Bioimpacts. 13(5):415-424.
doi: 10.34172/bi.2023.27576
Original Article
Cytotoxicity of WT1-reactive T cells against Wilms tumor: An implication for antigen-specific adoptive immunotherapy
Seyed Mostafa Monzavi Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, 1, 2, 3 
Amir Ali Hamidieh Conceptualization, Investigation, Writing – original draft, 4 
Mohammad Vasei Resources, Validation, Writing – original draft, 5
Jafar Ai Writing – original draft, 6
Naser Ahmadbeigi Resources, Validation, Writing – original draft, 7
Hamid Arshadi Resources, Writing – original draft, 3
Samad Muhammadnejad Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Writing – original draft, 4, 7, * 
Abdol-Mohammad Kajbafzadeh Conceptualization, Data curation, Investigation, Methodology, Resources, Validation, Writing – original draft, 2, 3, * 
Author information:
1Department of Applied Cell Sciences, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran
2Cancer Control Foundation, Iran University of Medical Sciences, Tehran, Iran
3Pediatric Urology and Regenerative Medicine Research Center, Gene, Cell & Tissue Research Institute, Tehran University of Medical Sciences, Tehran, Iran
4Pediatric Cell and Gene Therapy Research Center, Gene, Cell & Tissue Research Institute, Tehran University of Medical Sciences, Tehran, Iran
5Department of Pathology, Tehran University of Medical Sciences, Tehran, Iran
6Department of Tissue Engineering, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran
7Gene Therapy Research Center, Digestive Diseases Research Institute, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran
Abstract
Introduction:
T cells that recognize WT1 peptides have been shown to efficiently eliminate WT1-expressing tumor cells. This study was designed to investigate the feasibility of isolating WT1-reactive T cells from peripheral blood mononuclear cells (PBMCs) from healthy donors and patients with Wilms tumor, and to assess the cytotoxicity mediated by these cells against Wilms tumor cells (WiTu cells).
Methods:
WT1-reactive T cells were enriched and isolated by stimulating PBMCs with a WT1 peptide pool and interferon-γ capture-based immunomagnetic separation (IMS). Using the lactate dehydrogenase release assay, the in vitro cytotoxicity of the isolated cells and standard chemotherapy was evaluated on WiTu cells.
Results:
Higher proportions of WT1-reactive T cells were isolated from patients with Wilms tumor compared to those isolated from HDs. WT1-reactive T cells produced > 50% specific lysis when co-cultured with WT1+ WiTu cells at the highest effector-to-target (E:T) ratio in this study (i.e., 5:1), compared to <23% when co-cultured with WT1- WiTu cells at the same ratio. WT1-reactive T cells showed anti-tumoral activity in a dose-dependent manner and mediated significantly greater cytotoxicity than the non-WT1-reactive fraction of PBMCs on WT1+ WiTu cells. The cytotoxicity of standard chemotherapy was significantly lower than that of WT1-reactive T cells when co-cultured with WT1+ WiTu cells at E:T ratios of 2:1 and 5:1.
Conclusion:
WT1-reactive T cells can be effectively enriched from the PBMCs of patients with Wilms tumor. Ex vivo generated WT1-reactive T cells might be considered an adoptive immunotherapeutic option for WT1+ Wilms tumors.
Keywords: Adoptive immunotherapy, Cytotoxicity tests, Immunomagnetic separation, T-lymphocytes, Wilms tumor, WT1
Copyright and License Information
© 2023 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
T cells reactive against specific antigens (Ags) have emerged as potent targeted immunotherapies for certain cancers and hard-to-treat viral diseases.1,2 Although the immune system harbors different clones of Ag-reactive T cells to endogenously eliminate cancerous or virally infected cells,3 the body may be caught in a losing battle because of the small number of these cells. Hence, to augment Ag-specific T cell responses in vivo, large-scale in vitro (or ex vivo) enrichment and expansion of the relevant T cell lines may be a solution. This can be achieved via a number of methods including2-4 (1) extraction of Ag-reactive tumor-infiltrating lymphocytes from a tumor graft, (2) enrichment and isolation of endogenous Ag-reactive T cells from peripheral blood, and (3) genetic engineering of autologous T cells using gene vectors encoding a T cell receptor (TCR) or chimeric antigen receptor redirected against the antigen of interest.
Enrichment and isolation of endogenous Ag-reactive T cells from peripheral blood can be accomplished via2-6 (1) reactivation and immunoadsorption of Ag-reactive T cells using peptide-MHC multimers followed by immunomagnetic cell sorting/immunomagnetic separation (IMS) of the tagged cells; or (2) stimulation of Ag-reactive T cells to secrete cytokines using antigen-specific peptide pools, followed by IMS of the cytokine-secreting cells; or (3) co-culture of the peripheral blood mononuclear cells (PBMCs) with the Ag-loaded antigen-presenting cells and subsequent enrichment with multimeter-based or cytokine capture-based IMS. Among these, the relatively convenient and low-cost methodology is to stimulate Ag-reactive T cells with a specific antigen peptide pool and to enrich and isolate the responsive (reactive) cells based on cytokine secretion.
It has been argued that any mutant, overexpressed, or abnormally expressed protein in cancer cells or virally infected cells can serve as a potential target for Ag-specific immunotherapy.1,7,8 The protein encoded by the Wilms’ tumor gene 1 (WT1) located on chromosome 11p is a zinc finger transcription factor that plays an important role in cell growth, survival, and differentiation. It has been named after being identified as a strong candidate predisposition gene for Wilms tumor. The expression of this Ag is restricted to certain types of tissues, especially urogenital and hematopoietic organs. Although WT1 was initially considered a tumor suppressor, it was later recognized as a highly immunogenic oncogene.9,10 WT1 overexpression has been detected in leukemia and various types of solid tumors, and on this basis, it has become an attractive molecule for the development of targeted immunotherapies.7-9 In a clear-cut project supported by the US National Cancer Institute, WT1 has achieved the highest rank of priority among 75 cancer antigens for immunotherapy.7 T cells recognizing WT1 peptides have been efficient in eliminating WT1-expressing tumor cells.6,9,11,12 Hence, enrichment and characterization of WT1-reactive T cells would be highly desirable for cancers with a considerable number of WT1-expressing cells. In this study, we sought to investigate the feasibility of enrichment and expansion of WT1-reactive T cells from the peripheral blood of patients with Wilms tumor and healthy donors using the WT1 peptide pool and IFN-γ capture-based IMS, and to assess the in vitro cytotoxicity of these cells against Wilms tumor cells (WiTu cells).
