Bioimpacts. 13(4):333-346.
doi: 10.34172/bi.2022.24209
Original Article
Combination of pioglitazone and dendritic cell to optimize efficacy of immune cell therapy in CT26 tumor models
Samaneh Tokhanbigli 1, #  , Helia Alavifard 1, #
, Helia Alavifard 1, #  , Hamid Asadzadeh Aghdaei 1, Mohammad Reza Zali 2, Kaveh Baghaei 1, *
, Hamid Asadzadeh Aghdaei 1, Mohammad Reza Zali 2, Kaveh Baghaei 1, * 
Author information:
1Basic and Molecular Epidemiology of Gastrointestinal Disorders Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2Gastroenterology and Liver Diseases Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran
#These authors contributed equally to this work.
Abstract
Introduction:
The maturation faith of dendritic cells is restrained by the inflammatory environment and cytokines, such as interleukin-6 and its downstream component. Therefore, introducing the suitable antigen to dendritic cells is crucial. However, reducing the severity of the suppressive tumor microenvironment is indispensable. The present study examined the combination therapy of lymphocyte antigen 6 family member E (LY6E) pulsed mature dendritic cells (LPMDCs) and pioglitazone against colorectal cancer (CRC) to elevate the effectiveness of cancer treatment through probable role of pioglitazone on inhibiting IL-6/STAT3 pathway.
Methods:
Dendritic cells were generated from murine bone marrow and were pulsed with lymphocyte antigen 6 family member E peptide to assess antigen-specific T-cell proliferation and cytotoxicity assay with Annexin/PI. The effect of pioglitazone on interleukin (IL)-6/STAT3 was evaluated in vitro by real-time polymerase chain reaction (PCR). Afterward, the CRC model was established by subcutaneous injection of CT26, mouse colon carcinoma cell line, in female mice. After treatment, tumor, spleen, and lymph nodes samples were removed for histopathological, ELISA, and real-time PCR analysis.
Results:
In vitro results revealed the potential of lysate-pulsed dendritic cells in the proliferation of double-positive CD3-8 splenocytes and inducing immunogenic cell death responses, whereas pioglitazone declined the expression of IL-6/STAT3 in colorectal cell lines. In animal models, the recipient of LPMDCs combined with pioglitazone demonstrated high tumor-infiltrating lymphocytes. Elevating the IL-12 and interferon-gamma (IFN-γ) levels and prolonged survival in lysate-pulsed dendritic cell and combination groups were observed.
Conclusion:
Pioglitazone could efficiently ameliorate the immunosuppressive feature of the tumor microenvironment, mainly through IL-6. Accordingly, applying this drug combined with LPMDCs provoked substantial CD8 positive responses in tumor-challenged animal models.
Keywords: Tumor-associated antigen, Dendritic cells, Thiazolidinediones, Colorectal cancer, Lymphocyte antigen 6 family member E, Pioglitazone
Copyright and License Information
© 2023 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
The compromised antigen-presenting procedure and immunosuppressive status of the tumor microenvironment are closely related. These two mechanisms are the pillars of the processes interfering with immune surveillance and antitumor responses.1,2
Immunotherapy utilizing immune cells has evolved as a mainstay of contemporary oncology. Recognition, up-taking, and presentation of cancer-related antigens to T cells are controlled by dendritic cells (DCs) functions in the context of provoking cytotoxic T-lymphocytes (CTLs) responses.3 Hence, in immune cell therapy by DCs, it is imperative to introduce an appropriate tumor antigen to DCs.4,5 Identifying an overexpressed surface antigen is the first vital step in DC therapy.6,7 Nonetheless, many efforts have been conducted using DC loading with tumor lysates to provoke the CTL responses against tumor cells, considered blinded and non-specific strategies.8-10
The previous microarray-based survey indicated that the expression of lymphocyte antigen6 E (LY6E) is highly upregulated in two potentially lethal gastrointestinal (GI) cancers: those of colon and stomach, as well as pancreatic cancers.11,12 Evidence has evolved to support Lymphocyte antigen 6 family member E (LY6E) overexpression with worse overall survival of patients and gastric cancer (GC) with cancer cell survival, proliferation, and migration.13 In addition, the recent DC therapy-based study by LY6E verifies the efficiency of introducing designed LY6E peptides for the major histocompatibility complex (MHC) class I and II. At the same time, DCs exhilarate the CD8 positive immune responses against colorectal models.14
Immune cell therapy is one of the hopes of cancer treatment. Nevertheless, subduing the immunosuppressive state of tumor microenvironments would amend the efficacy of cancer immunotherapy.15 Interleukin (IL)-6 signaling and its downstream component, signal transducer, and activator of transcription 3 (STAT3) are generally assumed as a malevolent player in the tumor microenvironment, especially colorectal cancer (CRC).16 Conversely, the dark visage of IL-6 is mediated by STAT3 phosphorylation, followed by transcribing genes involved in tumor progression by inducing cell proliferation, differentiation, and survival.17 On the other hand, further studies demonstrated that the hyperactivation of the IL-6 axis in tumor microenvironments not only impairs the DC maturation but also promotes tumor formation by suppressing the differentiation of interferon-gamma (IFN-γ)18,19 and IL-1220,21 producing T helper type 1 (Th1) cells. Therefore, blockade of this signaling axis could be a promising approach to overcome the dysfunctionality of DCs and T cell-mediated immune responses.
As a thiazolidinedione family, pioglitazone is a specific peroxisome proliferator-activated receptor gamma (PPAR-γ) agonist. Previous studies have demonstrated that pioglitazone as a potent agonist of PPARγ could inhibit the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and IL-6 axis and consequently form cancer stem cells spheroids in breast cancer cells.22 In animal models, another study on breast cancer in vitro and in vivo revealed that administrating pioglitazone could inhibit tumor growth through the Janus kinase 2/STAT3 pathway. Moreover, the administration of pioglitazone exhibited antitumor and antimetastatic effects on pancreatic cell lines and xenograft models.23
Hence, LY6E as a common overexpressed antigen in CRC could fulfill the objective of multipurpose therapeutic anti-cancer strategies. This research devised a DC therapy based on a tumor-associated antigen (TAA)-peptide approach to provoke an effective CTL response against CRC animal models. Whereas IL-6 negatively regulates DC function toward tolerogenic DCs, the current study administered pioglitazone simultaneously to overcome the obstacles in the way of LY6E loaded DC therapy against CRC by suppressing IL-6/STAT3 signaling.
Materials and Methods
Animals
Female BALB/c mice, 6-8 weeks old, were purchased from the Lab Animal Center, Pasteur Institute (Tehran, Iran). They were divided into five experimental groups and maintained under similar conditions with 12 hours dark/light cycle and free access to standard rodent chow and water.
Animal care and processing were performed in conformity with the guidelines of animal experiments by the Ethics Committee of the Research Institute for Gastroenterology and Liver Diseases (RIGID) and Shahid Beheshti Medical University (SBMU) (Code of Ethics: IR.SBMU.RIGLD.REC.1398.019).
Effect of pioglitazone on HT29 and CT26 cell lines viability
HT29 human (ATCC® HTB-38TM) and CT26 mouse colon carcinoma (ATCC® CRL-2638TM) were cultivated in complete RPMI (Biosera, France) containing 10% FBS (Gibco, USA) and 1% Penicillin/streptomycin (Gibco, USA) in a humidified atmosphere of 5% CO2 at 37°C. MTT assay was performed to determine the appropriate drug dose. Briefly, after 70-80% confluency, each two-cell line were plated in 96-well culture plates (5000 cells/200 µL/well), rested overnight in a CO2 incubator. After 24 hours, treatment was given in respective concentrations, 10, 50, 100, 150, 200, 250, and 300 µM of pioglitazone for 24, 48, and 72 hours. Further, at each time point, the medium was replenished with a medium containing 10% MTT solution (Sigma, USA), and the cells were incubated for a further 4 hours. Finally, the medium was removed, and formazan crystals formed by the viable cells were dissolved in 200 µl of dimethyl sulfoxide (DMSO). An ELISA reader measured the absorbance at 570 nm with a reference wavelength of 630 nm. Consecutively, the half-maximal inhibitory concentration (IC50) was estimated.
RNA isolation and quantification of IL-6 and STAT3 gene expression
To determine the effect of pioglitazone on the IL-6/STAT3 pathway in vitro, the HT29 and CT26 cell lines were treated with 250 µM of pioglitazone for 48 hours. Using RNA Purification Mini Kit (FavorGen, Taiwan), total mRNA was extracted and analyzed for concentration and purity. The same procedure was carried out for tumor tissue. Subsequently, RNAs were utilized for cDNA synthesis, using RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, USA) and applied to the real-time polymerase chain reaction (PCR) (Applied Biosystems). The NCBI Reference Sequence Database were used to access available mRNA sequences for mouse STAT3, human STAT3, mouse IL-6, human IL-6, HPRT as mice housekeeping and GAPDH as human housekeeping. The primer pairs were designed by Primer3 software (Table 1). The gene expression was normalized against the housekeeping gene GAPDH mRNA expression. In animal tissue, the relative expression of IL-6 and STAT3 gene expression was determined by normalizing against the housekeeping gene mouse HPRT.
Table 1.
Primer sequences for evaluating gene expressions with real-time polymerase chain reaction analysis
|
Target genes
|
Oligonucleotide sequence (5’-3')
|
Gene ID
|
|
IL-6 (Homo sapiens) |
F: GATTCAATGAGGAGACTTGCC
R: GGTCAGGGGTGGTTATTGC |
3569 |
|
IL-6 (mus musculus) |
F: GATACCACTCCCAACAGACCT
R: GCCATTGCACAACTCTTTTCTC |
16193 |
|
STAT3 (Homo sapiens) |
F: ATTGACAAAGACTCTGGGGAC
R: CCCTCAGGGTCAAGTGTTTG |
6774 |
|
STAT3 (mus musculus) |
F: CAAAGAAAACATGGCTGGCA
R: TGAAACCCATGATGTACCCTT |
20848 |
|
GAPDH (Human housekeeping) |
F: TGAAGGTCGGAGTCAACGGATTTGGT
R: CATGTGGGCCATGAGGTCCACCAC |
2597 |
|
HPRT (Mice housekeeping) |
F: GTCCCAGCGTCGTGATTAGC
R: TCGAGCAAGTCTTTCAGTCCT |
3251 |
Differentiation and peptide pulsing of bone marrow-derived DCs
DCs were generated as previously described.24 Briefly, bone marrow was harvested from BALB/c mice femurs and tibiae. A single-cell suspension was obtained by passing cells through a 70 μm cell strainer. Bone marrow-derived DCs (2 × 106 cells/well) were cultured in non-tissue treated six-well plates with complete RPMI containing 10% FBS, 1% Penicillin/streptomycin, supplemented with 250 U/ml granulocyte-macrophage colony-stimulating factor (GM-CSF), 250 U/mL IL-4 (Peprotech, USA), 1% L-glutamine (Gibco, USA), and 50 µm 2-mercaptoethanol (Sigma-Aldrich, UK). On day 3, a fresh medium containing the supplements was added to each well, and on day 6, half of the medium was centrifuged. The cell pellet was dissolved in a fresh medium supplemented with cytokines. At this time point, the microscopic characteristics of DCs and their colony were detectable under the microscope.
