Bioimpacts. 13(4):347-353.
doi: 10.34172/bi.2023.24195
Short Communication
Cloth-based microfluidic devices integrated onto the patch as wearable colorimetric sensors for simultaneous sweat analysis
Bambang Kuswandi Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing, , * 
Lukman H Irsyad Formal analysis, Investigation, Methodology, Validation, Writing – original draft,
Ayik R. Puspaningtyas Data curation, Resources, Writing – review & editing, 
Author information:
Chemo and Biosensors Group, Faculty of Pharmacy, University of Jember, Jl. Kalimantan 37, Jember, East Java, 68121, Indonesia
Abstract
Introduction:
In this work, a flexible, and wearable point-of-care (POC) device integrated on a pain relief patch as wearable colorimetric sensors have been developed for sweat analysis, such as lactic acid, sodium ions, and pH simultaneously. Herein, the patch has still functioned as pain relief, while it allows for sweat monitoring during exercise, and in daily activities.
Methods:
It was constructed on cotton cloth using wax printing technology (batik stamp) as cloth-based microfluidic devices (CMDs). Here, it uses micro volumes of samples to perform the reaction in the sensing zones, where the sensitive reagents are immobilized so that it can collect and analyze the sweat (lactic acid, sodium ions, and pH) as the model for sweat analytes. The colorimetric analysis was conducted via a smartphone camera by using a free app (Color Grab) for a color image analysis that uses for quantitative analysis or naked eye for semi-qualitative analysis.
Results:
The ∆RGB value of the CMDS shows the excellent linear correlation vs analytes concentration, where the coefficient of correlations was found for lactic acid (R2 = 0.994), sodium ion (R2 = 0.998), and pH (R2 = 0.994). The ∆RGB value shows the appropriate color value for the linear correlation of the analyte target concentrations in the sweat samples. Here, the limit of detection (LOD) was found at 45.73 µg/mL for lactic acid and 56.46 µg/mL for sodium ions. The reproducibility was found at 0.79% and 0.89%, for lactic acid and sodium ions respectively.
Conclusion:
It was applied for sweat analysis during exercise, and the results show in agreement with the standard methods used in a clinical laboratory.
Keywords: Wearable device, Smart patch, Cloth-based microfluidic devices, Sweat analysis
Copyright and License Information
© 2023 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Nowadays, wearable devices provide personalized health monitoring via the user in their daily activities, for more realistic and real-time monitoring compared to a clinical laboratory. An important advantage is that the user becomes more aware of their health conditions that have a big impact on the preventative approach in healthcare. Chemical sensors and biosensors have various applications, including wearable devices for clinical analysis that could offer health information to the user. Wearable devices for healthcare monitoring have been implemented by many researchers for various applications for the detection of physiological parameters relevant to human health, such as bodily motion, skin temperature, heart rate, etc; many of these devices are commercially available.1-3 Here, obtaining chemical parameters from body fluids provides information on the user’s health condition at the molecular level.3
In body fluid monitoring, sweat analysis offers many benefits over other fluids, e.g., it is non-invasive, easily accessible, and simple.4 These benefits have motivated works on the development prototype of wearable sweat analysis based on colorimetric sensor,5 where quantification analysis only needs digital capture using a smartphone. Commonly, these sensors are developed as disposable sensors due to their irreversible nature. While using electrochemical sensors need the integration of a flexible electrochemical sensor and skin-friendly device, including on-site signal processing and calibration for reliable analysis.6-8 Due to the electronic components and their circuitry that are needed to be employed in these electrochemical sensors to provide accurate information. Hence, limited the application of this sensor for wearable devices in long-term clinical monitoring rather than for disposable testing.8 Therefore, most of the approaches in chemical sensors for the wearable sensor are developed based on colorimetric sensors, as they are more simple, small, and relatively low-cost compared to the electrochemical sensors.9 In the case of sweat analysis, the wearable sensors or on-skin sensor offers advantages in terms of small size, user-friendly, and convenience that provides an innovative approach for further development.5 Most of these wearable sensors, however, are a concern for biohazard issues. For instance, to detect chloride ions in sweat, silver chromate with dark-brown color can be used to create a white color precipitant, however, it is carcinogenic and irritant to the skin.3 Similarly for sweat analysis based on electrochemical sensors, when they attach to skin the electrode could cause blistering and burning. Therefore, these are not popular to be used for on-skin detection.3
Currently, microfluidic devices have been widely developed for clinical analysis as point-of-care (POC) devices using paper as microfluidic paper-based analytical devices (µPADs)10 or even textile materials, such as cloth-based microfluidic devices (CMDs).11 Due to benefit of vertical layering and the advantages of these materials, such as lightweight, flexible, capillary action, and simple colorimetric detection,12 POC based on these materials are promising to be developed for on-skin detection.13
Herein, a flexible, and wearable CMD integrated with a pain relief patch is proposed as a novel smart patch that has been developed for effective sweat analysis, such as lactic acid, sodium ions, and pH simultaneously. The CMD was designed and patterned on hydrophilic cotton cloth using wax printing technology (i.e., batik stamp) that can collect and direct sweat to the sensing zones. The CMD uses micro volumes of samples to perform the reaction in the sensing zones, where the sensitive reagents are immobilized. Cotton fabric has been employed as the hydrophilic platform because of its high porosity even without any pretreatment.14 The sensing zones are designed to detect lactic acid, sodium ions, and pH simultaneously as the model for sweat analytes. Here. the patch has still functioned as pain relief, while it allows for sweat monitoring during exercise, and in daily activities.
