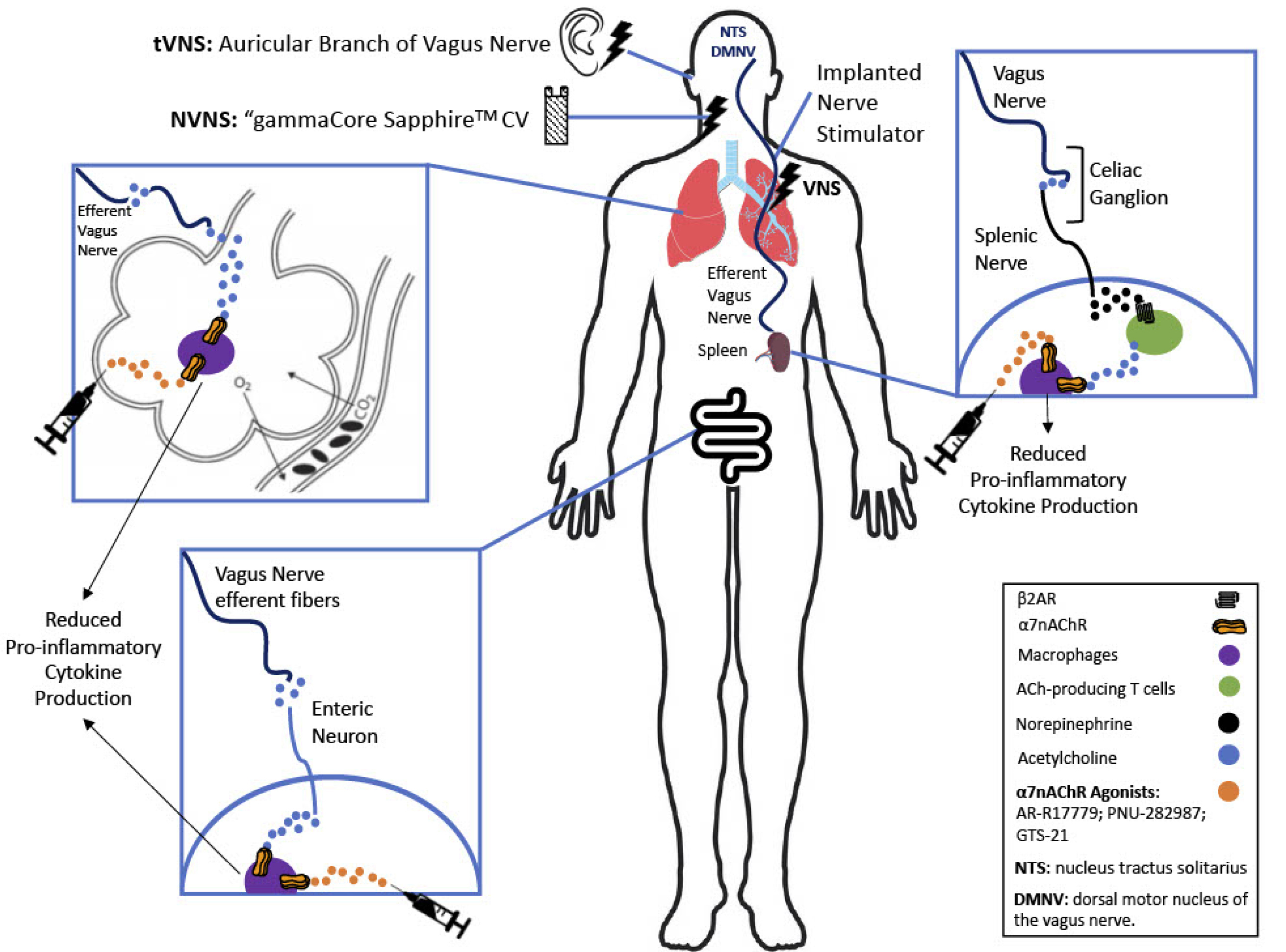

Figure 1.