To induce DC maturation, on day 9, generated DCs was treated with 100 ng/mL lipopolysaccharide (Sigma-Aldrich, UK), and the culture incubation was continued overnight. The next day DCs were harvested by pipetting and extensively washed with PBS and were incubated with previously designed peptide at a concentration of 10 µg/mL for 3-4 hours at 37°C.24
Lymphocyte proliferation assay
To measure proliferation, the splenocytes (SPLs) of BALB/c mice were labeled independently with CFSE (©CellTraceTM Cell Proliferation Kit, Thermo Fisher Scientific). Isolated SPL cells (1×106 cells/mL) by Ficoll-Paque PLUS (GE Healthcare, Life Sciences) were incubated with CFSE for 20 minutes at 37°C in the dark. After the incubation time, the culture medium containing 1% protein bovine serum albumin (BSA) was used for quenching the dye. The SPLs were co-cultured with the matured and LY6E pulsed DCs at a ratio of 10:1 (splenocyte: DC). After 96 hours, cells were collected and analyzed by flow cytometry according to the manufacturer’s instructions (BD FACScan flow cytometry system, San Jose, CA, USA).
DCs, and T cell characterization
For morphological examination of DCs, cells were washed, centrifuged, and fixed on microscope slides with methanol for 10 minutes. Subsequently, the cells were stained with Giemsa at 1:20 (Merck, Germany) for 30 minutes; the Giemsa solution was rinsed off by washing in PBS. To evaluate the phenotype of generated immature and mature DCs, CD40, CD11c, CD80 antibodies were used. After stimulation of SPLs with the matured and lymphocyte antigen 6 family member E (LY6E) pulsed mature dendritic cells (LPMDCs) in vitro and infiltration of T cells in tumor sites of treated animals, the population of CD3, CD4, CD8 were evaluated by flow cytometry. T-cell characterization was analyzed in vitro and in vivo after stimulation with matured DCs and their expression markers. All the utilized antibodies are listed in Table 2.
Table 2.
Antibodies used in flow cytometry assay
|
Antibody
|
Label
|
Producer
|
Cat No.
|
| CD40 |
FITC-conjugated |
eBioscience (Germany) |
102905 |
| CD11c |
FITC-conjugated |
BioLegend (USA) |
117305 |
| CD8 |
FITC-conjugated |
BioLegend (USA) |
100707 |
| CD80 |
PE-conjugated |
eBioscience (Germany) |
104707 |
| CD4 |
PE-conjugated |
BioLegend (USA) |
100509 |
| CD3 |
PreCP-Vio700 |
BioLegend (USA) |
100217 |
CRC model establishment and therapeutic experiments
The ectopic xenograft mouse model of CRC is established by CT26 cells subcutaneously injection in vivo. The CT26 was routinely cultured in RPMI-1640 medium containing 10% FBS and 1% penicillin/streptomycin at 37°C in a 5% CO2 incubator. Tumors were generated by subcutaneous injection of 1 × 106 CT26 cell /mouse into the right flank areas of adult BALB/c female mice. The mice were examined weekly to evaluate tumor development for 7-10 days until the tumor became palpable and measurable. The desired tumor volume for starting the treatment was considered 150-200 mm3 approximately.25
Therapeutic experiments
Animals were randomly allocated into five treatment groups, and each group comprised seven mice as follows: 1) control- phosphate-buffer saline (PBS) treated; 2) LPMDC; 3) pioglitazone-treated; 4) combination therapy treated (LPMDC + pioglitazone); 5) mature dendritic cell (MDC) treated (Fig. 1).
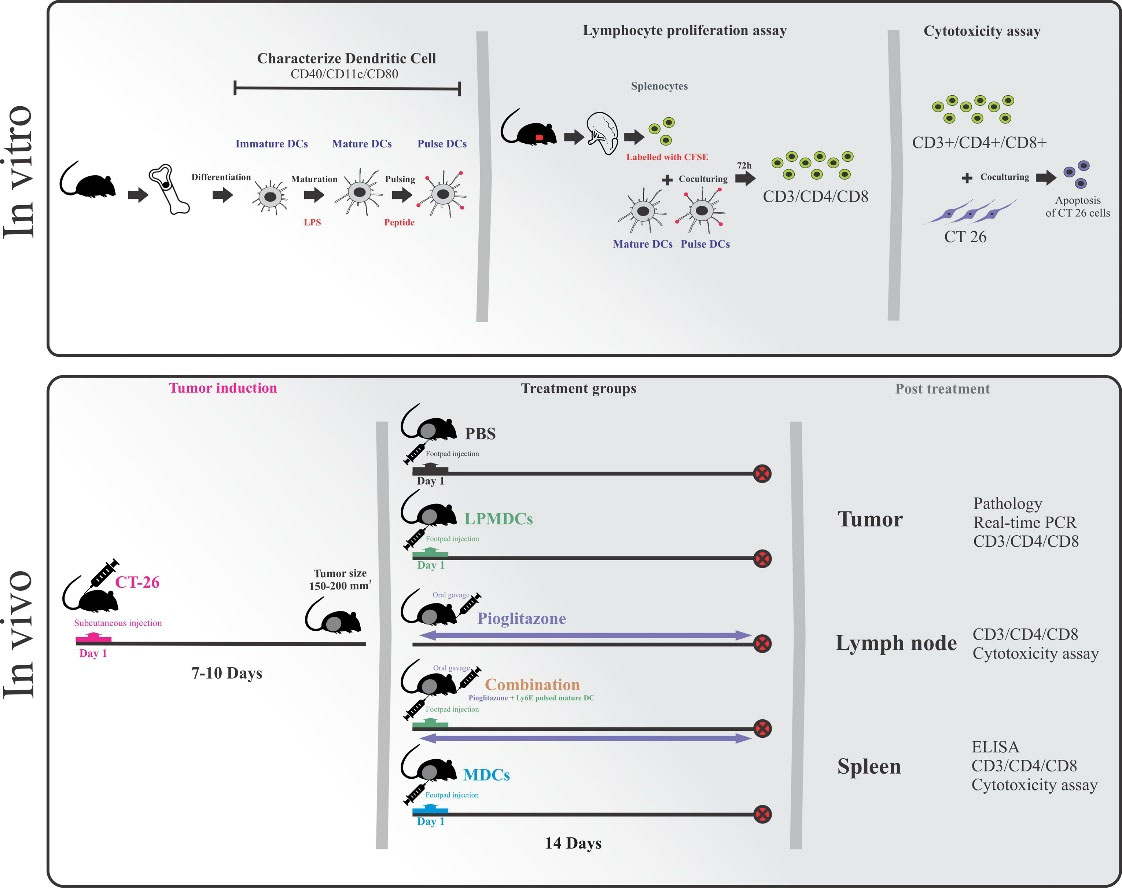
Fig. 1.
Schematic presentation of in vitro and in vivo experiments. Bone marrow-derived dendritic cells were matured and pulsed with designed LY6E peptides. The antigenic potential of LPMDCs and the cytotoxicity effect of primed SPLs were evaluated. The therapeutic potential of LPMDC and pioglitazone as monotherapy and in combination in CT26 challenged BALB/c mice (n= 7 mice in each group) as CRC models were assessed in vivo. PBS and mature DC treated groups were considered as control groups. After 14 days of treatment, all mice were anesthetized, and the tumor, lymph nodes, and spleen were extracted for further analyses. LPMDCs, LY6E pulsed mature DCs; CRC, colorectal cancer; PBS, phosphate-buffer saline; DC, dendritic cell; MDCs, mature DCs; combination, pioglitazone + LPMDCs.
.
Schematic presentation of in vitro and in vivo experiments. Bone marrow-derived dendritic cells were matured and pulsed with designed LY6E peptides. The antigenic potential of LPMDCs and the cytotoxicity effect of primed SPLs were evaluated. The therapeutic potential of LPMDC and pioglitazone as monotherapy and in combination in CT26 challenged BALB/c mice (n= 7 mice in each group) as CRC models were assessed in vivo. PBS and mature DC treated groups were considered as control groups. After 14 days of treatment, all mice were anesthetized, and the tumor, lymph nodes, and spleen were extracted for further analyses. LPMDCs, LY6E pulsed mature DCs; CRC, colorectal cancer; PBS, phosphate-buffer saline; DC, dendritic cell; MDCs, mature DCs; combination, pioglitazone + LPMDCs.
DCs (1×106 cells) were injected on the footpad area in three treated groups. The pioglitazone-treated group received 30mg/kg pioglitazone doses by oral gavage once a day for 14 days. Tumor length and width were measured every three days, and the percentages of inhibition in the growth of the tumors at each treatment group were calculated, respectively. On day 14, mice were euthanized using 100% carbon dioxide (CO2), and the tumor tissues were excised and weighed, and the spleen and lymph nodes were removed. The collected samples were subjected to histopathological, ELISA, Real-Time PCR analysis, and cytotoxicity assay.
Histopathological examination
To assess the presence of lymphocytes in tumor tissues, histopathological studies were conducted, the excised tumors from different groups were fixed in 10% paraformaldehyde and embedded in paraffin. Sections were stained with hematoxylin-eosin (H&E) staining using a standard protocol.
ELISA
Spleens were collected from all groups for assessing the cytokine responses. The SPLs were seeded at 96-well plates. They were stimulated with phytohaemagglutinin (PHA) (2% v/v), tumor lysate (30 µg/mL), and peptide (30 µg/mL) separately and incubated at 37 °C in 5% CO2. Unstimulated samples were prepared as the control group in the other wells. Cell culture supernatants were used for the estimation of cytokine production. The levels of IL-12 and INF-γ were assessed using the relevant ELISA kits (R&D Systems Inc., Minneapolis, USA) following the manufacturers' instructions. Briefly, 50 µL of Assay Diluent RD-14 and 50 µL of control and samples were added into ELISA plates and incubated for 2 hours at room temperature. The wells were aspirated and washed with 400 µL of wash buffer. After that, the wells were incubated for 2 hours at room temperature with 100 µL of mouse IL-12 p70 conjugate and mouse IFN-γ conjugate separately. All samples were tested in triplicate, and the optical density was determined at 450 nm and 630 nm.
