Bioimpacts. 13(5):383-392.
doi: 10.34172/bi.2022.26386
Original Article
Chemo-immune cell therapy by intratumoral injection of adoptive NK cells with capecitabine in gastric cancer xenograft model
Zeinab Ghazvinian 1  , Shahrokh Abdolahi 1, Mohammad Ahmadvand 2, Amir Hossein Emami 3, Samad Muhammadnejad 4, Hamid Asadzadeh Aghdaei 5, Jafar Ai 6, Mohammad Reza Zali 7, Iman Seyhoun 1, Javad Verdi 1, *
, Shahrokh Abdolahi 1, Mohammad Ahmadvand 2, Amir Hossein Emami 3, Samad Muhammadnejad 4, Hamid Asadzadeh Aghdaei 5, Jafar Ai 6, Mohammad Reza Zali 7, Iman Seyhoun 1, Javad Verdi 1, *  , Kaveh Baghaei 5, 7, *
, Kaveh Baghaei 5, 7, * 
Author information:
1Department of Applied Cell Sciences, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran
2Hematology-Oncology and Stem Cell Transplantation Research Center, Tehran University of Medical Sciences, Tehran, Iran
3Department of Internal Medicine, School of Medicine, Imam Khomeini Hospital Complex, Cancer Institute, Tehran University of Medical Sciences, Tehran, Iran
4Gene Therapy Research Center, Digestive Diseases Research Institute, Tehran University of Medical Sciences, Tehran, Iran
5Basic and Molecular Epidemiology of Gastrointestinal Disorders Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
6Department of Tissue Engineering, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran
7Gastroenterology and Liver Diseases Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Abstract
Introduction:
Gastric cancer is one of the most commonly known malignancies and is the fifth cancer-related death globally. Whereas natural killer (NK) cells play a critical role in tumor elimination; therefore, adoptive NK cell therapy has become a promising approach in cancer cytotherapy. Hence, this study investigated the chemo-immune cell therapy in MKN-45 derived xenograft gastric cancer model.
Methods:
Three groups of animals have received the following treatments separately: activated NK cells, capecitabine, the combination of capecitabine and activated NK cells, and one was considered as the control group. Morphometric properties of tumor samples were evaluated at the end of the study. NK cells infiltration was evaluated by immunohistochemistry (IHC) of hCD56. Mitotic count and treatment response was assessed by hematoxylin and eosin (H&E) staining. The proliferation ratio to apoptosis was determined by IHC assessment of Ki67 and caspase 3.
Results:
The results indicated that the NK cell therapy could effectively decrease the mitotic count in pathology assessment, but the tumor was not completely eradicated. In combination with metronomic chemotherapy (MC) of capecitabine, NK cell therapy demonstrated a significant difference in tumor morphometric properties compared to the control group. The proliferation ratio to apoptosis was also in line with pathology data.
Conclusion:
Although NK cell therapy could effectively decrease the mitotic count in vivo, the obtained findings indicated lesser potency than MC despite ex vivo activation. In order to enhance NK cell therapy effectiveness, suppressive features of the tumor microenvironment and inhibitory immune checkpoints blockade should be considered.
Keywords: Gastric cancer, Capecitabine, Adoptive NK cell therapy, Chemo-immune cell therapy, Metronomic chemotherapy
Copyright and License Information
© 2023 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Gastric cancer is the fifth cause of cancer-related death worldwide. Patients are usually diagnosed in the advanced stage, and they poorly respond to approved treatments.1 Chemotherapy is the primary conventional therapy for stomach cancer; however, immune cell therapy is the new cancer treatment landscape. The immune system of patient is employed to combat against cancerous cells in the immunotherapy context. Different approaches to immunotherapy are immune checkpoint blockade, monoclonal antibodies, and cancer vaccines. 2 However, cell therapy injects activated and viable cells into patients to fight the cancer cells and enhance cellular immunity directly. Natural killer (NK) cell adoptive based therapy is considered a promising cytotherapy in cancer.3,4 These cells are safe, rarely induce GVHD, and are well tolerated in recipients.5,6 Different studies corroborated the dysfunctionality of own patient’s NK cells in gastric cancer. Reportedly elevated Fas expression on circulating NK cells causes apoptotic NK cells in gastric cancer patients.7 Further studies had been shown that PGE2 derived from gastric tumor cells hampers the antitumor activity of patients’ NK cells.8 Activating receptors such as NKG2D are also decreased in gastric cancer patients' NK cells, so the NK cell activation is impaired.9 Adoptive immune cell therapy by activated lymphocytes such as NK cells has shown no serious side effects. It may extend the survival of gastric cancer patients.10 Nk cells play a pivotal role in suppressing gastric cancer initiation, progression, and distant metastasis in patients. However, their efficacy in combination with chemotherapeutic drugs needs more investigation.11 Many different chemotherapy drugs have been approved for different stages of gastric cancer treatment.12 Capecitabine, a thymidylate synthase inhibitor and the oral form of 5-fluorouracil (5-fu), has been approved for metastatic or locally advanced gastric cancer1000 mg/m2 twice daily for two weeks, cycling every 21 days.13 Capecitabine is a prodrug and enzymatically is converted to the active form (fluorouracil) preferentially in tumor cells, inhibiting DNA synthesis and decreasing tumor cell proliferation. The maximum tolerated dose (MTD) of capecitabine is 2500 mg/m2 total daily amount.14 MTD chemotherapy and metronomic chemotherapy (MC) are the two paths in cancer treatment. MC is frequently prescribed due to low but active concentration of drugs during a long period and low toxicity, orally and without hospital care. Capecitabine is prescribed metronomically in different cancers and gastric cancer.15,16 MC has been reported to inhibit angiogenesis directly through down-regulating VEGFR2-317 and indirectly alters the angiogenesis relating factors such as thrombospondin-1,18,19 consequently disrupting the cancer stem cell niche in the tumor center.20 In the context of chemo-immune cell therapy combination, a study in gastric cancer reported enhanced immune function and alleviated adverse chemotherapy effects by a variety of CIK/DC (dendritic cell)-CIK therapy with chemotherapy drugs.21 Adoptively activated NK cells have been demonstrated as antitumor activities in preclinical models of colorectal cancer, glioblastoma, and ovarian cancer.22-24 It is reported that cellular immunotherapy combined with immune checkpoint inhibitors could improve the survival of late-stage gastric cancer. Among different patterns of cell therapy, NK cell therapy induces less cytokine-release syndrome and can be a suitable candidate for gastric cancer cytotherapy.25 Still, most investigations should be considered to determine the efficacy of adoptive NK cell therapy combined with chemotherapy. However, the combination of monoclonal antibodies and adoptive NK cells that trigger the antibody-dependent cellular cytotoxicity along with capecitabine led to treatment response in metastatic lung cancer. However, the present study's central hypothesis, the combination of capecitabine and NK cell cytotherapy in a preclinical model of gastric cancer, has not been reported.26 The current study proposed improving treatment efficacy by diminishing the dose of the drug and directly combining adoptive NK cells injection to the tumor site.
Material and Methods
Material
The materials of the current study have been prepared as follows. The cell culture medium prepared by using RPMI 1640 (Gibco, United States) and FBS (Gibco; United States). In order to isolate human NK cells, premium Ficoll-Paque (GE Healthcare’s; United States) and human NK cell isolation kit (Miltenyi Biotec; Germany) have been used. NK cells’ characterization has been done by flow cytometry, using PE anti-human CD56 (Cat no: 362507; BioLegend; United States) and FITC anti-human CD3 (Cat no: 317305; BioLegend; United States). NK cells’ activation has been done by adding IL-2 (Miltenyi Biotec, Germany) to the cell culture medium. The cytotoxicity assay analyzed by Human IFNγ Quantikine ELISA Kit (Cat no: DIF50C; R&D Systems; United States) and Human TNF-alpha Quantikine ELISA Kit (Cat no: DTA00D; R&D Systems; United States). Immunohistochemistry assay of pathology sections have been assayed by using Purified anti-human CD56 (NCAM) Antibody (Cat no: 304601; BioLegend; United States), Ki67 antibody (Cat no: orb378204; Biorbyte; United Kingdom) and Caspase 3 antibody (Cat no: orb536309; Biorbyte; United Kingdom).
