Bioimpacts. 14(1):27652.
doi: 10.34172/bi.2023.27652
Original Article
Effect of Honokiol on culture time and survival of Alzheimer’s disease iPSC-derived neurons
Duong Thi Thuy Le Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing, 1, 2, * 
Cuong Manh Vu Data curation, Formal analysis, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing, 1 
Thuy Thi Bich Ly Writing – original draft, 1, 2
Nam Trung Nguyen Writing – original draft, 1, 2
Phuong Thi Mai Nguyen Formal analysis, Writing – review & editing, 1, 2
Ha Hoang Chu Supervision, 1, 2
Author information:
1Institute of Biotechnology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet Road, Hanoi, Vietnam
2Graduate University of Science and Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet Road, Hanoi, Vietnam
Abstract
Introduction:
Patient-derived induced pluripotent stem cells (iPSCs) have been widely used as disease models to test new therapeutic strategies. Moreover, the regenerative potential of stem cells can be improved with the use of biologically active compounds. Our study was designed to explore the effect of honokiol, a small polyphenol molecule extracted from Magnolia officinalis, on the survival and culture time of iPSC-derived neurons from a sporadic Alzheimer’s disease (AD) patient. This study aimed to generate iPSCs from peripheral blood mononuclear cells (PBMCs) of an AD patient using episomal plasmids with a nucleofector system and differentiate them into neurons. These iPSC-derived neurons were used to investigate the effect of honokiol extracted from M. officinalis on their survival and long-term cultures.
Methods:
IPSCs were generated from PBMCs of an AD patient by introducing Oct-3/4, Sox2, Klf4, L-Myc, and Lin28 using NucleofectorTM Technology. Differentiation of neurons derived from iPSCs was carried out using inducers and recognized by biomarkers. The viability of iPSC-derived neurons with the addition of honokiol extracted from the bark of M. officinalis was determined by the MTT analytical kit.
Results:
IPSCs were generated by reprogramming AD patient-derived PBMCs and subsequently converted into neurons. The survival and growth of iPSC-derived neurons were significantly enhanced by adding honokiol in the experiment conditions.
Conclusion:
AD iPSC-derived neurons had a high viability rate when cultured in the presence of honokiol. These results have shown that AD iPSC-derived neurons can be an excellent model for screening neurotrophic agents and improving the conditions for long-term cultures of human iPSC-derived neurons. Honokiol proves to be a potential candidate for cellular therapeutics against neurodegenerative disorders.
Keywords: Episomal plasmid, Induced pluripotent stem cells, PBMCs, Neuronal differentiation, Neurotrophic agent, Honokiol
Copyright and License Information
© 2024 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Their abilities to proliferate and differentiate make stem cells powerful medicinal products. Recent researches have focused on stem cell therapy for the treatment of various diseases such as neurological conditions, diabetes, myocardial infarction and leukemia. Induced pluripotent stem cells (iPSCs) resemble embryonic stem cells (ESCs) but have no effects on the host immune system and avoid ethical issues.1 Somatic cells are harvested from the patients and treated with factors responsible for reprogramming to obtain iPSCs,2-4 which can be further differentiated into various cell types in the body. In addition to transplantation therapy, iPSCs can be utilized for disease modeling and drug screening. Common sources of patient-derived cells for reprogramming include fibroblasts and peripheral blood mononuclear cells (PBMCs). iPSCs were first generated from skin fibroblasts; however, skin biopsy is invasive and might cause visible scars.5 As an alternative, mononuclear cells can be harvested from peripheral blood of the patient. Blood draw is minimally invasive and routinely performed.6 Numerous methods to reprogram somatic cells are available with specific advantages and disadvantages. The initial approach for generating iPSCs was integration of exogenous DNA into the host genome using retrovirus7 or lentivirus8 with high reprogramming efficiency. However, these methods might increase the risk of tumor formation and insertional mutagenesis, resulting in safety concerns in their application in a clinical setting.9,10 Safer techniques have been investigated such as episomal plasmids, Sendai virus vectors, electrical impulses (Nucleofector and Neon transfection system), chemical–based methods (Lipofectamine 3000) or elastin-like polypeptide (ELP)-based transfection and synthetic messenger RNA.10-12 Among these techniques, nucleofection has been demonstrated as a highly efficient gene transfection approach.13
The advent of iPSCs has enabled the generation of patient-specific Alzheimer’s disease (AD) models for pathogenesis studies and drug screening. AD is a form of dementia with cognitive and functional decline that can negatively affect the daily life of patients and eventually result in death. Biological hallmarks of AD were identified in mid-1980s as extracellular amyloid-β (Aβ) plaques and intracellular neurofibrillary tangles comprised of hyperphosphorylated and aggregated tau proteins.14 Patients can be categorized into early-onset AD, which is linked to mutations in PSEN1, PSEN2 and AβPP genes, and late-onset AD, which is less specific with no gene mutations.15 The neuroprotective effects of many antioxidants have been explored in iPSC-derived models of several neurodegenerative disorders, including AD. According to the oxidative stress theory of neuronal degeneration, the excess of free radicals results in neuronal death by means of increased mitochondrial stress.16 Induced by oxidative damage, mitochondrial defects associated with energy metabolism can lead to the disruption of calcium homeostasis, which has been observed in neurodegenerative diseases such as AD and Parkinson’s disease.16 Persistent elevation of intracellular calcium could cause severe phenotypes in neurons, eventually leading to neuronal death and degeneration.17 Targeting oxidative stress, therefore, provides an important approach to the prevention and treatment of neurodegenerative disorders. The AD brain is known to have substantially low antioxidant enzymatic activities, which makes it more prone to toxicity caused by Aβ.18 Therefore, antioxidant agents have gained interest in the search of novel drugs for AD treatment. Natural antioxidants exhibit neuroprotective effects through a range of mechanisms such as scavenging reactive oxygen species (ROS), interacting with transition metal ions, altering enzymatic activities, modulating intracellular signaling cascades and gene expression.16 Several groups have used AD iPSC-derived neurons to discover neuroprotective effects of natural antioxidants extracted from plants such as N-butylidenephthalide,19 apigenin,20 Thymoquinone.21 Balez et al found that apigenin, an antioxidant polyphenol found in parsley, artichoke and celery, could increase viability and ameliorate disease phenotypes such as neurite shortening and hyperexcitability in AD neurons.20
Honokiol, which is an abundant polyphenol in the bark of M. officinalis, exhibited neuroprotective capacity as an antioxidative, anti-excitotoxic, anti-neuroinflammatory and anti-Aβ agent.22 Intravenous administration of honokiol reduced lipid peroxidation and ROS production in the rat brain with focal cerebral ischemia-reperfusion injury (CIRI),23 and oral administration prevented oxidative stress in N-methyl-d-aspartic acid-treated mice.24 In CIRI mice’s microglia treated with honokiol, nuclear factor-κB activation, which plays a crucial role in inflammation, was suppressed through the reduction of p65 subunit level.25 In the same study, culture medium of glial cells showed lower levels of inflammatory factors such as nitric oxide, tumor necrosis factor-α and RANTES protein.25 Honokiol reduced cell death prompted by Aβ toxicity through suppressing ROS production, Ca2+ elevation and caspase-3 activation.22 In addition, honokiol has been demonstrated to traverse the blood-brain barrier as well as the blood-cerebrospinal fluid barrier.26,27 However, there have been no reports of neuronal survival promotion by honokiol in AD iPSC-derived neurons.