The cholinergic anti-inflammatory pathway (CAP) exerts its anti-inflammatory effect via efferent vagus nerve stimulation. The CAP then branches off in 3 directions: some vagal fibers innervate the celiac ganglion where they synapse with sympathetic neurons which project to the spleen where they innervate ACh producing T cells. The T cells then release ACh that binds to α7nAChR on macrophages. Activation of the α7nAChR on macrophages inhibits the synthesis and release of proinflammatory cytokines from the macrophages, altering them to the M2 anti-inflammatory phenotype. The second branch of the CAP involves vagal efferents that innervate lung tissue, releasing ACh that directly activates α7nAChR on alveolar macrophages, again converting them to the M2 anti-inflammatory phenotype. The third branch of the CAP involves vagal efferents that activate post-ganglionic parasympathetic enteric neurons in the gut, which release ACh that activates α7nAChR on resident macrophages in the gut again converting them to the M2 anti-inflammatory phenotype. The effect of the CAP can be mimicked with pharmacological administration of selective α7nAChR agonists (depicted by the hypodermic needle). Figure derived in part from Koopman et al, Wu et al, and Bonaz et al.
27-29
.
The cholinergic anti-inflammatory pathway (CAP) exerts its anti-inflammatory effect via efferent vagus nerve stimulation. The CAP then branches off in 3 directions: some vagal fibers innervate the celiac ganglion where they synapse with sympathetic neurons which project to the spleen where they innervate ACh producing T cells. The T cells then release ACh that binds to α7nAChR on macrophages. Activation of the α7nAChR on macrophages inhibits the synthesis and release of proinflammatory cytokines from the macrophages, altering them to the M2 anti-inflammatory phenotype. The second branch of the CAP involves vagal efferents that innervate lung tissue, releasing ACh that directly activates α7nAChR on alveolar macrophages, again converting them to the M2 anti-inflammatory phenotype. The third branch of the CAP involves vagal efferents that activate post-ganglionic parasympathetic enteric neurons in the gut, which release ACh that activates α7nAChR on resident macrophages in the gut again converting them to the M2 anti-inflammatory phenotype. The effect of the CAP can be mimicked with pharmacological administration of selective α7nAChR agonists (depicted by the hypodermic needle). Figure derived in part from Koopman et al, Wu et al, and Bonaz et al.
27-29
In animal models, activation of α7nAChRs on macrophages downregulates the production of proinflammatory cytokines primarily via the JAK2–STAT3 signaling pathway, and through prevention of activation of the NF-κB pathway.
13-16
The lability of ACh and the non-specificity of nicotine and ACh for the α7nAChR limits their use as therapeutic agents. However, there are α7nAChR selective agonists, such as AR-R17779,
17
PNU-282987,
18
and GTS-21,
19
that are potential therapeutic agents.
Vagus nerve stimulation
The CAP can be activated through external vagus nerve stimulation in two ways: Invasive vagus nerve stimulation, applied to the cervical branch of the vagus nerve, via neurosurgical intervention which is approved by the FDA for the treatment of depression and epilepsy in patients >12 years of age
20
; while transcutaneous vagus nerve stimulation (tVNS) of the auricular branch of the vagus nerve is suggested to be a non-invasive alternative means of vagal stimulation.
21
There are several ongoing clinical trials to assess the tVNS impact on different conditions such as stress response in major depression (NCT04448327), and pediatric inflammatory bowel disease (NCT03863704). Of note, there is an ongoing clinical trial to investigate whether transcutaneous electrical stimulation of the auricular branch of the vagus nerve will decrease the proinflammatory cytokine response in healthy individuals (NCT02910973).
Another type of non-invasive vagus nerve stimulation (NVNS) device, “gammaCore SapphireTMCV” developed by electroCore, Inc., which fits onto the neck and sends pulses to the vagus nerve, has been granted emergency use authorization (EUA) for the treatment of COVID-19 associated dyspnea (https://www.fda.gov/media/139968/download; accessed July 19, 2021).
Role of the spleen in CAP
Acetylcholine is primarily produced by neurons for use as a neurotransmitter, but non-neuronal cells, including T cells in the spleen, can also synthesize ACh. After splenectomy, vagus nerve stimulation is no longer able to reduce inflammation, therefore the spleen is vital for the CAP response.