Cell lines and Culture Media
MKN-45 as a human poorly differentiated stomach cancer cell line (accession number: CVCL_0434) was prepared from the Iranian Biological Resource Center (Tehran, Iran) and was cultured in a complete medium including RPMI 1640 medium complemented with 10% FBS, 2 mM glutamine, 100 U of penicillin, and 100 mg of streptomycin. The feeder layer was Epstein-Barr virus (EBV)–transformed lymphoblastic cell line (LCL) obtained from Iranian Biological Research Center and were maintained in the same complete medium.
Isolation, development, and characterization of human NK cells
NK cells were prepared from a healthy donor by receiving informed consent. In brief, peripheral blood mononuclear cells (PBMCs) have been isolated with Premium Ficoll-Paque (GE Healthcare’s, United States) density-gradient centrifugation from whole blood. NK cells were collected according to manufacture instructions by adverse selection, applying an isolation kit of human NK cell (Miltenyi Biotech, Germany). NK cells characterization with PE anti-human CD56+ and FITC anti-human CD3− markers was quantitatively evaluated (FACSCalibur Becton Dickinson, United States). Flow cytometry was implemented, the FlowJo software was performed to analyze the data.
NK cell expansion was done in the media containing the feeder cells. LCL transformed by EBV was developed by culturing mononuclear cells derived from peripheral blood in 100 μg/mL cyclosporin. NK cell expansion based on existing protocol27 for in vivo studies had been performed by utilizing 100 Gy-irradiated transformed LCL (at a ratio of 1:10) to activate NK cells proliferation. After five days without changing the media, NK cell colonies were formed and observed; every three days, half of the media was exchanged by a complete medium supplemented with IL-2 (500 IU/mL) till day 21.
Cell cytotoxicity assay
The cell cytotoxicity of human NK cells (effector cells) against MKN-45 cell line (target cells) were performed in vitro. The effector (E) to target (T) ratio E: T of 1:1, 5:1, and 10:1 were studied. Lactate dehydrogenase (LDH) assay performed in cell culture medium after 24 hours of incubation; the abundance of LDH release was considered a necrosis marker and cytotoxicity effect of NK cells.28
Cytokine release assay in vitro
Activated NK cells were analyzed for cytotoxic cytokine release by ELISA to confirm activation before injection. Based on protocol instruction, the IFNγ and TNFα levels were assessed by collecting the NK cells condition media.
Establishment of a subcutaneously implanted tumor model
Ten- to 12-week-old male BALB/c nude mice (pathogen-free) were prepared from Shahid Beheshti University, Avicenna Research Institute, and maintained in individually ventilated cages (IVC) at humidity (approximately 40%–50%) and proper temperature (25 ± 2°C). The light/dark cycle condition, 12 hours/12 hours was performed. The animals were randomly divided into four different groups (4 nude mice were assigned per group). MKN-45 cells were collected in the logarithmic expansion phase and pipette into a single cell suspension. The amount of 5×106 MKN-45 cell/200 µL phosphate-buffered saline (PBS) were injected subcutaneously in mice right flank. Twice a week, tumor sizes and body weights were measured. All the experiments were initiated when the tumor volume reached 100-200 mm. Animals are sedated with ketamine and xylazine hydrochloride before euthanasia and then CO2 utilized for termination. Animals with a tumor volume ≥1500 mm3 and actual bodyweight loss ≥20% were humanely terminated.
Capecitabine, NK cell dosage, and schedule of administration
The capecitabine active substance was kindly provided by Dr. Saremi Sh (Baran Chemical and Pharmaceutical Company). While capecitabine MTD dose is 2500 mg/m2 in humans,29 considering the body surface area, the dosage was calculated from human to mice by the following formula30:
Almost 70% of MTD was administered metronomically PO by sterilized gavage needle, once a day, five days per week; 7 days of discontinuation were considered (cycled three times) in drug and combination groups. Fresh isolated and expanded NK cells were injected intratumoral, directly to the margin of the tumor. Almost 7×106 cells were injected per mouse three times in drug discontinuation intervals and not simultaneously with drug administration in the combination group. The NK cell group was injected with 7×106 NK cells on days 0, 10, and 20. The control group received PBS orally by sterilized needle gavage (Fig. 1)
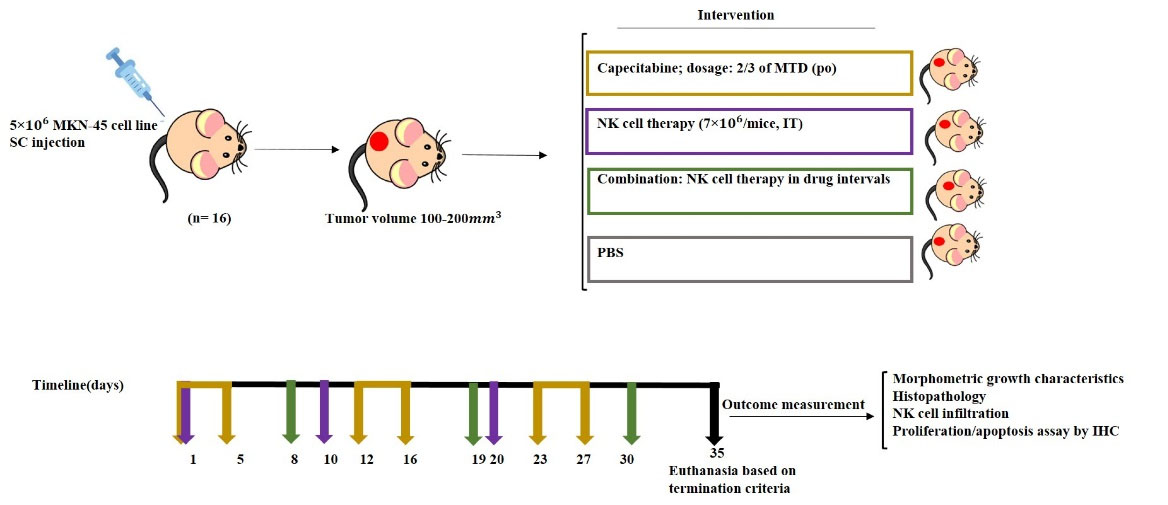
Fig. 1.
Schematic diagram of chemo-immune cell therapy of gastric cancer xenograft model. Three groups of animals have received the following treatments separately: capecitabine, activated NK cell, the combination of capecitabine and NK cells, and one was considered as the control group. SC, subcutaneous; IV, intravenous; PO, by mouth; IT, intratumoral.
.
Schematic diagram of chemo-immune cell therapy of gastric cancer xenograft model. Three groups of animals have received the following treatments separately: capecitabine, activated NK cell, the combination of capecitabine and NK cells, and one was considered as the control group. SC, subcutaneous; IV, intravenous; PO, by mouth; IT, intratumoral.
Investigating of tumor micro structure properties
Tumor volume was assessed using a digital caliper and computed by the mentioned formula: tumor volume = 1/2 (length × width 2). The following formula calculated relative tumor volume (RTV): RTV = (The measured day tumor volume)/ (The day 0 tumor volume) in order to assess the morphometric growth kinetics of the tumors. Tumor growth inhibition (TGI) was assessed by following formula: %TGI = [(1-(Vt1/Vto)/ (Cto/Ct1))/ (1-Cto/Ct1)] *100 to present anti-tumor activity of treatment. The mice were humanly euthanized at the end of the experiment, the tumors were removed and investigated.