Materials and Methods
Materials
Chlorophenol red (CPR), Fe(III)-tris (1,10 phenanthroline), potassium chromate, silver nitrate lactic acid, NaCl, and phosphate-buffered solution (1X PBS) consisting KCl, NaCl, KH2PO4, and Na2HPO4 as well as hydrochloric acid (HCl), and sodium hydroxide (NaOH), were obtained from Sigma-Aldrich (UK). To make phosphate buffer at pH 4 to pH 7 were adjusted with 0.1 M HCl or 0.1 NaOH accordingly. Wax for batik was ordered from local batik store (Rumah Batik Jember), and pain relief patch (Salonpas, Hisamitsu Pharma, Indonesia) and cotton cloths (Nofrill, Indonesia) ordered from Indomart (Jember, Indonesia). All chemicals used are analytical reagent grade.
Fabrication of CMDs
The smart patch is contained of vertically attached CMDs with three type sensing zones on the top of pain relief patch that is stacked on the skin by a medical dressing (Salonpas), while a hole in the patch was prepared by a hole puncher 10 mm in size (Fig. 1A). The smart patch is flexible, lightweight, and adhesive to make a contact with skin, which allows simultaneous colorimetric detection (Fig. 1B). The patterned cotton cloth-based microfluidics was conducted using a customized traditional batik stamp at 70oC for 1 minute to allow it to penetrate through the cloth thickness and to make hydrophobic barriers to guide the sweat flow. This wax stamp technology is made of brass (Fig. 2A) and was used for the simple and rapid production of the CMDs, just by simple stamping on the cotton cloth placed on a dedicated table to ovoid crumpled cloth (Fig. 2B). The CMDs size was 200 × 200 mm, have a middle circle around 15 mm in diameter, and 9 channels around 10 mm in length, 2 mm wide, and 9 corner circles around 5 mm in diameter, with a thickness of all channels around 0.8 mm (Fig. 2C). The microscopy image of CMDs is presented in Fig. S1, and the sample volume that can be accommodated in the sensing zone is up to 1.2 µL (Fig. S2). In addition, the material cost of the smart patch is inexpensive because it is made of inexpensive materials, i.e., cotton cloths and pain relief patches.
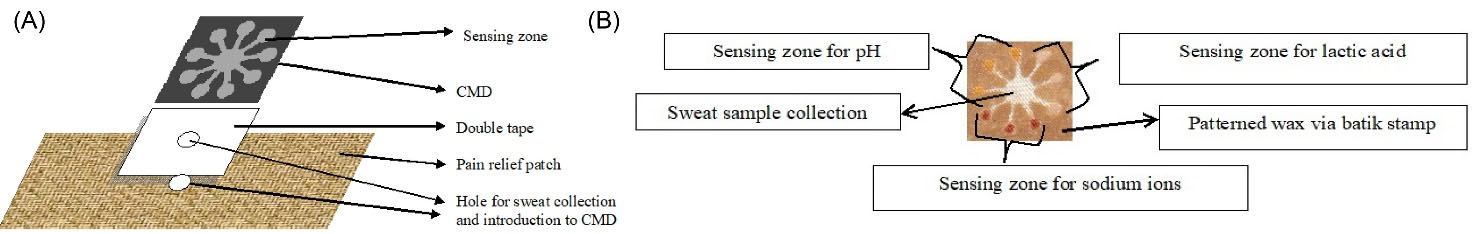
Fig. 1.
Design and construction of the smart patch integrated with a cloth-based microfluidic device (CMD) (A), and the CMDs as a point-of-care device (B).
.
Design and construction of the smart patch integrated with a cloth-based microfluidic device (CMD) (A), and the CMDs as a point-of-care device (B).

Fig. 2.
The batik stamp (A) is made of brass for rapid production of patterned cloth-based microfluidics (B), and the CMDs ready to be integrated into the patch (C).
