Bioimpacts. 13(2):89-96.
doi: 10.34172/bi.2023.24166
Original Article
Modulating the tumor microenvironment improves antitumor effect of anti-PD-L1 mAb in breast cancer
Xiuying Li Conceptualization, Formal analysis, Investigation, Methodology, Resources, Visualization, Writing – original draft, Writing – review & editing, 1, * 
Xianqin Luo Formal analysis, 2
Shunqin Hu Formal analysis, 3
Author information:
1Faculty of Life Science and Technology, Kunming University of Science and Technology, Kunming, China
2College of Traditional Chinese Medicine, Chongqing Medical University; Chongqing, China
3Department of Gynecology and Obstetrics, the First Affiliated Hospital of Kunming Medical University, Kunming, China
Abstract
Introduction:
Immune checkpoint inhibitors (ICIs) have provided noteworthy benefits in multiple cancer patients. However, the efficacy of monotherapy of ICIs was very limited. In this study, we endeavored to explore whether losartan can modulate the solid tumor microenvironment (TME) and improve the therapeutic efficacy of anti-PD-L1 mAb in 4T1 mouse breast tumor model and the underlying mechanism.
Methods:
The tumor-bearing mice were treated with control agents, losartan, anti-PD-L1 mAb or the dual agents. The blood and tumor tissues were respectively used for ELISA and immunohistochemical analysis. CD8-depletion and lung metastatic experiments were performed.
Results:
Compared to control group, losartan inhibited the expression of alpha-smooth muscle actin (α-SMA), deposition of collagen I in the tumor tissues. The concentration of transforming growth factor-β1 (TGF-β1) in the serum was low in the losartan treated group. Although losartan alone was ineffective, the combination of losartan and anti-PD-L1 mAb elicited dramatic antitumor effect. Immunohistochemical analysis revealed that there were more intra-tumoral infiltration of CD8+ T cells and increased granzyme B production in the combination therapy group. In addition, the size of spleen was smaller in the combination therapy group, compared to monotherapy. The CD8-depleting Abs abrogated the antitumor efficacy of losartan and anti-PD-L1 mAb in vivo. The combination of losartan and anti-PD-L1 mAb significantly inhibited 4T1 tumor cells lung metastatic in vivo.
Conclusion:
Our results indicated that losartan can modulate the tumor microenvironment, and improve the efficacy of anti-PD-L1 mAb.
Keywords: Anti-PD-L1 mAb, Losartan, Tumor microenvironment, Immunotherapy
Copyright and License Information
© 2023 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
The immune checkpoint inhibitors (ICIs), such as PD-L1 and PD-1 mAbs, can elicit strong and long-lasting immune response in a variety of solid cancers.1 Nevertheless, the efficacy of single-agent of PD-L1/PD-1 mAbs was only 20%-40% in most solid cancer types.2,3 Although there are multiple mechanisms involved in immune resistance of ICIs, the intra-tumor immunosuppressive microenvironment plays an important role in immune resistance of ICIs.4,5 Thus, it is urgent to develop innovative therapeutic strategy, such as combining immunotherapy with conventional treatments, to make more cancer patients to benefit from the ICIs. The tumor microenvironment (TME) is intricate, and formed by highly heterogeneous populations of stromal cells, vasculature and extracellular matrix (ECM). Emerging data has suggested that the tumor ECM is a key factor in drug resistance and immune suppression.2,4,5 Accordingly, blocking the immunosuppression of TME can reconstruct the normal antitumor immune defense, and enhance the therapeutic efficacy of ICIs.
The most abundant stromal cells in the TME are cancer-associated fibroblasts (CAFs). CAFs has the ability to promote tumor growth and immune evasion via diverse mechanisms.4,5 The structural components in the solid TME, such as collagen type I, II and IV, as well as fibronectin, are mainly produced by alpha-smooth muscle actin (α-SMA) positive CAFs.4,6 CAFs can also secret different kinds of soluble cytokines, such as SDF-1 and transforming growth factor-β (TGF-ß), to promote tumor cell proliferation and inhibit them apoptosis.5,7 On the other hand, CAFs hampered the infiltration of cytotoxic T lymphocytes, while attracting immunosuppressive regulatory T lymphocytes into tumor tissue.8,9 It is generally accepted that the dense collagen matrix can reduce blood perfusion and increase the interstitial fluid pressure, which hamper the therapeutic agents to enter into the tumor tissue.5,6 All these indicated that targeting CAFs could enhance immunotherapeutic effect.
The renin-angiotensin system (RAS) is a classical circulating or hormonal system, which regulate the blood pressure. Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II (AngII) receptor blockers (ARBs) all belong to the RAS blockers (RASBs). AngII signaling can convert quiescent myofibroblast into active-state through AngII receptor type-1 (AT1).10,11 A growing body of evidence suggests that RASBs exhibit potent antitumor activities, the favorable outcomes are associated with their use in some different tumor patients.12,13 Large epidemiological researches also indicated that RASBs have the potential protective effects against cancer risk.12 In the hypoxic microenvironment, the tumor cells can produce AngII through a hypoxia-lactate-chymase-dependent mechanism, which is different from the classical angiotensinogen-renin-ACE-AngII signal pathway.13
Losartan, one representative drug of ARBs, is an attractive component of combination therapy for breast cancer. The reasons were as follows. First, losartan is widely prescribed for patients with high blood pressure and easily repurposed by oncology. Second, losartan has anti-fibrotic properties, and can inhibit the activation of CAFs and collagen deposition in the tumor tissues.4,10,14 Third, the local Ang II is responsible for the formation of tumor immunosuppressive microenvironment.15 Finally, losartan has immunomodulatory function involving monocyte and macrophage activity.16 Based on these data we can deduce that losartan may modulate the immunological features of breast cancer microenvironment. The combination of losartan and ICIs may enhance the efficacy of ICIs.
