Bioimpacts. 12(5):471-476.
doi: 10.34172/bi.2022.24077
Short Communication
Functional properties of thermally tampered poly(ethylene oxide)
Niloofar Babanejad 1  , Umadevi Kandalam 2, Yadollah Omidi 1
, Umadevi Kandalam 2, Yadollah Omidi 1  , Hamid Omidian 1, *
, Hamid Omidian 1, * 
Author information:
1College of Pharmacy, Nova Southeastern University, Fort Lauderdale, FL, USA
2Woody L. Hunt School of Dental Medicine, Texas Tech University Health Sciences Center, El Paso, TX, USA
Abstract
Introduction:
Poly(ethylene oxide) (PEO) is the most common polymer used in commercial abuse-deterrent tablets. Due to its vulnerability to high-temperature manipulation, we investigated abuse-deterrent capability and the toxicity of this polymer upon thermal treatments at 80°C and 180°C for 1 hour.
Methods:
Tablets (200 mg PEO and 300 mg Avicel®) were directly compressed under 2000 lb. The thermally manipulated PEOs were evaluated for their viscosity, crushability, structural changes, and cell toxicity.
Results:
Our findings showed that 180°C-treated tablets underwent some degrees of oxidative degradation with profound toxicity in both mesenchymal stem cells and MG63 cells. The 180°C-treated tablets exhibited almost no resistance against crushing and were prone to abuse. While thermal processing of PEO at around its melting temperature is a common approach to enhance crush resistance of its dosage forms, thermal manipulation at close to the PEO’s oxidation temperature can lead to structural changes, dramatic loss of crush and extraction resistance, and significant cell toxicity.
Conclusion:
Similar to the low molecular weight PEO, when thermally manipulated at its thermo-oxidative temperature, the high molecular weight PEO loses its deterrence performance and causes severe cell toxicity.
Keywords: Abuse deterrent, Crush resistance, Extraction resistance, Thermal manipulation, Poly(ethylene oxide), Cytotoxicity
Copyright and License Information
© 2022 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Abuse deterrent formulations (ADFs) are described as dosage forms that impede the abuse of medications by making them hard to tampering or less satisfying.
1
Poly(ethylene oxide) (PEO) is commonly used in the manufacturing of the ADFs such as reformulated OxyContin (oxycodone HCl), reformulated Opana ER (oxymorphone HCl) (now off the market), and Nucynta ER (Tapentadol).
2
Some formulations are benefitting from the PEO’s solution properties while others are taking advantage of both solution and solid properties of the PEO.
3
Out of different grades, the high molecular weight PEOs (POLYOX Coagulant) can provide superior mechanical properties and solution viscosity leading to crush and extraction resistance, respectively. If not manipulated, the high molecular weight PEO can effectively prevent filterability and impede tablet abuse by intravenous injection.
4
Additionally, low molecular weight PEO polymers can provide superior crush resistance. This can be attributed to the excellent film-forming capability of low molecular weight PEO that enhances the adhesiveness as well as the integrity of the tablet composition.
5,6
When an oral ADF no longer offers the same euphoric feeling, abusers may manipulate the formulation to achieve a greater or faster euphoria.
6
Thermal manipulation is one of the major tools used by abusers to overcome crush resistance and extraction resistance of drug products.
7
Heating high molecular weight PEO close to its degradation temperature may change its chemical structure
8
and diminish crush resistance. Additionally, the high viscosity built up by PEO in water or hydroalcoholic solutions may be significantly lost if the original solid PEO is heated up to its degradation temperature before preparing its aqueous solutions.
6,7
It has been reported that the intravenous abuse of tablets containing high molecular weight PEO (~7 000 000 Da) leads to kidney injury (due to free hemoglobin toxicity), and thrombotic microangiopathy (TMA) in humans.
9
The toxicity related to the occurrence of a TMA was reported in 15 patients who injected Opana ER, thirteen intravenously, and one subcutaneously.
9,10
Although toxicity in humans was investigated upon intravenous exposure to the Opana ER tablet, the manipulation methods applied by the abusers to overcome the tablets’ deterrence properties have not been clarified. Furthermore, to the best of our knowledge, there is no report on the impact of thermal manipulation on the toxicity of high molecular weight PEO.
