Bioimpacts. 13(3):241-253.
doi: 10.34172/bi.2023.24252
Original Article
PCL-based nanoparticles for doxorubicin-ezetimibe co-delivery: A combination therapy for prostate cancer using a drug repurposing strategy
Mina Yousefnezhad Investigation, Methodology, Writing – original draft, Writing – review & editing, 1
Soodabeh Davaran Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing, 2, 3, * 
Mirzaagha Babazadeh Funding acquisition, Supervision, Writing – original draft, Writing – review & editing, 1
Abolfazl Akbarzadeh Methodology, Supervision, 4
Hamidreza Pazoki-Toroudi Methodology, Resources, 5
Author information:
1Department of Chemistry, Tabriz Branch, Islamic Azad University, Tabriz, Iran
2Department of Medicinal Chemistry, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
3Research Center for Pharmaceutical Nanotechnology, Tabriz University of Medical Sciences, Tabriz, Iran
4Department of Medical Nanotechnology, Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran
5Department of Physiology, Iran University of Medical Sciences, Tehran, Iran
Abstract
Introduction:
Drug repurposing is an effective strategy for identifying the use of approved drugs for new therapeutic purposes. This strategy has received particular attention in the development of cancer chemotherapy. Considering that a growing body of evidence suggesting the cholesterol-lowering drug ezetimibe (EZ) may prevent the progression of prostate cancer, we investigated the effect of EZ alone and in combination with doxorubicin (DOX) on prostate cancer treatment.
Methods:
In this study, DOX and EZ were encapsulated within a PCL-based biodegradable nanoparticle. The physicochemical properties of drug containing nanoparticle based on PCL-PEG-PCL triblock copolymer (PCEC) have been exactly determined. The encapsulation efficiency and release behavior of DOX and EZ were also studied at two different pHs and temperatures.
Results:
The average size of nanoparticles (NPs) observed by field emission scanning electron microscopy (FE-SEM) was around 82±23.80 nm, 59.7±18.7 nm, and 67.6±23.8 nm for EZ@PCEC, DOX@PCEC, and DOX+EZ@PCEC NPs, respectively, which had a spherical morphology. In addition, DLS measurement showed a monomodal size distribution of around 319.9, 166.8, and 203 nm hydrodynamic diameters and negative zeta potential (-30.3, -6.14, and -43.8) mV for EZ@PCEC, DOX@PCEC, and DOX+EZ@PCEC NPs, respectively. The drugs were released from the NPs sustainably in a pH and temperature-dependent manner. Based on the MTT assay results, PCEC copolymer exhibited negligible cytotoxicity on the PC3 cell line. Therefore, PCEC was a biocompatible and suitable nano-vehicle for this study. The cytotoxicity of the DOX-EZ-loaded NPs on the PC3 cell line was higher than that of NPs loaded with single drugs. All the data confirmed the synergistic effect of EZ in combination with DOX as an anticancer drug. Furthermore, fluorescent microscopy and DAPI staining were performed to show the cellular uptake, and morphological changes-induced apoptosis of treated cells.
Conclusion:
Overall, the data from the experiments represented the successful preparation of the nanocarriers with high encapsulation efficacy. The designed nanocarriers could serve as an ideal candidate for combination therapy of cancer. The results corroborated each other and presented successful EZ and DOX formulations containing PCEC NPs and their efficiency in treating prostate cancer.
Keywords: Doxorubicin, Ezetimibe, PCL-based nanoparticles, Prostate cancer, Combination therapy
Copyright and License Information
© 2023 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
According to the American Cancer Society, the most significant number of deaths are related to lung, prostate, and colorectal cancers in men and lung, breast, and colorectal cancers in women.
1
Furthermore, nearly 17 000 patients were diagnosed with prostate cancer (PCA) in the United States in 2019.
2
Chemotherapy, hormone therapy, tumor-targeting therapy, radiation therapy, and surgery are practical cancer treatment choices.
3-5
Among these mentioned treatment strategies, chemotherapy is widely used. However, it is accompanied by many side effects including nausea, vomiting, fatigue, pain, mouth ulcers, nerve damage, and skin reactions.
6
Co-delivery of different therapeutic agents might be a promising strategy in chemotherapy that provides an additive or synergistic effect.
7-10
To overcome the limitations of chemotherapeutic drugs and achieve better cancer therapeutic efficiency, it is necessary to design a novel drug delivery system. Nanotechnology is a promising approach in cancer treatment. Cancer nanotechnology enhances chemotherapy and reduces its adverse effects by guiding drugs to selectively target cancer cells. Another approach in the fight against cancer is the drug repurposing strategy. Drug repositioning or repurposing is the alternative use of existing drugs which are approved for one clinical use, in another disease or syndrome. The drug repurposing strategy is a cost-effective way of overcoming cancer therapeutic bottleneck. Using metformin as a cytostatic agent, thalidomide and derivatives in cancer therapy, cytokine-based therapies, statins as inhibitors of many GTPase oncogene activity, are all used as anti-cancer drugs in multiple tests.
11
Like statins, ezetimibe (EZ) is an LDL-cholesterol-lowering drug. In preclinical studies, not only statins, but also EZ, demonstrated antitumor activity in PCA cells and synergistic toxicity when combined with other anti-cancer drugs.
12
Recently, repurposing the efficacy of the currently used anti-viral drugs, such as remdesivir and favipiravir, has attracted a lot of attention to fight COVID-19.
13
Use of drugcombinations could increase the success rate of drug repurposing screens. Therefore, a better knowledge of these therapeutic modalities is needed for improved cancer therapy.
14
Combination therapy based on nano codelivery is an effective method to overcome chemotherapy limitations. Utilizing three advanced approaches in cancer treatment, including (i) drug codelivery using combination therapy, (ii) targeted delivery using nanocarriers, and (iii) repurposing strategy through which the effectiveness of chemotherapy can be significantly increased.
Among the potent anticancer agents, doxorubicin (DOX) is known as an effective agent against many different types of cancers. Thin-film hydration and an ultrasonic dispersion method were used to create DOX-loaded polymeric nanoparticles (NPs) based on poly(ε-caprolactone)-poly(ethyleneglycol)-poly(ε-caprolactone)(PCL-PEG) amphiphilic triblock copolymers. The results suggested that the DOX-loaded polymeric NPs based on the PCL-PEG-PCL triblock copolymer would be a promising nanosized drug delivery system for cancer therapy.
15
PCL/PEG/PCL NPs were also employed to load DOX by a pH-induced self-assembly method. In vitro release studies indicated that DOX release from NPs at pH 5.5 was faster than that at pH 7.0.
16
Many other studies have shown that DOX NPs can be used to treat a variety of cancers, including lung, breast, liver, and PCA.
17
EZ has poor aqueous solubility and low bioavailability. Due to the inherent problems of this potent drug, nanostructured lipid carriers have been developed via a high pressure homogenization technique.
18
For codelivery of DOX and EZ, an amphiphilic nano carrier is needed that increases the loading of hydrophobic drug (EZ) into the hydrophobic region of the NPs and hydrophilic DOX hydrochloride into the hydrophilic region of the nanoparticle. To prepare an effective carrier for codelivery of EZ and DOX, we synthesized poly(ε-caprolactone)-poly (ethyleneglycol)-poly(ε-caprolactone)(PCL-PEG-PCL) triblock copolymers. The structure of the copolymers was characterized by 1H-NMR, Fourier-transform infrared spectroscopy (FTIR), gel permeation chromatography (GPC) techniques. The NPs containing two drugs were prepared using a solvent evaporation method. The synergistic anti-PCA effect of DOX and EZ was evaluated by cytotoxicity assay in the PC3 PCA cell line.
Materials and Methods
Materials
Dimethyl sulfoxide (DMSO), dichloromethane (CH2Cl2), poly (ethylene glycol) (MW=2000), stannous octoate (Sn(OCT)2), ε-caprolactone (ε-CL), 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT), penicillin, and streptomycin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Materials used in biological protocols, including Roswell Park Memorial Institute 1640 growth medium (RPMI) and trypsin were purchased from Gibco BRL Life Technologies (Ireland). Fetal bovine serum (FBS) was obtained from Bioidea Co. A human prostate carcinoma cell line (PC3) was obtained from the Pasteur Institute of Iran (Tehran, Iran). DOX salts and EZ were purchased from EBEWE Pharmaceutical Co. (Austria) and Cipla USA Inc., respectively.
Preparation of poly(ε-caprolactone)-poly (ethyleneglycol)-poly(ε-caprolactone) (PCEC) triblock copolymer
PCEC triblock copolymer was synthesized by ring-opening polymerization of ε-CL initiated by PEG2000 (Fig. 1A).
19,20
PEG and PCL polymers with a ratio of 1:10 were transferred to a three-neck round-bottom flask put in a bath of silicone oil on a stirrer equipped with a heater and melted at a temperature of 130°C for a few minutes, in the presence of nitrogen. A thermometer was placed inside the oil bath to precisely control the temperature during the whole process. Then, tin (׀׀) 2-ethylhexanoate 1% (w/w) was added as a catalyst to start the polymerization reaction. The polymerization process lasted for 7 hours. The resulting polymer was cooled to room temperature and dissolved in dichloromethane, then poured into a cold diethyl ether to purify and isolate the remaining monomers. Diethyl ether played an anti-solvent role and precipitated the copolymer. Finally, the prepared copolymer was placed under the vacuum to evaporate the solvent and dry it.
19

Fig. 1.