Cytotoxicity assay
CT26 cells at a density of 1×104 cell/well were seeded in 96-well plates as target cells. After 16 hours, the cells were exposed with proliferative lymphocytes as effector cells for 5 hours in vitro and 16 hours for SPLs of treated animals in a 5:1 effector-to-target (E/T) ratio. The unexposed cells were provided as controls in every experiment. Briefly, all cells were collected and washed with cold BioLegend's cell staining buffer. Subsequently, BioLegend's FITC Annexin V Apoptosis Detection Kit with propidium iodide solution (PI) were used according to the manufacturers' instructions. the cells were re-suspended in Annexin V Binding Buffer at a concentration of 0.25-1.0 × 10 cells/mL. Briefly, 100 µL of cells were re-suspended in 5 µL of FITC Annexin V, and 10 µL of PI were incubated for 15 minutes at room temperature (25°C). The cytotoxicity was analyzed using a flow cytometry technique.
Analysis
Quantitative data from three separate experiments were performed (GraphPad Software, USA) using the student’s t test method and two-way ANOVA. Kaplan-Meier curves analyzed survival. The following formula calculated the tumor growth inhibition (TGI) rate: 1-(the mean tumor volume of the treated group at the time t/ the mean tumor volume of the control group at the time t) ×100. Data are presented as the mean ± standard error of the mean, and P<0.05 indicated a statistically significant difference. Whereas all the obtained flow cytometry data were analyzed using Flowjo software.
Results
Appropriate concentration of pioglitazone which profoundly suppressed IL-6/STAT3 expression in CT26 and HT29 cell lines
The impact of pioglitazone as a PPAR-γ agonist on the IL-6/STAT3 circuit in CRC has remained undefined. Therefore, we examined the IC50 of two treated colorectal cell lines, CT26 and HT29 mouse and human, respectively, following the exposure to pioglitazone in various concentrations. MTT assay, the representative of viable cell numbers in any given sample, indicated that in CT26 and HT29 cell lines after 48 hours of treatment at the concentration of 250µM, half of the cells were detected alive (Fig. 2A). Therefore, the treated cells were harvested and applied to real-time PCR at the mentioned concentration and time point. IL-6 and STAT3 expression kinetics upon exposure to pioglitazone for 48 hours to the mentioned concentration induced a substantial decrease in mRNA levels. In the CT26 cell line, pioglitazone significantly suppressed the expression levels of IL-6 and STAT3 (P value=0.002 and 0.0048, respectively) compared with that observed in the untreated cells. Furthermore, 250µM pioglitazone-treatment in the HT29 cell line notably reduced the IL-6 and STAT3 expression compared to the untreated group (P value=0.0014 and 0.0038 respectively; Fig. 2B). These data highlight the probable effect of pioglitazone on the IL-6/STAT3 pathway in CRC cell lines.
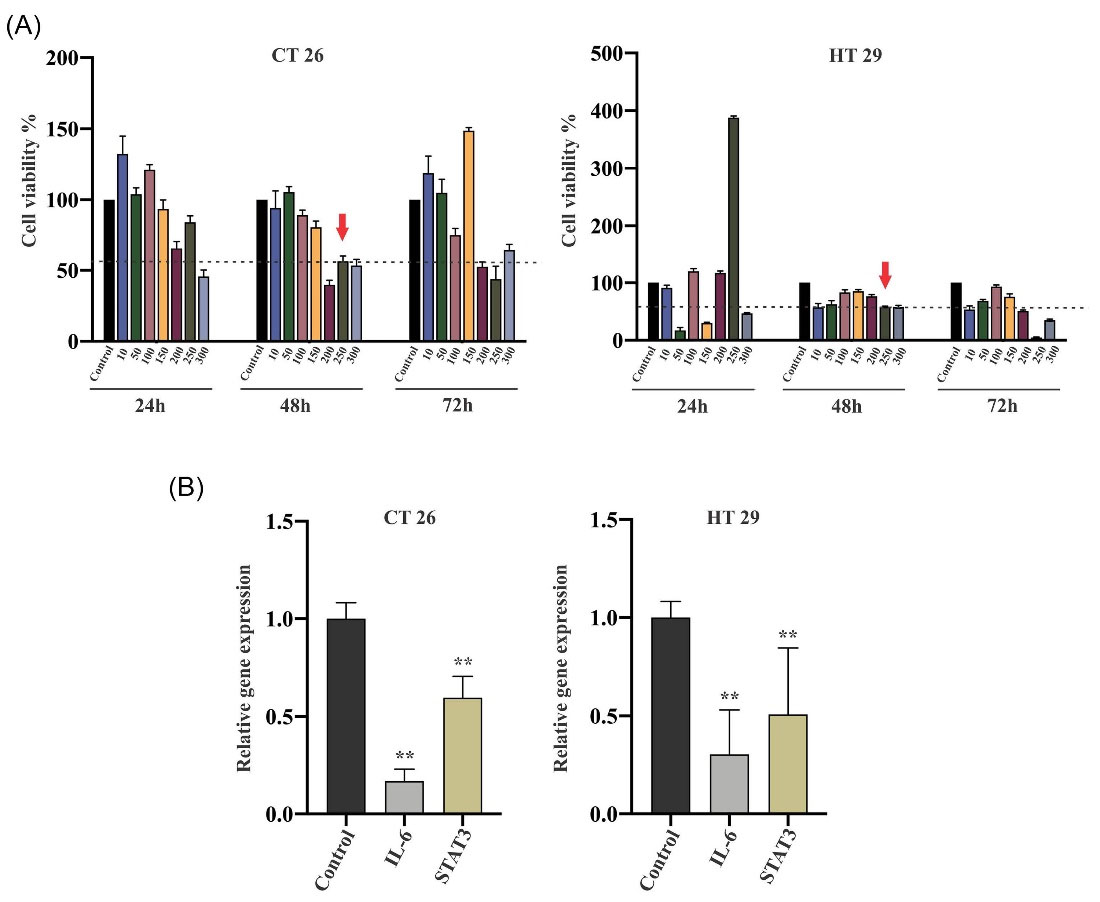
Fig. 2.
Effect of pioglitazone on colorectal cancer cell proliferation could be through the IL-6/STAT3 circuit. (A) The percentage of the cytotoxic effect of pioglitazone is determined by comparing the cell density of drug-treated cells to untreated controls. 50% of cells detected alive in CT26 and HT29 lines after 48 hours in 250µM drug concentration. (B) mRNA expression levels illustrated the inhibitory effect of pioglitazone on CT26 and HT29 cell lines. The data are representative of more than three independent experiments expressed as mean values ± SEM. Statistical data analysis was performed with the one-tailed student's t test. ** Indicates P <0.01 concerning untreated cells. IL, interleukin; STAT, signal transducer and activator of transcription; SEM, standard error of the mean.
.
Effect of pioglitazone on colorectal cancer cell proliferation could be through the IL-6/STAT3 circuit. (A) The percentage of the cytotoxic effect of pioglitazone is determined by comparing the cell density of drug-treated cells to untreated controls. 50% of cells detected alive in CT26 and HT29 lines after 48 hours in 250µM drug concentration. (B) mRNA expression levels illustrated the inhibitory effect of pioglitazone on CT26 and HT29 cell lines. The data are representative of more than three independent experiments expressed as mean values ± SEM. Statistical data analysis was performed with the one-tailed student's t test. ** Indicates P <0.01 concerning untreated cells. IL, interleukin; STAT, signal transducer and activator of transcription; SEM, standard error of the mean.
The proliferation and induced immunogenic cell death responses of LPMDCs in vitro confirmed the antigenic potential of the LY6E peptide
Staining with May-Grunwald and Giemsa and cell surface marker analysis in different stages are depicted in Fig. 3. According to established methods, acquired cellular morphology changes were distinguished in either adhered and suspended colonies under the microscope 72 hours after cytokine induction. Over time, dendritic protrusions were observed, and a typical MDC phenotype was detected 24 hours after lipopolysaccharide induction (Fig. 3A). Regarding the surface molecular expression, flow cytometry analysis confirmed the double-positive expression of CD80, CD11c, and CD80, CD40 after maturation (60% and 71.6% respectively; Fig. 3B). These results imply that the maturation process was successful with substantial efficiency.
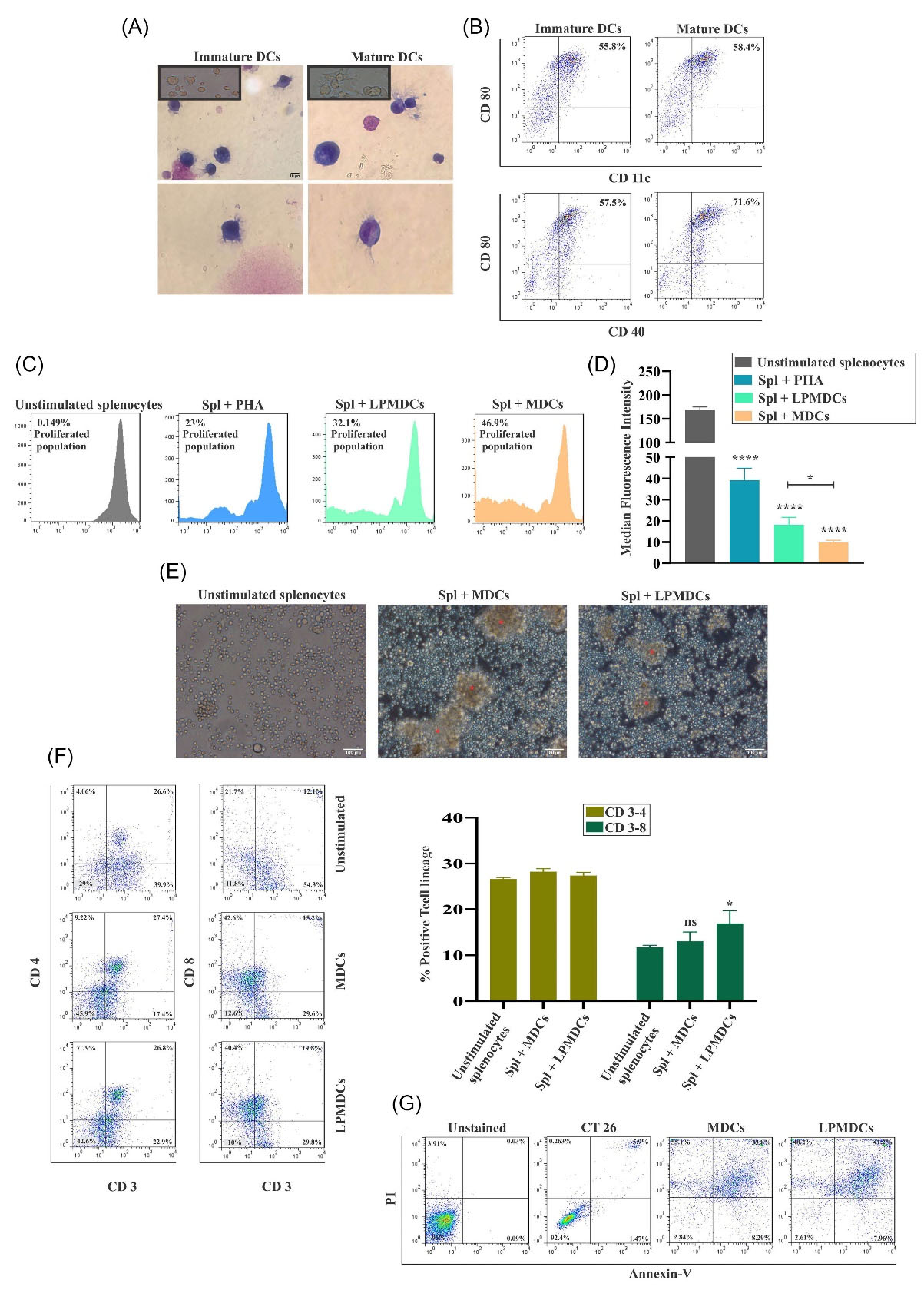

Fig. 3.