Materials and Methods
Materials
Four plasmids: pCXLE-hOCT3/4-shp53-F, pCXLE-hSK, pCXLE-hUL and pCXWB-EBNA1 carrying reprogramming factors: Oct-3/4, Sox2, Klf4, L-Myc and Lin28 were supplied by Addgene (Waterdown, MA, USA). P3 Primary Cell 4D-NucleofectorTM X Kit and4D-NucleofectorTM System were bought from Lonza (Cologne, Germany). The medium components including DMEM/F12, DMEM, Panexin NTS, 2 – mercaptoethanol, fetal bovine serum (FBS), Penicillin – Streptomycin (PS) were purchased from PAN Biotech (Aidenbach, Germany). TRIzol reagent, MitoTracker® solution, phosphate-buffered saline (PBS), FGF-2 and Paraformaldehyde (PFA) were obtained from Invitrogen (Waltham, MA, USA). Primers were purchased from Integrated DNA Technologies (Coralville, IA, USA).11 RevertAid First Strand cDNA Synthesis Kit was purchased from Thermo Fisher Scientific (Waltham, MA, USA) and PCR Master Mix kit was purchased from Promega (Madison, WI, USA). Honokiol was isolated and purified from M. officinalis with a purity > 97%. Honokiol’s purity was identified by comparison with standard purchased from Shanghai Aladdin Bio-Chem Technology (Shanghai, China). Honokiol’s structure was determined based on Fourier transformed infrared (FT-IR) (Nexus 670 ThermoNicolet Fourier Transform Infrared Spectrometer, Thermo Fisher Scientific, Waltham, MA, USA), liquid chromatography-mass spectroscopy (LC-MS) (the Agilent 1100 Series LC/MSD Trap SL, Agilent Technologies, Santa Clara, CA, USA), and nuclear magnetic resonance (NMR) spectral analyses (Bruker Corporation, Billerica, MA, USA).
Reprogramming of PBMCs to iPSCs by nucleofection
Peripheral blood sample was donated by a 69-year old male patient with clinically and genetically characterised AD by the National Geriatric Hospital (Vietnam) after obtaining informed consent. All procedures performed in study involving participants were in accordance with the ethical standards of the institutional and/or national research committee with number 2151/QĐ-VHL.
Then the PBMCs were isolated by density gradient centrifugation using Ficoll-Paque® -1077 (Sigma Aldrich, St. Louis, MO, USA) at 1000 × g for 30 minutes and 20°C. After the isolation procedure, PBMCs were seeded in DMEM supplemented with 10% FBS, 1% PS. To generate iPSCs, PBMCs were transduced with five episomal vectors pCXLE-hOCT3/4, pCXLE-hSK, pCXLE-hUL, pCXWB-EBNA1. Briefly, 1 × 106 cells were washed by DMEM/F12 medium and then electroporated using nucleofection kit P3 solution and Nucleofector 4D, program EO-100 (Lonza, Cologne, Germany). Transfected cells were plated in DMEM high-glucose medium. Cells transfected with GFP were used as controls. On day 7, the medium was replaced by the iPSC-specific medium (DMEM/F12, 20% pannexin, 4 ng/mL FGF-2, 100 nM β-mercaptoethanol, 1% PS). After 15-20 days, colonies which have ES-like appearance were observed under a microscope. Between day 20-30 post-transduction, cells were manually picked based on their morphology. iPSCs were cultured on 6-well plates coated with Matrigel and in mTESR1 medium. The cells were maintained at 37 °C in a humidified atmosphere containing 5% CO2, fed daily with medium until confluence reached 80-90%.
Immunocytochemistry analysis
Cells were first fixed with 4% paraformaldehyde for 5 minutes at room temperature. After washing with PBS (Thermo Fisher Scientific, Waltham, MA, USA), cells were incubated with 0.2% Triton X-100 in PBS for 30 min at room temperature. Subsequently, the iPSCs were incubated with antibodies for the pluripotency markers Oct-3/4 (Oct-3/4 is conjugated to Alexa Fluor® 594 (sc-5279, AF594)); Nanog (1E6C4) is conjugated to Alexa Fluor® 488 (sc-293121 AF488)); and nerve cellswere incubated with antibodies MAP-2 (MAP-2 antibody is conjugated to Alexa Fluor® 594 (sc-32791, AF 594))overnight at 4 °C. After the cells were rinsed with PBS for three times, nuclei were counterstained with DAPI. Cells were observed using a Nikon Ti E Eclipse microscope equipped with a Ti-TIRF illumination unit and confocal system.
Karyotype analysis
iPSCs were treated with KaryoMAX® ColcemidTM Solution (Thermo Fisher Scientific, Waltham, MA, USA) and using standard cytogenetic procedures. Chromosomes were analyzed by G-banding and were carried out by Hanoi Obstetrics & Gynecology hospital.
Neural induction
Neural progenitor cells (NPCs) were generated from the patient’s iPSCs using PSC Neural Induction Medium (NM) (Thermo Fisher Scientific, Waltham, MA, USA). Initially, iPSCs were seeded in 6-well plates and when the confluency of iPSCs reached to 20%, the cells had its medium replaced with neural induction medium (NIM) (1:1 vol/vol mixture of DMEM/F12 and neurobasal medium, 1× N-2 supplement, 1× B-27 supplement, 1× nonessential amino acids [NEAA], 2 mM L-glutamine, 50 U/mL penicillin/streptomycin, 100 μM β-mercaptoethanol, retinoic acid 5 μg/mL insulin), which was supplemented with 5 ng/mL basic fibroblast growth factor (bFGF). NIM was changed every 2 days. Neural cells were cultured in neural maintenance medium (1:1 vol/vol mixture of DMEM/F12 and neurobasal medium, 2 mM L-glutamine, 50 U/mL penicillin/ streptomycin, 1× N-2 supplement, 1× B-27 supplement, 1× NEAA) and supplemented with 10 ng/mL epidermal growth factor and 10 ng/mL bFGF.
DPPH radical scavenging assay
The antioxidant property of honokiol was investigated using the DPPH scavenging assay adapted from a method previously described.28 Honokiol was diluted in DMSO at various concentrations to prepare a sample solution. Ascorbic acid was used as a reference standard. The sample solution and ascorbic acid (9 μL) were added to 171 μL of DPPH solution (0.24 mg/mL). After a 20-minute incubation, the absorbance of the reaction mixture was measured at 517 nm by a UV-Vis spectrometer. The percentage of inhibition of DPPH radicals was calculated using the following equation:
where A0 is the absorbance of control, and A1 is the absorbance of the test or standard sample.
Comet assay
The in vitro genotoxicity of honokiol was evaluated using the alkaline comet assay based on a protocol previously described.29 After respective treatments with honokiol (10 μg/mL) or H2O2 (500 μM), the neurons were collected and washed with PBS before being resuspended in low melting agarose 1%. The suspension was spread on microscope slides which had been coated with a thin layer of normal melting agarose. The slides were refrigerated for 10 minutes to allow the low melting agarose to solidify, followed by overnight immersion in the lysis buffer at 4 °C. The slides were then immersed in the alkaline buffer for 1h, and electrophoresed for 30 minutes at 4 °C. After that, the slides were stained with DAPI and observed using a Nikon Ti E Eclipse microscope equipped with a Ti-TIRF illumination unit and confocal system.
Cell viability assay
AD iPSC-derived neurons were grown in 48-well plates at 24 hours. The medium was replaced by 200 μL of the formulation at pure honokiol concentrations of 0.1-10 μg/mL followed by incubation for 1 month at 37 °C and culture medium was changed every 3 days. Cell viability was assessed by MTT assay (Promega, Madison, WI, USA) according to manufacturer’s instructions. Cell viability was determined using the following equation:
Statistical analysis
Data were expressed as the mean ± standard deviation (SD). The data were statistically analyzed using Student’s t test. The differences between the samples were considered to be significant when P value is < 0.05.