Synthesis mechanism of PCEC and Preparation of drug- loaded PCL-PEG –PCL nanoparticles. (A) Scheme of the PCEC copolymer synthesis mechanism. (B) Preparation of EZ-loaded PCL-PEG-PCL NPs using single emulsion method. (C) Preparation of DOX loaded PCL-PEG –PCL NPs using double emulsion method. (D) Preparation of EZ+DOX-loaded PCL-PEG-PCL NPs using double emulsion method.
.
Synthesis mechanism of PCEC and Preparation of drug- loaded PCL-PEG –PCL nanoparticles. (A) Scheme of the PCEC copolymer synthesis mechanism. (B) Preparation of EZ-loaded PCL-PEG-PCL NPs using single emulsion method. (C) Preparation of DOX loaded PCL-PEG –PCL NPs using double emulsion method. (D) Preparation of EZ+DOX-loaded PCL-PEG-PCL NPs using double emulsion method.
Preparation of drug-loaded PCEC NPs
NPs were prepared using both double emulsion (W1/O/W2)
21
and simple emulsion (O/W) methods (Fig. 1B, 1C, 1D).
22
Preparation of EZ-loaded PCEC NPs (EZ@PCEC)
EZ-loaded PCEC NPs were prepared using a simple emulsion technique (Fig. 1B). For this purpose, 10 mg of EZ and 100 mg of PCEC were weighed and dissolved in 700 µL and 2 mL of ethanol and dichloromethane, respectively. Then, the solution was poured into a 30 mL aqueous solution of polyvinyl alcohol (PVA) 0.5 wt% and mixed at 12 000 rpm for 3 minutes. The solution was stirred at room temperature for another 5 hours for dichloromethane to evaporate. The prepared NPs were separated by centrifugation (Model 3-16L/Sigma Co.) at 9000 rpm for 20 minutes. The collected NPs were dried by freeze-drying (Model Alpha 1-4/Christ Co.), and the supernatant was utilized to measure the encapsulated drug concentration.
Preparation of DOX-loaded PCEC NPs (DOX@PCEC)
An aqueous solution of (DOX. HCl) (2000 ppm: 5 mL) was added to the organic solution (Oil) of 100 mg of copolymer in 2 mL dichloromethane. In order to avoid DOX decomposition in the presence of light, the suspension was kept in a dark environment. The first emulsion (W1/O) was prepared by homogenization (Silent Crusher M, Heidolph Instruments GmbH, Schwabach, Germany) at 11000 rpm for 3 minutes. Then W1/O emulsion was added to a 30 mL aqueous PVA solution (0.5wt %; W2), and the mixing was continued at 13000 rpm for 7 minutes to make W1/O/W2 emulsion. The W1/O/W2 emulsion was stirred at room temperature for 5 hours to evaporate the organic phase (Fig. 1C). The NPs were separated using a centrifuge, and the supernatant solution was utilized to measure the concentration of the encapsulated drug. NPs were dried by freeze-drying.
Preparation of DOX+EZ-loaded PCEC NPs (DOX+EZ@PCEC)
As depicted in Fig. 1D, an aqueous solution of (DOX. HCl) (2000 ppm:2.5 mL) was added to the organic solution (Oil) containing 100 mg triblock copolymer and 5 mg of EZ in 2 mL dichloromethane and 400 µL ethanol, respectively. The first emulsion (W1/O) was prepared by homogenization at 11 000 rpm for 3 minutes. The further preparation procedure follows the protocol explained in the previous section. The ratio of drugs in EZ+DOX@PCEC was 1:1.
Characterization of prepared triblock copolymer and NPs
The obtained copolymer was characterized through Fourier transform infrared spectroscopy (FT-IR, Tensor 270/Bruker, Germany). Proton nuclear magnetic resonance (1H-NMR) spectroscopy (in CDCl3) was recorded on an ultra-shield 400 spectrometer (Bruker, Germany) at 400 MHz. The molecular weight and polydispersity of PCEC copolymer were determined using GPC (Shimadzu LC-20A). The sample was dissolved in tetrahydrofuran (THF) at a concentration of 1-2 mg/2 mL for this purpose. At a rate of 1.0 mL/min, THF was eluted. The external and column temperature were kept at 35°C.The size and morphology of the drug-loaded PCEC NPs were determined by field emission scanning electron microscopy (FE-SEM) (MIRA3 FEG-SEM/TESCAN). Dried NPs were mounted on a tape, coated with a thin layer of gold, and images were obtained at a voltage of 15 kV. Moreover, the particle size and zeta potential of thedrug-loaded PCEC NPs were determined by dynamic light scattering (DLS) analysis using a zetasizer nano ZS90 (Malvern Instruments, UK).
Drug encapsulation efficiency and loading capacity
A ultraviolet-visible (UV-Vis) spectrophotometer (PU 8620/PHILIPS) was used to calculate the encapsulation efficiency (EE) and loading capacity (LC) of prepared EZ and DOX-loaded NPs at two wavelengths of 228 nm and 480 nm, respectively. The EE and LC were calculated using the following equations:
In vitro drug release
The release of DOX and EZ was investigated according to the sample and separate (SS) method
23
as follows: 4 mg of each drug-loaded nanocarrier (DOX@PCEC, EZ@PCEC, and DOX+ EZ@PCEC NPs) was dispersed in the release medium containing 2 mL of PBS and ethanol 96% with a ratio of 60:40 at two different pH values of 5.6 and 7.4. The samples were placed in an incubator under gentle stirring at various temperatures (40°C and 37°C) for particular time intervals. The supernatant was taken out at pre-determined time intervals to measure the amount of released drug and replaced with the same volume of fresh PBS to keep the sink condition. The concentrations of released DOX and EZ were measured by a UV-Vis spectrophotometer at 480 and 228 nm, respectively. The drug release experiments were done in triplicate, and the average data were reported. The cumulative release of drugs was calculated using the following equation, where Ci is the concentration of drug in the release medium at the time i, V is the total volume of release solution, Vs is the sample volume, and m is the mass of drug encapsulated in nanocarriers:
Cell culture
The PC3 cell line was cultured in RPMI-1640 medium supplemented with 10% FBS and penicillin/streptomycin 1%. The cells were treated with different concentrations of drug-loaded PCEC NPs and free drugs. The same volumes of the medium, without drug-loaded PCEC NPs or free drugs, were added to the 96-well plate as a control group. The culture was maintained in a 95% air-humidified atmosphere containing 5% CO2 at 37°C for 72 hours.
24-27
Cytotoxicity assay
The prostate PC3 cell line was cultured in an RPMI-1640 culture medium containing 10% FBS, 10 mL penicillin/streptomycin, and 2 mg sodium bicarbonate, and incubated in a 95% air-humidified atmosphere containing 5% CO2 at 37°C in sterile flasks. MTT assay was conducted to evaluate the cytotoxicity of free DOX, EZ, and DOX+EZ and drug-loaded NPs (DOX@PCEC, EZ@PCEC, and DOX+EZ@PCEC). PC3 cells were suspended in culture medium and seeded in two different 96-well plates in triplicate at a density of 104 cells/well for 24 hours. Then, free DOX, EZ, and DOX+EZ, and drug-loaded NPs of DOX@PCEC, EZ@PCEC, and DOX+EZ@PCEC with different drug concentrations (0, 0.39, 1.56, 3.12, 6.25, 12.5, 25, 50, and 100 μg/mL) were incubated with PC3 cells for 48 hours. Moreover, the cells were treated with blank nanocarriers with different PCEC copolymer concentrations to investigate the biocompatibility of nanocarriers. Cell-free wells without treatment were used as controls, and wells containing a cell-free medium were used as a blank for the Elisa Reader (Sunrise Instruments, Tekan). After 48 hours, the cell medium was taken out, and the wells were rinsed twice with sterilized PBS solution. Then, 150 µL fresh culture medium and 50 µL MTT solution were added to each well. Plates were kept in dark conditions to avoid MTT decomposition in the presence of light and incubated at 37°C for an additional 4 hours. Afterward, the medium containing MTT was removed from each well, and replaced by 200 µL of DMSO, which was incubated for 20 minutes to dissolve the formed blue formazan crystals. After shaking on a shaker for 5 minutes, the cell viability was determined using an Elisa Reader at 570 nm with a reference wavelength of 630 nm. All tests were done in triplicate. The following equation was used to convert OD to the percentage of live cells:
where OD (test) and OD (control) are the mean absorbance values of the tested groups and control groups (without any treatment), respectively.
Analysis of the combination effect
The combination index (CI) values were calculated utilizing CompuSyn v.1 software
28
according to Chou and Talalay’s equation given below
29
:
CIX was utilized to assess the synergistic effect of DOX and EZ combinations on PC3 cells in vitro, where (ICx)1 and (ICx)2 are the ICx of EZ-loaded NPs and DOX-loaded NPs, respectively, and (D)1 and (D)2 are the concentrations of EZ and DOX in the dual drug-loaded NPs at the ICx value.
Cellular uptake study
The fluorescence measurement (fluorimeter) method was utilized to evaluate the cellular uptake of various formulations of free drugs and drug-loaded NPs by the PC3 cell line. Briefly, PC3 cells seeded in 12-well plates (1×106 cell/well) were treated with two concentrations of 50 and 100 μg/mL of free drugs (single and dual) and drug-loaded NPs for 24 hours at 37°C. After incubation for 24 hours, the cells were washed with PBS three times, and placed in cold PBS, and measured by the fluorimeter vehicle at two conditions: (1) excitation wavelength 268 nm (uv) and emission wavelength (435-485 nm ), respectively; (2) excitation wavelength 470 nm (Blue) and emission wavelength (514-567 nm), respectively.
