Bioimpacts. 13(5):393-403.
doi: 10.34172/bi.2023.25282
Original Article
Autophagy induced macrophages by α-alumina(α-AL2O3) conjugated cysteine peptidase, enhances the cytotoxic activity of CD8+ T lymphocytes against Leishmania major
Fatemeh Beyzay Formal analysis, Investigation, Writing – original draft, 
Ahmad Zavaran Hosseini Conceptualization, Methodology, Supervision, Writing – review & editing, , * 
Ali Hazrati Formal analysis, Investigation, Writing – original draft,
Mozhdeh Karimi Formal analysis, Investigation, Writing – original draft,
Sara Soudi Conceptualization, Methodology, Supervision, Writing – review & editing, , * 
Author information:
Department of Immunology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
Abstract
Introduction:
Induction of a protective immune response against Leishmania major requires the activation of both TH1 and CD8+ T lymphocytes. Because L. major is an intra-phagosomal parasite, its antigens do not have access to MHC-I. The present study aimed to evaluate the effect of cysteine peptidase A (CPA)/cysteine peptidase B (CPB) conjugated to α-AL2O3 on autophagy induction in L. major infected macrophages and subsequent activation of cytotoxic CD8+ T lymphocytes.
Methods:
Recombinant CPA and CPB of L. major were produced in expression vectors and purified. Aldehyde functionalized α-AL2O3 were conjugated to hydrazine-modified CPA/CPB by a chemical bond was confirmed by Fourier-transform infrared spectroscopy (FTIR). The High efficient internalization of α-AL2O3 conjugated CPA/CPB to macrophages was confirmed using a fluorescence microscope and flowcytometry. Induction of the acidic autophagosome and LC3 conversion in macrophages was determined by acridine orange (AO) staining and western blot. Autophagy-activated macrophages were used for CD8+ T cell priming. Cytotoxic activity of the primed CD8+ T cell against L. major infected macrophages was measured using apoptosis assay.
Results:
α-AL2O3 conjugated CPA/CPB enhances macrophages antigen uptake and increases acidic vacuole formation and LC-3I to LC-3II conversion. Co-culture of autophagy-activated macrophages with CD8+ T cells augmented CD8+ T cells priming and proliferation more than in other study groups. These primed CD8+ T cells induce significant apoptotic death of L. major infected macrophages compared with non-primed CD8+ T cells.
Conclusion:
α-AL2O3 nanoparticles enhance the cross-presentation of L. major antigens to CD8+ T cells by inducing autophagy. This finding supports the positive role of autophagy and encourages the use of α-AL2O3 in vaccine design.
Keywords: Leishmania major, Cysteine peptidase, α-Alumina, Autophagy, Cytotoxic T cell
Copyright and License Information
© 2023 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Leishmaniasis is known as a group of globally widespread parasitic diseases caused by different species of Leishmania, which is capable of infecting a variety of mammals.1,2 In both human and animal models of leishmaniasis, immunity is predominantly mediated by T lymphocytes.3 Both helper and cytotoxic T cells play a significant role in the clearance of intracellular parasitic infections and generating antigen-specific memory T-cell responses to inhibit the establishment of the same infection after the second exposure. Post Leishmania exposure, the different populations of CD4+ T cells, including Th1, Th2, Th17, and Tregs in both local and systemic secondary lymphoid tissue.4,5 According to various preclinical and clinical studies, the predominance of the Th1 population indicates resistance to leishmaniasis and provides protection.6,7 The most important reason is the production of high levels of interferon-gamma (IFN-γ), tumor necrosis factor-α (TNF-α), and interleukin-2 (IL-2) cytokines, which activate ROS and nitric oxide-mediated parasite killing mechanisms.8 In contrast, induction of Tregs promoted latent leishmaniasis by IL-10 production while restricted exacerbation of infection through inhibiting Th2 development and IL-4 production.9,10 The similar dual role in Leishmania infection is seen in the Th17 population. Although most evidence demonstrated the counter-protective activity of Th17, some evidence confirmed the therapeutic and protective effect of IL-17 production.11 CD8+ T cells are another lymphocyte population participant in the host defense against Leishmania species.3 These lymphocytes increase innate immune cells' killing activity by IFN-γ secretion and eliminate Leishmania-infected cells by cytotoxic activity.3,12 Numerous studies have shown that the formation of predominant Th1 responses alone is sufficient to overcome leishmaniosis.13 The role of CD8+ T cells becomes prominent when the host immune system fails to induce an effective Th1 response or the innate immune cells, including macrophages, neutrophils, and natural killer (NK) cells, have weak killing activity.14 However, the protective role for CD8+ T cells during Leishmania infection is still controversial and largely depends on the infection model.15 In this regard, Uzonna et al demonstrated that infection with a low dose of L. major terminated to predominant Th2 immune response and inhibition of parasite dissemination and disease recovery depends on CD8+ T cell expansion.16 Other experiments confirmed the protective role of IFN-γ production and Fas dependent cytotoxicity of CD8+ T cells in secondary L. major infection and lesion healing.17,18 According to evidence, there is a significant correlation between the recovery of patients with leishmaniasis and the apoptosis rate of infected macrophages induced by CD8+ T cells.3 Based on these results, induction and activation of CD8+ T lymphocytes were targeted for vaccine development against Leishmania. Vaccination with DNA encoding LACK antigen, heat-killed Leishmania antigen, and recombinant Leishmania proteins promoted protective immune responses mediated by CD8+ T-cell.19,20 Although cytotoxic T lymphocytes are induced against Leishmania antigens during natural infection, there are obstacles to the efficient antigen presentation through MHC-I pathway. Leishmania enters the antigen-presenting cells through phagocytosis and is enclosed in a phagosomal membrane.21 Therefore, Leishmania antigens cannot interact with major histocompatibility complex (MHC)-I molecules through the classical presentation of endogenous peptides. Cross-presentation is the main way to deliver intra-phagosomal antigen to the MHC-I molecules.22 During cross-presentation, Leishmania proteins are degraded by intra-phagosomal proteases or escape to the cytosol and convert into antigenic peptides by immunoproteasomes. In both situations, antigenic peptides are loaded onto the intra-vacuolar MHC-I in an unusual place.23 Therefore, the cross-presentation phenomenon is particularly important in immunity against Leishmania. So to circumvent this phenomenon, different species of Leishmania reduce the induction of cytotoxic T lymphocytes by inhibiting cross-presentation.24 The Leishmania metalloprotease GP63 cleaves phagosomal SNAREs, notably vesicle-associated membrane protein 8, and inhibits phagolysosome biogenesis and cross-presentation.24 Also, lipophosphoglycan and GP63 prevent phagosome acidification and subsequent Leishmania protein degradation.25,26 Therefore, enhancing antigens cross-presentation via MHC-I molecules is particularly important for optimal CD8+ T cells stimulation. In contrast to cross-presentation, during autophagy, cytosolic components or organelles are surrounded by the auto-phagosome, directed to the lysosome, and degraded by enzymatic digestion.27 Autophagy affects the outcome of immune responses by affecting the development and function of immune cells.28 Different evidence demonstrated an effective function of autophagy in MHC-II cross-presentation of cytosolic antigens and MHC-I cross-presentation of exogenous antigens.29,30 Although the mode of action of autophagy in the cross-presentation of exogenous antigens to MHC-I is not well understood, experimental findings suggest that activation of autophagy in herpes simplex virus-1 infected macrophages, human cytomegalovirus infected dendritic cells and other viral infections, plays an important role in CD8+ T-cell priming.31,32 In addition, induction of cytotoxic T cell responses by autophagy inducing adjuvants or vaccines confirmed the direct relation between autophagy and MHC-I cross-presentation.33 Nano-particles are among the most important activators and inhibitors of autophagy. Antigen or drug carrier metallic nanoparticles and their derivatives, including Au-NPs, C60-NPs, α-Al2O3-NPs, FeO-NPs, Si-NPs and Zn-NPs, can stimulate autophagy-related immune responses while enhancing the entry of antigen/drugs into the cell.34,35 This new approach in immunotherapy has been considered in the treatment of cancer while neglected in parasitic infections.36,37 The present study was designed to evaluate the effectiveness of using antigen-carrying nanoparticles in activating cytotoxic CD8+ T lymphocytes against L. major infected macrophages. The alumina nanoparticle was chosen as the antigen carrier because various studies have confirmed the potential in delivering antigen to the autophagosome and modulating autophagy.37,38 In addition, aluminium based adjuvants, including aluminium oxide (Al2O3), are safe and non-cytotoxic ingredients of human vaccines.39
Cysteine peptidase A and B (CPA and CPB), as one of the main virulence factors of L. major and vaccine candidates, were applied as antigens for conjugation to alumina.40 Different cysteine peptidase vaccines based on DNA, recombinant, and purified protein immunization have been designed and used in animal models against leishmaniasis.41,42 Although Th1 immunity and IFN-γ production were induced post this vaccination, they could not support an effective protective immunity.43
This research considered enhancing the immune response against L. major cysteine CPA and CPB by inducing autophagy in macrophages. To induce autophagy, recombinant L. major CPA and CPB conjugated to α-AL2O3 nanoparticle using Aldehyde/Hydrazine reaction. Then the efficacy of CPA/CPB- α-AL2O3 entrance to macrophages was assessed. The optimum amount of CPA/CPB- α-AL2O3 was used for autophagy induction in BALB/c peritoneal macrophages. Induction of autophagy was analyzed in CPA/CPB- α-AL2O3 treated macrophages by measuring acidic vacuole formation and LC3-I/LC3-II conversion using acridine orange (AO) and western blotting. Autophagy-activated macrophages were used for CD8+ T cell priming. Finally, induction of CD8+ T cell cytotoxic activity against L. major infected macrophages was determined.