PEO can be readily formulated into tablets owing to its desirable compressibility and flow properties. PEO’s low melting temperature makes it a proper choice for extrusion, molding, as well as casting of solid dosage forms. Such temperature can help with better flow of PEO and hence help with enhancing tablet mechanical strength. We also hypothesized that temperatures close to melting point of the polymer has no undesirable effect on abuse deterrence and safety of such compositions. However, abusers have no restrictions as what to use to abuse a pharmaceutical product. These include high temperature tampering at close to thermal decomposition of the PEO, which occurs at around 180oC. Therefore, we selected thermal manipulation at 80oC (close to PEO melting temperature) and 180oC (PEO decomposition temperature) to further evaluate the polymer efficacy and safety after thermally tampered at such temperatures. We also hypothesized that thermal manipulation and hence thermo-oxidative degradation of high molecular weight PEO would result in considerable loss of PEO abuse-deterrent properties and generation of toxic byproducts. To test our hypothesis, we heated the solid high molecular weight PEO above its melting and thermal degradation temperatures. The thermally manipulated PEO products (solid and solution) were then assessed for their crush resistance, extraction resistance (syringeability), and toxicity. At the end, we compared cytotoxicity of the low and high molecular weight PEOs upon thermal manipulation.
Methods
PEO with 4000 kDa molecular weight (Dow Chemical Inc., Midland, Mi, USA) was spread over a watch glass and heated at 80°Cand 180°C for 1 hour in an air-circulated oven (VWR Oven F Air 2.3 CF, 89511-410, Germany). These samples were used in the evaluation of the heat effect on, (i) the PEO structural changes using FTIR spectrometer (Spectrum 100 FTIR, PerkinElmer, Shelton, CT, USA) and Differential Scanning Calorimetry, DSC 4000 (PerkinElmer, the Netherlands), (ii) the PEO rheological changes using a Cone & Plate Rheometer (Brookfield DV-III Ultra, Middleboro, MA, USA; 2.4 cm cone), (iii) the syringeability using a Texture Analyzer, Brookfield CT3 (Brookfield, Middleboro, MA, USA), and (iv) cytotoxicity of the PEO abuse-deterrent polymer. The Live/Dead Cell Assay (Molecular Probes, Invitrogen, Carlsbad, CA, USA) was used to find out the cytotoxic impacts of PEO (i.e., the heat-treated at 80°C and 180°C, and the control samples) on osteosarcoma MG63 cell line (ATCC No: CRL-1427) and mesenchymal stem cells derived from human gingival tissue (GMSCs) expanded from the frozen patient-derived primary cultures (IRB No 2018-241). The data were obtained from three individual experiments of each cell type. The cells were cultured in growth media containing Dulbecco's modified eagle medium (DMEM) (Thermo Fisher Scientific, Carlsbad, CA, USA) containing 10% Fetal bovine serum (Atlanta Biologics, Flowery Branch, GA, USA), 1% antibiotic and antimitotic solutions (Sigma-Aldrich, St. Louis, MO, USA). The cells were grown at a seeding density of 7.5×104 cells/well on flat bottom24-well cell culture plates. The cells were allowed to attach to 24-well plates overnight in the humidified incubator at 37°C and 5% CO2. Pilot experiments were conducted with various wt./vol of PEO (data not shown), and the max concentration (60 mg/mL) was selected at which no cytotoxicity observed in untreated PEO. At around 50% confluency, the cells were exposed to 60 mg/mL of sterilized control and heat-treated PEOs and incubated at 37°C for 24 hours. They were then washed (×2) with phosphate-buffered saline (PBS), followed by adding 200 µL (0.5 mM calcein and 0.25 mM ethidium homodimer) live/dead assay reagent. The cells were incubated in the dark at 25°C for 30 minutes and then observed under a fluorescence microscope, Olympus IX51 (Olympus Corp., Shinjuku City, Tokyo, Japan) at 10× magnification. For the analysis, a quantitative assessment was made based on the counting number of viable cells present in the field of view under 10× magnification, and thus, 4 field views were randomly selected. The average number of cells obtained from 4 views was calculated, and three replicas were used for each treatment group. The viability percentage was calculated, and the data were presented as the fold difference normalized with the control cells. Cytotoxicity experiments were performed in triplicates, and the data were introduced as the mean ± standard deviation (SD). Student t tests and two-way analysis of variance were used to determine the differences between the groups.