Materials and Methods
Materials
Materials used for this study included: PBMC isolation medium (Lymphosep®, Biowest, France, #L0560), RPMI-1640 medium (BioideaTM, Iran, #BI-100), collagenase I and IV (Gibco®, USA, #17100-017 and #17104-019, respectively), bovine serum albumin (BSA; Sigma-Aldrich®, USA, #9048-46-8), ethylenediamine tetra-acetic acid (EDTA; Merk, Germany, #108452), phosphate-buffered saline (PBS, Gibco®, UK, #70011036), WT1 peptide pool (PepTivator® WT1-premium grade, Miltenyi Biotec, Germany, #130-095-916), IFN-γ Secretion Assay - Cell Enrichment and Detection Kit (Miltenyi Biotec, Germany, #130-054-201), LS columns (Miltenyi Biotec, Germany, #130-042-401), CytoStimTM (Miltenyi Biotec, Germany, #130-092-172), TexMACSTM medium (Miltenyi Biotec, Germany, #130-097-196), recombinant human IL-2 (Miltenyi Biotec, Germany, #130-097-742), 24-, 48- and 96-well plates (SPL Life Sciences, South Korea, #30024, #30048 and #30096, respectively), pierce LDH cytotoxicity assay kit (Thermo Fisher Scientific Inc., USA, #PI88953), PerCP anti-human CD3 antibody (Clone UCHT1, BioLegend®, USA, #300428), APC anti-human CD4 antibody (Clone RPA-T4, BioLegend®, USA, #300514), FITC anti-human CD8 antibody (Clone SK1, BD Biosciences, USA, #347313), 7-amino-actinomycin D (7AAD; BD Biosciences, USA, #559925), anti-human WT1 antibody (Clone 6F-H2, Vitro Master Diagnóstica, Spain, #MAD-005671QD). Notably, the IFN-γ Secretion Assay - Cell Enrichment and Detection Kit (Miltenyi Biotec, Germany) comprises three components: (1) IFN-γ catch reagent (anti-IFN-γ antibody conjugated with anti-human CD45 antibody), (2) IFN-γ detection antibody (PE-conjugated anti-IFN-γ antibody), and (3) anti-PE MicroBeads UltraPure (colloidal superparamagnetic microbeads conjugated with anti-PE antibody). The drugs used for the cytotoxicity assay were vincristine sulfate (Gedeon Richter, Poland) and dactinomycin (Baxter Oncology GmbH, Germany).
Subjects
To isolate and expand the cells of interest, blood was collected from healthy blood donors and patients with Wilms tumor. According to the study design, sampling was planned for five subjects in each group. The criteria for inclusion of healthy donors included adult volunteers (18-55 years old) without any past medical history, especially no history of smoking, chronic diseases (e.g., endocrine disorders, asthma, autoimmune disorders, cardiovascular diseases), hematologic disorders, or any kind of cancer. In addition, pediatric patients (>2 years old) referred to the Children’s Medical Center, TUMS, Iran, with a heterogeneous solid mass in their kidney, as evidenced in the imaging workup, were included as candidates for blood and tumor specimen collection. Blood specimens of these patients were collected prior to chemotherapy and surgical resection of the kidney. The blood and tumor specimens were used for further processing, if the diagnosis of Wilms tumor was confirmed by the pathologist in our research team on the day of nephrectomy (the day of sampling). Patients with renal cell carcinoma, mesoblastic nephroma, and other causes of renal masses were excluded. However, the first five consecutive pediatric patients enrolled in this study were all diagnosed with Wilms tumor.
WT1-reactive T cell enrichment and isolation
Stimulation of Ag-specific T cells in a polyclonal T cell population using peptide pools and enrichment of cytokine-secreting cells using fluorochrome-conjugated cytokine capture matrix and anti-fluorochrome antibody-coated microbeads have been described previously, through four general steps.13-15 First, the T cells were stimulated using a specific antigen or polyclonal TCR stimulus. Next, a cytokine-specific catching reagent composed of the cytokine-specific “catch or capture” antibody conjugated with a CD45-specific monoclonal antibody was added to the culture and bound to CD45 at the surface of the T cells, while it mutually bound to the secreted cytokine adjacent to the surface of the cytokine-secreting cells. Then, a second cytokine-specific antibody conjugated to a fluorochrome was added to label the cytokine entrapped on the cytokine-secreting cell. Finally, the labeled cells can be used for sensitive analysis by flow cytometry or magnetically enriched via superparamagnetic particles conjugated to an anti-fluorochrome antibody (Fig. 1). The detailed protocol for the enrichment and isolation of WT1-reactive T cells was performed as follows:
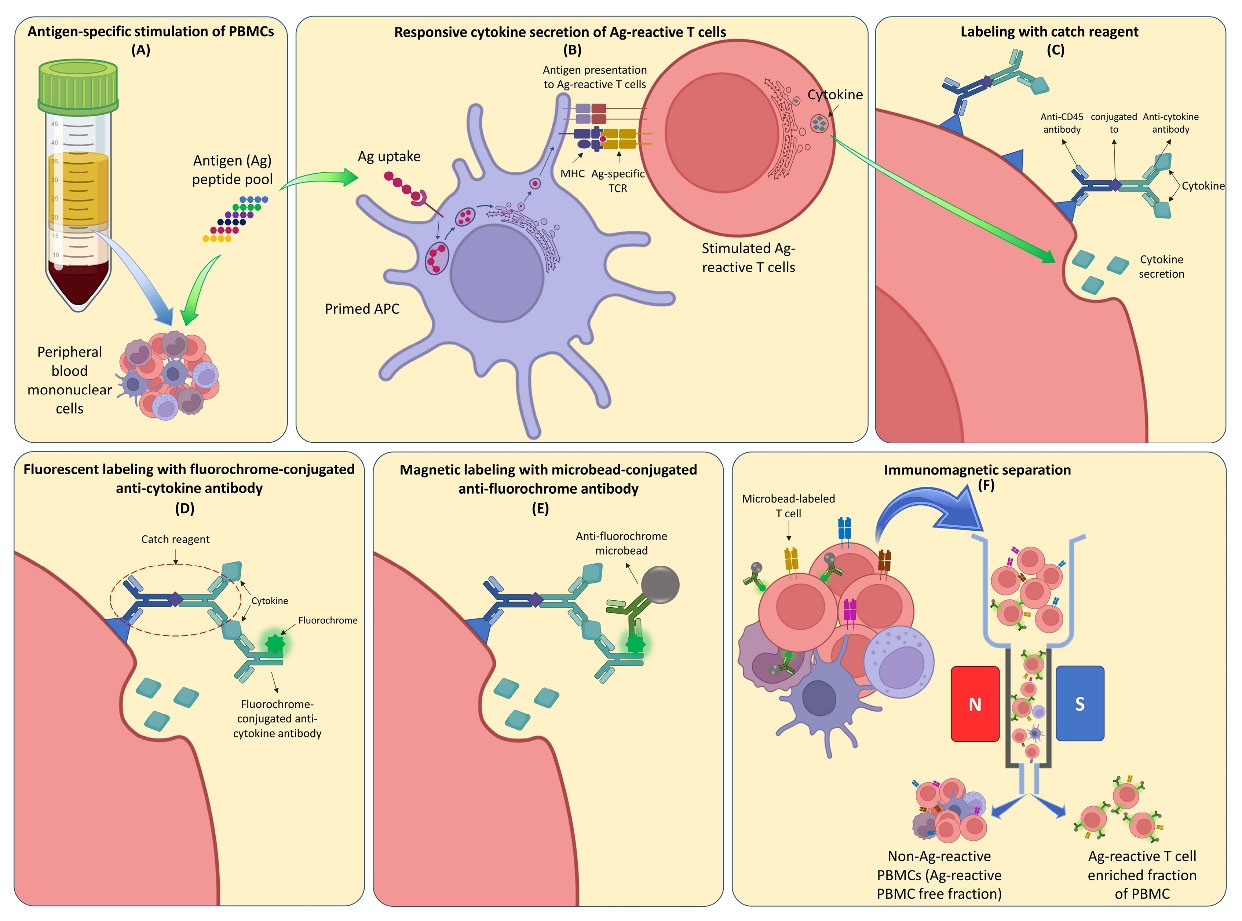
Fig. 1.