Materials and Methods
Materials
All cell culture media and components were provided from Gibco, Life Technologies (Paisley, UK). qPCR SYBER Green master mix was purchased from BioFACT (Daejeon, Korea), and Taq DNA Polymerase Master Mix RED was obtained from Ampliqon. Well plates, cell culture flasks, and pipettes were purchased from SPL Life Sciences (Pocheon, South Korea).Anti-LC3 antibody (MAP LC3α/β SC-398822 F2918), Annexin V Apoptosis Detection Kit PE (eBioscience), CD8+ T cell isolation kit, MiniMACS (Miltenyi Biotec Inc cat NO: 130-096-543), AO (Invitrogen, USA).
CPA, CPB protein preparation, and conjugation
For this purpose, L. major promastigotes MHRO: IR: 75: ER were isolated from infected BALB/c mice as described previously.44 CPB and CPA coding regions were amplified using previously designed primers and cloned in the pET28a expression vector.44 After determining the accuracy of the inserted sequence, pET28a-CPB, and pET28a-CPA recombinant vectors were introduced into E. coli BL21 (DE3) and related proteins were purified by affinity chromatography on a Ni+2 resin column. The eluted rCPA, and rCPB were concentrated with Amicon and dialyzed against PBS.The eluted proteins were conjugated to α-AL2O3 nanoparticles. The size and morphology of conjugated nanoparticles and proper conjugation were confirmed using transmission electronic microscopy (TEM) and Fourier.The method and results of recombinant protein production and conjugation were described in detail in the previously published paper.
Detection of acidic vesicular organelles
Peritoneal macrophages from BALB/c mice (Experiments were carried out according to the instruction of the laboratory animal ethical commission of Tarbiat Modares University) were obtained by the peritoneal lavage technique based on our previous study.44 AO staining was used to determine the acidic vesicular organelles which increase during autophagy induction. Briefly, macrophages were cultured at 105 cells per well of 6 well-plates and allowed to attach by an overnight incubation and were treated with 3-methyladenine (3-MA) (10 mM), rapamycin (50 nM ), α-AL2O3 (100 μg), α-AL2O3 conjugated with CPA (100 μg containing 8 μg CPA protein), α-AL2O3 conjugated with CPB (100 μg containing 8 μg CPB protein), CPA (8 μg), CPB protein (8 μg), for 48 hours. Then the cells were treated with 1 μg/mL AO (Invitrogen, USA) in PBS for 20 minutes. Red and green fluorescent vesicles were analyzed using a fluorescence microscope (Zeiss, Germany) and reported as a percentage of red to green fluorescent ratio by ImageJ software.
Determination of LC3I/LCII conversion by western blotting
To determine the effect of α-AL2O3 nanoparticles on the induction of autophagy in macrophages, conversion of the unlipidated form of LC3 (LC3-I) into the lipidated form (LC3-II) was assessed using western blotting.44 Peritoneal macrophages of different experimental groups were harvested, washed, and treated as mentioned above experimental groups. After 48h, macrophages were detached by ice-cold PBS and were centrifuged twice at 3000 rpm at 4°C. The pellet was suspended in lysis RIPA buffer at 4°C 5 times and then shifted to 95°C. The protein concentration of each sample was determined using BCA Protein Quantification Kit (Parstous Biotech). 10 µg of proteins from each sample were fractionated on 10% SDS–PAGE gels and transferred to nitrocellulose membrane by western blotting. The membrane was subjected to an anti-LC3 antibody (MAP LC3α/β SC-398822 F2918) overnight at 4°C, followed by anti-mouse IgG-HRP staining for 1 hour at room temperature. Finally, LC3protein visualization was performed by ECL kits.
Evaluation of the cytotoxic effect of α-AL2O3 on macrophage
To determine the cytotoxic effect of α-AL2O3 on macrophage cultures, the impact of different treatments on macrophage apoptosis and necrosis was assessed.For this purpose, Annexin V Apoptosis Detection Kit PE (eBioscience) was used. In brief, 105 macrophages were treated with α-AL2O3 (100 μg), α-AL2O3conjugated with CPA (100 μg containing 8 μg CPA protein), α-AL2O3 conjugated with CPB (100 μg containing 8 μg CPB protein), CPA (8 μg), CPB protein (8 μg). After 72 hours, the macrophages were washed and resuspended in the binding buffer (100 µL of calcium buffer containing 10 mM HEPES/NaOH, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2). Then, annexin-V (5 µl) was added to the cells, followed by 5 µL 7-AAD. The samples were incubated for 10 minutes in the dark at 4°C and then subjected to flowcytometry evaluation.
In vitro naive T CD8+ cells proliferation assay
Naive CD8+ T cells (CD44-, CD8+, CD28+) were enriched to more than 90% purity from the spleen by depletion of non-target cells using the Naïve CD8+ T cell isolation kit, MiniMACS (Miltenyi Biotec Inc cat NO: 130-096-543). Non-target cells, including T helper cells, B cells, NK cells, macrophages, granulocytes, endothelial cells, and erythroid cells, are magnetically labeled with a cocktail of biotin-conjugated monoclonal antibodies. Simultaneously, CD44 microbeads were added to label memory T cells and their separation. Purified naïve CD8+ T cells were washed with PBS and resuspended in PBS containing 1 mM carboxyfluorescein diacetate succinimidyl ester (CFSE). 45 The cell suspension was incubated at 37°C for 10 min and immediately washed with cold RPMI 1640/10% FCS before plating. 5×105 macrophages were incubated with α-AL2O3conjugated with CPA, α-AL2O3conjugated with CPB, CPA, and CPB protein for 6 h in the presence or absence of 3-MA, then washed three times and co-incubated for 60 hours with 106 CFSE labeled naïve CD8+ T cell. The percentages of divided naive CD8+ T cells were determined by flowcytometry analysis. Co-incubated non-treated macrophages with naïve CD8+ T cells and only naïve CD8+ T cell culture were used as control.
Killing activity of CD8+ T cells on L. major infected macrophages
For this purpose, 105 macrophages/wells of 6-well plates were cultured as described previously and were exposed to the stationary phase promastigotes of L. major in a 1:10 ratio. After one hour, the parasites were removed and replaced with 106 preactivated CD8+ T cells. To produce activated CD8+ T cells, the cells were co-cultured with pre-treated macrophages with α-AL2O3 conjugated with CPA, α-AL2O3 conjugated with CPB, CPA, and CPB protein. After 48 hours, activated CD8+ T cells were collected and added to L. major infected macrophages. The percentage of apoptosis of infected macrophages was measured to show the killing activity of pre-activated CD8+ T. The experiment was repeated three times. The results were reported as the mean percentage of apoptosis.
Statistical analysis
All in vitro experiments were repeated three times in triplicate. The obtained data were reported as mean ± SD and analyzed by SPSS software version 14.0. Statistical significance was set at the level of P ≤ 0.05.