In addition to the PEO powder's heat-treatment, tablet compacts containing 200 mg PEO and 300 mg Avicel® were prepared and heat-treated at 80°C and 180°C for 1 hour. A single station tablet press (Carver, 3851-0, Wabash, Indiana, USA) was used in the preparation of the directly compressed tablets using a 2000 lb. compressive force. The prepared tablets were then investigated for their crush resistance properties using a ball-mill (Retsch, Newtown, PA, USA) followed by determination of particle size distribution using a sifter, Cole Palmer SS-3CP (Cole Palmer, Vernon Hills, IL, USA) at 60 taps/min for 10 minutes.
To compare the results of this study with those we have published on low molecular weight PEO, we used same methodology as previously reported.
5
Results and Discussion
Fig. 1A shows the FTIR spectra of the control and heat-treated samples. The FTIR spectrum of the 180°C-treated PEO showed a degradation peak at 1720 cm-1. This peak was absent in the spectra of the control and the 80°C-treated PEO. These results indicate that the PEO oxidative degradation occurs at 180°C. The DSC thermogram of the PEO sample heated from room temperature to 300°C is shown in Fig. 1B. The PEO demonstrated a melting temperature of 83°C and the onset of oxidative degradation below 180°C, which is consistent with the FTIR results. Mechanical properties of the heat-treated tablets were assessed to find out the effect of heat on the crush resistance properties of the tablets. Fig. 1C exhibits the resultant powders after ball-milling. The yellow color of the 180°C-treated powders confirmed its oxidative degradation, while the 80°C-treated tablet showed an improved mechanical strength with no color change. In control tablets, 25% of particles were larger than 850 µm and this amount increased to 92% in 80°C-treated tablets (P < 0.01). The thermoplastic behavior of PEO causes improvement in the mechanical strength of the tablets under such thermal conditions. Notably, the mechanical strength decreased in 180°C-treated tablets, which was further evidenced by the fine particles collected after crushing the tables in a ball-mill (only 2% of particles were larger than 850 µm) (P > 0.05). The aqueous solutions of the control and 80°C-treated PEO demonstrated a pseudoplastic behavior; nevertheless, the 2% w/v aqueous solution of the 180°C-treated PEO lost its viscosity over a wide range of shear rates (Fig. 1D). According to the Casson model, the yield values for control and 80oC-heated PEO solutions are 1438.66±175.75 and 1114.23±135.21, respectively. Coefficient of Friction (COF) and yield stress showed an error (value is equal to zero) for 180°C-treated PEO and could not be used to estimate the yield stress. This can be attributed to the more solid behavior of 2% w/v control and 