22-25
As shown in Fig. 1, following vagal stimulation, the anti-inflammatory reflex travels through the sympathetic splenic nerve to the spleen. The splenic nerve, which uses norepinephrine as its neurotransmitter, activates beta-2 adrenergic receptors (β2AR) on acetylcholine-producing T cells (choline acetyltransferase positive T-cells (CHAT+)). This stimulates them to secrete ACh in the spleen, establishing an anti-inflammatory response through activation of the α7nAChR on macrophages,
13
inhibiting their secretion of proinflammatory cytokines.
9,26,27
Of note, this anti-inflammatory effect is not limited to macrophages in the spleen. As shown in Fig. 1, the innervation of vagus nerve into the other organs such as lungs and the gastrointestinal tract can exert a local anti-inflammatory effect.
28,29
Nonetheless, the spleen is the efferent vagus nerve main targeted organ for the anti-inflammatory effect.
24,25
Concluding remarks: CAP and COVID-19
Autopsies of COVID-19 patients show a high infiltration of macrophages within the bronchopneumonia area.
30
Furthermore, ACE2 expressing macrophages containing SARS-CoV-2 nucleoprotein antigen densely infiltrate the lymph nodes and spleen of COVID-19 patients, causing significant interlukin-6 (IL-6) production.
31
In severe COVID-19 cases, substantial serum IL-6 elevation has been observed.
32
The high production of IL-6, together with the macrophage activation syndrome,
33
may explain the high serum level of C-reactive protein,
34
which is normally undetectable in viral infections. Therefore, macrophage activation may be an exacerbating factor for severe COVID-19 infection, producing proinflammatory cytokines and contributing to the cytokine storm.
31,35
Anti-IL-6 or anti-IL-1 treatment of COVID-19 patients significantly improved patient symptoms.
33,36-39
Of note, a recent study has shown that vagus nerve stimulation inhibits the acute respiratory distress syndrome inflammatory response through activation of the α7nAChR, via the CAP.
14
Therefore, activation of the CAP through vagus nerve stimulation or pharmacological activation through selective α7nAChR agonists, may be a possible adjunctive therapy to ameliorate severe inflammation in COVID-19 patients by inhibiting production and release of proinflammatory cytokines by macrophages, thereby reducing the cytokine storm that is a major contributor to COVID-19 morbidity, without causing systemic effects of nicotinic cholinergic receptor stimulation.
Funding sources
None.
Ethical statement
Not applicable.
Competing interests
None delared.
Authors’ contribution
DM and RCS drafted the article, designed the figure, and approved the version to be published.
References
- Jackson CE. Cholinergic System. In: Kreutzer JS, J DeLuca, B Caplan, editors. Encyclopedia of Clinical Neuropsychology. New York, NY: Springer New York; 2011. p. 562-4.
- Halder N, Lal G. Cholinergic System and Its Therapeutic Importance in Inflammation and Autoimmunity. Front Immunol 2021; 12:660342. doi: 10.3389/fimmu.2021.660342 [Crossref] [ Google Scholar]
- Wessler I, Kilbinger H, Bittinger F, Unger R, Kirkpatrick C. The non-neuronal cholinergic system in humans: Expression, function and pathophysiology. Life Sci 2003; 72:2055-61. doi: 10.1016/S0024-3205(03)00083-3 [Crossref] [ Google Scholar]
- Yuan H, Silberstein SD. Vagus Nerve and Vagus Nerve Stimulation, a Comprehensive Review: Part I Headache. The Journal of Head and Face Pain 2016; 56:71-8. doi: 10.1111/head.12647 [Crossref] [ Google Scholar]