Results
α-AL2O3 conjugation increases acidic vesicles
To examine the effect of α-AL2O3 conjugation on induction of autophagy, peritoneal macrophages of BALB/c mice were treated with different forms of α-AL2O3 conjugates mentioned in the 2.2 section. As the lysosomotropic dye, AO gives a green fluorescent colour at physiological PH but becomes protonated and emits bright red fluorescence within an acidic environment. So, AO staining was used to detect the amount of autophagolysosome induction. For this purpose, the stained specimens were examined by fluorescence microscopy, and photographs of different sections of each sample were stored. At least three images from each experimental group were analyzed by ImageJ software, and the ratio of red to green fluorescent vesicles was calculated. The mean ratio of red to green fluorescent vesicles were 0.05, 0.01, 20, 3.25, 1.16, 0.77, and 0.43 for non-treated, 3-MA, rapamycin, α-AL2O3, α-AL2O3+ CPA, α-AL2O3+ CPB, CPA and CPB groups, respectively (Fig. 1I). Both visual examination and statistical analysis of mean ratios show a significant increase (P≤ 0.05) in the red colour vesicles in rapamycin (50nM, a well-known inhibitor of the PI3K-mTOR pathway) treated macrophages as autophagy inducer compared to other groups (Fig. 1C).19 α-AL2O3 and 3-MA, which potently inhibit autophagy-dependent protein degradation and suppress the formation of autophagosomes, showed the lowest amount of red vacuoles as the untreated group (Fig. 1A, B, D), Post hoc analysis with ANOVA showed that although CPA and CPB antigens induce the formation of autophagosomes (Fig. 1G, H)., their conjugated form with α-AL2O3 causes a significant increase (P≤ 0.05) in this process (Fig. 1E, F; Table 1).
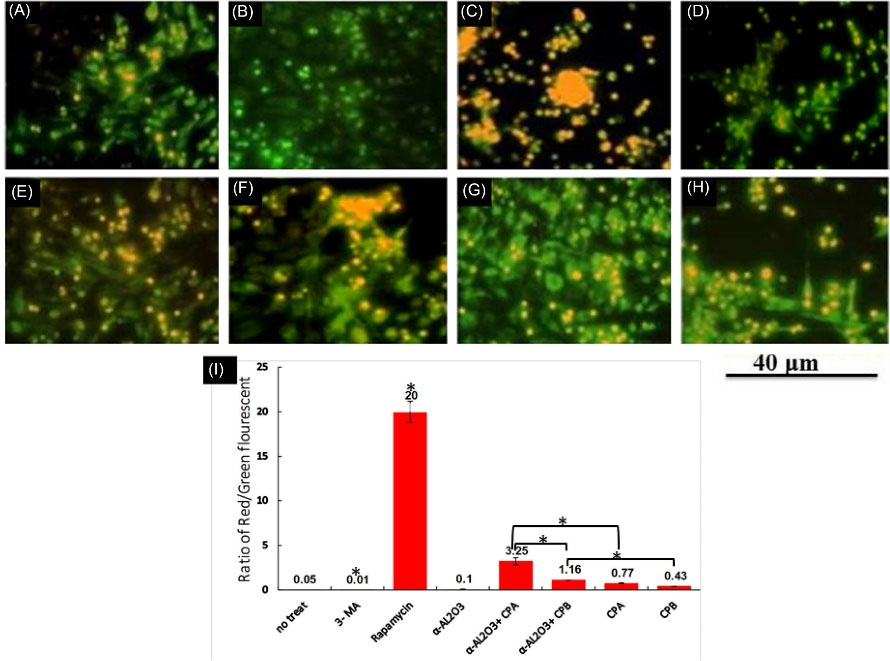
Fig. 1.
Fluorescence microscopy of stained peritoneal macrophages with acridine orange/ethidium bromide.Untreated macrophages (A) or macrophages treated with 3-MA(B), rapamycin (C), α-AL2O3 (D), α-AL2O3-CPA (E), α-AL2O3-CPB (F), CPA (G), CPB(H), for 48 h. This experiment was performed in triplicate. For each repetition, different microscopic images were prepared and examined. The mean ratio of red/ green fluorescent of experimental groups was estimated by ImageJ software (I). Statistically significant groups were determined using post hoc tests with ANOVA. Star (*) indicates that this group is statistically different (P ≤ 0.05) from other groups. The star on the bracket shows a statistically significant difference (P ≤ 0.05) between the two comparing groups.
.
Fluorescence microscopy of stained peritoneal macrophages with acridine orange/ethidium bromide.Untreated macrophages (A) or macrophages treated with 3-MA(B), rapamycin (C), α-AL2O3 (D), α-AL2O3-CPA (E), α-AL2O3-CPB (F), CPA (G), CPB(H), for 48 h. This experiment was performed in triplicate. For each repetition, different microscopic images were prepared and examined. The mean ratio of red/ green fluorescent of experimental groups was estimated by ImageJ software (I). Statistically significant groups were determined using post hoc tests with ANOVA. Star (*) indicates that this group is statistically different (P ≤ 0.05) from other groups. The star on the bracket shows a statistically significant difference (P ≤ 0.05) between the two comparing groups.
Table 1.
Percentage of macrophages that internalized the FITC labeled AL2O3-CPA/CPB at different concentrations by flowcytometry analysis
|
Concentration(μg/mL)
|
%Internalization α-AL2O3-CPA-FITC
|
%Internalization α-AL2O3-CPB-FITC
|
| 1 |
15% |
8% |
| 5 |
27% |
33.3% |
| 10 |
61% |
90.3% |
| 100 |
95% |
94.55% |
| 200 |
98% |
96.7% |
α-AL2O3 induces the conversion of LC3-I to LC3-II
Following autophagy induction, the unlipidated form of LC3 (LC3I) is converted to its lapidated form (LC3II), indicating the formation of autophagosomes. Although LC3II has more molecular weight than LC3I, it moves faster on the electrophoresis gel due to its spatial shape. So measurement of the conversion of LC3I to LC3II represents autophagy induction. As indicated in Fig. 2A, the LC3I to LC3II conversion was induced in all experimental groups compared with non-treated and 3-MA treated macrophages. Since the amount of protein used in western blotting was the same for all experimental groups, semi-quantitative analysis of the bands was done by mean grey value measurement by ImageJ software (Fig. 2B). Statistical analysis of densitometry results demonstrated a significant increase (P≤ 0.05) in LC3I to LC3II conversion in the α-AL2O3+ CPB treated macrophages compared to other experimental groups. There was no significant difference in LC3I/LC3II protein level between rapamycin and autophagy inducers with α-AL2O3, CPB, CPA, and α-AL2O3+ CPA treated groups. This experiment was performed in triplicate. Statistically significant (P≤ 0.05) groups were determined using the ANOVA test.
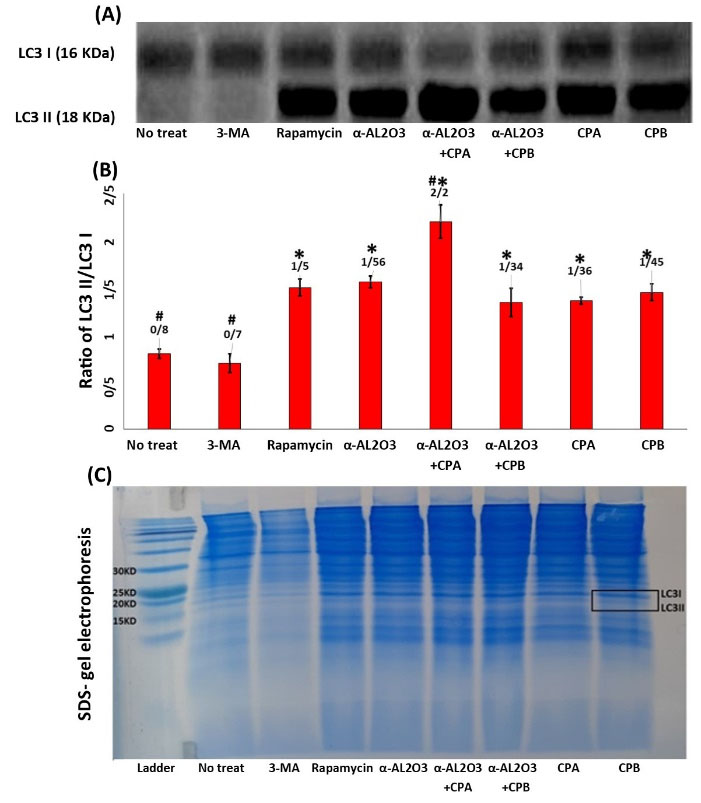

Fig. 2.