80°C-treated PEO solutions and the loss of yield stress in 180°C-treated PEO solution due to the oxidation. According to Fig. 1E, the control and 80°C-treated PEO solutions demonstrated resistance against syringeability with an average difficulty of 5.81±0.54 N and 4.93±0.3 N, respectively, while this number reduced significantly to 0.82±0.13 N for the 180°C-treated PEO solution (P < 0.01). These data are in accordance with that of the rheology study and suggest that faster and more effective thermal oxidation occurs at temperatures close to the degradation point of the PEO.
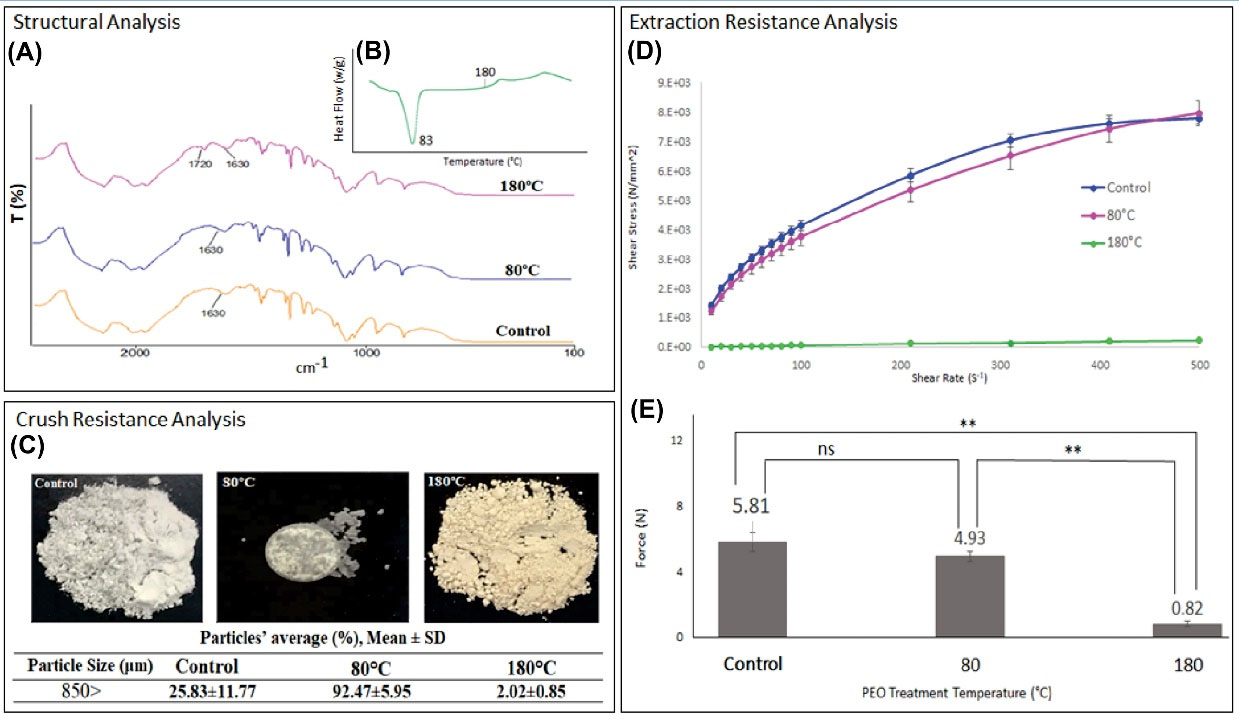
Fig. 1.
(A) FTIR spectra of PEO samples; control powder, 80°C-treated PEO powder, 180°C-treated PEO powder. (B) Differential Scanning Calorimetry thermogram of PEO powder heated from 25°C to 300°C at 25°C/min. (C) The resultant powder after crushing by the ball-mill (25 Hz, 5 min). (D) The rheolograms of the 2% w/v PEO solutions (control and heat-treated) at 25°C (n = 3). (E) Syringeability of 2% w/v PEO solutions prepared from control and heat-treated PEO. The experiments were performed at 25°C, n = 3. **P < 0.01, ns P > 0.05.
.
(A) FTIR spectra of PEO samples; control powder, 80°C-treated PEO powder, 180°C-treated PEO powder. (B) Differential Scanning Calorimetry thermogram of PEO powder heated from 25°C to 300°C at 25°C/min. (C) The resultant powder after crushing by the ball-mill (25 Hz, 5 min). (D) The rheolograms of the 2% w/v PEO solutions (control and heat-treated) at 25°C (n = 3). (E) Syringeability of 2% w/v PEO solutions prepared from control and heat-treated PEO. The experiments were performed at 25°C, n = 3. **P < 0.01, ns P > 0.05.
The live/dead cell is a combination of two markers, Calcein-AM (a green fluorescent marker), and ethidium homodimer, EthD-1 (a red fluorescent marker. The live cells can be detected via the presence of intracellular esterase activity. When the non-fluorescent cell-permeant calcein-AM is converted into the intensely fluorescent calcein, green fluorescence is emitted that is an indicator of the live cells. When the EthD-1 molecules can enter the cells through damaged cell membranes and bind with nucleic acids bright red fluorescence is emitted that is an indicator of the dead cells. In this study, we examined the PEO cytotoxicity on stem cells derived from human gingiva and osteoblast-like cells.
11,12
The live/dead cell assay data demonstrated no significant morphological changes in both cell types upon exposure to the untreated PEO (control). The overall results indicated no considerable reduction in the viability of MG63 cells and GMSCs upon the exposure to untreated PEO and 80ºC-treated PEO, while the 180ºC-treated PEO significantly reduced or abolished the cell proliferation in both cell types (Figs. 2A-F).