Cytokine secretion assay for immunomagnetic separation of antigen-reactive T cells. (A) A specific antigen (Ag) peptide pool is added to a culture of peripheral blood mononuclear cells. (B) The primed antigen presenting cells stimulate the Ag-reactive T cells. (B) & (C) The Ag-reactive T cells start to produce pro-inflammatory cytokines. (C) & (D) The Ag-reactive T cells are labeled with catch reagent which mutually binds to the secreted cytokine. (D) The cytokines are entrapped with fluorochrome-conjugated anti-cytokine antibodies. (E) The fluorochrome-conjugated anti-cytokine antibodies are labeled with anti-fluorochrome microbeads. (F) The magnetically labeled cells are enriched using immunomagnetic separation method.
.
Cytokine secretion assay for immunomagnetic separation of antigen-reactive T cells. (A) A specific antigen (Ag) peptide pool is added to a culture of peripheral blood mononuclear cells. (B) The primed antigen presenting cells stimulate the Ag-reactive T cells. (B) & (C) The Ag-reactive T cells start to produce pro-inflammatory cytokines. (C) & (D) The Ag-reactive T cells are labeled with catch reagent which mutually binds to the secreted cytokine. (D) The cytokines are entrapped with fluorochrome-conjugated anti-cytokine antibodies. (E) The fluorochrome-conjugated anti-cytokine antibodies are labeled with anti-fluorochrome microbeads. (F) The magnetically labeled cells are enriched using immunomagnetic separation method.
Initially, 1.5–2 × 107 PBMCs were isolated from a donor by density gradient centrifugation (with Lymphosep® medium), then washed, resuspended in RPMI-1640 medium, and centrifuged at 300 g for 10 minutes. After removing the supernatant, the PBMCs (cell pellet) were resuspended in culture medium (RPMI-1640 supplemented with 10% human autologous serum) and seeded at a density of 5 × 106 cells/cm2 in a multi-well plate. A fraction of PBMCs (at least 100 000 cells) was analyzed for CD3 expression by flow cytometry. To stimulate cytokine secretion in PBMCs, 20 μL WT1 peptide pool was added to the test culture well (0.6 nmol of each peptide/mL), followed by incubation at 37°C in a humidified 5% CO2 atmosphere for 4 hours. The simulated cells were collected in a 50-mL conical tube filled with 10 mL cold buffer and centrifuged at 300 g for 10 minutes at 4°C. The buffer consisted of PBS, 0.5% (w/v) BSA, and 2 mM EDTA. After complete aspiration of the supernatant, the cell pellet was resuspended in 80 μL of cold buffer, followed by the addition of 20 μL IFN-γ catch reagent, mixed well, and incubated on ice for 5 minutes. Ten milliliters of warm culture medium were added to the tube and the tube was incubated at 37°C for 45 minutes to allow cytokine secretion with intermittent rotation every 5 minutes to avoid sedimentation of the cells. The tube was then filled with an equal amount of cold buffer (10 mL) to stop the secretion phase. The tube was incubated on ice for 10 minutes to ensure that the sample was completely chilled and then centrifuged at 300 g for 10 minutes at 4°C, followed by complete removal of the supernatant. The cell pellet was resuspended in 80 μL of cold buffer, and then 20 μL of the detection antibody was added and mixed well, followed by incubation on ice for 10 minutes. The cells were then washed with 10 mL cold buffer and centrifuged at 300 g for 10 minutes at 4°C. After complete aspiration of the supernatant, the cell pellet was resuspended in 80 μL of cold buffer, the cells were enumerated, and a fraction (at least 100 000 viable cells) was collected for analysis using flow cytometry (FACSCaliburTM, BD Bioscience, USA). A total of 10 μL APC anti-human CD4, 20 μL FITC anti-human CD8 monoclonal antibodies, and a suitable amount of buffer were added to reach a total volume of 100 μL. The sample was incubated in a refrigerator for 10 minutes and subjected to flow cytometry. Ten minutes prior to sample acquisition, 5 μL 7AAD was added to exclude non-viable cells from flow cytometry analysis.
A suitable amount of cold buffer was added to the rest of the cell suspension to reach a volume of 80 μL, and 20 μL of anti-PE MicroBeads UltraPure was added, followed by incubation at 4°C for 15 minutes. The cells were then washed with 10 mL of cold buffer and centrifuged at 300 × g for 10 minutes at 4°C. After complete aspiration of the supernatant, the cell pellet was resuspended in 500 μL cold buffer. The cell suspension was applied onto a pre-rinsed LS column in the magnetic field of a MidiMACSTM separator (Miltenyi Biotec, Germany), while allowing unlabeled (non-reactive cells to WT1) cells to pass through the column, followed by washing the column with 3 mL buffer three times. After removing the column from the magnetic field, the column was filled with 5 mL buffer, and the labeled IFN-γ-secreting WT1-reactive T cells were flushed into a conical tube using a plunger. Unlabeled cells (non-WT1 reactive subset) were also collected for expansion and cytotoxicity assays. For post-enrichment flow cytometric analysis of cytokine-secreting cells, a fraction (>100 000 viable cells) was again counterstained with 10 μL APC anti-human CD4 and 20 μL FITC anti-human CD8 monoclonal antibodies. It is noteworthy that for each donor, a negative control culture (no WT1 stimulation) and a positive control culture (cells treated with 20 μL of CytoStimTM, an artificial superantigen) with the same starting PBMC count as the WT1-stimulated culture (test culture) and the same enrichment process were also established.
Expansion of WT1-reactive cells
Both subsets of PBMC (the subset enriched for WT1-reactive T cells and the subset containing non-reactive cells to WT1) were expanded in a 24-well plate containing 1 ml/well TexMACS medium supplemented with 300 IU/mL recombinant human IL-2. Cultures were replenished with fresh medium and cytokines every other 3–4 days and cells were sub-cultured when they reached confluence. The population doubling levels (PDLs) of the cells were calculated as follows: PDL = 3.32 × (logH - logS), where H is the number of cells at the time of harvest and S is the number of cells initially seeded.16
Cytotoxicity assay
To assess the cytotoxicity of the PBMC subset enriched for WT1-reactive T cells and the unlabeled non-WT1-reactive subset, they were co-cultured with WT1+ (WT1-expressing) WiTu cells, WT1- WiTu cells (as Ag-negative comparators), and K562 cells (a WT1-expressing cell line as Ag-positive comparator) at four different E:T ratios (i.e., 0.5, 1, 2, and 5) for 24 hours in 96-well plates containing 100 μL/well RPMI-1640 supplemented with 10% FBS. WT1+ and WT1- WiTu cells were derived from two different donors, as described in the next section. The sources of the effector cells on WT1+ and WT1- WiTu cells were from their corresponding donors (autologous), and the source of the effector cells on K562 cells was from the donor of WT1+ WiTu cells (allogeneic). WT1 positivity of the Wilms tumor samples was evaluated via immunohistochemical staining on formalin-fixed paraffin-embedded (FFPE) tissue slides of the primary tumors using anti-human WT1 monoclonal antibody. Using the same method and reagent, an FFPE tissue slide of a xenograft developed in an immunodeficient mouse after subcutaneous injection of 1 × 106 K562 cells was evaluated to confirm WT1 expression in the K562 cell line (Fig. 2). The results of the effector and target cell cocultures were compared with the cytotoxicity mediated by the drugs recommended in the SIOP chemotherapy regimen,17 against the target WT1+ and WT1- WiTu cells. For this purpose, target cells were cultured in wells containing 100 μL RPMI-1640 supplemented with 10% FBS, 7 ng/mL vincristine sulfate, and 7 ng/mL dactinomycin. These doses were chosen as per the previously reported plasma concentrations of these drugs in pharmacokinetic studies approximately 1 hour after systemic injection.18,19 All cytotoxicity assays, which were based on quantifying the specific lysis via measurement of lactate dehydrogenase (LDH) release using the Pierce LDH Cytotoxicity Assay Kit, were performed in triplicate. The level of LDH released in each experiment was quantified using a Stat Fax 2100 Microplate Reader (Awareness Technology, Palm City, FL, USA).