.
The batik stamp (A) is made of brass for rapid production of patterned cloth-based microfluidics (B), and the CMDs ready to be integrated into the patch (C).
Reagents immobilization
The sensing zones for lactic acid detection was immobilized with 1000 µg/mL Fe(III)-tris (1,10 phenanthroline), for sodium ion (Na+) detection was immobilized of mixed potassium chromate (5% or 5000 µg/mL) and silver nitrate (1000 µg/mL) in 1:1 volume ratio, and for pH detection was immobilized 2000 µg/mL CPR. Then, 0.7 μL of each sensitive reagent solution was immobilized via adsorption using casting technique sequentially by micropipette (Eppendorf, Germany). Thus, each reagent concentration used in each sensing zone were 0.7 µg/µL of Fe(III)-tris (1,10 phenanthroline), 3.5 µg/µL of potassium chromate and 0.7 µg/µL of silver nitrate, and 1.7 µg/µL of CPR.
Colorimetric measurement
Here, the colorimetric detection was performed on-skin that allows an integrated function to avoid manual procedures and the need for transferring sweat to reduce its evaporation (Fig. 1C). The wearable colorimetric sensors provide in situ quantitative analysis for simultaneous sweat analytes (i.e., lactic acid, sodium ions, and pH), where the color change can be captured by a smartphone camera (13 megapixels) of a Samsung Galaxy S5 smartphone (Samsung Electronics, Suwon, South Korea), along with Android 6.0, and analyzed by the App Color Grab 3.6.1 (Loomatix®) as the color image analysis for Android to capture digital color and presented as the RGB values. To support a reproducible and stable color image using a smartphone camera, it was aided by using a ring light called ‘Ringlight LED Clip-On for Smartphone” (XY, China) that attached to the smartphone.15 The set-up for taking pictures and the mobile application for producing RGB values are depicted in Fig. S3. By using this ring light, the smartphone flashlight was used along with additional light from the LED of a clip-on ring, with the same distance and background, so that all the colors taken should be in a light-controlled area. Thus, the ring light helps the smartphone camera to take pictures in the same conditions.
The clinical standard methods for sweat analysis
The clinical standard methods for sweat analysis used at Clinical Laboratory, Subandi Hospital, Jember as a comparison of the wearable sensors are demonstrated as follows. The lactic acid analysis was performed using UV/Vis spectrophotometer16 (U-3010/3310, Hitachi, Japan). A 50 μL sweat test containing lactic acid was added to 2 mL Fe(III)Cl3 (0.2%) solution and stirred until homogenous. The absorbance was measured at 390 nm against the reference solution (2 mL of a 0.2%FeCl3 solution). The reaction and measurements were performed at 25 ± 5°C, where the color of the solution was stable for 15 minutes. While the sodium ions were done using ISE17,18 (FC300B, Hanna Instrument, UK). Here, a 1 mL sweat test was added to a 10-mL volumetric flask, then add 1-mL ISA (ionic strength adjuster, 4 M NH4Cl, and 4 M NH4OH) and dilute to the volume with distilled-deionized water. Then the concentration of sodium ion can be determined using a calculated equation of the calibration line obtained. While a pH measurement was determined using a pH meter (Methrom 780, UK) by reading directly the pH value of the prepared sweat test. Here, a 1 mL sweat test added to a 10-mL volumetric flask and dilute to the volume with distilled-deionized water was used as a prepared sweat test.
Results and Discussion
Advantage of the novel wearable colorimetric sensors
The advantage of the wearable colorimetric sensor is consisting of a paint relief patch that was stacked to the skin, while the CMD was attached on the top of the patch with three analytes groups of triple sensing zones. When it is used, the smart patch would be stacked tightly on the skin (Fig. 3A). Sweat is secreted from the skin goes upward into the sweat collection zone above the patch and is directed into sensing zones in the CMD, where lactic acid, sodium ions, and pH, can be detected simultaneously (Fig. 3). Thus, it is better compared to others that used patches only for sweat analysis.5,19-21 It would result in the reaction of sweat with the reagent immobilized onto sensing zones, consequently changing the color of the sensing zone correspond to lactic acid, sodium ions, and pH in the sweat. By using color image analysis, the color change in the sensing zone is proportional to the concentration of target analytes in sweat.

Fig. 3.
An example of the smart patch is when it is used for sweat analysis (A), before (B), and after being used by the user (C).
.
An example of the smart patch is when it is used for sweat analysis (A), before (B), and after being used by the user (C).