Histopathology assessment
Histopathology assessment was performed using microscopic examination of the biopsy of tumor samples following the euthanasia of the mice. Histopathological responses following the treatment include treatment effect or residual tumor (R) (Table 1),31 and mitotic count (number of mitosis in 10 high power filed (10HPF) providing proliferation activity) that were evaluated by hematoxylin and eosin (H&E) staining as previously described by Meuten and colleagues.32 NK cell infiltration was evaluated by Immunohistochemistry (IHC) of human CD56+ using the protocol described previously.33 IHC of CD56+ NK cells was evaluated based on the Allred score, including proportion and intensity scores (Table 2).34 Active protein caspase three and Ki67, antibodies were evaluated for apoptosis induction and growth fraction. In brief critical steps of IHC are preparing paraffin block, adding appropriate antigen for retrieval, preparation of antibodies and suit reagents, washing and counterstaining.35
Table 1.
The Classification of Residual tumor (R) upon Treatment
|
Classification
|
Description
|
| R0 |
Resection for a tumor or complete remission. |
| R1 |
Cancer cells present less than 30% in tumor micro-structure in response to current treatment; diffused necrosis and apoptosis were assessed. |
| R2 |
Macroscopic residual tumor at the primary cancer site. More than 30% of tumors have diagnosed with fibrosis and apoptosis in response to the current treatment. |
| R3 |
The nonresponsive tumor following current treatment. |
Table 2.
Immunoreactive cell percentage and Intensity grade based on the Allred score
|
Positive cells percentage
|
Proportion grade
|
Intensity
|
Intensity grade
|
| No immunoreactivity |
0 |
Negative |
0 |
| ≤1% |
1 |
Weak |
1 |
| 1–10% |
2 |
Intermediate |
2 |
| 11–33% |
3 |
Strong |
3 |
| 34–66% |
4 |
|
|
| 67–100% |
5 |
|
|
Statistical analysis
Data are evaluated as mean ± standard deviation and GraphPad Prism 9 software package (GraphPad Software, Inc., San Diego, United States) utilized for analyzing, ANOVA and the t test were performed for comparing the data. P< 0.05 was considered significant.
Results
EBV-LCL feeder cells induced selective expansion of NK cells from mononuclear cells
PBMC isolated NK cells were hCD56+ and hCD3-, the biomarkers of NK cell surface. The acquired purity was 82 % (Fig. 2A). Isolated NK cells were then cultured in RPMI 1640 complete media, IL2 and EBV-LCL as feeder cells for 21 days. Clonal growth and round shape morphology has been recognized from day five till day 21 (Fig. 2B). IL-2 induces NK cells activation in vitro.36 Finally, on day 21, the research reached 250-fold expansions of NK cells. The viability of NK cells was more than 95% before injection, evaluated by Trypan blue staining.
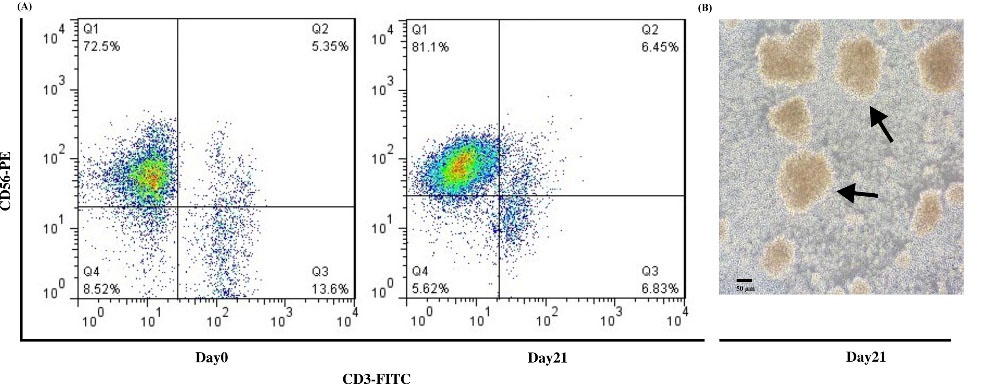
Fig. 2.
Isolation, Development, and Characterization of human NK cells. (A) The analysis of flow cytometry revealed CD56+ CD3- NK cells frequency in PBMC-derived NK cells more than 70% purity on day 0 and more than 80% purity on day 21 after MACS isolation. (B) The morphology of NK cells on day 21 before injection; arrows illustrate the round shape colony of NK cells. Scale bar: 50 μm, Magnification 200X.
.
Isolation, Development, and Characterization of human NK cells. (A) The analysis of flow cytometry revealed CD56+ CD3- NK cells frequency in PBMC-derived NK cells more than 70% purity on day 0 and more than 80% purity on day 21 after MACS isolation. (B) The morphology of NK cells on day 21 before injection; arrows illustrate the round shape colony of NK cells. Scale bar: 50 μm, Magnification 200X.
Significant cytotoxicity against target cells induced by ex vivo expanded NK cells
NK cells cytotoxicity (effector) against MKN-45 cell line (target) was evaluated by LDH release assay. NK cells and MKN-45 cell lines were co-cultured with the E: T ratio of 1:1, 5:1, and 10:1 for 24 hours. In consequence, the most prominent cytotoxicity of activated NK cells was achieved in the 10:1 group (P< 0.0001) (Fig. 3.A).
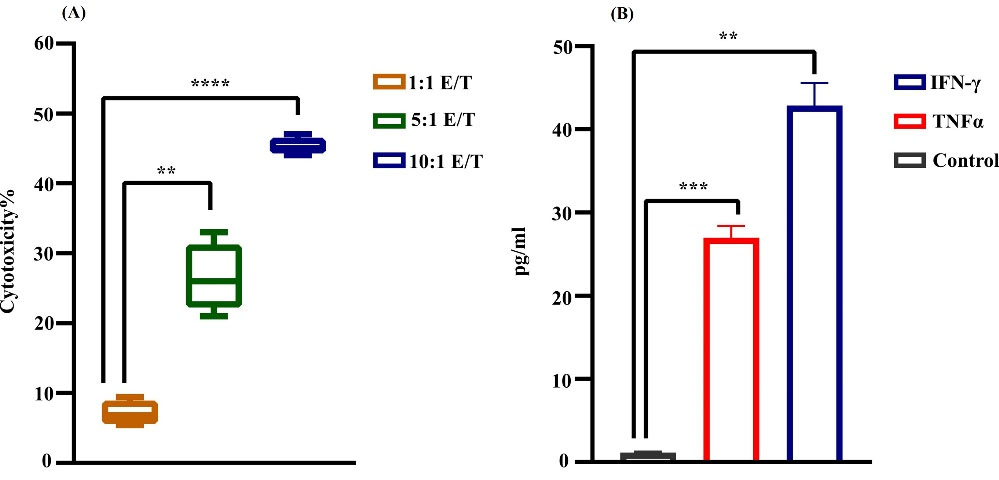
Fig. 3.
Cytotoxicity of NK cells. (A) LDH assay; activated NK cells toward MKN45 cells at different E: T ratios. The results demonstrated the most cytotoxic effect in 10:1 E: T (****P < 0.0001). Data reported as SEM for four groups independently. (B) Cytokine release assay of NK cells on day 21 by collecting the condition media. In comparison to control, the cytotoxic cytokine release of NK cells was ≈45 pg/mL and ≈27 pg/mL for IFNγ (**P < 0.01) and TNF α, respectively (***P < 0.001). Statistical significance was determined using a t-test (*P < 0.05).
.
Cytotoxicity of NK cells. (A) LDH assay; activated NK cells toward MKN45 cells at different E: T ratios. The results demonstrated the most cytotoxic effect in 10:1 E: T (****P < 0.0001). Data reported as SEM for four groups independently. (B) Cytokine release assay of NK cells on day 21 by collecting the condition media. In comparison to control, the cytotoxic cytokine release of NK cells was ≈45 pg/mL and ≈27 pg/mL for IFNγ (**P < 0.01) and TNF α, respectively (***P < 0.001). Statistical significance was determined using a t-test (*P < 0.05).