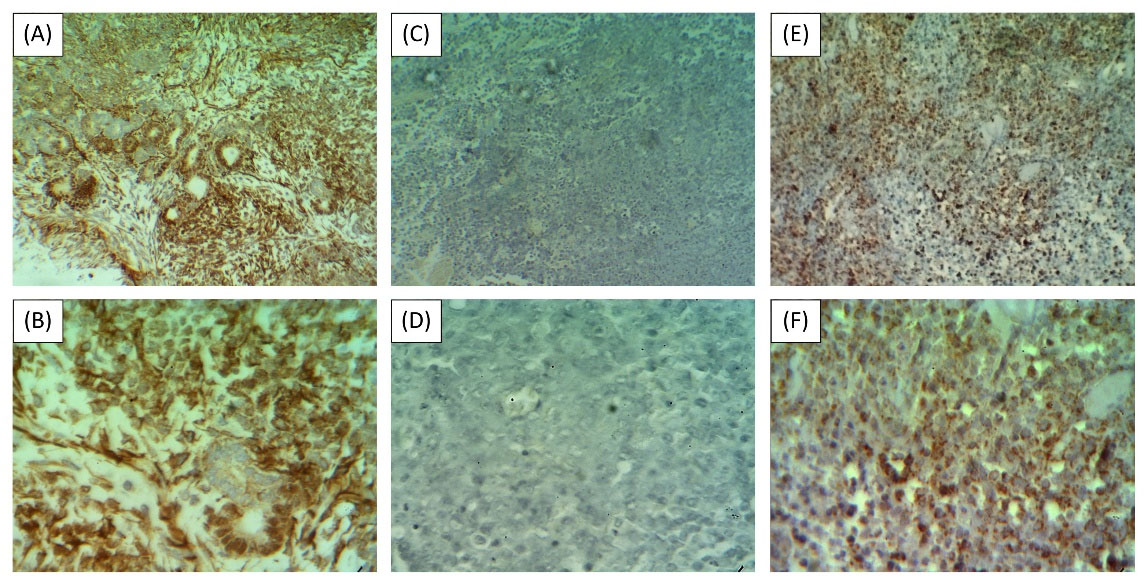
Fig. 2.
WT1 expression in the target cells of the cytotoxicity assay. (A) & (B) WT1+ Wilms tumor or test sample (WT1 IHC, ×100 and ×400, respectively); (C) & (D) WT1- Wilms tumor or antigen-negative comparator (WT1 IHC, ×100 and ×400, respectively); (E) & (F) K562-derived xenograft tumor or antigen-positive comparator (WT1 IHC, ×100 and ×400, respectively).
.
WT1 expression in the target cells of the cytotoxicity assay. (A) & (B) WT1+ Wilms tumor or test sample (WT1 IHC, ×100 and ×400, respectively); (C) & (D) WT1- Wilms tumor or antigen-negative comparator (WT1 IHC, ×100 and ×400, respectively); (E) & (F) K562-derived xenograft tumor or antigen-positive comparator (WT1 IHC, ×100 and ×400, respectively).
Isolation of Wilms tumor cells from tumor explants
Wilms tumor fragments resected from the parental tumor of each donor (WT1+ and WT1-) were incubated at 37°C in a humidified 5% CO2 atmosphere in a microtube (1 fragment per tube) containing 500 μL cocktail of collagenase I and IV (1:1). The tubes were agitated every 10 minutes for 2–3 hours. When the fragment was fully dissociated by visual inspection, the tube (containing the single-cell suspension) was centrifuged at 300 g for 10 minutes. After removal of the supernatant, WiTu cells were resuspended in RPMI-1640 and enumerated. This process was performed for three Wilms tumor fragments (weighing 2–3 mg) from each donor, and the cell yield of the fragments were revealed to be 1.06 (0.82–1.38) × 105 cells per mg on average (min-max).
Statistical analysis
Data analysis and presentation were performed using GraphPad Prism (GraphPad Software Inc., USA). Individual findings are reported as frequencies or percentages. For each group, data are presented as mean ± standard deviation (SD). Student’s t test was used to analyze the differences between two groups or between two E:T ratios. The significance level was set at P < 0.05.
Results
Subjects’ characteristics
Blood specimens were collected from five patients with histologically favorable Wilms tumor (Male:Female = 3:2) with a mean (SD) age of 5.2 (1.8) years and five healthy blood donors (Male:Female = 3:2) with a mean age of 32.8 (9.9) years. Immunophenotyping showed that 54.9 (6.6) and 51.6 (6) % of buffy coat-derived PBMCs were CD3+ T cells in patients with Wilms tumor and healthy donors, respectively, with no significant difference (Table 1).
Table 1.
Isolation yield of IFN-γ-secreting T cells reactive to WT1 out of 1 × 107 starting PBMC
|
Donor type
|
WT1-reactive T cellsa
, %
|
Starting CD3+
T cellsb
, %
|
WT1-reactive T cells (total) / starting CD3+
T cells, %
|
| WTP1 |
1.2 |
52.7 |
2.3 |
| WTP2 |
0.7 |
47.1 |
1.5 |
| WTP3 |
0.9 |
52.2 |
1.7 |
| WTP4 |
1.6 |
64.6 |
2.5 |
| WTP5 |
1.3 |
57.8 |
2.2 |
| HD1 |
0.6 |
44.2 |
1.4 |
| HD2 |
0.9 |
51.4 |
1.8 |
| HD3 |
0.9 |
48.3 |
1.9 |
| HD4 |
0.4 |
53.7 |
0.7 |
| HD5 |
0.7 |
60.2 |
1.2 |
| Average of WTPs |
1.1 |
54.9 |
2 |
| Average of HDs |
0.7 |
51.6 |
1.4 |
a The relative output of IFN-γ-secreting T cells reactive to WT1 after immunomagnetic separation.
b Frequency of CD3+ T cells in the starting buffy coat-derived PBMC of each donor.
Abbreviations: WTP, Wilms tumor patient; HD, healthy donor.
WT1-reactive T cell isolation yield
Following IMS, 4.8 (1.1) × 105 and 3.4 (1.2) × 105 cells were isolated (total isolated cells) per 1 × 107 WT1-stimulated PBMCs from patients with Wilms tumor and healthy donors, respectively. Through flow cytometry analysis, which comprised lymphocyte gating to exclude monocytes and debris, 7AAD - gating to exclude nonviable cells, and calibrating with the IFN-γ-secreting cells in the unstimulated samples, the populations of CD4+ and CD8+ IFN-γ-secreting T cell subsets were found to constitute 12.7 (2.5) and 9.4 (1.5) % of the total isolated cells (Fig. 3). These cumulatively translate to 22.1 (3.8) % of the isolated cells being WT1-reactive IFN-γ-secreting T cells. It can therefore be said that 1.1 (0.4) × 105 and 0.7 (0.2) × 105 WT1-reactive IFN-γ-secreting T cells were isolated per 1 × 107 stimulated PBMCs of patients with Wilms tumor and healthy donors, respectively (Table 1). The ratio of isolated WT1-reactive T cells to the starting CD3+ T cell subset of PBMCs was higher in patients with Wilms tumor than in healthy donors, although the difference only approached the level of significance (mean (SD), 2 (0.4) % vs. 1.4 (0.5) %; P = 0.057).