The mechanism of the detection method for target analytes, in this case, can be described as follows. The mechanism is based on the colorimetric reaction of lactic acid with Fe(III), where it is reduced to Fe(II),22 which in turn, changes color from weak orange to intense red. The Fe(II) produced is proportional to the concentration of lactate (L) in the sample. While the sodium ion is detected by a mixed reagent of potassium chromate (K2CrO4) and silver nitrate (AgNO3). In this case, yellowish potassium chromate turns red in the presence of silver ions as silver chromate (Ag2CrO4). Thus, in the presence of sodium ions or sodium chloride (NaCl) in sweat, the sodium makes a complex with nitrate (NaNO3) substituted from potassium nitrate, while silver makes a complex with chloride as silver chloride (AgCl) substituted from NaCl, and turn back the yellowish potassium chromate (K2CrO4).23 Hence, this reaction turns color from red to yellow. This color change is proportional to the concentration of sodium ions in the sweat sample. Thus, in this substitution reaction, potassium chromate is utilized as an indicator in the complex reaction between sodium ion and nitrate ion. For sweat pH detection, CPR was used as a pH indicator, since it has a pH range between 4.6-7.0, and changes its color from yellow to red from acid to neutral pH.24 Herewith, the optimized CPR concentration used in this case was found at 2000 µg/mL toward pH 4 to pH 7, where different CPR concentrations give different intensities of color change (Fig. S3). The mechanism detections of three target analytes, i.e. lactic acid, sodium ions, and pH can be summarized as follows:
(1)
(2)
(3)
Analytical characteristics
To evaluate the analytical performance of the wearable colorimetric sensors to detect sweat analytes using colorimetric detection, a series of standard solutions of lactic acid, sodium ion, and pH were tested on the smart patch (Fig. 4). The color change is sometimes not well distributed on the sensing zone (Fig. S4). This may be due to associated to the cotton fibrous nature and incomplete network inside cotton.9 It could be shorted out by preparing the saturated cotton (wet-out) and the close contact.25 The small size of the cotton collecting zone (i.d. 15 mm) needs only a volume of sweat in a minute (~15 µL) during exercise, which is 100 times less than the sampling volume (15 mL) in classical analysis.20 Here, a very small sampling volume would decrease the time and load of sweat collection for screening purposes.
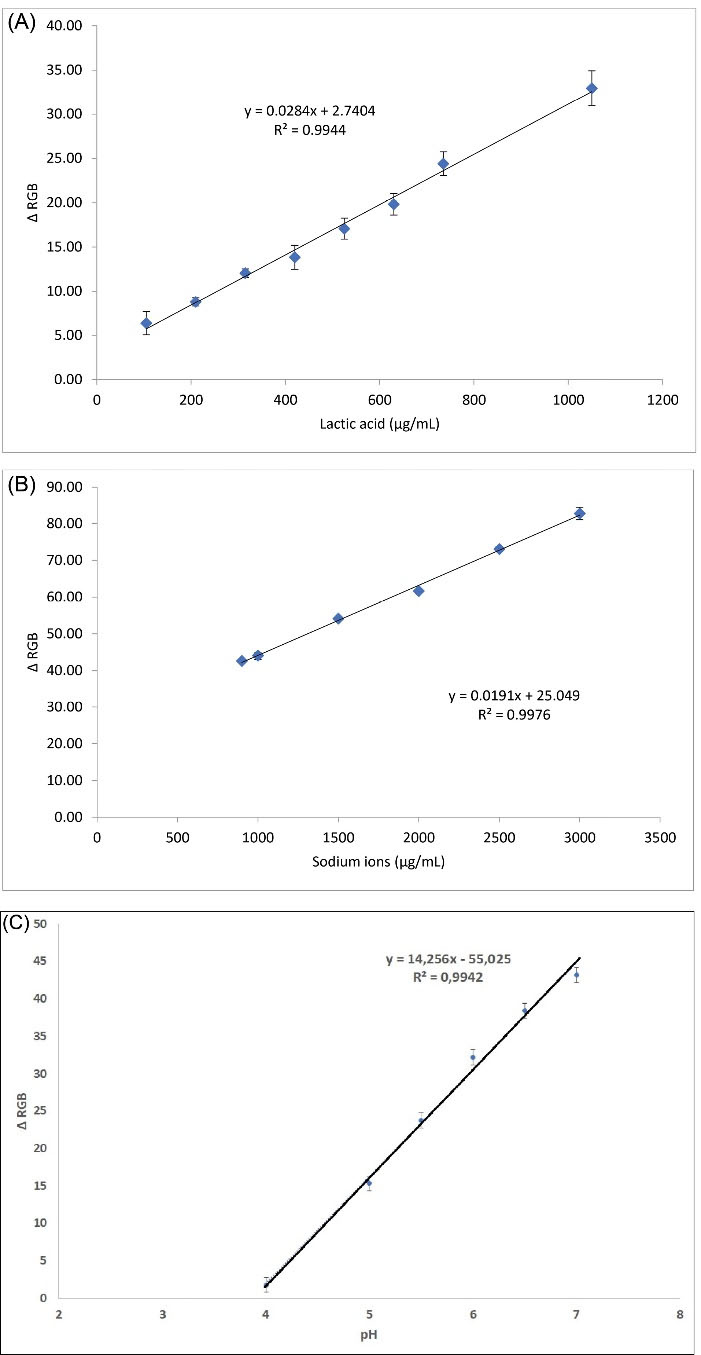
Fig. 4.