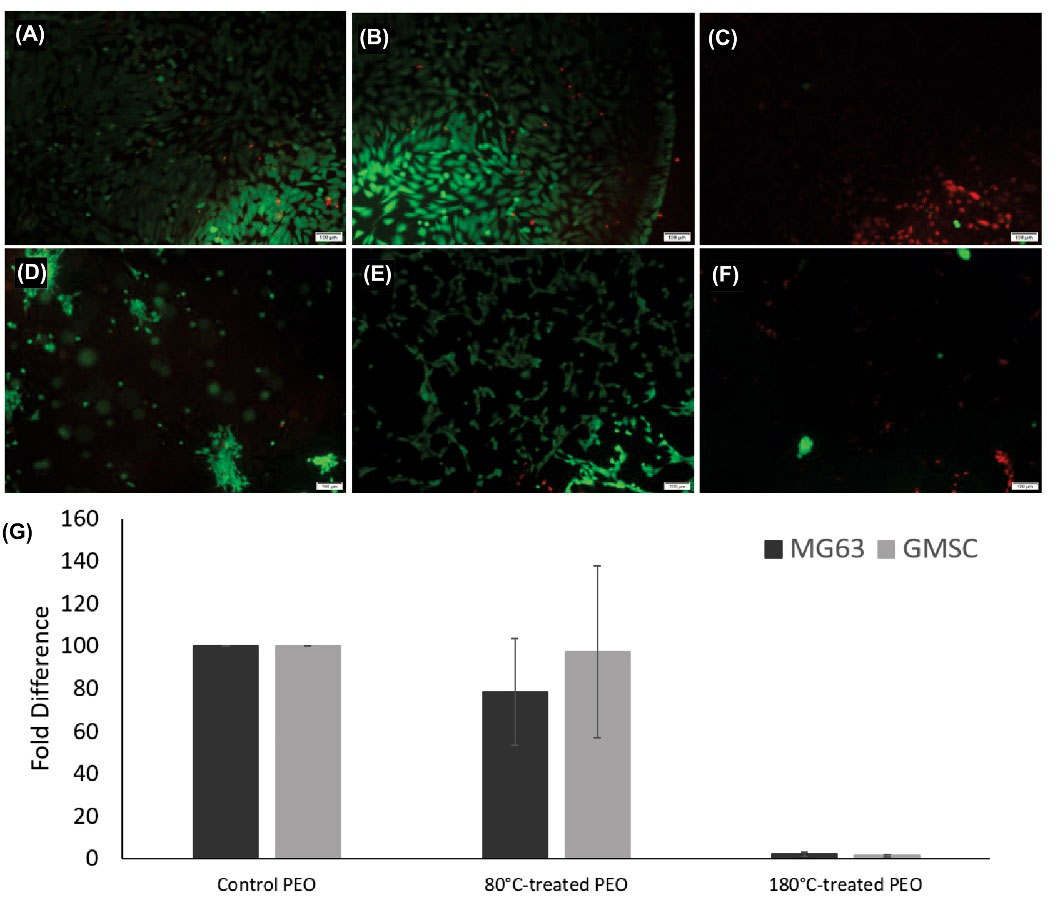
Fig. 2.
The fluorescent microscopy for the cytotoxicity test of MG63 cells, 24 h after the treatment. (A) The cells exposed to the control PEO sample. (B) The cells exposed to 80°C-treated PEO sample. (C) The cells exposed to 180°C-treated PEO sample. The green and red cells represent live and dead cells, respectively (scale bar 100 µM). The fluorescent microscopy for the cytotoxicity test of GMSCs, 24 h after the treatment. (D) The cells exposed to the control PEO sample. (E) The cells exposed to 80°C-treated PEO sample. (F) The cells exposed to 180°C-treated PEO sample. The green and red cells represent live and dead cells, respectively (scale bar 100 µM). (G) The quantitative analysis of live/dead cell assay in MG63 cells and GMSCs. The cells were exposed to PEO samples for 24 h, including control PEO, 80°C-treated PEO, and 180°C-treated PEO.
.
The fluorescent microscopy for the cytotoxicity test of MG63 cells, 24 h after the treatment. (A) The cells exposed to the control PEO sample. (B) The cells exposed to 80°C-treated PEO sample. (C) The cells exposed to 180°C-treated PEO sample. The green and red cells represent live and dead cells, respectively (scale bar 100 µM). The fluorescent microscopy for the cytotoxicity test of GMSCs, 24 h after the treatment. (D) The cells exposed to the control PEO sample. (E) The cells exposed to 80°C-treated PEO sample. (F) The cells exposed to 180°C-treated PEO sample. The green and red cells represent live and dead cells, respectively (scale bar 100 µM). (G) The quantitative analysis of live/dead cell assay in MG63 cells and GMSCs. The cells were exposed to PEO samples for 24 h, including control PEO, 80°C-treated PEO, and 180°C-treated PEO.