It is widely believed that 4T1 breast tumor cells are poorly immunogenic, and its tumor tissue is rich in CAFs and ECM, and resistant to checkpoint immunotherapy.17 Whether losartan can modulate the immunosuppressive microenvironment of breast tumor, and improve the therapeutic effect of anti-PD-L1 mAb is not known. Here, our data suggested that losartan could modulate the immunological features of breast cancer, which in turn acts in concert with anti-PD-L1 mAb to promote antitumor immunity. Our study provided a rational for the combined clinical testing of losartan and anti-PD-L1 mAb.
Materials and Methods
Materials
Antibodies against α-SMA and CD8 were purchased from Cell Signaling Technology (MA, USA). Granzyme B was purchased from eBioscience (CA, USA). Antibody against collagen Type I was purchased from Proteintech (WH, CHN). Losartan was purchased from MedChemExpress (SH, CHN). Rat IgG isotype, hematoxylin and 3, 3'-diaminobenzidine tetrahydrochloride (DAB) were purchased from Beyotime (NT, CHN). PD-L1 mAb (10F.9G2) and anti-CD8 antibody (2.43 clone) were purchased from Bio X Cell (NH, USA). Mouse TGF-β1 enzyme-linked immunosorbent assay (ELISA) kit was purchased from KeyGEN (NJ, China).
Cell culture
Murine 4T1 breast carcinoma cells were purchased from Chinese Academy of Sciences Kunming Cell Bank (KM, CHN). 4T1 cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with FBS (10%), streptomycin (100 µg/mL) and penicillin (100 µnits/mL). 4T1 cells were maintained in a humidified incubator at 5% CO2. The cells were harvested using trypsin, and passaged every 2–3 days to maintain exponential growth.
Xenograft mouse model
All animal experiments were approved by the Ethics Committee of Kunming University of Science and Technology (2020JC015), and carried out in accordance with China’s Guidelines for Care and Use of Laboratory Animals. Balb/C female mice (six- to seven-week-old) were purchased from the Hunan Slake Jingda Experimental Animal Co., Ltd (China). Mice (n = 10) were randomized into two groups: control and losartan groups. 4T1 cells (5 × 105) were injected subcutaneously in the right flanks of mice. The losartan was suspended in edible oil by ultrasonic treatment. Losartan (40 mg/kg)18 and edible oil (control group) were given on the same day (day 0) but before inoculation with 4T1 tumor cells, and given every day by oral gavage for 3 weeks. The mice were euthanized at the indicated time. Blood was obtained from retro-ocular artery, and centrifuged at 1500 g for 15 minutes to collect the serum for further analysis. The subcutaneous tumor was collected and used for immunohistochemical staining.
For analysis of the efficacy of combination therapy, the Balb/C mice were randomized into four groups (n = 7 mice/group): control, anti-PD-L1 mAb, losartan, and combination of losartan and anti-PD-L1 mAb. 4T1 cells (5 × 105) were injected subcutaneously into the right flanks of mice. The cancer-bearing mice were treated with rat IgG isotype, losartan, PD-L1 mAb, or the dual agents respectively. The administration of losartan was the same as before. Losartan was given every day throughout the experiment. PD-L1 mAb and same dose rat IgG isotype (200 µg/mouse) were given intraperitoneally on D7, 10, 13 and 16 after tumor inoculation.19 The caliper was used to measure the tumor diameters every 3 days. The tumor volume was calculated using the following formula: volume = width2 × length × 0.52. The tumor-bearing mice were euthanized one week after the last dose of anti-PD-L1 mA. The spleens were harvested and weighted. The tumor tissues were fixed with 4% paraformaldehyde and used for immunohistochemical staining.
Depletion of CD8 T cells in vivo
For depletion of CD8+ T lymphocytes,20 4T1 cells (5 × 105) were injected subcutaneously into the right flanks of mice (n = 5 per group). Anti-CD8 antibody (10 mg/kg) was injected intraperitoneally on the same day (day 0) but before inoculation with 4T1 tumor cells, then administered on day 7, 10, 13 and 16. The administration of losartan/anti-PD-L1 mA was same as before. The caliper was used to measure the tumor diameter every 3 days. The tumor volume was calculated.
ELISA for TGF-β1
The concentration of TGF-β1 in the serum was measured with a mouse TGF-β1 ELISA kit according to the manufacturer̛̛̛s instructions. The reaction was read at 450 nm.
Immunohistochemical analysis
The paraffin-embedded tumor sections (4 μm) were dewaxed in xylene and rehydrated with 50%-100% graded ethanol. Microwave-mediated antigen retrieval was carried out in Tri-EDTA buffer (pH 9.0) for 20 minutes. Hydrogen peroxide (3%) was used to inactivate the endogenous peroxidase activity. Tumor tissue sections were incubated with 5% goat serum at room temperature for 30 minutes to block the nonspecific sites, and incubated overnight at 4℃ with the following primary monoclonal antibodies: α-SMA (1:100), CD8 (1:50), Granzyme B (1:50), and collagen Type I (1:300). The immune complexes were detected with 3, 3'-diaminobenzidine tetrahydrochloride. The tumor sections were counterstained with hematoxylin.