The calibration curve of standard lactic acid concentration vs Δ RGB value (A), the calibration curve of standard sodium ions concentration vs Δ RGB value (B), and the calibration curve of pH vs Δ RGB value (C).
.
The calibration curve of standard lactic acid concentration vs Δ RGB value (A), the calibration curve of standard sodium ions concentration vs Δ RGB value (B), and the calibration curve of pH vs Δ RGB value (C).
Then the linear regression analysis was conducted for an average of each color of triple sensing zones for lactic acid, sodium ion, and pH respectively (Fig. 4), and the supported data is given in Tables S1 to S3. Herein, ∆RGB shows the excellent linear correlation vs analytes concentration, where the coefficient of correlation was found for lactic acid concentration (R2 = 0.994) (Fig. 4A), sodium ion concentration (R2 = 0.998) (Fig. 4B), and for pH (R2 = 0.994) (Fig. 4C). Herein, the ∆RGB value shows the appropriate color value for the linear correlation of the analyte target concentrations in the sweat samples. Based on the calibration curves, the limit of detection (LOD) was found at 45.73 µg/mL for lactic acid concentration and 56.46 µg/mL for sodium ions concentration. Here, the LOD is measured by 3 times of standard deviation (SD) of Δ RGB value as the analytical response from the blank. In the case of lactic acid, it was found that the SD value for the blank was 1.611. By multiplying with 3 as the blank signal, and by using the equation in Fig. 4A, the LOD was found at 45.7262 or 45.73 µg/mL. Similarly for LOD of sodium ions, as the SD value for the blank was 8.709, thus LOD was calculated to be 56.455 or 56.46 µg/mL
The reproducibility was found at 0. 79% and 0.89%, for lactic acid and sodium ions respectively. While the recovery of the detection was between 99.83 to 102.67%, and 99.67 to 101.65%, for lactic acid and sodium ions respectively. Moreover, no significant interference from urea at a ratio of 1:100. These reproducibility and recovery values, including the interferences, were excellent for this type of measurement.26 While the matrix effect, particularly interference from urea, since urea concentration in the normal sweat is around 22.2 mmol/L.27 The results show no interference of the urea toward lactic acid and sodium ion determination at ratio 1:10 (urea). Thus, it can be stated that no interference from the matrix.
In addition, the semi-quantitative and qualitative detections of these target analytes could also be performed by the naked eye using a reference color reader (Fig. 5A), where the validated color reader was based on the CMDs color produced toward various target analyte concentrations (Fig. S5). This reference color reader was tested on the wearable colorimetric sensors to detect the target analytes (lactic acid, sodium ion, and pH), where their concentrations follow normal sweat content.28 The good results of five sweat samples were obtained using this detection method. Thus, this naked-eye detection can be applied for simple and rapid detection of sweat analytes using wearable colorimetric sensors.