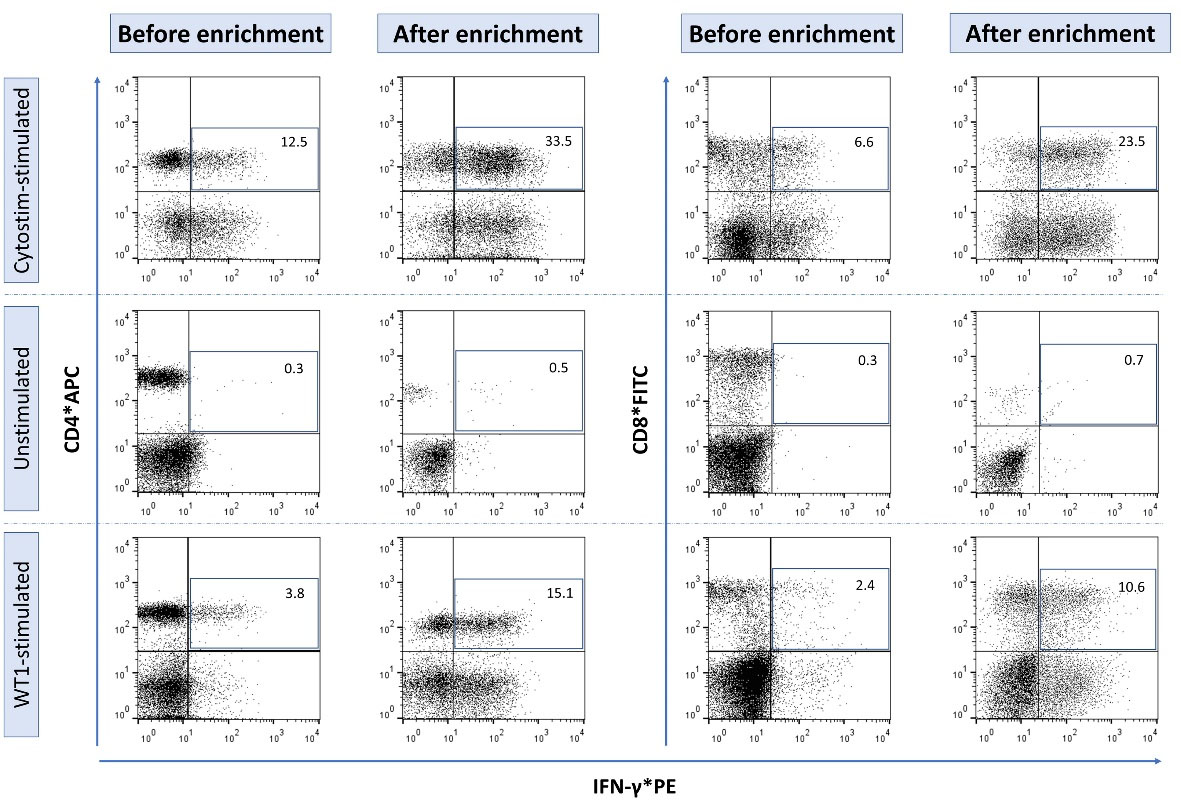
Fig. 3.
Flow cytometry analysis of IFN-γ secreting WT1-reactive T cells. The panels are illustrated in three rows representing the three study samples before and after enrichment with immunomagnetic separation: The test sample that is stimulated with WT1 peptide pool, the positive control sample that is stimulated with CytostimTM (positive control for flow cytometry analysis), and the negative control sample or unstimulated sample (negative control for flow cytometry analysis).
.
Flow cytometry analysis of IFN-γ secreting WT1-reactive T cells. The panels are illustrated in three rows representing the three study samples before and after enrichment with immunomagnetic separation: The test sample that is stimulated with WT1 peptide pool, the positive control sample that is stimulated with CytostimTM (positive control for flow cytometry analysis), and the negative control sample or unstimulated sample (negative control for flow cytometry analysis).
Cytotoxicity of WT1-reactive T cells
The isolated cells from two different donors (patients with histologically WT1+ and WT1- Wilms tumor) were expanded in cytokine-supplemented culture condition for two weeks, and the cells underwent 4–4.5 population doublings during this period. In order to evaluate the anti-tumoral effect of WT1-reactive T cells (effector cells) on WT1-expressing cancer cells, donor-derived WT1+ WiTu cells and K562 cells (as Ag-positive comparators) were used as target cells. Donor-derived WT1- WiTu cells were also evaluated as Ag-negative comparators. Following co-culture with WT1+ WiTu cells, WT1-reactive T cells produced over 50% specific lysis at an E:T ratio of 5:1 in the LDH release cytotoxicity assay (Fig. 4A). Nonetheless, for WT1- WiTu cells, this rate was less than 23% (Fig. 4B). WT1-reactive T cells showed anti-tumoral activity in a dose-dependent manner on both WT1+ WiTu and K562 cells; in addition, compared to the WT1-reactive-free PBMC subset, they produced significantly greater cytotoxicity (Fig. 4A & 4C). However, on WT1- WiTu cells, there was no significant difference between the cytotoxicity of both subsets of PBMC, i.e., the enriched subset for WT1-reactive T cells and the non-WT1-reactive subset (Fig. 4B). The cytotoxicity of WT1-reactive T cells on WT1+ WiTu cells was significantly higher than that of standard chemotherapy at E:T ratios of 2:1 and 5:1 (Fig. 4D); however, chemotherapy resulted in significantly greater cytotoxicity than WT1-reactive T cells on WT1- WiTu cells at E:T ratios of 0.5:1, 1:1, and 2:1, and was only comparable with the cytotoxicity mediated by these cells at an E:T ratio of 5:1 (Fig. 4E). The highest levels of cytotoxicity mediated by WT1-reactive T cells were observed when they were co-cultured with K562 cells, and the lowest levels were observed when they were co-cultured with WT1- WiTu cells at all E:T ratios (Fig. 4F).


Fig. 4.
Cytotoxicity mediated by WT1-reactive T cells compared with non-WT1-reactive PBMCs and standard chemotherapy on Wilms tumor and K562 cells. (A) Cytotoxicity mediated by WT1-reactive T cells compared with non-WT1-reactive PBMCs on WT1+ Wilms tumor cells; (B) Cytotoxicity mediated by WT1-reactive T cells compared with non-WT1-reactive PBMCs on WT1- Wilms tumor cells; (C) Cytotoxicity mediated by WT1-reactive T cells compared with non-WT1-reactive PBMCs on K562 cells; (D) Cytotoxicity mediated by WT1-reactive T cells compared with chemotherapy on WT1+ Wilms tumor cells; (E) Cytotoxicity mediated by WT1-reactive T cells compared with chemotherapy on WT1- Wilms tumor cells; (F) Comparison of the cytotoxicity mediated by WT1-reactive T cells on WT1+ and WT1- Wilms tumor and K562 cells. Symbols and whiskers represent mean and standard errors, respectively. The analysis of difference in (A), (B) and (C) panels are between the treatment groups at each E:T ratio. Ns, non-significant; * P < 0.05, ** P < 0.01, *** P < 0.001
.