Results
Generation of iPSCs from PBMCs
In our research, PBMCs were reprogrammed into iPSCs by using a non-integrating method. Nucleofector with the plasmid DNA cocktail which contained the four vectors in equimolar amounts. The colonies were formed after 3 weeks of transfection with ESC – like characteristics (Fig. 1C). Thus, the expression of pluripotency marker genes by electrophoresis from wells 1; 2; 3; 4 showed clear DNA bands such as Oct-3/4 (144 bp), c-MYC (328 bp), Nanog (287 bp), Sox2 (151 pb) (Fig. 1A). These results supported the roadmap to reprogram and provided reliable evidence of the successful reprogramming process.
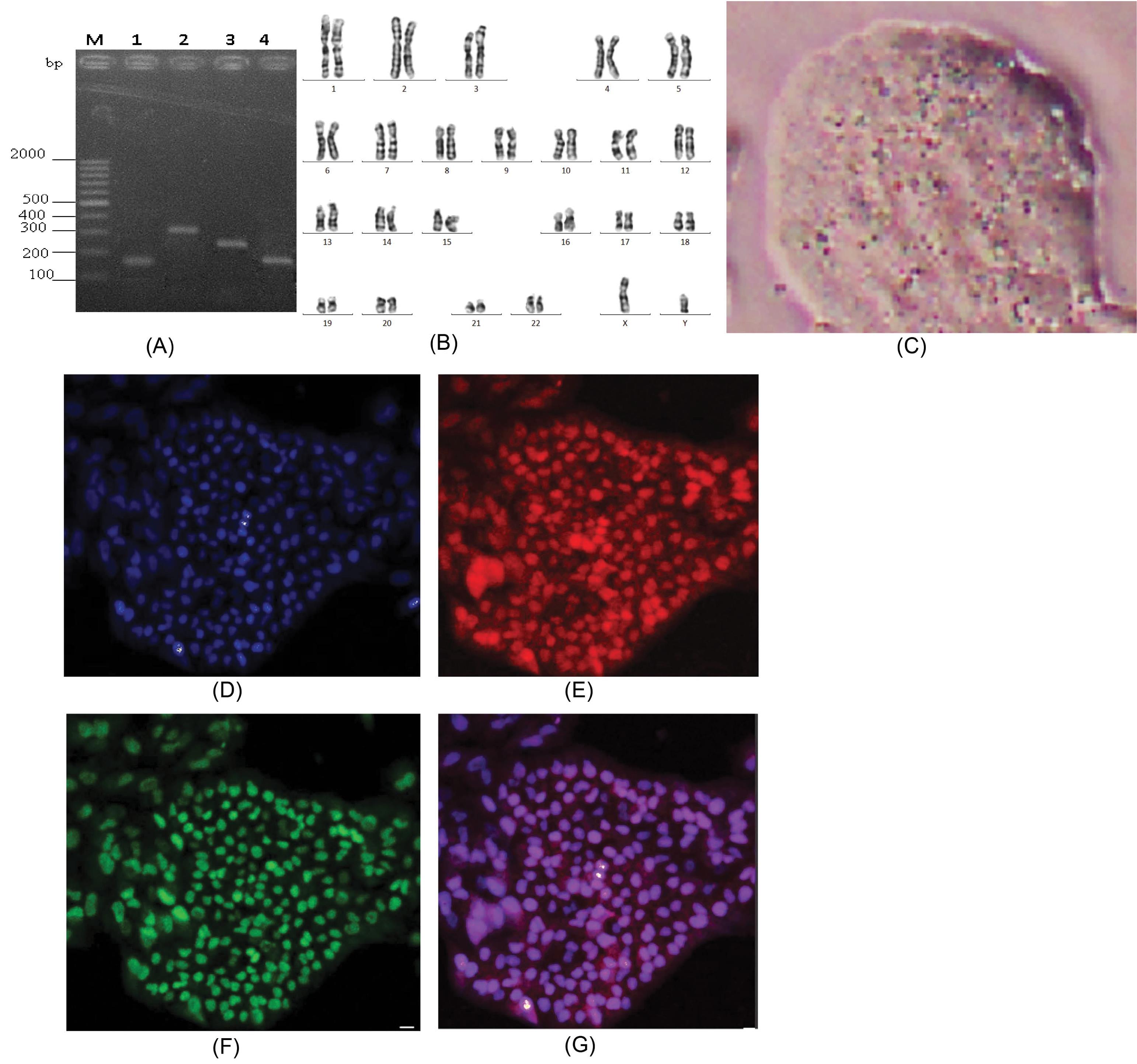
Fig. 1.
Characterization of induced pluripotent stem cells from peripheral blood mononuclear cells: (A) Expression of pluripotency marker genes: M: DNA marker, 1: Oct-3/4, 2: c – MYC, 3: Nanog, 4: Sox2. (B) Images of karyotyping of iPSC line; (C). iPSC colony; (D), (E), (F), (G) Immunofluorescence staining of pluripotency markers: (D) DAPI, (E) Oct-3/4, (F) Nanog, (G) Merged.
.
Characterization of induced pluripotent stem cells from peripheral blood mononuclear cells: (A) Expression of pluripotency marker genes: M: DNA marker, 1: Oct-3/4, 2: c – MYC, 3: Nanog, 4: Sox2. (B) Images of karyotyping of iPSC line; (C). iPSC colony; (D), (E), (F), (G) Immunofluorescence staining of pluripotency markers: (D) DAPI, (E) Oct-3/4, (F) Nanog, (G) Merged.
The karyotype of the iPSC line was examined using Giemsa-banding, which demonstrated a normal diploid 46, XY karyotype, with no observable abnormalities (Fig. 1B). Immunofluorescence analysis confirmed the expression of pluripotency markers Oct-3/4 (Fig. 1E) and Nanog (Fig. 1F), demonstrating the pluripotent nature of the iPSC line. Overall, these results showed that we successfully generated iPSCs from PBMCs by transfection with Yamanaka reprogramming factors in a feeder-free medium.
Generation and characterization of neurons from iPSCs
After deriving neuronal progenitor cells from hiPSCs, we induced the NPCs to differentiate into neural cells by culturing them in the neuron differentiation media. The cells were subsequently stained for MAP-2, a marker of neuronal differentiation. We found that the induced cells were stained positive for the neuronal marker (Fig. 2), implying that NPCs had differentiated into neurons.