Western blot analysis of LC3 protein expression in the lysate of macrophages of different experimental groups. Representation of protein level of LC3I (18 kDa) and LC3II (16 kDa) bands on a nitrocellulose membrane by western blotting (A). Densitometry analysis of LC3I/LC3II level by ImageJ software (B). After detection and imaging, the bands only appeared within the range of 15 kDa and 20 kDa in the blot. Then the part that included these bands was cropped and examined with ImageJ software. However, compared with SDS-electrophoresis gel and protein ladder, the bands that appeared were within the range of 15 kDa and 20 kDa, which are estimated to represent LC3I and LC3II at 16 and 18 kDa, respectively. To confirm this, two of the SDS- gel electrophoresis images of the experiment (macrophage extract) (C). This experiment was performed in triplicate. Statistically significant groups were determined using the ANOVA test. Star (*) indicates that this group is significantly different (P ≤ 0.05) from 3-MA. Hash (#) indicates that this group is significantly different (P ≤ 0.05) from Rapamycin.
.
Western blot analysis of LC3 protein expression in the lysate of macrophages of different experimental groups. Representation of protein level of LC3I (18 kDa) and LC3II (16 kDa) bands on a nitrocellulose membrane by western blotting (A). Densitometry analysis of LC3I/LC3II level by ImageJ software (B). After detection and imaging, the bands only appeared within the range of 15 kDa and 20 kDa in the blot. Then the part that included these bands was cropped and examined with ImageJ software. However, compared with SDS-electrophoresis gel and protein ladder, the bands that appeared were within the range of 15 kDa and 20 kDa, which are estimated to represent LC3I and LC3II at 16 and 18 kDa, respectively. To confirm this, two of the SDS- gel electrophoresis images of the experiment (macrophage extract) (C). This experiment was performed in triplicate. Statistically significant groups were determined using the ANOVA test. Star (*) indicates that this group is significantly different (P ≤ 0.05) from 3-MA. Hash (#) indicates that this group is significantly different (P ≤ 0.05) from Rapamycin.
Apoptosis increases in the presence of α-AL2O3
The rate of cell death (apoptosis and necrosis) was assessed using an apoptosis detection kit to evaluate the cytotoxic effect of different treatments on macrophages. For this purpose, 72 hours after treatment of macrophages with previously mentioned stimulators, cells were stained with annexin-V/7-AAD and analyzed by flow cytometry. An example of gating of macrophages and annexin-V/7-AAD positive cell populations is shown in Fig. 3. This experiment was performed three times, and the mean results were reported in Fig. 3B. As indicated in the result, non-treated macrophages represented about 0.06% of cell death in both apoptosis and necrosis. The increase in apoptosis and necrosis of other experimental groups, including macrophages treated with rapamycin, CPA, CPB, α-AL2O3-CPA/CPB, was very small and ineffective. While, the apoptosis rate in macrophages treated with 3-MA and α-AL2O3 was significantly (P≤ 0.05) increased by 20% and 6.8%, respectively. The results show that α-AL2O3 alone can induce apoptosis in macrophages, but its cytotoxic effect disappears after conjugation (P≤ 0.05).
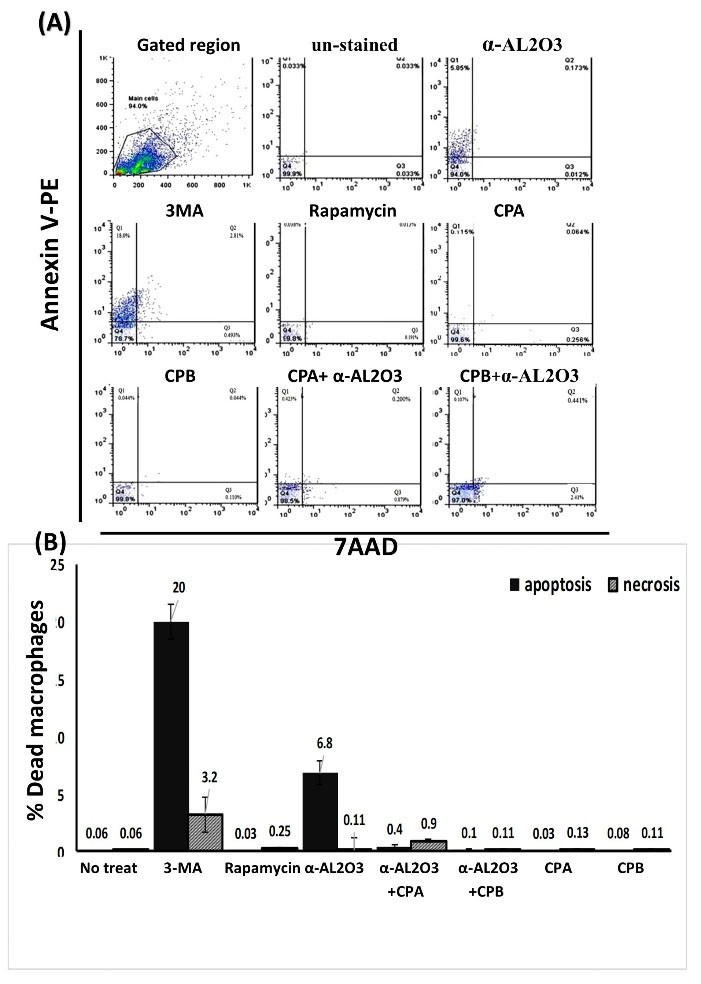
Fig. 3.
Flowcytometry analysis of annexin-v/ 7-AAD expression in macrophages of different experimental groups. Macrophages were treated with α-AL2O3, α-AL2O3-CPA/CPB, soluble CPA, CPB, 3-MA, and rapamycin for 72 h and examined for detection of apoptotic macrophages using Annexin V-PE apoptosis detection kit. Dot plot analysis represented the total population of early apoptotic cells (Q1), late apoptotic cells (Q2), necrotic cells (Q3), and viable cells (Q4) (A). The mean percent of apoptotic and necrotic macrophages of different experimental groups is shown in the bar chart (B). This experiment was performed in triplicate. Statistically significant groups were determined using the ANOVA test. Star (*) indicates that amount of necrosis in this group is significantly different (P≤0.05) from other groups. Hash (#) indicates that amount of apoptosis in this group is significantly different (p≤0.05) from other groups.
.
Flowcytometry analysis of annexin-v/ 7-AAD expression in macrophages of different experimental groups. Macrophages were treated with α-AL2O3, α-AL2O3-CPA/CPB, soluble CPA, CPB, 3-MA, and rapamycin for 72 h and examined for detection of apoptotic macrophages using Annexin V-PE apoptosis detection kit. Dot plot analysis represented the total population of early apoptotic cells (Q1), late apoptotic cells (Q2), necrotic cells (Q3), and viable cells (Q4) (A). The mean percent of apoptotic and necrotic macrophages of different experimental groups is shown in the bar chart (B). This experiment was performed in triplicate. Statistically significant groups were determined using the ANOVA test. Star (*) indicates that amount of necrosis in this group is significantly different (P≤0.05) from other groups. Hash (#) indicates that amount of apoptosis in this group is significantly different (p≤0.05) from other groups.
Peritoneal macrophages induce antigen-specific activation of CD8+ T Cells upon uptake of α-AL2O3-CPA/CPB in vitro
To evaluate the effect of different treatments on the ability of macrophages to stimulate CD8+ T cells, peritoneal macrophages were treated for 6h with different treatments and then co-cultured for an additional 60 h with CFSE-labeled naïve CD8+ T cells. Subsequently, CD8+ T cell proliferation was analyzed by flowcytometry. To determine the role of autophagy-induced macrophages in antigen presentation and activation of CD8+ T cell, both autophagy inhibitor (3-MA) and autophagy activator (rapamycin) were used. To evaluate cell proliferation, the fluorescent intensity of the cells was first recorded immediately after being labeled by CFSE. Changes in the fluorescent intensity of lymphocytes co-cultured with treated macrophages are compared to the initial position. According to the obtained results, macrophages treated with α-AL2O3-CPA/CPB induced proliferation of naïve CD8+ T cells more efficiently than macrophages treated with α-AL2O3, CPA, or CPB alone (P≤ 0.05). Post hoc analysis of obtained results indicated that the presence of 3-MA in the culture of AL2O3-CPA/CPB, CPA, and CPB stimulated macrophages reduced significantly (P≤ 0.05) the proliferation of CD8+ T cells compared with the non-3MA containing groups. Flow cytometry analysis of CFSE labeled CD8+ T cell stimulated with non-treated macrophages, and PHA is shown in Fig. 4A. A sample of the histogram of CFSE labeled CD8+ T cell after co-culture with different antigen primed macrophages is shown in Fig. 4B. This experiment was performed in triplicate, and the mean ± SD of the percentage of CD8+ T cell proliferation is represented in Fig. 4C.