In vitro expanded NK cells release cytotoxic cytokines
In order to evaluate the cytokine release of expanded NK cells, we assessed the IFNγ and TNFα release by ELISA test. In comparison to control, the cytotoxic cytokine release of NK cells was ≈45 pg/mL and ≈27 pg/mL for IFNγ (P < 00.0013) and TNFα, respectively (P < 0.0009). Active, viable NK cells/PBS were injected intratumorally into a cell line-derived gastric cancer xenograft model (Fig. 3B).
Effects of capecitabine and NK cells on morphometric growth properties of xenograft model of gastric cancer
To evaluate the effect of capecitabine chemotherapy in the metronomic approach combined with NK cell therapy, we further investigated the morphometric properties of xenograft cancer models. The morphometric features of tumor growth curve are shown in Fig. 4. The mean tumor volume in the chemotherapy and combination group is significantly lower than in cytotherapy and control groups (P<0.0001). However, no significant difference between the combination and chemotherapy groups was found (Fig. 4A). The percent of tumor growth inhibition in the combination group (P< 0.02) and MC group (P< 0.007) was significantly higher (≈20%, and 35%, respectively) than in the cytotherapy group. The result demonstrated no significant difference in %TGI between the combination and MC groups (Fig. 4B). Simultaneously with the end of experiment, animals were euthanized by the mentioned procedure, and implanted subcutaneously tumors were removed and investigated. The tumor weight mean was significantly higher in the control and cytotherapy groups (P< 0.0001) in contrast to the combination and MC groups (Fig. 4C).
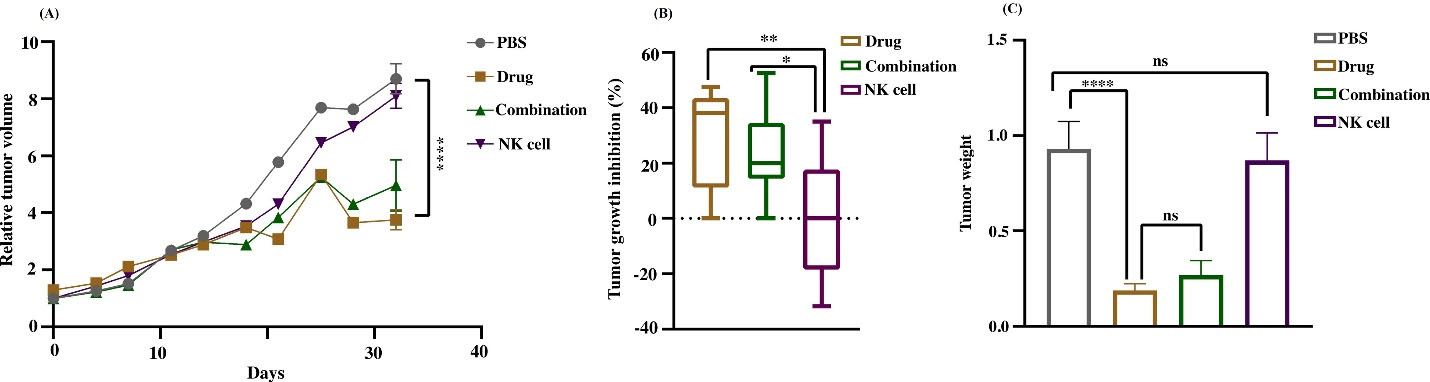
Fig. 4.
The characterization of tumor morphometric properties. (A) Relative tumor volume (RTV) curve versus the time elapsed demonstrated the variations of growth. The chemotherapy and combination groups mean tumor volume is significantly lower than cytotherapy and control groups (****P < 0.0001). (B) Tumor growth inhibition of different experiments modified by normalizing with control tumor. The percent of tumor growth inhibition in the combination group (*P < 0.05) and MC group (**P < 0.01) was significantly higher than cytotherapy group. (C) Tumor weight means were higher in the control and cytotherapy group (****P < 0.0001) than in the combination and MC group. Data reported as SEM. Statistical significance was evaluated utilizing one–way ANOVA with *P < 0.05.
.
The characterization of tumor morphometric properties. (A) Relative tumor volume (RTV) curve versus the time elapsed demonstrated the variations of growth. The chemotherapy and combination groups mean tumor volume is significantly lower than cytotherapy and control groups (****P < 0.0001). (B) Tumor growth inhibition of different experiments modified by normalizing with control tumor. The percent of tumor growth inhibition in the combination group (*P < 0.05) and MC group (**P < 0.01) was significantly higher than cytotherapy group. (C) Tumor weight means were higher in the control and cytotherapy group (****P < 0.0001) than in the combination and MC group. Data reported as SEM. Statistical significance was evaluated utilizing one–way ANOVA with *P < 0.05.
The effects of interventions on histopathological outcomes
Chemo-immune cell therapy improves the treatment response against the gastric cancer xenograft model. The MC and combination group treatment effect was R2 histologic response in almost 90% of tumor samples based on the pathological assessment. In other words, tumor samples demonstrated more than 30% response to current treatment and have diagnosed with necrosis and apoptosis in contrast to cytotherapy and the control group in which the response rate was very low or non-existence (R3) (Table 1, Fig. 5A). The result showed no significant difference in treatment response of MC and the combination group. The mitotic count was used to investigate the effects of interventions on tumor proliferation intensity. The highest anti-proliferative effect was seen in the MC group in contrast with the control group (P< 0.0004), with almost 20 mitotic cell counts per 10HPF against 45 mitotic cell counts in the control group. While minimal treatment effect was observed in the cytotherapy group, the mitotic count was remarkably lower than in the control group (P< 0.006), with almost 30 mitotic cell count/10HPF. However, the combination group had a higher anti-proliferative effect (P< 0.0002) in contrast to the control group with almost 20 mitotic count /10HPF; there was not any notable difference in mitotic count between MC and combination group, and the result was in line with other histopathologic data (Fig. 5B).
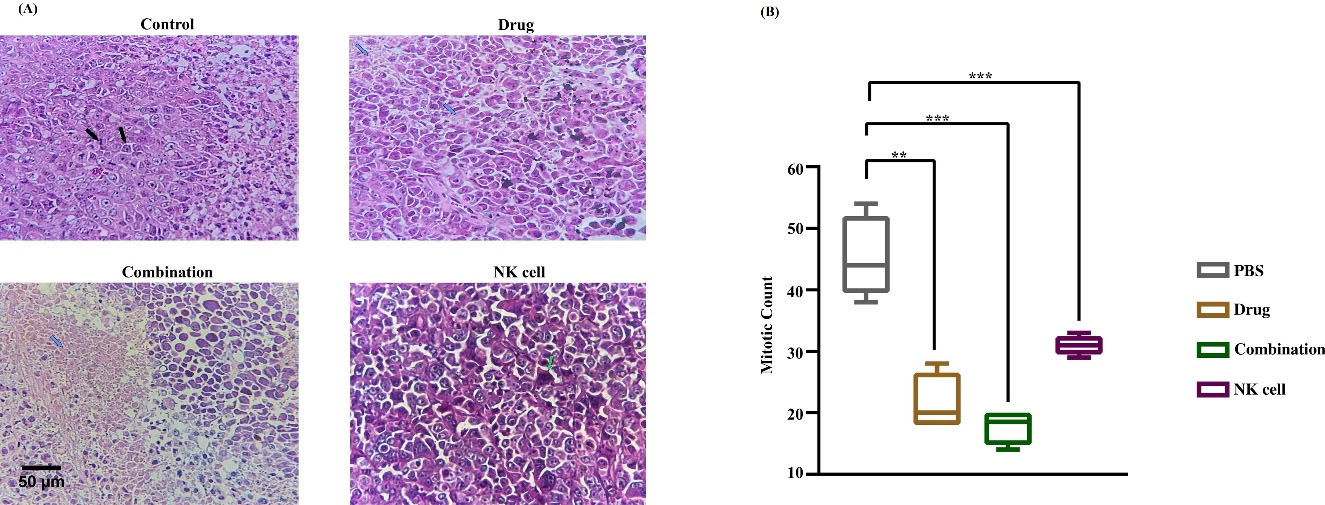
Fig. 5.