- Borovikova LV, Ivanova S, Nardi D, Zhang M, Yang H, Ombrellino M. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton Neurosci 2000; 85:141-7. doi: 10.1016/s1566-0702(00)00233-2 [Crossref] [ Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000; 405:458-62. doi: 10.1038/35013070 [Crossref] [ Google Scholar]
- Hoover DB. Cholinergic modulation of the immune system presents new approaches for treating inflammation. Pharmacol Ther 2017; 179:1-16. doi: 10.1016/j.pharmthera.2017.05.002 [Crossref] [ Google Scholar]
- van Westerloo DJ, Giebelen IA, Florquin S, Daalhuisen J, Bruno MJ, de Vos AF. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J Infect Dis 2005; 191:2138-48. doi: 10.1086/430323 [Crossref] [ Google Scholar]
- Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci U S A 2008; 105:11008-13. doi: 10.1073/pnas.0803237105 [Crossref] [ Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 2003; 421:384-8. doi: 10.1038/nature01339 [Crossref] [ Google Scholar]
- Bertrand D, Lee C-HL, Flood D, Marger F, Donnelly-Roberts D. Therapeutic Potential of α7 Nicotinic Acetylcholine Receptors. Pharm Rev 2015; 67:1025. doi: 10.1124/pr.113.008581 [Crossref] [ Google Scholar]
- van Maanen MA, Stoof SP, Larosa GJ, Vervoordeldonk MJ, Tak PP. Role of the cholinergic nervous system in rheumatoid arthritis: aggravation of arthritis in nicotinic acetylcholine receptor α7 subunit gene knockout mice. Ann Rheum Dis 2010; 69:1717-23. doi: 10.1136/ard.2009.118554 [Crossref] [ Google Scholar]
- van Maanen MA, Vervoordeldonk MJ, Tak PP. The cholinergic anti-inflammatory pathway: towards innovative treatment of rheumatoid arthritis. Nat Rev Rheumatol 2009; 5:229-32. doi: 10.1038/nrrheum.2009.31 [Crossref] [ Google Scholar]
- Li S, Qi D, Li J-N, Deng X-Y, Wang D-X. Vagus nerve stimulation enhances the cholinergic anti-inflammatory pathway to reduce lung injury in acute respiratory distress syndrome via STAT3. Cell Death Discov 2021; 7:63. doi: 10.1038/s41420-021-00431-1 [Crossref] [ Google Scholar]
- Ren C, Tong Y-L, Li J-C, Lu Z-Q, Yao Y-M. The Protective Effect of Alpha 7 Nicotinic Acetylcholine Receptor Activation on Critical Illness and Its Mechanism. Int J Biol Sci 2017; 13:46-56. doi: 10.7150/ijbs.16404 [Crossref] [ Google Scholar]
- Báez-Pagán CA, Delgado-Vélez M, Lasalde-Dominicci JA. Activation of the Macrophage α7 Nicotinic Acetylcholine Receptor and Control of Inflammation. J Neuroimmune Pharmacol 2015; 10:468-76. doi: 10.1007/s11481-015-9601-5 [Crossref] [ Google Scholar]
- Grandi A, Zini I, Flammini L, Cantoni AM, Vivo V, Ballabeni V. α(7) Nicotinic Agonist AR-R17779 Protects Mice against 2,4,6-Trinitrobenzene Sulfonic Acid-Induced Colitis in a Spleen-Dependent Way. Front Pharmacol 2017; 8:809. doi: 10.3389/fphar.2017.00809 [Crossref] [ Google Scholar]
- Pinheiro NM, Miranda C, Santana FR, Bittencourt-Mernak M, Arantes-Costa FM, Olivo C. Effects of VAChT reduction and α7nAChR stimulation by PNU-282987 in lung inflammation in a model of chronic allergic airway inflammation. Eur J Pharmacol 2020; 882:173239. doi: 10.1016/j.ejphar.2020.173239 [Crossref] [ Google Scholar]
- Kitagawa H, Takenouchi T, Azuma R, Wesnes KA, Kramer WG, Clody DE. Safety, pharmacokinetics, and effects on cognitive function of multiple doses of GTS-21 in healthy, male volunteers. Neuropsychopharmacology 2003; 28:542-51. doi: 10.1038/sj.npp.1300028 [Crossref] [ Google Scholar]
- Johnson RL, Wilson CG. A review of vagus nerve stimulation as a therapeutic intervention. J Inflamm Res 2018; 11:203-13. doi: 10.2147/JIR.S163248 [Crossref] [ Google Scholar]
- Ellrich J. Transcutaneous Auricular Vagus Nerve Stimulation. J Clin Neurophysiol 2019; 36:437-42. doi: 10.1097/wnp.0000000000000576 [Crossref] [ Google Scholar]