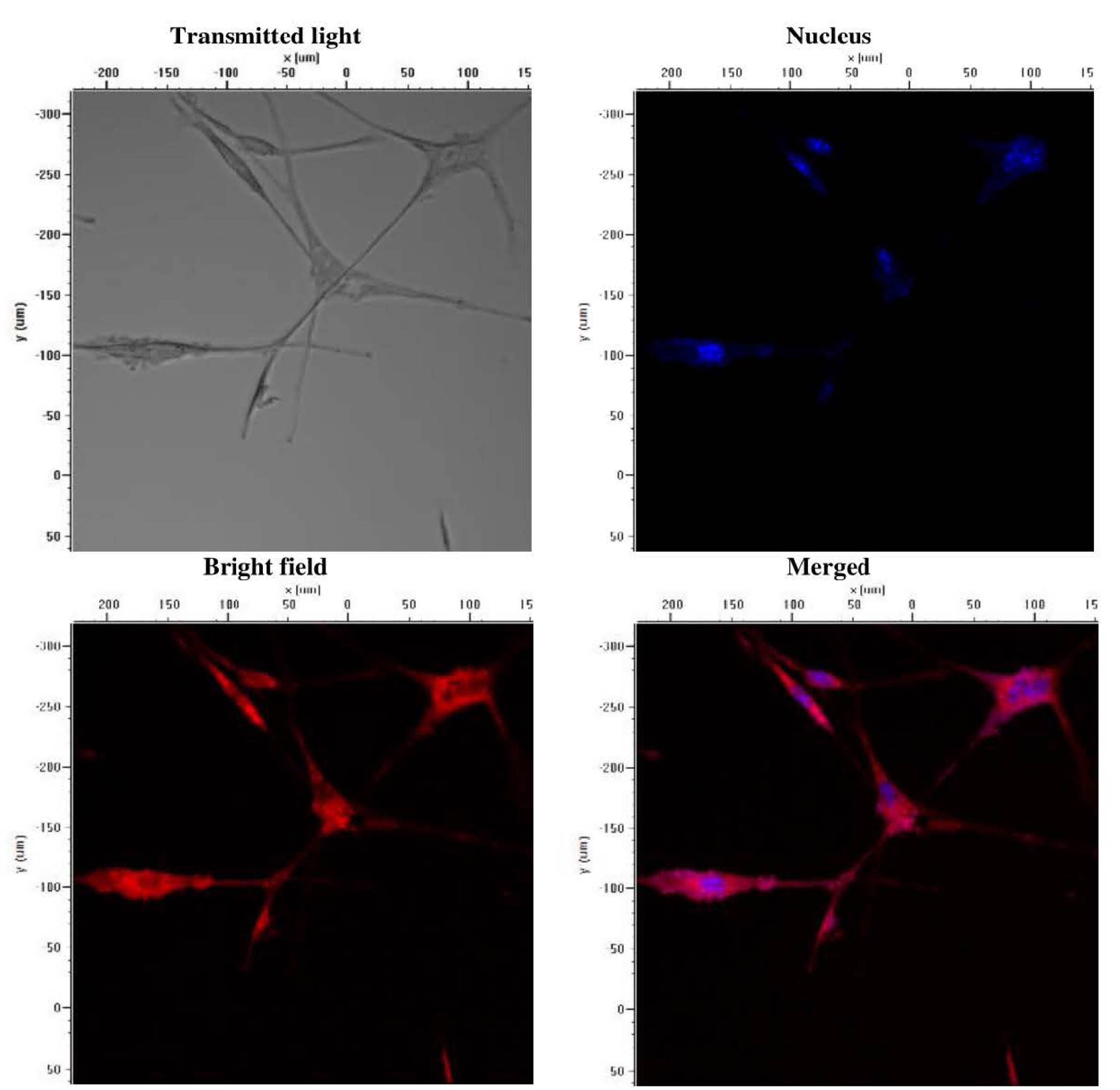
Fig. 2.
Immunofluorescence characterization of Alzheimer’s disease iPSC-derived neurons stained with DAPI (blue) and MAP-2 (Red).
.
Immunofluorescence characterization of Alzheimer’s disease iPSC-derived neurons stained with DAPI (blue) and MAP-2 (Red).
Analysis of honokiol’s radical scavenging activity and genotoxicity
Honokiol with molecular formula C18H18O2 and molecular weight of 266 g/mole was isolated from M. officinalis and its purity was confirmed by high-performance liquid chromatography (single peak) as well as by 1H and 13C NMR spectra and high resolution electron ionization mass spectrum (Supplementary file 1).
DPPH scavenging assay was conducted to investigate honokiol’s ability to inhibit free radicals. As shown in Fig. 3A, honokiol and ascorbic acid (reference standard) showed an increase in the inhibition of DPPH radicals in a concentration-dependent manner, reaching 62.75 and 80.92% inhibition at the concentration of 100 µg/mL respectively.
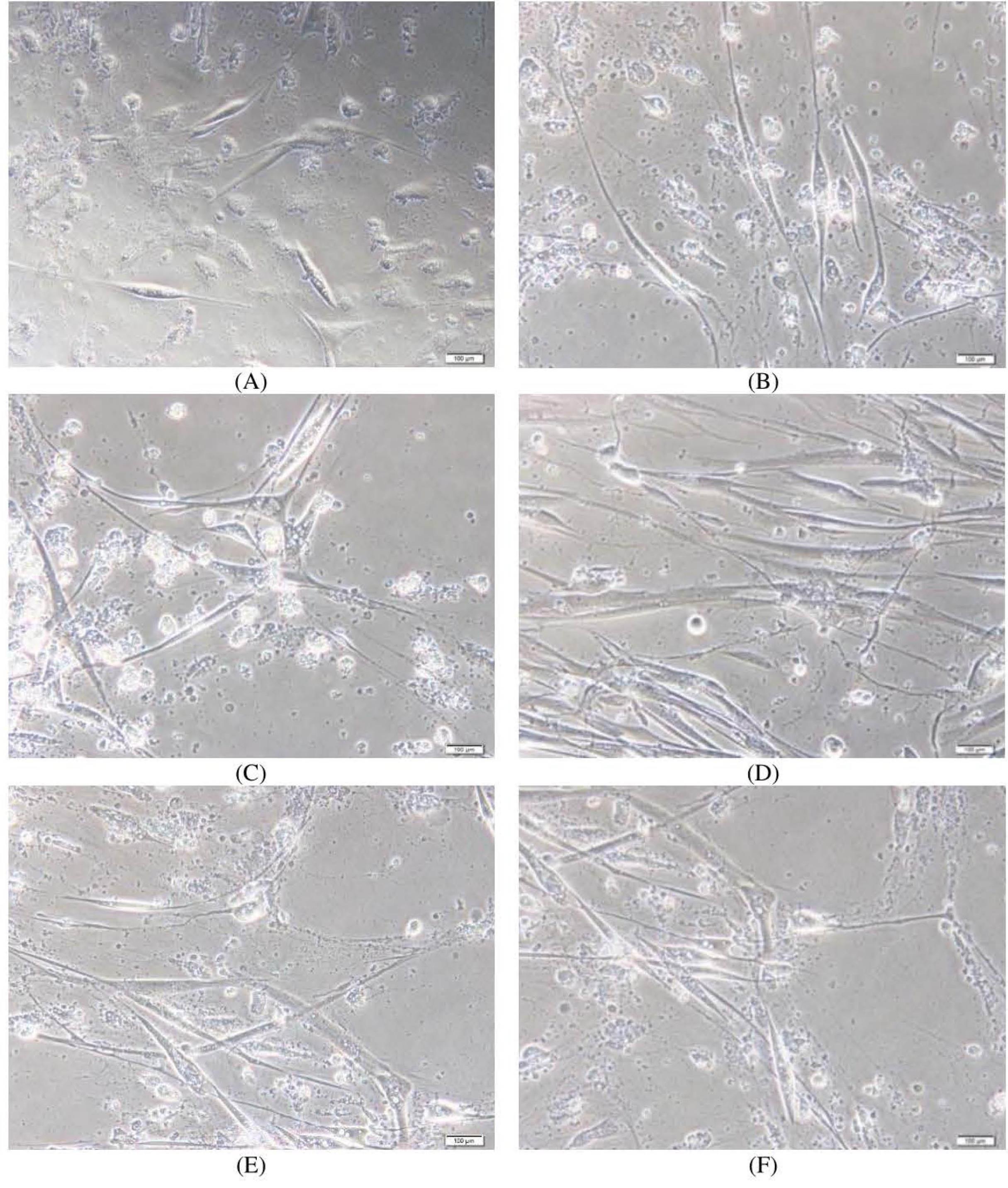
Fig. 3.
Analysis of honokiol’s antioxidant property and genotoxicity.(A) Chemical structure of honokiol; (B) DPPH scavenging assay results; (C) Comet assay results. DNA damage is represented as tail moment. Data presented are means ± SD; Student’s t-test: ns P > 0.05 versus the control group, *** P ≤ 0.001 versus the control group.
.
Analysis of honokiol’s antioxidant property and genotoxicity.(A) Chemical structure of honokiol; (B) DPPH scavenging assay results; (C) Comet assay results. DNA damage is represented as tail moment. Data presented are means ± SD; Student’s t-test: ns P > 0.05 versus the control group, *** P ≤ 0.001 versus the control group.
The IC50 values of honokiol and ascorbic acid were found to be 75.66 and 53.98 µg/mL respectively.