Confirming the generated LPMDC and the potential of primed SPLs with LPMDC eliciting strong immune responses
in vitro. (A) The morphology of the bone marrow-derived immature and MDCs stained with Giemsa under the light microscope, 100x magnification (scale bar represents 10 µm). (B) The surface molecules CD80 (PE), CD11c, and CD40 (FITC) were expressed at a high rate on bone marrow-derived MDCs. (C) Proliferated population of co-cultured CFSE stained SPLs with LPMDCs and MDCs for 72 hours confirmed the stimulation potency of LY6E. The ratio of reactive cells to stimulating cells was 10:1. PHA was exploited as a positive control. (D) The MFI statistic of stimulated SPLs illustrated significant proliferation in different groups concerning unstimulated SPLs. (E) Microscopic presentation of proliferating spl colonies (* in red) under an inverted microscope with ×20 magnification (scale bar represents 100 µm). (F) The primed SPLs were subjected to flow cytometry to measure the CD3-4 and CD3-8 DP population after 72 hours of co-culture with MDC and LPMDC. Concerning unstimulated SPLs, CD3-8 DP cells were statistically significant in LPMDCs. (G) Percentage of apoptotic cells indicated more significant apoptosis in the LPMDC group than the MDC group after 5 hours.The data are reported as mean values ± SEM for three independent experiments (n= 4 mice in each group). Statistical data analysis was performed with the one-tailed student's t test. ns not significant, * P < 0.05, and **** P < 0.0001. LPMDCs, LY6E pulsed mature DCs; Spls, splenocytes; MDCs, mature dendritic cells; CD, cluster of differentiation; PE, phycoerythrin; FITC, fluorescein isothiocyanate; CFSE, carboxyfluorescein succinimidyl ester; LY6E, lymphocyte antigen 6 family member E; PHA, polyhydroxyalkanoates; MFI, median fluorescence intensity; DP, double positive; SEM, standard error of the mean.
.
Confirming the generated LPMDC and the potential of primed SPLs with LPMDC eliciting strong immune responses
in vitro. (A) The morphology of the bone marrow-derived immature and MDCs stained with Giemsa under the light microscope, 100x magnification (scale bar represents 10 µm). (B) The surface molecules CD80 (PE), CD11c, and CD40 (FITC) were expressed at a high rate on bone marrow-derived MDCs. (C) Proliferated population of co-cultured CFSE stained SPLs with LPMDCs and MDCs for 72 hours confirmed the stimulation potency of LY6E. The ratio of reactive cells to stimulating cells was 10:1. PHA was exploited as a positive control. (D) The MFI statistic of stimulated SPLs illustrated significant proliferation in different groups concerning unstimulated SPLs. (E) Microscopic presentation of proliferating spl colonies (* in red) under an inverted microscope with ×20 magnification (scale bar represents 100 µm). (F) The primed SPLs were subjected to flow cytometry to measure the CD3-4 and CD3-8 DP population after 72 hours of co-culture with MDC and LPMDC. Concerning unstimulated SPLs, CD3-8 DP cells were statistically significant in LPMDCs. (G) Percentage of apoptotic cells indicated more significant apoptosis in the LPMDC group than the MDC group after 5 hours.The data are reported as mean values ± SEM for three independent experiments (n= 4 mice in each group). Statistical data analysis was performed with the one-tailed student's t test. ns not significant, * P < 0.05, and **** P < 0.0001. LPMDCs, LY6E pulsed mature DCs; Spls, splenocytes; MDCs, mature dendritic cells; CD, cluster of differentiation; PE, phycoerythrin; FITC, fluorescein isothiocyanate; CFSE, carboxyfluorescein succinimidyl ester; LY6E, lymphocyte antigen 6 family member E; PHA, polyhydroxyalkanoates; MFI, median fluorescence intensity; DP, double positive; SEM, standard error of the mean.
Furthermore, on functional testing, the dilution of CFSE dye overtime was used to illustrate the proliferation of SPLs co-cultured with LPMDCs. T cell proliferation is the result of the effective presentation of peptides by DCs. As presented in Fig. 3C, the increased population of SPLs in all groups was significant compared to unstimulated SPLs. LPMDCs co-cultured with SPLs induced 32.1% of the population to increase. Nonetheless, proliferation in SPLs adjacent to MDCs these data were higher with 46.9%. The Mean Fluorescence Intensity (MFI) graph analysis indicated that the MDCs had higher potency to proliferate the SPLs among other groups compared to their unstimulated counterpart (P value<0.0001; Fig. 3D). This difference between matured DC and LPMDCs illustrated significance in favor of MDC stimulated SPLs (P value=0.0171). Under the inverted microscope, the proliferating colonies of SPLs were detectable (Fig. 3E).
On the other hand, DCs are defined by the ability to present antigens to lymph node-resident naïve T-cells; therefore, after 4 days of co-culturing SPLs with MDCs and LPMDCs, they were stained with CD3, CD4, and CD8, the markers of T cells. As depicted in Fig 3F, both matured, and LPMDCs provoked the proliferation of CD4 and CD8 positive cells. The CD3-4 double-positive (DP) T cells in both MDCs and LPMDCs had no significant difference not compared to unstimulated SPLs nor in compared to each other. The target peptide is designed to present the antigen through the MHC-I pathway; only LPMDC stimulated SPLs with 19.8% demonstrated significant CD3-8 DP T cells compared to unstimulated SPLs. The MDC stimulated SPLs illustrated 15.2% CD3-8 DP T cells. These data indicated that the provoking MHCI peptide favors eliciting CD8-specific T cell responses more significantly among other groups (P-value=0.05; Fig. 3F). We next sought to confirm the cytotoxic responses against CT26 tumor cells generated by tumor antigen-primed T cells in vitro. The conducted cytotoxicity assay illustrated that 5 hours after co-culture of primed SPLs with CT26, 41.2% of CT26 cells were subjected to apoptosis in the stimulated SPLs with LPMDC, which was higher in contrast to stimulated SPLs with MDC with 33.8% (Fig. 3G).
Pioglitazone augmented the elicited anti-cancer responses by LY6E in tumor-bearing mice
Compelling evidence points to the clinical relevance of TILs at the tumor site and overall survival. Therefore, we evaluated the CD3-4 and CD3-8 DP T cells at the tumor site of different groups (Fig. 4A). Our data illustrated that the number of CD3-4 DP TILs at the tumor site in the combination group was higher among the other groups with 0.51% (P value =0.0042). Worth mentioning that the CD3-4 DP TILs in the recipient of LPMDCs only had a slight difference to the combination-treated group with 0.49%, which is statistically significant compared with the PBS group (P value =0.0357). In both the pioglitazone and MDC recipient, the CD3-4 TILs were 0.19% and 0.07%, respectively, insignificant with respect to the PBS group (P value =0.0798 and P value =0.243, respectively). The most critical T cells in tumor eradication are CD8 positive T cells. Therefore, these cells were quantified in the tumor.