- Gigliotti JC, Huang L, Ye H, Bajwa A, Chattrabhuti K, Lee S. Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway. J Am Soc Nephrol 2013; 24:1451-60. doi: 10.1681/asn.2013010084 [Crossref] [ Google Scholar]
- Huston JM, Rosas-Ballina M, Xue X, Dowling O, Ochani K, Ochani M. Cholinergic Neural Signals to the Spleen Down-Regulate Leukocyte Trafficking via CD11b. J Immunol 2009; 183:552. doi: 10.4049/jimmunol.0802684 [Crossref] [ Google Scholar]
- Ji H, Rabbi MF, Labis B, Pavlov VA, Tracey KJ, Ghia JE. Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis. Mucosal Immunol 2014; 7:335-47. doi: 10.1038/mi.2013.52 [Crossref] [ Google Scholar]
- Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. Journal of Experimental Medicine 2006; 203:1623-8. doi: 10.1084/jem.20052362 [Crossref] [ Google Scholar]
- Olofsson PS, Katz DA, Rosas-Ballina M, Levine YA, Ochani M, Valdés-Ferrer SI. α7 nicotinic acetylcholine receptor (α7nAChR) expression in bone marrow-derived non-T cells is required for the inflammatory reflex. Mol Med 2012; 18:539-43. doi: 10.2119/molmed.2011.00405 [Crossref] [ Google Scholar]
- Koopman FA, Schuurman PR, Vervoordeldonk MJ, Tak PP. Vagus nerve stimulation: A new bioelectronics approach to treat rheumatoid arthritis?. Best Pract Res Clin Rheumatol 2014; 28:625-35. doi: 10.1016/j.berh.2014.10.015 [Crossref] [ Google Scholar]
- Wu H, Li L, Su X. Vagus nerve through α7 nAChR modulates lung infection and inflammation: models, cells, and signals. BioMed Res Int 2014; 2014:283525. doi: 10.1155/2014/283525 [Crossref] [ Google Scholar]
- Bonaz B, Sinniger V, Pellissier S. The Vagus Nerve in the Neuro-Immune Axis: Implications in the Pathology of the Gastrointestinal Tract. Front Immunol 2017; 8:1452. doi: 10.3389/fimmu.2017.01452 [Crossref] [ Google Scholar]
- Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 Autopsies, Oklahoma, USA. Am J Clin Pathol 2020; 153:725-33. doi: 10.1093/ajcp/aqaa062 [Crossref] [ Google Scholar]
- Park MD. Macrophages: a Trojan horse in COVID-19?. Nat Rev Immunol 2020; 20:351. doi: 10.1038/s41577-020-0317-2 [Crossref] [ Google Scholar]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054-62. doi: 10.1016/S0140-6736(20)30566-3 [Crossref] [ Google Scholar]
- Otsuka R, Seino K-i. Macrophage activation syndrome and COVID-19. Inflamm Regen 2020; 40:19. doi: 10.1186/s41232-020-00131-w [Crossref] [ Google Scholar]
- Ali N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J Med Virol 2020; 92:2409-11. doi: 10.1002/jmv.26097 [Crossref] [ Google Scholar]
- Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med 2020; 383:2255-73. doi: 10.1056/NEJMra2026131 [Crossref] [ Google Scholar]
- Ulhaq ZS, Soraya GV. Anti-IL-6 receptor antibody treatment for severe COVID-19 and the potential implication of IL-6 gene polymorphisms in novel coronavirus pneumonia. Med Clin (Barc) 2020; 155:548-56. doi: 10.1016/j.medcli.2020.07.002 [Crossref] [ Google Scholar]
- Du P, Geng J, Wang F, Chen X, Huang Z, Wang Y. Role of IL-6 inhibitor in treatment of COVID-19-related cytokine release syndrome. Int J Med Sci 2021; 18:1356-62. doi: 10.7150/ijms.53564 [Crossref] [ Google Scholar]
- Kotak S, Khatri M, Malik M, Malik M, Hassan W, Amjad A. Use of Tocilizumab in COVID-19: A Systematic Review and Meta-Analysis of Current Evidence. Cureus 2020; 12:e10869. doi: 10.7759/cureus.10869 [Crossref] [ Google Scholar]
- Franzetti M, Forastieri A, Borsa N, Pandolfo A, Molteni C, Borghesi L. IL-1 Receptor Antagonist Anakinra in the Treatment of COVID-19 Acute Respiratory Distress Syndrome: A Retrospective, Observational Study. J Immunol 2021. doi: 10.4049/jimmunol.2001126 [Crossref]