Cytotoxicity mediated by WT1-reactive T cells compared with non-WT1-reactive PBMCs and standard chemotherapy on Wilms tumor and K562 cells. (A) Cytotoxicity mediated by WT1-reactive T cells compared with non-WT1-reactive PBMCs on WT1+ Wilms tumor cells; (B) Cytotoxicity mediated by WT1-reactive T cells compared with non-WT1-reactive PBMCs on WT1- Wilms tumor cells; (C) Cytotoxicity mediated by WT1-reactive T cells compared with non-WT1-reactive PBMCs on K562 cells; (D) Cytotoxicity mediated by WT1-reactive T cells compared with chemotherapy on WT1+ Wilms tumor cells; (E) Cytotoxicity mediated by WT1-reactive T cells compared with chemotherapy on WT1- Wilms tumor cells; (F) Comparison of the cytotoxicity mediated by WT1-reactive T cells on WT1+ and WT1- Wilms tumor and K562 cells. Symbols and whiskers represent mean and standard errors, respectively. The analysis of difference in (A), (B) and (C) panels are between the treatment groups at each E:T ratio. Ns, non-significant; * P < 0.05, ** P < 0.01, *** P < 0.001
Discussion
Targeted adoptive immunotherapy for cancer relies on ex vivo generation and expansion of Ag-specific T cell lines. In fact, tumor antigens recognizable to T lymphocytes are at the core of cancer immunotherapy.20,21 To isolate functional Ag-specific T cells from PBMC, magnetic-activated peptide-MHC multimer-guided enrichment and magnetic-activated cytokine capture-based sorting have been used as the two methods for reliably reproducible GMP-grade production.2,3,13,15 Using the latter method, WT1-reactive T cells were enriched and isolated from two groups of healthy donors and patients with Wilms tumor in this study. It was revealed that greater frequencies of these cells can be enriched from patients with Wilms tumor. Moreover, we found that the enriched and expanded WT1-reactive subset of PBMCs was able to mediate high levels of cytotoxicity against autologous WT1+ WiTu cells, whereas they produced significantly lower cytotoxicity against WT1- WiTu cells. Moreover, non-WT1-reactive PBMCs showed limited and comparable cytotoxic effects against both target cells. This means that the cells of interest in this study (WT1-reactive T cells) could exert their anti-tumoral effects in an antigen-directed manner. These findings demonstrate the feasibility of enrichment and ex vivo expansion of these cells and their anti-tumoral activity for targeted immunotherapy against WT1-expressing cancer cells in patients with Wilms tumor.
Wilms tumor is among the malignancies majorly composed of WT1-expressing tumor cells, which is recognized with over 80% WT1 positivity, especially in epithelial and blastemal components of the tumor bulk.22-24 There are also a number of other solid tumors (e.g., lung, breast, esophageal, colorectal, pancreatic, brain, laryngeal, endometrial, and ovarian cancers as well as neuroblastoma and osteo-, rhabdo-, and angio-sarcoma) and hematologic malignancies (e.g., leukemia and lymphoma) with high WT1 expression.9 The immune system of a patient with Wilms tumor highly expressing WT1 is constantly exposed to WT1+ tumor cells, and therefore, more WT1-specific T cells are in vivo stimulated and expanded (as compared with healthy donors).6 Wang et al similarly reported higher numbers of WT1-specific T cells in patients with acute myelogenous leukemia (AML) having high numbers of WT1-overexpressing cells compared with healthy donors.6 Casalegno-Garduño et al, likewise, detected high and enduring frequencies of WT1-specific T cells in patients with AML and myelodysplastic syndrome who had longer survival and continuous complete remission.25 Kyi et al, also, generated a modest number of functional WT1-specific T cells from patients with ovarian cancer using WT1 peptide pools.26
Cytokine-producing Ag-specific T cells are small clones of T cells that fated to be expanded via stimulation with a specific antigen (e.g., WT1). Following Ag-mediated stimulation, reactive T cells respond by secreting inflammatory cytokines (e.g., IFN-γ). Cytokine secretion assays function through the recognition and capture of an antibody matrix engulfing the secreted cytokine that forms on the cell surface. Hence, using anti-cytokine antibody conjugated with a fluorochrome and microbeads coated with anti-fluorochrome antibody, reactive cells can be isolated and enriched.2,13,27 It has been shown that with this method of isolation without the influence of long-term culture, a rapid and ready-to-treat Ag-specific cellular product can be generated.13,28 Ag-specific T cells can be generated and expanded not only from patients with cancer, but also from those with hard-to-treat viral diseases.1,2 For instance, a recent study reported the enrichment of high numbers of SARS-CoV2-specific T cells from COVID-19 convalescent donors.15
In this study, we found that the enriched subset of PBMCs containing high numbers of WT1-reactive T cells could induce substantial cytotoxicity against WT1+ WiTu and K562 cells, which was significantly greater than the non-WT1-reactive PBMC subset as the negative control. However, the cytotoxicity of WT1-reactive T cells against K562 cells was significantly higher than that against WT1+ WiTu cells. This may be partly attributed to HLA incompatibility between the effector and target cell sources in the co-culture of WT1-reactive T and K562 cells, whereas in the cytotoxicity assay on WT1+ WiTu cells, the source of WT1-reactive T cells was from the same donor (autologous). In other words, alloreactivity of the effector cells on the K562 cells, which had been derived from an unrelated donor with chronic myelogenous leukemia, has potentially augmented their direct cytotoxic effect on the tumor cells.29 Likewise, in a study conducted by Plantinga et al, WT1-reactive T cells stimulated with WT1-loaded dendritic cells robustly lysed line-697 cells (a WT1-expressing ALL cell line) and primary pediatric AML cell cultures.30
WT1-reactive T cells also exhibited more potent anti-tumor effects than chemotherapy against WT1+ WiTu cells, although this was not the case for WT1- WiTu cells. Correspondingly, WT1-reactive T cells induced greater specific lysis of WT1+ WiTu cells than WT1- cells. Similarly, in a study on renal cell carcinoma (RCC), Kashima et al showed that WT1-specific TCR T cells could inhibit growth of WT1+ RCC xenografts despite having no effect on WT1- RCC xenografts.31 In this context, activated specific adoptive immunotherapy targeting the WT1 has been shown to eliminate WT1-expressing cancer cells and additionally render the patients with WT1-positive cancer disease-free for long term.32,33 Administration of ex vivo generated allogeneic donor-derived WT1-reactive cytotoxic T cells showed longevity in blood circulation and exhibited robust antileukemic activity causing complete remission for over 2 years in patients with refractory poor-prognosis AML.12 Nonetheless, for the patients with recurrent ovarian cancer enrolled in a trial by Kyi et al, therapeutic response could not be established after administration of autologous WT1-specific T cells, perhaps due to the inadequate number of cells injected.26 For Wilms tumor, Shimodaira et al demonstrated that the induction of in vivo WT1-specific T cell response via ex vivo generated WT1-loaded dendritic cells can prompt a stable disease in a chemo-refractory patient.34 Using the same approach, long-term circulation of the WT1-reactive cytotoxic T cells in the blood and anti-tumor efficacy have been ascertained in patients with gastrointestinal cancer.35 Altogether, enrichment of WT1-reactive T cells out of the patient-derived PBMCs and subsequent multiplication to provide extra number of legions of WT1-directed warriors might be an adoptive immunotherapeutic option for patients with WT1-expressing tumors to prevent relapse after induction chemotherapy or to maintain remission after autologous hematopoietic stem cell transplantation as a selective donor lymphocyte transfusion.4,6,12,13
The present study had some limitations. First, to adhere the ethics principles of the study, we were not allowed to collect blood samples from healthy children; thus, there was a significant age difference between healthy donors and patients with Wilms tumor. Second, the cytotoxicity mediated by WT1-reactive T cells on K562 cells was affected by alloreactivity due to incompatibility between the blood donor and cell line donor. Third, although it might be considered that evaluation of the cytokine profile in culture medium provides supporting evidence for in vitro cytotoxicity findings, this study lacks these data. Finally, the PBMC subset responsive to the WT1 peptide pool was not fully enriched for IFN-γ-secreting cells only, and as illustrated in the flow cytometry figures, this subset also contained IFN-γ*PE-negative cells. However, this may not considerably affect the inferences, as the WT1-reactive T cells accumulated only in one subset (the subset that retains in the MACS column within the magnetic field). In addition, the PBMC subset enriched for WT-reactive T cells mediated higher cytotoxicity in comparison to the WT1-reactive free subset of the PBMC (unlabeled subset passed through the column). To overcome this limitation, a second or further column run can be used to increase the purity of IFN-γ-secreting cells.