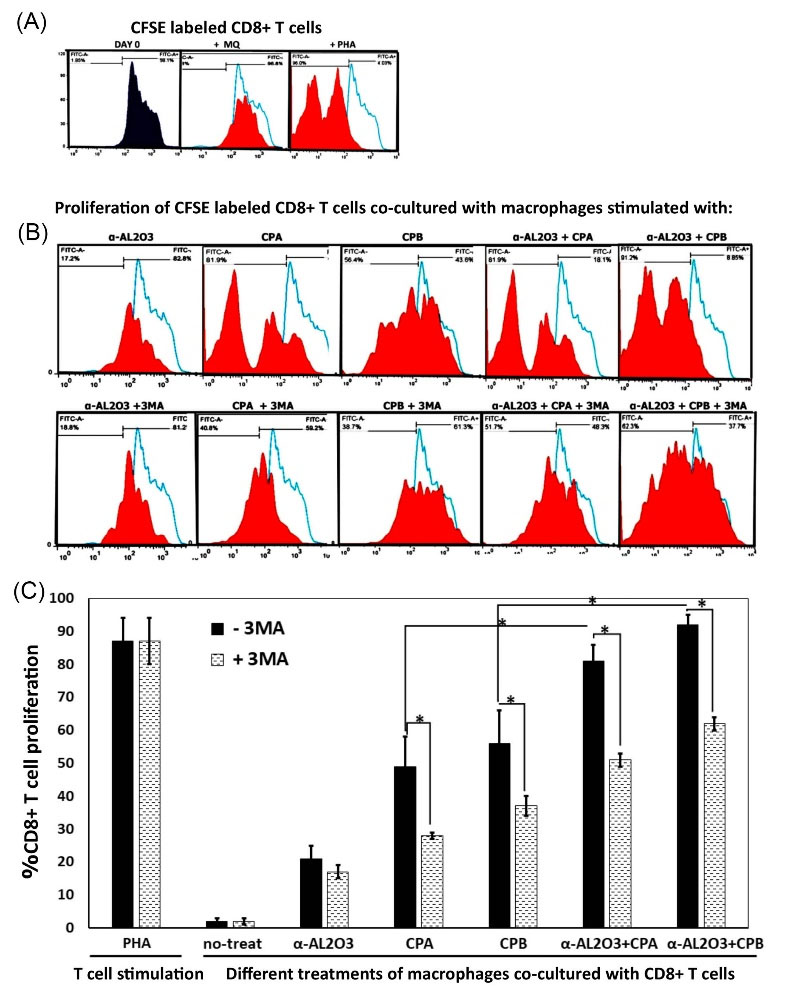

Fig. 4.
Flowcytometry analysis of the proliferation of CFSE labeled CD8+ T cells. Macrophages were treated with α-AL2O3, α-AL2O3-CPA/CPB, soluble CPA, CPB, 3-MA, and rapamycin for 6 h, then co-cultured with CFSE labeled CD8+ T lymphocytes.60 hours later, lymphocyte proliferation was determined using CFSE dilution analysis. Histogram analysis of CFSE labeled CD8+ T cells at day 0 of CFSE labeling and 48 hours post-PHA stimulation as the positive control (A & B). Histogram analysis of CFSE labeled CD8+ T cells proliferation after 60 hours of co-culturing with differently treated macrophages in the presence or absence of autophagy inhibitor (3-MA) (C). This experiment was performed in triplicate. The bar chart represented the mean percent of CD8+ T cells proliferation co-cultured with treated macrophages. Statistically significant groups were determined using post hoc tests with ANOVA. Star (*) indicates that this group is statistically different (P ≤ 0.05) from other groups. The star on the bracket shows a statistically significant difference (P ≤ 0.05) between the two comparing groups.
.
Flowcytometry analysis of the proliferation of CFSE labeled CD8+ T cells. Macrophages were treated with α-AL2O3, α-AL2O3-CPA/CPB, soluble CPA, CPB, 3-MA, and rapamycin for 6 h, then co-cultured with CFSE labeled CD8+ T lymphocytes.60 hours later, lymphocyte proliferation was determined using CFSE dilution analysis. Histogram analysis of CFSE labeled CD8+ T cells at day 0 of CFSE labeling and 48 hours post-PHA stimulation as the positive control (A & B). Histogram analysis of CFSE labeled CD8+ T cells proliferation after 60 hours of co-culturing with differently treated macrophages in the presence or absence of autophagy inhibitor (3-MA) (C). This experiment was performed in triplicate. The bar chart represented the mean percent of CD8+ T cells proliferation co-cultured with treated macrophages. Statistically significant groups were determined using post hoc tests with ANOVA. Star (*) indicates that this group is statistically different (P ≤ 0.05) from other groups. The star on the bracket shows a statistically significant difference (P ≤ 0.05) between the two comparing groups.
CD8+ T cells educated with α -AL2O3-CPA/CPB primed macrophages induce apoptosis of L. major infected macrophages
The cytotoxic ability of educated CD8+ T cells by nanoparticle/Ag-loaded macrophages was assessed by measurement of apoptosis of L. major infected macrophages. As indicated in Fig. 5, α -AL2O3 treated and non-treated macrophages could not affect the cytotoxic activity of CD8+ T cells. Whereas CPA, CPB, and α-AL2O3-CPA/CPB treated macrophages induce a significant (P≤ 0.05) increase in the cytotoxic activity of CD8+ T cells with apoptotic death of 32%, 53%, 74%, and 90% of L. major infected macrophages, respectively. The highest cytotoxic activity was observed in CD8+ T cells stimulated with macrophages treated with α-AL2O3 conjugated CPA or CPB. A decrease in apoptosis induction by CD8+ T cells stimulated with macrophages in the presence of autophagy inhibitor (3-MA) demonstrated the role of autophagy in CD8+ T cells activation.
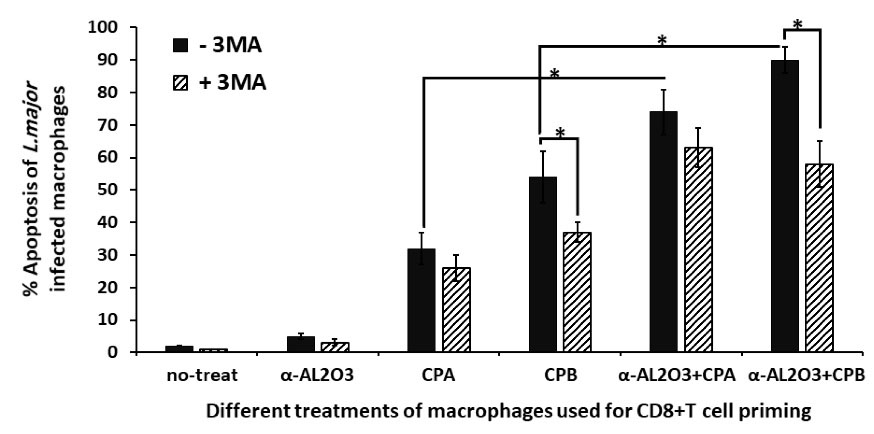
Fig. 5.
Cytotoxic activity of educated CD8+ T cells on L. major infected macrophages.CD8+ T lymphocytes were pre-activated with macrophages of different experimental groups, then co-cultured with L. major infected macrophages in a 10 to 1 ratio. 48 hours later, CD8+ T lymphocytes were removed, and apoptosis of L. major infected macrophages was determined. This experiment was performed in triplicate. The bar chart represented the mean ± SD percent of apoptosis of infected macrophages. A statistically significant difference (P≤0.05) between the two comparing groups was denoted by the star (*). The X-axis demonstrated different treatments of macrophages that applied for CD8+ T cells priming in the presence or absence of autophagy inhibitor (3-MA).
.