To confirm whether honokiol can induce genotoxicity in the iPSC-derived neurons, the comet assay was performed (Fig. 3B). There was no formation of tails (damaged DNA) in the honokiol-treated cells, which were similar to control cells. In contrast, H2O2-treated cells showed a significant increase in tail DNA. These results indicate that at the concentration of 10 μg/mL, honokiol is not genotoxic to the iPSC-derived neurons.
Effect of honokiol on the survival and culture time of iPSC-derived neurons
The results (Fig. 4) showed that honokiol enhanced the viability of AD iPSC-derived neurons in a dose-dependent manner (Figs. 4 and 5). After 35 days of culture, the control sample without honokiol had a high number of dead neurons. However, samples treated with different concentrations of honokiol had a lower number of dead neurons. Especially at the concentration of honokiol 5 µg/mL, the nerve cells still developed normally compared to other concentrations. But at higher concentrations, the number of viable cells began to decrease. The above observations are shown in Figs. 4 and 5. In the honokiol-treated samples, the neurons had longer neurite outgrowth than the control samples (Fig. 4). At the concentration of 10 µg/mL, the cell survival rate began to decrease. Thus, a reasonable concentration to increase neuronal survival in this experiment is in the range of 2-5 µg/mL.
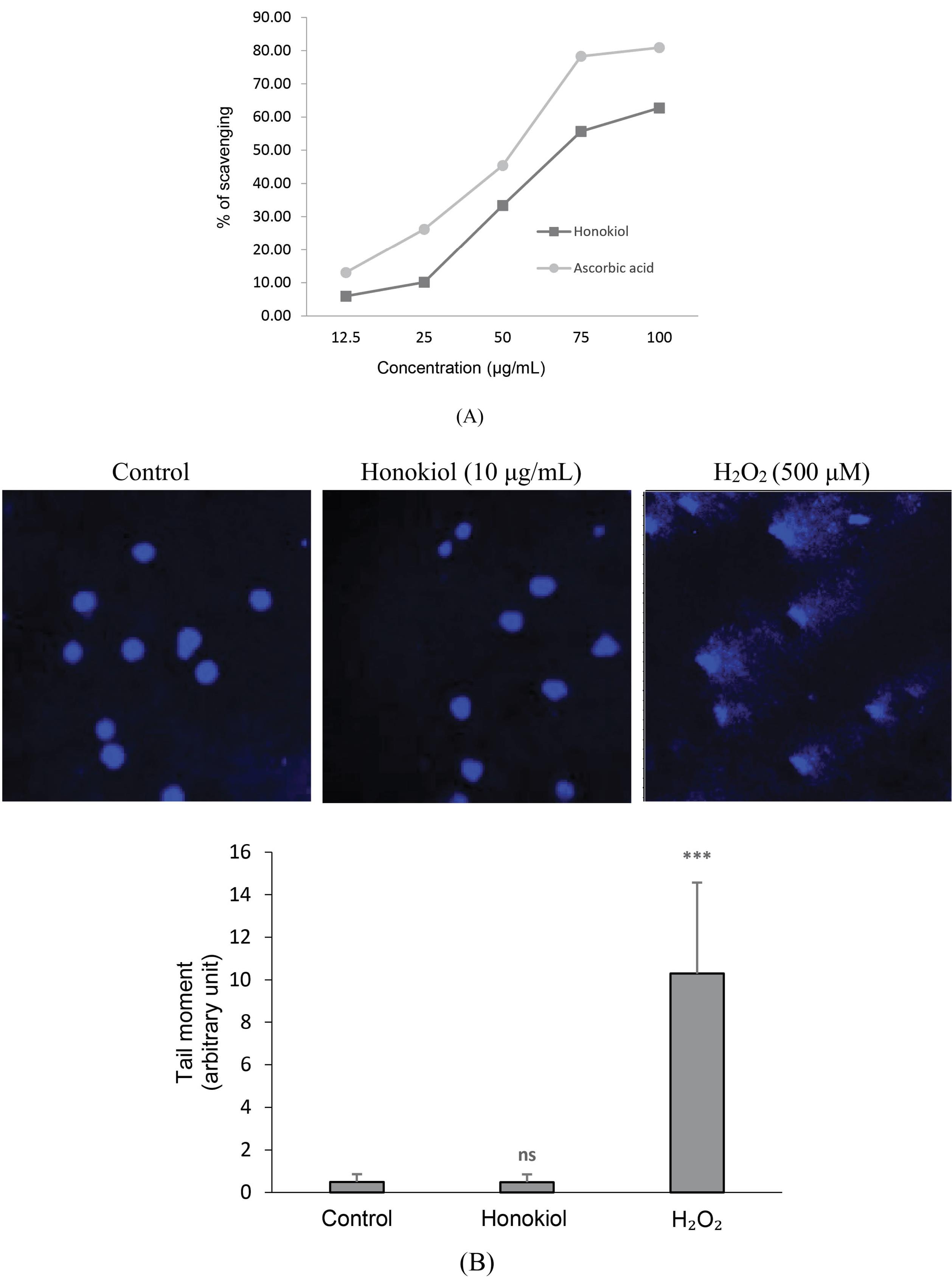
Fig. 4.
Neurite survival of Alzheimer’s disease iPSC-derived neurons by honokiol after 35 days of cell culture. (A) 0,5% DMSO; (B) 0.1 µg/mL; (C) 1 µg/mL; (D) 2,5 µg/mL; (E) 5 µg/mL; (F) 10 µg/mL.
.
Neurite survival of Alzheimer’s disease iPSC-derived neurons by honokiol after 35 days of cell culture. (A) 0,5% DMSO; (B) 0.1 µg/mL; (C) 1 µg/mL; (D) 2,5 µg/mL; (E) 5 µg/mL; (F) 10 µg/mL.
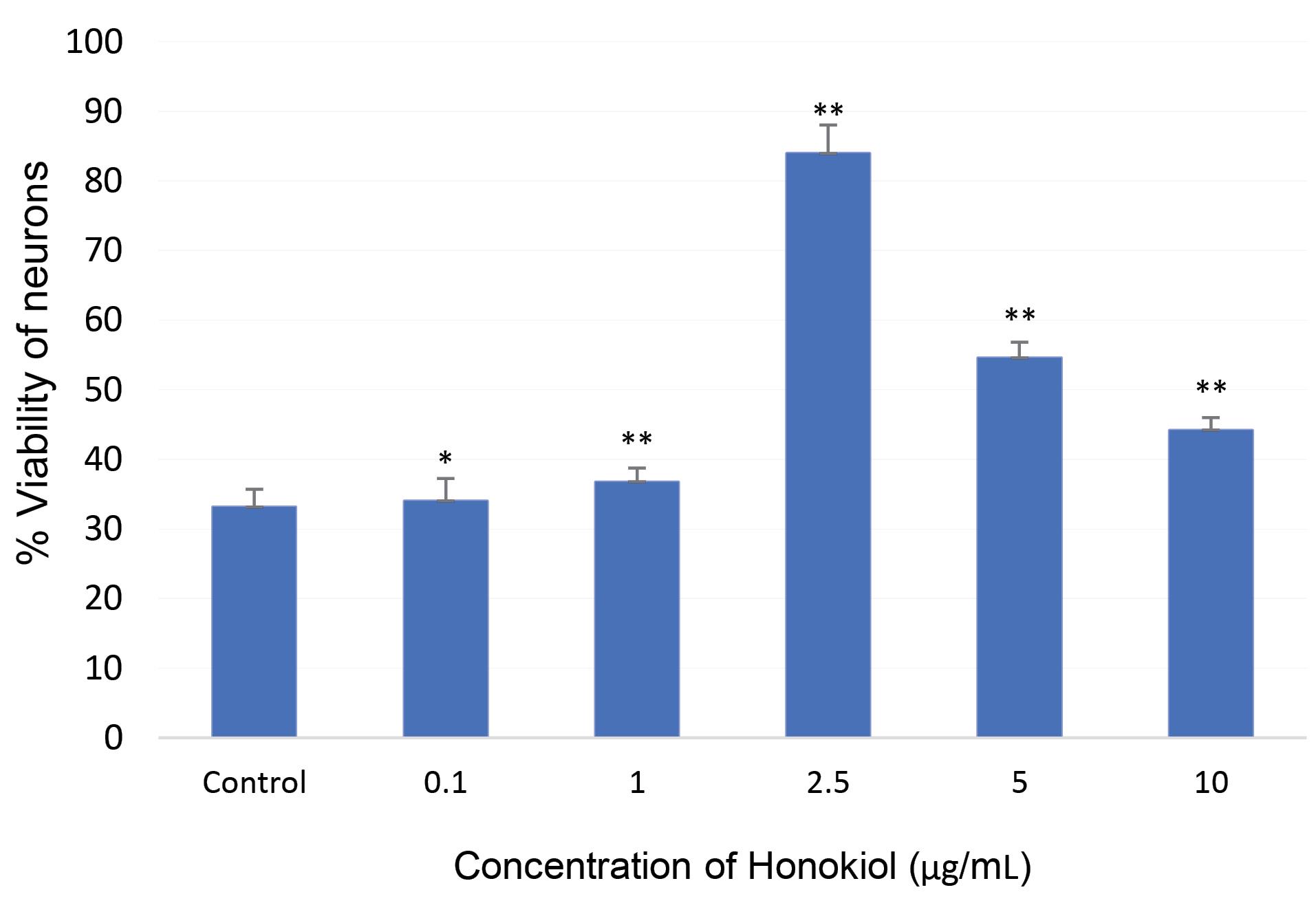
Fig. 5.