In vivo metastasis study
To investigate lung metastasis, 4T1 tumor cells (3 × 104) were injected into Balb/C mice via tail vein. Mice were randomized into four groups: control, anti-PD-L1 mAb, losartan, and combination of losartan and anti-PD-L1 mAb (n = 5 mice/group). The administration of rat IgG isotype, losartan, PD-L1 mAb, or the dual agents was same as before, except that losartan was given on the same day but before tumor cells inoculation throughout the experiment. PD-L1 mAb was given on D1, 4, 7 and 10 after tumor inoculation. The mice were killed on the 7th day after the last dose of anti-PD-L1 mAb. The lungs were harvested for hematoxylin-eosin staining.
Statistical analysis
The statistical significance of differences was analyzed by GraphPad software (Prism version 5). The results were expressed as means ± SEM (standard error of the mean). One-way analysis of variance (ANOVA) and Tukey post tests were used for multiple comparisons. Student’s t test was used for two-group comparisons. Differences were considered significant if P < 0.05. In figures, the significant symbols were used as ***P < 0.001; ** P < 0.01; * P < 0.05.
Results
Losartan normalized the tumor microenvironment
The angiotensin II type 1 receptor blockers (ARBs) have anti-fibrotic properties. ARBs can inhibit CAFs activation and collagen type I deposition in tumor tissue. To investigate whether losartan can modify the TME, we treated tumor-bearing mice with losartan, or an equal volume of edible oil (control) for 3 weeks. We found that tumor growth rates were similar for tumor-bearing mice, which were treated with losartan or control agent (Fig. 1A). The expression of α-SMA and collagen type I is the direct measures of CAFs activity, as well as solid stress due to consequences of CAFs activation.5,10 So, we measured the expression of α-SMA, and the deposition of collagen type I in the tumor tissues. For quantification the staining of α-SMA and collagen type I, five nonoverlapping fields were chosen at random, and analyzed (Image-Pro Plus 6.0). We observed that there was a significantly decrease in number of α-SMA+ cells in the tumor tissues of losartan-treated group (P = 0.001; Fig. 1B and C). Losartan treatment also significantly lowered the deposition of type 1 collagen (P = 0.0034, Fig. 1D and E).
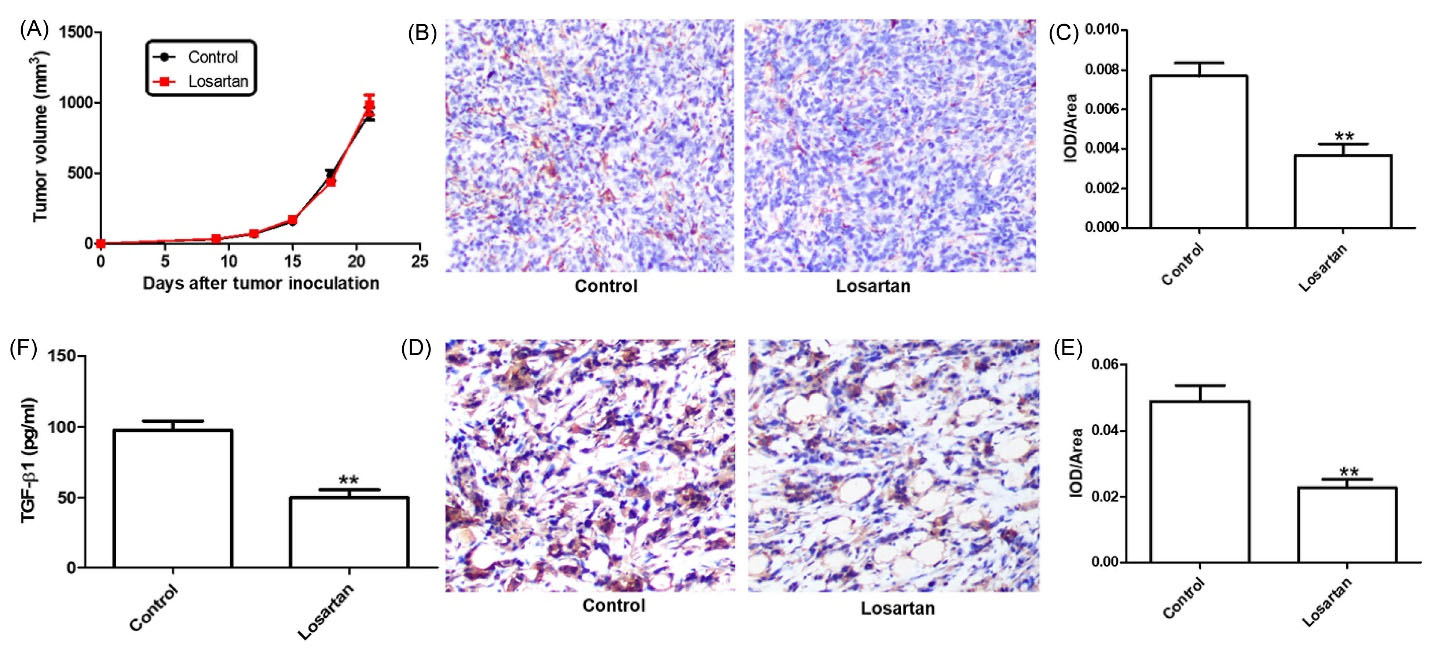
Fig. 1.