Histopathology findings. (A)Hematoxylin and Eosin (H and E) staining report. Black arrows pointed active mitosis, necrotic areas were pointed by blue arrows, and apoptotic bodies were pointed by green arrows. (B) Mitotic count as proliferation score assessed in 10HPF. The highest anti-proliferative effect was seen in MC and combination groups in contrast with control and cytotherapy groups (**P < 0.01). Data reported as SEM. The significancy was evaluated using a t test with *P < 0.05. Scale bar: 100 μm, 400X magnification.
.
Histopathology findings. (A)Hematoxylin and Eosin (H and E) staining report. Black arrows pointed active mitosis, necrotic areas were pointed by blue arrows, and apoptotic bodies were pointed by green arrows. (B) Mitotic count as proliferation score assessed in 10HPF. The highest anti-proliferative effect was seen in MC and combination groups in contrast with control and cytotherapy groups (**P < 0.01). Data reported as SEM. The significancy was evaluated using a t test with *P < 0.05. Scale bar: 100 μm, 400X magnification.
Infiltration assay of adoptive NK cells to the tumor micro-structure
While the NK cells were injected intratumorally, the surface marker of NK cells, hCD56, was evaluated by IHC to verify NK cell permeation to the tumor microenvironment. The control and MC group did not receive human NK cells, so expressing the hCD56 was definitely negative. Cytotherapy and combination groups demonstrated a different pattern of hCD56 expression due to the Allred score. The proportion score in the cytotherapy group indicated more than 30% immunoreactivity, and the intensity was intermediate positive (3 of 4 tumor samples). Still, the combination group demonstrated lower immunoreactivity, almost 10%, and the intensity was weakly positive (3 of 4 tumor samples). Evaluation of IHC slides demonstrated the aggregation of adoptive NK cells in the tumor margin. The cells could not properly infiltrate to tumor center in both experimental groups (Table 2, Fig. 6).
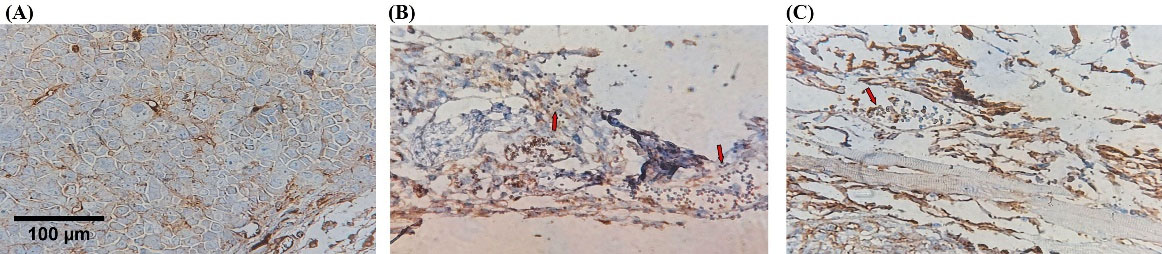
Fig. 6.
Adoptive NK cells infiltration injected intratumorally. IHC evaluated Tumor-infiltrating NK cells with anti-CD56 antibody in tumor microenvironment. Cytotherapy and combination groups demonstrated a different pattern of hCD56 expression (Red arrow showed immunoreactive cells). (A) Negative control, (B) Combination, (C) NK cell therapy. Scale bar: 100 μm, 400X magnification.
.
Adoptive NK cells infiltration injected intratumorally. IHC evaluated Tumor-infiltrating NK cells with anti-CD56 antibody in tumor microenvironment. Cytotherapy and combination groups demonstrated a different pattern of hCD56 expression (Red arrow showed immunoreactive cells). (A) Negative control, (B) Combination, (C) NK cell therapy. Scale bar: 100 μm, 400X magnification.
The ratio of proliferation and apoptosis of tumor cells
IHC assessed Ki67 and caspase3 to evaluate the proliferation ratio to apoptosis. Evaluation of proliferation ratio to apoptosis highlighted MC as more effective in treating gastric cancer xenograft tumors. The higher value of this ratio is consistent with increased proliferation and decreased apoptosis. The highest percentage was determined in the control group and NK cell therapy by almost 4.3% and 3.2%, demonstrating no significant difference. In the drug and combination group, the value determined nearly 1.4% and 1.16%, respectively, with no significant difference in line with other obtained data. The ratio was remarkably lower in the drug and combination group against the control and NK cell therapy group; almost 4%, 3%, 1%, and 1%, respectively. The low dose of capecitabine was significantly prominent in increasing apoptosis in MC and the combination group (Figs. 7 and 8).
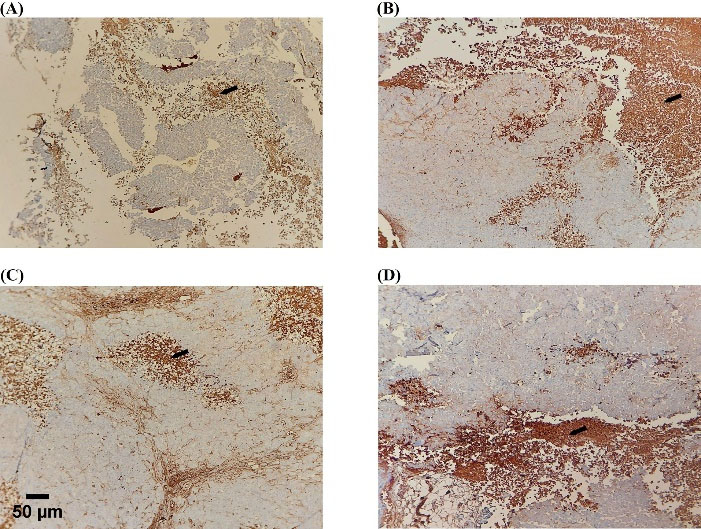
Fig. 7.
Apoptosis induction by capecitabine. Active caspase-3 (cytoplasmic staining) immunostaining was evaluated in the tumor section (black arrows; necrotic areas). The highest immune reactive cells were detected in drug and combination groups. (A) Control, (B) Drug, (C) Combination, (D) NK cell therapy. Scale bar: 50 μm, 200X magnification.
.
Apoptosis induction by capecitabine. Active caspase-3 (cytoplasmic staining) immunostaining was evaluated in the tumor section (black arrows; necrotic areas). The highest immune reactive cells were detected in drug and combination groups. (A) Control, (B) Drug, (C) Combination, (D) NK cell therapy. Scale bar: 50 μm, 200X magnification.
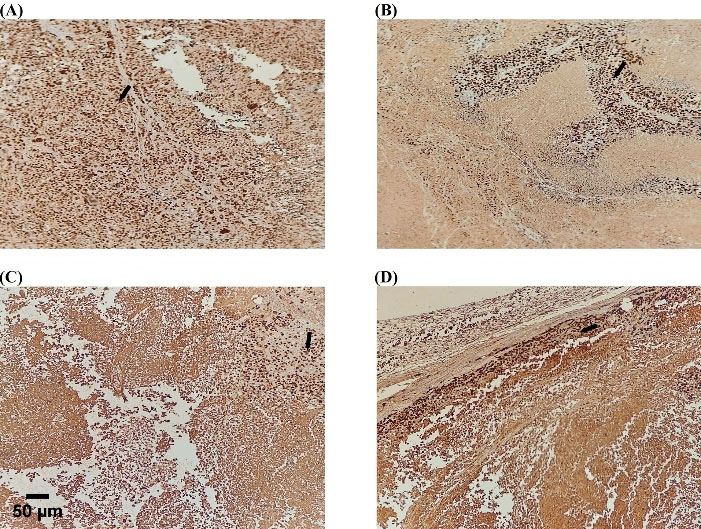
Fig. 8.