Effect of honokiol on cell viability of Alzheimer’s disease iPSC-derived neurons after 35 days of cell culture. Data presented are means ± SD; Student’s t test: * P ≤ 0.05 versus the control group, ** P ≤ 0.01 versus the control group.
.
Effect of honokiol on cell viability of Alzheimer’s disease iPSC-derived neurons after 35 days of cell culture. Data presented are means ± SD; Student’s t test: * P ≤ 0.05 versus the control group, ** P ≤ 0.01 versus the control group.
Discussion
Thanks to their resemblance to ECSs, iPSCs have proved to be a potential tool in medical science. Since their advent, many research groups have generated iPSCs from a variety of somatic cell types, typically fibroblasts.7,30 While it is clinically demanding to collect fibroblasts, peripheral blood cells are more easily obtainable as surgery is not needed to extract them.31 The most fundamental distinction between the PBMCs and fibroblasts is that the former cells first have to be converted into attached form in the course of reprogramming.32 In this experiment, Matrigel was utilised to support cells’ attachment and facilitate adherence. Afterwards, the attached cells can proliferate into reprogrammed iPSCs. Additionally, our study adopted a transfection method using plasmids to reprogram PBMCs instead of DNA-integrative retroviral and lentiviral vectors. This approach helps us to avoid the possible risks of contaminating iPSCs with random genomic insertions of viral vectors.33 Furthermore, by culturing iPSCs without feeder cells, we also ensured that iPSCs were not adversely affected by undefined or unknown constituentspresent in feeder cells’ medium supplements, typically fetal bovine serum or proprietary serum replacements.34,35
We confirmed the generation of iPSCs from PBMCs using PCR and immunohistochemistry for the expression of pluripotency marker genes, including Oct-3/4, c-MYC, Nanog, Sox2. The expression of pluripotency marker genes relates to the three sequential events of the somatic cell reprogramming process including early event (initiation phase), intermediate event (maturation phase) and final event (stabilization phase).36 C-Myc is regarded as the initiation phase marker that relates to cell proliferation.36 Nanog, referred as hallmark in the second transcriptional wave, is upregulated by the expressions of exogenous Oct-3/4 and Sox2. Soufi et al had also demonstrated that exogenous Oct-3/4, Sox2andKLF4 bind to inactive DNA regions and play roles as repressor of lineage-specific genes and activator/enhancer of stemness-specific genes.37 Moreover, chromosome integrity of iPS cells is a necessary condition after somatic cell reprogramming. In this study, iPSCs with a normal karyotype were competent to differentiate into different cell types.
Having established PBMC-derived iPSCs, we differentiated these cells into neurons and characterized them using immunofluorescence staining for MAP-2, a neuronal marker. MAP-2 is a microtubule-associated protein that plays an essential role in the maintenance of neuroarchitecture by stabilizing microtubules, stimulating tubulin assembly and acting as substrates for protein kinases and phosphatases.38 MAP-2 is predominantly found in the nervous system and is among the most plentiful proteins in brain.39 MAP-2 isoforms consist of 2 groups: high-molecular weight (HMW) forms, including MAP-2A and MAP-2B, and low-molecular weight forms, including MAP-2C and MAP-2D.40 The HMW forms are specifically expressed in neurons within the nervous system,38,41 and therefore chosen as a specific marker for neurons. We used the MAP-2 antibody which can detect MAP-2A and MAP-2B, thus characterizing neuron formation.
In this study, we have successfully converted AD’s patient-specific iPSCs into neurons.
Much of the understanding of neurological disorders has been gained from examining post-mortem neuronal tissues or animal disease models because of the limited access to patient’s living neurons. The application of iPSCs in disease modeling affords the opportunities to investigate patient-specific neuronal cells in vitro.
Advancement in iPSC-based neurons and differentiation protocols allow disease modeling using physiologically relevant cells. In this study, we investigated whether honokiol could improve the survival and growth of AD iPSC-derived neurons. Human iPSC-derived neurons are sensitive and require extended culturing time. Moreover, current hiPSC-derived neuron-based applications are often hindered by limitations in survival and long-term culture conditions. Therefore, modeling neuron disease using hiPSC-based approaches requires in vitro culture conditions to improve and to prolong the survival time. Thiry et al used a new coating substrate of cytocompatible dendritic polyglycerol amine (dPGA) for the long-term cultures of human iPSC-derived motor neurons.42 The results showed an improvement in the experimental conditions of the long-term cultures of human iPSC-derived motor neurons, which could allow improved survival studies, molecular identity, electrophysiological activity and single-cell RNA sequencing.42
Neurotrophic factors have proven to be promising candidates in treating neurodegenerative disorders such as AD. However, endogenous neurotrophic factors are high-molecular mass proteins, which can hardly traverse the blood-brain barrier or can be degraded by peptidase.43 Therefore, it is necessary to discover small molecules from natural products which can mimic the properties of neurotrophins. Honokiol, a natural biphenyl neolignan extracted from M. officinalis, has been demonstrated to traverse the blood-brain barrier as well as the blood-cerebrospinal fluid barrier.26,27
Honokiol has been shown to promote neuronal survival and outgrowth; however, studies about its neurotrophic ability have mainly focused on animal neuronal cells. These animal models may not accurately reflect electrophysiological properties of human neurons. To the best of our knowledge, our study is the first to attempt to evaluate honokiol’s neurotrophic function on human iPSC-derived neurons.
Honokiol also exerted its neuroprotective effects in rats with traumatic brain injury by inhibiting the expression of APAF-1, thus preventing neurons from undergoing apoptosis.44 Honokiol increased the viability of motor neurons NSC-34 cells in amyotrophic lateral sclerosis disease models both in vitro and in vivo.45 In the current research, studied concentrations of honokiol resulted in a significant increase in neuron survival after 35 days, when compared to neurons without honokiol. Moreover, it was shown that honokiol exerted no genotoxicity in the iPSC-derived neurons at the concentration of 10 μg/mL. The neuroprotective effect of honokiol could be due to its antioxidant property, which was examined in this study using DPPH scavenging assay. In the present study, honokiol promoted DPPH inhibition in a concentration-dependent manner within the concentration range of 12.5-100 μg/mL.