DAPI staining for apoptosis study
To investigate the apoptotic effect of free EZ and EZ@PCEC NPs on the PC3 cell line, the nucleus of the cells was stained with 4′,6-diamidino-2-phenylindole (DAPI): PC3 cells were seeded in a 6-well plate at a density of 2×105 cell/well and incubated for 24 hours. Then, after 48 hours, the culture medium was substituted with a fresh medium containing free drugs and drug-loaded nanocarriers at concentrations of 6 and 100 µg/mL. The medium was then discarded, and the cells were rinsed three times with PBS (pH 7.4). The cells were fixed with 1 mL of paraformaldehyde 4% (w/v) and incubated for 1 hour. Subsequently, the fixed cells were washed with fresh PBS and permeabilized by adding 0.5 mL of 0.1% (w/v) Triton x-100 and incubated for 5 minutes. The nuclei of the cells were stained with 1 µg/mL DAPI for 10 minutes after being rinsed with PBS. Lastly, the cells were imaged employing fluorescence microscopy (BioTek, USA, excited at 405 nm) at 400x magnification. The images were processed using ImageJ software.
30
The dynamic light scattering technique
The particle size and zeta potential of the drug-loaded PCEC NPs were determined by DLS analysis using a zetasizer nano ZS90 (Malvern Instruments, UK). For this reason, the NPs were dispersed in distilled water by sonication for 10 minutes.
15
Statistical analysis
GraphPad Prism 8 (GraphPad Software, Inc., La Jolla. GA) was employed for statistical analysis. Single-factor analysis for variance (ANOVA) was utilized to evaluate the statistical significance of the results. All the samples were analyzed in triplicate and expressed as means ± SD for n=3. The P-value determined the level of significance. P < 0.05 (*) was supposed to be statistically significant. On the other hand, P < 0.01 (**), P < 0.001 (***), and P < 0.0001 (****) were regarded as highly significant.
Results and Discussion
Preparation and characterization of PCEC NPs
PCEC triblock copolymer was synthesized by ring-opening polymerization of ε-CL initiated by PEG2000 (Figs. 2 and 3). Both DOX as an anticancer drug and EZ as an agent capable of inhibiting cholesterol uptake were loaded separately and combined into the obtained co-polymeric NPs by double emulsion (W1/Oil/W2) and simple emulsion methods.
21,23,31,32
Further in-vitro evaluation of prepared formulations was carried out in the presence of PC3 PCA cells. The following section discusses the structural characterization of these drug delivery systems, investigated using 1H-NMR, FT-IR, GPC, FE-SEM and DLS:
1H-NMR analysis
On an ultra-shield 400 spectrometer, 1H-NMR spectra (in CDCl3) were reported at 400 MHz. In Fig.2A, the specific absorption peaks can be seen. Methylene protons of (CH2)3, OCCH2, and CH2OOC in PCL chains were associated with peaks at 1.38 (b), 1.63 (b), 2.31 (c), and 4.04 (a) ppm, respectively. The methylene protons of PEG segments are responsible for the sharp peak at 3.62 (f) ppm. The methylene protons of O-CH2-CH2 in the PEG end unit were attributed to the weak peaks at 4.22(d) ppm and 3.85(e) ppm, respectively.
19,33,34
The PCEC copolymer developed successfully, according to the results.
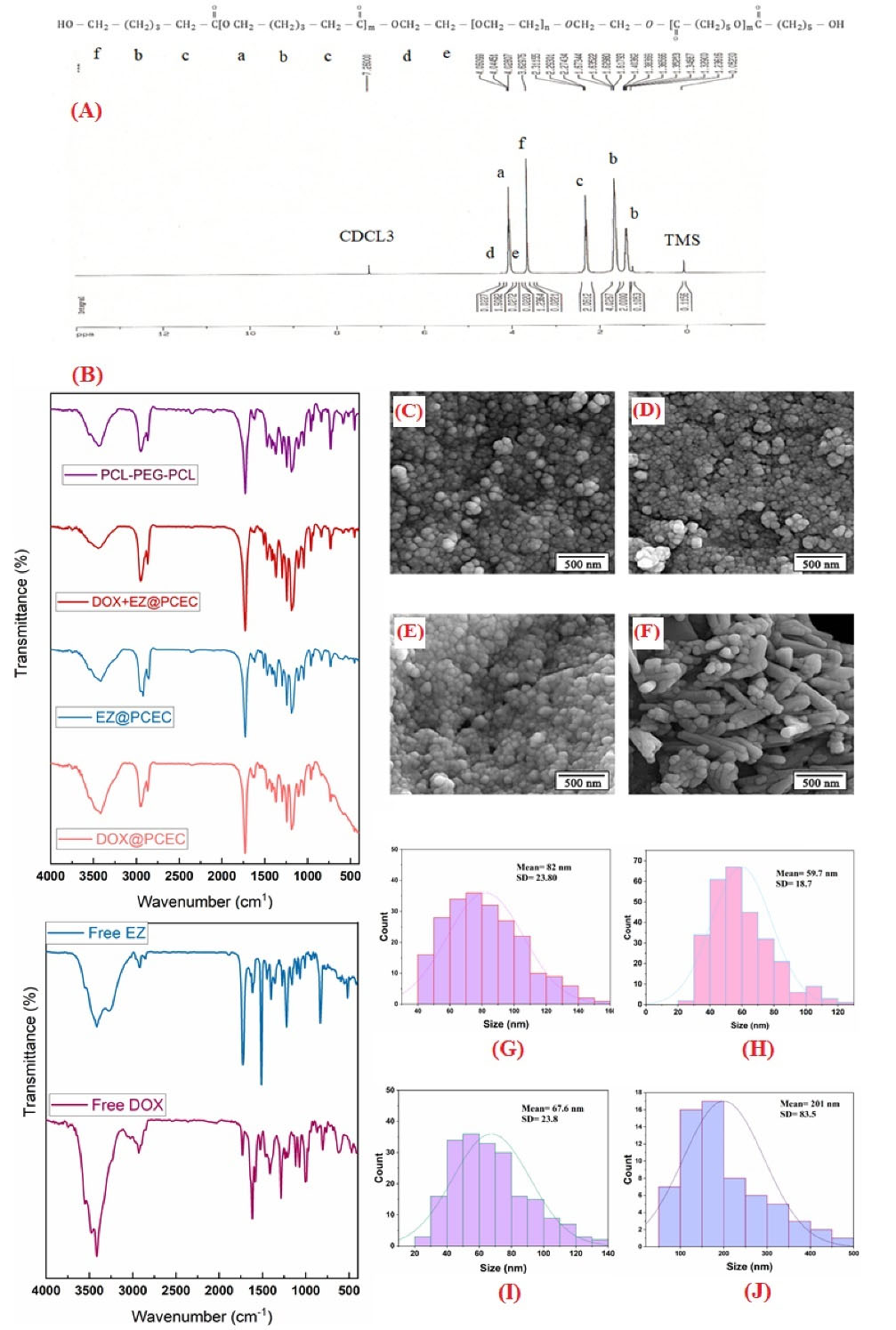
Fig. 2.
The structural and morphological characterizationof PCEC NPs.(A) 1H NMR spectrum of PCL-PEG-PCL copolymer. (B) Fourier transform infrared spectrum of PCL-PEG-PCL copolymer, DOX+EZ@PCEC, EZ@PCEC, DOX@PCEC NPs, free EZ and free DOX, respectively. FE-SEM images of (C) EZ@PCL-PEG-PCL NPs, (D) DOX@PCL-PEG-PCL NPs, (E) EZ+DOX@ PCL–PEG–PCL NPs, and (F) PCL-PEG-PCL copolymer before drug loading, (G) The corresponding diameter distribution of the EZ@PCL-PEG-PCL NPs, (H) The corresponding diameter distribution of the DOX @PCL-PEG-PCL NPs, (I) The corresponding diameter distribution of the EZ+DOX @PCL-PEG-PCL NPs, (J) The corresponding diameter distribution of the PCL-PEG-PCL copolymer before drug loading.
.
The structural and morphological characterizationof PCEC NPs.(A) 1H NMR spectrum of PCL-PEG-PCL copolymer. (B) Fourier transform infrared spectrum of PCL-PEG-PCL copolymer, DOX+EZ@PCEC, EZ@PCEC, DOX@PCEC NPs, free EZ and free DOX, respectively. FE-SEM images of (C) EZ@PCL-PEG-PCL NPs, (D) DOX@PCL-PEG-PCL NPs, (E) EZ+DOX@ PCL–PEG–PCL NPs, and (F) PCL-PEG-PCL copolymer before drug loading, (G) The corresponding diameter distribution of the EZ@PCL-PEG-PCL NPs, (H) The corresponding diameter distribution of the DOX @PCL-PEG-PCL NPs, (I) The corresponding diameter distribution of the EZ+DOX @PCL-PEG-PCL NPs, (J) The corresponding diameter distribution of the PCL-PEG-PCL copolymer before drug loading.
FT-IR spectroscopy
Fig. 2B represents the FT-IR spectrum of PCL-PEG-PCL copolymer, DOX+EZ@PCEC, EZ@PCEC, DOX@PCEC NPs, free EZ,
35
and free DOX,
36
respectively. In the spectrum of PCL-PEG-PCL copolymer, the absorption peaks at 1727 cm-1 belonged to C=O stretching vibrations of the ester carbonyl group. The peaks that appeared at 1188-1297 cm-1 are dedicated to the C-O-C stretching vibrations of the -O-CH2-CH2 repeated units present in the PEG structure and the -COO- bands' stretching vibrations, respectively. The absorption peaks at 2869 cm-1 and 2945 cm-1 belong to the C-H aliphatic stretch. The peak at 3437 cm-1 is due to the terminal hydroxyl group (-OH) in the copolymer.