Losartan treatment inhibited the activation of fibroblasts. (A) The influence of losartan on 4T1 tumor growth in syngeneic Balb/c mice (n=5). (B and C) the density of α-SMA+ cells and quantitative analysis. (D and E) the deposition of collagen I and quantitative analysis. (F) Serum was tested for TGF-β1 by ELISA analysis.
.
Losartan treatment inhibited the activation of fibroblasts. (A) The influence of losartan on 4T1 tumor growth in syngeneic Balb/c mice (n=5). (B and C) the density of α-SMA+ cells and quantitative analysis. (D and E) the deposition of collagen I and quantitative analysis. (F) Serum was tested for TGF-β1 by ELISA analysis.
The fibrotic cytokine TGF-β is a famous inducer of ECM production and α-SMA expression by CAFs.5,10 To determine if the losartan mediated the reduction of TGF-β1, ELISA was used to measure the levels of TGF-β1 in the serum. The level of TGF-β1 in the serum was lower in losartan-treated mice, compared to the control mice (Fig. 1F, P = 0.005). This suggested that losartan alleviated CAF-driven pathologies were partially mediated by downregulation of TGF-β1.
Combination treatment improved antitumor efficacy
Studies have indicated that CAFs implicated in immunosuppressive microenvironment.9,21 In line with this, it has been shown that the anticancer agents that target immunosuppressive microenvironment could enhance the antitumor immune response and improve the efficacy of immunotherapy.22,23 So, we hypothesized that losartan treatment might modifying the tumor immunosuppressive microenvironment, and convert them into a normal milieu. The xenograft tumor models were used to investigate the effect of losartan/anti-PD-L1 mAb on tumor growth. The cancer-bearing mice were treated with control agent, losartan, anti-PD-L1 mAb, or the two agents. We found that anti-PD-L1 mAb monotherapy resulted in substantial tumor control but only just significant. In contrast, the combination of losartan and anti-PD-L1 mAb obtained dramatic antitumor effect (Fig. 2A and 2B). This suggested that losartan increased the sensitivity of 4T1 breast cancer to anti-PD-L1 mAb immunotherapy, and hinder cancer progression further.
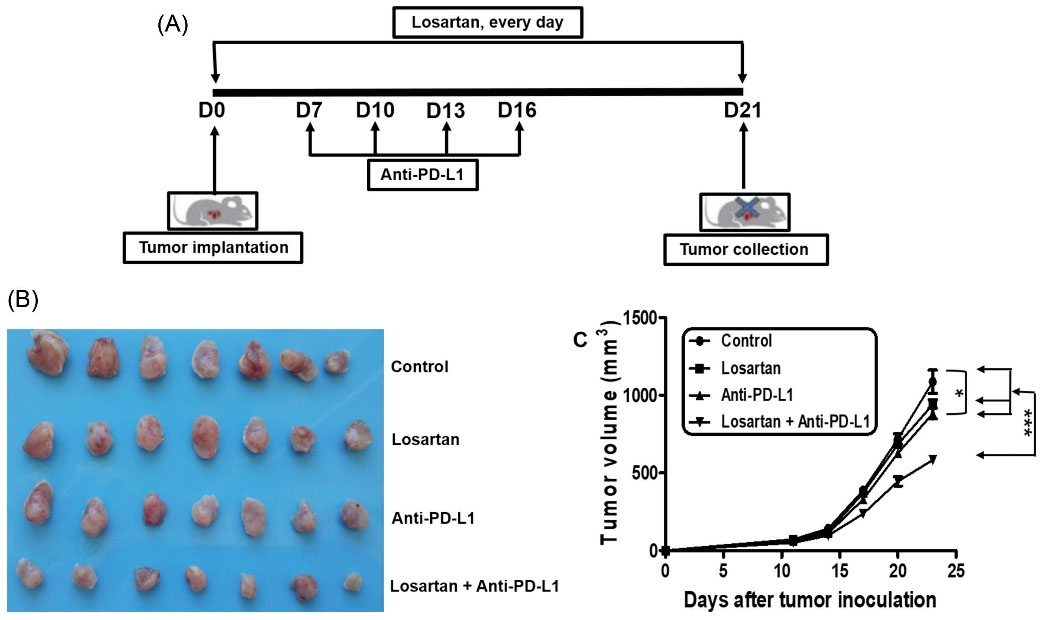
Fig. 2.
The combination of losartan and anti-PD-L1 mAb indicated an increased anti-tumor efficacy. (A) The scheme of treatments (n=7). (B) The tumor tissues were isolated from each treatment groups on day 23. (C) Tumor volume.
.
The combination of losartan and anti-PD-L1 mAb indicated an increased anti-tumor efficacy. (A) The scheme of treatments (n=7). (B) The tumor tissues were isolated from each treatment groups on day 23. (C) Tumor volume.