Despite its benefits, honokiol may display neurotoxicity at high doses. Although honokiol has been shown to promote neuronal survival and outgrowth at low doses, it has also been discovered to increase neuronal death at higher doses.46 This is in line with our findings about honokiol’s effects at high doses.
Conclusion
The models for researching diseases and discovering new therapies have shown to be more accurate than traditional disease models. The results showed that neurons derived from sporadic AD iPSCs were successfully generated. Pure honokiol isolated and purified from the bark of M. officinalis significantly affected neuronal survival and neurite outgrowth of AD iPSC-derived neurons. Thus, honokiol appears to exert neurotrophic effects on neuronal cells and to affect neurite outgrowth. These results have shown that AD iPSC-derived neurons can be an excellent model for screening neurotrophic agents and honokiol may be a potential therapeutic agent for neurodegenerative diseases.
Research Highlights
What is the current knowledge?
√ Alzheimer’s disease iPSC-derived neurons can be an excellent model for screening neurotrophic agents and personalized medicine.
√ Neurons derived from iPSCs often require culture conditions to improve and prolong survival in vitro. Therefore, substances capable of increasing cell survival need to be discovered or investigated.
What is new here?
√ Honokiol isolated and purified from the bark of M. officinalis in Vietnam significantly affected neuronal survival and neurite outgrowth of AD iPSC-derived neurons
Acknowledgments
The authors thank Dr. Le Thi Thuy Hien, the National Geriatric Hospital (Vietnam) for her help in collection of the blood sample.
Competing Interests
The authors declare they have no conflict of interest.
Ethical Statement
Peripheral blood sample in this study was donated by a 69-year old male patient with clinically and genetically characterised AD by the National Geriatric Hospital (Vietnam) after obtaining informed consent. All procedures performed in study involving participants were in accordance with the ethical standards of the institutional and/or national research committee with number 2151/QĐ-VHL.
Funding
This work was supported by grants from the Vietnam Academy of Science and Technology (VAST) with project number VAST02.02/20-21.
Supplementary Materials
Supplementary file 1 contains HPLC, 1H NMR, 13C NMR, MS and FTIR results of honokiol as well as Table S1.
(pdf)
References
- Aboul-Soud MAM, Alzahrani AJ, Mahmoud A. Induced Pluripotent Stem Cells (iPSCs)-Roles in Regenerative Therapies, Disease Modelling and Drug Screening. Cells 2021; 10:2319. doi: 10.3390/cells10092319 [Crossref] [ Google Scholar]
- Ebert AD, Yu J, Rose FF, Jr Jr. Ebert AD, Yu J, Rose FF, Jr, Mattis VB, Lorson CL, Thomson JA, et alInduced pluripotent stem cells from a spinal muscular atrophy patient. Nature 2009; 457:277-80. doi: 10.1038/nature07677 [Crossref] [ Google Scholar]
- Rowland HA, Hooper NM, Kellett KAB. Modelling Sporadic Alzheimer's Disease Using Induced Pluripotent Stem Cells. Neurochem Res 2018; 43:2179-98. doi: 10.1007/s11064-018-2663-z [Crossref] [ Google Scholar]
- Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A. Disease-specific induced pluripotent stem cells. Cell 2008; 134:877-86. doi: 10.1016/j.cell.2008.07.041 [Crossref] [ Google Scholar]
- Ohnuki M, Takahashi K. Present and future challenges of induced pluripotent stem cells. Philos Trans R Soc Lond B Biol Sci 2015; 370:20140367. doi: 10.1098/rstb.2014.0367 [Crossref] [ Google Scholar]
- Seki T, Yuasa S, Oda M, Egashira T, Yae K, Kusumoto D. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell 2010; 7:11-4. doi: 10.1016/j.stem.2010.06.003 [Crossref] [ Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131:861-72. doi: 10.1016/j.cell.2007.11.019 [Crossref] [ Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007; 318:1917-20. doi: 10.1126/science.1151526 [Crossref] [ Google Scholar]
- Duinsbergen D, Salvatori D, Eriksson M, Mikkers H. Tumors originating from induced pluripotent stem cells and methods for their prevention. Ann N Y Acad Sci 2009; 1176:197-204. doi: 10.1111/j.1749-6632.2009.04563.x [Crossref] [ Google Scholar]
- Haridhasapavalan KK, Borgohain MP, Dey C, Saha B, Narayan G, Kumar S. An insight into non-integrative gene delivery approaches to generate transgene-free induced pluripotent stem cells. Gene 2019; 686:146-59. doi: 10.1016/j.gene.2018.11.069 [Crossref] [ Google Scholar]
- Manzini S, Viiri LE, Marttila S, Aalto-Setälä K. A Comparative View on Easy to Deploy non-Integrating Methods for Patient-Specific iPSC Production. Stem Cell Rev Rep 2015; 11:900-8. doi: 10.1007/s12015-015-9619-3 [Crossref] [ Google Scholar]
- Lee CH, Ingrole RSJ, Gill HS. Generation of induced pluripotent stem cells using elastin like polypeptides as a non-viral gene delivery system. Biochim Biophys Acta Mol Basis Dis 2020; 1866:165405. doi: 10.1016/j.bbadis.2019.01.031 [Crossref] [ Google Scholar]
- Iversen N, Birkenes B, Torsdalen K, Djurovic S. Electroporation by nucleofector is the best nonviral transfection technique in human endothelial and smooth muscle cells. Genet Vaccines Ther 2005; 3:2. doi: 10.1186/1479-0556-3-2 [Crossref] [ Google Scholar]
- Soria Lopez JA, González HM, Léger GC. Alzheimer's disease. Handb Clin Neurol 2019; 167:231-55. doi: 10.1016/b978-0-12-804766-8.00013-3 [Crossref] [ Google Scholar]
- Robinson M, Lee BY, Hane FT. Recent Progress in Alzheimer's Disease Research, Part 2: Genetics and Epidemiology. J Alzheimers Dis 2017; 57:317-30. doi: 10.3233/jad-161149 [Crossref] [ Google Scholar]
- Albarracin SL, Stab B, Casas Z, Sutachan JJ, Samudio I, Gonzalez J. Effects of natural antioxidants in neurodegenerative disease. Nutr Neurosci 2012; 15:1-9. doi: 10.1179/1476830511y.0000000028 [Crossref] [ Google Scholar]
- Celsi F, Pizzo P, Brini M, Leo S, Fotino C, Pinton P. Mitochondria, calcium and cell death: a deadly triad in neurodegeneration. Biochim Biophys Acta 2009; 1787:335-44. doi: 10.1016/j.bbabio.2009.02.021 [Crossref] [ Google Scholar]
- Makhaeva GF, Lushchekina SV, Boltneva NP, Serebryakova OG, Rudakova EV, Ustyugov AA. 9-Substituted acridine derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors possessing antioxidant activity for Alzheimer's disease treatment. Bioorg Med Chem 2017; 25:5981-94. doi: 10.1016/j.bmc.2017.09.028 [Crossref] [ Google Scholar]
- Chang CY, Chen SM, Lu HE, Lai SM, Lai PS, Shen PW. N-butylidenephthalide attenuates Alzheimer's disease-like cytopathy in Down syndrome induced pluripotent stem cell-derived neurons. Sci Rep 2015; 5:8744. doi: 10.1038/srep08744 [Crossref] [ Google Scholar]