19,33,37
However, in comparison with the spectrum of drug-loaded NPs, we could not see any observable difference in the appearance of the spectrum after the loading of drugs, except for increasing the intensity of the characteristic carbonyl peak. Similar reports in other studies suggest that the drug was localized and entrapped within the nanocarrier.
38
The results of the FT-IR spectra confirmed the successful formation of the PCEC triblock copolymer.
FE-SEM analysis
The size and morphology of the drug-loaded PCEC NPs were determined by FE-SEM. Fig. 2 (C, D, E and F) demonstrates images of EZ@PCEC, DOX@PCEC, DOX+EZ@PCEC NPs, and PCEC copolymer, respectively. The results of the PCEC copolymer and nanoparticle morphology assessment revealed the uniformity in the size and shape with round topology. In NPs, after encapsulation of DOX and EZ by an emulsion process, the size of the particles decreased significantly. A suitable and stable system is created with the emulsification process due to the favourable contact between oil and water phases using an appropriate surfactant.
39,40
The evaluation of FE-SEM images by ImageJ confirmed the formation of nano-sized NPs. The measurements showed the average size of NPs to be 82±23.80 nm, 59.7±18.7 nm, and 67.6±23.8 nm for EZ@PCEC, DOX@PCEC, and DOX+EZ@PCEC NPs, respectively (Fig. 2G, 2H, and 2I). While the mean size of blank PCEC copolymer was 201±83.5 and without round morphology (Fig. 2J). As a result, the size of the NPs decreased significantly compared with the blank PCEC copolymer. Similar results were reported in other previous works.
19
Gel permeation chromatography
GPC is a size exclusion chromatography technique used to determine the prepared triblock copolymer’s molecular weight.
41,42
The samples’ molecular weight was calculated based on the molecular weight of the polystyrene standard (Table 1).
Table 1.
Molecular characteristics of the synthesized copolymer
|
Copolymer
|
CL/EG
c
feed
|
M
n
a
|
M
w
a
|
PDI
b
|
| PCL-PEG-PCL |
10/1 |
3953 |
6938 |
1.75529 |
aDetermined by GPC analysis using narrow molecular weight polystyrene standards.
b Mw/Mn= Polydispersity index of the polymers (PDI) determined by GPC analysis.
cCL=caprolacton, EG=Polyethylene glycol.
Dynamic light scattering technique
The particle size distribution and zeta potential of thedrug-loaded PCEC NPs in distilled water were determined by DLS analysis. DLS measurement showed a monomodal size distribution of around 319.9, 166.8, and 203 nm hydrodynamic diameters and negative zeta potential (-30.3, -6.14 and -43.8) mV for EZ@PCEC, DOX@PCEC, and DOX+EZ@PCEC NPs, respectively. In addition, negatively charged NPs are often more resistant to plasma macromolecular protein adsorption and are easier to disperse in the bloodstream compared to positively charged ones, which favour in vivo drug delivery.
15
Encapsulation efficiency and in vitro drug release
The simple and double emulsion technique was used to prepare DOX and EZ-loaded NPs. The feeding ratio of each drug to nano-carriers was 1 to 10. The loading capacity of DOX+EZ@PCEC for DOX and EZ was obtained at about 3.5% and 3.2%, respectively. At the same time, this measurement for DOX and EZ in DOX@PCEC and EZ@PCEC samples was calculated to be 6.0% and 9.6%, respectively. DOX and EZ encapsulation efficiencies in DOX+ EZ@PCEC NPs were 70% and 64%, respectively. Moreover, the drug encapsulation efficiency of DOX@ PCEC and EZ@ PCEC NPs was obtained at almost 60% and 96% for DOX and EZ, respectively.
The in vitro drug release of different formulations was investigated at two pH values and temperatures (Fig. 3): First, at physiological conditions (pH 7.4 at 37°C) and second, cancer tissue conditions (pH 5.6 at 40C)
43
. The results indicated an initial burst release of about 54% and 39.35% in the first 8 hours for EZ@PCEC and EZ+DOX@PCEC NPs at cancer tissue conditions (pH 5.6 at 40°C). Whereas under physiologic conditions, a sustained release was observed for EZ@ PCEC NPs. The release profile of EZ+DOX@PCEC NPs experienced an intersection between acidic and physiologic pH, which follows a steep upward slope. At the beginning of the study, the burst release of EZ may be attributed to the drugs that were physically absorbed on the surface of the nanocarriers.
44
The total release for single EZ and DOX-loaded NPs after 72 hours was 56.3 ± 1.7 and 11.32 ± 0.58, respectively. Dual drug-loaded nanocarriers after 72 hours showed a total release of 48.14 ± 1.4 and 9.45 ± 0.5 for EZ and DOX, respectively. These results indicated that release in single drug-loaded samples was higher than that in dual drugs incorporated formulations. It may be attributed to the low encapsulation efficiency of EZ in EZ+DOX@PCEC NPs compared with EZ@PCEC NPs.

Fig. 3.
In vitro cumulative release profiles of loaded drug formulations at two pH values and temperatures. (A) The release profile of EZ from EZ@PCEC NPs, (B) The release profile of EZ from EZ+DOX@PCEC NPs, (C) The release profile of DOX from DOX@ PCEC NPs, (D) The release profile of DOX from DOX+ EZ @ PCEC NPs.
.
In vitro cumulative release profiles of loaded drug formulations at two pH values and temperatures. (A) The release profile of EZ from EZ@PCEC NPs, (B) The release profile of EZ from EZ+DOX@PCEC NPs, (C) The release profile of DOX from DOX@ PCEC NPs, (D) The release profile of DOX from DOX+ EZ @ PCEC NPs.
On the other hand, the release profile of DOX and DOX+ EZ-loaded NPs showed no burst release of DOX and experienced a sustained and slow release compared to the EZ. Besides, the results illustrated that the general release of DOX in the single drug-loaded form was higher than the co-delivery formulation (11% at pH 5.6). It can be attributed to the formation of high levels of hydrogen bonds between DOX and NPs. The results also proved that the total release of the drug in cancer conditions (pH 5.6, 40°C) is more than release under physiological conditions (pH 7.4, 37°C), which was also reported by Abedi et al.
43,45
The same result was also reported for DOX-loaded NPs in other studies in which authors showed single drug-loaded nanocarriers executed higher release than dual drug-loaded ones.
44
Cytotoxicity assay
The MTT assay is a colorimetric method used to evaluate mitochondrial activity and quantify cell proliferation or cell death. This study used the MTT assay to evaluate the cytotoxic effects of PCEC formulations as biocompatible drug-loaded nanocarriers and free drugs of DOX and EZ on the PC3 PCA cell line (Fig. 4A). Finally, optical absorption results were analyzed using Graf pad prism software, and inhibition concentrations (IC50) for each specimen were then calculated (Table 2).
8
The results showed dose-dependent cytotoxicity for all formulations in which the cell viability was reduced by an increase in drug concentration.
15
Cell viability in the formulations of free drugs and drug-loaded NPs decreased with steep and slow slopes, respectively, except for the EZ@PCEC NPs. This could be due to different uptake mechanisms and cellular distribution of free DOX and DOX@PCEC NPs,
15
besides the release rate of DOX and EZ from NPs.
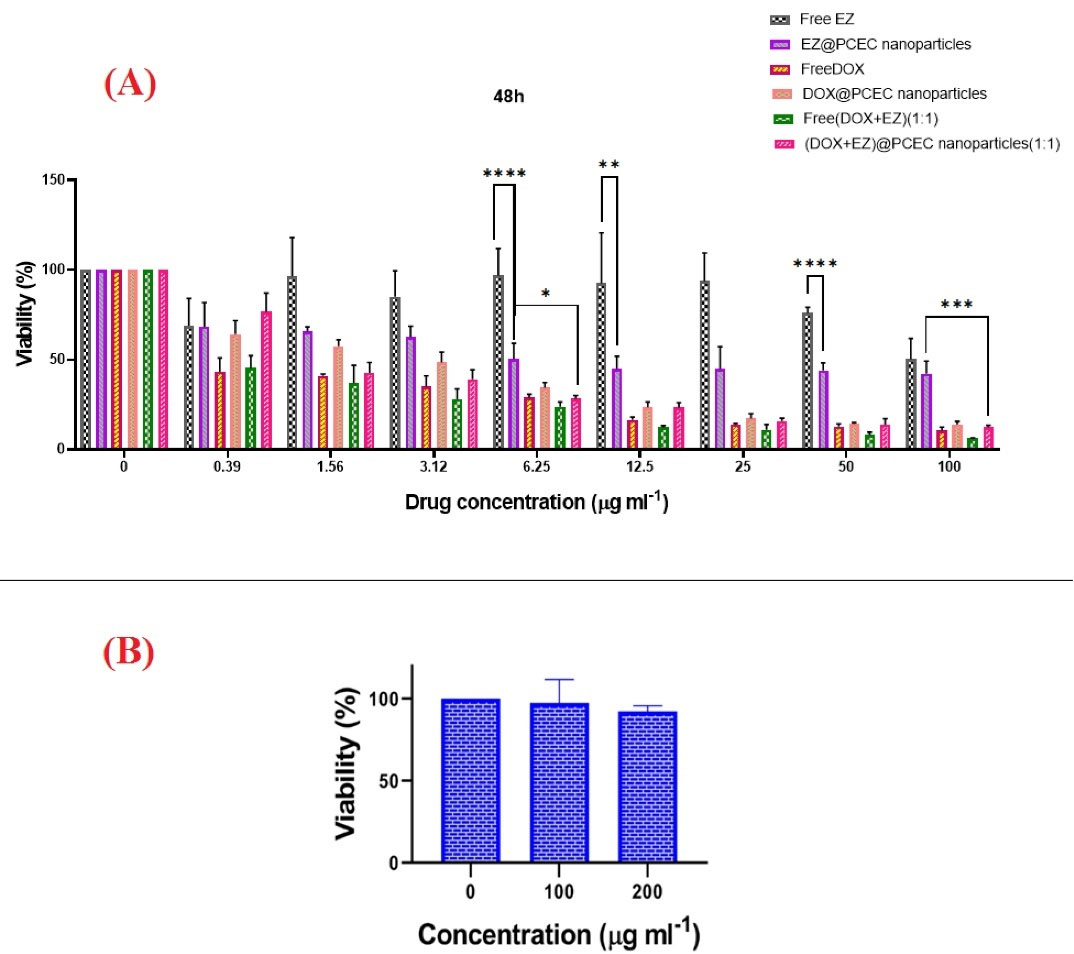
Fig. 4.