Losartan reverses the immunosuppressive tumor microenvironment
Due to large number of CAFs and high density of collagen, the TME can restrict T lymphocytes intra-tumoral distribution.9,10 Mobilization of CD8+ T lymphocytes in the cancer core is a prerequisite for favorable response to PD-L1 blockade.2,3,24 Granzyme B (GzmB), a marker of T cell activation, is secreted by cytotoxic T lymphocytes, and plays a critical role in CTLs-mediated elimination of tumor cells.25 To investigate the mechanism that losartan could sensitize breast tumor to anti-PD-L1 immunotherapy, we investigated the infiltration of CD8+ T lymphocytes and the level of GzmB in the tumor tissues by immunohistochemical analysis. For quantification of GzmB and CD8+ T cells staining, five nonoverlapping fields were selected at random. Image-Pro Plus 6.0 software was used to examined the slides, and the average positive area was determined. A larger number of CD8+ T lymphocytes that infiltrated deep into tumor tissue were observed in the combined group, whereas control, losartan or anti-PD-L1 mAb monotherapy had no statistical effect on the CD8+ T cells infiltration (Fig. 3A and 3D). The infiltration of CD8+ T cells was accompanied by increased produce of GzmB in the tumor tissues (Fig. 3B and 3E). In addition, the weight of spleens in the combined group was much less than the other groups (Fig. 3C and 3F), consistent with previous reports.26
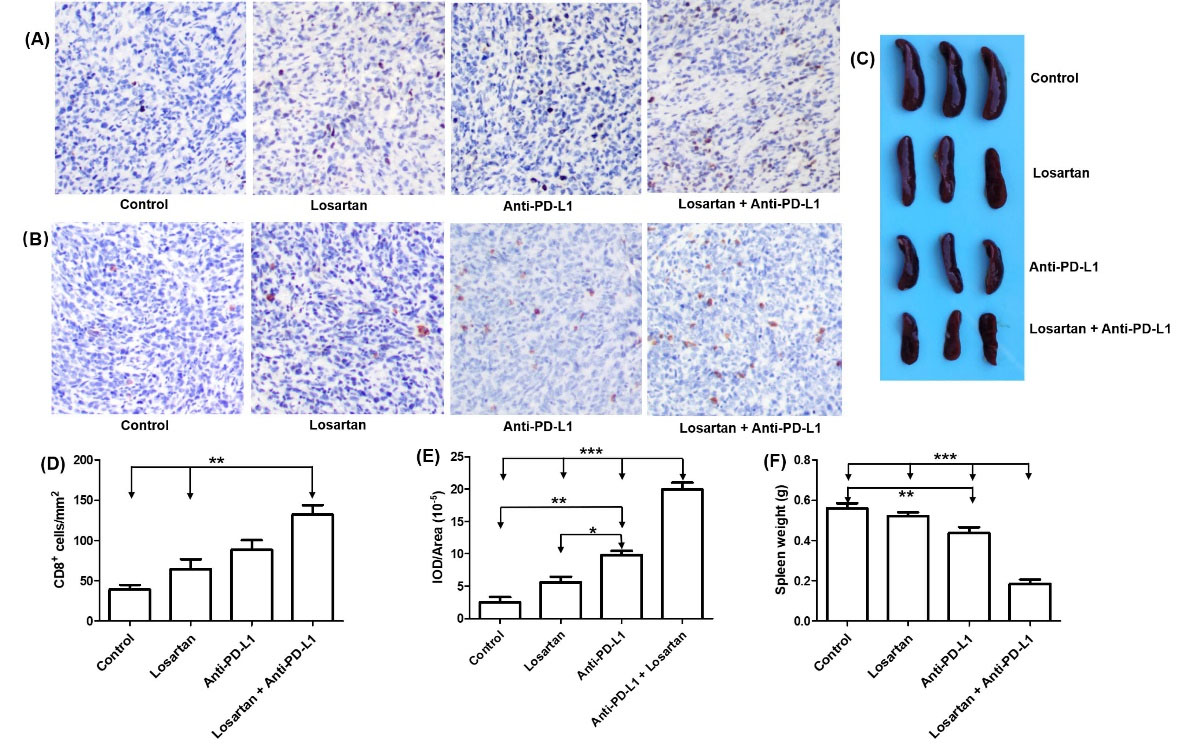
Fig. 3.
Losartan sensitized tumor to PD-L1 blockade. (A and D) Change in CD8+ T cells infiltration in tumor tissues, and quantitative analysis. (B and E) the change granzyme B in tumor tissues, and quantitative analysis. (C and F) Assessment of spleen weight in different treated group, and quantitative analysis.
.
Losartan sensitized tumor to PD-L1 blockade. (A and D) Change in CD8+ T cells infiltration in tumor tissues, and quantitative analysis. (B and E) the change granzyme B in tumor tissues, and quantitative analysis. (C and F) Assessment of spleen weight in different treated group, and quantitative analysis.
To assess whether CD8+ T lymphocytes was indispensable for the observed antitumor effect of the combination of losartan and anti-PD-L1 mAb, 4T1 tumor-bearing Balb/C mice were administered monoclonal antibody against CD8 during the course of treatment (Fig. 4A). We found that depletion of CD8+ T cells during the treatment period abrogated the antitumor efficacy of combination of losartan and anti-PD-L1 mAb (Fig. 4B). Taken together, these data suggested that CD8+ T lymphocytes were necessary for the antitumor effect of combination of losartan and anti-PD-L1 mAb.
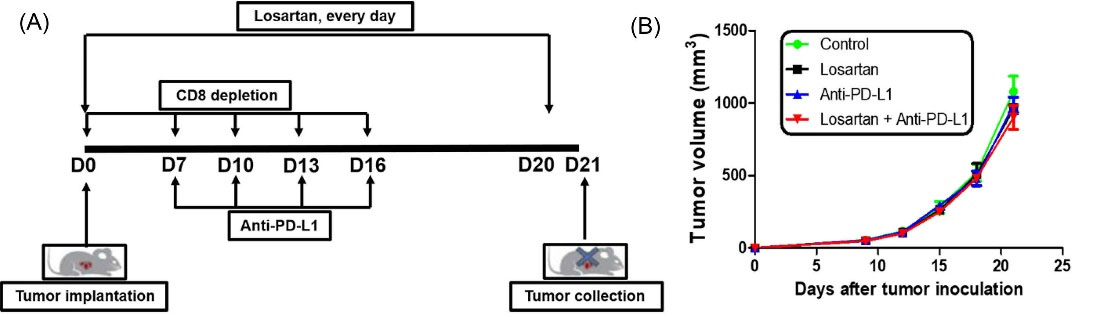
Fig. 4.