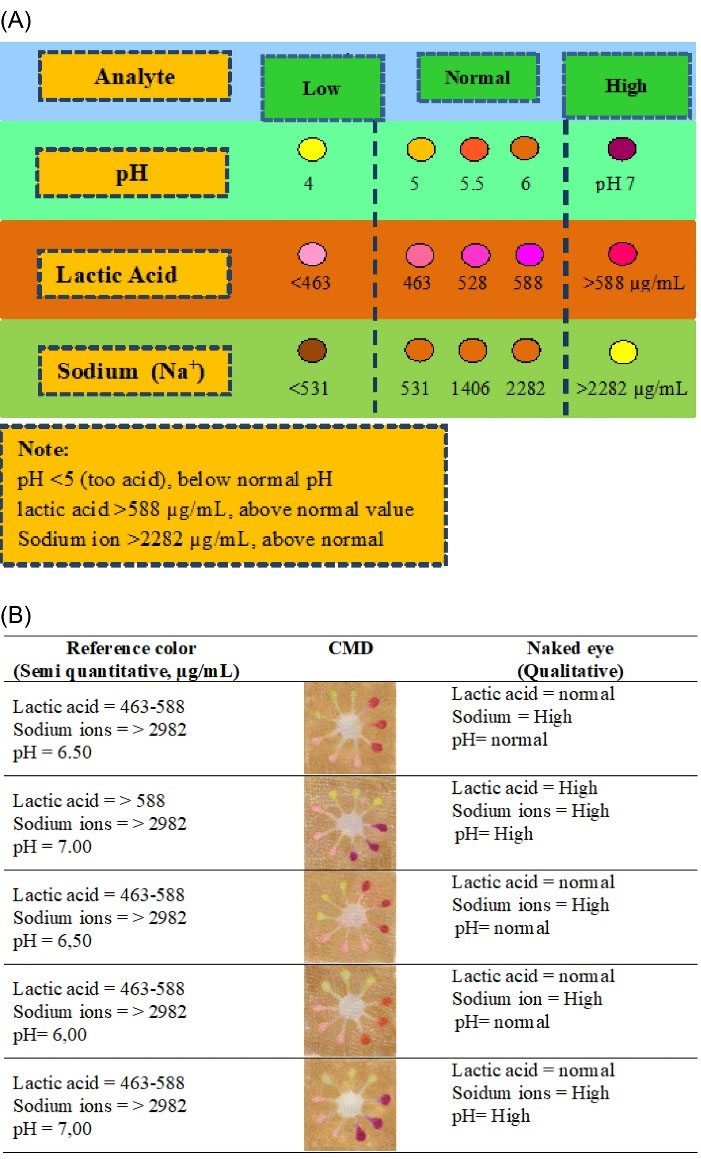
Fig. 5.
The color reference reading for naked-eye detection (A), and the results using real sweat samples (B).
.
The color reference reading for naked-eye detection (A), and the results using real sweat samples (B).
Application with the real samples
The wearable colorimetric sensors were used to screen the real sweat samples from 5 patients above at Clinical Laboratory, Subandi Hospital, Jember along with ethical review and approval. The typical wearable colorimetric sensors change after being tested during exercise is given in Fig. 5B, where it turns into relating colors in about 5 minutes, according to the patient’s perspiration rate, where the skin patch triple colors changed could distinguish for each target analyte detected.
Herein, the comparison results between the wearable colorimetric sensors and the clinical standard analysis are demonstrated in Table 1. The quantitative results of the wearable colorimetric sensors were compared with that of a standard clinical laboratory used for these sweat analyses, where the lactic acid analysis using UV/Vis spectrophotometer,16 sodium ions using ISE,17 and pH measurement using pH meter. The two methods show a good correlation for three analytes tested (R2 = 0.967 for lactic acid, R2 = 0.984 for sodium ion, and R2 = 0.953 for pH).26 These results show that the immobilized reagents in the sensing zones of CMDs allow for the quantitative colorimetric detection of sweat analytes. Therefore, the wearable colorimetric sensors are being compared with the spectrophotometric method of lactic acid, ISE (ion-selective electrode) for sodium ions detection, and pH meter for pH detection. Thus, it can be concluded that the wearable colorimetric sensors allow the detection of sweat analytes, while it has still functioned as a pain relief patch.
Table 1.
The comparison results of sweat analysis between the smart patch and the clinical laboratory
|
Smart patch
|
Clinical Lab
|
|
Lactic acid (µg/mL)
|
Sodium ion (µg/mL)
|
pH
|
UV/Vis Spectrophotometry (µg/mL)
|
ISE (µg/mL)
|
pH Meter
|
| 527.42 ± 4.71 |
3278.03 ± 25.38 |
6.50 ± 0.17 |
533.01 ± 3.25 |
3298.20 ± 20.40 |
6.35 ± 0.16 |
| 540.28 ± 4.25 |
3511.90 ± 21.34 |
7.00 ± 0.12 |
532.56 ± 4.13 |
3691.50 ± 28.50 |
6.90 ± 0.15 |
| 567.38 ± 3.84 |
3514.95 ± 38.52 |
6.50 ± 0.18 |
561.02 ± 3.52 |
3929.70 ± 30.40 |
6.27 ± 0.32 |
| 602.22 ± 2.95 |
3730.68 ± 45.54 |
6.00 ± 0.15 |
600.28 ± 2.73 |
3978.00 ± 32.50 |
6.02 ± 0.12 |
| 560.60 ± 5.23 |
3728.63 ± 27.23 |
7.00 ± 0.12 |
557.04 ± 3.21 |
3969.80 ± 21.60 |
6.80 ± 0.24 |
Note: All data are presented as the average of triplicate measurements ± SD.