As shown in Fig. 2G, the viability of the GMSC and MG63 cells exposed to 80ºC-treated PEO was comparable to that of control PEO. In MG63, there was an over 20% decrease in the cell proliferation in 80ºC-treated PEO compared to MG63 exposed to control PEO. The extent of reduction in cell proliferation was much smaller in GMSCs compared to MG63 cells. However, 180ºC-treated PEO significantly reduced (99%, P < 0.05) both GMSCs and MG63 cells’ survival. This test demonstrates that heating PEO to its degradation temperature may cause acute toxicity. Since the majority of the abuse-deterrent formulations take advantage of the unique properties of the PEO at room temperature, these data show the same polymer can potentially cause serious adverse effects when manipulated at temperatures close to its degradation.
PEO’s thermal degradation breaks carbon-oxygen bonds generate free radicals followed by radical recombination. The low molecular weight fractions include atomic and molecular hydrogen, hydroxyl, carbon, CHx, water molecule, and carbon monoxide molecules. The higher molecular weight fraction comprises segments of the macromolecular chain with a different number of (-CH2-O-) units.
13-15
Degradation of 180°C-treated PEO through each of the above mechanisms decreases deterrence properties and leads to cell toxicity (Table 1). Cell exposure to the 180°C-treated samples demonstrated the hallmark features of cell toxicity, which can be attributed to the byproducts of the PEO thermo-oxidative degradation. However, the control and the 80°C-treated PEO alone did not affect the cell viability. Table 1 summarizes the effect of thermal manipulation on crush resistance, extraction resistance, and toxicity of the high molecular weight PEO polymer. Collectively, we speculate that the tampering of the PEO at higher temperatures (i.e., 180°C) might result in the generation of toxic by-products, which might give a rise to the reactive oxygen species (ROS). The emergence of ROS, in turn, can interfere with the normal function of the cells by the induction of oxidative stress (e.g., proteins oxidation, lipids peroxidation, damage of nucleic acids, and inhibition of enzymatic activities) triggering apoptosis and/or necrosis. Such byproducts may also interact with the anti-apoptotic mechanisms of the cells such as direct/indirect interaction with intracellular antioxidant, glutathione, which might, in turn, interfere and deteriorate the intracellular redox equilibrium, resulting in an enhanced level of ROS and hence induction of cell death program.
16,17
Table 1.
Effect of heat on abuse deterrence and toxicity of the HMW PEO polymer
|
|
Control
|
80°C-Treated
|
180°C-Treated
|
Ref.
|
| Crush Resistance |
Crushable (fine particles) |
Non-crushable entities (larger granules may solidify into a plastic matrix with enhanced hardness) |
Crushable (very fine particles due to PEO degradation) |
18,19
|
| Extraction Resistance in an aqueous medium |
Viscous gel, high syringeability force due to the higher extent of inter-chain entanglements) |
Same as control |
Very runny liquid with a consistency like water due to the reduction in polymer molecular weight upon oxidation. |
20,21
|
| Toxicity |
Not-measurable; significantly different from that of 180°C-treated |
Very low; significantly different from that of 180°C-treated |
High toxic impacts possibly through (i) enhanced reactive oxygen species (ROS), and (ii) declined intracellular antioxidant glutathione |
16,17
|
Effect of PEO molecular weight
The GMSC and MG63 cell viability of control, 80ºC-treated and 180ºC-treated low molecular weight PEO
5
were statistically compared with the GMSC and MG63 cell viability of control, 80ºC-treated and 180ºC-treated high molecular weight PEO, respectively. Table 2 demonstrates these comparisons for the GMSC and MG63 cells. In both cell lines, the cell viability of control low and high molecular weight PEO were not statistically significant (P > 0.05). The cell viability of 80ºC-treated low and high molecular weight PEO were not statistically significant (P > 0.05). The cell viability of 180ºC-treated low and high molecular weight PEO were not statistically significant (P > 0.05). These statistical analyses indicate that low and high molecular weight PEO have similar effect on GMSC and MG63 cell viability.
Table 2.
Cytotoxicity of low and high molecular weight PEOs on the GMSC and MG63 cell lines
|
|
P
value* (GMSC)
|
P
value* (MG63)
|
| Control (LMW vs HMW) |
0.490926568 |
0.170402553 |
| 80ºC (LMW vs HMW) |
0.776462 |
0.812076891 |
| 180ºC (LMW vs HMW) |
0.303169 |
0.282631457 |
* t test: Null Hypothesis: The control, 80°C-treated and 180°C low and high molecular weight PEOs behave similar in cell viability (P < 0.05 statistically significant).