Proliferation inhibition by capecitabine. Single-color immunostaining for Ki-67(nuclear staining) in the tumor section (black arrows; active proliferation). The highest immune reactive cells were detected in the control and NK cell therapy groups. (A) Control, (B) Drug, (C) Combination, (D) NK cell therapy. Scale bar: 50 μm, 200X magnification.
.
Proliferation inhibition by capecitabine. Single-color immunostaining for Ki-67(nuclear staining) in the tumor section (black arrows; active proliferation). The highest immune reactive cells were detected in the control and NK cell therapy groups. (A) Control, (B) Drug, (C) Combination, (D) NK cell therapy. Scale bar: 50 μm, 200X magnification.
Discussion
Metronomic chemotherapy represents a new trend in cancer treatment because of its low toxicity, considerably tolerable, cost-effective, and easy access.37 In clinical studies, metronomically administered capecitabine demonstrated treatment safety and improved overall survival.38,39 While drug resistance is among the leading cause of mortality in cancer patients,40 this study was designed to improve treatment efficacy by combining adoptive NK cell therapy and capecitabine MC. Since there are dysfunctional peripheral NK cells in gastric cancer patients, and their number decreases with cancer progression, adoptive fresh NK cells combined with ongoing chemotherapy could be a promising approach to eliminate cancer.8 In the current study, capecitabine was administered metronomically, lower than the MTD dose, and fresh NK cells were injected intratumorally in drug intervals. A critical limitation for efficacious NK cytotherapy is infiltration in solid tumors. Physiologic stimuli such as hypoxia and lactic acid in the context of solid tumors, recruitment of suppressive myeloid-derived suppressor cells (MDSCs) by tumor cells,41 tumor-derived TGFβ,42 local immune suppression caused by inhibitory immune checkpoints on tumor cells’ surface such as programmed death-ligand 1 (PD-L1), and PD-L2 are among the most prominent preventer of NK cells infiltration to the tumor micro-structure.43
Therefore, to assess the NK cell functionality, regardless of systematic infiltration, the NK cells were directly injected intratumorally, and their efficacy was evaluated in this study. In contrast to this research, intratumorally injected NK cells in an orthotopic glioblastoma xenograft model previously showed significant therapeutic effects.44
Fresh activated NK cells were injected into the tumor site directly, and the drug was administered orally by sterilized needle gavage demonstrated in Fig. 1. Morphometric results showed the most efficacious treatment of MC in contrast to cytotherapy. MC treatment inhibited the tumor growth, and the combination had no significant difference with MC.
In the current study, none of the mice suffered from drug toxicity, there was no mortality due to drug side effects, and low doses of capecitabine were well tolerable. He et al reported that capecitabine MC is a kind of palliative treatment along with less side effects in aged patients that diagnosed with advanced gastric cancer.38
Tumor volume assessment in this study demonstrated no significant difference among control and NK cell therapy groups, and rapid progression of the tumor was observed similarly in both groups.Similar to the present study’s findings,Tseng et al reported that in animal models of hepatocellular carcinoma treated with parental NK cells or PBS, rapid disease progression developed, and tumor volume increased in both NK cell and control groups with no significant difference.4,5 Moreover, another study reported that human NK cells revealed no growth inhibition of the U87MG (the cell line of glioblastoma) in vitro compared to the control group. In contrast, treatment response was achieved when the NK cells were genetically manipulated.46 A recent study demonstrated that parental NK cells did not affect tumor eradication in a murine multiple myeloma model (MM). The tumor progressed in both control and NK cell therapy groups similarly.47 Although MM is a hematologic malignancy, but NK cell activation suppressor immune cells such as MDSCs, M2-like macrophages, regulatory B/T-cells are presented in tumor microenvironment.48 In the current study, the morphometric and histopathologic results demonstrated that the NK cells were not efficient enough to inhibit tumor growth. Adoptive NK cell low functionality in solid tumors could be considered in two aspects: the first due to tumor microenvironment and the second because of checkpoint features of NK cells. Due to chronic immune suppressive signals of the solid tumors microenvironment, NK cells are not acting properly. One possible consideration is those soluble modulators such as TGF-β and hypoxic conditions of solid tumors that inhibit NK cell activation.49,50
In pathology assessment, the mitotic count of tumor cells was lower in the MC group than in the control and cytotherapy group, in line with other obtained data. The combination group had no significant difference from the MC group. The mitotic count in the cytotherapy group was remarkably lower than control group. However, the NK cells did not affect the reduction of tumor volume, but in pathology, they were able to diminish mitotic count significantly but not enough to eradicate the tumor. The minimal effects of NK cells monotherapy against tumor caused low mitotic count and probably required continued therapy at higher doses. The cytotoxicity results demonstrated the higher cytotoxic effects of NK cells against the gastric cancer cell line by elevating the cell number up to tenfold. In addition, genetic modification or checkpoint manipulation of NK could improve the efficacy of cell therapy. The present study evaluated the cytokine production of NK cells before injection. IFNγ production was significantly higher than control which demonstrated NK cell activation. Zhang et al recently reported that although 5-Fu is generally adopted to directly kill the cancer cells in gastric cancer, it up-regulates the expression of inhibitory checkpoint expression PD-L1 and distantly suppresses the immune response.51 Ki67 and activated caspase3 were assessed to determine the proliferation and apoptosis in tumor sections by IHC. The ratio demonstrated the higher proliferation in the control and NK cell therapy group and more elevated apoptosis in the drug and combination group, consistent with other results. Reportedly, the MC by capecitabine inhibited the proliferation of different gastric cancer cell lines and induced apoptosis when the drug dosage was elevated.52 In the combination group, the intensity score of the NK cells population was lower than cytotherapy alone. In both cytotherapy and combination groups, NK cells did not properly infiltrate to tumor center, and their aggregation mainly was in the margins. Our previous study revealed that NK cells that were treated with anti-PD-1 antibody (Nivolumab) before injection would significantly inhibit the tumor growth in the MKN-45 gastric cancer xenograft model and NK cells infiltrate to tumor site more properly than NK cell monotherapy.53 One of the most recent studies explained the role of CIS as an internal checkpoint molecule in the development of NK cell therapy that promotes NK cell fitness in the suppressive tumor microenvironment.54 Thus, modulating the NK cells with prominent checkpoint inhibitors in the setting of cancer immune cell therapy should be considered.
Conclusion
Regarding the present study’s findings, it could be concluded that adoptive NK cell therapy combined with capecitabine MC was not more efficient than MC alone. The results demonstrated that although NK cells were active before injection, they were not sufficiently efficacious to improve the tumor eradication; because the tumors in the combination group continued to progress even after chemotherapy. Moreover, low doses of capecitabine decreased the proliferation rate of tumoral cells. However, in clinic, discontinuing the patient’s standard care is not ethic, new strategies should be found out to improve the treatment efficacy with minimal side effects. Immune-suppressive tumor microenvironment and inhibitory immune checkpoints made the tumor none responsive to this kind of combined chemo-immune cell therapy that should be further investigated.
Research Highlights
What is the current knowledge?
√ NK cells play a critical role in tumor elimination.
√ NK cells are safe, rarely induce GVHD, and are well tolerated in recipients.
√ NK cells play a fundamental role in suppressing gastric cancer development and metastasis.
√ NK cells efficacy in combination with chemotherapeutic drugs needs additional investigation.
What is new here?
√ NK cell therapy combined with chemotherapy.
√ Combination of capecitabine and NK cell cytotherapy in gastric cancer cell line derived model has not been reported.
√ Regardless of systematic infiltration, the NK cells were directly injected intratumorally, and their efficacy was evaluated.
√ Regarding this study’s findings, it is concluded that adoptive NK cell therapy combined with capecitabine MC was not more efficient than MC alone.
Funding
The Shahid Beheshti University of Medical Science supported this work with an award number1055; Dr. Kaveh Baghaei is a grant-receiving researcher.
Competing interests
The authors declare no competing interests.