Conclusion
Herein, the novel wearable colorimetric sensors were demonstrated as an alternative device for sweat analysis and its advantages in the diagnostic sweat screening. The important part of the wearable colorimetric sensors is the CMD, where colorimetric detection was performed. The simplicity of the colorimetric detection allows it to be integrated into the sweat analysis using a flexible, lightweight, and low-cost pain relief patch. Furthermore, colorimetric detection can be visually detected by the naked eye. It is also promising for eliminating some critical issues in classical laboratory diagnoses, such as laborious operation, long time, and high expense. Thus, it opens up new alternatives in health screening via sweat analysis, including other POC applications, such as the detection of clinically important analytes in sweat related to diseases.
Research Highlights
What is the current knowledge?
√ During the last decade, cloth-based microfluidic devices offer promising wearable devices for clinical analysis of biological fluids, i.e. sweat.
√ The application of cloth-based microfluidic combined with pain relief patches as a wearable device is a novel approach.
What is new here?
√ The proposed novel wearable device is a simple, disposable, efficient, and low-cost device for sweat analysis.
√ There is no need for costly instrumentation since it can be detected using the naked eye or smartphone camera.
√ This wearable device can be applied for sweat analysis of the user, such as lactic acid, sodium ions, and pH simultaneously for user health monitoring.
Acknowledgments
This work was supported by The Ministry of Education, Culture, Research and Technology, the Republic of Indonesia through the WCR Grant 2021.
Competing Interests
The authors declare that they have no competing interests.
Ethical Statement
All the experiments in this work were done in compliance with the guidelines and regulations of the Ethical Committee of Clinical Research, the University of Jember, and all experiments were performed based on institutional guidelines. In addition, informed consent for participation in real sample analysis was obtained. At the same time, all the human individuals involved were informed about the research and they voluntarily agreed.
Funding
The WCR Grant 2021, The Ministry of Research, Technology and Higher Education, the Republic of Indonesia.
Supplementary Materials
Supplementary file 1 contains Tables S1-S3 and Figures S1-S5.
(pdf)
References
- Villar R, Beltrame T, Hughson RL. Validation of the Hexoskin Wearable Vest during Lying, Sitting, Standing, and Walking Activities. Appl PhysiolNutrMetab 2015; 40:1019-1024. doi: 10.1139/apnm-2015-0140 [Crossref] [ Google Scholar]
- Dadosky A, Overbeck H, Barbetta L, Bertke K, Corl M, Daly K. Telemanagement of Heart Failure Patients Across the Post-Acute Care Continuum. Telemed J E Health 2018; 24:360-366. doi: 10.1089/tmj.2017.0058 [Crossref] [ Google Scholar]
- Bandodkar AJ, Jeerapan I, Wang J. Wearable Chemical Sensors: Present Challenges and Future Prospects. ACS Sensors 2016; 1:464-482. doi: 10.1021/acssensors.6b00250 [Crossref] [ Google Scholar]
- Heikenfeld J. Non-Invasive Analyte Access and Sensing through Eccrine Sweat: Challenges and Outlook circa 2016. Electroanalysis 2016; 28:1242-1249. doi: 10.1002/elan.201600018 [Crossref] [ Google Scholar]
- Koh A, Kang D, Xue Y, Lee S, Pielak RM, Kim J. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci Transl Med 2016; 8:366ra165. doi: 10.1126/scitranslmed.aaf2593 [Crossref] [ Google Scholar]
- Sonner Z, Wilder E, Gaillard T, Kasting G, Heikenfeld J. Integrated sudomotor axon reflex sweat stimulation for continuous sweat analyte analysis with individuals at rest. Lab Chip 2017; 17:2550-2560. doi: 10.1039/c7lc00364a [Crossref] [ Google Scholar]
- Imani S, Bandodkar AJ, Mohan AMV, Kumar R, Yu S, Wang J. a wearable chemical-electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat Commun 2016; 7:1-7. doi: 10.1038/ncomms11650 [Crossref] [ Google Scholar]
- Gao W, Emaminejad S, Nyein HYY, Challa S, Chen K, Peck A. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016; 529:509-514. doi: 10.1038/nature16521 [Crossref] [ Google Scholar]