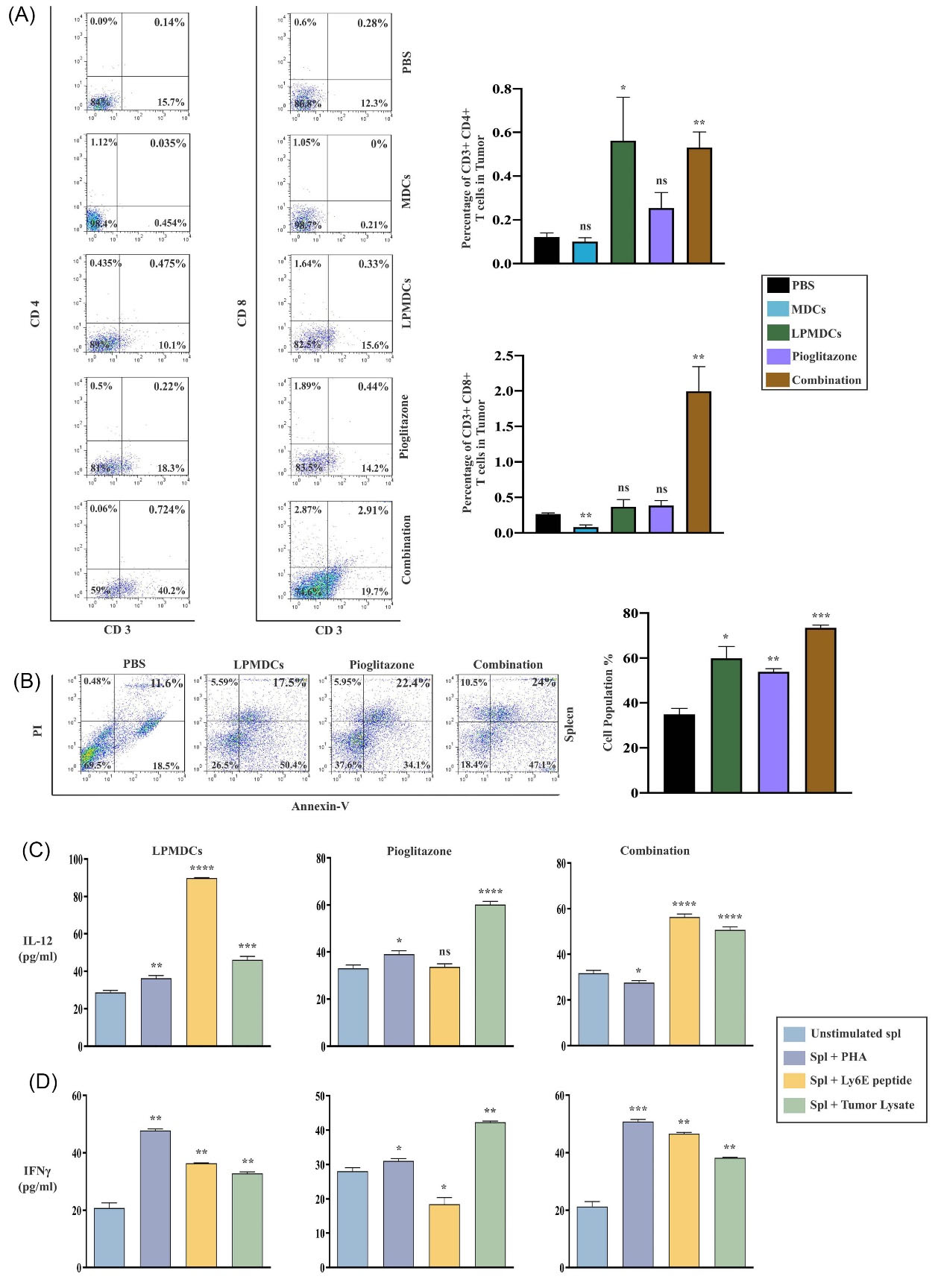

Fig. 4.
Pioglitazone, in combination with LPMDC, enhances the CTL responses, apoptosis, and inflammatory cytokines secretion. (A) CD3-4, CD3-8 DP T cells recruitment into tumor was measured in all treatment groups by flow cytometry and then compared to the PBS (control/untreated) group. (B) The CT26 cells were co-cultured with SPLs of tumor-bearing mice from different groups in a 1:10 ratio, respectively, for 16 hours. The most apoptotic cells were detected in the combination-treated group concerning PBS. Production of (C) IL-12 and (D) IFN-γ from SPLs after stimulation with PHA (positive control), LY6E peptide, and tumor lysate (to elicit specific responses). In both combination and LPMDC-treated groups, the elevation of IL-12 and IFN-γ were significantly increased after 72 hours concerning unstimulated SPLs indicating the successful treatment. The data are represented as the mean values ± SEM of four independent experiments (n = 4 mice in each group). Statistical data analysis was performed with the one-tailed Student's t-test. ns not significant, * P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001. LPMDCs, LY6E pulsed mature DCs; CTL, cytotoxic T lymphocytes; CD, cluster of differentiation; DP, double positive; PBS, phosphate-buffered saline; SPLs, splenocytes; IL, Interleukin; IFN-γ, interferon-gamma; PHA, polyhydroxyalkanoates; LY6E, lymphocyte antigen 6 family member E; SEM, standard error of the mean; n, number.
.
Pioglitazone, in combination with LPMDC, enhances the CTL responses, apoptosis, and inflammatory cytokines secretion. (A) CD3-4, CD3-8 DP T cells recruitment into tumor was measured in all treatment groups by flow cytometry and then compared to the PBS (control/untreated) group. (B) The CT26 cells were co-cultured with SPLs of tumor-bearing mice from different groups in a 1:10 ratio, respectively, for 16 hours. The most apoptotic cells were detected in the combination-treated group concerning PBS. Production of (C) IL-12 and (D) IFN-γ from SPLs after stimulation with PHA (positive control), LY6E peptide, and tumor lysate (to elicit specific responses). In both combination and LPMDC-treated groups, the elevation of IL-12 and IFN-γ were significantly increased after 72 hours concerning unstimulated SPLs indicating the successful treatment. The data are represented as the mean values ± SEM of four independent experiments (n = 4 mice in each group). Statistical data analysis was performed with the one-tailed Student's t-test. ns not significant, * P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001. LPMDCs, LY6E pulsed mature DCs; CTL, cytotoxic T lymphocytes; CD, cluster of differentiation; DP, double positive; PBS, phosphate-buffered saline; SPLs, splenocytes; IL, Interleukin; IFN-γ, interferon-gamma; PHA, polyhydroxyalkanoates; LY6E, lymphocyte antigen 6 family member E; SEM, standard error of the mean; n, number.
Interestingly, compared to the PBS-treated group, CD3-8 DP TILs in the combination group demonstrated greater frequency in dissected tumors with approximately 3% concerning the PBS group (P value =0.0078). In addition, CD3-8 DP TILs were increased in LPMDC and pioglitazone-treated groups with 0.36% and 0.38%, respectively. Nevertheless, this increase was not statistically meaningful. Contrary to in vitro results, the MDC-treated group indicated shallow and inconsequential infiltration of CD3-8 DP TILs with 0.08%.
Furthermore, the cytotoxic efficacy of elicited CTL responses in further confrontation with tumor cells was evaluated in vitro. Harvested SPLs from the combination group induced the highest percentage of early-late apoptotic cells (71.1 %) above the other groups. The detected apoptotic cells in pioglitazone and LPMDC-treated groups were 56.5% and 67.9 %, respectively. These results verified that the combination of pioglitazone with LPMDC significantly increased the susceptibility of CT26 cells to apoptosis in contrast to other treated groups (Fig. 4B).
Moreover, the levels of IL-12 and IFN-γ as the inflammatory cytokines were studied in SPLs of groups subjected to treatment. Therefore, SPLs were co-cultured with tumor antigen peptide, LY6E, and CT26 extracted tumor lysate. In this regard, after adding LY6E peptide, IL-12 production in combination and LPMDC-treated groups elevated significantly (P value <0.0001). In combination and LPMDC-treated groups addition of tumor lysate substantially stimulated SPLs to produce IL-12 (P value <0.0001 and P value = 0.0002, respectively). However, in the pioglitazone group, only tumor lysate stimulated SPLs for IL-12 production (P value <0.0001) (Fig. 4C). The SPLs of combination and LPMDC-treated groups showed greater IFN-γ production in response to the addition of LY6E peptide (P value <0.0013 and P value = 0.0016; respectively). Nonetheless, the LY6E peptide did not induce IFN-γ production in SPLs from the pioglitazone-treated group. IL-12 and IFN-γ levels significantly raised after tumor lysate treatment. Still, as expected, no enhancement of IL-12 and IFN-γ was observed in response to the LY6E peptide in the pioglitazone-treated group (Fig. 4D).
The density of tumor-infiltrating immune cells in combination group indicated the provoked immune responses and superior tumor growth inhibition effect
The presence and compactness of tumor-infiltrating immune cells in H&E-stained sections were examined to evaluate the efficiency of LPMDC in combination with pioglitazone. Regardless of the lymphocyte subsets, the density of infiltrated immune cells in H&E-stained sections in the combination group was strongly positive with a dense population. Although in LPMDC and pioglitazone-treated groups, the infiltrated cells were lower than the combination group, the presence of mononuclear immune cells was substantial. These results suggest the successful antitumor immune responses elicited by the treatments. In both MDC and PBS treated groups, the presence of immune cells was hardly detectable (Fig. 5A).
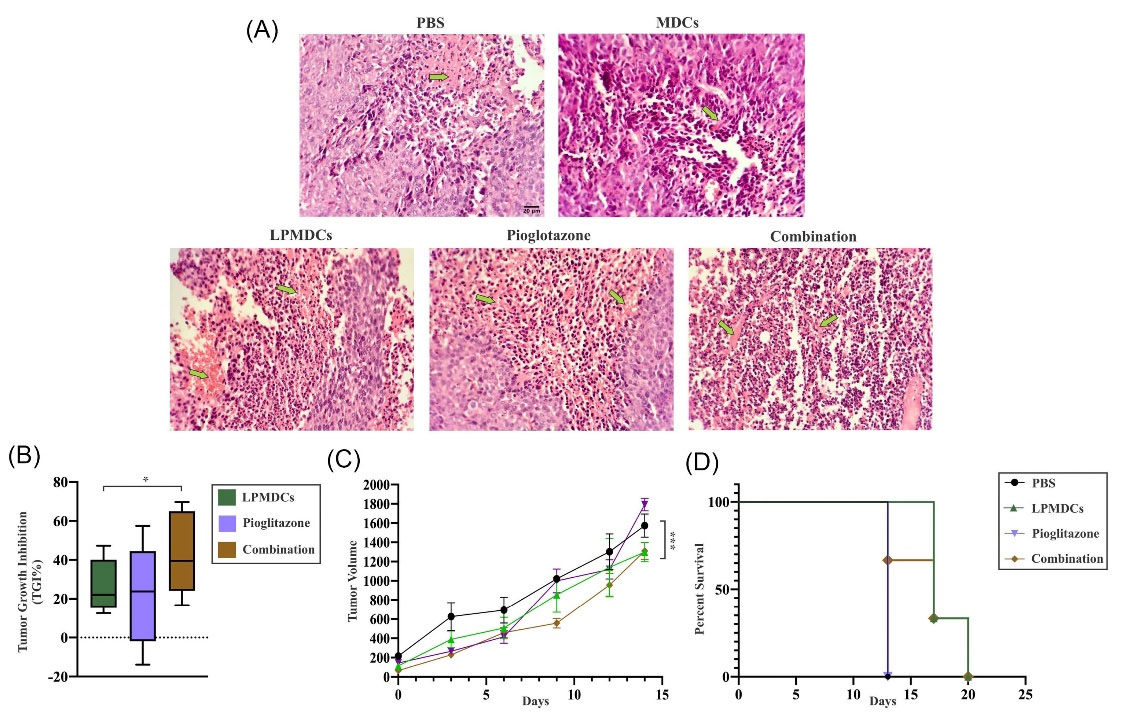
Fig. 5.