Cytotoxic activity of educated CD8+ T cells on L. major infected macrophages.CD8+ T lymphocytes were pre-activated with macrophages of different experimental groups, then co-cultured with L. major infected macrophages in a 10 to 1 ratio. 48 hours later, CD8+ T lymphocytes were removed, and apoptosis of L. major infected macrophages was determined. This experiment was performed in triplicate. The bar chart represented the mean ± SD percent of apoptosis of infected macrophages. A statistically significant difference (P≤0.05) between the two comparing groups was denoted by the star (*). The X-axis demonstrated different treatments of macrophages that applied for CD8+ T cells priming in the presence or absence of autophagy inhibitor (3-MA).
Discussion
Induction of a protective immune response against L. major requires the activation of both Th1 and cytotoxic T lymphocyte populations. Therefore, efficient antigen presentation is required from both MHC-I and MHC-II pathways. Because Leishmania is a mandatory intracellular parasite, it is usually captured, infected, and removed by phagocytes (such as macrophages) and lives inside their phagosomes.46 As mentioned, because L. major is an intra-phagosomal parasite, its antigens do not have access to MHC-I classically. Cross-presentation of antigens is the cell's strategy to make intra-phagosomal antigens available to MHC-I and intra-cytosolic antigens to MHC-II.47 As mentioned before, autophagy facilitates the cross-presentation of antigens as a cellular hemostatic process. Although autophagy is the host's natural response to infection-induced stress, the involvement of pathogens in autophagy as an escape mechanism necessitates manipulating this process for infection control. Nanoparticles, including metallic nanoparticles, are effective tools for inducing or inhibiting autophagy. In the present study, α-AL2O3 nanoparticles were used as Leishmania CPA/CPB antigens carrier and inducer of autophagy in macrophages as the main host of L. major. Autophagy-activated macrophages were then used to activate CD8+ T lymphocytes. Finally, the apoptosis detection kit measured the cytotoxic activity of CD8+ T cells against L. major infected macrophages. Recombinant CPA/CPB antigens production and conjugation to α-AL2O3 were produced and qualified according to the previously published paper.44 A study by Li et al showed that α-AL2O3 nanoparticles effectively induce autophagy.38 Also, other studies have shown that alum can induce NLRP3-mediated inflammasome and IL-1β, which results in an increase in autophagy.48 In addition, the ability of different concentrations of antigen and their α-AL2O3 conjugates to enter the macrophage was determined in the mentioned paper. The amount of antigens and α-AL2O3 that were not toxic to macrophages and entered efficiently were selected for treatment of macrophages in the current study. There were no studies on induction of autophagy by CPA and CPB in macrophages before this work, but Williams reported their effects in facilitating effective differentiation in L. Mexicana through autophagy.40 BALB/c isolated macrophages were grouped and treated with 3-MA, rapamycin, α-AL2O3 conjugated with CPA, α-AL2O3 conjugated with CPB, CPA, and CPB protein for 48 hours. To evaluate the effect of treatments on autophagy induction, both AO staining and LC3 conversion were used. Since the results of both methods are qualitative, they were semi-quantified with the help of ImageJ software for a more detailed evaluation. The ratios obtained from these two methods were not the same because the ratio of change in acidic vacuoles is not necessarily the same as the ratio of change in LC-3 conversion. Regarding the finding of AO staining, it should be noted that not all active lysosomal vacuoles have resulted from autophagy, while LC-3 conversion is directly related to autophagy.49 However, both experiments confirmed the acidic vacuole formation and significant changes in LC-3 conversion in rapamycin, CPA/CPB, and its α-AL2O3 conjugated form compared to 3-MA and no-treat groups. It seems that an exaggerated significant increase in acidic vacuole content in the rapamycin group is related to vacuolar fragmentation under the TOR network activation by rapamycin.50 However, both rapamycin and α-AL2O3 show the same LC3 conversion ratio. According to obtained results, both CPA and CPB antigens can trigger LC3 conversion similar to α-AL2O3 or rapamycin. Although the mechanism of induction of autophagy by CPA /CPB antigens is unknown, it may have promoted the autophagy process through the intervention in the autophagy pathway or interaction with macrophage TLR receptors.51-53 Different studies demonstrated the ability of α-Al2O3 to induction autophagy. Although the CPA/CPB conjugated with α-Al2O3 shows higher acidic vacuole formation compared with CPA/CPB alone, the increase in LC3 conversion was only observed in α-Al2O3+CPA compared with CPA alone. These differences indicate that the CPA/CPB antigens target the autophagy pathway differently from the α-Al2O3 conjugated form. After confirmation of the autophagy enhancement in treated macrophages, the effect of these treatments on macrophage viability was determined using an apoptosis detection kit.
As shown in Fig. 3, a significant apoptotic death was observed in 3-MA and α-Al2O3 treated macrophages, while other treatments had no significant effect on viability. The increase in apoptosis in the presence of the 3-MA seems to be due to the lack of a pro-survival role of autophagy and the loss of inhibition from the apoptotic pathway.54 Regarding the toxicity of α-Al2O3, several studies have shown the effect of α-Al2O3 on apoptosis and necrosis of cells in size and concentration-dependent manner.55 Finally, autophagy-activated macrophages co-cultured with CD8+ T cells. According to the flowcytometry analysis of CFSE labeled CD8+ T cells, α-Al2O3, CPA, CPB, α-Al2O3+CPA, and α-Al2O3+CPB stimulated CD8+ T cells priming and proliferation. The higher CD8+ T cells priming ability is related to α-Al2O3+CPA and α-Al2O3+CPB treated macrophages, indicating the higher enhancement of MHC-I antigen presentation compared with CPA or CPB alone.56 As expected from the LC3 conversion results, CPA/CPB treatment alone could induce MHC-I antigen presentation and CD8+ T cells priming. In all experimental groups, the amount of CD8+ T cell proliferation showed a significant reduction in the presence of 3-MA as an autophagy inhibitor.
However, the CD8+ T cell proliferation in the presence of 3-MA never stopped. This finding suggests that the applied treatments used different ways to enhance antigen cross-presentation, including autophagy.22 The killing activity of CD8+ T cells against L. major infected macrophages can be mediated by granule-dependent cytotoxicity or Fas-FasL interaction. The result of both Fas-FasL interaction and perforin-granzyme B secretion terminated to induction of apoptosis in infected macrophages.12,57 Therefore, the apoptosis rate of L. major infected macrophages was determined after the co-culture with activated CD8+ T cells. According to obtained results, there was a direct correlation between CD8+ T cell activation and apoptosis induction. Both CPA and CPB antigens treated macrophages could prime cytotoxic activity of CD8+ T cells. However, a higher rate of apoptotic induction was observed in the population of CD8+ T cells that α-Al2O3+CPA and α-Al2O3+CPB treated macrophages triggered. Although we did not track the presentation of CPA/CPB epitopes on the macrophage MHC-I molecules in this experiment, the difference between the results obtained from CPA and CPB priming confirmed the antigen-specific responses of the CD8+ T cells. Significant reduction in the apoptotic rate of L. major infected macrophages in 3-MA treated groups emphasized the involvement of the autophagy pathway on MHC-I cross-presentation and CD8+ T cells cytotoxicity. The current research findings are inconsistent with previous studies on the role of α-Al2O3 as an efficient antigen delivery tool and enhancer of MHC-I cross-presentation to CD8+ T lymphocytes.38 According to the present study results, as a perspective, after extracting macrophages and activating them by α-Al2O3 conjugated with CPA/CPB, they can be injected into mice with Leishmania infection and study the infection and recovery process in them.
Conclusion
CPA and CPB are among the virulent factors and vaccine candidates against L. major infection. This study tried to improve the MHC-I cross-presentation of antigens to CD8+ T lymphocytes by conjugating these antigens to α-Al2O3. While CPA and CPB antigens trigger autophagy and MHC-I cross-presentation, its α-Al2O3conjugated forms augmented this process, leading to higher CD8+ T cells cytotoxic activity. Applying 3-MA in combination with different antigenic treatments showed that inhibiting autophagy reduces antigen cross-presentation and CD8+ T lymphocyte stimulation but does not disappear completely. It is suggested that antigens use different methods of cross-presentation to CD8+ T lymphocytes which are not limited to autophagy. Unequal levels of LC3 conversion and CD8+ T cells priming by CPA and CPB antigens reflected the distinctive mode of their intervention in autophagy and cross-presentation. Evaluation of the effect of α-Al2O3 conjugated with CPA/CPB on the induction of CD4+ T lymphocyte responses and the outcome of vaccination in the L. major susceptible and resistance mouse model is missing in this study, which will be considered in the future.