- Nilghaz A, Bagherbaigi S, Lam CL, Mousavi SM, Cόrcoles EP, Wicaksono DHB. Multiple semi-quantitative colorimetric assays in compact embeddable microfluidic cloth-based analytical device (ΜCAD) for effective point-of-care diagnostic. Microfluid Nanofluidics 2015; 19:317-333. doi: 10.1007/s10404-015-1545-9 [Crossref] [ Google Scholar]
- Aydindogan E, Guler Celik E, Timur S. Paper-based analytical methods for smartphone sensing with functional nanoparticles: bridges from smart surfaces to global health. Anal Chem 2018; 90:12325-12333. doi: 10.1021/acs.analchem.8b03120 [Crossref] [ Google Scholar]
- Nilghaz A, Ballerini DR, Shen W. Exploration of microfluidic devices based on multi-filament threads and textiles: a review. Biomicrofluidics 2013; 7:051501. doi: 10.1063/1.4820413 [Crossref] [ Google Scholar]
- López-Marzo AM, Merkoçi A. Paper-based sensors and assays: a success of the engineering design and the convergence of knowledge areas. Lab Chip 2016; 16:3150-3176. doi: 10.1039/c6lc00737f [Crossref] [ Google Scholar]
- Evans D, Papadimitriou KI, Vasilakis N, Pantelidis P, Kelleher P, Morgan H, Prodromakis T. A novel microfluidic point-of-care biosensor system on printed circuit board for cytokine detection. Sensors (Basel) 2018; 18:1-14. doi: 10.3390/s18114011 [Crossref] [ Google Scholar]
- Nilghaz A, Wicaksono DHB, Gustiono D, Abdul Majid FA, Supriyanto E, Abdul Kadir MR. Flexible microfluidic cloth-based analytical devices using a low-cost wax patterning technique. Lab Chip 2012; 12:209-218. doi: 10.1039/c1lc20764d [Crossref] [ Google Scholar]
- Kuswandi B, Andriani N, Nugraha AS. Simple monitoring of pH and urea in whole blood using wearable smart woman pad. BioImpacts 2022; 12:43-50. doi: 10.34172/bi.2021.41 [Crossref] [ Google Scholar]
- Borshchevskaya LN, Gordeeva TL, Kalinina AN, Sineokii SP. Spectrophotometric Determination of Lactic Acid. J Anal Chem 2016; 71:755-758. doi: 10.1134/S1061934816080037 [Crossref] [ Google Scholar]
- Nielsen SS. Sodium Determination Using Ion-Selective Electrodes, Mohr Titration, and Test Strips. Cham: Springer; 2017. P. 161–170. 10.1007/978-3-319-44127-6_19.
- Levy GB. Determination of sodium with ion-selective electrodes. Clin Chem 1981; 27:1435-1438. doi: 10.1093/clinchem/27.8.1435 [Crossref] [ Google Scholar]
- Diamond D, Tomczak M, Barrett R, Porter A, Alizadeh A, McCaul M. A wearable patch for continuous monitoring of sweat electrolytes during exertion. Lab Chip 2018; 18:2632-2641. doi: 10.1039/c8lc00510a [Crossref] [ Google Scholar]
- Zhang Y, Guo H, Kim SB, Wu Y, Ostojich D, Park SH. Passive sweat collection and colorimetric analysis of biomarkers relevant to kidney disorders using a soft microfluidic system. Lab Chip 2019; 19:1545-1555. doi: 10.1039/c9lc00103d [Crossref] [ Google Scholar]
- Mu X, Xin X, Fan C, Li X, Tian X, Xu KF. A Paper-Based Skin Patch for the Diagnostic Screening of Cystic Fibrosis. Chem Commun 2015; 51:6365-6368. doi: 10.1039/c5cc00717h [Crossref] [ Google Scholar]
- Zucchini F, Giuliano Terme S. United States Patent S371 (c)( 1; 2016.
- Siddiqui MR, AlOthman ZA, Rahman N. Analytical Techniques in Pharmaceutical Analysis: A Review. Arabian Journal of Chemistry 2017: 10: S1409–S1421. 10.1016/j.arabjc.2013.04.016.
- Oncescu V, O’Dell D, Erickson D. Smartphone Based Health Accessory for Colorimetric Detection of Biomarkers in Sweat and Saliva. Lab Chip 2013; 13:3232. doi: 10.1039/c3lc50431j [Crossref] [ Google Scholar]
- Wicaksono DH, Syazwani IN, Ratnarathorn N, Sadir S, Shahir S, Ruckthong L. Cotton Fiber-Based Assay with Time-Based Microfluidic Absorption Sampling for Point-of-Care Applications. Bioanalysis 2019; 11:855-73. doi: 10.4155/bio-2018-0190 [Crossref] [ Google Scholar]
- Miller J, Miller J. Statistics and Chemometrics for Analytical Chemistry. 6th ed. Harlow: Pearson Education; 2010.
- Huang CT, Chen ML, Huang LL, Mao IF. Uric Acid and Urea in Human Sweat. Chin J Physiol 2002; 45:109-115. [ Google Scholar]
- Jadoon S, Karim S, Akram MR, Kalsoom Khan A, Zia MA, Siddiqi AR, Murtaza G. Recent developments in sweat analysis and its applications. Int J Anal Chem 2015; 2015:164974. doi: 10.1155/2015/164974 [Crossref] [ Google Scholar]