H&E sections, TGI, tumor volume, and survival rates of treated groups. (A) The presence and density of tumor-infiltrating immune cells in CT26 induced tumors of treated groups (n= 4 mice in each group) was detectable and abundant. Light Microscopic presentation of infiltrated immune cells at tumor sites with 20x magnification (Scale bar represents 20 µm). Green arrows showed necrotic areas. (B, C, D) The indication of data implies that LPMDC and pioglitazone in combination significantly inhibited the growth of the induced tumor with greater prolonged survival. Tumor-bearing mice were sacrificed due to the 2000 mm3 tumor volume limitation at different time points. The data are represented as the mean values ± SEM of three independent experiments (n = 3 mice in each group). Statistical data analysis was performed with the two-way ANOVA. * P < 0.05, *** P < 0.001.
.
H&E sections, TGI, tumor volume, and survival rates of treated groups. (A) The presence and density of tumor-infiltrating immune cells in CT26 induced tumors of treated groups (n= 4 mice in each group) was detectable and abundant. Light Microscopic presentation of infiltrated immune cells at tumor sites with 20x magnification (Scale bar represents 20 µm). Green arrows showed necrotic areas. (B, C, D) The indication of data implies that LPMDC and pioglitazone in combination significantly inhibited the growth of the induced tumor with greater prolonged survival. Tumor-bearing mice were sacrificed due to the 2000 mm3 tumor volume limitation at different time points. The data are represented as the mean values ± SEM of three independent experiments (n = 3 mice in each group). Statistical data analysis was performed with the two-way ANOVA. * P < 0.05, *** P < 0.001.
On the other hand, to evaluate the effect of treatment on induced tumors, the TGI and survival rate were investigated. The inhibitory effect of tumor growth in LPMDC combined with pioglitazone was higher than the other groups (P value = 0.0357) (Fig. 5B). Moreover, the combination and LPMDC-treated groups showed a decrease in their tumor volumes compared to the control group at the end of the experiment (P value = 0.0004) (Fig. 5C). Furthermore, the two mentioned groups also had a prolonged survival rate compared to the PBS group. Based on the 2000 mm3 tumor volume limitation, all the mice in all the groups were euthanized at different time points (Fig. 5D). These data highlight that LPMDC combined with pioglitazone demonstrated a special effect in the excitation of immune responses against CRC.
Pioglitazone alleviated the IL-6 expression at tumor site
Regarding the suppressive effect of pioglitazone on the IL-6/STAT3 pathway in vitro, dissected tumors in combination and pioglitazone administrated groups were subjected to Real-Time PCR. The results demonstrated that pioglitazone diminished the expression levels of IL-6 in both combination (the recipient of LPMDCs and pioglitazone) and pioglitazone administrated groups (P value =0.011 and P value =0.012, respectively). Despite the in vitro results, the administration of pioglitazone did not reduce the expression levels of STAT3 in neither of the groups. In combination and pioglitazone-treated groups, the expression of STAT3 illustrated up-regulation (P value =0.0225 and 0.0103, respectively) (Fig. 6).
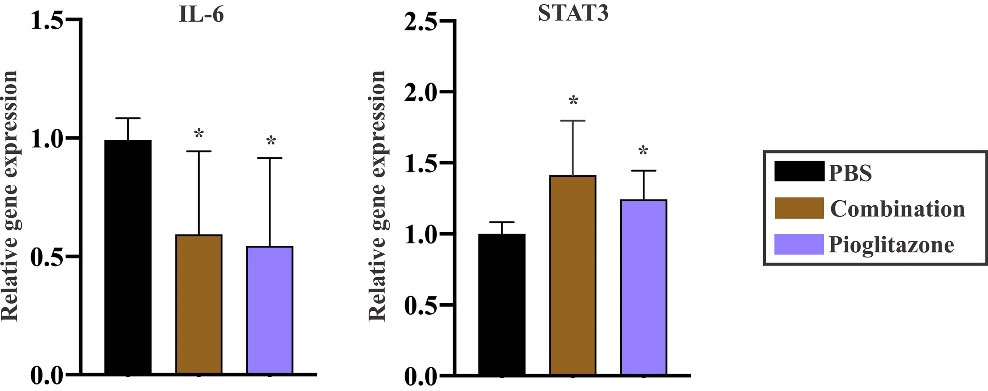
Fig. 6.
Effect of pioglitazone on regulation of IL-6 and STAT3 in CRC models. mRNA expression levels illustrated the inhibitory effect of pioglitazone on IL-6 in combination and pioglitazone administrated groups (n= 4 mice in each group). The data demonstrated that although pioglitazone restrained the expression of IL-6, it did not influence the expression of STAT3. The data are the mean ± SEM. Statistical data analysis was performed with the one-tailed Student's t-test, * P < 0.05 and ** P < 0.01 concerning the PBS group. IL, interleukin; CRC, colorectal cancer; combination, pioglitazone + LPMDCs; STAT, signal transducer and activator of transcription; SEM, standard error of the mean; PBS, phosphate-buffered saline.
.
Effect of pioglitazone on regulation of IL-6 and STAT3 in CRC models. mRNA expression levels illustrated the inhibitory effect of pioglitazone on IL-6 in combination and pioglitazone administrated groups (n= 4 mice in each group). The data demonstrated that although pioglitazone restrained the expression of IL-6, it did not influence the expression of STAT3. The data are the mean ± SEM. Statistical data analysis was performed with the one-tailed Student's t-test, * P < 0.05 and ** P < 0.01 concerning the PBS group. IL, interleukin; CRC, colorectal cancer; combination, pioglitazone + LPMDCs; STAT, signal transducer and activator of transcription; SEM, standard error of the mean; PBS, phosphate-buffered saline.
Discussion
Dendritic cell-based therapy aims to excite the CTL responses against the target tumor, primarily depending on introducing the appropriate TAA-peptide to DCs.26 The researchers previously reported that human and mice DC pulsed with LY6E activating MHC class I and II simultaneously elicited active and specific responses against CRC and GC cell lines in vitro and CRC challenged animal models in vivo.14,24 Overexpression of LY6E in patients of different cancers, namely CRC, and the indispensable role of LY6E in immune regulation, drug resistance, tumorigenesis, and poor survival has been proven.12,27
Many endeavors have been made to introduce a proper peptide to DC in treating cancers, from different clinical trials28-33 to FDA-approved sipuleucel-T for prostate cancer.34 Therefore, regarding the obtained results from our previous studies, this study improved the designed LY6E peptide specific for MHC class I to exclusively stimulate CD8 positive T cells.
In this regard, the obtained in vitro results confirmed the potential of LPMDCs in eliciting the CD8 positive T cells in proliferated naïve splenocytes and inducing the immunogenic cell death in the CT26 cell line. Nevertheless, the only inconsistency of the obtained in vitro results with the other reported studies is the increased population of naïve splenocytes co-cultured with MDCs and LPMDCs. Surprisingly, regardless of the proliferation results, the population of CD8 positive T-cell was markedly higher in naïve splenocytes co-cultured with LPMDCs concerning MDCs in the present study, indicating that LPMDC primes more CD8 positive T cells. In line with this study’s findings, DCs transduced with lentiviral vectors encoding full-length MAGE-A3 gene induced marked proliferation compared to the immature DC group. However, this difference was not significant in mature DCs and DCs expressing the MAGE-A3 gene.35 Furthermore, the proliferation results of another study revealed that the transfected DCs with a recombinant adeno-associated virus carrying the α-fetoprotein gene (rAAV/AFP) had a significant difference with increased T cells in the untreated DC group.36 However, these studies have concentrated on the proliferation capability of tumor lysate in inducing T cell proliferation concerning designed peptides.
Additionally, the in vitro results demonstrated that LPMDC induced the proliferating CD3-4 DP T cells in naïve splenocytes. Conversely, these proliferations were statistically insignificant. In line with these data in extracted splenocytes of treated groups with LPMDC and pioglitazone, the population of CD3-4 did not exhibit a significant increase (data not shown). It is proposed that CD4 TILs do not impact the patients’ survival, and only CD8 positive TILs are associated with immunological conditions of patients and prolonged survival.37 Notably, CD3-4 DP T cells were detected in tumor sites both in LPMDC and combination group with significant difference compared to the recipient of PBS group. Accordingly, the primed splenocytes with LPMDCs exerted more substantial immunogenic cell death in CT26 cells with 41%. Different studies based on peptide-pulsed DCs demonstrated less cytotoxic activity against their target tumors in vitro. The recent study showed that primed naïve splenocytes with placental gp96 loaded dendritic cells induced approximately 30% immunogenic cell death in melanoma and lung carcinoma cell lines in a 1:10 ratio.38 DCs transduced with CML28 encoding antigen-induced approximately 15% immunogenic cell death in HepG2 cell line. T cell priming with CML28 expressing DCs induced approximately 30% immunogenic cell death in antigen-positive HLA-I-matched cell lines, HepG2 and MCF-7, to confirm the HLA-restricted cell lysis.39 Therefore, compared to these studies, primed SPLs with LY6E exerted more robust immunogenic responses.
On the other side, to overcome the obstacle of DCs and T cells penetration inside the tumor, inhibition of IL-6/STAT3 by pioglitazone has been tailored for improving the current DC therapy. IL-6 is the critical regulator cytokine in the development and progression.16,40 In animal models, various studies have proven that IL-6 downregulates the IL-12 production from DCs in the tumor microenvironment in favor of attenuating CD4 T cell responses.21 Furthermore, utilizing the monoclonal antibodies against IL-6 receptor-induced tumor-specific responses inhibits tumor growth.41,42
Approvingly, the thiazolidinedione family inhibits the IL-6/STAT3 circuit in different diseases in vitro and in vivo. Therefore, the present study speculated that using pioglitazone to tackle the immunosuppressive effect of IL-6 improves the function of LPMDC at the tumor site. The in vitro results of the present study demonstrated the possibility that pioglitazone reduces the cell viability in CRC cell lines by downregulation of the IL6/STAT3 axis in mRNA levels. Which could be considered as additional target for pioglitazone. The restraining effect of pioglitazone on proliferation and inducing apoptosis in CRC and other cancers through PPAR𝛾 and different pathways have been described.23,43-45 In oral squamous carcinoma (HSC-3), prostate adenocarcinoma (PC-3), multiple myeloma (RPMI8226), chronic myelogenous leukemia (K562), and Gastric adenosquamous carcinoma (MKN1 and MKN45), cell lines treated with pioglitazone had an inhibitory effect on STAT3 and Survivin expression and inducing apoptosis.46 The capability of LPMDC in priming the CD8 positive T cells and the cytotoxic effect of these T cells against CT26 cell line, whereas the inhibitory potential of pioglitazone on the IL-6/STAT3 circuit were encouraging enough to extend these in vitro findings to the CRC pre-clinical models. It is generally accepted that dendritic cell-based immunotherapy's ultimate goal is to elicit type-1 helper immune responses and penetration of CD8 positive T cells into tumor sites. In CT26 induced CRC tumor models, this research demonstrated that although the CD8 positive T cells in the LPMDC group increased substantially, the presence of CD8 positive T cells at the tumor site markedly elevated in the combination-treated group. This significant difference emphasizesthat pioglitazone successfully alleviated the IL-6-induced immunosuppressive microenvironment in favor of the high infiltration of CD8 positive T cells at the tumor site. Hence, presumably combining the pioglitazone with DC therapy could increase the infiltrating primed T cells to the tumor site.