- Balez R, Steiner N, Engel M, Muñoz SS, Lum JS, Wu Y. Neuroprotective effects of apigenin against inflammation, neuronal excitability and apoptosis in an induced pluripotent stem cell model of Alzheimer's disease. Sci Rep 2016; 6:31450. doi: 10.1038/srep31450 [Crossref] [ Google Scholar]
- Alhibshi AH, Odawara A, Suzuki I. Neuroprotective efficacy of thymoquinone against amyloid beta-induced neurotoxicity in human induced pluripotent stem cell-derived cholinergic neurons. Biochem Biophys Rep 2019; 17:122-6. doi: 10.1016/j.bbrep.2018.12.005 [Crossref] [ Google Scholar]
- Hoi CP, Ho YP, Baum L, Chow AH. Neuroprotective effect of honokiol and magnolol, compounds from Magnolia officinalis, on beta-amyloid-induced toxicity in PC12 cells. Phytother Res 2010; 24:1538-42. doi: 10.1002/ptr.3178 [Crossref] [ Google Scholar]
- Liou KT, Shen YC, Chen CF, Tsao CM, Tsai SK. Honokiol protects rat brain from focal cerebral ischemia-reperfusion injury by inhibiting neutrophil infiltration and reactive oxygen species production. Brain Res 2003; 992:159-66. doi: 10.1016/j.brainres.2003.08.026 [Crossref] [ Google Scholar]
- Cui HS, Huang LS, Sok DE, Shin J, Kwon BM, Youn UJ. Protective action of honokiol, administered orally, against oxidative stress in brain of mice challenged with NMDA. Phytomedicine 2007; 14:696-700. doi: 10.1016/j.phymed.2007.03.005 [Crossref] [ Google Scholar]
- Zhang P, Liu X, Zhu Y, Chen S, Zhou D, Wang Y. Honokiol inhibits the inflammatory reaction during cerebral ischemia reperfusion by suppressing NF-κB activation and cytokine production of glial cells. Neurosci Lett 2013; 534:123-7. doi: 10.1016/j.neulet.2012.11.052 [Crossref] [ Google Scholar]
- Lin JW, Chen JT, Hong CY, Lin YL, Wang KT, Yao CJ. Honokiol traverses the blood-brain barrier and induces apoptosis of neuroblastoma cells via an intrinsic bax-mitochondrion-cytochrome c-caspase protease pathway. Neuro Oncol 2012; 14:302-14. doi: 10.1093/neuonc/nor217 [Crossref] [ Google Scholar]
- Wang X, Duan X, Yang G, Zhang X, Deng L, Zheng H. Honokiol crosses BBB and BCSFB, and inhibits brain tumor growth in rat 9L intracerebral gliosarcoma model and human U251 xenograft glioma model. PLoS One 2011; 6:e18490. doi: 10.1371/journal.pone.0018490 [Crossref] [ Google Scholar]
- Adebiyi OE, Olayemi FO, Ning-Hua T, Guang-Zhi ZJB-SUJoB, Sciences A. In vitro antioxidant activity, total phenolic and flavonoid contents of ethanol extract of stem and leaf of Grewia carpinifolia. 2017; 6:10-4.
- Lu Y, Liu Y, Yang C. Evaluating In Vitro DNA Damage Using Comet Assay. J Vis Exp 2017. 10.3791/56450.
- Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hämäläinen R. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 2009; 458:766-70. doi: 10.1038/nature07863 [Crossref] [ Google Scholar]
- Staerk J, Dawlaty MM, Gao Q, Maetzel D, Hanna J, Sommer CA. Reprogramming of human peripheral blood cells to induced pluripotent stem cells. Cell Stem Cell 2010; 7:20-4. doi: 10.1016/j.stem.2010.06.002 [Crossref] [ Google Scholar]
- Liu Q, Du J, Fan J, Li W, Guo W, Feng H. Generation and Characterization of Induced Pluripotent Stem Cells from Mononuclear Cells in Schizophrenic Patients. Cell J 2019; 21:161-8. doi: 10.22074/cellj.2019.5871 [Crossref] [ Google Scholar]
- Hamada A, Akagi E, Yamasaki S, Nakatao H, Obayashi F, Ohtaka M. Induction of integration-free human-induced pluripotent stem cells under serum- and feeder-free conditions. In Vitro Cell Dev Biol Anim 2020; 56:85-95. doi: 10.1007/s11626-019-00412-w [Crossref] [ Google Scholar]
- Price PJ. Best practices for media selection for mammalian cells. In Vitro Cell Dev Biol Anim 2017; 53:673-81. doi: 10.1007/s11626-017-0186-6 [Crossref] [ Google Scholar]
- Nims RW, Harbell JW. Best practices for the use and evaluation of animal serum as a component of cell culture medium. In Vitro Cell Dev Biol Anim 2017; 53:682-90. doi: 10.1007/s11626-017-0184-8 [Crossref] [ Google Scholar]
- David L, Polo JM. Phases of reprogramming. Stem Cell Res 2014; 12:754-61. doi: 10.1016/j.scr.2014.03.007 [Crossref] [ Google Scholar]
- Soufi A, Donahue G, Zaret KS. Facilitators and impediments of the pluripotency reprogramming factors' initial engagement with the genome. Cell 2012; 151:994-1004. doi: 10.1016/j.cell.2012.09.045 [Crossref] [ Google Scholar]
- Sánchez C, Díaz-Nido J, Avila J. Phosphorylation of microtubule-associated protein 2 (MAP2) and its relevance for the regulation of the neuronal cytoskeleton function. Prog Neurobiol 2000; 61:133-68. doi: 10.1016/s0301-0082(99)00046-5 [Crossref] [ Google Scholar]
- Schoenfeld TA, Obar RA. Diverse distribution and function of fibrous microtubule-associated proteins in the nervous system. Int Rev Cytol 1994; 151:67-137. doi: 10.1016/s0074-7696(08)62631-5 [Crossref] [ Google Scholar]
- Garner CC, Matus A. Different forms of microtubule-associated protein 2 are encoded by separate mRNA transcripts. J Cell Biol 1988; 106:779-83. doi: 10.1083/jcb.106.3.779 [Crossref] [ Google Scholar]
- Charrière-Bertrand C, Garner C, Tardy M, Nunez J. Expression of various microtubule-associated protein 2 forms in the developing mouse brain and in cultured neurons and astrocytes. J Neurochem 1991; 56:385-91. doi: 10.1111/j.1471-4159.1991.tb08163.x [Crossref] [ Google Scholar]
- Thiry L, Clément JP, Haag R, Kennedy TE, Stifani S. Optimization of Long-Term Human iPSC-Derived Spinal Motor Neuron Culture Using a Dendritic Polyglycerol Amine-Based Substrate. ASN Neuro 2022; 14:17590914211073381. doi: 10.1177/17590914211073381 [Crossref] [ Google Scholar]
- Fukuyama Y, Kubo M, Harada K. The search for, and chemistry and mechanism of, neurotrophic natural products. J Nat Med 2020; 74:648-71. doi: 10.1007/s11418-020-01431-8 [Crossref] [ Google Scholar]
- Ermis IS, Deveci E. Investigation of the biochemical, histopathological, and immunohistochemical effects of Honokiol on the changes in the choroid plexus after traumatic brain injury in rats. Anal Quant Cytopathol Histpathol 2021. 43: 417-25.
- Zhou Y, Tang J, Lan J, Zhang Y, Wang H, Chen Q. Honokiol alleviated neurodegeneration by reducing oxidative stress and improving mitochondrial function in mutant SOD1 cellular and mouse models of amyotrophic lateral sclerosis. Acta Pharm Sin B 2023; 13:577-97. doi: 10.1016/j.apsb.2022.07.019 [Crossref] [ Google Scholar]
- Fukuyama Y, Nakade K, Minoshima Y, Yokoyama R, Zhai H, Mitsumoto Y. Neurotrophic activity of honokiol on the cultures of fetal rat cortical neurons. Bioorg Med Chem Lett 2002; 12:1163-6. doi: 10.1016/s0960-894x(02)00112-9 [Crossref] [ Google Scholar]