The anti-tumor activity of the combination of losartan and PD-L1 mAb was dependent on CD8+ T cells. (A) Scheme of treatments (n=5). (B) The tumor growth kinetics of each treatment group was analyzed.
.
The anti-tumor activity of the combination of losartan and PD-L1 mAb was dependent on CD8+ T cells. (A) Scheme of treatments (n=5). (B) The tumor growth kinetics of each treatment group was analyzed.
Losartan inhibited tumor cells lung metastasis in combination with anti-PD-L1 mAb
Since the metastasis is the main cause of cancer-related deaths, we tested whether the combination of the losartan and anti-PD-L1 mAb improved animal survival in lung metastatic experiments. We injected the 4T1 tumor cells via tail vein. We found that anti-PD-L1 mAb monotherapy had no effect on 4T1 tumor cells lung metastasis. Consistent with other study,16 4T1 pulmonary metastatic burden was reduced in losartan monotherapy. More important, the combination treatment reduced both metastatic tumor number and metastatic tumor size (Fig. 5A). The reduction in lung metastasis was confirmed via hematoxylin-eosin staining (Fig. 5B and 5C). The most metastatic nodules in combination treatment group were micro-metastatic, while the most metastatic nodules in other three groups were macro-metastatic. More important, the metastatic index was significantly lowest in the combined treatment group compared to the other groups. Overall, these data suggested that losartan not only effectively suppressed 4T1 tumor cells lung metastasis; it also could enhance anti-PD-L1 mAb immunotherapy outcomes in models of immunotherapy-refractory breast cancer.

Fig. 5.
The anti-tumor activity of the combination of losartan and PD-L1 mAb was dependent on CD8+ T cells. (A) Scheme of treatments (n=5). (B) The tumor growth kinetics of each treatment group was analyzed.
.
The anti-tumor activity of the combination of losartan and PD-L1 mAb was dependent on CD8+ T cells. (A) Scheme of treatments (n=5). (B) The tumor growth kinetics of each treatment group was analyzed.
Discussion
Currently, ICIs have achieved unprecedented success in clinical in a small fraction of tumor patients. Unfortunately, monotherapy of ICIs has only obtained limited clinical benefit for the main malignant cancer patients due to the various immune escape mechanisms.27,28 Accordingly, there is an ongoing effort to increase the effectiveness of cancer immunotherapy. Main studies on immunotherapy resistance of cancer cells mainly focused on the cancer cell itself. However, it is becoming increasingly clear that cancer microenvironment plays a crucial role in resistance to immunotherapy.
The increased collagen level in the CAFs rich tumor tissue was resistant to PD-1/PD-L1 blockade via inhibiting CD8+ T cells to infiltrate, and induce CD8+ T cell exhaustion.29,30 Losartan can reduce CAFs activation, lead to decrease of α-SMA positive CAFs and collagen deposition during tumor development.4,10 Losartan could also modify tumor immunosuppressive microenvironment. The breast cancer tissue was rich in CAFs, and 4T1 breast tumor is resistant to ICIs immunotherapy.31 Therefore, the goal of this study was to investigate if losartan could modulate the cancer microenvironment, and enhance the efficacy of anti-PD-L1 mAb in breast cancer.
The substantial evidence suggests that the tumor cells secreted high amounts of TGF-β1. The increased level of TGF-β1 in the circulating plasma is associated with the advanced stage of the tumors.32 TGF-β1 is a famous inducer of ECM production and α-SMA expression by CAFs.10 In this study, we observed that losartan treatment significantly decreased the number of α-SMA+ cells, and lowered the deposition of type 1 collagen in the tumor tissues. The level of TGF-β1 in the serum was low in the tumor-bearing mice, which were treated with losartan. We did not observe direct antitumor effect from losartan treatment in vivo. Instead, we found that losartan treatment could improve the therapeutic effect of anti-PD-L1 mAb. A larger number of CD8+ T lymphocytes that infiltrated deep into tumor tissue were considered as a crucial factor that predicted a favorable response to ICIs.33 We found that the combination treatment could promote CD8+ T cells infiltration, this accompanied by more GzmB in the tumor tissues. Further, we found that the weight and size of spleens in the combination group was much less than the other groups. All these indicated that the efficacy of combination therapy was dependent on CD8+ T cells.
The main cause of cancer-related deaths is the metastasis. Study has indicated that losartan could inhibit tumor cell metastasis. Our study also found that losartan not only effectively suppressed 4T1 tumor cells lung metastasis; it also enhanced the efficacy of anti-PD-L1 mAb.