Conclusion
Adoptive transfer of ex vivo generated WT1-specific T cells might be considered an option for treating WT1+ Wilms tumor, as in the current study, these cells could be effectively enriched from peripheral blood of donors through an optimized cytokine capture-based magnetic-activated cell sorting and mediated notable cytotoxicity against WT1+ WiTu cells. Further preclinical and clinical studies are required to evaluate the efficacy and safety of WT1-specific T cell therapy for Wilms tumor.
Research Highlights
What is the current knowledge?
√ T cells recognizing tumor antigens have been shown efficient in targeted elimination of antigen-expressing tumor cells.
√ T cells reactive against specific antigens can be isolated via IMS of cytokine-secreting cells pre-stimulated with antigen-peptide pools.
√ The overexpression of the WT1 protein has been detected in leukemia and various types of solid tumors including Wilms tumor.
What is new here?
√ WT1-reactive T cells can be effectively enriched from PBMCs of patients with Wilms tumor.
√ WT1-reactive T cells showed anti-tumoral activity in a dose-dependent manner and mediated notable cytotoxicity against WT1+ Wilms tumor and K562 cells.
√ WT1-reactive T cells caused greater specific lysis when co-cultured with WT1+ cells than when co-cultured with WT1- cells.
Acknowledgments
The authors would like to thank the laboratory staff of the Pediatric Urology and Regenerative Medicine Research Center and the Gene Therapy Research Center, Tehran University of Medical Sciences, for their kind cooperation. The authors would also like to acknowledge Dr. Farshid Noorbakhsh and Dr. Alireza Shoae-Hassani for their advice on study design. The authors would also like to acknowledge the Cancer Control Foundation, Iran University of Medical Sciences, Tehran, Iran for their kind support (CCF-98049). Some of the graphical elements used in the graphical abstract and figure 1 were created using BioRender.com.
Competing Interests
The authors declare no conflict of interest.
Ethical Statement
All experimental protocols involving human subjects were approved by the Institutional Review Board (Ethics code: IR.TUMS.CHMC.REC.1397.038) and performed in accordance with the Declaration of Helsinki. Donors (or their legal guardians) provided written informed consent. Animal experiments were conducted according to the institutional standards for the care and use of laboratory animals at the Tehran University of Medical Sciences (adopted from AAALAC international guidelines).
Funding
The results presented in this article have been derived from a PhD thesis performed at the Tehran University of Medical Sciences (TUMS). The project was supported by an institutional grant (TUMS-97-01-84-38268) and a supportive grant from Cancer Control Foundation, Iran University of Medical Sciences, Tehran, Iran (CCF-98049).
References
- Maus MV, Fraietta JA, Levine BL, Kalos M, Zhao Y, June CH. Adoptive immunotherapy for cancer or viruses. Annu Rev Immunol 2014; 32:189-225. doi: 10.1146/annurev-immunol-032713-120136 [Crossref] [ Google Scholar]
- Monzavi SM, Naderi M, Ahmadbeigi N, Kajbafzadeh AM, Muhammadnejad S. An outlook on antigen-specific adoptive immunotherapy for viral infections with a focus on COVID-19. Cell Immunol 2021; 367:104398. doi: 10.1016/j.cellimm.2021.104398 [Crossref] [ Google Scholar]
- Yee C, Lizee G, Schueneman AJ. Endogenous T-Cell Therapy: Clinical Experience. Cancer J 2015; 21:492-500. doi: 10.1097/PPO.0000000000000158 [Crossref] [ Google Scholar]
- Yee C, Lizee GA. Personalized Therapy: Tumor Antigen Discovery for Adoptive Cellular Therapy. Cancer J 2017; 23:144-8. doi: 10.1097/PPO.0000000000000255 [Crossref] [ Google Scholar]
- Ho WY, Nguyen HN, Wolfl M, Kuball J, Greenberg PD. In vitro methods for generating CD8+ T-cell clones for immunotherapy from the naive repertoire. J Immunol Methods 2006; 310:40-52. doi: 10.1016/j.jim.2005.11.023 [Crossref] [ Google Scholar]
- Wang X, Schmitt A, Chen B, Xu X, Mani J, Linnebacher M. Streptamer-based selection of WT1-specific CD8+ T cells for specific donor lymphocyte infusions. Exp Hematol 2010; 38:1066-73. doi: 10.1016/j.exphem.2010.07.002 [Crossref] [ Google Scholar]
- Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 2009; 15:5323-37. doi: 10.1158/1078-0432.CCR-09-0737 [Crossref] [ Google Scholar]
- Leung W, Heslop HE. Adoptive Immunotherapy with Antigen-Specific T Cells Expressing a Native TCR. Cancer Immunol Res 2019; 7:528-33. doi: 10.1158/2326-6066.CIR-18-0888 [Crossref] [ Google Scholar]
- Sugiyama H. WT1 (Wilms' tumor gene 1): biology and cancer immunotherapy. Jpn J Clin Oncol 2010; 40:377-87. doi: 10.1093/jjco/hyp194 [Crossref] [ Google Scholar]
- Hastie ND. Wilms' tumour 1 (WT1) in development, homeostasis and disease. Development 2017; 144:2862-72. doi: 10.1242/dev.153163 [Crossref] [ Google Scholar]
- Weber G, Karbach J, Kuci S, Kreyenberg H, Willasch A, Koscielniak E. WT1 peptide-specific T cells generated from peripheral blood of healthy donors: possible implications for adoptive immunotherapy after allogeneic stem cell transplantation. Leukemia 2009; 23:1634-42. doi: 10.1038/leu.2009.70 [Crossref] [ Google Scholar]
- Chapuis AG, Ragnarsson GB, Nguyen HN, Chaney CN, Pufnock JS, Schmitt TM. Transferred WT1-reactive CD8+ T cells can mediate antileukemic activity and persist in post-transplant patients. Sci Transl Med 2013; 5:174ra27. doi: 10.1126/scitranslmed.3004916 [Crossref] [ Google Scholar]
- Campbell JD, Foerster A, Lasmanowicz V, Niemoller M, Scheffold A, Fahrendorff M. Rapid detection, enrichment and propagation of specific T cell subsets based on cytokine secretion. Clin Exp Immunol 2011; 163:1-10. doi: 10.1111/j.1365-2249.2010.04261.x [Crossref] [ Google Scholar]
- Schmied S, Gostick E, Price DA, Abken H, Assenmacher M, Richter A. Analysis of the functional WT1-specific T-cell repertoire in healthy donors reveals a discrepancy between CD4(+) and CD8(+) memory formation. Immunology 2015; 145:558-69. doi: 10.1111/imm.12472 [Crossref] [ Google Scholar]
- Cooper RS, Fraser AR, Smith L, Burgoyne P, Imlach SN, Jarvis LM. Rapid GMP-Compliant Expansion of SARS-CoV-2-Specific T Cells From Convalescent Donors for Use as an Allogeneic Cell Therapy for COVID-19. Front Immunol 2020; 11:598402. doi: 10.3389/fimmu.2020.598402 [Crossref] [ Google Scholar]
- Hayflick L. Subculturing human diploid fibroblast cultures. In: Kruse PF, MK Patterson, editors. Tissue Culture: Methods and Applications. London, UK: Elsevier; 1973. p. 220-3.
