Nazanin Kianinejad is a chemical engineer currently pursuing her Ph.D. in Pharmaceutical Sciences at
Nova Southeastern University, College of Pharmacy (USA). Nazanin’s research interests include targeted delivery using nanoparticles for cancer treatment.
Young M. Kwon is an associate professor of Pharmaceutical Sciences at Nova Southeastern University,
College of Pharmacy (USA). Dr. Kwon’s research interests include targeted delivery and controlled release of biological molecules.
Abstract
Summary
The delivery of chemotherapies to brain tumors faces the difficult task of crossing the blood-brain barrier (BBB).1-4 The brain capillary endothelial cells (BCECs) along with other cell lines, such as astrocytes and pericytes, form the BBB. This highly selective semipermeable barrier separates the blood from the brain parenchyma. The BBB controls the movement of drug molecules in a selective manner5 and maintains central nervous system (CNS) homeostasis. Depending on the properties of drugs such as their hydrophilic-lipophilic balance (HLB), some can cross the BBB through passive diffusion.6 However, this approach alone has not led to successful drug developments due to low net diffusion rates and systemic toxicity. Although the use of nanomedicine has been proposed to overcome these drawbacks, many recent studies still rely on the so-called ‘enhanced permeability and retention (EPR)’ effect though there is a realization in the field of drug delivery that EPR effect may not be sufficient for successful drug delivery to brain tumors. Since, compared to many other solid tumors, brain tumors pose additional challenges such as more restrictive blood-tumor barrier as well as the well-developed lymphatic drainage, the selection of functional moieties on the nanocarriers under consideration must be carried out with care to propose better solutions to this challenge.
Keywords: Blood-brain-barrier, Targeted delivery, EPR effect, Lymphatic drainage, Brain tumors, Nanomedicine
Copyright and License Information
© 2023 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Many research studies that focus on directing chemotherapeutics to solid tumors using nanocarriers rely on a long-standing passive targeting approach called ‘enhanced permeability and retention (EPR) effect’.7 The endothelial linings of the blood vessels in normal tissues would inhibit the diffusion of nanoparticles (NPs). On the other hand, an actively growing tumor involves rapid neovascularization, resulting in a tissue architecture that distinctively differ from its normal counterpart, which is characterized by 1) faulty endothelial linings with gaps and; 2) lack of lymphatic drainage.7,8 The EPR effect suggests that macromolecular drug carriers can, in theory, accumulate in a solid tumor tissue passively based on such pathologic characteristics, and may serve as a tentative model for reduced toxicity via such passive targeting of cytotoxic agents.
However, this approach has been less effective inpractice than in theory due to the fact that some tumors have well-developed lymphatic drainage.8,9 In particular, in the case of brain tumors, brain has a powerful lymphatic draining system that may effectively clear the particles that find their way to the tumor site even if there was an enhanced permeability effect.10-12 Therefore, high levels of therapeutics may fail to retain sufficiently in tumor sites, reducing the reliability of the EPR effect. Specific targeting of tumor cells may help to retain NPs in the tumor site and avoid their rapid removal by the brain lymphatic drainage. The delivery process of carrying the chemotherapeutic agent across the blood-brain barrier (BBB), and then homing onto the tumor site, is called “dual-targeting”. Dual-targeting systems are intended to increase the efficiency of the drug, and also to significantly reduce systemic side effects. These formulations of NP therapeutics are expected to exhibit an increased ability in crossing the BBB and targeting tumor cells. This urgent need highlights a need for a much better understanding of the transportation potential of the brain capillary endothelial cells (BCECs) and the BBB. By employing a specific dual-targeting strategy, the drug delivery systems (DDSs) to the brain based on the nanovesicle formulation can display significant BBB crossing and specific targeting of glioma cells. Vesicle systems such as niosomes and liposomes with bilayer membranes and interior cavities are favored in drug delivery design due to their loading capacity, wide surface area, and the ability to incorporate into hydrophilic and lipophilic components. These carriers can be conjugated with multiple attachments.6 This system should be superior to the EPR because it specifically and actively allows the drug to cross the BBB, and also allows the selectivity of the drug towards cancer cells to enhance drug concentration at the desired site (Fig. 1).