Mutations may evoke neoplastic phenotypes in normal cells. The accumulations of mutation alone generally do not result in cancer formation. The interaction and crosstalk between incipient cancer cells and the supporting cells which form the TME endow incipient cancer cells to acquire the traits that enable them to become tumorigenic and ultimately malignant. This indicates that TME plays a critical role in cancer development.34,35 CAFs are crucial in modulating the delivery of therapeutic agents via secreting CAF-specific proteins, cytokines, growth factors, and producing an ECM. Quiescent fibroblasts become activated in TME, and the activated fibroblasts are key regulators of the paracrine signaling between stromal and cancer cells.35,36 In addition, other studies also indicated that modifying the TME can improve the therapeutic effect.37-39 According to these studies, losartan was given on the same day (day 0) but before inoculation with 4T1 tumor cells.
Research Highlights
What is the current knowledge?
√ Losartan is the widely used anti-hypertensive agent in clinic.
√ Losartan has anti-fibrotic properties, and could inhibit the activation of CAFs and collagen deposition in the tumor tissues.
√ 4T1 breast tumor cells are poorly immunogenic, and resistant to checkpoint immunotherapy.
What is new here?
√ Losartan reverses the immunosuppressive tumor microenvironment and enhance the efficacy of anti-PD-L1 mAb.
√ Losartan inhibited tumor cells lung metastasis in combination with anti-PD-L1 mAb.
√ The antitumor efficacy of the combination of losartan and PD-L1 mAb was depended on CD8+ T cells.
Conclusion
This study demonstrated that losartan sensitized 4T1 breast cancer to anti-PD-L1 mAb immunotherapy via modulating the TME, and further hindered cancer progression. This combination was a rational therapeutic approach to elicit T-cell-mediated immune responses in breast cancer tissue.
Funding sources
Not applicable.
Ethical statement
All animal experiments were approved by the Ethics Committee of Kunming University of Science and Technology, and carried out in accordance with China’s Guidelines for Care and Use of Laboratory Animals.
Competing interests
There is not any conflict of interest.
References
- Li X, Song W, Shao C, Shi Y, Han W. Emerging predictors of the response to the blockade of immune checkpoints in cancer therapy. Cell Mol Immunol 2019; 16:28-39. doi: 10.1038/s41423-018-0086-z [Crossref] [ Google Scholar]
- Topalian S, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443-2454. doi: 10.1056/NEJMoa1200690 [Crossref] [ Google Scholar]
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R. Safety and tumor responses with lambrolizumab (antiPD-1) in melanoma. N Engl J Med 2013; 369:134-144. doi: 10.1056/NEJMoa1305133 [Crossref] [ Google Scholar]
- Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci USA 2011; 108:2909-2914. doi: 10.1073/pnas.1018892108 [Crossref] [ Google Scholar]
- Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer 2016; 16:582-98. doi: 10.1038/nrc.2016.73 [Crossref] [ Google Scholar]
- Yazdani S, Bansal R, Prakash J. Drug targeting to myofibroblasts: implications for fibrosis and cancer. Adv Drug Deliv Rev 2017; 121:101-116. doi: 10.1016/j.addr.2017.07.010 [Crossref] [ Google Scholar]
- Valkenburg KC, de Groot AE, Pienta KJ. Targeting the tumour stroma to improve cancer therapy. Nat Rev Clin Oncol 2018; 15:366-81. doi: 10.1038/s41571-018-0007-1 [Crossref] [ Google Scholar]
- Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 2018; 33:463-479.e10. 10.1016/j.ccell.2018.01.011.
- Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci USA 2013; 110:20212-20217. doi: 10.1073/pnas.1320318110 [Crossref] [ Google Scholar]
- Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun 2013; 4:2516. doi: 10.1038/ncomms3516 [Crossref] [ Google Scholar]
- Xia Y, Yan J, Jin X, Entman ML, Wang Y. The chemokine receptor CXCR6 contributes to recruitment of bone marrow-derived fibroblast precursors in renal fibrosis. Kidney Int 2014; 86:327-337. doi: 10.1038/ki.2014.64 [Crossref] [ Google Scholar]
- Htoo PT, Stürmer T, Jonsson-Funk M, Pate V, Simpson RJ Jr, Lund JL. Renin-angiotensin-aldosterone system-based antihypertensive agents and the risk of colorectal cancer among medicare beneficiaries. Epidemiology 2019; 30:867-875. doi: 10.1097/EDE.0000000000001065 [Crossref] [ Google Scholar]
- George AJ, Thomas WG, Hannan RD. The renin-angiotensin system and cancer: old dog, new tricks. Nat Rev Cancer 2010; 10:745-59. doi: 10.1038/nrc2945 [Crossref] [ Google Scholar]
- Zhao Y, Cao J, Melamed A, Worley M, Gockley A, Jones D. Losartan treatment enhances chemotherapy efficacy and reduces ascites in ovarian cancer models by normalizing the tumor stroma. Proc Natl Acad Sci USA 2019; 116:2210-2219. doi: 10.1073/pnas.181835711 [Crossref] [ Google Scholar]
- Xie GZ, Cheng T, Lin J, Zhang L, Zheng J, Liu Y. Local angiotensin II contributes to tumor resistance to checkpoint immunotherapy. J Immunother Cancer 2018; 6:88. doi: 10.1186/s40425-018-0401-3 [Crossref] [ Google Scholar]