- Israels T, Moreira C, Scanlan T, Molyneux L, Kampondeni S, Hesseling P. SIOP PODC: clinical guidelines for the management of children with Wilms tumour in a low income setting. Pediatr Blood Cancer 2013; 60:5-11. doi: 10.1002/pbc.24321 [Crossref] [ Google Scholar]
- Veal GJ, Cole M, Errington J, Parry A, Hale J, Pearson AD. Pharmacokinetics of dactinomycin in a pediatric patient population: a United Kingdom Children's Cancer Study Group Study. Clin Cancer Res 2005; 11:5893-9. doi: 10.1158/1078-0432.CCR-04-2546 [Crossref] [ Google Scholar]
- van de Velde ME, Panetta JC, Wilhelm AJ, van den Berg MH, van der Sluis IM, van den Bos C, et al. Population Pharmacokinetics of Vincristine Related to Infusion Duration and Peripheral Neuropathy in Pediatric Oncology Patients. Cancers (Basel) 2020; 12. 10.3390/cancers12071789.
- Stambrook PJ, Maher J, Farzaneh F. Cancer Immunotherapy: Whence and Whither. Mol Cancer Res 2017; 15:635-50. doi: 10.1158/1541-7786.MCR-16-0427 [Crossref] [ Google Scholar]
- Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer 2014; 14:135-46. doi: 10.1038/nrc3670 [Crossref] [ Google Scholar]
- Ooms A, Vujanic GM, D'Hooghe E, Collini P, L'Hermine-Coulomb A, Vokuhl C, et al. Renal Tumors of Childhood-A Histopathologic Pattern-Based Diagnostic Approach. Cancers (Basel) 2020; 12. 10.3390/cancers12030729.
- Chen BF, Tzen CY, Liang DC, Liu HC, Huang YW, Fan CC. Immunohistochemical expression of Wilms' tumor 1 protein in nephroblastoma. J Chin Med Assoc 2004; 67:506-10. [ Google Scholar]
- Pode-Shakked N, Shukrun R, Mark-Danieli M, Tsvetkov P, Bahar S, Pri-Chen S. The isolation and characterization of renal cancer initiating cells from human Wilms' tumour xenografts unveils new therapeutic targets. EMBO Mol Med 2013; 5:18-37. doi: 10.1002/emmm.201201516 [Crossref] [ Google Scholar]
- Casalegno-Garduno R, Schmitt A, Spitschak A, Greiner J, Wang L, Hilgendorf I. Immune responses to WT1 in patients with AML or MDS after chemotherapy and allogeneic stem cell transplantation. Int J Cancer 2016; 138:1792-801. doi: 10.1002/ijc.29909 [Crossref] [ Google Scholar]
- Kyi C, Doubrovina E, Zhou Q, Kravetz S, Iasonos A, Aghajanian C, et al. Phase I dose escalation safety and feasibility study of autologous WT1-sensitized T cells for the treatment of patients with recurrent ovarian cancer. J Immunother Cancer 2021; 9. 10.1136/jitc-2021-002752.
- Campbell JD. Detection and enrichment of antigen-specific CD4+ and CD8+ T cells based on cytokine secretion. Methods 2003; 31:150-9. doi: 10.1016/s1046-2023(03)00125-7 [Crossref] [ Google Scholar]
- Zhu F, Shah N, Xu H, Schneider D, Orentas R, Dropulic B. Closed-system manufacturing of CD19 and dual-targeted CD20/19 chimeric antigen receptor T cells using the CliniMACS Prodigy device at an academic medical center. Cytotherapy 2018; 20:394-406. doi: 10.1016/j.jcyt.2017.09.005 [Crossref] [ Google Scholar]
- Guedan S, Luu M, Ammar D, Barbao P, Bonini C, Bousso P, et al. Time 2EVOLVE: predicting efficacy of engineered T-cells - how far is the bench from the bedside? J Immunother Cancer 2022; 10. 10.1136/jitc-2021-003487.
- Plantinga M, Lo Presti V, de Haar CG, Dunnebach E, Madrigal A, Lindemans CA. Clinical Grade Production of Wilms' Tumor-1 Loaded Cord Blood-Derived Dendritic Cells to Prevent Relapse in Pediatric AML After Cord Blood Transplantation. Front Immunol 2020; 11:559152. doi: 10.3389/fimmu.2020.559152 [Crossref] [ Google Scholar]
- Kashima S, Maeda T, Masuda K, Nagano S, Inoue T, Takeda M. Cytotoxic T Lymphocytes Regenerated from iPS Cells Have Therapeutic Efficacy in a Patient-Derived Xenograft Solid Tumor Model. iScience 2020; 23:100998. doi: 10.1016/j.isci.2020.100998 [Crossref] [ Google Scholar]
- Van Driessche A, Berneman ZN, Van Tendeloo VF. Active specific immunotherapy targeting the Wilms' tumor protein 1 (WT1) for patients with hematological malignancies and solid tumors: lessons from early clinical trials. Oncologist 2012; 17:250-9. doi: 10.1634/theoncologist.2011-0240 [Crossref] [ Google Scholar]
- Chapuis AG, Egan DN, Bar M, Schmitt TM, McAfee MS, Paulson KG. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat Med 2019; 25:1064-72. doi: 10.1038/s41591-019-0472-9 [Crossref] [ Google Scholar]
- Shimodaira S, Hirabayashi K, Yanagisawa R, Higuchi Y, Sano K, Koizumi T. Dendritic Cell-Based Cancer Immunotherapy Targeting Wilms' Tumor 1 for Pediatric Cancer. In: van den Heuvel-Eibrink MM, editor. Wilms Tumor. Brisbane, Australia: Codon Publications; 2016. p. 113-30.
- Koya T, Date I, Kawaguchi H, Watanabe A, Sakamoto T, Togi M, et al. Dendritic Cells Pre-Pulsed with Wilms' Tumor 1 in Optimized Culture for Cancer Vaccination. Pharmaceutics 2020; 12. 10.3390/pharmaceutics12040305.