Research Highlights
What is the current knowledge?
√ Many nanomaterials were reported to change the basal level of autophagy.
√ Correlation between α-AL2O3 nanoparticles and mitochondria dysfunction that leads to autophagy increasement.
√ AL2O3 induces autophagy effectively and exhibits potent antitumor capability.
What is new here?
√ Alpha-alumina conjugated to cysteine peptidase A and B antigens of L. major, can internalize to macrophages and induce autophagy.
√ Autophagy-induced macrophages activate CD8+ T lymphocyte proliferation and cytotoxic properties.
√ Activated CD8+ T lymphocyte induces apoptotic death of L. major infected macrophages.
Acknowledgments
This research was supported by Tarbiat Modares University, Faculty of Medical Sciences.
Competing Interests
The authors declare no conflict of interest.
Ethical Statement
Not applicable. This paper does not involve research on humans.
Funding
This research was supported by Tarbiat Modares University, Faculty of Medical Sciences.
References
- Maraghi S, Mardanshah O, Rafiei A, Samarbafzadeh A, Vazirianzadeh B. Identification of cutaneous leishmaniasis agents in four geographical regions of Khuzestan province using Nested PCR. Iran J Parasitol 2016; 11:65-72. [ Google Scholar]
- Mutiso JM, Macharia JC, Kiio MN, Ichagichu JM, Rikoi H, Gicheru MM. Development of Leishmania vaccines: predicting the future from past and present experience. J Biomed Res 2013; 27:85. doi: 10.7555/JBR.27.20120064 [Crossref] [ Google Scholar]
- Ruiz J, Becker I. CD8 cytotoxic T cells in cutaneous leishmaniasis. Parasite Immunol 2007; 29:671-8. doi: 10.1111/j.1365-3024.2007.00991.x [Crossref] [ Google Scholar]
- Glennie ND, Volk SW, Scott P. Skin-resident CD4+ T cells protect against Leishmania major by recruiting and activating inflammatory monocytes. PLoS Pathog 2017; 13:e1006349. doi: 10.1371/journal.ppat.1006349 [Crossref] [ Google Scholar]
- Alexander J, Brombacher F. T helper1/t helper2 cells and resistance/susceptibility to leishmania infection: is this paradigm still relevant?. Front Immunol 2012; 3:80. doi: 10.3389/fimmu.2012.00080 [Crossref] [ Google Scholar]
- Shahi M, Mohajery M, Shamsian SAA, Nahrevanian H, Yazdanpanah SMJ. Comparison of Th1 and Th2 responses in non-healing and healing patients with cutaneous leishmaniasis. Rep Biochem Mol Biol 2013; 1:43. [ Google Scholar]
- McMahon‐Pratt D, Alexander J. Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or the visceral disease?. Immunol Rev 2004; 201:206-24. doi: 10.1111/j.0105-2896.2004.00190.x [Crossref] [ Google Scholar]
- Horta MF, Mendes BP, Roma EH, Noronha FSM, Macêdo JP, Oliveira LS. Reactive oxygen species and nitric oxide in cutaneous leishmaniasis. J Parasitol Res 2012; 2012:203818. doi: 10.1155/2012/203818 [Crossref] [ Google Scholar]
- Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+ CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 2002; 420:502-7. doi: 10.1038/nature01152 [Crossref] [ Google Scholar]
- Aseffa A, Gumy A, Launois P, MacDonald HR, Louis JA, Tacchini-Cottier F. The early IL-4 response to Leishmania major and the resulting Th2 cell maturation steering progressive disease in BALB/c mice are subject to the control of regulatory CD4+ CD25+ T cells. J Immunol 2002; 169:3232-41. doi: 10.4049/jimmunol.169.6.3232 [Crossref] [ Google Scholar]
- Banerjee A, Bhattacharya P, Joshi AB, Ismail N, Dey R, Nakhasi HL. Role of pro-inflammatory cytokine IL-17 in Leishmania pathogenesis and in protective immunity by Leishmania vaccines. Cell Immunol 2016; 309:37-41. doi: 10.1016/j.cellimm.2016.07.004 [Crossref] [ Google Scholar]
- Novais FO, Scott P. CD8+ T cells in cutaneous leishmaniasis: the good, the bad, and the ugly. Semin Immunopathol 2015; 37:251-9. doi: 10.1007/s00281-015-0475-7 [Crossref] [ Google Scholar]
- Aebischer T, Moody SF, Handman E. Persistence of virulent Leishmania major in murine cutaneous leishmaniasis: a possible hazard for the host. Infect Immun 1993; 61:220-6. doi: 10.1128/iai.61.1.220-226.1993 [Crossref] [ Google Scholar]
- Belkaid Y, Von Stebut E, Mendez S, Lira R, Caler E, Bertholet S. CD8+ T cells are required for primary immunity in C57BL/6 mice following low-dose, intradermal challenge with Leishmania major. J Immunol 2002; 168:3992-4000. doi: 10.4049/jimmunol.168.8.3992 [Crossref] [ Google Scholar]
- Stager S, Rafati S. CD8+ T cells in Leishmania infections: friends or foes?. Front Immunol 2012; 3:5. doi: 10.3389/fimmu.2012.00005 [Crossref] [ Google Scholar]
- Uzonna JE, Joyce KL, Scott P. Low dose Leishmania major promotes a transient T helper cell type 2 response that is down-regulated by interferon γ–producing CD8+ T cells. J Exp Med 2004; 199:1559-66. doi: 10.1084/jem.20040172 [Crossref] [ Google Scholar]
- Müller I, Kropf P, Louis JA, Milon G. Expansion of gamma interferon-producing CD8+ T cells following secondary infection of mice immune to Leishmania major. Infect Immun 1994; 62:2575-81. doi: 10.1128/iai.62.6.2575-2581.1994 [Crossref] [ Google Scholar]
- Conceição‐Silva F, Hahne M, Schröter M, Louis J, Tschopp J. The resolution of lesions induced by Leishmania major in mice requires a functional Fas (APO‐1, CD 95) pathway of cytotoxicity. Eur J Immunol 1998; 28:237-45. doi: 10.1002/(SICI)1521-4141(199801)28:01<237::AID-IMMU237>3.0.CO;2-O [Crossref] [ Google Scholar]
- Jayakumar A, Castilho TM, Park E, Goldsmith-Pestana K, Blackwell JM, McMahon-Pratt D. TLR1/2 activation during heterologous prime-boost vaccination (DNA-MVA) enhances CD8+ T Cell responses providing protection against Leishmania (Viannia). PLoS Negl Trop Dis 2011; 5:e1204. doi: 10.1371/journal.pntd.0001204 [Crossref] [ Google Scholar]
- Gurunathan S, Sacks DL, Brown DR, Reiner SL, Charest H, Glaichenhaus N. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J Exp Med 1997; 186:1137-47. doi: 10.1084/jem.186.7.1137 [Crossref] [ Google Scholar]
- Walker DM, Oghumu S, Gupta G, McGwire BS, Drew ME, Satoskar AR. Mechanisms of cellular invasion by intracellular parasites. Cell Mol Life Sci 2014; 71:1245-63. doi: 10.1007/s00018-013-1491-1 [Crossref] [ Google Scholar]
- Embgenbroich M, Burgdorf S. Current concepts of antigen cross-presentation. Front Immunol 2018; 9:1643. doi: 10.3389/fimmu.2018.01643 [Crossref] [ Google Scholar]
- Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol 2012; 12:557-69. doi: 10.1038/nri3254 [Crossref] [ Google Scholar]
- Matheoud D, Moradin N, Bellemare-Pelletier A, Shio MT, Hong WJ, Olivier M. Leishmania evades host immunity by inhibiting antigen cross-presentation through direct cleavage of the SNARE VAMP8. Cell Host Microbe 2013; 14:15-25. doi: 10.1016/j.chom.2013.06.003 [Crossref] [ Google Scholar]
- Vinet AF, Fukuda M, Turco SJ, Descoteaux A. The Leishmania donovani lipophosphoglycan excludes the vesicular proton-ATPase from phagosomes by impairing the recruitment of synaptotagmin V. PLoS Pathog 2009; 5:e1000628. doi: 10.1371/journal.ppat.1000628 [Crossref] [ Google Scholar]