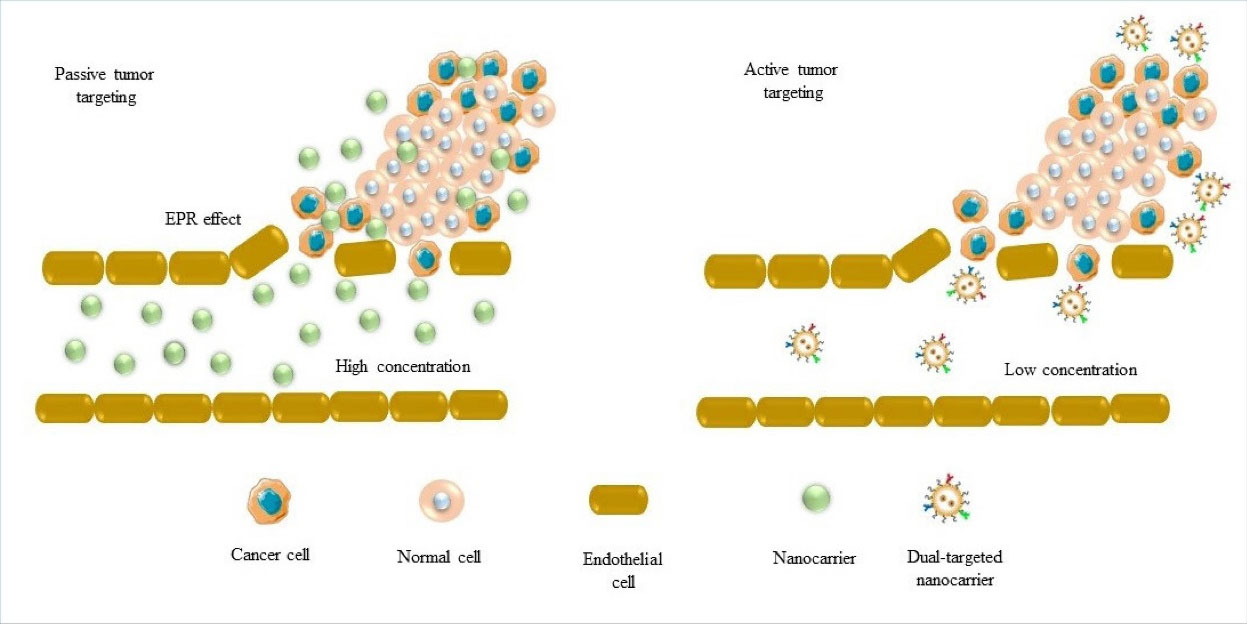
Fig. 1.
Different types of targeting brain tumors using nanoparticles. Passive targeting based on EPR effect and active targeting that localizes nanoparticles in specific intracellular spaces and promotes their retention in the tumor site. Redrawn from Gandhi et al.1
.
Different types of targeting brain tumors using nanoparticles. Passive targeting based on EPR effect and active targeting that localizes nanoparticles in specific intracellular spaces and promotes their retention in the tumor site. Redrawn from Gandhi et al.1
An example of dual-targeting of brain tumors is a nanovesicle system conjugated with an angiopep-2 (ANG) peptide on the surface. The investigators took advantage of the observation that the low-density lipoprotein receptor-related protein-1 (LRP-1) is highly expressed on the blood-tumor barrier of gliomas.14 LRP-1 has been used for macromolecular transport across BBB by ligand binding followed by receptor-mediated transcytosis.15 A peptide called “angiopep-2, or ANG” that consists of 19 amino acids (TFFYGGSRGKRNNFKTEEY) has shown enhanced NP permeability across the BBB mediated by LRP-1 transcytosis of nanoscale drug delivery systems.16 After crossing the BBB, the nanovesicles decorated with mAb, ligand, and so on specific to the overexpressed surface targets of glioma cells such as folate receptors (FRs)17 may have a chance to resist clearance by lymphatic drainage. Therefore, such dual-targeted nanovesicle systems may overcome the BBB and target the glioma cells specifically through direct attachment, thereby attenuating the brain lymphatic drainage.
Future direction in nanovesicles formulation (i.e., liposomes, niosomes) for targeting the DDSs are focused on increasing cellular trafficking performance and enhancing their ability to cross the BBB. This can be achieved using dual-targeted nanovesicle DDSs to the brain cancer cells using novel targeting moieties.18,19 Having NPs attached directly to the tumor is necessary to overcome the scenario of suboptimal drug delivery and neurotoxicity to the normal cells when the NPs were used without appropriate direct targeting moiety. In designing a DDS ideal for delivery of chemotherapies into the brain, important performance criteria must be met. First, this system must be safe and non-toxic. This usually means that the system should be comprised of biocompatible materials such as lipids, biodegradable polymers, and/or non-ionic surfactants. Second, the system must exhibit sufficient stability in blood. Third, the system should have the capacity for controlled release of the drug. Lastly, the targeting moieties should not only drive the NPs into the brain, but also to the tumor site within the brain.
Today, the advances in anticancer DDS and brain drug targeting have already produced several tools available to deliver NPs into the brain. Research effort should be focused to fine-tune the delivery systems as we learn more about the details of how NPs interact with different components under the pathophysiologic environment. Hopefully such effort will help in better translation of new findings into clinical applications in the future.
Authors’ contribution
Nazanin Kianinejad: conceptualization, draft preparation, writing and reviewing.
Young M. Kwon: conceptualization, supervision, writing and reviewing, project administration.
Funding sources
This study was supported by Nova Southeastern University Health Professions Division grant # 334632.
Ethical statement
Not applicable.
Competing interests
None declared.