In vitro Cytotoxicity of DOX and EZ formulations and PCEC in PC3 cell line. (A) Cell viability of PC3 cell after treatment with various doses of free DOX, free EZ, free DOX+EZ, DOX@PCEC NPs, EZ@PCEC NPs, and DOX+EZ@PCEC NPs for 48 hours. (B) Cell viability results of PC3 treated with different doses of PCEC for 48 hours. Comparison among groups was conducted by one-way ANOVA followed by Tukey’s HSD analysis, P < 0.05 (*), P < 0.01 (**), P < 0.001 (***), P < 0.0001(****).
.
In vitro Cytotoxicity of DOX and EZ formulations and PCEC in PC3 cell line. (A) Cell viability of PC3 cell after treatment with various doses of free DOX, free EZ, free DOX+EZ, DOX@PCEC NPs, EZ@PCEC NPs, and DOX+EZ@PCEC NPs for 48 hours. (B) Cell viability results of PC3 treated with different doses of PCEC for 48 hours. Comparison among groups was conducted by one-way ANOVA followed by Tukey’s HSD analysis, P < 0.05 (*), P < 0.01 (**), P < 0.001 (***), P < 0.0001(****).
Table 2.
IC50 values and dose-effect parameters for free drugs and drug-loaded NPs in PC3 cell line
|
Specimen
|
IC
50
|
m
|
Dm (µg/ml)
|
r
|
| Free EZ |
- |
0.17032 |
759465 |
0.25695 |
| Free DOX |
0.4653 |
0.39357 |
0.37098 |
0.96359 |
| Free EZ+DOX |
0.4021 |
0.49305 |
0.38173 |
0.98557 |
| EZ@PCEC NPs |
13.45 |
0.22758 |
13.9774 |
0.94012 |
| DOX@ PCEC NPs |
1.924 |
0.50765 |
1.75958 |
0.97681 |
| (EZ+DOX)@PCEC NPs |
1.543 |
0.54453 |
1.57958 |
0.97001 |
The data were collected from MTT assay and were subjected to the automated calculation of m, Dm, and r parameters using CompuSyne software.
The free DOX+EZ (ratio 1:1) exhibited more significant antitumor activity compared to DOX+EZ (ratio 1:1) @PCEC NPs, which could be attributed to the diffusion mechanism that free drugs use to enter the cancer cells, as well as the slow release of DOX+EZ from its polymeric carrier.
Furthermore, the comparison of cytotoxicity between DOX@PCEC, DOX+EZ@PCEC, and EZ@PCEC NPs showed a significant difference in cell viability of PC3 treated with EZ@PCEC NPs in all of their concentrations.
The MTT assay results demonstrated that EZ@PCEC NPs with a concentration of 13.45 µg/mL had a cytotoxic effect on 50% of PC3 cells. Moreover, calculated IC50 showed that 0.4653 µg/mL of free DOX and 1.924 µg/mL of DOX@PCEC NPs were able to induce cytotoxic effects in 50% of PC3 cells. Besides, the results revealed that 0.4021 µg/mL of free DOX+EZ and 1.543 µg/mL of EZ+DOX@PCEC NPs could be followed by the death of 50% of the PC3 cell line in PCA. The EZ+DOX@PCEC NPs and EZ+DOX formulations were more effective than the single free drugs and single drug-loaded NPs at their highest concentrations after 48 hours. The biocompatibility of the PCEC copolymer was also confirmed using the MTT assay, as it did not affect the growth of the PC3 cell line (Fig. 4B).
The combination effect analysis was also calculated by CI. CI < 1, CI=1, and CI >1 show synergistic, additive, and antagonistic effects, respectively.
44
Dose-effect parameters are given in Table 2. These parameters include m, Dm, and r, which represent the slope of the median–effect plot (shape parameter), the dose of the median–effect (potency parameter like IC50), and the linear correlation coefficient of the median-effect plot (conformity parameter), respectively.
The resultant values of the CI for free drugs and drug-loaded NPs at the actual experimental point, along with various effect levels (Fa) and types of effect, were calculated using CompuSyne software and are presented in Table 3.
Table 3.
The combination index (CI) values for free drug and drug-loaded NPs calculated using CompuSyne software in various concentrations
|
Concentration (µg/mL)
|
Fa
|
CI value*
|
Effect type
|
|
Interaction type of free drug
|
| 0.39 |
0.54635 |
0.32772 |
Synergistic effect |
| 1.56 |
0.63104 |
0.53768 |
Synergistic effect |
| 3.12 |
0.72168 |
0.37354 |
Synergistic effect |
| 6.25 |
0.76139 |
0.44171 |
Synergistic effect |
| 12.5 |
0.87705 |
0.11439 |
Strong Synergistic effect |
| 25 |
0.89087 |
0.16242 |
Strong Synergistic effect |
| 50 |
0.91856 |
0.14285 |
Strong Synergistic effect |
| 100 |
0.93859 |
0.13203 |
Strong Synergistic effect |
|
Interaction type of drug-loaded NP
S
|
| 0.39 |
0.23375 |
3.72114 |
Antagonistic effect |
| 1.56 |
0.57419 |
0.26099 |
Strong Synergistic effect |
| 3.12 |
0.60935 |
0.38511 |
Synergistic effect |
| 6.25 |
0.71751 |
0.28685 |
Strong Synergistic effect |
| 12.5 |
0.76646 |
0.34420 |
Synergistic effect |
| 25 |
0.84204 |
0.26349 |
Strong Synergistic effect |
| 50 |
0.86099 |
0.39187 |
Synergistic effect |
| 100 |
0.87516 |
0.61383 |
Synergistic effect |
*(CI˃ 1), (0.7˂ CI ˂1), (0.3˂ CI ˂0.7), and (CI ˂ 0.3) indicating antagonistic, medium synergistic, synergistic, and strong synergistic effect, respectively.
In addition, the CI plot (Fa-CI plot) of the obtained results was depicted in Fig. 5, in which the CI values were plotted against the corresponding effect levels.
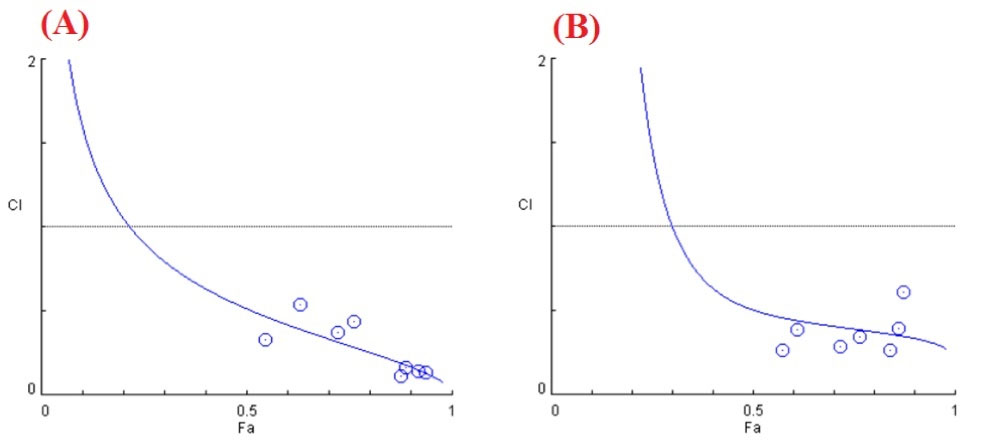
Fig. 5.
Combination index curves (Fa-CI plot) for (A) free drug and (B) drug@PCEC NPs were plotted as a function of the fraction inhibition (Fa) of cell viability/growth by computer simulation (CompuSyn software).
.
Combination index curves (Fa-CI plot) for (A) free drug and (B) drug@PCEC NPs were plotted as a function of the fraction inhibition (Fa) of cell viability/growth by computer simulation (CompuSyn software).
According to Chou and Talalay’s equation, the CI value was calculated to be 0.45 for EZ+DOX@PCEC NPs. This result indicated that EZ+DOX@PCEC NPs could act synergistically with the drug ratio of 1:1 in vitro. The MTT results showed that drug-carrying NPs increased drug solubility, caused selective drug delivery, modified the drug release kinetics, and provided a prolonged sustained release of drugs. It is noteworthy that free drugs in cell culture medium can rapidly release their effects after being transported into cells through passive diffusion. On the other hand, the drug incorporated NPs that internalized the cells through endocytosis and exhibited their anticancer activity after the drug was released from the NPs. Similar cytotoxicity results were also reported in other studies in which the free drugs resulted in higher cytotoxicity than drug-loaded NPs.