In line with this study’s results, the mRNA levels of IL-6 have statistically decreased in both combination and pioglitazone administrated groups highlighting the inhibitory effect of pioglitazone on IL-6. However, the pioglitazone did not extend the survival rate nor inhibit the tumor volume in the pioglitazone-treated group. Nonetheless, the increased survival rate and decrease in tumor volume were observed in LPMDC and combination groups. As strong immune responses were observed in the combination-treated group, the TGI was higher in this group. According to some reports, in xenograft models of cancers, administration of TZDs, namely pioglitazone, has abolished cell proliferation and tumor size up to 90% in lung cancer and decreased the number of aberrant cases of cryptal foci in colon cancer models.47-49 In line with the current study findings in the pioglitazone-treated group, TILs infiltration was also perceived into tumor sites.
Furthermore, the high levels of the IL-12 and IFN-γ cytokines in the LPMDC and combination group confirmed the potential of LY6E in extracting the CTL responses. The high density of TILs is the marker of enhanced antitumor immunity50 and is a sign of good prognosis in human cancers.51,52 The pathology results also agree with these findings in which the density of TILs was high in combination and the other treated groups compared to MDC and PBS administered ones. Moreover, at the end of in vivo experiment, the extracted splenocytes from the combination group exerted more cytotoxic effects on the CT26 cell line, indicating that combining DC therapy with pioglitazone improves the antitumor immune responses. Different reports suggest specific immune responses in tumor-challenged models to indicate successful DC-based vaccination. After immunizing mice with either MUC1-positive tumor cells or EphA2 derived peptide-pulsed dendritic cells, extracted splenocytes from CRC models induced immunogenic tumor cell death similar to our in vivo study.53,54 Although the splenocytes of immunized mice with only one administration of DC loaded with melanoma lysate exerted 10-20% cytotoxicity, confirming the strong elicited immune responses in this study with LY6E.55
In sum, the current research reports that pioglitazone could alleviate the immunosuppressive status of the tumor microenvironment, the major obstacle in the way of DC activation and consequently excitation of CTL responses against the target tumor. In the present study, combining pioglitazone with LY6E loaded DCs boosted the DC therapy effect and specific tumor responses against CRC models even with one dose of LPMDC administration, highlighting a new approach in immune cell therapy against cancers.
Conclusion
Based on the pooled results put forth herein, this study concludes that the major hurdle in the way of immune therapy is believed to be the TME. Therefore, tackling this problem seems crucial to fulfilling immune cell therapy's anticipated goals, i.e., DC therapy. The present study results revealed that DC therapy with LY6E combined with pioglitazone to overcome the immunosuppressive feature of TME through the IL-6/STA3 circuit increased the efficacy of LY6E based DC therapy against CRC by increasing the TILs in the tumor site and the survival rate. Furthermore, the exact mechanism of pioglitazone on this signaling requires more investigation.
Research Highlights
What is the current knowledge?
√ Dendritic cell-based therapy is promising approach to induce antigen-specific CTL responses in CRC patients.
√ IL‐6/STAT3 signaling pathway in tumor microenvironments reduce the maturation of DC and promotes tumor formation.
√ Targeting IL-6 may improve the efficacy of immunotherapies for cancer patients.
What is new here?
√ Combination therapy of LY6E pulsed DCs and pioglitazone has been successfully increased the effectiveness of colorectal cancer treatment by targeting IL-6/STA3 circuit.
Acknowledgments
The authors would like to thank the Research Institute of Gastroenterology and Liver Diseases of the Shahid Beheshti Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Competing interests
The authors declare no competing interests.
Ethical Statement
The Institutional Ethical Review Committee approved the study protocol of the Research Institute for Gastroenterology and Liver Diseases (RIGLD) and Shahid Beheshti University of Medical Sciences (SBMU) (Code of Ethics: IR.SBMU.RIGLD.REC.1398.019).
Funding
This study was financially supported by a research grant (1048) from the Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
- Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer 2014; 14:135-46. doi: 10.1038/nrc3670 [Crossref] [ Google Scholar]
- Pitt JM, Marabelle A, Eggermont A, Soria JC, Kroemer G, Zitvogel L. Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol 2016; 27:1482-92. doi: 10.1093/annonc/mdw168 [Crossref] [ Google Scholar]
- Durgeau A, Virk Y, Corgnac S, Mami-Chouaib F. Recent Advances in Targeting CD8 T-Cell Immunity for More Effective Cancer Immunotherapy. Front Immunol 2018; 9:14. doi: 10.3389/fimmu.2018.00014 [Crossref] [ Google Scholar]
- Palucka AK, Ueno H, Fay J, Banchereau J. Dendritic cells: a critical player in cancer therapy?. J Immunother 2008; 31:793-805. doi: 10.1097/CJI.0b013e31818403bc [Crossref] [ Google Scholar]
- Gardner A, Ruffell B. Dendritic Cells and Cancer Immunity. Trends Immunol 2016; 37:855-65. doi: 10.1016/j.it.2016.09.006 [Crossref] [ Google Scholar]
- Finn OJ. Human tumor antigens, immunosurveillance, and cancer vaccines. Immunol Res 2006; 36:73-82. doi: 10.1385/ir:36:1:73 [Crossref] [ Google Scholar]
- Parmiani G, De Filippo A, Novellino L, Castelli C. Unique human tumor antigens: immunobiology and use in clinical trials. J Immunol 2007; 178:1975-9. doi: 10.4049/jimmunol.178.4.1975 [Crossref] [ Google Scholar]
- Rainone V, Martelli C, Ottobrini L, Biasin M, Texido G, Degrassi A. Correction: immunological characterization of whole tumour lysate-loaded dendritic cells for cancer immunotherapy. Plos one 2016; 11:e0151008. doi: 10.1371/journal.pone.0146622 [Crossref] [ Google Scholar]
- Bachleitner-Hofmann T, Strohschneider M, Krieger P, Sachet M, Dubsky P, Hayden H. Heat shock treatment of tumor lysate-pulsed dendritic cells enhances their capacity to elicit antitumor T cell responses against medullary thyroid carcinoma. J Clin Endocrinol Metab 2006; 91:4571-7. doi: 10.1210/jc.2006-0971 [Crossref] [ Google Scholar]
- Hatfield P, Merrick AE, West E, O'Donnell D, Selby P, Vile R. Optimization of dendritic cell loading with tumor cell lysates for cancer immunotherapy. J Immunother 2008; 31:620-32. doi: 10.1097/CJI.0b013e31818213df [Crossref] [ Google Scholar]
- Baghaei K, Hosseinkhan N, Asadzadeh Aghdaei H, Zali MR. Investigation of a common gene expression signature in gastrointestinal cancers using systems biology approaches. Mol Biosyst 2017; 13:2277-88. doi: 10.1039/c7mb00450h [Crossref] [ Google Scholar]
- Luo L, McGarvey P, Madhavan S, Kumar R, Gusev Y, Upadhyay G. Distinct lymphocyte antigens 6 (Ly6) family members Ly6D, Ly6E, Ly6K and Ly6H drive tumorigenesis and clinical outcome. Oncotarget 2016; 7:11165. doi: 10.18632/oncotarget.7163 [Crossref] [ Google Scholar]
- Lv Y, Song Y, Ni C, Wang S, Chen Z, Shi X. Overexpression of Lymphocyte Antigen 6 Complex, Locus E in Gastric Cancer Promotes Cancer Cell Growth and Metastasis. Cell Physiol Biochem 2018; 45:1219-29. doi: 10.1159/000487453 [Crossref] [ Google Scholar]
- Tokhanbigli S, Asadirad A, Baghaei K, Piccin A, Yarian F, Parsamanesh G. Dendritic Cell-Based Therapy Using LY6E Peptide with a Putative Role Against Colorectal Cancer. Immunotargets Ther 2020; 9:95-104. doi: 10.2147/itt.S245913 [Crossref] [ Google Scholar]
- Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol 2007; 25:267-96. doi: 10.1146/annurev.immunol.25.022106.141609 [Crossref] [ Google Scholar]
- Waldner MJ, Foersch S, Neurath MF. Interleukin-6--a key regulator of colorectal cancer development. Int J Biol Sci 2012; 8:1248-53. doi: 10.7150/ijbs.4614 [Crossref] [ Google Scholar]
- Johnson DE, O'Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol 2018; 15:234-48. doi: 10.1038/nrclinonc.2018.8 [Crossref] [ Google Scholar]
- Tsukamoto H, Fujieda K, Hirayama M, Ikeda T, Yuno A, Matsumura K. Soluble IL6R Expressed by Myeloid Cells Reduces Tumor-Specific Th1 Differentiation and Drives Tumor Progression. Cancer Res 2017; 77:2279-91. doi: 10.1158/0008-5472.Can-16-2446 [Crossref] [ Google Scholar]
- Tsukamoto H, Nishikata R, Senju S, Nishimura Y. Myeloid-derived suppressor cells attenuate TH1 development through IL-6 production to promote tumor progression. Cancer Immunol Res 2013; 1:64-76. doi: 10.1158/2326-6066.Cir-13-0030 [Crossref] [ Google Scholar]
- Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 1993; 260:547-9. doi: 10.1126/science.8097338 [Crossref] [ Google Scholar]
- Ohno Y, Kitamura H, Takahashi N, Ohtake J, Kaneumi S, Sumida K. IL-6 down-regulates HLA class II expression and IL-12 production of human dendritic cells to impair activation of antigen-specific CD4(+) T cells. Cancer Immunol Immunother 2016; 65:193-204. doi: 10.1007/s00262-015-1791-4 [Crossref] [ Google Scholar]
- Papi A, Guarnieri T, Storci G, Santini D, Ceccarelli C, Taffurelli M. Nuclear receptors agonists exert opposing effects on the inflammation dependent survival of breast cancer stem cells. Cell Death & Differentiation 2012; 19:1208-19. doi: 10.1038/cdd.2011.207 [Crossref] [ Google Scholar]
- Ninomiya I, Yamazaki K, Oyama K, Hayashi H, Tajima H, Kitagawa H. Pioglitazone inhibits the proliferation and metastasis of human pancreatic cancer cells. Oncol Lett 2014; 8:2709-14. doi: 10.3892/ol.2014.2553 [Crossref] [ Google Scholar]
- Tokhanbigli S, Parsamanesh G, Baghaei K, Yarian F, Asadirad A, Hashemi SM. Antigenic Potency of LY6E in Stimulating Dendritic Cells to Elicit Tumor-Specific Responses Against Human Colorectal and Gastric Cancer Cell Lines. Int J Pept Res Ther 2021; 27:1001-8. doi: 10.2147/ITT.S245913 [Crossref] [ Google Scholar]
- Rezaei R, Baghaei K, Hashemi SM, Zali MR, Ghanbarian H, Amani D. Tumor-derived exosomes enriched by miRNA-124 promote anti-tumor immune response in CT-26 tumor-bearing mice. Front Med (Lausanne) 2021; 8. 10.3389/fmed.2021.619939.