PEO is among rare polymers that its melting temperature is almost independent of its molecular weight. In other words, both low and very high molecular weight PEO polymers display a very narrow melting point. Our results also showed that low and high molecular weight PEO polymers undergo thermo-oxidative degradation at around the same temperature. Since both melting and thermal decomposition of PEO polymers are governed by the intermolecular forces between the PEO chains, it is likely that unique crystal structure of PEOs dictates its thermal behavior, which collectively and similarly affect their structure, performance, abuse deterrence, and cytotoxicity.
Conclusion
This study demonstrates that heating abuse deterrent products containing high molecular weight PEO at temperatures close to the polymer decomposition range can considerably affect its functional characteristics such as viscosity, syringeability, crush and extraction resistance due to the change in the PEO structure. In particular, heating PEO-based products within PEO’s decomposition temperature results in severe cell toxicity. This study also showed that cytotoxicity of the thermally-manipulated PEO is independent of its molecular weight.
Research Highlights
What is the current knowledge?
√ The nontoxic-biocompatible HMW PEO can provide crush and extraction resistance in abuse-deterrent formulations under non-aggressive conditions.
√ Compositions containing HMW PEO heated at around PEO’s melting temperature offer improved abuse deterrence, however, they become easily abusable when heated at around the PEO’s decomposition temperature.
What is new here?
√ HMW PEO heated at around its decomposition temperature is cytotoxic.
√ Cytotoxicity of the thermally-manipulated PEO is independent of its molecular weight.
√ Manufacturers and legal authorities are advised to include cytotoxicity and genotoxicity (recommended) in the evaluation of abuse-deterrent formulations after thermal manipulation.
Acknowledgments
The authors would like to acknowledge Nova Southeastern University for funding this work.
Funding sources
This work was supported by the Health Professions Division of Nova Southeastern University (HPD grant number, 334601, 2019). The sponsor was not involved in the study design, data collection, analysis, data interpretation, paper writing, or decision to submit the article for publication.
Ethical statement
There is none to be disclosed.
Competing interests
The authors declare that there is no conflict of interest.
Authors’ contribution
HO designed the study. NB collected the data and did abuse deterrent study and analyzed the data. UK did the cytotoxicity study and analyzed the data. NB wrote the manuscript. YO, and HO edited the manuscript.
References
-
FDA. Understanding Abuse Deterrent Opioids; https://www.fda.gov/downloads/AboutFDA/WorkingatFDA/FellowshipInternshipGraduateFacultyPrograms/PharmacyStudentExperientialProgramCDER/UCM532123.pdf. 2020.
- Omidian H, Mastropietro DJ, Muppalaneni S, inventors; MEC Device Pharma International LLC (Fort Lauderdale, FL, US), assignee. Deterring abuse of pharmaceutical products and alcohol; US Patent Application 16/596304. US; 2020.
- Kopecky EA, Fleming AB, Levy-Cooperman N, O'Connor M, E MS. Oral Human Abuse Potential of Oxycodone DETERx((R)) (Xtampza((R)) ER). J Clin Pharmacol 2017; 57:500-12. doi: 10.1002/jcph.833 [Crossref] [ Google Scholar]
- Upadhye SB, Rajabi-Siahboomi AR. Properties and Applications of Polyethylene Oxide and Ethylcellulose for Tamper Resistance and Controlled Drug Delivery. Melt Extrusion: Materials, Technology and Drug Product Design 2013; 9:145-58. doi: 10.1007/978-1-4614-8432-5_6 [Crossref] [ Google Scholar]
- Babanejad N, Kandalam U, Ahmad R, Omidi Y, Omidian H. Abuse-deterrent properties and cytotoxicity of poly (ethylene oxide) after thermal tampering. Int J Pharm 2021; 600:120481. doi: 10.1016/j.ijpharm.2021.120481 [Crossref] [ Google Scholar]
- Omidian H, Muppalaneni S, Joshi Y, Mastropietro DJ, inventors; MEC Device Pharma, LLC, assignee. Compositions for deterring abuse of pharmaceutical products and alcohol; US Patent Application 15/770193. US; 2018.