- Conceição-Silva F, Morgado FN. Leishmania spp-host interaction: there is always an onset, but is there an end?. Front Cell Infect Microbiol 2019; 9:330. doi: 10.3389/fcimb.2019.00330 [Crossref] [ Google Scholar]
- Khandia R, Dadar M, Munjal A, Dhama K, Karthik K, Tiwari R. A comprehensive review of autophagy and its various roles in infectious, non-infectious, and lifestyle diseases: current knowledge and prospects for disease prevention, novel drug design, and therapy. Cells 2019; 8:674. doi: 10.3390/cells8070674 [Crossref] [ Google Scholar]
- Jiang G-M, Tan Y, Wang H, Peng L, Chen H-T, Meng X-J. The relationship between autophagy and the immune system and its applications for tumor immunotherapy. Mol Cancer 2019; 18:1-22. doi: 10.1186/s12943-019-0944-z [Crossref] [ Google Scholar]
- Patterson NL, Mintern JD. Intersection of autophagy with pathways of antigen presentation. Protein Cell 2012; 3:911-20. doi: 10.1007/s13238-012-2097-3 [Crossref] [ Google Scholar]
- Das M, Kaveri SV, Bayry J. Cross-presentation of antigens by dendritic cells: role of autophagy. Oncotarget 2015; 6:28527. doi: 10.18632/oncotarget.5268 [Crossref] [ Google Scholar]
- Tey S-K, Khanna R. Tey S-K, Khanna RAutophagy mediates transporter associated with antigen processing-independent presentation of viral epitopes through MHC class I pathway. Blood 2012; 120:994-1004. doi: 10.1182/blood-2012-01-402404 [Crossref] [ Google Scholar]
- English L, Chemali M, Duron J, Rondeau C, Laplante A, Gingras D. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat Immunol 2009; 10:480. doi: 10.1038/ni.1720 [Crossref] [ Google Scholar]
- Tao S, Drexler I. Targeting Autophagy in Innate Immune Cells: Angel or Demon During Infection and Vaccination?. Front Immunol 2020; 11:460. doi: 10.3389/fimmu.2020.00460 [Crossref] [ Google Scholar]
- Wei F, Duan Y. Crosstalk between autophagy and nanomaterials: internalization, activation, termination. Adv Biosyst 2019; 3:1800259. doi: 10.1002/adbi.201800259 [Crossref] [ Google Scholar]
- Cordani M, Somoza Á. Targeting autophagy using metallic nanoparticles: a promising strategy for cancer treatment. Cell Mol Life Sci 2019; 76:1215-42. doi: 10.1007/s00018-018-2973-y [Crossref] [ Google Scholar]
- Panzarini E, Inguscio V, Tenuzzo BA, Carata E, Dini L. Nanomaterials and autophagy: new insights in cancer treatment. Cancers 2013; 5:296-319. doi: 10.3390/cancers5010296 [Crossref] [ Google Scholar]
- Guo L, He N, Zhao Y, Liu T, Deng Y. Autophagy modulated by inorganic nanomaterials. Theranostics 2020; 10:3206. doi: 10.7150/thno.40414 [Crossref] [ Google Scholar]
- Li H, Li Y, Jiao J, Hu H-M. Alpha-alumina nanoparticles induce efficient autophagy-dependent cross-presentation and potent antitumour response. Nat Nanotechnol 2011; 6:645-50. doi: 10.1038/nnano.2011.153 [Crossref] [ Google Scholar]
- Bilyy R, Paryzhak S, Turcheniuk K, Dumych T, Barras A, Boukherroub R. Aluminum oxide nanowires as safe and effective adjuvants for next-generation vaccines. Materials Today 2019; 22:58-66. doi: 10.1016/j.mattod.2018.10.034 [Crossref] [ Google Scholar]
- Williams RA, Tetley L, Mottram JC, Coombs GH. Cysteine peptidases CPA and CPB are vital for autophagy and differentiation in Leishmania mexicana. Microb Cell 2006; 61:655-74. doi: 10.15698/mic2018.09.646 [Crossref] [ Google Scholar]
- Rafati S, Baba AA, Bakhshayesh M, Vafa M. Vaccination of BALB/c mice with Leishmania major amastigote‐specific cysteine proteinase. Clin Exp Immunol 2000; 120:134-8. doi: 10.1046/j.1365-2249.2000.01160.x [Crossref] [ Google Scholar]
- Rafati S, Nakhaee A, Taheri T, Taslimi Y, Darabi H, Eravani D. Protective vaccination against experimental canine visceral leishmaniasis using a combination of DNA and protein immunization with cysteine proteinases type I and II of Linfantum. Vaccine 2005; 23:3716-25. doi: 10.1016/j.vaccine.2005.02.009 [Crossref] [ Google Scholar]
- Soudi S, Hosseini A, Hashemi S. Co‐administration of rectal BCG and autoclaved Leishmania major induce protection in susceptible Balb/c mice. Parasite Immunol 2011; 33:561-71. doi: 10.1111/j.1365-3024.2011.01318.x [Crossref] [ Google Scholar]
- Beyzay F, Hosseini AZ, Soudi S. Alpha alumina nanoparticle conjugation to cysteine peptidase A and B: an efficient method for autophagy induction. Avicenna J Med Biotechnol 2017; 9:71. [ Google Scholar]
- Ten Brinke A, Marek-Trzonkowska N, Mansilla MJ, Turksma AW, Piekarska K, Iwaszkiewicz-Grześ D. Monitoring T-cell responses in translational studies: optimization of dye-based proliferation assay for evaluation of antigen-specific responses. Cell 2010; 140:313-26. doi: 10.1016/j.cell.2010.01.028 [Crossref] [ Google Scholar]
- Podinovskaia M, Descoteaux A. Leishmania and the macrophage: a multifaceted interaction. Future microbiol 2015; 10:111-29. doi: 10.2217/fmb.14.103 [Crossref] [ Google Scholar]
- Nyambura LW, Jarmalavicius S, Walden P. Impact of Leishmania donovani infection on the HLA I self peptide repertoire of human macrophages. PloS One 2018; 13:e0200297. doi: 10.1371/journal.pone.0200297 [Crossref] [ Google Scholar]
- Shi C-S, Shenderov K, Huang N-N, Kabat J, Abu-Asab M, Fitzgerald KA. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol 2012; 13:255-63. doi: 10.1038/ni.2215 [Crossref] [ Google Scholar]
- Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell 2010; 140:313-26. doi: 10.1016/j.cell.2010.01.028 [Crossref] [ Google Scholar]
- Stauffer B, Powers T. Target of rapamycin signaling mediates vacuolar fragmentation. Curr Genet 2017; 63:35-42. doi: 10.1007/s00294-016-0616-0 [Crossref] [ Google Scholar]
- Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll‐like receptors control autophagy. EMBO J 2008; 27:1110-21. doi: 10.1038/emboj.2008.31 [Crossref] [ Google Scholar]
- Oh JE, Lee HK. Pattern recognition receptors and autophagy. Front Immunol 2014; 5:300. doi: 10.3389/fimmu.2014.00300 [Crossref] [ Google Scholar]
- Whitaker SM, Colmenares M, Pestana KG, McMahon-Pratt D. Leishmania pifanoi proteoglycolipid complex P8 induces macrophage cytokine production through Toll-like receptor 4. Infect Immun 2008; 76:2149-56. doi: 10.1128/IAI.01528-07 [Crossref] [ Google Scholar]
- Li M, Gao P, Zhang J. Crosstalk between autophagy and apoptosis: potential and emerging therapeutic targets for cardiac diseases. Int J Mol Sci 2016; 17:332. doi: 10.3390/ijms17030332 [Crossref] [ Google Scholar]
- Olivier V, Duval J-L, Hindie M, Pouletaut P, Nagel M-D. Comparative particle-induced cytotoxicity toward macrophages and fibroblasts. Cell Biol Toxicol 2003; 19:145-59. doi: 10.1023/a:1024723326036 [Crossref] [ Google Scholar]
- Mayer A, Zhang Y, Perelson AS, Wingreen NS. Regulation of T cell expansion by antigen presentation dynamics. Proc Natl Acad Sci U S A 2019; 116:5914-9. doi: 10.1073/pnas.1812800116 [Crossref] [ Google Scholar]
- Campos TM, Costa R, Passos S, Carvalho LP. Cytotoxic activity in cutaneous leishmaniasis. Mem Inst Oswaldo Cruz 2017; 112:733-40. doi: 10.1590/0074-02760170109 [Crossref] [ Google Scholar]