Ethical Statement
All animal procedures were carried out under the approved protocol according to the Ethics Committee of Tehran University of Medical Science (Ethic Code IR.TUMS.VCR.REC.1398.873). All methods and experiments were performed under relevant guidelines and regulations and the ARRIVE guidelines.
References
- Akhavan A, Binesh F, Seifaddiny A. Results of combination chemotherapy and radiation therapy in non-metastatic gastric cancer in Yazd–Iran. Indian J Cancer 2015; 52:40. doi: 10.4103/0019-509X.175583 [Crossref] [ Google Scholar]
- Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin 2020; 70:86-104. doi: 10.3322/caac.21596 [Crossref] [ Google Scholar]
- Gulubova M, Manolova I, Kyurkchiev D, Julianov A, Altunkova IJA. Decrease in intrahepatic CD56+ lymphocytes in gastric and colorectal cancer patients with liver metastases. APMIS 2009; 117:870-9. doi: 10.1111/j.1600-0463.2009.02547.x [Crossref] [ Google Scholar]
- Peng L-s, Zhang J-y, Teng Y-s, Zhao Y-l, Wang T-t, Mao F-y. Tumor-associated monocytes/macrophages impair NK-cell function via TGFβ1 in human gastric cancer. Cancer Immunol Res 2017; 5:248-56. doi: 10.1158/2326-6066.CIR-16-0152 [Crossref] [ Google Scholar]
- Li Y, Yin J, Li T, Huang S, Yan H, Leavenworth J. NK cell-based cancer immunotherapy: from basic biology to clinical application. Sci China Life Sci 2015; 58:1233-45. doi: 10.1007/s11427-015-4970-9 [Crossref] [ Google Scholar]
- Sakamoto N, Ishikawa T, Kokura S, Okayama T, Oka K, Ideno M. Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer. J Transl Med 2015; 13:1-13. doi: 10.1186/s12967-015-0632-8 [Crossref] [ Google Scholar]
- Saito H, Takaya S, Osaki T, Ikeguchi M. Increased apoptosis and elevated Fas expression in circulating natural killer cells in gastric cancer patients. Gastric Cancer 2013; 16:473-9. doi: 10.1007/s10120-012-0210-1 [Crossref] [ Google Scholar]
- Li T, Zhang Q, Jiang Y, Yu J, Hu Y, Mou T. Gastric cancer cells inhibit natural killer cell proliferation and induce apoptosis via prostaglandin E2. Oncoimmunology 2016; 5:e1069936. doi: 10.1080/2162402X.2015.1069936 [Crossref] [ Google Scholar]
- González S, López-Soto A, Suarez-Alvarez B, López-Vázquez A, López-Larrea C. NKG2D ligands: key targets of the immune response. Trends Immunol 2008; 29:397-403. doi: 10.1016/j.it.2008.04.007 [Crossref] [ Google Scholar]
- Takimoto R, Kamigaki T, Okada S, Matsuda E, Ibe H, Oguma E. Efficacy of adoptive immune-cell therapy in patients with advanced gastric cancer: a retrospective study. Anticancer Res 2017; 37:3947-54. doi: 10.21873/anticanres.11778 [Crossref] [ Google Scholar]
- Du Y, Wei Y. Therapeutic potential of natural killer cells in gastric cancer. Front Immunol 2019; 9:3095. doi: 10.3389/fimmu.2018.03095 [Crossref] [ Google Scholar]
- Johnston FM, Beckman MJCor. Updates on management of gastric cancer. Curr Oncol Rep 2019; 21:1-9. doi: 10.1007/s11912-019-0820-4 [Crossref] [ Google Scholar]
- Chen J, Xiong J, Wang J, Zheng L, Gao Y, Guan Z. Capecitabine/cisplatin versus 5‐fluorouracil/cisplatin in Chinese patients with advanced and metastatic gastric cancer: Re‐analysis of efficacy and safety data from the ML17032 phase III clinical trial. Asia Pac J Clin Oncol 2018; 14:e310-e6. doi: 10.1111/ajco.12832 [Crossref] [ Google Scholar]
- Goodin S. Oral chemotherapeutic agents: Understanding mechanisms of action and drug interactions. Am J Health Syst Pharm 2007; 64:S15-S24. doi: 10.2146/ajhp070034 [Crossref] [ Google Scholar]
- Bocci G, Kerbel RS. Pharmacokinetics of metronomic chemotherapy: a neglected but crucial aspect. Nat Rev Clin Oncol 2016; 13:659-73. doi: 10.1038/nrclinonc.2016.64 [Crossref] [ Google Scholar]
- Hanahan D, Bergers G, Bergsland E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest 2000; 105:1045-7. doi: 10.1172/JCI9872 [Crossref] [ Google Scholar]
- Park M, Kim JY, Kim J, Lee J-H, Kwon Y-G, Kim Y-M. Low-dose metronomic doxorubicin inhibits mobilization and differentiation of endothelial progenitor cells through REDD1-mediated VEGFR-2 downregulation. BMB Rep 2021; 54:470. doi: 10.5483/BMBRep.2021.54.9.096 [Crossref] [ Google Scholar]
- Park M, Kim J, Kim T, Kim S, Park W, Ha K-S, et al. REDD1 is a determinant of low-dose metronomic doxorubicin-elicited endothelial cell dysfunction through downregulation of VEGFR-2/3 expression. Exp Mol Med 2021; 1-11. 10.1038/s12276-021-00690-z.
- Pramanik R, Tyagi A, Agarwala S, Vishnubhatla S, Dhawan D, Bakhshi S. Evaluation of vascular endothelial growth factor (VEGF) and thrombospondin-1 as biomarkers of metronomic chemotherapy in progressive pediatric solid malignancies. Indian Pediatr 2020; 57:508-11. [ Google Scholar]
- Benayoun L, Gingis‐Velitski S, Voloshin T, Segal E, Segev R, Munster M. Tumor‐initiating cells of various tumor types exhibit differential angiogenic properties and react differently to antiangiogenic drugs. Stem cells 2012; 30:1831-41. doi: 10.1002/stem.1170 [Crossref] [ Google Scholar]
- Mu Y, Zhou C-H, Chen S-F, Ding J, Zhang Y-X, Yang Y-P. Effectiveness and safety of chemotherapy combined with cytokine-induced killer cell/dendritic cell–cytokine-induced killer cell therapy for treatment of gastric cancer in China: A systematic review and meta-analysis. Cytotherapy 2016; 18:1162-77. doi: 10.1016/j.jcyt.2016.05.015 [Crossref] [ Google Scholar]
- Geller MA, Knorr DA, Hermanson DA, Pribyl L, Bendzick L, Mccullar V. Intraperitoneal delivery of human natural killer cells for treatment of ovarian cancer in a mouse xenograft model. Cytotherapy 2013; 15:1297-306. doi: 10.1016/j.jcyt.2013.05.022 [Crossref] [ Google Scholar]
- Navarro AG, Kmiecik J, Leiss L, Zelkowski M, Engelsen A, Bruserud Ø. NK cells with KIR2DS2 immunogenotype have a functional activation advantage to efficiently kill glioblastoma and prolong animal survival. J Immunol 2014; 193:6192-206. doi: 10.4049/jimmunol.1400859 [Crossref] [ Google Scholar]
- Veluchamy JP, Lopez-Lastra S, Spanholtz J, Bohme F, Kok N, Heideman DAM. In vivo efficacy of umbilical cord blood stem cell-derived NK cells in the treatment of metastatic colorectal cancer. Front Immunol 2017; 8:87. doi: 10.3389/fimmu.2017.00087 [Crossref] [ Google Scholar]
- Faghfuri E, Shadbad MA, Faghfouri AH, Soozangar NJI. Cellular immunotherapy in gastric cancer: adoptive cell therapy and dendritic cell-based vaccination. Immunotherapy 2022; 14:475-88. doi: 10.2217/imt-2021-0285 [Crossref] [ Google Scholar]
- Takada M, Terunuma H, Deng X, Dewan MZ, Saji S, Kuroi K. Refractory lung metastasis from breast cancer treated with multidisciplinary therapy including an immunological approach. Breast Cancer 2011; 18:64-7. doi: 10.1007/s12282-010-0198-5 [Crossref] [ Google Scholar]
- Berg M, Lundqvist A, McCoy Jr P, Samsel L, Fan Y, Tawab A. Clinical-grade ex vivo-expanded human natural killer cells up-regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumor cells. Cytotherapy 2009; 11:341-55. doi: 10.1080/14653240902807034 [Crossref] [ Google Scholar]
- Chan FK-M, Moriwaki K, De Rosa MJ. Detection of necrosis by release of lactate dehydrogenase activity. Immune Homeostasis 2013; 65-70. 10.1007/978-1-62703-290-2_7.