- Joshi Y, Muppalaneni S, Omidian A, Mastropietro DJ, Omidian H. Determining Abuse Deterrence Performance of Poly (ethylene oxide) Using a Factorial Design. Adv Pharm Bull 2018; 8:495-505. doi: 10.15171/apb.2018.058 [Crossref] [ Google Scholar]
- Vrandecic NS, Erceg M, Jakic M, Klaric I. Kinetic analysis of thermal degradation of poly(ethylene glycol) and poly(ethylene oxide)s of different molecular weight. Thermochimica Acta 2010; 498:71-80. doi: 10.1016/j.tca.2009.10.005 [Crossref] [ Google Scholar]
- Hunt R, Yalamanoglu A, Tumlin J, Schiller T, Baek JH, Wu A. A mechanistic investigation of thrombotic microangiopathy associated with IV abuse of Opana ER. Blood 2017; 129:896-905. doi: 10.1182/blood-2016-08-736579 [Crossref] [ Google Scholar]
- Marder E, Kirschke D, Robbins D, Dunn J, Jones TF, Racoosin J. Thrombotic Thrombocytopenic Purpura (TTP)-Like Illness Associated with Intravenous Opana ER Abuse - Tennessee, 2012. Mmwr-Morbidity and Mortality Weekly Report 2013; 62:1-4. [ Google Scholar]
- Hassan W, Dong Y, Wang W. Encapsulation and 3D culture of human adipose-derived stem cells in an in-situ crosslinked hybrid hydrogel composed of PEG-based hyperbranched copolymer and hyaluronic acid. Stem Cell Res Ther 2013; 4:32. doi: 10.1186/scrt182 [Crossref] [ Google Scholar]
- Zivkovic L, Akar B, Roux BM, Spremo Potparevic B, Bajic V, Brey EM. Investigation of DNA damage in cells exposed to poly (lactic-co-glycolic acid) microspheres. J Biomed Mater Res A 2017; 105:284-91. doi: 10.1002/jbm.a.35849 [Crossref] [ Google Scholar]
- Cai XQ, Hao JJ, Zhang XY, Yu BZ, Ren JM, Luo C. The polyhydroxylated fullerene derivative C-60(OH)(24) protects mice from ionizing-radiation-induced immune and mitochondrial dysfunction. Toxicol Appl Pharmacol 2010; 243:27-34. doi: 10.1016/j.taap.2009.11.009 [Crossref] [ Google Scholar]
- Pulskamp K, Diabate S, Krug HF. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol Lett 2007; 168:58-74. doi: 10.1016/j.toxlet.2006.11.001 [Crossref] [ Google Scholar]
- Zhang XY, Qi HX, Wang SQ, Feng L, Ji Y, Tao L. Cellular responses of aniline oligomers: a preliminary study. Toxicol Res 2012; 1:201-5. doi: 10.1039/c2tx20035j [Crossref] [ Google Scholar]
- Liu GQ, Li YS, Yang L, Wei Y, Wang X, Wang ZM. Cytotoxicity study of polyethylene glycol derivatives. Rsc Adv 2017; 7:18252-9. doi: 10.1039/c7ra00861a [Crossref] [ Google Scholar]
- Sharma P, Bhushan Jha A, Shanker Dubey R, Pessarakli M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J Bot 2012; 2012:217037. doi: 10.1155/2012/217037 [Crossref] [ Google Scholar]
- de Sainte Claire P. Degradation of PEO in the Solid State: A Theoretical Kinetic Model. Macromolecules 2009; 42:3469-82. doi: 10.1021/ma802469u [Crossref] [ Google Scholar]
- Choukourov A, Grinevich A, Polonskyi O, Hanus J, Kousal J, Slavinska D. Vacuum Thermal Degradation of Poly(ethylene oxide). Journal of Physical Chemistry B 2009; 113:2984-9. doi: 10.1021/jp8107107 [Crossref] [ Google Scholar]
- Crowley MM, Zhang F, Koleng JJ, McGinity JW. Stability of polyethylene oxide in matrix tablets prepared by hot-melt extrusion. Biomaterials 2002; 23:4241-8. doi: 10.1016/S0142-9612(02)00187-4 [Crossref] [ Google Scholar]
- Meruva S, Donovan MD. Polyethylene Oxide (PEO) Molecular Weight Effects on Abuse-Deterrent Properties of Matrix Tablets. AAPS PharmSciTech 2019; 21:28. doi: 10.1208/s12249-019-1565-y [Crossref] [ Google Scholar]