References
- Gandhi H, Sharma AK, Mahant S, Kapoor DN. Recent advancements in brain tumor targeting using magnetic nanoparticles. Ther Deliv 2020; 11:97-112. doi: 10.4155/tde-2019-0077 [Crossref] [ Google Scholar]
- Jafari B, Pourseif MM, Barar J, Rafi MA, Omidi Y. Peptide-mediated drug delivery across the blood-brain barrier for targeting brain tumors. Expert Opin Drug Deliv 2019; 16:583-605. doi: 10.1080/17425247.2019.1614911 [Crossref] [ Google Scholar]
- Sun C, Ding Y, Zhou L, Shi D, Sun L, Webster TJ, Shen Y. Noninvasive nanoparticle strategies for brain tumor targeting. Nanomed Nanotechnol Biol Med 2017; 13:2605-2621. doi: 10.1016/j.nano.2017.07.009 [Crossref] [ Google Scholar]
- Wei X, Chen X, Ying M, Lu W. Brain tumor-targeted drug delivery strategies. Acta Pharm Sin B 2014; 4:193-201. doi: 10.1016/j.apsb.2014.03.001 [Crossref] [ Google Scholar]
- Aldape K, Brindle KM, Chesler L, Chopra R, Gajjar A, Gilbert MR. Challenges to curing primary brain tumours. Nat Rev Clin Oncol 2019; 16:509-520. doi: 10.1038/s41571-019-0177-5 [Crossref] [ Google Scholar]
- Omidi Y, Kianinejad N, Kwon Y, Omidian H. Drug delivery and targeting to brain tumors: considerations for crossing the blood-brain barrier. Expert Rev Clin Pharmacol 2021; 14:357-381. doi: 10.1080/17512433.2021.1887729 [Crossref] [ Google Scholar]
- Matsumura, Y. and H. Maeda. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 1986; 46(12 Pt 1): p. 6387-92.
- Nichols, J.W. and Y.H. Bae. EPR: Evidence and fallacy. J Control Release 2014; 190: p. 451-64. 10.1016/j.jconrel.2014.03.057.
- Danhier F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine?. J Control Release 2016; 244:108-121. doi: 10.1016/j.jconrel.2016.11.015 [Crossref] [ Google Scholar]
- Clapham R, O'Sullivan E, Weller RO, Carare RO. Cervical lymph nodes are found in direct relationship with the internal carotid artery: significance for the lymphatic drainage of the brain. Clin Anat 2010; 23:43-7. doi: 10.1002/ca.20887 [Crossref] [ Google Scholar]
- Dissing-Olesen L, Hong S, Stevens B. New brain lymphatic vessels drain old concepts. EBioMedicine 2015; 2:776-777. doi: 10.1016/j.ebiom.2015.08.019 [Crossref] [ Google Scholar]
- Sun BL, Wang LH, Yang T, Sun JY, Mao LL, Yang MF. Lymphatic drainage system of the brain: A novel target for intervention of neurological diseases. Prog Neurobiol 2018; 163-164:118-143. doi: 10.1016/j.pneurobio.2017.08.007 [Crossref] [ Google Scholar]
- Werengowska-Ciećwierz K, Wiśniewski M, Terzyk AP, Furmaniak S. The chemistry of bioconjugation in nanoparticles-based drug delivery system. Adv Condens Matter Phys 2015; 2015:198175. doi: 10.1155/2015/198175 [Crossref] [ Google Scholar]
- Bragagni M, Mennini N, Furlanetto S, Orlandini S, Ghelardini C, Mura P. Development and characterization of functionalized niosomes for brain targeting of dynorphin-B. Eur J Pharm Biopharm 2014; 87:73-79. doi: 10.1016/j.ejpb.2014.01.006 [Crossref] [ Google Scholar]
- May P, Herz J, Bock H. Molecular mechanisms of lipoprotein receptor signalling. Cell Mol Life Sci CMLS 2005; 62:2325-2338. doi: 10.1007/s00018-005-5231-z [Crossref] [ Google Scholar]
- McCord E, Pawar S, Koneru T, Tatiparti K, Sau S, Iyer AK. Folate Receptors' Expression in Gliomas May Possess Potential Nanoparticle-Based Drug Delivery Opportunities. ACS Omega 2021; 6(6):4111-8. doi: 10.1021/acsomega.0c05500 [Crossref] [ Google Scholar]
- Elechalawar CK, Bhattacharya D, Ahmed MT, Gora H, Sridharan K, Chaturbedy P. Dual targeting of folate receptor-expressing glioma tumor-associated macrophages and epithelial cells in the brain using a carbon nanosphere–cationic folate nanoconjugate. Nanoscale Adv 2019; 1:3555-3567. doi: 10.1039/C9NA00056A [Crossref] [ Google Scholar]
- Li M, Shi K, Tang X, Wei J, Cun X, Chen X. pH-sensitive folic acid and dNP2 peptide dual-modified liposome for enhanced targeted chemotherapy of glioma. Eur J Pharm Sci 2018; 124:240-248. doi: 10.1016/j.ejps.2018.07.055 [Crossref] [ Google Scholar]
- Gao JQ, Lv Q, Li LM, Tang XJ, Li FZ, Hu YL. Glioma targeting and blood–brain barrier penetration by dual-targeting doxorubincin liposomes. Biomaterials 2013; 34:5628-39. doi: 10.1016/j.biomaterials.2013.03.097 [Crossref] [ Google Scholar]