15,46-49
As a conclusion, all data confirmed that the EZ as a cholesterol-lowering drug with DOX as an anticancer drug could synergistically affect PCA cells.
Cellular uptake of free drugs and drug-loaded NPs
The effect of EZ on the intracellular uptake of free DOX+EZ and DOX+EZ@PCEC NPs was evaluated on the PC3 cell line by fluorimeter (Fig. 6). For this purpose, PC3 as an aggressive prostate carcinoma cell line was treated with the free drugs, drug-loaded NPs, and PCEC, incubated for 24 hours in two concentrations of 50 and 100 (µg/mL). The results indicated the increased uptake of all formulations of drugs and drug-loaded NPs, incubated for 24 hours compared with the control groups with the same concentrations. As shown in Fig. 6, the cellular uptake in dual drug-loaded NPs was higher than that in other formulations. This may be due to the CI effect of DOX+EZ@PCEC NPs on the PC3 cell line. Comparing two formulations of free DOX and DOX@PCEC NPs,the cellular uptake of free DOX was higher than that of DOX@PCEC NPs, which was in agreement with other studies.
15
This might be because DOX@PCEC NPs were internalized by the cells via the endocytic pathway. In contrast, free DOX can be transported into cells through passive diffusion.
15,31
Finally, the fluorimeter results confirmed that the formulation of dual drugs@PCEC NPs had a significant cellular uptake effect, and EZ as a cholesterol-lowering drug had a remarkable effect on cell uptake.
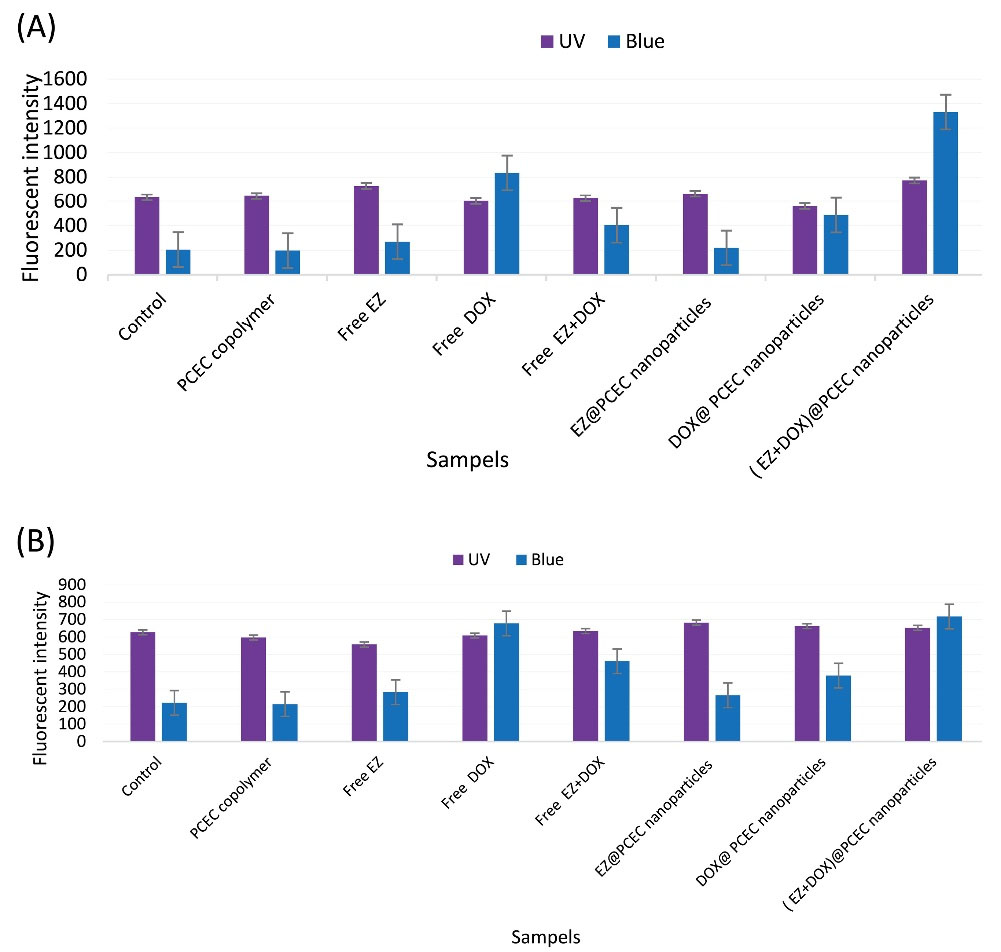
Fig. 6.
Cellular uptake of various formulations of PCEC copolymer, free EZ, free DOX, free DOX+EZ, EZ@PCEC NPs, DOX@ PCEC NPs and ( EZ+DOX)@PCEC NPs at two concentrations of (a) 100 and (b) 50 (µg/mL) after 24 hours. Uv and Blue = the fluorescent intensities of each group at two canditions: 1- excitation wavelength 268 nm (uv) and emission wavelength (435-485 nm) respectively; 2- excitation wavelength 470 nm (Blue) and emission wavelength (514-567 nm), respectively.
.
Cellular uptake of various formulations of PCEC copolymer, free EZ, free DOX, free DOX+EZ, EZ@PCEC NPs, DOX@ PCEC NPs and ( EZ+DOX)@PCEC NPs at two concentrations of (a) 100 and (b) 50 (µg/mL) after 24 hours. Uv and Blue = the fluorescent intensities of each group at two canditions: 1- excitation wavelength 268 nm (uv) and emission wavelength (435-485 nm) respectively; 2- excitation wavelength 470 nm (Blue) and emission wavelength (514-567 nm), respectively.
DAPI staining for the study of apoptosis
DAPI staining was used to evaluate the morphological changes induced by PC3 cell line apoptosis (Fig. 7).
30,44
In this regard, the chromatin morphological changes and nucleus density of PC3 cells, treated with free EZ, EZ@PCEC NPs, and PCEC were observed after 48 hours using fluorescence microscopy. The results showed that the cells treated with nanocarriers indicated no significant morphological changes compared with control cells and maintained their healthy and evenly shapes. On the other hand, the sign of apoptosis, containing cell shrinkage, loss of cell-cell contact, nuclear fragmentation, and chromatin condensation was observed in images of cells treated with the free drug and drug-loaded nanocarriers.
30
The cells treated with EZ@PCEC NPs showed remarkable morphological changes compared with free EZ. Specifically, free EZ, among other groups, had no intangible effect on cells. Besides, the density of cells in the EZ@PCEC NPs group was lower than that in other formulations. As reported in the MTT assay results, EZ@PCEC NPs showed a higher potential in cancer cells’ death compared with free EZ, which was also clearly observed in the results of DAPI staining. Therefore, EZ@PCEC NPs are promising candidates for anticancer applications.
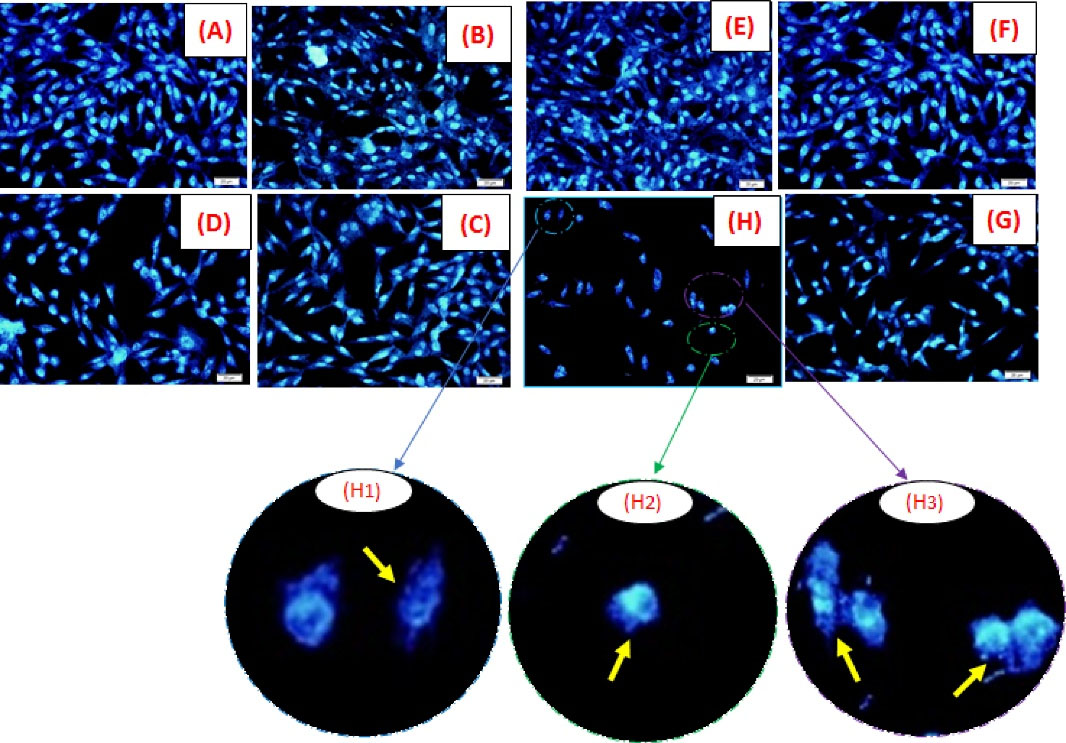
Fig. 7.