- Hennessy BT, Gauthier AM, Michaud LB, Hortobagyi G, Valero V. Lower dose capecitabine has a more favorable therapeutic index in metastatic breast cancer: retrospective analysis of patients treated at MD Anderson Cancer Center and a review of capecitabine toxicity in the literature. Ann Oncol 2005; 16:1289-96. doi: 10.1093/annonc/mdi253 [Crossref] [ Google Scholar]
- Morino K, Neschen S, Bilz S, Sono S. IRS-1 serine phosphorylation is a key molecular event in the pathogenesis of fat-induced insulin resistance in skeletal muscle in vivo. Diabetes 2005; 54:A339. doi: 10.1093/annonc/mdi253 [Crossref] [ Google Scholar]
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. Thyroid cancer staging. In: AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010.
- Meuten DJ, Moore FM, George JW. Mitotic count and the field of view area: time to standardize. Vet Pathol 2016. 10.1177/0300985815593349.
- Kim S-W, Roh J, Park C-S. Immunohistochemistry for pathologists: protocols, pitfalls, and tips. J Pathol Transl Med 2016; 50:411. doi: 10.4132/jptm.2016.08.08 [Crossref] [ Google Scholar]
- Parvin T, Das C, Choudhury M, Chattopadhyay BK, Mukhopadhyay M. Prognostic utility of cyclin D1 in invasive breast carcinoma. Indian J Surg Oncol 2019; 10:167-73. doi: 10.1007/s13193018-0839-2 [Crossref] [ Google Scholar]
- O’Hurley G, Sjöstedt E, Rahman A, Li B, Kampf C, Pontén F. Garbage in, garbage out: a critical evaluation of strategies used for validation of immunohistochemical biomarkersMol. Oncol 2014; 8:783-98. doi: 10.1016/j.molonc.2014.03.008 [Crossref] [ Google Scholar]
- Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005; 105:3051-7. doi: 10.1182/blood-2004-07-2974 [Crossref] [ Google Scholar]
- El Darsa H, El Sayed R, Abdel-Rahman O. What is the real value of metronomic chemotherapy in the treatment of gastrointestinal cancer? Expert Opin Pharmacother 2021. 10.1080/14656566.2021.1940953.
- He S, Shen J, Hong L, Niu L, Niu D. Capecitabine “metronomic” chemotherapy for palliative treatment of elderly patients with advanced gastric cancer after fluoropyrimidine-based chemotherapy. Medical Oncology 2012; 29:100-6. doi: 10.1007/s12032-010-9791-x [Crossref] [ Google Scholar]
- Roberto M, Romiti A, Onesti CE, D’Antonio C, Milano A, Falcone R. A metronomic schedule as salvage chemotherapy for upper gastrointestinal tract cancer. Anticancer Drugs 2016; 27:106-11. doi: 10.1097/CAD.0000000000000308 [Crossref] [ Google Scholar]
- Riesco-Martinez M, Parra K, Saluja R, Francia G, Emmenegger U. Resistance to metronomic chemotherapy and ways to overcome it. Cancer Lett 2017; 400:311-8. doi: 10.1016/j.canlet.2017.02.027 [Crossref] [ Google Scholar]
- Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med 2011; 208:1949-62. doi: 10.1084/jem.20101956 [Crossref] [ Google Scholar]
- Pasche BJJocp. Role of transforming growth factor beta in cancer. J Cell Physiol 2001; 186:153-68. doi: 10.1002/1097-4652(200002)186:2<153::AID-JCP1016>3.0.CO;2-J [Crossref] [ Google Scholar]
- Melero I, Rouzaut A, Motz GT, Coukos G. T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discov 2014; 4:522-6. doi: 10.1158/2159-8290.CD-13-0985 [Crossref] [ Google Scholar]
- Lee SJ, Kang WY, Yoon Y, Jin JY, Song HJ, Her JH, et al. Natural killer (NK) cells inhibit systemic metastasis of glioblastoma cells and have therapeutic effects against glioblastomas in the brain. BMC Cancer2015. 15: 1-13. 10.1186/s12885-015-2034-y.
- Tseng H-c, Xiong W, Badeti S, Yang Y, Ma M, Liu T. Efficacy of anti-CD147 chimeric antigen receptors targeting hepatocellular carcinomaNat. Commun 2020; 11:1-15. doi: 10.1038/s41467-020-18444-2 [Crossref] [ Google Scholar]
- Murakami T, Nakazawa T, Natsume A, Nishimura F, Nakamura M, Matsuda R. Novel human NK cell line carrying CAR targeting EGFRvIII induces antitumor effects in glioblastoma cells. Anticancer Res 2018; 38:5049-56. doi: 10.21873/anticanres.12824 [Crossref] [ Google Scholar]
- Goodridge JP, Bjordahl R, Mahmood S, Reiser J, Gaidarova S, Blum R. FT576: multi-specific off-the-shelf CAR-NK cell therapy engineered for enhanced persistence, avoidance of self-fratricide and optimized mab combination therapy to prevent antigenic escape and elicit a deep and durable response in multiple myeloma. Blood 2020; 136:4-5. [ Google Scholar]
- Uckun FMJC. Overcoming the immunosuppressive tumor microenvironment in multiple myeloma. Cancers (Basel) 2021; 13:2018. doi: 10.3390/cancers13092018 [Crossref] [ Google Scholar]
- Batlle E, Massagué J. Transforming growth factor-β signaling in immunity and cancer. Immunity 2019; 50:924-40. doi: 10.1016/j.immuni.2019.03.024 [Crossref] [ Google Scholar]
- Vitale M, Cantoni C, Pietra G, Mingari MC, Moretta L. Effect of tumor cells and tumor microenvironment on NK‐cell function. Eur J Immunol 2014; 44:1582-92. doi: 10.1002/eji.201344272 [Crossref] [ Google Scholar]
- Zhang M, Fan Y, Che X, Hou K, Zhang C, Li C. 5-FU-induced upregulation of exosomal PD-L1 causes immunosuppression in advanced gastric cancer patients. Front Oncol 2020; 10:492. doi: 10.3389/fonc.2020.00492 [Crossref] [ Google Scholar]
- Yuan F, Shi H, Ji J, Cai Q, Chen X, Yu Y. Capecitabine metronomic chemotherapy inhibits the proliferation of gastric cancer cells through anti-angiogenesis. Oncol Rep 2015; 33:1753-62. doi: 10.3892/or.2015.3765 [Crossref] [ Google Scholar]
- Abdolahi S, Ghazvinian Z, Muhammadnejad S, Ahmadvand M, Aghdaei HA, Ebrahimi-Barough S, et al. Adaptive NK Cell Therapy Modulated by Anti-PD-1 Antibody in Gastric Cancer Model. Front Pharmacol 2021; 12. 10.3389/fphar.2021.733075.
- Daher, M., Basar, R., Gokdemir, E., Baran, N., Uprety, N., Nunez Cortes, A. K., et al. Targeting a cytokine checkpoint enhances the fitness of armored cord blood CAR-NK cells Blood 2021; 137(5), 624–636. 10.1182/blood.2020007748.