- Regan DP, Coy JW, Chahal KK, Chow L, Kurihara JN, Guth AM. The angiotensin receptor blocker losartan suppresses growth of pulmonary metastases via AT1R-independent inhibition of CCR2 signaling and monocyte recruitment. J Immunol 2019; 202:3087-3102. doi: 10.4049/jimmunol.1800619 [Crossref] [ Google Scholar]
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017; 541:321-330. doi: 10.1038/nature21349 [Crossref] [ Google Scholar]
- Ma Y, Xia Z, Ye C, Lu c, Zhou S, Pan J. AGTR1 promotes lymph node metastasis in breast cancer by upregulating CXCR4/SDF-1 alpha and inducing cell migration and invasion. Aging (Albany NY) 2019; 11:3969-3992. doi: 10.18632/aging.102032 [Crossref] [ Google Scholar]
- Peng DH, Rodriguez BL, Diao LX, Chen L, Wang J, Byers LA. Collagen promotes anti-PD-1/PD-L1 resistance in cancer through LAIR1-dependent CD8+ T cell exhaustion. Nat Commun 2020; 11:4520. doi: 10.1038/s41467-020-18298-8 [Crossref] [ Google Scholar]
- Wang Y, Su LJ, Morin MD, Jones BT, Mifune Y, Shi H. Adjuvant effect of the novel TLR1/TLR2 agonist Diprovocim synergizes with anti–PD-L1 to eliminate melanoma in mice. Proc Natl Acad Sci USA 2018; 115:E8698-E8706. doi: 10.1073/pnas [Crossref] [ Google Scholar]
- Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 2010; 330:827-830. doi: 10.1126/science.1195300 [Crossref] [ Google Scholar]
- Wallin JJ, Bendell JC, Funke R, Sznol M, Korski K, Jones S. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun 2016; 7:12624. doi: 10.1038/ncomms12624 [Crossref] [ Google Scholar]
- Manegold C, Dingemans AC, Gray JE, Nakagawa K, Nicolson M, Peters S. The potential of combined immunotherapy and antiangiogenesis for the synergistic treatment of advanced NSCLC. J Thorac Oncol 201 7; 12:194-207. doi: 10.1016/j.jtho.2016.10.003 [Crossref] [ Google Scholar]
- Keenan TE, Burke KP, Van Allen EM. Genomic correlates of response to immune checkpoint blockade. Nat Med 2019; 25:389-402. doi: 10.1038/s41591-019-0382-x [Crossref] [ Google Scholar]
- Lieberman J, Fan Z. Nuclear war: the granzyme A-bomb. Curr Opin Immunol 200 3; 15:553-559. doi: 10.1016/s0952-7915(03)00108-0 [Crossref] [ Google Scholar]
- Kumar S, Davra V, Obr AE, Geng K, Wood TL, De Lorenzo MS. Crk adaptor protein promotes PD-L1 expression, EMT and immune evasion in a murine model of triple-negative breast cancer. Oncoimmunology 2018; 7:e1376155. doi: 10.1080/2162402X.2017.1376155 [Crossref] [ Google Scholar]
- Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015; 348:74-80. doi: 10.1126/science.aaa6204 [Crossref] [ Google Scholar]
- Melero I, Berman DM, Aznar MA, Korman AJ, Pérez Gracia JL. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer 2015; 15:457-72. doi: 10.1038/nrc3973 [Crossref] [ Google Scholar]
- Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest 2012; 122:899-910. doi: 10.1172/JCI45817 [Crossref] [ Google Scholar]
- Michel MC, Foster C, Brunner HR, Liu L. A systematic comparison of the properties of clinically used angiotensin II type 1 receptor antagonists. Pharmacol Rev 2013; 65:809-848. doi: 10.1124/pr.112.007278 [Crossref] [ Google Scholar]
- Luo H, Tu G, Liu Z, Liu M. Cancer-associated fibroblasts: a multifaceted driver of breast cancer progression. Cancer Lett 2015; 361:155-63. doi: 10.1016/j.canlet.2015.02.018 [Crossref] [ Google Scholar]
- Krasagakis K, Tholke D, Farthmann B, Eberle J, Mansmann U, Orfanos CE. Elevated plasma levels of transforming growth factor (TGF)-beta1 and TGF-beta2 in patients with disseminated malignant melanoma. Br J Cancer 1998; 77:1 492-494. doi: 10.1038/bjc.1998.245 [Crossref] [ Google Scholar]
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer 2005; 5:263-74. doi: 10.1038/nrc1586 [Crossref] [ Google Scholar]
- Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell 2018; 175:313-326. doi: 10.1016/j.cell.2018.09.035 [Crossref] [ Google Scholar]
- Belli C, Trapani D, Viale G. Targeting the microenvironment in solid tumors. Cancer Treat Rev 2018; 65:22-32. doi: 10.1016/j.ctrv.2018.02.004 [Crossref] [ Google Scholar]
- Kalluri R. The biology and function of fibroblasts in cancer. Nature Reviews Cancer 2016; 16:582-598. doi: 10.1038/nrc.2016.73 [Crossref] [ Google Scholar]
- Chauhan VP, Chen IX, Tong R, Ng MR, Martin JD, Naxerova K. Reprogramming the microenvironment with tumor selective angiotensin blockers enhances cancer immunotherapy. Proc Natl Acad Sci USA 2019; 116:10674-10680. doi: 10.1073/pnas.1819889116 [Crossref] [ Google Scholar]
- Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci USA 2011; 108:2909-2914. doi: 10.1073/pnas.1018892108 [Crossref] [ Google Scholar]
- Wadsworth BJ, Cederberg RA, Lee CM, Firmino NS, Franks SE, Pan J. Angiotensin II type 1 receptor blocker telmisartan inhibits the development of transient hypoxia and improves tumour response to radiation. Cancer Lett 2020; 493:31-40. doi: 10.1016/j.canlet.2020.07.015 [Crossref] [ Google Scholar]