Nuclear morphology alteration of PC3 cell lineat two different concentrations. Fluorescence microscopy images showing nuclear morphology of PC3 cell line after 48-hour treatment at concentrationsof 6 (µg/mL)with(A)Untreated cells as control, (B) copolymer PCEC, (C) free EZ, (D) EZ@PCEC NPs and concentration 100 (µg/mL) with (E) untreated cells as control, (F) copolymer PCEC, (G) free EZ, (H) EZ@PCEC NPs. Images of DAPI-stained cells were taken at a 400× magnification. (H1), (H2), (H3) represented nuclear fading, nuclear shrinkage, and nuclear fragmentation, respectively.
45
At concentration of 100 µg/mL, the density of cells reduced as a result of apaptotic cells engulfed by neighbor cells.
30
Scale bars indicated 20 µm.
.
Nuclear morphology alteration of PC3 cell lineat two different concentrations. Fluorescence microscopy images showing nuclear morphology of PC3 cell line after 48-hour treatment at concentrationsof 6 (µg/mL)with(A)Untreated cells as control, (B) copolymer PCEC, (C) free EZ, (D) EZ@PCEC NPs and concentration 100 (µg/mL) with (E) untreated cells as control, (F) copolymer PCEC, (G) free EZ, (H) EZ@PCEC NPs. Images of DAPI-stained cells were taken at a 400× magnification. (H1), (H2), (H3) represented nuclear fading, nuclear shrinkage, and nuclear fragmentation, respectively.
45
At concentration of 100 µg/mL, the density of cells reduced as a result of apaptotic cells engulfed by neighbor cells.
30
Scale bars indicated 20 µm.
Research Highlights
What is the current knowledge?
√ Cancer nanotechnology enhances chemotherapy and reduces its adverse effects by guiding drugs to selectively target cancer cells.
√ DOX is an anthracycline drug, which has been used alone or in combination therapy for PCA.
√ EZ is a cholesterol uptake-blocking drug.
What is new here?
√ Another approach in the fight against cancer is the drug repurposing strategy.
√ DOX and EZ were encapsulated within a PCL-based biodegradable hydrogel in order to treat cancer.
√ The results presented successful EZ and DOX formulations containing PCEC NPs in treating PCA.
Conclusion
Lately, triblock copolymer-based nanocarriers and a synergistic combination of two or more drugs have shown promising potential to overcome the side effects of current chemotherapy and pave the path to achieving the desired results. In this study, PCEC wassynthesizedbyring-opening polymerization and characterized by 1H-NMR, FT-IR, and GPC. The EZ was loaded with PCEC NPs by the simple emulsion method. Moreover, DOX and a combination of DOX and EZ were loaded onto NPs by the double emulsion technique. FE-SEM evaluated the morphology and size of the resultant NPs. The particle size distribution and zeta potential of thedrug-loaded PCEC NPs in distilled water were determined by DLS analysis. The DOX and EZ’s encapsulation efficiency were calculated, and an in vitro release study showed that prepared nanocarriers showed a slow and sustained release. The cytotoxicity of NPs and free drugs was evaluated by the MTT assay using PCA PC3 cell lines. In vitro cytotoxicity assay showed that the PCEC did not affect the growth of PC3 cells; therefore, it is an appropriate and biocompatible candidate for formulating nanocarriers. The cytotoxic activity of the dual drugs in both free form and loaded on NPs against PC3 cells was better than their single formulations. Furthermore, the IC50 results showed that the EZ as a cholesterol-lowering drug and DOX as an anticancer drug incorporated in PCEC had synergistic effects on PCA. Furthermore, the fluorimeter results confirmed that the formulation of dual drugs@PCEC NPs has a significant cellular uptake effect. EZ as a cholesterol-lowering drug has a remarkable effect on cell uptake. The results of DAPI staining also confirmed that EZ@PCEC NPs are promising candidates for anticancer applications. The results corroborated each other and presented successful EZ and DOX formulations containing PCEC NPs and their efficiency in treating PCA.
Acknowledgement
We would like to thank the Drug Applied Research Center (DARC), Tabriz University of Medical Sciences (Tabriz, Iran), and Iran National Science Foundation (INSF, Granted Research Chair Awards) for their support and help. All of the experiments in this study were conducted at DARC with supporting from the Iran National Science Foundation.
Funding
None.
Ethical Statement
Not applicable.
Conflict of interests
None.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69:7-34. doi: 10.3322/caac.21551 [Crossref] [ Google Scholar]
-
Li K, Zhan W, Chen Y, Jha RK, Chen X. Docetaxel and doxorubicin codelivery by nanocarriers for synergistic treatment of prostate cancer. Front Pharmacol 2019; 10. 10.3389/fphar.2019.01436.
- Assi T, El Rassy E, Tabchi S, Ibrahim T, Moussa T, Chebib R. Treatment of cancer patients in their last month of life: aimless chemotherapy. Support Care Cancer 2016; 24:1603-8. doi: 10.1007/s00520-015-2959-3 [Crossref] [ Google Scholar]
- Hu T, Zhou R, Zhao Y, Wu G. Integrin alpha6/Akt/Erk signaling is essential for human breast cancer resistance to radiotherapy. Sci Rep 2016; 6:33376. doi: 10.1038/srep33376 [Crossref] [ Google Scholar]
- James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016; 387:1163-77. doi: 10.1016/s0140-6736(15)01037-5 [Crossref] [ Google Scholar]
- Wang BL, Shen YM, Zhang QW, Li YL, Luo M, Liu Z. Codelivery of curcumin and doxorubicin by MPEG-PCL results in improved efficacy of systemically administered chemotherapy in mice with lung cancer. Int J Nanomedicine 2013; 8:3521-31. doi: 10.2147/IJN.S45250 [Crossref] [ Google Scholar]
- Pan J, Rostamizadeh K, Filipczak N, Torchilin VP. Polymeric Co-Delivery Systems in Cancer Treatment: An Overview on Component Drugs' Dosage Ratio Effect. Molecules 2019; 24:1035. doi: 10.3390/molecules24061035 [Crossref] [ Google Scholar]
- Rahimi M, Safa KD, Salehi R. Co-delivery of doxorubicin and methotrexate by dendritic chitosan-g-mPEG as a magnetic nanocarrier for multi-drug delivery in combination chemotherapy. Polymer Chemistry 2017; 8:7333-50. doi: 10.1039/c7py01701d [Crossref] [ Google Scholar]
- Carvalho BG, Vit FF, Carvalho HF, Han SW, de la Torre LG. Recent advances in co-delivery nanosystems for synergistic action in cancer treatment. J Mater Chem B 2021; 9:1208-37. doi: 10.1039/D0TB02168G [Crossref] [ Google Scholar]
- Rahimi M, Safa KD, Alizadeh E, Salehi R. Dendritic chitosan as a magnetic and biocompatible nanocarrier for the simultaneous delivery of doxorubicin and methotrexate to MCF-7 cell line. New Journal of Chemistry 2017; 41:3177-89. doi: 10.1039/c6nj04107h [Crossref] [ Google Scholar]
- Schein CH. Repurposing approved drugs for cancer therapy. Br Med Bull 2021; 137:13-27. [ Google Scholar]
- Papanagnou P, Stivarou T, Papageorgiou I, Papadopoulos GE, Pappas A. Marketed drugs used for the management of hypercholesterolemia as anticancer armament. Onco Targets Ther 2017; 10:4393. [ Google Scholar]
- Singh TU, Parida S, Lingaraju MC, Kesavan M, Kumar D, Singh RK. Drug repurposing approach to fight COVID-19. Pharm Rep 2020; 72:1479-1508. doi: 10.1007/s43440-020-00155-6 [Crossref] [ Google Scholar]
- Correia AS, Gartner F, Vale N. Drug combination and repurposing for cancer therapy: the example of breast cancer. Heliyon 2021; 7:e05948. doi: 10.1016/j.heliyon.2021.e05948 [Crossref] [ Google Scholar]
- Zhang L, Chen Z, Wang H, Wu S, Zhao K, Sun H. Preparation and evaluation of PCL–PEG–PCL polymeric nanoparticles for doxorubicin delivery against breast cancer. RSC Adv 2016; 6:54727-37. doi: 10.1039/c6ra04687h [Crossref] [ Google Scholar]
- Gou M, Zheng X, Men K, Zhang J, Zheng L, Wang X. Poly(epsilon-caprolactone)/poly(ethylene glycol)/poly(epsilon-caprolactone) nanoparticles: preparation, characterization, and application in doxorubicin delivery. J Phys Chem B 2009; 113:12928-33. doi: 10.1021/jp905781g [Crossref] [ Google Scholar]
- Sabir F, Asad MI, Qindeel M, Afzal I, Dar MJ, Shah KU. Polymeric Nanogels as Versatile Nanoplatforms for Biomedical Applications. Journal of Nanomaterials 2019; 2019:1526186. doi: 10.1155/2019/1526186 [Crossref] [ Google Scholar]
- Agrawal YO, Mahajan UB, Agnihotri VV, Nilange MS, Mahajan HS, Sharma C. Ezetimibe-loaded nanostructured lipid carrier based formulation ameliorates Hyperlipidaemia in an experimental model of high fat diet. Molecules 2021; 26:1485. doi: 10.3390/molecules26051485 [Crossref] [ Google Scholar]
- Jia W, Gu Y, Gou M, Dai M, Li X, Kan B. Preparation of biodegradable polycaprolactone/poly (ethylene glycol)/polycaprolactone (PCEC) nanoparticles. Drug Deliv 2008; 15:409-16. doi: 10.1080/10717540802321727 [Crossref] [ Google Scholar]
- Gökçe Kocabay Ö, İsmail O. Preparation and optimization of biodegradable self-assembled PCL-PEG-PCL nano-sized micelles for drug delivery systems. International Journal of Polymeric Materials and Polymeric Biomaterials 2020; 70:328-37. doi: 10.1080/00914037.2020.1713784 [Crossref] [ Google Scholar]
- Fathi Karkan S, Davaran S, Akbarzadeh A. Cisplatin-loaded superparamagnetic nanoparticles modified with PCL-PEG copolymers as a treatment of A549 lung cancer cells. Nanomedicine Research Journal 2019; 4:209-19. doi: 10.22034/nmrj.2019.04.002 [Crossref] [ Google Scholar]
- Cohen-Sela E, Teitlboim S, Chorny M, Koroukhov N, Danenberg HD, Gao J. Single and double emulsion manufacturing techniques of an amphiphilic drug in PLGA nanoparticles: formulations of mithramycin and bioactivity. J Pharm Sci 2009; 98:1452-62. doi: 10.1002/jps.21527 [Crossref] [ Google Scholar]
- D’Souza S. a review of in vitro drug release test methods for nano-sized dosage forms. Advances in Pharmaceutics 2014; 2014:304757. doi: 10.1155/2014/304757 [Crossref] [ Google Scholar]
- Orzechowska EJ, Girstun A, Staron K, Trzcinska-Danielewicz J. Synergy of BID with doxorubicin in the killing of cancer cells. Oncol Rep 2015; 33:2143-50. doi: 10.3892/or.2015.3841 [Crossref] [ Google Scholar]
- Jin J, Sui B, Gou J, Liu J, Tang X, Xu H. PSMA ligand conjugated PCL-PEG polymeric micelles targeted to prostate cancer cells. PloS One 2014; 9:e112200. doi: 10.1371/journal.pone.0112200 [Crossref] [ Google Scholar]
- Masters JR. Human cancer cell lines: fact and fantasy. Nat Rev Mol Cell Biol 2000; 1:233-6. doi: 10.1038/35043102 [Crossref] [ Google Scholar]
- Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest Urol 1979; 17:16-23. [ Google Scholar]
- Chou T, Martin N. CompuSyn for drug combinations and for general dose-effect analysis, software and user’s guide: A computer program for quantitation of synergism and antagonism in drug combinations, and the determination of IC50 and ED50 and LD50 values. Paramus, NJ:ComboSyn Inc Paramus, NJ; 2005.
- Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 2006; 58:621-81. doi: 10.1124/pr.58.3.10 [Crossref] [ Google Scholar]
- Moghaddam SV, Abedi F, Alizadeh E, Baradaran B, Annabi N, Akbarzadeh A. Lysine-embedded cellulose-based nanosystem for efficient dual-delivery of chemotherapeutics in combination cancer therapy. Carbohydrate Polymers 2020; 250:116861. doi: 10.1016/j.carbpol.2020.116861 [Crossref] [ Google Scholar]
-
Lv L, Qiu K, Yu X, Chen C, Qin F, Shi Y, et al. Amphiphilic Copolymeric Micelles for Doxorubicin and Curcumin Co-Delivery to Reverse Multidrug Resistance in Breast Cancer. J Biomed Nanotechnol 2016. 12: 973-85. 10.1166/jbn.2016.2231.
- Hammersley D, Signy M. Ezetimibe: an update on its clinical usefulness in specific patient groups. Ther Adv Chronic Dis 2017; 8:4-11. [ Google Scholar]
- Shakiba E, Khazaei S, Hajialyani M, Astinchap B, Fattahi A. Preparation and in vitro characterization of retinoic acid-loaded poly(epsilon-caprolactone)-poly(ethylene glycol)-poly(epsilon-caprolactone) micelles. Res Pharm Sci 2017; 12:465-78. doi: 10.4103/1735-5362.217427 [Crossref] [ Google Scholar]
- Feng R, Song Z, Zhai G. Preparation and in vivo pharmacokinetics of curcumin-loaded PCL-PEG-PCL triblock copolymeric nanoparticles. Int J Nanomedicine 2012; 7:4089-98. doi: 10.2147/IJN.S33607 [Crossref] [ Google Scholar]
- Kunam V, Suryadevara V, Garikapati DR, Mandava VBR, Sasidhar RLC. Solubility and dissolution rate enhancement of ezetimibe by solid dispersion and pelletization techniques using soluplus as carrier. Int J Appl Pharmaceutics 2019; 11:57-64. doi: 10.22159/ijap.2019v11i4.32274 [Crossref] [ Google Scholar]
- Bansal R, Singh R, Kaur K. Quantitative analysis of doxorubicin hydrochloride and arterolane maleate by mid IR spectroscopy using transmission and reflectance modes. BMC Chem 2021; 15:27. doi: 10.1186/s13065-021-00752-3 [Crossref] [ Google Scholar]
- Asadi N, Annabi N, Mostafavi E, Anzabi M, Khalilov R, Saghfi S. Synthesis, characterization and in vitro evaluation of magnetic nanoparticles modified with PCL-PEG-PCL for controlled delivery of 5FU. Artif Cells Nanomed Biotechnol 2018; 46:938-45. doi: 10.1080/21691401.2018.1439839 [Crossref] [ Google Scholar]
-
Butt AM, Amin MCIM, Katas H, Sarisuta N, Witoonsaridsilp W, Benjakul R. In vitro characterization of pluronic F127 and D--tocopheryl polyethylene glycol 1000 succinate mixed micelles as nanocarriers for targeted anticancer-drug delivery. Journal of Nanomaterials 2012; 2012; 916573. 10.1155/2012/916573.
- Idris H. Experimental Studies of the Two Phase Flow and Monodispersed Pickering Emulsions Stabilized by LaponiteRD in a Microfluidic T-Junction. Institutt for fysikk; 2014.
- Xu J, Zhang S, Machado A, Lecommandoux S, Sandre O, Gu F. Controllable microfluidic production of drug-loaded PLGA nanoparticles using partially water-miscible mixed solvent microdroplets as a precursor. Sci Rep 2017; 7:4794. doi: 10.1038/s41598-017-05184-5 [Crossref] [ Google Scholar]
- Wu K, Yu L, Ding J. Synthesis of PCL–PEG–PCL Triblock Copolymer via Organocatalytic Ring-Opening Polymerization and Its Application as an Injectable Hydrogel—An Interdisciplinary Learning Trial. J Chem Educ 2020; 97:4158-65. doi: 10.1021/acs.jchemed.0c00325 [Crossref] [ Google Scholar]
- Danafar H, Khurana V. Preparation and characterization of PCL-PEG-PCL polymersomes for delivery of clavulanic acid. Cogent Medicine 2016; 3:1235245. doi: 10.1080/2331205x.2016.1235245 [Crossref] [ Google Scholar]
- Abedi F, Davaran S, Hekmati M, Akbarzadeh A, Baradaran B, Moghaddam SV. An improved method in fabrication of smart dual-responsive nanogels for controlled release of doxorubicin and curcumin in HT-29 colon cancer cells. J Nanobiotechnology 2021; 19:18. doi: 10.1186/s12951-020-00764-6 [Crossref] [ Google Scholar]
- Rahimi M, Karimian R, Mostafidi E, Bahojb Noruzi E, Taghizadeh S, Shokouhi B. Highly branched amine-functionalized p-sulfonatocalix[4]arene decorated with human plasma proteins as a smart, targeted, and stealthy nano-vehicle for the combination chemotherapy of MCF7 cells. New Journal of Chemistry 2018; 42:13010-24. doi: 10.1039/c8nj01790e [Crossref] [ Google Scholar]
- Rahimi M, Shafiei-Irannejad V, K DS, Salehi R. Multi-branched ionic liquid-chitosan as a smart and biocompatible nano-vehicle for combination chemotherapy with stealth and targeted properties. Carbohydr Polym 2018; 196:299-312. doi: 10.1016/j.carbpol.2018.05.059 [Crossref] [ Google Scholar]
-
Alibolandi M, Sadeghi F, Abnous K, Atyabi F, Ramezani M, Hadizadeh F. The chemotherapeutic potential of doxorubicin-loaded PEG-b-PLGA nanopolymersomes in mouse breast cancer model. Eur J Pharm Biopharm 2015. 94: 521-31.10.1016/j.ejpb.2015.07.005.
- Tian B, Ding Y, Han J, Zhang J, Han Y, Han J. N-Acetyl-D-glucosamine decorated polymeric nanoparticles for targeted delivery of doxorubicin: synthesis, characterization and in vitro evaluation. Colloids Surf B Biointerfaces 2015; 130:246-54. [ Google Scholar]
- Wang CH, Wang CH, Hsiue GH. Polymeric micelles with a pH-responsive structure as intracellular drug carriers. J Control Release 2005; 108:140-9. doi: 10.1016/j.jconrel.2005.07.017 [Crossref] [ Google Scholar]
- Wei R, Cheng L, Zheng M, Cheng R, Meng F, Deng C. Reduction-responsive disassemblable core-cross-linked micelles based on poly(ethylene glycol)-b-poly(N-2-hydroxypropyl methacrylamide)-lipoic acid conjugates for triggered intracellular anticancer drug release. Biomacromolecules 2012; 13:2429-38. doi: 10.1021/bm3006819 [Crossref] [ Google Scholar]