- Garg AD, Coulie PG, Van den Eynde BJ, Agostinis P. Integrating Next-Generation Dendritic Cell Vaccines into the Current Cancer Immunotherapy Landscape. Trends Immunol 2017; 38:577-93. doi: 10.1016/j.it.2017.05.006 [Crossref] [ Google Scholar]
- Upadhyay G. Emerging Role of Lymphocyte Antigen-6 Family of Genes in Cancer and Immune Cells. Front Immunol 2019; 10:819. doi: 10.3389/fimmu.2019.00819 [Crossref] [ Google Scholar]
- Kimura H, Matsui Y, Ishikawa A, Nakajima T, Iizasa T. Randomized controlled phase III trial of adjuvant chemoimmunotherapy with activated cytotoxic T cells and dendritic cells from regional lymph nodes of patients with lung cancer. Cancer Immunol Immunother 2018; 67:1231-8. doi: 10.1007/s00262-018-2180-6 [Crossref] [ Google Scholar]
- Schreibelt G, Bol KF, Westdorp H, Wimmers F, Aarntzen EH, Duiveman-de Boer T. Effective Clinical Responses in Metastatic Melanoma Patients after Vaccination with Primary Myeloid Dendritic Cells. Clin Cancer Res 2016; 22:2155-66. doi: 10.1158/1078-0432.Ccr-15-2205 [Crossref] [ Google Scholar]
- Vo MC, Nguyen-Pham TN, Lee HJ, Jaya Lakshmi T, Yang S, Jung SH. Combination therapy with dendritic cells and lenalidomide is an effective approach to enhance antitumor immunity in a mouse colon cancer model. Oncotarget 2017; 8:27252-62. doi: 10.18632/oncotarget.15917 [Crossref] [ Google Scholar]
- Rodriguez J, Castañón E, Perez-Gracia JL, Rodriguez I, Viudez A, Alfaro C. A randomized phase II clinical trial of dendritic cell vaccination following complete resection of colon cancer liver metastasis. J Immunother Cancer 2018; 6:96. doi: 10.1186/s40425-018-0405-z [Crossref] [ Google Scholar]
- Wilgenhof S, Corthals J, Heirman C, van Baren N, Lucas S, Kvistborg P. Phase II Study of Autologous Monocyte-Derived mRNA Electroporated Dendritic Cells (TriMixDC-MEL) Plus Ipilimumab in Patients With Pretreated Advanced Melanoma. J Clin Oncol 2016; 34:1330-8. doi: 10.1200/jco.2015.63.4121 [Crossref] [ Google Scholar]
- Lu L, Yan H, Shyam-Sundar V, Janowitz T. Cross-sectional and longitudinal analysis of cancer vaccination trials registered on the US Clinical Trials Database demonstrates paucity of immunological trial endpoints and decline in registration since 2008. Drug Des Devel Ther 2014; 8:1539-53. doi: 10.2147/dddt.S65963 [Crossref] [ Google Scholar]
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363:411-22. doi: 10.1056/NEJMoa1001294 [Crossref] [ Google Scholar]
- Lin L, Wei J, Chen Y, Huang A, Li KK, Zhang W. Induction of antigen-specific immune responses by dendritic cells transduced with a recombinant lentiviral vector encoding MAGE-A3 gene. J Cancer Res Clin Oncol 2014; 140:281-9. doi: 10.1007/s00432-013-1552-8 [Crossref] [ Google Scholar]
- Zhou J, Ma P, Li J, Song W. Comparative analysis of cytotoxic T lymphocyte response induced by dendritic cells pulsed with recombinant adeno-associated virus carrying α-fetoprotein gene or cancer cell lysate. Mol Med Rep 2015; 11:3174-80. doi: 10.3892/mmr.2014.3059 [Crossref] [ Google Scholar]
- Pagès F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol 2009; 27:5944-51. doi: 10.1200/jco.2008.19.6147 [Crossref] [ Google Scholar]
- Zheng H, Liu L, Zhang H, Kan F, Wang S, Li Y. Dendritic cells pulsed with placental gp96 promote tumor-reactive immune responses. PLoS One 2019; 14:e0211490. doi: 10.1371/journal.pone.0211490 [Crossref] [ Google Scholar]
- Xie LH, Sin FW, Cheng SC, Cheung YK, Chan KT, Xie Y. Activation of cytotoxic T lymphocytes against CML28-bearing tumors by dendritic cells transduced with a recombinant adeno-associated virus encoding the CML28 gene. Cancer Immunol Immunother 2008; 57:1029-38. doi: 10.1007/s00262-007-0434-9 [Crossref] [ Google Scholar]
- Lin Y, He Z, Ye J, Liu Z, She X, Gao X. Progress in Understanding the IL-6/STAT3 Pathway in Colorectal Cancer. Onco Targets Ther 2020; 13:13023-32. doi: 10.2147/ott.S278013 [Crossref] [ Google Scholar]
- Narita Y, Kitamura H, Wakita D, Sumida K, Masuko K, Terada S. The key role of IL-6-arginase cascade for inducing dendritic cell-dependent CD4(+) T cell dysfunction in tumor-bearing mice. J Immunol 2013; 190:812-20. doi: 10.4049/jimmunol.1103797 [Crossref] [ Google Scholar]
- Sumida K, Wakita D, Narita Y, Masuko K, Terada S, Watanabe K. Anti-IL-6 receptor mAb eliminates myeloid-derived suppressor cells and inhibits tumor growth by enhancing T-cell responses. Eur J Immunol 2012; 42:2060-72. doi: 10.1002/eji.201142335 [Crossref] [ Google Scholar]
- Takano S, Kubota T, Nishibori H, Hasegawa H, Ishii Y, Nitori N. Pioglitazone, a ligand for peroxisome proliferator-activated receptor-gamma acts as an inhibitor of colon cancer liver metastasis. Anticancer Res 2008; 28:3593-9. doi: 10.1038/bjc.2012.130 [Crossref] [ Google Scholar]
- Shen D, Deng C, Zhang M. Peroxisome proliferator-activated receptor γ agonists inhibit the proliferation and invasion of human colon cancer cells. Postgraduate Medical Journal 2007; 83:414-9. doi: 10.1136/pmj.2006.052761 [Crossref] [ Google Scholar]
- Ciaramella V, Sasso FC, Di Liello R, Della Corte CM, Barra G, Viscardi G. Activity and molecular targets of pioglitazone via blockade of proliferation, invasiveness and bioenergetics in human NSCLC. J Exp Clin Cancer Res 2019; 38:1-13. doi: 10.1186/s13046-019-1176-1 [Crossref] [ Google Scholar]
- Tsubaki M, Takeda T, Tomonari Y, Kawashima K, Itoh T, Imano M, et al. Pioglitazone inhibits cancer cell growth through STAT3 inhibition and enhanced AIF expression via a PPARγ‐independent pathway. J Cell Physiol 2018. 233: 3638-47. 10.1002/jcp.26225.
- Li MY, Kong AW, Yuan H, Ma LT, Hsin MK, Wan IY. Pioglitazone prevents smoking carcinogen-induced lung tumor development in mice. Curr Cancer Drug Targets 2012; 12:597-606. doi: 10.2174/156800912801784848 [Crossref] [ Google Scholar]
- Fu H, Zhang J, Pan J, Zhang Q, Lu Y, Wen W. Chemoprevention of lung carcinogenesis by the combination of aerosolized budesonide and oral pioglitazone in A/J mice. Mol Carcinog 2011; 50:913-21. doi: 10.1002/mc.20751 [Crossref] [ Google Scholar]
- Osawa E, Nakajima A, Wada K, Ishimine S, Fujisawa N, Kawamori T. Peroxisome proliferator-activated receptor gamma ligands suppress colon carcinogenesis induced by azoxymethane in mice. Gastroenterology 2003; 124:361-7. doi: 10.1053/gast.2003.50067 [Crossref] [ Google Scholar]
- Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res 2011; 71:1263-71. doi: 10.1158/0008-5472.Can-10-2907 [Crossref] [ Google Scholar]
- Zhang Z, Huang J, Zhang C, Yang H, Qiu H, Li J. Infiltration of dendritic cells and T lymphocytes predicts favorable outcome in epithelial ovarian cancer. Cancer Gene Ther 2015; 22:198-206. doi: 10.1038/cgt.2015.7 [Crossref] [ Google Scholar]
- Fridman WH, Dieu-Nosjean MC, Pagès F, Cremer I, Damotte D, Sautès-Fridman C. The immune microenvironment of human tumors: general significance and clinical impact. Cancer Microenviron 2013; 6:117-22. doi: 10.1007/s12307-012-0124-9 [Crossref] [ Google Scholar]
- Chen D, Xia J, Tanaka Y, Chen H, Koido S, Wernet O. Immunotherapy of spontaneous mammary carcinoma with fusions of dendritic cells and mucin 1‐positive carcinoma cells. Immunology 2003; 109:300-7. doi: 10.1046/j.1365-2567.2003.01656.x [Crossref] [ Google Scholar]
- Yamaguchi S, Tatsumi T, Takehara T, Sasakawa A, Hikita H, Kohga K. Dendritic cell-based vaccines suppress metastatic liver tumor via activation of local innate and acquired immunity. Cancer Immunol Immunother 2008; 57:1861-9. doi: 10.1007/s00262-008-0514-5 [Crossref] [ Google Scholar]
- Siders WM, Vergilis KL, Johnson C, Shields J, Kaplan JM. Induction of specific antitumor immunity in the mouse with the electrofusion product of tumor cells and dendritic cells. Mol Ther 2003; 7:498-505. doi: 10.1016/s1525-0016(03)00044-3 [Crossref] [ Google Scholar]